Abstract
Extracellular vesicles (EVs) are secreted by both eukaryotes and prokaryotes, and are present in all biological fluids of vertebrates, where they transfer DNA, RNA, proteins, lipids, and metabolites from donor to recipient cells in cell-to-cell communication. Some EV components can also indicate the type and biological status of their parent cells and serve as diagnostic targets for liquid biopsy. EVs can also natively carry or be modified to contain therapeutic agents (e.g., nucleic acids, proteins, polysaccharides, and small molecules) by physical, chemical, or bioengineering strategies. Due to their excellent biocompatibility and stability, EVs are ideal nanocarriers for bioactive ingredients to induce signal transduction, immunoregulation, or other therapeutic effects, which can be targeted to specific cell types. Herein, we review EV classification, intercellular communication, isolation, and characterization strategies as they apply to EV therapeutics. This review focuses on recent advances in EV applications as therapeutic carriers from in vitro research towards in vivo animal models and early clinical applications, using representative examples in the fields of cancer chemotherapeutic drug, cancer vaccine, infectious disease vaccines, regenerative medicine and gene therapy. Finally, we discuss current challenges for EV therapeutics and their future development.
Key words: Extracellular vesicle, Therapeutic agent, Delivery carrier, Outer membrane vesicle, Cancer therapy, Infectious disease vaccine, Regenerative medicine, Gene therapy
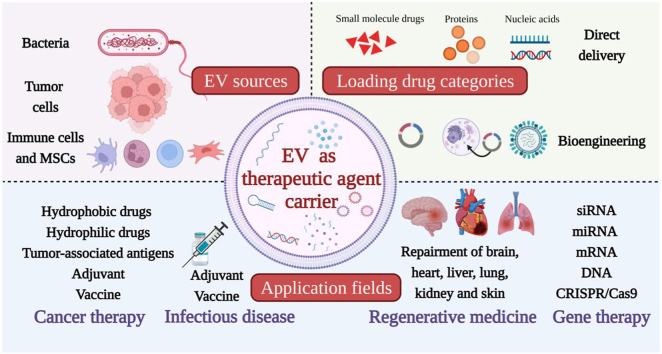
Graphical abstract
Different sources’ extracellular vesicles (EVs) can load various therapeutic agents to play the excellent therapeutic role in the fields of cancer therapy, infectious disease vaccines, regenerative medicine and gene therapy.
1. Introduction
Extracellular vesicles (EVs) are natural nanoscale phospholipid bilayer structures that are actively released by eukaryotic prokaryotic cells1,2. At their discovery in the late 1980s, EVs were considered to be cellular “junk”3, but research and methodological developments have since led researchers to realize that EVs play critical roles in cell-to-cell communications that regulate both homeostatic and disease processes through their actions on immune function, tissue repair, and cell growth. Recent advances have significantly increased understanding of EV interactions and effects upon their recipient cells.
EVs are detectable in all vertebrate body fluids and contain DNA, RNA, proteins, lipids, and metabolites from their parental cells, some of which are specific for their parental cell type or its physiologic or disease status4. Analysis of these cargoes can therefore provide evidence for early disease diagnosis and real-time evaluation of disease severity to allow prognostic evaluation and treatment monitoring. EVs also can also function as excellent nanocarriers for drug delivery. Synthetic nanocarriers fabricated from cationic polymers, cyclodextrin, lipids, etc. have been examined for delivery of therapeutic agents5, but their clinical application can be hindered by their toxicity, immunogenicity, low loading efficiency, and preferential accumulation in the liver and spleen6. EVs, by contrast, have strong potential in applications ranging from cancer therapy to regenerative medicine due to their high biocompatibility and low toxicity and immunogenicity. EVs can also evade the mononuclear phagocytic system to show relatively stable in the circulation and penetrate multiple biological barriers to improve their accumulation at targeted sites7. EVs can also be modified by bioengineering approaches to achieve targeted delivery of their therapeutic cargoes to specific sites8.
In this review, we summarize recent developments, advances, and representative examples that apply to the development of EV therapeutic biomedical applications.
2. Classification
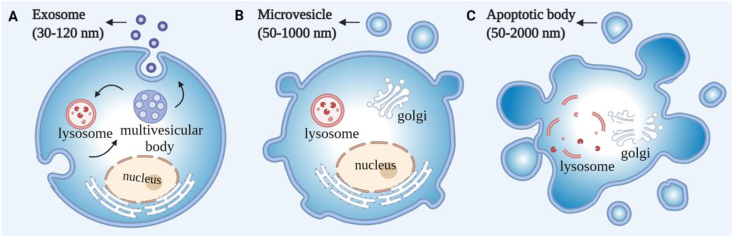
EVs are generally classified into three major subpopulations by their diameters and biogenesis mechanisms: exosomes (30–120 nm), microvesicles (50–1000 nm), and apoptotic bodies (50–2000 nm)9 (Fig. 1). Exosomes are generated by inward budding of the endosome membrane to form multivesicular bodies (MVBs) that fuse with the plasma membrane to release mature exosomes via a closely regulated process. Conversely, microvesicles and apoptotic bodies are generated by outward budding of the plasma membrane by different processes in viable cells and in cells undergoing proGrammed death, respectively10. However, all three EV populations can overlap in size, surface markers, and composition to prevent reliable isolation of pure EV samples of specific subtypes by methods in current use. Given this lack of precision, we use the generic term “EV” rather than exosome or microvesicle in this review when describing results of studies that report the innate or engineered properties of these vesicles.
Figure 1.
Biogenesis and classification of extracellular vesicle subtypes. (A) Exosomes are released by the fusion of multivesicular bodies with the plasma membrane. (B) Microvesicles are generated by outward budding of the plasma membrane. (C) Apoptotic bodies are produced by the budding of the cell undergoing programmed death.
This does not imply that these EVs carry the same markers and mediate similar functions. One study that analyzed the proteomes of large and small EVs (100–800 nm vs. 30–150 nm) identified multiple proteins that were preferentially enriched in large EVs (ATP5F1A/B, DHX9, GOT2, HSPA5, HSPD1, MDH2, STOML2) and small EVs (CD9, CD44, CD63, CD81, CD82, PDCD6IP, SDCBP, TSG101)11. However, this study did not attempt to determine if the EVs derived from the cytoplasmic or endosomal membrane (ectosomes vs. exosomes), although gene ontology analysis indicated the endosome associated proteins were overrepresented in the small EV fraction, as would be expected due to the size range of exosomes. Another study that attempted to identify markers differentially expressed in the proteomes of exosomes and endosomes of HeLa cells reported that EVs that express CD9 and CD81 but little CD63 primarily derive from the plasma membrane, while those that express CD63 but little CD9 predominantly derive from the endosomal membrane12. However, this has yet to be replicated in other cell types and under different conditions. EV subtype classification and analysis is also further complicated by potential differential contributions from distinct exosome subtypes, since one group has reported that exosomes can be further divided into three subtypes ([exomeres ∼35 nm], and small [60–80 nm] and large [90–120 nm] exosomes) that differ in their physical properties (diameter, zeta potential, and stiffness) and cargo compositions13.
Despite the lack of precision in EV subtype isolation, several studies have reported that EVs from different sources carry specific bioactive components from their parental cells that can confer specific functional properties. EVs are thus often frequently classified based on their cell or tissue source and associated regulatory activity or application. EVs derived from immune cells represent one example. DC-derived EVs are of great interest for vaccine applications since they carry peptide: MHC complexes and their co-stimulatory molecules, and integrins and other proteins involved in regulating adaptive and innate immune responses14. EVs secreted by NK cells carry NK marker and cytotoxic molecules (e.g., CD16, CD56, granzyme and perforin)15 that can promote NK cell proliferation and induce cytotoxic responses16. Macrophage-derived EVs carry factors that can regulate pro-inflammatory responses and induce macrophage polarization. By contrast, MSC-derived EVs, which can be produced at large scale17, can promote tissue repair and maintain tissue homeostasis by delivering trophic factors, signal molecules, regulatory RNAs, proteins, and other factors18. That can exert therapeutic effects on their recipient cells. EVs secreted by diseased cells can also carry factors that permit them to serve as the basis of vaccine strategies. This included tumor derived EVs, that can serve as candidates for cell-free cancer vaccines. However, safety is a priority when using EVs derived from infected or malignant cells, since these EVs can transfer material that can initiate an infection or play important roles in tumor progression and immune responses19. Bacteria also secrete vesicles similar to vertebrate EVs, and EVs secreted by bioengineered bacteria can function as excellent vaccine carriers since they usually display multiple pathogen-associated molecular patterns that can function as adjuvants to stimulate a robust immune response to the pathogenic-specific factors they carry.
3. EV roles in pathological and physiological conditions
3.1. Mechanisms of EVs-mediated intercellular communication
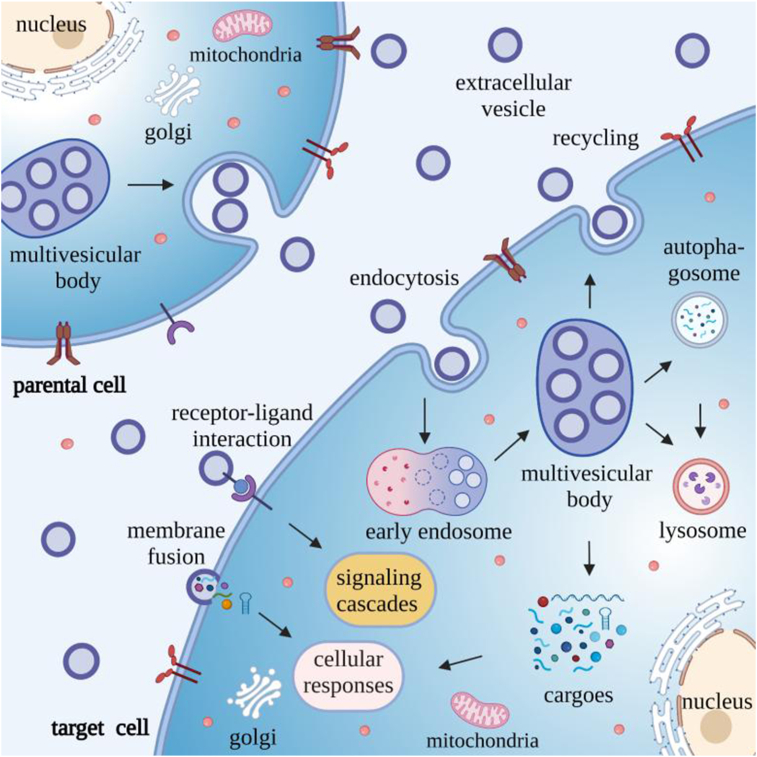
The targeted delivery of EV cargoes to recipient cells to play essential roles in intercellular signal transduction and tissue homeostasis. EV-mediated cell-to-cell communication is mediated by three primary mechanism: receptor–ligand interactions, direct membrane fusion and endocytosis20 (Fig. 2), which can respectively induce signaling cascades, transfer regulatory factors into the plasma membrane and cytosol, or release such factors into the cytosol after EV uptake by endosomes.
Figure 2.
EVs-mediated intercellular communication. EV interact with target cells by three mechanisms to facilitate intracellular communication: 1) direct membrane fusion, which release their contents into the cytoplasm of the recipient cell to where they can exert regulatory effects; 2) receptor–ligand interactions, which can induce signaling cascades; and 3) endocytosis, where EVs first accumulate in endosomes to form MVBs, which then fuse with lysosomes/autophagosomes for degradation, fuse with the plasma membrane for recycling, or release their contents into the cytosol to allow the captured EV cargoes to exert regulatory functions.
EVs can mediate immunomodulatory effects without endocytosis since EV ligands can initiate downstream signaling cascades via interactions with surface receptors on target cells. For example, major histocompatibility complex (MHC) present on EVs derived from dendritic cells (DCs) can directly interact with the T cell receptor to affect immune responses21. Similarly, EVs released by metastatic melanoma cells that express high levels of the proGrammed cell death ligand-1 (PD-L1) can suppress the function of CD8+ T cells and facilitate cancer growth by interacting with proGrammed cell death protein-1 (PD-1) expressed on these cells22.
Multiple studies have, however, shown that EVs can fuse with recipient cells to transfer their membrane and vesicular cargoes. An early study that incubated recipient cells with EVs that were membrane-labeled with a fluorescent lipid dye found that the EV label was transferred to the recipient cells via membrane fusion23. Subsequent studies found that EV fusion events released EV cargoes into the cytosol that could have regulatory effects, as exemplified by one early study that determined that EV miRNA cargo transfer could repress mRNA translation in recipient cells24.
