Abstract
Materials and Methods
A search was performed for the literature on cinnabar and realgar in PubMed, the Chinese Pharmacopeia, Google, and other sources. The search included studies using single herbs, traditional formulations, or novel dosage forms.
Results
Cinnabar and cinnabar formulas exhibit good efficacy for sedation, sleep improvement, anxiety alleviation, and brain protection. However, previous studies on neurotransmitters have reached different conclusions, and detailed pharmacological mechanisms are lacking. Realgar and its formulas exert promising antitumor activity through regulation of cell cycle arrest, intrinsic and extrinsic apoptosis, induction of differentiation, autophagy, metabolic reprogramming, matrix metalloproteinase-9 (MMP-9) signaling, and reactive oxygen species (ROS) generation. In addition, realgar can be used to treat a variety of refractory diseases by regulating immunity and exerting antibacterial, antiviral, and other effects. However, the existing pharmacological research on the use of realgar for epidemic prevention is insufficient, and animal experiments and research at the cellular level are lacking. Inappropriate applications of cinnabar and realgar can cause toxicity, including neurotoxicity, liver toxicity, kidney toxicity, and genotoxicity. The toxicological mechanism is complex, and molecular-level research is limited. For clinical applications, theory and clinical experience must be combined to guide scientific and rational drug use and to achieve reduced toxicity and increased efficacy through the use of modern preparation methods or combined drugs. Notably, when cinnabar and realgar are used to treat targeted diseases, these agents have a bidirectional effect of “treatment” and “toxicity” on the central nervous system in pathological and normal states. The pharmacological and toxicological mechanisms need to be elucidated in greater detail in the future. Overall, systematic research is needed to provide a basis for better promotion of the rational use of cinnabar and realgar in the clinic.
Conclusion
Mineral medicines are multicomponent, multiactivity, and multitargeted substances. The pharmacology and mechanisms of the toxicity and action of realgar and cinnabar are extremely complex. A number of Chinese medicinal preparations of realgar and cinnabar have demonstrated unique efficacy in the treatment of refractory diseases.
1. Introduction
Mineral medicines include raw minerals (cinnabar, calamine, natural copper, realgar, gypsum, etc.), processed products of raw mineral materials (calomelas, mirabilite, etc.), and animal bones or fossils of animal bones (bone or teeth fossils of large mammals) and are characteristic of traditional medicines used in China.
Throughout history, mineral medicines have played an important role in traditional Chinese medicine (TCM). TCM has a long application history with the accumulation of rich clinical experiences and is still widely used today. The earliest record of the roles of mineral medicines is documented in The Classic of Mountains and Seas, which states that taking mineral medicines can “make people healthy and live longer.” In addition, the modern 2020 edition of the Pharmacopoeia of the People's Republic of China (2020ChP) [1] affirms the wide range of clinical applications of mineral medicines in internal medicine, orthopedics, gynecology, and otorhinolaryngology. Cinnabar and realgar are two mineral medicines commonly used in the clinic with confirmed curative effects. However, their safety is of great public concern. Therefore, these medicines will be the focus of this minireview.
Cinnabar is described by the 2020ChP [1] as follows: “This product is the sulfide mineral cinnabar of the cinnabar family, which mainly contains Mercury sulfide (≥96%), which has the effects of clearing away the heart-fire and sedative, relieving uneasiness of body and mind, improving eyesight, and detoxifying.” Cinnabar is a critical sedative component of numerous Chinese prescriptions [2], such as Zhusha Anshen Pill (ASAS) and Baizi Yangxin Pill (BZYX). In addition, modern research has found that cinnabar has antianxiety, tranquillizing, antioxidative stress, and anti-brain-damage effects, and the mineral is used in compound preparations for the treatment of insomnia, anxiety disorders, brain trauma, stroke, and neuroinflammation [3].
Realgar is described by the 2020ChP [1] as a sulfide mineral of the realgar family that mainly contains arsenic disulfide (As₂S₂) and has the functions of “detoxifying and killing insects, drying dampness and removing phlegm, and treating malaria.” Modern research has further revealed that realgar can be combined with other drugs to treat a variety of blood system diseases [4], including acute promyelocytic leukemia (APL) [5], myelodysplastic syndrome (MDS) [6–9], and lymphoma. Realgar has also shown significant pharmacological effects against primary or metastatic cancers, such as breast cancer (cells) [4, 10, 11], cervical cancer [12], lung cancer [13], osteosarcoma [14], gastric cancer [15], oral cancer (cells) [16], and liver cancer [17], in nonclinical studies. In addition, realgar exhibits good immunoregulatory, antiviral, and antiepidemic effects, which may provide new ideas for the treatment of viral infectious diseases.
In recent years, the occurrence of phytotoxicity incidents caused by the improper use of mineral drugs has increased the concern regarding their toxicity. Cinnabar and realgar have become popular discussion topics because their main components (Mercury and arsenic) are known toxic metals/metalloids. Some have even questioned the necessity of heavy metal minerals, such as cinnabar and realgar, in medicinal formulas.
Several studies have found that different forms of Mercury can be absorbed through the gastrointestinal tract, respiratory tract, and skin and that excess levels of Mercury and its compounds have acute or chronic toxic effects on the human body [18–23]. Arsenic toxicity is associated with liver tumors, diabetes, and cardiovascular, neurological, and other diseases [24–26]. However, some in vivo experiments in mice have indicated that cinnabar does not significantly affect transporter genes in the liver and kidneys [27, 28]. This contradiction underscores the need for further research on the pharmacology and toxicology of cinnabar and realgar.
When discussing Mercury toxicity and arsenic toxicity, it is necessary to distinguish the chemical forms. In traditional Chinese medicine, Mercury and arsenic are used orally in sulfide forms [29]. The chemical forms of cinnabar and realgar are significant determinants of their activity and toxicity. Both cinnabar and realgar are far less toxic than the well-known Mercury [21, 30–32] and arsenic [33]. In addition, studies have demonstrated that cinnabar (α-HgS) differs from environmental Mercury (HgCl2, MeHg) based on its bioavailability, intestinal transport levels, distribution, metabolism, elimination, and toxicity [29, 32]. Similarly, realgar (As₂S₂, As4S4) differs from sodium arsenate (As5+) and sodium arsenite (As3+) in structure, disposition, efficacy, and toxicity [29].
Thousands of years of clinical practice have suggested the effectiveness of cinnabar and realgar. However, the exact pharmacodynamic and toxic mechanisms of these minerals and how to optimize their clinical applications remain unclear; an in-depth research with modern research techniques is needed. A literature search of the use of Chinese materia medica in the treatment of diseases reveals that cinnabar and realgar are seldom used alone. These agents are commonly used in multiherb/metal mixtures. Therefore, we recommend that analyzing the mechanisms of clinically effective formulations at the molecular, cellular, and organismal levels represents a potentially effective method for assessing the value of traditional medicines.
In this article, we briefly summarize the main pharmacological and toxicological characteristics and usage specifications of the mineral medicines cinnabar, realgar, and their commonly used preparations, aiming to provide a theoretical basis for the rational and safe clinical use of cinnabar and realgar.
2. Cinnabar
For thousands of years, cinnabar has been used to treat different diseases [2]. According to the 2020ChP [1], approximately 10%–30% of Chinese compound prescriptions contain cinnabar. Representative prescriptions include ASAS and Baizi Yangxin Pill. According to traditional Chinese medicine, cinnabar taken orally can clear away heart fire, exert sedative effects, relieve uneasiness in the body and mind, improve eyesight, and detoxify. Modern research has shown that cinnabar can improve sleep, combat anxiety and oxidative stress, and protect the brain from damage.
However, the use of cinnabar in clinical practice has been controversial because it contains the heavy metal Mercury. Epidemiological investigations and animal experiments have shown that excessive intake of cinnabar can exert toxic effects on the kidneys, liver, and nervous system. Therefore, the pharmacology and toxicology of cinnabar and traditional medicines containing cinnabar have attracted attention.
2.1. Pharmacological Research on Cinnabar Alone
2.1.1. Sedative and Anxiolytic Mechanisms
Cinnabar has long been used in combination with other Chinese materia medica as a sedative for more than 2000 years [2]. The sedative effect of cinnabar has been verified in animal experiments. For example, studies have shown that, in mice administered low-dose cinnabar (10 mg/kg/d) for 11 weeks, motor activity is decreased, and pentobarbital-induced sleeping time is prolonged, suggesting that cinnabar has sedative effects [34]. Wang et al. confirmed the anxiolytic effect of cinnabar on anxiety-like behaviors in mice using the elevated plus maze test; this neuropharmacological effect may have been similar to the sedative and soothing effects of cinnabar. The results suggest that cinnabar exerts anxiolytic effects when administered chronically at effective doses and is associated with reduced brain serotonin (5-HT) levels [35].
2.1.2. Antioxidative Stress and Brain Injury Protection Mechanisms
Oxidative stress plays a key role in neuronal death and underlies neurodegenerative diseases. Thus, antioxidants can play important roles in treating several neurodegenerative diseases [36]. Reactive oxygen species (ROS) are markers of oxidative stress that can indicate the state of redox balance in the body.
One study confirmed that cinnabar can reduce the disappearance of antioxidant enzymes and the overproduction of ROS through regulation of the 5-HT metabolism pathway under hypoxic conditions at the cellular level and in zebrafish in vivo, ultimately alleviating hypoxia-induced oxidative stress and significantly improving the survival of neuronal cells under high-ROS conditions [37]. This finding highlights one of the potential mechanisms by which cinnabar exerts neuroprotective effects.
In the endoplasmic reticulum (ER) stress response, protein kinase RNA-like ER kinase (PERK) induces the activation of C/EBP homologous protein (CHOP). Activated CHOP then induces apoptosis via the apoptosis receptor BCL2 family proteins and the death receptor caspase family proteins of the downstream mitochondria-dependent pathway [38]. Low cinnabar concentrations induce antioxidative and antiapoptotic effects on cells and inhibit intracellular stress responses. The possible mechanism involves blockade of ER stress-induced apoptosis via downregulation of PERK expression and indirect downregulation of CHOP activation [39]. In an emerging microbiome study, cinnabar reduced neuronal stress and inflammation through the gut–brain axis by altering the microbiome structure via reductions in Verrucomicrobiaceae and increases in Enterobacteriaceae, suggesting that HgS-containing traditional medicines can mechanistically target gut microbiota to exert their therapeutic effects [40]. The pharmacological mechanism of cinnabar is presented in Figure 1.
Figure 1.

Pharmacological mechanism of cinnabar.
