Abstract
Objective:
Fetal electrocardiogram (ECG) ST-changes are associated with fetal cardiac hypoxia. Our objective was to evaluate ST-changes by maternal diabetic status and stage of labor.
Methods:
Secondary analysis of a multi-centered randomized-controlled trial in which laboring patients with singleton gestations underwent fetal ECG scalp electrode placement and were randomly assigned to masked or unmasked ST-segment readings. Our primary outcome was the frequency of fetal ECG tracings with ST-changes by stage of labor. ECG tracings were categorized into mutually exclusive groups (ST-depression, ST-elevation without ST-depression or no ST-changes). We compared participants with pre-gestational diabetes mellitus (DM), gestational DM (GDM), and no DM.
Results:
Of the 5,436 eligible individuals in the first stage of labor (95 with pre-gestational DM and 370 with GDM) 4,427 progressed to the second stage. ST-depression occurred more frequently in the first stage of labor in participants with pre-gestational DM (15%, aOR 2.20, 95% CI 1.14-4.24) and with GDM (9.5%, aOR 1.51, 95% CI 1.02-2.25) as compared with participants without DM (5.7%). The frequency of ST-elevation was similar in participants with pregestational DM (33%, aOR 0.79, 95% CI 0.48-1.30) and GDM (33.2%, aOR 0.91, 95% CI 0.71-1.17) as compared with those without DM (34.2%). In the second stage, ST-depression did not occur in participants with pre-gestational DM (0%) and occurred more frequently in participants with GDM (3.5%, aOR 2.01, 95% CI 1.02-3.98) as compared with those without DM (2.0%). ST-elevation occurred more frequently in participants with pregestational DM (30%, aOR 1.81, 95% CI 1.02-3.22) but not with GDM (19.0%, aOR 1.06, 95% CI 0.77-1.47) as compared with those without DM (17.8%).
Conclusion:
ST-changes in fetal ECG occur more frequently in fetuses of diabetic mothers during labor.
ClinicalTrials.gov number, NCT01131260
Keywords: maternal diabetes, fetal heart, fetal electrocardiogram
Precis:
ST-changes in fetal electrocardiogram, a marker of fetal cardiac hypoxia, occur more frequently in fetuses of diabetic parturients
Introduction:
Fetal electrocardiogram (ECG) is a technique used to evaluate the fetal heart. In animal models, waveform analysis, specifically changes in the ST-segment (ST elevation or depression), provides a measure of myocardial hypoxia1 2 3 4 and these ECG changes are thought to precede cerebral hypoxic damage 5. These findings, among others, led to evaluation of the clinical application of fetal ECG waveform analysis as an adjunct to continuous fetal heart rate monitoring among laboring patients. To date, six randomized controlled trials have been conducted to evaluate whether fetal ECG waveform analysis results in improved perinatal outcomes 6 7 8 9 10 11; these studies have had varying results.
Fetal hypertrophic cardiomyopathy (HCM) and/or fetal cardiac dysfunction have been reported in 30-70% of fetuses of patients with pre-gestational diabetes mellitus (DM) 12 13 14 15 16 17 and 9-50% in fetuses of patients with gestational diabetes mellitus (GDM) 18 19. In pregnant patients with DM, the fetal HCM or cardiac dysfunction typically develops in the third trimester18. As fetal echocardiography is not routinely performed late in pregnancy, the condition is often undiagnosed outside of a study protocol. Fortunately, the clinical course is benign for the majority of these infants and most experience rapid post-natal resolution of the cardiac findings20. However, the clinical course is not universally favorable and a minority of these infants experience adverse events including perinatal demise21 22 23.
We hypothesize that HCM or cardiac dysfunction, both of which occur commonly among fetuses of mothers with DM12-17, may be associated with diminished fetal cardiac oxygenation during labor and concomitant changes in the fetal ECG waveform. Such information, compromised cardiac function in a fetus during labor, may have clinical implications for labor management. An understanding of when these changes may occur (first or second stage of labor) may also provide insight into the fetal response to labor as it progresses from the first to the second stage. The objective of this analysis was to evaluate ST-changes by maternal diabetic status and stage of labor.
