Abstract
Significant under-utilization of breast cancer chemoprevention remains, despite guidelines stating that physicians should recommend chemoprevention with anti-estrogen therapy to high-risk women. We randomized women, age 35-75 years, who met high-risk criteria for breast cancer, without a personal history of breast cancer or prior chemoprevention use, to standard educational materials alone or combined with a web-based decision aid. All healthcare providers, including primary care providers and breast specialists, were given access to a web-based decision support tool. The primary endpoint was chemoprevention uptake at 6 months. Secondary outcomes included decision antecedents (perceived breast cancer risk/worry, chemoprevention knowledge, self-efficacy) and decision quality (decision conflict, chemoprevention informed choice) based upon patient surveys administered at baseline, 1 and 6 months after randomization. Among 282 evaluable high-risk women enrolled from November 2016 to March 2020, mean age was 57 years (SD, 9.9) and mean 5-year invasive breast cancer risk was 2.98% (SD, 1.42). There was no significant difference in chemoprevention uptake at 6 months between the intervention and control groups (2.1% vs. 3.5%). Comparing the intervention and control arms at 1 month, there were significant differences among high-risk women in accurate breast cancer risk perceptions (56% vs. 39%, p=0.017), adequate chemoprevention knowledge (49% vs. 27%, p<0.001), mean decision conflict (34.0 vs. 47.0, p<0.001), and informed choice (41% vs. 23%, p=0.003). These differences were no longer significant at 6 months. Although our decision support tools did not result in a significant increase in chemoprevention uptake, we did observe improvements in decision antecedents and decision quality measures.
INTRODUCTION
Breast cancer is the most common malignancy and second leading cause of cancer-related death among women in the U.S. with an estimated 281,550 new cases and 43,600 deaths due to breast cancer in 2021.[1] Chemoprevention with selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene, and aromatase inhibitors (AIs), such as anastrozole and exemestane, given for 5 years have been shown in randomized controlled trials to reduce breast cancer incidence by 40-65% among high-risk women.[2-6] Based upon the strength of this evidence, the U.S. Preventive Services Task Force (USPSTF) and other professional organizations state that physicians should recommend SERMs or AIs to high-risk women for breast cancer risk reduction.[7-9] However, these guidelines have not been widely adopted in primary care, with less than 5% of high-risk women offered anti-estrogen therapy agreeing to take these medications.[10]
Approximately 15% of women in the U.S., age 35-79 years, meet high-risk criteria for breast cancer and are potentially eligible for chemoprevention.[11] There are a number of established breast cancer risk assessment tools, such as the Gail model, which account for a woman’s age, race/ethnicity, first-degree family history of breast cancer, benign breast biopsies, and reproductive factors.[12] Eligibility criteria for participation in the U.S. chemoprevention trials included women with a 5-year invasive breast cancer risk ≥1.67% according to the Gail model or history of lobular carcinoma in situ (LCIS).[2, 4]
Barriers to chemoprevention uptake include the lack of routine breast cancer risk assessment in the primary care setting, insufficient knowledge about SERMs and AIs among high-risk women and primary care providers (PCPs), time constraints, competing comorbidities, and concerns about side effects.[10, 13] In order to address these barriers, we have developed web-based clinical decision support (CDS) tools, RealRisks and BNAV (Breast cancer risk NAVigation), for patients and healthcare providers, respectively, which are designed to be used outside of the clinical encounter to engage patients and providers in shared decision-making about breast cancer chemoprevention. In an initial pilot study with a pretest-posttest design, exposure to RealRisks was associated with a significant improvement in breast cancer risk perceptions and chemoprevention knowledge, with nearly a quarter of high-risk women expressing interest in taking chemoprevention.[14] In the current study, we aimed to test the effects of the CDS tools on chemoprevention uptake, with all healthcare providers given access to BNAV and high-risk patients randomized to standard educational materials alone or in combination with RealRisks. We hypothesized that exposure to these CDS tools would lead to increased chemoprevention uptake among high-risk women.
MATERIALS AND METHODS
Study Design
We enrolled 50 healthcare providers and 300 high-risk women (unit of randomization), who were randomized 1:1 and stratified by Hispanic ethnicity and menopausal status to the web-based decision aid, RealRisks, combined with standard educational materials or a control arm of standard educational materials alone. All healthcare providers at Columbia University Irving Medical Center (CUIMC), including those enrolled and not enrolled in our trial, were given access to the BNAV decision support tool via the Ambulatory Medicine dashboard within our electronic health record (EHR). Both patients and providers were enrolled from the primary care clinics (internal medicine, family medicine, gynecology) and breast clinics/mammography at CUIMC in New York City. For more details, the trial protocol is previously described.[15] Written informed consent was obtained from all enrolled patients and healthcare providers. This study was approved by the institutional review board at CUIMC in accordance with the Belmont Report, the randomized controlled trial was conducted in accordance with CONSORT guidelines, and registered at clinicaltrials.gov (ClinicalTrials.gov identifier NCT03069742).
Study Sample
Patient eligibility criteria included: 1) women, age 35-75 years; 2) 5-year invasive breast cancer risk ≥1.67% according to the Gail model or a personal history of LCIS; 3) no prior SERM or AI use; 4) no personal history of breast cancer; 5) English- or Spanish-speaking; 6) healthcare provider at CUIMC; 7) access to the internet. Healthcare providers included PCPs, such as internists, family practitioners, and gynecologists, and specialists, such as breast surgeons, medical oncologists, and nurse practitioners, who saw high-risk women in their clinics at CUIMC.
Recruitment
High-risk women were identified through a variety of strategies[16]: 1) in-person recruitment and breast cancer risk assessment during screening mammography[17]; 2) collection of breast cancer risk factors from the EHR among women undergoing screening mammography[18]; 3) identification of women with high-risk benign breast lesions, such as atypical hyperplasia (AH) and LCIS, via ICD-9/10 diagnostic codes[19]; 4) screening clinic schedules of enrolled providers with direct referral of patients; 5) self-referrals from high-risk women responding to recruitment flyers and online resources. Potential healthcare providers were identified through faculty email listservs for our target clinics.
