Dear editor,
Concerns are being raised about whether the long-standing coronavirus disease 2019 (COVID-19) pandemic will increase the burden of multidrug-resistant organisms (MDROs).1 The healthcare practices may have been disrupted and overloaded owing to the lack and exhaustion of frontline healthcare workers (HCWs), particularly during the COVID-19 crisis.2 This situation could cause the overuse of antibiotics and inappropriate environmental management, given the move away from priorities of antimicrobial stewardship programs (ASP) and contact precautions.3 On the contrary, unprecedentedly strict implementation of non-pharmacological interventions (NPIs) may help reduce the intra-hospital spread of MDROs through excellent hand hygiene and the use of personal protection equipment. The inconsistent results from previous studies, which were conducted in the early stages of the COVID-19 pandemic and included relatively short observation periods,3, 4, 5, 6, 7 warrant close monitoring of the epidemiology trends of MDROs in various. We aimed to investigate the changing trends in new case and bacteremia with MDROs before and after the COVID-19 pandemic in South Korea, where the frequency of MDROs is high.8
This multicenter retrospective cohort study included six medical university-affiliated hospitals that were located in three different provinces of South Korea. We collected the monthly numbers of new case (first identification in clinical specimens or stool) and first bacteremia of extensively drug-resistant Acinetobacter baumanii (XDR-ABA), difficult-to-treat Pseudomonas aeruginosa (DTR-PAE), vancomycin-resistant Enterococci (VRE), and carbapenem-resistant Enterobacterales (CRE) as well as numbers of stool examinations for VRE and CRE between January 2012 and December 2021. The VRE or CRE stool tests were categorized into two kinds of data: (1) total negative results, best reflecting the efforts to detect VRE and CRE, and (2) first examination after hospitalization, including positive and negative results (except for the stool tests performed after VRE or CRE was detected in a clinical sample), which would represent the active surveillance performance appropriately. We divided the case of MDROs into pre- and post-COVID-19 periods, using the cutoff date of January 2020. The study was approved by the local Institutional Review Board from each hospital with the waiver of the informed consent (YUHS:3–2022–0176, KUAM:2022AN0027, KNUH:2022–01–002, and HUCH:2022–01–016).
Isolates were categorized as XDR when they showed non-susceptibility to ≥1 antimicrobial agent in all but ≤2 antimicrobial categories. Isolate were categorized as DTR when there was no susceptibility to the antimicrobial categories of carbapenems, antipseudomonal β-lactams, and fluoroquinolones. We included CRE isolates that were not susceptible to any carbapenem, regardless of carbapenemase-producing strains (CPE).9
We applied the Bayesian structural time series (BSTS) model, which is the state-space analysis proposed by Google, Inc.,10 using the R-language (version 4.1.3). The BSTS model can determine the causalities and regression of an intervention using its counterfactual prediction function by artificial control of what would have taken place had the intervention not occurred and the observed data.10 We expressed the relative ([observed–predicted]/predicted × 100) causal effects as percentages and 95% credible prediction intervals (CIs). The autoregressive integrated moving average (ARIMA) model was adjusted for the stool examinations in VRE or CRE using SAS (version 9.4) to minimize the impact of test numbers on the new case. A two-tailed p-value < 0·05 was considered statistically significant.
