Abstract
Geniposide is a naturally sourced active ingredient that has diverse pharmacological effects and great potential in improving or treating different kinds of diseases. In recent years, more and more studies have confirmed that geniposide can improve glucose and lipid metabolism disorder, which is an increasingly prevalent health problem causing various metabolic diseases globally. Our review aims to summarize basic information on the pharmacological effects of geniposide on glucolipid metabolism. Geniposide increases glucose utilization and insulin production, protects pancreatic islet β cells, inhibits insulin resistance and hepatic glucose production, and suppresses gluconeogenesis. While in the aspect of lipid metabolism, geniposide can promote lipolysis, inhibit lipogenesis, and regulate lipid transport. Geniposide ameliorates lipid and glucose metabolic disorders, improving the entire glycolipid metabolism network in a three-dimensional manner at the level of molecular mechanism. Growing evidence revealed that geniposide may serve as an effective drug to combat metabolic diseases for the time to come.
Keywords: geniposide, lipid metabolism, glucose metabolism, Gardenia Jasminoides Ellis, metabolic disease, glucolipid metabolism, naturally sourced active ingredient, pharmacological evidence
Introduction
Glucose and lipid metabolism imbalance is a high-risk etiology leading to various complications, including obesity, diabetes, hyperlipemia, non-alcohol fatty liver disease (NAFLD), and cardiovascular diseases.1 We are currently in the midst of a global metabolic disease epidemic, with its prevalence increasing with age.2,3 There has been an increasing interest in investigating effective strategies to control and treat comorbidities associated with glucose and lipid metabolism disorders.4 A variety of plants and natural active ingredients derived from plants have been used to combat diseases associated with glycolipid metabolism disorders.5 Geniposide is one such naturally active ingredient derived from the fruits of Gardenia Jasminoides Ellis (GJE, popularly called Zhizi in China) and has traditionally been commonly used for hundreds of years in traditional Chinese medicine.6
There is an increasing number of pharmacological evidence proving that geniposide exerts various biological activities, including neuroprotective, antidiabetic, hepatoprotective, anti-inflammatory, analgesic, antidepressant-like, cardioprotective, antioxidant, immune-regulatory, antithrombotic, and anti-tumoral effects.7–11 The anti-inflammatory, hepatoprotective, antidiabetic, and antioxidant properties of geniposide were reviewed before.9,10,12–15 The most recognized pharmacological effects of geniposide are anti-inflammatory and antioxidant effects;9,13 however, in recent years, a growing number of studies explored the role of geniposide in regulating glucolipid metabolism. How geniposide regulates the glucolipid metabolism, the specific mechanism, the disadvantages and future research directions have not been elucidated. There are few reviews on the improvement of glucolipid metabolism disorders and metabolic diseases using geniposide. As a consequence, a comprehensive review focusing on geniposide-regulated glucolipid metabolism is necessary to advance the knowledge on geniposide. This review will serve as a reference for researchers to further understand the pharmacological effects of geniposide and develop more valuable applications in the future.
Geniposide at a Glance
Structure and Physicochemical Properties
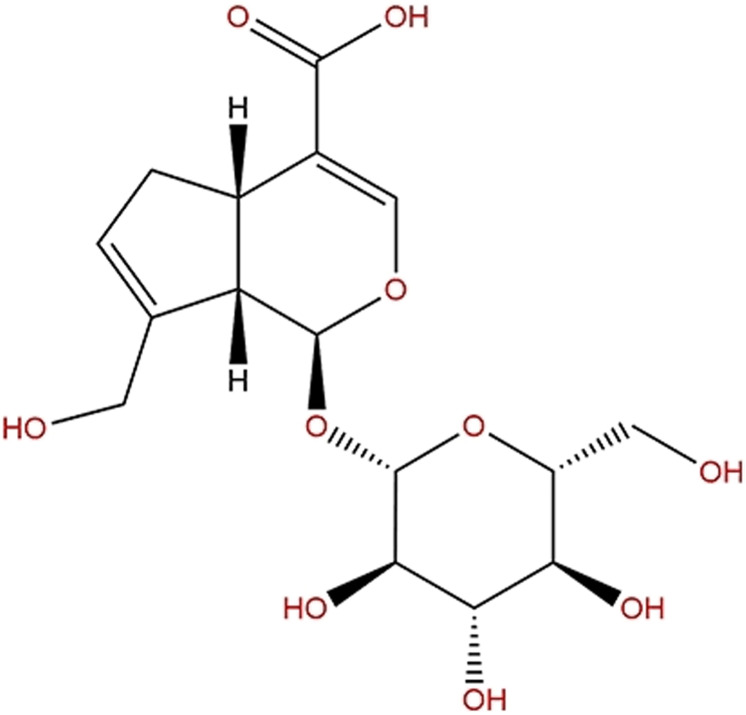
Geniposide (methyl (1S,4aS,7aS)-1-(β-D-glucopyranosyloxy)-7-(hydroxylmethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate; C17H24O10) is regarded as an iridoid glycoside, namely genipin 1-O-β-D-glucopyranoside (Figure 1).10 From another perspective, geniposide is also regarded as a C11 methyl ester of geniposidic acid. Its molecular weight is 388,366. Because of its chemical structure, it is also considered a glycoside containing one molecule of genipin and glucose. Geniposide is a white or pale yellow powder with a density of 1.49 g/cm3 and a melting point of 161.53°C.7 It is easily dissolved in water, soluble in ethanol, and undissolved in petroleum ether.6
Figure 1.
Chemical structure of geniposide.
Sources
Geniposide is mainly sourced from GJE, but it is also detected in other commonly used Chinese herbal medicines, such as Eucommia Ulmoides Oliv (EUO), Rehmannia Officinalis (RO), and Radix Scrophulariae (RS) (Figure 2).16 Among these, GJE contains about 3.3–8.56% geniposide, while RO only contains 0.205–0.4381%, EUO contains 0.0173–0.5811%, and RS contains 0.0699–0.1135% of geniposide. Therefore, GJE is the main geniposide source.17 The 2015 edition of the Chinese Pharmacopoeia stipulates that geniposide abundance in GJE is no less than 1.8%. Geniposide content in GJE on the market reaches 6%.18
Figure 2.
Main sources of geniposide. (A). Gardenia Jasminoides Ellis (B). Eucommia Ulmoides Oliv (EUO) (C). Rehmannia Officinalis (RO) (D). Radix Scrophulariae (RS).
Pharmacokinetics
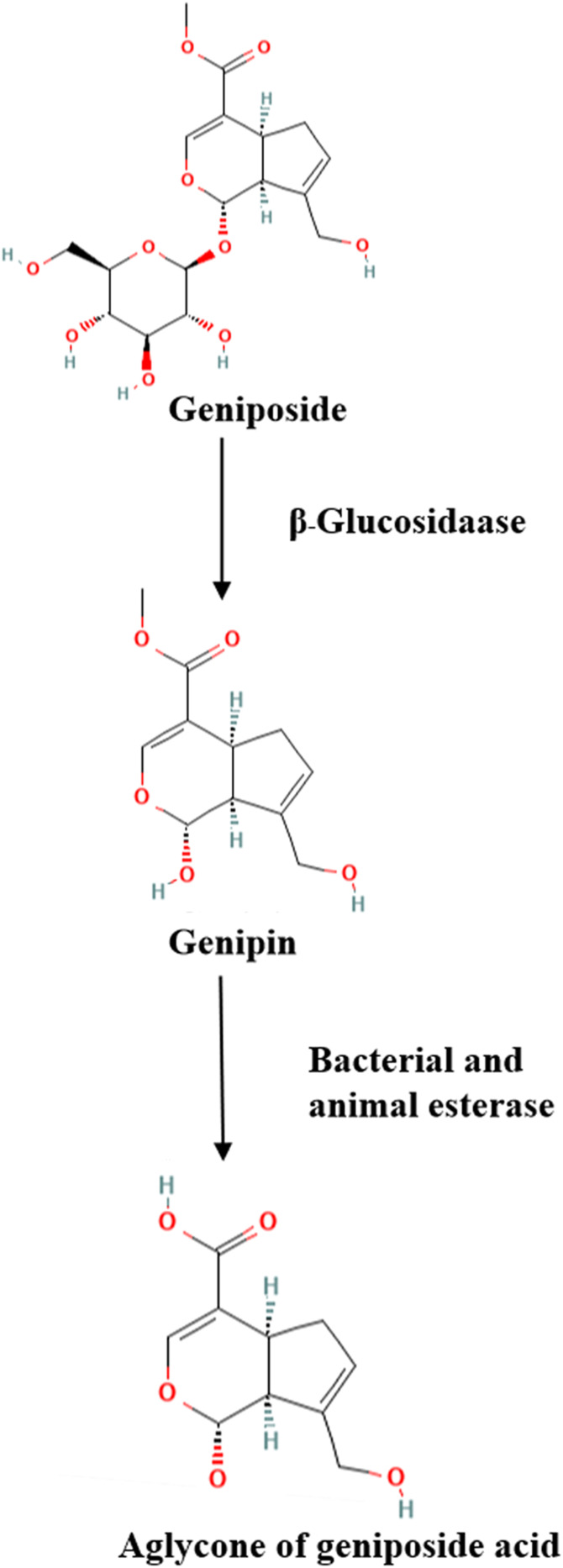
We investigated that the concentration of geniposide reached a peak in the liver and spleen 30 min after oral administration, and was detected in the kidney and brain 2 h later.19 The absolute bioavailability of geniposide was 9.67% after it was metabolized within 12 h. The bioavailability of geniposide is strongly linked to different administration methods. In addition, geniposide can be converted to genipin by an intestinal microbiota enzyme (β-glucosidase) (Figure 3). Geniposide metabolism involves methylation, glucosylation, decarboxylation, taurine conjugation, hydrolysis, demethylation, hydrogenation, hydroxylation, cysteine S-conjugation through dehydration, sulfate conjugation, and other related complex reactions.20,21 Geniposide is mainly excreted in its original form through the kidneys. The excretion level accounts for a proportion of over 90% of the initial dosage after 10 h.22
Figure 3.
