Abstract
Background
To evaluate prescription trends and clinical factors of the sodium-glucose cotransporter 2 inhibitors (SGLT2i) use according to the presence of atherosclerotic cardiovascular disease (ASCVD) or heart failure (HF) in Korean patients with type 2 diabetes mellitus (T2DM).
Methods
Prescription patterns of SGLT2i use between 2015 and 2019 were determined using the Korean National Health Insurance Service database of claims.
Results
Of all patients with T2DM (n=4,736,493), the annual prescription rate of SGLT2i increased every year in patients with ASCVD (from 2.2% to 10.7%) or HF (from 2.0% to 11.1%). After the first hospitalization for ASCVD (n=518,572), 13.7% (n=71,259) of patients initiated SGLT2i with a median of 10.6 months. After hospitalization for HF (n=372,853), 11.2% (n=41,717) of patients initiated SGLT2i after a median of 8.8 months. In multivariate regression for hospitalization, older age (per 10 years, odds ratio [OR], 0.57; 95% confidence interval [CI], 0.56 to 0.57), lower household income (OR, 0.93; 95% CI, 0.92 to 0.95), rural residents (OR, 0.95; 95% CI, 0.93 to 0.97), and dipeptidyl peptidase-4 inhibitor (DPP-4i) users (OR, 0.82; 95% CI, 0.81 to 0.84) were associated with lesser initiation of SGLT2i in ASCVD. Additionally, female gender (OR, 0.97; 95% CI, 0.95 to 0.99) was associated with lesser initiation of SGLT2i in HF.
Conclusion
The prescription rate of SGLT2i increased gradually up to 2019 but was suboptimal in patients with ASCVD or HF. After the first hospitalization for ASCVD or HF, older age, female gender, low household income, rural residents, and DPP-4i users were less likely to initiate SGLT2i.
Keywords: Asians; Cardiovascular diseases; Diabetes mellitus, type 2; Healthcare disparities; Heart failure; Sodium-glucose transporter 2 inhibitors
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is commonly complicated by atherosclerotic cardiovascular disease (ASCVD) and heart failure (HF). Patients with T2DM and cardiovascular disease (CVD) have a higher risk for recurrent CVD events than those with prior CVD without diabetes [1]. Among antidiabetic drugs, sodium-glucose cotransporter 2 inhibitor (SGLT2i) and glucagon-like peptide-1 (GLP-1) analogues reduced the risk of major adverse cardiovascular events (MACE) [2-5]. Particularly, SGLT2i reduced CVD risk with the largest benefit for reducing hospitalization for HF [6]. In addition, cardiovascular (CV) benefits independent of the presence of ASCVD, glycemic control status, or baseline renal function have been observed [7]. Accordingly, recent recommendations in the main guidelines for diabetes treatment are shifting their importance from glycemic control to prevention and management of MACE according to the presence or risk of ASCVD. The updated American Diabetes Association guidelines [8] and Korean Diabetes Association guidelines [9] now recommend prioritizing antidiabetic drugs with a proven benefit for CVD in patients with established ASCVD or HF.
According to the United States and Europe nationwide cohort study [10-12], the proportion of SGLT2i use has increased globally in patients with T2DM and in those with concomitant T2DM and CVD. Among United States patients with concomitant T2DM and established CVD between 2014 and 2018, the proportion of those taking SGLT2i had increased from 2.0% in 2014 to 7.2% in 2018 [10]. Austria region-wide diabetes registry data between 2012 and 2018 (n=10,875) showed that the overall prescription rate of SGLT2i in patients with T2DM had increased from 3.7% in 2012 to 11.7% in 2018 [11]. A Danish nationwide population-based study compared the initiation rate between SGLT2i and dipeptidyl peptidase-4 inhibitor (DPP-4i) between 2014 and 2017 and found that SGLT2i initiators had increased 3.6-fold in 2017 compared to that in 2014 (from 53/100,000 to 172/100,000 per year) [12].
Several factors can affect the use of SGLT2i in eligible patients with CV risk. According to medical and pharmacy claims data from the United States between 2013 and 2016 [13], SGLT2i users were younger and more likely to have commercial health insurance. The United States claims database of commercially health insured population for T2DM between 2015 and 2019 (n=934,737) showed that black and female patients and those with low socioeconomic status were independently associated with lower rates of SGLT2i use [14].
It is important to decide which subjects to prescribe and start SGLT2i treatment to expect clinical benefits. However, there are scarce population-based data on when SGLT2i is initiated after the first onset of ASCVD or HF. In addition, few studies have analyzed clinical factors affecting the prescription of SGLT2i as secondary prevention therapy. SGLT2i has been covered by the Korean National Health Insurance Service (KNHIS) and reimbursed under regulation since 2015 in Korea. Korean population-based healthcare databases provide an opportunity to characterize SGLT2i utilization trends in the Korean population and describe all individuals with an incident case of SGLT2i use after the first onset of ASCVD or HF. Therefore, the aim of this study was to examine trends in annual prescription rate of SGLT2i and compare the overall prescription rate to other cardioprotective medications and antidiabetic drugs according to the presence of ASCVD or HF. Additionally, clinical characteristics and prescribing patterns of other antidiabetic and cardioproctive drugs were compared to determine whether SGLT2i was prescribed or not after the first hospitalization for ASCVD or HF. Clinical factors associated with SGLT2i initiation after the onset of ASCVD or HF were also assessed.