Study Intervention
All participating patients received standard educational materials in paper/electronic format in English or Spanish, consisting of a brochure for the CUIMC high-risk breast clinic and information on breast cancer chemoprevention from the Susan G. Komen foundation. Patients in the intervention group received the RealRisks patient-facing decision aid (DA), which is comprised of interactive modules on risk (definitions of risk and breast cancer risk factors) and chemoprevention (information on SERMs and AIs and risk/benefit profiles), which are tailored to a woman’s personalized breast cancer risk. Each module presents information as a narrative in graphic novel format (“light”) and detailed text explanations with visual aids (“dense”). Additional features to account for varying health and computer literacy include text to hover over to view definitions of key terms in the narrative, audio buttons, Spanish translations, and explanatory videos to navigate through the tool. The breast cancer risk module collects self-reported breast cancer risk factors to calculate absolute 5-year and lifetime invasive breast cancer risk according to the Gail model, with an interactive “clicking” game to demonstrate personalized breast cancer risk using a pictograph of 100 figures.[20] The chemoprevention module demonstrates the patient’s individualized risks and benefits of SERMs and AIs and includes a “preference elicitation” game where patients input their chemoprevention intention and weigh factors that are important in decision-making. After completing the RealRisks modules, a PDF summary of the patient’s personalized breast cancer risk information is generated, as an action plan to be presented at the next scheduled clinic visit with her healthcare provider.[15]
All healthcare providers had access to the BNAV tool through a link-out in the EHR Ambulatory Medicine dashboard at CUIMC. BNAV consists of educational modules on breast cancer risk and chemoprevention, as well as breast cancer screening, genetic testing, and risk communication. Providers with patients randomized to the intervention arm who completed RealRisks are able to view personalized risk reports through a secure patient list within BNAV. More details on the CDS tools are previously described.[15]
Data Collection
Study questionnaires were administered to patients at baseline, 1 and 6 months after randomization. The baseline questionnaire included questions on sociodemographics (age, race/ethnicity, educational level), breast cancer risk factors (age of menarche, age of first live birth, menopausal status, benign breast disease, first-degree family history of breast cancer), and validated measures for health literacy[21], subjective numeracy[22], and acculturation.[23] Decision antecedents, such as perceived breast cancer risk[24], breast cancer worry[25, 26], chemoprevention knowledge[27], and decision self-efficacy[28] were assessed at all 3 timepoints. Decision quality measures, such as decision conflict[29] and informed choice[27] were assessed at 1 and 6 months post-randomization.
Healthcare providers completed a baseline survey on demographics and practice characteristics, including medical specialty, number of years since completing medical training and using an EHR, use of breast cancer risk assessment tools and experience prescribing chemoprevention with SERMs or AIs, and a validated measure of the provider’s confidence in communicating risk to their patients.[30]
Outcomes
The primary outcome was chemoprevention uptake with initiation of a SERM or AI within 6 months after randomization, as assessed by EHR log and self-report. Secondary endpoints included validated patient-reported outcomes: 1) perceived breast cancer risk[24]; 2) breast cancer worry[25, 26]; 3) chemoprevention knowledge[27]; 4) self-efficacy[28]; 5) decision conflict[29]; 6) informed choice.[27]
Statistical Analyses
The primary endpoint is chemoprevention uptake at 6 months between the intervention and control arms. With a sample size of 300 patients (150 per arm), we had >80% power to detect a difference in chemoprevention uptake at 6 months of 1% in the control arm and 10% in the active intervention arm, assuming a type 1 error of 5% and a 10% unevaluable rate (effective sample size of 270). The primary analysis was based upon intent-to-treat principle. Frequency distributions and summary descriptive statistics were calculated for all baseline characteristics and secondary endpoints for both study arms. We determined associations between study arms and categorical outcome variables using chi-squared tests and continuous outcome variables using t-tests or non-parametric analog. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.
RESULTS
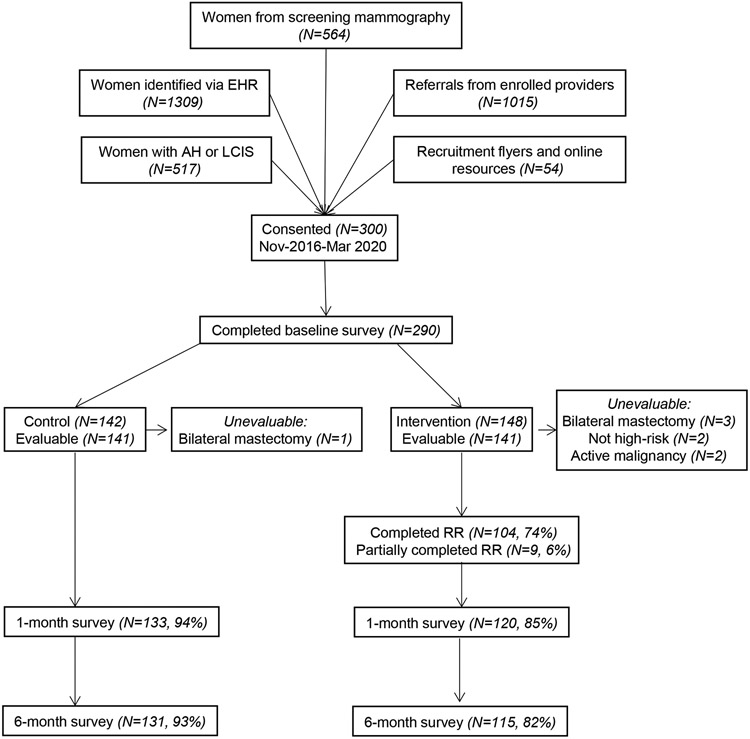
From November 2016 to March 2020 (Figure 1), we screened 3459 patients from 5 different recruitment sources: 1) high-risk women identified via the EHR (N=1309, 37.8%), 2) referrals from enrolled providers (N=1015, 29.3%), 3) women from screening mammography (N=564, 16.3%), 4) women with AH or LCIS (N=517, 15.0%), 5) women responding to recruitment flyers and online resources (N=54, 1.6%).[16] Among the 300 consented patients, 134 (44.7%) were referrals from enrolled providers, 82 (27.3%) were from screening mammography, 35 (11.7%) were identified via the EHR, 31 (10.3%) had AH or LCIS, and 18 (6.0%) responded to recruitment flyers and online resources, with 290 (96.7%) completing the baseline survey. Among these women, 8 were found to be ineligible (4 due to bilateral mastectomies, 2 not meeting high-risk criteria for breast cancer, 2 with active malignancy), leaving 282 evaluable patients: 253 (90%) completed the 1-month survey and 246 (87%) completed the 6-month survey, with slightly better retention in the control arm. In the intervention group, 74% of women completed all of the modules of RealRisks and 6% partially completed based upon usage logs. Among 660 healthcare providers screened in the primary care and breast clinics at CUIMC, 50 were consented and 45 completed their baseline survey.
