Abstract
Adverse childhood experiences have been consistently linked with physical and mental health disorders in adulthood that may be mediated, in part, via the effects of such exposures on biological aging. Using recently developed “epigenetic clocks”, which provide an estimate of biological age, several studies have demonstrated a link between the cumulative exposure to childhood adversities and accelerated epigenetic aging. However, not all childhood adversities are equivalent and less is known about how distinct dimensions of childhood adversity relate to epigenetic aging metrics. Using two measures of childhood adversity exposure, we assess how the dimensions of Maltreatment and Household Dysfunction relate to epigenetic aging using two “second-generation” clocks, GrimAge and PhenoAge, in a cohort of unmedicated somatically healthy adults with moderate to severe major depression (n = 82). Our results demonstrate that the dimension of Maltreatment is associated with epigenetic age acceleration (EAA) using the PhenoAge but not the GrimAge clock. This association was observed using both the Childhood Trauma questionnaire (CTQ; β = 0.272, p = 0.013) and the Adverse Childhood Experiences (ACEs) questionnaire (β = 0.307, p = 0.005) and remained significant when adjusting for exposure to the dimension of Household Dysfunction (β = 0.322, p = 0.009). In contrast, the dimension of Household Dysfunction is associated with epigenetic age deceleration (β = −0.194, p = 0.083) which achieved significance after adjusting for exposure to the dimension of Maltreatment (β = −0.304, p = 0.022). This study is the first to investigate these effects among individuals with Major Depressive Disorder and suggests that these dimensions of adversity may be associated with disease via distinct biological mechanisms.
Subject terms: Epigenetics in the nervous system, Depression
Introduction
A wealth of evidence suggests that exposure to childhood adversity is associated with increased risk of chronic somatic disease [1–3], premature mortality [4], and psychopathology in adulthood [5, 6], including Major Depressive Disorder (MDD). While the biological mechanisms linking childhood adversity to MDD, morbidity [7, 8] and mortality have not been fully elucidated several studies have suggested that modulation of biological aging (as assessed by telomere length [9–12], mitochondrial function [13], and pubertal timing [14]), is one mechanism by which childhood adversity is embedded into the individual and contributes to both somatic and mental health consequences in adulthood.
More recently, studies utilizing “epigenetic clocks” [15–17] have demonstrated the link between exposure to childhood adversity and biological aging. To date, several DNA methylation-based epigenetic clocks have been developed using a variety of methods, with the “first-generation” clocks (Horvath [18] and Hannum [19]) trained to predict chronological age and the “second generation” clocks (PhenoAge [20] and GrimAge [21]) trained to predict health outcomes and time to death. The deviation between an individuals’ chronological age and epigenetic age can be used to calculate relative epigenetic age acceleration or epigenetic age deceleration and has been widely studied in a variety of somatic [22–26] and psychiatric conditions [11, 27–29] including studying the effects of childhood adversity [16, 30, 31].
Using the “cumulative risk” model [32, 33], which simply tallies the number of adversities experienced to create a cumulative risk score, several studies have demonstrated a link between the number of adversities experienced and epigenetic age acceleration. A more recent model, the “dimensional model” of adversity and psychopathology [32, 34–37] hypothesizes that individual adverse experiences may be condensed into dimensions of experience which share common pathophysiological mechanisms.
Historically, exposures assessed using the Adverse Childhood Experiences (ACEs) questionnaire (as we do in this study) have been grouped into two categories: Maltreatment and Household Dysfunction [38, 39] and later work confirmed these dimensions as explanatory factors [40]. These dimensions differentiate between exposures that are directed towards the child (Maltreatment; which includes all forms of abuse and neglect) versus those that affect the child “indirectly” via their environment (Household Dysfunction; including exposures such as living in a household with substance use, mental illness, etc.). While no studies to date have examined the relationship between these dimensions and biological aging metrics, some studies have suggested they may have unique patterns of association with psychiatric symptoms [41].
Several dimensions of adversity may exist and a model from McLaughlin and colleagues [32] hypothesized that adversity could be categorized into the dimensions of Threat (which includes exposure to abuse and reflects potential harm to the individual) and Deprivation (which includes experiences of physical/emotional neglect as well as food insecurity and cognitive deprivation early in life). Prior work by Sumner et al. [42] in a cohort of children/adolescents demonstrated that exposure to threat-related adversity was associated with accelerated epigenetic aging (using the Horvath clock) as well as pubertal stage [42]. In contrast, exposure to deprivation-related adversity had the opposite effect and was associated with decelerated biological aging (as measured by pubertal stage but not epigenetic aging).
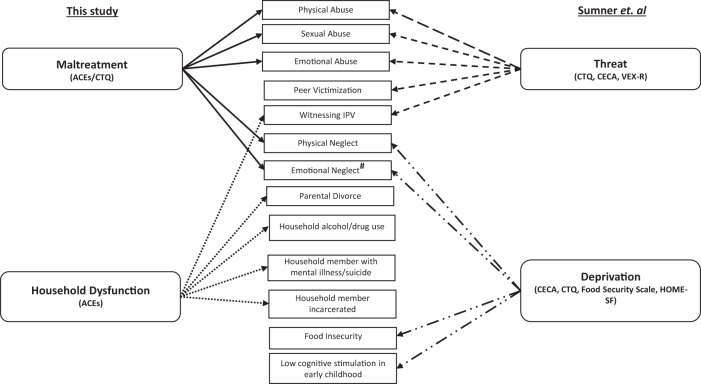
Recent work has demonstrated that exposure to childhood adversity is associated with greater epigenetic aging in adults with MDD compared to those without a history of such exposures [43]. However, there have been no studies to date which have specifically investigated how distinct dimensions of childhood adversity are associated with epigenetic aging in adults with MDD. While there are some overlapping features between the dimensions of Threat/Deprivation with Maltreatment/Household Dysfunction they are not identical (Fig. 1). We assess the dimensions of Maltreatment/Household Dysfunction in this study given the use of the CTQ and ACEs questionnaire from which we cannot re-create the dimensions of Threat/Deprivation. Here, we test the hypothesis that amongst adults with MDD, the dimensions of Maltreatment and Household Dysfunction differentially affect biological aging as measured by two “second-generation” epigenetic clocks, PhenoAge and GrimAge, which are capable of capturing variations in the risk of disease and death.
Fig. 1. Comparison of dimensions of childhood adversity.