METHODS
Data sources
Data from the KNHIS datasets of claims from 2015 to 2019, including diagnosis, demographic factors, prescription records, and comorbidities, were analyzed. The KNHIS, a single-payer system for all residents in Korea, covers 97.1% of Koreans (approximately 50 million individuals) [15]. The KNHIS datasets include an eligibility database (age, sex, socioeconomic variables, type of eligibility, household income level), a medical treatment claims database (based on medical bills that were claimed by medical service providers for their medical expenses), and a medical care institution database (type of medical care institutions). Adult patients aged more than 20 years were included in this study. These datasets have been established since 2002. Written informed consent by the patients was waived due to a retrospective nature of our study. This study was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (IRB no. GNUCH 2021-08-021).
Study population and definitions
The diagnosis was classified using the International Classification of Disease, 10th Revision (ICD-10). Operational definition of diabetes and its related comorbidities were applied for further analysis (Supplementary Table 1). Patients with T2DM were defined when ICD-10 codes for T2DM (E11–E14) as the principal diagnosis or up to a fourth additional diagnosis were present with a prescription of at least one antidiabetic drug in a given year [16]. The presence of hypertension was defined according to at least one claim in a given year for the prescription of antihypertensive drugs under ICD-10 codes I10–I13 and I15 [16]. The presence of dyslipidemia was defined as at least one claim for lipid-lowering agents in a given year under the corresponding ICD-10 code (E78) [16]. Drugs corresponding to each class are summarized in Supplementary Table 2. Urban residents were defined as cases where prescriptions were made in seven metropolitan cities, including Seoul. In the classification of medical institutions, a hospital was defined as a case of more than a general hospital, including secondary and tertiary hospitals. Household income level was recruited through the annual contribution amount for health insurance, and dichotomized at the lower 25th percentile or divided into quartiles. ASCVD comprised ischemic heart disease and stroke. Ischemic heart disease was defined as the presence of ICD-10 codes of I20–I25 and/or the accompanied procedure for percutaneous coronary intervention or coronary artery bypass graft [16]. Stroke was defined as the presence of ICD-10 code of I50 with computed tomography/magnetic resonance imaging scan [16].
Baseline demographic variables, socioeconomic factors, concomitant use of other medications (antidiabetic drugs, CV medications), and comorbidities were defined differently according to prevalent and incident cases. For prevalent cases, the presence of ASCVD or HF was determined based on whether there were claim data with corresponding ICD-10 codes within the whole study period or a given year, regardless of hospitalization. The use of each class of drugs was defined as whether drugs were prescribed at least once in a given year. Demographic variables such as age, sex, region, and prescribed medical institutions were determined based on when the first claim data were sent to Health Insurance Review Agency (HIRA) for each diagnostic code (e.g., T2DM, ASCVD, HF) in a given year. Meanwhile, for incident cases, a 12-year washout period (from January 2002 to December 2014) was set to confirm incident cases during 2015 to 2019. In addition, the presence of ASCVD or HF was determined when the billing data with each corresponding ICD-10 code was present with hospitalization during the study period. The use of each medication, demographic factors, income levels, residents, prescribed medical institutions, and comorbidities were determined based on the first claim data sent to HIRA after the first diagnosis and hospitalization for ASCVD or HF during 2015 to 2019.
Statistical analysis
Baseline characteristics are presented as mean with standard deviation or numbers and percentages. Clinical parameters between SGLT2i users and non-users were compared using one-way analysis of variance for continuous variables and the chi-square test for categorical variables. Multivariate logistic regression analysis was conducted to evaluate associations between the initiation of SGLT2i after the first hospitalization for ASCVD or HF and clinical parameters including age, gender, residents, income levels, concomitant antidiabetic drugs, comorbidities, and CV medications. These results are presented as odds ratio (OR) and 95% confidence interval (CI). All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 3.2.5 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.Rproject.org). All statistical tests were two-tailed, and significance level was set at P<0.05.
RESULTS
Prevalent case
Among all Korean patients with T2DM enrolled between 2015 to 2019 (n=4,736,493), 1,720,996 (36.1%) patients had ASCVD (ICD-10 code corresponding to ASCVD exists at least once), and 783,378 (16.5%) patients had HF (Supplementary Table 3). Annual proportion of patients with concomitant T2DM and ASCVD ranged from 24.3% to 24.5% (Supplementary Fig. 1). Annual prescription rate of SGLT2i in total T2DM patients increased steadily from 2.4% (n=74,805) in 2015 to 11.0% (n=435,179) in 2019. These trends were consistent independent of the presence of ASCVD (from 2.2% to 10.7%) or HF (from 2.0% to 11.1%) (Fig. 1).
Fig. 1.
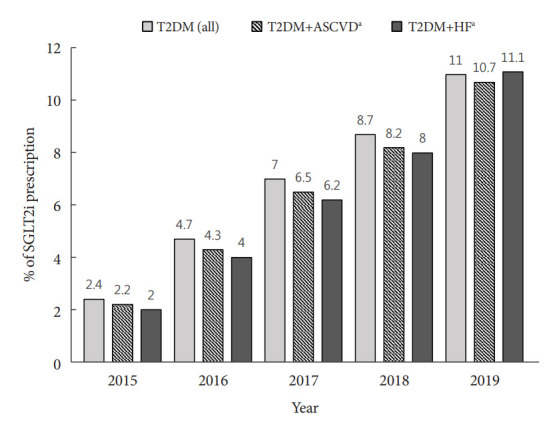
Annual prescription rate of sodium-glucose cotransporter 2 inhibitor (SGLT2i) in patients with type 2 diabetes mellitus (T2DM) according to the presence of atherosclerotic cardiovascular disease (ASCVD) or heart failure (HF). aThe presence of ASCVD or HF was determined whether the corresponding International Classification of Disease, 10th Revision (ICD-10) code presents in a given year regardless of hospitalization.