Figure 1.
CONSORT diagram of recruitment and retention of women at high-risk for breast cancer to a randomized controlled trial of web-based decision support for breast cancer chemoprevention
Baseline characteristics of evaluable patients and providers are presented in Tables 1 and 2, respectively. The mean age was 57.4 years (SD, 9.9), with the majority (60%) being postmenopausal. The study population was racially/ethnically diverse with 59% non-Hispanic White, 14% non-Hispanic Black, and 21% Hispanic. In terms of breast cancer risk factors, about two-thirds had a history of benign breast disease and 64% of women had a first-degree family history of breast cancer. Overall, the mean 5-year invasive breast cancer risk was 2.98% (SD, 1.42). Among the 45 evaluable providers, the mean age was 44 years (SD, 13.3). The majority were female (73%), non-Hispanic White (69%), internists (67%), and were more than 5 years since completing medical training (60%). Most providers (82%) had been using the EHR for over 5 years and were willing to try new technologies. In terms of practice patterns for breast cancer risk assessment and prescribing of chemoprevention, less than half (44%) had ever used the Gail model and about a quarter (24%) had ever prescribed anti-estrogen therapy for breast cancer chemoprevention. The most common reasons for not prescribing chemoprevention included preferring to refer high-risk patients to a specialist (66%), not comfortable prescribing chemoprevention (55%), and not identifying high-risk patients in whom chemoprevention was indicated (24%). On a scale of 1-6, their mean confidence in risk communication was 3.80 (SD, 0.10).
Table 1.
Baseline characteristics of evaluable high-risk women
| Patient Characteristics | Intervention (N=141) |
Control (N=141) |
Total (N=282) |
|||
|---|---|---|---|---|---|---|
| Age, years | ||||||
| Mean (SD) | 58.43 (10.40) | 56.42 (9.22) | 57.43 (9.86) | |||
| Menopausal status, N (%) | ||||||
| Pre/Perimenopausal | 54 | 38.3% | 58 | 41.1% | 112 | 39.7% |
| Postmenopausal | 87 | 61.7% | 83 | 58.9% | 170 | 60.3% |
| Race/ethnicity, N (%) | ||||||
| Non-Hispanic White | 80 | 56.7% | 85 | 60.3% | 165 | 58.5% |
| Non-Hispanic Black | 19 | 13.5% | 21 | 14.9% | 40 | 14.2% |
| Hispanic | 33 | 23.4% | 26 | 18.4% | 59 | 20.9% |
| Other | 9 | 6.4% | 9 | 6.4% | 18 | 6.4% |
| Highest Level of Education, N (%) | ||||||
| High school or less | 25 | 17.7% | 18 | 12.8% | 43 | 15.2% |
| Some college or bachelors | 51 | 36.2% | 53 | 37.6% | 104 | 36.9% |
| Graduate or professional degree | 46 | 32.6% | 52 | 36.9% | 98 | 34.8% |
| Unknown | 19 | 13.5% | 18 | 12.8% | 37 | 13.1% |
| Benign breast disease, N (%) | 97 | 68.8% | 91 | 64.5% | 188 | 66.7% |
| Atypical hyperplasia | 29 | 20.6% | 31 | 22.0% | 60 | 21.3% |
| Lobular carcinoma in situ (LCIS) | 4 | 2.8% | 11 | 7.8% | 15 | 5.3% |
| First-degree family history of breast cancer, N (%) | 84 | 59.6% | 96 | 68.0% | 180 | 63.8% |
| Age of menarche, N (%) | ||||||
| 7-11 years | 36 | 25.5% | 36 | 25.5% | 72 | 25.5% |
| 12-13 years | 76 | 53.9% | 77 | 54.6% | 153 | 54.3% |
| 14+ years | 27 | 19.1% | 27 | 19.1% | 54 | 19.1% |
| Unknown | 2 | 1.4% | 1 | 0.7% | 3 | 1.1% |
| Age of first birth, N (%) | ||||||
| No births | 41 | 29.1% | 33 | 23.4% | 74 | 26.2% |
| <20 years | 13 | 9.2% | 24 | 17.0% | 37 | 13.1% |
| 20-24 years | 22 | 15.6% | 19 | 13.5% | 41 | 14.5% |
| 25-29 years | 17 | 12.1% | 17 | 12.1% | 34 | 12.1% |
| 30+ years | 48 | 34.0% | 48 | 34.0% | 96 | 34.0% |
| * 5-year invasive breast cancer risk, % | ||||||
| Mean (SD) | 2.95 (1.53) | 3.00 (1.30) | 2.98 (1.42) | |||
| * Lifetime invasive breast cancer risk, % | ||||||
| Mean (SD) | 15.81 (9.26) | 17.48 (8.08) | 16.64 (8.72) | |||
| Adequate health literacy, N (%) | 115 | 83.3% | 118 | 83.7% | 233 | 83.2% |
| Mean subjective numeracy (SD) [range, 1-6] | 4.08 (1.42) | 4.13 (1.54) | 4.11 (1.48) | |||
| Mean acculturation (SD) [range, 1-5] | 3.81 (1.69) | 4.03 (1.56) | 3.92 (1.63) |
Calculated according to the Gail breast cancer risk assessment tool, excluding women with a history of lobular carcinoma in situ
Table 2.
