Graphical abstract
Keywords: Pomelo, Grapefruit, Flavonoid profiles, UHPLC-ESI MS/MS, Antioxidant activity, In vitro enzyme inhibition
Highlight
-
•
Fourteen flavonoid compounds were detected in pomelo and grapefruit pulp.
-
•
The flavonoid profiles in pomelo and grapefruit pulp had varietal difference.
-
•
Flavonoids of pomelo and grapefruit showed strong cellular antioxidant activity.
-
•
Flavonoids of pomelo and grapefruit are good inhibitors of pancreatic lipase.
Abstract
Previous results indicated that the flavonoid profiles might have varietal differences in pomelo, but detailed information is unknown. We previously isolated 4 new flavonoids, cigranoside C, D, E, F, in Citrus grandis Shatianyu pulp. However, their distribution in different pomelo cultivars remains to be explored. Therefore, the flavonoid profiles and in vitro bioactivity of the pulp from 5 pomelo and 1 grapefruit cultivars commonly consumed in China were investigated. Fourteen flavonoids were identified, cigranoside C, D, E were detected in these pomelo and grapefruit. Naringin and cigranoside C were the major flavonoids in grapefruit, Guanximiyu-W, Guanximiyu-R and Liangpingyu, while melitidin and rhoifolin was the predominant flavonoid in Shatianyu and Yuhuanyu, respectively. Pomelo and grapefruit showed strong antioxidant activity, and were potent inhibitors of pancreatic lipase with IC50 values of 11.4–72.6 mg fruit/mL except Shatianyu. Thus, pomelo and grapefruit are natural antioxidants and possess anti-obesity potential.
1. Introduction
Flavonoids are the most common group of polyphenols in fruits and can contribute to reducing the risk of many chronic diseases, such as metabolic syndrome, type 2 diabetes mellitus and cardiovascular diseases, possibly due to their antioxidant activity and digestive enzyme inhibitory activity (Liu, 2003, Sun et al., 2019, Wu et al., 2017, Zhu et al., 2014). Citrus fruits, greatly popular in the word, are abundant in flavonoids, especially flavanones in aglycone or glycoside forms (Khan and Dangles, 2014, Lu et al., 2020). Pomelo (Citrus grandis (L.) Osbeck) is a kind of Citrus fruits and cultivated widely in southern China. C. grandis cvs. Guanximiyu, Shatianyu, Liangpingyu and Yuhuanyu are representative pomelo cultivars in China (Zhang et al., 2011). Grapefruit (C. paradise Mcfad), a hybrid of sweet orange (C. sinensis) and sweet pomelo (C. maxima Burm) mainly distributed in South Africa and the European Union, is also highly appreciated by Chinese consumers.
Earlier studies have preliminarily revealed the flavonoid profiles of the pulp of pomelo and grapefruit. Mäkynen et al. (2013) detected 6 flavonoids, including naringin, naringenin, hesperidin, hesperetin, dihydrochalcone and neohesperidin, in the pulp of pomelo cultivars from Thailand and found that naringin was their main flavonoid. Xi et al. (2014) revealed that naringin was the predominant flavonoid in pomelo, while naringin and neohesperidin were the predominant flavonoids in grapefruits. In our previous study, we isolated 4 new flavonoids (cigranoside C, D, E, F), together with 2 firstly reported flavonoids (neoeriocitrin and bergamjuicin) in pomelo. Besides, the main flavonoid we isolated from Shatianyu pulp was melitidin (Deng et al., 2021). These different results from our lab and others indicated that the flavonoid profiles in pomelo pulp might have varietal difference. Furthermore, the distribution of the 6 newly isolated flavonoids from Shatianyu in different pomelo cultivars remains to be explored. Therefore, it is necessary to further analyze the compositions and contents of flavonoids among different pomelo cultivars.
Antioxidant activity and digestive enzymes (pancreatic lipase, α-amylase and α-glucosidase) inhibitory effects are the important effects of flavonoids, which account for their many health benefits. The bioactivities of flavonoids are closely related to their molecular structure (Liu et al., 2017, Salahuddin et al., 2020, Su et al., 2014). Previous studies showed that the flavonoid extracts of citrus peels (Huang et al., 2020) or the digesta of citrus fruits (Sun, Tao, Huang, Ye and Sun, 2019) exhibited CAA activity and pancreatic lipase inhibitory activity. The differences in antioxidant and enzyme inhibitory activity of flavonoids from different pomelo pulp are still unknown.
In order to clarify the varietal differences of pomelo pulp in flavonoid profiles and bioactivity, 5 representative pomelo cultivars together with a grapefruit cultivar commonly consumed in China were analyzed in the present study to determine their compositions and contents of flavonoids in the pulp; and to compare their differences in antioxidant activity and inhibitory activity to α-amylase, α-glucosidase and pancreatic lipase.
2. Materials and methods
2.1. Materials
Shatianyu was purchased from Meizhou county, Guangdong province, China, in December 2018. Liangpingyu was obtained from Liangping county, Chongqing province, China, in November 2018. Guanximiyu with red (Guangximiyu-R) and white (Guangximiyu-W) pulp were purchased from Pinghe county, Fujian province, China, in October 2018. Yuhuanyu was obtained from Yuhuan county, Zhejiang province, China, in November 2018. Grapefruits were collected from the local supermarket in Guangzhou in October 2018.
