Abstract
Background
Cryptococcus neoformans (C. neoformans) is commonly presented in immunocompromised individuals and causes cryptococcosis mostly in the respiratory and/or central nervous system. Liver cryptococcosis is exceedingly rare and sometimes difficult to diagnose through conventional assays.
Case Presentation
The present study reports a rare case of liver cryptococcosis characterized by increased serum carbohydrate antigen 19–9 (CA19-9) level and intrahepatic multiple nodules without other symptoms in an immunocompetent woman. Her cancer family history and imaging examinations initially suspected metastatic liver malignancy. But no sign of the malignant tumor was found after endoscopy, 18-fluorine fluorodeoxyglucose positron emission tomography-computed tomography, and liver biopsy. The histopathology of the liver biopsy specimen indicated chronic inflammatory granuloma and then infectious diseases were suspected. However, traditional microbiologic testing failed to identify any potential pathogen. Eventually, metagenomic next-generation sequencing (mNGS) was applied to identify the definite diagnosis of liver cryptococcosis by acquiring the genome sequence of C. neoformans. Fortunately, after 6-month diagnostic anti-fungal therapy of fluconazole, the liver nodules effectively faded away and the serum CA19-9 level gradually regressed to the normal range.
Conclusion
We identified a rare case of hepatic cryptococcosis by mNGS in an immunocompetent patient. When conventional methods have difficulties in the diagnosis of a specific pathogen, mNGS has the advantage of early and accurate identification of potential pathogens from the specimen.
Keywords: liver nodule, Cryptococcus neoformans, diagnosis, metagenomic next-generation sequencing
Introduction
Cryptococcus neoformans (C. neoformans) is an opportunistic pathogenetic fungus existing in pigeon excrement and soil that can cause cryptococcosis. Cryptococcosis usually occurs in the immunocompromised population such as acute immunodeficiency syndrome, organ transplantation, immunosuppressants or corticosteroid treatment, malignancy, and diabetes. Meningitis and pneumonia are common clinical manifestations in infected patients.1–3 Infections in the skin, bone marrow, gastrointestinal tract, and liver are relatively rare but have been reported in some cases.3 Indian Ink staining and cryptococcal antigen (Cr-Ag) are helpful but limited in the diagnosis of Cryptococcal neoformans disease.4 Chen et al reported a patient with cryptococcal meningitis manifested with abnormal walking, difficult leg lifting, and frequent falling, and multiple conventional tests, including Indian Ink staining and Cr-Ag, failed to identify the pathogen.5 Therefore, single-cell sequencing (scS) was used to test the cerebrospinal fluid and Cryptococcus gattii sensu stricto was identified.5 The present study reports a rare case of liver cryptococcosis presented with increased serum carbohydrate antigen 19–9 (CA19-9) level and intrahepatic multiple nodules without other symptoms in an immunocompetent patient. Despite the negative result of the lateral flow assay (LFA) serum Cr-Ag, metagenomic next-generation sequencing (mNGS) was applied to identify the definite diagnosis of liver cryptococcosis by acquiring the genome sequence of C. neoformans.
Case Presentation
A 48-year-old woman with a 2-month history of elevated serum CA19-9 levels (50.05 IU/mL, reference range <25IU/mL) without any symptoms was referred to our hospital. The patient was in good health without other chronic diseases before admission. She denied a history of alcohol abuse, drug addiction, and regular medication intake. Notably, her family history showed that her father dies of hepatocellular carcinoma.
On admission to our hospital, no positive findings were noted in the patient after a careful physical examination. The laboratory investigation disclosed the elevated CA19-9 level (46.45 IU/mL, reference range <25 IU/mL), serum alkaline phosphatase level (145 IU/L, reference range 30–120 IU/L), and Limulus test (87 pg/mL, reference range <10 pg/mL). The white blood count, coagulation factors, erythrocyte sedimentation rate, procalcitonin, C-reactive protein carcinoembryonic antigen, alpha fetal protein, and carbohydrate antigen 125 levels were normal. The serology for viral hepatitis (hepatitis B and C) and human immunodeficiency virus was negative. The autoantibodies were also negative, and the serum immunoglobulins were within the normal range.