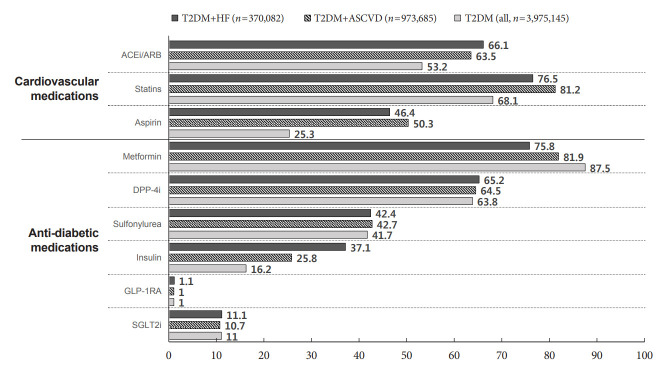
Among all patients with T2DM (n=3,975,145) based on the latest data in 2019, 370,082 patients had concomitant T2DM and HF, 973,685 patients had concomitant T2DM and ASCVD, respectively (Fig. 2). Angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blockade (ARB) was used more frequently in patients with T2DM accompanied ASCVD (63.5%; n=618,100) or HF (66.1%; n=244,629) than in all patients with T2DM (53.2%; n=2,114,946). Similarly, statins and aspirin were used more in patients with ASCVD (81.2% were on statins, 50.3% were on aspirin) or HF (76.5% were on statins, 46.4% were on aspirin) than in all patients with T2DM. Regarding antidiabetic drugs used in all patients with T2DM, 11% (n=435,179) of patients were prescribed SGLT2i, and 63.8% (n=2,536,481) were prescribed DPP-4i. Meanwhile, SGLT2 prescription rates were similar in patients accompanied by ASCVD (10.7%; n=103,761) or HF (11.1%; n=41,008) compared to all patients (11.0%; n=435,179). Among patients with concomitant T2DM and ASCVD in 2019, there was no regional difference in the prescription rate of SGLT2i (Supplementary Fig. 2).
Fig. 2.
Use of cardiovascular and antidiabetic medications among patients with type 2 diabetes mellitus (T2DM) and atherosclerotic cardiovascular disease (ASCVD) or heart failure (HF) in 2019. Data were presented as percentage (%). ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
When prescription rates of SGLT2i were compared according to age, gender, and household income levels as of 2019, male gender (12.2% vs. 8.7% in females, P<0.001) and younger patients (26.0% in 30 to 49 years old vs. 15.0% in 50 to 69 years old and 5.9% in 70 years old or higher, P<0.001) were prescribed SGLT2i more frequently in patients with T2DM and ASCVD. However, the prescription rate of SGLT2i was not significantly different according to household income level (10.6% vs. 10.6%, P=0.647) (Supplementary Fig. 3).
Incident case: ASCVD
During 2015 to 2019, a total of 518,572 patients were hospitalized with the first diagnosis of ASCVD, and 71,259 patients (13.7%) initiated SGLT2i treatment with a median of 10.6 months after discharge. Baseline demographic, socioeconomic, and medication differences between those who were prescribed an SGLT2i and those who were not are summarized in Table 1. SGLT2i was initially prescribed the most by hospitals (n=52,332, 73.4%), followed by local clinics (n=18,497, 26.0%). Patients who initiated SGLT2i after the first hospitalization for ASCVD were younger (mean 61.6 years vs. 69.8 years, P<0.001). In addition, patients who initiated SGLT2i after the onset of ASCVD also had higher proportions of male gender (63.4% vs. 54.6%, P<0.001) and urban residents (42.3% vs. 40.7%, P<0.001) than those who did not initiate. Overall, 7.4% (n=38,192) of patients who were first hospitalized for ASCVD between 2015 and 2019 initiated SGLT2i therapy within a year, which increased from 2.5% (n=2,272) in 2015 to 12.9% (n=13,455) in 2019 (Fig. 3).
Table 1.
Baseline characteristics according to initiation of SGLT2i prescription after the first hospitalization for atherosclerotic cardiovascular disease
| Characteristic | All | SGLT2i treatment |
P value | |
|---|---|---|---|---|
| No | Yes | |||
| Number | 518,572 | 447,313 | 71,259 | |
| Age, yr | 68.7±12.1 | 69.8±11.8 | 61.6±11.7 | <0.001 |
| Male sex | 289,347 (55.8) | 244,192 (54.6) | 45,155 (63.4) | <0.001 |
| Time to initiation, mo | - | - | 10.6 (1.2–26.8) | |
| Urban residents | 212,152 (40.9) | 182,017 (40.7) | 30,135 (42.3) | <0.001 |
| Incomes (low 25%) | 152,749 (29.5) | 132,390 (29.6) | 20,359 (28.6) | <0.001 |
| Prescription | ||||
| Hospitals | - | - | 52,332 (73.4) | |
| Clinics | - | - | 18,497 (26.0) | |
| Etc. | - | - | 430 (0.6) | |
| Comorbidities | ||||
| Hypertension | 425,037 (82.0) | 367,326 (82.1) | 57,711 (81.0) | <0.001 |
| Dyslipidemia | 393,162 (75.8) | 330,498 (73.9) | 62,664 (87.9) | <0.001 |
| Antidiabetic drugsa | ||||
| Metformin | 360,462 (69.5) | 301,817 (67.5) | 58,645 (82.3) | <0.001 |
| DPP-4i | 235,352 (45.4) | 202,327 (45.2) | 33025 (46.4) | <0.001 |
| Insulin | 134,654 (26.0) | 121,148 (27.1) | 13,506 (19.0) | <0.001 |
| Sulfonylureas | 192,135 (37.1) | 160,143 (35.8) | 31,992 (44.9) | <0.001 |
| TZD | 30,799 (5.9) | 25,912 (5.8) | 4,887 (6.9) | <0.001 |
| AGI | 14,107 (2.7) | 12,545 (2.8) | 1,562 (2.2) | <0.001 |
| Meglitinide | 3,693 (0.7) | 3,455 (0.8) | 238 (0.3) | <0.001 |
| SGLT2i | 14,516 (2.8) | 0 | 14,516 (20.4) | <0.001 |
| GLP-1RA | 833 (0.2) | 598 (0.1) | 235 (0.3) | <0.001 |
| Cardiovascular medications | ||||
| ACEi/ARB | 347,273 (67.0) | 296,864 (66.4) | 50,409 (70.7) | <0.001 |
| Statins | 393,010 (75.8) | 330,815 (74.0) | 62,195 (87.3) | <0.001 |
| ASA | 341,707 (65.9) | 289,442 (64.7) | 52,265 (73.4) | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (range).
