Abstract
Purpose
About 20–25% of patients experience weight regain (WR) or insufficient weight loss (IWL) after bariatric metabolic surgery (BS). Therefore, we aimed to retrospectively assess the effectiveness of adjunct treatment with the GLP-1 receptor agonist semaglutide in non-diabetic patients with WR or IWL after BS.
Materials and Methods
Post-bariatric patients without type 2 diabetes (T2D) with WR or IWL (n = 44) were included in the analysis. The primary endpoint was weight loss 3 and 6 months after initiation of adjunct treatment. Secondary endpoints included change in BMI, HbA1c, lipid profile, hs-CRP, and liver enzymes.
Results
Patients started semaglutide 64.7 ± 47.6 months (mean ± SD) after BS. At initiation of semaglutide, WR after post-bariatric weight nadir was 12.3 ± 14.4% (mean ± SD). Total weight loss during semaglutide treatment was − 6.0 ± 4.3% (mean ± SD, p < 0.001) after 3 months (3.2 months, IQR 3.0–3.5, n = 38) and − 10.3 ± 5.5% (mean ± SD, p < 0.001) after 6 months (5.8 months, IQR 5.8–6.4, n = 20). At 3 months, categorical weight loss was > 5% in 61% of patients, > 10% in 16% of patients, and > 15% in 2% of patients. Triglycerides (OR = 0.99; p < 0.05), ALT (OR = 0.87; p = 0.05), and AST (OR = 0.89; p < 0.05) at baseline were negatively associated with weight loss of at least 5% at 3 months’ follow-up (p < 0.05).
Conclusion
Treatment options to manage post-bariatric excess weight (regain) are scarce. Our results imply a clear benefit of adjunct treatment with semaglutide in post-bariatric patients. However, these results need to be confirmed in a prospective randomized controlled trial to close the gap between lifestyle intervention and revision surgery in patients with IWL or WR after BS.
Graphical abstract
Keywords: Semaglutide, GLP-1 receptor agonist, Weight regain, Bariatric surgery
Introduction/Purpose
There is an urgent need to develop novel strategies to treat insufficient weight loss or weight regain in post-bariatric patients. Despite the overall safety and efficacy of bariatric metabolic surgery (BS), outcomes vary considerably for individual patients [1]. Approximately 20–25% of patients experience considerable weight regain (WR) defined as regain of weight that occurs after achievement of an initial successful weight loss (defined as EWL% > 50%) or insufficient weight loss (IWL) defined as < 50% EWL at 18 months after BS [2, 3]. As a consequence, patients may only experience partial remission of comorbidities; e.g., a large number of patients who experience complete T2D remission in the early period after surgery suffer a relapse on long-term follow-up [4]. In the Swedish Obese Subjects (SOS) study, the first long-term prospective trial to provide information on the effects of BS, 10% of RYGB patients did not maintain ≥ 5% weight loss at 10-year follow-up [1, 5, 6]. The rate of conversion surgery due to failure of the initial procedure is almost twice after sleeve gastrectomy (SG) than after Roux-en-Y bypass (RYGB) [7]. Conversion surgery usually implies a greater degree of malabsorption and higher rate of morbidity, reoperations, and readmission [8].
The Endocrine Society’s clinical practice guidelines on the management of the post-bariatric surgery patient recommend that pharmacotherapy should be included in the multidisciplinary treatment of WR [1, 9]. However, most studies evaluating pharmacotherapy for long-term treatment of overweight and obesity have excluded participants with a history of BS [10, 11]. Thus, there are no approved weight management drugs for use in patients who had BS, even though several studies have suggested that the combination of pharmacotherapy and lifestyle modification results in more weight loss than either approach used alone [1, 12, 13]. So far, there is limited data about the efficacy of pharmacotherapy to treat insufficient weight loss or weight regain in the post-bariatric population. Overall, the currently available FDA-approved weight loss drugs have been associated with relatively modest weight loss in post-bariatric patients [14, 15].
In 2021, the long-acting human GLP-1 receptor agonist (GLP-1 RA) semaglutide was approved for the treatment of obesity in the USA and in 2022 in Europe. In non-surgical patients with obesity, semaglutide induces clinically important weight loss and improvement in cardiometabolic risk factors [16]. So far, there are no data on the effectiveness of semaglutide in the management of IWL and WR in non-diabetic patients after BS.
The aim of the present study was to assess the potential effects of semaglutide 0.5 mg once-weekly in non-diabetic patients with WR and IWL as adjunct therapy after BS in a retrospective setting.
Materials and Methods
Study Population
A total of 53 adult patients (≥ 18 years) who underwent either sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) according to the S3 Leitlinie (Guidelines) Chirurgie der Adipositas [17] were included in this study. Patients with second step procedures prior to adjunct treatment with semaglutide were considered having SG (n = 1). Between 2020 and 2022, patients attended our obesity outpatient clinic, which is certified by the European Accreditation Council for Bariatric Surgery as a center of excellence for obesity and metabolic surgery.
