Abstract
Background
Venoarterial extracorporeal membrane oxygenation (va-ECMO) is an advanced life support for critically ill patients with refractory cardiogenic shock. This temporary support bridges time for recovery, permanent assist, or transplantation in patients with high risk of mortality. However, the benefit of this modality is still subject of discussion and despite the continuous development of critical care medicine, severe cardiogenic shock remains associated with high mortality. Therefore, this work aims to analyze the current literature regarding in-hospital mortality and complication rates of va-ECMO in patients with cardiogenic shock.
Methods
We conducted a systematic review and meta-analysis of the most recent literature to analyze the outcomes of va-ECMO support. Using the PRISMA guidelines, Medline (PubMed) and Scopus (Elsevier) databases were systematically searched up to May 2022. Meta-analytic pooled estimation of publications variables was performed using a weighted random effects model for study size.
Results
Thirty-two studies comprising 12756 patients were included in the final analysis. Between 1994 and 2019, 62% (pooled estimate, 8493/12756) of patients died in the hospital. More than one-third of patients died during ECMO support. The most frequent complications were renal failure (51%, 693/1351) with the need for renal replacement therapy (44%, 4879/11186) and bleeding (49%, 1971/4523), bearing the potential for permanent injury or death. Univariate meta-regression analyses identified age over 60 years, shorter ECMO duration and presence of infection as variables associated with in-hospital mortality, while the studies reporting a higher incidence of cannulation site bleeding were unexpectedly associated with a reduced in-hospital mortality.
Conclusions
Extracorporeal membrane oxygenation is an invasive life support with a high risk of complications. We identified a pooled in-hospital mortality of 62% with patient age, infection and ECMO support duration being associated with a higher mortality. Protocols and techniques must be developed to reduce the rate of adverse events. Finally, randomized trials are necessary to demonstrate the effectiveness of va-ECMO in cardiogenic shock.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-022-01067-9.
Keywords: Adverse events, Cardiogenic shock, Complications, Extracorporeal life support, ECMO, Mortality, Venoarterial extracorporeal membrane oxygenation
Background
Extracorporeal membrane oxygenation (ECMO) is an advanced life support modality for critically ill patients with refractory respiratory or cardiac failure. The first reports of prolonged extracorporeal oxygenation of a patient with severe respiratory failure date from 1971 and present the beginning of ECMO support as we know it today [1, 2]. This temporary support for cardiorespiratory failure bridges time for recovery, permanent assistance, or transplantation. It is used as a last resort in severe respiratory failure as a venovenous (vv-ECMO) and in a cardiogenic shock as a venoarterial (va-ECMO) configuration [3]. In the last decades, ECMO support has been used increasingly in a variety of clinical presentations, like bridging to lung or heart transplant, resuscitation of patients with severe traumas, or extracorporeal-assisted rewarming (ECAR) of accidental hypothermia. The Extracorporeal Life Support Organization (ELSO) recommends the initiation of ECMO support in case of cardiorespiratory failure with a high risk of mortality (80%)[4].
Based on the data from 543 ELSO centers, more than 170,000 ECMOs were employed until the end of 2021. The number of ECMO support cases increased gradually in the last 10 years, especially since the outbreak of coronavirus disease 2019 (COVID-19). The reported survival rate to discharge or transfer of all adult ECMO patients was 49%, with 58% in case of respiratory failure and 45% for cardiac failure [5]. However, these data originate only from the ELSO registered centers, missing the data from other centers and introducing a potential selection bias.
The overall benefit, adverse events, and mortality rate are still the subject of discussion. Despite the continuous development of critical care, severe cardiogenic shock is still associated with high mortality [6–9]. Although an increasing number of studies, with a larger number of patients, report on adverse events associated with ECMO support, the approximate rates of complications are still very heterogeneous, in part because of small study populations [10]. Multiple studies strived to evaluate the potential benefit of ECMO support, but due to methodological issues, its efficacy remains controversial [11–13].
Given the above, our study aims to summarize the evidence and provide a comprehensive review of va-ECMO support outcomes in adult patients with refractory cardiogenic shock. We conducted a meta-analysis to examine mortality and complication rates in published studies, and we provide a summary of the demographic and clinical characteristics of critically ill patients undergoing ECMO support.
Materials and methods
We conducted a systematic review and meta-analysis of all studies reporting on va-ECMO support, complying with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Additional file 1: Table S1) [14]. This study is registered in the International prospective register of systematic reviews (PROSPERO) under number CRD42022326365.
The primary endpoint was the estimation of in-hospital mortality associated with the use of va-ECMO in patients with cardiogenic shock. Secondary endpoints included analysis of data on individual adverse events and mortality (in-hospital mortality, brain death, and death during ECMO support). The included studies comprised patients who underwent va-ECMO support, reporting on the incidence of adverse events and mortality. As our review and meta-analysis primarily aims at describing patient outcomes, we did not cover any potential comparators to the applied interventions, Additional file 1: Table S2.
Search
A systematic literature search was performed in Medline (PubMed) and Scopus (Elsevier) databases (data range up to May 1, 2022), using the combination of the following terms: ECMO, ECLS, ELS, extracorporeal, membrane, oxygen, life, support; fatal, death, mortality; complications and adverse (Additional file 1: Table S3). To ensure completeness of the search, we also searched the reference lists of the included studies, gray literature, and Google Scholar. In case of the full text of the study being not available, the authors of the studies were contacted. We included all studies reporting on (1) va-ECMO only, (2) both adverse events and in-hospital mortality, and (3) more than 100 patients with the patient follow-up to discharge from the hospital. Excluded were (1) all studies reporting on less than 100 patients, (2) reporting selectively on patients under 16 years, and (3) duplicate publications. Furthermore, we excluded studies with the main focus on extracorporeal support as a bridge to transplantation and durable mechanical circulatory support (e.g., ventricular assist device) or extracorporeal cardiopulmonary resuscitation (eCPR) as these groups of patients’ present extremes in means of patient outcome. To avoid overlapping of patients with original studies, systematic reviews and meta-analyses were excluded. Studies reporting on the results from the same institutions or ELSO registry were also excluded, as the overlapping of patients with the submitting center could not be excluded. Finally, we excluded studies with a main focus other than cardiogenic shock (i.e., transport of ECMO patients), or reported in other languages than English. Based on the methodology used in prior systematic reviews and meta-analyses, we chose a study sample size cutoff of 100 patients, to exclude the influence of case reports and small studies [15]. All study and data restrictions are presented in Additional file 1: Table S2.
The title and abstract screening were performed by 2 independent assessors (SR, DJ). Full-text articles of selected studies were reviewed and included if they met the inclusion eligibility criteria. In case of insufficient clarity in data presentation and presumable unreliable information, the study was excluded from the analysis. Any potential conflict in study selection was solved by reaching consensus in the research team.
Data extraction and synthesis
Two authors (SR, DJ) independently extracted relevant data regarding the basic study characteristics, patient demographics, reported complications, and mortality including the ECMO support technical information. Detailed information on the data extraction and synthesis is available in Additional file 1: Table S4. The definitions for reported outcomes were the ones adopted by the investigators of the included studies.
For comparison and to standardize the results of included studies, we performed simple calculations: (1) in case if only the female sex was reported, the number of male patients was calculated from the total number of patients, (2) percentage was computed into original values and original values into percentage where needed, (3) in case if outcome reported for compared groups, the overall sum was computed, and (4) ECMO support duration reported in hours was computed into days. All calculations were performed by 2 authors (SR, CO) independently.
Quality assessment
The methodological quality of studies was evaluated with the Newcastle–Ottawa scale for assessing the quality of nonrandomized studies in meta-analyses [16]. A study was considered to be of good quality if scored with 7 out of 9 Newcastle–Ottawa scale stars, fair if it achieved 5, and low-quality with less than 5 stars (Table 1). Two authors (SR, CO) independently evaluated the methodological quality of the studies; disagreements were resolved through consensus within the research team.
Table 1.