Baseline characteristics of healthcare providers
| Provider Characteristics | Total (N=45) |
|---|---|
| Age, years | |
| Mean (SD) | 44 (13.3) |
| Gender, N (%) | |
| Male | 12 (27) |
| Female | 33 (73) |
| Race/ethnicity, N (%) | |
| Non-Hispanic White | 31 (69) |
| Non-Hispanic Black | 4 (9) |
| Hispanic | 4 (9) |
| Asian | 5 (11) |
| Other Race | 1 (2) |
| Medical specialty, N (%) | |
| Internal medicine | 30 (67) |
| Family medicine | 4 (9) |
| Gynecology | 6 (13) |
| Other | 5 (11) |
| Number of years since completed training, N (%) | |
| Have not yet finished training | 10 (22) |
| <5 | 8 (18) |
| 5-10 | 6 (13) |
| 11-15 | 4 (9) |
| 16-20 | 8 (18) |
| >20 | 9 (20) |
| Willingness to try new technology (range, 0-100) | |
| Mean (SD) | 78.2 (19.6) |
| Number of years using EHR, N (%) | |
| <5 | 8 (18) |
| 5-10 | 25 (56) |
| 11-15 | 10 (22) |
| 16-20 | 2 (4) |
| Ever used the Gail model, N (%) | |
| Yes | 20 (44) |
| Used the Gail model for which patients (multiple responses) | |
| Women age 60 years or older | 4 (20) |
| Women with a breast cancer family history | 10 (50) |
| Women who may be at higher than average risk for breast cancer | 10 (50) |
| I do not regularly use breast cancer risk assessment tools | 7 (35) |
| No | 25 (56) |
| Reasons for not using the Gail model (multiple responses) | |
| I do not see patients in whom breast cancer risk calculation is indicated | 1 (4) |
| I do not have enough time with my patients to use breast cancer risk assessment tools | 6 (24) |
| I do not think that the results of breast cancer risk assessment tools would change my management | 4 (16) |
| I am not sufficiently familiar with breast cancer risk assessment tools | 20 (80) |
| Ever prescribed chemoprevention, N (%) | |
| Yes | 11 (24) |
| About how many patients | |
| 1-5 | 11 (100) |
| No | 33 (73) |
| Reasons for not prescribing chemoprevention (multiple responses) | |
| I have not identified a patient in whom chemoprevention was indicated | 8 (24) |
| I do not believe that chemoprevention benefits most women who are eligible to receive it | 0 |
| I am not comfortable prescribing chemoprevention | 18 (55) |
| I do not have time to discuss chemoprevention with my patients | 4 (12) |
| I would prefer to refer my high-risk patients to a specialist to discuss chemoprevention | 22 (66) |
| Mean confidence in risk communication [range, 1 - 6] (SD) | 3.80 (0.10) |
In terms of the primary endpoint (Table 3), chemoprevention uptake at 6 months was not significantly different between the intervention and control arms (2.1% vs. 3.5%, p=0.735). For secondary endpoints, decision antecedents such as breast cancer risk perceptions, breast cancer worry, self-efficacy, and chemoprevention knowledge were not significantly different at baseline between the intervention and control arms. About a third of women had accurate breast cancer risk perceptions and about half over-estimated their risk at baseline. After exposure to RealRisks, accurate breast cancer risk perceptions improved in the intervention group compared to the control group with short-term follow-up (56% vs. 39%, p=0.017). At baseline, adequate chemoprevention knowledge was low in both groups (18%), which improved in the intervention arm compared to the control arm at 1 month (49% vs. 17%, p<0.001). There were no significant differences in breast cancer worry and self-efficacy between the arms. In terms of decision quality measures, mean decision conflict score was lower in the intervention group compared to the control group with short-term follow-up (34.0 vs. 47.0, p<0.001). In addition, chemoprevention informed choice was higher in the intervention compared to the control arm (41% vs. 23%, p=0.003). Differences between the intervention and control arms in all of these patient-reported outcomes were not sustained at 6 months.
Table 3.