2.1.3. Research on Cinnabar Formulas
Given its good curative effect, cinnabar has been widely used as a sedative ingredient in TCM prescriptions. However, the mechanisms of cinnabar and its various formulations have been the subject of few studies. Some pharmacological studies on the use of cinnabar and its formulations for the treatment of insomnia have been reported in the Chinese literature but are not available in PubMed. One study found that the formula ASAS, which includes cinnabar as the main therapeutic ingredient, exhibits good sedative and antianxiety effects. A study on ASAS in rats with conditioned fear showed that this pill can antagonize conditioned fear; the antagonistic effect is mainly reflected in the fading stage of fear memory and may be involved in regulating the content of monoamine neurotransmitters and the expression of c-Fos protein in the basolateral amygdala. This mechanism increases the efficiency of fear extinction and improves sleep in rats [41]. The mechanism is related to hippocampal neuron protection, hippocampal synaptic structure regulation, and functional plasticity [42]. In the sleep phase, moderate and high doses of a decoction of ASAS obviously decrease the duration of wakefulness and increase the total sleep time. A moderate dose of the decoction obviously increases the duration of slow-wavestage-1 sleep, whereas high doses of the decoction obviously increase the duration of slow-wavestage-2 sleep. Although low doses do not decrease the duration of wake, they can increase the duration of slow-wavestage-2 sleep [43]. The mechanism may be related to inhibition of the production of 5-HT and norepinephrine (NE) monoamine neurotransmitters in the ventrolateral preoptic area [44] that increase the content of γ-aminobutyric acid in the brain [45].
2.2. Toxicological Research on Cinnabar
Cinnabar is a natural medicinal substance containing metallic Mercury [46]. Regarding toxicology, cinnabar has known toxic effects on the kidneys, liver, and nervous system. Wei et al. [47] applied a nuclear magnetic resonance metabolomics method and found that cinnabar altered biochemical indexes with time-anddose-dependent effects. Proteomics has shown that therapeutic and toxic doses of cinnabar affect different pathways and potential targets in the mouse cerebral cortex [48]. In addition, a study found that the cerebellum is more sensitive to cinnabar treatment than the cerebrum [19]. Some studies have revealed that exposure to a high dose (1.0 g/kg/day for 7 or 14 consecutive days) of cinnabar or HgS causes ototoxicity [20] and neurotoxicity [21, 49], whereas 0.01 g/(kg day) causes no toxic effects [21, 49].
Cinnabar toxicity may occur because high dose or long-term use of Chinese compound preparations containing cinnabar leads to the accumulation of Mercury in the kidneys, resulting in kidney damage, such as kidney inflammation and mild fibrosis [22]. Cinnabar may also cause proximal tubular damage, which may be related to the activation of the renal tubular apoptosis pathway and the expression of organic anion transporters (OATs) and the expression of tubular basal transporters organic anion transporter OAT1 and OAT3 [23, 50]. One study found that the gut microbiota is a potential target for the dual effects of cinnabar and that oligopeptide transporter 1 may represent an important transporter of cinnabar into the intestinal epithelium [32]. Under the influence of human intestinal bacteria, cinnabar is transformed into nontoxic products (mercuric polysulfides) rather than methylmercury [51].
Although long-term use of cinnabar can cause a series of toxic side effects, scholars have found that cinnabar is indeed much less toxic than other mercury-containing compounds, such as HgCl2 [30, 32] and MeHg [21, 31].
This reduced toxicity may be related to the downregulation of oligopeptide transporter 1 mRNA and protein expression by cinnabar [32], which affects the intestinal absorption and transport of cinnabar; its dissolution, absorption, distribution, and accumulation in vivo; and the molecular configuration of Mercury compounds, particle size, and biological activity of the gastrointestinal tract [52–54].
The toxic effects of cinnabar have varied greatly in different experiments. In addition to disparities in the administered dose, different methods of cinnabar processing may also have contributed to the different toxicities. Ancient traditional Chinese medicine practitioners stressed that the washing (called Shui-Fei) method during cinnabar processing was extremely sensible, as it avoided high temperatures and reduced the levels of soluble toxic elements. However, different researchers have used different medicinal materials in their toxicological studies on cinnabar, and only a few studies have emphasized the use of washing methods, which may be another important reason explaining the inconsistently experimental results. Given that the detoxification methods of cinnabar and realgar are similar, the final statement is unified.
3. Realgar
Realgar contains >90% arsenic sulfide (As₂S₂) and is widely used externally and internally in many traditional medicine recipes in China. The 2020ChP [1] contains more than 30 types of formulas containing realgar with representative formulas including the Niuhuang Jiedu Pill, Niuhuang Qingxin Pill, and Angong Niuhuang Pill. The functions of realgar are mostly “detoxifying and killing insects, drying dampness and removing phlegm, and treating malaria” [1]. Realgar is used for various diseases, such as “carbuncles, swelling, sores, snake bites, ascariasis, epilepsy, and malaria” [1].
Recent studies have found that realgar exhibits significant anticancer activity. The possible antitumor mechanism may be closely related to the regulation of cell cycle arrest, intrinsic and extrinsic apoptosis, induction of differentiation, autophagy, metabolic reprogramming, matrix metalloproteinase-9 (MMP-9) signaling, and ROS generation [4, 10].
However, 90% of realgar is As₂S₂, which exhibits toxic effects characteristic of heavy metals; thus, the clinical application of realgar is controversial. Epidemiological investigations and animal experiments have shown that long-term arsenic exposure can lead to language, cognitive, behavioral, and hearing and motor impairments, as well as mental retardation and neurological deficits in severe cases. Even when arsenic exposure ceases, central nervous system function does not quickly return to normal [55]. Therefore, the pharmacology and toxicology of realgar and traditional medicines containing realgar have attracted extensive attention.
3.1. Pharmacological Research on Realgar
3.1.1. Pharmacological Research on Realgar Alone
(1) Antitumor Mechanism. Induction of tumor cell apoptosis is the main mechanism of action of realgar in the treatment of tumors, and these effects can be achieved through a variety of ways. Studies have demonstrated a link between oxidative stress and tumor cell apoptosis [56]. To improve the activity of realgar, some researchers have used intrinsic biotransformation pathways in microorganisms to obtain a realgar transformation solution (RTS). RTS activates p53-mediated cell cycle arrest and apoptotic pathways by inhibiting the cellular antioxidant defense system and massive accumulation of ROS in tumor cells and by inducing apoptosis and interrupting G2/M progression in HepG2 cells to inhibit the growth of tumor cells and promote apoptosis [17]. Similar mechanistic studies have shown that the expression of proapoptotic proteins, including caspase-7 and caspase-8, is activated under the action of As₂S₂ to trigger tumor cell apoptosis [10]. The results of another study suggest that As₂S₂ inhibits cell viability and induces apoptosis and cell cycle arrest in both MCF-7 and MDA-MB-231 cell lines by regulating the expression of key proteins involved in related pathways [4].
Cell cycle-regulated gene mutations play important roles in tumorigenesis. Cell cycle arrest provides cells with additional time to repair damage, thereby reducing the occurrence of mutations and tumor development. In a molecular study on the use of As₂S₂ in the treatment of APL, As₂S₂ was found to induce the accumulation of NB4-R1 cells in S and G(2)/M phases [57]. This effect may be related to the regulation of the expression of related proteins, including cyclin B1 and cell division cycle protein 2 [10].
Induction of differentiation is a common strategy in tumor therapy. Realgar induces multilineage differentiation in the human promyelocytic leukemia cell line HL-60, thereby exerting anticancer effects. Myeloid differentiation of HL-60 cells is induced via the p38 mitogen-activated protein kinase-related signaling pathway [58], and monocytic differentiation of HL-60 cells is induced via serine/threonine protein phosphatase-related pathways [59]. Realgar simultaneously induces apoptosis and differentiation in both the all-trans retinoic acid- (ATRA-) sensitive NB4 and ATRA-resistant MR2 cell lines [60]. The following specific mechanisms may be involved: (1) binding of realgar to the pML/RARα fusion protein, making it ineffective; (2) enhancement of the level of cellular ubiquitin by realgar; (3) promotion of pML/RARα fusion protein ubiquitination; (4) formation of a covalent enzyme system; and (5) entry into the protease system for degradation into short peptides and loss of activity [5].
Autophagy is an important cell biological process induced by multiple forms of cellular stress, including nutrient or growth factor deprivation, hypoxia, and ROS [61]. Excessive levels of autophagy damage body tissues and cells and disrupt homeostasis [62]. Realgar can induce autophagy in endometrial cancer JEC cells as one of its anticancer mechanisms [63]. In addition, realgar preparations can induce autophagy via upregulation of LC3 and p62/SQSTM1 [64] and inhibit the Akt/mTOR signaling pathway [14].
Under hypoxic conditions, hypoxia-inducible factor (HIF-1) promotes the metabolic reprogramming of tumor cells and induces acidification of the tumor extracellular environment, which helps tumor cells obtain energy and survive [65]. However, nanorealgar inhibits tumor growth in vitro and in vivo by repressing metabolic reprogramming. This inhibitory effect is potentially related to the downregulation of HIF-1α expression via the PI3K/Akt/mTOR pathway [13].
MMP-9 is one of the most complex matrix metalloproteinases. In tumor cells, MMP-9 contributes to the remodeling of extracellular matrix proteins, providing a microenvironment for tumorigenesis. MMP-9 degrades basal type IV collagen near tumor cells and then invades other normal tissues, thereby inducing cancer invasion and metastasis [66]. However, As₂S₂ treatment inhibits MMP-9 protein expression [10].
Studies have shown that As₂S₂ inhibits the proliferation of colon cancer cells by regulating the nuclear factor of activated T cells (NFAT) pathway [15, 67]. Its inhibitory effect is related to a signaling pathway related to vascular endothelial growth factor 2 [68].
These results suggest that realgar preparations have broad-spectrum scavenging effects in tumor cells. In cells and tissues, regulation is a complex process involving multiple signaling pathways, and the pathways in which realgar is involved require further study.
(2) Immunoregulatory and Antibacterial Mechanisms. Severe systemic lupus erythematosus (SSLE) refers to a manifestation of systemic lupus erythematosus (SLE) involving important organs. Patients with SSLE often experience multiple complications simultaneously, treatment difficulties, and worse prognoses [69]. Studies have shown that realgar can be used to treat severe forms of SLE, such as lupus nephritis, and that the mechanism may involve downregulation of the expression of phosphorylated signal transducer and activator of transcription 1 [70].
Realgar promotes apoptosis of B cells from MRL/lpr mice by increasing Ca2+ concentrations, reducing surface molecule activation, stimulating surface factor expression, and restraining its activation to reduce the production of antibodies, reduce MRL/lpr mouse autoimmune injury, and slow down the SLE course [71]. In addition, in Caenorhabditis elegans, realgar can alleviate the infection of wild-type N2, glp-4 mutant, and daf-2 mutant nematode strains by inducing both immune and protective responses and significantly increasing antibacterial effector levels, which leads to pathogen elimination [72, 73].
