Abstract
Frontal alpha asymmetry (FAA) is widely examined in EEG research, yet a procedural consensus on its assessment is lacking. In this study, we tested a latent factorial approach to measure FAA. We assessed resting-state FAA at broad, low, and high alpha bands (8–13; 8–10.5; 11–13 Hz) using mastoids as reference electrodes and Current Source Density (CSD) transformation (N=139 non-clinical participants). From mastoid-referenced data, we extracted a frontal alpha asymmetry factor (FAAf) and a parietal factor (PAAf) subjecting all asymmetry indices to a varimax-rotated, principal component analysis. We explored split-half reliability and discriminant validity of the mastoid factors and the mastoid and CSD raw asymmetry indices (F3/4, F7/8, P3/4, P7/8). Both factor and raw scores reached an excellent split-half reliability (>.99), but only the FAAf reached the maximum discriminant validity from parietal scores. Next, we explored the correlations of latent factor and raw FAA scores with symptoms of depression, anxiety, and personality traits to determine which associations were driven by FAA after variance from parietal activity was removed. After correcting for false discovery rate, only FAAf at the low alpha band was negatively associated with depression symptoms (a latent CES-D factor) and significantly diverged from PAAf’s association with depression symptoms. With respect to personality traits, only CSD-transformed F7/8 was positively correlated with Conscientiousness and significantly diverged from the correlations between Conscientiousness and P3/4 and P7/8. Overall, the latent factor approach shows promise for isolating functionally distinct resting-state EEG signatures, although further research is needed to examine construct validity.
Keywords: EEG frontal alpha asymmetry factor, alpha frequency bands, reference montages, internalizing symptoms, personality traits
1. Introduction
The alpha rhythm, recorded in the 8–13 Hz band of the ongoing electroencephalogram (EEG; Berger, 1929), is a neural oscillation commonly thought to measure low cortical activity (Goldman, Stern, Engel & Cohen, 2002; Laufs et al., 2003a, 2003b). Frontal alpha asymmetry (FAA) is an index of the difference in alpha power between left and right frontal EEG electrodes, such that increased FAA indicates relatively greater left frontal cortical activity and is thought to reflect greater approach motivation (Coan & Allen, 2004; Davidson, 1992, 1994, 1998; Harmon-Jones & Allen, 1997; Harmon-Jones, Gable & Peterson, 2010; Smith, Reznik, Stewart & Allen, 2017); whereas reduced FAA, which indicates relatively greater right frontal cortical activity, is thought to reflect greater avoidance motivation (Davidson, 1992; Sutton & Davidson, 1997). FAA has been considered to be a trait-like feature (Allen, Coan & Nazarian, 2004) and a potential correlate of internalizing disorders and personality traits via approach and avoidance motivation. Specifically, reduced approach motivation (reduced left frontal activity) has been associated with depression (Harmon-Jones & Gable, 2018; Nusslock et al., 2011; Smith, Cavanagh & Allen, 2018), and an enhanced avoidance motivation (enhanced right frontal activity) has been associated with anxiety symptoms (Mathersul, Williams, Hopkinson & Kemp, 2008).
1.1. FAA and internalizing symptoms
FAA has been widely studied in association with internalizing symptoms, particularly depression (Coan & Allen, 2004). However, findings of two recent meta-analyses (Thibodeau, Jorgensen & Kim, 2006; Van Der Vinne, Vollebregt, Van Putten & Arns, 2017) and reviews on FAA in depression (Bruder, Steward & McGrath, 2017; Kaiser, Gnjezda, Knasmüller & Aichhorn, 2018) are mixed. Some have argued that reduced FAA is a biomarker for depression (Greco et al., 2021) and a potential vulnerability factor to first onset of depression (Nusslock et al., 2011; Pössel, Fritz & Seemann, 2008; Stewart & Allen, 2018). Still others have suggested it is manifested by remitted depressed individuals (Allen & Reznik, 2015; Stewart, Bismark, Towers, Coan, & Allen, 2010) and may predict the recurrence of depression (Allen, McKnight, Moreno, Demaree, & Delgado, 2009) and therapeutic response to antidepressant treatment (Arns et al., 2016; Bruder et al., 2008; Tenke et al., 2011, 2017). In addition, recent research provided the first evidence of FAA intergenerational transmission among mother-infant dyads, such that FAA between mothers and their infants is highly associated and a reduced FAA in infants is associated with maternal depressive symptoms (Hill et al., 2020). However, other researchers have not found reduced FAA in individuals with lifetime depression (Debener et al., 2000; Reid, Duke, & Allen, 1998) and some researchers have evidenced its low heritability (Anokhin, Heath & Myers, 2006). Findings have also been mixed in non-clinical samples. Some studies have linked reduced FAA to higher depression symptoms (De Raedt, Franck, Fannes & Verstraeten, 2008; Diego, Field & Hernandez-Reif, 2001), whereas others have found non-significant effects (Gold, Fachner & Erkkilä, 2013; Mathersul et al., 2008). These inconsistent findings have led researchers in the field to argue that more research is needed to establish more conclusive evidence of FAA being associated with depression (Jesulola, Sharpley, Bitsika, Agnew & Wilson, 2015; Olbrich & Arns, 2013).
The association between FAA and anxiety has been more consistent, with more studies finding reduced FAA among individuals with anxiety (Kaiser et al., 2018; Thibodeau et al., 2006). Research on non-clinical samples has also found associations between reduced FAA and anxiety symptoms (Gold et al 2013; Mathersul et al., 2008; Nitschke, Heller, Palmieri & Miller, 1999; Adolph & Margraf, 2017). Despite more research supporting the relation between FAA and anxiety (compared to depression), some studies have not replicated this effect (Gordon, Palmer & Cooper, 2010; Kentgen et al., 2000).
1.2. FAA and personality
In addition to associations with internalizing symptoms, FAA has also been linked to personality traits. FAA is considered to be a stable, trait-like feature (Allen et al., 2004; Jacobs & Snyder, 1996). Indeed, one study found that 60% of the variance in FAA was attributable to stable traits, such as negative affectivity and approach-avoidance tendencies (Hagemann, Naumann, Thayer & Bartussek, 2002). Moreover, researchers have observed a link between Neuroticism and greater FAA variability across time (Minnix & Kline, 2004). However, studies that have examined Neuroticism and Extraversion in association with FAA have reported inconclusive results (Kuper, Käckenmester & Wacker, 2019) and others have failed to find an association between FAA and Neuroticism-Extraversion (Hagemann et al., 1999; Schmidtke & Heller 2004), or Neuroticism alone (Pauli, Wiedemann & Nickola, 1999). Additionally, a meta-analysis summarizing these results found a null effect size between Extraversion and FAA (Wacker, Chavanon, & Stemmler, 2010). The association between FAA and other Big Five personality traits (i.e., Openness, Agreeableness and Conscientiousness) have been less explored in research. In one study by Kuper and colleagues (2019), no correlation was found between FAA and Conscientiousness. In a separate study, enhanced FAA was related to the anticipation of uncertain stimuli in high-Openness individuals (Käckenmester, Kroencke & Wacker, 2018). Given the inconsistent findings described above, it is clear that further research is needed to better understand how FAA might correspond to other stable traits.
1.3. Moderating variables in FAA
The inconsistency of findings in this area has led researchers to examine the role of potential moderating variables such as EEG recording period, operationalization of depression, age, handedness, gender, medication and the clinical homogeneity across samples (e.g., severity of disease and comorbidity; Kaiser et al., 2018; Nusslock et al., 2018; Thibodeau et al., 2006). In the present paper we examine three methodological moderators that should be taken into account in measuring FAA: the electrode site used to detect FAA, the alpha band recorded, and the reference/transformation method in analyzing FAA.
Electrode site.
This methodological moderator has received relatively little attention in FAA literature. The majority of research on FAA, internalizing psychopathology, and personality has used mid-frontal asymmetry (F3/4) or lateral frontal asymmetry (F7/8) as the most common FAA indices. In two meta-analyses on depression research, the mid-frontal site was the most commonly used FAA index, which has shown the strongest effect (Jakobi, 2009; Thibodeau et al., 2006). However, this result could be attributed to the fact that a large number of studies described in the meta-analyses exclusively reported effects at F3/4. Conversely, some studies have found an association between depression and reduced FAA in both F3/4 and F7/8 indices (Barnhofer et al., 2007; Chan, Han, Sze, Wong & Cheung, 2013; Henriques & Davidson 1991; Keune, Bostanov, Hautzinger & Kotchoubey, 2013) or only at F7/8 (Jacobs & Snyder, 1996). Findings have also varied by site in anxiety research (Kaiser et al., 2018; Thibodeau et al., 2006). Various studies have found anxiety to be related to reduced FAA at F3/4 (Mathersul et al., 2008; Nitschke et al., 1999; Petruzzello & Landers, 1994; Wiedemann et al., 1999) and F7/8 (Tomarken & Davidson, 1994) or F7/8 alone (Gold et al., 2013).
Regarding research on FAA and personality traits, a recent meta-analysis observed that F3/4 is the most commonly used index to assess FAA. In fact, the F3/4 have been observed in 61 studies, whereas F7/8 was used in 25 (Kuper et al., 2019). Moderator analyses indicated that there was no difference between the F3/4 and F7/8 in terms of their effect sizes on personality traits (Kuper et al., 2019). Thus, it is still unclear which scalp site should be used to assess FAA.
Alpha bands.
Evidence suggests that different alpha bandwidths are linked to different cognitive processes and are functionally independent (Petsche et al., 1997). They also respond differently to a variety of tasks such as anticipation of emotive stimuli (Onoda et al., 2007) and cognitive challenge in adults with ADHD at rest (Hale et al., 2009). In particular, high alpha is associated with memory retrieval whereas low alpha is associated with attentive processes (Klimesch, Sauseng & Hanslmayr, 2007) and positively correlates with cognitive abilities (Gianotti et al., 2007). Moreover, individual variations, as gender and age, might affect alpha total (Segrave et al., 2011) and FAA intergenerational transmission among mother-infant dyads is stronger for high alpha versus low alpha (Hill et al., 2020). In light of these findings, some researchers have chosen to investigate not only total, but also high and low alpha band in order to provide a more sensitive measure of alpha asymmetry (Jaworska, Blier, Fusee & Knott, 2012; Segrave et al., 2011).
In depression and anxiety studies, the majority of researchers have focused on the broad alpha band (8–13 Hz). The studies that have explored different alpha bands have reported mixed results. Some studies observed a reduced FAA on depression only in the high alpha band (Jaworska et al., 2012), others evidenced a reduced FAA in the low alpha band in depressed patients with psychomotor retardation (Cantisani et al., 2015) or during presentation of happy and sad faces stimuli (Koller-Schlaud, Ströhle, Bärwolf, Behr & Rentzsch, 2020). Conversely, other authors reported significant findings at broad alpha band (Cantisani et al., 2016) and non-significant findings at all three alpha bands (Kaiser, Doppelmayr & Iglseder, 2018; Segrave et al., 2011). Regarding anxiety studies, low and broad alpha bands have manifested similar results (Nusslock et al., 2018). None of the personality traits studies have examined low or high alpha.
Reference/Transformation.
