Abstract
Quisqualis indica L. of Combretaceae family is a traditional medicine that is widely used for various gastrointestinal discomfort including stomach pain, constipation, and digestive problem. In this study, the potential repeated dose toxicity and genotoxicity of a standardized Quisqualis indica L. extract (HU033) were determined under good laboratory practice conditions. For the repeated dose toxicity test, HU033 was orally administered to Sprague–Dawley (SD) rats at doses of 500, 1000, and 2000 mg/kg/day for 13 consecutive weeks. The genotoxicity of HU033 was determined with a standard battery of genotoxicity test, including an in vitro bacterial reverse mutation test, an in vitro chromosomal aberration test, and an in vivo micronucleus test. After 13 weeks of repeated dose of HU033 by oral administration, there was no treatment related adverse clinical sign including food consumption, organ weights, and histopathological findings or significant decrement in bodyweight. The no-observed-adverse-effect level of HU033 was higher than 2000 mg/kg in both male and female SD rats. No target organs were identified. In addition, no evidence of HU033 genotoxicity was detected based on results from the bacterial reverse mutation test, chromosomal aberration test, and micronucleus test. Based on results of this study, HU033 could be safely used in food and medical products within the tested dose range.
Keywords: Quisqualis indica L., Sub-chronic toxicity, NOAEL, Genotoxicity, 13-week
Introduction
Plant extract-derived substances have been recognized as major sources of herbal medicines. They are known to contain bioactive compounds with pharmacological properties that can provide novel drug candidates [1, 2]. The World Health Organization (WHO) has reported that about 80% of the global population relies on traditionally used medicinal plants for their primary health care [3]. Among numerous advantages of herbal medicines, the most common reasons for using them are affordability, correspondence to a patient’s ideology, relief from adverse effects of allopathic medicines, and effective public availability of health information [4–6].
Quisqualis indica L. (QI) is a type of tropical vines belonging to the family Combretaceae with different names by region, including Rangoon Creeper (English), Madhumalti (Hindi), Radha Manoharam (Telgu), and Vilayati chambeli (Marathi) [7]. Traditionally, extracts from different parts of QI were recognized as a complementary remedy for diverse disease conditions including bacterial infection, inflammation, pyrexia, and intestinal discomfort [8–12]. Moreover, it has been recently reported that QI extract can improve benign prostatic hyperplasia (BPH) by regulating prostate cell proliferation and ameliorate low urinary tract symptoms in a testosterone-induced BPH model [13, 14].
Although plant-derived medicines are often believed to be safer than synthetic drugs, the natural origin of products does not always guarantee the safety from potential toxicity [15]. In addition, there are several reports of toxicity related to indiscriminate use of natural medicines, including hepatotoxicity, nephrotoxicity, and mutagenicity [16–18]. To date, there have been various research studies on pharmacological activities of various parts of QI and derivatives in non-clinical/clinical studies. However, validated data on the safety of QI extract are insufficient. In addition, toxin components in plant species can vary depending on confounding factors, including environmental stresses and different parts (leaves, stem, root, and seeds) of the plant. It is necessary to evaluate the safety of plant derived material to ensure safe use of herbal medicines and further development of novel candidates. Thus, the objective of the present study was to qualitatively and quantitatively determine the potential 13-week oral toxicity and genotoxicity of a standardized QI seed ethanol extract (HU033) under good laboratory practice (GLP) conditions. The 13-week oral toxicity test and battery of genotoxicity studies were conducted in accordance with the guidelines by the Korea Ministry of Food and Drug Safety (No. 2017-71) and OECD (No. 471, 473, and 474) respectively.
Materials and methods
Preparation of test material
HU033 was prepared according to a previous report [14]. In brief, dried seeds of QI (Voucher no. HU033/SKJA150427) from a local herbal market (Ansan, Korea) were prepared as a powder (50 kg) and extracted by reflux with 70% ethanol for 6 h at 80 °C. After complete evaporation of organic solvent, total soluble solids content (HU033) was mixed with maltodextrin at a 1:1 mass ratio and spray-dried (ODA-25, SeoGang Engineering, Cheonan, Korea) to obtain the final extract. The standardization of HU033 was done with quisqualic acid content (1%) using a validated HPLC analysis [14].
Experimental animals
All experiments were conducted at Biotoxtech (Cheongju, Korea), an institution certified to conduct non-clinical studies under the GLP regulations. Sprague–Dawley (SD) rats of each sex (5 weeks old: male, 111.3–130.3 g; female, 99.8–123.6 g, n = 40 for each sex) and CrljOri:CD1 (ICR) mice (7 weeks old male mice weighing 30.1–32.4 g, n = 25) were purchased from Orient Bio Inc. (Seongnam, Korea). Animals were used after one week of acclimation. They were housed in an environment with controlled temperature (19–25 °C) and humidity (30–70%) and a light/dark cycle of 12 h/12 h with free access to a standard rodent chow and sterilized tap water ad libitum. All animal study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Biotoxtech (sub-chronic oral toxicity protocol #170827; micronucleus test protocol #170768). All animal studies were conducted in accordance with GLP Regulation and Test Guidelines of Standards for Toxicity Studies of Drugs issued by the Korean Food and Drug Administration.
13-week repeated oral dose toxicity study
All SD rats were randomly allocated into four groups (n = 10/group) for each sex and administered with either HU033 (at dose of 500, 1000, or 2000 mg/kg/day) or its vehicle at a volume of 10 mL/kg for 13 consecutive weeks. The present dose range was selected based on the result from the preliminary 2-week dose range finding study, which showed no adverse effect up to 5000 mg/kg/day (data not shown). The high dose was 70-fold higher than the maximum recommended clinical dose of HU033. Food and water consumption, body weight, and clinical signs were observed during the experimental period. All animals were observed twice daily for mortality, general condition, and clinical signs. Body weights were obtained for individuals just prior to dosing on day 1 and once a week thereafter. Food and water consumption were measured on the initial day of administration and then each week during the experimental period. After 13 weeks of repeated oral administration, all animals were euthanized with carbon dioxide inhalation. Postmortem samples were collected for further analysis, including serum biochemical analysis, urinalysis, and histopathological evaluation. The physicochemical property of urine was assessed using urine test strips (Comburtest®M stick, Roche, Germany) and an automatic analyzer (Cobas u411, Roche, Germany). Blood hematology and biochemistry analysis were done with a hematological autoanalyzer (ADVIA 2120i, SIEMENS, Germany) and an automated serum analyzer (7180, HITACHI, Japan), respectively. For histopathological evaluation, organs and tissues were collected and paraffin fixed followed by examination with a light microscope.