(3) Prevention and Treatment of Epidemics. Epidemic diseases are highly contagious and dangerous. Historically, realgar has played an important role in the prevention and treatment of epidemics in China in past dynasties. However, pharmacological research on realgar use for epidemic prevention and treatment is scarce. According to a recent literature search, realgar was the drug most frequently used by physicians for epidemic prevention in past dynasties [74]. Wearing colorful sachets containing realgar, drinking realgar wine, and sprinkling realgar on walls and doors during the Dragon Boat Festival represent traditional public uses. Realgar works primarily by killing pathogens (disinfecting) and expelling pathogens (detoxifying) [75].
External use through various methods (i.e., burning, externally coating, wearing, stuffing, sneezing, and tears) can block the transmission of the epidemic pathogen. Realgar can also be taken internally in the form of pills, medicinal liquors, etc. and can be combined with cinnabar for epidemic prevention. Ruan [76] and others believed that coronavirus disease 2019 (COVID-19) was caused by a “cold-damp pestilential pathogen” invading the human body according to climatic, seasonal, and regional characteristics. Based on syndrome differentiation, realgar and other pungent and warm (Xin Wen) products were chosen to relieve the cold-damp poison. Similarly, Changchun University of Traditional Chinese Medicine also provided guidance that the new strains of coronavirus, mainly Omicron, that broke out in China in 2022 were caused by a cold-damp pestilential pathogen and suggested that pungent and warm (Xin Wen) products should be used. Compared with the epidemic prevention and treatment methods of modern medicine, those of traditional Chinese medicine have broad-spectrum immune effects and strong universality, are forward-looking, and achieve the purpose of disease prevention. Research on the prevention and treatment of diseases with realgar may provide new ideas for the use of TCM for the prevention and treatment of modern epidemics. However, the antiepidemic mechanism of action of realgar requires further study.
(4) Antiviral Mechanism. In an experiment using acyclovir as a positive control, nanorealgar showed good anti-herpes simplex virus type II activity when administered in 3 modes (pretreatment, treatment, and direct inactivation) [77]. The antiviral mechanism may involve destruction of the enzyme system required for virus proliferation by the realgar nanoparticles, so that the virus cannot proliferate in living cells. Realgar nanoparticles may also directly kill the virus by directly destroying the virus envelope protein, thereby reducing virus viability [77]. The pharmacological mechanism of realgar is presented in Figure 2.
Figure 2.
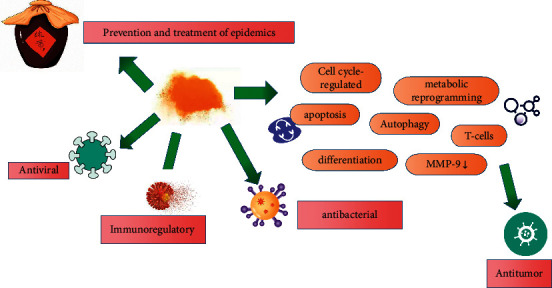
Pharmacological mechanism of realgar.
3.1.2. Research on Realgar Formulas
(1) Antitumor Mechanisms. Since the 1970s, researchers in the field of blood diseases in China have successively applied realgar-Indigo naturalis formula (RIF) as the active ingredient in the clinical treatment of APL, achieving definite curative effects. RIF, a typical representative of realgar anticancer preparations, has now been approved for marketing in China.
A molecular-level study indicated that realgar is the principal component of the formula, whereas tanshinone IIA and indirubin serve as adjuvant ingredients [78]. Clinical trials have confirmed that oral arsenic (RIF) provides an outcome similar to that of intravenous arsenic trioxide (ATO) [79, 80]. More detailed clinical trials have shown that RIF is also safe and effective in newly diagnosed pediatric APL patients [81]. Furthermore, compared with ATO plus all-trans retinoic acid (ATRA), simultaneous use of oral RIF plus ATRA greatly reduces the clinical expenses [82, 83] and hospital stays [84–87] of APL patients during induction and remission therapy. According to long-termfollow-up results, RIF provides excellent long-term survival advantages [88]. The possible mechanisms of action include [78] (1) synergistic effects on human APL cell differentiation in vitro, (2) enhanced ubiquitination and degradation of the PML-RARα oncoprotein, (3) relief of transcriptional suppression, and (4) G1/G0 arrest of APL cells with effects on key regulators of cell cycle progression. The main component realgar cooperates with other components in RIF to increase the absorption rate by inducing upregulation of the transmembrane protein aquaglyceroporin 9. Further studies have shown that RIF can significantly inhibit the growth of APL NB4 cells [89] and the proliferation and viability of the K562 cell line [90].
Clinical studies have shown that the Chinese medicine Qinghuang Powder (QHP) (daily dose of 0.1 g of realgar), the main component of which is realgar, is effective and safe for the treatment of patients with MDS and that reasonable adjustment of the daily dose of realgar can improve the efficacy without increasing the clinical toxicity [8, 9]. In vitro studies have shown that realgar, as the main drug contained in QHP, can induce cell differentiation in AML cells that progress from MDS, which might explain one of the mechanisms of QHP in the treatment of MDS [91].
Different DNA methylation subtypes may be present in MDS-metachromatic leukodystrophy (MLD) patients. A clinical study found that Chinese herbal medicine containing realgar has good effects in the treatment of MDS-MLD patients with the hypomethylation subtype [7]. The therapeutic mechanism may involve an increase in DNA methyltransferase expression in MDS [6]. After genotyping 43 MDS patients via ultradeep targeted sequencing, Zhao et al. [92] found that QHP was effective and safe, especially in those with genetic mutations in SF3B1, DNMT3A, U2AF1, and/or ASXL1. Another study revealed that As₂S₂ can reduce BCR-ABL protein levels in chronic myelogenous leukemia cells without affecting its transcription level. The mechanism of action involves binding of As₂S₂ to the RING finger domain of CBL (a RING-type E3 ligase) to inhibit its self-ubiquitination/degradation, thereby ubiquitinating the BCR-ABL protein [93].
These findings suggest that traditional Chinese medicine products containing realgar represent potential drugs with multiple activities. The active ingredients of realgar can be combined with some antitumor drugs to synergistically enhance the inhibition of tumor cells and reverse antitumor drug resistance [8], thus enhancing therapeutic efficacy [94].
(2) Antiviral and Anti-Inflammatory Mechanisms. Modern physicians have also applied realgar to various conditions. Professor Liangchun, a master of traditional Chinese medicine, used the traditional Chinese medicine formula Duo-Tan-Ding-Jing-San created with realgar to treat viral infectious diseases such as Japanese encephalitis, pneumonia, and meningitis and achieved good clinical results [95].
The realgar-containing Chinese medicine formula Liu Shen Wan (LSW) can significantly inhibit the influenza virus at different stages of viral replication in vitro. The antiviral effect is attributable to downregulation of the expression of inflammatory cytokines induced by the influenza virus via regulation of the activity of the TLR4/NF-кB signaling pathway [96]. Further research has shown that LSW significantly ameliorates lung injury caused by viral and secondary bacterial infections [97].
3.2. Toxicological Research on Realgar
Studies have shown that the disturbance of the intestinal flora and the severity of intestinal inflammation in realgar-treated mice are related to the realgar dose [98]. Therefore, in clinical applications, realgar should be used at a low dose in combination with other drugs to reduce intestinal inflammation [98].
One study performed untargeted lipidomics and other analyses on realgar-exposed mice to identify markers of the toxicity of realgar exposure to the nervous system [99]. The study found that the arsenic contained in realgar passed through the blood-brain barrier and accumulated in the brain, causing abnormal changes in the cerebral cortex [99]. In addition, increased oxidative damage and lipid dysfunction are also responsible for the neurotoxicity of realgar [99]. The specific mechanism involves excessive activation of Nrf2 regulated by the upstream signaling molecules ERK1/2 and p38MAPK [100].
Metabolomics studies have revealed that oral administration of realgar disrupts endogenous metabolites and related metabolic pathways in mice [101]. Follow-up kidney proteomic studies have revealed that apoptosis and oxidative stress might be related to realgar-induced nephrotoxicity in mice [102].
Cytochrome P450 enzymes are extremely important enzyme transport systems in the body. The main function of these enzymes is to metabolize exogenous substances (such as drugs) and endogenous substances. However, some studies have shown that higher doses of realgar can inhibit the enzymatic activity of cytochrome P450 enzymes, which may be related to the accumulation of arsenic from realgar [103]. Realgar exposure in mice activates innate immune-mediated inflammatory responses and disrupts the homeostasis of bile acids in the liver, plasma, and urine, causing immune-inflammatory infiltration in the liver [104]. A cross-sectional study of 1556 adults from the area surrounding a realgar plant showed that chronic arsenic exposure impairs cognitive function in adults and that this impairment is positively dose-related [105].
Although the toxicity of realgar nanoparticles is lower than that of realgar, studies have shown that realgar nanoparticles can induce free fatty acid and triglyceride accumulation, resulting in hepatotoxicity, and the metabolic markers of nanoparticle-treated subjects are different from those of traditional realgar-treated subjects [106].
Long-term or high-dose consumption of realgar can lead to the accumulation of heavy metals in the body, causing damage to the liver, blood, and nervous system. However, some clinical studies have found that higher concentrations of As₂S₂ in peripheral blood can enhance the clinical efficacy of realgar in the treatment of MDS-MLD patients [107]. Therefore, it is hypothesized that the pharmacological activity and toxicity of realgar may differ according to the activity of different cells. Realgar has a protective effect on nerve cells under pathological conditions but is harmful to normal nerve cells.
4. Research on Formulas Containing Both Cinnabar and Realgar
Compared with single mineral medicines, traditional Chinese medicine formulas containing multiple mineral medicines are more widely used in clinical practice. The mechanisms of action of mineral medicines or mineral-containing medicines in the treatment of diseases are complex but are related to the multicomponent, multitarget, and multichannel treatment characteristics of the medicines; these features also represent the characteristics and advantages of TCM for the treatment of diseases in general. Cinnabar and realgar are often used together in traditional Chinese medicine compounds.
4.1. Basic Medical Research
An-Gong-Niu-Huang Wan (AGNH) is a famous traditional Chinese medicine used to treat cerebral emergencies, such as traumatic brain injury, hemorrhage, brain infection, ischemia, and stroke, with a history of use of more than 3,000 years [1]. Modern research has suggested that AGNH has neuroprotective effects by reducing infarction volume, preserving blood-brain barrier integrity, and improving neurological functions against cerebral ischemia-reperfusion injury [108].
Based on skepticism about mineral medicines, some scholars have tried to remove cinnabar and realgar from AGNH. Interestingly, removing realgar and/or cinnabar from AGNH abolishes the neuroprotective effects [108]. In one study, histopathological analysis of mice that received AGNH orally showed that AGNH caused mild or no injuries to the liver and kidneys [109].