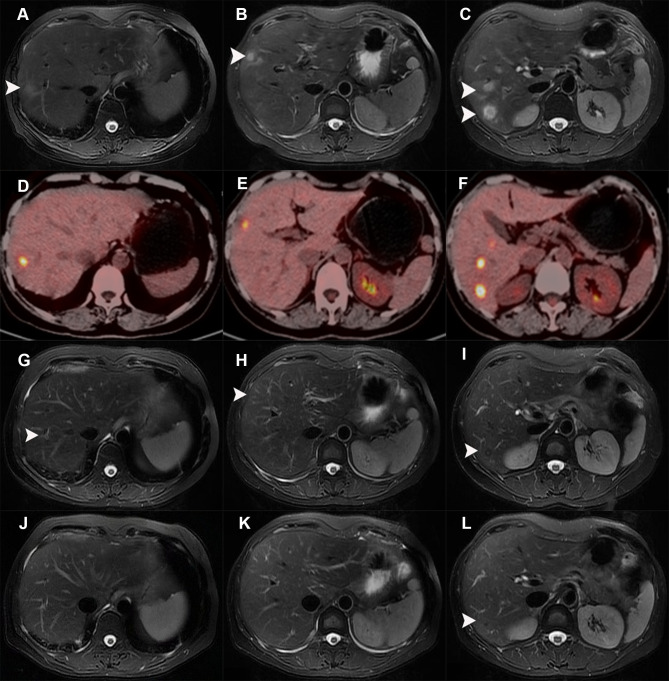
An abdominal contrast-enhanced magnetic resonance (MR) scan was performed. Multiple abnormal signal nodules were found in the liver. The T2-weighted image (Figure 1A-C) and diffusion-weighted image (DWI, b value = 800 mm/s2) (not shown) showed several hyperintense round-like lesions. The diameter of the largest nodule is approximately 20mm (Figure 1C). Contrast-enhanced MR showed no enhancement of nodules in the arterial phase (see Supplementary Figure 1A-C), while the nodules were slightly hypointense to normal liver parenchyma with peripheral ring-like slight hyperintense area in the portal venous phase (see Supplementary Figure 1D-F) and with peripheral ring-like obvious hyperintense area in the delayed phase (see Supplementary Figure 1G-I). The MR scan suggested that metastatic tumors or infectious diseases should be both considered.
Figure 1.
The radiological manifestations of liver nodules during the disease process. (A-C) MR images (T2-weighted) showed several round-like hyperintense nodules (white arrows) in the right lobe of the liver. (D-F) PET-CT displayed multiple lesions with increased uptake of nuclear species in the liver. (G-I) MR images (T2-weighted) showed the size of the nodules (white arrows) in the liver was smaller than before after 2 months of antifungal treatment. (J-L) MR images (T2-weighted) showed the liver nodules (white arrows) almost disappeared after 6 months of treatment.
Contrast-enhanced ultrasound of the liver revealed multiple hypoechoic lesions with blurry margins and a little blood-flow signal, while it depicted the arterial-phase enhancement of lesions, stronger than the surrounding liver (wash-in), and hypoechoic intensity compared to the surrounding liver (wash-out) in the venous phase after intravenous administration of ultrasound contrast agent (SonoVue 2mL). 18-fluorine fluorodeoxyglucose positron emission tomography-computed tomography (18-FDG PET-CT) displayed multiple low-dense lesions of various sizes with obviously increased uptake of nuclear species, which indicated the metastatic liver malignancy could not be excluded. (Figure 1D-F). Although gastrointestinal tumors are the main resources of liver metastasis, no significant signs of malignancy were detected in the gastrointestinal tract after the PET-CT scan. Moreover, no significant lesions were observed after the gastroscopy and colonoscopy examination.
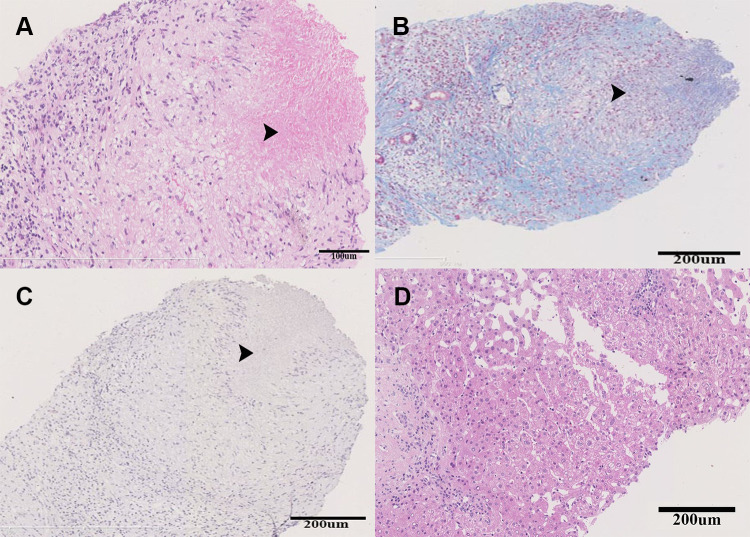
To determine the nature of liver nodules, the patient received a percutaneous liver biopsy guided by ultrasound. The Hematoxylin and Eosin staining of the specimen obtained from the biggest nodule showed granulomatous inflammation with central caseous necrosis and a few multinucleated giant cells (Figure 2A). Masson triple staining indicated that the necrotic area was surrounded by fiber (Figure 2B). The periodic acid-Schiff (PAS), D-PAS, and acid-fast staining (Figure 2C) were all negative. The specimen of para-nodule was presented with chronic inflammatory changes (Figure 2D). No malignant cells were found in the specimens and then the infectious diseases were highly suspected, which need to be further validated.
