Graphical abstract
Abbreviations: CAP, Cider Apple Pomace; Δ/Δ, drying rate; W, moisture content (kg water/kg dried matter); dm, dried matter; Deff, effective diffusivity (m2/s); T, drying time (s); x, direction of the water transport (m); k, mass transfer coefficient (kg water/m2s); , dry solid density (kg dm/m3); aw, water activity; L, the thickness of the CAP layer (m); , the relative humidity of the drying air; Var, percentage of explained variance; , standard deviation of the estimation; , standard deviation of the experimental data; D0, pre-exponential factor (m2/s); Ea, activation energy (kJ/mol); R, ideal gas constant (kJ/mol K); T, temperature of each experiment (K); TPC, Total Phenolic Content (TPC); VC, Vitamin C; AA, ascorbic acid; AC, Antioxidant Capacity; FRAP, Ferric Reducing Ability Power; AIR, Alcohol Insoluble Residue; SC, Swelling Capacity; WRC, Water Retention Capacity; ORC, Oil Retention Capacity; PPO, Polyphenoloxidase
Keywords: By-product, Drying kinetics, Antioxidant properties, Alcohol insoluble residue, Fiber properties
Highlights
-
•
Influence of cider apple pomace drying temperature was studied.
-
•
Temperature affected drying kinetics and antioxidant and dietary fibre properties.
-
•
Antioxidant properties were best preserved when drying in the range of 80–100 °C.
-
•
The best properties of alcohol insoluble residue were found when drying at 40–60 °C.
Abstract
Apple pomace, the by-product of the cider industry, contains a high content of antioxidant compounds and dietary fiber. Drying would allow its preservation for a later use. The aim of this study was to evaluate the effect of the drying temperature on the drying kinetics, antioxidant properties and the fiber characteristics. For this, drying experiments were performed at different temperatures (40–120 °C). The increase in temperature enhanced the drying rate, as was shown by the effective diffusivity and mass transfer coefficient identified by modelling. The influence of temperature was quantified through the activation energy (38.21 kJ/mol). Regarding the retention of antioxidant properties, the best results were found at 80–100 °C while 40–60 °C was the best temperature range for the fiber characteristics. Therefore, 80 °C could be an adequate temperature for drying of cider apple pomace, as it represents a good balance between kinetics, and antioxidant and fiber properties.
1. Introduction
Cider is an alcoholic beverage obtained from the fermentation of apple juice. According to the European Cider & Fruit Wine Association (AICV), more than 1,000,000 tons of apple are used for cider production per year (AICV & Global Data, 2020). This activity generates a large volume of by-product, consisting mainly of pulp, peel, core, stems, exhausted soft tissue and seeds (Diñeiro García et al., 2009), which cause environmental and economic issues. The extraction of compounds of interest or the direct incorporation of some fractions of this by-product as a functional ingredient could represent interesting alternative means of increasing its value.
Apple pomace has been shown to have strong antioxidant properties, with a polyphenol composition similar to that in apples including hydrocinnamic acids, dihydrochalcons and flavonols (da Silva et al., 2021, Lavelli and Corti, 2011) as well as ascorbic acid (Hernández-Carranza et al., 2016). It has also been considered to be a good source of dietary fiber, including significant amounts of pectin with interesting functional properties, better even than other fruit pomaces (Wang et al., 2019). So, its use as a functional ingredient can contribute to reducing the risks of diabetes, hypercholesterolemia or obesity (Mateos-Aparicio et al., 2020).
The valorization of this by-product needs a prior stabilization step. In this sense, drying reduces the moisture content, slows down degradative reactions and cuts storage and transport costs. Hot air drying is the most widely used drying technique. However, this operation involves long processing times, which can induce structural damage and a loss of bioactive compounds. Thus, drying can cause the degradation of the original antioxidant compounds (Méndez-Lagunas et al., 2017) or the formation of new ones (Szychowski et al., 2018). Moreover, the functional properties of the fiber can also be altered as a result of structural changes induced in the matrix formed by the polysaccharide chains (Femenia et al., 2009, Garau et al., 2007).
Many studies into apple pomace drying have assessed the influence of temperature on drying kinetics, but have not considered its effect either on compounds of interest or structure (Kara and Doymaz, 2015, Wang et al., 2007). There can also be found another set of studies that assesses the effects of drying process on quality, but by considering drying as a general process, not taking into account the influence of the drying conditions applied, such as the temperature used. In this sense, Diñeiro García et al. (2009) studied the main phenolic compounds in dried apple pomace at only one drying temperature, 60 °C. Sudha et al. (2016) identified the main phytochemicals of apple pomace as well as its possible incorporation into bakery products as dried apple pomace; however, only the pomace dried at 55 °C was considered. Other studies used a single drying temperature of 70 °C to assess the advantages of dietary supplementation with dried apple pomace (Kosmala et al., 2011) and its composition of polyphenols (Birtic et al., 2019). Finally, there are studies that compare the results obtained with different drying methods, but only one combination of process variables is tested. Thus, Rana et al. (2015) assessed the influence of oven drying at 60 °C, sun drying and freeze-drying on dietary fiber and antioxidant compounds. Lavelli & Corti (2011) compared the effect of vacuum drying at 40 °C and hot air drying at 60 °C on the phenolic compounds of apple pomace subsequent to a lengthy storage.
However, to our knowledge, there is no study which jointly analyzes the influence of the temperature of the convective drying of apple pomace on both kinetics and quality parameters. Therefore, the general aim of this study was to assess the effect of the temperature applied during the convective drying of apple pomace obtained from the cider industry on the drying kinetics, antioxidant properties and alcohol insoluble residue yield and characteristics.
2. Materials and methods
2.1. Raw material
Cider apple pomace (CAP) was obtained from a local cellar (Villaviciosa, Spain) and included a mixture of the apple varieties from the Protected Geographical Indication “Cider of Asturias”. The samples were taken directly after the pressing operation in which the apple juice is collected for the purposes of cider making. They consisted of a mix of pulp, peels, stems and seeds. They were immediately vacuum packed and frozen at −28 °C until their later processing. Prior to the drying experiments, the samples were defrosted in controlled conditions maintaining the vacuum conditions in the plastic bags to avoid water content changes. Then, the bags were opened, and the stems and seeds were removed. The moisture content was measured by differential weighting after maintaining the samples at 70 °C in a vacuum oven until constant weight.
