Highlights
-
•
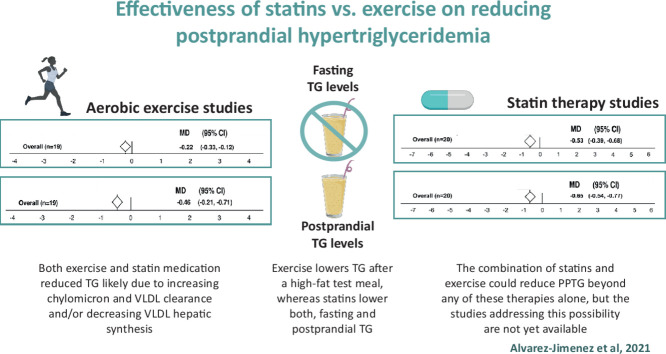
Both exercise and statin treatment decrease postprandial triglyceridemia (PPTG), probably by increasing chylomicron and very low-density lipoprotein-triglyceride (VLDL-TG) clearance and/or reducing liver VLDL-TG synthesis.
-
•
Exercise lowers blood triglyceride (TG) after a high-fat test meal, whereas statins lower both fasting and postprandial blood TG.
-
•
The combination of statins and exercise could reduce PPTG more than any of these therapies alone, but studies addressing this possibility are not yet available.
Keywords: Aerobic exercise, Cardiovascular disease, Hydroxymethylglutaryl-CoA reductase inhibitor, Meta-analysis, Metabolic syndrome
Abstract
Background
Individuals at risk of suffering cardiovascular disease (CVD) present with larger increases in blood triglyceride (TG) concentration after a high-fat meal than do healthy individuals. These postprandial hypertriglyceride levels are an independent risk factor for CVD. Prescription of statins and a bout of prolonged exercise are both effective in lowering postprandial hypertriglyceride levels. We aimed to evaluate the comparative effectiveness of statins vs. a bout of aerobic exercise in reducing fasting and postprandial TG (PPTG) concentrations in individuals at high risk of developing CVD.
Methods
Thirty-seven studies from a systematic literature search of the PubMed, EMBASE, and Cochrane databases were included in this review. The selected studies conducted trials involving statin therapy (n = 20) or a bout of aerobic exercise (n = 19) and measured their impact on PPTG levels as the outcome. Two studies analyzed both treatments and were included in duplicate. The meta-analysis was constructed using a random-effects model to calculate the mean difference (MD). The Student t test was used to compare the data sets for statins vs. exercise.
Results
Overall, statin and exercise interventions showed similar reductions in PPTG levels, with an MD of –0.65 mmol/L for statins (95% confidence interval (95%CI): –0.54 to –0.77; p < 0.001) and –0.46 mmol/L for exercise (95%CI: –0.21 to –0.71; p < 0.01). However, statins lowered fasting TG levels more than exercise (MD = –1.54 mmol/L, 95%CI: –2.25 to –0.83; p = 0.009).
Conclusion
Although aerobic exercise is effective in lowering blood TG levels, statins seem to be more efficient, especially in the fasted state. A combination of exercise and statins might reveal a valuable approach to the treatment and prevention of CVD. More studies are required to determine the underlying mechanisms and the possible additive effects of these interventions.
Graphical abstract
1. Introduction
Dyslipidemia (elevated plasma cholesterol, elevated triglyceride (TG) levels, and/or reduced high-density lipoprotein cholesterol levels) contribute to the development of atherosclerosis and cardiovascular disease (CVD). The risk for atherogenic plaque formation increases after ingestion of meals with high-fat content due to the rise in the remnant of TG-rich lipoproteins (i.e., chylomicrons and very low-density lipoprotein-triglyceride (VLDL-TG) levels).1 In healthy men, a high-fat meal reduces flow-mediated vasodilation in association with increases in serum TG levels, suggesting that hypertriglyceridemia could impair endothelial function.2 Furthermore, postprandial TG (PPTG) levels 6–8 h after a high-fat meal are an independent predictor of the presence of coronary artery disease.3 Upon ingestion of a high-fat meal, individuals with metabolic syndrome (MetS) and dyslipidemia produce more hepatic VLDL-TG and chylomicrons than healthy individuals.4,5 Thus, interventions geared to reducing PPTG levels could be clinically effective in populations with adverse atherogenic postprandial profiles.
Statins (i.e., inhibitors of hydroxymethylglutaryl coenzyme A reductase) are prescribed for the prevention of coronary heart disease in patients with dyslipidemia. Statins stabilize the atherosclerotic plaque, reverse endothelial dysfunction, and decrease thrombogenicity.6 The mechanisms of action of statins include reducing liver cholesterol synthesis, which culminates in a reduction in blood low-density lipoprotein (LDL) cholesterol levels. The intracellular hepatic cholesterol levels are under feedback-regulated control,7 and when they are lowered by statins, LDL receptors are externalized to the hepatocyte surface to retrieve cholesterol from LDL cholesterol and circulating TG-rich lipoproteins. Thus, statins, in addition to reducing blood LDL cholesterol levels, have the secondary but important role of lowering blood TG levels.8 Statins lower PPTG levels more than other antihypertriglyceridemic agents, such as niacin,9 but fibrates lower it to a similar10 or lower extent.11 Publications on the effects of statins on PPTG levels have been accumulating in the past 2 decades, and now there are enough studies to assess the strength of this secondary but clinically relevant effect of statins.12, 13, 14
Another useful intervention for reducing the area under the PPTG curve after a high-fat meal is a bout of aerobic exercise.15 The mechanisms of this reduction have not been fully elucidated, but they may be mediated by the exercise-induced activation of endothelial lipoprotein lipase (LPL),16, 17, 18 which clear circulating lipoproteins. Although most exercise studies report reductions in PPTG levels after a high-fat test meal, the variability in the results is large. Factors such as exercise mode (i.e., resistance vs. endurance), exercise dose (i.e., duration and intensity),19,20 the induced caloric deficit,21 and the timing between exercise and the high-fat meal22 can affect the PPTG response to exercise.15 Although, most exercise studies have been conducted in healthy individuals, a few reports suggest that individuals with MetS and CVD could also benefit from exercise that lowers PPTG levels.23, 24, 25 An updated account of the effects of exercise on PPTG levels in this population at risk of developing CVD is currently lacking in the literature.
We recently studied the combined effects of a bout of exercise and statin medication on PPTG levels in individuals with MetS.13,14 The exercise intervention did not lower PPTG levels; thus, it was not possible to study the combinatory effects of both therapies (i.e., exercise plus statins). However, there are enough data in the literature to assess the impact of statins or exercise on PPTG levels. Therefore, the purpose of this meta-analysis was to compare the effects of statins with the effects of aerobic exercise on the fasting TG and PPTG-level responses in individuals with CVD risk, thus providing clinicians and exercise physiologists with the most current information, allowing them to decide on the most effective therapy for reducing the atherogenic hypertriglyceridemia that occurs after meals.
2. Methods
2.1. Data sources and search strategy
A literature search was conducted in July 2020 in the following databases: PubMed (National Institutes of Health, National Library of Medicine), EMBASE, and Cochrane. The search was restricted to studies in humans, studies written in English, and studies published before June 2020. Eligible studies included only randomized controlled trials approved by ethics committees. We selected studies using repeated-measures crossover randomized trials. Title, abstract, and keyword search fields were examined in 2 search actions: (1) “Statin” OR “HMG-CoA reductase” and (2) “Exercise” OR “physical activity”. These search actions were combined with the following terms to restrict the search to studies conducting a high-fat test meal: AND “postprandial lipemia” OR/AND “postprandial triglyceridemia” OR/AND “oral fat tolerance test” OR/AND “high-fat meal”. These search actions were further combined with the following terms to restrict the search to individuals with cardiovascular risk factors: AND “overweight” OR/AND “obesity” OR/AND “abdominal obesity” OR/AND “dyslipidemia” OR/AND “hypercholesterolemia” OR/AND “metabolic syndrome” OR/AND “hypertension” OR/AND “cardiovascular diseases” OR/AND “coronary heart disease” OR/AND “type 2 diabetes mellitus” OR/AND “T2DM”.
