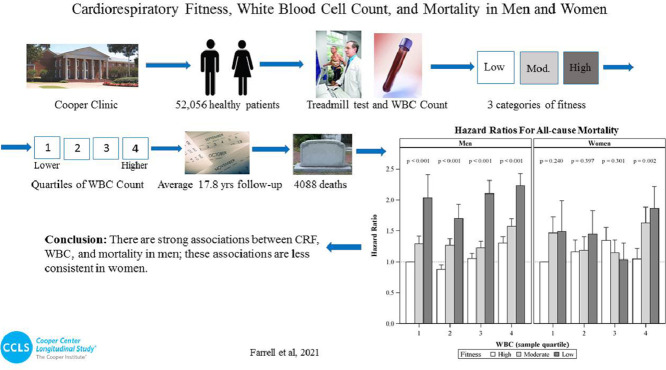
Highlights
-
•
We evaluated the joint associations of cardiorespiratory fitness and white blood cell count, an inflammatory marker, with mortality risk in 52,056 men and women.
-
•
In men, higher levels of fitness were protective against all-cause, cardiovascular disease, and cancer mortality in those with low-normal, normal, and high-normal levels of inflammation.
-
•
In women, higher levels of fitness were protective against all-cause, cardiovascular disease, and cancer mortality in those with high-normal levels of inflammation only.
-
•
In order to decrease mortality risk, all men and women should strive to meet or exceed current public health guidelines for physical activity.
Keywords: Complete blood count, Epidemiology, Inflammation, Physical fitness
Abstract
Background
We examined the associations of cardiorespiratory fitness (CRF) and white blood cell count (WBC) with mortality outcomes.
Methods
A total of 52,056 apparently healthy adults completed a comprehensive health examination, including a maximal treadmill test and blood chemistry analyses. CRF was categorized as high, moderate, or low by age and sex; WBC was categorized as sex-specific quartiles.
Results
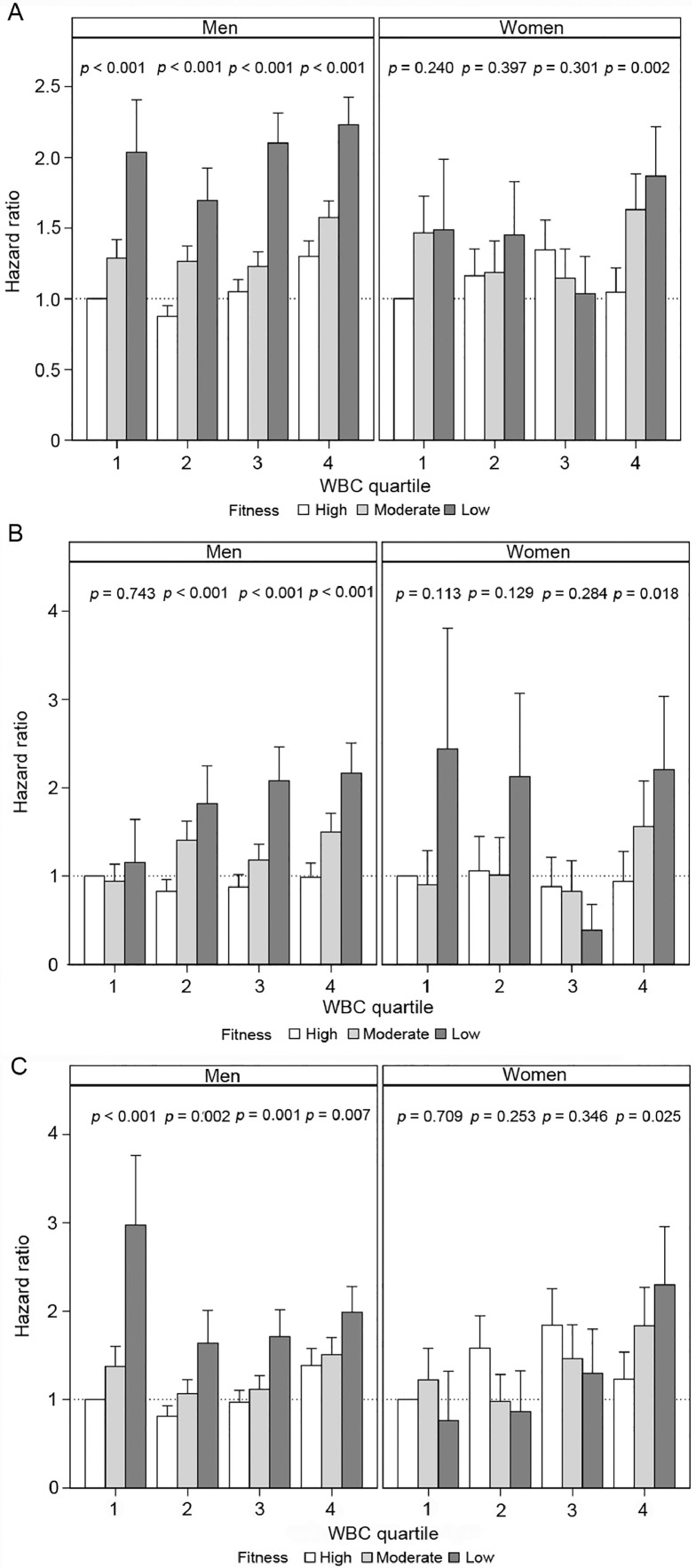
During 17.8 ± 9.5 years (mean ± SD) of follow-up, a total of 4088 deaths occurred. When regressed jointly, significantly decreased all-cause mortality across CRF categories was observed within each quartile of WBC in men. Within WBC Quartile 1, all-cause mortality hazard ratios (HRs) with a 95% confidence interval (95%CI) were 1.0 (referent), 1.29 (95%CI: 1.06‒1.57), and 2.03 (95%CI: 1.42‒2.92) for high, moderate, and low CRF categories, respectively (p for trend < 0.001). Similar trends were observed in the remaining 3 quartiles. With the exception of cardiovascular disease (CVD) mortality within Quartile 1 (p for trend = 0.743), there were also similar trends across CRF categories within WBC quartiles in men for both CVD and cancer mortality (p for trend < 0.01 for all). For women, there were no significant trends across CRF categories for mortality outcomes within Quartiles 1–3. However, we observed significantly decreased all-cause mortality across CRF categories within WBC Quartile 4 (HR = 1.05 (95%CI: 0.76‒1.44), HR = 1.63 (95%CI:1.20‒2.21), and HR = 1.87 (95%CI:1.29‒2.69) for high, moderate, and low CRF, respectively (p for trend = 0.002)). Similar trends in women were observed for CVD and cancer mortality within WBC Quartile 4 only.