Patients with WR defined as continuous WR after an initial successful weight loss (defined as EWL > 50%) and IWL defined as achieving a nadir weight with EWL < 50% after surgery were prescribed semaglutide in addition to autonomous lifestyle modification for weight management following BS [2, 3]. All patients gave their informed consent to off-label drug use prior to prescription. Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks and increased to 0.5 mg/week thereafter according to individual tolerability. Contraindications for use were a history of acute/chronic pancreatitis and pancreatic cancer, positive family history of multiple endocrine neoplasia, current pregnancy/breastfeeding, and glomerular filtration rate below 15 mL/min. Exclusion criteria included incomplete records, presence of T2D, and revision surgery during follow-up. Of an initial population of 53 patients, 9 patients were excluded from the final analysis (n = 44): Two patients were excluded due to coexisting T2D, and two patients discontinued treatment with semaglutide due to revision surgery or pregnancy occurring during follow-up. In 3 patients, treatment was discontinued 4 weeks after treatment initiation due to lack of effectiveness (defined as total weight loss < 2 kg within 4 weeks), and 2 patients were lost to follow-up.
Study Design
Follow-up data were retrospectively collected from 44 patients at baseline. To provide reasonable comparability between the cases, the available data were allocated 3 “visits” by time in relation to initiation of semaglutide once-weekly. In addition to baseline data 64.7 ± 47.6 months (mean ± SD) after surgery, data from visit 1 (n = 44) were analyzed 1 month after treatment initiation, data from visit 2 (n = 38) 3 months after treatment initiation, and data from visit 3 (n = 20) 6 months after treatment initiation with semaglutide.
Variables
Data on height (cm), weight (kg), body mass index (BMI; kg/m2), sex, age, type of surgery, platelets (103/µL), CRP (mg/L), hs-CRP (mg/dL), hemoglobin (g/dL), albumin (g/L), aspartate aminotransferase (AST; U/L), alanine aminotransferase (ALT; U/L), gamma-glutamyl transpeptidase (GGT; U/L), total cholesterol (TC; mg/dL), triglycerides (mg/dL), high-density lipoprotein cholesterol (HDL; mg/dL), low-density lipoprotein cholesterol (LDL; mg/dL), lipase (U/L), and HbA1c (%) were analyzed at baseline and during follow-up. Percent of total weight loss (%TWL) was calculated by dividing the difference between initial weight and postoperative weight by initial weight multiplied by 100 [18]. Clinically significant weight loss following medical treatment adjunct to BS was defined as > 5% decrease in body weight as per clinical guidelines [19, 20]. Side effects were pulled from electronic medical records.
Statistical Methods
Standard descriptive statistics were used for all study end points. Distributions of continuous variables were described with mean and standard deviation (SD). Categorical variables were described with absolute and relative frequencies. Continuous variables at baseline were compared using Student’s t-test. Categorical variables were compared using chi-square statistics. Continues data between multiple visits were compared using one-way ANOVA with Dunnett’s test for multiple comparisons. Weight loss outcomes between subgroups and multiple visits were compared using two-way ANOVA with Šídák’s test for multiple comparisons. Univariate logistic regression analysis was conducted to identify significant factors of successful weight loss defined as relative weight loss of ≥ 5% at 3 months’ follow-up compared to the baseline visit. Odds ratio (OR) with 95% confidence intervals (CI) and p values were calculated. p values below 0.05 were considered statistically significant.
Results
Patient Characteristics at Baseline
Baseline characteristics are presented in Table 1. Age was 46.4 ± 8.8 years (mean ± SD), and 75.4% of patients were female. 65.9% of patients underwent SG, and 34.1% underwent RYGB as initial weight loss procedure. BMI prior to surgery was 49.4 ± 8.9 kg/m2 (mean ± SD), total weight loss after surgery was –21.5 ± 10.3% (mean ± SD), and maximum weight loss following BS was − 29.1 ± 11.9% (mean ± SD). Postoperative WR after post-bariatric weight nadir and before adjunct semaglutide was 12.3 ± 14.4% (mean ± SD).
Table 1.
Anthropometric and biochemical characteristics at baseline by type of surgery
| RYGB (N = 15) | SG (N = 29) | Total (N = 44) | p value | |
|---|---|---|---|---|
| Age [year] | 46.8 (7.3) | 46.3 (9.6) | 46.4 (8.8) | 0.859 |
| Sex (females) | 12 (80%) | 20 (69%) | 32 (73%) | 0.436 |
| Body weight before BS [kg] | 136.3 (17.5) | 150.5 (38.7) | 145.7 (33.5) | 0.185 |
| BMI before BS [kg/m2] | 48.0 (5.9) | 50.1 (10.1) | 49.4 (8.9) | 0.467 |
| Body weight nadir post BS [kg] | 92.5 (18.9) | 107.9 (27.2) | 102.9 (25.6) | 0.065 |
| BMI nadir post BS [kg/m2] | 32.5 (6.0) | 35.8 (6.5) | 34.7 (6.5) | 0.118 |
| Time from BS to weight loss nadir [months] | 27.8 (20.1) | 28.2 (45.9) | 28.0 (39.1) | 0.973 |