Here we show dimensions of adversity assessed in this study (Maltreatment/Household Dysfunction) compared to the dimensions of Threat/Deprivation as assessed by Sumner et al. In parentheses, we show the scales utilized to create these composite scores. Individual items incorporated into each of these domains are shown in the center with arrows indicating to which dimensions they belong. Threat and Deprivation have been assessed using a multi-modal approach, while our study assessed exposures with the commonly utilized Childhood Trauma Questionnaire (CTQ) and Adverse Childhood Experiences (ACEs) questionnaire. We observed that Threat and Maltreatment are highly overlapping with the major differences being inclusion of neglect items and exclusion of witnessing IPV item into the dimension of Maltreatment. Peer victimization (PV) was not assessed in this study as we did not utilize the Violence Exposure Scale-Revised (VEX-R) which provides a measure of different types of peer victimizations experiences. Household Dysfunction and Deprivation were assessed utilizing different scales. #Emotional neglect was assessed in distinct ways between these studies. Specifically, the ACEs/CTQ assesses subjective appraisals of emotional neglect. In prior studies which utilized the Threat/Deprivation framework, emotional neglect was specifically assessed using the Childhood Experiences of Care and Abuse (CECA) which assesses neglectful behaviors (rather than an appraisal of the distant experience of neglect). Cognitive stimulation was assessed using Home Observation Measurement of the Environment (HOME-SF).
Methods
Ethics statement
This research was approved by the Institutional Review Board of the University of California, San Francisco.
Recruitment procedures and study participants
One hundred subjects with MDD were recruited by flyers, bulletin board notices, Craigslist postings, newspaper ads, and clinical referrals and informed consent was obtained from all subjects. All subjects were diagnosed with MDD without psychotic symptoms according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID), which was the version in use at the beginning of this study, and diagnosis was verified by clinical interview with a board-certified psychiatrist. Depressive symptomatology was evaluated with the 17-item Hamilton Score for Depression Rating Scale (HDRS), with a current score of ≥17 being an inclusion criterion. Depressed subjects were excluded for presence of the following: bipolar disorders, psychotic symptoms, history of psychosis outside of a mood episode, any eating disorder or post-traumatic stress disorder (PTSD) within one month of entering the study, and substance abuse or dependence (including alcohol) within six months of entering the study. The study participants had no acute illnesses/infections, chronic inflammatory disorders, neurological disorders, or other major medical condition. All subjects were free of psychotropic medications, including antidepressants, and other potentially interfering medications and had not had any vaccinations for at least 6 weeks prior to enrollment in the study, and none was taking vitamin supplements above the US recommended daily allowances. Short-acting sedative-hypnotics were allowed as needed for sleep up to a maximum of 3 times per week, but none within 1 week prior to blood draws. Prior to each study visit, all subjects had to pass a urine toxicology screen for drugs of abuse and a urine test for pregnancy for women of child-bearing potential.
DNA preparation and analysis of methylation
Blood samples were drawn in the morning following an overnight fast. Whole blood was collected in acid citrate dextrose tubes for preparation of DNA. Aliquoted samples were stored frozen at −80 °C until use. DNA was extracted from whole blood using QIAmp DNA purification kits (Qiagen, Redwood City, CA), followed by quality check using a Tapestation (Agilent). Identification and analysis of methylated CpGs used protocols used by our group previously [44]. Genomic DNA (500 ng) was treated with sodium bisulfite using the Zymo EZ96 DNA Methylation Kit (Zymo Research, Orange, CA, USA), and genome-wide DNA methylation patterns were profiled using the Infinium HumanMethylation450 BeadChip array (Illumina, Inc., San Diego, CA, USA). Noob background correction [45] was used to pre-process the data prior to submitting it to the DNAm Age website https://dnamage.genetics.ucla.edu for analysis [18].
Childhood exposure to adversity
Early life adversity was measured using two scales, the Childhood Trauma Questionnaire (CTQ) [46] and the ACE-10 questionnaire [47]. The CTQ is a validated self-report questionnaire that assesses five types of maltreatment between birth and 18 years: sexual, physical, and emotional abuse as well as emotional and physical neglect [46]. Items are rated on a 5-point scale from “Never True” to “Very Often True”. In order to assess the effects of types of maltreatment we created a composite Abusive Maltreatment score (sum of scores on sexual, physical, and emotional abuse subscales) and a composite Neglectful Maltreatment score (sum of scores on physical and emotional neglect subscales). A total All Maltreatment (total CTQ) score was created by summing the Abusive Maltreatment score and the Neglectful Maltreatment score [48]. The ACE scale was also used to assess early life adversity, where items are rated based on their absence (score = 0) or presence (score = 1). This scale consists of 10 items: five items for subtypes of maltreatment (physical, sexual, or emotional abuse, physical or emotional neglect which is similar to the total CTQ score) and five items measuring a distinct domain of experience, not assessed by the CTQ, referred to as household dysfunction (parental separation or divorce, witnessing domestic violence, and incarceration, substance abuse, or mental illness of a household member) occurring between birth and 18 years. We create two composite scores based on prior studies [40, 49] which found a two-factor solution for the ACEs survey: Maltreatment, and Household Dysfunction. A total Maltreatment score (ACEs) was created by summing the first five items (physical, sexual, and emotional abuse as well as emotional and physical neglect) which is similar to the All Maltreatment score (Total CTQ score). A total Household Dysfunction score was created by summing the last five items (parental separation, incarceration, mental illness, witnessing IPV, and substance use) which is not assessed by the CTQ. A Total ACE score was created by summing the Maltreatment score and Household Dysfunction score (sum of all 10 items on the ACEs questionnaire). We utilized the CTQ as well as the ACE in our analyses for several reasons. First, the CTQ has been previously used in other studies to assess the effect of Maltreatment exposure [50] as well as abuse versus neglect [43, 48] (both forms of maltreatment) and we aimed to determine whether we could reproduce in MDD participants the results previously identified in non-depressed participants. Additionally, we include the ACEs questionnaire which not only assesses the domain of Maltreatment (identical to the exposures assessed in the CTQ), but also assesses the unique dimension of Household Dysfunction. Prior studies have suggested that these distinct domains can lead to specific kinds of psychopathology [51, 52], which may be mediated in part via distinct pathophysiological mechanisms. Creation of other composite scores is detailed in the Supplementary Material.