Characteristics of 32 included studies (n = 12,756)
| Authors | Country | Study period | Prospective study | Patients | Age (years) | BMI (kg/m2) | ECMO duration (days) | Pre-ECMO cardiac arrest | Population | Patient groups | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aso et al. [21] | Japan | 2010–2013 | No | 4658 | 64.8 | 2187 | Cardiogenic shock | Noneb | Good | ||
| Aubin et al. [54] | Germany | 2011–2015 | No | 160 | 56 | 4a | 102 | Refractory circulatory failure or cardiac arrest | Survivors and non-survivors | Fair | |
| Bonacchi et al. [38] | Multicentric | 2004–2018 | Yes | 209 | 67.5 | 25.8 | 5.3 | 13 | Postcardiotomy cardiogenic shock | Survivors and non-survivors | Fair |
| Cakici et al. [35] | Turkey | 2010–2015 | No | 148 | 56.6 | 25.2 | 5a | Cardiogenic shock | Distal perfusion catheter and arterial side-graft technique | Good | |
| Choi et al. [55] | Korea | 2004–2018 | No | 253 | 181 | AMI patients with cardiogenic shock | ECMO used before and after revascularization, and ECPR | Good | |||
| Elsharkawy et al. [56] | USA | 1995–2005 | No | 233 | Postcardiotomy patients | Survivors and non-survivors | Fair | ||||
| Fux et al. [57] | Sweden | 2006–2015 | No | 105 | 62a | 26.2a | 7a | 31 | Refractory postcardiotomy cardiogenic shock | Survivors and non-survivors | Good |
| Karatolios et al. [58] | Germany | 2014–2019 | No | 123 | 61.25 | 28.42 | 5.4a | 72 | Cardiogenic shock due to AMI, dilated cardiomyopathy, or myocarditis | Impella and va-ECMOc | Good |
| Laimoud et al. [39] | Saudi Arabia | 2015–2019 | No | 106 | 40.2 | 26.5 | Cardiogenic shock | Survivors and non-survivors | Good | ||
| Lan et al. [59] | Taiwan | 1994–2008 | No | 607 | 53.8 | Postcardiotomy cardiogenic shock, acute myocarditis, cardiomyopathy, AMI, and acute rejection after heart transplantation | Survivors and non-survivors | Good | |||
| Li et al. [60] | China | 2011–2012 | No | 123 | 56.2 | 4.4 | Postcardiotomy cardiogenic shock | Survivors and non-survivors | Good | ||
| Liao et al. [24] | China | 2008–2019 | No | 179 | 4.8 | 54 | Low cardiac output after open-heart surgery, AMI, cardiomyopathy, pulmonary embolism, myocarditis | Presence of lower limb ischemia | Good | ||
| Liem et al. [40] | USA | 2010–2018 | No | 102 | 52 | 11.2 | Cardiogenic shock | ECPR and cardiogenic shockb | Fair | ||
| Loforte et al. [25] | Italy | 2006–2012 | No | 228 | 58.3 | 10.9 | 29 | Postcardiotomy cardiogenic shock | Postcardiotomy, donor graft failure, AMI, heart failure, myocarditis | Good | |
| Lunz et al. [22] | Germany | 2010–2016 | No | 223 | 58.1a | 26.1a | 3a | 146 | Femoral Va-ECMO approach for low cardiac output, cardiac failure during coronary intervention and resuscitation | Presence of limb ischemia | Fair |
| Masha et al. [36] | USA | 2012–2016 | No | 223 | 144 | Cardiac arrest, cardiogenic shock, AMI, support of heart transplant or left ventricular assist device | Survivors and non-survivors | Good | |||
| Mazzeffi et al. [61] | USA | 2010–2015 | No | 121 | Postcardiotomy shock, other cardiogenic shock, or respiratory failure with cardiac dysfunction | ACT and aPTT | Good | ||||
| McCloskey et al. [62] | USA | 2000–2017 | No | 187 | 47 | 29.7 | 3.1a | Failed weaning from CPB, AMI, refractory arrhythmia, pulmonary embolism, decompensated heart failure and others | Survivors and non-survivors | Good | |
| Papadopoulos et al. [63] | Germany | 2001–2013 | No | 360 | 62 | 7 | 50 | Postcardiotomy cardiogenic shock | None | Fair | |
| Radakovic et al. [64] | Germany | 2010–2019 | No | 158 | Postcardiotomy shock, cardiac arrest, failed weaning from CPB, refractory cardiogenic shock, right-heart failure, and left-heart failure | Central and peripheral cannulation | Good | ||||
| Rastan et al. [65] | Germany | 1996–2008 | No | 517 | 63.5 | 3.3 | 32 | Postcardiotomy cardiogenic shock | Survivors and non-survivors | Fair | |
| Ro et al. [66] | Korea | 2005–2012 | No | 253 | 58.8 | 3a | 80 | Cardiogenic shock | Presence of IABP | Good | |
| Roth et al. [67] | Germany | 2011–2018 | No | 344 | 59 | 7.6 | Cardiogenic shock | Presence of thromboembolic complications | Good | ||
| Rubino et al. [37] | UK | 2008–2016 | No | 101 | 57.1 | 5a | Va-ECMO after cardiac surgery | Survivors and non-survivors | Good | ||
| Salna et al. [68] | USA | 2008–2019 | No | 431 | 60a | 28.3a | 4.5a | 42 | Postcardiotomy shock, AMI, acute decompensated heart failure, ECPR, primary graft dysfunction, unstable arrhythmia, pulmonary embolism, and others | Body mass index categories | Good |
| Son et al. [69] | USA | 2014–2018 | No | 105 | 54.9 | 5.6 | 48 | Cardiogenic shock or ECPR | Presence of acute limb ischemia | Good | |
| Toivonen et al. [70] | Multicentric | 2010–2018 | No | 781 | Postcardiotomy cardiogenic shock | Presence of neurologic injury | Good | ||||
| Vigneshwar et al. [71] | Multicentric | 2013–2018 | No | 789 | 54.8 | 29.8 | 4.3a | 119 | Postcardiotomy shock, cardiomyopathy, cardiac arrest, AMI, and acute rejection of heart transplant | Survivors and non-survivors | Good |
| Wood et al. [72] | USA | 2011–2018 | No | 203 | Cardiogenic shock, postcardiotomy shock, ECPR, acute pulmonary embolism and sepsis | Presence of anticoagulation | Good | ||||
| Wu et al. [73] | Taiwan | 2003–2009 | No | 110 | 60 | 6 | Postcardiotomy cardiogenic shock | Survivors and non-survivors | Fair | ||
| Yau et al. [74] | USA | 2011–2016 | No | 154 | 55 | 3.7a | Patients with femoral va-ECMO | None | Good | ||
| Zhigalov et al. [23] | Germany | 2009–2019 | No | 462 | 66.2 | 27.9 | 4a | 115 | Cardiogenic shock or ECPR | Presence of postcardiotomy | Good |
aMedian value. NOS: Newcastle–Ottawa scale score, AMI: acute myocardial infarction, va-ECMO: venoarterial extracorporeal membrane oxygenation, ECPR: extracorporeal cardiopulmonary reanimation, CPB: cardiopulmonary bypass, ACT: activated clotting time, aPTT: activated partial thromboplastin time, IABP: intra-aortic balloon pump
bAuthors included only the group of patients with cardiogenic shock in the analysis
cAuthors included only the group of patients with VA-ECMO in the analysis
Statistical assessment
Statistical analysis and visualizations were performed with “meta”, “metafor” and “dmetar” packages of R software environment version 4.0.0 (R Core Team 2020: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). For the pooling of single proportions, we used the inverse variance methods with logit transformation. Confidence intervals for individual studies were estimated with Clopper–Pearson method. The heterogeneity between-study and its possible causes were explored by Cochran’s Q test and t2 statistics, the Baujat plot, graphic display of heterogeneity analysis—GOSH [17, 18], and quantified with the I2 statistic. The univariate meta-regression analysis was used to identify potential predictors. Further subgroup analyses were performed to evaluate whether the prespecified study characteristics could account for the overall in-hospital mortality (i.e., prospective, or retrospective data collection, study setting, geographical region, publication year, period, and duration of data collection, reporting on less or more of 200 patients, the proportion of male patients, including of ECPR patients and their fraction). Publication bias was assessed using trim and fill [19], contour-enhanced funnel plot [20], and Egger’s test. To confirm the consistency of the main analysis, the sensitivity analysis was performed by excluding the potential effect of influence study on the results of the meta-analysis. A significance level of 0.05 was applied.