| Time from BS to initiation of semaglutide treatment [months] | 78.8 (37.8) | 57.4 (51.1) | 64.7 (47.6) | 0.160 |
| Time from weight nadir to initiation of semaglutide treatment [months] | 50.8 (32.5) | 29.2 (32.7) | 36.2 (33.8) | 0.048 |
| Total weight loss from BS to nadir [%] | − 32.7 (10.3) | − 27.4 (12.4) | − 29.1 (11.9) | 0.172 |
| Weight regain from nadir to initiation of semaglutide treatment [%] | 17.4 (15.8) | 9.8 (13.2) | 12.3 (14.4) | 0.103 |
| Weight prior to initiation of semaglutide treatment [kg] | 106.5 (18.2) | 117.1 (27.7) | 113.5 (25.2) | 0.187 |
| BMI prior to initiation of semaglutide treatment [kg/m2] | 37.3 (6.4) | 38.9 (6.5) | 38.3 (6.4) | 0.465 |
| Hb prior to initiation of semaglutide treatment [g/dL] | 12.9 (1.2) | 13.7 (1.3) | 13.4 (1.3) | 0.066 |
| Platelets prior to initiation of semaglutide treatment [Mrd/L] | 288.8 (93.7) | 256.7 (55.2) | 267.7 (71.3) | 0.160 |
| HbA1c prior to initiation of semaglutide treatment [%] | 5.4 (0.4) | 5.3 (0.3) | 5.3 (0.4) | 0.424 |
| Albumin prior to initiation of semaglutide treatment [g/L] | 39.3 (2.2) | 38.5 (3.6) | 38.8 (3.2) | 0.402 |
| Total cholesterol prior to initiation of semaglutide treatment [mg/dL] | 176.1 (28.8) | 185.8 (38.8) | 182.4 (35.6) | 0.398 |
| Triglycerides prior to initiation of semaglutide treatment [mg/dL] | 149.9 (81.5) | 151.6 (62.8) | 151.0 (68.8) | 0.940 |
| HDL-cholesterol prior to initiation of semaglutide treatment [mg/dL] | 61.3 (20.9) | 52.3 (10.1) | 55.3 (15.1) | 0.060 |
| LDL-cholesterol prior to initiation of semaglutide treatment [mg/dL] | 84.9 (17.2) | 100.8 (35.1) | 95.4 (30.9) | 0.107 |
| Total cholesterol/HDL-cholesterol ratio prior to initiation of semaglutide treatment | 3.0 (1.0) | 3.7 (0.9) | 3.4 (1.0) | 0.047 |
| AST prior to initiation of semaglutide treatment [U/L] | 25.1 (6.7) | 19.9 (5.8) | 21.7 (6.5) | 0.011 |
| ALT prior to initiation of semaglutide treatment [U/L] | 27.2 (11.4) | 21.2 (8.8) | 23.2 (10.1) | 0.060 |
| GGT prior to initiation of semaglutide treatment [U/L] | 17.1 (10.2) | 22.4 (16.7) | 20.6 (14.9) | 0.260 |
| CRP prior to initiation of semaglutide treatment [mg/L] | 2.2 (2.1) | 5.0 (6.2) | 4.1 (5.4) | 0.134 |
| hs-CRP prior to initiation of semaglutide treatment [mg/dL] | 0.2 (0.2) | 0.4 (0.5) | 0.3 (0.4) | 0.134 |
| Lipase prior to initiation of semaglutide treatment [U/L] | 42.7 (11.9) | 42.5 (14.2) | 42.5 (13.3) | 0.966 |
| Total weight loss following BS to initiation of semaglutide treatment [%] | 22.0 (8.2) | 21.2 (11.4) | 21.5 (10.3) | 0.803 |
| Prediabetes prior to initiation of semaglutide treatment | 5 (33%) | 3 (10%) | 8 (18%) | 0.061 |
Data are reported as mean (SD) and N (%). N, number of individuals; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; BS, bariatric metabolic surgery; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; CRP, C-reactive protein; hs-CRP, high sensitive C-reactive protein
Weight Loss Outcomes
Patients initiated semaglutide 64.7 ± 47.6 months (mean ± SD) after bariatric metabolic surgery at a BMI of 38.3 ± 6.4 kg (mean ± SD). After 3 months (3.2, IQR 3.0–3.5, n = 38) of treatment with semaglutide, total weight loss was − 6.0 ± 4.3% (mean ± SD, p < 0.001) and after 6 months (5.8, IQR 5.8–6.4, n = 20), total weight loss added up to − 10.3 ± 5.5% (mean ± SD, p < 0.001).
After 3 months on semaglutide treatment, 61% of patients reached categorical weight loss > 5%, 16% of patients > 10% weight loss, and 2% of patients > 15% weight loss. After 6 months of adjunct semaglutide treatment, 85% of patients reached > 5% weight loss, 45% of patients reached > 10% weight loss, and 5% of patients reached > 15% weight loss.
Female patients presented a more pronounced weight loss at 3 months’ (− 7.10 ± 3.95%) and 6 months’ follow-up (− 11.04 ± 5.74%) after initiation of adjunct semaglutide treatment once-weekly compared to male patients (− 2.44 ± 3.77% and − 5.90 ± 2.87%, respectively). This difference in weight loss outcomes was statistically significant for the 3-month follow-up visit (p = 0.005) (data not shown).
68.2% of patients were classified as having WR, and 31.8% of patients were classified as having IWL. Differentiating between these two groups, no significant differences in weight loss response were found (data not shown).
Side effects included nausea during the first 2 weeks of treatment in two patients. In 1 patient, increase in pancreatic lipase levels was observed, which resolved spontaneously 6 months after treatment initiation.
Weight Loss Outcomes Depending on Type of Surgery
With regard to type of surgery, there were no significant differences in semaglutide-induced weight loss after 3 months (p = 0.7) and 6 months (p = 0.8) between patients who had undergone SG and RYGB. BMI and change in BMI prior to initiation of semaglutide were not significantly different between the subgroups. In both subgroups, patients experienced a significant weight loss as early as 1 month post initiation of semaglutide (p < 0.001). Further reductions in body weight occurred at visit 2 (p < 0.001) and visit 3 (p < 0.001) compared to baseline (Fig. 1).