The third FAA moderator that we examine in this work is reference/transformation. Smith and colleagues (2017) discussed the effects of reference/transformation on EEG data and highlighted the importance of seeking a relatively inactive reference in order to record the EEG activity without interference from other scalp sources (Hagemann, Naumann & Thayer, 2001). From the comparison across different reference montages, the Cz appeared to be the least adequate solution (Smith et al., 2017). The suggested solution to overcome this issue was to employ a spatial filter: the reference-free Current Source Density (CSD) transformation (Tenke & Kayser, 2012; Kayser & Tenke, 2006). The CSD transformation computes the second spatial derivative of voltage between nearby electrode sites. This method permits to increase the contribution of local electrode activity lowering the influence from distal sources. Evidence has supported the CSD transformation as an effective method to record the frontal activity while reducing the effect of non-frontal sources (Stewart et al., 2010; Stewart, Coan, Towers & Allen 2014; Velo, Stewart, Hasler, Towers & Allen, 2012). The authors presented the CSD as the elective method to study FAA but noted that a 64-channel cap should be employed to guarantee its effectiveness (Smith et al., 2017).
A recent review on FAA in depression (Kaiser et al., 2018) encouraged researchers to consider the role of reference montage in their work. Studies using mastoid reference when measuring FAA reported a reduced FAA in depression (Kemp et al., 2010; Keune et al., 2013; Liu, Sarapas & Shankman, 2013; Reid et al., 2008) and anxiety (Adolph & Margraf, 2017; Gold et al., 2013), while others did not find an effect, either for depression alone (Gold et al., 2013; Jaworska et al., 2012; Quinn, Rennie, Harris & Kemp, 2014), or for anxiety and depression (Gordon et al., 2010; Kaiser et al., 2016). When comparing different reference montages, some authors (Stewart et al. 2010; 2014) found that depression was related to reduced FAA only when FAA was CSD-transformed, and not when other transformations (Cz, average or linked mastoid) were used. Others studies reporting only CSD-transformed FAA data showed similar findings in a female sub-sample (Stewart & Allen, 2018); however, this effect has not always been found (Brzezicka, Kamiński, Kamińska, Wołyńczyk-Gmaj & Sedek, 2017).
The only study examining anxiety with CSD transformation demonstrated that elevated worry is related to enhanced FAA, and that elevated anxiety and reduced worry is related to reduced FAA (Smith, Zambrano-Vazquez & Allen, 2016). To our knowledge, no studies have examined CSD-transformed FAA with personality traits.
1.4. Aims of the study
In light of the findings described above, reduced FAA might represent an individual difference that corresponds not only to internalizing diagnoses (MDD and GAD), but also to subthreshold symptomatology and personality traits. In fact, Davidson (1998) argued that frontal alpha asymmetry indicates a diathesis that predisposes individuals toward different affective styles and may constitute a risk factor for developing clinical psychopathologies, such as depression and anxiety. Therefore, effectively identifying FAA variations could be an useful marker for prevention screening or to assess treatment response. However, it appears there is no procedural consensus for how to quantify FAA. This is problematic because it increases the risk of choosing whichever electrode sites, alpha band, or reference/transformation methods yield the strongest effect, which contributes to questionable research practices such as hypothesizing after results are known (i.e., “HARKing”). In turn, the publication of these findings, obtained through differing methods, makes it more difficult for researchers to replicate effects (Olbrich & Arns, 2013). We hypothesize that the electrode site activation is influenced by the reference montage and the alpha band examined.
With the aim of removing shared variance with parietal electrode sites using a factor analytic approach, we propose a novel method for operationalizing FAA. We believe that the alpha signal is most accurately captured across the frontal areas rather than isolated to a single, specific site. Thus, we propose an empirically-derived frontal factor that could represent a frontal alpha asymmetry score. This can be achieved through principal component analysis (PCA), a statistical technique employed to reduce the dimensionality of a dataset, minimizing information loss and at the same time increasing interpretability (Ringnér, 2008). Several studies have employed a data-driven approach to identify an EEG factor using EEG alpha power measures, especially for CSD-transformed data through a principal component analysis, taking into account spatial and frequencies characteristics (e.g., Barry & De Blasio, 2018; Panier et al., 2020; Tenke & Kayser, 2005). Our purpose is to apply a similar method to frontal alpha asymmetry data.
We also aimed to compare the reliability of the novel FAA factor with the traditional alpha asymmetry scores by examining split-half reliability and discriminant validity from parietal counterparts. Several studies have explored EEG alpha power reliability in terms of convergent and discriminant validity (Smith et al., 2020) and test-retest reliability (Smit, Posthuma, Boomsma & De Geus, 2005; Tenke et al., 2017; Tomarken, Davidson, Wheeler, & Kinney, 1992); however, fewer have explored alpha asymmetry reliability (Hagemann et al., 2001; Hill et al., 2020; Towers & Allen, 2009).
Therefore, in light of the arguments described above, this study has been developed to achieve five aims. Aim 1 is to apply a principal component analysis to identify a frontal alpha asymmetry factor (FAAf) including all 32 electrodes and alpha frequencies (8, 9, 10, 11, 12, 13 Hz) for mastoid-referenced and CSD-transformed data. We hypothesized that a FAA factor would emerge from the analysis, capturing unique variance that is empirically separable from signal attributable to nearby parietal regions (i.e., parietal alpha asymmetry [PAAf] factor). We compared this new approach to the measures that have been traditionally used to operationalize FAA (mastoid-referenced and CSD-transformed F3/4 and F78 at broad, low, and high alpha). We hypothesized that using principal components analysis to derive FAAf would empirically create an index that better isolates frontal activity, allowing for generalization of findings and encouragement of replication efforts.
Aim 2 is to analyze split-half reliability using the Spearman-Brown prophecy formula for factor and raw FAA scores across mastoid/CSD and frequency alpha bands and to evaluate the discriminant validity of frontal scores from parietal scores. We hypothesized that both factor and raw scores would demonstrate good reliability but that the FAAf would demonstrate better discriminant validity from parietal scores compared to raw FAA scores.
Aim 3 is to assess the associations among factors and raw FAA scores with internalizing symptoms. Building upon Aim 1, the current aim fills a present gap in the literature by comparing commonly used depression and anxiety measures (depression: CES-D, IDAS-II and DASS-21; anxiety: GAD-7 and DASS-21) in their relation to FAAf and raw data in a non-clinical sample. In addition to the latent factor created for frontal asymmetry, we conducted a factor analysis on item-level response scores to create Depression and Anxiety symptoms factors. We hypothesized that heightened depression and anxiety symptoms would be related to reduced FAA.
Moreover, through an exploratory perspective, we aimed to compare both latent factors and raw indicators at different alpha bandwidths (low alpha 8–10.5 Hz; high alpha 11–13 Hz; broad alpha 8–13 Hz), to clarify if they differed in terms of their relations to relevant criteria or if they can be used interchangeably.
Aim 4 is to explore relations between factor and raw FAA indices and FFM personality trait factors. This is largely an exploratory aim, as there is a relative lack of research assessing FAA in the context of personality traits. Given its theoretical overlap with depression and anxiety symptoms, we hypothesized that high Neuroticism would relate to reduced FAA.
Finally, Aim 5 of the current study is to assess similarities and divergences across the correlational profiles of each FAA signal with internalizing symptoms and personality measures in order to examine which signals share similar patterns of association with these theoretically relevant criteria. To achieve this aim, we compared the profile similarity of the raw F3/4 and F7/8 with P3/4 and P7/8, then the similarity of FAA with PAA factors to assess whether they diverged in their associations with the measures of interest. This is guided by an exploratory interest in understanding how much the association with constructs is found in raw scores, which share a variance with parietal scores, or a frontal factor in which its shared variance with parietal factor is removed.
In conclusion, our purpose is to provide a valid and reliable FAA measure that can effectively detect variation in internalizing symptoms and personality and could serve as an indicator that might identify individuals vulnerable to developing internalizing psychopathologies.
2. Method
2.1. Participants
The current study included a total of 139 undergraduate non-clinical participants, 54.7% women (n= 76) and 45.3% men (n = 63), ranging in age from 18 to 24 years old (M =19.2, SD =1.23). They were not selected on any screening measures. Continental nationality of the sample was North American (84.3%, n = 117), South American (10.8%, n = 15) and other (4.9%, n = 7). Participants were 10.8% Hispanic/Latino (n = 15) and 84.3% not Hispanic/Latino (n = 117). Across the full sample, 5 participants declined to respond regarding their age (3.6%) and 7 participants regarding their ethnicity (4.9%). Written informed consent was obtained from all participants and they were compensated with either course credit or monetary payment. This research was formally approved by the local Institutional Review Board. The number of participants varied across analyses. EEG recording and factor analysis was performed on 139 subjects. Correlations between FAA and relevant external criteria ranged from 104 to 114 subjects (N = 104 for Depression and Anxiety symptoms factors; N = 109 for FFMRF Big Five measures; N = 114 for IDAS-II and DASS measures; N = 104 for CES-D and GAD-7).
2.2. Self-report measures
Center for Epidemiologic Studies Depression Scale (CES-D).
The CES-D (Radloff, 1977) is a 20-item measure with scores ranging from 0 to 60. Responders are asked to rate the frequency with which they have experienced 20 symptoms over the past week (e.g., feelings of helplessness and hopelessness, depressed mood, loss of energy) using a 4-point Likert scale. A score of 16 or above suggests a high level of depressive symptoms. The CES-D has demonstrated good reliability and validity across different demographic groups (Radloff, 1977). In the current sample, the CES-D showed excellent reliability (Cronbach’s alpha = .91).
Generalized Anxiety Disorder 7-item scale (GAD-7).
The GAD-7 (Spitzer, Kroenke, Williams, & Lowe, 2006) is a short self-report questionnaire based on DSM-IV-TR diagnostic criteria that evaluates general anxiety disorder symptoms over the past two weeks with 7 items in a 4-point Likert scale. Scores on the GAD-7 range from 0 to 21. Cut points of 5, 10, and 15 might be interpreted as representing mild, moderate, and severe levels of anxiety on the GAD-7. The GAD-7 has been shown to be valid and reliable in past research (Spitzer et al., 2006). In the current sample, the GAD-7 demonstrated good reliability (Cronbach’s alpha = .89).
Inventory of Depression and Anxiety Symptoms (IDAS-II).
The IDAS-II (Watson et al., 2012) is a 99-item measure assessing current depression and other psychiatric symptoms over the past two weeks using a 5-point Likert scale. Cut points of 53, 74, 85 might be interpreted as representing mild, moderate and severe depression (Stasik-O’Brien et al., 2019). The IDAS-II is a reliable self-report measure to detect depressive symptoms given its good internal consistency, convergent and discriminant validity, and test-retest reliability (Watson et al., 2012). In the current sample, the IDAS-II general depression scale demonstrated good reliability (Cronbach’s alpha = .89).
Depression Anxiety Stress Scales (DASS-21).