Results
Search results and description of studies
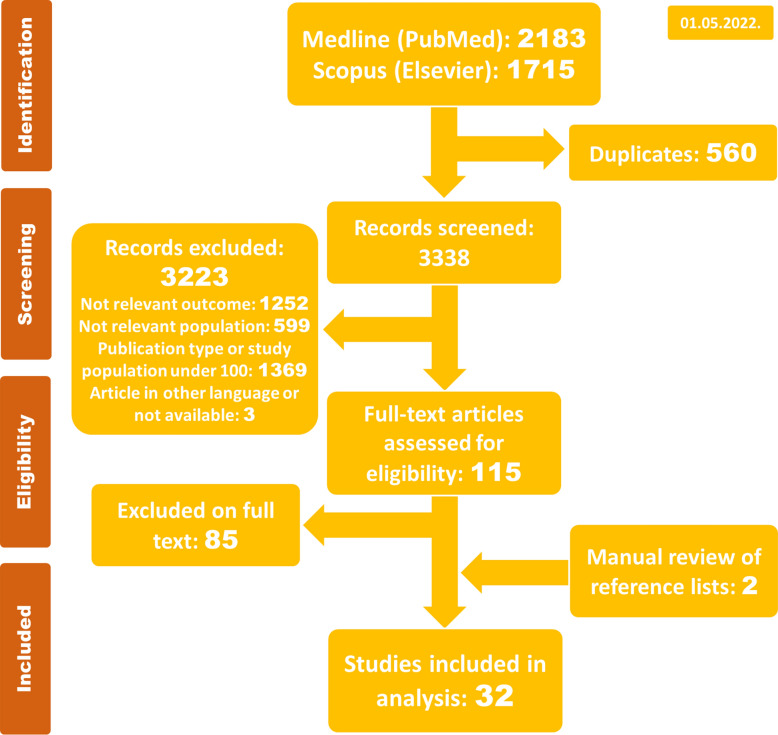
The systematic search yielded 2183 references in Medline via PubMed and 1715 in Scopus (Elsevier) database (May 1, 2022). After duplicates removal, a total of 3338 articles were selected for the titles and abstracts screening. In a second step, 3223 papers were excluded: 1369 due to publication type or patient population under 100, 599 addressed populations not relevant for the present analysis, and 1252 addressed irrelevant outcomes (Additional file 1: Table S2). A flow chart of the search process is illustrated in Fig. 1. The main excluded studies are presented in Additional file 1: Table S5. Thereby, 115 publications were selected for full-text screening, of which 85 were excluded once they reported a non-relevant outcome or used the same or similar patient data as other publications. Finally, our systematic assessment of studies comprised 32 publications, including 2 publications retrieved from the manual search of references [21, 22].
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart of the search process
The main features of the included studies are presented in Table 1. Analyzed articles reflect the situation from USA (n = 9), Germany (n = 8), Taiwan (n = 2), Korea (n = 2), China (n = 2), Italy (n = 1), Japan (n = 1), Saudi Arabia (n = 1), Sweden (n = 1), Turkey (n = 1) and United Kingdom (n = 1), and three multicentric publications. Twenty-five of the 32 studies reported on in-hospital mortality and more than 2 complications, while 7 addressed 2 or fewer complications. Only one study used the ELSO definition of bleeding [23]. The reviewed publications obtained the data on ECMO support outcomes mostly through prospective or retrospective databases, hospital records, administrative claims databases, and local or national registers. Finally, the methodological quality assessment of studies showed no low-quality study, 8 studies were rated as fair, and 24 as being of good quality (Table 1).
Patient population and outcomes
In the period from 1994 to 2019, a total of 12,756 patients received va-ECMO due to refractory cardiogenic shock. The analyzed population comprised of 69% male adult patients, with a pooled mean age of 61.1 years and a mean BMI of 28.2 kg/m2. ECMO support was employed for an average of 5.3 days. In total 3421 (pooled 30.2%) patients experienced cardiac arrest before or during ECMO support implantation, and only two studies [22, 24] reported on SOFA score (ranging from 11 to 12.5) and one on SAPS II score [25].
In total 8493 of 12,756 patients died during hospital stay. More than one-third of patients died during ECMO support (37.4% of all and 53.2% of all deceased patients). In-hospital mortality ranged from 40% to 75%, and 154 patients were diagnosed with brain death (pooled 13.1%, [95% CI 9.4; 17.8]), Additional file 1: Table S6.
The need for renal replacement therapy and the presence of limb ischemia were the most often reported adverse events (23 studies), followed by any bleeding and death on-support (15 and 14 studies, respectively), Table 2 and Additional file 1: Table S6.
Table 2.
Reporting of ECMO-related outcomes and complication rate
| Outcome | Number of studies reporting data (events/population) | Pooled rate (95%CI) | I2 (p value) | Reported range (%) |
|---|---|---|---|---|
| Mortality | ||||
| Brain death | 4 (154/1042) | 13.1 (9.4; 17.8) | 65% (0.035) | 7.6–16.6 |
| Death during ECMO | 14 (2909/7783) | 42.9 (38.3; 47.7) | 91% (< 0.001) | 33.4–53.5 |
| In-hospital mortality | 32 (8493/12756) | 62.2 (58.8; 65.5) | 92% (< 0.001) | 40.3–75.2 |
| Stroke | ||||
| CNS complications (not specified) | 7 (360/2450) | 12.5 (13.9; 16.8) | 82% (< 0.001) | 5.4–19.1 |
| Cerebral bleeding/hemorrhagic stroke | 12 (263/3969) | 5.6 (3.4; 9.0) | 92% (< 0.001) | 2.7–25.4 |
| Ischemic stroke | 13 (414/4371) | 9.8 (7.2; 13.1) | 89% (< 0.001) | 1.4–26.9 |
| Stroke (not otherwise specified) | 10 (206/2704) | 8.6 (5.3; 13.5) | 91% (< 0.001) | 2.3–25.5 |
| Renal failure | ||||
| Renal failure | 7 (693/1351) | 50.5 (31.7; 69.2) | 97% (< 0.001) | 9.5–85.7 |
| Renal replacement therapy | 23 (4879/11186) | 44.3 (39.2; 49.5) | 96% (< 0.001) | 10.3–70.5 |
| Infections | ||||
| Infection (not otherwise specified) | 4 (290/1395) | 18.8 (14.3; 24.2) | 80% (0.002) | 13.0–24.7 |
| Pneumonia | 8 (617/2298) | 23.7 (16.2; 33.3) | 95% (< 0.001) | 7.9–61.0 |
| Sepsis | 9 (489/2529) | 17.8 (14.3; 21.9) | 83% (< 0.001) | 6.4–28.2 |
| MODS | 3 (171/584) | 24.4 (13.3; 40.4) | 93% (< 0.001) | 8.9–37.3 |
| Bleeding | ||||
| Any bleeding | 15 (1971/4523) | 48.5 (40.6; 56.4) | 96% (< 0.001) | 17.8–97.0 |
| Surgical site bleeding | 2 (66/390) | 14.9 (3.7; 44.4) | 96% (< 0.001) | 7.4–27.3 |
| Cannulation site bleeding | 8 (224/1464) | 16.8 (11.1; 24.7) | 91% (< 0.001) | 3.9–28.4 |
| Gastrointestinal bleeding | 6 (74/1335) | 5.9 (3.3; 10.5) | 84% (< 0.001) | 0.9–12.3 |
| Cardiac tamponade | 3 (42/406) | 10.5 (7.9; 13.9) | 0% (0.423) | 8.4–12.7 |
| Pulmonary hemorrhage | 2 (24/305) | 8.4 (3.5; 18.7) | 79% (0.029) | 5.4–12.7 |
| Disseminated intravascular coagulation | 1 (26/148) | – | – | – |
| Hemolysis | 1 (6/462) | – | – | – |
| Thrombosis | ||||
| Any thrombosis | 5 (187/921) | 13.4 (6.7; 25.1) | 93% (< 0.001) | 2.0–34.0 |
| Limb ischemia | 23 (868/5932) | 12.2 (8.7; 16.9) | 96% (< 0.001) | 3.7–43.9 |
| Limb amputation | 5 (19/1368) | 1.5 (1.0; 2.3) | 0% (0.733) | 0.4–1.9 |
| Arterial thrombosis | 1 (63/344) | – | – | – |
| Venous thrombosis | 1 (11/344) | – | – | – |
| Pulmonary embolism | 1 (3/203) | – | – | – |
| Mechanical complications | ||||
| Circuit component clots | 3 (12/771) | 1.8 (0.5; 6.7) | 81% (0.006) | 0.9–5.7 |
| Oxygenator replacements | 4 (111/1253) | 9.0 (3.7; 20.3) | 92% (< 0.001) | 0.0–25.6 |
ECMO Extracorporeal membrane oxygenation, CNS Central nervous system, MODS Multiple organ dysfunction syndrome
Renal failure and any kind of bleeding, where the most often experienced adverse events (pooled 50.5% [95%CI 31.7; 69.2] and 48.5% [95%CI 40.6; 56.4], respectively), followed by the need for renal replacement therapy (44.3% [95%CI 39.2; 49.5]). However, differentiation of bleeding severity was impossible due to distinct variations in definitions, from any bleeding to bleeding requiring reoperation and blood product transfusion. Surgical and cannulation site bleeding were reported with 14.9% (95%CI 3.7; 44.4) and 16.8% (95%CI 11.1; 24.7), respectively. Thrombosis, as reported in five studies, had a pooled prevalence of 13.4% (95%CI 6.7; 25.1). Finally, pneumonia and sepsis had an incidence of 23.7% (95%CI 16.2; 33.3) and 17.8% (95%CI 14.3; 21.9), respectively (Table 2 and Additional file 1: Table S4).