Methods:
We conducted a secondary analysis of the trial, “A Randomized Trial of Intrapartum Fetal ECG ST-Segment Analysis,” which was conducted at 26 hospitals within the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network from 2010 through 2013. Details of the study design and methods have been published elsewhere11. Institutional review board approval was secured at each participating hospital. Briefly, in the parent trial, laboring patients with a singleton gestation who were 36 1/7 weeks gestation or greater who had cervical dilation between 2-7 cm were eligible to participate. Consenting individuals had fetal electrocardiogram scalp electrodes (Neoventa S31, Neoventa Medical) placed and were randomly assigned to masked or unmasked ST-segment readings. In the masked group, the scalp electrode functioned as a normal fetal heart rate monitor. In the open group, the fetal heart rate tracing was displayed in addition to computer-generated and displayed fetal electrocardiographic ST-segment analysis. To perform ST-segment analysis, the Neoventa system continuously monitors baseline fetal ECG data and each time a ST elevation occurs--either baseline (>0.05 units above the T/QRS ratio baseline and lasting greater than 10 minutes) or episodic (>0.10 units above and lasting 10 minutes or less) or depression (in the form of a biphasic T-wave) occurs, a notification of a ST-event was automatically displayed as an alert on the monitor. These alerts were to be used as an adjunct to the interpretation of fetal heart rate tracing when an uncertain tracing was detected. Management was dictated by pre-specified guidelines11. The ST-segment changes for each labor tracing were saved in a digital file, with the files being combined and used for this analysis.
In this analysis, we included individuals with available ECG-waveform analysis and excluded those found to have major fetal anomalies. Our primary outcome was the frequency of fetal ECG tracings with ST-changes by stage of labor compared according to whether individuals had the diagnosis of pregestational DM, GDM or no DM. ECG tracings were categorized into mutually exclusive groups (ST-depression, ST-elevation without ST-depression or no ST-changes). We defined ST-depression as any waveform analysis that contained one or more ST-depressions. We defined ST-elevation as any waveform analysis that contained one or more ST-elevations without ST-depression and defined no ST-changes as any waveform analysis in which no ST-events were detected. Maternal demographic and clinical characteristics were determined by patient interview and chart review as indicated. Chronic hypertension was defined as the diagnosis or treatment of hypertension at any point prior to 20 weeks of gestation. Hypertensive disease of pregnancy included the diagnosis of gestational hypertension or preeclampsia at any time after 20 weeks of gestation. The first stage of labor was defined by labor prior to cervical dilation of 10 cm and the second stage of labor was defined as labor after cervical dilation of 10 cm.
Maternal demographic and clinical characteristics were compared between participants with pregestational DM or GDM, each with no DM, using the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables. For fetal electrocardiogram outcomes, separate analyses were performed for the first and second stage of labor for each comparison. Unadjusted comparisons of ST- depression and ST- elevation with no ST- changes were performed with the chi-square test, or Fisher exact test when appropriate. We developed parsimonious multivariable logistic regression models that included baseline characteristics associated with diabetic status in the univariable analysis (P<0.05) and iteratively removed variables until only those with P values less than 0.05 were retained. Duration of fetal monitoring for each stage of labor was included in the models to control for the length of observation and time in labor. Analyses were performed using SAS 9.4. A P-value of <0.05 was chosen to denote significance. No adjustments were made for multiple comparisons. No imputation for missing data was performed.
Results:
Our study included 5,436 eligible patients in the first stage of labor and 4,427 who then progressed to the second stage of labor (Figure 1). Of these, 95 participants had pregestational DM (28 Type 1 and 67 Type 2) and 370 had GDM. In univariable analyses, participants with DM were more likely to be older, heavier, Hispanic or Black, publicly insured and have hypertensive disorders as displayed in Table 1.
Figure 1.