Wu Jutong, a warm-disease scientist of the Qing Dynasty, called Angong Niuhuang Pill the first of the “three classic antipyretic preparations” and believed that it had good heat-clearing, detoxifying, and sedating effects. Modern research suggests that cinnabar and realgar in the Angong Niuhuang Pill may play the main roles in “clearing heat and detoxifying” by helping inhibit the excessive release of inflammatory mediators [110]. This formula has significant effects against acute cerebrovascular disease, the Japanese epidemics of encephalitis and cerebrospinal meningitis, acute jaundice and primary hepatitis, renal failure, severe pneumonia, and infantile measles [110]. Tang et al.'s study on a fever rat model showed that realgar overinduced stress proteins (HSP70, HO-1) to improve positive stress levels in the body and inhibited overrelease of some inflammatory mediators (IL-1beta) to reduce inflammatory reactions under pathological conditions and inhibit the storm of inflammatory factors, thereby helping remove internal toxins and prevent damage to important organs [111].
Some scholars [112] have conducted similar research on Hua-Feng-Dan (HFD). Traditional HFD (containing 10% cinnabar and 10% realgar) improves behavioral dysfunction and attenuates microglial activation, rescuing the loss of dopamine (DA) neurons. However, a HFD lacking cinnabar and realgar is ineffective. This phenomenon reveals that cinnabar and realgar are active ingredients in HFD that exert useful effects in a neurodegenerative model and on the gut microbiota.
4.2. Clinical Applications
AGNH and HFD are still widely used clinically in their traditional and new forms. These agents can be used alone or in combination with other drugs for synergistic treatment.
Some meta-analyses of clinical studies on AGNH have revealed that AGNH has been widely used for acute cerebral infarction and intracerebral hemorrhage [113], traumatic brain damage [114], persistent vegetative state [115], and other severe cranial brain diseases. Moreover, studies have found that it can effectively promote postoperative recovery and exert respiratory care effects in chronic obstructive pulmonary disease patients after cardiac surgery [116]. However, there are relatively few clinical reports on the therapeutic effects of HFD, and all of the existing reports were published in China. HFD is often used in combination with other drugs in clinical applications and has good therapeutic effects on epilepsy [117], stroke [118], peripheral facial paralysis [119], and idiopathic facial paralysis [120].
With the introduction of modern biopharmaceutical technologies, innovations in the dosage forms of traditional Chinese medicine, such as capsules or liquid forms, have been reported. Based on these innovations, the pharmacological effects of the medicines are relatively clear, and these formulations have achieved outstanding curative effects that have been widely recognized in the medical field in recent years.
Traditional Chinese medicine injections are unique dosage forms in mainland China [121] that are administered by intramuscular injection or intravenous infusion. Qingkailing (QKL) injection is based upon the traditional Chinese medicine formulation AGNH, which is widely used in the treatment of many diseases given its good clinical efficacy. The main target diseases include acute cerebrovascular diseases and respiratory system infections, such as upper respiratory tract infection [122], pneumonia caused by respiratory syncytial virus [123], and acute stroke [124]. In the 2003 SARS incident, QKL was used as the basic drug for the treatment of atypical pneumonia with the integration of traditional Chinese and Western medicines and was combined with other drugs for comprehensive treatment [125]. During the coronavirus disease (COVID)-19 pandemic, Beijing also included QKL capsules in its clinical treatment plan [126].
In a previous study, QKL (or combined treatment) had a better effect on most outcome indicators compared with control, but the occurrence of adverse reactions cannot be ignored [127]. The current evidence, while being weak, indicates that QKL carries a low risk of adverse drug reactions and adverse events; however, some of the adverse events may be ascribed to improper use of the drug [128]. In the future, macromolecular substances should be removed as thoroughly as possible in production [121]. Serious care should be taken when QKL is administered to children, and QKL should not be combined with cephalosporin [121]. In addition, studies have shown that the delivery of Qingkailing by nebulization shows an equivalent or better curative effect with fewer side effects than injection of QKL in the treatment of pneumonia, respiratory tract infection, and tracheitis [129].
Xingnaojing injection (XNJ) is the only Chinese herbal injection approved for emergency treatment of stroke in China [130]. In the treatment of ischemic stroke, XNJ results in a significantly better overall response rate and better improvement of clinical symptoms than conventional treatment alone [130, 131]. In addition, XNJ can be used alone or as an adjuvant therapy to reduce brain damage and improve nerve health for the treatment of acute cerebral hemorrhage [132] and traumatic craniocerebral injury [133, 134]. During the COVID-19 epidemic, the National Health Commission of the People's Republic of China listed XNJ as a recommended traditional Chinese medicine injection in the “Guidelines for the Diagnosis and Management of COVID-19 (8th Edition)” [135]. For patients with severe symptoms, the Angong Niuhuang Pill is also recommended [135].
The above findings clearly show that the application of new technologies and new processes in development and production processes has enabled innovations and improvements of TCM preparations. This new technology has not only driven the development of traditional Chinese medicine, but also (and more importantly) promoted the internationalization of traditional Chinese medicine. In the Chinese Pharmacopeia (2020) [1], 24 recipes contain both cinnabar and realgar, such as Jufang Zhibao Dan for the treatment of febrile diseases and Qingyu Piwen Dan for heat stroke. Elucidation of the pharmacology, safety, and clinical application of AGNH and HFD and their novel formulations is critical to help assess the benefits and risks of these metal-containing traditional medicines.
5. Safe Application of Cinnabar and Realgar
Chinese materia medica containing cinnabar and realgar are generally relatively nontoxic at therapeutic doses under the pharmacopeia guidelines [1]. Important factors affecting the toxicity of cinnabar and realgar include the processing method, dose, compatibility, and attenuation.
5.1. Processing Method
In TCM, cinnabar and realgar are subjected to grinding and washing (called Shui-Fei) at least 3-4 times, which is crucial for the safe use of cinnabar and realgar in medicine.
Ancient physicians emphasized several important purposes of concocting mineral medicines using the grinding and washing method: to remove impurities, to reduce soluble elements as much as possible, to control the temperature to avoid the formation of new toxic and harmful substances, and to make the drug powder extremely fine and improve its bioavailability. Modern research illustrates that, as a TCM processing method, water grinding reduces toxicity [136, 137].
5.2. Appropriate Doses
The 1995 edition of the Chinese Pharmacopoeia reduced the daily dose of cinnabar from 0.3 to 1.5 g to 0.1∼0.5 g, and this standard is still used today [1]. In the 2000–2020 editions of the Pharmacopoeia of the People's Republic of China [1], the dose of realgar was 0.05∼0.1 g. Studies have shown that the toxicity of cinnabar [19] and realgar [138] is concentration- and time-dependent. Therefore, mineral medicines should not be used in large quantities, nor should they be used in small amounts for a long time. It is also necessary to pay attention to the administration period and special populations. Use should be limited to special populations, such as children, pregnant women, and those with liver and kidney insufficiency.
5.3. Toxicity-Attenuating Compatibility
In TCM, minerals are not applied alone but mixed with herbs and/or animal products. Chemical reactions may occur due to the mixing of agents, and it is assumed that the various components promote each other to achieve the desired therapeutic effect and reduce toxicity [29]. Studies have confirmed that the toxicity of cinnabar is low and that the herbal medicines in cinnabar formulas can attenuate the damage caused by cinnabar to body systems [29, 139, 140], which may be related to the absorption, distribution, and excretion of Mercury [28, 138]. Similarly, when realgar is used in a formula, the other herbal medicines in the formula can significantly reduce realgar toxicity by reducing blood arsenic levels [29, 141].
Metabolomics studies have confirmed that the other herbs present in AGNH alleviate inflammatory cell infiltration and damage in the liver and kidneys as well as the serum metabolic profile disruptions induced by cinnabar and realgar insults [139, 142]. The mechanism may involve protective effects of AGNH's herbal constituents against the accumulation of Hg and As and the hepatorenal toxicity induced by cinnabar and realgar via downregulation of the expression of uptake transporters and upregulation of the expression of efflux transporters in hepatorenal tissues [141]. Similar studies on LSW [143] and Niuhuang Jiedu tablets [144] have also demonstrated that other herbal ingredients in the formulas can reduce the damage caused by realgar to some extent.
6. Summary and Outlook
The above review demonstrates that, despite great progress in research on the pharmacological and toxicological mechanisms of cinnabar and realgar, limited pharmacological studies have assessed cinnabar's sedative and soothing effects and realgar's anti-inflammatory, antioxidant, and epidemic-preventing effects. Notably, the compositions of metal mineral medicines are complex, as these medicines contain a variety of compounds. However, research on the physiological effects of the other components is relatively limited. In addition, because the body itself contains various trace elements, it is difficult for the current analytical technologies to achieve accurate detection in vivo, which is the key hindrance to in-depth research on the absorption and metabolism of metal-based mineral drugs. Researching pharmacology, toxicology, and mechanisms of action is an important method to study medicinal properties. However, most of the research conducted to date has focused on the overall efficacy and toxicity of cinnabar, realgar, or their formulations. Many researchers are observing and testing some macro-indicators, but research to clarify the mechanisms of action at the molecular-level remains lacking. There is no sufficient scientific basis for rational clinical drug use, and there is an urgent need to conduct more in-depth research on the molecular mechanisms of the pharmacological and toxic effects of cinnabar and realgar.
To date, research on the toxicity of mineral drugs has mostly adopted modern toxicology methods to analyze the toxicity to normal organisms. However, due to neglect of the toxicity-reducing compatibility theory of traditional Chinese medicine, the toxicity of mineral medicines has been exaggerated.
To improve efficacy and reduce adverse effects, TCM often combines mineral medicines with herbs, animal products, and/or other minerals in products called formulas. Increasing evidence demonstrates that treatment regimens for various illnesses contain multiple drugs with distinct but related mechanisms. These drugs can often amplify the therapeutic efficacy of the other drugs, enabling multitargeted synergistic therapy and leading to maximal therapeutic efficacy with minimal adverse effects. However, the essential compounds have not been identified in most formulas, and the precise mechanisms of the formulas remain to be addressed using molecular approaches. When the specific mechanisms of action and toxicity mechanisms of two-drug formulas are unclear, the use of each single drug may not completely explain the pharmacodynamic and toxicity mechanisms of the two drugs together. Each drug may need to be combined with other compatible drugs to completely explain the possible mechanisms of action, and pharmacodynamic research may need to be conducted to achieve attenuation and antiviral effects.