Schematic diagram of the metabolic effect of geniposide by β-glucosidase and esterase.
Main Pharmacological Functions of Geniposide
Geniposide exerts abundant and complicated pharmacological effects, such as anti-inflammatory, antioxidant, hepatoprotective, neuroprotective, analgesic, antidiabetic, antidepressant-like, immune-regulatory, cardioprotective, antithrombotic, and antitumoral effects.8 These pharmacological effects have laid a foundation for its application in the improvement of a variety of diseases, such as cardiovascular diseases, diabetes and diabetic complications, hepatic diseases, Parkinson’s disease, Alzheimer’s disease, and ischemia and reperfusion injury. Some of the potential benefits include the influence on the normal and healthy operation of the nervous, endocrine, circulatory, digestive, urinary, muscle, and other different bodily systems.
In particular, the anti-inflammatory and antioxidant effects of geniposide have been widely studied. Geniposide not only fights inflammation-related diseases like swelling, pain, liver disease, Alzheimer’s disease, and others but also inhibits classic inflammatory-related pathways such as NF-κB, MAPK, and TLR4 signaling pathways.10 Geniposide could delay cell injury via upregulating endogenous antioxidative enzymes. Geniposide can also increase the activity of some important antioxidant enzymes and pathways including hepatic lipid peroxidation (LPO), glutathione-S-transferase (GST), glutathione (GSH), glutathione peroxidase (GPx), and copper- and zinc-containing superoxide dismutase (CuZn-SOD), and protects against oxidative stress injury.23–25
Pharmacological Effects of Geniposide in Glucose and Lipid Metabolism
Effects on Glucose Metabolism
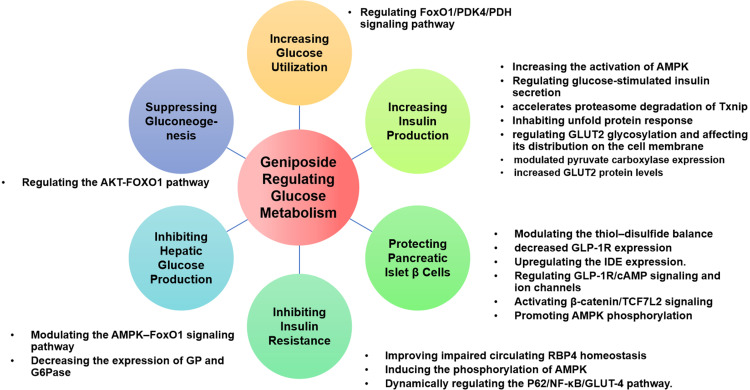
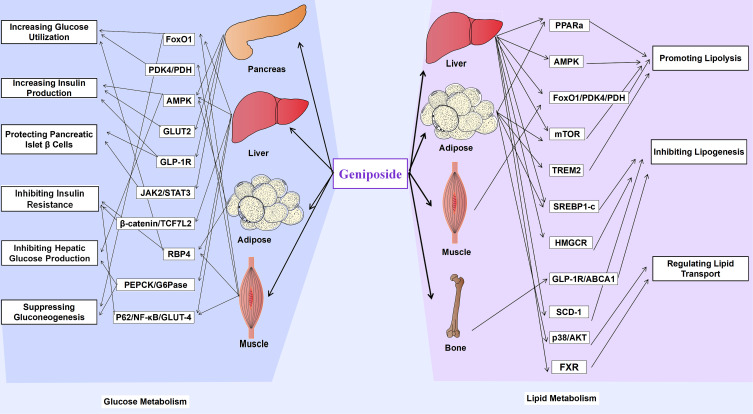
It is well-known that an imbalanced glucose metabolism triggers many metabolic diseases. An adequate blood glucose level must be maintained at all time. Sources of blood glucose are intestinal absorption, liver glycogenolysis, and gluconeogenesis.26 Glucose-consuming pathways include glucose uptake by various tissues and organs for oxidation, glycogen synthesis, and conversion into other sugars, fats, or amino acids. The balance of blood glucose is mainly regulated by hormones, including insulin, glucagon, adrenaline, and glucocorticoid.27 The interaction between major glucose metabolic pathways, such as glycolysis, gluconeogenesis, glycogenesis, and glycogenolysis, maintains the homeostasis of hepatic glucose metabolism.28 Figure 4 summarizes the pharmacological effects of geniposide on glucose metabolism and the details are exhibited in Table 1.
Figure 4.
Pharmacological effects of geniposide on Glucose Metabolism.
Table 1.
In vivo/In vitro Studies of Geniposide in Glucose Metabolism
| Effect on Glucose Metabolism | In vivo/In vitro | Model | Dose and Duration | Experimental Outcome | References |
|---|---|---|---|---|---|
| Increasing the Utilization of glucose | In vivo | C57BL/6 wild-type mice were fed a high-fat diet for 9 weeks | Geniposide 25mg/kg for 1 week | ↑glucose utilization ↑fast-twitch muscle phenotype ↓the mRNA/protein levels of PDK4 in skeletal muscle ↓the mRNA/protein levels of FoxO1/PDK4/p-PDH/GAS |
[30] |
| In Vitro | Mouse C2C12 myoblasts treated with 2% horse serum to replace FBS in culure medium for 48 h | Geniposide 0.4mg/mL for 12 h | ↑glucose utilization ↑fast-twitch muscle phenotype ↓the mRNA/protein levels of FoxO1/PDK4/p-PDH/GAS |
[30] | |
| Increasing Insulin Production | In Vitro | Rat INS-1 insulinoma cells exposed to 10 mol/L geniposide for 1 h | Geniposide 0.01/0.1/1/10/100μM for 1 h | ↑insulin secretion ↑glucose-stimulated insulin secretion |
[33] |
| Rat INS-1 pancreatic β cell exposed to 5.5,11,33 mM glucose for 20 min | Geniposide 10μM for 2 h | ↑insulin secretion ↑glucose uptake ↓content of ATP ↓the mRNA and protein levels of pyruvate carboxylase gene |
[34] | ||
| Rat INS-1 pancreatic β cell exposed to 5.5 mM glucose for 1 h | Geniposide 10μM for 1 h | ↑the phosphorylation of PDK1 ↑the phosphorylation of Akt473 and GSK3β ↑the protein level of Glut2 |
[35] | ||
| INS-1 pancreatic β cell exposed to 5/11/25 mmol/l glucose for 1 h | Geniposide 10μM for 2 h | ↑the glycosylation of Glut2 ↑the mRNA and protein levels of GnT-IVa, galectin-9, clathrin |
[36] | ||
| INS-1 pancreatic β cell exposed to 5/25 mM glucose for 1 h | Geniposide 10 μM for 2 h | ↑insulin secretion and ATP content ↓glucose uptake ↑ACC phosphorylation |
[37] | ||
| Protecting Pancreatic Islet β Cells | In Vitro | INS-1 pancreatic β cell exposed to 5/11/25 mM glucose for 24 h | Geniposide 10 μM for 1 h | ↑Insulin serection ↓The accumulation of H2O2 ↑the protein levels of protein disulfide isomerase ↓the protein levels of endoplasmic reticulum oxidoreductin 1 (ERO1) ↓the content of thiol group in INS-1 cells. |
[38] |
| Rat INS-1E insulinoma cell were incubated with 2.0 and 5.0 μM HIAPP | Geniposide 1.0/10/100μm for 2 h | ↑cell viability ↑the proten levels of insulin-degrading enzyme ↑the aggregation of HIAPP |
[39] | ||
| INS-1 pancreatic β cell incubated5/11/25 mM glucose for 24.48 or 72 h | Geniposide 10μm for 5 minutes, 24 h, 48 h, and 72 h | ↓glucose-induced impairment of insulin release ↑the phosphorylation of AMPK ↓the protein levels of HO-1 ↑the Bcl-2/BAX protein ratio ↑the cleavage of Caspase-3 |
[40] | ||
| INS-1 pancreatic β cell exposed to 16.7 mM glucose/0.2mM palmitate for 24 h | Geniposide 10 μM for 24 h | ↑cell viability ↑the protein expression of HO‐1, Bcl‐2 ↓the protein expression of Bax ↑PERK/eIF2α/IRE1α phosphorylation (Unfolded protein response) |
[40] | ||
| INS-1 pancreatic β cell exposed to 25 mM glucose for 24 h | Geniposide 10 μM for 24 h | ↓ protein levels of Txnip ↓Insulin serection ↑glucose and ATP content |
[41] | ||
| INS-1 pancreatic β cell exposed to 25mM glucose for 12 h | Geniposide 10 μM for 2 h | ↓PERK/eIF2α/IRE1α phosphorylation (Unfolded protein response) |
[42] | ||
| INS-1 rat insulinoma cell were incubated with palmitate for 18 h |
Geniposide 1μm for 2 h | ↓palmitate-induced cell apoptosis ↓the protein levels of caspase-3 ↓the phosphorylation of Akt (Thr308), Akt (ser473) and Foxo1 ↓the protein levels of PDX-1 |
[44] | ||
| Rat Islets cells were stimulated with 2.8 mM or 8.3 mM glucose for 0.5 h | Geniposide 10㎛ for 0.5 h | ↑insulin secretion ↑cAMP accumulation ↓ Kv channels ↑action potential duration ↑currents through voltage-dependent Ca2 channels |
[39] | ||