In vitro bacterial reverse mutation test
The Ames test was conducted in accordance with the OECD test guideline No. 471 for the testing of chemicals [19]. Positive controls for the mutagenicity test were 2-nitrofluorene (Sigma-Aldrich Co., St Louis, MO, USA), 2-aminoanthracene (Sigma-Aldrich), sodium azide (Sigma-Aldrich), 9-aminoacridine (Sigma-Aldrich), and 4-nitroquinoline N-oxide (Sigma-Aldrich). HU033 was weighed and dissolved in water for injection (JW Pharmaceutical Co., Ltd., Republic of Korea). It was serially diluted to make 0, 313, 628, 1250, 2500, and 5000 μg/plate. Histidine auxotroph mutants of Salmonella typhimurium strains (TA98, TA100, TA1535, and TA1537) and a tryptophan auxotroph mutant of Escherichia coli strain (WP2uvrA) were purchased from Molecular Toxicology Inc. (Boone, NC, USA) and cultured in 2.5% nutrient broth No. 2 (Oxoid Ltd, Basingstoke, UK) medium. The mutagenicity test was performed by mixing the serial concentration of test substance and tester strains (1 × 109 cells/mL) in the presence or absence of S9 mixture (Oriental Yeast Co., Ltd., Tokyo, Japan). After 20 min of incubation at 37 °C, the mixture was mixed with top Bacto agar (BD, USA) and a minimal amount of histidine-biotin (for TA98, TA100, TA1535 and TA1537) or tryptophan (for WP2uvrA) and then poured onto the surface of 15 mL of solidified bottom agar on Petri dishes (Thermo Fisher Scientific, Waltham, MA, USA). After 72 h of incubation, the number of revertant colonies was counted and compared to that of the control.
In vitro chromosomal aberration test
The in vitro chromosome aberration test was conducted using CHL/IU (Chinese Hamster Lung) cells in accordance with the OECD test guideline No. 473 for the testing of chemicals [20] and the MFDS Standards Guidelines for Toxicity Test of Pharmaceuticals (MFDS Notification, 2017-71) [21].
A preliminary dose range-finding test was performed to determine the toxicity of HU033 in Chinese hamster lung (CHL/IU) cells by calculating the relative population of doubling (RPD) in substance-treated culture. The RPD value was determined using the following formula:
The mutagenicity of HU033 was determined by evaluating its ability to induce chromosomal aberrations in CHL/IU cells purchased from the American Type Culture Collection (Manassas, VA, USA). These cells were maintained in Eagle’s minimum essential medium (Lonza Walkersville Inc., USA) supplemented with 10% fetal bovine serum (Gibco, USA). Cells were cultured in cell culture flasks (culture surface 75 cm2) at 37 ± 1 °C with 5% CO2 atmosphere in a humidified incubator (MCO 20AIC, SANYO, Japan). Benzo[a]pyrene (20 μg/mL) and mitomycin C (0.1 μg/mL) were used as positive controls in the presence or absence of the S9 mixture, respectively. The negative control was sterile water. Dose ranges in this study were determined according to the cytotoxicity observed in a dose-range finding test (data not shown). After the dose-range finding test, HU033 concentrations were 625, 1250, 2500, and 5000 μg/mL (short-term treatment and continuous treatment) in the absence of the S9 mixture. In the presence of the S9 mixture, HU033 concentrations were 625, 1250, 2500, and 5000 μg/mL (short-term treatment). Each 60 mm plate was seeded with cells at density of 5 × 104 cells/mL in complete culture medium and incubated for 24 h. After removing the complete culture medium, HU033 solution, S9 mixture, and complete culture medium were added to the plate. Cells were exposed to HU033 solution for 6 h (short-term treatment) or 24 h (continuous treatment). Cell cultures were then treated with 0.2 μg/mL colcemid solution (Gibco, USA). Metaphasic cells were harvested by gentle shaking and centrifugation. Cell pellets were resuspended in a 0.075 mol/L potassium chloride solution for hypotonic treatment and then fixed with a fixative solution (methanol:acetic acid = 3:1). A few drops of the cell pellet suspension were placed onto pre-cleaned glass microscope slides and air-dried. These slides were stained with 3% Giemsa stain solution. One slide was prepared for each culture. More than 300 metaphases per smear were analyzed under the microscope at 600 × magnification (BX51, Olympus, Shinjuku, Japan). Duplicate cultures were used for each dose. Structural aberrations in metaphasic chromosomes were categorized into chromosome types of breaks (csb), exchanges (cse), or gaps (csg), chromatid types of breaks (ctb), exchanges (cte), or gaps (ctg), fragmentation (frg), and total cells with structural aberrations including (gap+) or excluding (gap−) multiple aberration. The numerical aberration in metaphasic chromosomes was then categorized into polyploidy (pol) and endo-reduplication (end). Cells showing one or more chromosomal abnormalities were marked as abnormal cells. The type of each chromosomal abnormality was recorded accordingly.
In vivo micronucleus test
The in vivo micronucleus test was conducted in accordance with the OECD guidelines for the Testing of Chemicals, 474 Mammalian Erythrocyte Micronucleus Test [22], and the MFDS Standards Guidelines for Toxicity Test of Pharmaceuticals (MFDS Notification, 2017-71) [21]. Seven-week-old male ICR mice were obtained from a specific pathogen-free colony at ORIENTBIO Co. Ltd. (Seongnam, Korea). These animals were housed in a room with a temperature of 23 ± 3 °C and a relative humidity of 55 ± 15% under artificial lighting with a luminous intensity of 150–300 lx from 07:00 to 21:00 h and with 10–15 air changes per hour. Animals were permitted ad libitum access to an irradiation-sterilized pellet diet for laboratory animals (Teklad-certified irradiated global 18% protein rodent diet, 2918C, Envigo RMS, USA). Groundwater disinfected using an ultraviolet sterilizer followed by ultrafiltration was provided ad libitum via polycarbonate water bottles. After a 7-day period of quarantine and acclimatization, these ICR mice were randomly assigned to one of five groups (n = 5 for each group). In the preliminary test, three males and three females per group were administered at doses of 2000 and 5000 mg/kg/day of HU033 for two consecutive days. These animals were observed for 3 days (including the day of administration). There was no substantial difference in the toxicity profile between sexes (data not shown). Based on results of the preliminary test, HU033 was administered once daily for 2 days via gavage to each mouse at dose of 1000, 2000, or 5000 mg/kg/day. Daily application volume (10 mL/kg) was calculated in advance based on the body weight of each mouse on the day of treatment. The negative control group received an equivalent volume of sterile distilled water. Mitomycin C in normal saline was administered via intraperitoneal injection at 2 mg/kg/day as a positive control. During the study, all animals were observed twice daily for any clinical signs of toxicity and mortality. Twenty-four hours after the second treatment, mice were sacrificed by cervical dislocation. Their femurs were then obtained. Bone marrows were collected by flushing femurs with 200 μL FBS. Bone marrow cells were then centrifuged at 1000 rpm for 5 min at 4 °C and smeared on a clean slide glass. Smeared slides were air-dried, fixed in methanol, and then stained with 3% Giemsa solution for 30 min. These stained slides were observed under a fluorescence microscope at 600 × magnification (BX51, Olympus). Numbers of micronucleated polychromatic erythrocytes (MNPCE) and polychromatic erythrocytes (PCE) among red blood cells were counted. A genotoxic index was expressed as the average number of MNPCEs in 4000 PCEs per mouse. A cytotoxic index was expressed as the average ratio of PCE to RBCs by counting a total of 500 RBCs.
Statistical analyses
All statistical analyses were carried out using the SAS program version 9.1.3 (SAS Institute Inc., Cary, NC, USA). Data are expressed as mean ± standard deviation (SD). Differences in parameters (body weights, food consumption, and hematological data) from the control and test substance-treated groups were analyzed by one-way analysis of variance (ANOVA) following Bartlett’s test to determine the homogeneity of variance (p < 0.05, two-sided). If the variance was not homogeneous, data were analyzed using the nonparametric Kruskal–Wallis test. If statistical significance was observed (p < 0.05), the control and treated groups were compared using either Dunnett’s multiple comparison test (homogeneous data, p < 0.05, two-sided) or Steel’s multiple comparison test (heterogeneous data, p < 0.05, two-sided).