Neurological complications were reported in more than half of the studies, with cerebral bleeding occurring in 5.6% (95%CI 3.4; 9.0) and ischemic stroke in 9.8% (95%CI 7.2; 13.1) of patients. The type of stroke was not specified in ten studies (pooled 8.6% [95%CI 5.3; 13.5]) and not-specified neurological complications were reported in 12.5% (95%CI 13.9; 16.8) of patients.
Regarding mechanical ECMO data, a centrifugal pump was used in all 11 studies, and 9 studies reported the use of UFH coated circuits. Information on the anticoagulation regime was provided in 17 studies (UFH), with the target ACT ranging from 140 to 220, and aPTT from 40 to 80 s, see Additional file 1: Table S7. None of the studies reported on the use of argatroban or other types of anticoagulation as primary anticoagulation strategy. Mechanical complications were rather seldom reported and affected 123 of 1821 patients, with most of them requiring oxygenator replacement (111 patients), Table 2.
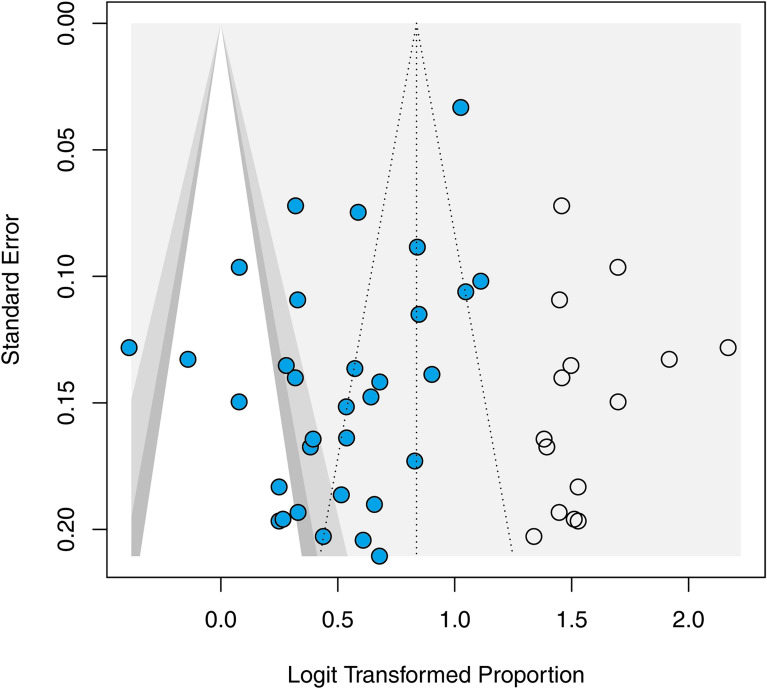
The publication bias of the studies included in the meta-analysis was confirmed using a funnel plot (Fig. 2), and with the linear regression test of funnel plot asymmetry (p = 0.021).
Fig. 2.
Funnel plot with the trim-and-fill method. Solid circles present the analysis of included studies. Open circles indicate missing studies imputed by the trim-and-fill method
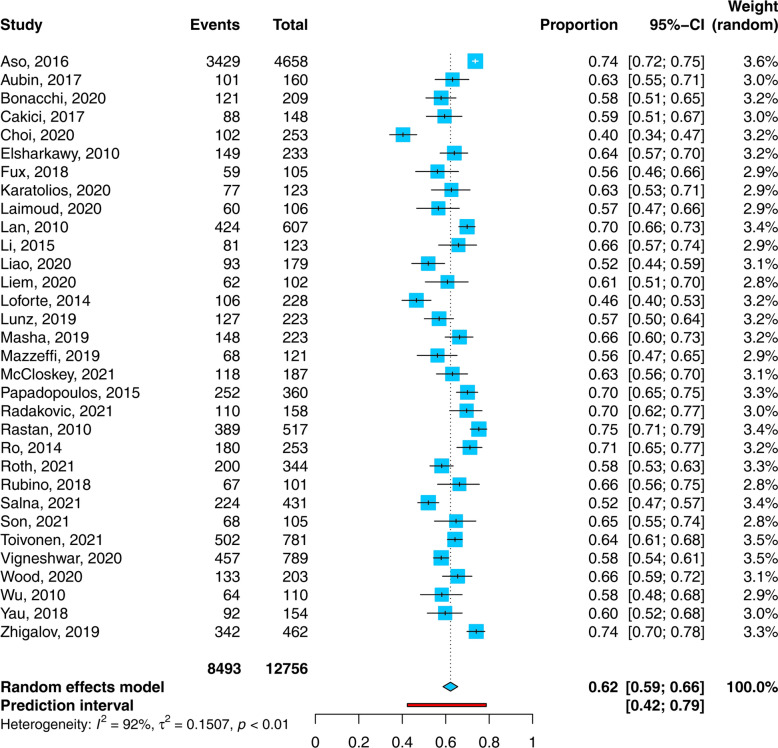
Pooled in-hospital mortality of patients with cardiogenic shock receiving ECMO support (32 studies, 12,756 patients) was 62.2% (95% CI 58.8; 65.5) with a heterogeneity of I2 = 92% (95%CI 89.9; 93.8; Q = 391.3, Tau2 = 0.151, p < 0.001), Fig. 3. Influence analysis revealed one influential study [21], Additional file 1: Figure S1. After its exclusion, the remaining 31 studies had 8098 patients with 5064 events and the pooled in-hospital mortality did not change (62%, 95%CI 59; 65, I2 = 87%), Additional file 1: Table S8. Subgroup analyses did not show a significant influence of analyzed parameters on the overall in-hospital mortality (Additional file 1: Table S9).
Fig. 3.
Forest plot: proportion of non-survivors among cardiogenic shock patients requiring extracorporeal membrane oxygenation support
Univariable meta-regression analyses identified age over 60 years (n = 23; b = 0.333; p = 0.013), shorter ECMO duration (n = 22; b = − 0.066; p = 0.048) and presence of infection (n = 4; b = 0.033; p = 0.017) being associated with in-hospital mortality. The cannulation site bleeding (n = 8; b = − 0.029; p < 0.001) was associated with reduced in-hospital mortality, Additional file 1: Table S9.