EVs can transfer their cargoes to exert regulatory effects via a receptor-independent fusion with the plasma membrane25. However, mounting evidence indicates that EV endocytosis is the primary means of EV-mediated cell-to-cell communication and can occur via five different mechanisms: clathrin-, caveolin-, and lipid-raft-mediated endocytosis, macropinocytosis, and phagocytosis26. Most EV recipient cells employ clathrin-mediated endocytosis for EV uptake, in a process that involves clathrin assembly around membrane-bound EVs, followed by membrane bending and invagination to form clathrin-coated vesicles, that bud inward and undergo scission from the plasma membrane to enter the cytoplasm, and fuse with early endosomes after release of their clathrin shells27. However, there is evidence that EV uptake can also occur through other mechanisms. For example, caveolin-dependent endocytosis can also produce small plasma membrane invaginations that produce intracellular vesicles28. Caveolin-1, the primary protein component of these vesicles, is also reported to regulate EV endocytosis and internalization29, but there is conflicting data on the relevance of caveolin-dependent endocytosis for EV uptake. One group has reported that caveolin-1 can promote EV uptake in epithelial cells30, while another has indicated that caveolin-1 inhibits EV uptake in glioblastoma cells and fibroblasts via a signal transduction-mediated process31. Lipid raft-associated membrane invagination events may also permit EV uptake and subsequent fusion with early endosomes32. Macropinocytosis, a process in which the plasma membrane deforms to envelop extracellular fluid, has also been shown to regulate EV entry into the cytosol through a mechanism that requires cholesterol, Na+/H+ exchange, and phosphatidylinositol-3-kinase (PI3K) activity33. Finally, EV uptake can also occur via phagocytosis, which primarily occurs in immune cells such as macrophages and DCs, through a receptor-mediated actin polymerization process that induces membrane invagination to engulf the contacted material and target it to the phagosome pathway34. Several of these mechanisms have also been observed to regulate EV entry into the same cells, to complicate interpretation of this process35.
EVs which have fused with early endosomes after entering recipient cells, can accumulate in the endosomal compartment to form multivesicular bodies and most of them may be targeted to lysosomes/autophagosomes, leading to EV degradation, which could provide metabolites to the recipient cells; fuse with plasma membrane to induce extracellular EV release for recycling, or these EVs may fuse with the endosomal membrane to release their contents into the cytosol to exert effector functions36 (Fig. 2). This final process is still poorly understood but required for regulatory EV cargo transfers that participate in various pathological or physiological responses in recipient cells37. However, more is known about factors that influence the efficiency of EV internalization. Surface factors present on EVs and their potential recipient cells can regulate EV uptake, and conditions that enhance the cleavage of these factors, including temperature, can inhibit EV uptake20. Microenvironmental pH has also been reported to influence EV release and uptake, partially by altering the lipid composition of the EV membrane to enhance their fusion efficiency with the membranes of recipient cells23.
3.2. EV roles in physiological conditions
EVs are ubiquitous and can transfer membrane proteins, signaling molecules, nucleic acids, and other materials from their parental cells to regulate multiple physiologic processes. For example, EVs secreted by antigen-presenting cells (APCs) carry MHC proteins and signaling molecules that can present antigenic peptides to T cells to induce effector activity38, although this immune response induced is not as effective as that induced by APCs and the reason for this difference is not clear39. EVs can also carry bacterial components from macrophages infected with an intracellular pathogen (e.g., Mycobacterium avium) to uninfected macrophages to activate them via a toll-like receptor ligand dependent mechanism40. In addition to these immune effects, EVs also regulate intercellular communication associated with other critical processes, including angiogenesis, cell proliferation and apoptosis, tissue homeostasis and remodeling, and reproduction and development41,42. For example, EVs secreted by platelets, which regulate the clotting response following tissue injury, contain α-granules, coagulation factors, growth factors, and RNA species that play important roles in wound healing through their actions to modulate coagulation, inflammation, cell growth, and stem cell proliferation, migration, and differentiation43. Similarly, EVs secreted by Schwann cells carry miRNAs that promote cell-to-cell communication and enhance peripheral nerve regeneration after nerve damage44.
3.3. EV roles in pathological conditions
EVs secreted by injured or diseased cells and tissues have also been implicated in initiating or promoting pathological responses associated with malignant, chronic, and infectious disease states. Tumor-derived EVs derived from different cell types in the tumor microenvironment (TME), including cancer, stromal, and immune cells, carry genetic material and regulatory factors that participate in various pathological changes, including TME remodeling, the immune response to cancer antigens, therapy resistance, and tumor invasion, metastasis, and migration45,46. These EVs can express FasL, NKG2D, TGF-β, and PD-L1 to induce T cells apoptosis and suppress NK cells cytotoxicity to facilitate tumor escape from immune survellance47. The TME is a highly complex and dynamic system where metabolic remodeling may provide the energy or additional materials required for tumor survival, growth, and migration, and non-coding RNAs present in tumor-derived EVs may influence these mechanisms48. TME stromal cell EVs can also influence tumor pathology by transferring their cargoes to neighboring cells to triggering intracellular signaling events that inhibit apoptosis and promote tumor proliferation49. Conversely, EVs secreted by immune cells can inhibit tumor development by depleting mesenchymal tumor stromal cells via altering signaling pathways that elicit mesenchymal-to-epithelial transition to promote TME development50.
EVs also play important roles in tissue injury in neurodegenerative disorders, including the transport of specific proteins that accumulate and form protein aggregates that are common pathological characteristics of such disorders and can contribute to disease pathology51. For example, EVs secreted by central nervous system tissue of patients with Parkinson's disease contain elevated levels of α-synuclein, the primary component of characteristic protein aggregates associated with this neurodegenerative condition52. EV α-synuclein levels also correlate with disease severity and may enhance neuronal injury, since normal neuronal cells exhibit greater apoptosis when exposed to EV containing α-synuclein oligomers versus free α-synuclein oligomers, which may promote the spread of neurological injury53.
EVs have also been reported to mediate the pathology of several other chronic conditions, including cardiovascular, ocular, and endocrine diseases38,54,55, as well as infectious diseases. For example, EVs released from damaged endothelial cells (ECs) carry multiple bioactive molecules that can increase apoptosis, clot and atherosclerotic plaque formation at recipient ECs56, while EVs secreted by infected cells can transfer pathogen-derived factors to promote systemic infection or influence the host immune response57. Notably, overlaps among host pathways involved in viral packaging and exosome biogenesis have been proposed to permit EVs secreted by cells infected with hepatitis C virus (HCV) or HIV-1 to package and transfer these viral genomes to recipient cells to induce productive infections58. Serum EVs from patients with HCV infections carry replication competent viral RNA complexed with factors that can promote HCV replication or stabilize its replication complex (Ago2, miR122, and HSP90), and can transfer the HCV RNA genome to recipient cells to promote infection59. These carrier EVs may express few if any viral proteins, unlike HCV virions, and thus avoid a systemic antibody-mediated virus neutralization response60. HIV can also employ EV transfer to evade an antibody neutralization response. EV-mediated transfer of the HIV virulence factor Nef can induce a pre-activation state in recipient CD4+T to increase their susceptibility to HIV infection and apoptosis61. Nef expression can also activate PI3K signaling to alter endosomal vesicular formation and trafficking to downregulate MHCI expression and cytotoxic immune responses against HIV-infected CD4+T cells62.
4. EV isolation and quality control requirement for EV therapeutics
EVs, lipoprotein complexes, protein aggregates, and other materials present in biological samples exhibit similar physical properties that complicate the isolation of high purity EVs required for clinical EV applications. Segregation of specific EV subpopulations can be even more difficult since these groups exhibit substantial size overlap and morphological similarity, and lack distinctive markers to permit their specific differential capture63. Nevertheless, accurate EV characterization can be important for EV applications, since different EV subtypes may express different factors and thus therapeutic effects may depend upon the reproducible isolation of specific EV subtypes that contain the desired regulatory factors.
4.1. Isolation strategies
Several approaches have been used to isolate EVs from biological samples, and each requires trade-offs between purity, yield, and integrity of isolated EV fractions (Table 1).
Table 1.
Principles and characteristics of isolation methods of EVs.
| Type | Method | Principle | Advantage | Disadvantage |
|---|---|---|---|---|
| By density | Ultracentrifugation | Different centrifugal processes to isolate EVs based on density and mass | Easy to operate; Low cost | Low purity; Low throughput; Time-consuming |
| By size | Ultrafiltration | Using the filter membrane to remove large bioparticles | High purity; Time-saving | Clogging the nanopores; Damaging the structure and dissoluting EVs; |
| Size exclusion chromatography | Large particles such as EVs are unable to pass through column thus rapidly eluting | High purity; Preserving the structure | The contaminations co-eluting with EVs | |
| By solubility | Polymer precipitation | Changing the solubility of the solution | Easy to operate; Low-cost; Getting concentrated and high-yield EVs | Poor specificity; The contaminations co-precipitating with EVs |
| By immunoaffinity | Immunoaffinity magnetic beads; Immunoaffinity chromatography; Plate-mounted immunoaffinity | The surface markers of EVs interacting with antibody | High specificity; High purity | High-cost; Low throughput; Relying on reliable markers |
| Emerging methods | Microfluidics | Using various methods achieve microscale isolation based on their physical and biochemical properties | High purity; Low sample consumption; Low cost | Lack of standardization; Clogging the probe |
| Asymmetric flow field-flow fractionation | Based on the particles density and hydrodynamic properties | Label-free; Gentle; Getting EVs subpopulations | Complex processes; Low throughput | |
| Nano-flow cytometry (nano-FCM) | Based on the particles of polydispersity, charge characteristics and surface markers | High throughput; High resolution | High cost; Professional personnel |
Ultracentrifugation (UC) remains the gold standard64,65, and most popular EV, isolation technique66, and employs differential centrifugation to remove cells, debris, organelles, and large particles from EV source materials before applying high-speed centrifugation to precipitate EVs. UC is low throughput and time-consuming, however, and the EV fractions it produces exhibit highly variable purity, since EVs can co-precipitate with several factors not removed during sample clarification, including protein, DNA and RNA aggregates, lipoproteins, and others materials67. Ultrafiltration (UF) approaches that employ size-exclusion membranes to remove large bioparticles from biological samples can be faster and produce higher purity EV fractions than UC68, although trapping of large sample components may clog membrane pores and reduce EV yields and strong shear forces encountered during filtration can rupture EVs or damage their structural integrity69. Size exclusion chromatography (SEC) can also be used to separate EVs from particles that differ in size70. In this approach, EV containing biological specimens are fractioned over columns packed with porous beads that have an exclusion diameter less than that of the targeted EV population, and EVs are rapidly eluted while smaller materials are retained on the column71. SEC can preserve the structure and maintain the function of EVs, although sample components similar in size to EVs are also isolated in the EV SEC fraction72. EV precipitation methods primarily employ polyethylene glycol (PEG) to differentially precipitate larger, less soluble sample components like EVs73. PEG-based precipitation is simple, inexpensive, and gentle74 as it requires only a low-speed centrifugation step to produce concentrated and high-yield EV samples74. However, these EV fractions have low purity since EVs co-precipitate with multiple non-specific factors, including large particulates and molecular aggregates75. Immunoaffinity precipitation methods using receptors or antibodies that recognize EVs-specific factors are an effective means of obtaining high purity EV isolates63,76, but EV yields are usually much lower than obtained with PEG-based precipitation methods, other factors can still be pulled down by interaction with the precipitation matrix, and this approach is much more expensive than PEG precipitation77. Several EV isolation approaches have also recently been proposed that use different characteristics for EV segregation than standard EV isolation methods. These include microfluidic approaches that can achieve microscale isolation of EVs based on their physical and biochemical properties78; asymmetric flow field-flow fractionation (AF4) and nano-flow cytometry (nano-FCM)65,79. However, it is unclear if these approaches can be employed for large-scale isolations required to formulate future EV therapeutics, and both they and the current EV isolation studies will require extensive validation studies before they can be employed to isolate EVs for this purpose.
4.2. Characterization strategies
EVs must be characterized after isolation or before and after loading therapeutic agents to ensure that they meet the requirements of their intended application. Various strategies are available to characterize EV size and morphology, and protein, nucleic acid, and lipid composition (Table 2).
Table 2.