Patient-reported outcomes assessed at baseline, 1 month, and 6 months
| Patient Outcome Measures | Baseline (N=280) |
P- value |
1 month (N=253) |
P- value |
6 months (N=246) |
P- value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||||||||
| (N=139) | (N=141) | (N=120) | (N=133) | (N=115) | (N=131) | ||||||||||
| Primary Endpoint | |||||||||||||||
| Chemoprevention uptake, N (%) | 3 | 2.1% | 5 | 3.5% | 0.735 | ||||||||||
| Decision Antecedents | |||||||||||||||
| Breast cancer risk perception | |||||||||||||||
| Accurate breast cancer risk perception, N (%) | 51 | 38% | 43 | 32% | 0.332 | 64 | 56% | 50 | 39% | 0.017 | 44 | 39% | 45 | 36% | 0.785 |
| Underestimate, N (%) | 17 | 13% | 25 | 19% | 9 | 8% | 21 | 16% | 14 | 13% | 19 | 15% | |||
| Overestimate, N (%) | 67 | 50% | 67 | 50% | 42 | 37% | 57 | 45% | 54 | 48% | 61 | 49% | |||
| Breast cancer worry, mean (SD) [range, 1-7] | 3.18 (1.56) | 3.04 (1.36) | 0.447 | 3.10 (1.63) | 3.30 (1.66) | 0.295 | 3.08 (1.51) | 3.04 (1.28) | 0.841 | ||||||
| Self-efficacy, mean (SD) [range, 0-100] | 82.73 (17.51) | 83.00 (18.42) | 0.797 | 84.01 (18.63) | 83.05 (19.80) | 0.864 | |||||||||
| Chemoprevention knowledge | |||||||||||||||
| Adequate knowledge, N (%) | 26 | 19% | 24 | 17% | 0.713 | 58 | 49% | 36 | 27% | <.001 | 43 | 37% | 44 | 34% | |
| Decision Quality | |||||||||||||||
| Decision conflict, mean (SD) [range, 0-100] | 33.97 (27.59) | 47.03 (27.76) | <.001 | 34.38 (27.54) | 41.16 (30.40) | 0.072 | |||||||||
| Chemoprevention attitudes, N (%) | |||||||||||||||
| Positive | 16 | 14% | 20 | 15% | 0.953 | 14 | 12% | 18 | 14% | 0.445 | |||||
| Neutral | 38 | 33% | 44 | 33% | 21 | 18% | 31 | 24% | |||||||
| Negative | 62 | 53% | 69 | 52% | 79 | 69% | 79 | 62% | |||||||
| Chemoprevention intention, N (%) | |||||||||||||||
| Likely | 21 | 18% | 31 | 23% | 0.282 | ||||||||||
| Not Likely | 97 | 82% | 102 | 77% | |||||||||||
| Informed Choice, N (%) | 48 | 41% | 31 | 23% | 0.003 | 39 | 34% | 36 | 28% | 0.333 | |||||
DISCUSSION
There was no significant difference in chemoprevention uptake at 6 months after exposure to RealRisks plus standard educational materials and BNAV vs. standard educational materials and BNAV, with uptake of SERMs or AIs remaining below 5% in both groups. With short-term follow-up, exposure to RealRisks was associated with a significant improvement in decision antecedents, such as accurate breast cancer risk perceptions and adequate chemoprevention knowledge, and decision quality, namely reduced decision conflict and increased informed choice. However, these differences were no longer significant at 6 months.
Our finding of low chemoprevention uptake among high-risk women is consistent with the prior literature. Intervention trials of CDS tools targeting both patients and providers designed to increase chemoprevention uptake have met with limited success. A web-based DA called Guide to Decide which informed high-risk postmenopausal women about the risks and benefits of SERMs yielded only 0.5% uptake.[31] The Ready, Set, GO GAIL! project involved systematic screening of over 5700 women by PCPs using the Gail model.[32] About 15.2% of women were identified as high-risk, with 14.7% referred for specialized risk counseling, 6.4% completing the consultation, and 2% starting a SERM. The BreastCARE intervention used a tablet-based patient-facing tool that generated individualized breast cancer risk reports for patients and their providers.[33] In a randomized controlled trial of the BreastCARE intervention compared to usual care among 1235 women (age 40-74 years) screened in the primary care setting, more high-risk women were referred for specialized risk counseling with the intervention compared to the control arm (18.8% vs. 4.1%). However, discussions about chemoprevention were still limited (1% vs. 0%).
Our study is unique in that we implemented multiple strategies for identifying women at high-risk for breast cancer in the primary care setting[17, 18, 34] and we targeted a racially/ethnically diverse patient population with a patient-facing DA, which is available in English and Spanish and has been rigorously tested among women of varying health literacy and numeracy.[14, 20, 35] Given that breast cancer chemoprevention is not widely adopted in the primary care setting, more effective tools are needed to inform both patients and providers about the risks and benefits of SERMs and AIs for primary prevention. Our web-based CDS tools, RealRisks and BNAV, were designed to address these chemoprevention knowledge gaps in patients and providers, respectively, and help them engage in informed shared decision-making.
Among our enrolled providers, we found that less than half had ever used the Gail model for breast cancer risk assessment and less than a quarter had ever prescribed SERMs or AIs for chemoprevention. The majority did not feel comfortable prescribing SERMs or AIs and preferred to refer high-risk patients to specialists to discuss chemoprevention. Studies that involved breast cancer risk counseling by specialists reported chemoprevention uptake rates ranging from 11% to 58%.[36-42] Therefore, higher uptake may be achieved with healthcare providers that have sufficient knowledge about breast cancer risk and prevention strategies. Since many community practices may not have access to high-risk clinics, PCPs who are at the frontline of chronic disease prevention will need to be trained on how to identify women who meet high-risk criteria and feel comfortable with prescribing SERMs and AIs for chemoprevention. This professional education may need to occur early during medical school or residency training or through continuing medical education (CME).
PCPs often screen for atherosclerotic cardiovascular disease (ASCVD) risk factors and prescribe aspirin and statins to patients who meet high-risk criteria for primary prevention of cardiovascular events. For example, statins are recommended in individuals at intermediate to high-risk for CVD, with a 10-year risk of 7.5% or higher according to the 2013 American College of Cardiology/American Heart Association (ACA/AHA) ASCVD risk calculator.[43] Compared to the low uptake of chemoprevention among high-risk women[10], uptake of statins for the prevention of cardiovascular events among high-risk individuals was over 40%.[44] The reason for these differences are likely multifactorial, including greater awareness of statins among patients and providers, greater comfort level for assessing ASCVD risk and prescribing of statins among PCPs, and availability of a surrogate marker with serum LDL cholesterol to assess ASCVD risk and response to statins. Breast cancer risk assessment with a number of well-validated risk calculators, such as the Gail model[12], may not be widely implemented in the primary care clinics. Therefore, more efficient and automated methods for breast cancer risk assessment are needed to reduce clinical burden to PCPs, who need to manage multiple chronic conditions. Currently, there are no surrogate biomarkers to assess breast cancer risk and response to chemoprevention available in the clinical setting, although various circulating biomarkers and mammographic density are under investigation.[45]
Concerns about side effects is another major barrier to chemoprevention uptake. In addition to menopausal symptoms with both SERMs and AIs, rare but serious side effects of tamoxifen include an increased risk of thromboembolic events and endometrial cancer[2, 4], whereas AIs have been associated with increased musculoskeletal symptoms, osteoporosis, hyperlipidemia, and hypertension among high-risk postmenopausal women.[5, 6] Low-dose tamoxifen at 5mg daily for 3 years (compared to the standard dose of 20mg daily for 5 years) was recently shown in a randomized controlled trial of women with atypical hyperplasia, lobular or ductal carcinoma in situ to reduce breast cancer incidence by about 50%.[46] It remains to be seen whether low-dose tamoxifen will gain greater acceptance among these high-risk women.