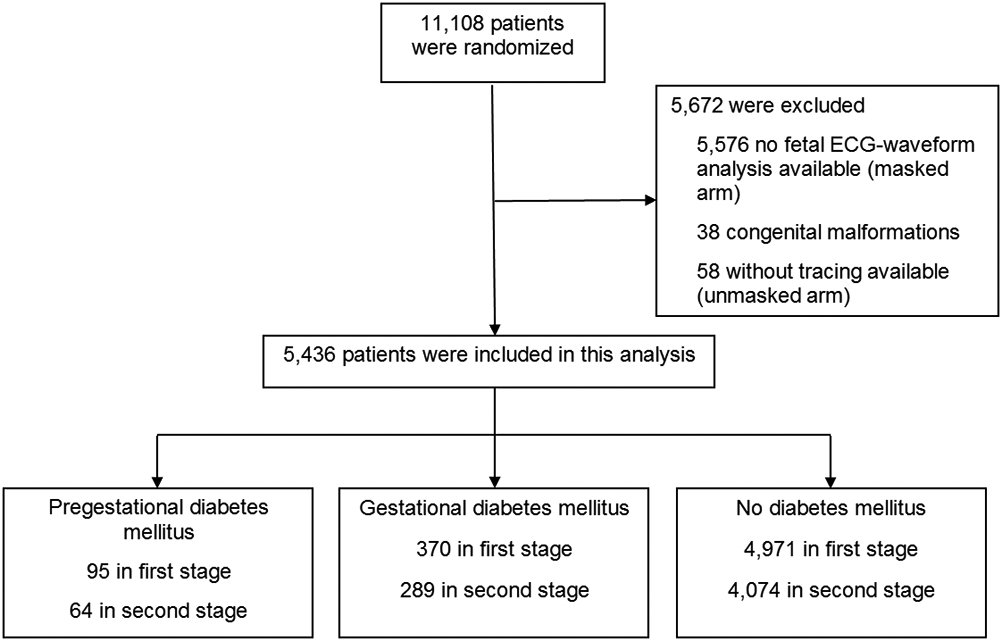
Participant Inclusion and Exclusion
Table 1.
Maternal demographic and clinical characteristics
| Pregestational DM N=95 |
P-valuea | GDM N=370 |
P-valueb | No DM (Referent) N=4971 |
|
|---|---|---|---|---|---|
| Maternal age (years) | 30.1 ± 7.1 | <0.001 | 29.9 ± 6.1 | <0.001 | 27.1 ± 5.8 |
| Race/ethnicity | 0.003 | <0.001 | |||
| Black | 22 (23) | 69 (18.7) | 1132 (22.8) | ||
| Hispanic | 42 (44) | 173 (46.8) | 1449 (29.2) | ||
| White/Other | 31 (33) | 128 (34.6) | 2390 (48.1) | ||
| Nulliparous | 36 (38) | 0.32 | 141 (38.1) | 0.06 | 2139 (43.0) |
| Public insurance/none | 74 (78) | <0.001 | 260 (70.5) | <0.001 | 2939 (59.1) |
| Education (years) | 11.9 ± 2.9 | 0.001 | 12.2 ± 3.2 | <0.001 | 12.9 ± 2.6 |
| BMI at admission (kg/m2) | 37.7 ± 8.2 | <0.001 | 35.6 ± 7.3 | <0.001 | 32.4 ± 6.9 |
| Tobacco use | 12 (13) | 0.09 | 27 (7.3) | 0.67 | 393 (7.9) |
| Illicit drug use | 4 (4) | 0.33 | 4 (1.1) | 0.06 | 133 (2.7) |
| Chronic hypertension | 21 (22) | <0.001 | 33 (8.9) | <0.001 | 154 (3.1) |
| Gestational hypertensive disorders | 27 (28) | <0.001 | 53 (14.3) | 0.03 | 535 (10.8) |
| Duration of labor | |||||
| First stage (hours) | 4.7 (2.7-7.5) | <0.001 | 3.4 (1.8-6.1) | 0.003 | 3.0 (1.5-5.2) |
| Second stage (minutes) | 36 (11-70) | 0.98 | 26 (13-64) | 0.74 | 27 (14-60) |
BMI = body mass index; DM = diabetes mellitus; GDM = gestational diabetes mellitus.
Data presented as mean ± standard deviation, median (interquartile range), or n (%). Significant associations (P<0.05) are in bold.
Pregestational DM versus no DM
GDM versus no DM
In unadjusted analyses, during the first stage of labor the frequency of fetal ECG tracings with ST-depression was higher in fetuses of participants with pregestational DM (15%, P<0.001), and GDM (9.5%, P=0.004) as compared with no DM (5.7%). However, the frequency of fetal ECG tracings with ST-elevation without depression was comparable in those with pregestational DM (33%, P=0.70) and GDM (33.2%, P=0.86), compared with no DM (34.2%).