Principles and characteristics of characterization methods of EVs.
| Type | Method | Principle | Advantage | Disadvantage |
|---|---|---|---|---|
| By physical property (size and morphology) | TEM; Cryo-EM; SEM | Electron radiation | High resolution | High cost; Low throughout; Complex sample processing; Not quantitative |
| AFM | Measuring the force between the probe and sample | High resolution | High cost; Low throughput; Not quantitative | |
| DLS | Measuring the scatter light from EVs in Brownian motion | Easy to operate; Low cost | Not quantitative; Not suitable for polydisperse sample | |
| NTA | Capturing the Brownian motion of individual particle | Quantitative; Suitable for monodisperse and polydisperse samples | Affecting by the instrument parameter settings | |
| Imaging FCM | Based on FCM and fluorescence imaging | Sensitive; High throughput; Low sample volume | High cost; Professional personnel | |
| Nano-FCM | FCM based on nanopore | Quantitative; Low sample volume | High cost; Professional personnel | |
| CLSM | Microscopy imaging after fluorescent label | High resolution; Dynamic visualization | Not quantitative; High cost | |
| TRPS | Based on the changes of resistance pulses of a single particle through a pore | Quantitative; Low sample volume | Clogging the pore by large particle | |
| TSPR | Based on free electrons collectively oscillate under the Incident light field | Quantitative; Low sample volume | Noise interference by containments | |
| By compositional property (protein) | ELISA | Immunoaffinity | High throughput; Fast | High cost; Low specific |
| SDS-PAGE | Characteristic absorption in the visible spectrum | Easy to operate; Fast | Not quantitative; Low detection limit | |
| WB | Immunoaffinity | Quantitative; Specific | High cost; Time-consuming | |
| MS | q/e analysis of small fragments | High specific; Quantitative | High cost; Professional personnel | |
| By compositional property (nucleic acid) | UV‒Vis | The characteristic absorption peaks | Low cost; Easy to operate; Fast | Low specific |
| qPCR | Amplification of specific genes | High throughput; Low sample volume | Only suitable for known genes | |
| microarray | Based on the principle of base pairing | High throughput; Specific | High cost; Professional personnel | |
| NGS | Fluorescence sequencing after RNA reverse transcription | Sensitive; Specific | Low throughput; High cost | |
| By compositional property (lipid) | GC–MS, LC‒MS | q/e analysis of small fragments | High specific; Quantitative | High cost; Professional personnel |
EV imaging methods used for high-resolution analysis of EV size and morphological properties include transmission electron microscopy (TEM), cryo-electron microscopy (Cryo-EM), scanning electron microscopy (SEM), and atomic force microscopy (AFM). Dynamic light scattering (DLS) is used to measure the Brownian motion of suspended particles isolated EV samples to estimate the polydispersity index (PDI) of their particle diameters80. Nanoparticle tracking analysis (NTA), a DLS-based method, is one of the most common techniques used to calculate EV size distributions and concentrations81. Imaging flow cytometry (IFCM) can more accurately assess EV concentration than NTA, since IFCM allows for single-particle quantification based on EV’ immunophenotype marker and size rather than bulk quantification82. However, EV sizes and concentrations are not frequently estimated by IFCM or several other alternate analysis methods, including nano-FCM, confocal laser scanning microscopy (CLSM), tunable resistance pulse sensing (TRPS) and transmission surface plasmon resonance (TSPR) and frequency-locked optical whispering evanescent resonance (FLOWER).
EVs can also be characterized by analysis of biomarkers positively and negatively associated with EVs, with the International Society for Extracellular Vesicles (ISEV) recommending at least one protein belonging to three distinct categories be analyzed to evaluate the nature and relative purity of EV factions83. This includes the analysis of a transmembrane or GPI-anchored proteins that denote the existence of a lipid bilayer, a cytosolic protein that indicate the detected vesicles contain a lumen, and a commonly co-isolated negative control protein that serves as an indicator of sample contamination. Specific factors of interest must also be characterized to ensure that the EV fraction meets the specific need of its research or clinical application (e.g., expresses a surface factor that will be employed for targeted delivery of a therapeutic agent). EV protein composition has been analyzed by standard quantitative techniques, including enzyme-linked immunosorbent assay (ELISA), sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blot and mass spectrometry (MS) analyses84. EV characterization studies have also employed UV–Vis spectrometry and capillary electrophoresis to quantify nucleic acid levels and size distributions, quantitative real-time polymerase chain reaction (qPCR) to quantify individual nucleic acid targets, and microarray analyses and next-generation sequencing (NGS), which can sensitively detect low abundance transcripts, to survey the expression of a broad array of genes. Less research has been performed to characterize EV lipids, but gas chromatography (GC)‒MS and liquid chromatography (LC)‒MS studies have been employed for EV lipidomic studies85.
Reliable EV isolation and characterization protocols are critical for consistent isolation of EVs that have innate therapeutic properties or that have been modified by bioengineering or drug loading to function as therapeutic agents. Several issues still need to be addressed to refine these processes. EV preparation procedures should be streamlined to reduce isolation times and enhance the integrity, stability, and functionality of EV preparations. Most EV characterization methods are expensive, complex, and time-consuming. Improved procedures are therefore needed to achieve high-purity isolations of specific EVs or EV subtypes. Some therapeutic applications may also require selective purification of distinct EV subtypes, which can be challenging due to potential overlaps in their physical properties and surface factors. New EV characterization methods should thus rapidly and accurately distinguish target EV subpopulations by distinctive differences in their physicochemical or biological properties to generate EV preparations with reproducible cell targeting and drug loading properties to produce consistent therapeutic effects. Such new characterization methods are also needed to facilitate the development of improved EV separation methods. Substantial effort is now focused on developing new methods to meet these needs.
5. EVs as therapeutic agent carriers
Some drugs and biomolecules with promising therapeutic effects have low aqueous solubility, are subject degradation, have toxic side effects, and/or lack specificity for the targeted cells or tissues, resulting in poor in vivo bioavailability and therapeutic effects. Researchers have thus developed synthetic delivery vehicles based on cyclodextrin, cationic polymers, polymeric nanoparticles, or liposomes or modified viruses to limit the undesired properties of therapeutic molecules86,87. However, these carriers can exhibit relatively high immunogenicity, short circulation times, and preferential accumulation in highly vascularized tissues, including the spleen and liver, rather than disease sites6,88 partially due to phagocytic cell uptake. Modified viruses, which are mainly used as nucleic acids delivery vectors, can improve uptake rates and may be permit some degree of target specificity, but their inherent immunogenicity renders them highly susceptible to host immune responses that can decrease their efficacy and increase their safety risks89.
By contrast, EVs have several advantages. Most EVs used in therapeutic applications are derived from human cell lines or primary cultures and thus have low immunogenicity and high biocompatibility since they do not display exogenous factors targeted by the immune system, including the mononuclear phagocytic system, and tend to have greater stability in the circulation due to their important roles in endocrine signaling events90. EVs are non-replicating and thus have greater safety profiles than viruses, but like viruses can natively carry surface factors that allow tissue- or cell-selective uptake to increase the effective dose in a target tissue while reducing systemic side effects91. EVs can also efficiently transit biological barriers, including blood vessels and the blood–brain barrier (BBB)—a critical feature for drug delivery vehicles92—to permit targeted or untargeted delivery of their therapeutic factors and thereby increase bioavailability across such barriers to reduce doses required for therapeutic effects and systemic side effects. In the following sections, we review how these EV properties have been employed in EV applications for cancer therapy, vaccine, regenerative medicine and gene delivery applications.
5.1. Cancer chemotherapeutic drug carriers
EVs serve as excellent carriers for both polar and non-polar chemotherapeutic drugs, since they can transport hydrophobic drugs in their lipid bilayer and hydrophilic drugs in their lumen (Fig. 3A). EVs also contain transmembrane and membrane-anchored proteins that can promote endocytosis to improve the efficiency of intracellular delivery of their chemotherapeutic drug93. Further, surface factors present on EVs derived from specific cells, including cells bioengineered to express factors that confer specific cell trophisms, can be used to enhance delivery of EV-loaded chemotherapeutics to specific cells or tissues. This can increase the selective drug concentrations and bioavailability in target tissues while reducing cytotoxic side effects caused by drug actions at other systemic locations94. Several groups have now used EVs to deliver a broad array of chemotherapeutics, including paclitaxel (PTX), 5-fluorouracil (5-FU), doxorubicin (Dox), celastrol (CEL), β-elemene, curcumin (Cur), and sorafenib (SRF)95,96, to take advantage of their beneficial drug delivery characteristics.
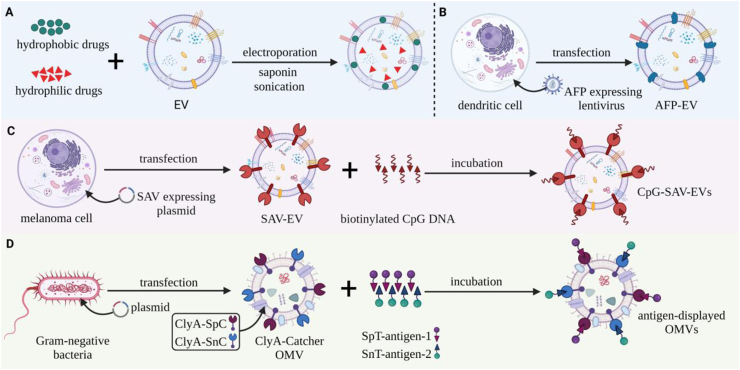
Figure 3.
Strategies for loading EVs with different cargoes for cancer therapy. (A) Hydrophobic drugs (green circles) can be loaded into EV lipid bilayers by direct incubation, while hydrophilic drugs (red triangles) can loaded into the EV lumen by EVs by electroporation, sonication, and saponin-mediated membrane permeation. (B) Dendritic cells engineered to express proteins with specific cell tropism (e.g., α-fetoprotein; AFP) by transfection with lentivirus expression vectors can produce EVs useful for cell- or tissue-selective EV targeting. (C) Melanoma cells transfected with streptavidin (SAV) expression vectors produce SAV-modified EVs that can be incubated with biotinylated CpG DNA to produce a CpG-SAV-EV adjuvant. (D) Gram-negative bacteria transfected by plasmids that express two ClyA-“catcher” fusion proteins (ClyA-SpC (red) and ClyA-SnC (blue)) can be used to produce OMVs expressing these catcher activities on their outer membrane. Incubation of these OMVs with proteins modified with the corresponding tags (SpT and SnT, red and blue triangles) permits the formation of an isopeptide bond between them to allow the stable display of these proteins on the resulting EVs.
For example, in an attempt to improve bioavailability during the administration of chemotherapy drugs, one study loaded EVs isolated from cow milk with PTX, a first-line broad-spectrum chemotherapeutic drug that exhibits poor aqueous solubility, substantial toxicity, and rapid degradation97. In this study, PTX was directly loaded into the lipid bilayer of these EVs by directly mixing the EV isolates with the PTX solution at a 10:1 volume ratio, after which EVs were precipitated and PBS washed to remove unbound drug, sterile filtered and stored at −80 °C until oral administration98. Notably, these EVs showed excellent stability in a simulated gastrointestinal environment, and mice treated with oral doses of these EVs exhibited greater inhibition of tumor growth and lower systemic and immunogenic toxicity than mice treated with free PTX by intravenous injection98.
The properties of native EVs have also been employed to promote target EV delivery across other biological barriers than gastrointestinal lining, using synthetic vesicles designed to mimic EVs. This includes the BBB, which prevents >98% of small molecule drugs from entering tumor cells within brain tissue92, since EVs display better delivery effects than free drugs and liposome-mediated drug delivery. In one study, EVs and synthetic EV analogs (bioinspired nanovesicles: BNVs) loaded with DOX were shown to cross the BBB to demonstrate excellent tumor suppression effects in mice and zebrafish models of glioblastoma. BNVs used in this study were generated by serial extrusion of bEnd.3 brain-derived endothelial cells to produce 500-fold more BNVs than EVs that could be isolated from the same number of cultured cells. Interestingly, both exhibited similar sonication-induced drug-loading capacities and pharmacokinetic parameters, with both exhibiting greater DOX bioavailability with a longer half-life and reduced systemic clearance than free DOX. Treatment of a mouse glioblastoma model with either DOX-loaded EVs or BNVs also significantly decreased tumor volume versus mice treated with free DOX or liposome-encapsulated DOX, without inducing weight loss or other sides effects associated with the latter treatments. Thus, EVs and BNVs derived from brain endothelial cells demonstrate promise for targeted drug delivery across the BBB, and EVs and BNVs derived from other cell types may also show promise for targeted drug delivery to other tissues and cell types.
In addition to using the inherent properties of EVs or BNVs isolated from unmodified biological sources, groups have also directly or indirectly modified EVs to express specific proteins to achieve targeted delivery of chemotherapeutic drugs. For example, one group recently employed EVs modified to express a HER2 affibody-LAMP2-EGFP fusion protein to target the efficient delivery of miR-21i and 5-FU to colon cancer cells99. These proteins were chosen because LAMP2 was abundantly expressed on the EV surface, HER2 promoted EV targeting to colon cancer cells, and EGFP enhanced EV uptake. EVs were loaded with both 5-FU and miR-21i, an inhibitor of 5-FU resistance, to enhance the therapeutic effect of these modified EVs. This design was found to facilitate EV uptake by colon cancer cells, enhance 5-FU cytotoxicity in 5-FU-resistant colon cancer cells, and did not cause toxicity in the hematological system and major organs in mice models.