Fig. 1.
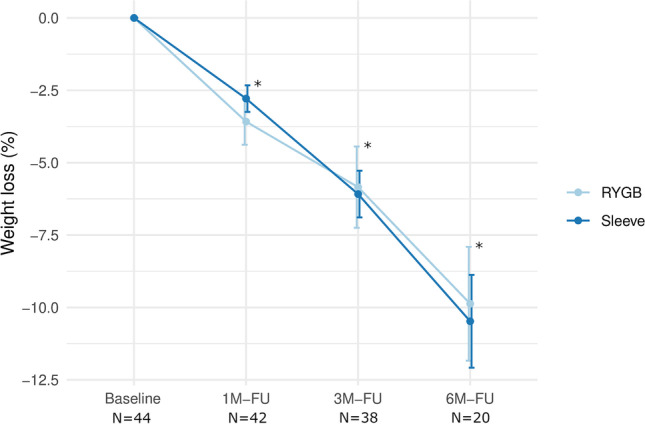
Weight loss over time following adjunct treatment with semaglutide once-weekly by type of surgery. SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; FU, follow-up; N, number of individuals. Results are expressed as means and standard deviation. *Significantly different from baseline regardless of surgical group (p < 0.001)
Effects on Cardiovascular Surrogate Markers
Prior to semaglutide treatment, HbA1c was 5.3 ± 0.4% (mean ± SD); 8 patients suffered from prediabetes at baseline. Over the follow-up period, HbA1c remained stable. Also, there was no significant change in total cholesterol, HDL- and LDL-cholesterol, triglycerides, hs-CRP, and liver enzymes (Table 2).
Table 2.
Anthropometric and biochemical characteristics at baseline and follow-up visits
| Baseline (N = 44) | 3 M-FU (N = 38) | p value | 6 M-FU (N = 20) | p value | |
|---|---|---|---|---|---|
| Body weight [kg] | 113.5 (25.2) | 106.5 (24.5) | 0.357 | 105.7 (25.1) | 0.419 |
| BMI [kg/m2] | 38.3 (6.4) | 36.0 (6.1) | 0.147 | 36.2 (6.7) | 0.380 |
| Hb [g/dL] | 13.4 (1.3) | 13.6 (1.2) | 0.703 | 13.4 (1.2) | 0.999 |
| Platelets [Mrd/L] | 267.7 (71.3) | 269.5 (60.8) | 0.992 | 282.0 (111.9) | 0.729 |
| HbA1c [%] | 5.3 (0.4) | 5.2 (0.3) | 0.310 | 5.2 (0.2) | 0.446 |
| Albumin [g/L] | 38.8 (3.2) | 39.3 (2.9) | 0.999 | 38.5 (2.1) | 0.999 |
| Total cholesterol [mg/dL] | 182.4 (35.6) | 182.1 (37.0) | 0.999 | 170.3 (28.6) | 0.502 |
| Triglycerides [mg/dL] | 151.0 (68.8) | 142.9 (64.2) | 0.792 | 114.6 (38.7) | 0.062 |
| HDL-cholesterol [mg/dL] | 55.3 (15.1) | 51.7 (14.3) | 0.471 | 50.4 (16.7) | 0.394 |
| LDL-cholesterol [mg/dL] | 95.4 (30.9) | 98.7 (33.8) | 0.859 | 89.5 (27.9) | 0.723 |
| Total cholesterol/HDL-cholesterol ratio | 3.4 (1.0) | 3.8 (1.5) | 0.346 | 3.9 (1.9) | 0.328 |
| AST [U/L] | 21.7 (6.5) | 24.0 (10.8) | 0.555 | 24.3 (17.5) | 0.600 |
| ALT [U/L] | 23.2 (10.1) | 21.1 (12.2) | 0.645 | 20.8 (13.8) | 0.678 |
| GGT [U/L] | 20.6 (14.9) | 18.7 (13.7) | 0.796 | 16.8 (16.4) | 0.549 |
| CRP [mg/L] | 4.1 (5.4) | 4.0 (5.6) | 0.998 | 5.8 (13.5) | 0.640 |
| hs-CRP [mg/dL] | 0.3 (0.4) | 0.3 (0.5) | 0.999 | 0.2 (0.3) | 0.603 |
| Lipase [U/L] | 42.5 (13.3) | 43.5 (14.5) | 0.928 | 44.4 (12.6) | 0.834 |
| Total weight loss [%] | − 6.0 (4.3) | < 0.001 | − 10.3 (5.5) | < 0.001 | |
| Prediabetes | 8 (18%) | 2 (6%) | 0.098 | 1 (5%) | 0.179 |
Data are reported as mean (SD). N, number of individuals; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; CRP, C-reactive protein; hs-CRP, high sensitive C-reactive protein
Logistic Regression Analysis for Weight Loss of at Least 5% at 3 Months’ Follow-up
Triglycerides (OR = 0.99; p < 0.05), ALT (OR = 0.87; p = 0.05), and AST (OR = 0.89; p < 0.05) at baseline were negatively associated with weight loss of at least 5% at 3 months’ follow-up. Results of logistic regression analysis are shown in Table 3.
Table 3.