The DASS-21 (Lovibond & Lovibond, 1995) is the short version of the DASS. It is composed of 21 items on a 4-point Likert scale that assess the severity and frequency of depression, anxiety and stress symptoms in the past week (with seven items assessing each). The DASS-21 has shown good internal consistency and reliable discrimination between the three scales (Lovibond & Lovibond, 1995). Cut points of 5–6, 7–10, 11–13 and over 14 might be interpreted as representing mild, moderate, severe and extremely severe levels of depression. Cut points of 4–5, 6–7, 8–9 and over 10 might be interpreted as representing mild, moderate, severe and extremely severe levels of anxiety. In the current sample, the depression and anxiety subscales exhibited good to excellent reliability (Cronbach’s alpha DASS-21 depression = .91; Cronbach’s alpha DASS-21 anxiety = .80).
Five-Factor Model Rating Form (FFMRF).
The FFMRF (Mullins-Sweatt, Jamerson, Samuel, Olson & Widiger, 2006) is a 30-item self-report scale assessing the Big Five personality domains: Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness. Participants are asked to rate themselves on each item using a five-point Likert scale ranging from extremely low (1) to extremely high (5). In the current sample, reliability ranged from adequate to good (Neuroticism = .78; Extraversion = .79; Openness = .66; Agreeableness = .71; Conscientiousness = .79).
2.3. Procedure
Upon arrival, participants were seated in a comfortable chair in an isolated room and the EEG electrodes were applied. Resting state was recorded while participants were instructed to look at a white fixation cross on a black computer screen and relax, refraining from moving and closing their eyes. We recorded only the eyes-open condition because researchers have demonstrated that alpha power does not differ between eyes open or closed (e.g., Henriques & Davidson, 1990; Henriques & Davidson, 1991; Reid et al., 1998). Presentation software (Neurobehavioral Systems, Inc., Berkeley, CA) was used to control the timing of the resting state period, which lasted for two minutes. Some research has suggested that the measurement of resting frontal alpha asymmetry may require less recording time than the standard eight minutes (Towers & Allen, 2009). In fact, to approach an internal consistency reliability of .90, researchers recommend an acceptable epochs set size N = 75–125 for mastoid-referenced data. Given that our averaged epochs were approximately 600 for each EEG channel, we have exceeded this minimum criterion (see Table 1 in Supplemental Materials; Towers & Allen, 2009). During the same lab session, participants were asked to complete a battery of questionnaires through an online procedure. The order of EEG and questionnaire completion were counterbalanced across participants. This study is a part of a more extended study on psychopathological symptoms and EEG patterns (Ait Oumeziane, Jones & Foti, 2019; Hill, Lane & Foti, 2019). The resting EEG was always collected first, followed by two reward tasks and one emotional viewing tasks. The recording session lasted 1.5 hours including the capping process.
2.4. EEG recording
EEG activity was recorded using an actiCAP and the actiCHamp amplifier system (Brain Products GmbH, Munich, Germany), which uses active electrodes that amplify the signal at the scalp before transmission through the cable, thereby protecting against interference. The EEG signal was digitized at 24-bit resolution at a sampling rate of 500 Hz. Recordings were taken from 32 scalp electrodes based on the International 10–20 electrode system (Fp1, Fz, F3, F7, FT9, FC5, FC1, C3, T7, TP9, CP5, CP1, Pz, P3, P7, O1, Oz, O2, P4, P8, TP10, CP6, CP2, Cz, C4, T8, FT10, FC6, FC2, F4, F8, Fp2) and a ground electrode at Fpz. Horizontal and vertical eye movement were captured with two auxiliary electrodes placed 1 cm above and below the left eye, forming a bipolar channel to record the electrooculogram. Electrode impedances were kept below 30 kOhms. Brain Vision Analyzer (Brain Products, Munich, Germany) was used for offline analysis.
In our procedure, we employed the mastoid (TP9/TP10) and the CSD transformation as a reference montage. After the CSD transformation, which is the recommended reference scheme (Hagemann et al., 2001; Tenke & Kayser 2012; Velo et al., 2012), the mastoid reference scheme is preferred as compared to Cz because it has been found to be superior in terms of psychometric proprieties (Allen et al., 2004; Reid et al., 1998; Tomarken et al., 1992). Even though the CSD transformation is recommended for 64 channel-caps (Smith et al., 2017) and we used a 32 channel-cap, we applied the CSD transformation because evidence in the literature has found that selecting less flexible splines (m=4) for a low-density EEG montage can largely retain the topography of an high-density montage (Kayser & Tenke, 2015). Thus, we applied the CSD transformation with the following characteristics: Oder of splines: 4; Maximal Degree of Legendre Polynomials: 10; Default Lambda 1e-5.
2.5. Data reduction and analysis
Electroencephalogram FAA.
The following procedure to calculate FAA was performed according to published recommendations (Smith et al., 2017). To obtain mastoid-referenced data we re-referenced data to the mastoid average (TP9/10) and band-pass filtered from 0.01–100 Hz using Butterworth zero phase filters. Using a regression method, we corrected the signal offline for electrooculogram (EOG) artifacts (Gratton, Coles, & Donchin, 1983). We segmented data in 1 s epochs (50% overlap) and individual channels were rejected trial-wise using a semi-automated procedure, with artifacts automatically defined as a step of 50 μV, a 200 μV change within 200-ms intervals, or a change <0.5 μV within 100-ms intervals and manually identified visually. We applied the Fast Fourier Transform (Cooley & Tukey, 1965), with a Hamming window of 100% length and 50% overlap. Alpha power at each site was log-transformed to approximate a normal distribution (Williams et al., 2005). To obtain CSD-transformed data we re-referenced data after the artifact rejection step; thus, we applied CSD transformation, the Fast Fourier Transform, and averaged epochs. This is an accepted procedure since CSD is reference-free and the result will be equivalent regardless of the reference montage applied before. Epochs were averaged (see Table 1 Supplemental Materials for descriptive statistics of number of averaged epochs), and power density values in the broad alpha (8–13 Hz), the low alpha (8–10.5 Hz) and the high alpha (11–13 Hz) frequency ranges were extracted for each channel separately. For principal component analysis, we further extracted separate frequency bands at 8 Hz (7.5–8.5 Hz), 9 (8.5–9.5 Hz), 10 (9.5–10.5 Hz), 11 (10.5–11.5 Hz), 12 (11.5–12.5 Hz), 13 (12.5–13.5 Hz). In addition, for each previously described broad, low, high, and separate frequency bands, we extracted distinct alpha bands for odd and even segments to calculate split-half reliability analysis. These scores were then used to calculate asymmetry values such that the left hemisphere value (e.g., F3) was subtracted from the right homologous electrode (e.g., F4) (i.e., (ln(F4)-ln(F3)) for either reference montage. Cortical alpha power is inversely associated with the cortical activity (Oakes et al., 2004; Scheeringa et al., 2008); thus, lower FAA (reduced left frontal cortical activity) is indicated by negative values whereas higher FAA (increased left frontal cortical activity) is indicated by positive values; consequently, the symmetrical activity across hemispheres is indicated by a zero score (Coan & Allen, 2004).
Factor analysis of FAA, internalizing symptoms and personality traits.
For single electrode analysis, we focused on mid-frontal (F3/4) and lateral frontal (F7/8) asymmetry indices, in line with the existing literature (e.g., Allen & Cohen, 2010; Thibodeau et al., 2006). In order to create factors representing FAAf and PAAf (with the goal of removing any shared variance due to parietal activity from the frontal factor), following Tenke and Kayser’s analysis (2005), we performed a principal component analysis, Varimax rotation with Kaiser normalization, based on correlation matrix, using all asymmetry indices (O1/2 P3/4 P7/8 TP9/10 CP5/6 CP1/2 C3/4 Fp1/2 F3/4 FT9/10 F7/8 FC5/6 FC1/2 T7/8) and all frequency alpha band (8, 9, 10, 11, 12, 13 Hz), obtaining a total of 84 scores for mastoid reference data (i.e., O21 8Hz; O21 9Hz etc.). We repeated this procedure for CSD reference data. We used a correlation-based PCA because alpha asymmetry scores are not on the same scale (see Table 2 in Supplemental materials for description) and researchers recommend to use standardized data (correlation) in this case (Jolliffe & Cadima, 2016).
With respect to internalizing symptoms and personality traits we performed an exploratory factor analysis with oblimin rotation (Delta parameter = 0) and maximum likelihood estimation. We conducted three factor analyses: we included all depression items to extract one depression symptoms factor (CES-D, IDAS-II and DASS-21); all anxiety items to extract an anxiety symptoms factor (GAD-7 and DASS-21) and the Five-Factor Model Rating Form to extract the five personality traits. In addition, we calculated the latent factor of each questionnaire, in order to further explore if they differ in the association with FAAf and raw scores.
Split-half reliability and discriminant validity.
Following Hill and colleagues’ procedure (2020) we estimated split-half reliability using the Spearman–Brown prophecy formula (Anastasi & Urbina, 1997) for all data split by even and odd segments. In particular, we calculated reliability for F3/4 and F7/8 for both mastoid and CSD-transformed data and FAAf. For the latter, we calculated a frontal factor for all odd segments and a frontal factor for all even segments, which we then compared using the Spearman-Brown formula. Reliability estimates were categorized as good or excellent over .80 and .90, respectively (Cicchetti, 1994). In addition, we explored discriminant validity between frontal and parietal scores through a correlation matrix. We were interested in comparing the absence of correlation between FAAf and PAAf (as determined by Varimax rotation) with mastoid and CSD raw scores to evidence how much the frontal traditional scores discriminate from parietal influence.
Associations between FAA and relevant external criteria.
Next, we examined zero-order correlations between depression symptoms, anxiety symptoms, and personality traits factors with the FAAf, PAAf, and raw frontal and parietal scores (F3/4, F7/8, P3/4, P7/8) at broad, low, and high alpha band for mastoid and CSD reference. Correlational profile similarities were analyzed with respect to the FAAf and PAAf factors and the raw indices. Profile similarities (which are calculated as double-entry intraclass correlations) refer to the degree of consistency that two empirical constructs show with one another based on their scores across an array of independent measures (Furr, 2010). The goal in using this method was to determine whether the FAAf factor and the raw FAA indicators showed a similar pattern of association with theoretically relevant criteria, and to compare frontal asymmetry correlation profiles with parietal asymmetry correlation profiles to evaluate whether the latent factor approach led to more discriminant patterns in correlations compared to its raw indicator counterpart. Profile similarities range from zero to one, with values closer to one indicating a high degree of similarity.
Because there is no current recommended cut-off value in terms of statistically significant profile similarities, we supplemented these analyses with Steiger’s (1980) test of dependent rs to determine whether there were statistically significant differences between the factors and raw FAA indices in terms of their correlations with internalizing symptoms and personality traits. Given the large number of comparisons being examined in the present study and the potential for being under-powered given our sample size, false discovery rate analyses using a Bonferroni-type approach described by Benjamini and Hochberg (1995) with a q-value of .05 were conducted for the correlations and tests of dependent rs to decrease the likelihood of reporting statistical significance that may have been the result of multiple comparisons.
3. Results
Latent alpha asymmetry factors.