5.2. Cancer vaccine platforms
EVs can also carry tumor-associated antigens (TAAs) and may therefore serve as strong candidates for antigenic factors in cancer vaccines. EVs can have desirable properties as vaccine agents, since they can carry specific antigens expressed by their parental cells, and be effectively modified to adjust their immunogenic properties, including any adverse immunosuppressive associated with their parental cells. EVs also retain functional stability after short-term storage at 4 °C and thawing from frozen storage. EV RNA and protein levels and uptake efficiency were reported to be stable for seven, five, and 3 day at 4 °C, respectively, with EV uptake efficiency remaining stable for at least 7 days at −20 °C and 14 days and −80 °C100. Several currently available techniques can also be employed to protect the bioactivity of EV-based vaccines, including freezing, spray drying, and freeze-drying, which should facilitate the transportation, storage, and routine handling of EVs vaccines for clinical applications101.
Studies now indicate that EVs derived from DCs and tumor cells can serve as vaccines for cancer immunotherapy102,103. EVs derived from DCs, the most potent professional APCs, can transfer peptide-MHC complexes to the plasma membranes of recipient DCs to evoke T cell activation responses21,104. One study engineered DCs to secrete EVs that abundantly expressed the liver protein α-fetoprotein (AFP EVs) to induce strong antigen-specific immune responses to attenuate tumor growth and prolong survival in mice with hepatocellular cancers revealing antigenic and pathological heterogeneity (Fig. 3B). Mice treated with AFP EVs had increased levels of CD8+ T cell proliferation and IFN-γ and IL-2 and decreased levels of regulatory T (Treg) cells and IL-10 and TGF-β in their tumors, indicating a potent effect to remodel their TME immune cell compositions. EVs secreted by tumor cells can natively express antigenic epitopes that stimulate cells to promote anti-tumor-specific immune responses105. However, modified EVs can function as adjuvants, materials used in most vaccines to enhance the immune response to their target antigen(s), and which play a critical role in effective presentation of tumor antigens in cancer vaccines106. Melanoma-derived EVs engineered to simultaneously deliver antigen and adjuvant to recipient DCs have been used to enhance their ability to achieve an effective cancer immunotherapy response. Melanoma cells were modified to express streptavidin fused to EV-trophic lactadherin to produce EVs that expressed streptavidin on their outer membrane (SAV-EVs). These SAV-EVs were then incubated with biotinylated CpG DNA to produce CpG-SAV-EVs that delivered tumor antigens and CpG adjuvant to recipient DCs. Notably, CpG-SAV-EV immunization increased DC activation, tumor antigen presentation, and anti-tumor effects, and mouse survival time more than co-administration EVs and CpG DNA107 (Fig. 3C).
Bacterial outer membrane vesicles (OMVs), the bacterial analogs of EVs described in the “Infectious Disease Platform” section of this review, that exhibit native adjuvant activity can also be engineered to express tumor-specific antigens to induce strong immune responses required for effective cancer vaccine applications108,109. For example, one group modified OMVs to express basic fibroblast growth factor (BFGF), which has multiple functions to promote cancer cell survival, proliferation, invasion and tumor growth, and found that mice immunized with these modified OMVs developed a persistent anti-BFGF auto-antibody response that antagonized these pro-tumorigenic effects and induced tumor regression110. Another group recently described a versatile Plug-and-Display OMV-based vaccine platform that permits OMVs modified with two distinct “catcher” proteins to be subsequently modified with a variety of proteins. In this approach, proteins modified by either of two specific peptide tags are specifically recognized by their complementary catcher protein to spontaneously form an isopeptide bond, allowing OMVs multiple tumor-specific antigens to be rapidly attached to the surface catcher-modified OMVs at the same time by a by a simple incubation step111,112 (Fig. 3D). Mice injected with melanoma cells and then vaccinated with catcher-decorated OMVs displaying melanoma-associated TRP2 protein demonstrated a near complete attenuation of lung metastasis in conjunction with robust indication of a tumor-specific immune response111. A similar response was also observed in different mouse metastasis model, although metastasis reductions observed with OMVs displaying each of the two analyzed single tumor antigens were less pronounced than those observed with OMVs displaying both antigens111.
Notably, this OMV platform provides a flexible and convenient means to generate cancer vaccines by addition of tagged cancer-specific proteins to a standard preformulated OMV stock material. The ability to rapidly produce monovalent or multivalent cancer vaccines using this approach has major implications for the development of personalized cancer vaccines. This Plug-and-Display approach also has strong potential utility for OMV-based infectious disease vaccines, and for therapeutics that employ human EVs.
5.3. Infectious disease vaccine platforms
Emerging and re-emerging infectious diseases are constant threats to human health and remain a massive burden in some developing countries, but drug resistance and pathogen variations can hinder disease treatment and containment efforts. Vaccination is the most cost-effective and practical public health intervention to prevent and control infectious disease.
Vaccination is also a vital measure to produce herd immunity to infectious diseases that are subject to global outbreaks that can lead to epidemics and pandemics, and it is essential that such vaccines are designed in a manner that allows their rapid development and production. Several EVs properties, including their stability and potential cell/tissue targeting abilities, are of great interest to researchers developing new delivery vehicles for a variety of therapeutic agents. However, the ability of EVs to elicit protective immune responses is also of substantial interest for new vaccines to a spectrum of infectious diseases caused by bacterial, fungi, viral, and parasitic human pathogens113, 114, 115, although most effort has focused on the use of OMVs for bacterial vaccines.
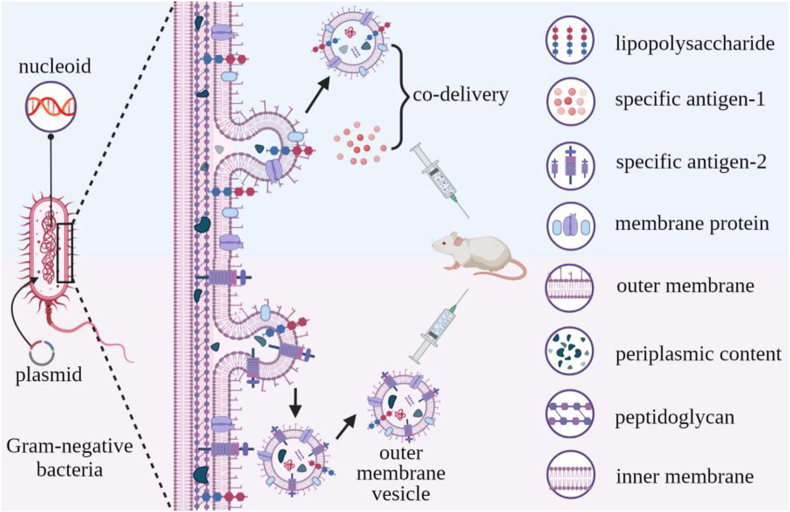
Similar to eukaryotic cells, bacteria spontaneously release membrane-defined vesicles (OMVs) into their extracellular environment through an outward membrane budding process116. Most OMV studies have used OMVs from Gram-negative bacteria since it is not clear how OMVs penetrate the thick cell walls of Gram-positive bacteria OMVs117,118, and the mechanism(s) responsible for OMV secretion is not clear even in Gram-negative bacteria119. OMVs are spherical 20–250 nm diameter vesicles that are bounded by a lipid bilayer that contains lipopolysaccharide (LPS) and membrane proteins and which carry a variety of bioactive molecules, including DNA, RNAs, proteins, and peptidoglycan from the periplasm and cytoplasm120 (Fig. 4), including factors that can induce protective immune responses121.
Figure 4.
OMV formation, structure, and function. Gram-negative bacteria secrete OMVs by outward budding of their outer membrane. OMV potentially antigenic or pro-inflammatory factors, including their surface lipopolysaccharide and membrane proteins, and luminal cargoes of DNA, RNA, peptidoglycan and others factors, allowing them to serve as adjuvants that can be engineered display specific antigens and serve an antigen/adjuvant co-delivery platform for vaccine development.
OMVs have several properties that allow them to function as excellent adjuvants, including their vesicular structure and size, which allows easy entry the lymphatic system, and their transport of multiple pathogen-associated molecular patterns (PAMPs), including LPS, CpG rich DNA, peptidoglycan, and others that can stimulate potent immune responses109. Co-injection of OMVs with an antigen (e.g., ovalbumin) can induce more robust innate and adaptive immune responses than co-administering antigen with CpG DNA or aluminum hydroxide adjuvants2. OMVs are promising vaccine platforms, particularly for microbial infections, with a comparison of OMV, whole-cell, and acellular vaccines for Bordetella pertussis infection found that the OMV vaccine produced a broad humoral response and the highest antibody titers122,123.
Several potential issues should be considered when using bacterial OMVs, however, including their antigen repertoire their potential to induce LPS toxicity and other side effects. OMVs isolated from bacteria that express some variants of LPS can induce pyroptosis that can lead to sepsis at high concentration2, while OMVs derived from a bacterial strain that expresses a less toxic form of LPS were more effecting in activating DCs than heat-inactivated or live-attenuated bacteria. OMVs can also be bioengineered for greater biocompatibility, lower toxicity, and to alter their interactions with the immune system124. For example, one study that engineered Escherichia coli BL21 to express only the biosynthetic precursor of LPS to reduce OMV toxicity, found that its OMVs still stimulated toll-like receptor four signaling to function as adjuvants and revealed strong immunogenicity and low toxicity while producing a balanced Th1/Th2 humoral immune response125. Modification of these OMVs to link an influenza A-derived peptide to the OMV membrane protein ClyA also provided complete protection against influenza-induced mortality when three mouse strains were immunized with these OMVs prior to a challenge with a lethal dose of influenza A125.
OMVs also represent a convenient means for delivery of polysaccharides employed in vaccines. Polysaccharides can represent attractive vaccine substrates but their isolation and conjugation processes can be complex, time-consuming, low yield, and expensive when applied to either natural or chemically synthesized material126. For example, poly-N-acetyl-d-glucosamine (PNAG) is a promising vaccine target generated by bacterial, fungal, and protozoan cells, but to achieve effective immunogenicity PNAG glycoform variants must be conjugated to a protein carrier127. However, antibodies generated to PNAG variants exhibit poor microbial killing and in vivo protection, and while deacetylated PNAG (dPNAG) glycoform variants can stimulate protective immune responses128 the extraction or chemical synthesis of dPNAG variants and their conjugation to carrier proteins can be difficult and expensive. These challenges can be addressed by bioengineering OMVs to carry targeted polysaccharide antigens, and plasmid transfer into non-pathogenic E coli. Strains have been used to produce glycosylated OMVs (glycOMVs) that carried recombinant PNAG (rPNAG) or dPNAG (rdPNAG) variants. Mice immunized with rdPNAG-glycOMVs had the highest specific antibody titers and the longest overall survival when challenged with a lethal dose of Staphylococcus aureus when compared to mice injected with PBS, empty OMVs, and rPNAG-glycOMVs129. Similar results were obtained in mice immunized with rdPNAG-glycOMVs and challenged with a lethal dose of Francisella tularensis, indicating that vaccination with rPNAG-glycOMVs had broad activity to eliminate pathogens that express PNAG.
However, relatively little research has been focused on the development of OMV vaccines, despite the successful use of an OMV vaccine for Neisseria meningitidis for more than three decades. First generation N. meningitidis OMV vaccines generated by extracting OMVs with detergents to remove LPS and decrease endotoxin activity were found to be effective against epidemic N. meningitidis outbreaks in Cuba, Norway, and New Zealand, demonstrating greater than 70% efficiency in these populations. The immunogenicity of these OMV vaccines relied upon an immunodominant antigen, PorA, that exhibits high strain-to-strain variability, and resulted in vaccine strain-specificity130. Subsequently, bacteria were bioengineered to produce OMVs vaccines in which multivalent OMVs display six different PorA subtypes, and evoked a strong humoral immune response and protective effect in a phase Ⅰ trial131. Recently, studies have investigated the potential utility of B. pertussis OMV vaccines since current vaccines do not evoke the same immune response as infection, exhibit waning immunity, and provide individual protection without preventing transmission132. No clinical studies have yet been performed with B. pertussis OMV vaccine candidates, but mouse studies have demonstrated promising results that indicate that B. pertussis OMVs can produce protection similar to challenge with heat-killed B. pertussis bacilli, which persists for up to 9 months, but induce less pro-inflammatory cytokines to address the adverse inflammatory response encountered with whole-cell vaccination.