Univariate logistic regression analysis to identify significant factors of successful weight loss defined as relative weight loss of ≥ 5% at 3 months’ follow-up compared to the baseline visit
| Baseline visit data | OR | 95% CI | p value |
|---|---|---|---|
| Age [year] | 0.97 | 0.89–1.04 | 0.399 |
| Sex [f] | 4.44 | 0.95–25.09 | 0.067 |
| Body weight [kg] | 1.00 | 0.98–1.03 | 0.798 |
| BMI [kg/m2] | 1.10 | 0.99–1.26 | 0.110 |
| Hb [g/dL] | 0.69 | 0.37–1.15 | 0.180 |
| Platelets [Mrd/L] | 1.01 | 1.00–1.02 | 0.099 |
| HbA1c [%] | 6.01 | 0.85–69.77 | 0.102 |
| Albumin [g/L] | 0.77 | 0.55–1.01 | 0.081 |
| Total Cholesterol [mg/dL] | 1.00 | 0.98–1.02 | 0.766 |
| Triglycerides [mg/dL] | 0.99 | 0.97–1.00 | 0.016* |
| HDL-cholesterol [mg/dL] | 1.01 | 0.97–1.06 | 0.503 |
| LDL-cholesterol [mg/dL] | 1.00 | 0.98–1.03 | 0.771 |
| Total cholesterol/HDL-cholesterol ratio | 0.72 | 0.35–1.42 | 0.353 |
| AST [U/L] | 0.89 | 0.78–0.99 | 0.047* |
| ALT [U/L] | 0.87 | 0.78–0.95 | 0.005** |
| GGT [U/L] | 0.99 | 0.94–1.04 | 0.627 |
| CRP [mg/L] | 1.06 | 0.92–1.31 | 0.498 |
| hs-CRP [mg/dL] | 1.75 | 0.35–18.65 | 0.552 |
| Lipase [U/L] | 0.97 | 0.92–1.02 | 0.200 |
| Type of surgery [SG] | 0.94 | 0.23–3.68 | 0.927 |
| BMI before BS [kg/m2] | 0.98 | 0.91–1.06 | 0.657 |
| BMI nadir post BS [kg/m2] | 1.07 | 0.97–1.22 | 0.206 |
| Time from BS to weight loss nadir [months] | 0.98 | 0.94–1.00 | 0.301 |
| Time from BS to initiation of semaglutide treatment [months] | 1.00 | 0.98–1.01 | 0.751 |
| Total weight loss from BS to nadir [%] | 1.04 | 0.98–1.10 | 0.248 |
| Weight regain from nadir to initiation of semaglutide treatment [%] | 1.01 | 0.96–1.06 | 0.747 |
Odds ratio (OR) with 95% confidence intervals (CI) and p values. F, female; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; CRP, C-reactive protein; hs-CRP, high sensitive C-reactive protein; SG, sleeve gastrectomy; BS, bariatric metabolic surgery
Conclusion
Here, we report on the effectiveness of adjunct medical treatment with semaglutide once-weekly in non-diabetic patients with WR or IWL after bariatric metabolic surgery. After 3 months (3.2, IQR 3.0–3.5, n = 38) of treatment with semaglutide, total weight loss was − 6.0 ± 4.3% (mean ± SD, p < 0.001) and after 6 months (5.8, IQR 5.8–6.4, n = 20), total weight loss added up to − 10.3 ± 5.5% (mean ± SD, p < 0.001). After 6 months of adjunct semaglutide treatment, 85% of patients reached > 5% weight loss, 45% of patients reached > 10% weight loss, and 5% of patients reached > 15% weight loss (Fig. 2). Side effects included gastrointestinal symptoms within 14 days of treatment initiation like nausea and a feeling of fullness, which was usually mild and did not lead to treatment discontinuation.
Fig. 2.
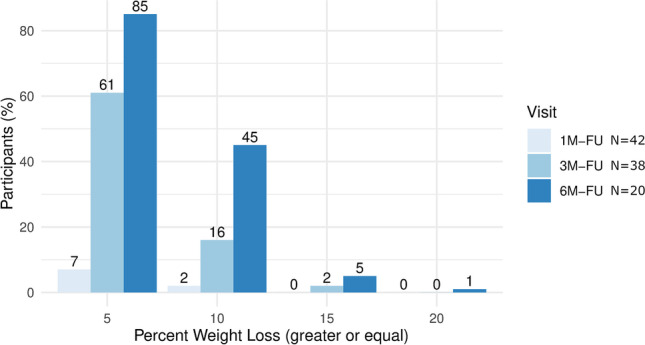
Percentages of patients who reached weight loss of at least 5%, 10%, 15%, and 20%, respectively, following adjunct treatment with semaglutide once-weekly for 1, 3, and 6 months. FU, follow-up; N, number of individuals
Analogous to the results of the semaglutide Phase III trial STEP-1 (the Semaglutide Treatment Effect in People with Obesity), post-bariatric patients of our cohort showed more than a 2% reduction in body weight within only the first 4 weeks of treatment initiation with semaglutide and continued to lose weight throughout the 6-month follow-up period [16]. Since semaglutide 2.4 mg once-weekly, which is a FDA/EMA-approved weight loss drug in the USA and Europe, was not yet approved for the treatment of obesity at the time the study was conducted, patients of our cohort received a maximum dose of 0.5 mg once-weekly.