| In vivo | C57BL/6 male mice were fed a high-fat diet for 12 weeks | Geniposide solution was prepared in 0.9% NaCl and delivered by oral gavage at dosage of 100 mg/kg daily | ↓pancreatic islet β cell apoptosis ↑the protein and mRNA levels of TCF7L2 ↑the mRNA levels of insulin,PDX1,IL1β, and CyclinD1 ↑β-catenin/TCF7L2 signaling ↑β-cell regeneration |
[46] | |
| Inhibiting Insulin Resistance | In vivo | Spontaneously obese Type 2 diabetic (TSOD) mices | 0.1%/0.3% geniposide for 8 weeks | ↓Plasma Glucose levels in Oral Glucose Tolerance Test | [47] |
| Male C57BL/6 J mice fed with High-Fat Diet for 8 weeks | Geniposde 25 or 50 mg/kg for 8 weeks | ↓the body weight gain ↓the random blood glucose and fasting blood glucose levels ↓the area under the curve of glucose tolerance tests and insulin tolerance tests ↓hepatic glycogen content and serum insulin ↑the phosphorylations of hepatic IR, Akt (S473) and GSK3β in liver and GAS ↓the mRNA/protein levels of FoxO1 and PDK4 ↑the mRNA levels and protein levels of Glut4 ↓serum RBP4 levels and the mRNA/protein levels of RBP4 and TTR |
[31] | ||
| In Vitro | 3T3-L1 adipocyte cells cultured with 33 mM glucose and 100 nM insulin for 48 h | Geniposide 10㎛ for 2 h | ↑Glucose uptake ↓the protein levels of p-IRS-1, IRS-1, GLUT −1, and IR-β ↑Txnip deregulation ↑the phosphorylation of AMPK |
[49] | |
| HepG2 cell treated with 50, 100, 200 or 500 nmol/l insulin for 48h | Geniposide 62.5 mg/l for 20 h | ↓supernatant glucose centent ↑the mRNA and protein levels of Glut4 ↑Autophagy (↑the protein levels of LC3,P62) ↑the protein levels of P62,P65 |
[48] | ||
| Primary mouse hepatocytes were isolated from the C57BL/6 J mice |
Geniposide 50 mg/l for 24 h | ↓the mRNA/protein levels of RBP4 and TTR ↓RBP4 levels in the culture medium |
[31] | ||
| Inhibiting Hepatic Glucose Production | In Vitro | HepG2 treated with 5.5mM D-glucose | Geniposide 0.1/1/10/100㎛ for 6 h | ↓Glucose production ↑the phosphorylation of AMPK, ACC, and FoxO1 ↓The G6Pase and PEPCK activities |
[50] |
| Suppressing Gluconeogenesis | In vivo | C57BL/6 male mice were fed a high-fat diet | Geniposide (100 or 10 mg/kg) was injected intraperitoneally every day for two weeks | ↓Glucose tolerance | [51] |
| C57BL/6 male mice were fed a high-fat diet | Geniposide (100, 200, and 400 mg/kg) for two weeks | ↓plasma glucose, body weight, TC, TG and insulin levels ↓GP and G6Pase activities and mRNA/protein expression |
[52] | ||
| In Vitro | L02 treated with 1 mmol/L insulin plus 25 mmol/L glucose for 24 h | Geniposide 1/10/100μmol/L for 24 h | ↓Glucose production ↓The G6Pase and PEPCK activities ↑DEX induced FOXO1 nuclear accumulation ↑phosphorylation of AKT |
[51] |
Abbreviations: FoxO1, forkhead box O1; PDK4, pyruvate dehydrogenase kinase 4; PDH, Propane Dehydrogenation; GAS, gasoline; ATP, Adenosine Triphosphate; PDK1, pyruvate dehydrogenase kinase 1; Akt, AKT serine/threonine kinase; GSK3β, glycogen synthase kinase 3 beta; GLUT2, glucose transporter 2; GnT-IVa, glucosaminyl (N-acetyl) transferase family member 7; ERO1, endoplasmic reticulum oxidoreductin 1; IAPP, amyloid polypeptide; AMPK, activated protein kinase; HO-1, heme oxygenase 1; Bcl-2, B cell leukemia/lymphoma 2; BAX, BCL2 associated X, apoptosis regulator; Txnip, thioredoxin-interacting protein; PERK, Protein kinase R (PKR)-like endoplasmic reticulum kinase; eIF2α, eukaryotic translation initiation factor 2 subunit alpha; IRE1α, Inositol-requiring enzyme-1α; PDX-1, Pancreatic duodenal homeobox-1; ACaCa, acetyl-CoA carboxylase; TC, total cholesterol; TG, total triglyceride.
Increasing Glucose Utilization
Skeletal muscle, which is the main organ participating in the uptake and metabolism of glucose, contains slow-twitch and fast-twitch muscle fibers. Fast-twitch fibers generate adenosine triphosphate (ATP) primarily through glycolysis, whereas slow-twitch myofibers rich in mitochondria have high oxidative capacity.29 Geniposide improves glucose homeostasis by promoting a slow-to-fast myofiber switch and glucose utilization. Further studies exposed that geniposide exerts the above effects by regulating forkhead box O1 (FoxO1)/ pyruvate dehydrogenase kinase 4 (PDK4), which controls respiratory substrate selection through pyruvate dehydrogenase.30 From another point of view, in a 2022 study, it was revealed that geniposide regulates respiratory substrate selection, promotes glucose uptake in skeletal muscles, and suppresses glycogen storage by disturbing the synthesis, secretion, and homeostasis of retinol-binding protein 4 (RBP4).31
Increasing Insulin Production
Glucose-stimulated insulin secretion (GSIS) is essential to the maintenance of a stable level of blood glucose.32 Guo et al33 first reported that geniposide could increase GSIS, and the results indicated that glucagon-like peptide 1 receptors (GLP-1R) plays an important role in the geniposide-regulated GSIS. Geniposide regulates GSIS possibly via pyruvate carboxylase-mediated glucose metabolism in pancreatic β cells.34 Exploration from another point of view revealed that GSIS is phosphatidylinositol 3 kinase- dependent and geniposide increases the expression of glucose transporter 2 (GLUT2) in total cell lysates under normal glucose conditions.35 An in-depth study focusing on GLUT2, indicated that it may be related to the regulation of glucosaminyl (N-acetyl) transferase family member 7 (GNT-IVa)-mediated glycosylation of GLUT2 and the residence of glycosylated GLUT2 on the pancreatic β cell membrane.36 A 2021 study found that 5’AMP-activated protein kinase (AMPK) activation also plays an essential role in geniposide-regulated GSIS in pancreatic β cells.37
Protecting Pancreatic Islet β Cells
Diabetes is characterized by pancreatic islet β cell dysfunction or loss. One therapeutic strategy for improving blood glucose homeostasis is to prevent pancreatic islet β cells from failure and promote new pancreatic islet β cell formation.27
Geniposide is a promising pancreatic islet β cell protector, which prevents pancreatic islet β cells from exhaustion and injury resulting from excessive insulin secretion under high glucose conditions. Geniposide administration is a possible way of balancing the oxidative stress of pancreatic β cells by regulating the expression of protein disulfide isomerase (PDI) and endoplasmic reticulum oxidoreductin 1 (ERO1).38 Extensive islet amyloid polypeptide (IAPP) deposits are thought to contribute to pancreatic β cell dysfunction, either by direct cytotoxicity or by reducing the pancreatic islet β cell mass, resulting in impaired insulin secretion. Geniposide prevents human IAPP-induced cytotoxicity in INS-1E cells through the upregulation of (insulin degrading enzyme) IDE.39 Liu et al40 suggested that AMPK plays a crucial role in how geniposide antagonizes high glucose-induced pancreatic β cell injury.