OMVs have also been studied as vaccines for endemic and emerging human viruses, including influenza A H1N1, MERS-CoV, and Zika133, including a multivalent vaccine for both Influenza A H1N1 Virus and MERS-CoV134. A SARS-CoV-2 OMV could also be developed using a similar approach, and the ability to rapidly modify some OMV vaccine platforms could be particularly useful when developing new vaccines to emerging SAR-CoV-2 variants of concern135. However, most companies and research institutions developing COVID-19 vaccines have focused on protein, DNA, RNA, and viral vector vaccines136, which can each have distinct challenges. Limitation of virus vaccines have been summarized above, and protein vaccines must retain the conformation of their target protein in the presence of adjuvant. Naked DNA and RNA vaccines frequently exhibit low in vivo stability and uptake, and other nucleic acid and protein delivery methods, including virus-like particles, nanoparticles, and liposomes, typically induce weak immune responses and require adjuvants to promote protective immune responses137. Characteristics of recent nucleic acid vaccines that employ liposomes are not well known, although current SARS-CoV-2 RNA vaccines require low-temperature storage prior to use, and have short windows of activity after being thawed for use. Notably, however, most of these limitations do not apply to OMV vaccines, as discussed above.
In summary, OMVs can function as excellent antigen/adjuvant co-delivery vehicles that can directly activate the strong innate immune response without the safety risks associated with heat-inactivated or live-attenuated pathogens, or diseased cells. Bioengineering also allows streamlined construction of OMVs that can both function as adjuvants and present multiple antigens (e.g., polysaccharides, proteins, and nucleic acids) to serve as multivalent vaccines against an array of targeted pathogens or cancers138.
5.4. Regenerative medicine
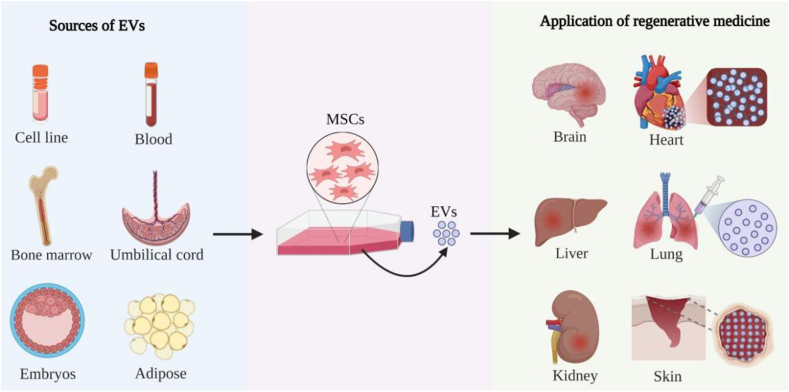
Regenerative medicine applications attempt to repair or regenerate damaged, diseased or missing cells, tissues and organs to restore normal function. Mesenchymal stem/stromal cells (MSC)s are of great interest for regenerative medicine applications since they can differentiate to multiple lineages, self-renew, modulate immune responses, and can be isolated and cultured from multiple sources, cell lines, bone marrow, umbilical cord and adipose tissue, and others139. Many MSC effects can be attributed to paracrine signaling mechanisms where EVs function as key effectors140. Similar to MSCs, MSC-derived EVs (MSC-EVs) can deliver proteins, nucleic acids, and signaling molecules that maintain pluripotency, induce regenerative phenotypes, inhibit apoptotic reactions, and regulate immune responses, to promote regenerative repair of wounded cells and tissues140. However, unlike MSCs, MSC-EVs have simple storage and handling requirements, are less immunogenic, readily cross biological barriers due to their small size, and have fewer safety concerns (e.g., cannot self-replicate and thus are not associated with a risk of neoplastic transformation)141,142. MSC-EVs represent a powerful tool to repair tissue damage associated with several chronic or degenerative diseases that can affect brain, heart, liver, lung, kidney, skin, and bone143, 144, 145 (Fig. 5).
Figure 5.
MSC-EV applications in regenerative medicine. MSCs obtained from commercial cell lines, bone marrow, blood, umbilical cord, embryonic, and adipose tissue can be cultured to isolate EVs to repair cell and tissue damage in the brain, heart, liver, lung, kidney, and skin. Examples cited in this review include the use of MSC-EVs to regenerate cardiomyocytes damaged after myocardial infarction, repair lung tissue damaged by pneumonia, ARDS, acute lung injury, or pulmonary fibrosis, or promote the repair large skin wounds in animal models.
Recent MSC-EVs applications have shown great feasibility for regenerative medicine approaches intended to repair other tissue injuries. For example, surgical open-chest procedures used to place cardiac patches that transfer therapeutics to cardiac injury sites cause substantial trauma and adverse impacts. Recently, however, a minimally invasive procedure that sprays MSC-EVs and a U.S. Food and Drug Administration (FDA)-approved fibrin scaffold directly onto the heart through a small thoracic incision has been reported to repair and regenerate damaged cardiac tissue146. Notably, this MSCs-EVs spray method extended MSC-EV retention, enhanced their cardiomyocyte uptake, improved cardiac function, enhanced angiomyogenesis, and diminished the infarct size to support cardiac regeneration.
MSC-EVs have recently been evaluated for treatment of COVID-19 associated tissue damage. Some individuals with severe SARS-CoV-2 infections develop diffused alveolar injury, endothelial cell damage, and bilateral interstitial pneumonia, which can lead to pulmonary fibrosis and acute respiratory distress syndrome (ARDS)147. New treatments are thus needed treat or repair this tissue damage. Multiple clinical trials have been registered (https://www.clinicaltrials.gov/) to investigate the safety and efficacy of MSCs-EV therapies for this purpose, but only one has been published to date. This trial reported that severe COVID-19 patients who received a single intravenous dose of MSCs-EVs revealed clinical and oxygenation status improvements without treatment-associated mortality or safety concerns during the 14-day post-treatment evaluation interval148. Further studies are needed to validate the safety and utility of MSC-EV therapy for severe COVID-19 cases, but the results of this study agree with the results of a systematic review of 39 studies that examined the use of EV treatments in animal models of pneumonia, ARDS, acute lung injury, or pulmonary fibrosis149. This review concluded that EV therapy had multiple beneficial effects to attenuate tissue damage and prolong survival, which included attenuating inflammation, promoting the repair of damaged alveolar and microvascular endothelial tissue, and attenuating or preventing pulmonary fibrosis. MSC-EVs thus appear likely to have great therapeutic to attenuate and repair COVID-19-related lung injuries by enhancing tissue regeneration via multiple pathways, which could greatly improve the current treatment landscape.
Cell-free, EV-laden biomaterial scaffolds are another area of growing interest in regenerative medicine 150, since some of these scaffolds are reported to regulate immune responses that promote tissue regeneration. One study found that immune cells were primarily responsible for in vivo uptake of scaffold-associated EVs, with the EVs and the scaffold matrix respectively functioning to recruit and train immune cells and synergistically induce macrophage and regulatory T cell responses to repair mouse severe skin wounds that would not otherwise heal151. Negatively charged MSCs-EVs were immobilized onto a positively charged fibrous polyester matrix by electrostatic interaction to prolong EV retention and allow the continuous uptake of these EVs by immune cells recruited to the injury site. This MSCs-EV uptake accelerated M2 macrophage polarization associated with tissue repair, partially by activating CD4+ T helper two cells (TH2) and regulatory T cells (Treg) to secrete cytokines and growth factors that favored M2 macrophage polarization.
5.5. EVs as carriers for gene therapy agents
Gene therapy offers the potential to resolve or attenuate otherwise incurable chronic diseases by in situ correction of a defective gene responsible for the pathology. Considerable effort has, however, focused on the development of safe gene delivery vectors since one of the first gene therapy patients died from a toxic response to the therapeutic vector. Both viral and non-viral vehicles are employed for gene delivery, and each has advantages and disadvantages. Virus-based gene delivery approaches primarily employ adenoviruses, adeno-associated viruses, lentiviruses, and retroviruses that have high loading and transfer efficiency, but which cannot fully escape immune surveillance, are expensive to employ, and can have significant safety risks152,153. Non-viral vectors use synthetic materials that can generally have lower safety risks, but have can have poor loading and targeting efficiency and may still have relatively high immunogenicity154.
EVs of considerable interest as gene delivery vehicles since they play important roles in cell-to-cell communication events and therefore have high biocompatibility, low immunogenicity, can protect their cargoes from degradation in the extracellular space and circulation, and target specific cells or tissues. EV safety profiles are also better than viruses, since they cannot replicate and their composition can be controlled by careful selection or genetic modification of their parental cells. Gene engineering or other modification approaches can also be used to introduce new therapeutic cargoes or alter their target them to specific cells or tissues. Recent studies have shown exciting progress in using EVs to deliver CRISPR/Cas complexes for in situ gene editing as well as, and RNA and DNA cargoes with therapeutic activity.
5.5.1. EV delivery of CRISPR/Cas9 therapies
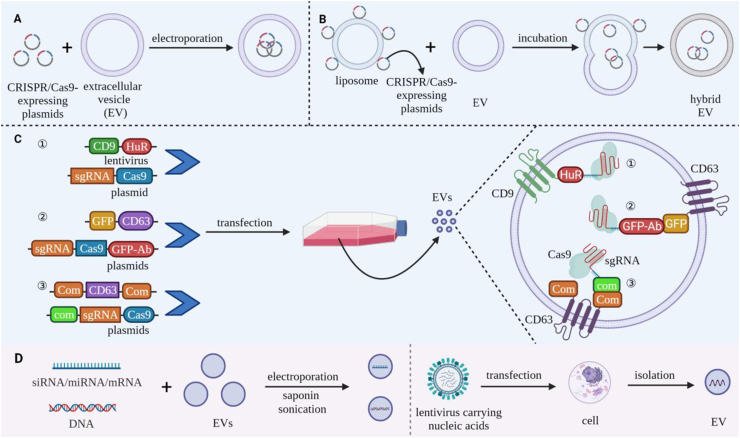
CRISPR/Cas9 complexes contain Cas9 protein and a single guide RNA (sgRNA) that recognizes a complementary DNA sequence, causing Cas9 to efficiently bind and cleave this DNA target for precise gene editing155. However, CRISPR/Cas9 gene therapy approaches require efficient delivery of CRISPR/Cas9 complexes into targeted cells, and current delivery vehicles exhibit relatively high immunogenicity and poor transfer efficiency and specificity156. EV electroporation has been used to package CRISPR/Cas9-expressing plasmids into EVs for delivery to ovarian cancer tumors in SKOV3 xenograft mice (Fig. 6A). EVs derived from cancer cells were more effective in mediating genome editing in these tumors than those derived from epithelial cells in this study, likely due to the tropism of the cancer-derived EVs, although the use of tumor-derived EVs raises potential safety concerns157. Directly loading EVs with a CRISPR/Cas9 plasmid by electroporation or sonication was not useful, since small EVs (50–150 nm diameter) were not effective carriers due to their limited volume and larger EVs that could encapsulate the CRISPR/Cas9 plasmids had low loading efficiency. However, hybrid EVs, produced by fusing EVs with liposomes, can package large nucleic acid molecules to address this issue158 (Fig. 6B). Hybrid EVs have been loaded with CRISPR/Cas9 expression plasmid by mixing negatively charged EVs with positively charged liposomes that have bound plasmid DNA via electrostatic interaction159, since subsequent incubation of these vesicles induced their fusion. This hybrid EV approach successfully delivered CRISPR/Cas9 plasmids into MSCs that were not effectively transfected by liposomes159.
Figure 6.
Strategies for loading bioactive components into EVs for gene therapy. (A‒C) Load approaches for CRISPR/Cas9 gene editing systems. (A) Electroporation-mediate loading of Cas9/sgRNA-expressing plasmids into large-diameter EVs. (B) EV fusion with liposomes that are surface loaded with Cas9/sgRNA-expressing by electrostatic interaction, which transfers these plasmids to the lumen of the resulting hybrid EVs. (C) Transfection of parental cells with vectors that express fusion proteins that induce the EV enrichment of recombinant Cas9/sgRNA complexes. Approaches reported to date include: ① CD9-HuR fusion protein-mediated capture of CRISPR/Cas9 complexes containing an miR-155-tagged sgRNA. ② CD63-GFP fusion-protein mediated capture of CRISPR/Cas9 complexes containing a Cas9-GFP nanobody fusion protein. ③ CD63-com fusion protein capture of CRISPR/Cas9 complexes that contain sgRNA modified with the com aptamer (D) EV loading with nucleic acids (e.g., siRNA, miRNA, mRNA, and DNA). By electroporation, sonication, or saponin-mediated membrane permeation, or during EV biogenesis in parental cells following lentivirus transfection.