In our initial pilot study of RealRisks, our high-risk population, which was mainly identified through screening mammography, had a median age of 64 years (range, 49-74) and a median 5-year invasive breast cancer risk of 2.2% (range, 1.75-5.60).[14] For the current randomized controlled trial, we specifically tried to target younger, healthier women and those who were at higher risk for breast cancer, since this is the patient population that is more likely to benefit from chemoprevention. Current USPSTF and American Society of Clinical Oncology (ASCO) guidelines recommend targeting women who have a 5-year invasive breast cancer risk of 3% or higher for chemoprevention, a higher threshold compared to eligibility criteria in the chemoprevention trials since these women are more likely to have a favorable risk-benefit profile with SERMs and AIs.[7, 8] The mean 5-year invasive breast cancer risk in our study population was under 3%. Therefore, we may have been able to achieve higher chemoprevention uptake by targeting women at higher risk for breast cancer. For example, women with high-risk benign breast lesions, such as AH or LCIS derive up to a 70-80% relative risk reduction in breast cancer with SERMs and AIs.[2, 5, 6, 47, 48]
Although we did not observe a significant change in chemoprevention uptake at 6 months, we did observe significant improvement with short-term follow-up in decision antecedents, such as accurate breast cancer risk perceptions and adequate chemoprevention knowledge, and decision quality, with reduced decision conflict and increased informed choice. The chemoprevention trials of SERMs and AIs only demonstrated a reduction in breast cancer incidence among high-risk women, but no significant reduction in breast cancer-specific or all-cause mortality.[2-6] Due to this body of evidence, professional organizations recommend that clinicians engage in informed shared decision-making about chemoprevention with high-risk women, rather than prescribing SERMs and AIs to all eligible women.[7-9, 49] Engaging in shared decision-making about chemoprevention may be challenging due to a lack of knowledge about SERMs and AIs and time constraints during the clinical encounter among PCPs. During a National Cancer Institute, Division of Cancer Prevention Think Tank[50], specific emphasis was placed on risk-stratified cancer prevention and targeting high-risk women with AH and LCIS using “physician education or patient decision aids with a goal of achieving informed decision-making.” Therefore, improving chemoprevention informed choice may be a more appropriate measure than actual uptake of SERMs or AIs among high-risk women.
Strengths of our study include the relatively large sample size, the racially/ethnically diverse patient population, and rigorous randomized controlled study design with the use of well-validated measures. We did not specifically exclude high-risk women who were breast-feeding, taking oral contraceptives, or hormone replacement therapy and did not collect this information at baseline. However, we believe the number of these women enrolled would be small, since we were targeting women who were considering anti-estrogen therapy for chemoprevention at the time of enrollment. We did exclude the following patients from our analysis given that they are not candidates for chemoprevention: women with prior bilateral mastectomy, those who did not meet high-risk criteria, and those with active malignancy, Limitations of our study include the relatively short-term follow-up of 6 months, given that decisions about cancer prevention among high-risk individuals may require longer follow-up compared to treatment decisions among cancer patients. We conducted this trial at a single academic institution with access to a high-risk breast clinic, so our results may not be generalizable to other geographic or clinical settings. All of the healthcare providers at CUIMC, including those enrolled and not enrolled in our trial, had access to the BNAV decision support tool via the EHR, which may have dampened the effect of our study intervention. However, chemoprevention uptake in our control arm remained low at under 5%. We also had higher than anticipated loss to follow-up, particularly in the intervention arm, and the completion rate of RealRisks was under 80%. Although we tried to make the DA accessible to diverse patients, access to technology and poor computer literacy may have posed additional barriers to certain high-risk women. We also did not directly link patient access to RealRisks with scheduled clinic appointments with their providers, which may have further dampened the impact of the study intervention. Only 33% of patients had a clinical encounter scheduled with their healthcare provider before or within a month of the 6-month evaluation. In order to reinforce long-term compliance to chemoprevention for up to 5 years, a more sustainable patient intervention, such as automated reminders for patients to revisit the RealRisks decision aid prior to their clinic visit, which is integrated into clinic workflow may be necessary.
In a follow-up study (clinicaltrials.gov identifier, NCT04496739), we are conducting an ongoing cluster randomized controlled trial of RealRisks and BNAV among high-risk women with AH or LCIS and their healthcare providers, respectively. The primary outcome is patient-reported chemoprevention informed choice at 6 months and secondary endpoints include chemoprevention uptake and early discontinuation with up to 5 years of follow-up. The study intervention is also tied to a required 6-month clinical encounter, since patients are sent reminders to revisit RealRisks prior to their clinic visit with the enrolled healthcare provider. With cluster randomization at the site level, this trial will be able to assess the effectiveness and implementation of this multi-level intervention in the clinical setting.