Elevated thioredoxin-interacting protein (Txnip) levels induce β cell apoptosis and dysfunction.41 Geniposide improves GSIS by accelerating Txnip degradation. A further study proved that geniposide-related Txnip degradation attenuates the early-stage apoptosis of pancreatic β cells. Geniposide regulates Txnip degradation and GSIS through endoplasmic reticulum (ER) stress by accelerating the phosphorylation of Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) and Inositol-requiring enzyme-1α (IRE1α).42
The main roles of GLP-1R are stimulation of GSIS, induction of pancreatic β-cell proliferation, inhibition of postprandial glucagon release, and delay in gastric emptying.43 Geniposide inhibits the early stage of lipotoxicity-induced β-cell apoptosis, and GLP-1R plays a critical role by counteracting lipotoxicity in INS-1 pancreatic islet β cells.44 Cui et al45 analyzed the microarray data of INS-1 cells treated with geniposide and identified key lncRNAs and mRNAs in a 2021 study. They found that the lncRNA NONRATT027738 interacts with all three hub mRNAs (Pomc, Htr2a, and Agtr1a). There is also other research focusing on the role of GLP-1R in the beneficial effects of geniposide-medicated pancreatic islet β cell protection. Geniposide potentiates insulin secretion via activating GLP-1R and adenylyl cyclase/cAMP signaling pathways. Researchers also observed Ca+2 channel activation by geniposide.39
Apart from inhibiting the damage and apoptosis of pancreatic islet β cells, another therapeutic strategy for the protection of pancreatic β cells is to promote their regeneration. Geniposide promotes pancreatic islet β cell regeneration in vivo to balance blood glucose levels and the mechanism includes triggering duct cell differentiation by enhancing TCF7L2 expression and activating the Janus-activated kinase 2 (JAK2) / signal transducer and activator of transcription 3 (STAT3) pathway.46
Improving Insulin Resistance (IR)
IR is an important factor, which can lead to the onset of type 2 diabetes. IR occurs when cells in muscles, fat, and liver stop responding to insulin. Geniposide alleviates abnormal glucose tolerance and hyperinsulinemia, indicating that it has an IR-alleviating effect.47 Geniposide promotes autophagy in HepG2 cells and significantly improves IR, which may be associated with the dynamic regulation of the P62/NF-κB/GLUT-4 pathway.48 Zhao et al49 pointed out that geniposide improves insulin signaling deficiency possibly through AMPK-mediated Txnip degradation in 3T3-L1 adipocytes. A regulatory mechanism study considered that geniposide could improve systemic insulin sensitivity by regulating circulating RBP4 levels.31
Inhibiting Hepatic Glucose Production
The liver plays a crucial role in maintaining blood glucose homeostasis by coordinating glucose storage, utilization, and production. The ability of the liver to store glycogen is typically diminished in diabetic subjects. Thus, promoting hepatic glycogen synthesis and suppression of hepatic glucose production could be more effective in the improvement of overall glycemic control. Geniposide significantly inhibits hepatic glucose production in a dose-dependent manner and the inhibitory effect is partly through AMPK activation.50 Geniposide simultaneously stimulates glycogen synthesis in mice induced by a high-fat diet and streptozotocin injection and HepG2 cells.
Suppressing Gluconeogenesis
Hepatic gluconeogenesis is an important factor in regulating plasma glucose levels. It may be the major source of fasting blood glucose and it has been regarded as one of the main contributors to hyperglycemia in diabetes mellitus. Phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) are the key regulatory enzymes of gluconeogenesis.50 Geniposide significantly decreases the expression of glycogen phosphorylase and G6Pase at mRNA and protein levels, as well as their activity, in a dose-dependent manner.51 The activities of PEPCK and G6Pase were significantly suppressed by geniposide. Geniposide may reduce blood glucose levels and suppress hepatic gluconeogenesis by regulating the AKT serine/threonine kinase (AKT)-FOXO1 pathway.52
Effects on Lipid Metabolism
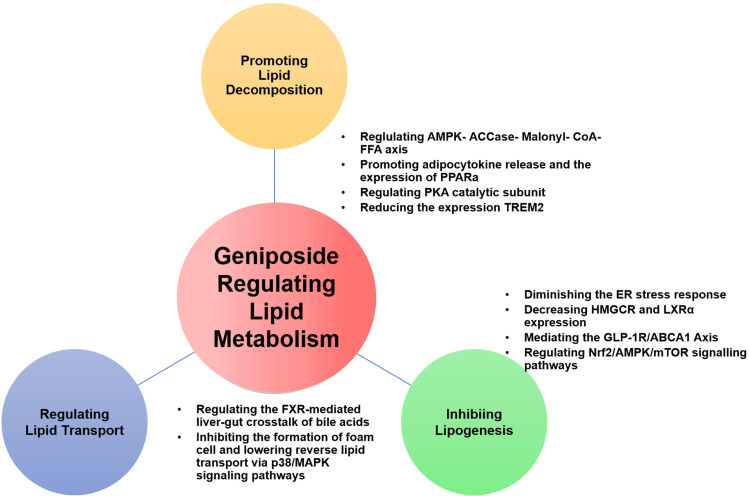
Lipid metabolism disorder is one of the main risk factors of metabolic diseases, which is characterized by abnormal content or type of triglycerides, fatty acids, or cholesterol in the serum or surrounding tissues.53 Lipid metabolism includes three main aspects as follows: Lipolysis, lipogenesis, and lipid transportation. Regulating lipid metabolism and restoring lipid homeostasis is one of the methods for the prevention and treatment of dyslipidemia and related metabolic diseases.54 Figure 5 summarizes the pharmacological effects of geniposide on lipid metabolism and the details are exhibited in Table 2.
Figure 5.
Pharmacological effects of geniposide on Lipid Metabolism.
Table 2.
In vivo/In vitro Studies of Geniposide in Lipid Metabolism
| Effect on lipid metabolism | In vivo/In vitro | Model | Dose and Duration | Experimental Outcome | References |
|---|---|---|---|---|---|
| Promoting lipidolysis | In vivo | Spontaneously obese Type 2 diabetic (TSOD) mice | 0.1%/0.3% geniposide for 8 weeks | ↓body weight and visceral fat accumulation ↓abnormal lipid metabolism and intrahepatic lipid accumulation ↑the mRNA levels of PPARα |
[47] |
| C57BL/6 male mice | Geniposide 25mg/kg for 4weeks | ↓body temperature ↓the mRNA levels of genes related to thermogenesis, adipose browning, and mitochondrial function ↓ lipid deposition and UCP1 density ↓cold tolerance ↓mRNA expressions of thermogenesis-related genes: UCP1, PRDM16, CIDEA, and DIO2 in iBAT and iWAT |
[64] | ||
| Male Sprague–Dawley rats induced by a high-fat emulsion 10 mL/kg for six weeks | Geniposide 25, 50 or 100 mg/kg of was given via gavage for 6 weeks | ↓the liver weights ↓serum and liver levels of TG, TC, FFA, and serum LDL-C ↑serum HDL-C ↓the liver MDA levels↑the liver SOD and GSH-Px activity ↓the fatty deposition in hepatocytes ↓the protein levels of CYP2E1 and TNFa ↑he protein levels of PPARα |
[57] | ||
| In Vitro | HepG2 cells treated with o.5mM palmitic acid for 48h | Geniposide 10/50/100㎛ for 48h | ↓TG/TC concentration ↑the mRNA levels of PPARa |
[47] | |
| 3T3-L1 adipocyte cells treated with 1 μg/mL insulin, 500 μM IBMX, 1 μM DEX, 1 nM T3, and 5 μM rosiglitazone for 48 h | Geniposide 20 mg/mL for 24 h | ↓the mRNA expressions of thermogenic genes (UCP1, PRDM16, CIDEA, DIO2, PGC1α, ELVOL3) and browning markers in adipocytes ↓ the protein levels of UCP1 and PRDM16 in adipocytes ↓ The OCRs of adipocytes ↓ the transcription activity of UCP1 |
[64] | ||
| Inhibitin Lipogenesis | In vivo | Male Wistar rats fed a high-fat diet for 8 weeks | Geniposide 0.001mL/g for 4 weeks | ↓Weight and fat weight ↓Liver TG, FFA ↓the activity of ALT and AST ↑the protein levels of AMPK in the liver ↓the content of FAS, ACCase, and Mal-ony1-CoA in the liver |
[65] |
| C57BL/6J mice fed a high-fat diet for 16 weeks | Geniposide 90 mg/kg for 4 weeks | ↓body weight, liver weight, HDL-C, LDL-C, serum ALT, serum AST, fasting blood glucose, and HOMA-IR ↑HDL-C |
[66] | ||
| SPF rat intramuscularly injected 5 mg/kg Dexamethasone sodium phosphat into the gluteus maximus for 14 weeks |
Geniposide 50/100 mg/kg for 16 weeks | ↓loss of the bone trabecula ↑the protein levels of RUNX2 and OPN in the bone trabecula of the proximal femurs ↑the protein levels of ABCA1 ↑the protein levels of GLP-1R |
[69] | ||