Two reviewers (LAJ and RMR) independently carried out the identification, screening, eligibility, and inclusion of studies, with disagreement being settled in joint meetings.
2.2. Study selection
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement guidelines.26 The inclusion criteria for the studies were as follows: (1) the study included the triglyceridemic response after a high-fat test meal as the dependent variable; (2) the study included treatment with statins or aerobic exercise as the independent variable; (3) the study had to report both fasting and PPTG measurements; (4) the study was a controlled trial; (5) the study included participants who had at least 1 MetS factor (i.e., abdominal obesity, hypertension, fasting dyslipidemia, or hyperglycemia27), familial hypercholesterolemia, coronary artery disease, or type 2 diabetes mellitus; and (6) the study provided means and standard deviations (or standard errors, medians, or interquartile ranges) for both treatment and control trials, or these data could be derived from graphs.
2.3. Data extraction
We found a total of 37 studies that fulfilled the criteria. Of these studies, 20 analyzed the effects of statin treatments,8,12, 13, 14,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 and 19 studied the effects of exercise interventions.9,13,14,20,44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 Two studies analyzed both treatments and were included in duplicate.13,14 Study quality was evaluated according to the Physiotherapy Evidence Database (PEDro) scale.59
2.4. Data analysis
The mean difference (MD) of each study was calculated to estimate an overall summary MD, with a 95% confidence interval (95%CI). Data were analyzed using a random-effects model due to the variability in experimental factors. Heterogeneity among studies was tested using the Q statistic and was quantified by I2. Interpretation was as follows: I2: 0%–40% = low heterogeneity; 30%–60% = moderate heterogeneity; 50%–90% = substantial heterogeneity; 75%–100% = considerable heterogeneity.60,61 z tests were used to compare summary variables. All meta-analyses were performed using Comprehensive MetaAnalysis (CMA) Version 3.3.070 software (Biostat, Englewood, NJ, USA). We also ran paired and unpaired Student t tests to assess within- (fasting vs. PPTG levels) and between- (statins vs. exercise) differences between sets of studies. Last, we used an unpaired Student t test to assess whether the subjects’ characteristics between sets of studies (statins vs. exercise) differed.62 The correlation between fasting and postprandial variations in response to statins was evaluated using the Pearson correlation coefficient. For this statistical analysis, we used Excel (Microsoft Office 2010; Microsoft, Redmond, WA, USA).
3. Results
3.1. Literature search
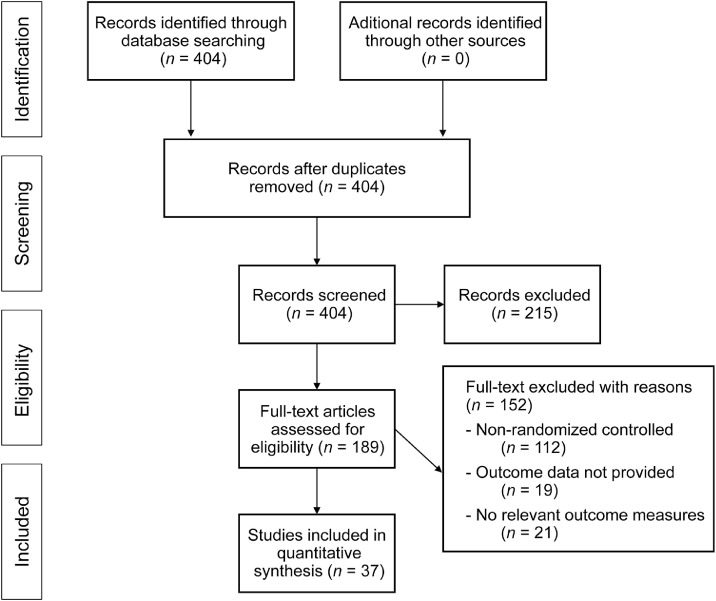
The initial online search yielded 404 potential studies. After screening (reviewing the title and abstract), 367 studies were dismissed according to the inclusion criteria (PRISMA flow chart) (Fig. 1). Upon further analysis, 20 studies using statins as an intervention and 19 using exercise as an intervention were included in the final meta-analysis. The included studies enrolled 595 individuals (436 males and 159 females; 27% female). A total of 371 participants had a statin intervention and 224 had an exercise intervention. Most studies (34 of 37) used a repeated-measures crossover, randomized control trial design, which reduced most sources of experimental bias. As shown in Table 1, the studies achieved a mean PEDro score of 7.4 of 11.
Fig. 1.
Meta-analysis PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Table 1.
Quality metrics of included studies.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Overall PEDro |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simo et al. (1993)8 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Contacos et al. (1998)28 | Y | Y | Y | Y | Y | Y | Y | Y | – | Y | Y | 10 |
| Battula et al. (2000)30 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Twickler et al. (2000)29 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Sheu et al. (2001)31 | Y | Y | Y | Y | Y | Y | Y | Y | – | Y | Y | 10 |
| Wilmink et al. (2001)32 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Dane-Stewart et al. (2002)33 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Guerin et al. (2002)34 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Schaefer et al. (2002)36 | Y | Y | Y | Y | – | – | – | Y | – | Y | Y | 6 |
| Parhofer et al. (2003)12 | Y | Y | Y | Y | – | – | – | Y | Y | Y | Y | 7 |
| Verseyden et al. (2003)37 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Castro Cabezas et al. (2004)38 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| van Wijk et al. (2005)39 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Olijhoek et al. (2008)40 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Arao et al. (2009)35 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Hajer et al. (2009)41 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Nagashima et al. (2011)43 | Y | Y | – | Y | Y | Y | – | Y | Y | Y | Y | 9 |
| Lee et al. (2012)42 | Y | Y | – | Y | – | – | – | Y | – | – | Y | 5 |
| Mora-Rodriguez et al. (2020)13 | Y | Y | Y | Y | Y | Y | Y | Y | – | Y | Y | 10 |
| Alvarez-Jimenez et al. (2021)14 | Y | Y | Y | Y | Y | Y | Y | Y | – | Y | Y | 10 |
| Gill et al. (2004)44 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Zhang et al. (2004)45 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Zhang et al. (2006)46 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Gill et al. (2007)47 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Zhang et al. (2007)20 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Burton et al. (2008)48 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Mestek et al. (2008)49 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Miyashita et al. (2008)50 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Plaisance et al. (2008)9 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Miyashita et al. (2010)51 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Ho et al. (2011)52 | Y | Y | – | Y | – | – | – | Y | – | Y | Y | 6 |
| Hurren et al. (2011)53 | Y | – | – | Y | – | – | – | Y | Y | Y | Y | 6 |
| Hurren et al. (2011)54 | Y | Y | – | Y | – | – | – | Y | – | – | Y | 5 |
| Davitt et al. (2013)58 | Y | Y | – | Y | – | – | – | Y | – | – | Y | 5 |
| Freese et al. (2015)55 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Emerson et al. (2016)56 | Y | Y | – | Y | – | – | – | Y | Y | Y | Y | 7 |
| Kashiwabara et al. (2018)57 | Y | Y | – | Y | – | – | – | Y | – | Y | Y | 6 |
| Mora-Rodriguez et al. (2020)13 | Y | Y | – | Y | – | – | – | Y | – | Y | Y | 6 |
| Alvarez-Jimenez et al. (2021)14 | Y | Y | – | Y | – | – | – | Y | – | – | Y | 5 |
Notes: Items in the PEDro scale: 1 = eligibility criteria were specified; 2 = subjects were randomly allocated to groups; 3 = allocation was concealed; 4 = the groups were similar at baseline; 5 = there was blinding of all subjects; 6 = there was blinding of all therapists who administered the therapy; 7 = there was blinding of all assessors who measured at least 1 key outcome; 8 = measures of at least 1 key outcome were obtained from more than 85% of the subjects initially allocated to groups; 9 = all subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least 1 key outcome were analyzed by “intention to treat”; 10 = the results of between-group statistical comparisons are reported for at least 1 key outcome; 11 = the study provides both point measures and measures of variability for at least 1 key outcome.
Abbreviations: PEDro = Physiotherapy Evidence Database; Y = yes.