Conclusion
There are strong joint associations between CRF, WBC, and all-cause, CVD, and cancer mortality in men; these associations are less consistent in women.
Graphical Abstract
1. Introduction
Cardiovascular disease (CVD) and cancer have long been the 2 leading causes of death in adults in the United States, accounting for 859,125 and 600,920 deaths, respectively, in 2017.1 A low level of cardiorespiratory fitness (CRF) is well-established as a major risk factor for all-cause,2, 3, 4 CVD,5, 6, 7 and cancer8, 9, 10 mortality in various populations. In recent years, inflammation has been identified as a common factor in the etiology of these mortality risks. The normal inflammatory response is temporary and is characterized by activation of immune and nonimmune cells that protect the host from a variety of threats, such as bacteria, viruses, other infections, and toxins.11 Activation of these cells eliminates pathogens and promotes tissue repair and recovery. Under normal conditions, once these processes are completed, the host returns to homeostasis. In recent years, a condition known as systemic chronic inflammation has been shown to be prevalent, particularly in middle-aged and older populations. It is a persistent, low-grade type of inflammation and has been linked to a number of morbidity outcomes, such as metabolic syndrome, type 2 diabetes, CVD, and some cancers.11
Circulating white blood cell count (WBC) is a simple, readily available, inexpensive, and widely used indicator of nonspecific inflammation. Positive associations between higher WBC and all-cause,12,13 CVD,7,14,15 and cancer16,17 mortality have been observed in numerous studies. For example, Weijenberg et al.18 reported on the association of WBC with the risk for coronary heart disease as well as all-cause mortality in a sample of 884 older men in the Zutphen Study. The adjusted relative risk (RR) of mortality was significantly greater for coronary heart disease (RR = 1.32) and all-cause (RR = 1.25) mortality, respectively, in men with higher WBCs. In the National Health and Nutrition Examination Survey (NHANES) II, Erlinger et al.17 examined the association between WBC and cancer mortality in 7674 participants who were followed for 16 years. The survey found a significantly higher adjusted mortality RR of 2.23 in the highest vs. lowest WBC quartile.
Several lifestyle-related factors have been associated with increased levels of inflammatory markers. These include excessive alcohol intake,19 poor diet,20 abdominal obesity,21,22 tobacco use,23 and physical inactivity.24,25 From a pharmacological perspective, aspirin,26 statins,27 and interleukin-1β antagonists28 have been examined for their utility relative to decreasing inflammatory markers and, more importantly, to decreasing various morbidity and mortality outcomes.
Although there are numerous studies regarding the associations between CRF and mortality, as well as between WBC and mortality, there is scant evidence pertaining to the joint associations of these variables with mortality. We hypothesized that higher levels of CRF are associated with reduced mortality risk across the spectrum of inflammation. These types of analyses should provide valuable additional information beyond that of examining each exposure separately. Thus, the primary purpose of this investigation was to examine the joint relationship of CRF and WBC with all-cause, CVD, and cancer mortality in a cohort of apparently healthy men and women.
2. Methods
The Cooper Center Longitudinal Study (CCLS) is a prospective epidemiological study of health behaviors, health characteristics, and chronic disease biomarkers in adult men and women. A total of 71,712 participants were considered for inclusion in the present study if they completed a comprehensive physical examination at the Cooper Clinic in Dallas, TX, between 1978 and 2015. Cooper Clinic patients are most often self-referred, are referred by their primary care physicians, or are executives referred by their companies. Prior to their examinations, participants provided written informed consent for the examination and for participation in the CCLS. The CCLS undergoes annual review and approval by the Institutional Review Board of The Cooper Institute.
2.1. Exclusions
Patients with previously diagnosed CVD or cancer at baseline (n = 1705), those with body mass indexes (BMIs) < 18.5 kg/m2 (n = 886), those who failed to achieve 85% of predicted maximal heart rate during maximal treadmill exercise testing (n = 2277), those with abnormal electrocardiogram (n = 4958), those with an abnormal blood pressure responses to exercise or exertional angina (n = 77), those with less than 1 year of follow-up (n = 1375), those missing smoking status (n = 8013), and those with WBC < 2000/μL or > 12,000/μL (n = 365) were excluded from the analyses. These exclusions resulted in a final analytic sample of 52,056 participants (37,052 men and 15,004 women).
2.2. Exam procedures
Patients were advised to refrain from smoking on the day of their examination and to refrain from physical activity (PA) the day prior. A clinical evaluation was performed that included an examination by a physician, blood chemistry assessment, health history, anthropometry, resting blood pressure, and electrocardiogram, and a maximal graded treadmill exercise test.29 All procedures were administered by trained technicians who followed standardized measurement protocols. Blood samples were drawn from an antecubital vein in the early morning following a 12-h fast and then analyzed using standard automated laboratory techniques. The key blood chemistry component of interest was WBC, but blood glucose, total cholesterol, high-density lipoprotein (HDL), low density lipoprotein (LDL), and triglycerides were also measured. The triglyceride-to-HDL ratio was calculated by dividing the triglyceride value by the HDL value. Height and weight were measured without shoes using a stadiometer and standard physician's scale. BMI was calculated as body weight in kilograms divided by height in meters squared. Seated resting blood pressure was acquired by using a mercury sphygmomanometer following the American Heart Association protocol. Current smoking status was obtained from a standardized questionnaire and grouped categorically for analysis (yes/no).