| Male wild-type (WT) and Nrf2−/− C57BL/6 mice fed Tyloxapol 500 mg/kg for 18 h | Geniposide 50/70/100 mg/kg for 19 h | ↓TC, TG, LDL and VLDL ↑HDL ↓the contents of MMP-9, ApoC3, VCAM-1, ICAM-1 and MCP-1 ↓the contents of TNF-α, IL-1β and IL-6 ↑the protein levels of Nrf2 and PPARα into nucleus ↑the expression of HO-1 ↑the phosphorylation of ACC, AKT, AMPKα, AMPKβ ↓the levels of P-mTORC, P-S6K, P-S6 and SREBP-1c |
[70] | ||
| In Vitro | HepG2 cells treated with Oleate (0.1 mmol/L) and SIM (10 μmol/L) for 24 h or 28 h | Geniposide 10μmol/L for 24 or 48 h | ↓the accumulation of lipid droplets ↓TC and TG content in the culture medium of HepG2 cells ↑the mRNA expression of ABCA and AMPK ↓the mRNA expression of CYP7A1, LXRα ↓the protein expression of HMGCR |
[68] | |
| MC3T3-E1 cell incubated osteogenic induction medium for 15 days | Geniposide 10 μM or 25 μM for 15 days | ↓cell differentiation ↑the protein expression of RUNX2 and OPN ↓DEX-induced cholesterol accumulation ↑the protein levels of ABCA1 and ApoA-I ↑the protein levels of GLP-1R |
[69] | ||
| HepG2 cells dealt with different concentrations of geniposide (0, 65, 130, 260, 390 and 520 μmol/L) for 24 hours with or without OA (660 μmol/L) and PA (330 μmol/L) | Geniposide 65, 130, or 260 μmol/L for 1 h | ↓lipid accumulation ↑the protein levels of Nrf2, PPARα and PPARγ ↑the protein content of HO-1 in cytoplasm ↑the phosphorylation of ACC, AKT, AMPKα, AMPKβ, GSK 3β ↓the protein levels of PI3K, P-mTORC, P-S6K, P-S6, SREBP-1c and HMGB1 |
[72] | ||
| RegulatingLipid Transport | In vivo | 1. C57BL/6 male mice with high cholesterol diet ApoE−/− 2. mouse with nomal chow diet |
Geniposide 50mg/kg/D for 13 weeks | ↓serum TC/TG levels ↓hepatic TC/TG levels ↓the hepatic mRNA levels of HMGCR ↑the hepatic protein/mRNA levels of CYP7a1, CYP27a1, CYP7b1 and CYP8b1 ↓the hepatic protein/mRNA levels of FXR, MAFG, and SHP ↑the hepatic protein/mRNA levels of HNF-4α and LRH-1 ↓the protein/mRNA levels of FXR, I-BABP and ASBT in the ileum |
[73] |
| ApoE−/− mouse fed a high-fat diet for 16 weeks |
50/100 mg/kg for unknown weeks | ↓serum TC, TG, LDL-C, HDL-C and the development of atherosclerosis ↓the protein expressions levels of ABCA1, ABCG1 SR-A, CD36, and SR-B1 ↓p38MAPK and AKT phosphorylation |
[72] | ||
| In Vitro | HepG2 cells treated with 2.5 μm GW4064 for 12/24 h | Geniposide 100 μm for12/24 h | ↓the protein/mRNA levels of FXR and MAFG ↑the protein/mRNA levels of HNF-4α and LRH-1 |
[73] | |
| Caco-2 cells treated with 2.5 μm GW4064 for 12/24 h | Geniposide 100 μm for12/24 h | ↑the protein/mRNA levels of FXR and I-BABP ↓the protein/mRNA levels of ASBT |
[73] | ||
| RAW264.7 macrophage was exposed to 200mM LPA for24 h | Geniposide 50, 100 or 200 μg/mg for 24 h | ↓oil red O staining and cholesterol/cholesterol ester quantitation assay ↑the mRNA and protein expression levels of ABCA1 and SR-B1 ↓the mRNA and protein levels of SR-A expression ↓p38MAPK and AKT phosphorylation |
[70] |
Abbreviations: PPARα, peroxisome proliferators-activated receptors α; UCP1, Uncoupling protein 1; PRDM16, PR/SET Domain 16; CIDEA, Cell Death Inducing DFFA Like Effector A; DIO2, Iodothyronine deiodinase 2; AST, aspartate aminotransferase; AMPK, activated protein kinase; TC, total cholesterol; TG, total triglyceride; Akt, AKT serine/threonine kinase; ACaCa, acetyl-CoA carboxylase; FASN, fatty acid synthase; SFAs, saturated fatty acids; SCD1, stearoyl-CoA desaturase 1; chREBP, carbohydrate-responsive element-binding protein; LDL-c, low-density lipoprotein cholesterol; VLDL, very-low-density lipoproteins; HDL, High-density lipoproteins; VCAM-1, vascular cell adhesion molecule 1;ICAM-1, Intercellular Adhesion Molecule 1; MCP-1, Monocyte chemoattractant protein-1; CYP7A1, Cytochrome P450 Family 7 Subfamily A Member 1; LXRα, Liver X receptor alpha; PI3K, Phosphoinositide 3-kinases; mTORC, mammalian target of rapamycin complex 1; S6K, S6 kinase beta-1; P-S6, Phospho-S6; SREBP-1c, Sterol regulatory element-binding transcription factor 1; HMGB1, High mobility group box 1; SR-B1, The scavenger receptor, class B type 1; FXR, Farnesoid X receptor; MAFG, MAF BZIP Transcription Factor G; ASBT, apical sodium–bile acid transporter; MAPK, mitogen-activated protein kinase.
Promoting Lipolysis
Fat is mostly stored in adipose and other tissues or other non-adipose tissues. Excessive accumulation of triacylglycerols (TAGs) and cholesterol esters (CEs) leads to abnormal lipid metabolism. The lipid droplets (LDs) in the cytoplasm are mainly rich in TAGs and CEs, which are considered dynamic TAG storage pools and take part in several aspects of lipid metabolism.55
The primary oxidative pathway for energy production in the liver is β-oxidation which takes place in the mitochondria, the predominant regulators of which are the transcription factors peroxisome proliferators-activated receptors α (PPARα) and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (Pgc1α).56 Geniposide exerts suppressive effects on hepatic lipid accumulation in rats fed with a high-fat diet and the underlying mechanism may be related to the regulation of adipocytokine release and PPARα expression.57 Geniposide improves the expression of NF-E2-related factor 2 (Nrf2), PPARγ, PPARα, and heme oxygenase 1 (HO-1) and regulates the AMPK/mTORC signaling pathway in mice. Genipin, the aglycone of geniposide, inhibits intracellular lipid accumulation and significantly increases PPARα mRNA expression.47 In the mitochondrion, FAs are oxidatively decomposed by carnitine palmitoyltransferase 1 (CPT1) to provide energy for other physiological processes. Sirtuin 1 (SIRT1), FoxO1, and PGC1α also control the activity of the related enzymes. Geniposide treatment alters the expression of FoxO1, PDK4, p-PDH and PDH at mRNA/protein level in the gastrocnemius of HFD-fed mice.31 In agreement with this finding, the inhibitory effects of geniposide on the FoxO1/PDK4/PDH signaling pathway have also been reported.30
Lipophagy (lysosome-mediated autophagy) and cytoplasmic lipolysis are two pathways to decompose TAGs and CEs in LDs. Geniposide increases the levels of autophagy in plaque macrophages via inhibiting the triggering receptors expressed on myeloid cells 2 (TREM2)/mechanistic target of rapamycin kinase (mTOR) axis.58 It may block the development of atherosclerosis through this mechanism. The more widely studied effect of geniposide on inflammation and oxidative stress through enhancing autophagy has been extensively studied recently, although further research is warranted.59–63
Activation of thermogenic adipocytes has great significance in the treatment of lipid metabolism disorders and is related to plenty of metabolic diseases. There is a new study published in 2021 different from the previous studies on the effect of geniposide on lipid metabolism. Thermogenic adipocyte is acknowledged to be a major regulator of energy homeostasis by affecting energy expenditure and glucolipid metabolism. Li et al64 suggested that geniposide is an inhibitor of fat thermogenesis in adipocytes through regulating the PKA signaling pathway, indicating that adipocyte thermogenic capacity may be inessential for geniposide to exert its effects in obesity, and the metabolic advantage of geniposide exerted on other organs, except for adipose tissues, can indemnify for the effect of geniposide-induced thermogenic activity of adipocytes. Further research on how geniposide regulates lipolysis through influencing thermogenesis in adipocytes is warranted.