First, we examined the descriptive statistics of all channel*frequencies in order to exclude channel(s) with missing data. We retained all channels with no missing data, excluding from the PCA TP9/10 and FC1/2 (see Table 2 in Supplemental Materials for descriptive statistics). We confirmed the assumptions required for PCA (Watkins, 2021): we observed an adequate linearity, sampling adequacy with Kayser-Meyer-Olkin and Bartlett’s test and we found no significant outliers with Mahalanobis distance. Next, we conducted a PCA, varimax-rotated correlation matrix with unrestricted factor extraction. This resulted in a 13-factor extraction, with a high dispersion of scores across different factors, which were composed by different channels across all frequencies (see Table 3 in Supplemental Materials). Therefore, we decided to restrict factor extraction at two components in the PCA analysis. We hypothesized to identify a frontal and a parietal factor.
Factor loadings for mastoid-referenced data are presented in Table 4 in Supplemental Materials. The PCA resulted in the hypothesized factors, with FAA indices (F7/8, F3/4, FC5/6 and FC1/2) loading onto frontal alpha asymmetry factor (which we named the FAA factor – FAAf; average factor loadings .646) and the PAA indices (O2/1, P3/4; P7/8, CP5/6, CP1/2, T7/8) loading onto the parietal alpha asymmetry factor (which we named the PAA factor – PAAf; average factor loading .667). C3/4 similarly loaded on the frontal and parietal factors, whereas FT 9/10 did not load on any factor. No difference emerged between different frequency alpha bands. Although they were not statistically different, previous research has suggested that frequency alpha bands are functionally independent (Petsche, Kaplan, Von Stein & Filz, 1997) and that FAA’s relations to depression symptoms may differ by alpha band (Cantisani et al., 2015; Jaworska et al., 2012). Therefore, we calculated factors at low alpha band (8–10 Hz) and high alpha band (11–13 Hz) to further explore if differences would emerge in their relations to external criteria (see Table 4 in Supplemental Materials).
Split-half reliability and discriminant validity.
Results from the Spearman-Brown prophecy formula are presented in Table 1. All factors showed a similar pattern of distribution and reached an adequate measure of Kayser-Meyer-Olkin and Bartlett’s test1. All scores reached an excellent reliability (>.90). Zero-order correlations between frontal and parietal factors and raw data are presented in Tables 2 and 3. As frontal and parietal factors have been extracted with varimax rotation, their correlation is zero. For CSD-transformed raw scores, F3/4 and F7/8 demonstrated similar discriminant validity from parietal scores. One exception was in the low alpha band, in which F4/3 demonstrated a significantly smaller correlation with P3/4 compared with P7/8. For CSD-transformed scores the average correlation between F3/4 and parietal scores was r = .140; the average correlation between F7/8 and parietal scores was r = .214. Across mastoid data, F7/8 demonstrated more discriminant validity from P3/4 and P7/8 compared to F3/4. Specifically, for mastoid-referenced scores, the average correlation between F3/4 and parietal scores was r = .383; the average correlation between F7/8 and parietal scores was r = .187. One exception was for P3/4 in low alpha band, which demonstrated a smaller correlation with F7/8 compared to F3/4, but not statistically significantly so.
Table 1.
Spearman brown coefficient for factor and raw frontal alpha asymmetry scores
| Broad data | Low alpha | High alpha | |
|---|---|---|---|
|
| |||
| FAAf | .990 | .982 | .989 |
| F3/4 | .994 | .991 | .981 |
| F7/8 | .998 | .996 | .994 |
| F3/4 csd | .998 | .996 | .994 |
| F7/8 csd | .999 | .997 | .996 |
Table 2.
Zero-order correlations of factor and raw mastoid-referenced scores
| FAAf | FAAf low | FAAf high | PAAf | PAAf low | PAAf high | F3/4 low | F7/8 low | F3/4 | F7/8 | F/34 high | F7/8 high | P3/4 low | P7/8 low | P3/4 | P7/8 | P3/4 high | P7/8 high | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| FAAf | 1 | |||||||||||||||||
| FAAf low | .955** | 1 | ||||||||||||||||
| FAAf high | .954** | .834** | 1 | |||||||||||||||
| PAAf | 0 | .030 | .021 | 1 | ||||||||||||||
| PAAf low | .002 | 0 | .056 | .949** | 1 | |||||||||||||
| PAAf high | .026 | .078 | 0 | .942** | .795** | 1 | ||||||||||||
| F3/4 low | .661** | .746** | .557** | .269** | .202* | .305** | 1 | |||||||||||
| F7/8 low | .755** | .698** | .684** | −.045 | −.024 | .001 | .415** | 1 | ||||||||||
| F3/4 | .718** | .752** | .656** | .296** | .230** | .333** | .967** | .467** | 1 | |||||||||
| F7/8 | .780** | .695** | .736** | −.055 | −.024 | −.019 | .417** | .983** | .487** | 1 | ||||||||
| F3/4 high | .740** | .669** | .771** | .265** | .229** | .281** | .795** | .478** | .911** | .532** | 1 | |||||||
| F7/8 high | .771** | .637** | .782** | −.076 | −.026 | −.061 | .369** | .888** | .461** | .951** | .577** | 1 | ||||||
| P3/4 low | .223** | .215* | .239** | .772** | .831** | .650** | .316** | .237** | .335** | .226** | .317** | .191* | 1 | |||||
| P7/8 low | .121 | .134 | .141 | .863** | .853** | .780** | .327** | .134 | .346** | .129 | .307** | .101 | .751** | 1 | ||||
| P3/4 | .258** | .254** | .254** | .806** | .766** | .787** | .370** | .253** | .411** | .246** | .407** | .215* | .927** | .734** | 1 | |||
| P7/8 | .154 | .171* | .164 | .869** | .812** | .836** | .363** | .146 | .394** | .147 | .361** | .127 | .712** | .977** | .749** | 1 | ||
| P3/4 high | .275** | .277** | .247** | .744** | .604** | .837** | .398** | .244** | .457** | .244** | .456** | .221** | .715** | .622** | .914** | .689** | 1 | |
| P7/8 high | .181* | .206* | .172* | .836** | .723** | .864** | .392** | .140 | .432** | .149 | .399** | .142 | .613** | .883** | .708** | .957** | .730** | 1 |
Note. PAAf = parietal alpha asymmetry factor; FAAf = frontal alpha asymmetry factor; low= low alpha band (8–10 Hz); high=high alpha band (11–13 Hz)
indicates statistical significance at p < .05
indicates statistical significance at p < .001.
Bolded coefficients represent corresponding frontal and parietal electrode sites and latent factors.
Table 3.
Zero-order correlations of raw CSD-transformed scores
| F3/4csd low | F7/8csd low | F3/4csd | F7/8csd | F3/4csd high | F7/8csd high | P3/4csd low | P7/8csd low | P3/4csd | P7/8csd | P3/4csd high | P7/8csd high | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| F3/4csd low | 1 | |||||||||||
| F7/8csd low | .314** | 1 | ||||||||||
| F3/4csd | .967** | .290** | 1 | |||||||||
| F7/8csd | .286** | .982** | .280** | 1 | ||||||||
| F3/4csd high | .942** | .309** | .833** | .262** | 1 | |||||||
| F7/8csd high | .323** | .960** | .282** | .894** | .347** | 1 | ||||||
| P3/4csd low | .028 | .207* | .051 | .179* | .082 | .121 | 1 | |||||
| P7/8csd low | .143 | .252** | .147 | .242** | .140 | .207* | .399** | 1 | ||||
| P3/4csd | .047 | .190* | .095 | .174* | .148 | .133 | .936** | .375** | 1 | |||
| P7/8csd | .143 | .249** | .156 | .247** | .160 | .226** | .401** | .969** | .410** | 1 | ||
| P3/4csd high | .079 | .155 | .142 | .157 | .211* | .148 | .918** | .333** | .741** | .298** | 1 | |
| P7/8csd high | .169* | .240** | .192* | .252** | .209* | .259** | .366** | .933** | .297** | .829** | .369** | 1 |
Note. low= low alpha band (8–10 Hz); high=high alpha band (11–13 Hz)
indicates statistical significance at p < .05
indicates statistical significance at p < .001.
Bolded coefficients represent corresponding frontal and parietal electrode sites and latent factors.
Latent depression symptoms, anxiety symptoms and Big Five personality trait factors.
Similarly to the procedures for alpha asymmetry factors, we first tested the assumptions that should be met before performing a factor analysis. No outliers tested with Mahalanobis distance were found for internalizing symptoms and personality traits, the assumption of linearity was confirmed, and the questionnaire data were normally distributed (Kline, 2005), with the exception of IDAS-II 13 and 52 which exceeded Kline’s range and were excluded from the factor analysis (see Tables 5, 6, 7 in Supplemental Materials for descriptive statistics). Factor loadings for depression, anxiety and Big Five personality trait items are presented in Tables 8, 9, 10 in Supplemental Materials. For depression and anxiety symptoms measures, we also calculated specific factors for each questionnaire.
FAA correlations and profile similarities with internalizing symptoms and personality traits.
In the present sample, 26.8% of participants met suggested thresholds for clinically significant depression and 30.9% for clinically significant anxiety, respectively 2.9% and 9.5% of participants indicated experiencing “severe” levels of depression and anxiety symptoms (see Table 11 in Supplemental Materials for descriptive statistics of internalizing symptoms).
Correlations between the factors and raw asymmetry scores in association with theoretically relevant constructs are presented in Table 4. Given the relatively small sample size, false discovery rate corrections (Benjamini & Hochberg, 1995) were applied for all correlations with criterion measures within each alpha band to obtain more conservative results. After examining correlations with depression symptoms factor, anxiety symptoms factor and five personality traits factors, we further explored correlations with single depression or anxiety symptoms questionnaires.
Table 4.
Zero-order correlations between raw, single electrode asymmetry scores and depression, anxiety, and basic personality measures
| Depression | Anxiety | Neuroticism | Conscientiousness | Agreableness | Extraversion | Openness | CES-D | DASS dep | IDAS-II dep | GAD7 | DASS anx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAAf | −.172 | −.131 | .110 | .186 | −.027 | −.076 | .086 | −.205 ** | −.123 | −.089 | −.150 | −.097 |
| FAAf low | −.196 * | −.144 | .155 | .190 * | −.051 | −.049 | .095 | −.233 ** DC1 | −.163 | −.098 | −.157 | −.099 |
| FAAf high | −.129 | −.116 | .040 | .153 | −.010 | −.097 | .065 | −.160 | −.065 | −.058 | −.135 | −.096 |
| F3/4 | −.149 | −.137 | .077 | .023 | −.086 | −.139 | −.090 | −.156 | −.105 | −.101 | −.139 | −.105 |
| F7/8 | −.164 | −.120 | .187 | .208 ** | −.139 | −.074 | .014 | −.186 | −.181 | −.162 | −.155 | −.077 |
| F3/4 low | −.155 | −.134 | .126 | .028 | −.089 | −.100 | −.068 | −.150 | −.068 | −.091 | −.145 | −.105 |
| F7/8 low | −.190 | −.130 | −.221 ** | .226 ** | −.154 | −.071 | .038 | −.164 | −.139 | −.142 | −.145 | −.074 |
| F3/4 high | −.105 | −.107 | −.026 | .020 | −.040 | −.186 | −.096 | −.109 | .001 | −.059 | −.117 | −.088 |
| F7/8 high | −.106 | −.088 | .100 | .151 | −.093 | −.064 | −.011 | −.112 | −.062 | −.100 | −.116 | −.055 |
| F3/4 csd | .002 | −.012 | .034 | .040 | −.035 | −.022 | −.012 | .016 | .061 | .031 | .007 | −.037 |
| F7/8 csd | −.029 | −.083 | .113 | .216 ** | −.094 | −.025 | .012 | −.009 | −.055 | −.008 | −.108 | −.037 |
| F3/4 csd low | .016 | .003 | .049 | .030 | −.040 | .000 | .022 | .007 | .057 | .003 | −.012 | −.055 |
| F7/8 csd low | −.016 | −.082 | .125 | .230 ** DC2 | −.111 | −.028 | .015 | −.024 | −.045 | −.027 | −.109 | −.041 |
| F3/4 csd low | −.004 | −.011 | −.008 | .043 | .001 | −.080 | −.066 | .010 | .047 | −.019 | −.008 | −.070 |
| F7/8 csd low | −.040 | −.073 | .080 | .178 | −.041 | −.028 | .021 | −.038 | −.030 | −.046 | −.097 | −.041 |
Note. PAAf = parietal alpha asymmetry factor; FAAf = frontal alpha asymmetry factor; low= low alpha band (8–10 Hz); high=high alpha band (11–13 Hz)
indicates statistical significance at p < .05
indicates statistical significance at p < .05 after correcting for false discovery rate
indicates that correlations differ significantly between FAAf and PAAf at low alpha band
indicates that correlations differ significantly between F7/8 CSD and both P3/4 CSD and P7/8 CSD at low alpha band.