2.2. Drying experiments
The drying process was performed in a convective dryer (Binder FD 260 model, Germany). The experiments were carried out at five different air temperatures (40, 60, 80, 100 and 120 °C) with a constant air velocity (0.9 ± 0.1 m/s). Every condition was carried out in quadruplicate. For each run, 180 g of sample was placed as a thin layer (0.003 m) in aluminium trays. During drying, the weight was recorded every 10 min with an analytical balance (±0.01 g) (Mettler Toledo, model PB3002-S, Switzerland). The process ended when CAP samples reached constant weight (3 consecutive weight differences of less than 0.1 g). Then dried samples were ground, sieved (particle size of less than 200 µm), vacuum packed and stored in the dark until further analysis.
2.3. Modelling of drying kinetics
The modelling of the experimental data was carried out to quantify the influence of air temperature on the drying kinetics. For this purpose, a diffusion-based model was considered. Some assumptions were considered: the temperature and initial moisture content was uniform; the water diffusivity during drying was constant; samples were isotropic and homogenous, the moisture flux was unidimensional and the samples behaviour as a finite slab geometry (Eq. (1)).
| (1) |
where is the effective diffusivity (m2/s) and is the direction of the water transport (m).
Due to the low air velocity used (0.9 ± 0.1 m/s) in this study, both internal and external resistances to mass transfer were considered. In this sense, the external resistance was introduced into the model through the boundary condition shown in Eq. (2).
| (2) |
where is the mass transfer coefficient (kg water/m2s) and represents a measurement of the mass transport in the air-sample interface, is the dry solid density (kg dm/m3), is the water activity, is the thickness of the CAP layer (m), and is the relative humidity of the drying air for each drying experiment. Sorption data reported by Lavelli and Kerr (2012) following a GAB equation fit (Eq. (1)) were used to estimate the equilibrium conditions.
| (3) |
Being C = 8.2, K = 1.0 and m0 = 3.87 kg w/100 kg dm the GAB parameter values.
The diffusion-based model was solved in Matlab 2015B (The MathWorks, Inc., Natick, USA) using a finite difference method (Garcia-Perez et al., 2012). The SIMPLEX method was used to identify and values, which minimize the objective function, the sum of the squared difference between the experimental and calculated moisture content. The goodness of the fit was evaluated from the estimation of the percentage of explained variance by the model () (Eq. (4)):
| (4) |
where is the standard deviation of the estimation, and is the standard deviation of the experimental data.
The influence of temperature on effective diffusivity was quantified using an Arrhenius type equation (Eq. (5)):
| (5) |
where is a pre-exponential factor (m2/s), is the activation energy (kJ/mol), is the ideal gas constant (kJ/mol K) and is the temperature of each experiment (K). For this last variable, two different situations were considered. First, considering the air drying temperature (Wang et al., 2007, Kara and Doymaz, 2015) and second considering the average temperature of the samples during drying. To this end, the evolution of temperature of samples was recorded each 10 min during the process with the help of 6 thermocouples type K attached to a data logger Comark Diligence (mod. EV N2014, Comarck instruments Ltd. Norwich, Uk).
2.4. Antioxidant properties
2.4.1. Extracts
The total phenolic content, vitamin C and antioxidant capacity of CAP were determined through an ethanolic extract from both the fresh and dried samples. For this purpose, 1 g of sample was vigorously mixed with 20 mL of pure ethanol (Labkem, Barcelona, Spain), using Ultra-Turrax (T25 Digital, IKA, Germany) at 16,707 G. After centrifuging at 1,466 G for 10 min (Medifriger BL-S, P. Selecta, Spain), the supernatant of the samples was filtered using a glass microfiber filter (1.2 μm pore).
For the purposes of determining the polyphenol profile, the extraction was carried out by mixing 2 g of dry CAP in 15 mL of acetone/water (70/30 v/v) solution for 5 min in an ultrasonic bath (25 °C). The extracts were pooled, centrifuged (10 min at 10 °C and 17,000G) and brought up to 50 mL with the extractant mixture. An aliquot of this extract was filtered through 0.22 µm PVDF membrane (Teknokroma, Barcelona,Spain) and analyzed.
2.4.2. Total phenolic content (TPC)
The TPC was estimated following the procedure described by Gao et al. (2000). For this purpose, 100 µL of the ethanolic extract of the sample, 200 µL of Folin-Ciocalteu and 2 mL of distilled water were mixed. The mixture was maintained at room temperature for 3 min, and then 1 mL of 20 % sodium carbonate (Labkem, Barcelona, Spain) solution was added. After one hour of incubation at room temperature, the sample’s absorbance was measured in a spectrophotometer (Helios, Gamma, Thermo Spectronic, Cambridge, UK) at 765 nm. The results were expressed as an equivalent concentration of gallic acid thanks to a calibration curve made with solutions of known gallic acid (Sigma-Aldrich, Madrid, Spain) concentration. The tests were performed in triplicate for each drying experiment.
2.4.3. Polyphenol profile
The HPLC determinations were performed by means of a Waters system equipped with a 717plus automatic injector, a multisolvent low-pressure pump (Waters 600E), a 2,996 DAD and an Empower v 3.0 software data module. The polyphenols were separated onto a Nucleosil C120 C18 column (250 × 0.46 mm I.D., 3 µm from Macherey-Nagel, Panreac, Barcelona, Spain), at 30 °C. The mobile phase was made up of 2 % acetic acid (solvent A) and methanol (solvent B). The gradient elution was as reported by Diñeiro García et al. (2009). The flow rate was 0.8 mL/min and the injection volume was 10 µL.
The quantification was carried out by the external standards method: chlorogenic acid was used as standard for hydroxycinnamic acids (λ = 320 nm), quercitrin for flavonol glycosides (λ = 350 nm) and phloridzin for dihydrochalcones (λ = 280 nm). All of the compounds were obtained from Sigma-Aldrich (St. Louis, MO, USA). The samples from each drying condition were analyzed in triplicate.
2.4.4. Vitamin C (VC)
The vitamin C content was measured by means of the procedure described by Jagota and Dani (1982). Thus, 0.5 mL of the sample extract was mixed with 0.5 mL of a 7.5 % trichloroacetic acid solution (Panreac, Barcelona, Spain). The mixture was homogenised and incubated for 5 min at 4 °C and then filtered through a pore of 0.45 µm. Then, 2 mL of distilled water and 200 µL of a diluted solution (1:10 v/v) of the Folin-Ciocalteu (Sigma-Aldrich, Madrid, Spain) reagent were added to 200 µL of the prepared solution. The mixture was incubated at room temperature for 10 min before measuring the absorbance at 760 nm in the spectrophotometer. Previously, a calibration curve was prepared with ethanolic solutions of a known concentration of ascorbic acid (Sigma-Aldrich, Madrid, Spain). Three replicates were performed for each analyzed sample.