Inhibiting Lipogenesis
Excessive lipid synthesis is a major cause of lipid metabolism disorders. The de novo lipogenesis, FAs obtained from acetyl-CoA to fatty acyl-CoA transformation, is principally regulated by acetyl-CoA carboxylase (ACaCa) and fatty acid synthase (FASN). The central enzymatic node of saturated fatty acids (SFAs) transformed into monounsaturated fatty acids (MUFAs) is stearoyl-CoA desaturase 1 (SCD1), which is regulated by sterol regulatory element-binding transcription factor 1 (SREBP-1c), carbohydrate-responsive element-binding protein (chREBP), and LXR. Transcriptional regulation of Acc and FASN happens mainly via SREBP-1c and chREBP. Geniposide is highly effective in inhibiting lipogenesis and free fatty acid (FFA) levels in hepatic tissues were decreased significantly after geniposide treatment, whereas TG, FASN, ACaCa, and malonyl-CoA levels were significantly reduced in the geniposide group.65 Geniposide could significantly suppress hepatic total triglyceride (TG), total cholesterol (TC), and FFA in NAFLD rodents.66 Geniposide also decreases the expression of SREBP-1c.67
Cholesterol plays a central role in lipid metabolism. TC and/or low-density lipoprotein cholesterol (LDL-c) levels ultimately depend on cholesterol hypersecretion. Significantly, geniposide inhibited TC and LDL production in different animal models. High mobility group box 1 (HMGCR), squalene monooxygenase, and SREBP are all crucial players in cholesterol synthesis (the first two are rate-limiting enzymes in the biosynthetic pathway, and the latter is a master transcriptional regulator of cholesterol synthesis). Geniposide inhibited TC, TG, LDL, and very-low-density lipoprotein (VLDL) production, SREBP1-c expression, and HMGCR expression at the mRNA/protein level in vivo.67,68 Zheng Y et al published a study focusing on cholesterol metabolism in osteoporosis in 2021, they found that geniposide can effectively ameliorate dexamethasone-induced cholesterol accumulation via activating the GLP-1R/ABCA1 axis in MC3T3-E1 cells.69 Geniposide has significant beneficial effects on cholesterol metabolism and lipid accumulation in HepG2 cells. After HepG2 cells were treated with geniposide, the expression of ABCA1 and AMPK mRNA significantly increased, while that of Cytochrome P450 Family 7 Subfamily A Member 1 (CYP7A1) and Liver X receptor alpha (LXRA) mRNA was significantly reduced.68 Geniposide and chlorogenic acid combination can significantly reduce hepatic TG, TC, and FFA via SCD-1 suppression in the liver.66
Regulating Lipid Transport
Lipid transportation in the blood depends on plasma lipoproteins. High-density lipoproteins (HDL) snatch the cholesterol from other lipoproteins or the peripheral tissues and bring it back to the liver, which is called reverse cholesterol transport (RCT). In the circulation, VLDL, produced in the liver, release TGs and FFA.71 Hepatic lipase removes TGs from intermediate-density lipoprotein, forming LDL. LDL has a high cholesterol content and it can be removed from circulation through binding to LDL receptors in extrahepatic tissues and the liver.72
Geniposide attenuates the development of atherosclerosis and reduces serum TC, TG, and LDL levels in ApoE-/- mice.70 In vitro and in vivo experiments have revealed that geniposide can modify the efflux-related proteins and lipoproteins to regulate cholesterol uptake, thus balancing the lipid transport levels and eventually inhibiting the formation of foam cells. These advantages seem to be mediated by the downregulation of P-p38 mitogen-activated protein kinase (MAPK) and P-AKT.
There are two main methods of assuaging hypercholesterolemia. The first is to increase the transformation of cholesterol into bile acids in the liver and the other is enhanced RCT, through which cholesterol in the plasma can be returned to the liver for catabolism. On the one hand, geniposide improves the hepatic synthesis of bile acids via passivating the negative feedback regulation of bile acids regulated by Farnesoid X receptor (FXR).73 On the other hand, geniposide facilitates the RCT, more cholesterol is directed from the circulation back to the liver, exerting the hypolipidemic effect of geniposide indirectly.73 Hwa-Young Lee74 displayed an unusual yet very clear design solution, their study was conducted using the Eucommia ulmoides Oliver extract, and its active constituents, aucubin and geniposide. They found that geniposide reduces hepatic lipid accumulation and secretion of apolipoprotein B, and they believe the mechanism may be linked to ER stress and hepatic dyslipidemia.
Discussion
Glucolipid metabolism is complex and variable, often involving multiple pathological reactions. Over the past few decades, a growing number of animal and cell model studies have revealed the potential of geniposide to ameliorate glucolipid metabolism disorders. We summarized basic information about various in vitro and in vivo studies on the pharmacological effects of geniposide on glucolipid metabolism in this review.
In terms of glucose metabolism, geniposide can increase glucose utilization and insulin production, protect pancreatic islet β-cells, inhibit IR and hepatic glucose production, and suppress gluconeogenesis. In the aspect of lipid metabolism, geniposide can promote lipid decomposition, inhibit lipogenesis, and regulate lipid transport. Geniposide regulates a wide range of factors related to glucose and lipid metabolism, including metabolic regulators AMPK, Glut2\Glut4\GLP-1R, which are indispensable in glucose metabolism, and PPARα/SREBP1c/ LXR/FXR, which regulate lipid metabolism. Such multi-pathway regulation improves the entire glycolipid metabolism network in a three-dimensional manner. However, there are still some challenges that need to be addressed.
First of all, the current research on geniposide is restricted to whole animal and cell experiments and has not included any clinical trials. Therefore, further clinical studies are essential to determine the role of geniposide in clinical therapy.
Secondly, thorough studies and precise targets are required. Studies on the effect of geniposide on glucolipid metabolism are sometimes unconvincing and have not been verified via rigorous experiments using knockout mice or cell lines. There is insufficient evidence supporting the results and conclusions of these studies. At present, research focusing on the pathological mechanisms of geniposide has not included a specific target, and future studies should be more accurate.
Furthermore, the efficacy and toxicity of geniposide need to be confirmed. We investigated several studies on geniposide toxicity that were published in 2021, and found that chronic oral toxicity in rats resulted in an impact on serum biochemical, urinary, and hematological parameters, and affected related organ weights. However, studies which reported that geniposide improves glucose and lipid metabolism have not investigated the potential toxicity of geniposide. Further studies are warranted to verify the feasibility of geniposide as a drug and develop a safe procedure for its administration. Its efficacy should also be compared with that of the current major drugs for metabolic diseases.
Conclusion
Geniposide ameliorates lipid and glucose metabolic disorders, improving the entire glycolipid metabolism network in a three-dimensional manner at the level of molecular mechanism (Figure 6). Accumulating studies, related to the effect of geniposide on glucose and lipid metabolism, have been performed. While full-scale progress has been made, the underlying mechanism of glucolipid metabolism has not yet been utterly elucidated. Future research is warranted to explain both long- and short-term effects of geniposide on glucose and lipid metabolism. Our review affords the first systematic summary of research examining the effect of geniposide on glucolipid metabolism, which will be beneficial in the development of metabolic diseases therapy in the future and in obtaining more reproducible and reliable data.
Figure 6.
Molecular mechanisms involved in the regulation of glucolipid metabolism by geniposide.