In the second stage of labor, there were no tracings with ST-depression in fetuses of participants with pregestational DM, and there were few tracings with ST-depression in fetuses of those with GDM (3.5%, P=0.08) compared with no DM (2.0%) in unadjusted analyses. However, during the second stage of labor, tracings with ST-elevation without depression occurred with greater frequency among fetuses of participants with pregestational DM (30%, P=0.02), but not GDM (19.0%, P=0.50) as compared with those with no DM (17.8%) in unadjusted analyses. It should be noted that the duration of the second stage of labor was fairly short with a median of 36, 26 and 27 minutes for participants with pregestational DM, GDM and no DM, respectively, and results should be interpreted in this context.
Multivariable models were adjusted for maternal race and ethnicity, insurance type, education, and duration of fetal ECG monitoring. In the adjusted model, fetal ECG tracings with ST-depression occurred significantly more frequently in the first stage of labor in participants with pregestational DM (aOR 2.20, 95% CI 1.14-4.24) and with GDM (aOR 1.51, 95% CI 1.02-2.25), as compared with those with no DM. In the second stage of labor, fetal ECG tracings with ST-depression occurred more frequently in participants with GDM as compared with no DM (aOR 2.01, 95% CI 1.02-3.98). Fetal ECG tracings with ST-elevation occurred more frequently in those with pregestational DM (aOR 1.81, 95% CI 1.02-3.22) but not with GDM (aOR 1.06, 95% CI 0.77-1.47), as compared with no DM.
Discussion:
Our analysis demonstrated that fetal ECG tracings with ST-segment changes occurred more frequently among fetuses of participants with pregestational DM and GDM as compared with those with no DM. The findings varied by the specific type of ST-segment change and by stage of labor. Specifically, in multivariable analyses, the odds of fetal ECG tracings with ST-depression were significantly higher among fetuses of those with pregestational DM (aOR 2.20, 95% CI 1.14-4.24) and GDM (aOR 1.51, 95% CI 1.02-2.25) during the first stage of labor. During the second stage of labor, the odds of fetal ECG tracing with ST-elevation (without depression) were significantly higher among fetuses of participants with pregestational DM (aOR 1.81, 95% CI 1.02-3.22) and the odds of ST-depression was significantly higher among fetuses of participants with GDM (aOR 2.01, 95% CI 1.02-3.98). Taken together, these findings demonstrate that ST-changes in fetal ECG, a marker of fetal cardiac hypoxia, occur more frequently in fetuses of diabetic participants and suggest that the effects of maternal diabetes on the fetal heart are not limited to structural abnormalities.
In many respects, our findings are similar to the one previous analysis that evaluated ST-events in fetuses of patients with DM 24. Yli et al performed a secondary analysis of 1,088 participants (75 with pregestational DM, 338 with GDM and 675 with no DM) drawn from two separate clinical trials, Yli found that fetal ECG tracings with ST elevation without depression occurred frequently among fetuses of all participants. The prevalence in patients with pregestational DM was 45%, 44% with GDM and 40% with no DM. These findings were similar to our analysis for the first stage of labor with a prevalence of 33%, 33% and 34% for fetuses of participants with pregestational DM, GDM and no DM, respectively. Yli found ST-depression occurred more frequently in fetus of patients with pregestational DM with an odds ratio of 2.6 (95% CI 1.4-4.7) in multivariable analyses adjusted for trial, birth weight, and nulliparity. We similarly found an increased odds of fetal ECG-tracings with ST-depression in fetuses of participants with pregestational DM.
However, Yli’s results differed from ours, in that she did not find a statistically significant relationship in adjusted analyses between ST-depression and fetuses of individuals with GDM. We did find this association but it was limited only to the second stage of labor. As Yli’s analysis was not stratified by stage of labor, and the number of events were small, the difference in the analytic approach may explain our discordant results.
It is not entirely clear why ST-changes may occur differentially in the first and second stage of labor in fetuses of patients with pregestational DM as compared to fetuses of those with GDM. It is possible that fetus of patients with GDM have a milder hypertrophic cardiac phenotype than fetuses of those with pregestational DM and that they do not demonstrate ST-changes until later in the labor course. However, fetal echocardiogram findings would be required to substantiate this supposition. In addition, it is important to note that patients who went on to the second stage of labor (n=4,427) represent a subset of the patients who were included in this analysis for the first stage of labor (n=5,436). Those that did not enter into the second stage of labor were delivered by cesarean delivery either due to labor dystocia or fetal heart rate concerns and thus represent a different population than those in the first stage of labor. It is possible that fetuses with ST-depression in the first stage were less likely to continue on to the second stage. It is also possible that the short duration of the second stage (approximately 30 minutes on average) as compared to the longer duration of the first stage (3-4 hours), contributed to the reduced frequency of ST-depression in the second stage amongst patients with pregestational DM, GDM and no DM.