None of the parietal scores were correlated with constructs of interest. See Supplemental Materials for additional tables (Tables 12–17) of zero-order correlations and profile similarities between factors, raw mastoid scores, and raw CSD scores with depression symptoms, anxiety symptoms, and personality traits.
Three constructs of interests emerged in correlation with FAA measures: depression symptoms, Neuroticism and Conscientiousness. The general latent depression symptoms factor was negatively associated with FAAf at low alpha band. Depression symptoms measured with the latent CES-D factor were negatively associated with FAAf at broad and low alpha band. Both associations were statistically significant after correcting for false discovery rate. Additionally, FAAf low alpha band had a significantly different correlation with the CES-D than its parietal counterpart. Neuroticism was negatively correlated with F7/8 at low alpha band, but this association was not significantly different from Neuroticism’s correlations with P3/4 and P7/8. Conscientiousness was positively correlated with F7/8 mastoid and CSD at broad and low alpha bands, as well as with FAAf at low alpha band; however, only F7/8 CSD-transformed FAA at low alpha band demonstrated a significantly different correlation with Conscientiousness from parietal (P3/4, P7/8) scores. In sum, considering only correlations surviving FDR correction and significantly diverging from parietal counterparts, only FAAf at low alpha band negatively correlated with the CES-D Depression symptoms factor and significantly diverged from PAAf in its relation to CES-D Depression. With respect to personality traits, only CSD-transformed F7/8 correlated with Conscientiousness and significantly diverged from P3/4 and P7/8 in terms of its relation to Conscientiousness.
4. Discussion
Guided by the purpose of identifying a reliable FAA index that included FAA measured at multiple electrode sites while also eliminating variance from parietal activity, we examined a novel way to measure frontal alpha asymmetry through a principal component analysis to derive a FAA factor index that removes shared variance with parietal regions. The FAAf, demonstrating less overlap with parietal noise, could serve as an useful indicator of FAA, given that it is extracted with varimax rotation, which does not include any shared variance from the parietal neural signal.
Split-half reliability and discriminant validity.
Mastoid-referenced alpha asymmetry raw scores demonstrated excellent split-half reliability, confirming previous findings (Hill et al., 2020; Towers & Allen, 2009). Additionally, our findings demonstrated excellent split-half reliability of the FAAf and the CSD-transformed F7/8 and F3/4 scores, indicating that the FAAf demonstrated comparable reliability to FAA measures currently utilized in the field.
Further, we analyzed the discriminant validity of FAA factor and raw alpha asymmetry frontal scores with respect to parietal counterparts. Given that the FAAf was empirically derived to isolate frontal asymmetry signal from parietal activity, this measure provides significant discriminant validity in comparison to traditional measures. Discriminant validity was further supported given the statistically significantly different effect sizes between frontal and parietal factors with respect to CES-D depression. Our findings indicated that the second-best approach to discriminate from parietal scores are the CSD-transformed raw electrode scores, confirming the CSD transformation as an effective method for increasing the contribution of local electrode activity while reducing influence from distal sources (Hagemann et al., 2001; Tenke & Kayser 2012; Velo et al., 2012). Mastoid raw scores, on the other hand, demonstrated more overlap with the frontal and parietal counterparts as evidenced by their correlations with each other and lack of differences in their correlational profiles with relevant criteria. This comparison indicates that the mastoid-referenced frontal factor (FAAf) and CSD-transformed raw electrodes scores are less likely to be influenced by distal generators.
FAA correlations and profile similarities with internalizing symptoms and personality traits.
As third and fourth aims, we explored the association between depression and anxiety symptoms and the five factor personality traits across FAA factor and raw scores. Three constructs emerged in correlation with FAA scores: Depression with the FAAf, Conscientiousness across FAA scores, and Neuroticism with the F7/8 raw score at low alpha band.
In line with literature on non-clinical populations (De Raedt et al., 2008; Diego et al., 2001), we found a reduced FAA among participants with higher levels of depression symptoms (CES-D). This is also consistent with meta-analytic findings demonstrating an effect between FAA and both clinical and non-clinical depression (Jakobi, 2009), as well as longitudinal findings that FAA predicted depression symptoms among healthy subjects (Nusslock et al., 2011; Pössel et al, 2008; Stewart & Allen, 2018). However, we found this effect only with the FAAf, whereas the cited authors observed this correlation using FAA raw indices. Given the higher correlation between CES-D with FAAf compared with raw FAA scores, it appears that the effect was mostly driven by variance that is unique to frontal activity. Additionally, the relation between the depression factor and FAA appears to mostly have been driven by CES-D depression.
Although the CES-D is used less frequently than other measures for assessing clinical depression (for example, in comparison with the BDI-II), previous work has found that both CES-D and BDI-II evidence satisfactory levels of specificity and sensitivity in detecting depressive symptoms in a college-age sample (Shean & Baldwin, 2008). In addition, the CES-D was developed specifically for use in general populations (rather than clinical populations; Radloff, 1977) which may be why it was particularly sensitive to depressive symptoms in our non-clinical sample compared to other measures. Moreover, comparing to IDASS-II and DASS21, it may be possible that the CES-D demonstrated the strongest effect in our sample due to its response format, which might be more effective than other measures in detecting depressive symptoms. In fact, it requests participants to respond using a more concrete Likert scale compared to the more subjective rating scale of the other measures (i.e., asking a participant how often they felt/behaved a certain way over the past week on a 1–7 day scale versus “Not at all” to “Extremely” on the IDASS-II or “Did not apply to me at all” to “Applied to me very much” on the DASS21). Our findings suggest that FAA could be considered a marker of non-clinical depression symptoms. Notably, this effect was found only for the FAAf.
With respect to personality traits, we observed a reduced FAA in high trait Neuroticism participants, in accordance with previous findings (Kuper et al., 2019). However, this result was only found at F7/8 raw score low alpha band. Findings on Neuroticism seems less robust and confirming this, a recent systematic review did not support the validity of resting asymmetry as a marker for approach-avoidance personality traits and parallel measure such as Neuroticism (Vecchio & De Pascalis, 2020). More interesting are results on Conscientiousness, we reported an enhanced FAA in high Conscientiousness participants across different FAA indices. To our knowledge, this is the first study to report this association for CSD-transformed data. Notably, our findings contradict prior research findings suggesting no correlation between raw FAA and Conscientiousness (Kuper et al., 2019). This is particularly interesting given that Conscientiousness was the most consistent significant effect found in relation to FAA across both transformations of F7/8 and the FAAf. The contrast with previous findings could potentially be explained by the different operationalizations and measures of Conscientiousness used between the two studies (Kuper et al., 2019) and warrant further research to replicate these findings.
The positive correlation between FAA and Conscientiousness could be of interest because literature is mainly focused on exploring reduced FAA, its related constructs, and how it could be considered a risk factor for psychopathology (Nusslock et al., 2011; Pössel et al., 2008; Stewart & Allen, 2018). Conversely, these findings suggest that more effort should be made to explore increased FAA and its related constructs to explore whether it could constitute a potential protective factor. The existing literature has provided some evidence to support this hypothesis. For example, it is well-known that an increased FAA correlates with approach motivation (Coan & Allen, 2004; Davidson, 1992, 1994, 1998; Harmon-Jones & Allen, 1997; Harmon-Jones et al., 2010; Smith et al., 2017). This is consistent with the findings from the current study given previous findings that Conscientiousness is positively correlated with approach motivation (Briki, 2018) and negatively correlates with avoidance motivation (Mitchell et al., 2007). In addition, Conscientiousness has been found to be associated with good psychological health during the lifespan (Bogg & Roberts, 2013; Friedman & Kern, 2014). Thus, we encourage researchers to explore if Conscientiousness, and an increased FAA, might play the role of a protective factor against psychopathological symptoms. In general, further research on the latent FAAf index is needed to determine if these effects replicate in other samples and if this could be a more consistently applied index of FAA in the literature.
As a final aim, we examined if correlations between the FAA, internalizing symptoms, and basic personality traits are specifically found in frontal areas, or if effects emerge with frontal scores that absorb activity from multiple distributed neural systems. Profile similarities were calculated to determine whether the FAAf bore less similar correlations to internalizing symptoms and personality traits when compared to its parietal counterpart than raw FAA indices. We found that the associations between FAAf low alpha with the CES-D Depression factor and CSD-transformed F7/8 low alpha with Conscientiousness showed a significantly different effect size from their parietal counterparts. Other associations did not significantly differ from parietal correlations.
Across effect sizes in the present study, correlations at the low alpha band were stronger with depression symptoms, anxiety symptoms, and personality traits. Although the principal components analysis did not identify differences between alpha bands, these differential effects support previous recommendations for resting EEG research that alpha band should be included in the FAA research protocol (Kaiser et al., 2018). We recommend that researchers analyze and report effect sizes at low and high alpha, in addition to broad alpha, to provide context and transparency to their findings and to prevent “cherry-picking” alpha bands to choose the strongest effect size. This is particularly salient given research that has found significant differences in effects at different alpha bands of FAA indices (Cantisani et al., 2015; Jaworska et al., 2012).
Finally, it is noteworthy to mention that none of the effect sizes obtained in this study (even those attaining statistical significance) were large; however, this is common in cross-method correlational research. In addition, these effect sizes are consistent with previous meta-analytic effect size estimates between FAA and trait Neuroticism (Kuper et al., 2019), depression, and anxiety (Thibodeau et al., 2006).
Limitations and Future Directions
Although the sample size of the present study is relatively large compared with other EEG studies, it is still not highly powered enough to adequately detect small effects. Thus, more research with larger samples is needed to further explore these questions. It is important to note, however, that this sample size nonetheless allowed us to examine a novel assessment of frontal alpha asymmetry. Additionally, for all correlations between our criterion measures and asymmetry measures (both raw scores and factors), false discovery rate correction was used to minimize the potential for reporting significant findings that were the result of multiple comparisons.