Statistical analysis
Of the original 100 subjects with MDD recruited, four were excluded due to missing data for CTQ, ten were excluded due to lack of data on epigenetic aging measures (not assayed), two were excluded as they had initiated antidepressant treatment prior to their blood draw, one was excluded due to lack of data on household income (used as a covariate), and one was excluded due to missing data on smoking habits (used as a covariate; n = 82). Additionally, one subject was excluded in our analysis using the ACEs survey due to missing data on ACEs exposures (n = 81). PhenoAge and GrimAge acceleration—defined as the residuals resulting from regressing PhenoAge and GrimAge on chronological age (AgeAccelPheno and AgeAccelGrim, respectively)—were calculated for all participants. DNA methylation assays (described above) were carried out in two batches and epigenetic age acceleration variables were calculated within each batch. Linear regression models were used to determine the associations between the epigenetic clocks and All Maltreatment score as well as Abusive Maltreatment and Neglectful Maltreatment score. Separate linear regression models were used to identify associations between the epigenetic clocks and total ACE score, as well as Maltreatment and Household Dysfunction scores. Several a priori covariates were included in sequentially adjusted linear regression models including gender, race/ethnicity (White, Black, Latino, Other), BMI, smoking status (never, former, or current), household income (≤$49 000, $50 000-$99 999, $100 000-$200 000, or ≥$200 000) [53] and DNAm-based estimates of blood cell composition. Specifically, the DNAm-based estimates of blood cell composition included [54, 55] naïve CD8 T cells, CD8pCD28nCD45Ran “exhausted T cells”, B cells, CD4 T cells, NK cells, Monocytes, and Granulocytes (as calculated by the DNAm Age website [18]). In addition, to assess the unique associations between the domains of adversity and biological aging, we controlled for the other domains of adversity for each exposure. Specifically, for our analysis examining the relationship between Abusive Maltreatment and epigenetic aging we include Neglectful Maltreatment in the model and vice versa. In our analysis of the relationship between Maltreatment score (ACEs) and epigenetic aging, we include Household Dysfunction in the model and vice-versa. All data were assessed for normality prior to analyses and Blom transformation [56] was applied to AgeAccelGrim to achieve normality. Inspection of the data revealed that one value for AgeAccelPheno was approximately three standard deviations away from the mean AgeAccelPheno. To reduce disproportionate influence on the analysis of this point, the dataset was winsorized (using R package DescTools) and winsorized models are presented in our sensitivity analysis. Results did not differ appreciably from those of nonwinsorized data. We first carried out analyses with CTQ given that prior studies have examined associations between this measure of adversity and the “second-generation” clocks [48, 50] and subsequently assessed associations with the ACEs questionnaire.
Results
Participant characteristics
Our final sample included 82 individuals with MDD who had data on early life adversity (as measured by the CTQ) and both epigenetic age clocks and demographic variables used as covariates. One of these subjects was missing ACE data, leaving 81 subjects for those analyses; The mean age of the sample was 36.9 years and was 53% female. Additional participant characteristics are noted in Table 1.
Table 1.
Participant characteristics.
| Sociodemographic characteristics | |
| Age | 37.1 (13.1) [20–68] |
| Female sex [n(%)] | 44 (53.7) |
| Race/Ethnicity | |
| White | 42 (51.2) |
| Black | 8 (9.8) |
| Latino | 8 (9.8) |
| Other | 24 (29.3) |
| Income [n(%)] | |
| ≤49,000 | 54 (65.9) |
| 50,000–99,999 | 15 (18.3) |
| 100,000–199,999 | 11 (13.4) |
| ≥200,000 | 2 (0.024) |
| Lifestyle characteristics | |
| Body Mass Index | 25.5 (4.2) [18.8–36.3] |
| Smoking History [n(%)] | |
| Never | 44 (53.7) |
| Former | 22 (26.8) |
| Current | 16 (19.5) |
| Characterization of Major Depressive Disorder | |
| Depressive Symptoms Score (IDS) | 25.1 (5.31) [12–37] |
| Hamilton Depression Rating Sclae (HDRS) | 19.8 (3.28) [13–34] |
| Duration of Current Depressive Episode (days) | 2258.3 (3663.8) [24–18536] |
| Chronicity of Lifetime Depression (months) | 154.7 (145.8) [3.41–609] |
| Number of Depressive Episodes | 3.59 (2.68) [1–12] |
| Lifetime Days of Depression | 4707.8 (4438.5) [104–18536.1] |
| Characterization of Adversity Exposure | |
| Childhood Trauma Questionnaire | |
| All Maltreatment Score (Total CTQ) | 42.5 (12.4) [25–82] |
| Physical Abuse | 6.9 (2.3) [5–15] |
| Emotional Abuse | 10.4 (4.3) [5–25] |
| Sexual Abuse | 6.3 (3.5) [5–23] |
| Physical Neglect | 6.9 (2.6) [5–15] |
| Emotional Neglect | 12.1 (4.9) [5–23] |
| Adverse Childhood Experiences Survey | |
| Total ACE | 2.8 (2.2) [0–10] |
| Maltreatment Score | 1.4 (1.5) [0–5] |
| Yes | 53 (65.4) |
| No | 28 (34.6) |
| Household Dysfunction Score | 1.4 (1.3) [0–5] |
| Yes | 57 (70.3) |
| No | 24 (29.7) |
Mean (sd) [range] presented unless otherwise indicated.
Abusive maltreatment but not neglectful maltreatment is associated with accelerated epigenetic aging
Using the CTQ, we examined the relationship between the All Maltreatment score (total CTQ score) and accelerated epigenetic aging, as has been previously done [42, 43, 48, 57]. We observed that this All Maltreatment score (which reflects exposure to all forms of abuse and neglect) was positively associated with AgeAccelPheno (β = 0.272 p = 0.013) but not AgeAccelGrim (β = 0.007 p = 0.949) (Table 2). This All Maltreatment score (total CTQ score) was not associated with depression severity (β = −0.053 p = 0.638). Based on significant results between the All Maltreatment score and PhenoAge but not GrimAge, we examined AgeAccelPheno as our primary outcome but present the regression results for AgeAccelGrim for completeness. We next assessed whether Abusive Maltreatment and Neglectful Maltreatment were differentially associated with epigenetic aging. Notably, we observed that Abusive Maltreatment was positively associated with AgeAccelPheno (β = 0.288 p = 0.009) (Table 2) which survived adjustment for several covariates including blood cell composition. In contrast, Neglectful Maltreatment was not associated with accelerated epigenetic aging as measured by either clock (β = 0.187 p = 0.093). (Table 2; Scatterplots are shown in Supplementary Fig. 1), even after adjustment for several covariates. We observed that Abusive Maltreatment and Neglectful Maltreatment scores were significantly correlated with each other (r = 0.570, p < 0.001), consistent with co-occurrence of adversity types. Given this co-occurrence, we evaluated the specific effects of these composite scores by including both forms of adversity in the model. The effect size for the Abusive Maltreatment score was somewhat reduced when controlling for co-occurring Neglectful Maltreatment score (β = 0.265 p = 0.068), while the effect size for the Neglectful Maltreatment score was greatly reduced when controlling for the Abusive Maltreatment score (β = 0.050 p = 0.709), suggesting that abuse was the main contributor to the association between the All Maltreatment score and accelerated epigenetic aging.