In both Yli’s analysis and ours, ST-elevation occurred relatively frequently among fetuses of participants with and without DM. These findings are similar to those in animal studies. In the sheep model, repeated umbilical cord occlusion was applied to create intermittent hypoxia to simulate decreased placental oxygenation during contractions 2. Fetuses who experienced occlusion every 5 minutes demonstrated predominantly ST-segment elevation with recovery between each occlusion. However, those fetuses that were subject to more frequent periods of occlusion, every 2.5 minutes, demonstrated progressive deterioration in the ECG tracing with ST-depression noted after prolonged periods 2. Similarly, prolonged umbilical cord occlusion in sheep prompted ST-depression in the form of biphasic T-waves25. A similar progression from ST-elevation to ST-depression was observed when fetal lambs were exposed to progressive degrees of hypoxia 1. It is possible that ST-elevation that occurs frequently during labor represents transient myocardial hypoxia with recovery, and those fetuses that experience ST-depression no longer have sufficient myocardial reserve to compensate for the myocardial hypoxic event. In contrast to animal models, in this secondary analysis of a clinical trial, we do not have direct measurements of fetal hypoxemia or cardiac hypoxia at the time of ST-events to substantiate our findings.
Our study is limited by lack of fetal echocardiography data and lack of more detailed data regarding diabetes control. Factors including patient hemoglobin A1C levels, insulin requirements, fetal macrosomia and polyhdramnios may be associated with fetal hypertrophic cardiomyopathy and fetal ECG findings.
Our analysis has several strengths, among them its large size and prospective data collection by trained research personnel. In addition, the parent trial took place across 26 hospitals in the United States and represents a racially and ethnically diverse patient population, making the findings broadly generalizable. Our analysis is limited in that we do not have data regarding maternal diabetic treatment or diabetic control. We also do not have fetal echocardiogram data to determine the underlying fetal cardiac structure or function that may be associated with the increased frequency of ST-changes among fetuses of diabetic patients. The prevalence of fetal hypertrophic cardiomyopathy and/or cardiac dysfunction is relatively high among fetuses of diabetic patients [1-8], but we were unable to directly assess the fetal heart for the presence or absence of hypertrophy in this secondary analysis. Thus, the association between diabetic hypertrophic cardiomyopathy and fetal ST-changes remains speculative. Other factors, including maternal vascular abnormalities and altered placental perfusion may contribute to fetal cardiac hypoperfusion as well.
In summary, our analysis demonstrated that in adjusted models, ST-changes occurred more frequently in fetuses of participants with pregestational DM in both the first and second stages of labor and with GDM in the second stage of labor as compared with fetuses of those who did not have DM. The findings of the study suggest that the effects of diabetes on the fetal heart are not limited to structural abnormalities and may warrant further study.
Table 2.
Fetal electrocardiogram outcomes by stage of labor and diabetic status
| First stage of labor |
Pregestational DM N=95 |
OR (95% CI) | aOR (95% CI) |
GDM N=370 |
OR (95% CI) | aOR (95% CI) |
No DM (Referent) N=4971 |
|---|---|---|---|---|---|---|---|
| ST depression | 14 (15) | 2.96 (1.62, 5.42) | 2.20 (1.14, 4.24) | 35 (9.5) | 1.74 (1.20, 2.55) | 1.51 (1.02, 2.25) | 283 (5.7) |
| ST elevation (no depression) | 31 (33) | 1.09 (0.69, 1.72) | 0.79 (0.48, 1.30) | 123 (33.2) | 1.02 (0.81, 1.29) | 0.91 (0.71, 1.17) | 1698 (34.2) |
| Second stage of labor |
Pregestational DM N=64 |
GDM N=289 |
No DM (Referent) N=4074 |
||||
| ST depression | 0 (0) | n/a | n/a | 10 (3.5) | 1.80 (0.92, 3.52) | 2.01 (1.02, 3.98) | 81 (2.0) |
| ST elevation (no depression) | 19 (30) | 1.91 (1.11, 3.28) | 1.81 (1.02, 3.22) | 55 (19.0) | 1.11 (0.82, 1.51) | 1.06 (0.77, 1.47) | 723 (17.8) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DM = diabetes mellitus; GDM = gestational diabetes mellitus; N/A, not available; OR, odds ratio
Data presented as n (%), unless otherwise noted. Statistically significant associations (P<0.05) are displayed in bold. Odds ratios adjusted for maternal race and ethnicity, insurance type, education, and duration of fetal ECG monitoring. No DM is the referent group.