3.2. Characteristics of the selected studies
A detailed description of each study is provided in Table 2 (statins) and Table 3 (a bout of exercise). Briefly, the mean age of the participants in the statin and exercise studies was 52 ± 6 years and 45 ± 6 years (mean ± SD), respectively (p = 0.03). Subjects in the exercise studies presented a higher body mass index (BMI) (BMI = 31 ± 3 kg/m2) in comparison to the statin participants (BMI = 27 ± 2 kg/m2; p < 0.001). Each study used a different high-fat test meal because standardization has not been fully developed.63 However, the content of fat as a percent of the total energy of the meal was similar in the studies (i.e., statin vs. exercise; p = 0.21). Studies using the aerobic exercise intervention used primarily a bout of low-intensity (60% maximal oxygen consumption (VO2max)) prolonged (>45 min) continuous exercise. In the studies using the statin intervention, the dose of statin varied according to the pharmacological bioavailability of the various statins used (lovastatin, pravastatin, cerivastatin, simvastatin, atorvastatin, or pitavastatin).
Table 2.
Characteristics of statins studies.
| Study | n (M/F) | Age (year) | BMI (kg/m2) | Cardiovascular disease risk | Treatment duration (week) | Statin | Daily dose (mg) | Fat-meal content (%) | Fat-meal energy (kcal) |
|---|---|---|---|---|---|---|---|---|---|
| Simo et al. (1993)8 | 11M | 42 ± 7 | 26 ± 3 | Dyslipidemic | 6 | Lovastatin | 40 | 69 | n.a. |
| Contacos et al. (1998)28 | 12M/7F | 56 ± 12 | 27 ± 5 | MetS | 6 | Pravastatin | 40 | 90 | n.a. |
| Battula et al. (2000)30 | 2M/6F | 67 ± 11 | 29 ± 3 | Dyslipidemic, T2DM | 4 | Cerivastatin | 0.3 | 55 | 1100 |
| Twickler et al. (2000)29 | 4M/3F | 47 ± 7 | 26 ± 2 | FCHL | 16 | Simvastatin | 80 | 40 | n.a. |
| Sheu et al. (2001)31 | 8M/16F | 62 ± 10 | 26 ± 4 | MetS | 16 | Simvastatin | 20 | 33 | 2190 |
| Wilmink et al. (2001)32 | 15M | 25 ± 4 | 22 ± 3 | Hypertense | 3 | Cerivastatin | 0.4 | 40 | n.a. |
| Dane-Stewart et al. (2002)33 | 15M/3F | n.a. | 27 ± 6 | CHD, overweight | 12 | Atorvastatin | 80 | 47 | 437 |
| Guerin et al. (2002)34 | 11M | 55 ± 10 | 27 ± 3 | Overweight, dyslipidemic | 6 | Atorvastatin | 40 | 48 | 1200 |
| Schaefer et al. (2002)36 | 74M/14F | 62 ± 9 | 28 ± 3 | CHD, overweight, dyslipidemic | 4 | Atorvastatin | 40 | 57 | 880 |
| Parhofer et al. (2003)12 | 8M/2F | 40 ± 9 | 27 ± 3 | MetS | 4 | Atorvastatin | 10 | 87 | 1305 |
| Verseyden et al. (2003)37 | 6M/6F | 43 ± 17 | 26 ± 2 | FCHL, MetS | 12 | Atorvastatin | 80 | n.a. | n.a. |
| Castro Cabezas et al. (2004)38 | 10M/8F | 45 ± 8 | 26 ± 2 | FCHL, MetS | 16 | Atorvastatin | 80 | n.a. | n.a. |
| van Wijk et al. (2005)39 | 12M/6F | 49 ± 6 | 24 ± 4 | PTCA | 5 | Simvastatin | 80 | 40 | n.a. |
| Olijhoek et al. (2008)40 | 19M | 54 ± 7 | 30 ± 3 | MetS | 6 | Simvastatin | 80 | 40 | 925 max |
| Arao et al. (2009)35 | 16M | 63 ± 8 | 23 ± 3 | CHD, dyslipidemic | 24 | Pitavastatin | 2 | 33 | 680 |
| Hajer et al. (2009)41 | 19M | 54 ± 7 | 30 ± 3 | MetS | 6 | Simvastatin | 80 | 40 | 925 max |
| Nagashima et al. (2011)43 | 12M | 48 ± 9 | 28 ± 3 | MetS | 2 | Pitavastatin | 2 | 35 | 342 |
| Lee et al. (2012)42 | 10M/18F | 63 ± 8 | 26 ± 2 | Dyslipidemic | 8 | Atorvastatin | 20 | 30 | 750 |
| Mora-Rodriguez et al. (2020)13 | 9M/1F | 61 ± 7 | 30 ± 4 | MetS | 1 | Various | – | 36 | 995 |
| Alvarez-Jimenez et al. (2021)14 | 7M/1F | 61 ± 7 | 30 ± 4 | MetS | 1 | Various | – | 38 | 862 |
Note: Data are expressed as mean ± SD.
Abbreviations: BMI = body mass index; CHD = coronary artery disease; F = female; FCHL = familiar hypercholesterolemia; M = male; MetS = metabolic syndrome; n.a. = not available; PTCA = percutaneous transluminal coronary angioplasty; T2DM = type 2 diabetes mellitus.
Table 3.
Characteristics of exercise trials.
| Study | n (M/F) | Age (year) | BMI (kg/m2) | Cardiovascular disease risk | Exercise mode/duration | VO2max (mL/kg/min) | VO2max (%) | Fat-meal content (%) | Fat-meal energy (kcal) | Time exer- meal (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gill et al. (2004)44 | 10M | 46 ± 10 | 32 ± 5 | Obese | Treadmill walk for 90 s | 41 ± 7 | 50 | 40 | 1028 | >12 |
| Zhang et al. (2004)45 | 10M | 40 ± 6 | 30 ± 5 | HyperTG | Treadmill jog for 60 s | 37 ± 6 | 60 | 83 | 980 | 12 |
| Zhang et al. (2006)46 | 10M | 40 ± 7 | 31 ± 4 | MetS | Treadmill jog for 60 s | 37 ± 5 | 60 | 83 | 980 | 11 |
| Gill et al. (2007)47 | 10M | 49 ± 11 | 30 ± 3 | T2DM | Treadmill walk for 90 s | 35 ± 4 | 50 | 49 | 1028 | >12 |
| Zhang et al. (2007)20 | 10M | 35 ± 5 | 30 ± 3 | MetS | Treadmill walk for 60 s | 36 ± 4 | 60 | 83 | 980 | 12 |
| Burton et al. (2008)48 | 13M | 40 ± 8 | 31 ± 3 | Obese, hypertense | Treadmill walk for 90 s | 39 ± 6 | 50 | 21 | 798 | <1 |
| Mestek et al. (2008)49 | 14M | 43 ± 9 | 34 ± 6 | MetS | Treadmill walk until 500 kcal | 26 ± 6 | 60–70 | 83 | 1000 | 8-12 |
| Miyashita et al. (2008)50 | 8M | 26 ± 3 | 29 ± 4 | Overweight, hypertense | Cycled for 30 s | n.a. | 60 | 35 | 771 | >12 |
| Plaisance et al. (2008)9 | 15M | 46 ± 8 | 34 ± 3 | MetS | Treadmill walk until 500 kcal | 28 ± 5 | 60–70 | 83 | 1000 | <1 |
| Miyashita et al. (2010)51 | 10M | 46 ± 6 | 32 ± 3 | Obese, hypertense | Cycled for 30 s | 30 ± 6 | 60 | 35 | 831 | >12 |
| Ho et al. (2011)52 | 2M/20F | 59 ± 5 | 32 ± 6 | Obese, HyperTG | Treadmill walk for 30 s | n.a. | 60 | 35 | n.a. | >12 |
| Hurren et al. (2011)53 | 8M | 47 ± 9 | 29 ± 2 | Overweight, HyperTG | Treadmill walk for 90 s | 37 ± 5 | 60 | 66 | 1520 | <1 |
| Hurren et al. (2011)54 | 8M | 49 ± 10 | 31 ± 3 | Obese | Treadmill walk for 90 s | 34 ± 6 | 60 | 50 | 1504 | <1 |
| Davitt et al. (2013)58 | 12F | 24 ± 2 | 37 ± 2 | Obese | Treadmill walk for 60 s | 25 ± 1 | 60–65 | 36 | 1076 | <1 |
| Freese et al. (2015)55 | 22F | 52 ± 11 | 31 ± 7 | MetS | Four all-out sprints for 30 s | n.a. | n.a. | 44 | 980 | >12 |
| Emerson et al. (2016)56 | 12M | 24 ± 5 | 28 ± 2 | Overweight, HyperTG | Treadmill walk for 60 s | 39 ± 8 | 60 | 69 | 1118 | 12 |
| Kashiwabara et al. (2018)57 | 12F | 70 ± 5 | 25 ± 3 | HyperTG | Continuous walking for 30 s | n.a. | n.a. | 35 | 549 | 2 |
| Mora-Rodriguez et al. (2020)13 | 9M/1F | 61 ± 7 | 30 ± 4 | MetS | Interval cycling for 41 s | 31 ± 6 | 40–70–85 | 36 | 995 | <1 |
| Alvarez-Jimenez et al. (2021)14 | 7M/1F | 61 ± 7 | 30 ± 4 | MetS | Interval cycling for 41 s | 31 ± 6 | 40–70–85 | 38 | 862 | >12 |
Note: Data are presented as mean ± SD.