Results
Clinical signs, body weights, and food consumption
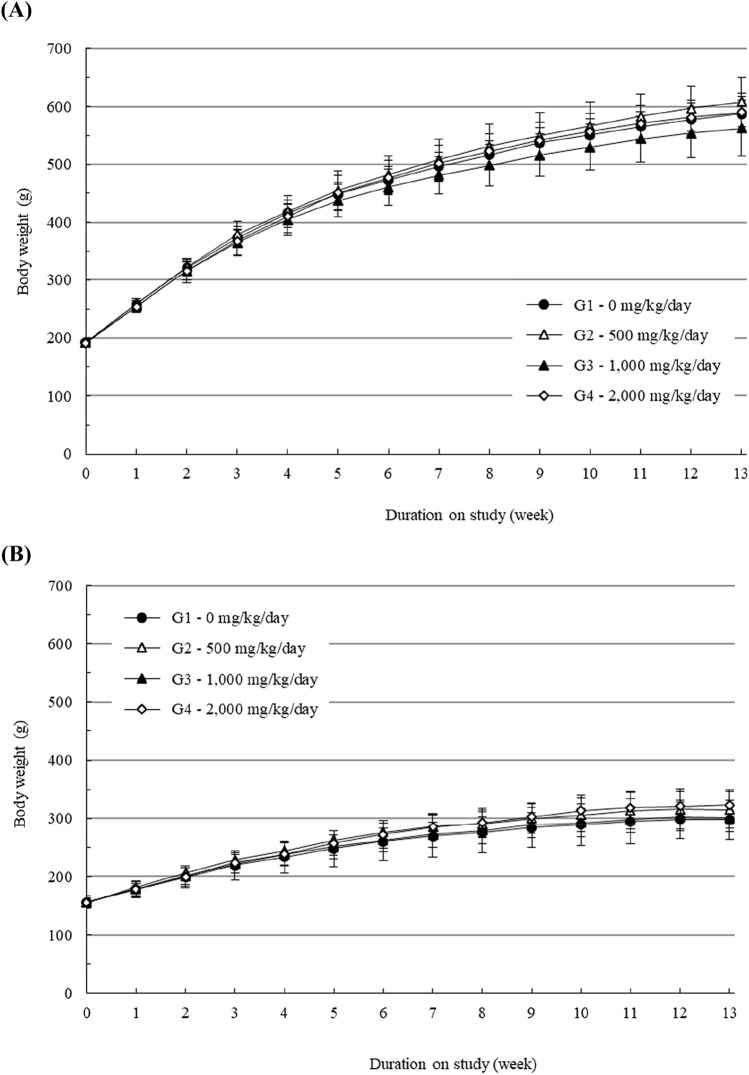
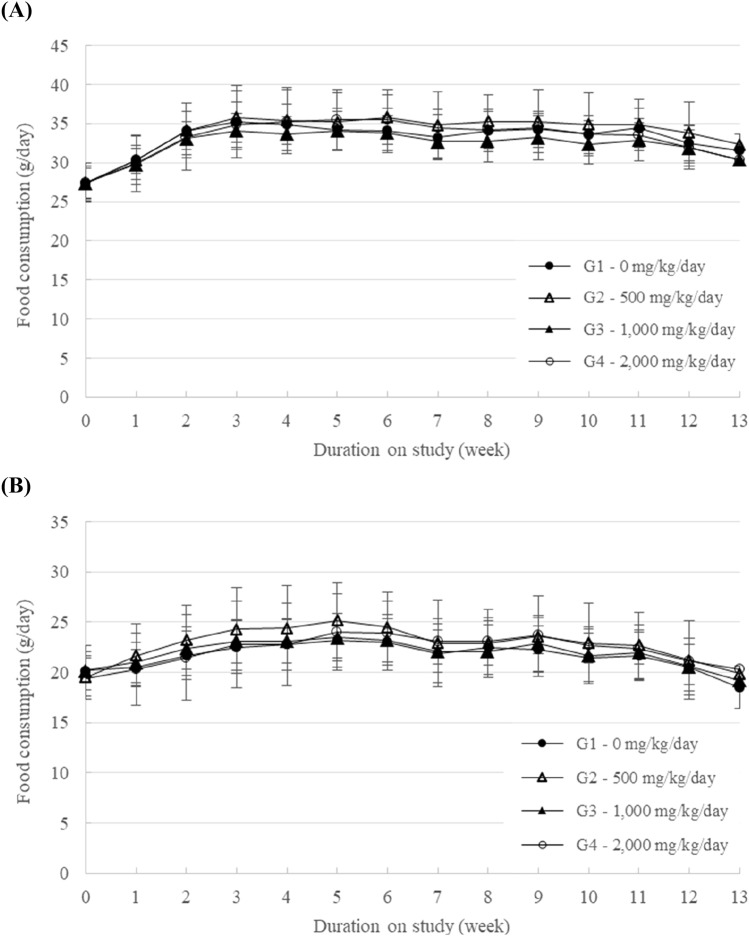
During the experimental period, mortality and abnormal clinical signs were not found in male or female rats at dose up to 2000 mg/kg/day group (data not shown). In addition, there was no significant difference in body weight or food consumption in male or female groups at any tested doses (Figs. 1, 2). Ophthalmic examination did not reveal any treatment related toxicological changes in any animals treated with HU033 either (data not shown).
Fig. 1.
Body weight changes of (A) male rats and (B) female rats treated with a standardized extract of Quisqualis indica L. (HU033) in the 13-week repeated oral dose toxicity study. Results are presented as the mean ± SD (n = 10)
Fig. 2.
Food consumption of (A) male rats and (B) female rats treated with HU033 in the 13-week repeated oral dose toxicity study. Results are presented as the mean ± SD (n = 10)
Urinalysis
No significant differences were observed in results of urinalysis (Table 1). Some values and frequencies of parameters showed increase and decrease tendencies, respectively. However, these findings were incidental. They were of no toxicological significance.
Table 1.