Key points:
Fetal hypertrophic cardiomyopathy (HCM) and cardiac dysfunction occur frequently among fetuses of diabetic patients
Fetal ECG changes such as ST elevation and depression reflect cardiac hypoxia
Fetuses of diabetic patients demonstrate higher prevalence of fetal ECG tracings with ST changes
Acknowledgements
The authors thank Ashley Salazar, RN, MSN, WHNP, for assistance with protocol development and coordination between clinical research centers; Elizabeth Thom, PhD, for protocol development and oversight; and Michael W. Varner, MD, Catherine Y. Spong, MD, and Uma M. Reddy, MD, MPH for protocol development, oversight and outcome review.
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD34208, HD53097, HD40545, HD40560, HD27869, HD40485, HD40512, HD27915, HD40544, HD40500, HD68282, HD68268, HD27917, HD21410, HD36801] and by funding from Neoventa Medical. Comments and views expressed in this article are those of the authors and do not necessarily represent views of the NICHD. Neoventa Medical did not participate in the monitoring of the study; data collection, management, or analysis; or manuscript preparation.
Appendix
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
Northwestern University, Chicago, IL - G. Mallett, A. Peaceman, N. Dekker, A. Roy, M. Vanecko, E. Irwin, M. Dinsmoor (NorthShore University HealthSystem), K. Paychek (NorthShore University HealthSystem)
University of Utah Health Sciences Center, Salt Lake City, UT - K. Hill, S. Timothy, A. Sowles, E. Clark, M. Varner, J. Stratford (McKay-Dee Hospital), S. Quinn, P. Reed (deceased, Intermountain Medical Center), M. Gertsch (UVRMC), M. Love (St. Mark's Hospital)
University of Texas Medical Branch, Galveston, TX - A. Salazar, J. Sikes, B. Aguillon, M. Wilson-Jimenez, G. Hankins, G. Olson, M. Costantine, T. Wen, S. Nilsen, H. Harirah, L. Pacheco, S. Jain, S. Clark, M. Munn
The University of Texas Health Science Center at Houston, McGovern Medical School-Children’s Memorial Hermann Hospital, Houston, TX - F. Ortiz, B. Sibai (LBJ General Hospital), P. Givens, M. Phillips, L. Garcia (LBJ General Hospital), B. Rech
University of North Carolina at Chapel Hill, Chapel Hill, NC - K. Clark, S. Timlin, M. Kearney, L. Hitchings, S. Brody
University of Alabama at Birmingham, Birmingham, AL - J. Biggio, S. Harris, A. Todd, G. Adams, J. Grant, L. Merin, M. Lee
Columbia University, New York, NY - R. Wapner, S. Bousleiman, A. Mermelstein, C. Torres, G. Kaur, B. Leopanto (Drexel U), C. Tocci (Drexel U), J. Benson (Christiana H), S. Forester (Christiana H), C. Boutros (St. Peter's UH), M. Lake (St. Peter's UH)
The Ohio State University, Columbus, OH - J. Iams, F. Johnson, K. Strafford, R. Ozug, K. Fennig, T. Dible, K. Snow
MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH - C. Milluzzi, B. Mercer, W. Dalton, B. Stetzer, K. Kushner, L. Polito
Brown University, Providence, RI - D. Allard, B. Hughes, D. Cermik, B. Wallin, K. Grant, L. Beati, J. Rousseau
University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO - K. Hale, R. Gibbs, N. Behrendt, M. Donnelly, S. Andrews, G. Moore, J. Hurt
Stanford University, Stanford, CA - K. Kushniruk, M. Norton, A. Monk, E. Kogut, C. Willson, K. Harney, J. Kassis, K. Milan
Wayne State University, Detroit, MI - N. Hauff, D. Driscoll, P. Lockhart
University of Pittsburgh, Pittsburgh, PA - H. Simhan, M. Cotroneo, H. Birkland
The George Washington University Biostatistics Center, Washington, DC - E. Thom, D. Mapp, L. Powers-Happ, M. Dingman, L.S. Firrell, B. Broderick, A. Shaver, V. Donohue
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD - C. Spong, U. Reddy, S. Tolivaisa
MFMU Network Steering Committee Chair (Medical University of South Carolina, Charleston, SC) - J.P. VanDorsten, M.D.