For clinical applications, it is necessary to combine theory and clinical experience to guide scientific and rational drug use and achieve the purposes of reducing toxicity and increasing efficacy through the use of modern preparation methods or drug combinations. Processing has a significant effect on the content of toxic impurities in mineral medicines. The processing parameters of the washing method should be further standardized, and the amount of water added and the numbers of repeated grinding operations should be clarified to obtain decoctions of uniform quality. The development of nanopreparations, microbial leaching preparations, new crystal preparations, and compound preparations represents the current focus of research on mineral pharmaceutical preparations. How to effectively improve drug solubility, bioavailability, and targeting, adjust sustained release and controlled release, and reduce the occurrence of adverse reactions is the key issue to be considered with these new processing methods.
The next steps are to explore the effective traditional formulas through the combination of minerals and other drugs, to elucidate the mechanisms of these formulas, to upgrade the formulas into modern dosage forms, and to describe the traditional theories of traditional Chinese medicine, such as synergistic treatments and toxin counteraction with toxin therapy, in the modern language of molecular biology. The complementary combination of systems biology and theoretical research on TCM will greatly promote the pace of traditional Chinese medicine internationalization.
Acknowledgments
This study was supported by Jilin Province Health Science and Technology Capacity Improvement Project (No. 2021JC070) and the Disciplinary Layout Project of Natural Science Foundation of Jilin Province (No. 20200201412JC) and the National Natural Science Foundation of China (No. 82205190) and the Natural Science Foundation of Henan Province (No.202300410251) and the Henan Province Traditional Chinese Medicine Scientific Research Special Project (No.2019JDZX2033) and the Jilin Province Science and Technology Development Plan Project (No.20190201124JC).
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
The authors read and approved the final manuscript.
Supplementary Materials
The authors review the accumulating evidence and discuss the pharmacology, toxicology, formulas, and safe use of cinnabar (HgS) and realgar (As₂S₂).
References
- 1. Pharmacopedia of China . Beijing, China: People’s Press; 2020. [Google Scholar]
- 2.Zhou X., Wang Q., Yang X. Progresses on mechanisms of pharmacological and toxicological effects of cinnabar. Zhongguo Zhongyao Zazhi . 2009;34(22):2843–2847. [PubMed] [Google Scholar]
- 3.Chen C. J., Wu S. K., Wang Y. B., Hou J. F., Ma L., Sun X. Y. Recent researches of synthetic mercury sulfide in traditional medicine system. Zhongguo Zhongyao Zazhi . 2012;37(19):2968–2970. [PubMed] [Google Scholar]
- 4.Zhao Y., Yuan B., Onda K., et al. Anticancer efficacies of arsenic disulfide through apoptosis induction, cell cycle arrest, and pro-survival signal inhibition in human breast cancer cells. American Journal of Cancer Research . 2018;8(3):366–386. [PMC free article] [PubMed] [Google Scholar]
- 5.Hai Y., Wang X., Song P., et al. Realgar transforming solution-induced differentiation of NB4 cell by the degradation of PML/RARα partially through the ubiquitin-proteasome pathway. Archives of Pharmacal Research . 2019;42(8):684–694. doi: 10.1007/s12272-019-01170-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q. B., Liu Z. T., Wang H. Z., Guo X. Q., Xu Y. G., Hu X. M. Arsenic disulfide promoted hypomethylation by increasing DNA methyltransferases expression in myelodysplastic syndrome. Drug Design, Development and Therapy . 2020;14:1641–1650. doi: 10.2147/dddt.s239158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q. B., Du Y., Zhang S. S., Liu Z. T., Ma R., Xu Y. G. Clinical response to traditional Chinese herbs containing realgar (As(2)S(2)) is related to DNA methylation patterns in bone marrow DNA from patients with myelodysplastic syndrome with multilineage dysplasia. Cancer Management and Research . 2021;13:55–63. doi: 10.2147/cmar.s280886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng Z. Y., Zhu S. R., Wang M. J., et al. Relation of blood arsenic concentration with effect and safety of arsenic-containing Qinghuang powder in patients with myelodysplastic syndrome. Chinese Journal of Integrative Medicine . 2019;25(7):497–501. doi: 10.1007/s11655-019-3070-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q., Deng Z., Zhu S., Zhao P., Wang M., Hu X. Study on the clinical safe and effective methods of arsenic-containingcompound-qinghuang powder in the treatment of myelodysplastic syndrome. Evidence-Based Complementary and Alternative Medicine . 2017;2017:6. doi: 10.1155/2017/2095682.2095682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y., Onda K., Sugiyama K., et al. Antitumor effects of arsenic disulfide on the viability, migratory ability, apoptosis and autophagy of breast cancer cells. Oncology Reports . 2019;41(1):27–42. doi: 10.3892/or.2018.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiaoxia X., Jing S., Dongbin X., et al. Realgar nanoparticles inhibit migration, invasion and metastasis in a mouse model of breast cancer by suppressing matrix metalloproteinases and angiogenesis. Current Drug Delivery . 2020;17(2):148–158. doi: 10.2174/1567201817666200115105633. [DOI] [PubMed] [Google Scholar]
- 12.Ding W., Ji T., Xiong W., Li T., Pu D., Liu R. Realgar, a traditional Chinese medicine, induces apoptosis of HPV16-positive cervical cells through a HPV16 E7-related pathway. Drug Design, Development and Therapy . 2018;12:3459–3469. doi: 10.2147/dddt.s172525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F. R., Zhao Y. F., Hu X. W., et al. Nano-realgar suppresses lung cancer stem cell growth by repressing metabolic reprogramming. Gene . 2021;788 doi: 10.1016/j.gene.2021.145666.145666 [DOI] [PubMed] [Google Scholar]
- 14.Wang G., Zhang T., Sun W., et al. Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma. Free Radical Biology and Medicine . 2017;106:24–37. doi: 10.1016/j.freeradbiomed.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Kang T., Zhang L., Tong Y., Ding W., Chen S. NFATc3 mediates the sensitivity of gastric cancer cells to arsenic sulfide. Oncotarget . 2017;8(32) doi: 10.18632/oncotarget.17175.52735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bujňáková Z., Kello M., Kováč J., et al. Preparation of As(4)S(4)/Fe(3)O(4) nanosuspensions and in-vitro verification of their anticancer activity. Materials Science and Engineering: C . 2020;110 doi: 10.1016/j.msec.2020.110683.110683 [DOI] [PubMed] [Google Scholar]
- 17.Song P., Chen P., Wang D., et al. Realgar transforming solution displays anticancer potential against human hepatocellular carcinoma HepG2 cells by inducing ROS. International Journal of Oncology . 2017;50(2):660–670. doi: 10.3892/ijo.2016.3831. [DOI] [PubMed] [Google Scholar]
- 18.Biswas S., Bellare J. Ayurvedic processing of α-HgS gives novel physicochemistry and distinct toxicokinetics in zebrafish. Chemosphere . 2020;251 doi: 10.1016/j.chemosphere.2020.126295.126295 [DOI] [PubMed] [Google Scholar]
- 19.Wei L., Xue R., Zhang P., Wu Y., Li X., Pei F. (1)H NMR-based metabolomics and neurotoxicity study of cerebrum and cerebellum in rats treated with cinnabar, a traditional Chinese medicine. OMICS: A Journal of Integrative Biology . 2015;19(8):490–498. doi: 10.1089/omi.2015.0042. [DOI] [PubMed] [Google Scholar]
- 20.Chuu J. J., Hsu C. J., Lin-Shiau S. Y. Abnormal auditory brainstem responses for mice treated with mercurial compounds: involvement of excessive nitric oxide. Toxicology . 2001;162(1):11–22. doi: 10.1016/s0300-483x(01)00348-1. [DOI] [PubMed] [Google Scholar]
- 21.Chuu J. J., Liu S. H., Lin-Shiau S. Y. Differential neurotoxic effects of methylmercury and mercuric sulfide in rats. Toxicology Letters . 2007;169(2):109–120. doi: 10.1016/j.toxlet.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Wang D., Wu J., et al. Cinnabar induces renal inflammation and fibrogenesis in rats. BioMed Research International . 2015;2015:10. doi: 10.1155/2015/280958.280958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu W. H., Zhang N., Qi J. F., Sun C., Wang Y. H., Lin M. Arsenic and mercury containing traditional Chinese medicine (realgar and cinnabar) strongly inhibit organic anion transporters, Oat1 and Oat3, in vivo in mice. BioMed Research International . 2015;2015:7. doi: 10.1155/2015/863971.863971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H., Shi X., Liu K. J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Molecular and Cellular Biochemistry . 2004;255(1-2):67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Waalkes M. P. Liver is a target of arsenic carcinogenesis. Toxicological Sciences . 2008;105(1):24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyler C. R., Allan A. M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Current Environmental Health Reports . 2014;1(2):132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu S. F., Wu Q., Zhang B. B., et al. Comparison of mercury sulfides with mercury chloride and methylmercury on hepatic P450, phase-2 and transporter gene expression in mice. Journal of Trace Elements in Medicine & Biology . 2016;37:37–43. doi: 10.1016/j.jtemb.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Sui Y., Yang H., Tian X. Z., Liu J., Shi J. Z. Effect of Zhusha Anshen pill, cinnabar, HgS, HgCl2 and MeHg on gene expression of renal transporters in mice. Zhongguo Zhongyao Zazhi . 2015;40(3):506–510. [PubMed] [Google Scholar]
- 29.Liu J., Wei L. X., Wang Q., et al. A review of cinnabar (HgS) and/or realgar (As(4)S(4))-containing traditional medicines. Journal of Ethnopharmacology . 2018;210:340–350. doi: 10.1016/j.jep.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Zhou S., Ma H., Shi J. S., Lu Y. F. Investigation of the differential transport mechanism of cinnabar and mercury containing compounds. Environmental Toxicology and Pharmacology . 2019;66:83–90. doi: 10.1016/j.etap.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Liu J., Shi J. Z., Yu L. M., Goyer R. A., Waalkes M. P. Mercury in traditional medicines: is cinnabar toxicologically similar to common mercurials. Experimental Biology and Medicine . 2008;233(7):810–817. doi: 10.3181/0712-mr-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q., He X., Zhou S., Shi F., Lu Y. Role of PEPT1in the transport of cinnabar in Caco-2 cells. Toxicology in Vitro . 2020;63 doi: 10.1016/j.tiv.2019.104747.104747 [DOI] [PubMed] [Google Scholar]