CRF was estimated on the basis of the duration of the physician-supervised maximal treadmill exercise test by using a modified Balke protocol, as described previously.30 Exercise duration based on this protocol has been shown to correlate closely with laboratory measures of maximal oxygen uptake (VO2max) in men (r = 0.92) and women (r = 0.94).31 To standardize exercise-test performance, we computed maximal metabolic equivalent (MET) (1 MET = 3.5 mL O2 uptake/kg body weight/min) levels of CRF from the final treadmill speed and grade.32 The treadmill speed and grade are calibrated on a regular basis. Self-reported PA was assessed via questionnaire. Patients were asked to provide detailed information regarding activity mode, frequency, duration, and intensity over the previous 3 months. PA frequency and duration were used to calculate the average number of minutes per week for each reported mode of PA. The Compendium of Physical Activities33 was then used to estimate an MET value for each reported activity. MET values were then multiplied by duration for each activity and summed across activities in order to estimate total MET-minutes per week of PA.
2.3. Mortality surveillance
Participants were followed for mortality from the date of their examinations to the date of death for decedents or to December 31, 2016, for survivors. Vital status was ascertained using the National Death Index Plus service. Outcomes included all-cause, CVD, and cancer mortality. CVD deaths were identified using the International Classification of Diseases (ICD), the 9th and 10th Editions, Revised. CVD deaths were identified and ascertained using the ICD, the 9th revision (Codes 140–208) for deaths occurring before 1999 and 10th revision (Codes C00-C97) for deaths between 1999 and 2016. Similarly, cancer deaths were classified with ICD-9 codes (140–239) and ICD-10 codes (C00-C97).
2.4. Statistical analysis
CRF was classified by age and sex into standard categories based on grouped fitness categories of the CCLS overall: high (Quintiles 4–5), moderate (Quintiles 2–3), or low (Quintile 1).2 Baseline characteristics of participants by sex were summarized by ordered CRF category and by WBC sample quartile. Unadjusted trends were tested using the nonparametric Jonckheere-Terpstra method. All-cause, CVD, and cancer mortality hazard ratios (HRs) were estimated separately for men and women by using Cox proportional hazards regression of attained age on joint primary exposures, adjusted for smoking, systolic blood pressure, total cholesterol, glucose, triglyceride-to-HDL ratio, BMI, and, in women, age ≥ 50 years as a proxy for menopausal status. Primary exposures included main effects and interaction of the CRF and WBC classifications. Men and women were modeled separately due to differences in longevity and to avoid a number of interaction terms (e.g., sex × current smoking) that would have been necessary in a combined model. Using attained age as the time scale provided tight control over the age dependence of mortality outcomes, and provision was made for exclusion from risk until baseline. Proportional hazards assumptions were confirmed by testing the correlation between weighted Schoenfeld residuals and ranked failure times. All analyses were programmed in SAS/STAT, Version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
The age of the sample was 45.2 ± 9.5 years (mean ± SD). Characteristics of men and women according to CRF and WBC categories are presented in Table 1 and Table 2, respectively. For both sexes, more favorable values were observed across ordered CRF and WBC strata. During 17.8 ± 9.5 years of follow-up, 4088 all-cause deaths occurred, including 1130 CVD and 1444 cancer deaths. Maximal METs as well as mean PA values corresponding to low, moderate, and high CRF categories by sex and age group are reported in Table 3.
Table 1.
Characteristics of 37,052 men and 15,004 women according to CRF level; the Cooper Center Longitudinal Study, 1978–2016.
| Low CRF (Quintile 1) | Moderate CRF (Quintiles 2–3) | High CRF (Quintiles 4–5) | p for trend | ||
|---|---|---|---|---|---|
| Men | n = 37,052 | n = 4782 | n = 14,858 | n = 17,412 | |
| Age (year) | 45.2 ± 9.2 | 44.0 ± 8.9 | 45.1 ± 9.1 | 45.5 ± 9.4 | <0.001 |
| BMI (kg/m2) | 27.2 ± 4.0 | 31.5 ± 5.6 | 27.8 ± 3.5 | 25.5 ± 2.7 | <0.001 |
| CRF (METs) | 11.6 ± 2.3 | 8.5 ± 1.1 | 10.6 ± 1.1 | 13.4 ± 1.8 | <0.001 |
| Physical activity (MET-min/week) (missing 10,228) | 1150 ± 1354 | 483 ± 1002 | 785 ± 1069 | 1561 ± 1467 | <0.001 |
| Systolic BP (mmHg) | 121.5 ± 12.8 | 124.4 ± 13.5 | 121.8 ± 12.6 | 120.4 ± 12.5 | <0.001 |
| Total cholesterol (mmol/L) | 5.2 ± 1.0 | 5.4 ± 1.1 | 5.3 ± 1.0 | 5.1 ± 1.0 | <0.001 |
| Triglyceride-to-HDL ratio | 3.1 ± 2.2 | 4.4 ± 2.7 | 3.5 ± 2.3 | 2.4 ± 1.7 | <0.001 |
| Glucose (mmol/L) | 5.5 ± 0.9 | 5.8 ± 1.3 | 5.5 ± 1.0 | 5.4 ± 0.6 | <0.001 |
| WBC (109/L) | 6.0 ± 1.4 | 6.8 ± 1.6 | 6.2 ± 1.4 | 5.6 ± 1.2 | <0.001 |
| Current smoking (n (%)) | 6688 (18.1) | 1349 (28.2) | 3230 (21.7) | 2109 (12.1) | <0.001 |
| Total number of deaths | 3409 | 698 | 1495 | 1216 | |
| All-cause mortality rate (per 1000 man-years) | 5.0 | 8.0 | 5.4 | 3.8 | |
| Women | n = 15,004 | n = 1374 | n = 4613 | n = 9017 | |
| Age (year) | 45.2 ± 10.1 | 44.7 ± 10.2 | 45.1 ± 10.2 | 45.4 ± 10.0 | <0.001 |
| BMI (kg/m2) | 24.2 ± 4.6 | 30.1 ± 7.0 | 25.3 ± 4.4 | 22.8 ± 3.0 | <0.001 |
| CRF (METs) | 9.7 ± 2.0 | 6.6 ± 0.9 | 8.4 ± 1.0 | 10.9 ± 1.6 | <0.001 |
| Physical activity (MET-min/week) (missing 3709) | 1196 ± 1382 | 464 ± 908 | 718 ± 1114 | 1509 ± 1445 | <0.001 |
| Systolic BP (mmHg) | 112.7 ± 14.0 | 117.2 ± 15.4 | 113.5 ± 14.2 | 111.6 ± 13.5 | <0.001 |
| Total cholesterol (mmol/L) | 5.1 ± 1.0 | 5.3 ± 1.0 | 5.2 ± 1.0 | 5.0 ± 0.9 | <0.001 |
| Triglyceride-to-HDL ratio | 1.6 ± 1.3 | 2.4 ± 1.7 | 1.9 ± 1.6 | 1.4 ± 0.9 | <0.001 |
| Glucose (mmol/L) | 5.1 ± 0.7 | 5.4 ± 1.2 | 5.2 ± 0.8 | 5.1 ± 0.6 | <0.001 |
| WBC (109/L) | 5.9 ± 1.5 | 6.8 ± 1.6 | 6.2 ± 1.6 | 5.6 ± 1.4 | <0.001 |
| Current smoking (n( %)) | 1291 (8.6) | 213 (15.5) | 548 (11.9) | 530 (5.9) | <0.001 |
| Total number of deaths | 679 | 109 | 265 | 305 | |
| All-cause mortality rate (per 1000 woman-years) | 2.8 | 4.5 | 3.4 | 2.2 |
Note: Variables age through WBC are presented as mean ± SD.