Abbreviations: BMI = body mass index; hyperTG = hypertriglyceridemic; F = female; FM = fat mass; M = male; MetS = metabolic syndrome; n.a. = not available; T2DM = type 2 diabetes mellitus; VO2max = maximal oxygen consumption.
3.3. Data analysis
3.3.1. Statin effect
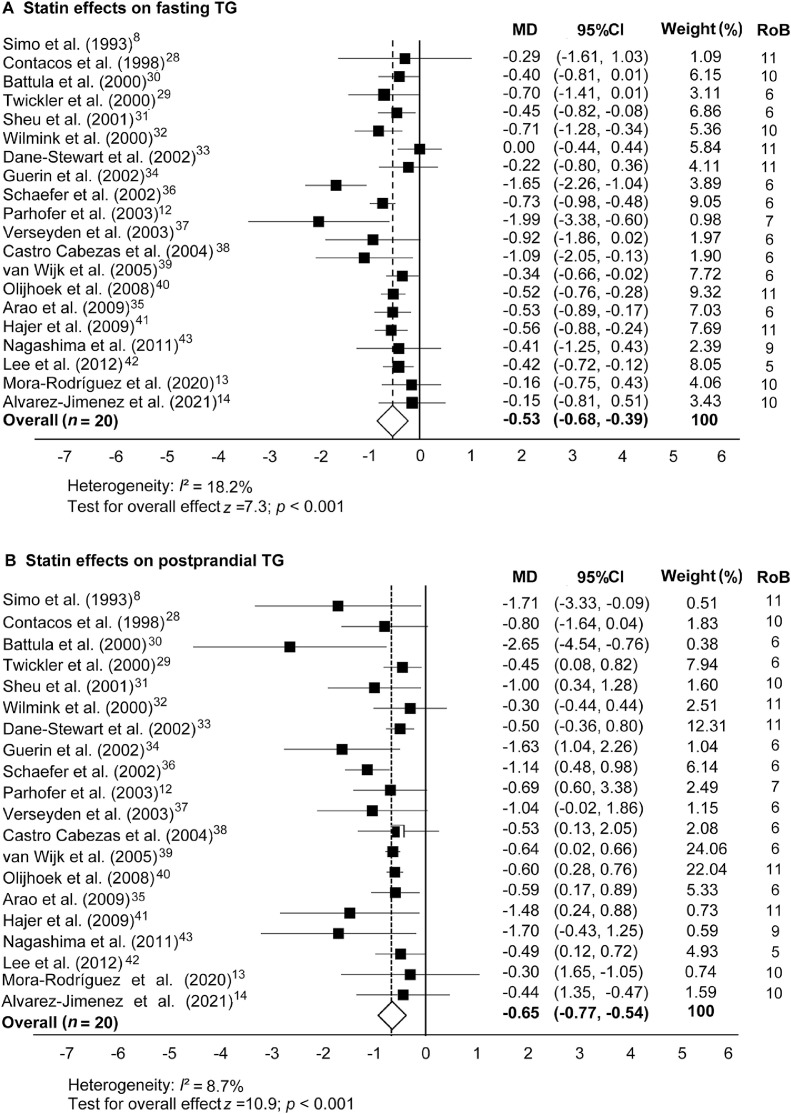
Fig. 2 shows the overall results of the statin treatment data set on fasting TG and PPTG levels. Statistically significant reductions were found for fasting TG (MD = –0.53 mmol/L, 95%CI: −0.68 to −0.39; p < 0.001) and PPTG concentrations (MD = –0.65 mmol/L, 95%CI: −0.77 to −0.54; p < 0.001). The Student t test showed that the effect of statins on lowering fasting TG levels was similar to their effect on lowering PPTG levels (p = 0.503).
Fig. 2.
Statin effects on (A) fasting and (B) postprandial blood TG concentrations in comparison to a nonmedicated control trial. Data are MD and 95%CI. 95%CI = 95% confidence interval; MD = mean difference; RoB = risk of bias; TG = triglyceride.
3.3.2. Exercise effect
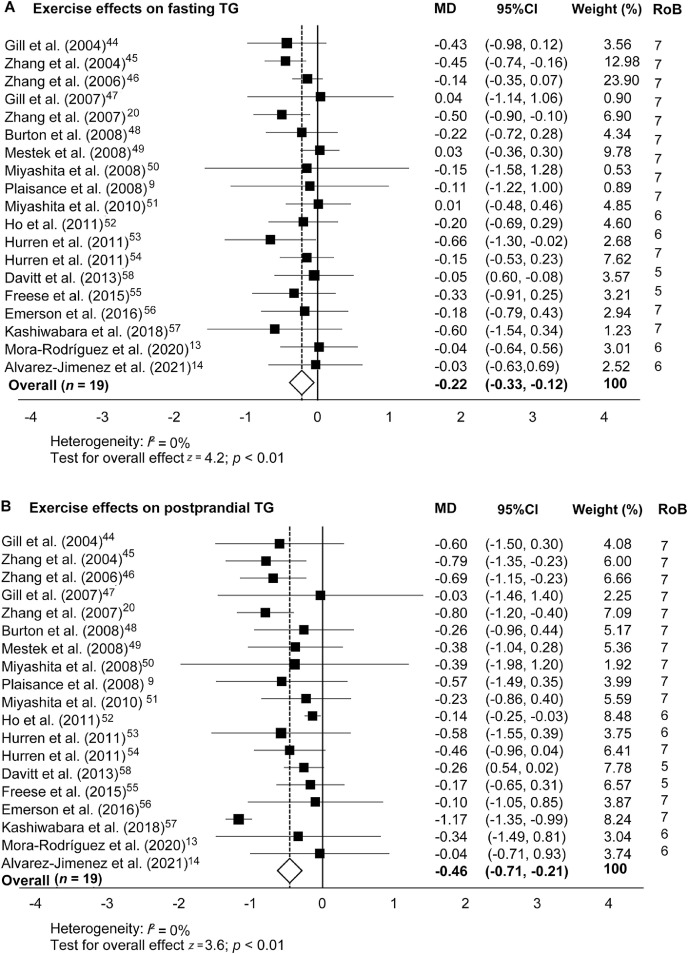
The effects of a bout of exercise on fasting and PPTG levels are shown in Fig. 3. Exercise induced an MD of –0.46 mmol/L overall mean reduction (95%CI: −0.71 to −0.21) on PPTG levels (Fig. 3B). However, the overall effects of exercise on fasting TG levels were less pronounced (MD = –0.22 mmol/L, 95%CI: –0.33 to –0.12; p < 0.01) but were still statistically significant (z = 4.2; p < 0.01). The Student t test revealed that the effect of exercise on reducing fasting TG levels was 0.24 mmol/L smaller than the effect on reducing PPTG levels (p = 0.015).
Fig. 3.
Exercise effects on (A) fasting and (B) postprandial blood TG concentrations in comparison to a nonexercise control trial. Data are MD and 95%CI. 95%CI = 95% confidence interval; MD = mean difference; RoB = risk of bias; TG = triglyceride.