Figure 2.
The histopathology of the liver specimen obtained from the biggest nodule. The black arrows indicated the granulomatous inflammation with central caseous necrosis. (A) Hematoxylin & eosin staining, (B) Masson triple staining, (C) acid-fast staining, and (D) Hematoxylin & eosin staining of the para-nodule liver specimen.
The patient denied a history of tuberculosis (TB), diabetes mellitus, or exposure to bird excrement. The chest CT showed no evidence of obvious inflammation. The flow cytometry analysis of T lymphocytes (CD3 cell count 1493 cells/μL, CD4 cell count 830 cells/μL, and CD8 cell count 640 cells/μL) showed that the patient had no apparent immune deficiency. We further conducted infection-related laboratory tests. The T-SPOT.TB, (1,3)-β-D glucan test, the LFA serum Cr-Ag, and the aspergillus antigen test were all negative. Therefore, the traditional tests did not show any evidence of a specific pathogen. The mNGS of the liver specimen was conducted and the result revealed the identification of the sequence of C. neoformans, but the reads number was 1 and the reads per million ratios were 3.11%, which was the only etiological evidence.
Considering the delay in antifungal therapy related to poorer clinical outcomes, a diagnostic treatment with oral fluconazole (400 mg/day) was administrated after the informed consent of the patient, then follow-up regularly. After 2 months of treatment, the size of the nodules in the liver examined by MR significantly decreased compared to that of baseline, and the diameter of the biggest nodule was approximately 7mm (Figure 1G-I). At the follow-up of 3 months, the dose of fluconazole was subsequently reduced to 200 mg/day due to moderately elevated aminotransferase, and the liver function tests gradually recovered within the next month. After 6 months of treatment, the liver nodules examined by MR almost disappeared (Figure 1J-L), and the serum CA19-9 and Limulus test levels regressed to the normal range.
Discussion
Cryptococcosis mainly affects immunocompromised patients, but an increasing number of cases have been reported in immunocompetent individuals.6–9The liver is not a common site of C. neoformans infection, but several cases of hepatobiliary cryptococcosis have been reported as a manifestation of disseminated or isolated cryptococcosis in immunocompromised patients.6,7,9–11 In these previous reported hepatobiliary cryptococcosis cases, obstructive jaundice and low-attenuated mass extending along the hepatoduodenal ligament with bilateral intrahepatic bile duct dilation were dominant manifestations and the diagnosis of cholangiocarcinoma or primary sclerosing cholangitis (PSC) was suspicious but C. neoformans infection was confirmed by a microscopic examination or a bile culture.6,7,9–11
In our report, the patient, who presented with increased serum CA19-9 level, family cancer history and intrahepatic multiple nodules was initially suspected of having metastatic liver malignancy. But no sign of the malignant tumor was found after comprehensive examinations including endoscopy, PET-CT, and liver biopsy. And then infectious diseases were suspected according to the histological findings. However, conventional microbiologic testing failed to identify the pathogen. Eventually, mNGS was applied to identify the definite diagnosis of liver cryptococcosis by acquiring the genome sequence of C. neoformans in the liver specimen despite only one sequence. To our knowledge, this is the first report of liver cryptococcosis confirmed by the method of mNGS.
In recent years, mNGS is emerging as a powerful method incorporated into the diagnosis of complicated infectious diseases.12 The advantages of mNGS are that it screens the potential pathogens of the clinical specimen comprehensively and unbiasedly,13 as well as simultaneously tests various pathogens, including bacteria, viruses, and fungus.14 In the present case, only one sequence of C. neoformans was successfully detected by mNGS. Such a low abundance of detected sequences is still convincing for the following reasons. Firstly, the process of liver puncture and subsequent tests were all aseptic, the mNGS result should be highly dependable. Secondly, mNGS has been proven to possess high sensitivity in detecting very low abundance Cryptococcus (0.2–19.7 reads per million)13 which might indicate a low burden of the pathogen in tissue samples.15 Thirdly, only 3 sequences of Chlamydia psittaci by the method of mNGS were detected in the blood sample in a case reported recently, while the conditions of the patient improved after the adjustment to a specific antibiotic (minocycline) for psittacosis, which further supported the diagnosis.16 Fourthly, the prompt response to diagnostic antifungal treatment in our case, in turn, validated the results of mNGS. However, the scS technology generates enough sequence reads and it is considered more effective than mNGS for identifying unexpected pathogens in clinical specimens in the previous report.5 We can assume that the scS technology is applied in this case, and perhaps more convincing conclusions can be drawn.