As the field stands now, there is no recommended protocol for how to operationalize FAA with regard to electrode sites and frequency range. For this reason, studies have reported effects associated with a large range of different sites and alpha bands. We would argue that a better step would be to create an empirically-derived FAA factor using the methods described in this paper, to parse out any shared variance that may be coming from other brain regions and to use signal from multiple electrode sites rather than relying on a single electrode. As the construct validity analyses indicate, the FAA factor is highly similar to the raw scores that researchers currently use in terms of its relations to relevant criteria, but it also has the additional advantage to isolate frontal activity from signal in the parietal region. If using raw indicators, our findings indicate that researchers should use CSD transformation to obtain the most spatially distinct score.
In order to further examine FAAf, we also invite researchers to calculate and compare factors across different reference montages (i.e., as Cz, linked mastoids, linked ears, nose, average ears etc.). Although we hypothesize that FAAf would be equally sensitive in detecting FAA variation regardless of reference methodology because the calculation of a factor would clean the reference effect, this is an empirical question. We encourage researchers to attempt to replicate these findings across different datasets to continue to explore the utility of the FAAf in frontal asymmetry research.
In conclusion, the FAA factor (FAAf) is a promising method to measure alpha asymmetry given findings supporting its reliability, discriminant validity from a PCA-derived parietal factor, and construct validity in terms of its relations with internalizing symptoms and relevant personality traits. The FAAf provides researchers with the ability to examine correlations with constructs of interest in a way that maximizes the contribution of frontal, rather than parietal, activity. It may also be a valuable and sensitive index to detect potential health and risk factors.
Supplementary Material
Figure 1.
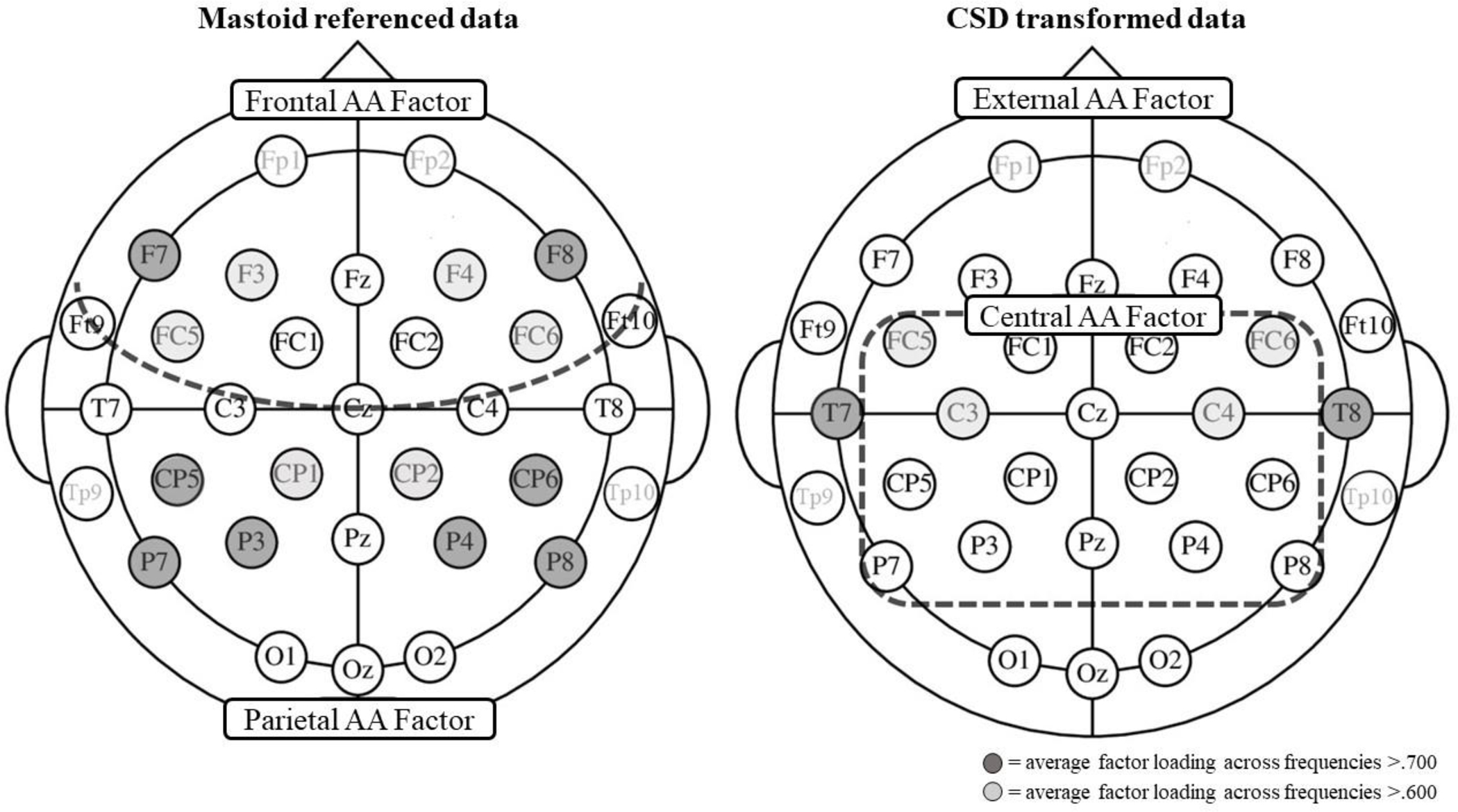
Topography of alpha asymmetry factor scores depicted separately for mastoids-referenced and CSD-transformed data
Note. FAAf = frontal alpha asymmetry factor; PAAf = parietal alpha asymmetry factor; CAAf = central alpha asymmetry factor; EAAf = external alpha asymmetry factor. Dashed line indicates separation of factors; channels colored with dark grey showed an average factor loading across frequencies >.700 (e.g., alpha asymmetry score of F7/8 at 8, 9, 10, 11, 12, 13 Hz on FAA); channels colored with light grey showed an average factor loading across frequencies >.600; channels Fp1/2 and Tp9/10 were excluded from the PCA.
Figure 2.
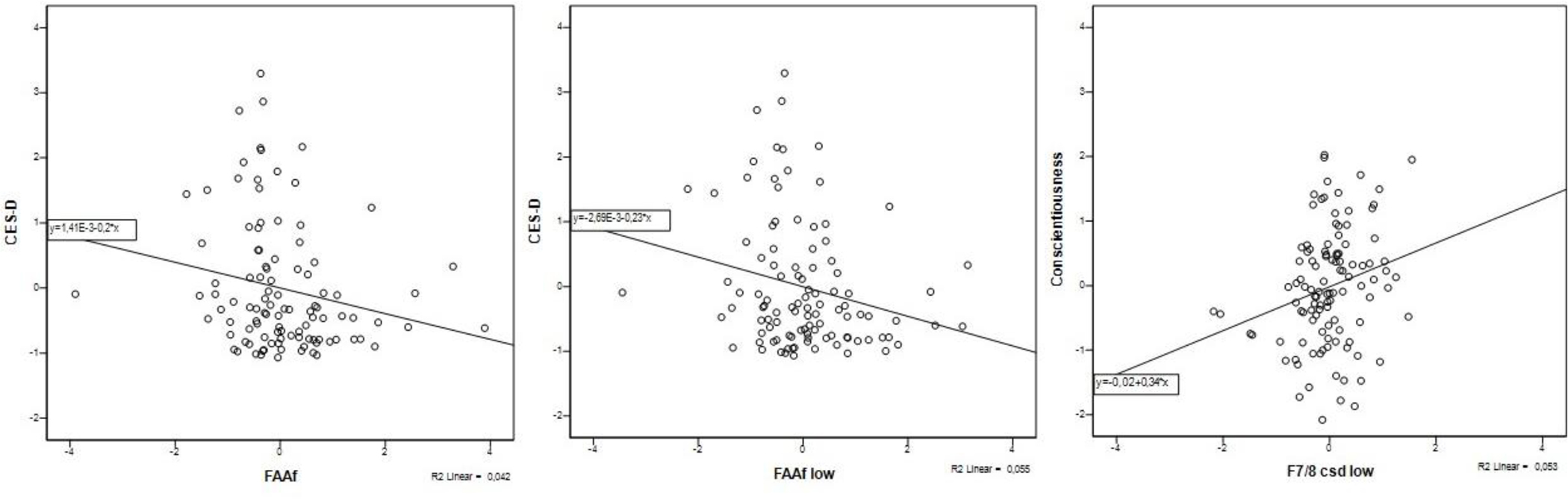
Association between FAAf and FAAf low with CES-D and F7/8 csd low with Conscientiousness
Note. FAAf = frontal alpha asymmetry factor 8–13 Hz; FAAf low= frontal alpha asymmetry factor 8–10 Hz; F7/8 csd low =frontal alpha asymmetry score at F7/8 csd 8–10 Hz; CES-D = depression score.
Acknowledgments.
Kaylin Hill was supported in part from a NIMH training grant (T32-MH18921) during completion of this work.
Footnotes
Broad factor: Odd - KMO 0.764. Bartlett’s test 20455.62** (2556); Even - KMO 0.762; Bartlett’s test 20414.98**(2556). Low factor: Odd - KMO 0.787. Bartlett’s test 10824.21** (903); Even: KMO 0.787; Bartlett’s test 10866.10** (903). High factor: Odd - KMO 0.735. Bartlett’s test 8576.37** (630); Even: KMO 0.748; Bartlett’s test 8679.98** (630).