Urinalysis results of male and female rats treated with HU033 in the 13-week repeated oral dose toxicity study
| Dose (mg/kg/day) | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 500 | 1000 | 2000 | 0 | 500 | 1000 | 2000 | |
| No. of animals | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Volume (mL) | ||||||||
| Mean | 9.7 | 11.5 | 13.9 | 11.7 | 5.3 | 5.8 | 5.4 | 5.5 |
| SD | 1.4 | 2.9 | 4.9 | 3 | 1.6 | 2.1 | 1 | 2.2 |
| Color | ||||||||
| Pale yellow | 3 | 1 | 1 | |||||
| Yellow | 5 | 2 | 4 | 5 | 4 | 5 | 5 | 5 |
| Transparency | ||||||||
| Clear | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 |
| Mild turbidity | 1 | 1 | ||||||
| pH | ||||||||
| 6 | ||||||||
| 6.5 | 1 | |||||||
| 7 | 1 | |||||||
| 8 | 4 | 5 | 4 | 4 | 4 | 3 | 2 | 1 |
| 9 | 1 | 1 | 2 | 3 | 4 | |||
| Protein (mg/dL) | ||||||||
| – | 1 | 3 | 1 | 5 | 5 | 4 | 5 | |
| 25 | 4 | 2 | 4 | 5 | 1 | |||
| Glucose | ||||||||
| Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Ketone body | ||||||||
| – | 1 | 5 | 3 | 4 | 2 | 1 | ||
| 5 | 3 | 2 | 4 | 1 | 3 | 3 | 5 | |
| 15 | 1 | 1 | 1 | |||||
| 150 | ||||||||
| Bilirubin (mg/dL) | ||||||||
| – | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Occult blood (Ery/μL) | ||||||||
| – | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 |
| 50 | 1 | |||||||
| Casta | ||||||||
| 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Epithelial cella | ||||||||
| 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Leukocytea | ||||||||
| 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Erythrocytea | ||||||||
| 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Specific gravity | ||||||||
| 1.021–1.030 | 1 | 1 | 1 | |||||
| 1.031–1.040 | 1 | 2 | 1 | |||||
| 1.041–1.050 | 1 | 2 | 2 | 2 | 3 | 1 | 1 | 2 |
| 1.051–1.060 | 2 | 3 | 1 | 3 | 2 | 3 | 1 | |
| > 1.060 | 2 | 2 | ||||||
SD standard deviation
aSediment
Organ weight and histopathology
There were no significant differences in absolute or relative organ weights between HU033 treated groups and the control group (all p > 0.05; Table 2). There were no abnormalities of the external surface, internal organs, or tissues related to HU033 administration. Significant lesions increased or intensified in HU033 treated groups in comparison with the control group were not found either (data now shown).
Table 2.
Relative organ weights of male and female rats treated with HU033 in the 13-week repeated oral dose toxicity study (results are presented as the mean ± SD, n = 10)
| Dose (mg/kg/day) | ||||
|---|---|---|---|---|
| 0 | 500 | 1000 | 2000 | |
| No. of rats | 10 | 10 | 10 | 10 |
| Male | ||||
| Brain | 0.39 ± 0.03 | 0.38 ± 0.03 | 0.41 ± 0.03 | 0.38 ± 0.03 |
| Pituitary | 0.0022 ± 0.0002 | 0.0024 ± 0.0003 | 0.0024 ± 0.0002 | 0.0022 ± 0.0002 |
| Thyroid | 0.0058 ± 0.0007 | 0.0057 ± 0.0009 | 0.0061 ± 0.0008 | 0.0058 ± 0.0008 |
| Thymus | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.02 |
| Heart | 0.29 ± 0.02 | 0.31 ± 0.02 | 0.31 ± 0.03 | 0.30 ± 0.03 |
| Lung | 0.32 ± 0.02 | 0.31 ± 0.02 | 0.32 ± 0.02 | 0.30 ± 0.02 |
| Liver | 2.68 ± 0.14 | 2.67 ± 0.24 | 2.76 ± 0.19 | 2.76 ± 0.17 |
| Spleen | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.16 ± 0.03 | 0.16 ± 0.02 |
| Kidney | 0.60 ± 0.04 | 0.60 ± 0.05 | 0.64 ± 0.05 | 0.65 ± 0.04 |
| Adrenal | 0.0125 ± 0.0022 | 0.0125 ± 0.0019 | 0.0120 ± 0.0020 | 0.0120 ± 0.0033 |
| Testis | 0.66 ± 0.07 | 0.60 ± 0.16 | 0.71 ± 0.08 | 0.66 ± 0.10 |
| Epididymis | 0.28 ± 0.02 | 0.26 ± 0.06 | 0.30 ± 0.04 | 0.27 ± 0.03 |
| Prostate | 0.11 ± 0.02 | 0.12 ± 0.03 | 0.11 ± 0.02 | 0.12 ± 0.03 |
| Female | ||||
| Brain | 0.70 ± 0.08 | 0.63 ± 0.06 | 0.68 ± 0.05 | 0.65 ± 0.06 |
| Pituitary | 0.0059 ± 0.0009 | 0.0052 ± 0.0009 | 0.0058 ± 0.0011 | 0.0052 ± 0.0007 |
| Thyroid | 0.0074 ± 0.0013 | 0.0077 ± 0.0011 | 0.0079 ± 0.0015 | 0.0076 ± 0.0014 |
| Thymus | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.10 ± 0.02 |
| Heart | 0.34 ± 0.04 | 0.33 ± 0.03 | 0.34 ± 0.02 | 0.32 ± 0.02 |
| Lung | 0.42 ± 0.02 | 0.40 ± 0.04 | 0.40 ± 0.02 | 0.40 ± 0.02 |
| Liver | 2.44 ± 0.20 | 2.43 ± 0.11 | 2.47 ± 0.22 | 2.43 ± 0.12 |
| Spleen | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.02 |
| Kidney | 0.63 ± 0.05 | 0.64 ± 0.05 | 0.66 ± 0.05 | 0.64 ± 0.04 |
| Adrenal | 0.0248 ± 0.0022 | 0.0224 ± 0.0037 | 0.0249 ± 0.0049 | 0.0231 ± 0.0030 |
| Ovary | 0.309 ± 0.0081 | 0.0278 ± 0.0046 | 0.0278 ± 0.0058 | 0.0281 ± 0.0052 |
| Uterus | 0.21 ± 0.05 | 0.21 ± 0.06 | 0.22 ± 0.06 | 0.25 ± 0.06 |
Blood hematology and serum biochemistry
Treatment-related changes of hematological and serum biochemical parameters were not observed in HU033 treated groups compared to those of the control group (all p > 0.05; Tables 3, 4).
Table 3.