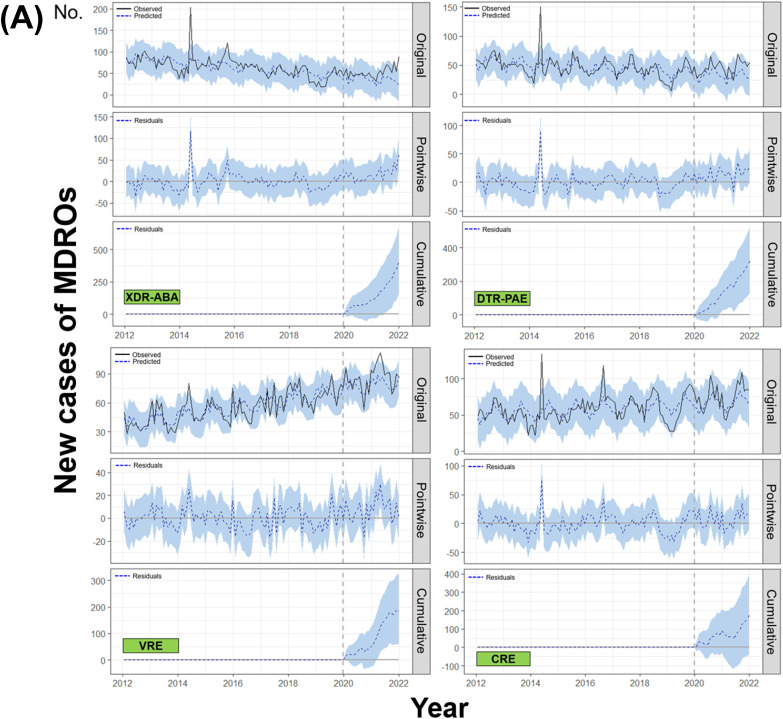
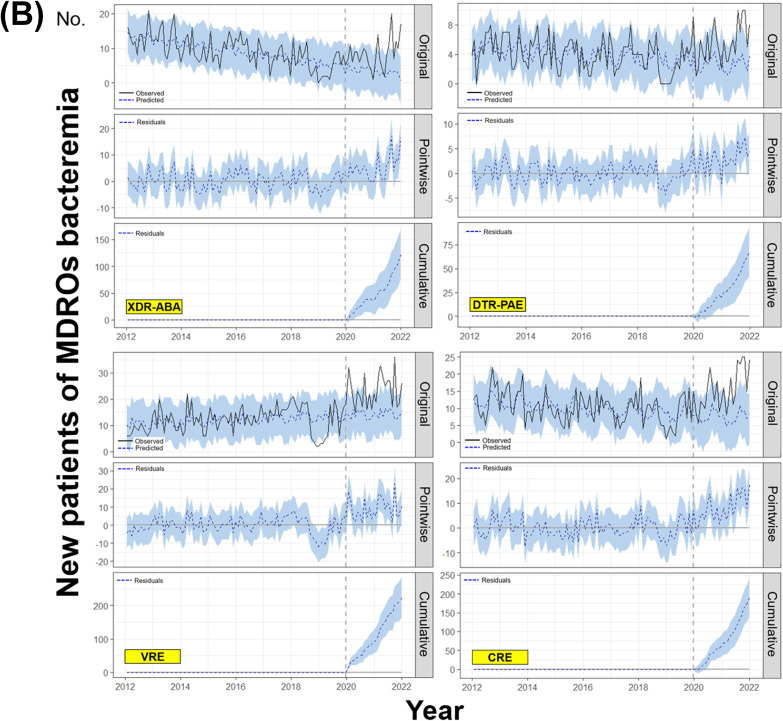
The BSTS models revealed that both new case and first bacteremia of XDR-ABA (relative effects; 47% [95% CI:19–79%], p = 0.003 and 150% [93–211%], p = 0.001, respectively) and DTR-PAE (41% [17–67%], p = 0.002 and 103% [65–145%], p = 0.001, respectively) had significantly increased in the post-COVID period. In addition, the observed values of VRE were significantly higher than the predicted values after the COVID-19 pandemic for both new case (10% [3–17%], p = 0.007) and first bacteremia (65% [48–83%], p = 0.001). The first CRE bacteremia numbers also showed a significant increase in the post-COVID-19 era (106% [78–135%], p = 0.001). However, the observed data for new case of total CRE (p = 0.056) did not differ from the predicted values (Table 1 and Fig. 1 ). After adjustment for stool test numbers, which were decreased in the post-COVID-19 period, the new case (32% in model 3 adjusted for total negative and first examination) and first bacteremia (12%) of VRE showed a significant increase compared to the predicted values (all p < 0.001). The reduction in stool examinations did not affect the number of first bacteremia of CRE (4%, p = 0.025) (Table 2 ).
Table 1.
Changes in new case and first bacteremia with MDROs during the pre- and post-COVID pandemic using the Bayesian structural time series model.