There is limited data about the use of GLP-1 RA in post-bariatric WR or IWL, which were found to be more effective for treating post-bariatric weight regain than non-GLP-1 RA–based pharmacotherapies regardless of surgery type [21]. According to a retrospective review comparing amphetamine-derived phentermine with phentermine-topiramate, phentermine and phentermine-topiramate in addition to diet and exercise in post-RYGB and LAGB patients induced weight loss of 6.35 kg (12.8% excess weight loss) and 3.81 kg (12.9% EWL) within 90 days, respectively [14]. Zoss et al. reported weight loss of 8.0 ± 2.0 kg in patients with gastric banding treated with the lipase inhibitor orlistat 120 mg three times a day (TID) for 8 months [15]. A 12-month treatment with the GLP-1 RA exenatide twice a day (BID) resulted in more significant weight loss (− 14 kg) and diabetes resolution in a patient with gastric banding [22]. Also, a recent study in 117 patients without T2D suggests that post-bariatric surgery patients can lose a significant amount of weight (− 6.3 ± 7.7 kg, p < 0.05) within 4 months while taking liraglutide 3.0 mg regardless of the type of surgery they had [19]. Data from Pajecki et al. of 15 patients with weight regain after different surgical procedures point toward the same direction. Notably, all patients treated with liraglutide (max. dose 1.8 mg/day) reported improvement in satiety [23]. However, according to our data, the effectiveness of semaglutide seems to be superior to liraglutide 3.0 mg in post-bariatric WR or IWL. Differentiating between patients with WR and IWL, no significant differences in weight loss response were found. With respect to sex, female patients presented a more pronounced weight loss, which was statistically significant for the 3-month follow-up visit. Women seem to perform better in terms of EWL% following BS [24]. In contrast, a recent matched-pair cohort analysis of 707 men and women demonstrated that BS results in comparable short- and mid-term efficacy in men and women, and is associated with similar rate and severity of postoperative complications between sexes [25]. With respect to pharmacotherapy, especially GLP-1-based weight loss pharmacotherapy, the data are more consistent supporting the hypothesis of a gender‐dimorphic response with more pronounced weight reduction in females [26]. There is evidence that central GLP‐1 effects could be modulated by sex steroids; e.g., female rats are more sensitive than males to the anorexigenic effect of a centrally administered GLP‐1 receptor agonist. The anorexigenic effect of estrogen and modulation of GLP‐1 activity could involve the ventral tegmental area (VTA) and the nucleus accumbens (NAc) [26, 27]. Further studies are urgently needed to identify gender-related differences in efficacy and toxicity of GLP-1 RA in the pre- and post-bariatric population for a tailored approach to obesity management.
WR appears to differ by surgical procedure and tends to be higher in patients with SG [28]. Some reports have suggested that RYGB increases postprandial GLP-1 to a greater extent than SG [29]. Even though one could argue that enhancing the GLP-1 response could therefore be beneficial in patients who had SG, we did not observe any differences in the effectiveness of semaglutide once-weekly between SG and RYGB. This is in line with data from Wharton et al. who did not detect any differences in the effectiveness of liraglutide 3 mg 1 year after surgery [19]. In contrast, the observed response to adjuvant weight loss medication with phentermine, phentermine-topiramate extended release, lorcaserin, or naltrexone/bupropion was significantly better in gastric bypass and gastric banding patients compared with SG according to an observational study in 209 patients 1 year following BS. Furthermore, adjuvant pharmacotherapy was more effective in patients with higher BMI [30]. In our cohort, BMI categories prior to initiation of semaglutide and change in BMI were not significantly different between the surgical groups. Hence, we can only speculate that differences in initial BMI and change in BMI following pharmacotherapy would have driven greater weight loss in those patients with SG. According to available data in non-surgical patients, early treatment response (1–3 months) to the weight loss drug seems to be a good predictor of long-term weight maintenance [31–34]. Metabolic predictors of weight loss could not have been identified in patients treated with exenatide twice daily subcutaneously [35]. Predictors of response to pharmacotherapy in our cohort might be triglycerides, ALT, and AST at baseline, which were negatively associated with weight loss of at least 5% at 3 months’ follow-up. Even though there usually is remarkable variability in individual capacity for weight loss in response to pharmacological agents [36], these results might suggest that patients with poor metabolic long-term outcomes following BS are less likely to benefit from add-on pharmacotherapy.
Our study has a few limitations. Here we report a retrospective analysis of a small number of patients lacking a control group. At 6 months’ follow-up, only 20 patients have been included into the analysis. Differences in dose titration and time point of treatment initiation may have impacted the variability in weight loss.
To enhance outcomes for BS patients, close postoperative follow-up is required to address IWL or WR preferably early. Our study highlights a new and safe concept to pharmacologically treat IWL or WR along with lifestyle intervention following BS. Prospective randomized controlled trials are needed to evaluate larger cohorts of patients to determine if semaglutide once-weekly may close the gap between lifestyle intervention and revision surgery to treat weight recidivism or insufficient weight loss after BS.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Ethics Approval
For this type of study, formal consent is not required.
Consent to Participate
Informed consent does not apply.
Conflict of Interest
Anne Lautenbach, Tobias B. Huber, Sebastian M. Meyhöfer, Jens Aberle: The authors have received honoraria for speaking at symposia, financial support for attending symposia, financial support for educational programs, and consultation from Novo Nordisk.
Marie Wernecke, Fabian Stoll, Jonas Wagner, Svenja Meyhöfer: The authors declare that they have no conflict of interest.
Footnotes
Key Points
• There is an urgent need to develop strategies to treat IWL or WR following BS.
• Treatment with semaglutide in post-bariatric patients is of clear benefit.