References
- Adolph D, & Margraf J (2017). The differential relationship between trait anxiety, depression, and resting frontal α-asymmetry. Journal of Neural Transmission, 124(3), 379–386. 10.1007/s00702-016-1664-9. [DOI] [PubMed] [Google Scholar]
- Ait Oumeziane BA, Jones O, & Foti D (2019). Neural sensitivity to social and monetary reward in depression: Clarifying general and domain-specific deficits. Frontiers in behavioral neuroscience, 13. 10.3389/fnbeh.2019.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, & Cohen MX (2010). Deconstructing the “resting” state: exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Frontiers in human neuroscience, 4, 232. 10.3389/fnhum.2010.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, & Reznik SJ (2015). Frontal EEG asymmetry as a promising marker of depression vulnerability: Summary and methodological considerations. Current opinion in psychology, 4, 93–97. 10.1016/j.copsyc.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological psychology, 67(1–2), 183–218. 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Allen JJ, McKnight KM, Moreno FA, Demaree HA, & Delgado PL (2009). Alteration of frontal EEG asymmetry during tryptophan depletion predicts future depression. Journal of affective disorders, 115(1–2), 189–195. 10.1016/j.jad.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasi A, Urbina S (1997): Psychological Testing, 7th ed. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- Anokhin AP, Heath AC, & Myers E (2006). Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological psychology, 71(3), 289–295. 10.1016/j.biopsycho.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M, Bruder G, Hegerl U, Spooner C, Palmer DM, Etkin A, … & Gordon E (2016). EEG alpha asymmetry as a gender-specific predictor of outcome to acute treatment with different antidepressant medications in the randomized iSPOT-D study. Clinical Neurophysiology, 127(1), 509–519. 10.1016/j.clinph.2015.05.032 [DOI] [PubMed] [Google Scholar]
- Barnhofer T, Duggan D, Crane C, Hepburn S, Fennell MJ, & Williams JMG (2007). Effects of meditation on frontal α-asymmetry in previously suicidal individuals. Neuroreport, 18(7), 709–712. 10.1097/WNR.0b013e3280d943cd [DOI] [PubMed] [Google Scholar]
- Barry RJ, & De Blasio FM (2018). EEG frequency PCA in EEG-ERP dynamics. Psychophysiology, 55(5), e13042. 10.1111/psyp.13042 [DOI] [PubMed] [Google Scholar]
- Benjamini Y & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistics Society, 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berger H (1929). Über das elektrenkephalogramm des menschen. European archives of psychiatry and clinical neuroscience, 87(1), 527–570. 10.1007/BF01797193 [DOI] [Google Scholar]
- Bogg T, & Roberts BW (2013). The case for conscientiousness: Evidence and implications for a personality trait marker of health and longevity. Annals of Behavioral Medicine, 45(3), 278–288. 10.1007/s12160-012-9454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briki W (2018). Trait self-control: Why people with a higher approach (avoidance) temperament can experience higher (lower) subjective wellbeing. Personality and Individual Differences, 120, 112–117. 10.1016/j.paid.2017.08.039 [DOI] [Google Scholar]
- Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, & Tenke CE (2008). EEG alpha measures predict therapeutic response to an SSRI antidepressant: Pre and post treatment findings. Biological psychiatry, 63(12), 1171. 10.1016/j.biopsych.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, & McGrath PJ (2017). Right brain, left brain in depressive disorders: clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neuroscience & Biobehavioral Reviews, 78, 178–191. 10.1016/j.neubiorev.2017.04.021 [DOI] [PubMed] [Google Scholar]
- Brzezicka A, Kamiński J, Kamińska OK, Wołyńczyk-Gmaj D, & Sedek G (2017). Frontal EEG alpha band asymmetry as a predictor of reasoning deficiency in depressed people. Cognition and emotion, 31(5), 868–878. 10.1080/02699931.2016.1170669 [DOI] [PubMed] [Google Scholar]
- Cantisani A, Koenig T, Horn H, Müller T, Strik W, & Walther S (2015). Psychomotor retardation is linked to frontal alpha asymmetry in major depression. Journal of affective disorders, 188, 167–172. 10.1016/j.jad.2015.08.018 [DOI] [PubMed] [Google Scholar]
- Cantisani A, König T, Stegmayer K, Federspiel A, Horn H, Müller TJ, … & Walther S (2016). EEG marker of inhibitory brain activity correlates with resting-state cerebral blood flow in the reward system in major depression. European archives of psychiatry and clinical neuroscience, 266(8), 755–764. 10.1007/s00406-015-0652-7 [DOI] [PubMed] [Google Scholar]
- Chan AS, Han YM, Sze SL, Wong QY, & Cheung MC (2013). A randomized controlled neurophysiological study of a chinese chan-based mind-body intervention in patients with major depressive disorder. Evidence-based complementary and alternative medicine : eCAM, 2013, 812096. 10.1155/2013/812096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290. 10.1037/1040-3590.6.4.284 [DOI] [Google Scholar]
- Coan JA, & Allen JJ (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological psychology, 67(1–2), 7–50. 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Cooley JW, & Tukey JW (1965). An algorithm for the machine calculation of complex Fourier series. Mathematics of computation, 19(90), 297–301. 10.2307/2003354 [DOI] [Google Scholar]
- Davidson RJ (1992). Anterior cerebral asymmetry and the nature of emotion. Brain and cognition, 20(1), 125–151. 10.1016/0278-2626(92)90065-T [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1994). Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology, 6(4), 741–758. 10.1017/S0954579400004764 [DOI] [Google Scholar]
- Davidson RJ (1998). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion, 12(3), 307–330. 10.1080/026999398379628 [DOI] [Google Scholar]
- De Raedt R, Franck E, Fannes K, & Verstraeten E (2008). Is the relationship between frontal EEG alpha asymmetry and depression mediated by implicit or explicit self-esteem?. Biological Psychology, 77(1), 89–92. 10.1016/j.biopsycho.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, & Kayser J (2000). Is resting anterior EEG alpha asymmetry a trait marker for depression?. Neuropsychobiology, 41(1), 31–37. 10.1159/000026630 [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, & Hernandez-Reif M (2001). CES-D depression scores are correlated with frontal EEG alpha asymmetry. Depression and Anxiety, 13(1), 32–37. [DOI] [PubMed] [Google Scholar]
- Friedman HS, & Kern ML (2014). Personality, well-being, and health. Annual review of psychology, 65, 719–742. 10.1146/annurev-psych-010213-115123 [DOI] [PubMed] [Google Scholar]
- Furr RM (2010). The double-entry intraclass correlation as an index of profile similarity: Meaning, limitations, and alternatives. Statistical Developments and Applications, 92(1), 1–15. 10.1080/00223890903379134 [DOI] [PubMed] [Google Scholar]
- Gianotti LR, Künig G, Lehmann D, Faber PL, Pascual-Marqui RD, Kochi K, & Schreiter-Gasser U (2007). Correlation between disease severity and brain electric LORETA tomography in Alzheimer’s disease. Clinical Neurophysiology, 118(1), 186–196. 10.1016/j.clinph.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Greco C, Matarazzo O, Cordasco G, Vinciarelli A, Callejas Z, & Esposito A (2021). Discriminative power of EEG-based biomarkers in major depressive disorder: A systematic review. IEEE Access. 10.1109/ACCESS.2021.3103047 [DOI] [Google Scholar]
- Gold C, Fachner J, & Erkkilä J (2013). Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scandinavian Journal of Psychology, 54(2), 118–126. 10.1111/sjop.12022 [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr, & Cohen MS (2002). Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport, 13(18), 2487–2492. 10.1097/01.wnr.0000047685.08940.d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E, Palmer DM, & Cooper N (2010). EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clinical EEG and neuroscience, 41(4), 178–183. 10.1177/155005941004100404 [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, & Thayer JF (2001). The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology, 38(5), 847–857. 10.1111/1469-8986.3850847 [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Lürken A, Becker G, Maier S, & Bartussek D (1999). EEG asymmetry, dispositional mood and personality. Personality and Individual Differences, 27(3), 541–568. 10.1016/S0191-8869(98)00263-3 [DOI] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, & Bartussek D (2002). Does resting electroencephalograph asymmetry reflect a trait? An application of latent state-trait theory. Journal of personality and social psychology, 82(4), 619–641. 10.1037/0022-3514.82.4.619 [DOI] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Hanada G, Macion J, McCracken JT, McGough JJ, & Loo SK (2009). Atypical alpha asymmetry in adults with ADHD. Neuropsychologia, 47(10), 2082–2088. 10.1016/j.neuropsychologia.2009.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJ (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of abnormal psychology, 106(1), 159–163. 10.1037//0021-843x.106.1.159 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Gable PA (2018). On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology, 55(1), e12879. 10.1111/psyp.12879 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, & Peterson CK (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological psychology, 84(3), 451–462. 10.1016/j.biopsycho.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1990). Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology, 99(1), 22–31. 10.1037/0021-843X.99.1.22 [DOI] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1991). Left frontal hypoactivation in depression. Journal of abnormal psychology, 100(4), 535–545. 10.1037/0021-843X.100.4.535 [DOI] [PubMed] [Google Scholar]
- Hill KE, Lane SP, & Foti D (2019). Block-wise and trial-wise analyses of the Late Positive Potential reveal distinct affective trajectories as a function of neuroticism. Brain research. 1720, 146292. 10.1016/j.brainres.2019.06.011 [DOI] [PubMed] [Google Scholar]
- Hill KE, Neo WS, Hernandez A, Hamrick LR, Kelleher BL, & Foti D (2020). Intergenerational transmission of frontal alpha asymmetry among mother–infant dyads. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 420–428. 10.1016/j.bpsc.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GD, & Snyder D (1996). Frontal brain asymmetry predicts affective style in men. Behavioral Neuroscience, 110(1), 3–6. 10.1037//0735-7044.110.1.3 [DOI] [PubMed] [Google Scholar]
- Jakobi UE (2009). A Meta-analysis about EEG-Alpha-Asymmetries and Depression in Adults: Proof of a vulnerability marker for depression?. VDM Publishing. [Google Scholar]
- Jaworska N, Blier P, Fusee W, & Knott V (2012). Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. Journal of psychiatric research, 46(11), 1483–1491. 10.1016/j.jpsychires.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesulola E, Sharpley CF, Bitsika V, Agnew LL, & Wilson P (2015). Frontal alpha asymmetry as a pathway to behavioural withdrawal in depression: Research findings and issues. Behavioural Brain Research, 292, 56–67. 10.1016/j.bbr.2015.05.058 [DOI] [PubMed] [Google Scholar]
- Jolliffe IT, & Cadima J (2016). Principal component analysis: a review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 374(2065), 20150202. 10.1098/rsta.2015.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käckenmester W, Kroencke L, & Wacker J (2018). Frontal asymmetry predicts the incentive value of perceptual information. International Journal of Psychophysiology, 134, 22–29. 10.1016/j.ijpsycho.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Kaiser AK, Doppelmayr M, & Iglseder B (2018). Electroencephalogram alpha asymmetry in geriatric depression. Zeitschrift Für Gerontologie Und Geriatrie, 51(2), 200–205. 10.1007/s00391-016-1108-z [DOI] [PubMed] [Google Scholar]
- Kaiser AK, Gnjezda MT, Knasmüller S, & Aichhorn W (2018). Electroencephalogram alpha asymmetry in patients with depressive disorders: current perspectives. Neuropsychiatric disease and treatment, 14, 1493–1504. 10.2147/NDT.S137776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, & Tenke CE (2006). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical neurophysiology, 117(2), 348–368. 10.1016/j.clinph.2005.08.034 [DOI] [PubMed] [Google Scholar]
- Kayser J, & Tenke CE (2015). Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. International Journal of Psychophysiology, 97(3), 189–209. 10.1016/j.ijpsycho.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg WHIM, Arns M, … & Bryant RA (2010). Disorder specificity despite comorbidity: resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological psychology, 85(2), 350–354. 10.1016/j.biopsycho.2010.08.001 [DOI] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, & Bruder GE (2000). Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology, 109(4), 797–802. 10.1037/0021-843X.109.4.797 [DOI] [PubMed] [Google Scholar]