2.4.5. Antioxidant capacity (AC)
The AC of fresh and dried CAP was determined following the Ferric Reducing Ability Power (FRAP) method described by Benzie and Strain (1996). The FRAP reagent was prepared from 10 mM TPTZ (Sigma-Aldrich, Madrid, Spain) diluted in 40 mM HCl (Panreac, Barcelona, Spain), 20 mM of FeCl3 6·H2O (Labkem, Barcelona, Spain) and 0.3 M acetate buffer (Panreac, Barcelona, Spain), in equal parts, and incubated for 30 min at 37 °C. Then, 900 µL of the reagent was mixed with 30 µL of distilled water and 30 µL of sample extract. The mix was incubated again for 30 min at 37 °C before measuring the absorbance at 595 nm in a spectrophotometer. The results were read through a calibration curve, previously determined with a known concentration of ethanolic solutions of Trolox (Sigma-Aldrich, Madrid, Spain). Each measurement was taken in triplicate.
2.5. Alcohol insoluble residue (AIR)
The AIR was obtained based on the procedure described by Femenia et al. (1998), with minor modifications. For this purpose, several washing cycles of the samples were performed with boiling ethanol, two of which used 85 % (v/v) ethanol and one 96 % (v/v). Finally, the last wash used acetone. Then, the solid was dried at 60 °C for 24 h and weighed. The AIR yields in both fresh and dried CAP were determined, and their different functional properties were measured in triplicate following the method described by Garau et al. (2007).
2.5.1. Swelling capacity (SC)
The SC was determined by placing 0.20 ± 0.01 g of AIR into a graduated test tube and then adding 10 mL of distilled water. The initial volume occupied by the AIR at room temperature was measured, as was the volume after 24 h. The SC was calculated by the difference of these volumes and the results were expressed as mL/g of AIR.
2.5.2. Water retention capacity (WRC)
The WRC was measured by mixing 0.20 ± 0.01 g of AIR with 10 mL of distilled water. After 24 h, the samples were centrifuged at 9,167 G, for 15 min at 25 °C. The excess of supernatant was decanted, and the amount of water retained by AIR was measured by differential weight. The results were expressed as kg water/kg AIR.
2.5.3. Oil retention capacity (ORC)
To estimate the ORC, 10 mL of vegetal oil was added to 0.20 ± 0.01 g of AIR. After 24 h at room temperature, the samples were centrifuged at 9,167 G, for 15 min at 25 °C. The excess oil was eliminated, and the weights of the solid samples were taken. The oil retained in the AIR was estimated through the difference with the initial weight. The results were expressed as kg oil/kg AIR.
2.6. Statistical analysis
Analyses of variance (one way ANOVA) (p < 0.05) were carried out and the LSD (Least Significant Difference) intervals were determined through Statgraphics Centurion XVI (Statpoint Technologies Inc., Warrenton, VA, USA) for the parameters considered. In this sense, it was possible to determine the significance of the differences (p < 0.05) between the drying time, and the values, as well as the quality parameters (TPC, polyphenol profile, VC, AC, AIR yield, SC, WRC and ORC) obtained at the different drying temperatures tested.
3. Results and discussion
3.1. Effect of temperature on drying kinetics
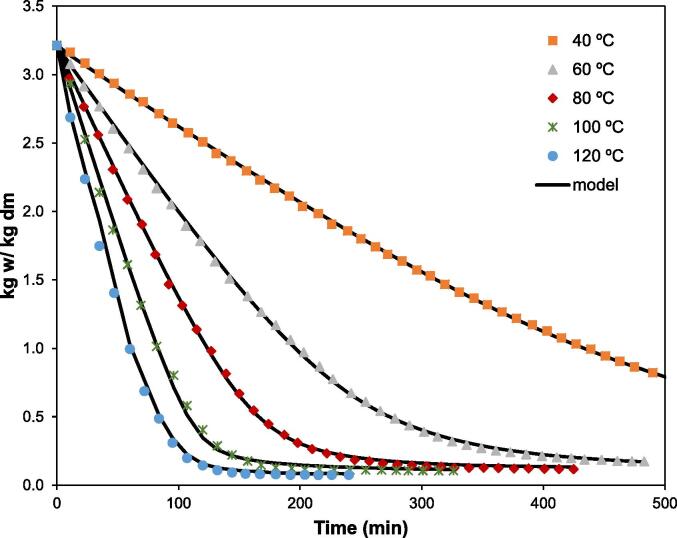
The initial moisture content of CAP was 0.76 ± 0.02 kg water/kg dm. As expected, air temperature significantly affected the drying kinetics of CAP (Fig. 1). In this way, the drying time required to reach a water content of 0.15 ± 0.01 kg w/kg dm was shortened by 91 % when the drying was performed at 120 °C compared to when it was carried out at 40 °C (Table 1). These drying time values were in the range of those previously reported by Wang et al. (2007) for thin layer apple pomace drying at similar temperatures. The increase in the drying temperature enhanced the heat transfer at the air-sample interface. This increased the vapour pressure inside the sample favouring the evaporation of the water molecules and their movement.
Fig. 1.
Experimental and modelled drying kinetics of cider apple pomace (CAP) at different temperatures.
Table 1.
Drying time (min) required to reach 0.15 ± 0.01 kg w/kg dm. Effective water diffusivity (), mass transfer coefficient () and percentage of variance () at the different temperatures (mean ± SD).
| Temperature (°C) |
|||||
|---|---|---|---|---|---|
| 40 | 60 | 80 | 100 | 120 | |
| Drying time (min) | 1,400 ± 73a | 471 ± 25c | 260 ± 17c | 173 ± 18d | 129 ± 18d |
| (10−10 m2/s) | 5 ± 1a | 13 ± 4a | 33 ± 11a | 62 ± 27b | 91 ± 31b |
| (10−4 kg w/m2 s) | 2.97 ± 0.07a | 6.3 ± 0.1b | 11 ± 0.4c | 16.7 ± 0.9d | 25 ± 2e |
| 0.9966 | 0.9982 | 0.9988 | 0.9995 | 0.9998 | |
Different letters in the same row indicate significant differences at p < 0.05.