Discussion
The present systematic review and meta-analysis investigated the use of va-ECMO support in patients with refractory cardiogenic shock, including 32 studies with 12,756 patients. Our work presents the largest up-to-date analysis on va-ECMO outcomes in adult patients. We found a pooled in-hospital mortality as high as 62.2%, a slightly higher than reported currently by ELSO [5]. Moreover, we demonstrated an age over 60 years, a shorter ECMO duration and the presence of an infection being associated with an increased in-hospital mortality.
ECMO support and all-cause in-hospital mortality
Despite several randomized controlled trials being under way (ECLS-SHOCK-NCT03637205; EUROSHOCK-NCT03813134; ECMO-CS-NCT02301819; and ANCHOR-NCT04184635), the current evidence is based mostly on retrospective studies, systematic reviews, and meta-analyses. Moreover, previous meta-analyses included a rather smaller number of patients and reported on significant heterogeneity in the results [7, 26]. A meta-analysis from 2015 reported an in-hospital survival of 40.2% for patients receiving va-ECMO in cardiogenic shock and cardiac arrest (16 studies with 841 patients) [26]. The complication rates were particularly high for renal impairment (47.4%), infection (25.1%) and neurologic deficits (13.3%) [26]. More recently, another meta-analysis on outcomes of va-ECMO for refractory cardiogenic shock (5292 patients) reported a 43.0% in-hospital, a 36.7% 1-year, and a 29.9% 5-year survival [7]. At a first glance, our in-hospital mortality of 62% seems higher than the above-mentioned works. However, the current ELSO International Summary of Statistics report, including 50,371 ECMO runs in adults, reported a comparable 58.2% in-hospital mortality [5]. The on-support mortality was comparable as well (44.4% vs. 43% in our work). Furthermore, a recent ELSO database analysis of the association between mechanical unloading and va-ECMO outcomes (12,734 patients, mean age 53.7 years, median ECMO duration 4 days) reported an on-support and an in-hospital mortality of 46.2% and 58.6%, respectively [27]. The discrepancy of in-hospital mortality to our work may be partly explained by the selection of patients with peripheral cannulation only, their age, or the limitations inherent to the ELSO registry itself. Therefore, with our large data set, we were able to confirm the earlier observed high mortality.
Nearly one-third of the patients (pooled 30.2%, 95% CI 21.47; 40.7) experienced cardiac arrest before or during ECMO implantation. Surprisingly, cardiac arrest did not change mortality, which is a rather encouraging finding (Additional file 1: Table S9). These findings should support clinicians to indicate ECPR in well-selected patients and warrants the further emergence of ECPR.
Regardless of decades of research, the efficacy of ECMO support in cardiogenic shock has still to be proven. A retrospective trial from the US revealed a low mortality rate of 49% in about 800 ECMO runs in patients experiencing cardiogenic shock [11]. Surprisingly, in a matched cohort the mortality rate of patients without ECMO was as low as 4%. However, this study is limited by matching methods and missing the variables describing the clinical severity in both groups. It could be that the patients receiving ECMO support were simply sicker. This is in contrast to the recent ARREST trial, where Yannopoulous et al. observed a strikingly higher rate of survival to hospital discharge with va-ECMO support in an ongoing cardiac arrest compared to the standard advanced life support (43% vs. 7%) [28]. Whether such a benefit or otherwise of va-ECMO therapy compared to the standard of care is possible in cardiogenic shock (without cardiac arrest) will be evaluated in the ongoing prospective randomized trials EURO-SHOCK and ECLS-SHOCK.
Furthermore, a recent international survey from 60 countries demonstrated various therapy approaches in cardiogenic shock [29]. In the case of acute myocardial infarction, about 42% use percutaneous coronary intervention (PCI) if an electrocardiogram is suggestive of ischemia, one-third perform PCI to all patients in cardiogenic shock, whereas one-fifth only if the universal definition of myocardial infarction criteria are fulfilled. Given these different institutional approaches, the comparison of mechanical cardiac support in cardiogenic shock to therapy without it is hindered. However, the early use of revascularization therapy reduced the initially high mortality rates, but in-hospital mortality remains significant (27–51%) [30]. Despite an overall increase in PCIs, Amsterdam et al. recently even described a potential rise of mortality from 27% to 30% due to increasing patient complexity and care being more often delivered by less experienced lower volume centers [31]. Finally, an early invasive hemodynamic assessment may help the identification of cardiogenic shock phenotype, which is important for further treatment, since distinct etiologies may respond differently to medical and device-based management. Hopefully, the undergoing prospective trials will be able to shed light on these important issues.
Complications and adverse events during va-ECMO
Current literature comprises mostly smaller studies offering a wide range of reported complications, commonly without any standardization which may be attributed to the presence of different criteria for the identification and reporting of adverse events. However, the pooled rate of adverse events in our data should more accurately reflect the rate of expectable complications.
Comparable to literature, hemorrhage was the second most frequent complication in nearly half of the patients, which is in line with the largest meta-analysis so far, reporting any kind of hemorrhage in 40% [15]. Going into greater detail confirmed the cannulation and surgical area being the most common sites of bleeding [32–34]. However, the latter findings may be weakened by the fact that only one study reported on the use of the ELSO bleeding definition [23], despite the definition´s existence of more than 8 years.
Recently, a study from Turkey demonstrated a need for continuous renal replacement therapy in about one-quarter of 148 patients with refractory cardiogenic shock. In contrast to that, we found rates nearly as twice as high (44.3% vs. 24.4%) representing the most common complication [35]. Moreover, the same group reported renal failure in only 9.5%, which is inconsistent (1) with the higher rate of the continuous renal replacement therapy in the same study and (2) our findings in 1351 patients (renal failure in 50.5%). This may be due to the retrospective nature of the above-mentioned study. Furthermore, these authors used different definitions of renal failure. Masha et al. defined acute renal failure as serum creatinine increase of more than 1.5 mg/dl with or without renal replacement therapy [36]; Rubino et al. as renal impairment requiring continuous renal replacement therapy [37]; and Zhigalov et al. as a new renal dysfunction requiring renal replacement therapy or a rise in serum creatinine (greater than three times baseline or greater than 5 mg/dL) [23]. Other authors defined renal failure as an acute kidney injury or organ dysfunction, failing to provide a more detailed definition [35, 38–40]. Finally, our findings are in line with an earlier meta-analysis on ECMO in cardiogenic shock in nearly three-time smaller patient sample compared to ours [10]. Cheng et al. reported a pooled estimate rate for acute kidney injury of 55.6%, and need for continuous renal replacement therapy of 46.0%, respectively [10]. The marginal alteration in pooled prevalence may be explained by the greater availability of evidence due to increased number of studies, selection of studies with larger patient samples, and an overall sample of patients in the present analysis. Moreover, the potential impact of technological advances and critical care medicine development cannot be completely excluded.
The low rate of thrombosis is most probably due to an underestimation of thrombotic events and lacking of regular radiological investigations or post-mortem examinations in the majority of the predominantly retrospective studies [41].
Risk factors for mortality
Univariable meta-regression analyses identified age over 60 years, shorter ECMO duration and presence of infection as variables associated with in-hospital mortality. The studies reporting a higher incidence of cannulation site bleeding were unexpectedly associated with a reduced in-hospital mortality.
The role of the age in va-ECMO support of patients with cardiogenic shock remains controversial. According to the ELSO guidelines, there is no defined age cutoff, but an age-related risk should be considered [4, 42]. In the case of a COVID-19-related ECMO indication, ELSO defined an age of more than 65 years as a relative and an even higher age as an absolute contraindication for ECMO initiation [43]. A recent retrospective study pointed out the importance of a patient-oriented and individualized approach in decision-making related to ECMO support initiation, arguing against the use of any age-related cutoffs [44]. In our work, an age of more than 60 years (median age of reported population), was associated with increased mortality (Additional file 1: Figure S2). However, as age alone should not be a risk factor, decision-making should be focused on the severity of the disease in combination with comorbidities, frailty, and rehabilitation potential [44, 45].
We found a shorter ECMO support duration being associated with an increased mortality, which is presumably rather a consequence than a mortality influencing factor itself. Most likely the share of patients with shorter than average ECMO runs will experience the more severe underlying pathologies. A further possible explanation may be the presence of the immortal time bias, as patients dying early on ECMO support may not have had enough time for organ recovery [46]. Moreover, the sickest patients may die anyway, regardless of ECMO support. Finally, despite the different setting in vv-ECMO, these findings are consistent with a meta-analysis reporting on vv-ECMO [47].