Footnotes
The authors did not report any potential financial conflicts of interest.
Presented in part at the Society for Maternal-Fetal Medicine's 39th Annual Pregnancy Meeting, February 11-16, 2019, Las Vegas, Nevada.
See Appendix for a list of other members of the NICHD MFMU Network
Contributor Information
Beth A. Plunkett, Departments of Obstetrics and Gynecology of Northwestern University, Chicago, IL.
Steven J. Weiner, George Washington University Biostatistics Center, Washington, DC.
George R. Saade, University of Texas Medical Branch, Galveston, TX.
Michael A. Belfort, University of Utah Health Sciences Center, Salt Lake City, UT.
Sean C. Blackwell, University of Texas Health Science Center at Houston, McGovern Medical School-Children’s Memorial Hermann Hospital, Houston, TX.
John M. Thorp, Jr., University of North Carolina at Chapel Hill, Chapel Hill, NC.
Alan T.N. Tita, University of Alabama at Birmingham, Birmingham, AL.
Russell S. Miller, Columbia University, New York, NY.
David S. McKenna, The Ohio State University, Columbus, OH.
Edward K.S. Chien, MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH.
Dwight J. Rouse, Brown University, Providence, RI.
Yasser Y. El-Sayed, Stanford University, Stanford, CA.
Yoram Sorokin, Wayne State University, Detroit, MI.
Steve N. Caritis, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Hökegård KH, Eriksson BO, Kjellmer I, Magno R, Rosén KG. Myocardial metabolism in relation to electrocardiographic changes and cardiac function during graded hypoxia in the fetal lamb. Acta Physiol Scand. 1981;113(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2.Westgate JA, Bennet L, Brabyn C, Williams CE, Gunn AJ. ST waveform changes during repeated umbilical cord occlusions in near-term fetal sheep. Am J Obstet Gynecol. 2001;184(4):743–751. [DOI] [PubMed] [Google Scholar]
- 3.Hokegard KH, Karlsson K, Kjellmer I, Rosen KG. ECG-changes in the fetal lamb during asphyxia in relation to beta-adrenoceptor stimulation and blockade. Acta Physiol Scand. 1979;105(2):195–203. [DOI] [PubMed] [Google Scholar]
- 4.Hokegard KH, Rosen KG. Alterations in the electrocardiogram of the fetal lamb as a sign of fetal asphyxia. A comparison between the scalp lead and the precordial lead. Acta Obstet Gynecol Scand. 1980;59(5):411–415. [DOI] [PubMed] [Google Scholar]
- 5.Rosén KG, Hökegård KH, Kjellmer I. A study of the relationship between the electrocardiogram and hemodynamics in the fetal lamb during asphyxia. Acta Physiol Scand. 1976;98(3):275–284. [DOI] [PubMed] [Google Scholar]
- 6.Westgate J, Harris M, Curnow JS, Greene KR. Randomised trial of cardiotocography alone or with ST waveform analysis for intrapartum monitoring. Lancet. 1992;340(8813):194–198. [DOI] [PubMed] [Google Scholar]
- 7.Ojala K, Vaarasmaki M, Makikallio K, Valkama M, Tekay A. A comparison of intrapartum automated fetal electrocardiography and conventional cardiotocography--a randomised controlled study. BJOG. 2006;113(4):419–423. [DOI] [PubMed] [Google Scholar]
- 8.Vayssiere C, David E, Meyer N, et al. A French randomized controlled trial of ST-segment analysis in a population with abnormal cardiotocograms during labor. Am J Obstet Gynecol. 2007;197(3):299 e291–296. [DOI] [PubMed] [Google Scholar]
- 9.Westerhuis ME, Visser GH, Moons KG, et al. Cardiotocography plus ST analysis of fetal electrocardiogram compared with cardiotocography only for intrapartum monitoring: a randomized controlled trial. Obstet Gynecol. 2010;115(6):1173–1180. [DOI] [PubMed] [Google Scholar]