Abbreviations: BMI = body mass index; BP = blood pressure; CRF = cardiorespiratory fitness; HDL = high-density lipoprotein; MET = metabolic equivalent; WBC = white blood cell count.
Table 2.
Characteristics of 37,052 men and 15,004 women according to quartiles of WBC; the Cooper Center Longitudinal Study, 1978–2016.
| Q1 (<5.0 × 109/L) | Q2 (5.0–5.699 × 109/L) | Q3 (5.7–6.8 × 109/L) | Q4 (>6.8 × 109/L) | p for trend | ||
|---|---|---|---|---|---|---|
| Men | n = 37,052 | n = 8318 | n = 9242 | n = 9792 | n = 9700 | |
| Age (year) | 45.2 ± 9.2 | 45.5 ± 9.0 | 45.1 ± 9.1 | 45.1 ± 9.2 | 45.0 ± 9.5 | 0.017 |
| BMI (kg/m2) | 27.2 ± 4.0 | 26.2 ± 3.3 | 26.9 ± 3.7 | 27.4 ± 4.0 | 28.3 ± 4.6 | <0.001 |
| CRF (METs) | 11.6 ± 2.3 | 12.5 ± 2.3 | 12.0 ± 2.3 | 11.5 ± 2.2 | 10.7 ± 2.1 | <0.001 |
| Physical activity (MET-min/week) (missing 6116) | 1150 ± 1354 | 1366 ± 1435 | 1193 ± 1412 | 1113 ± 1336 | 914 ± 1165 | <0.001 |
| Systolic BP (mmHg) | 121.5 ± 12.8 | 120.4 ± 12.3 | 121.0 ± 12.6 | 121.8 ± 12.8 | 122.4 ± 13.2 | <0.001 |
| Total cholesterol (mmol/L) | 5.2 ± 1.0 | 5.1 ± 1.0 | 5.2 ± 1.0 | 5.3 ± 1.0 | 5.4 ± 1.0 | <0.001 |
| Triglyceride-to-HDL ratio | 3.1 ± 2.2 | 2.3 ± 1.7 | 2.8 ± 2.1 | 3.2 ± 2.3 | 3.7 ± 2.5 | <0.001 |
| Glucose (mmol/L) | 5.5 ± 16.0 | 5.4 ± 0.7 | 5.5 ± 0.8 | 5.5 ± 1.0 | 5.6 ± 1.0 | <0.001 |
| WBC (109/L) | 6.0 ± 1.4 | 4.4 ± 0.5 | 5.4 ± 0.2 | 6.2 ± 0.3 | 7.9 ± 1.1 | <0.001 |
| Current smoking (n(%)) | 6688 (18.1) | 904 (10.9) | 1256 (13.6) | 1605 (16.4) | 2923 (30.1) | <0.001 |
| Total number of deaths | 3049 | 457 | 630 | 876 | 1446 | |
| All-cause mortality rate (per 1000 person-years) | 5.0 | 3.3 | 3.7 | 4.7 | 7.4 | |
| Women | n = 15,004 | n = 4357 | n = 3460 | n = 3493 | n = 3694 | |
| Age (year) | 45.2 ± 10.1 | 46.2 ± 9.6 | 45.2 ± 10.0 | 45.1 ± 10.2 | 44.2 ± 10.6 | <0.001 |
| BMI (kg/m2) | 24.2 ± 4.6 | 23.0 ± 3.4 | 23.8 ± 4.0 | 24.5 ± 4.4 | 25.8 ± 5.7 | <0.001 |
| CRF (METs) | 9.7 ± 2.0 | 10.2 ± 2.0 | 9.9 ± 2.0 | 9.6 ± 2.0 | 9.1 ± 2.0 | <0.001 |
| Physical activity (MET-min/week) (missing 2325) | 1196 ± 1382 | 1348 ± 1421 | 1202 ± 1310 | 1178 ± 1530 | 1009 ± 1217 | <0.001 |
| Systolic BP (mmHg) | 112.7 ± 14.0 | 111.7 ± 13.6 | 112.1 ± 13.8 | 113.1 ± 14.2 | 114.1 ± 14.4 | <0.001 |
| Total cholesterol (mmol/L) | 5.1 ± 1.0 | 5.1 ± 0.9 | 5.1 ± 1.0 | 5.1 ± 0.9 | 5.1 ± 1.0 | <0.001 |
| Triglyceride-to-HDL ratio | 1.6 ± 1.3 | 1.3 ± 0.9 | 1.5 ± 1.1 | 1.7 ± 1.3 | 2.1 ± 1.8 | <0.001 |
| Glucose (mmol/L) | 5.1 ± 0.7 | 5.1 ± 0.6 | 5.1 ± 0.6 | 5.2 ± 0.7 | 5.2 ± 0.9 | <0.001 |
| WBC (109/L) | 5.9 ± 1.5 | 4.3 ± 0.5 | 5.4 ± 0.2 | 6.2 ± 0.3 | 8.0 ± 1.1 | <0.001 |
| Current smoking (n (%)) | 1291 (8.6) | 162 (3.7) | 192 (5.5) | 303 (8.7) | 634 (17.2) | <0.001 |
| Total number of deaths | 679 | 2.8 | 2.2 | 2.4 | 2.7 | 3.8 |
| All-cause mortality rate (per 1000 woman-years) | 2.8 | 2.2 | 2.4 | 2.7 | 4.8 |
Note: Variables age through WBC are presented as mean ± SD.