3.2. Modelling of experimental data
In order to quantify the influence of temperature on drying kinetics, the effective moisture diffusivity () and mass transfer coefficient () were identified by fitting the previously described model to the experimental data. This model provided an accurate description of the drying kinetics, as shown by the high percentages of the explained variance () values (Table 1), over 99.9 % in every case. Moreover, both the experimental and calculated moisture contents followed the same trend (Fig. 1).
The identified values of both kinetic parameters, and , increased as the air temperature rose. These values agree with those reported by Wang et al. (2007) or Kara and Doymaz (2015). Thus, the higher the drying temperature, the more energy available in the medium for moisture transport, which accelerates the water movement inside the samples, measured by . Moreover, the greater temperature gradient between the drying air and CAP samples enhances the heat transfer, which improves the moisture transport between the sample surface and surrounding air, which is measured by .
The influence of the air temperature on the was quantified from the estimation of the activation energy () by means of an Arrhenius equation (Eq. (5)) being found a good agreement (r2 0.9891). The identified for the CAP, 38.21 kJ/mol, was greater than that reported by Wang et al. (2007), 24.51 kJ/mol, or Kara and Doymaz (2015), 29.65 kJ/mol, for apple pomace drying. This could be attributed to the different apple varieties used in the present study, corresponding with local varieties included in the PGI “Cider of Asturias”.
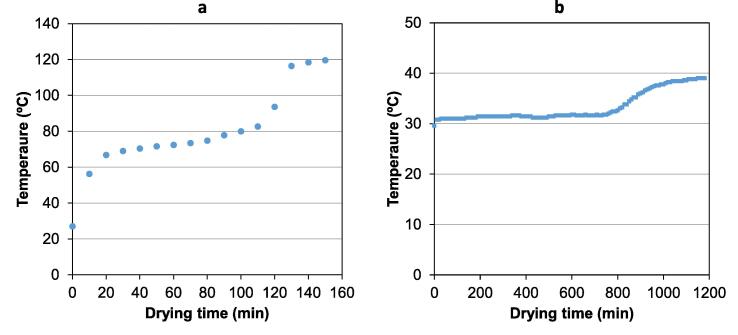
Moreover, the influence of actual sample temperature on Deff was also estimated. To do this, a study of the evolution of internal temperature of CAP samples during the drying at the different drying air temperatures considered was carried out (Simal et al., 1993). Thus, at the start of the drying process, an initial heating period was observed (Fig. 2) until sample temperature approximately achieve the wet-bulb temperature (Eijkelboom et al., 2022). Afterwards, a second period was identified in which the temperature raised slightly. In this period, the evaporation of moisture content partially compensated the energy supplied by the drying air. The decrease of drying rate because the decreasing of moisture content explain the increase of sample temperature in this period. Finally, a fast increase of temperature took place at the longer drying time. This can be attributed to the low moisture content of sample at this stage and, therefore, the low evaporation rate. Thus, considering the average temperature inside the product, the activation energy was calculated. The figure identified was 65.05 kJ/mol (r2 0.9898), which was greater than the identified considering the drying air temperature. This fact pointed out the great influence of temperature in the drying rate of CAP samples.
Fig. 2.
Evolution of the temperature inside the cider apple pomace (CAP) during drying with an air temperature of 120 °C (a) and 40 °C (b).
3.3. Antioxidant properties
3.3.1. Total phenolic content (TPC)
Phenolic compounds are associated with health benefits due to their antioxidant activity. In the case of CAP, drying significantly reduced the amount of TPC, the retention being dependent on the drying temperature (Table 2). Thus, the highest retention was found in samples dried at 80 °C, while those dried at a higher or lower temperature exhibited lower values of TPC. High temperatures can cause irreversible changes in the chemical structure of heat sensitive phenolic compounds. These changes harm the reactivity of the aromatic rings that interact with the Folin-Ciocalteu reagent (Bustos et al., 2018), explaining the low TPC retention observed at the highest temperature tested (Table 2). Furthermore, the cellular disruption produced by drying can release oxidative enzymes, such as polyphenoloxidase (PPO) (Méndez-Lagunas et al., 2017). At drying temperatures below 50 °C, these oxidative enzymes are not deactivated, causing the oxidation of phenolic compounds. Moreover, the long drying time required in these conditions can favour the oxidation reactions which is coherent with the significant TPC reduction observed in the experiments carried out at 40 °C (Table 2). Therefore, as the results suggest, drying at a temperature close to 80 °C could represent a good compromise between temperature and drying time, which would provide the best TPC retention. It has also been reported that this drying temperature was the most adequate for TPC retention in the case of apple drying (Henríquez et al., 2010, Vega-Gálvez et al., 2012).
Table 2.
Percentage of retention of the total phenolic content (TPC), vitamin C (VC) and antioxidant capacity (AC), and content of the main polyphenol compounds (mg/kg, dry weight) of dried cider apple pomace at the different temperatures (mean ± SD).
| Temperature (°C) |
|||||
|---|---|---|---|---|---|
| 40 | 60 | 80 | 100 | 120 | |
| TPC | 22 ± 5a | 55 ± 4c | 66 ± 11d | 57 ± 6c | 45 ± 10b |
| VC | 21 ± 5a | 31 ± 4b | 46 ± 7d | 37 ± 8c | 43 ± 4 cd |
| AC | 28 ± 5a | 30 ± 3ab | 35 ± 6bc | 41 ± 7d | 36 ± 4c |
| Dihydrochalcones | |||||
| Unk DHC-A | 15 ± 1a | 21 ± 2b | 40 ± 2c | 63 ± 2d | 61.2 ± 0.3d |
| Unk DHC-B | 15 ± 2a | 27 ± 1b | 55 ± 3c | 102 ± 7e | 76.1 ± 1.3d |
| Phloretin 2′-xyloglucoside | 40 ± 2a | 63 ± 5b | 128 ± 9c | 274 ± 7d | 338.6 ± 0.6e |
| Phloridzin | 137 ± 42a | 215.0 ± 0.3a | 338 ± 30b | 678 ± 43c | 801.8 ± 16.9d |
| Hydroxycinnamic acids | |||||
| Chlorogenic acid | 107 ± 10a | 142 ± 7a | 267 ± 17b | 402 ± 2c | 514.9 ± 33.4d |
| Flavonols | |||||
| Hyperin | 161 ± 17a | 164 ± 2a | 176 ± 12a | 268 ± 1c | 244 ± 4b |
| Rutin + Isoquercitrin | 88 ± 14a | 82.6 ± 0.7a | 86 ± 4a | 153 ± 4c | 133 ± 2b |
| Reynoutrin | 47 ± 2a | 51.2 ± 0.3ab | 54 ± 3b | 80 ± 4c | 78 ± 2c |
| Avicularin | 130 ± 13a | 142.0 ± 0.2ab | 153 ± 11b | 208 ± 9c | 163.7 ± 0.1b |
| Quercitrin | 85 ± 2a | 95.7 ± 0.1b | 98 ± 2b | 142 ± 4d | 126.9 ± 0.1c |
Different letters in the same row indicate significant differences stablished from least significance difference (LSD) intervals (p < 0.05).