In summary, we did not observe increased chemoprevention uptake with our web-based CDS tools, but we did find significant short-term improvement in chemoprevention informed choice and other decision measures. The persistent under-utilization of breast cancer chemoprevention is likely multifactorial due to: 1) lack of routine breast cancer risk assessment in the primary care setting; 2) lack of awareness of the risks and benefits of chemoprevention among high-risk patients and their providers; 3) lack of established breast cancer risk thresholds to maximize benefits and minimize harms of chemoprevention; 4) fear of adverse effects; and 5) lack of established surrogate biomarkers in the clinical setting to assess breast cancer risk and response to chemoprevention.[51-54] Addressing these barriers will likely require a multi-pronged approach, including: 1) incorporating more efficient breast cancer risk assessment using clinical data from the EHR[18]; 2) developing CDS tools for chemoprevention decision-making which are integrated into clinic workflow; 3) developing models to better predict the risks and benefits of SERMS and AIs with personalized risk-benefit profiles[55]; 4) developing novel dosing regimens (e.g., low-dose oral tamoxifen[46]) and routes of administration (e.g., topical preparations[56]) of established chemopreventive agents, as well as novel agents with favorable toxicity profiles in the prevention setting; 5) developing novel surrogate biomarkers which are modifiable with chemoprevention and predict long-term clinical outcomes. Future studies should focus on targeting specific high-risk populations for chemoprevention, such as women with AH or LCIS, and better integrating breast cancer risk assessment and CDS tools into clinic workflow via patient portals and EHRs.
Prevention Relevance.
In this randomized controlled trial of decision support for 300 high-risk women and 50 healthcare providers, we did not observe a significant increase in chemoprevention uptake, which remained low at under 5%. However, these decision support tools may increase knowledge and informed choice about breast cancer chemoprevention.
AKNOWLEDGEMENTS
This work was supported by the National Institutes of Health, National Cancer Institute R01CA177995 (K.D. Crew, R. Kukafka), R01CA226060 (K.D. Crew, R. Kukafka), P30 CA013696 (K.D. Crew), K01 CA241393 (T. Jones); National Center for Advancing Translational Sciences UL1 TR000040 (K.D. Crew); The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer Statistics, 2021. CA Cancer J Clin 2021, 71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A et al. : Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005, 97(22):1652–1662. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, Forbes JF, Investigators I-I: Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015, 16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN et al. : Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010, 3(6):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J et al. : Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011, 364(25):2381–2391. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S et al. : Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 2014, 383(9922):1041–1048. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HD, Smith ME, Griffin JC, Fu R: Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013, 158(8):604–614. [DOI] [PubMed] [Google Scholar]
- 8.Visvanathan K, Chlebowski RT, Hurley P, Col NF, Ropka M, Collyar D et al. : American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol 2009, 27(19):3235–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.nccn.org [Internet]. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Risk Reduction; cited January 31, 2022. Available from: http://www.nccn.org/professionals/>physician_gls/pdf/breast_risk.pdf [Google Scholar]
- 10.Ropka ME, Keim J, Philbrick JT: Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 2010, 28(18):3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH: Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst 2003, 95(7):526–532. [DOI] [PubMed] [Google Scholar]
- 12.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, Wieand HS: Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 1999, 91(18):1541–1548. [DOI] [PubMed] [Google Scholar]
- 13.Ravdin PM: The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila) 2010, 3(6):686–688. [DOI] [PubMed] [Google Scholar]
- 14.Kukafka R, Fang J, Vanegas A, Silverman T, Crew KD: Pilot study of decision support tools on breast cancer chemoprevention for high-risk women and healthcare providers in the primary care setting. BMC Med Inform Decis Mak 2018, 18(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crew KD, Silverman TB, Vanegas A, Trivedi MS, Dimond J, Mata J et al. : Study protocol: Randomized controlled trial of web-based decision support tools for high-risk women and healthcare providers to increase breast cancer chemoprevention. Contemp Clin Trials Commun 2019, 16:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuinness JE, Bhatkhande G, Amenta J, Silverman T, Mata J, Guzman A et al. : Strategies to Identify and Recruit Women at High Risk for Breast Cancer to a Randomized Controlled Trial of Web-based Decision Support Tools. Cancer Prev Res (Phila) 2022:OF1–OF8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuinness JE, Ueng W, Trivedi MS, Yi HS, David R, Vanegas A et al. : Factors Associated with False Positive Results on Screening Mammography in a Population of Predominantly Hispanic Women. Cancer Epidemiol Biomarkers Prev 2018, 27(4):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, McGuinness JE, Sin M, Silverman T, Kukafka R, Crew KD: Identifying Women at High Risk for Breast Cancer Using Data From the Electronic Health Record Compared With Self-Report. JCO Clin Cancer Inform 2019, 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi MS, Coe AM, Vanegas A, Kukafka R, Crew KD: Chemoprevention uptake among women with atypical hyperplasia and lobular and ductal carcinoma in situ. Cancer Prevention Research 2017, 10(8):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kukafka R, Yi H, Xiao T, Thomas P, Aguirre A, Smalletz C et al. : Why Breast Cancer Risk by the Numbers Is Not Enough: Evaluation of a Decision Aid in Multi-Ethnic, Low-Numerate Women. J Med Internet Res 2015, 17(7):e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J: Development of a brief test to measure functional health literacy. Patient Educ Couns 1999, 38(1):33–42. [DOI] [PubMed] [Google Scholar]
- 22.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM: Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making 2007, 27(5):672–680. [DOI] [PubMed] [Google Scholar]