Bioengineering can also produce modified EVs that promote the delivery of gene cargoes. For example, CRISPR/Cas9 components have been enriched in EV cargoes (Fig. 6C) by fusing EV membrane proteins with proteins that bound specific tags. In one study, the EV membrane protein CD9 was fused with HuR, an RNA binding protein with high affinity for miR-155, to enrich recombinant Cas9 mRNA and sgRNA transcripts tagged with miR-155 sequence. Cells were transfected with vectors expressing miR-155-tagged sgRNA and Cas9 transcripts and CD9-HuR. EVs produced by these cells were enriched in the tagged sgRNA and Cas9 transcripts and reduced both in vitro and in vivo expression of the sgRNA-targeted gene160. Similarly, vectors expressing the EV membrane protein CD63 fused with GFP and Cas9 fused with a GFP-specific nanobody were used to enhance EV enrichment of Cas9161. Recently, a vector expressing a recombinant CD63 protein modified at both termini with the aptamer binding protein com was also used to promote EV enrichment of CRISPR/Cas9 ribonucleotide complexes containing an sgRNA that was tagged with the com aptamer to efficient gene editing162.
5.5.2. RNA-based therapies
RNA-based therapeutics have primarily relied on the delivery of miRNA, siRNA, or mRNA cargoes that directly or indirectly inhibit the activity of a defective or dysfunctional gene or restore the normal activity or a target gene. EVs are gaining popularity for these approaches since RNA cargoes are protected from degradation, can traverse biological barriers, and be targeted to specific cell types. Common methods of RNA transfer are summarized in Fig. 6D. Recent EV preclinical studies have primarily focused on the delivery of miRNAs therapeutics to attenuate a diverse array of diseases, although such approaches may lack specificity and have off-target effects since any given miRNA may regulate hundreds of mRNAs163.
For example, EVs engineered to express miR-31 are reported to inhibit the expression of hypoxia-inducible factor 1-alpha inhibitor (HIF1AN) and epithelial membrane protein-1 (EMP-1) to enhance wound healing in diabetic Sprague Dawley rats by promoting angiogenesis, fibrogenesis, and re-epithelization164. Intramuscular injection of tumor-derived EVs loaded with three miRNAs (Let-7i, miR-142, and miR-155), which enhanced in vitro DC maturation and T cell proliferation and cytotoxicity, was also found to decrease tumor growth and increase survival in a mouse breast cancer model, which was associated with a shift from poorly differentiated to well-differentiated tumor phenotypes and an increase in tumor cell necrosis165.
Similar studies have been performed with siRNAs, short synthetic RNAs designed to bind a complementary RNA sequence specific for a unique mRNA target. For example, one study used genetic engineering to fuse a neuron-specific RVG peptide to the EV membrane protein LAMP and employed electroporation to load these EVs with a siRNA specific for beta-secretase 1 (BACE1), a therapeutic target in Alzheimer's disease. These modified EVs were found to efficiently cross the BBB after intravenous injection into a mouse model of Alzheimer's Disease, resulting in ∼60% less BACE1 mRNA and protein in the brains of these mice166. Similarly, injecting tumors in a mouse model of pancreatic cancer with EVs loaded with a siRNA specific for PAK4, whose overexpression promotes cell proliferation, migration and invasion167,168, decreased tumor growth and increased mouse survival corresponding to tumor PAK4 decreases and necrosis increases169.
EVs have also been employed to deliver mRNAs to induce therapeutic protein expression in targeted recipient cells. For example, intravenous injection of low-density lipoprotein receptor (Ldlr) deficient mice with EVs loaded with Ldlr mRNA restored liver Ldlr protein expression reduce liver lipid deposition and pro-inflammatory and pro-fibrotic gene expression and decrease atherosclerotic plaque formation following a high-fat diet challenge170.
5.5.3. DNA-based therapies
Relatively few studies have examined the use of EVs to package and deliver DNA versus RNA to target cells171, and efficient DNA loading into EVs appears to be a limiting factor in the development of therapeutics that rely upon DNA expression vectors. DNA and length and EV volume are reported to limit EV loading by electroporation, with short linear DNA (<1000 bp) being more efficiency loaded than longer linear or circular DNAs, and exosome-like EVs exhibiting reduced loading capacities than larger microvesicle-like EVs172. DNA-loaded EVs produced in this study were found to transfer their DNA cargoes to recipient cells, but gene expression was not observed following DNA transfer. It may thus be necessary to carefully refine electroporation conditions since this process may promote EV aggregation and changes in EV morphology that could affect the recovery of functional EVs.
Sonication has been used to load small DNA fragments into EVs with high loading efficiency, but these EVs have not shown encouraging therapeutic effects in vivo171, likely due to disruption of EV integrity during the loading process173. EV treatment with surfactant saponin reagents has been used to increase EV membrane permeability without destroying the lipid bilayer structure and may be useful in DNA loading, but few studies have examined the ability of saponin reagents to load DNA into EVs. Further, given its hemolytic activity, saponin concentrations should be kept low and residual saponin should be removed from the loaded EVs174. Finally, for all these methods, care must be taken in selecting an appropriate separation strategy following the DNA loading procedure, since EVs may co-precipitate with unincorporated DNA to compromise DNA loading estimates.
6. Challenges and future perspectives
We have reviewed how these EV properties have been employed in EV applications with representative examples provided in Table 3. EVs research has made great progress in since EVs were first described 30 years ago, but further work needs to be done to address remaining challenges that can limit the development and use of EV-based clinical applications. Studies have defined the basic processes involved in EV biogenesis and some of the mechanisms that regulate their participation in cell–cell communication, but additional studies are required to clarify the exact mechanisms involved and if this information can be used to promote selective biogenesis, secretion, or isolation of desired EV subsets. Several EV features must also be considered when designing an EV-based therapeutic application.
Table 3.
Representative examples of EVs used as therapeutic agent carriers.
| Application | Therapeutic agent | Donor cell | Loading strategy | Advantage | Ref. |
|---|---|---|---|---|---|
| Cancer therapy | PTX | Cow milk | Incubation | Exhibiting low systemic toxicity and excellent stability | 98 |
| 5-FU, miR-21i and Her2 affibody-LAMP2-EGFP | Colorectal cancer (HCT-1165FR) | Electroporation | Targeting cancer cells overexpressing Her2; Facilitating cellular uptake and improving the cytotoxicity for 5-FU-resistant cells | 99 | |
| AFP antigen | Dendritic cells | Lentivirus | Disseminating antigenic material among DCs | 105 | |
| Tumor specific antigen and CpG DNA adjuvant | Melanoma (B16) | Incubation | Delivering CpG-EVs tumor specific antigen; Exerting stronger anti-tumor effects than co-delivery | 107 | |
| BFGF antigen | E. coli | Transfecion (plasmid) | Producing persistent anti-BFGF auto-antibodies | 110 | |
| DOX | Glioblastoma (bEnd.3) | Sonication | Crossing the BBB; Escaping lysosomal degradation; Low cytotoxicity and exhibiting excellent tumor suppression effect | 175 | |
| Infectious disease vaccine | Adjuvant | B. pseudomallei (strain Bp82) | – | Low toxicity and strong immunostimulation | 2 |
| The specific antigen peptide | B. pertussis (strain B1917) | – | Eliciting high antibody level and inducing broad humoral response | 122,123 | |
| Lipid IVa instead of full LPS | E. coli | Transduction (phage) | Serving as the adjuvant to show high immunogenicity and low toxicity | 125 | |
| dPNAG polysaccharide antigen | E. coli (strain BL21) | – | Broadly eliminating pathogens expressing PNAG on the surface | 129 | |
| PorA N. meningitidis antigen | N. meningitidis (strain PL16215 or PL10124) | – | Evoking strong humoral immune response and produce a powerful protective effect | 131 | |
| Regenerative medicine | Proteins and nucleic acids with repaired and regenerative functions | Mesenchymal Stem cells (main) | – | Enhance lung tissue regeneration in multiple pathways | 150 |
| Proteins and nucleic acids with repaired and regenerative Functions, fibrinogen and thrombin | Mesenchymal stem cells | Co-delivery | Extending the retention and promote uptake of EVs | 151 | |
| Proteins and nucleic acids with repaired and regenerative functions, fibrous polyester materials | Mesenchymal stem cells | Incubation | Prolong the retention of EVs; Recruit and active uptake EVs of immune cells | 151 | |
| Gene therapy | CRISPR/Cas9 | Ovarian cancer (SKOV3) IVA | Electroporation (CRISPR/Cas9-expressing plasmid) | Achieving to load large molecule nucleic acids; CRISPR/Cas9 selectively accumulate in cancer cell | 157 |
| CRISPR/Cas9 | 293T | Incubation (EVs fuse with liposome carrying CRISPR/Cas9-expressing plasmid) | Higher loading efficiency than electroporation | 158 | |
| CRISPR/Cas9 | 293T | Transfection (CD9-HuR (plasmid) and sgRNA-Cas9 (lentivirus)) | Improving gene editing efficiency, safety and flexibility | 160 | |
| CRISPR/Cas9 | 293T | Transfection (GFP-CD63 (plasmid) and sgRNA-Cas9-GFP Ab (plasmid) | Improving gene editing efficiency, safety and flexibility | 161 | |
| CRISPR/Cas9 | 293T | Transfection (com-sgRNA (plasmid), Com-CD63-Com (plasmid) and Cas9-com (plasmid)) | Improving gene editing efficiency, safety and flexibility | 162 | |
| miR-31 | 293T | Transfection (lentivirus) | Promoting the wound healing; safety | 164 | |
| miRNA (Let-7i, miR-142 and miR-155) | Breast cancer (41T) | Electroporation | Modulating immune response and tumor microenvironment to reduce tumor burden | 165 | |
| Neuron-specific RVG peptide and miRNA | Not available | Electroporation | Crossing BBB, target specific cells | 166 | |
| siRNA | Pancreatic cancer (PANC-1) | Electroporation | Lower toxicity and equal treatment efficiency comparing with transfection reagent | 169 | |
| Low-density lipoprotein receptor (Ldlr) mRNA | Liver cell (AML12) | Transfection (plasmid) | Mainly targeting the liver then producing ample Ldlr protein | 170 | |
| DNA | Not available | Sonication or saponin | High loading efficiency | 171 | |
| Linear DNA | 293T or HUVEC | Electroporation | Every large size EV contains hundreds of DNA | 172 |
EV selection is a critical factor in all EV applications, since different EVs may be appropriate for different therapeutic applications, and multiple factors can influence the selection of the EV source, including the inherent and/or engineered targeting and regulatory properties of the final EVs, their relative immunogenicity, and their ease of production or isolation. EVs secreted by primary cells or cells lines may natively express desired regulatory factors but lack target specificity, if required, or selectively target a desired cell population but lack desired therapeutic activity, and thus require manipulation thorough genetic engineering or other approaches to confer necessary properties or attenuate undesired activities. Scale and reproducibility considerations may also influence the selection of native or synthetic EV production methods.
For example, EVs derived from immune cells, and particularly DCs, are frequently used for cancer therapeutics since they can confer unique immunomodulatory properties to recipient tumor cells. EV-based vaccines usually employ EVs that carry, or can be modified to carry, both antigens and adjuvants. OMVs are an attractive choice but may require modification to avoid excessive inflammation. Tumor-derived EVs are rarely used in most EV applications, including cancer vaccines, due to safety concerns that these EVs might transfer factors that could promote tumor growth and the establishment of pre-metastatic niches. However, EV derived from microbial pathogens, which naturally express both pathogen-specific antigens and factors that can serve as adjuvants, have been used in vaccines against their source organisms. Finally, EVs secreted by mesenchymal stem cells (MSCs) are frequently used in regenerative medicine since their cargoes have been reported to promote tissue repair and exert anti-inflammatory effects.
Different EV isolation strategies may also affect their purity, subtype enrichment and structural integrity of EV samples. Further study is thus required to define how EV isolation methods and cell culture conditions, viability, differentiation state, or other factors, influence the isolation of EV subtypes, and their integrity, surface markers, cargoes for specific applications. Standard culture conditions and precise isolation procedures defined in such studies are needed to produce stable, reproducible, and high purity EV isolates with consistent functional activities. Scale-up studies will also be required to validate that change in culture and isolation volumes do not alter EV characteristics. Further studies will also be required to evaluate if drug loading strategies or bioengineering approaches alter the original characteristics of these EVs and whether additional modifications are required to reduce their immunogenicity or toxicity prior to their translation into clinical applications.
Finally, different drug loading and surface modification approaches can influence EV loading capacity and integrity to alter EV-based drug delivery. It is therefore important to optimize both EV loading procedures for different therapeutic agents and EV modification approaches used to target specific cell and tissue targets to maximize the bioavailability of EV therapeutics at desired sites and to reduce therapeutic doses and systemic side effects.
Acknowledgments
This work was supported by Tulane Weatherhead Endowment Fund (USA). All figures were created with BioRender.com.