Table 2.
Associations of Maltreatment (CTQ) with biological aging.
| AgeAccelPheno | AgeAccelGrim | |||||
|---|---|---|---|---|---|---|
| b (95% CI) | β | p-value | b (95% CI) | β | p-value | |
| All Maltreatment | ||||||
| Model 1 | 0.12 [0.026, 0.214] | 0.272 | 0.013 | 0.0006 [−0.017, 0.018] | 0.007 | 0.949 |
| Model 2 | 0.122 [0.017, 0.226] | 0.277 | 0.023 | 0.0008 [−0.016, 0.017] | 0.011 | 0.92 |
| Abusive Maltreatment | ||||||
| Model 1 | 0.208 [0.054, 0.362] | 0.288 | 0.009 | 0.008 [−0.021, 0.037] | 0.061 | 0.586 |
| Model 2 | 0.212 [0.038, 0.386] | 0.293 | 0.018 | 0.003 [−0.024, 0.031] | 0.025 | 0.816 |
| Model 3a | 0.192 [−0.014, 0.398] | 0.265 | 0.068 | 0.005 [−0.028, 0.038] | 0.0398 | 0.752 |
| Neglectful Maltreatment | ||||||
| Model 1 | 0.157 [−0.027, 0.341] | 0.187 | 0.093 | −0.009 [−0.042, 0.025] | −0.057 | 0.61 |
| Model 2 | 0.153 [−0.042, 0.349] | 0.182 | 0.122 | −0.001 [−0.031, 0.029] | −0.007 | 0.943 |
| Model 3b | 0.042 [−0.184, 0.269] | 0.05 | 0.709 | −0.004 [−0.040, 0.032] | −0.027 | 0.821 |
Model 1. Unadjusted model.
Model 2. Adjusted for gender, BMI, smoking status, race, household income and DNAm-based markers of immune cell composition.
aModel 3. Model 2 further adjusted for Neglectful Maltreatment score.
bModel 3. Model 2 further adjusted for Abusive Maltreatment score.
Household dysfunction is associated with decelerated epigenetic aging
Next, we utilized the ACEs questionnaire to assess the effects Maltreatment (identical to the total CTQ score) and Household Dysfunction on epigenetic aging [58]. First, we assessed the relationship between the Total ACE score (which combines Maltreatment and Household Dysfunction scores) and epigenetic aging (Table 3). Total ACE score was not significantly related to either AgeAccelPheno (β = 0.091 p = 0.421) or AgeAccelGrim (β = 0.032 p = 0.778).
Table 3.
Associations of ACE scores (Total, Maltreatment, Household Dysfunction) with Biological Aging.
| AgeAccelPheno | AgeAccelGrim | |||||
|---|---|---|---|---|---|---|
| b (95% CI) | β | p-value | b (95% CI) | β | p-value | |
| Total ACE score | ||||||
| Model 1 | 0.230 [−0.336, 0.797] | 0.091 | 0.421 | 0.015 [−0.088, 0.117] | 0.032 | 0.778 |
| Model 2 | 0.165 [−0.520, 0.850] | 0.065 | 0.632 | −0.020 [−0.122, 0.082] | −0.044 | 0.697 |
| Maltreatment | ||||||
| Model 1 | 1.16 [0.353, 1.96] | 0.307 | 0.005 | .0005 [−0.147, 0.157] | 0.008 | 0.944 |
| Model 2 | 1.05 [0.123, 1.97] | 0.277 | 0.027 | −0.04 [−0.185, 0.099] | −0.063 | 0.552 |
| Model 3a | 1.21 [0.309, 2.12] | 0.322 | 0.009 | −0.044 [−0.190, 0.101] | −0.065 | 0.544 |
| Household Dysfunction | ||||||
| Model 1 | −0.820 [−1.75, 0.111] | −0.194 | 0.083 | 0.034 [−0.137, 0.204] | 0.044 | 0.697 |
| Model 2 | −1.06 [−2.18, 0.071] | −0.249 | 0.066 | 0.005 [−0.167, 0.178] | 0.007 | 0.95 |
| Model 3b | −1.29 [−2.38, −0.194] | −0.304 | 0.022 | 0.014 [−0.161, 0.189] | 0.018 | 0.875 |
Note the opposite direction of association between epigenetic age acceleration and maltreatment or household dysfunction score.
Model 1. Unadjusted model.
Model 2. Adjusted for gender, BMI, smoking status, race, household income and DNAm-based markers of immune cell composition.
aModel 3. Model 2 further adjusted for Household Dysfunction score.
bModel 3. Model 2 further adjusted for Maltreatment score.
Most individuals (n = 37) endorsed both Maltreatment and Household Dysfunction experiences, while a smaller number endorsed only Maltreatment experiences (n = 16) or only Household Dysfunction experiences (n = 20) and a minority reporting no exposures at all (n = 8). Similar to the All Maltreatment score (total CTQ score), the ACEs Maltreatment score was positively associated with AgeAccelPheno (β = 0.307 p = 0.005) but not AgeAccelGrim (β = 0.008 p = 0.944) (Table 3; Scatterplots are shown in Supplementary Fig. 2). Maltreatment measures (CTQ and ACEs) were highly correlated (r = 0.701 p < 0.0001). Given the co-occurrence of Maltreatment and Household Dysfunction (r = 0.230, p = 0.039), we controlled for the dimension of Household Dysfunction and observed that the relationship between the ACEs Maltreatment score and AgeAccelPheno remained significant. Further analyses revealed that depressed individuals with an ACEs Maltreatment score of one or more (“Any”) showed greater epigenetic age acceleration as measured by AgeAccelPheno, than individuals who had a score of zero (“None”; p = 0.014, Cohen’s d = 0.6; Supplementary Table 1). This remained significant after controlling for the co-occurring Household Dysfunction score.
In contrast, the Household Dysfunction score was negatively associated with AgeAccelPheno after controlling for the effects of co-occurring Maltreatment (β = −0.304 p = 0.022), suggesting that Household Dysfunction is associated with decelerated epigenetic aging and may have negated the effect of Maltreatment when examining the Total ACE score (Table 3). Further analyses revealed that individuals with a Household Dysfunction score of one or greater (“Any”) showed reduced epigenetic age acceleration compared to individuals with a score of zero (“None”; p = 0.008, Cohen’s d = 0.6; Supplementary Table 2). These group differences persisted after controlling for co-occurring Maltreatment score. No significant group differences were observed with AgeAccelGrim (data not shown).