Hematological parameters of male/female rats treated with HU033 in the 13-week repeated oral dose toxicity study (results are presented as the mean ± SD, n = 10)
| Parameters | Dose (mg/kg/day) | |||
|---|---|---|---|---|
| 0 | 500 | 1000 | 2000 | |
| No. of rats | 10 | 10 | 10 | 10 |
| Male | ||||
| RBC (× 106cells/μL) | 8.38 ± 0.41 | 8.48 ± 0.36 | 8.61 ± 0.50 | 8.61 ± 0.35 |
| HGB (g/dL) | 14.6 ± 0.7 | 14.5 ± 0.4 | 14.4 ± 0.7 | 14.7 ± 0.4 |
| HCT (%) | 44.1 ± 2.0 | 43.9 ± 1.2 | 43.6 ± 2.1 | 44.4 ± 1.0 |
| MCV (fL) | 52.7 ± 1.6 | 51.8 ± 1.7 | 50.8 ± 1.6 | 51.7 ± 2.5 |
| MCH (pg) | 17.5 ± 0.6 | 17.1 ± 0.7 | 16.8 ± 0.5 | 17.1 ± 0.9 |
| MCHC (g/dL) | 33.2 ± 0.5 | 33.0 ± 0.4 | 33.0 ± 0.4 | 33.2 ± 0.4 |
| PLT (× 103 cells/μL) | 925 ± 123 | 885 ± 74 | 863 ± 92 | 869 ± 75 |
| WBC (× 103 cells/μL) | 7.45 ± 1.55 | 8.85 ± 2.85 | 7.68 ± 2.44 | 8.68 ± 2.52 |
| NEU (%) | 22.8 ± 7.2 | 20.1 ± 4.8 | 19.6 ± 8.3 | 22.4 ± 8.2 |
| LYM (%) | 72.0 ± 6.9 | 74.9 ± 4.9 | 75.4 ± 8.7 | 72.6 ± 8.0 |
| MONO (%) | 2.3 ± 0.7 | 2.2 ± 0.7 | 2.1 ± 0.4 | 2.4 ± 0.6 |
| EOS (%) | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.5 | 1.1 ± 0.4 |
| BASO (%) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Female | ||||
| RBC (× 106cells/μL) | 7.86 ± 0.24 | 7.99 ± 0.27 | 8.11 ± 0.25 | 7.94 ± 0.45 |
| HGB (g/dL) | 14.7 ± 0.6 | 14.7 ± 0.3 | 14.8 ± 0.3 | 14.6 ± 0.6 |
| HCT (%) | 42.6 ± 1.7 | 43.1 ± 1.4 | 43.1 ± 0.9 | 42.9 ± 1.7 |
| MCV (fL) | 54.2 ± 1.0 | 53.9 ± 1.0 | 53.1 ± 1.0 | 54.1 ± 1.7 |
| MCH (pg) | 18.7 ± 0.4 | 18.5 ± 0.4 | 18.3 ± 0.5 | 18.5 ± 0.6 |
| MCHC (g/dL) | 34.4 ± 0.3 | 34.3 ± 0.5 | 34.4 ± 0.5 | 34.1 ± 0.4 |
| PLT (× 103 cells/μL) | 928 ± 84 | 870 ± 77 | 843 ± 71 | 861 ± 72 |
| WBC (× 103 cells/μL) | 4.70 ± 1.45 | 5.03 ± 1.82 | 5.12 ± 2.40 | 4.41 ± 1.32 |
| NEU (%) | 18.1 ± 7.9 | 17.4 ± 7.3 | 11.9 ± 4.2 | 17.4 ± 7.2 |
| LYM (%) | 77.6 ± 7.8 | 77.4 ± 8.3 | 83.7 ± 4.4 | 77.9 ± 7.5 |
| MONO (%) | 1.9 ± 0.4 | 2.1 ± 0.7 | 2.0 ± 0.5 | 2.1 ± 0.8 |
| EOS (%) | 1.3 ± 0.5 | 1.7 ± 0.7 | 1.1 ± 0.5 | 1.5 ± 0.5 |
| BASO (%) | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
RBC red blood cell count, HGB hemoglobin, HCT hematocrit, MCV mean corpuscular volume, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, PLT platelet, WBC white blood cell count, NEU neutrophils, LYM lymphocytes, MONO monocyte, EOS eosinophils, BASO basophils
Table 4.
Serum biochemistry values of male and female rats treated with HU033 in the 13-week repeated oral dose toxicity study (results are presented as the mean ± SD, n = 10)
| Parameters | Dose (mg/kg/day) | |||
|---|---|---|---|---|
| 0 | 500 | 1000 | 2000 | |
| No. of rats | 10 | 10 | 10 | 10 |
| Male | ||||
| ALT (U/L) | 29.6 ± 6.9 | 26.1 ± 6.2 | 27.0 ± 7.1 | 24.4 ± 7.9 |
| AST (U/L) | 76.6 ± 19.3 | 77.8 ± 22.5 | 71.7 ± 14.9 | 75.5 ± 16.0 |
| ALP (U/L) | 291.1 ± 88.2 | 244.9 ± 32.5 | 252.4 ± 45.2 | 246.4 ± 42.9 |
| GGT (U/L) | 0.27 ± 0.14 | 0.27 ± 0.12 | 0.27 ± 0.08 | 0.35 ± 0.13 |
| Glu (mg/dL) | 143 ± 17 | 139 ± 8 | 154 ± 16 | 148 ± 10 |
| BUN (mg/dL) | 12.4 ± 1.5 | 11.7 ± 1.4 | 11.1 ± 1.8 | 12.0 ± 1.8 |
| Crea (mg/dL) | 0.41 ± 0.02 | 0.39 ± 0.03 | 0.39 ± 0.06 | 0.40 ± 0.03 |
| T-chol (mg/dL) | 83 ± 13 | 79 ± 17 | 78 ± 33 | 74 ± 14 |
| TG (mg/dL) | 51 ± 24 | 67 ± 28 | 54 ± 13 | 70 ± 23 |
| TP (mg/dL) | 5.9 ± 0.3 | 5.9 ± 0.3 | 5.9 ± 0.2 | 5.9 ± 0.3 |
| Alb (g/dL) | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.2 | 2.4 ± 0.1 |
| P (mg/dL) | 5.78 ± 0.65 | 5.53 ± 0.74 | 5.32 ± 0.87 | 5.63 ± 0.90 |
| Ca (mg/dL) | 9.9 ± 0.8 | 10.2 ± 0.3 | 10.1 ± 0.4 | 10.0 ± 0.5 |
| Female | ||||
| ALT (U/L) | 28.3 ± 11.9 | 48.6 ± 50.4 | 28.3 ± 7.3 | 29.7 ± 18.8 |
| AST (U/L) | 76.3 ± 23.6 | 80.7 ± 36.5 | 61.5 ± 9.2 | 79.9 ± 54.4 |
| ALP (U/L) | 142.8 ± 39.9 | 142.9 ± 36.9 | 119.5 ± 36.7 | 149.7 ± 37.2 |
| GGT (U/L) | 0.66 ± 0.23 | 0.72 ± 0.19 | 0.61 ± 0.31 | 0.64 ± 0.24 |
| Glu (mg/dL) | 133 ± 10 | 144 ± 12 | 139 ± 11 | 140 ± 13 |
| BUN (mg/dL) | 15.1 ± 1.3 | 13.1 ± 2.0 | 14.1 ± 2.2 | 13.6 ± 1.4 |
| Crea (mg/dL) | 0.49 ± 0.03 | 0.45 ± 0.03 | 0.46 ± 0.05 | 0.45 ± 0.04 |
| T-chol (mg/dL) | 91 ± 12 | 82 ± 8 | 91 ± 14 | 87 ± 12 |
| TG (mg/dL) | 21 ± 17 | 18 ± 7 | 25 ± 9 | 25 ± 10 |
| TP (mg/dL) | 6.3 ± 0.4 | 6.1 ± 0.2 | 6.3 ± 0.5 | 6.0 ± 0.4 |
| Alb (g/dL) | 2.8 ± 0.3 | 2.7 ± 0.1 | 2.9 ± 0.4 | 2.6 ± 0.2 |
| P (mg/dL) | 4.45 ± 0.58 | 4.28 ± 0.68 | 4.14 ± 0.60 | 4.36 ± 0.68 |
| Ca (mg/dL) | 9.8 ± 0.4 | 9.9 ± 0.3 | 9.9 ± 0.2 | 9.5 ± 0.5 |
ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gamma glutamyl transpeptidase, BUN blood urea nitrogen, Crea creatinine, TP total protein, Alb albumin, T-chlo total cholesterol, TG triglycerides, P phosphorus, Glu glucose, Ca calcium
Reverse mutation test
Bacterial reverse mutation test results are presented in Table 5. All positive control drugs increased the number of revertant colonies for all tested mutant bacterial strains. There were no increases in the number of revertant colonies in groups treated with HU-033 at all doses tested (0, 313, 628, 1250, 2500, and 5000 μg/plate) compared to the negative control group in the absence or presence of S9 metabolic activation, supported the non-mutagenic potential of HU-300.
Table 5.