SGLT2i, sodium-glucose cotransporter 2 inhibitor; DPP-4i, dipeptidyl peptidase-4 inhibitor; TZD, thiazolidinedione; AGI, alpha-glucosidase inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade; ASA, aspirin.
Drug that was first prescribed and billed after hospitalization for atherosclerotic cardiovascular disease.
Fig. 3.
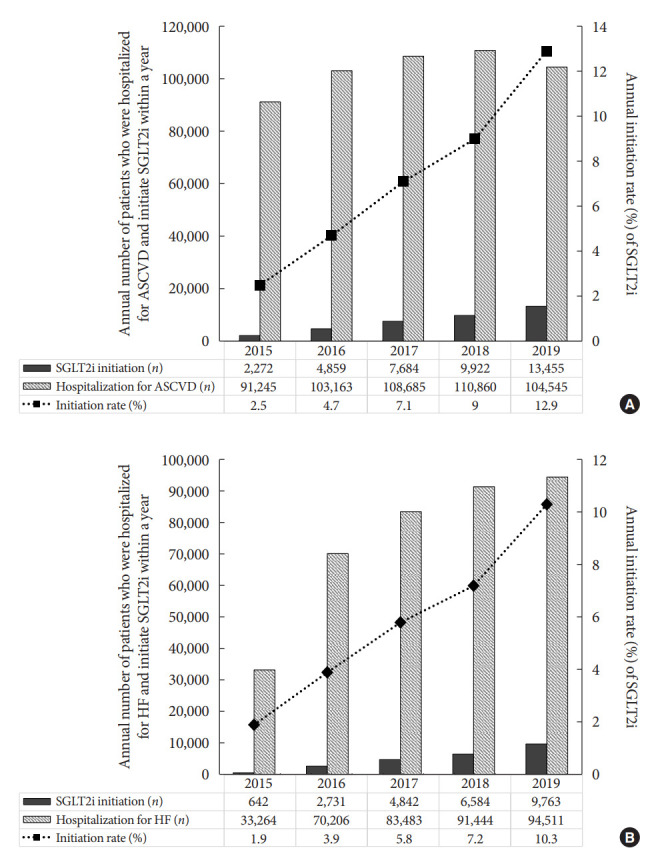
Annual initiation rate of sodium-glucose cotransporter 2 inhibitor (SGLT2i) within a year after the first hospitalization for (A) atherosclerotic cardiovascular disease (ASCVD) or (B) heart failure (HF).
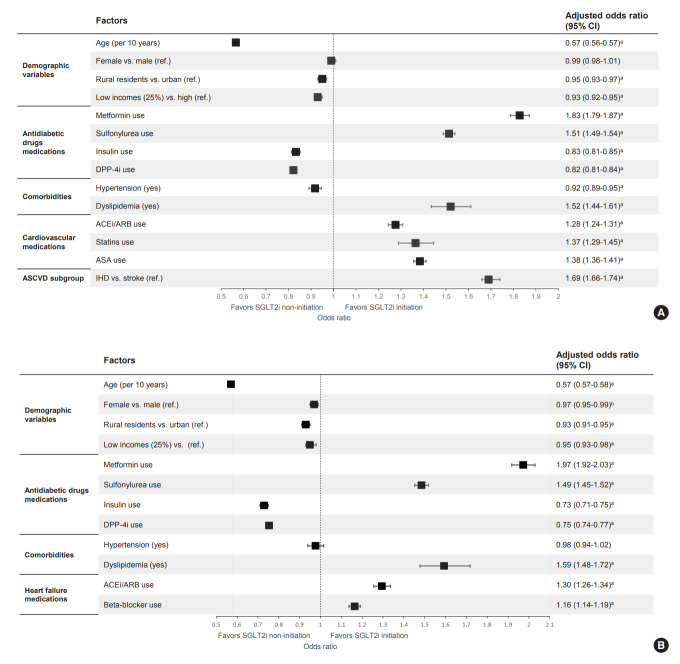
In multivariate analyses (Fig. 4A, Supplementary Table 4), older age (per 10 years, OR, 0.57; 95% CI, 0.56 to 0.57; P<0.001), rural residents (OR, 0.95; 95% CI, 0.93 to 0.97; P<0.001), lower income level (OR, 0.93; 95% CI, 0.92 to 0.95; P<0.001), the use of DPP-4i (OR, 0.82; 95% CI, 0.81 to 0.84; P<0.001) and the presence of hypertension (OR, 0.92; 95% CI, 0.89 to 0.95; P<0.001) were independently associated with lesser initiation of SGLT2i after the first hospitalization for ASCVD. Meanwhile, the presence of dyslipidemia (OR, 1.52; 95% CI, 1.44 to 1.61; P<0.001), use of metformin (OR, 1.83; 95% CI, 1.79 to 1.87; P<0.001), sulfonylurea (OR, 1.51; 95% CI, 1.49 to 1.54; P<0.001), ACEi/ARB (OR, 1.28; 95% CI, 1.24 to 1.31; P<0.001), statins (OR, 1.37; 95% CI, 1.29 to 1.45; P<0.001), and aspirin (OR, 1.38; 95% CI, 1.36 to 1.41; P<0.001) were also associated with SGLT2i use. Additionally, ischemic heart disease as a cause of ASCVD (OR, 1.69; 95% CI, 1.66 to 1.74; P<0.001) was associated with SGLT2i use compared to stroke.