While we utilized the validated two-factor solution of Maltreatment and Household Dysfunction to identify differential associations with epigenetic aging, some studies have suggested that witnessing interpersonal violence (IPV) loads on to the dimension of Maltreatment rather than Household Dysfunction [49, 59]. We created a modified Maltreatment score which included IPV and removed it from the Household Dysfunction score (Supplementary Methods). The modified Maltreatment score was positively associated with AgeAccelPheno after controlling for the modified Household Dysfunction score (β = 0.317 p = 0.013). Additionally, the modified Household Dysfunction score was negatively associated with AgeAccelPheno after controlling for the modified Maltreatment score (β = −0.327 p = 0.012; Supplementary Table 3). The effect sizes were larger using the modified Household Dysfunction score, compared to the initial model [45, 56].
Associations between childhood adversity and first-generation epigenetic clocks
Given that exposure to childhood adversity has been associated with premature morbidity/mortality [60], our primary analyses examined the association between these exposures and the “second generation” clocks. However, we also explored associations between childhood adversity and two “first-generation” epigenetic clocks (Horvath and Hannum). We found no significant associations between Maltreatment scores (CTQ and ACE) or Household Dysfunction scores and epigenetic age acceleration with either clock (Supplementary Table 4 and Supplementary Table 5).
Sensitivity analysis
To ensure that our results were not due to the undue influence of potential outliers, winsorized models are also presented. We continue to observe that the Abusive Maltreatment score was associated with accelerated epigenetic aging as measured by AgeAccelPheno while the Neglectful Maltreatment score showed no association (Supplementary Table 6, Supplementary Table 7, Supplementary Fig. 3). Additionally, we continue to see that the Household Dysfunction score was associated with decelerated epigenetic aging as measured by AgeAccelPheno (Supplementary Table 8, Supplementary Table 9, Supplementary Fig. 4). Group differences between depressed individuals exposed to Maltreatment or Household Dysfunction compared to non-exposed depressed individuals persisted in the winsorized models (Supplementary Table 10 and Supplementary Table 11).
Discussion
Exposure to childhood adversity has been associated with the development of physical [61] and mental health issues in adulthood [6, 62, 63] and may be mediated, in part, via effects on biological aging. Here, we assess how different dimensions of childhood adversity are associated with epigenetic aging amongst unmedicated somatically healthy individuals with moderate to severe MDD using two recently developed metrics of epigenetic aging-PhenoAge and GrimAge. We demonstrate that greater experiences of maltreatment (driven by the abusive, but not neglectful, forms of maltreatment) are associated with accelerated epigenetic aging (as measured by PhenoAge but not GrimAge) in adulthood. In contrast, the experience of household dysfunction is associated with decelerated epigenetic aging, as measured by the PhenoAge clock. These results provide support for the idea that childhood adversity encompasses distinct dimensions of experience that may be associated with vulnerability to disease via unique biological mechanisms.
To date, a single study has examined the association of childhood adversity exposure with epigenetic aging in adults with Major Depressive Disorder. The work by Han et al. [43] utilized an epigenetic clock developed to gauge chronological age to demonstrate that amongst adults with MDD, exposure to childhood trauma was associated with greater epigenetic aging. Our results build on these findings to demonstrate that in addition to “dose,” the “type” of adversity is an important factor which has distinct associations with epigenetic aging. Furthermore, we demonstrate these associations using a “second-generation” clock which stands out for its ability to capture biological disturbances associated with aging. Although we focus here on these “second-generation” clocks, we did explore associations between childhood adversity and “first-generation” clocks (Horvath and Hannum clocks) and found no significant associations between adversity measures and epigenetic aging. Possible reasons for the lack of association are discussed further below.
Our work adds to a body of literature studying the effects of childhood adversity on epigenetic aging in adults without psychiatric diagnoses. Our results provide further support for a dimensional approach in studying the effects of childhood adversity on outcomes later in life. Recently, McCrory et al. [64] demonstrated that childhood adversity (specifically poverty) resulted in approximately 1.2 to 2 years accelerated epigenetic aging as measured by the GrimAge and Pace of Aging clock, although it is unclear how their assessment of childhood poverty relates to our measure of Household Dysfunction. Additionally, Hamlat et al. [48] demonstrated that childhood adversity (measured by the CTQ) was associated with accelerated epigenetic aging using the GrimAge clock (but not with the PhenoAge, Horvath, or Hannum clocks). Similar to our own results (although with GrimAge and not PhenoAge), they demonstrated that this association was driven by the experience of abuse rather than neglect. Reasons for the differential associations with epigenetic clocks are discussed below.
The 10 ACEs assessed in our study have typically been conceptualized into the two constructs of Maltreatment and Household Dysfunction [38, 39] and this factor structure has been confirmed in large scale studies [40]. While these types of experiences differ on the basis of whether they are “directly” experienced (Maltreatment) or “indirectly” experienced (Household Dysfunction), how this might lead to opposite patterns of association with epigenetic aging is unclear. We can speculate that directly experienced adversities represent exposures which are more endangering to the individual while those which are indirectly experienced (i.e., household member incarceration, drug use, mental illness, etc.) may reflect limited access to resources. These findings can be understood in the context of life history theory [65, 66] which suggests that the effects of these exposures on aging are evolutionary adaptations. Those which directly affect and thus endanger the individual (Maltreatment) results in accelerated aging as a way of prioritizing reproduction. In contrast, those that are indirectly experienced in the environment of the individual and are associated with limited access to resources and care (Household Dysfunction) results in decelerated aging in order to delay reproduction until favorable conditions arise.
While the biological underpinnings of this differential association are not clear, studies looking at outcomes have shown that these exposures may have differential associations with psychiatric symptoms [41, 52]. These findings suggest that these exposures could alter unique biological pathways. Notably, the work of Ridout et al. [67] has attempted to understand how exposure to resource poor conditions and disruption of the maternal-infant bond (which we hypothesize resembles the dimension of Household Dysfunction) might contribute to decelerated aging. Using a non-human primate model, they demonstrate that exposure to such conditions in childhood resulted in longer telomeres in adulthood, which may be connected via increased production of Glucagon-Like Peptide-1 (GLP-1). Additional studies are needed to better understand the biological basis for the differential effects of these exposures.