| MDROs | Variables | COVID-19 pandemic |
|||||
|---|---|---|---|---|---|---|---|
| Before (2012∼2019) | After (2020∼2021) |
||||||
| Observed (N) | Observed (N) | Predicted (N) | Absolute effect (N) | Relative effect (%) | p-value | ||
| VRE | New case | ||||||
| Total | 54.9 | 86.0 | 78.2 (72.3–84·1) | 7.7 (2.4–13.6) | 10 (3–17) | 0.007 | |
| VR-E. faecium | 24.5 | 54.0 | 33.7 (28.9–38·3) | 20.3 (16.2–24.8) | 60 (48–73) | 0.001 | |
| New bacteremia | |||||||
| Total | 12.2 | 23.5 | 14.3 (12.2–16·9) | 9.2 (6.8–11.8) | 65 (48–83) | 0.001 | |
| VR-E. faecium | 9.5 | 18.8 | 11.4 (9.2–13·0) | 7.4 (5.3–9.6) | 64 (46–84) | 0.001 | |
| CRE | New case | ||||||
| Total | 57.9 | 74.3 | 67.2 (58.1–75·9) | 7.2 (−1.5–16.6) | 11 (−2–25) | 0.056 | |
| CR-Klebsiella spp. | 36.4 | 51.6 | 49.7 (42.7–56·2) | 1.9 (−4.6–9.1) | 4 (−9–18) | 0.301 | |
| CR-E. coli | 14.5 | 16.0 | 14.0 (11.1–17·3) | 2.0 (−0.7–4.8) | 14 (−5–35) | 0.073 | |
| New bacteremia | |||||||
| Total | 10.2 | 15.4 | 7.5 (5.3–9·5) | 7.9 (5.8–10.1) | 106 (78–135) | 0.001 | |
| CR-Klebsiella spp. | 5.2 | 10.0 | 4.7 (3.2–6·1) | 5.2 (3.8–6.8) | 111 (81–143) | 0.001 | |
| CR-E. coli | 3.9 | 3.8 | 2.4 (1.4–3·4) | 1.4 (0.5–2.5) | 59 (19–102) | 0.005 | |
| XDR-ABA | New case | 64.7 | 52.8 | 35.8 (24.8–46·4) | 17.0 (6.7–28.2) | 47 (19–79) | 0.003 |
| New bacteremia | 9.1 | 8.5 | 3.4 (1.3–5·3) | 5.1 (3.2–7.1) | 150 (93–211) | 0.001 | |
| DTR-PAE | New case | 46.3 | 45.9 | 32.7 (23·8–41.2) | 13.3 (5.4–21.8) | 41 (17–67) | 0.002 |
| New bacteremia | 4.0 | 5.5 | 2.7 (1.6–3.7) | 2.8 (1.8–3.9) | 103 (65–145) | 0.001 | |
Data are expressed as numbers or numbers (95% credible prediction intervals).
Fig. 1.
Changes in numbers of new case and first bacteremia patients of several MDROs before and after the COVID-19 pandemic evaluated by the Bayesian structural time series model (A) New case (B) First bacteremia.1
Table 2.
Multivariable analyses adjusted by numbers of VRE or CRE stool tests in the autoregressive integrated moving average (ARIMA) model.
| MDROs | Variables | Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|---|
| Estimate (SE) | p-value | Estimate (SE) | p-value | Estimate (SE) | p-value | ||
| VRE | New case | ||||||
| Total | 29.85 (7.24) | <0.001 | 32.36 (5.50) | <0.001 | 31.76 (5.45) | <0.001 | |
| VR-E. faecium | 25.43 (4.72) | <0.001 | 24.45 (4.76) | <0.001 | 25.06 (4.89) | <0.001 | |
| New bacteremia | |||||||
| Total | 11.98 (2.07) | <0.001 | 11.88 (2.01) | <0.001 | 11.97 (2.06) | <0.001 | |
| VR-E. faecium | 9.83 (1.75) | <0.001 | 9.84 (1.69) | <0.001 | 9.81 (1.71) | <0.001 | |
| CRE | New case | ||||||
| Total | 12.39 (7.44) | 0.099 | 12.40 (7.53) | 0.103 | 12.53 (7.67) | 0.106 | |
| CR-Klebsiella spp. | 18.73 (10.34) | 0.074 | 18.93 (5.50) | <0.001 | 15.77 (7.02) | 0.027 | |
| CR-E. coli | 0.92 (1.66) | 0.582 | 0.96 (1.63) | 0.558 | 0.98 (1.63) | 0.549 | |
| New bacteremia | |||||||
| Total | 4.51 (1.93) | 0.022 | 4.51 (1.93) | 0.022 | 4.45 (1.96) | 0.025 | |
| CR-Klebsiella spp. | 4.51 (1.45) | 0.002 | 4.52 (1.44) | 0.002 | 4.52 (1.45) | 0.003 | |
| CR-E. coli | 0.15 (0.61) | 0.803 | 0.15 (0.62) | 0.813 | 0.15 (0.62) | 0.809 | |
The estimate (standard error) is expressed as a percentage. Model 1: adjusted for total negative results of stool VRE or CRE tests (category 1); model 2: adjusted for total stool VRE or CRE tests performed as the first examination after admission (category 2); model 3: adjusted for categories 1 and 2.