- 33.Lu Y. F., Wu Q., Yan J. W., Shi J. Z., Liu J., Shi J. S. Realgar, cinnabar and An-Gong-Niu-Huang Wan are much less chronically nephrotoxic than common arsenicals and mercurials. Experimental Biology and Medicine . 2011;236(2):233–239. doi: 10.1258/ebm.2010.010247. [DOI] [PubMed] [Google Scholar]
- 34.Huang C. F., Liu S. H., Lin-Shiau S. Y. Neurotoxicological effects of cinnabar (a Chinese mineral medicine, HgS) in mice. Toxicology and Applied Pharmacology . 2007;224(2):192–201. doi: 10.1016/j.taap.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q., Yang X., Zhang B., Yang X., Wang K. The anxiolytic effect of cinnabar involves changes of serotonin levels. European Journal of Pharmacology . 2007;565(1-3):132–137. doi: 10.1016/j.ejphar.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Pandey A. K., Patnaik R., Muresanu D. F., Sharma A., Sharma H. S. Quercetin in hypoxia-induced oxidative stress: novel target for neuroprotection. International Review of Neurobiology . 2012;102:107–146. doi: 10.1016/B978-0-12-386986-9.00005-3. [DOI] [PubMed] [Google Scholar]
- 37.He Q., Ma J., Kalavagunta P. K., et al. HgS inhibits oxidative stress caused by hypoxia through regulation of 5-HT metabolism pathway. International Journal of Molecular Sciences . 2019;20(6):p. 1364. doi: 10.3390/ijms20061364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu H., Tian M., Ding C., Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Frontiers in Immunology . 2018;9:p. 3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma H. H., Ding Y. N., Wang A., et al. Cinnabar protects serum-nutrient starvation induced apoptosis by improving intracellular oxidative stress and inhibiting the expression of CHOP and PERK. Biochemistry and Biophysics Reports . 2021;27 doi: 10.1016/j.bbrep.2021.101055.101055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu A. L., Song S., Li Y., et al. Mercury sulfide-containing Hua-Feng-Dan and 70 W (Rannasangpei) protect against LPS plus MPTP-induced neurotoxicity and disturbance of gut microbiota in mice. Journal of Ethnopharmacology . 2020;254 doi: 10.1016/j.jep.2020.112674.112674 [DOI] [PubMed] [Google Scholar]
- 41.Guo Wencheng L. B., Zhang L. W. Y., Huang Lili L. I. T. Effect of ZhushaAnshen pill on expression of monoamine neurotransmitters and c⁃Fos protein in neurons of rats with conditioned fear. Journal of Guangdong Pharmaceutical University . 2021;37(01):84–90. [Google Scholar]
- 42.Yue L., Tingli C. J. S. Effect of Zhusha Anshen pill on synaptic plasticity of hippocampus in rats with conditional fear. Shanghai Journal of Traditional Chinese Medicine . 2020;54(02):97–101+107. [Google Scholar]
- 43.Yang J., Guangwei W., Tingli L. Influence of decoction of “Zhusha anshen pill” on sleep phase in insomia rats. Shanghai Journal of Traditional Chinese Medicine . 2008;42(12):74–76. [Google Scholar]
- 44.Tingli L. B. The effect of Zhusha Anshen Pill on monoamine neurotransmitters in rat VLPO brain region. Chinese Journal of Drug Dependence . 2018;27(06):425–430. [Google Scholar]
- 45.Tie Y., Hanyu C., Fengli C. Effect of aqueous decoction of Zhusha Anshin Pill on sleep phase and γ-aminobutyric acid content in the brain of mice. Guangdong Medical Journal . 2016;37(03):351–353. [Google Scholar]
- 46.Ding T., Luo J. Y., Han X., Yang S. H., Lin R. C., Yang M. H. Advances of toxicity evaluation of cinnabar and compatibility necessity analysis. Zhongguo Zhongyao Zazhi . 2016;41(24):4533–4540. doi: 10.4268/cjcmm20162409. [DOI] [PubMed] [Google Scholar]
- 47.Wei L., Liao P., Wu H., et al. Toxicological effects of cinnabar in rats by NMR-based metabolic profiling of urine and serum. Toxicology and Applied Pharmacology . 2008;227(3):417–429. doi: 10.1016/j.taap.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Yang M., Wang L., Zhang T., et al. Different proteomic profiles of cinnabar upon therapeutic and toxic exposure reveal distinctive biological manifestations. Journal of Ethnopharmacology . 2020;253 doi: 10.1016/j.jep.2020.112668.112668 [DOI] [PubMed] [Google Scholar]
- 49.Chuu J. J., Young Y. H., Liu S. H., Lin-Shiau S. Y. Neurotoxicity of mercury sulfide in the vestibular ocular reflex system of Guinea pigs. Naunyn-Schmiedeberg’s Archives of Pharmacology . 2001;364(3):249–258. doi: 10.1007/s002100000279. [DOI] [PubMed] [Google Scholar]
- 50.Shen Q. Q., Wang J. J., Roy D., et al. Organic anion transporter 1 and 3 contribute to traditional Chinese medicine-induced nephrotoxicity. Chinese Journal of Natural Medicines . 2020;18(3):196–205. doi: 10.1016/s1875-5364(20)30021-2. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X., Wang L., Sun X., et al. Cinnabar is not converted into methylmercury by human intestinal bacteria. Journal of Ethnopharmacology . 2011;135(1):110–115. doi: 10.1016/j.jep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 52.Dong W., Liu J., Wei L., Jingfeng Y., Chernick M., Hinton D. E. Developmental toxicity from exposure to various forms of mercury compounds in medaka fish (Oryzias latipes) embryos. PeerJ . 2016;4 doi: 10.7717/peerj.2282.e2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Y., Yang D., Song X., Wang S., Song M., Hang T. Bioaccessibility and health risk assessment of mercury in cinnabar containing traditional Chinese medicines. Journal of Trace Elements in Medicine & Biology . 2017;44:17–25. doi: 10.1016/j.jtemb.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Z. Y., Li C., Zhang M., et al. Dissolution, absorption and bioaccumulation in gastrointestinal tract of mercury in HgS-containing traditional medicines Cinnabar and Zuotai. Zhongguo Zhongyao Zazhi . 2015;40(12):2455–2460. [PubMed] [Google Scholar]
- 55.Manju R., Hegde A. M., Parlees P., Keshan A. Environmental arsenic contamination and its effect on intelligence quotient of school children in a historic gold mining area hutti, north Karnataka, India: a pilot study. Journal of Neurosciences in Rural Practice . 2017;8(3):364–367. doi: 10.4103/jnrp.jnrp_501_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo C. L., Wang L. J., Zhao Y., et al. A novel bromophenol derivative BOS-102 induces cell cycle arrest and apoptosis in human A549 lung cancer cells via ROS-mediated PI3K/Akt and the MAPK signaling pathway. Marine Drugs . 2018;16(2):p. 43. doi: 10.3390/md16020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., He P. C., Qi J., Liu Y. F., Zhang M. Tetra-arsenictetra-sulfide induces cell cycle arrest and apoptosis in retinoic acid-resistant acute promyelocytic leukemia cells. Biomedical Reports . 2015;3(4):583–587. doi: 10.3892/br.2015.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang N., Wang L. W., Gou B. D., Zhang T. L., Wang K. Realgar-induced differentiation is associated with MAPK pathways in HL-60 cells. Cell Biology International . 2008;32(12):1497–1505. doi: 10.1016/j.cellbi.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Luo L. Y., Huang J., Gou B. D., Zhang T. L., Wang K. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by arsenic sulphide: involvement of serine/threonine protein phosphatases. Leukemia Research . 2006;30(11):1399–1405. doi: 10.1016/j.leukres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Chen S., Fang Y., Ma L., Liu S., Li X. Realgar-induced apoptosis and differentiation in all-trans retinoic acid (ATRA)-sensitive NB4 and ATRA-resistant MR2 cells. International Journal of Oncology . 2012;40(4):1089–1096. doi: 10.3892/ijo.2011.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Molecular Cell . 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murrow L., Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annual Review of Pathology: Mechanisms of Disease . 2013;8(1):105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Z., Xu K., Xu Y., et al. Involvement of autophagy in realgar quantum dots (RQDs) inhibition of human endometrial cancer JEC cells. PeerJ . 2020;8 doi: 10.7717/peerj.9754.e9754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X., Chen B., Zhao L., et al. Autophagy enhanced antitumor effect in K562 and K562/ADM cells using realgar transforming solution. Biomedicine & Pharmacotherapy . 2018;98:252–264. doi: 10.1016/j.biopha.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Sebastian C. Tracking down the origin of cancer: metabolic reprogramming as a driver of stemness and tumorigenesis. Critical Reviews in Oncogenesis . 2014;19(5):363–382. doi: 10.1615/critrevoncog.2014011844. [DOI] [PubMed] [Google Scholar]
- 66.Mondal S., Adhikari N., Banerjee S., Amin S. A., Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. European Journal of Medicinal Chemistry . 2020;194 doi: 10.1016/j.ejmech.2020.112260.112260 [DOI] [PubMed] [Google Scholar]
- 67.Ding W., Tong Y., Zhang X., Pan M., Chen S. Study of arsenic sulfide in solid tumor cells reveals regulation of nuclear factors of activated T-cells by PML and p53. Scientific Reports . 2016;6(1) doi: 10.1038/srep19793.19793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song P., Hai Y., Wang X., et al. Realgar transforming solution suppresses angiogenesis and tumor growth by inhibiting VEGF receptor 2 signaling in vein endothelial cells. Archives of Pharmacal Research . 2018;41(4):467–480. doi: 10.1007/s12272-018-1014-6. [DOI] [PubMed] [Google Scholar]
- 69.Signorini V., Elefante E., Zucchi D., Trentin F., Bortoluzzi A., Tani C. One year in review 2020: systemic lupus erythematosus. Clinical & Experimental Rheumatology . 2020;38(4):592–601. [PubMed] [Google Scholar]
- 70.Xu W., Chen Z., Shen X., Pi C. Reno-protective effect of realgar nanoparticles on lupus nephritis of MRL/lpr mice through STAT1. Iranian Journal of Immunology . 2019;16(2):170–181. doi: 10.22034/IJI.2019.80260. [DOI] [PubMed] [Google Scholar]
- 71.Wei-Dong X. Y. L., Zheng-Feng L. X. L., Xiao-Dong S. J. Y. Realgar nano-particlesdown-regulated the expression of CD40 in B cells from MRL/lpr mice and promote B cell apoptosis through rising their Ca2+ concentration. Chinese journal of immunology . 2013;29(12):1249–1253. [Google Scholar]