3.3.2. Polyphenol profiles
The TPC analysis represents a global measurement of the polyphenol content of the samples. However, the drying temperature can affect each particular group of polyphenols differently. For this reason, the content of the main components of three groups of polyphenols (dihydrochalcones, hydroxycinnamic acids and flavonols) were also measured (Table 2). The polyphenol profile obtained was similar to that reported by Schieber et al. (2003) for apple pomace dried in a three-stage drum dryer at 50–60 °C.
Overall, as can be observed in Table 2, the lower the drying temperature, the smaller the content of the different compounds. Thus, the highest contents were observed at drying temperatures in the range of 80–120 °C, depending on the specific compound. The results were slightly different to those obtained for TPC, whose maximum retention was achieved at 80 °C. This could be attributed to the low specificity of the Folin-Ciocalteu reagent, since it can react with any reducing substance and does not allow the behaviour of individual compounds to be identified (Lang and da Lindemann, 2019).
As regards the more specific results obtained by HPLC, two phloretin glycosides (phloretin-2′-xyloglucoside and phloridzin) and two more indeterminate compounds were found in the dihydrochalcon group. Phloridzin was the predominant compound in the polyphenol profile, its content ranging from 136.5 ± 42.1 to 801.8 ± 16.9 mg/kg dm. These values were lower than those obtained by Lavelli and Corti (2011); this was probably due not only to the presence of seeds in their experiments, which contain a large amount of phloridzin (Schieber et al., 2003), but also to the shorter drying time.
As for the group of hydroxycinnamic acids, the only compound detected was chlorogenic acid. This polyphenol is the main substrate of the PPO, causing its oxidation (Birtic et al., 2019). Drying at moderate temperatures does not inactivate the enzyme and the residual activity could be extended during the process, leading to significant losses. This has been previously reported in other fruits (Madrau et al., 2009).
In the case of flavonols, the maximum content was found in the samples dried at 100 °C. These values were very similar to those obtained by Schieber et al. (2001) working on dried apples at 110 °C. In this case, querecetin glycosides are not direct substrates of PPO because the enzyme does not act directly on glycosides (Madrau et al., 2009). However, it has been proven that apple quercetin glycosides exhibit good resistance to thermal degradation, thus showing minimal losses during baking at 175 °C (Rupasinghe et al., 2008).
3.3.3. Vitamin C
Vitamin C (VC) is widely used as a quality indicator of the intensity of drying processes and can be affected by high temperatures, longer drying times and even by acting as a protector of polyphenols against oxidation. The initial content of the CAP sample before drying was 21.4 ± 3.2 mg AA/100 g dm, a value in the range of those obtained in apple pomace (Hernández-Carranza et al., 2016). After drying, the VC content dropped significantly, the retention being between 21 and 46 % of the initial content depending on the drying temperature applied (Table 2). Similarly to TPC, the maximum VC retention was found in the samples dried at 80 °C, being lower at higher and lower drying temperatures. When analyzing the hot air drying of apple slices at temperatures in the range of 60 to 90 °C, Rajoriya et al. (2019) found the best VC retentions in those samples dried at 80 and 90 °C, which can be attributed to shorter drying times that prevent oxidation. This good time–temperature balance when drying at temperatures close to 80 °C has also been observed by other authors (Deng et al., 2018).
3.3.4. Antioxidant capacity
A global AC measurement of CAP was also taken to complement the TPC, polyphenol profile and VC measurements. Thus, AC includes possible synergetic or antagonistic effects of the different compounds (García-Alonso et al., 2004). The initial AC measured by the FRAP assay was 32 ± 7 mg Trolox/100 g dm, which was significantly reduced after drying. The percentage of retention varied in line with the temperature, ranging from 28 to 46 % (Table 2). The maximum retention was obtained in CAP samples dried at 100 °C. The results exhibited a strong correlation with the content of quercetin glycosides at the drying temperatures tested (correlation coefficient of 0.9199). Thus, the samples dried at 100 °C had the highest content of these flavonols (Table 2), and the maximum AC retention (Table 2). Lee et al. (2003) reported that quercetin glycosides account for 34.7 % of the AC of apple, being one of the compounds that contribute the most to this property. Diñero García et al. (2009) were able to predict the AC from the polyphenol profile. They found a marked influence of the compounds rutin + isoquericitrin and hyperin. In the present study, a significant correlation of these compounds with AC was also found (correlation coefficient of 0.8537 and 0.8964, respectively).
Other compounds, such as vitamin C, whose maximum retention was found in CAP dried at 80 °C, or chlorogenic acid and phloretin glycosides, whose maximum content was observed in CAP dried at 120 °C, also influenced the AC (Diñeiro García et al., 2009, Lee et al., 2003). In addition, some studies have reported that melanoids, derived from Maillard reactions, can be formed at high temperatures which can increase antioxidant capacity (Vega-Gálvez et al., 2012). All this results agreed to the maximum AC retained in those samples dried at 100 °C.
Thus, in the case of the CAP samples, the long exposure to the action of oxygen and enzymes that take place when drying at moderate temperatures (40–60 °C) contribute to a greater reduction in AC, while, higher temperature (100–120 °C) can damage compounds that contribute to the AC. However, in this last case, high temperature also induces the shortening of the drying process, which can contribute to the preservation of antioxidant properties. This has also been reported when analyzing the antioxidant capacity of other products (Martín-Gómez et al., 2020). Therefore, from the results achieved it can be concluded that using drying temperatures in the range of 80–100 °C is the best means of preserving the antioxidant components in the CAP samples.