According to the clinical practice guideline for the management of cryptococcal disease,17 the antifungal treatment, including amphotericin B (AmB) deoxycholate, flucytosine, fluconazole, and itraconazole, should be scheduled in patients with different immune status and infectious sites. Recommendation 67 states,
If CNS disease is ruled out, fungemia is not present, infection occurs at a single site, and there are no immunosuppressive risk factors, consider fluconazole (400 mg [6 mg/kg] per day orally) for 6–12 months.17
Indeed, in the present case, 6-month treatment of fluconazole led to the fade of the nodules without any notable side effects.
In summary, a rare liver cryptococcosis case mimicked metastatic liver cancer was identified in an immunocompetent adult. When pathogen infection was suspected after comprehensive examinations, the awareness of the possibility of rare pathogens, such as C. neoformans, should be improved. Meanwhile, the case also highlighted the importance and the superiority of mNGS in the diagnosis of infectious liver nodules.
Funding Statement
The work was sponsored by the Shanghai Sailing Program (grant number 20YF1428500) and the National Natural Science Foundation of China (grant number 82002185).
Patient Consent Statement
Written informed consent has been provided by the patient to have the case details and any accompanying images published. The study has been approved by the ethics committees of Ruijin hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gushiken AC, Saharia KK, Baddley JW. Cryptococcosis. Infect Dis Clin North Am. 2021;35(2):493–514. doi: 10.1016/j.idc.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 2.Li SS, Mody CH. Cryptococcus. Proc Am Thorac Soc. 2010;7(3):186–196. doi: 10.1513/pats.200907-063AL [DOI] [PubMed] [Google Scholar]
- 3.Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30(1):179–206. doi: 10.1016/j.idc.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsley MD, Mekha N, Baggett HC, et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis. 2011;53(4):321–325. doi: 10.1093/cid/cir379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Hong N, Hu S, et al. Molecular identification of Cryptococcus gattii from cerebrospinal fluid using single-cell sequencing: a case study. J Infect. 2020;81(4):634–638. doi: 10.1016/j.jinf.2020.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai X, Liu K, Liang Y, Yu H, Lv F, Liang X. Isolated biliary cryptococcosis manifesting as obstructive jaundice in an immunocompetent adult. Int J Med Sci. 2012;9(3):200–206. doi: 10.7150/ijms.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nara S, Sano T, Ojima H, et al. Liver cryptococcosis manifesting as obstructive jaundice in a young immunocompetent man: report of a case. Surg Today. 2008;38(3):271–274. doi: 10.1007/s00595-007-3605-6 [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Osawa R, Kubota Y, et al. Early diagnosis of Cryptococcus neoformans var. grubii meningitis using multiplex PCR assay in an immunocompetent patient. J Infect Chemother. 2021;27(12):1765–1768. doi: 10.1016/j.jiac.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Du L, Cai W, Wu Y, Lv F. Isolated hepatobiliary cryptococcosis manifesting as obstructive jaundice in an immunocompetent child: case report and review of the literature. Eur J Pediatr. 2014;173(12):1569–1572. doi: 10.1007/s00431-013-2132-2 [DOI] [PubMed] [Google Scholar]
- 10.Hoshiai S, Hiyama T, Kawasaki H, et al. Mass-forming hepatic cryptococcosis: a mimicker of metastatic tumors. Abdom Radiol. 2020;45(7):2268–2273. doi: 10.1007/s00261-020-02437-2 [DOI] [PubMed] [Google Scholar]
- 11.Utili R, Tripodi MF, Ragone E, et al. Hepatic cryptococcosis in a heart transplant recipient. Transpl Infect Dis. 2004;6(1):33–36. doi: 10.1111/j.1399-3062.2004.00041.x [DOI] [PubMed] [Google Scholar]
- 12.Schlaberg R, Chiu CY, Miller S, et al. Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection. Arch Pathol Lab Med. 2017;141(6):776–786. doi: 10.5858/arpa.2016-0539-RA [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran PS, Cresswell FV, Meya DB, et al. Detection of Cryptococcus DNA by Metagenomic Next-generation Sequencing in Symptomatic Cryptococcal Antigenemia. Clin Infect Dis. 2019;68(11):1978–1979. doi: 10.1093/cid/ciy1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect. 2018;76(3):225–240. doi: 10.1016/j.jinf.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Qiu Y, Zeng W, Wei X, Zhang J. Metagenomic next-generation sequencing for the early diagnosis of talaromycosis in HIV-uninfected patients: five cases report. BMC Infect Dis. 2021;21(1):865. doi: 10.1186/s12879-021-06551-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Li S, Tan W, Wang H, Xu H, Wang D. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerg Microbes Infect. 2021;10(1):1418–1428. doi: 10.1080/22221751.2021.1948358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]