Given that we cannot create true Threat/Deprivation scores given the use of the CTQ/ACEs questionnaire, our results are not directly comparable to the findings of Sumner et al. [42]. However, the pattern of associations observed with Maltreatment and Household Dysfunction mirrors the findings observed with the dimensions of Threat and Deprivation [32, 34–36, 42, 68]. Given this, our results suggest that Maltreatment reflects threat-related exposure and is associated with accelerated epigenetic aging. While the Maltreatment score incorporates some deprivation-related exposures (subjective recall of emotional and physical neglect), our results suggest that the associations between Maltreatment and accelerated epigenetic aging is driven by Abusive Maltreatment rather than Neglectful Maltreatment and thus shows an association with aging consistent with a threat-related exposure. The lack of an expected association between Neglectful Maltreatment and epigenetic aging may be due to the possibility that these items (emotional/physical neglect) do not capture the full range of deprivation experiences. Prior studies examining the experience of deprivation included not only physical/emotional neglect but also food insecurity and low cognitive stimulation.
We hypothesize that the experiences captured by the Household Dysfunction score more fully captures deprivation-related experiences. This score assesses experiences such as living in a home with household members experiencing substance use, incarceration, divorce, etc. and may reflect early environments in which there is a lack of expected inputs, neglect, as well as limited cognitive stimulation early in life [36]. Notably, when we removed the IPV item from the Household Dysfunction score and incorporated this into the Maltreatment score, we observed larger effect sizes. Witnessing IPV has been conceptualized as a threatening experience and this finding further supports the idea that Household Dysfunction reflects deprivation based exposures. Unfortunately, we were unable to create Threat and Deprivation scores due to methodological differences in how adversity was measured [35, 42, 69, 70].
While a wealth of evidence has shown associations between childhood adversity and epigenetic aging amongst a variety of populations, no single epigenetic clock has shown a consistent association with childhood adversity exposure. We hypothesize that this is related to differences in the study populations assessed, assessments of childhood adversity used, as well as the clocks reported on in each study. We note that Han et. al. examined associations between trauma and epigenetic aging in a community-based sample (distinct from our depressed cohort) and utilized a clock of their own derivation (distinct from the method employed in this study). While our results show associations between the All Maltreatment (Total CTQ) score and PhenoAge, we note that Hamlat et al. observed similar associations with GrimAge. While speculative, this could be because their study specifically examined aging in a cohort of stressed female caregivers without MDD whose overall trauma exposure was lower than that of the depressed participants in this study. Additionally, their cohort of subjects was observed to be older than the average age of individuals in the current study and not necessarily medication free. Furthermore, our results contrast from those of the TILDA study which found associations between childhood poverty and the GrimAge clock. We note that this study assessed these markers in individuals aged 50–87 years old. While it is not clear how this would translate into associations with different clocks, we note that each clock is unique with regards to their derivation methods and it has been noted that there is little overlap in methylation sites contained within the epigenetic clocks [20, 71]. It is conceivable that the experience of adversity may differentially affect aging processes captured by these methylation sites in depressed versus non-depressed populations and with varying degrees of adversity. Additionally, it is possible that the associations observed with GrimAge in these studies is related to the advanced age and/or possible somatic dysfunction/medical co-morbidity of these subjects. Thus, changes in epigenetic aging may be better captured by the GrimAge clock in these cohorts given its superior ability to predict time to death and may be poorly suited to the younger, medically healthy cohort utilized in this study.
Strengths of our study include the use of rigorously diagnosed, moderate to severely depressed individuals. We also note that our sample is unique in that it specifically recruited individuals who were not treated with medications (including antidepressants), which may exert epigenetic effects and confound epigenetic age assessments [72]. Additionally, our study incorporated only somatically healthy individuals with MDD which limits the effect of medical co-morbidity on epigenetic age assessment.
Our study has several limitations including our modest sample size and predominantly Caucasian sample, which requires our results to be validated in a larger, more diverse cohorts. Additionally, given our retrospective assessment of childhood adversity, we cannot assess the timing or duration of the reported exposures. Our study was unable to account for childhood SES which may be an important covariate. While SES may be associated with increased risk for experiencing threat and deprivation, it may also be associated with other unknown toxic exposures that may also contribute to altered epigenetic aging. Furthermore, we note that these results are specific to individuals with MDD and studies in other populations will be needed. In addition, we do not assess any psychological, behavioral, or biological mediators which may mediate the effects of childhood adversity on epigenetic aging. Given methodological differences, we cannot assess the dimensions of Threat and Deprivation as prior studies have and future studies should utilize a multi-modal approach to measurement of childhood adversity to create multiple dimensions of adversity and understand the relationships between these dimensions.
Taken together, these findings demonstrate that amongst healthy, unmedicated individuals with Major Depressive Disorder, dimensions of adversity can become embedded into the host in distinct ways that predict biological aging. Specifically, experiences of maltreatment (driven by abuse) contribute to accelerated epigenetic aging while experiences of household dysfunction contribute to decelerated epigenetic aging. Future studies should further investigate how these distinct dimensions of adversity differentially affect epigenetic modifications to identify biological pathways that are affected. A greater appreciation of dimensions of adversity will provide greater understanding of the mechanisms by which childhood adversity is embedded into the individual and guide the development of novel interventions to limit the long-lasting consequences of childhood adversity.
Supplementary information
Acknowledgements
The authors acknowledge the assistance of Phuong Hong, Stacy Ann Miller, Elissa Hamlat, Ph.D., the UCSF CTSI Clinical Research Center staff, the PNE Lab research assistants, volunteers and research participants. This study was funded by grants from the Institute of Mental Health (NIMH) (R01-MH083784), the Tinberg family, UCSF Academic Senate, and UCSF Research Evaluation and Allocation Committee. This project was also supported by National Institutes of Health/National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI Grant UL1 RR024131 and TL-1 TR001871. Clinical Trial Registry Number (NCT00285935). None of the funding agencies had a role in the design/conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for the protection of human subjects as prescribed in AR 70–25.
Author contributions
RR, OW, SH conceptualized the project. RR, EP performed data analysis. RR produced figures/tables and wrote manuscript. All authors contributed to and edited the manuscript.
Code availability
R code available by contacting the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-022-02198-0.