Abbreviations: BMI = body mass index; BP = blood pressure; CRF = cardiorespiratory fitness; HDL = high-density lipoprotein; MET = metabolic equivalent; WBC = white blood cell count.
Table 3.
Maximal MET values (upper) and MET-min per week (mean ± SD; lower) corresponding to CRF categories for men and women by age group; the Cooper Center Longitudinal Study, 1978–2016.
| Low CRF | Moderate CRF | High CRF | |
|---|---|---|---|
| Men (year) | |||
| 20‒29 | <10.3 824 ± 1460 |
10.3‒12.6 1083 ± 1389 |
>12.6 2003 ± 2215 |
| 30‒39 | <10.3 514 ± 785 |
10.3‒12.6 812 ± 1014 |
>12.6 1475 ± 1353 |
| 40‒49 | <9.4 469 ± 1106 |
9.4‒12.2 774 ± 1006 |
>12.2 1574 ± 1402 |
| 50‒59 | <8.5 406 ± 834 |
8.5‒10.8 748 ± 1183 |
>10.8 1552 ± 1466 |
| 60‒69 | <6.7 485 ± 1374 |
6.7‒9.4 729 ± 1019 |
>9.4 1533 ± 1587 |
| 70‒79 | <6.7 567 ± 706 |
6.7‒9.4 1028 ± 1280 |
>9.4 1853 ± 1986 |
| Women (year) | |||
| 20‒29 | <8.1 730 ± 908 |
8.1‒10.3 1007 ± 1446 |
>10.3 1906 ± 1759 |
| 30‒39 | <8.1 496 ± 1226 |
8.1‒10.3 781 ± 1554 |
>10.3 1631 ± 1460 |
| 40‒49 | <7.2 426 ± 658 |
7.2‒9.4 707 ± 917 |
>9.4 1506 ± 1334 |
| 50‒59 | <6.3 402 ± 779 |
6.3‒8.1 627 ± 854 |
>8.1 1414 ± 1551 |
| 60‒69 | <5.8 453 ± 997 |
5.8‒7.6 656 ± 844 |
>7.6 1246 ± 1169 |
| 70‒79 | <5.8 739 ± 800 |
5.8‒7.6 762 ± 910 |
>7.6 1232 ± 1133 |
Notes: low CRF = Quintile 1; moderate CRF = Quintiles 2–3; high CRF = Quintiles 4–5; 1 MET = 3.5 mL O2/kg/min.
Abbreviations: CRF = cardiorespiratory fitness; MET = metabolic equivalent.
3.1. Mortality outcomes in men
Joint associations of CRF and WBC with adjusted all-cause, CVD, and cancer mortality in men are presented in Fig. 1A, 1B, and 1C, respectively. For all-cause mortality (Fig. 1A), we observed significantly higher risk across ordered CRF categories within each WBC quartile. More specifically, within WBC Quartile 1, HR were 1.0 (referent), 1.29 (95%CI: 1.06‒1.57), and 2.03 (95%CI: 1.42‒2.92) for high, moderate, and low CRF categories, respectively. Similar trends were observed within Quartiles 2–4 (p for trend < 0.001 for all). With the exception of CVD mortality within Quartile 1 (p for trend = 0.743), there were also similar trends across CRF categories within all WBC quartiles in men for both CVD (Fig. 1B) and cancer (Fig. 1C) mortality.
Fig. 1.
(A) All-cause mortality; (B) cardiovascular disease; and (C) cancer mortality hazard ratios relative to low fit. White blood cell count (WBC) Quartile 1, in men and women with standard error bars, referent value (dotted), and inset fitness trend tests within WBC quartiles. Interaction p values were (A) 0.42 (men) and 0.48 (women); (B) 0.57 (men) and 0.61 (women); and (C) 0.54 (men) and 0.38 (women), supporting the hypothesis that the relationship of fitness and all-cause mortality is independent of WBC (the Cooper Center Longitudinal Study (CCLS), 1978–2016).
3.2. Mortality outcomes in women
Joint associations of CRF and WBC with adjusted all-cause, CVD, and cancer mortality in women are presented in Fig. 1A, 1B, and 1C, respectively. For all-cause mortality (Fig. 1A), we did not observe any significant trends across CRF categories within WBC Quartiles 1–3. However, a significant mortality trend was observed across CRF categories within WBC Quartile 4, with HR of 1.05 (95%CI:0.76‒1.44), 1.63 (95%CI:1.20‒2.21), and 1.87 (95%CI:1.29‒2.69) for high, moderate, and low CRF, respectively (p for trend = 0.002). Similarly, when examining CVD (Fig. 1B) and cancer (Fig. 1C) mortality, a significant trend across CRF categories was observed within WBC Quartile 4 only (p for trend < 0.03 for both).
4. Discussion
To the authors’ knowledge, this is the first study to examine joint associations of CRF and WBC with mortality outcomes in both men and women. Approximately 63% of total deaths resulted from CVD and cancer, which is consistent with nationwide mortality data over the past few decades.34 Although low CRF and inflammatory markers (e.g., WBC) have been associated with increased mortality, little is known regarding the association of CRF with mortality within various levels of inflammation. Pletnikoff et al.35 reported on 2270 Finnish men with a mean age of 52.8 years and no cancer history at baseline. In a multivariable model, when compared to men with normal WBC (<5.4 × 109/L) and high CRF (>30.1 mL/kg/min), those with high WBC and low CRF had a 1.85-fold higher risk for cancer death (p < 0.01).