3.3.3. Comparison between statin and exercise effect on blood TG levels
The Student t test indicated that the effect of statins on lowering fasting TG was larger than the exercise effect (MD = –1.54 mmol/L, 95%CI: –2.25 to –0.83; p = 0.009). However, the effect of statins compared to exercise on lowering PPTG levels was not significantly different (p = 0.425).
4. Discussion
This meta-analysis gathered data from 37 randomized controlled trials and included data from 595 participants. It addressed the comparative effect of statin vs. exercise treatments on fasting blood TG levels and on elevations in blood TG levels following a high-fat test meal (i.e., PPTG levels). PPTG levels have been shown to be a high-risk atherogenic situation predictive of coronary artery disease incidence;2,3 therefore, reducing its level is clinically relevant to individuals with increased risk of CVD (e.g., individuals with metabolic syndrome factors, obesity, type 2 diabetes, or coronary artery disease). Studies included in our meta-analysis attempted to reduce hypertriglyceridemia either by using statins (Fig. 2) or by a bout of prolonged aerobic exercise (Fig. 3). Both the statin and the exercise data sets revealed significant reductions in plasma TG concentrations in the overall mean in comparison to control trials (i.e., either no medication or no exercise). The effects were observed in fasting TG as well as in PPTG levels. Thus, both therapies (statins and exercise) can reduce blood TG levels in individuals at risk of developing CVDs.
4.1. Statin effects on PPTG
When statins were used, the mean reductions in fasting and PPTG concentrations were similar (i.e., 0.53 mmol/L vs. 0.65 mmol/L; p = 0.503) and averaged a 26% to 27% reduction compared to the control trials. The risk reduction for developing CVDs when lowering PPTG levels by this magnitude has not been previously investigated. However, a similar reduction in fasting blood cholesterol reduces mortality and morbidity risks by 22%.64 Other authors have reported a strong positive association between the effects of statins on lowering fasting TG levels and their effects on lowering PPTG levels (r = 0.487; p = 0.05).65,66 When we tested that correlation in our meta-analysis using the 20 studies that involved the statin intervention, the correlation did not reach statistical significance (r = 0.335; p = 0.148). The risk for atherogenic plaque formation is higher after ingestion of a high-fat meal than after an overnight fast; thus, the reported 27% reduction in PPTG levels with statins is suggestive of a new use for statins in reducing risk of mortality due to CVD by lowering PPTG levels.
4.2. Mechanisms of action of statins
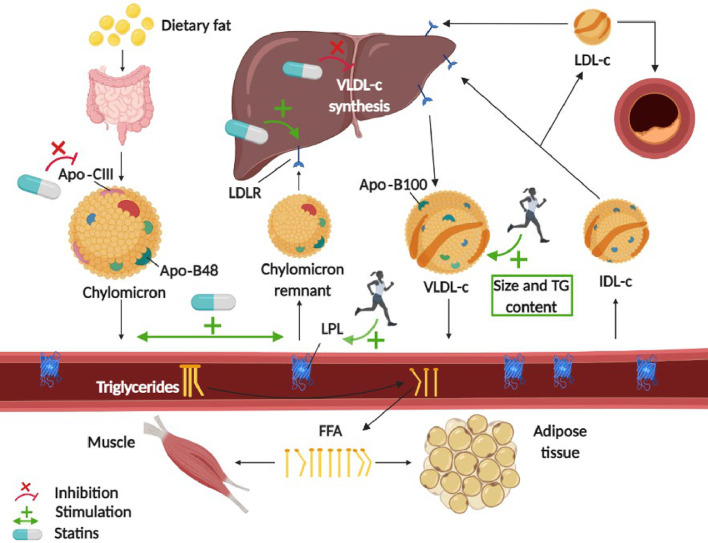
The main action of statins is to decrease liver cholesterol synthesis. This reduction induces the liver to upregulate LDL-receptor activity7 to seek cholesterol in the blood circulation. These receptors not only reduce circulating LDL cholesterol concentrations but also enhance the clearance of circulating TG-rich lipoproteins (i.e., VLDL cholesterol and chylomicrons).67 Some of the less well-known actions of statins include the postprandial metabolism of apolipoproteins. Statins reduce the incorporation of apolipoprotein-CIII in chylomicrons, liberating the inhibitory actions of apolipoprotein-CIII on LPL. By liberating LPL actions, statins may indirectly increase lipolysis.68,69 Statins also stimulate chylomicron-remnant catabolism, as reflected by a decrease in apolipoprotein-B48,68,70 an apolipoprotein that forms in the intestine during the digestion of a fatty meal. These actions of statins, summarized in Fig. 4, are likely to be the cause of the effectiveness of statin pharmacological treatments for lowering fasting TG and PPTG levels.
Fig. 4.
Possible mechanisms of action of statins and aerobic exercise in lowering postprandial triglyceridemia. Green arrows indicate stimulation; red lines indicate inhibition. The statin mechanisms encompass gut-derived apolipoproteins alteration, upregulation of hepatic LDLR and LPL in peripheral tissues, decrease in liver cholesterol synthesis, and increase in circulating triglyceride-rich lipoproteins clearance. The exercise mechanisms involve the activation of skeletal muscle LPL and the increase in triglyceride content of liver-derived VLDL-c particles. Apo = apolipoprotein; FFA = free fatty acid; IDL-c = intermediate-density lipoprotein cholesterol; LDL = low-density lipoprotein; LDL-c = low-density lipoprotein cholesterol; LDLR = low-density lipoprotein receptor; LPL = lipoprotein lipase; TG = triglyceride; VLDL-c = very low-density lipoprotein cholesterol.
4.3. Exercise effects on PPTG
In healthy individuals, a bout of prolonged aerobic exercise (involving high rates of oxygen delivery and consumption) lowers fasting blood TG levels and raises high-density lipoprotein-c concentrations.71 The TG-lowering effect of exercise appears to be more powerful after ingestion of a fatty meal (high atherogenic risk) than after an overnight fast (Fig. 3). A bout of exercise reduces PPTG levels not only in healthy individuals but also in individuals with dyslipidemia,52 MetS,20 and other CVD risk factors.58 The 19 studies involving exercise interventions included in our meta-analysis suggest that a bout of aerobic exercise decreases PPTG levels by 18% in individuals at risk of suffering CVDs compared to individuals in a nonexercise control situation. Thus, our data suggest that exercise is also an effective therapy acting for lowering PPTG levels.
4.4. Mechanisms of action of exercise
The most frequently proposed mechanism for exercise-reducing PPTG levels is the activation of skeletal muscle LPL, which induces the clearance of PPTGs (Fig. 4). Activation of muscle LPL peaks 4–18 h after exercise;17,72 thus, most studies separate exercise from the high-fat test meal by 8–12 h. However, 7 exercise studies included in our meta-analysis waited fewer than 3 h between the high-fat test meal and exercise and found reduced PPTG concentrations. This finding suggests that other mechanisms beyond exercise activation of muscle LPL may be at play73 and could involve exercise effects on hepatic metabolism. Some studies have found that exercise increases the TG content of liver-formed VLDL cholesterol particles,74,75 which may increase its susceptibility to hydrolysis by LPL76 (Fig. 4). On the other hand, studies using stable isotopes suggest that a bout of aerobic77,78 or resistance79 exercise reduces fasting hepatic VLDL-TG secretion which, in turn, could increase chylomicron clearance.73,80
4.5. Comparison of statin vs. exercise interventions
The PPTG reductions in the statin trials (27%) and the exercise trials (18%) were not significantly different statistically. This suggests that for reducing the atherogenic risk in the postprandial state, both statin and exercise interventions could be recommended in a population at risk of developing CVDs. However, we detected that statins and exercise have different effects on fasting TG levels. Statins lowered fasting levels of TGs, and that reduction was maintained during PPTG levels. Reductions in fasting TG levels caused by statins probably contributed to the reduction in PPTG levels. This confirms that statins have a predominant effect in reducing liver VLDL-TG secretion because most of the elevation in blood TGs after ingestion of a fat-rich meal test comes from endogenous liver production.58 In contrast, exercise did little to reduce fasting TG levels, although it significantly reduced TG levels when the high-fat test meal was ingested (i.e., PPTG area under the curve). In fact, the Student t test revealed that exercise-caused reductions in fasting TG levels were significantly lower than the reductions in fasting TG levels obtained with statin treatments (10% vs. 26% reduction, respectively; p = 0.004). This finding suggests that the effect of exercise on LPL activation is visible only when a large supply of lipoproteins transits the vasculature after a high-fat meal. The intensity of exercise performed in most of the studies included in our meta-analysis (i.e., around 60% VO2max) was not conducive to episodes of undue fatigue, nor did it represent a risk, even for patients with treated coronary or peripheral artery diseases. However, some side effects, especially at the muscular level, have been reported with statin treatments, which can occasionally result in rhabdomyolysis.81 Nevertheless, none of the articles included in our meta-analysis mentioned side effects.