3.4. Alcohol insoluble residue (AIR)
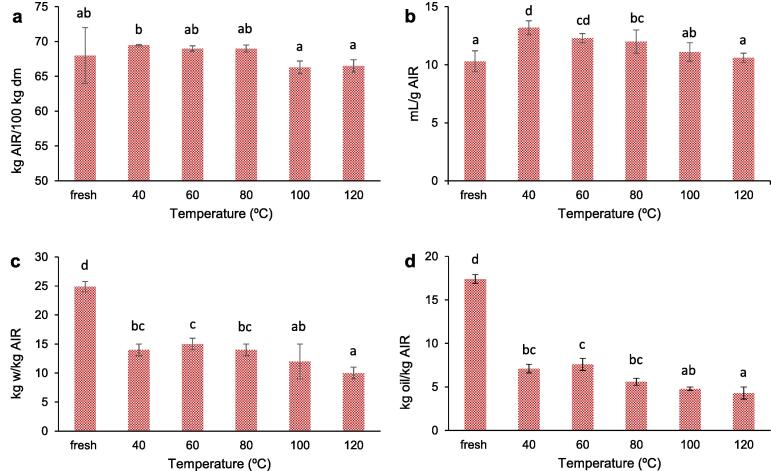
Apple pomace has large quantities of components, such as pectin or lignin that contribute to making this by-product a good source of fiber. The alcohol insoluble residue (AIR), achieved by ethanolic extraction, is just a concentrate of cell wall polymers and constitutes an approximate measurement of the fiber content. The AIR yield in the fresh CAP was 68 ± 4 % (Fig. 3a) showing the potential of this by-product as a potential source of fiber (Benítez et al., 2017). A similar value of 67.2 % was reported by Kosmala et al. (2011). This value was lower than that obtained by Borsini et al. (2021) for artichoke bracts (85 %), but higher than that of artichoke stems (63 %) or orange peel (53 %) (Mello et al., 2020).
Fig. 3.
Yield (kg AIR/100 kg dm) (a), swelling capacity (SC) in mL/g AIR (b), water retention capacity (WRC) in kg water/kg AIR (c) and oil retention capacity (ORC) in kg oil/kg AIR (d) of the fresh and dried cider apple pomace at the different temperatures.
After drying, no significant changes in AIR yield were observed, regardless of the drying temperature applied. This was also seen by Mello et al., 2020, Garau et al., 2007 when analyzing dried orange peel. However, drying can affect the physicochemical properties, provoking modifications in the functional properties (Garau et al., 2007). Therefore, in order to evaluate these possible changes, the swelling capacity (SC), the water retention capacity (WRC) and the oil retention capacity (ORC) of the fresh and dried at different temperatures CAP samples were studied.
3.4.1. Swelling capacity (SC)
The SC is related with the satiating effect of fiber. Thus, water can join the capillary structures of AIR due both to the force of surface tension as well as to the interaction with other components through hydrogen bonds or dipole forms, thus producing the swelling of the structure. The fresh CAP samples had a SC value of 10.3 mL/g AIR dm (Fig. 3b), which is in the range of the values obtained in other studies on fresh apple pomace or apple peel (Henríquez et al., 2010). This value increased after drying, especially in samples dried at 40 °C. However, the higher the drying temperature, the lower the SC, and there were no significant differences between fresh and dried at the highest temperatures (100 and 120 °C).
3.4.2. Water retention capacity (WRC)
The WRC is the ability of the matrix to retain water after applying an external force. WRC and SC determine the hydration properties of the AIR, and they are related with the increase in the stool weight and the velocity of the digestive tract (Meng et al., 2019). The WRC of the fresh CAP was 24.9 ± 0.9 kg w/kg AIR (Fig. 3c), suggesting that AIR can hold 25 times more water than its own weight. This value was slightly higher than that found by others when studying different apple products (Zlatanović et al., 2019).
After drying, WRC was significantly reduced: the higher the drying temperature, the lower the WRC (Fig. 3c). This behaviour has been previously reported by other authors (Borsini et al., 2021, Femenia et al., 2009). High temperatures can cause cellular damage to polysaccharides and impair the ability to retain water (Rana et al., 2015). At the same time, a more rigid structure is created that causes the collapse of channels and does not allow them to collect and retain water (Ghanem et al., 2020).
3.4.3. Oil retention capacity (ORC)
The ORC provides information about the fat retention ability of AIR (Femenia et al., 2009). This property is of special relevance because AIR can be used for several purposes: to prevent phase separation during food preservation (Nieto-Calvache et al., 2019), to reduce the absorption of lipids in the intestine and favour faecal lipid excretion, reducing serum cholesterol levels (Mateos-Aparicio et al., 2020).
The ORC of the fresh CAP samples was 17.4 ± 0.5 kg oil/kg AIR. After drying, it decreased significantly, with values in the range of 7.6 and 4.3 kg oil/kg AIR (Fig. 3d). The higher the drying temperature, the lower the ORC. As in the case of WRC, drying could damage the cell wall polysaccharides, such as lignin, which plays an important role in oil retention (Rana et al., 2015). It is possible that a rise in the temperature applied could lead to greater damage being done. The ORC value of AIR obtained in this study was similar to that found by Henríquez et al. (2010) for dried (110–140 °C) apple peel (5.5 and 6.0 kg oil/kg dm, respectively), and higher than that reported for apple pomace (Rana et al., 2015).
Therefore, drying at a temperature in the range of 40–60 °C provided dried CAP samples with the best AIR yield and properties.
4. Conclusions
The drying temperature significantly affected the drying kinetics, antioxidant properties and alcohol insoluble residue of cider apple pomace. The higher the temperature, the faster the drying process. The high activation energy identified (38.21 kJ/mol) indicated that temperature exerted a marked influence on moisture diffusion. The antioxidant properties of dried CAP, such as the content of polyphenols or vitamin C or the antioxidant capacity, were also influenced by drying temperature, with the range 80–100 °C being the most adequate for preservation purposes. On the contrary, the AIR yield and its properties, such as swelling capacity, water and oil retention capacity were better preserved when drying took place at lower temperatures, in the range of 40–60 °C.
Therefore, the drying temperature has to be chosen by considering what use the dried CAP is to be put to, fortifying either the antioxidant properties or the AIR properties. Thus, drying at temperatures close to 80 °C may represent a compromise, constituting a good balance between the drying time and the preservation of the quality attributes.