It is well established that CRF is associated with reduced all-cause, CVD, and cancer mortality.2,6,8,36 It has also been established that CRF is inversely associated with inflammatory markers such as C-reactive protein (CRP), WBC, tumor necrosis factor, and interleukin-6.37, 38, 39 A novel finding in the current study is that with the exception noted above, moderate to high levels of CRF provided protection from all-cause, CVD, and cancer mortality in men within each of the WBC quartiles. Thus, regardless of the level of inflammation present, achieving a moderate-to-high level of CRF is necessary to minimize mortality risk in men. However, as previously mentioned, among women this pattern of reduced mortality across CRF categories within WBC quartiles was observed in Quartile 4 only. It is unclear why our results were not as consistent in women as in men. One possible explanation is that body-fat distribution patterns are different between men and women, with the former tending to have an android (upper body) and the latter tending to have a gynoid (lower body) fat distribution. Unfortunately, we do not have sufficient data in our sample regarding waist and hip girth to substantiate this explanation. Using BMI as a proxy for adiposity in our sample, the women are leaner (mean BMI = 24.2 kg/m2) than the general population of women in the United States. On the other hand, the men's average BMI (27.2 kg/m2) in our sample is more representative of the general population of U.S. men.40 These differences in adiposity status between men and women in our sample vs. the general population, as well as hormonal differences between men and women, may account partially for our disparate results in men and women.
4.1. Strategies for achieving moderate CRF
When discussing strategies for improving CRF, it is important to distinguish PA from CRF. The former is a behavior, whereas the latter is a biological characteristic. The correlation between self-reported PA and objective measurement of CRF is modest;41 both PA and CRF are independent predictors of mortality.42,43 Achieving at least a moderate level of CRF should be a reasonable goal for most adults who meet the 2018 Physical Activity Guidelines for Americans;44 that is, accumulating 500–1000 MET-min/week. Indeed, our data support the notion that moderate CRF is associated with meeting these guidelines. For example, moderate CRF in the age 40–49 category for men and women was associated with mean values of 774 MET-min/week and 707 MET-min/week, respectively (Table 3), which corresponds to the guideline of accumulating 500–1000 MET-min/week. Training studies using aerobic exercise have also resulted in significant gains in CRF. For example, in the HEalth, RIsk factors, exercise Training And GEnetics (HERITAGE) Family Study,45 which included 288 inactive subjects with a mean VO2max of 29 mL/kg/min at baseline, a 20-week supervised aerobic training program resulted in an increased VO2max of 16.3%. As an added benefit, of the participants with metabolic syndrome at baseline, 32.7% no longer had metabolic syndrome following the intervention. Improvements in metabolic syndrome status were due most commonly to decreased blood triglycerides and resting blood pressure. There were no differences across sex or race with regard to the efficacy of exercise in treating metabolic syndrome. The results from HERITAGE are encouraging, but that study showed that there is a significant degree (47%) of heritability of CRF and correspondingly large between-person variations in response to a structured exercise training program. All HERITAGE subjects were unfit at baseline but performed the same exercise program and had excellent adherence; the increases in VO2max among subjects ranged from 0 to 1000 mL/min. Additionally, there was 2.5 times more variance between families than within families regarding the VO2max response to training, further strengthening the evidence that genetics contribute significantly to CRF.46 Thus, for some initially low-fit individuals, meeting current PA guidelines may not always result in reaching a moderate level of CRF. However, independent of improvements in VO2max, it has been well established that increased levels of PA are associated with improvement in a number of cardiometabolic risk factors, such as resting blood pressure, blood glucose, HDL, and triglyceride levels, as well as improved insulin sensitivity, and adiposity level. Therefore, PA needs to be viewed as an extremely beneficial overall behavior and not just as a means of improving CRF. It should also be noted that although meeting current PA guidelines would appear to be a realistic goal for most adults, the most recent accelerometer data from NHANES have shown that less than 5% of men and women ages 20 and older are meeting those guidelines.47
In recent years, studies using high-intensity interval training (HIIT) have shown more substantial increases in VO2max in persons who were physically inactive at baseline.48 A recently published randomized controlled trial (RCT) (The Generation 100 Study) of 1567 elderly Norwegian adults evaluated the effects of 5 years of supervised exercise training on mortality risk.49 Participants were assigned to 2 weekly sessions of either HIIT or moderate-intensity continuous training or to a control group that was instructed to follow national guidelines for physical activity. In all 3 groups, the expected age-related decline in CRF over the 5-year period was inhibited. Peak VO2 was significantly higher in the HIIT group than in the control group post-training. The primary outcome in the study was all-cause mortality. Although no differences were found in all-cause mortality risk across groups when using the control as the referent, HIIT was associated with a 49% reduction in mortality risk when using the moderate-intensity continuous training group as the referent. The authors concluded that future guidelines for PA in older adults should include a recommendation that at least part of the PA program should include HIIT.
In cross-sectional studies, inverse associations between CRF and inflammation are consistent, but results from training studies have been somewhat inconsistent. The Inflammation in Peripheral Vascular Interventions trial randomized control trial50 examined the effects of 4 months of aerobic exercise training among previously inactive middle-aged adults with elevated baseline CRP. Despite a significant improvement (12%) in VO2max in the exercise-training group as a whole, training was associated with a reduction in CRP only in those who lost weight and body fat. On the other hand, a study by Milani et al.51 showed that participation in a cardiac rehabilitation program was associated with a 36% reduction in CRP in the absence of changes in body weight. However, because the program included many components other than exercise (e.g., smoking cessation and dietary counseling with an emphasis on the Mediterranean diet), it was not possible to separate out which component(s) of the program were responsible for the significant reduction in CRP. Thus, additional training studies are necessary to determine whether exercise training by itself results in favorable changes in inflammation markers.