4.6. Limitations
Our meta-analysis is not free of limitations. First, the high-fat test meals were not standardized among the studies. In 2011, Kolovou and coworkers63 proposed to standardize the high-fat test meal to 75 g of fat, 25 g of carbohydrates, and 10 g of protein. However, most of the studies in our meta-analysis that occurred after 2011 (Table 1) did not follow this recommendation. The absolute amount of fat ingested in the high-fat test meal could affect the statin or exercise actions through a saturation effect or by insufficient interaction. However, the same high-fat test meal was used in the experimental and control trials of each study, which gives internal consistency to the studies. Second, the set of studies using exercise treatments also suffered from a lack of standardization in the exercise dose. Exercise duration ranged from 30 to 90 min; and, not unexpectedly, caloric expenditure varied up to 3-fold. Furthermore, the time that elapsed between ingestion of the high-fat test meal and the exercise intervention varied among studies. Third, it should be noted that the BMIs of the participants in the exercise studies were higher than the BMIs of participants in the statin studies (i.e., 31 kg/m2 vs. 27 kg/m2, respectively). Higher BMIs have been correlated with nonalcoholic fatty liver disease, which affects TG metabolism in fasting TG and PPTG levels.82 Thus, it is possible that the improvements in both fasting TG and PPTG levels may be biased by the initial condition of the subjects.83 Finally, the type of statins and the doses administered were different among the included studies, which could explain the differences in the strength and variability of the responses observed in the studies.84 The use of frameworks like Consolidated Standards of Reporting Trials (CONSORT)85 should be used to standardize the way researchers report the results of their studies.
5. Conclusion
In the studies we reviewed, both exercise and statin medication reduced PPTG levels, a result that was likely to be due to increased chylomicron and VLDL clearance and/or decreased VLDL cholesterol hepatic synthesis. The magnitude of the effect was similar, although the heterogeneity of the responses was larger when statins were used to lower PPTG levels. Expecting exercise to reduce blood TGs to the levels required to manage patients with severe hyperlipidemia may be unrealistic. However, using exercise as a coadjutant therapy seems a sensible hypothesis. It is unknown whether a combination of statins and exercise would generate additive effects on PPTG levels by increasing chylomicron and VLDL-TG catabolism or by reducing its secretion.14 Additional studies are needed to assess whether the beneficial effects of exercise and statin treatments can be combined to reduce PPTG and, if so, improve the clinical treatment and prevention of CVDs.
Acknowledgments
Acknowledgment
This work was partially funded by a grant from the Spanish Ministry of Economy and Competitiveness (DEP-2017-83244-R) and the European Economic Community.
Authors’ contributions
RMR developed the outline of the review, reviewed the literature, and wrote the manuscript; LAJ and JFO also reviewed the literature, contributed to figure preparation and data extraction, and provided suggestions for the original draft and revisions of the manuscript; AMC, MRJ, and FMP read the manuscript and provided suggestions for the original draft and revisions. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests .
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.Zilversmit DB. Atherogenesis: A postprandial phenomenon. Circulation. 1979;60:473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 2.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 3.Patsch JR, Miesenbock G, Hopferwieser T, et al. Relation of triglyceride metabolism and coronary artery disease: Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336–1345. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- 4.Shojaee-Moradie F, Ma Y, Lou S, Hovorka R, Umpleby AM. Prandial hypertriglyceridemia in metabolic syndrome is due to an overproduction of both chylomicron and vldl triacylglycerol. Diabetes. 2013;62:4063–4069. doi: 10.2337/db13-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 6.Paciaroni M, Hennerici M, Agnelli G, Bogousslavsky J. Statins and stroke prevention. Cerebrovasc Dis. 2007;24:170–182. doi: 10.1159/000104474. [DOI] [PubMed] [Google Scholar]
- 7.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 8.Simo IE, Yakichuk JA, Ooi TC. Effect of gemfibrozil and lovastatin on postprandial lipoprotein clearance in the hypoalphalipoproteinemia and hypertriglyceridemia syndrome. Atherosclerosis. 1993;100:55–64. doi: 10.1016/0021-9150(93)90067-5. [DOI] [PubMed] [Google Scholar]
- 9.Plaisance EP, Mestek ML, Mahurin AJ, Taylor JK, Moncada-Jimenez J, Grandjean PW. Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am J Clin Nutr. 2008;88:30–37. doi: 10.1093/ajcn/88.1.30. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Abbasi F, Lamendola C, Leary E, Reaven GM. Comparison in patients with type 2 diabetes of fibric acid versus hepatic hydroxymethyl glutaryl-coenzyme a reductase inhibitor treatment of combined dyslipidemia. Metabolism. 2002;51:1355–1359. doi: 10.1053/meta.2002.34713. [DOI] [PubMed] [Google Scholar]
- 11.Westphal S, Wiens L, Güttler K, Dierkes J, Luley C. Chylomicron remnants of various sizes are lowered more effectively by fenofibrate than by atorvastatin in patients with combined hyperlipidemia. Atherosclerosis. 2003;171:369–377. doi: 10.1016/j.atherosclerosis.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Parhofer KG, Laubach E, Barrett PH. Effect of atorvastatin on postprandial lipoprotein metabolism in hypertriglyceridemic patients. J Lipid Res. 2003;44:1192–1198. doi: 10.1194/jlr.M300011-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Mora-Rodriguez R, Ortega JF, Morales-Palomo F, Ramirez-Jimenez M, Moreno-Cabañas A. Effects of statin therapy and exercise on postprandial triglycerides in overweight individuals with hypercholesterolaemia. Br J Clin Pharmacol. 2020;86:1089–1099. doi: 10.1111/bcp.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Jimenez L, Moreno-Cabañas A, Ramirez-Jimenez M, Morales-Palomo F, Ortega JF, Mora-Rodriguez R. Effects of statins and exercise on postprandial lipoproteins in metabolic syndrome vs. metabolically healthy individuals. Br J Clin Pharmacol. 2021;87:955–964. doi: 10.1111/bcp.14447. [DOI] [PubMed] [Google Scholar]
- 15.Maraki MI, Sidossis LS. The latest on the effect of prior exercise on postprandial lipaemia. Sports Med. 2013;43:463–481. doi: 10.1007/s40279-013-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol. 1998;275:E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 17.Seip RL, Mair K, Cole TG, Semenkovich CF. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Physiol. 1997;272:E255–E261. doi: 10.1152/ajpendo.1997.272.2.E255. [DOI] [PubMed] [Google Scholar]
- 18.Harrison M, Moyna NM, Zderic TW, et al. Lipoprotein particle distribution and skeletal muscle lipoprotein lipase activity after acute exercise. Lipids Health Dis. 2012;11:64. doi: 10.1186/1476-511X-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annuzzi G, Jansson E, Kaijser L, Holmquist L, Carlson LA. Increased removal rate of exogenous triglycerides after prolonged exercise in man: Time course and effect of exercise duration. Metabolism. 1987;36:438–443. doi: 10.1016/0026-0495(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JQ, Ji LL, Fogt DL, Fretwell VS. Effect of exercise duration on postprandial hypertriglyceridemia in men with metabolic syndrome. J Appl Physiol (1985) 2007;103:1339–1345. doi: 10.1152/japplphysiol.00181.2007. [DOI] [PubMed] [Google Scholar]