Two recent studies presented the protective effect of the COVID-19 pandemic against extended-spectrum β-lactamase-producing E. coli isolates and CPE using data until December 2020 in France.4 , 6 The authors attributed these results to the decrease in antibiotic usage or testing of urine samples and hospital visits due to the overall lockdown as well as increased implementation of NPIs.4 , 6 It seems unclear whether these beneficial effects would extend beyond developed countries or after a period of well-executed strong national policies mitigating COVID-19 and beyond β-lactamase-producing MDROs because other studies have reported conflicting results.3 , 7
Our study showed that the occurrence of the most problematic MDROs had increased after the COVID-19 pandemic, especially in case of bacteremia. Surprisingly, the MDR-ABA and DTR-PAE, which had been steadily decreasing before the pandemic, showed an increasing trend in both new case and first bacteremia after COVID-19 (Figs. S1 and S2). Our results are similar to the trends of national data from the Korea Centers for Disease Control and Prevention. The total number of CRE infections and colonization, designated as a legal infectious disease in June 2017 and converted to a surveillance system with mandatory reporting within 24 h in South Korea, significantly increased since the COVID-19 pandemic (Fig. S3).
As a limitation, we did not quantify the performance rate of various elements of NPIs and ASP as well as an overload of HCWs at several hospitals. As the duration of the pandemic increases, the rate of implementation of initially strong policies would decrease. Taken together, our findings are concerning because the effects of MDROs on global health are critical, and it is difficult and time-consuming to reduce the resistance to the same levels as before. Hence, we need to actively and continuously monitor the increase in infections with MDROs as well as occurrence of new resistance in various regions.
Footnotes
The vertical dotted lines in all graphs represent January 2020, the beginning of the COVID-19 pandemic. The straight and dotted lines in the original panels indicate the observed and predicted values using the BSTS model, respectively. The dotted lines in the pointwise impact panels indicate the differences between the observed and predicted values, which are called residuals. The dotted lines in the cumulative impact panels represent the accumulated residuals.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.09.026.
Appendix. Supplementary materials
References
- 1.Pelfrene E., Botgros R., Cavaleri M. Antimicrobial multidrug resistance in the era of COVID-19: a forgotten plight? Antimicrob Resist Infect Control. 2021;10:21. doi: 10.1186/s13756-021-00893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karan A., Wadhera R.K. Healthcare system stress due to COVID-19: evading an evolving crisis. J Hosp Med. 2021;16:127. doi: 10.12788/jhm.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bork J.T., Leekha S., Claeys K., Seung H., Tripoli M., Amoroso A., et al. Change in hospital antibiotic use and acquisition of multidrug-resistant gram-negative organisms after the onset of coronavirus disease 2019. Infect Control Hosp Epidemiol. 2021;42:1115–1117. doi: 10.1017/ice.2020.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemenand O., Coeffic T., Thibaut S., Colomb Cotinat M., Caillon J., Birgand G. Decreasing proportion of extended-spectrum beta-lactamase among E. coli infections during the COVID-19 pandemic in France. J Infect. 2021;83:664–670. doi: 10.1016/j.jinf.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Taylor L. COVID-19: antimicrobial misuse in Americas sees drug resistant infections surge, says WHO. BMJ. 2021;375:n2845. doi: 10.1136/bmj.n2845. [DOI] [PubMed] [Google Scholar]
- 6.Duverger C., Monteil C., Souyri V., Fournier S. Decrease of carbapenemase-producing enterobacteriaceae incidence during the first year of the COVID-19 pandemic. J Infect. 2022;85:90–122. doi: 10.1016/j.jinf.2022.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segala F.V., Bavaro D.F., Di Gennaro F., Salvati F., Marotta C., Saracino A., et al. Impact of SARS-CoV-2 epidemic on antimicrobial resistance: a literature review. Viruses. 2021;13 doi: 10.3390/v13112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H., Yoon E.J., Kim D., Jeong S.H., Won E.J., Shin J.H., et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.42.1800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 10.Brodersen K.H., Gallusser F., Koehler J., Remy N., Scott S.L. Inferring causal impactusing Bayesian structural time-series models. Ann Appl Stat. 2015;9:247–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.