CRediT authorship contribution statement
B. Llavata: Methodology, Formal analysis, Investigation, Resources, Writing – original draft, Visualization. A. Picinelli: Methodology, Resources, Writing – original draft. S. Simal: Validation, Writing – review & editing, Funding acquisition. J.A. Cárcel: Conceptualization, Validation, Investigation, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support from the grant PID2019‐106148RRC42 and PID2019‐106148RRC43 funded by MCIN/AEI/10.13039/501100011033, the grant for open access charge of the Universitat Politècnica de València and the PhD grant of Beatriz Llavata from the Universitat Politècnica de València (PAID-01-19).
References
- AICV & Global Data. (2020). European Cider Trends 2020. 1–13. https://aicv.org/files/attachments/.407/AICV_Cider_Trends_2020.pdf.
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Birtic, S., Régis, S., Le Bourvellec, C., & Renard, C. M. G. C. (2019). Impact of air-drying on polyphenol extractability from apple pomace. Food Chemistry, 296(July 2018), 142–149. https://doi.org/10.1016/j.foodchem.2019.05.131. [DOI] [PubMed]
- Borsini A.A., Llavata B., Umaña M., Cárcel J.A. Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods. 2021;10(2):1–13. doi: 10.3390/foods10020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos M.C., Rocha-Parra D., Sampedro I., De Pascual-Teresa S., León A.E. The influence of different air-drying conditions on bioactive compounds and antioxidant activity of berries. Journal of Agricultural and Food Chemistry. 2018;66(11):2714–2723. doi: 10.1021/acs.jafc.7b05395. [DOI] [PubMed] [Google Scholar]
- da Silva L.C., Viganó J., de Souza Mesquita L.M., Dias A.L.B., de Souza M.C., Sanches V.L.…Rostagno M.A. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L.Z., Yang X.H., Mujumdar A.S., Zhao J.H., Wang D., Zhang Q.…Xiao H.W. Red pepper (Capsicum annuum L.) drying: Effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure. Drying Technology. 2018;36(8):893–907. doi: 10.1080/07373937.2017.1361439. [DOI] [Google Scholar]
- Diñeiro García Y., Valles B.S., Picinelli Lobo A. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chemistry. 2009;117(4):731–738. doi: 10.1016/j.foodchem.2009.04.049. [DOI] [Google Scholar]
- Eijkelboom, N.M.; Swinkels, A.C.M., de Ruiter, J., Schutyser, M.A.I. (2022). Temperature development of drying sessile single droplets. In 22nd International Drying Symposium. Worcester, Massachusetts, USA, DOI: https://doi.org/10.55900/jajdjacx.
- Femenia A., Robertson J.A., Waldron K.W., Selvendran R.R. Cauliflower (Brassica oleracea L), globe artichoke (Cynara scolymus) and chicory witloof (Cichorium intybus) processing by-products as sources of dietary fibre. Journal of the Science of Food and Agriculture. 1998;77(4):511–518. doi: 10.1002/(SICI)1097-0010(199808)77:4<511::AID-JSFA74>3.0.CO;2-2. [DOI] [Google Scholar]
- Femenia A., Sastre-Serrano G., Simal S., Garau M.C., Eim V.S., Rosselló C. Effects of air-drying temperature on the cell walls of kiwifruit processed at different stages of ripening. LWT - Food Science and Technology. 2009;42(1):106–112. doi: 10.1016/j.lwt.2008.05.022. [DOI] [Google Scholar]
- Gao X., Bjork L., Trajkovski V., Uggla M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. Journal of the Science of Food and Agriculture. 2000;80(14):2021–2027. doi: 10.1002/1097-0010(200011)80:14<2021::AID-JSFA745>3.0.CO;2-2. [DOI] [Google Scholar]
- Garau M.C., Simal S., Rosselló C., Femenia A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chemistry. 2007;104(3):1014–1024. doi: 10.1016/j.foodchem.2007.01.009. [DOI] [Google Scholar]
- García-Alonso M., De Pascual-Teresa S., Santos-Buelga C., Rivas-Gonzalo J.C. Evaluation of the antioxidant properties of fruits. Food Chemistry. 2004;84(1):13–18. doi: 10.1016/S0308-8146(03)00160-2. [DOI] [Google Scholar]
- Garcia-Perez J.V., Carcel J.A., Riera E., Rosselló C., Mulet A. Intensification of low-temperature drying by using ultrasound. Drying Technology. 2012;30(11–12):1199–1208. doi: 10.1080/07373937.2012.675533. [DOI] [Google Scholar]
- Ghanem N., Mihoubi D., Bonazzi C., Kechaou N., Boudhrioua N. Drying characteristics of lemon by-product (Citrus limon. v. lunari): Effects of drying modes on quality attributes kinetics’. Waste and Biomass Valorization. 2020;11(1):303–322. doi: 10.1007/s12649-018-0381-z. [DOI] [Google Scholar]
- Henríquez C., Speisky H., Chiffelle I., Valenzuela T., Araya M., Simpson R., Almonacid S. Development of an ingredient containing apple peel, as a source of polyphenols and dietary fiber. Journal of Food Science. 2010;75(6) doi: 10.1111/j.1750-3841.2010.01700.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Carranza P., Ávila-Sosa R., Guerrero-Beltrán J.A., Navarro-Cruz A.R., Corona-Jiménez E., Ochoa-Velasco C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. Journal of Food Processing and Preservation. 2016;40(1):103–115. doi: 10.1111/jfpp.12588. [DOI] [Google Scholar]
- Jagota S.K., Dani H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Analytical Biochemistry. 1982;127(1):178–182. doi: 10.1016/0003-2697(82)90162-2. [DOI] [PubMed] [Google Scholar]
- Kara C., Doymaz İ. Effective moisture diffusivity determination and mathematical modelling of drying curves of apple pomace. Heat and Mass Transfer/Waerme- Und Stoffuebertragung. 2015;51(7):983–989. doi: 10.1007/s00231-014-1470-6. [DOI] [Google Scholar]
- Kosmala M., Kolodziejczyk K., Zduńczyk Z., Juśkiewicz J., Boros D. Chemical composition of natural and polyphenol-free apple pomace and the effect of this dietary ingredient on intestinal fermentation and serum lipid parameters in rats. Journal of Agricultural and Food Chemistry. 2011;59(17):9177–9185. doi: 10.1021/jf201950y. [DOI] [PubMed] [Google Scholar]
- Lang, G. H., Lindemann, I. da S., Ferreira, C. D., Hoffmann, J. F., Vanier, N. L., & de Oliveira, M. (2019). Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chemistry, 287(September 2018), 197–204. https://doi.org/10.1016/j.foodchem.2019.02.028. [DOI] [PubMed]
- Lavelli V., Corti S. Phloridzin and other phytochemicals in apple pomace: Stability evaluation upon dehydration and storage of dried product. Food Chemistry. 2011;129(4):1578–1583. doi: 10.1016/j.foodchem.2011.06.011. [DOI] [Google Scholar]
- Lavelli V., Kerr W. Apple pomace is a good matrix for phytochemical retention. Journal of Agricultural and Food Chemistry. 2012;60(22):5660–5666. doi: 10.1021/jf3010993. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Kim Y.J., Kim D.O., Lee H.J., Lee C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. Journal of Agricultural and Food Chemistry. 2003;51(22):6516–6520. doi: 10.1021/jf034475w. [DOI] [PubMed] [Google Scholar]
- Madrau M.A., Piscopo A., Sanguinetti A.M., Del Caro A., Poiana M., Romeo F.V., Piga A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. European Food Research and Technology. 2009;228(3):441–448. doi: 10.1007/s00217-008-0951-6. [DOI] [Google Scholar]
- Martín-Gómez, J., Varo, M. Á., Mérida, J., & Serratosa, M. P. (2020). Influence of drying processes on anthocyanin profiles, total phenolic compounds and antioxidant activities of blueberry (Vaccinium corymbosum). LWT, 120(December 2019), 108931. https://doi.org/10.1016/j.lwt.2019.108931.