• Our study highlights a new and safe concept to pharmacologically treat IWL or WR.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Svenja Meyhöfer and Jens Aberle have contributed equally to this work.
References
- 1.Kalarchian M, Turk M, Elliott J, et al. Lifestyle management for enhancing outcomes after bariatric surgery. Curr Diabetes Reports 2014 1410 [Internet]. Springer; 2014 [cited 2021 Sep 4];14:1–9. Available from: https://link.springer.com/article/10.1007/s11892-014-0540-y [DOI] [PubMed]
- 2.El Ansari W, Elhag W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps-a scoping review. Obes Surg [Internet]. Obes Surg; 2021 [cited 2021 Sep 4];31:1755–66. Available from: https://pubmed.ncbi.nlm.nih.gov/33555451/ [DOI] [PMC free article] [PubMed]
- 3.Nedelcu M, Khwaja HA, Rogula TG. Weight regain after bariatric surgery-how should it be defined? Surg Obes Relat Dis [Internet]. Surg Obes Relat Dis; 2016 [cited 2021 Sep 5];12:1129–30. Available from: https://pubmed.ncbi.nlm.nih.gov/27350180/ [DOI] [PubMed]
- 4.Aminian A, Vidal J, Salminen P, et al. Late relapse of diabetes after bariatric surgery: not rare, but not a failure. Diabetes Care [Internet]. Diabetes Care; 2020 [cited 2022 Jan 1];43:534–40. Available from: https://pubmed.ncbi.nlm.nih.gov/31974105/ [DOI] [PubMed]
- 5.Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med [Internet]. 2004;351:2683–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15616203 [DOI] [PubMed]
- 6.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. http://dx.doi.org/ [Internet]. Massachusetts Medical Society ; 2009 [cited 2021 Sep 5];357:741–52. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa066254 [DOI] [PubMed]
- 7.Altieri MS, Yang J, Nie L, et al. Rate of revisions or conversion after bariatric surgery over 10 years in the state of New York. Surg Obes Relat Dis [Internet]. Elsevier; 2018 [cited 2021 Sep 5];14:500–7. Available from: http://www.soard.org/article/S155072891731105X/fulltext [DOI] [PubMed]
- 8.Zhang L, Tan WH, Chang R, et al. Perioperative risk and complications of revisional bariatric surgery compared to primary Roux-en-Y gastric bypass. Surg Endosc [Internet]. Surg Endosc; 2015 [cited 2021 Sep 5];29:1316–20. Available from: https://pubmed.ncbi.nlm.nih.gov/25294534/ [DOI] [PubMed]
- 9.Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an endocrine society clinical practice guideline. J Clin Endocrinol Metab Endocrine Society. 2010;95:4823–4843. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 10.Padwal RS, Rucker D, Li SK, et al. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev [Internet]. Wiley; 2003 [cited 2021 May 17];2003. Available from: https://pubmed.ncbi.nlm.nih.gov/15266516/
- 11.Rucker D, Padwal R, Li SK, et al. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ [Internet]. BMJ; 2007 [cited 2021 Sep 4];335:1194–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18006966/ [DOI] [PMC free article] [PubMed]
- 12.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med [Internet]. N Engl J Med; 2005 [cited 2021 Sep 4];353:2111–20. Available from: https://pubmed.ncbi.nlm.nih.gov/16291981/ [DOI] [PubMed]
- 13.Berkovitz RI, Wadden TA, Tershakovec AM, et al. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA [Internet]. JAMA; 2003 [cited 2021 Sep 4];289:1805–12. Available from: https://pubmed.ncbi.nlm.nih.gov/12684359/ [DOI] [PubMed]
- 14.Schwartz J, Chaudhry UI, Suzo A, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight recidivism or weight loss plateau post-bariatric surgery: a retrospective review. Obes Surg [Internet]. Obes Surg; 2016 [cited 2021 Sep 5];26:452–8. Available from: https://pubmed.ncbi.nlm.nih.gov/26615406/ [DOI] [PubMed]
- 15.Zoss I, Piec G, Horber FF. Impact of orlistat therapy on weight reduction in morbidly obese patients after implantation of the Swedish adjustable gastric band. Obes Surg [Internet]. Obes Surg; 2002 [cited 2021 Sep 4];12:113–7. Available from: https://pubmed.ncbi.nlm.nih.gov/11868286/ [DOI] [PubMed]
- 16.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med [Internet]. Massachusetts Medical Society; 2021 [cited 2021 May 17];384:989–1002. Available from: https://pubmed.ncbi.nlm.nih.gov/33567185/ [DOI] [PubMed]
- 17.Zusammenarbeit I. Chirurgische Arbeitsgemeinschaft für Adipositastherapie (CA-ADIP) Deutsche Adipositas-Gesellschaft (DAG) Deutsche Gesellschaft für Psychosomatische Medizin und Psychotherapie Deutsche Gesellschaft für Ernährungsmedizin S3-Leitlinie: Chirurgie der Adipositas [Internet]. 2010. Available from: www.adipositas-gesellschaft.de
- 18.Brethauer SA, Kim J, El Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis [Internet]. Surg Obes Relat Dis; 2015 [cited 2021 Dec 12];11:489–506. Available from: https://pubmed.ncbi.nlm.nih.gov/26093765/ [DOI] [PubMed]