- 10.Amer-Wahlin I, Hellsten C, Noren H, et al. Cardiotocography only versus cardiotocography plus ST analysis of fetal electrocardiogram for intrapartum fetal monitoring: a Swedish randomised controlled trial. Lancet. 2001;358(9281):534–538. [DOI] [PubMed] [Google Scholar]
- 11.Belfort MA, Saade GR, Thom E, et al. A Randomized Trial of Intrapartum Fetal ECG ST-Segment Analysis. N Engl J Med. 2015;373(7):632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielinsky P, da Costa MH, Oliveira LT, Bonow FP, da Silva NI, Hagemann LL. [Natural history of myocardial hypertrophy and its association with hyperinsulinism in infants of diabetic mothers]. Arq Bras Cardiol. 1997;69(6):389–394. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Sulaiman RM, Subaih B. Congenital heart disease in infants of diabetic mothers: echocardiographic study. Pediatr Cardiol. 2004;25(2):137–140. [DOI] [PubMed] [Google Scholar]
- 14.El-Ganzoury MM, El-Masry SA, El-Farrash RA, Anwar M, Abd Ellatife RZ. Infants of diabetic mothers: echocardiographic measurements and cord blood IGF-I and IGFBP-1. Pediatr Diabetes. 2012;13(2):189–196. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez AB, Young L, Doll JA, Morgan GM, Crawford SE, Plunkett BA. Elevated neonatal insulin-like growth factor I is associated with fetal hypertrophic cardiomyopathy in diabetic women. Am J Obstet Gynecol. 2014;211(3):290.e291–297. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan PQ, Rowland TW, Shah BL, McGravey VJ, Reiter EO. Maternal diabetic control and hypertrophic cardiomyopathy in infants of diabetic mothers. Clin Pediatr (Phila). 1986;25(5):266–271. [DOI] [PubMed] [Google Scholar]
- 17.Sanhal CY, Daglar HK, Kara O, Uygur D, Yucel A. Assessment of fetal myocardial performance index in women with pregestational and gestational diabetes mellitus. J Obstet Gynaecol Res. 2017;43(1):65–72. [DOI] [PubMed] [Google Scholar]
- 18.Palmieri CR, Simoes MA, Silva JC, Santos AD, Silva MR, Ferreira B. Prevalence of Hypertrophic Cardiomyopathy in Fetuses of Mothers with Gestational Diabetes before Initiating Treatment. Rev Bras Ginecol Obstet. 2017;39(1):9–13. [DOI] [PubMed] [Google Scholar]
- 19.Akbariasbagh P, Shariat M, Akbariasbagh N, Ebrahim B. Cardiovascular Malformations in Infants of Diabetic Mothers: A Retrospective Case-Control Study. Acta Med Iran. 2017;55(2):103–108. [PubMed] [Google Scholar]
- 20.Rijpert M, Breur JM, Evers IM, et al. Cardiac function in 7-8-year-old offspring of women with type 1 diabetes. Exp Diabetes Res. 2011;2011:564316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sardesai MG, Gray AA, McGrath MM, Ford SE. Fatal hypertrophic cardiomyopathy in the fetus of a woman with diabetes. Obstet Gynecol. 2001;98(5 Pt 2):925–927. [DOI] [PubMed] [Google Scholar]
- 22.Prefumo F, Celentano C, Presti F, De Biasio P, Venturini PL. Acute presentation of fetal hypertrophic cardiomyopathy in a type 1 diabetic pregnancy. Diabetes Care. 2005;28(8):2084. [DOI] [PubMed] [Google Scholar]
- 23.Vincent M, Benbrik N, Romefort B, Colombel A, Bezieau S, Isidor B. Three patients presenting with severe macrosomia and congenital hypertrophic cardiomyopathy: a case series. J Med Case Rep. 2017;11(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yli BM, Källén K, Stray-Pedersen B, Amer-Wåhlin I. Intrapartum fetal ECG and diabetes. J Matern Fetal Neonatal Med. 2008;21(4):231–238. [DOI] [PubMed] [Google Scholar]
- 25.Welin AK, Blad S, Hagberg H, Rosen KG, Kjellmer I, Mallard C. Electrocardiographic changes following umbilical cord occlusion in the midgestation fetal sheep. Acta Obstet Gynecol Scand. 2005;84(2):122–128. [DOI] [PubMed] [Google Scholar]