Our meta-analysis revealed the presence of infection as another risk factor for increased mortality which is based on the data from only four studies. Even with scant evidence on infection during ECMO support, the ELSO registry analysis reported a prevalence of 10–12% [48–50]. Our higher pooled incidence of 18% may be explained by a still small patient sample and seldom reporting on infections as complications of ECMO support. Clearly, further research should focus on more detailed reporting of infections (local and systemic) and its influence on outcomes.
Finally, studies with lower overall mortality reported a higher incidence of cannulation site bleeding. This is surprising as one would assume the opposite. However, as longer va-ECMO is needed, the possibility of adverse events in general may increase, including cannulation site bleeding. Moreover, the delayed minor bleeding during the ECMO course may be associated with a longer anticoagulation exposure and cumulative risk of hemorrhage. The meta-regression did not identify further factors that could be associated with mortality (Additional file 1: Table S9).
Strengths and limitations
The strengths of this work include robust inclusion and clear exclusion criteria. Our work included 32 publications with 12756 patients from almost all continents with at least fair quality of data. Moreover, we controlled for potential overlapping of patients within different studies, by excluding the studies from the same institutions and from the ELSO registry. Despite all the benefits international registers might provide, these results may not completely represent the real-life situation worldwide, as notification and selection bias may affect studies based on a big database. The inclusion of patients in the ELSO register is voluntary, and sites participating in the network are not a random sample of all centers utilizing ECMO support, but selected centers which guaranteed their membership by paying the membership fee [51]. Recent research of the Society of Thoracic Surgeons databases suggested that selected participant centers may improve quality and outcomes, simply by the feedback of collected data, consequently increasing institutional awareness and self-examination, making these systematically different from nonparticipant centers [52]. Therefore, our study presents the result of a predefined and systematically conducted search of two large international scientific databases, where authors can make their work available to the widest audience, independent of their status. Finally, this work is reported according to the recommendations of the PRISMA checklist, addressing all 27 items (Additional file 1: Table S1).
Nevertheless, this work has several limitations. The quality of our results is as strong as all included studies, given the retrospective and single-center nature of most of the studies. Publication and retrieval bias may have arisen, as studies may neither be available, nor published in the searched databases. Moreover, we excluded all studies reporting on less than 100 patients, to reduce the influence of case reports and small studies on the overall outcome. The majority of studies reported on adverse events missed to precisely define the outcome of interest, making a comparison between the studies at least complex. The ELSO definition of major and minor bleeding was used in only one study. Moreover, recommendations on reporting on outcomes and adverse events during ECMO are still missing, in contrast to the minimum reporting criteria for cardiopulmonary bypass [53]. The diversity of study questions led to different variable reporting, so well-established scores to evaluate the severity of the underlying disease were rather seldom reported (SOFA score, APACHE II, SAPS II or III). Furthermore, the majority of reported factors included between 6 and 12 data points. This may reduce the strength of the potential association between examined factors and in-hospital mortality.
Heterogeneity is a well-known limitation of observational retrospective studies, and the high heterogeneity levels observed implied the increased variance of analyzed studies. Therefore, the result of our analysis should be interpreted with caution, as the meta-analytic portion of our work may be limited by heterogeneity observed across studies.
Moreover, we cannot clarify if the observed rates of some adverse events reflect the consequence of ECMO itself or the severity of underlying disease independent of ECMO. It is most likely that complications such as cannulation site bleeding, limb ischemia, and amputation are more directly related to ECMO procedure, whereas renal failure, infections, and stroke may be consequences of cardiogenic shock, and joint effects of comorbidities and critical illness.
Conclusions
Despite the high rates of mortality in refractory cardiogenic shock, ECMO support can prolong the therapeutic window potentially allowing the heart to recover. This large meta-analysis comprising 12756 patients identified a pooled in-hospital mortality of 62%. Furthermore, patient age, presence of infection and shorter ECMO support duration have been shown to be independently associated with an increased in-hospital mortality. Moreover, adverse events during ECMO support are frequent and with potential for permanent injury or death. Renal failure with the need for renal replacement therapy and bleeding occurrence are the complications with the highest incidence. Protocols and techniques must be developed to reduce the rate of adverse events. Furthermore, use of pre-defined reporting criteria on ECMO is warranted. Finally, randomized trials are necessary to demonstrate the effectiveness of va-ECMO in cardiogenic shock.
Supplementary Information
Additional file1: Table S1. Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015 checklist: recommended items to address in a systematic review protocol. Table S2. PICOS criteria for inclusion and exclusion of publications. Table S3. Search strategy. Table S4. Detailed information on the data extraction and synthesis. Table S5. Main excluded studies. Table S6. Reported ECMO adverse events in the included studies (n = 32). Table S7. Patient anticoagulation and ECMO characteristics of the included studies (n = 32). Table S8. Influence analysis of studies reporting on in-hospital ECMO mortality. Table S9. Univariable meta-regression analyses. Figure S1. Influence analysis of studies reporting on in-hospital mortality. Figure S2. Meta-regression: scattered-plot of the relationship between age and in-hospital mortality.
Abbreviations
- ACT
Activated clotting time
- aPTT
Activated partial thromboplastin time
- AMI
Acute myocardial infarction
- CNS
Central nervous system
- CPB
Cardiopulmonary bypass
- ECMO
Extracorporeal membrane oxygenation
- ECPR
Extracorporeal cardiopulmonary resuscitation
- ELSO
Extracorporeal life support organization
- DIC
Disseminated intravascular coagulation
- IABP
Intra-aortic balloon pump
- MODS
Multiple organ dysfunction syndrome
- NOS
Newcastle–Ottawa scale score
- va-ECMO
Venoarterial extracorporeal membrane oxygenation
- UFH
Unfractionated heparin
Author contributions
Conceptualization, SR, BZ and BT; data curation, SR, ZB, MPK and DJ; formal analysis, SR and ZB; investigation, SR, MPK, CO, DJ and BT; methodology, SR, ZB and BT; project administration, SR and BT; resources, SR and BT; software, SR and ZB; supervision, SR and BT; validation, SR, RB, ZB and BT; visualization, SR, ZB and BT; writing—original draft, SR, RB, DJ, ZB, MPK, OK and BT; writing—review and editing, SR, RB, DJ, ZB, MPK, OK and BT. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The additional data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rajsic Sasa and Treml Benedikt are contributed equally to this work
Contributor Information
Sasa Rajsic, Email: sasa.rajsic@i-med.ac.at.
Benedikt Treml, Email: benedikt.treml@i-med.ac.at.
Zoran Bukumiric, Email: zoran.bukumiric@med.bg.ac.rs.
References
- 1.Featherstone PJ, Ball CM. The early history of extracorporeal membrane oxygenation. Anaesth Intensive Care. 2018;46(6):555–557. doi: 10.1177/0310057X1804600601. [DOI] [PubMed] [Google Scholar]
- 2.Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome) use of the Bramson membrane lung. New Engl J Med. 1972;286(12):629–634. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 3.Brasseur A, Scolletta S, Lorusso R, Taccone FS. Hybrid extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(Suppl 5):S707–S715. doi: 10.21037/jtd.2018.03.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek GJ. Extracorporeal life support: the ELSO red book. 5th edition. ed. Ann Arbor, Michigan: Extracorporeal Life Support Organization; 2017. 831 p.
- 5.Extracorporeal Life Support Organization (ELSO). Registry Report on Extracorporeal Life Support, International Summary. 2022. https://www.elso.org/Registry/InternationalSummaryandReports/InternationalSummary.aspx.