- Keune PM, Bostanov V, Hautzinger M, & Kotchoubey B (2013). Approaching dysphoric mood: state-effects of mindfulness meditation on frontal brain asymmetry. Biological Psychology, 93(1), 105–113. 10.1016/j.biopsycho.2013.01.016 [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, & Hanslmayr S (2007). EEG alpha oscillations: the inhibition–timing hypothesis. Brain research reviews, 53(1), 63–88. 10.1111/j.1469-8986.1992.tb02021.x [DOI] [PubMed] [Google Scholar]
- Kline RB (2005). Principles and practice of structural equation modeling 2nd ed. New York: Guilford, 3. [Google Scholar]
- Koller-Schlaud K, Ströhle A, Bärwolf E, Behr J, & Rentzsch J (2020). EEG Frontal Asymmetry and Theta Power in Unipolar and Bipolar Depression. Journal of Affective Disorders, 276, 501–510. 10.1016/j.jad.2020.07.011 [DOI] [PubMed] [Google Scholar]
- Kuper N, Käckenmester W & Wacker J (2019). Resting frontal EEG asymmetry and personality traits: A meta-analysis. European Journal of Personality, 33, 154–175. 10.1002/per.2197 [DOI] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, & Krakow K (2003b). EEG-correlated fMRI of human alpha activity. Neuroimage, 19(4), 1463–1476. 10.1016/S1053-8119(03)00286-6 [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, & Kleinschmidt A (2003a). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the national academy of sciences, 100(19), 11053–11058. 10.1073/pnas.1831638100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sarapas C, & Shankman SA (2016). Anticipatory reward deficits in melancholia. Journal of abnormal psychology, 125(5), 631–640. 10.1037/abn0000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour research and therapy, 33(3), 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, & Kemp AH (2008). Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion, 8(4), 560–572. 10.1037/a0012811 [DOI] [PubMed] [Google Scholar]
- Minnix JA, & Kline JP (2004). Neuroticism predicts resting frontal EEG asymmetry variability. Personality and Individual Differences, 36(4), 823–832. 10.1016/S0191-8869(03)00155-7 [DOI] [Google Scholar]
- Mitchell JT, Kimbrel NA, Hundt NE, Cobb AR, Nelson-Gray RO, & Lootens CM (2007). An analysis of reinforcement sensitivity theory and the five-factor model. European Journal of Personality: Published for the European Association of Personality Psychology, 21(7), 869–887. 10.1002/per.644 [DOI] [Google Scholar]
- Mullins-Sweatt SN, Jamerson JE, Samuel DB, Olson DR, & Widiger TA (2006). Psychometric properties of an abbreviated instrument of the five-factor model. Assessment, 13(2), 119–137. 10.1177/1073191106286748 [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, & Miller GA (1999). Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology, 36(5), 628–637. 10.1111/1469-8986.3650628 [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, & Abramson LY (2011). Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology, 120(2), 497. 10.1037/a0022940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, McMenamin BW, Greischar LL, Davidson RJ, & Kovacs M (2018). Comorbid anxiety moderates the relationship between depression history and prefrontal EEG asymmetry. Psychophysiology, 55(1), e12953. 10.1111/psyp.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, … & Davidson RJ (2004). Functional coupling of simultaneous electrical and metabolic activity in the human brain. Human brain mapping, 21(4), 257–270. 10.1002/hbm.20004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich S, & Arns M (2013). EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. International Review of Psychiatry, 25(5), 604–618. 10.3109/09540261.2013.816269 [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Shishida K, Hashizume A, Ueda K, Yamashita H, & Yamawaki S (2007). Anticipation of affective images and event-related desynchronization (ERD) of alpha activity: an MEG study. Brain research, 1151, 134–141. 10.1016/j.brainres.2007.03.026 [DOI] [PubMed] [Google Scholar]
- Panier LY, Bruder GE, Svob C, Wickramaratne P, Gameroff MJ, Weissman MM, … & Kayser J (2020). Predicting Depression Symptoms in Families at Risk for Depression: Interrelations of Posterior EEG Alpha and Religion/Spirituality. Journal of Affective Disorders, 274, 969–976. 10.1016/j.jad.2020.05.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli P, Wiedemann G, & Nickola M (1999). Pain sensitivity, cerebral laterality, and negative affect. Pain, 80(1–2), 359–364. 10.1016/S0304-3959(98)00231-0 [DOI] [PubMed] [Google Scholar]
- Petruzzello SJ, & Landers DM (1994). State anxiety reduction and exercise: Does hemispheric activation reflect such changes? Medicine & Science in Sports & Exercise, 26(8), 1028–1035. 10.1249/00005768-199408000-00015 [DOI] [PubMed] [Google Scholar]
- Petsche H, Kaplan S, Von Stein A, & Filz O (1997). The possible meaning of the upper and lower alpha frequency ranges for cognitive and creative tasks. International journal of psychophysiology, 26(1–3), 77–97. 10.1016/S0167-8760(97)00757-5 [DOI] [PubMed] [Google Scholar]
- Pössel P, Lo H, Fritz A, & Seemann S (2008). A longitudinal study of cortical EEG activity in adolescents. Biological Psychology, 78(2), 173–178. 10.1016/j.biopsycho.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Quinn CR, Rennie CJ, Harris AW, & Kemp AH (2014). The impact of melancholia versus non-melancholia on resting-state, EEG alpha asymmetry: Electrophysiological evidence for depression heterogeneity. Psychiatry research, 215(3), 614–617. 10.1016/j.psychres.2013.12.049 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Reid SA, Duke LM, & Allen JJ (1998). Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology, 35(4), 389–404. 10.1111/1469-8986.3540389 [DOI] [PubMed] [Google Scholar]
- Ringnér M (2008). What is principal component analysis?. Nature biotechnology, 26(3), 303–304. 10.1038/nbt0308-303 [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, & Hagoort P (2008). Frontal theta EEG activity correlates negatively with the default mode network in resting state. International journal of psychophysiology, 67(3), 242–251. 10.1016/j.ijpsycho.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Schmidtke JI, & Heller W (2004). Personality, affect and EEG: predicting patterns of regional brain activity related to extraversion and neuroticism. Personality and Individual Differences, 36(3), 717–732. 10.1016/S0191-8869(03)00129-6 [DOI] [Google Scholar]
- Segrave RA, Cooper NR, Thomson RH, Croft RJ, Sheppard DM, & Fitzgerald PB (2011). Individualized alpha activity and frontal asymmetry in major depression. Clinical EEG and Neuroscience, 42(1), 45–52. 10.1177/155005941104200110 [DOI] [PubMed] [Google Scholar]
- Shean G, & Baldwin G (2008). Sensitivity and specificity of depression questionnaires in a college-age sample. The Journal of genetic psychology, 169(3), 281–292. 10.3200/GNTP.169.3.281-292 [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, & De Geus EJC (2005). Heritability of background EEG across the power spectrum. Psychophysiology, 42(6), 691–697. 10.1111/j.1469-8986.2005.00352.x [DOI] [PubMed] [Google Scholar]
- Smith EE, Cavanagh JF, & Allen JJ (2018). Intracranial source activity (eLORETA) related to scalp-level asymmetry scores and depression status. Psychophysiology, 55(1), e13019. 10.1111/psyp.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Reznik SJ, Stewart JL, & Allen JJ (2017). Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. International Journal of Psychophysiology, 111, 98–114. 10.1016/j.ijpsycho.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Tenke CE, Deldin PJ, Trivedi MH, Weissman MM, Auerbach RP, … & Kayser J (2020). Frontal theta and posterior alpha in resting EEG: A critical examination of convergent and discriminant validity. Psychophysiology, 57(2), e13483. 10.1111/psyp.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Zambrano-Vazquez L, & Allen JJ (2016). Patterns of alpha asymmetry in those with elevated worry, trait anxiety, and obsessive-compulsive symptoms: A test of the worry and avoidance models of alpha asymmetry. Neuropsychologia, 85, 118–126. 10.1016/j.neuropsychologia.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine, 166(10), 1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Stasik-O’Brien SM, Brock RL, Chmielewski M, Naragon-Gainey K, Koffel E, McDade-Montez E, … & Watson D (2019). Clinical utility of the Inventory of Depression and Anxiety Symptoms (IDAS). Assessment, 26(5), 944–960. 10.1177/1073191118790036 [DOI] [PubMed] [Google Scholar]
- Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87, 245–251. 10.1037/0033-2909.87.2.245 [DOI] [Google Scholar]
- Stewart JL, & Allen JJ (2018). Resting frontal brain asymmetry is linked to future depressive symptoms in women. Biological psychology, 136, 161–167. 10.1016/j.biopsycho.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, & Allen JJ (2010). Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of abnormal psychology, 119(3), 502–512. 10.1037/a0019196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, & Allen JJ (2014). Resting and task-elicited prefrontal EEG alpha asymmetry in depression: Support for the capability model. Psychophysiology, 51(5), 446–455. 10.1111/psyp.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, & Davidson RJ (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological science, 8(3), 204–210. 10.1111/j.1467-9280.1997.tb00413.x [DOI] [Google Scholar]
- Tenke CE, & Kayser J (2012). Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical neurophysiology, 123(12), 2328–2345. 10.1016/j.clinph.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, & Kayser J (2005). Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA). Clinical Neurophysiology, 116(12), 2826–2846. 10.1016/j.clinph.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Pechtel P, Webb CA, Dillon DG, Goer F, … & Bruder GE (2017). Demonstrating test-retest reliability of electrophysiological measures for healthy adults in a multisite study of biomarkers of antidepressant treatment response. Psychophysiology, 54(1), 34–50. 10.1111/psyp.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, … & Bruder GE (2011). Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biological psychiatry, 70(4), 388–394. 10.1016/j.biopsych.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, & Kim S (2006). Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of abnormal psychology, 115(4), 715–729. 10.1037/0021-843X.115.4.715 [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, & Davidson RJ (1994). Frontal brain activation in repressors and nonrepressors. Journal of abnormal psychology, 103(2), 339–349. 10.1037//0021-843x.103.2.339 [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, & Kinney L (1992). Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology, 29(5), 576–592. 10.1111/j.1469-8986.1992.tb02034.x [DOI] [PubMed] [Google Scholar]
- Towers DN, & Allen JJ (2009). A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology, 46(1), 132–142. 10.1111/j.1469-8986.2008.00759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vinne N, Vollebregt MA, Van Putten MJ, & Arns M (2017). Frontal alpha asymmetry as a diagnostic marker in depression: Fact or fiction? A meta-analysis. Neuroimage: clinical, 16, 79–87. 10.1016/j.nicl.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio A, & De Pascalis V (2020). EEG Resting Asymmetries and Frequency Oscillations in Approach/Avoidance Personality Traits: A Systematic Review. Symmetry, 12(10), 1712. 10.3390/sym12101712 [DOI] [Google Scholar]
- Velo JR, Stewart JL, Hasler BP, Towers DN, & Allen JJ (2012). Should it matter when we record? Time of year and time of day as factors influencing frontal EEG asymmetry. Biological psychology, 91(2), 283–291. 10.1016/j.biopsycho.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, & Stemmler G (2010). Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality, 44(2), 167–179. 10.1016/j.jrp.2009.12.004 [DOI] [Google Scholar]
- Watkins MW (2021). A step-by-step guide to exploratory factor analysis with SPSS. New York: Routledge. [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … & Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, & Buchkremer G (1999). Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Archives of general psychiatry, 56(1), 78–84. 10.1001/archpsyc.56.1.78 [DOI] [PubMed] [Google Scholar]
- Williams LM, Simms E, Clark CR, Paul RH, Rowe D, & Gordon E (2005). The test-retest reliability of a standardized neurocognitive and neurophysiological test battery:“neuromarker”. International Journal of Neuroscience, 115(12), 1605–1630. 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