- 72.Ma W., Yue J., Liang S., et al. Realgar increases defenses against infection by Enterococcus faecalis in Caenorhabditis elegans. Journal of Ethnopharmacology . 2021;268 doi: 10.1016/j.jep.2020.113559.113559 [DOI] [PubMed] [Google Scholar]
- 73.Liu D., Zhi D., Zhou T., et al. Realgar bioleaching solution is a less toxic arsenic agent in suppressing the Ras/MAPK pathway in Caenorhabditis elegans. Environmental Toxicology and Pharmacology . 2013;35(2):292–299. doi: 10.1016/j.etap.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Meng-Na J. R., Liu Zheng F. Q., Zhang M.-J. L. Y., Yan-hong Q. Data mining of preventing pestilence by aromatic therapy in dictionary of traditional Chinese medicine prescriptions. Guiding Journal of Traditional Chinese Medicine and Pharmacy . 2021;27(11):217–221. [Google Scholar]
- 75.Wu X. Q., Zhang W. N., Hao M. Z., et al. How Chinese herbal medicine prevents epidemics: from ancient pestilences to COVID-19 pandemic. The American Journal of Chinese Medicine . 2021;49(5):1017–1044. doi: 10.1142/s0192415x2150049x. [DOI] [PubMed] [Google Scholar]
- 76.Xiaomin R. Y. F. D. X. Y. Z. Discussion on thoughts for treatment of coronavirus disease 2019 based on cold-damp epidemics. Journal of Guangzhou University of Traditional Chinese Medicine . 2020;37(6):1003–1007. [Google Scholar]
- 77.Wang D., Wang L., Xu R., Wu X., Li Y. Antiviral activity of nano-realgar against herpes simplex virus type II in vitro. Zhong Nan Da Xue Xue Bao Yi Xue Ban . 2019;44(10):1143–1150. doi: 10.11817/j.issn.1672-7347.2019.180552. [DOI] [PubMed] [Google Scholar]
- 78.Wang L., Zhou G. B., Liu P., et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proceedings of the National Academy of Sciences of the USA . 2008;105(12):4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu H. H., Wu D. P., Du X., et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. The Lancet Oncology . 2018;19(7):871–879. doi: 10.1016/s1470-2045(18)30295-x. [DOI] [PubMed] [Google Scholar]
- 80.Zhu H. H., Huang X. J. Oral arsenic and retinoic acid for non-high-risk acute promyelocytic leukemia. New England Journal of Medicine . 2014;371(23):2239–2241. doi: 10.1056/nejmc1412035. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L., Yang X. M., Chen J., et al. Population pharmacokinetics and safety of oral tetra-arsenictetra-sulfide formula in pediatric acute promyelocytic leukemia. Drug Design, Development and Therapy . 2021;15:1633–1640. doi: 10.2147/dddt.s305244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu H. H., Hu J., Gu X. F. Arsenic as traditional Chinese medicine provides new hope for overcoming high treatment costs of acute promyelocytic leukemia. Journal of Global Oncology . 2016;2(6):442–443. doi: 10.1200/jgo.2016.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang H., Liang G. W., Huang X. J., et al. Reduced medical costs and hospital days when using oral arsenic plus ATRA as the first-line treatment of acute promyelocytic leukemia. Leukemia Research . 2015;39(12):1319–1324. doi: 10.1016/j.leukres.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Yang M. H., Wan W. Q., Luo J. S., et al. Multicenter randomized trial of arsenic trioxide and realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: interim results of the SCCLG-APL clinical study. American Journal of Hematology . 2018;93(12):1467–1473. doi: 10.1002/ajh.25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lou Y., Lu Y., Ye X., et al. PML-RARA monitoring in newly diagnosed acute promyelocytic leukemia treated with an entirely oral chemotherapy-free postremission approach: a multiple institution experience. Hematological Oncology . 2020;38(4):618–621. doi: 10.1002/hon.2766. [DOI] [PubMed] [Google Scholar]
- 86.Zhu H. H., Guo Z. P., Jia J. S., Jiang Q., Jiang H., Huang X. J. The impact of oral arsenic and all-trans-retinoic acid on coagulopathy in acute promyelocytic leukemia. Leukemia Research . 2018;65:14–19. doi: 10.1016/j.leukres.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 87.Shi X., Li S., Tang S., Lu Y. Successful treatment of acute promyelocytic leukemia in a 92-year-old man using all-trans retinoic acid combined with oral arsenic: a case report. Medicine . 2021;100(22) doi: 10.1097/md.0000000000026144.e26144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu H. H., Wu D. P., Jin J., et al. Long-term survival of acute promyelocytic leukaemia patients treated with arsenic and retinoic acid. British Journal of Haematology . 2016;174(5):820–822. doi: 10.1111/bjh.13809. [DOI] [PubMed] [Google Scholar]
- 89.Xie Q., Yu L., Wang X., et al. A novel realgar-indigo naturalis formula more effectively induces apoptosis in NB4 cells. Pakistan journal of pharmaceutical sciences . 2019;32(3):957–962. [PubMed] [Google Scholar]
- 90.Zhang M., Guo L., Lin L. F., et al. Absorption characteristics of combination medication of realgar and indigo naturalis: in vitro transport across MDCK-MDR1 cells and in vivo pharmacokinetics in mice after oral administration. Evidence-Based Complementary and Alternative Medicine . 2018;2018:10. doi: 10.1155/2018/6493630.6493630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu X. M., Yuan B., Tanaka S., et al. Involvement of oxidative stress associated with glutathione depletion and p38 mitogen-activated protein kinase activation in arsenic disulfide-induced differentiation in HL-60 cells. Leukemia and Lymphoma . 2014;55(2):392–404. doi: 10.3109/10428194.2013.802779. [DOI] [PubMed] [Google Scholar]
- 92.Zhao P., Liang J. B., Deng Z. Y., et al. Association of gene mutations with response to arsenic-containing compound Qinghuang powder in patients with myelodysplastic syndromes. Chinese Journal of Integrative Medicine . 2019;25(6):409–415. doi: 10.1007/s11655-018-2977-3. [DOI] [PubMed] [Google Scholar]
- 93.Mao J. H., Sun X. Y., Liu J. X., et al. As4S4 targets RING-type E3 ligase c-CBL to induce degradation of BCR-ABL in chronic myelogenous leukemia. Proceedings of the National Academy of Sciences of the USA . 2010;107(50) doi: 10.1073/pnas.1016311108.21683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang W., Peng Z. L., Dai Y. J., Wang D. L., Huang P., Huang H. P. (-)-Epigallocatechin-3-gallate encapsulated realgar nanoparticles exhibit enhanced anticancer therapeutic efficacy against acute promyelocytic leukemia. Drug Delivery . 2019;26(1):1058–1067. doi: 10.1080/10717544.2019.1672830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liangchun Z. Duotan dingjing powder. 2012. https://www.google.com/search?rlz=1C1GCEB_enIN1005IN1005&q=Dotan+Dingjing+Powder&spell=1&sa=X&ved=2ahUKEwj47fTJ_P_5AhUYSGwGHZe5DHUQBSgAegQIARA1&biw=1366&bih=600&dpr=1 .
- 96.Ma Q., Huang W., Zhao J., Yang Z. Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo. Journal of Ethnopharmacology . 2020;252 doi: 10.1016/j.jep.2020.112584.112584 [DOI] [PubMed] [Google Scholar]
- 97.Zhao J., Wang Y., Huang X., et al. Liu Shen Wan inhibits influenza virus-induced secondary Staphylococcus aureus infection in vivo and in vitro. Journal of Ethnopharmacology . 2021;277 doi: 10.1016/j.jep.2021.114066.114066 [DOI] [PubMed] [Google Scholar]
- 98.Sun Y. T., Xu H. H., Nie Y., et al. Preliminary study of Realgar and arsenic trioxide on gut microbiota of mice. Zhongguo Zhongyao Zazhi . 2020;45(1):142–148. doi: 10.19540/j.cnki.cjcmm.20190813.403. [DOI] [PubMed] [Google Scholar]
- 99.Zhang W., Huo T., Li A., et al. Identification of neurotoxicity markers induced by realgar exposure in the mouse cerebral cortex using lipidomics. Journal of Hazardous Materials . 2020;389 doi: 10.1016/j.jhazmat.2019.121567.121567 [DOI] [PubMed] [Google Scholar]
- 100.Meng Y., Feng R., Yang Z., Liu T., Huo T., Jiang H. Oxidative stress induced by realgar in neurons: p38 MAPK and ERK1/2 perturb autophagy and induce the p62-Keap1-Nrf2 feedback loop to activate the Nrf2 signalling pathway. Journal of Ethnopharmacology . 2022;282 doi: 10.1016/j.jep.2021.114582.114582 [DOI] [PubMed] [Google Scholar]
- 101.Zhang S., Li C., Feng T., et al. A metabolic profiling study of realgar-induced acute kidney injury in mice. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.706249.706249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang S., Li C., Feng T., et al. Proteomics analysis in the kidney of mice following oral feeding Realgar. Journal of Ethnopharmacology . 2021;275 doi: 10.1016/j.jep.2021.114118.114118 [DOI] [PubMed] [Google Scholar]
- 103.Xu H. H., Hao F. R., Wang M. X., et al. Influences of realgar-indigo naturalis, a traditional Chinese medicine formula, on the main CYP450 activities in rats using a cocktail method. Evidence-Based Complementary and Alternative Medicine . 2017;2017:9. doi: 10.1155/2017/2374624.2374624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li A., Wu X., Yang J., et al. Sub-chronic exposure to realgar induces liver injury via upregulating the TXNIP/NLRP3 pathway and disturbing bile acid homeostasis in mice. Journal of Ethnopharmacology . 2021;281 doi: 10.1016/j.jep.2021.114584.114584 [DOI] [PubMed] [Google Scholar]
- 105.Wang X., Huang X., Zhou L., et al. Association of arsenic exposure and cognitive impairment: a population-basedcross-sectional study in China. Neurotoxicology . 2021;82:100–107. doi: 10.1016/j.neuro.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 106.Zhang M. H., Chen J. Q., Guo H. M., et al. Combination of LC/MS and GC/MS based metabolomics to study the hepatotoxic effect of realgar nanoparticles in rats. Chinese Journal of Natural Medicines . 2017;15(9):684–694. doi: 10.1016/s1875-5364(17)30098-5. [DOI] [PubMed] [Google Scholar]
- 107.Wang D. X., Xu Y. G., Du Y., et al. Arsenic concentration in peripheral blood is correlated with efficacy of a traditional Chinese medicine regimen containing realgar for the treatment of myelodysplastic syndrome. Journal of Traditional Chinese Medicine . 2021;41(4):630–635. doi: 10.19852/j.cnki.jtcm.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 108.Tsoi B., Wang S., Gao C., et al. Realgar and cinnabar are essential components contributing to neuroprotection of Angong Niuhuang Wan with no hepatorenal toxicity in transient ischemic brain injury. Toxicology and Applied Pharmacology . 2019;377 doi: 10.1016/j.taap.2019.114613.114613 [DOI] [PubMed] [Google Scholar]