- 6.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction. Circulation. 2009;119(9):1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson-Smith AR, Bogdanova Y, Roydhouse S, Phan K, Tian DH, Yan TD, et al. Outcomes of venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock: systematic review and meta-analysis. Annals Cardiothorac Surg. 2019;8(1):1–8. doi: 10.21037/acs.2018.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napp LC, Kühn C, Bauersachs J. ECMO in cardiac arrest and cardiogenic shock. Herz. 2017;42(1):27–44. doi: 10.1007/s00059-016-4523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayer GT, Baker JN, Parks KA. Heart rescue: the role of mechanical circulatory support in the management of severe refractory cardiogenic shock. Curr Opin Crit Care. 2012;18(5):409–416. doi: 10.1097/MCC.0b013e328357f1e6. [DOI] [PubMed] [Google Scholar]
- 10.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 11.El Sibai R, Bachir R, El Sayed M. Outcomes in cardiogenic shock patients with extracorporeal membrane oxygenation use: a matched cohort study in hospitals across the United States. Biomed Res Int. 2018;2018:2428648. doi: 10.1155/2018/2428648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjar LA, Teboul JL. Mechanical circulatory support devices for cardiogenic shock: state of the art. Crit Care. 2019;23(1):76. doi: 10.1186/s13054-019-2368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zavalichi MA, Nistor I, Nedelcu AE, Zavalichi SD, Georgescu CMA, Stătescu C, et al. Extracorporeal membrane oxygenation in cardiogenic shock due to acute myocardial infarction: a systematic review. Biomed Res Int. 2020;2020:6126534. doi: 10.1155/2020/6126534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Critic Care Resusc. 2013;15(3):172–178. [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, OConnell D, Peterson J, Welch V, Losos M, , et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. New York: Oxford; 2000. [Google Scholar]
- 17.Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21(18):2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 18.Olkin I, Dahabreh IJ, Trikalinos TA. GOSH - a graphical display of study heterogeneity. Res Synth Methods. 2012;3(3):214–223. doi: 10.1002/jrsm.1053. [DOI] [PubMed] [Google Scholar]
- 19.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care. 2016;20:80. doi: 10.1186/s13054-016-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunz D, Philipp A, Müller T, Pfister K, Foltan M, Rupprecht L, et al. Ischemia-related vascular complications of percutaneously initiated venoarterial extracorporeal membrane oxygenation: indication setting, risk factors, manifestation and outcome. J Crit Care. 2019;52:58–62. doi: 10.1016/j.jcrc.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhigalov K, Sá MPBO, Safonov D, Zagitov I, Alofesh A, Pavlova V, et al. Clinical outcomes of venoarterial extracorporeal life support in 462 patients: single-center experience. Artif Organs. 2020;44(6):620–627. doi: 10.1111/aor.13625. [DOI] [PubMed] [Google Scholar]
- 24.Liao X, Cheng Z, Wang L, Li B, Huang W, Wen J, et al. Vascular complications of lower limb ischemia in patients with femoral venoarterial extracorporeal membrane oxygenation. Heart Surg Forum. 2020;23(3):E305–E309. doi: 10.1532/hsf.2969. [DOI] [PubMed] [Google Scholar]
- 25.Loforte A, Marinelli G, Musumeci F, Folesani G, Pilato E, Martin Suarez S, et al. Extracorporeal membrane oxygenation support in refractory cardiogenic shock: treatment strategies and analysis of risk factors. Artif Organs. 2014;38(7):E129–E141. doi: 10.1111/aor.12317. [DOI] [PubMed] [Google Scholar]
- 26.Xie A, Phan K, Tsai YC, Yan TD, Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a meta-analysis. J Cardiothorac Vasc Anesth. 2015;29(3):637–645. doi: 10.1053/j.jvca.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Grandin EW, Nunez JI, Willar B, Kennedy K, Rycus P, Tonna JE, et al. Mechanical left ventricular unloading in patients undergoing venoarterial extracorporeal membrane oxygenation. J Am Coll Cardiol. 2022;79(13):1239–1250. doi: 10.1016/j.jacc.2022.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavazzi G, Rossello X, Grand J, Gierlotka M, Sionis A, Ahrens I, et al. Epidemiology, monitoring, and treatment strategy in cardiogenic shock a multinational cross-sectional survey of ESC-acute cardiovascular care association research section. Eur Heart J Acute Cardiovasc Care. 2022 doi: 10.1093/ehjacc/zuac087. [DOI] [PubMed] [Google Scholar]
- 30.Diepen SV, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American heart association. Circulation. 2017;136(16):232–268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 31.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6(1):97. doi: 10.1186/s13613-016-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oude Lansink-Hartgring A, de Vries AJ, Droogh JM, van den Bergh WM. Hemorrhagic complications during extracorporeal membrane oxygenation—the role of anticoagulation and platelets. J Crit Care. 2019;54:239–243. doi: 10.1016/j.jcrc.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Rajsic S, Breitkopf R, Oezpeker UC, Bukumirić Z, Dobesberger M, Treml B. The role of excessive anticoagulation and missing hyperinflammation in ECMO-associated bleeding. J Clin Med. 2022;11(9):2314. doi: 10.3390/jcm11092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cakici M, Ozcinar E, Baran C, Bermede AO, Sarıcaoglu MC, Inan MB, et al. A retrospective cohort analysis of percutaneous versus side-graft perfusion techniques for veno-arterial extracorporeal membrane oxygenation in patients with refractory cardiogenic shock. Perfusion. 2017;32(5):363–371. doi: 10.1177/0267659116683792. [DOI] [PubMed] [Google Scholar]
- 36.Masha L, Peerbhai S, Boone D, Shobayo F, Ghotra A, Akkanti B, et al. Yellow means caution: correlations between liver injury and mortality with the use of VA-ECMO. ASAIO J. 2019;65(8):812–818. doi: 10.1097/MAT.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 37.Rubino A, Costanzo D, Stanszus D, Valchanov K, Jenkins D, Sertic F, et al. Central Veno-arterial extracorporeal membrane oxygenation (C-VA-ECMO) after cardiothoracic surgery: a single-center experience. J Cardiothorac Vasc Anesth. 2018;32(3):1169–1174. doi: 10.1053/j.jvca.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Bonacchi M, Cabrucci F, Bugetti M, Dokollari A, Parise O, Sani G, et al. Outcomes' predictors in post-cardiac surgery extracorporeal life support. An observational prospective cohort study. International J Surg. 2020;82:56–63. doi: 10.1016/j.ijsu.2020.07.063. [DOI] [PubMed] [Google Scholar]
- 39.Laimoud M, Alanazi M. The clinical significance of blood lactate levels in evaluation of adult patients with veno-arterial extracorporeal membrane oxygenation. The Egyptian Heart J. 2020;72(1):74. doi: 10.1186/s43044-020-00108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liem S, Cavarocchi NC, Hirose H. Comparing in-patient extracorporeal cardiopulmonary resuscitation to standard cardiac treatment group of extracorporeal membrane oxygenation patients: 8 years of experience at a single institution. Perfusion. 2020;35(1):73–81. doi: 10.1177/0267659119860735. [DOI] [PubMed] [Google Scholar]
- 41.Rastan AJ, Lachmann N, Walther T, Doll N, Gradistanac T, Gommert JF, et al. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO) Int J Artif Organs. 2006;29(12):1121–1131. doi: 10.1177/039139880602901205. [DOI] [PubMed] [Google Scholar]
- 42.Tonna JE, Abrams D, Brodie D, Greenwood JC, Mateo-Sidron RUBIOJA, Usman A, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO) ASAIO J. 2021;67(6):601–610. doi: 10.1097/MAT.0000000000001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66(7):707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treml B, Breitkopf R, Bukumirić Z, Bachler M, Boesch J, Rajsic S. ECMO predictors of mortality: a 10-year referral centre experience. J Clin Med. 2022;11(5):1224. doi: 10.3390/jcm11051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso-Fernandez-Gatta M, Merchan-Gomez S, Toranzo-Nieto I, Gonzalez-Cebrian M, Diego-Nieto A, Barrio A, et al. Short-term mechanical circulatory support in elderly patients. Artif Organs. 2022;46(5):867–877. doi: 10.1111/aor.14117. [DOI] [PubMed] [Google Scholar]
- 46.Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009;37(11):2939–2945. doi: 10.1097/CCM.0b013e3181b7fbbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Critic Care. 2021;25(1):211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50(1):9–16. doi: 10.1016/j.ijantimicag.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Vogel AM, Lew DF, Kao LS, Lally KP. Defining risk for infectious complications on extracorporeal life support. J Pediatr Surg. 2011;46(12):2260–2264. doi: 10.1016/j.jpedsurg.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Critic Care Med. 2011;12(3):277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 51.Extracorporeal Life Support Organization (ELSO). Become A Member of Extracorporeal Life Support Organization. 2022. https://www.elso.org/AboutUs/JoinELSO.aspx. Accessed 15 Aug 2022.