4.2. Diet and inflammatory markers
We were unable to assess dietary intake in the current study, but it is important to note that diet has been shown to be associated with inflammatory markers. Whereas a high intake of meat and heavily processed foods is associated with increased levels of inflammatory markers and mortality,52 a greater intake of plant-based foods is inversely associated with these outcomes. For example, the Mediterranean-style diet emphasizes fruits and vegetables, whole grains, and olive oil, as well as nuts and seeds. This dietary approach was shown to improve endothelial function and insulin resistance, as well as the inflammatory markers of CRP and interleukin-6 over a 2-year period in 180 patients with metabolic syndrome.53 However, WBC was not measured in that study.
4.3. Randomized controlled trials regarding inflammation and health outcomes
The association between systemic chronic inflammation and adverse health outcomes has been well described in recent years, but the strongest evidence has been derived from RCTs targeting pro-inflammatory cytokines. For example, a 2015 meta-analysis of 8 RCTs using antitumor necrosis factor-α therapy showed that reduction of antitumor necrosis factor-α was significantly associated with increased insulin sensitivity and decreased risk of developing Alzheimer's disease in patients with rheumatoid arthritis.54 Results from a double-blind RCT using the interleukin-1-β inhibitor canakinumab in 10,061 adults with a history of myocardial infarction and elevated CRP levels were published in 2017. When compared to patients treated with placebo, those who were treated with canakinumab showed a significant reduction in CRP, along with lower risk of nonfatal myocardial infarction, nonfatal stroke CVD death, and unstable angina.55 Thus, it would appear that reducing levels of inflammatory markers in those with elevated values is significantly associated with beneficial health outcomes. However, at this time it is not known whether specifically targeting a reduction in WBC in patients such as those in the current study with high-normal to mildly elevated WBCs is beneficial. Until that issue is resolved, it seems prudent to focus primarily on promoting increased levels of PA and weight loss for individuals with low CRF and/or obesity to reduce mortality risk.
4.4. Strengths and limitations
Among the strengths of the current study are a large and well-characterized cohort of men and women, an extensive follow-up with a relatively large number of all-cause, CVD, and cancer deaths for analysis, and the use of objective measures for CRF and WBC exposures. We adjusted for several confounders and excluded men and women with markers for pre-existing subclinical disease that could influence mortality, i.e., underweight BMI status, those with very high or very low WBCs, those with abnormal electrocardiograms or blood pressure responses to exercise, as well as those with <1 year of follow-up. This study also has limitations. The cohort is primarily non-Hispanic white and from middle-to-upper socioeconomic strata; therefore, these findings must be cautiously interpreted when generalized to other populations. However, the homogeneity of the sample strengthens the internal validity by reducing potential confounding. We also point out that the median-estimated VO2max for CCLS men and women is very similar to the values in the NHANES study.56 For example, the median-estimated VO2max for CCLS vs. NHANES men in the 40- and 49-year age group is 40.1 mL/kg/min and 40.9 mL/kg/min, respectively. We also did not have sufficient data to include medication use or dietary intake information in this analysis, both of which are potential confounders. Finally, it would be interesting to include CRP as another inflammatory exposure in this study, but routine measurement did not begin until 1997, which limited the available follow-up time for mortal events.
5. Conclusion
Among men within each WBC quartile, there were inverse associations between CRF and mortality risk, suggesting that higher levels of CRF are protective against mortality even for men with higher levels of inflammation. For women, our results were not as consistent. Within WBC quartiles, we found significant inverse joint associations between CRF and all-cause, CVD, and cancer mortality risk among women in Quartile 4 only. Our overall results add to a large body of knowledge suggesting that adults undergo a measurement of CRF per the 2016 American Heart Association Position Statement.57 Adults are urged to meet the 2018 Physical Activity Guidelines for Americans. These data also suggest that measurement of WBC may have value in determining mortality risk status, particularly in men, but also in women in the high-normal/slightly elevated range of the WBC distribution.
Acknowledgment
The authors thank Kenneth H. Cooper, MD, MPH, for establishing the Cooper Center Longitudinal Study, as well the Cooper Clinic physicians and technicians for data collection.
Authors’ contributions
SWF was responsible for study concept and design and drafted the manuscript; DL conducted the statistical analysis, was the primary source for data interpretation, and edited the manuscript; KS assisted with data interpretation and edited the manuscript; CEB was responsible for data acquisition, assisted with data interpretation, and edited the manuscript; LFD, AP, and WLH assisted with data interpretation and edited the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics–2020 Update: A report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Laukkanen JA, Lakka TA, Rauramaa R, et al. Cardiovascular fitness as a predictor of mortality in men. Arch Intern Med. 2001;161:825–831. doi: 10.1001/archinte.161.6.825. [DOI] [PubMed] [Google Scholar]
- 5.Nes BM, Vatten LJ, Nauman J, Janszky I, Wisloff U. A simple nonexercise model of cardiorespiratory fitness predicts long-term mortality. Med Sci Sports Exerc. 2014;46:1159–1165. doi: 10.1249/MSS.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 6.Artero EG, Jackson AS, Sui X, et al. Longitudinal algorithms to estimate cardiorespiratory fitness: Associations with nonfatal cardiovascular disease and disease-specific mortality. J Am Coll Cardiol. 2014;63:2289–2296. doi: 10.1016/j.jacc.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 8.Farrell SW, Cortese GM, LaMonte MJ, Blair SN. Cardiorespiratory fitness, different measures of adiposity, and cancer mortality in men. Obesity (Silver Spring) 2007;15:3140–3149. doi: 10.1038/oby.2007.374. [DOI] [PubMed] [Google Scholar]
- 9.Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: A systematic review and meta-analysis. Ann Oncol. 2015;26:272–278. doi: 10.1093/annonc/mdu250. [DOI] [PubMed] [Google Scholar]
- 10.Thompson AM, Church TS, Janssen I, Katzmarzyk PT, Earnest CP, Blair SN. Cardiorespiratory fitness as a predictor of cancer mortality among men with pre-diabetes and diabetes. Diabetes Care. 2008;31:764–769. doi: 10.2337/dc07-1648. [DOI] [PubMed] [Google Scholar]