- 21.Maraki M, Sidossis LS. Effects of energy balance on postprandial triacylglycerol metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:608–617. doi: 10.1097/MCO.0b013e32833f1aae. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JQ, Thomas TR, Ball SD. Effect of exercise timing on postprandial lipemia and HDL cholesterol subfractions. J Appl Physiol (1985) 1998;85:1516–1522. doi: 10.1152/jappl.1998.85.4.1516. [DOI] [PubMed] [Google Scholar]
- 23.Freese EC, Gist NH, Cureton KJ. Effect of prior exercise on postprandial lipemia: An updated quantitative review. J Appl Physiol (1985) 2014;116:67–75. doi: 10.1152/japplphysiol.00623.2013. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16:132. doi: 10.1186/s12944-017-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon B, Chen S, Durstine JL. The effects of exercise training on the traditional lipid profile and beyond. Curr Sports Med Rep. 2014;13:253–259. doi: 10.1249/JSR.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 28.Contacos C, Barter PJ, Vrga L, Sullivan DR. Cholesteryl ester transfer in hypercholesterolaemia: Fasting and postprandial studies with and without pravastatin. Atherosclerosis. 1998;141:87–98. doi: 10.1016/s0021-9150(98)00151-8. [DOI] [PubMed] [Google Scholar]
- 29.Twickler TB, Dallinga-Thie GM, de Valk HW, et al. High dose of simvastatin normalizes postprandial remnant-like particle response in patients with heterozygous familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:2422–2427. doi: 10.1161/01.atv.20.11.2422. [DOI] [PubMed] [Google Scholar]
- 30.Battula SB, Fitzsimons O, Moreno S, et al. Postprandial apolipoprotein b48-and b100-containing lipoproteins in type 2 diabetes: Do statins have a specific effect on triglyceride metabolism? Metabolism. 2000;49:1049–1054. doi: 10.1053/meta.2000.7744. [DOI] [PubMed] [Google Scholar]
- 31.Sheu WH, Jeng CY, Lee WJ, Lin SY, Pei D, Chen YT. Simvastatin treatment on postprandial hypertriglyceridemia in type 2 diabetes mellitus patients with combined hyperlipidemia. Metabolism. 2001;50:355–359. doi: 10.1053/meta.2001.21026. [DOI] [PubMed] [Google Scholar]
- 32.Wilmink HW, Twickler MB, Banga JD, et al. Effect of statin versus fibrate on postprandial endothelial dysfunction: Role of remnant-like particles. Cardiovasc Res. 2001;50:577–582. doi: 10.1016/s0008-6363(01)00227-9. [DOI] [PubMed] [Google Scholar]
- 33.Dane-Stewart CA, Watts GF, Mamo JC, et al. Effect of simvastatin on markers of triglyceride-rich lipoproteins in familial hypercholesterolaemia. Eur J Clin Invest. 2002;32:493–499. doi: 10.1046/j.1365-2362.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 34.Guerin M, Egger P, Le Goff W, Soudant C, Dupuis R, Chapman MJ. Atorvastatin reduces postprandial accumulation and cholesteryl ester transfer protein-mediated remodeling of triglyceride-rich lipoprotein subspecies in type iib hyperlipidemia. J Clin Endocrinol Metab. 2002;87:4991–5000. doi: 10.1210/jc.2002-020298. [DOI] [PubMed] [Google Scholar]
- 35.Arao K, Yasu T, Umemoto T, et al. Effects of pitavastatin on fasting and postprandial endothelial function and blood rheology in patients with stable coronary artery disease. Circ J. 2009;73:1523–1530. doi: 10.1253/circj.cj-08-0917. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer EJ, McNamara JR, Tayler T, et al. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. Am J Cardiol. 2002;90:689–696. doi: 10.1016/s0002-9149(02)02591-2. [DOI] [PubMed] [Google Scholar]
- 37.Verseyden C, Meijssen S, van Dijk H, Jansen H, Castro Cabezas M. Effects of atorvastatin on fasting and postprandial complement component 3 response in familial combined hyperlipidemia. J Lipid Res. 2003;44:2100–2108. doi: 10.1194/jlr.M300201-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Castro Cabezas M, Verseyden C, Meijssen S, Jansen H, Erkelens DW. Effects of atorvastatin on the clearance of triglyceride-rich lipoproteins in familial combined hyperlipidemia. J Clin Endocrinol Metab. 2004;89:5972–5980. doi: 10.1210/jc.2003-031329. [DOI] [PubMed] [Google Scholar]
- 39.van Wijk JP, Buirma R, van Tol A, et al. Effects of increasing doses of simvastatin on fasting lipoprotein subfractions, and the effect of high-dose simvastatin on postprandial chylomicron remnant clearance in normotriglyceridemic patients with premature coronary sclerosis. Atherosclerosis. 2005;178:147–155. doi: 10.1016/j.atherosclerosis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Olijhoek JK, Hajer GR, van der Graaf Y, Dallinga-Thie GM, Visseren FL. The effects of low-dose simvastatin and ezetimibe compared to high-dose simvastatin alone on post-fat load endothelial function in patients with metabolic syndrome: A randomized double-blind crossover trial. J Cardiovasc Pharmacol. 2008;52:145–150. doi: 10.1097/FJC.0b013e31817ffe76. [DOI] [PubMed] [Google Scholar]
- 41.Hajer GR, Dallinga-Thie GM, van Vark-van der Zee LC, Visseren FL. The effect of statin alone or in combination with ezetimibe on postprandial lipoprotein composition in obese metabolic syndrome patients. Atherosclerosis. 2009;202:216–224. doi: 10.1016/j.atherosclerosis.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Park S, Kang SM, Jang Y, Chung N, Choi D. Effect of atorvastatin monotherapy and low-dose atorvastatin/ezetimibe combination on fasting and postprandial triglycerides in combined hyperlipedemia. J Cardiovasc Pharmacol Ther. 2012;17:65–71. doi: 10.1177/1074248411399762. [DOI] [PubMed] [Google Scholar]
- 43.Nagashima H, Endo M. Pitavastatin prevents postprandial endothelial dysfunction via reduction of the serum triglyceride level in obese male subjects. Heart Vessels. 2011;26:428–434. doi: 10.1007/s00380-010-0071-7. [DOI] [PubMed] [Google Scholar]
- 44.Gill JM, Al-Mamari A, Ferrell WR, et al. Effects of prior moderate exercise on postprandial metabolism and vascular function in lean and centrally obese men. J Am Coll Cardiol. 2004;44:2375–2382. doi: 10.1016/j.jacc.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JQ, Ji LL, Nunez G, Feathers S, Hart CL, Yao WX. Effect of exercise timing on postprandial lipemia in hypertriglyceridemic men. Can J Appl Physiol. 2004;29:590–603. doi: 10.1139/h04-038. [DOI] [PubMed] [Google Scholar]
- 46.Zhang JQ, Ji LL, Fretwell VS, Nunez G. Effect of exercise on postprandial lipemia in men with hypertriglyceridemia. Eur J Appl Physiol. 2006;98:575–582. doi: 10.1007/s00421-006-0304-8. [DOI] [PubMed] [Google Scholar]
- 47.Gill JM, Al-Mamari A, Ferrell WR, et al. Effect of prior moderate exercise on postprandial metabolism in men with type 2 diabetes: Heterogeneity of responses. Atherosclerosis. 2007;194:134–143. doi: 10.1016/j.atherosclerosis.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Burton FL, Malkova D, Caslake MJ, Gill JM. Energy replacement attenuates the effects of prior moderate exercise on postprandial metabolism in overweight/obese men. Int J Obes (Lond) 2008;32:481–489. doi: 10.1038/sj.ijo.0803754. [DOI] [PubMed] [Google Scholar]
- 49.Mestek ML, Plaisance EP, Ratcliff LA, Taylor JK, Wee SO, Grandjean PW. Aerobic exercise and postprandial lipemia in men with the metabolic syndrome. Med Sci Sports Exerc. 2008;40:2105–2111. doi: 10.1249/MSS.0b013e3181822ebd. [DOI] [PubMed] [Google Scholar]
- 50.Miyashita M. Effects of continuous versus accumulated activity patterns on postprandial triacylglycerol concentrations in obese men. Int J Obes (Lond) 2008;32:1271–1278. doi: 10.1038/ijo.2008.73. [DOI] [PubMed] [Google Scholar]