- Mateos-Aparicio, I., De la Peña Armada, R., Pérez-Cózar, M. L., Rupérez, P., Redondo-Cuenca, A., & Villanueva-Suárez, M. J. (2020). Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effectsin high-fat fed Wistar rats. Bioactive Carbohydrates and Dietary Fibre, 23(November 2019). https://doi.org/10.1016/j.bcdf.2020.100219.
- Mello R.E., Fontana A., Mulet A., Correa J.L.G., Cárcel J.A. Ultrasound-assisted drying of orange peel in atmospheric freeze-dryer and convective dryer operated at moderate temperature. Drying Technology. 2020;38(1–2):259–267. doi: 10.1080/07373937.2019.1645685. [DOI] [Google Scholar]
- Méndez-Lagunas L., Rodríguez-Ramírez J., Cruz-Gracida M., Sandoval-Torres S., Barriada-Bernal G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chemistry. 2017;230:174–181. doi: 10.1016/j.foodchem.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Meng X., Liu F., Xiao Y., Cao J., Wang M., Duan X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chemistry: X. 2019;3(May):100029. doi: 10.1016/j.fochx.2019.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Calvache J.E., de Escalada Pla M., Gerschenson L.N. Dietary fibre concentrates produced from papaya by-products for agroindustrial waste valorisation. International Journal of Food Science and Technology. 2019;54(4):1074–1080. doi: 10.1111/ijfs.13962. [DOI] [Google Scholar]
- Rajoriya D., Shewale S.R., Hebbar H.U. Refractance window drying of apple slices: Mass transfer phenomena and quality parameters. Food and Bioprocess Technology. 2019;12(10):1646–1658. doi: 10.1007/s11947-019-02334-7. [DOI] [Google Scholar]
- Rana S., Gupta S., Rana A., Bhushan S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Science and Human Wellness. 2015;4(4):180–187. doi: 10.1016/j.fshw.2015.10.001. [DOI] [Google Scholar]
- Rupasinghe H.P.V., Wang L., Huber G.M., Pitts N.L. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chemistry. 2008;107(3):1217–1224. doi: 10.1016/j.foodchem.2007.09.057. [DOI] [Google Scholar]
- Schieber A., Hilt P., Endreß H.U., Rentschler C., Carle R. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innovative Food Science and Emerging Technologies. 2003;4(1):99–107. doi: 10.1016/S1466-8564(02)00087-5. [DOI] [Google Scholar]
- Schieber, A., Keller, P., & Carle, R. (2001). Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. 910, 265–273. https://doi.org/10.1016/s0021-9673(00)01217-6. [DOI] [PubMed]
- Simal S., Berna A., Mulet A., Rossello C. A method for the calculation of the heat transfer coefficient in potato drying. Journal of the Science of Food and Agriculture. 1993;63:365–367. [Google Scholar]
- Sudha M.L., Dharmesh S.M., Pynam H., Bhimangouder S.V., Eipson S.W., Somasundaram R., Nanjarajurs S.M. Antioxidant and cyto/DNA protective properties of apple pomace enriched bakery products. Journal of Food Science and Technology. 2016;53(4):1909–1918. doi: 10.1007/s13197-015-2151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski, P. J., Lech, K., Sendra-Nadal, E., Hernández, F., Figiel, A., Wojdyło, A., & Carbonell-Barrachina, Á. A. (2018). Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying method. Food Chemistry, 255(August 2017), 157–164. https://doi.org/10.1016/j.foodchem.2018.02.075. [DOI] [PubMed]
- Vega-Gálvez A., Ah-Hen K., Chacana M., Vergara J., Martínez-Monzó J., García-Segovia P.…Di Scala K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chemistry. 2012;132(1):51–59. doi: 10.1016/j.foodchem.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Wang, S., Gu, B. J., & Ganjyal, G. M. (2019). Impacts of the inclusion of various fruit pomace types on the expansion of corn starch extrudates. LWT, 110(June 2018), 223–230. https://doi.org/10.1016/j.lwt.2019.03.094.
- Wang Z., Sun J., Liao X., Chen F., Zhao G., Wu J., Hu X. Mathematical modeling on hot air drying of thin layer apple pomace. Food Research International. 2007;40(1):39–46. doi: 10.1016/j.foodres.2006.07.017. [DOI] [Google Scholar]
- Zlatanović S., Kalušević A., Micić D., Laličić-Petronijević J., Tomić N., Ostojić S., Gorjanović S. Functionality and storability of cookies fortified at the industrial scale with up to 75% of apple pomace flour produced by dehydration. Foods. 2019;8(11) doi: 10.3390/foods8110561. [DOI] [PMC free article] [PubMed] [Google Scholar]