- 23.Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ: Development of a short acculturation scale for Hispanics. Hispanic J Behav Sci 1987, 9:183–205. [Google Scholar]
- 24.Graves KD, Huerta E, Cullen J, Kaufman E, Sheppard V, Luta G et al. : Perceived risk of breast cancer among Latinas attending community clinics: risk comprehension and relationship with mammography adherence. Cancer Causes & Control 2008, 19(10):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy AG, Shea J, Williams SV, Quistberg A, Armstrong K: Measuring perceptions of breast cancer risk. Cancer Epidemiology and Prevention Biomarkers 2006, 15(10):1893–1898. [DOI] [PubMed] [Google Scholar]
- 26.Lerman C, Kash K, Stefanek M: Younger women at increased risk for breast cancer: perceived risk, psychological well-being, and surveillance behavior. Journal of the National Cancer Institute Monographs 1994(16):171–176. [PubMed] [Google Scholar]
- 27.Korfage IJ, Fuhrel-Forbis A, Ubel PA, Zikmund-Fisher BJ, Greene SM, McClure JB et al. : Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Research 2013, 15(5):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.decisionaid.ohri.ca [Internet]. O’Connor A: User manual-decision self-efficacy scale. Patient Decision Aids, Ottawa Hospital Research Institute (OHIR) 1995; cited 2015 Feb 4. Available from: https://decisionaid.ohri.ca/docs/develop/Tools/Decision_SelfEfficacy.pdf [Google Scholar]
- 29.O'Connor AM: Validation of a decisional conflict scale. Medical decision making 1995, 15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 30.Han PK, Joekes K, Elwyn G, Mazor KM, Thomson R, Sedgwick P et al. : Development and evaluation of a risk communication curriculum for medical students. Patient education and counseling 2014, 94(1):43–49. [DOI] [PubMed] [Google Scholar]
- 31.Fagerlin A, Dillard AJ, Smith DM, Zikmund-Fisher BJ, Pitsch R, McClure JB et al. : Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat 2011, 127(3):681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL: Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract 2011, 7(2):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan CP, Livaudais-Toman J, Tice JA, Kerlikowske K, Gregorich SE, Perez-Stable EJ et al. : A randomized, controlled trial to increase discussion of breast cancer in primary care. Cancer Epidemiol Biomarkers Prev 2014, 23(7):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivedi MS, Coe AM, Vanegas A, Kukafka R, Crew KD: Chemoprevention Uptake among Women with Atypical Hyperplasia and Lobular and Ductal Carcinoma In Situ. Cancer Prev Res (Phila) 2017, 10(8):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coe AM, Ueng W, Vargas JM, David R, Vanegas A, Infante K et al. : Usability Testing of a Web-Based Decision Aid for Breast Cancer Risk Assessment Among Multi-Ethnic Women. AMIA Annu Symp Proc 2016, 2016:411–420. [PMC free article] [PubMed] [Google Scholar]
- 36.Sprague BL, Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA: Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res Treat 2010, 124(2):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM: Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol 2004, 22(24):4951–4957. [DOI] [PubMed] [Google Scholar]
- 38.Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M: Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer 2004, 100(9):1800–1806. [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe KA, Snyder C, Seidel J, Hanna D, Lynch HT, Narod S: The use of preventive measures among healthy women who carry a BRCA1 or BRCA2 mutation. Fam Cancer 2005, 4(2):97–103. [DOI] [PubMed] [Google Scholar]
- 40.Salant T, Ganschow PS, Olopade OI, Lauderdale DS: "Why take it if you don't have anything?" breast cancer risk perceptions and prevention choices at a public hospital. J Gen Intern Med 2006, 21(7):779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldenberg VK, Seewaldt VL, Scott V, Bean GR, Broadwater G, Fabian C et al. : Atypia in random periareolar fine-needle aspiration affects the decision of women at high risk to take tamoxifen for breast cancer chemoprevention. Cancer Epidemiol Biomarkers Prev 2007, 16(5):1032–1034. [DOI] [PubMed] [Google Scholar]
- 42.Rondanina G, Puntoni M, Severi G, Varricchio C, Zunino A, Feroce I et al. : Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol 2008, 26(9):1537–1543. [DOI] [PubMed] [Google Scholar]
- 43.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R et al. : 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 44.Pokharel Y, Tang F, Jones PG, Nambi V, Bittner VA, Hira RS et al. : Adoption of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guideline in Cardiology Practices Nationwide. JAMA Cardiol 2017, 2(4):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crew KD: Addressing barriers to uptake of breast cancer chemoprevention for patients and providers. Am Soc Clin Oncol Educ Book 2015:e50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L et al. : Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol 2019, 37(19):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K: Atypical hyperplasia of the breast--risk assessment and management options. N Engl J Med 2015, 372(1):78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A et al. : Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015, 16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wise J: NICE recommends preventive drugs for breast cancer. Bmj 2013, 346:f4116. [DOI] [PubMed] [Google Scholar]
- 50.McCaskill-Stevens W, Pearson DC, Kramer BS, Ford LG, Lippman SM: Identifying and Creating the Next Generation of Community-Based Cancer Prevention Studies: Summary of a National Cancer Institute Think Tank. Cancer Prev Res (Phila) 2017, 10(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogel VG: The Burdens and Uncertainties of Doing What One Should Do. Cancer Prev Res (Phila) 2017, 10(8):431–433. [DOI] [PubMed] [Google Scholar]
- 52.Vogel VG: Breast cancer risk reduction: Notable achievements and remaining challenges. Breast J 2020, 26(1):86–91. [DOI] [PubMed] [Google Scholar]
- 53.Vogel VG: Implementation of Risk-reducing Strategies for Breast Cancer is Long Overdue. Cancer Prev Res (Phila) 2021, 14(1):1–4. [DOI] [PubMed] [Google Scholar]
- 54.Wickerham DL, Vogel VG: Breast cancer chemoprevention: the saga of underuse continues. J Natl Cancer Inst 2015, 107(1):399. [DOI] [PubMed] [Google Scholar]
- 55.Freedman AN, Yu B, Gail MH, Costantino JP, Graubard BI, Vogel VG et al. : Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol 2011, 29(17):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazzeroni M, Serrano D, Dunn BK, Heckman-Stoddard BM, Lee O, Khan S et al. : Oral low dose and topical tamoxifen for breast cancer prevention: modern approaches for an old drug. Breast Cancer Res 2012, 14(5):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.