Result of the bacterial reverse mutation test of HU033 in the absence or presence of S9 metabolic activation
| Strain | Test substance | Dose (μg/plate) | No. of revertant colony counts | |
|---|---|---|---|---|
| S9 (−) | S9 ( +) | |||
| TA98 | HU033 | 0 | 21.7 ± 3.3 | 35.5 ± 0.5 |
| 313 | 21.8 ± 2.1 | 37.7 ± 1.5 | ||
| 628 | 24.2 ± 4.3 | 35 ± 0.9 | ||
| 1250 | 22.3 ± 1 | 31.7 ± 1.2 | ||
| 2500 | 26 ± 4.5 | 34.7 ± 1.2 | ||
| 5000 | 26 ± 3.1 | 30.8 ± 0.8 | ||
| 2-Nitrofluorene | 5.0 | 718 ± 18.7 | – | |
| 2-Aminoanthracene | 1.0 | – | 368.7 ± 49.2 | |
| TA100 | HU033 | 0 | 103.8 ± 2.1 | 111.5 ± 4.7 |
| 313 | 100.2 ± 4.4 | 107.8 ± 7.9 | ||
| 628 | 96.8 ± 7.5 | 112.8 ± 5.1 | ||
| 1250 | 108.8 ± 6.1 | 116.3 ± 4.8 | ||
| 2500 | 104.7 ± 3.4 | 123.3 ± 8.6 | ||
| 5000 | 101.2 ± 3.3 | 131 ± 3.8 | ||
| Sodium azide | 1.5 | 715.3 ± 16.8 | – | |
| 2-Aminoanthracene | 2.0 | – | 963.7 ± 27.9 | |
| TA1535 | HU033 | 0 | 15.2 ± 0.8 | 11.7 ± 1 |
| 313 | 14.2 ± 2.3 | 13.7 ± 2 | ||
| 628 | 16 ± 2.8 | 13.2 ± 1.3 | ||
| 1250 | 15 ± 1.8 | 13.8 ± 1.7 | ||
| 2500 | 17 ± 1.8 | 15.5 ± 1 | ||
| 5000 | 20.8 ± 1.2 | 18 ± 0.9 | ||
| Sodium azide | 1.5 | 577 ± 13.1 | – | |
| 2-Aminoanthracene | 3.0 | – | 172.8 ± 6.9 | |
| TA1537 | HU033 | 0 | 9.7 ± 0.5 | 21.3 ± 0.8 |
| 313 | 11.2 ± 1.8 | 20.8 ± 0.8 | ||
| 628 | 10.8 ± 1.2 | 21.7 ± 1.4 | ||
| 1250 | 10.7 ± 1 | 20.3 ± 1.5 | ||
| 2500 | 12.3 ± 2.3 | 21 ± 1.3 | ||
| 5000 | 11.7 ± 2.1 | 19.7 ± 0.8 | ||
| 9-Aminoacridine | 80.0 | 583.3 ± 11.5 | – | |
| 2-Aminoanthracene | 3.0 | – | 224.7 ± 16 | |
| WP2uvrA | HU033 | 0 | 120 ± 17.5 | 142.3 ± 5.8 |
| 313 | 133 ± 9.5 | 141.8 ± 5.8 | ||
| 628 | 132.3 ± 15.1 | 141.8 ± 6.7 | ||
| 1250 | 143.5 ± 6.6 | 156.3 ± 18.5 | ||
| 2500 | 139.5 ± 8.2 | 163.3 ± 7.1 | ||
| 5000 | 144.3 ± 20.4 | 174.7 ± 4 | ||
| 4-Nitroquinoline N-oxide | 0.1 | 692.5 ± 54.8 | – | |
| 2-Aminoanthracene | 2.0 | – | 541.2 ± 45 | |
In vitro chromosome aberration test
Positive control drugs mitomycin C (MMC) and benzo[a]pyrene (B[a]P) increased chromosomal abnormalities in CHL/IU cells up to more than 80% (Table 6). In contrast, frequencies of CHL/IU cells showing aberrations in chromosomal structure and chromosome numbers were not significantly increased when compared to those of the relevant negative control at all HU033 doses (625, 1250, 2500, and 5000 μg/mL) tested.
Table 6.
Results of chromosomal aberration test of HU033 in the absence or presence of S9 metabolic activation
| Trt-Rec time (hr) | S9 mix | Drug | Dose (μg/mL) | RPD (%) | No. of cells with structural aberrations | No. of cells with numerical aberrations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ctb | csb | cte | cse | frg | Gap | Total(%) | End | Pol | Total (%) | |||||||
| ctg | csg | Gap − | Gap + | |||||||||||||
| 6–18 | − | HU033 | 0 | 100 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| 625 | 99.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 1250 | 99.5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | |||
| 2500 | 77.7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | |||
| 5000 | 68.9 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 4 | 0 | 1 | 1 | |||
| MMC | 0.1 | 59.9 | 6 | 0 | 37 | 1 | 0 | 1 | 0 | 43* | 44 | 0 | 1 | 1 | ||
| + | HU033 | 0 | 100 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | |
| 625 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 1250 | 87.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 2500 | 83.2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | |||
| 5000 | 68.2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |||
| B[a]P | 20 | 51.3 | 10 | 1 | 62 | 1 | 0 | 0 | 0 | 67* | 67 | 0 | 0 | 0 | ||
| 24–0 | − | HU033 | 0 | 100 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| 625 | 83.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 1250 | 77.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | |||
| 2500 | 53.0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |||
| 5000 | 47.6 | 3 | 1 | 0 | 0 | 0 | 3 | 0 | 3 | 6 | 0 | 1 | 1 | |||
| MMC | 0.1 | 52.2 | 15 | 0 | 70 | 2 | 0 | 1 | 0 | 79* | 80 | 0 | 1 | 1 | ||
Trt-Rec treatment-recovery, RPD relative population doubling, ctb chromatid break, csb chromosome break, cte chromatid exchange, cse chromosome exchange, frg fragmentation, ctg chromatid gap, csg chromosome gap, gap− total number of cells with structural aberrations excluding gap, gap + total number of cells with structural aberrations including gap, end endo-reduplication, pol polyploidy
Significant difference from negative control, *p < 0.01
In vivo micronucleus test
As shown in Table 7, treatment-related body weight changes and death were not observed in any groups treated with HU033 at dose of 1000, 2000, or 5000 mg/kg. PCE/(PCE + NCE) ratio, a bone marrow cytotoxicity index, was about 30% for all HU033 treated groups. This ratio was comparable to that of the negative control group. In addition, significant HU033 related increases in MNPCEs frequencies in 4000 PCEs were not observed at any dose level compared with the negative control group (Table 8).
Table 7.
Body weights of HU033 treated male ICR mice
| Group | Dose (mg/kg) | Route | Before 1st dosing | 1 day after 2nd dosing |
|---|---|---|---|---|
| Negative control (water) | 0 | PO | 34.8 ± 1.44 | 34.8 ± 1.52 |
| HU033 | 1000 | PO | 34.7 ± 1.48 | 34.6 ± 1.66 |
| 2000 | PO | 34.5 ± 1.08 | 34.3 ± 0.69 | |
| 5000 | PO | 34.4 ± 1.17 | 33.7 ± 1.40 | |
| Positive control (MMC) | 2 | IP | 34.3 ± 1.14 | 34.2 ± 0.80 |
Table 8.