References
- 1.Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, et al. Childhood adversity and adult chronic disease an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48:345–9. doi: 10.1016/j.amepre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer T, Ulke C, Beutel M, Binder H, Brähler E, Johar H, et al. The relation between childhood adversity and adult obesity in a population-based study in women and men. Sci Rep. 2021;11:14068. doi: 10.1038/s41598-021-93242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deschênes SS, Kivimaki M, Schmitz N. Adverse childhood experiences and the risk of coronary heart disease in adulthood: examining potential psychological, biological, and behavioral mediators in the Whitehall II Cohort Study. J Am Heart Assoc. 2020;10:e019013. doi: 10.1161/JAHA.120.019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rod NH, Bengtsson J, Budtz-Jørgensen E, Clipet-Jensen C, Taylor-Robinson D, Andersen A-MN, et al. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. Lancet. 2020;396:489–97. doi: 10.1016/S0140-6736(20)30621-8. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiat. 2012;69:1151–60. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101–19. doi: 10.1017/S0033291797005588. [DOI] [PubMed] [Google Scholar]
- 7.Bomhof-Roordink H, Seldenrijk A, Hout HPJ, van, Marwijk HWJ, van, Diamant M, Penninx BWJH. Associations between life stress and subclinical cardiovascular disease are partly mediated by depressive and anxiety symptoms. J Psychosom Res. 2015;78:332–9. doi: 10.1016/j.jpsychores.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 8.van Reedt Dortland AKB, Giltay EJ, van Veen T, Zitman FG, Penninx BWJH. Personality traits and childhood trauma as correlates of metabolic risk factors: The Netherlands Study of Depression and Anxiety (NESDA) Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;36:85–91. doi: 10.1016/j.pnpbp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiat. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, et al. Early life adversity and telomere length: a meta-analysis. Mol Psychiatr. 2018;23:858–71. doi: 10.1038/mp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BWJH. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatr. 2014;19:895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 12.Verhoeven JE, Oppen P, van, Puterman E, Elzinga B, Penninx BWJH. The association of early and recent psychosocial life stress with leukocyte telomere length. Psychosom Med. 2015;77:882–91. doi: 10.1097/PSY.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Fang J, Wan Y, Su P, Tao F. Association of early-life adversity with measures of accelerated biological aging among children in China. Jama Netw Open. 2020;3:e2013588. doi: 10.1001/jamanetworkopen.2020.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleil ME, Spieker SJ, Gregorich SE, Thomas AS, Hiatt RA, Appelhans BM, et al. Early life adversity and pubertal timing: implications for cardiometabolic health. J Pediatr Psychol. 2020;46:36–48. doi: 10.1093/jpepsy/jsaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, et al. Exposure to violence accelerates epigenetic aging in children. Sci Rep. 2017;7:8962. doi: 10.1038/s41598-017-09235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang R, Howe LD, Suderman M, Relton CL, Crawford AA, Houtepen LC. Adverse childhood experiences, DNA methylation age acceleration, and cortisol in UK children: a prospective population-based cohort study. Clin Epigenetics. 2020;12:55. doi: 10.1186/s13148-020-00844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, et al. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrino. 2018;92:123–34. doi: 10.1016/j.psyneuen.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:3156. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–67. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–91. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–27. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce BT, Gao T, Zheng Y, Ma J, Hwang S-J, Liu L, et al. Epigenetic age acceleration reflects long-term cardiovascular health. Circ Res. 2021;129:770–81. doi: 10.1161/CIRCRESAHA.121.318965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshandel D, Chen Z, Canty AJ, Bull SB, Natarajan R, Paterson AD, et al. DNA methylation age calculators reveal association with diabetic neuropathy in type 1 diabetes. Clin Epigenetics. 2020;12:52. doi: 10.1186/s13148-020-00840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (atherosclerosis risk in communities) Circulation Genom Precis Med. 2018;11:e001937. doi: 10.1161/CIRCGEN.117.001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pottinger TD, Khan SS, Zheng Y, Zhang W, Tindle HA, Allison M, et al. Association of cardiovascular health and epigenetic age acceleration. Clin Epigenetics. 2021;13:42. doi: 10.1186/s13148-021-01028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Maden SK, Luebeck GE, Li CI, Newcomb PA, Ulrich CM, et al. Dysfunctional epigenetic aging of the normal colon and colorectal cancer risk. Clin Epigenetics. 2020;12:5. doi: 10.1186/s13148-019-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhoeven JE, Yang R, Wolkowitz OM, Bersani FS, Lindqvist D, Mellon SH, et al. Epigenetic age in male combat-exposed war veterans: associations with posttraumatic stress disorder status. Mol Neuropsychiatry. 2018;4:90–99. doi: 10.1159/000491431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whalley HC, Gibson J, Marioni R, Walker RM, Clarke T-K, Howard DM, et al. Accelerated epigenetic ageing in major depressive disorder. BioRxiv 2017. 10.1101/210666.
- 29.Lima CN, Soares J, Walss-Bass C, Quevedo J, Fries G. Association between accelerated epigenetic aging and poorer functional status in bipolar disorder. Biol Psychiat. 2021;89:S223–S224. doi: 10.1016/j.biopsych.2021.02.564. [DOI] [Google Scholar]
- 30.Marini S, Davis KA, Soare TW, Zhu Y, Suderman MJ, Simpkin AJ, et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrino. 2020;113:104484. doi: 10.1016/j.psyneuen.2019.104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copeland WE, Shanahan L, McGinnis EW, Aberg KA, Oord EJCG. Early adversities accelerate epigenetic aging into adulthood: a 10‐year, within‐subject analysis. J Child Psychol Psyc. 2022. 10.1111/jcpp.13575. [DOI] [PMC free article] [PubMed]
- 32.McLaughlin KA, Sheridan MA. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016;25:239–45. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bull. 2013;139:1342–96. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- 34.Miller AB, Sheridan MA, Hanson JL, McLaughlin KA, Bates JE, Lansford JE, et al. Dimensions of deprivation and threat, psychopathology, and potential mediators: a multi-year longitudinal analysis. J Abnorm Psychol. 2018;127:160–70. doi: 10.1037/abn0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18:580–5. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–91. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho TC, King LS. Mechanisms of neuroplasticity linking early adversity to depression: developmental considerations. Transl Psychiat. 2021;11:517. doi: 10.1038/s41398-021-01639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) Study. Am J Prev Med. 2019;56:774–86. doi: 10.1016/j.amepre.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, et al. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–8. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 40.Afifi TO, Salmon S, Garcés I, Struck S, Fortier J, Taillieu T, et al. Confirmatory factor analysis of adverse childhood experiences (ACEs) among a community-based sample of parents and adolescents. Bmc Pediatr. 2020;20:178. doi: 10.1186/s12887-020-02063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayyah MD, Merrick JS, Larson MD, Narayan AJ. Childhood adversity subtypes and young adulthood mental health problems: Unpacking effects of maltreatment, family dysfunction, and peer victimization. Child Youth Serv Rev. 2022;137:106455. doi: 10.1016/j.childyouth.2022.106455. [DOI] [Google Scholar]