- 11.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Jiang CQ, Xu L, et al. White blood cell count and all-cause and cause-specific mortality in the Guangzhou biobank cohort study. BMC Public Health. 2018;18:1232. doi: 10.1186/s12889-018-6073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KI, Lee J, Heo NJ, et al. Differential white blood cell count and all-cause mortality in the Korean elderly. Exp Gerontol. 2013;48:103–108. doi: 10.1016/j.exger.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: In-depth review and update. Tex Heart Inst J. 2013;40:17–29. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DW, Giles WH, Croft JB. White blood cell count: An independent predictor of coronary heart disease mortality among a national cohort. J Clin Epidemiol. 2001;54:316–322. doi: 10.1016/s0895-4356(00)00296-1. [DOI] [PubMed] [Google Scholar]
- 16.Shankar A, Wang JJ, Rochtchina E, Yu MC, Kefford R, Mitchell P. Association between circulating white blood cell count and cancer mortality: A population-based cohort study. Arch Intern Med. 2006;166:188–194. doi: 10.1001/archinte.166.2.188. [DOI] [PubMed] [Google Scholar]
- 17.Erlinger TP, Muntner P, Helzlsouer KJ. WBC count and the risk of cancer mortality in a national sample of U.S. adults: Results from the Second National Health and Nutrition Examination Survey mortality study. Cancer Epidemiol Biomarkers Prev. 2004;13:1052–1056. [PubMed] [Google Scholar]
- 18.Weijenberg MP, Feskens EJ, Kromhout D. White blood cell count and the risk of coronary heart disease and all-cause mortality in elderly men. Arterioscler Thromb Vasc Biol. 1996;16:499–503. doi: 10.1161/01.atv.16.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 20.Sureda A, Bibiloni MDM, Julibert A, et al. Adherence to the Mediterranean diet and inflammatory markers. Nutrients. 2018;10:62. doi: 10.3390/nu10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: A systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Faber DR, van der Graaf Y, Westerink J, Visseren FL. Increased visceral adipose tissue mass is associated with increased C-reactive protein in patients with manifest vascular diseases. Atherosclerosis. 2010;212:274–280. doi: 10.1016/j.atherosclerosis.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 24.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older U.S. adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes RA, Ritti-Dias RM, Balagopal PB, et al. Self-initiated physical activity is associated with high sensitivity C-reactive protein: A longitudinal study in 5030 adults. Atherosclerosis. 2018;273:131–135. doi: 10.1016/j.atherosclerosis.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. The Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 29.DeFina LF, Radford NB, Barlow CE, et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4:174–181. doi: 10.1001/jamacardio.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 31.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 32.American College of Sports Medicine (ACSM) 9th ed. Lippincott Williams & Wilkins; Baltimore, MD: 2014. ACSM's guidelines for exercise testing and prescription. [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Ward EM, Siegel RL. Jemal A. Temporal trends in mortality in the United States, 1969-2013. JAMA. 2015;314:1731–1739. doi: 10.1001/jama.2015.12319. [DOI] [PubMed] [Google Scholar]
- 35.Pletnikoff PP, Laukkanen JA, Tuomainen TP, Kurl S. The joint impact of prediagnostic inflammatory markers and cardiorespiratory fitness on the risk of cancer mortality. Scand J Med Sci Sports. 2018;28:613–620. doi: 10.1111/sms.12952. [DOI] [PubMed] [Google Scholar]
- 36.Farrell SW, Barlow CE, Willis BL, et al. Cardiorespiratory fitness, different measures of adiposity, and cardiovascular disease mortality risk in women. J Womens Health (Larchmt) 2020;29:319–326. doi: 10.1089/jwh.2019.7793. [DOI] [PubMed] [Google Scholar]
- 37.Kim DJ, Noh JH, Lee BW, et al. A white blood cell count in the normal concentration range is independently related to cardiorespiratory fitness in apparently healthy Korean men. Metabolism. 2005;54:1448–1452. doi: 10.1016/j.metabol.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137–145. doi: 10.1097/HCR.0b013e3182122827. [DOI] [PubMed] [Google Scholar]
- 39.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 40.Flegal KM, Ogden CL, Fryar C, Afful J, Klein R, Huang DT. Comparisons of self-reported and measured height and weight, BMI, and obesity prevalence from national surveys: 1999–2016. Obesity (Silver Spring) 2019;27:1711–1719. doi: 10.1002/oby.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dvorak RV, Tchernof A, Starling RD, Ades PA, DiPietro L, Poehlman ET. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: A doubly labeled water study. J Clin Endocrinol Metab. 2000;85:957–963. doi: 10.1210/jcem.85.3.6432. [DOI] [PubMed] [Google Scholar]
- 42.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(Suppl. 6):S379–S399. doi: 10.1097/00005768-200106001-00007. discussion S419–20. [DOI] [PubMed] [Google Scholar]
- 43.Williams PT. Physical fitness and activity as separate heart disease risk factors: A meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: Evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 46.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2max response to exercise training: Results from the HERITAGE Family Study. J Appl Physiol (1985) 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 47.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 48.Wen D, Utesch T, Wu J, et al. Effects of different protocols of high intensity interval training for VO2max improvements in adults: A meta-analysis of randomised controlled trials. J Sci Med Sport. 2019;22:941–947. doi: 10.1016/j.jsams.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Stensvold D, Viken H, Steinshamn SL, et al. Effect of exercise training for five years on all cause mortality in older adults-the Generation 100 study: Randomised controlled trial. BMJ. 2020;371:m3485. doi: 10.1136/bmj.m3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Church TS, Earnest CP, Thompson AM, et al. Exercise without weight loss does not reduce C-reactive protein: The INFLAME study. Med Sci Sports Exerc. 2010;42:708–716. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: A prospective study of over half a million people. Arch Intern Med. 2009;169:562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 54.Burska AN, Sakthiswary R, Sattar N. Effects of tumour necrosis factor antagonists on insulin sensitivity/resistance in rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 56.Wang CY, Haskell WL, Farrell SW, et al. Cardiorespiratory fitness levels among US adults 20–49 years of age: Findings from the 1999-2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171:426–435. doi: 10.1093/aje/kwp412. [DOI] [PubMed] [Google Scholar]
- 57.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.