Abbreviations
NAFLD, non-alcohol fatty liver disease; GJE, Gardenia Jasminoides Ellis; EUO, Eucommia Ulmoides Oliv; RO, Rehmannia Officinalis; RS, Radix Scrophulariae; LPO, hepatic lipid peroxidation; GST, glutathione-S-transferase; GSH, glutathione; GPx, glutathione peroxidase; CuZn-SOD, copper- and zinc-containing superoxide dismutase; ATP, adenosine triphosphate; RBP4, retinol-binding protein 4; GSIS, Glucose-stimulated insulin secretion; GLP-1R, glucagon-like peptide 1 receptors; GLUT2, glucose transporter 2; AMPK, activated protein kinase; PDI, protein disulfide isomerase; ERO1, endoplasmic reticulum oxidoreductin 1; IAPP, amyloid polypeptide; Txnip, thioredoxin-interacting protein; ER, endoplasmic reticulum; IR, Insulin Resistance; RBP4, retinol-binding protein 4; TAGs, triacylglycerols; CEs, cholesterol esters; LDs, lipid droplets; ACaCa, acetyl-CoA carboxylase; FASN, fatty acid synthase; SFAs, saturated fatty acids; MUFAs, monounsaturated fatty acids; SCD1, stearoyl-CoA desaturase 1; chREBP, carbohydrate-responsive element-binding protein; LDL-c, low-density lipoprotein cholesterol; VLDL, very-low-density lipoproteins; HDL, High-density lipoproteins; RCT, reverse cholesterol transport.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rana S, Ali S, Wani HA, et al. Metabolic syndrome and underlying genetic determinants-A systematic review. J Diabetes Metab Disord. 2022;21(1):1095–1104. doi: 10.1007/s40200-022-01009-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikegami H, Hiromine Y, Noso S. Insulin-dependent diabetes mellitus in older adults: current status and future prospects. Geriatr Gerontol Int. 2022;8:595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrack JA, Dougherty RJ, Corkum A, et al. Impact of time in motion on blood pressure regulation among patients with metabolic syndrome. Curr Hypertens Rep. 2022. doi: 10.1007/s11906-022-01202-8 [DOI] [PubMed] [Google Scholar]
- 4.Hasebe T, Hasebe N. Impact of risk factors related to metabolic syndrome on acute myocardial infarction in younger patients. Hypertens Res. 2022;45:287–299. [DOI] [PubMed] [Google Scholar]
- 5.Rahman MM, Dhar PS. Exploring the plant-derived bioactive substances as antidiabetic agent: an extensive review. Bio Pharmacother. 2022;152:113–217. doi: 10.1016/j.biopha.2022.113217 [DOI] [PubMed] [Google Scholar]
- 6.Tian J, Qin S, Han J, et al. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J Ethnopharmacol. 2022;289:114984. doi: 10.1016/j.jep.2022.114984 [DOI] [PubMed] [Google Scholar]
- 7.Shan M, Yu S, Yan H, et al. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules. 2017;22(10):255–278. doi: 10.3390/molecules22101689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv S, Ding Y, Zhao H, et al. Therapeutic potential and effective components of the Chinese herb gardeniae fructus in the treatment of senile disease. Aging Dis. 2018;9(6):1153–1164. doi: 10.14336/AD.2018.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan X, Lin L, Cui B, et al. Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: more friend than foe. Pharmacol Res. 2020;159:104–145. doi: 10.1016/j.phrs.2020.104945 [DOI] [PubMed] [Google Scholar]
- 10.Ran D, Hong W, Yan W, et al. Properties and molecular mechanisms underlying geniposide-mediated therapeutic effects in chronic inflammatory diseases. J Ethnopharmacol. 2021;273:113–158. doi: 10.1016/j.jep.2021.113958 [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Wu Q, Chen Y, et al. Updated pharmacological effects, molecular mechanisms, and therapeutic potential of natural product geniposide. Molecules. 2022;27(10):7900–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou YX, Zhang RQ, Rahman K, et al. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid Based Complement Alternat Med. 2019;2019:492. doi: 10.1155/2019/4925682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Li L, Wu H, et al. Antioxidative property and molecular mechanisms underlying geniposide-mediated therapeutic effects in diabetes mellitus and cardiovascular disease. Oxid Med Cell Longev. 2019;2019:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Liu K, Shi M, et al. Therapeutic potential of catalpol and geniposide in Alzheimer’s and Parkinson’s diseases: a snapshot of their underlying mechanisms. Brain Res Bull. 2021;174:281–295. doi: 10.1016/j.brainresbull.2021.06.020 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Zhang F, Hu Q, et al. The emerging possibility of the use of geniposide in the treatment of cerebral diseases: a review. Chin Med. 2021;16(1):86–102. doi: 10.1186/s13020-021-00486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang A, Sun H, Yuan Y, et al. An in vivo analysis of the therapeutic and synergistic properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on its active constituents. Fitoterapia. 2011;82(8):1160–1168. doi: 10.1016/j.fitote.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Zhou Y, Yin F, et al. Quality control and producing areas differentiation of Gardeniae Fructus for eight bioactive constituents by HPLC-DAD-ESI/MS. Phytomedicine. 2014;21(4):551–559. doi: 10.1016/j.phymed.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 18.Fu XM, Yang C, Wu ZG, et al. 不同采收期栀子中8个有效成分含量动态变化与颜色的相关性研究. [Correlation between color and content of eight components of Gardeniae Fructus at different harvest time]. Zhongguo Zhong Yao Za Zhi. 2020;45(13):3191–3202. Chinese. doi: 10.19540/j.cnki.cjcmm.20200229.202 [DOI] [PubMed] [Google Scholar]
- 19.Chang R, Liu J, Luo Y, et al. Isoflavones’ effects on pharmacokinetic profiles of main iridoids from gardeniae fructus in rats. J Pharm Anal. 2020;10(6):571–580. doi: 10.1016/j.jpha.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Feng F. Evaluation of the Hepatotoxicity of the Zhi-Zi-Hou-Po Decoction by Combining UPLC-Q-Exactive-MS-Based Metabolomics and HPLC-MS/MS-based geniposide tissue distribution. Molecules. 2019;24(3):123–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong SH, Jang JH, Cho HY, et al. Simultaneous determination of three iridoid glycosides of Rehmannia glutinosa in rat biological samples using a validated hydrophilic interaction-UHPLC-MS/MS method in pharmacokinetic and in vitro studies. J Sep Sci. 2020;43(22):4148–4161. doi: 10.1002/jssc.202000809 [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Li J, Yang X, et al. Comparative pharmacokinetics of geniposidic acid, genipin-1-β-gentiobioside, geniposide, genipin, and crocetin in rats after oral administration of crude gardeniae fructus and its three processed products using LC-MS/MS. Evid Based Complement Alternat Med. 2020;2020:164–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Bi L, Jin L, et al. Geniposide ameliorates liver fibrosis through reducing oxidative stress and inflammatory response, inhibiting apoptosis and modulating overall metabolism. Front Pharmacol. 2021;12:772–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen L, Ahn SH, Nguyen UT, et al. Geniposide, a principal component of gardeniae fructus, protects skin from diesel exhaust particulate matter-induced oxidative damage. Evid Based Complement Alternat Med. 2021;2021:884–958. doi: 10.1155/2021/8847358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Wang X, Shen X, et al. Geniposide possesses the protective effect on myocardial injury by inhibiting oxidative stress and ferroptosis via activation of the Grsf1/GPx4 Axis. Front Pharmacol. 2022;13:879. doi: 10.3389/fphar.2022.879870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheitasi I, Savari F, Akbari G, et al. Molecular mechanisms of hawthorn extracts in multiple organs disorders in underlying of diabetes: a review. Int J Endocrinol. 2022;2022:725–768. doi: 10.1155/2022/2002768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuttle KR, Agarwal R, Alpers CE, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102:248–260. doi: 10.1016/j.kint.2022.05.012 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Hei H, Zhang S, et al. PGC-1α participates in tumor chemoresistance by regulating glucose metabolism and mitochondrial function. Mol Cell Biochem. 2022. doi: 10.1007/s11010-022-04477-2 [DOI] [PubMed] [Google Scholar]
- 29.Sponton CH, de Lima-Junior JC, Leiria LO. What puts the heat on thermogenic fat: metabolism of fuel substrates. Trends Endocrinol Metab. 2022;1:965–996. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Pan H, Zhang X, et al. Geniposide improves glucose homeostasis via regulating FoxO1/PDK4 in skeletal muscle. J Agric Food Chem. 2019;67(16):4483–4492. doi: 10.1021/acs.jafc.9b00402 [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Song C, Nie C, et al. A novel regulatory mechanism of geniposide for improving glucose homeostasis mediated by circulating RBP4. Phytomedicine. 2022;95:153–162. doi: 10.1016/j.phymed.2021.153862 [DOI] [PubMed] [Google Scholar]
- 32.Ishihara H. Metabolism-secretion coupling in glucose-stimulated insulin secretion. Diabetol Int. 2022;13(3):463–470. doi: 10.1007/s13340-022-00576-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo LX, Xia ZN, Gao X, et al. Glucagon-like peptide 1 receptor plays a critical role in geniposide-regulated insulin secretion in INS-1 cells. Acta Pharmacol Sin. 2012;33(2):237–241. doi: 10.1038/aps.2011.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Guo L, Yin F, et al. Geniposide regulates glucose-stimulated insulin secretion possibly through controlling glucose metabolism in INS-1 cells. PLoS One. 2013;8(10):e78315. doi: 10.1371/journal.pone.0078315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo LX, Liu JH, Yin F. Regulation of insulin secretion by geniposide: possible involvement of phosphatidylinositol 3-phosphate kinase. Eur Rev Med Pharmacol Sci. 2014;18(9):1287–1294. [PubMed] [Google Scholar]