Fig. 4.
Factors associated with sodium-glucose cotransporter 2 inhibitors (SGLT2i) initiation after the first hospitalization for (A) atherosclerotic cardiovascular disease (ASCVD) or (B) heart failure (HF) in patients with type 2 diabetes mellitus. DPP-4i, dipeptidyl peptidase-4 inhibitor; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade; ASA, aspirin; IHD, ischemic heart disease; CI, confidence interval. aP<0.001, bP<0.01.
Incident case: HF
Of 372,853 patients hospitalized with the first diagnosis of HF during 2015 to 2019, 41,717 (11.2%) patients initiated SGLT2i treatment with a median of 8.8 months after discharge. Baseline demographic, socioeconomic, and medication differences between those who were prescribed an SGLT2i and those who were not are summarized in Table 2. Similarly, for incident ASCVD cases, SGLT2i users were younger (63.4 years vs. 71.8 years, P<0.001) and male-dominant (59.4% vs. 51.8%, P<0.001). They were more likely to be urban residents (41.8% vs. 39.9%, P<0.001). Hypertension (83.9% vs. 83.3%, P=0.002) and dyslipidemia (84.0% vs. 65.9%, P<0.001) were more common in patients who were prescribed an SGLT2i than in those who were not. Insulin was prescribed less in patients who used an SGLT2i than in those who did not (20.6% vs. 31.6%, P<0.001). Overall, 6.6% (n=24,562) of patients who were first hospitalized for HF between 2015 and 2019 initiated SGLT2i therapy within a year, which increased from 1.9% (n=642) in 2015 to 10.3% (n=9,763) in 2019 (Fig. 3).
Table 2.
Baseline characteristics according to initiation of SGLT2i prescription after the first hospitalization for heart failure
| Characteristic | All | SGLT2i treatment |
P value | |
|---|---|---|---|---|
| No | Yes | |||
| Number | 372,853 | 331,136 | 41,717 | |
| Age, yr | 70.8±12.3 | 71.8±12.0 | 63.4±12.5 | <0.001 |
| Male sex | 196,458 (52.7) | 171,668 (51.84) | 24,790 (59.42) | <0.001 |
| Time to initiation, mo | - | - | 8.8 (0.3–22.5) | |
| Urban residents | 149,452 (40.1) | 132,026 (39.9) | 17,426 (41.8) | <0.001 |
| Incomes (low 25%) | 114,650 (30.8) | 101,692 (30.7) | 12,958 (31.1) | 0.142 |
| Prescription | ||||
| Hospitals | 31,419 (75.3) | |||
| Clinics | 10,048 (24.1) | |||
| Etc. | 250 (0.6) | |||
| Comorbidities | ||||
| Hypertension | 310,925 (83.4) | 275,917 (83.3) | 35,008 (83.9) | 0.002 |
| Dyslipidemia | 253,174 (67.9) | 218,124 (65.9) | 35,050 (84.0) | <0.001 |
| Antidiabetic drugsa | ||||
| Metformin | 235,753 (63.2) | 202,742 (61.2) | 33,011 (79.1) | <0.001 |
| Sulfonylureas | 130,540 (35.0) | 112,159 (33.9) | 18,381 (44.1) | <0.001 |
| DPP-4i | 171,984 (46.1) | 152,549 (46.1) | 19,435 (46.6) | 0.045 |
| Insulin | 113,093 (30.3) | 104,491 (31.6) | 8,602 (20.6) | <0.001 |
| SGLT2i | 9,841 (2.6) | 0 | 9,841 (23.6) | <0.001 |
| TZD | 23,525 (6.3) | 20,409 (6.2) | 3,116 (7.5) | <0.001 |
| AGI | 9,370 (2.5) | 8,566 (2.6) | 804 (1.9) | <0.001 |
| Meglitinide | 2,671 (0.7) | 2,554 (0.8) | 117(0.3) | <0.001 |
| HF medications | ||||
| ACEi/ARB | 247,070 (66.3) | 217,021 (65.5) | 30,049 (72.0) | <0.001 |
| Statins | 251,300 (67.4) | 216,675 (65.4) | 34,625 (83.0) | <0.001 |
| ASA | 196,245 (52.6) | 170,509 (51.5) | 25,736 (61.7) | <0.001 |
Values are presented as mean±standard deviation, number (%), or median (range).
SGLT2i, sodium-glucose cotransporter 2 inhibitor; DPP-4i, dipeptidyl peptidase-4 inhibitor; TZD, thiazolidinedione; AGI, alpha-glucosidase inhibitor; HF, heart failure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade; ASA, aspirin.
Drug that was first prescribed and billed after hospitalization for heart failure.
Older age (per 10 years; OR, 0.57; 95% CI, 0.57 to 0.58; P<0.001), female gender (OR, 0.97; 95% CI, 0.95 to 0.99; P=0.004), rural resident (OR, 0.93; 95% CI, 0.91 to 0.95; P<0.001), and lower income level (OR, 0.95; 95% CI, 0.93 to 0.98; P<0.001) were independently associated with lesser initiation of SGLT2i after the first hospitalization for HF. Additionally, the use of DPP-4i (OR, 0.75; 95% CI, 0.74 to 0.77; P<0.001) and insulin (OR, 0.73; 95% CI, 0.71 to 0.75; P<0.001) were associated with SGLT2i non-users. Meanwhile, the use of metformin (OR, 1.97; 95% CI, 1.92 to 2.03; P<0.001), sulfonylurea (OR, 1.49; 95% CI, 1.45 to 1.52; P<0.001), ACEi/ARB (OR, 1.30; 95% CI, 1.26 to 1.34; P<0.001), and beta-blockers (OR, 1.16; 95% CI, 1.14 to 1.19; P<0.001) were associated with SGLT2i use (Fig. 4B, Supplementary Table 5).