- 19.Wharton S, Kuk JL, Luszczynski M, et al, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post‐bariatric surgery. Clin Obes [Internet]. Wiley-Blackwell; 2019 [cited 2021 Sep 4];9. Available from: /pmc/articles/PMC6771702/ [DOI] [PMC free article] [PubMed]
- 20.Interdisziplinäre S3-Leitlinie “Prävention und Therapie der Adipositas”: Version 2.0 (April 2014) [Internet]. 2014. Available from: http://www.adipositas-gesellschaft.de
- 21.Gazda CL, Clark JD, Lingvay I, et al. Pharmacotherapies for post-bariatric weight regain: real-world comparative outcomes. Obesity (Silver Spring) [Internet]. Obesity (Silver Spring); 2021 [cited 2022 May 19];29:829–36. Available from: https://pubmed.ncbi.nlm.nih.gov/33818009/ [DOI] [PubMed]
- 22.Rothkopf MM, Bilof ML, Haverstick LP, et al. Synergistic weight loss and diabetes resolution with exenatide administration after laparoscopic gastric banding. Surg Obes Relat Dis [Internet]. Surg Obes Relat Dis; 2009 [cited 2021 Sep 4];5:128–31. Available from: https://pubmed.ncbi.nlm.nih.gov/18996762/ [DOI] [PubMed]
- 23.Pajecki D, Halpern A, Cercato C, et al. Short-term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir [Internet]. Rev Col Bras Cir; 2013 [cited 2021 Sep 5];40:191–5. Available from: https://pubmed.ncbi.nlm.nih.gov/23912365/ [DOI] [PubMed]
- 24.Risi R, Rossini G, Tozzi R, et al. Sex difference in the safety and efficacy of bariatric procedures: a systematic review and meta-analysis. Surg Obes Relat Dis [Internet]. Surg Obes Relat Dis; 2022 [cited 2022 Jun 15]; Available from: https://pubmed.ncbi.nlm.nih.gov/35668018/ [DOI] [PubMed]
- 25.Mousapour P, Tasdighi E, Khalaj A, et al. Sex disparity in laparoscopic bariatric surgery outcomes: a matched-pair cohort analysis. Sci Rep [Internet]. Sci Rep; 2021 [cited 2022 Jun 15];11. Available from: https://pubmed.ncbi.nlm.nih.gov/34140595/ [DOI] [PMC free article] [PubMed]
- 26.Cataldi M, Muscogiuri G, Savastano S, et al. Gender-related issues in the pharmacology of new anti-obesity drugs. Obes Rev [Internet]. John Wiley & Sons, Ltd; 2019 [cited 2022 Jun 15];20:375–84. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/obr.12805 [DOI] [PubMed]
- 27.Rentzeperi E, Pegiou S, Koufakis T, et al Sex differences in response to treatment with glucagon-like peptide 1 receptor agonists: opportunities for a tailored approach to diabetes and obesity care. J Pers Med [Internet]. Multidisciplinary Digital Publishing Institute (MDPI); 2022 [cited 2022 Jun 15];12:454. Available from: /pmc/articles/PMC8950819/ [DOI] [PMC free article] [PubMed]
- 28.King WC, Hinerman AS, Courcoulas AP. Weight regain after bariatric surgery: a systematic literature review and comparison across studies using a large reference sample. Surg Obes Relat Dis [Internet]. Surg Obes Relat Dis; 2020 [cited 2021 Dec 12];16:1133–44. Available from: https://pubmed.ncbi.nlm.nih.gov/32446593/ [DOI] [PubMed]
- 29.Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology [Internet]. Endocrinology; 2017 [cited 2021 Dec 28];158:4139–51. Available from: https://pubmed.ncbi.nlm.nih.gov/29040429/ [DOI] [PMC free article] [PubMed]
- 30.Nor Hanipah Z, Nasr EC, Bucak E, et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Relat Dis [Internet]. Surg Obes Relat Dis; 2018 [cited 2022 Jan 12];14:93–8. Available from: https://pubmed.ncbi.nlm.nih.gov/29287757/ [DOI] [PubMed]
- 31.Maccora C, Ciuoli C, Goracci A, et al. One month weight loss predicts the efficacy of liraglutide in obese patients: data from a single center. Endocr Pract [Internet]. Endocr Pract; 2020 [cited 2021 Dec 28];26:235–40. Available from: https://pubmed.ncbi.nlm.nih.gov/31682516/ [DOI] [PubMed]
- 32.Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring) [Internet]. Obesity (Silver Spring); 2016 [cited 2021 Dec 27];24:2278–88. Available from: https://pubmed.ncbi.nlm.nih.gov/27804269/ [DOI] [PMC free article] [PubMed]
- 33.Finer N, Ryan DH, Renz CL, et al. Prediction of response to sibutramine therapy in obese non-diabetic and diabetic patients. Diabetes Obes Metab [Internet]. Diabetes Obes Metab; 2006 [cited 2021 Dec 28];8:206–13. Available from: https://pubmed.ncbi.nlm.nih.gov/16448525/ [DOI] [PubMed]
- 34.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dushay J, Eleftheria M;, Flier M, et al. Patterns and predictors of weight loss with exenatide treatment in overweight and obese women. medRxiv [Internet]. Cold Spring Harbor Laboratory Press; 2020 [cited 2021 Dec 28];2020.06.11.20128645. Available from: https://www.medrxiv.org/content/10.1101/2020.06.11.20128645v1
- 36.Dent R, McPherson R, Harper ME. Factors affecting weight loss variability in obesity. Metabolism. W.B. Saunders; 2020;113:154388. [DOI] [PubMed]