- 36.Jiang XQ, Shen SL, Li WZ, et al. 京尼平苷调节胰腺β细胞GLUT2糖基化的分子机制. [Molecular mechanism of geniposide in regulating GLUT2 glycosylation in pancreatic β cells]. Zhongguo Zhong Yao Za Zhi. 2021;46(14):3643–3649. Chinese. doi: 10.19540/j.cnki.cjcmm.20200118.401 [DOI] [PubMed] [Google Scholar]
- 37.Hao Y, Liu C, Yin F, et al. 5’-AMP-activated protein kinase plays an essential role in geniposide-regulated glucose-stimulated insulin secretion in rat pancreatic INS-1 β cells. J Nat Med. 2017;71(1):123–130. doi: 10.1007/s11418-016-1038-5 [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Hao Y, Yin F, et al. Geniposide balances the redox signaling to mediate glucose-stimulated insulin secretion in pancreatic β-cells. Diabetes Metab Syndr Obes. 2020;13:509–520. doi: 10.2147/DMSO.S240794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Yin F, Liu J, et al. Geniposide protects pancreatic INS-1E β cells from hIAPP-induced cell damage: potential involvement of insulin degrading-enzyme. Cell Biol Int. 2015;39(4):373–378. doi: 10.1002/cbin.10394 [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Hao Y. Yin, et al. Geniposide protects pancreatic β cells from high glucose-mediated injury by activation of AMP-activated protein kinase. Cell Biol Int. 2017;41(5):544–554. doi: 10.1002/cbin.10758 [DOI] [PubMed] [Google Scholar]
- 41.Liu CY, Hao YN, Yin F, et al. Geniposide accelerates proteasome degradation of Txnip to inhibit insulin secretion in pancreatic β-cells. J Endocrinol Invest. 2017;40(5):505–512. doi: 10.1007/s40618-016-0591-9 [DOI] [PubMed] [Google Scholar]
- 42.Hao Y, Shen S, Yin F, et al. Unfolded protein response is involved in geniposide-regulating glucose-stimulated insulin secretion in INS-1 cells. Cell Biochem Funct. 2019;37(5):368–376. doi: 10.1002/cbf.3414 [DOI] [PubMed] [Google Scholar]
- 43.Hjørne AP, Modvig IM, Holst JJ. The sensory mechanisms of nutrient-induced GLP-1 Secretion. Metabolites. 2022;12(5):234–263. doi: 10.3390/metabo12050420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Yin F, Xiao H, et al. Glucagon-like peptide 1 receptor plays an essential role in geniposide attenuating lipotoxicity-induced β-cell apoptosis. Toxicol In Vitro. 2012;26(7):1093–1097. doi: 10.1016/j.tiv.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 45.Cui LJ, Bai T, Zhi LP, et al. Analysis of long noncoding RNA-associated competing endogenous RNA network in glucagon-like peptide-1 receptor agonist-mediated protection in β cells. World J Diabetes. 2020;11(9):374–390. doi: 10.4239/wjd.v11.i9.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao DD, Yang L, Wang Y, et al. Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway. Cell Death Dis. 2015;6(5):e1746. doi: 10.1038/cddis.2015.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kojima K, Shimada T, Nagareda Y, et al. Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol Pharm Bull. 2011;34(10):1613–1618. doi: 10.1248/bpb.34.1613 [DOI] [PubMed] [Google Scholar]
- 48.Jiang H, Ma Y, Yan J, et al. Geniposide promotes autophagy to inhibit insulin resistance in HepG2 cells via P62/NF‑κB/GLUT‑4. Mol Med Rep. 2017;16(5):7237–7244. doi: 10.3892/mmr.2017.7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao W, Pu M, Shen S, et al. Geniposide improves insulin resistance through AMPK-mediated Txnip protein degradation in 3T3-L1 adipocytes. Acta Biochim Biophys Sin (Shanghai). 2021;53(2):160–169. doi: 10.1093/abbs/gmaa157 [DOI] [PubMed] [Google Scholar]
- 50.Guo L, Zheng X, Liu J, et al. Geniposide suppresses hepatic glucose production via AMPK in HepG2 Cells. Biol Pharm Bull. 2016;39(4):484–491. doi: 10.1248/bpb.b15-00591 [DOI] [PubMed] [Google Scholar]
- 51.Yang SQ, Chen YD, Li H, et al. Geniposide and gentiopicroside suppress hepatic gluconeogenesis via regulation of AKT-FOXO1 Pathway. Arch Med Res. 2018;49(5):314–322. doi: 10.1016/j.arcmed.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 52.Wu SY, Wang GF, Liu ZQ, et al. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin. 2009;30(2):202–208. doi: 10.1038/aps.2008.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew N, Ng CH, Truong E, et al. Non-alcoholic steatohepatitis drug development pipeline: an update. Semin Liver Dis. 2022;42:356–365. [DOI] [PubMed] [Google Scholar]
- 54.Deng KQ, Huang X, Lei F, et al. Role of hepatic lipid species in the progression of nonalcoholic fatty liver disease. Am J Physiol Cell Physiol. 2022;23:244–298. [DOI] [PubMed] [Google Scholar]
- 55.Rodrigues JE, Martinho A, Santa C, et al. Systematic review and meta-analysis of mass spectrometry proteomics applied to human peripheral fluids to assess potential biomarkers of schizophrenia. Int J Mol Sci. 2022;23(9):201–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Todisco S, Santarsiero A, Convertini P, et al. PPAR alpha as a metabolic modulator of the liver: role in the pathogenesis of nonalcoholic steatohepatitis (NASH). Biology. 2022;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma T, Huang C, Zong G, et al. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J Pharm Pharmacol. 2011;63(4):587–593. doi: 10.1111/j.2042-7158.2011.01256.x [DOI] [PubMed] [Google Scholar]
- 58.Xu YL, Liu XY, Cheng SB, et al. Geniposide enhances macrophage autophagy through downregulation of trem2 in atherosclerosis. Am J Chin Med. 2020;48(8):1821–1840. doi: 10.1142/S0192415X20500913 [DOI] [PubMed] [Google Scholar]
- 59.Fu C, Zhang X, Lu Y, et al. Geniposide inhibits NLRP3 inflammasome activation via autophagy in BV-2 microglial cells exposed to oxygen-glucose deprivation/reoxygenation. Int Immunopharmacol. 2020;84:106–147. doi: 10.1016/j.intimp.2020.106547 [DOI] [PubMed] [Google Scholar]
- 60.Luo X, Wu S, Jiang Y, et al. Inhibition of autophagy by geniposide protects against myocardial ischemia/reperfusion injury. Int Immunopharmacol. 2020;85:106–159. doi: 10.1016/j.intimp.2020.106609 [DOI] [PubMed] [Google Scholar]
- 61.Dusabimana T, Park EJ, Je J, et al. Geniposide improves diabetic nephropathy by enhancing ULK1-mediated autophagy and reducing oxidative stress through AMPK activation. Int J Mol Sci. 2021;22(4):109–124. doi: 10.3390/ijms22041651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu DX, Zhang D, Hu WM, et al. Geniposide protection against Aβ(1-42) toxicity correlates with mTOR inhibition and enhancement of autophagy. J Integr Neurosci. 2021;20(1):67–75. doi: 10.31083/j.jin.2021.01.242 [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Pan Y, Liu Y, et al. A new geniposidic acid derivative exerts antiaging effects through antioxidative stress and autophagy induction. Antioxidants. 2021;10(6):897–923. doi: 10.3390/antiox10060897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y, Zhang K, Liu J, et al. Geniposide suppresses thermogenesis via regulating PKA catalytic subunit in adipocytes. Toxicology. 2021;464:153–214. doi: 10.1016/j.tox.2021.153014 [DOI] [PubMed] [Google Scholar]
- 65.Liang HQ, Lin MT, Zhao X, et al. 栀子苷改善大鼠非酒精性脂肪性肝病游离脂肪酸代谢的机制研究. [Mechanism of geniposide in improving free fatty acid metabolism in rats with non-alcoholic fatty liver disease]. Zhongguo Zhong Yao Za Zhi. 2016;41(3):470–475. Chinese. doi: 10.4268/cjcmm20160319 [DOI] [PubMed] [Google Scholar]
- 66.Chen C, Xin X, Liu Q, et al. Geniposide and chlorogenic acid combination improves non-alcoholic fatty liver disease involving the potent suppression of elevated hepatic SCD-1. Front Pharmacol. 2021;12:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen B, Feng H, Cheng J, et al. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J Cell Mol Med. 2020;24(9):5097–5108. doi: 10.1111/jcmm.15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leng E, Xiao Y, Mo Z, et al. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complement Altern Med. 2018;18(1):122–167. doi: 10.1186/s12906-018-2189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y, Xiao Y, Zhang D, et al. Geniposide Ameliorated Dexamethasone-Induced Cholesterol Accumulation in Osteoblasts by Mediating the GLP-1R/ABCA1 Axis. Cells-Basel. 2021;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen D, Zhao D, Yang X, et al. Geniposide against atherosclerosis by inhibiting the formation of foam cell and lowering reverse lipid transport via p38/MAPK signaling pathways. Eur J Pharmacol. 2019;864:172–198. doi: 10.1016/j.ejphar.2019.172728 [DOI] [PubMed] [Google Scholar]
- 71.Newman CB. Effects of endocrine disorders on lipids and lipoproteins. Best Pract Res Clin Endocrinol Metab. 2022;101–167. doi: 10.1016/j.beem.2022.101667 [DOI] [PubMed] [Google Scholar]
- 72.Ribas-Latre A, Eckel-Mahan K. Nutrients and the circadian clock: a partnership controlling adipose tissue function and health. Nutrients. 2022;14(10):456–489. doi: 10.3390/nu14102084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Li Y, Sun C, et al. Geniposide reduces cholesterol accumulation and increases its excretion by regulating the FXR-mediated liver-gut crosstalk of bile acids. Pharmacol Res. 2020;152:104–131. doi: 10.1016/j.phrs.2020.104631 [DOI] [PubMed] [Google Scholar]
- 74.Lee HY, Lee GH, Lee MR, et al. Eucommia ulmoides Oliver extract, aucubin, and geniposide enhance lysosomal activity to regulate ER stress and hepatic lipid accumulation. PLoS One. 2013;8(12):813–849. doi: 10.1371/journal.pone.0081349 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]