Author contributions
Shan Liu and Xue Wu searched the literatures and wrote the manuscript. Chandra Sutapa and Christopher Lyon edited the language and provided the new idea. Bo Ning provided the idea and drew the figures and tables. Li Jiang and Jia Fan drew the figures. Tony Y. Hu conceived the study and provided the guidance of the whole study.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Prior J.T., Davitt C., Kurtz J., Gellings P., McLachlan J.B., Morici L.A. Bacterial-derived outer membrane vesicles are potent adjuvants that drive humoral and cellular immune responses. Pharmaceutics. 2021;13:131. doi: 10.3390/pharmaceutics13020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 4.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Q., Jiang C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm Sin B. 2020;10:979–986. doi: 10.1016/j.apsb.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cataldi M., Vigliotti C., Mosca T., Cammarota M., Capone D. Emerging role of the spleen in the pharmacokinetics of monoclonal antibodies, nanoparticles and exosomes. Int J Mol Sci. 2017;18:1248. doi: 10.3390/ijms18061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian T., Zhang H.X., He C.P., Fan S., Zhu Y.L., Qi C., et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Pang J., Wang Q., Yan L., Wang L., Xing Z., et al. Delivering antisense oligonucleotides across the blood-brain barrier by tumor cell-derived small apoptotic bodies. Adv Sci (Weinh) 2021;8:2004929. doi: 10.1002/advs.202004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willms E., Cabañas C., Mäger I., Wood M.J.A., Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J Proteonomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Rai A., Fang H., Claridge B., Simpson R.J., Greening D.W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu M., Nevo N., Jouve M., Valenzuela J.I., Maurin M., Verweij F.J., et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12:4389. doi: 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan W., Jiang S. Immune cell-derived exosomes in the cancer-immunity cycle. Trends Cancer. 2020;6:506–517. doi: 10.1016/j.trecan.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Jong A.Y., Wu C.H., Li J., Sun J., Fabbri M., Wayne A.S., et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6:1294368. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L., Kalimuthu S., Gangadaran P., Oh J.M., Lee H.W., Baek S.H., et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7:2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Tan X., Tan Y., Li Q., Ma J., Wang G. Mesenchymal stem cell derived exosomes in cancer progression, metastasis and drug delivery: a comprehensive review. J Cancer. 2018;9:3129–3137. doi: 10.7150/jca.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T., Zhu Y., Zhao R., Wei X., Xin X. Visualization of exosomes from mesenchymal stem cells in vivo by magnetic resonance imaging. Magn Reson Imaging. 2020;68:75–82. doi: 10.1016/j.mri.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Sun X., Zhao J., Yang Y., Cai X., Xu J., et al. Exosomes: a novel strategy for treatment and prevention of diseases. Front Pharmacol. 2017;8:300. doi: 10.3389/fphar.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 22.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L., Karlsson J.M., et al. Mechanism of transfer of functional micrornas between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pironti G., Strachan R.T., Abraham D., Mon-Wei Yu S., Chen M., Chen W., et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation. 2015;131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulcahy L.A., Pink R.C., Carter D.R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:10.3402. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiss A.L., Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J Cell Mol Med. 2009;13:1228–1237. doi: 10.1111/j.1582-4934.2009.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni K., Wang C., Carnino J.M., Jin Y. The evolving role of Caveolin-1: a critical regulator of extracellular vesicles. Med Sci (Basel) 2020;8:46. doi: 10.3390/medsci8040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanbo A., Kawanishi E., Yoshida R., Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87:10334–10347. doi: 10.1128/JVI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svensson K.J., Christianson H.C., Wittrup A., Bourseau-Guilmain E., Lindqvist E., Svensson L.M., et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delenclos M., Trendafilova T., Mahesh D., Baine A.M., Moussaud S., Yan I.K., et al. Investigation of endocytic pathways for the internalization of exosome-associated oligomeric alpha-synuclein. Front Neurosci. 2017;11:172. doi: 10.3389/fnins.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa Verdera H., Gitz-Francois J.J., Schiffelers R.M., Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–108. doi: 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y., Tu C., Zhang J., Wang J. Inhibition of multiple myeloma-derived exosomes uptake suppresses the functional response in bone marrow stromal cell. Int J Oncol. 2019;54:1061–1070. doi: 10.3892/ijo.2019.4685. [DOI] [PubMed] [Google Scholar]
- 36.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 37.Bissig C., Gruenberg J. Alix and the multivesicular endosome: alix in wonderland. Trends Cell Biol. 2014;24:19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Jiang F., Jiang Y., Wang Y., Li Z., Shi X., et al. Roles of exosomes in ocular diseases. Int J Nanomed. 2020;15:10519–10538. doi: 10.2147/IJN.S277190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent-Schneider H., Stumptner-Cuvelette P., Lankar D., Pain S., Raposo G., Benaroch P., et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific t cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 40.Bhatnagar S., Schorey J.S. Exosomes released from infected macrophages contain mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon R., Debnath C., Lai A., Guanzon D., Bhatnagar S., Kshetrapal P.K., et al. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. 2019;160:249–275. doi: 10.1210/en.2018-00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Jiao G., Ren S., Zhang X., Li C., Wu W., et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson J., Wu Y.W., Blyth C., Lichtfuss G., Goubran H., Burnouf T. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39:598–612. doi: 10.1016/j.tibtech.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Wu X., Wang L., Cong M., Shen M., He Q., Ding F., et al. Extracellular vesicles from skin precursor-derived schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann Transl Med. 2020;8:1640. doi: 10.21037/atm-20-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li I., Nabet B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. 2019;18:32. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang E., Wang X., Gong Z., Yu M., Wu H., Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Targeted Ther. 2020;5:242. doi: 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q., Li Y., Liu Y., Xu W., Zhu X. Exosomal non-coding RNAs-mediated crosstalk in the tumor microenvironment. Front Cell Dev Biol. 2021;9:646864. doi: 10.3389/fcell.2021.646864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan S., Xia L., Yi P., Han Y., Tang L., Pan Q., et al. Exosomal miRNAs in tumor microenvironment. J Exp Clin Cancer Res. 2020;39:67. doi: 10.1186/s13046-020-01570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neviani P., Wise P.M., Murtadha M., Liu C.W., Wu C.H., Jong A.Y., et al. Natural killer-derived exosomal MIR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019;79:1151–1164. doi: 10.1158/0008-5472.CAN-18-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beatriz M., Vilaca R., Lopes C. Exosomes: innocent bystanders or critical culprits in neurodegenerative diseases. Front Cell Dev Biol. 2021;9:635104. doi: 10.3389/fcell.2021.635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinnell J.R., Cui M., Tieu K. Exosomes in Parkinson disease. J Neurochem. 2021;157:413–428. doi: 10.1111/jnc.15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo M., Wang J., Zhao Y., Feng Y., Han S., Dong Q., et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. 2020;143:1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng D., Huo M., Li B., Wang W., Piao H., Wang Y., et al. The role of exosomes and exosomal microrna in cardiovascular disease. Front Cell Dev Biol. 2020;8:616161. doi: 10.3389/fcell.2020.616161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y., Liu D., Feng Q., Liu Z. Diabetic nephropathy: perspective on extracellular vesicles. Front Immunol. 2020;11:943. doi: 10.3389/fimmu.2020.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giro O., Jimenez A., Pane A., Badimon L., Ortega E., Chiva-Blanch G. Extracellular vesicles in atherothrombosis and cardiovascular disease: friends and foes. Atherosclerosis. 2021;330:61–75. doi: 10.1016/j.atherosclerosis.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Meckes D.G., Jr., Shair K.H., Marquitz A.R., Kung C.P., Edwards R.H., Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L., Ju Y., Chen S., Ren L. Recent progress on exosomes in RNA virus infection. Viruses. 2021;13:256. doi: 10.3390/v13020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bukong T.N., Momen-Heravi F., Kodys K., Bala S., Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen T.C., Hsieh C.H., Sarnow P. Supporting role for GTPase Rab27a in hepatitis C virus RNA replication through a novel miR-122-mediated effect. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sole C., Moline T., Vidal M., Ordi-Ros J., Cortes-Hernandez J. An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells. 2019;8:773. doi: 10.3390/cells8080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pawlak E.N., Dikeakos J.D. HIV-1 Nef: a master manipulator of the membrane trafficking machinery mediating immune evasion. Biochim Biophys Acta. 2015;1850:733–741. doi: 10.1016/j.bbagen.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 65.Gandham S., Su X., Wood J., Nocera A.L., Alli S.C., Milane L., et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38:1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardiner C., Vizio D.D., Sahoo S., Théry C., Witwer K.W., Wauben M., et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bang C., Batkai S., Dangwal S., Gupta S.K., Foinquinos A., Holzmann A., et al. Cardiac fibroblast-derived microrna passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Müller D., Cattaneo S., Meier F., Welz R., de Mello A.J. Nanoparticle separation with a miniaturized asymmetrical flow field-flow fractionation cartridge. Front Chem. 2015;3:45. doi: 10.3389/fchem.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 70.Böing A.N., van der Pol E., Grootemaat A.E., Coumans F.A., Sturk A., Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller L., Hong C.S., Stolz D.B., Watkins S.C., Whiteside T.L. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014;411:55–65. doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gámez-Valero A., Monguió-Tortajada M., Carreras-Planella L., Franquesa Ml, Beyer K., Borràs F.E. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deregibus M.C., Figliolini F., D'Antico S., Manzini P.M., Pasquino C., De Lena M., et al. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38:1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weng Y., Sui Z., Shan Y., Hu Y., Chen Y., Zhang L., et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640–4646. doi: 10.1039/c6an00892e. [DOI] [PubMed] [Google Scholar]
- 75.Zarovni N., Corrado A., Guazzi P., Zocco D., Lari E., Radano G., et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 76.Tauro B.J., Greening D.W., Mathias R.A., Ji H., Mathivanan S., Scott A.M., et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Greening D.W., Xu R., Ji H., Tauro B.J., Simpson R.J. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z., Wu H.J., Fine D., Schmulen J., Hu Y., Godin B., et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–2882. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi D., Montermini L., Jeong H., Sharma S., Meehan B., Rak J. Mapping subpopulations of cancer cell-derived extracellular vesicles and particles by nano-flow cytometry. ACS Nano. 2019;13:10499–10511. doi: 10.1021/acsnano.9b04480. [DOI] [PubMed] [Google Scholar]
- 80.Maguire C.M., Rosslein M., Wick P., Prina-Mello A. Characterisation of particles in solution—a perspective on light scattering and comparative technologies. Sci Technol Adv Mater. 2018;19:732–745. doi: 10.1080/14686996.2018.1517587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panagopoulou M.S., Wark A.W., Birch D.J.S., Gregory C.D. Phenotypic analysis of extracellular vesicles: a review on the applications of fluorescence. J Extracell Vesicles. 2020;9:1710020. doi: 10.1080/20013078.2019.1710020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coughlan C., Bruce K.D., Burgy O., Boyd T.D., Michel C.R., Garcia-Perez J.E., et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr Protoc Cell Biol. 2020;88:e110. doi: 10.1002/cpcb.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehryab F., Rabbani S., Shahhosseini S., Shekari F., Fatahi Y., Baharvand H., et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. doi: 10.1016/j.actbio.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 85.Li S., Yi M., Dong B., Tan X., Luo S., Wu K. The role of exosomes in liquid biopsy for cancer diagnosis and prognosis prediction. Int J Cancer. 2021;148:2640–2651. doi: 10.1002/ijc.33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan C., Wang J., Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: recent advances. Biotechnol Adv. 2021;48:107727. doi: 10.1016/j.biotechadv.2021.107727. [DOI] [PubMed] [Google Scholar]
- 87.Danhier F., Feron O., Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 88.Tsoi K.M., MacParland S.A., Ma X.Z., Spetzler V.N., Echeverri J., Ouyang B., et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15:1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yip B.H. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules. 2020;10:839. doi: 10.3390/biom10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elliott R.O., He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021;13:122. doi: 10.3390/pharmaceutics13010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yim N., Ryu S.W., Choi K., Lee K.R., Lee S., Choi H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang T., Martin P., Fogarty B., Brown A., Schurman K., Phipps R., et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm Res (N Y) 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luan X., Sansanaphongpricha K., Myers I., Chen H., Yuan H., Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim G., Lee Y., Ha J., Han S., Lee M. Engineering exosomes for pulmonary delivery of peptides and drugs to inflammatory lung cells by inhalation. J Control Release. 2021;330:684–695. doi: 10.1016/j.jconrel.2020.12.053. [DOI] [PubMed] [Google Scholar]