- 52.Grover FL, Shroyer AL, Hammermeister K, Edwards FH, Ferguson TB, Jr, Dziuban SW, Jr, et al. A decade's experience with quality improvement in cardiac surgery using the Veterans Affairs and Society of Thoracic Surgeons national databases. Ann Surg. 2001;234(4):464–472. doi: 10.1097/00000658-200110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landis RC, Arrowsmith JE, Baker RA, de Somer F, Dobkowski WB, Fisher G, et al. Consensus statement: defining minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum. 2008;11(5):E316–E322. doi: 10.1532/HSF98. [DOI] [PubMed] [Google Scholar]
- 54.Aubin H, Petrov G, Dalyanoglu H, Richter M, Saeed D, Akhyari P, et al. Four-year experience of providing mobile extracorporeal life support to out-of-center patients within a suprainstitutional network-outcome of 160 consecutively treated patients. Resuscitation. 2017;121:151–157. doi: 10.1016/j.resuscitation.2017.08.237. [DOI] [PubMed] [Google Scholar]
- 55.Choi KH, Yang JH, Hong D, Park TK, Lee JM, Song YB, et al. Optimal timing of venoarterial-extracorporeal membrane oxygenation in acute myocardial infarction patients suffering from refractory cardiogenic shock. Circ J. 2020;84(9):1502–1510. doi: 10.1253/circj.CJ-20-0259. [DOI] [PubMed] [Google Scholar]
- 56.Elsharkawy HA, Li L, Esa WA, Sessler DI, Bashour CA. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24(6):946–951. doi: 10.1053/j.jvca.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Fux T, Holm M, Corbascio M, Lund LH, van der Linden J. Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock: risk factors for mortality. J Thorac Cardiovasc Surg. 2018;156(5):1894–902.e3. doi: 10.1016/j.jtcvs.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 58.Karatolios K, Chatzis G, Markus B, Luesebrink U, Ahrens H, Divchev D, et al. Comparison of mechanical circulatory support with venoarterial extracorporeal membrane oxygenation or Impella for patients with cardiogenic shock: a propensity-matched analysis. Clin Res Cardiol. 2021;110(9):1404–1411. doi: 10.1007/s00392-020-01777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lan C, Tsai PR, Chen YS, Ko WJ. Prognostic factors for adult patients receiving extracorporeal membrane oxygenation as mechanical circulatory support–a 14-year experience at a medical center. Artif Organs. 2010;34(2):E59–64. doi: 10.1111/j.1525-1594.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- 60.Li CL, Wang H, Jia M, Ma N, Meng X, Hou XT. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. 2015;149(5):1445–1450. doi: 10.1016/j.jtcvs.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 61.Mazzeffi MA, Tanaka K, Roberts A, Rector R, Menaker J, Kon Z, et al. Bleeding, thrombosis, and transfusion with two heparin anticoagulation protocols in venoarterial ECMO patients. J Cardiothorac Vasc Anesth. 2019;33(5):1216–1220. doi: 10.1053/j.jvca.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 62.McCloskey CG, Engoren MC. Transfusion and its association with mortality in patients receiving veno-arterial extracorporeal membrane oxygenation. J Crit Care. 2022;68:42–47. doi: 10.1016/j.jcrc.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 63.Papadopoulos N, Marinos S, El-Sayed Ahmad A, Keller H, Meybohm P, Zacharowski K, et al. Risk factors associated with adverse outcome following extracorporeal life support: analysis from 360 consecutive patients. Perfusion. 2015;30(4):284–290. doi: 10.1177/0267659114542458. [DOI] [PubMed] [Google Scholar]
- 64.Radakovic D, Hamouda K, Penov K, Bening C, Sayed S, Gietzen C, et al. Central versus peripheral arterial cannulation for veno-arterial extracorporeal membrane oxygenation in post-cardiotomy patients. ASAIO J. 2021;67(1):67–73. doi: 10.1097/MAT.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 65.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139(2):302–311. doi: 10.1016/j.jtcvs.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 66.Ro SK, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Extracorporeal life support for cardiogenic shock: influence of concomitant intra-aortic balloon counterpulsation. Eur J Cardio Thorac Surg. 2014;46(2):186–192. doi: 10.1093/ejcts/ezu005. [DOI] [PubMed] [Google Scholar]
- 67.Roth S, Jansen C, M'Pembele R, Stroda A, Boeken U, Akhyari P, et al. Fibrinogen-albumin-ratio is an independent predictor of thromboembolic complications in patients undergoing VA-ECMO. Sci Rep. 2021;11(1):16648. doi: 10.1038/s41598-021-95689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salna M, Fried J, Kaku Y, Brodie D, Sayer G, Uriel N, et al. Obesity is not a contraindication to veno-arterial extracorporeal life support. Eur J Cardio Thorac Surg. 2021;60(4):831–838. doi: 10.1093/ejcts/ezab165. [DOI] [PubMed] [Google Scholar]
- 69.Son AY, Khanh LN, Joung HS, Guerra A, Karim AS, McGregor R, et al. Limb ischemia and bleeding in patients requiring venoarterial extracorporeal membrane oxygenation. J Vasc Surg. 2021;73(2):593–600. doi: 10.1016/j.jvs.2020.05.071. [DOI] [PubMed] [Google Scholar]
- 70.Toivonen F, Biancari F, Dalén M, Dell'Aquila AM, Jónsson K, Fiore A, et al. Neurologic injury in patients treated with extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock. J Cardiothorac Vasc Anesth. 2021;35(9):2669–2680. doi: 10.1053/j.jvca.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Vigneshwar NG, Kohtz PD, Lucas MT, Bronsert M, JW M, FM M, et al. Clinical predictors of in-hospital mortality in venoarterial extracorporeal membrane oxygenation. J Cardiac Surg. 2020;35(10):2512–2521. doi: 10.1111/jocs.14758. [DOI] [PubMed] [Google Scholar]
- 72.Wood KL, Ayers B, Gosev I, Kumar N, Melvin AL, Barrus B, et al. Venoarterial-extracorporeal membrane oxygenation without routine systemic anticoagulation decreases adverse events. Ann Thorac Surg. 2020;109(5):1458–1466. doi: 10.1016/j.athoracsur.2019.08.040. [DOI] [PubMed] [Google Scholar]
- 73.Wu MY, Lin PJ, Lee MY, Tsai FC, Chu JJ, Chang YS, et al. Using extracorporeal life support to resuscitate adult postcardiotomy cardiogenic shock: treatment strategies and predictors of short-term and midterm survival. Resuscitation. 2010;81(9):1111–1116. doi: 10.1016/j.resuscitation.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 74.Yau P, Xia Y, Shariff S, Jakobleff WA, Forest S, Lipsitz EC, et al. Factors associated with ipsilateral limb ischemia in patients undergoing femoral cannulation extracorporeal membrane oxygenation. Ann Vasc Surg. 2019;54:60–65. doi: 10.1016/j.avsg.2018.08.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file1: Table S1. Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015 checklist: recommended items to address in a systematic review protocol. Table S2. PICOS criteria for inclusion and exclusion of publications. Table S3. Search strategy. Table S4. Detailed information on the data extraction and synthesis. Table S5. Main excluded studies. Table S6. Reported ECMO adverse events in the included studies (n = 32). Table S7. Patient anticoagulation and ECMO characteristics of the included studies (n = 32). Table S8. Influence analysis of studies reporting on in-hospital ECMO mortality. Table S9. Univariable meta-regression analyses. Figure S1. Influence analysis of studies reporting on in-hospital mortality. Figure S2. Meta-regression: scattered-plot of the relationship between age and in-hospital mortality.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. The additional data sets used and analyzed during the current study are available from the corresponding author on reasonable request.