- 109.Lu Y. F., Yan J. W., Wu Q., Shi J. Z., Liu J., Shi J. S. Realgar- and cinnabar-containing an-gong-niu-huang wan (AGNH) is much less acutely toxic than sodium arsenite and mercuric chloride. Chemico-Biological Interactions . 2011;189(1-2):134–140. doi: 10.1016/j.cbi.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Lin P. Y., Tang Y. S., Wang N. S. Effects of cinnabar and realgar in angong niuhuang powder on heat shock protein, nitric oxide synthase and inflammatory cytokines in contusion cerebral edema. Zhong Yao Cai . 2006;29(5):458–461. [PubMed] [Google Scholar]
- 111.Tang Y. S., Wang N. S., Zhang Y. Q., Ye S. M., Ou W. P. Effects of realgar and prescription containing realgar on stress response proteins, inflammatory mediators and complements in fever rat models. Zhong Yao Cai . 2009;32(1):73–78. [PubMed] [Google Scholar]
- 112.Chen C., Zhang B. B., Hu A. L., Li H., Liu J., Zhang F. Protective role of cinnabar and realgar in Hua-Feng-Dan against LPS plus rotenone-induced neurotoxicity and disturbance of gut microbiota in rats. Journal of Ethnopharmacology . 2020;247 doi: 10.1016/j.jep.2019.112299.112299 [DOI] [PubMed] [Google Scholar]
- 113.Liu H., Yan Y., Pang P., et al. Angong Niuhuang Pill as adjuvant therapy for treating acute cerebral infarction and intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Journal of Ethnopharmacology . 2019;237:307–313. doi: 10.1016/j.jep.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 114.Zhao P. C., Huang Z. S., Xu S. N., Zhang Y. M. Adjuvant treatment with Angong Niuhuang pills in treating traumatic brain damage: a meta-analysis of randomized controlled trials. Annals of Palliative Medicine . 2021;10(2):1569–1577. doi: 10.21037/apm-20-1331. [DOI] [PubMed] [Google Scholar]
- 115.Song H., Chen X., Yu Y., Zhang L. Xingnao Kaiqiao acupuncture combined with Angong Niuhuang Wan for a patient under persistent vegetative state: a case report. Frontiers of Medicine . 2018;12(3):334–339. doi: 10.1007/s11684-017-0539-2. [DOI] [PubMed] [Google Scholar]
- 116.Lv L., Zheng J., Zhang Y., et al. Respiratory nursing care with Angong Niuhuang pill for patients with chronic obstructive pulmonary disease following cardiac surgery. Japan Journal of Nursing Science . 2021;18(1) doi: 10.1111/jjns.12344.e12344 [DOI] [PubMed] [Google Scholar]
- 117.Fan Y. X., Guo D. Q., Wang M. L. Clinical study of Huafengdan combined with sodium valproate in treatment of epilepsy. Drugs & Clinic . 2022;37(5):999–1003. [Google Scholar]
- 118.Xu Y., Li S. Y., Li Y. N., et al. Efficacy and safety of huafeng dan combined with basic therapy in the treatment of stroke: A meta-analysis. China Pharmaceuticals . 2022;31(8):112–116. [Google Scholar]
- 119.Chen J., Dai M., Gui Y. Clinical observation of acupuncture combined with Huafeng Elixir (化风丹)in treatment of 35 cases of peripheral facial paralysis of wind-cold type. Journal of Traditional Chinese Medicine . 2021;62(15):1332–1337. [Google Scholar]
- 120.Wang J. L., Tang Y., Wang C. Y. Clinical observation on Huafeng Dan (化风丹) combined with mecobalamine and acupuncture in treating 36 cases of idiopathic facial paralysis of wind-phlegm obstructing collateral type. Journal of Traditional Chinese Medicine . 2019;60(17):1488–1492. [Google Scholar]
- 121.Li Y., Duan J., Xia H., Shu B., Duan W. Macromolecular substances as a dangerous factor in traditional Chinese medicine injections were determined by size-exclusive chromatography. Toxicology Research . 2020;9(3):323–330. doi: 10.1093/toxres/tfaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao S. C., Wei R. L., Xie Y. M., Wang L. X., Wang Q., Yi D. H. Analysis of Qingkailing Injection in treatment of combined medication features of 2 147 cases of upper respiratory tract infection. Zhongguo Zhongyao Zazhi . 2019;44(23):5207–5216. doi: 10.19540/j.cnki.cjcmm.20191115.501. [DOI] [PubMed] [Google Scholar]
- 123.He S., Li W. S., Luo Y. J., Ye C. L., Zhang Z. Y. Qingkailing injection for treatment of children pneumonia induced by respiratory syncytial virus: a meta-analysis of randomized controlled trials. Chinese Journal of Integrative Medicine . 2018;24(4):288–295. doi: 10.1007/s11655-017-2763-7. [DOI] [PubMed] [Google Scholar]
- 124.Wu J., Zhang X., Zhang B. Qingkailing injection for the treatment of acute stroke: a systematic review and meta-analysis. Journal of Traditional Chinese Medicine . 2014;34(2):131–139. doi: 10.1016/s0254-6272(14)60066-2. [DOI] [PubMed] [Google Scholar]
- 125.Edition RTSnPDO. Progress in research on prevention and treatment of sars with traditional chinese medicine. 2003. https://scholar.google.co.in/scholar?q=Progress+in+Research+on+Prevention+and+Treatment+of+SARS+with+Traditional+Chinese+Medicine&hl=en&as_sdt=0&as_vis=1&oi=scholart .
- 126.Li X. H., Liu Q. Q. Chinese medicine prevention and treatment scheme for COVID-19 in Beijing (trial fifth edition) Beijing Journal of Traditional Chinese Medicine . 2020;39(07):655–656. [Google Scholar]
- 127.Wu B. L., He W. X., Ke M., Shang-Guan X. F., He G. F., Huang R. A retrospective analysis on 1330 adverse event reports of Qingkailing in China: further perception of its risks and rational use. Current Medical Science . 2018;38(6):1103–1108. doi: 10.1007/s11596-018-1990-2. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J., Wang S., Xu L., et al. Multiple perspectives of qingkailing injection-fraction-single compound in revealing the hepatotoxicity of baicalin and hyodeoxycholic acid. Journal of Ethnopharmacology . 2018;215:147–155. doi: 10.1016/j.jep.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 129.Miao X., Zhou J., Li J., Liao Y., Zheng Y. Chinese medicine in inhalation therapy: a review of clinical application and formulation development. Current Pharmaceutical Design . 2015;21(27):3917–3931. doi: 10.2174/1381612821666150820110550. [DOI] [PubMed] [Google Scholar]
- 130.Tian Z. Y., Feng L. D., Xie Y., et al. Chinese herbal medicine xingnaojing injection for acute ischemic stroke: an overview of systematic reviews and meta-analyses. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.659408.659408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang L., Fan X., Chen Y., Liang X., Shen W., Zhang Y. Efficacy and safety of xingnaojing injection for emergency treatment of acute ischemic stroke: a systematic review and meta-analysis. Frontiers in Pharmacology . 2022;13 doi: 10.3389/fphar.2022.839305.839305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Z. R., Zhao Y. H., Sun R., Zhang Y. R. Effects of Xingnaojing on serum high-sensitivityC-reactive protein and neuron-specific enolase in patients with acute cerebral hemorrhage: a protocol of systematic review and meta-analysis. Medicine . 2020;99(45) doi: 10.1097/md.0000000000021379.e21379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.An Z., Yin Y., Zhang L., et al. Effect of ulinastatin combined with xingnaojing injection on severe traumatic craniocerebral injury and its influence on oxidative stress response and inflammatory response. BioMed Research International . 2022;2022:6. doi: 10.1155/2022/2621732.2621732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen L., Jiang H., Xing G., et al. Effects of Yunanan Baiyao adjunct therapy on postoperative recovery and clinical prognosis of patients with traumatic brain injury: a randomized controlled trial. Phytomedicine . 2021;89 doi: 10.1016/j.phymed.2021.153593.153593 [DOI] [PubMed] [Google Scholar]
- 135.Wu Y., Zhong P. Clinical progress on management of pneumonia due to COVID-19 with Chinese traditional patent medicines. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.655063.655063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han T., Zhang H., Xu W., et al. Study on the mechanism of reducing hepatotoxicity of water-grinding realgar by metabolomics, morphology, and chemical analysis. Evidence-Based Complementary and Alternative Medicine . 2021;2021:14. doi: 10.1155/2021/8538287.8538287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tingguo G. J. Y. T. J. J. X. H. H. The impact of water processing on the content of soluble sulphur and mercury in cinnabar. Chinese Archives of Traditional Chinese Medicine . 2015;33(5):1113–1115. [Google Scholar]
- 138.Kang F., Wu K., He H., et al. Comparative toxicology study of Cinnabar, Zhusha Anshenwan, methylmercury and mercuric chloride. Zhongguo Zhongyao Zazhi . 2010;35(4):499–503. doi: 10.4268/cjcmm20100421. [DOI] [PubMed] [Google Scholar]
- 139.Xia F., Li A., Chai Y., et al. UPLC/Q-TOFMS-Based metabolomics approach to reveal the protective role of other herbs in an-gong-niu-huang wan against the hepatorenal toxicity of cinnabar and realgar. Frontiers in Pharmacology . 2018;9:p. 618. doi: 10.3389/fphar.2018.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su G., Chen G., An X., Wang H., Pei Y. H. Metabolic profiling analysis of the alleviation effect of treatment with baicalin on cinnabar induced toxicity in rats urine and serum. Frontiers in Pharmacology . 2017;8:p. 271. doi: 10.3389/fphar.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang S., Xiao X., Li A., Li P. The herbal constituents in an-gong-niu-huang wan (AGNH) protect against cinnabar- and realgar-induced hepatorenal toxicity and accumulations of mercury and arsenic in mice. Evidence-Based Complementary and Alternative Medicine . 2021;2021:9. doi: 10.1155/2021/5566078.5566078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li A., Zhang J. Y., Xiao X., et al. Hepatorenal protective effects of medicinal herbs in An-Gong-Niu-Huang Wan (AGNH) against cinnabar- and realgar-induced oxidative stress and inflammatory damage in mice. Food and Chemical Toxicology . 2018;119:445–456. doi: 10.1016/j.fct.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 143.Wang J., Ding L., Zhou J., et al. Target lipidomics approach to reveal the resolution of inflammation induced by Chinese medicine combination in Liu-Shen-Wan against realgar overexposure to rats. Journal of Ethnopharmacology . 2020;249 doi: 10.1016/j.jep.2019.112171.112171 [DOI] [PubMed] [Google Scholar]
- 144.Xu W., Pei Y., Xu S., Wang H., Jin P. Metabolic profiling analysis of the alleviation effect of the fractions of Niuhuang Jiedu tablet on realgar induced toxicity in rats. Evidence-Based Complementary and Alternative Medicine . 2018;2018:13. doi: 10.1155/2018/2154603.2154603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The authors review the accumulating evidence and discuss the pharmacology, toxicology, formulas, and safe use of cinnabar (HgS) and realgar (As₂S₂).