- 42.Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol Psychiat. 2019;85:268–78. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han LKM, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, et al. Epigenetic aging in major depressive disorder. Am J Psychiat. 2018;175:774–82. doi: 10.1176/appi.ajp.2018.17060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang R, Wu GWY, Verhoeven JE, Gautam A, Reus VI, Kang JI, et al. A DNA methylation clock associated with age-related illnesses and mortality is accelerated in men with combat PTSD. Mol Psychiatr. 2021;26:4999–5009. doi: 10.1038/s41380-020-0755-z. [DOI] [PubMed] [Google Scholar]
- 45.Triche TJ, Weisenberger DJ, Berg DVD, Laird PW, Siegmund KD. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 2013;41:e90–e90. doi: 10.1093/nar/gkt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl. 2003;27:169–90. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 47.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 48.Hamlat EJ, Prather AA, Horvath S, Belsky J, Epel ES. Early life adversity, pubertal timing, and epigenetic age acceleration in adulthood. Dev Psychobiol. 2021. 10.1002/dev.22085. [DOI] [PMC free article] [PubMed]
- 49.Mersky JP, Janczewski CE, Topitzes J. Rethinking the measurement of adversity: moving toward second-generation research on adverse childhood experiences. Child Maltreatment. 2016;22:58–68. doi: 10.1177/1077559516679513. [DOI] [PubMed] [Google Scholar]
- 50.Harvanek ZM, Fogelman N, Xu K, Sinha R. Psychological and biological resilience modulates the effects of stress on epigenetic aging. Transl Psychiat. 2021;11:601. doi: 10.1038/s41398-021-01735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins DJ, McCabe MP. Maltreatment and family dysfunction in childhood and the subsequent adjustment of children and adults. J Fam Violence. 2003;18:107–20. doi: 10.1023/A:1022841215113. [DOI] [Google Scholar]
- 52.Ryan KD, Kilmer RP, Cauce AM, Watanabe H, Hoyt DR. Psychological consequences of child maltreatment in homeless adolescents: untangling the unique effects of maltreatment and family environment. Child Abus Negl. 2000;24:333–52. doi: 10.1016/S0145-2134(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 53.Lawrence KG, Kresovich JK, O’Brien KM, Hoang TT, Xu Z, Taylor JA, et al. Association of neighborhood deprivation with epigenetic aging using 4 clock metrics. Jama Netw Open. 2020;3:e2024329. doi: 10.1001/jamanetworkopen.2020.24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. Bmc Bioinforma. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houseman EA, Molitor J, Marsit CJ. Reference-free cell mixture adjustments in analysis of DNA methylation data. Bioinformatics. 2014;30:1431–9. doi: 10.1093/bioinformatics/btu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.David FN, Blom G. Statistical estimates and transformed beta-variables. Biometrika. 1960;47:210. doi: 10.1093/biomet/47.1-2.210-b. [DOI] [Google Scholar]
- 57.Colich NL, Rosen ML, Williams ES, McLaughlin KA. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. BioRxiv 2019. 10.1101/642405. [DOI] [PMC free article] [PubMed]
- 58.Westermair AL, Stoll AM, Greggersen W, Kahl KG, Hüppe M, Schweiger U. All unhappy childhoods are unhappy in their own way—differential impact of dimensions of adverse childhood experiences on adult mental health and health behavior. Front Psychiatry. 2018;9:198. doi: 10.3389/fpsyt.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ford DC, Merrick MT, Parks SE, Breiding MJ, Gilbert LK, Edwards VJ, et al. Examination of the factorial structure of adverse childhood experiences and recommendations for three subscale scores. Psychol Violence. 2014;4:432–44. doi: 10.1037/a0037723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly-Irving M, Lepage B, Dedieu D, Bartley M, Blane D, Grosclaude P, et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol. 2013;28:721–34. doi: 10.1007/s10654-013-9832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beach SRH, Ong ML, Lei M-K, Klopack E, Carter SE, Simons RL, et al. Childhood adversity is linked to adult health among African Americans via adolescent weight gain and effects are genetically moderated. Dev Psychopathol. 2021;33:803–20. doi: 10.1017/S0954579420000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication ii: associations with persistence of DSM-IV disorders. Arch Gen Psychiat. 2010;67:124–32. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Putnam KT, Harris WW, Putnam FW. Synergistic childhood adversities and complex adult psychopathology: synergistic childhood adversities. J Trauma Stress. 2013;26:435–42. doi: 10.1002/jts.21833. [DOI] [PubMed] [Google Scholar]
- 64.McCrory C, Fiorito G, O’Halloran AM, Polidoro S, Vineis P, Kenny RA. Early life adversity and age acceleration at mid-life and older ages indexed using the next-generation GrimAge and Pace of Aging epigenetic clocks. Psychoneuroendocrino. 2022;137:105643. doi: 10.1016/j.psyneuen.2021.105643. [DOI] [PubMed] [Google Scholar]
- 65.Belsky J, Shalev I. Contextual adversity, telomere erosion, pubertal development, and health: Two models of accelerated aging, or one? Dev Psychopathol. 2016;28:1367–83. doi: 10.1017/S0954579416000900. [DOI] [PubMed] [Google Scholar]
- 66.Chisholm JS, Quinlivan JA, Petersen RW, Coall DA. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum Nat. 2005;16:233–65. doi: 10.1007/s12110-005-1009-0. [DOI] [PubMed] [Google Scholar]
- 67.Ridout KK, Syed SA, Kao H-T, Porton B, Rozenboym AV, Tang J, et al. Relationships between telomere length, plasma glucagon-like peptide 1, and insulin in early-life stress–exposed nonhuman primates. Biol Psychiatry Glob Open Sci. 2022;2:54–60. doi: 10.1016/j.bpsgos.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambert HK, King KM, Monahan KC, McLaughlin KA. Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Dev Psychopathol. 2017;29:929–40. doi: 10.1017/S0954579416000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berman IS, McLaughlin KA, Tottenham N, Godfrey K, Seeman T, Loucks E, et al. Measuring early life adversity: a dimensional approach. Dev Psychopathol. 2022;34:499–511. doi: 10.1017/S0954579421001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogel SC, Perry RE, Brandes-Aitken A, Braren S, Blair C. Deprivation and threat as developmental mediators in the relation between early life socioeconomic status and executive functioning outcomes in early childhood. Dev Cogn Neuros-neth. 2020;47:100907. doi: 10.1016/j.dcn.2020.100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergsma T, Rogaeva E. DNA methylation clocks and their predictive capacity for aging phenotypes and healthspan. Neurosci Insights. 2020;15:2633105520942221. doi: 10.1177/2633105520942221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menke A, Binder EB. Epigenetic alterations in depression and antidepressant treatment. Dialogues Clin Neurosci. 2014;16:395–404. doi: 10.31887/DCNS.2014.16.3/amenke. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
R code available by contacting the authors.