DISCUSSION
In this nationwide Korean population cohort study, the rate of SGLT2i prescription in patients with T2DM increased steadily. However, the rate was not different according to the presence of ASCVD or HF. Additionally, the overall prescription rate of SGLT2i use as secondary prevention therapy was insufficient compared to other CV medications. Among patients who were first diagnosed and hospitalized for ASCVD or HF, older age, female gender, DPP-4i users, rural residents, and low-income households were independently associated with lower initiation rates of SGLT2i treatment.
According to this nationwide population-based cohort data, although the rate of SGLT2i use among patients with T2DM had steadily increased every year, the proportion of SGLT2i use in eligible patients with high CV risk was suboptimal. Globally, national cohort studies through 2019 have shown that CVD presence or risk has little effect on the decision to use SGLT2i in patients with T2DM. A United States cohort study has demonstrated that the use of antidiabetic drugs with proven CV benefits (SGLT2i, GLP-1 analogues) is suboptimal compared to other cardioprotective medications such as statins or ACEi in T2DM patients with high CV risk [17]. According to a Danish population-based study, among all patients with T2DM who initiated SGLT2i, a minor increase was observed in proportion with ASCVD by year (from 28% in 2014 to 30% in 2017) [12]. In addition, 1- and 2-year cumulative incidence proportions of SGLT2i initiation after CVD onset were only 10.2% and 10.7%, respectively [18]. However, considering the fact that updated guidelines [8,9] have recently emphasized the selection of antidiabetic drugs according to CV risk and trends of gradual increase in the initiation rate of SGLT2i therapy since 2015, future studies with an additional follow-up are needed to determine whether the guideline-recommended SGLT2i therapy is applied according to the presence or risk of CVD.
In patients who were first diagnosed and hospitalized for ASCVD or HF between 2015 and 2019, 13.7% (with ASCVD) and 11.2% (with HF) of patients initiated SGLT2i therapy. Among SGLT2i users, 53.6% (n=38,192) of patients with ASCVD (median 10.6 months) and 58.9% (n=24,562) of those with HF (median 8.8 months) were prescribed within a year after the first hospitalization. A previous study showed that the time to initiation of SGLT2i after the onset of CVD was gradually shortened from 2012 (4.6 years to reach 15% of SGLT2i/GLP-1 analogues use) to 2018 (0.5 years to reach 15% of use) in a Danish population [18]. Meanwhile, Sun and Yan [19] have reported that early initiation of SGLT2i within 12 months is associated with significantly lower MACE, especially in patients with known ASCVD or additional CV risk factors. In addition, a recent randomized control study demonstrated that early initiation of sotagliflozin (dual SGLT1/2 inhibitor) after the hospitalization for worsening HF lower the risk of death from CVD and hospitalization for HF [20]. Considering the safety and potential efficacy, it may be necessary to consider early initiation of SGLT2i after hospitalization for ASCVD or HF for eligible high-risk patients.
Elderly patients were less likely to initiate SGLT2i treatment after the first diagnosis and hospitalization for ASCVD or HF. Older patients with T2DM are at a particularly high-risk of CVD [21]. However, other conditions that can increase the risk of frailty [22], fracture [23], and renal failure [24] also coexist in elderly patients. Although SGLT2i is well-tolerated, there are concerns related to volume depletion by the diuretic effect of SGLT2i that can lead to postural hypotension and syncope, especially in patients with older age. However, since CV protective effects of SGLT2i are maintained in both older and younger patients [25,26], it is necessary to actively prescribe SGLT2i with careful monitoring and management of concurrent conditions.
This study confirmed that the rate of SGLT2i use was low in females, consistent with findings of other nationwide cohort studies. Although whether poorer provider communication [27] and/or slower adoption of new guideline-directed therapies in females [14] might affect gender inequity remains unknown, concerns about side effects associated with a higher risk of genital infections [28], especially in women, might have acted as one of the barriers to prescribing SGLT2i in the high-risk group with CVD.
We also found that the use of DPP-4i was independently associated with a lower rate of SGLT2i initiation after the onset of ASCVD or HF. Globally, DPP-4i, which has neutral effects on CV outcomes, is still more widely used than SGLT2i even in CVD, HF, and chronic kidney disease [10,29]. Based on the United States claims data, patients with a history of CVD, HF, and nephropathy who could get the most benefit from SGLT2i therapy were less likely to initiate SGLT2i compared to DPP-4i [30]. In Korea, DPP-4i occupies a high proportion of total antidiabetic drugs market shares. The combination of metformin and DPP-4i is the most common dual combination therapy since 2014, while DPP-4i is the third most frequently prescribed drug after metformin and sulfonylurea among monotherapy [31]. Because the combination therapy of SGLT2i and DPP-4i was not covered by national health insurance, healthcare providers were required to consider out-of-pocket payments to initiate or switch to SGLT2i therapy. Institutional efforts to expand the insurance coverage for a combination of antidiabetic therapy are also required to ease the decision in choosing antidiabetic drugs according to CV risk.
Patients with lower income levels or rural residents were less likely to initiate SGLT2i treatment after the onset of ASCVD or HF. Income level was a strong predictor of unmet healthcare needs [29], and lower income households are associated with a reduced rate of SGLT2i treatment in the United States population [32]. Meanwhile, although low-income households were exempted from cost or had access to discounted copayment rates in Korea, the unfavorable gap in accessibility to hospitals according to income levels continued [33] due to limited benefit coverage [34].