- 95.Song H., Liu B., Dong B., Xu J., Zhou H., Na S., et al. Exosome-based delivery of natural products in cancer therapy. Front Cell Dev Biol. 2021;9:650426. doi: 10.3389/fcell.2021.650426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oskouie M.N., Aghili Moghaddam N.S., Butler A.E., Zamani P., Sahebkar A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J Cell Physiol. 2019;234:8182–8191. doi: 10.1002/jcp.27615. [DOI] [PubMed] [Google Scholar]
- 97.Munagala R., Aqil F., Jeyabalan J., Gupta R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agrawal A.K., Aqil F., Jeyabalan J., Spencer W.A., Beck J., Gachuki B.W., et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Liang G., Zhu Y., Ali D.J., Tian T., Xu H., Si K., et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18:10. doi: 10.1186/s12951-019-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu J.Y., Li Y.J., Hu X.B., Huang S., Xiang D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: a comparative evaluation of storage conditions. Drug Deliv. 2021;28:162–170. doi: 10.1080/10717544.2020.1869866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nikfarjam S., Rezaie J., Kashanchi F., Jafari R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J Exp Clin Cancer Res. 2020;39:258. doi: 10.1186/s13046-020-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naseri M., Bozorgmehr M., Zöller M., Ranaei Pirmardan E., Madjd Z. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. OncoImmunology. 2020;9:1779991. doi: 10.1080/2162402X.2020.1779991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chiang C., Coukos G., Kandalaft L. Whole tumor antigen vaccines: where are we? Vaccines. 2015;3:344–372. doi: 10.3390/vaccines3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Batista-Duharte A., Lindblad E.B., Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett. 2011;203:97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y., Fang Z., Li R., Huang X., Liu Q. Design of outer membrane vesicles as cancer vaccines: a new toolkit for cancer therapy. Cancers. 2019;11:1314. doi: 10.3390/cancers11091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li M., Zhou H., Yang C., Wu Y., Zhou X., Liu H., et al. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release. 2020;323:253–268. doi: 10.1016/j.jconrel.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 110.Huang W., Shu C., Hua L., Zhao Y., Xie H., Qi J., et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–312. doi: 10.1016/j.actbio.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 111.Cheng K., Zhao R., Li Y., Qi Y., Wang Y., Zhang Y., et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nat Commun. 2021;12:2041. doi: 10.1038/s41467-021-22308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schreurs M.W., Eggert A.A., de Boer A.J., Vissers J.L., van Hall T., Offringa R., et al. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 2000;60:6995–7001. [PubMed] [Google Scholar]
- 113.Baker S.M., Settles E.W., Davitt C., Gellings P., Kikendall N., Hoffmann J., et al. Burkholderia pseudomallei OMVS derived from infection mimicking conditions elicit similar protection to a live-attenuated vaccine. NPJ Vaccines. 2021;6:18. doi: 10.1038/s41541-021-00281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinky, Gupta S., Krishnakumar V., Sharma Y., Dinda A.K., Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev Rep. 2021;17:33–43. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drurey C., Coakley G., Maizels R.M. Extracellular vesicles: new targets for vaccines against helminth parasites. Int J Parasitol. 2020;50:623–633. doi: 10.1016/j.ijpara.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Avila-Calderón E.D., Ruiz-Palma M.D.S., Aguilera-Arreola M.G., Velázquez-Guadarrama N., Ruiz E.A., Gomez-Lunar Z., et al. Outer membrane vesicles of Gram-negative bacteria: an outlook on biogenesis. Front Microbiol. 2021;12:557902. doi: 10.3389/fmicb.2021.557902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shockman G.D., Barrett J.F. Structure, function, and assembly of cell walls of Gram-positive bacteria. Annu Rev Microbiol. 1983;37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- 118.Brown L., Wolf J.M., Prados-Rosales R., Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gill S., Catchpole R., Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev. 2019;43:273–303. doi: 10.1093/femsre/fuy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Micoli F., MacLennan C.A. Outer membrane vesicle vaccines. Semin Immunol. 2020;50:101433. doi: 10.1016/j.smim.2020.101433. [DOI] [PubMed] [Google Scholar]
- 122.Raeven R.H., van der Maas L., Tilstra W., Uittenbogaard J.P., Bindels T.H., Kuipers B., et al. Immunoproteomic profiling of bordetella pertussis outer membrane vesicle vaccine reveals broad and balanced humoral immunogenicity. J Proteome Res. 2015;14:2929–2942. doi: 10.1021/acs.jproteome.5b00258. [DOI] [PubMed] [Google Scholar]
- 123.Gerritzen M.J.H., Martens D.E., Wijffels R.H., van der Pol L., Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv. 2017;35:565–574. doi: 10.1016/j.biotechadv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Fantappiè L., de Santis M., Chiarot E., Carboni F., Bensi G., Jousson O., et al. Antibody-mediated immunity induced by engineered escherichia coli omvs carrying heterologous antigens in their lumen. J Extracell Vesicles. 2014;3:24015. doi: 10.3402/jev.v3.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watkins H.C., Rappazzo C.G., Higgins J.S., Sun X., Brock N., Chau A., et al. Safe recombinant outer membrane vesicles that display M2e elicit heterologous influenza protection. Mol Ther. 2017;25:989–1002. doi: 10.1016/j.ymthe.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frasch C.E. Preparation of bacterial polysaccharide-protein conjugates: analytical and manufacturing challenges. Vaccine. 2009;27:6468–6470. doi: 10.1016/j.vaccine.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 127.Maira-Litran T., Kropec A., Goldmann D.A., Pier G.B. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-n-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2005;73:6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Skurnik D., Cywes-Bentley C., Pier G.B. The exceptionally broad-based potential of active and passive vaccination targeting the conserved microbial surface polysaccharide pnag. Expert Rev Vaccines. 2016;15:1041–1053. doi: 10.1586/14760584.2016.1159135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stevenson T.C., Cywes-Bentley C., Moeller T.D., Weyant K.B., Putnam D., Chang Y.F., et al. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc Natl Acad Sci U S A. 2018;115:E3106–E3115. doi: 10.1073/pnas.1718341115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holst J., Martin D., Arnold R., Huergo C.C., Oster P., O'Hallahan J., et al. Properties and clinical performance of vaccines containing outer membrane vesicles from neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 131.Claassen I., Meylis J., van der Ley P., Peeters C., Brons H., Robert J., et al. Production, characterization and control of a neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–1008. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 132.Balhuizen M.D., Veldhuizen E.J.A., Haagsman H.P. Outer membrane vesicle induction and isolation for vaccine development. Front Microbiol. 2021;12:629090. doi: 10.3389/fmicb.2021.629090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martins P., Machado D., Theizen T.H., Guarnieri J.P.O., Bernardes B.G., Gomide G.P., et al. Outer membrane vesicles from neisseria meningitidis (proteossome) used for nanostructured Zika virus vaccine production. Sci Rep. 2018;8:8290. doi: 10.1038/s41598-018-26508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shehata M.M., Mostafa A., Teubner L., Mahmoud S.H., Kandeil A., Elshesheny R., et al. Bacterial outer membrane vesicles (OMVs)-based dual vaccine for influenza a H1N1 virus and mers-cov. Vaccines (Basel) 2019;7:46. doi: 10.3390/vaccines7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wise J. COVID-19: delta variant doubles risk of hospital admission compared with alpha variant, study shows. BMJ. 2021;374:n2152. doi: 10.1136/bmj.n2152. [DOI] [PubMed] [Google Scholar]
- 136.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19:810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 137.Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gan Y., Li C., Peng X., Wu S., Li Y., Tan J.P.K., et al. Fight bacteria with bacteria: bacterial membrane vesicles as vaccines and delivery nanocarriers against bacterial infections. Nanomedicine. 2021;35:102398. doi: 10.1016/j.nano.2021.102398. [DOI] [PubMed] [Google Scholar]
- 139.Samsonraj R.M., Raghunath M., Nurcombe V., Hui J.H., van Wijnen A.J., Cool S.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao T., Sun F., Liu J., Ding T., She J., Mao F., et al. Emerging role of mesenchymal stem cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Ther. 2019;14:482–494. doi: 10.2174/1574888X14666190228103230. [DOI] [PubMed] [Google Scholar]
- 141.Zhao A.G., Shah K., Cromer B., Sumer H. Mesenchymal stem cell-derived extracellular vesicles and their therapeutic potential. Stem Cell Int. 2020;2020:8825771. doi: 10.1155/2020/8825771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yeo R.W., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh B.J., et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 143.Wang M., Wang C., Chen M., Xi Y., Cheng W., Mao C., et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13:10279–10293. doi: 10.1021/acsnano.9b03656. [DOI] [PubMed] [Google Scholar]
- 144.Fan J., Lee C.S., Kim S., Chen C., Aghaloo T., Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. 2020;14:11973–11984. doi: 10.1021/acsnano.0c05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hade M.D., Suire C.N., Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10:1959. doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yao J., Huang K., Zhu D., Chen T., Jiang Y., Zhang J., et al. A minimally invasive exosome spray repairs heart after myocardial infarction. ACS Nano. 2021;15:11099–11111. doi: 10.1021/acsnano.1c00628. [DOI] [PubMed] [Google Scholar]
- 147.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khalaj K., Figueira R.L., Antounians L., Lauriti G., Zani A. Systematic review of extracellular vesicle-based treatments for lung injury: are EVs a potential therapy for COVID-19? J Extracell Vesicles. 2020;9:1795365. doi: 10.1080/20013078.2020.1795365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang J., Xiong J., Yang L., Zhang J., Sun S., Liang Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale. 2021;13:8740–8750. doi: 10.1039/d1nr01314a. [DOI] [PubMed] [Google Scholar]
- 151.Su N., Hao Y., Wang F., Hou W., Chen H., Luo Y. Mesenchymal stromal exosome-functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Sci Adv. 2021;7 doi: 10.1126/sciadv.abf7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Escors D., Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp. 2010;58:107–119. doi: 10.1007/s00005-010-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rabinowitz J., Chan Y.K., Samulski R.J. Adeno-associated virus (AAV) versus immune response. Viruses. 2019;11:102. doi: 10.3390/v11020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duan L., Xu L., Xu X., Qin Z., Zhou X., Xiao Y., et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387–1397. doi: 10.1039/d0nr07622h. [DOI] [PubMed] [Google Scholar]
- 155.Zhan T., Rindtorff N., Betge J., Ebert M.P., Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2019;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 156.Kim E.J., Kang K.H., Ju J.H. CRISPR-Cas9: a promising tool for gene editing on induced pluripotent stem cells. Korean J Intern Med. 2017;32:42–61. doi: 10.3904/kjim.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim S.M., Yang Y., Oh S.J., Hong Y., Seo M., Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 158.Sato Y.T., Umezaki K., Sawada S., Mukai S.A., Sasaki Y., Harada N., et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in mscs. Adv Sci (Weinh) 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2018;19:19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 161.Ye Y., Zhang X., Xie F., Xu B., Xie P., Yang T., et al. An engineered exosome for delivering sgRNA: Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater Sci. 2020;8:2966–2976. doi: 10.1039/d0bm00427h. [DOI] [PubMed] [Google Scholar]
- 162.Yao X., Lyu P., Yoo K., Yadav M.K., Singh R., Atala A., et al. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu T.X., Rothenberg M.E. Microrna. J Allergy Clin Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang J., Yu M., Yin W., Liang B., Li A., Li J., et al. Development of a novel rnai therapy: engineered mir-31 exosomes promoted the healing of diabetic wounds. Bioact Mater. 2021;6:2841–2853. doi: 10.1016/j.bioactmat.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Khani A.T., Sharifzad F., Mardpour S., Hassan Z.M., Ebrahimi M. Tumor extracellular vesicles loaded with exogenous Let-7i and miR-142 can modulate both immune response and tumor microenvironment to initiate a powerful anti-tumor response. Cancer Lett. 2021;501:200–209. doi: 10.1016/j.canlet.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 166.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 167.He L.F., Xu H.W., Chen M., Xian Z.R., Wen X.F., Chen M.N., et al. Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget. 2017;8:17573–17585. doi: 10.18632/oncotarget.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thillai K., Sarker D., Wells C. PAK4 pathway as a potential therapeutic target in pancreatic cancer. Future Oncol. 2018;14:579–582. doi: 10.2217/fon-2017-0458. [DOI] [PubMed] [Google Scholar]
- 169.Xu L., Faruqu F.N., Lim Y.M., Lim K.Y., Liam-Or R., Walters A.A., et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials. 2021;264:120369. doi: 10.1016/j.biomaterials.2020.120369. [DOI] [PubMed] [Google Scholar]
- 170.Li Z., Zhao P., Zhang Y., Wang J., Wang C., Liu Y., et al. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11:2953–2965. doi: 10.7150/thno.49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Orefice N.S. Development of new strategies using extracellular vesicles loaded with exogenous nucleic acid. Pharmaceutics. 2020;12:705. doi: 10.3390/pharmaceutics12080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lamichhane T.N., Raiker R.S., Jay S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm. 2015;12:3650–3657. doi: 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Podolak I., Galanty A., Sobolewska D. Saponins as cytotoxic agents: a review. Phytochemistry Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu J.Y., Li Y.J., Hu X.B., Huang S., Luo S., Tang T., et al. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: a head-to-head comparison. J Control Release. 2021;336:510–521. doi: 10.1016/j.jconrel.2021.07.004. [DOI] [PubMed] [Google Scholar]