Summary of the micronucleus test using male ICR mice
| Group | Dose (mg/kg) | PCE/(PCE + NCE) (%) | MNPCE/4000 PCE (%) |
|---|---|---|---|
| Negative control (water) | 0 | 32.3 ± 4.13 | 0.060 ± 0.029 |
| HU033 | 1000 | 31.5 ± 2.29 | 0.035 ± 0.042 |
| 2000 | 29.0 ± 6.42 | 0.045 ± 0.027 | |
| 5000 | 33.2 ± 1.58 | 0.055 ± 0.048 | |
| Positive control (MMC) | 2 | 30.6 ± 3.38 | 5.555* ± 0.571 |
MNPCE micronucleated polychromatic erythrocyte, PCE polychromatic erythrocyte, NCE normochromatic erythrocyte
Significant difference from negative control, * p < 0.01
Discussion
Due to decades of traditional use and numerous empirical data, herbal materials are often believed to be safer than modern synthetic pharmaceuticals [23]. As the use of herbal materials has been expanded from its traditional complementary use to resource for developing drugs development to find a novel therapeutic intervention, the importance of securing safety profiles of herbal materials is increasing. However, scientifically confirmed safety of herbal medicines in general is still lacking. Only 15% of randomized controlled trials evaluating herbal medicines have provided information on safety or side effects of herbal materials [24]. Despite their traditional use, several herbal materials from traditional medicine have potential genotoxicity [25–27]. Thus, the present study was carried out to evaluate the potential toxicity of HU033 in terms of its sub-chronic oral toxicity and genotoxicity.
It has been reported that the QI extract contains various phytoconstituents such as tannins, trigonelline, l-proline, l-aspragine, quisqualic acid, rutin, and cysteine synthase that are responsible for its various pharmacological activities [28]. In the present study, HU033, an ethanol extract of QI seeds, was used. It has been reported to contain asparagine, quisqualic acid, arginine, and glutamic acid [14]. Among these constituents, quisqualic acid, a relatively dominant constituent, was selected as marker compound for standardization. Its content was 1% of total seed extract.
The potential sub-chronic oral toxicity of HU033 was determined after 13-week consecutive oral administration to SD rats. Gross toxicity, NOAEL, and histopathology were assessed. In this study, repeated oral administration of HU033 in the 13-week repeated oral dose toxicity study did not result in death or any significant treatment-related adverse effects such as clinical signs, bodyweight, or food/water consumption. Extracts from all parts of QI plants have been reported to possess various biological effects. Especially, the seed extract of QI has been found to possess a potent cytotoxic anthelimintic activity [29]. Quisqualic acid, a marker compound from HU033, is one of the most potent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor agonists. It might lead to excitotoxicity [30]. In this study, consecutive 13-week of repeated dosing of HU033 in rats at dose up to 2000 mg/kg did not induce any adverse clinical signs including neurological signs or mortality. In repeated dose toxicity studies, it is recommended to establish a recovery group to ascertain the delayed onset, continuation or recovery of the toxic action of the substance. In the present 13-week repeated dose toxicity study, considering the relatively lower dose in 13-week study when compared to the preliminary 2-week DRF study, which showed no adverse effect at a dose of 5000 mg/kg, the recovery group was omitted. Despite there was no adverse effect observed in the 13-week toxicity study in all group tested, missing recovery group in this study might hinder proper understanding of the possible delayed toxicity of HU033.
Previously, it has been demonstrated that HU033 can attenuate benign prostate hyperplasia (BPS) state in testosterone propionate-induced BPS rat model by reducing prostate size and proliferation [14, 31]. However, abnormal changes in reproductive organ weights, necropsy, and histopathological findings were not observed in any dose groups of this study, indicating that effects of HU033 on sex-related hormones and reproductive organs in healthy state were negligible. In addition, the high dose (2000 mg/kg/day) in this study was 70-fold higher than the maximum recommended clinical dose (2000 mg/day) of HU033. Furthermore, in previous reports, 4-week repeated oral administration of HU033 did not induce any toxic effects at a dose of 2000 mg/kg/day in F344 rats [13]. Based on these results, it could be presumed that repeated oral administration of HU033 is safe without causing evident toxicities in rats at dose up to 2000 mg/kg/day.
The absence of treatment related mortality and clinical sings cannot guarantee the safety of herbal materials with respect to genotoxicity. Genotoxicity testing includes tests that measure substances’ capability to damage DNA or cellular chromosomes. These tests were conducted to recognize potential genotoxic carcinogens. To the authors' best knowledge, this is the first report on the genotoxicity of QI seed ethanol extract using a battery of genotoxic tests, including reverse mutation, chromosomal aberration, and micronucleus tests.
The Ames test, a reverse mutation test, is recognized as an initial screening test to assess mutagenic potential of candidate, especially related to point mutations, including addition, deletion, and substitution of one or a few DNA base pairs [19]. In the present study, HU033 treatment at dose up to 5000 μg/plate did not induce increment in the mean number of revertant colonies for histidine or tryptophan auxotrophic strains tested in the presence or absence of metabolic activation conditions.
The genotoxic potential, especially clastogenicity, of HU033 was tested using chromosomal aberration test. Diverse chromosomal aberrations have been reported in genetic diseases. Aberrations in tumor related genes from somatic cells are known to be involved in cancer initiation [32]. In the present study, various types of structural aberrations, mostly cte, were detected in the genotoxic positive control, MMC and B[a]P treated CHL/IU cells. Meanwhile, there was no statistical difference in the frequency of aberrations in the structure or number of chromosomes between HU033-treated groups at all dose levels and the negative control irrespective of metabolic activation. Based on these results, HU033 did not induce chromosomal aberrations in CHL/IU cells at tested doses.
The micronucleus test is a frequently used in vivo test system that can assess chromosomal damage caused by mutagen exposure, which can support genotoxicity profiles of candidates with weight of evidence [33]. In this study, we confirmed the safety profile of HU033 in the bone marrow with a dose up to 5000 mg/kg. After confirming that the dose caused no signs of toxicity in ICR mice, bone marrow cells were harvested from mice femurs. In this study, the PCE/(PCE + NCE) ratio was evidently increased in the positive control, indicating an enhanced cytotoxicity. On the contrary, HU033 dose-related increments of PCE/(PCE + NCE) ratio and the number of MNPCE were not found at any HU033 dose levels.
In conclusion, results of this study confirmed the safety profile of HU033 in repeated oral toxicity and genotoxicity study. The NOAEL of HU033 in the 13-week repeated oral dose toxicity study was higher than 2000 mg/kg. Potential genotoxicity of HU033 was not detected. This study provided validated safety information for HU033. Thus, HU033 could be safely used in functional foods and medical products within the test dose range.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Agri-Bio Industry Technology Development Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (2016190262).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Je-Won Ko, Email: rheoda@cnu.ac.kr.
Tae-Won Kim, Email: taewonkim@cnu.ac.kr.