This study has some limitations. Due to the nature of an insurance claims-based database, although the presence or initiation of each prescription was identified, adherence to or duration of treatment was unavailable. In addition, the actual usage rate might have been underestimated when the prescription was not covered by or claimed by the KNHIS. We were unable to identify clinical factors such as glycosylated hemoglobin or renal function. The use of SGLT2i is limited in T2DM patients with impaired renal function, especially in those with concomitant CVD. Until August 2019 in Korea, the use of SGLT2i was approved in patients with preserved renal function (estimated glomerular filtration rate [eGFR] >60 mL/min/1.73 m2). Meanwhile, the overall prevalence of chronic kidney disease (albuminuria or low eGFR [<60 mL/min/1.73 m2]) in patients diagnosed with CVD was 17.3%, which was higher than the general population (8.2%), according to the Korean National Health and Nutrition Examination Survey in 2011 to 2013 [35]. We could not account for intolerance, contraindications, comorbidities, or other barriers to choosing medical therapy. In addition, more detailed clinician- and patient-related factors and interactions that might affect the decision were not evaluated. Despite these limitations, this nationwide population-based data minimized the selection bias and offered nationwide prescription trends. In addition, this study offers a clue to provide more detailed and appropriate treatment for patients with T2DM who are vulnerable to or at high-risk for CVD in the future.
In conclusion, this Korean population-based cohort study demonstrated that the rate of SGLT2i use in patients with T2DM had increased steadily from 2015 to 2019. However, the accompanying ASCVD or HF did not affect the prescription rate of SGLT2i, and the prescription rate of SGLT2i was insufficient compared to that of other CV protective medications. There were age, gender, socioeconomic, and medication-related inequalities in prescribing an SGLT2i after the hospitalization for ASCVD or HF. More attention and efforts are needed to address factors affecting inequality in the prescription of SGLT2i with proven CV protection. Considering benefits and risks, it is necessary to actively consider the prescription of SGLT2i in the high-risk group for CVD.
Acknowledgments
This work was performed through cooperation with the Korea Health Industry Development Institute (KHIDI)-AZ Diabetes Research Program and the Korean Diabetes Association (KDA).
Footnotes
CONFLICTS OF INTEREST
Seung-Hyun Ko has been executive editor of the Diabetes & Metabolism Journal since 2022. She was not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: Y.S.Y., S.H.K., B.W.L., T.J.O., S.C., K. Y.H.
Acquisition, analysis, or interpretation of data: K.D.H., J.H.K., M.K.M., J.S.P., J.H.C.
Drafting the work or revising: J.H.B., Y.S.Y.
Final approval of the manuscript: S.H.K., K.Y.H.
FUNDING
None
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2022.0002.
Operational definitions of type 2 diabetes mellitus and its related comorbidities
Lists of each class of drugs
Baseline characteristics of the study population (2015 to 2019)
Factors associated with SGLT2i initiation among patients with type 2 diabetes mellitus and ASCVD
Factors associated with SGLT2i initiation among patients with type 2 diabetes mellitus and heart failure
Prevalence of atherosclerotic cardiovascular disease (ASCVD) in Korean patients with type 2 diabetes mellitus (T2DM). The presence of ASCVD were determined according to whether International Classification of Disease, 10th revision (ICD-10) code corresponding to ASCVD present in each given year.
Prescription rate of sodium-glucose cotransporter 2 inhibitor by region in type 2 diabetes mellitus with established atherosclerotic cardiovascular disease in 2019.
Proportion of those prescribed sodium-glucose cotransporter 2 inhibitor (SGLT2i) in patients with type 2 diabetes mellitus (T2DM) or accompanied atherosclerotic cardiovascular disease (ASCVD) according to gender, age, and income level in 2019. NS, not significant. aP<0.0001.
REFERENCES
- 1.Hubbard D, Colantonio LD, Rosenson RS, Brown TM, Jackson EA, Huang L, et al. Risk for recurrent cardiovascular disease events among patients with diabetes and chronic kidney disease. Cardiovasc Diabetol. 2021;20:58. doi: 10.1186/s12933-021-01247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019;394:121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 6.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–58. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–24. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 9.Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021;45:461–81. doi: 10.4093/dmj.2021.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz M, Ustyugova A, Sawalhi-Leckenby N, de Souza S, Zhang L, Gunnarsson E, et al. Utilization of glucose-lowering drugs in patients with T2DM and established CVD in US: a descriptive study using Optum clinformatics data. J Am Coll Cardiol. 2020;75(11 Suppl 1):2017. [Google Scholar]
- 11.Engler C, Leo M, Pfeifer B, Juchum M, Chen-Koenig D, Poelzl K, et al. Long-term trends in the prescription of antidiabetic drugs: real-world evidence from the Diabetes Registry Tyrol 2012-2018. BMJ Open Diabetes Res Care. 2020;8:e001279. doi: 10.1136/bmjdrc-2020-001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudsen JS, Baggesen LM, Lajer M, Nurkanovic L, Ustyugova A, Sorensen HT, et al. Changes in SGLT2i and GLP-1RA real-world initiator profiles following cardiovascular outcome trials: a Danish nationwide population-based study. PLoS One. 2020;15:e0229621. doi: 10.1371/journal.pone.0229621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCoy RG, Dykhoff HJ, Sangaralingham L, Ross JS, Karaca-Mandic P, Montori VM, et al. Adoption of new glucose-lowering medications in the U.S.: the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21:702–12. doi: 10.1089/dia.2019.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4:e216139. doi: 10.1001/jamanetworkopen.2021.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance System. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YH, Han K, Ko SH, Ko KS, Lee KU, Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold SV, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Mues KE, et al. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140:618–20. doi: 10.1161/CIRCULATIONAHA.119.041730. [DOI] [PubMed] [Google Scholar]