- 51.Miyashita M, Sasai H, Tanaka K. Post-prandial capillary triacylglycerol responses to moderate exercise in centrally obese middle-aged men. J Sports Sci. 2010;28:1269–1275. doi: 10.1080/02640414.2010.498485. [DOI] [PubMed] [Google Scholar]
- 52.Ho SS, Dhaliwal SS, Hills A, Pal S. Acute exercise improves postprandial cardiovascular risk factors in overweight and obese individuals. Atherosclerosis. 2011;214:178–184. doi: 10.1016/j.atherosclerosis.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Hurren NM, Balanos GM, Blannin AK. Is the beneficial effect of prior exercise on postprandial lipaemia partly due to redistribution of blood flow? Clin Sci (Lond) 2011;120:537–548. doi: 10.1042/CS20100460. [DOI] [PubMed] [Google Scholar]
- 54.Hurren NM, Eves FF, Blannin AK. Is the effect of prior exercise on postprandial lipaemia the same for a moderate-fat meal as it is for a high-fat meal? Br J Nutr. 2011;105:506–516. doi: 10.1017/S0007114510003995. [DOI] [PubMed] [Google Scholar]
- 55.Freese EC, Gist NH, Acitelli RM, et al. Acute and chronic effects of sprint interval exercise on postprandial lipemia in women at-risk for the metabolic syndrome. J Appl Physiol (1985) 2015;118:872–879. doi: 10.1152/japplphysiol.00380.2014. [DOI] [PubMed] [Google Scholar]
- 56.Emerson SR, Kurti SP, Snyder BS, Sitaraman K, Haub MD, Rosenkranz SK. Effects of thirty and sixty minutes of moderate-intensity aerobic exercise on postprandial lipemia and inflammation in overweight men: A randomized cross-over study. J Int Soc Sports Nutr. 2016;13:26. doi: 10.1186/s12970-016-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashiwabara K, Kidokoro T, Yanaoka T, Burns SF, Stensel DJ, Miyashita M. Different patterns of walking and postprandial triglycerides in older women. Med Sci Sports Exerc. 2018;50:79–87. doi: 10.1249/MSS.0000000000001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davitt PM, Arent SM, Tuazon MA, Golem DL, Henderson GC. Postprandial triglyceride and free fatty acid metabolism in obese women after either endurance or resistance exercise. J Appl Physiol (1985) 2013;114:1743–1754. doi: 10.1152/japplphysiol.00095.2013. [DOI] [PubMed] [Google Scholar]
- 59.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: A survey of the physiotherapy evidence database (PEDRO) Aust J Physiother. 2002;48:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 60.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 62.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. John Wiley & Sons; New York, NY: 2009. Introduction to meta-analysis. [Google Scholar]
- 63.Kolovou GD, Mikhailidis DP, Kovar J, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr Vasc Pharmacol. 2011;9:258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 64.Jeong SM, Choi S, Kim K, et al. Association of change in total cholesterol level with mortality: A population-based study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassader M, Gambino R, Musso G, et al. Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients. Lipids. 2001;36:1117–1124. doi: 10.1007/s11745-001-0822-5. [DOI] [PubMed] [Google Scholar]
- 66.Kolovou GD, Anagnostopoulou KK, Salpea KD, Daskalopoulou SS, Mikhailidis DP. The effect of statins on postprandial lipemia. Curr Drug Targets. 2007;8:551–560. doi: 10.2174/138945007780362809. [DOI] [PubMed] [Google Scholar]
- 67.Burnett JR, Barrett PH, Vicini P, et al. The HMG-COA reductase inhibitor atorvastatin increases the fractional clearance rate of postprandial triglyceride-rich lipoproteins in miniature pigs. Arterioscler Thromb Vasc Biol. 1998;18:1906–1914. doi: 10.1161/01.atv.18.12.1906. [DOI] [PubMed] [Google Scholar]
- 68.Chan DC, Watts GF, Somaratne R, Wasserman SM, Scott R, Barrett PHR. Comparative effects of pcsk9 (proprotein convertase subtilisin/kexin type 9) inhibition and statins on postprandial triglyceride-rich lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 2018;38:1644–1655. doi: 10.1161/ATVBAHA.118.310882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoonjans K, Peinado-Onsurbe J, Fruchart JC, Tailleux A, Fiévet C, Auwerx J. 3-hydroxy-3-methylglutaryl coa reductase inhibitors reduce serum triglyceride levels through modulation of apolipoprotein C-III and lipoprotein lipase. FEBS Lett. 1999;452:160–164. doi: 10.1016/s0014-5793(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 70.Parhofer KG, Barrett PH, Schwandt P. Atorvastatin improves postprandial lipoprotein metabolism in normolipidemlic subjects. J Clin Endocrinol Metab. 2000;85:4224–4230. doi: 10.1210/jcem.85.11.6978. [DOI] [PubMed] [Google Scholar]
- 71.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–S445. doi: 10.1097/00005768-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 72.Tsetsonis NV, Hardman AE, Mastana SS. Acute effects of exercise on postprandial lipemia: A comparative study in trained and untrained middle-aged women. Am J Clin Nutr. 1997;65:525–533. doi: 10.1093/ajcn/65.2.525. [DOI] [PubMed] [Google Scholar]
- 73.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–E362. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 74.Al-Shayji IA, Caslake MJ, Gill JM. Effects of moderate exercise on vldl(1) and intralipid kinetics in overweight/obese middle-aged men. Am J Physiol Endocrinol Metab. 2012;302:E349–E355. doi: 10.1152/ajpendo.00498.2011. [DOI] [PubMed] [Google Scholar]
- 75.Magkos F. Basal very low-density lipoprotein metabolism in response to exercise: Mechanisms of hypotriacylglycerolemia. Prog Lipid Res. 2009;48:171–190. doi: 10.1016/j.plipres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Fisher RM, Coppack SW, Humphreys SM, Gibbons GF, Frayn KN. Human triacylglycerol-rich lipoprotein subfractions as substrates for lipoprotein lipase. Clin Chim Acta. 1995;236:7–17. doi: 10.1016/0009-8981(95)06032-3. [DOI] [PubMed] [Google Scholar]
- 77.Tsekouras YE, Yanni AE, Bougatsas D, Kavouras SA, Sidossis LS. A single bout of brisk walking increases basal very low-density lipoprotein triacylglycerol clearance in young men. Metabolism. 2007;56:1037–1043. doi: 10.1016/j.metabol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 78.Bellou E, Magkos F, Kouka T, et al. Effect of high-intensity interval exercise on basal triglyceride metabolism in non-obese men. Appl Physiol Nutr Metab. 2013;38:823–829. doi: 10.1139/apnm-2012-0468. [DOI] [PubMed] [Google Scholar]
- 79.Magkos F, Tsekouras YE, Prentzas KI, et al. Acute exercise-induced changes in basal vldl-triglyceride kinetics leading to hypotriglyceridemia manifest more readily after resistance than endurance exercise. J Appl Physiol (1985) 2008;105:1228–1236. doi: 10.1152/japplphysiol.90761.2008. [DOI] [PubMed] [Google Scholar]
- 80.Gill JM, Al-Mamari A, Ferrell WR, et al. Effects of a moderate exercise session on postprandial lipoproteins, apolipoproteins and lipoprotein remnants in middle-aged men. Atherosclerosis. 2006;185:87–96. doi: 10.1016/j.atherosclerosis.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 81.Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15:757–769. doi: 10.1038/s41569-018-0098-5. [DOI] [PubMed] [Google Scholar]
- 82.Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2016;130:93–104. doi: 10.1042/CS20150447. [DOI] [PubMed] [Google Scholar]
- 83.Higgins JPT, Thomas J, Chandler J, et al. 2nd ed. John Wiley & Sons; Chichester, UK: 2019. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 84.Eltonsy S, Doiron MD, Simard P, et al. Comparing the effect of combining exercise with rosuvastatin versus atorvastatin on lipid profile and functional capacity: A retrospective cohort study. Biomed Res Int. 2020;2020 doi: 10.1155/2020/7026530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.