References
- 1.Rakotoarivelo NH, Rakotoarivony F, Ramarosandratana AV, Jeannoda VH, Kuhlman AR, Randrianasolo A, Bussmann RW. Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J Ethnobiol Ethnomed. 2015;11:68. doi: 10.1186/s13002-015-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011;8(1):1–10. doi: 10.4314/ajtcam.v8i1.60483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosihuzzaman M. Herbal medicine in healthcare-an overview. Nat Prod Commun. 2012;7(6):807–812. doi: 10.1177/1934578x1200700628. [DOI] [PubMed] [Google Scholar]
- 4.Cohen PA, Ernst E. Safety of herbal supplements: a guide for cardiologists. Cardiovasc Ther. 2010;28(4):246–253. doi: 10.1111/j.1755-5922.2010.00193.x. [DOI] [PubMed] [Google Scholar]
- 5.Loya AM, González-Stuart A, Rivera JO. Prevalence of polypharmacy, polyherbacy, nutritional supplement use and potential product interactions among older adults living on the United States-Mexico border: a descriptive, questionnaire-based study. Drugs Aging. 2009;26(5):423–436. doi: 10.2165/00002512-200926050-00006. [DOI] [PubMed] [Google Scholar]
- 6.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm R, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulshreshtha M, Srivastava G, Singh MP. Pharmacognostical, anti-oxidant activity and high performance thin layer chromatography studies on leaves of Quisqualis indica Linn. Curr Tradit Med. 2018;4(1):53–67. doi: 10.2174/2215083804666180118095645. [DOI] [Google Scholar]
- 8.Barik BS, Das S, Hussain T. Pharmacognostic properties of Quisqualis indica Linn: against human pathogenic microorganisms: an insight review. Eur J Med Plants. 2020;31(20):87–103. doi: 10.9734/ejmp/2020/v31i2030369. [DOI] [Google Scholar]
- 9.Kaiser MA, Islam MR, Rahman MS, Hossain MK, Rashid MA. Total phenolic content, free radical scavenging activity and reducing power of Quisqualis indica Linn. Dhaka Univ J Pharm Sci. 2009;8(2):173–175. doi: 10.3329/dujps.v8i2.6034. [DOI] [Google Scholar]
- 10.Singh VV, Jain J, Mishra AK. Determination of antipyretic and antioxidant activity of Cassia occidentalis Linn methanolic seed extract. Pharmacogn J. 2017;9(6):913–916. doi: 10.5530/pj.2017.6.143. [DOI] [Google Scholar]
- 11.Pal P, Singh A. In-vitro antioxidant and anti-inflammatory activities of Quisqualis indica Linn. Leaves Extract J Adv Biol. 2019;21(1):1–13. doi: 10.9734/jabb/2019/v21i130082. [DOI] [Google Scholar]
- 12.Gupta S, Singh A (2017) Antimicrobial, analgesic and anti-inflammatory activity reported on Tamarindus indica Linn root extract. Pharmacogn J. 10.5530/pj.2017.3.70
- 13.Wijerathne CU, Park HS, Jeong HY, Song JW, Moon OS, Seo YW, Won YS, Son HY, Lim JH, Yeon SH, Kwun HJ. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Bio Pharm Bull. 2017;40:2125–2133. doi: 10.1248/bpb.b17-00468. [DOI] [PubMed] [Google Scholar]
- 14.Baek JM, Kim HJ, Nam MW, Park HJ, Yeon SH, Oh MH, Yoon JS, Kwon HJ, Lee KP, Lim JH. Standardized seed extract of Quisqualis indica (HU033) attenuates testosterone propionate-induced benign prostatic hyperplasia via α1-adrenergic receptors and androgen/estrogen signaling. Prev Nutr Food Sci. 2019;24(4):492. doi: 10.3746/pnf.2019.24.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Oliveira AM, do Nascimento MF, Ferreira MRA, de Moura DF, dos Santos Souza TG, da Silva GC, da Silva Ramo EH, Paiva PMG, de Medeiros PL, da Silva TG, Soares LAL (2016) Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (Moraceae) in mice. J Ethnopharmacol 194:162‒168. 10.1016/j.jep.2016.09.004 [DOI] [PubMed]
- 16.Furbee RB, Barlotta KS, Allen MK, Holstege CP. Hepatotoxicity associated with herbal products. Clin Lab Med. 2006;26(1):227–241. doi: 10.1016/j.cll.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Asif M. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012 doi: 10.4103/2277-9175.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuete V, Tamokouu JDD (2014) Mutagenicity and carcinogenicity of African medicinal plants. In: Toxicological survey of African medicinal Plants, 1st edn. Elsevier, Netherlands, pp 277‒322
- 19.Organization for Economic Cooperation and Development (OECD) (1997) OECD guideline for testing of chemicals: bacterial reverse mutation test No. 471. OECD, Paris
- 20.OECD (1997) OECD guideline for testing of chemicals: in vitro mammalian chromosome aberration test No. 473. OECD, Paris
- 21.Ministry of Food and Drug Safety (MFDS) (2017) Guidelines for toxicity tests of pharmaceuticals. No. 2017-2071. MFDS, Sejong, Republic of Korea
- 22.OECD (1996) OECD guideline for testing of chemicals: mammalian erythrocyte micronucleus test No. 474. OECD, Paris
- 23.Jordan SA, Cunningham DG, Marles RJ. Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol. 2010;243(2):198–216. doi: 10.1016/j.taap.2009. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Douglas RM. Chinese herbal medicines in the treatment of acute respiratory infections: a review of randomized and controlled clinical trials. Med J Aust. 1998;169:579–582. doi: 10.5694/j.1326-5377.1998.tb123423.x. [DOI] [PubMed] [Google Scholar]
- 25.Ansah C, Khan A, Gooderham NJ. In vitro genotoxicity of the West African anti-malarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Toxicology. 2005;208(1):141–147. doi: 10.1016/j.tox.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Zan MA, Ferraz A, Richter MF, Picada JN, de Andrade HH, Lehmann M, Dihl RR, Nunes E, Semedo J, Da Silva J (2013) In vivo genotoxicity evaluation of an artichoke (Cynara scolymus L.) aqueous extract. J Food Sci 78:367‒371. 10.1111/1750-3841.12034 [DOI] [PubMed]
- 27.Fateh AH, Mohamed Z, Chik Z, Alsalahi A, Zain SRM, Alshawsh MA. Mutagenicity and genotoxicity effects of Verbena officinalis leaves extract in Sprague-Dawley rats. J Ethnopharmacol. 2019;235:88–99. doi: 10.1016/j.jep.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Lin TC, Ma YT, Wu J, Hsu FL. Tannins and related compounds from Quisqualis indica. J Chin Chem Soc. 1997;44(2):151–155. doi: 10.1002/jccs.199700025. [DOI] [Google Scholar]
- 29.Ekawardhani S, Anggoro UT, Krissanti I. Anthelmintic potential of medicinal plants against Ancylostoma caninum. Vet Med Int. 2021;2021:3879099. doi: 10.1155/2021/3879099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Robert A, Vogensen SB, Howe JR. The relationship between agonist potency and AMPA receptor kinetics. Biophys J. 2006;91(4):1336–1346. doi: 10.1529/biophysj.106.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DG, Kwon HJ, Lim JH, Kim JH, Lee KP. Quisqualis indica extract ameliorates low urinary tract symptoms in testosterone propionate-induced benign prostatic hyperplasia rats. Lab Anim Res. 2020;36(1):1–10. doi: 10.1186/s42826-020-00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson SL, Compton DA. Chromosomes and cancer cells. Chromosome Res. 2011;19(3):433–444. doi: 10.1007/s10577-010-9179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi M. The micronucleus test—most widely used in vivo genotoxicity test. Genes Environ. 2016;38(1):1–6. doi: 10.1186/s41021-016-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]