- 18.Funck KL, Knudsen JS, Hansen TK, Thomsen RW, Grove EL. Real-world use of cardioprotective glucose-lowering drugs in patients with type 2 diabetes and cardiovascular disease: a Danish nationwide cohort study, 2012 to 2019. Diabetes Obes Metab. 2021;23:520–9. doi: 10.1111/dom.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W, Yan B. Impact of early initiation of SGLT2 inhibitor on cardiovascular outcomes in diabetic patients with known atherosclerotic cardiovascular disease or risk factors: propensity score matched analysis. Eur Heart J. 2021;42(Suppl 1):ehab724–2653. [Google Scholar]
- 20.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–28. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 21.Halter JB, Musi N, McFarland Horne F, Crandall JP, Goldberg A, Harkless L, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes. 2014;63:2578–89. doi: 10.2337/db14-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanase T, Yanagita I, Muta K, Nawata H. Frailty in elderly diabetes patients. Endocr J. 2018;65:1–11. doi: 10.1507/endocrj.EJ17-0390. [DOI] [PubMed] [Google Scholar]
- 23.Dufour AB, Kiel DP, Williams SA, Weiss RJ, Samelson EJ. Risk factors for incident fracture in older adults with type 2 diabetes: the Framingham Heart Study. Diabetes Care. 2021;44:1547–55. doi: 10.2337/dc20-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–8. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 25.Cahn A, Mosenzon O, Wiviott SD, Rozenberg A, Yanuv I, Goodrich EL, et al. Efficacy and safety of dapagliflozin in the elderly: analysis from the DECLARE-TIMI 58 Study. Diabetes Care. 2020;43:468–75. doi: 10.2337/dc19-1476. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro P, Bergenstal RM, Toural E, Inzucchi SE, Zinman B, Hantel S, et al. Efficacy and safety of empagliflozin in older patients in the EMPA-REG OUTCOME® trial. Age Ageing. 2019;48:859–66. doi: 10.1093/ageing/afz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okunrintemi V, Valero-Elizondo J, Patrick B, Salami J, Tibuakuu M, Ahmad S, et al. Gender differences in patient-reported outcomes among adults with atherosclerotic cardiovascular disease. J Am Heart Assoc. 2018;7:e010498. doi: 10.1161/JAHA.118.010498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh TJ, Moon JY, Hur KY, Ko SH, Kim HJ, Kim T, et al. Sodium-glucose cotransporter-2 inhibitor for renal function preservation in patients with type 2 diabetes mellitus: a Korean Diabetes Association and Korean Society of Nephrology consensus statement. Diabetes Metab J. 2020;44:489–97. doi: 10.4093/dmj.2020.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang C, Mortensen MB, Lauridsen KG, Bruun JM. Trends in antidiabetic drug utilization and expenditure in Denmark: a 22-year nationwide study. Diabetes Obes Metab. 2020;22:167–72. doi: 10.1111/dom.13877. [DOI] [PubMed] [Google Scholar]
- 30.McCoy RG, Van Houten HK, Karaca-Mandic P, Ross JS, Montori VM, Shah ND. Second-line therapy for type 2 diabetes management: the treatment/benefit paradox of cardiovascular and kidney comorbidities. Diabetes Care. 2021;44:2302–11. doi: 10.2337/dc20-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BY, Won JC, Lee JH, Kim HS, Park JH, Ha KH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019;43:487–94. doi: 10.4093/dmj.2019.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCoy RG, Van Houten HK, Deng Y, Mandic PK, Ross JS, Montori VM, et al. Comparison of diabetes medications used by adults with commercial insurance vs medicare advantage, 2016 to 2019. JAMA Netw Open. 2021;4:e2035792. doi: 10.1001/jamanetworkopen.2020.35792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DH. Analysis on accessibility to medical services and reinterpreting coverage rate of the National Health Insurance by income level. Korean J Insur. 2018;114:85–112. [Google Scholar]
- 34.Heo J, Oh J, Kim J, Lee M, Lee JS, Kwon S, et al. Poverty in the midst of plenty: unmet needs and distribution of health care resources in South Korea. PLoS One. 2012;7:e51004. doi: 10.1371/journal.pone.0051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JI, Baek H, Jung HH. Prevalence of chronic kidney disease in Korea: the Korean National Health and Nutritional Examination Survey 2011-2013. J Korean Med Sci. 2016;31:915–23. doi: 10.3346/jkms.2016.31.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Operational definitions of type 2 diabetes mellitus and its related comorbidities
Lists of each class of drugs
Baseline characteristics of the study population (2015 to 2019)
Factors associated with SGLT2i initiation among patients with type 2 diabetes mellitus and ASCVD
Factors associated with SGLT2i initiation among patients with type 2 diabetes mellitus and heart failure
Prevalence of atherosclerotic cardiovascular disease (ASCVD) in Korean patients with type 2 diabetes mellitus (T2DM). The presence of ASCVD were determined according to whether International Classification of Disease, 10th revision (ICD-10) code corresponding to ASCVD present in each given year.
Prescription rate of sodium-glucose cotransporter 2 inhibitor by region in type 2 diabetes mellitus with established atherosclerotic cardiovascular disease in 2019.
Proportion of those prescribed sodium-glucose cotransporter 2 inhibitor (SGLT2i) in patients with type 2 diabetes mellitus (T2DM) or accompanied atherosclerotic cardiovascular disease (ASCVD) according to gender, age, and income level in 2019. NS, not significant. aP<0.0001.