2.2. Chemicals and reagents
Cigranoside A, B, C, D, E, F, bergamjuicin, neoeriocitrin, melitidin, rhoifolin, and naringin were prepared in our laboratory (Deng et al., 2021). Hesperidin, neohesperidin, narirutin, isoquercitrin, (+)-catechin, quercetin, gallic acid, Folin–Ciocalteu reagent, DCFH-DA, Trolox, AAPH, fluorescein sodium, 4-methylumbelliferyl oleate (4-MUO), α-glucosidase, α-amylase and pancreatic lipase, and 4-nitrophenyl-α-d-glucopyranoside (pNPG) were obtained from Sigma Aldrich Co. (St. Louis, MO, USA). HBSS, new bovine calf serum and DMEM (H) medium were obtained from Gibco Life Technologies (Grand Island, NY, USA). HepG2 human liver cancer cells were purchased from the ATCC (Rockville, MD, USA). Acetonitrile and glacial acetic acid in HPLC-grade were purchased from Thermo Fisher Scientific (Suwanee, GA, USA).
2.3. Extraction of phenolics
Phenolics were extracted following the method of Zhang et al (2013). The pulp of grapefruit and pomelo obtained by peeling were homogenized using a blender (WBL2521H, Midea Group Co., Ltd., Foshan, Guangdong, China). Subsequently, each pulp sample (100 g) with 80% aqueous acetone (1:2, w/v) were further homogenized at 5000 rpm for 5 min in an ice bath using an STSRH-300 homogenizer (Shanghai Sotin Intelligent Equipment Co., Ltd., Shanghai, China). The homogenates were centrifuged at 4000g for 10 min at 4 °C (TG16, Shanghai Lu Xiangyi Centrifuge Instrument Co. Ltd., China). Then, the residue was repeated the above extraction steps and the pooled supernatants were condensed to dry at 45 °C using a rotary evaporator (N-1300V, Tokyo Rika Machinery Co., Ltd.). Finally, the condensed phenolics were dissolved with 25 mL distilled water and stored at −20 °C for further analysis.
2.4. Measurement of total phenolic contents (TPC)
The Folin-Ciocalteu colorimetric method (Dewanto et al., 2002) was used to determine the TPC and the results were presented as mg gallic acid equivalents (GAE)/100 g fresh weight (FW) of the pulp sample.
2.5. Measurement of total flavonoid contents (TFC)
The TFC was measured according to the sodium borohydride/chloranil-based (SBC) assay (He et al., 2008) and the results were presented as mg catechin equivalents (CE)/100 g FW of the pulp sample.
2.6. Analysis of flavonoid compositions
The separation of phenolic extracts of different pomelo and grapefruit cultivars was conducted on a Thermo Scientific Dionex UltiMate 3000 UHPLC (Tempe, Arizona, USA) with a Waters HSS C18 column (1.8 μm, 2.1 × 100 mm, MA, USA). Acetonitrile (solvent A) and 0.4% aqueous acetic acid (v/v, solvent B) were used as the mobile phase. The elution of flavonoid compounds using the following conditions: 0–10 min, 5–8% A; 10–20 min, 8–12% A, 20–22 min, 12–14% A, 22–52 min, 14% A. Other analysis conditions were as follows: injection volume, 2 μL; flow rate, 1 mL/min; column temperature, 30 °C; detection wavelength, 280 nm.
ESI-MS analysis was performed on a Thermo Scientific TSQ Endura Triple Quad LC/MS/MS (Suwanee, GA, USA) equipped with an ion trap mass spectrometer and a diode array detector. The negative mode was chosen to conduct electrospray ionization using the following conditions: spray needle voltage, 4000 V; capillary temperature, 350 °C; dry gas, 10 L/min; collision energy, 10–30 V; mass spectra, m/z 100–1000.
2.7. Analysis of antioxidant activity
The oxygen radical absorbance capacity (ORAC) was measured according to the method of Huang et al. (2002) and the results were shown as μmol Trolox equivalents (TE)/100 g FW of the pulp sample. The cellular antioxidant activity (CAA) was determined using the method reported by Wolfe and Liu (2007) and the results were shown as μmol quercetin equivalents (QE)/100 g FW of the pulp sample.
2.8. In vitro enzymes inhibition assays
2.8.1. α-Amylase inhibition
The α-amylase inhibitory activity was measured according to the method reported by Salahuddin et al. (2020). A 96-well microplate was seeded with diluted samples (40 μL), α-amylase (0.25 U/mL, 40 μL) and PBS (20 μL), respectively, and incubated at 37 °C for 3 min. Then, soluble starch solution (1 mg/mL, 20 μL) was added, and the microplate was incubated at 37 °C for another 4 min. After the enzyme reaction was stopped by adding HCL (1 mol/L, 20 μL), 60 μL of iodine reagent containing 5 mmol/L potassium iodine and 5 mmol/L iodine was added to the microplate. Then, the absorbance was taken at 650 nm. The α-amylase inhibition (%) was determined as follows: [1-(A2-A1)/(A4-A3)]*100%, where A1 is the absorbance of the samples in the above measurement; A2 is the absorbance of the measurement in which the enzyme was replaced by PBS; A3 is the absorbance of the measurement in which the samples were replaced by PBS; A4 is the absorbance of the measurement in which both the samples and enzyme were replaced by PBS.
2.8.2. α-Glucosidase inhibition
The α-glucosidase inhibitory activity was determined following the method of Lin et al. (2015). A 96-well microplate was seeded with 20 µl of samples, 40 μL of PBS and 10 μL of α-glucosidase (0.2 U/mL), respectively, and incubated at 37 °C for 15 min. Then, 20 μL of 5 mmol/L pNPG was added, and the microplate was incubated at 37 °C for another 6 min. After the enzyme reaction was stopped by adding 100 μL of 200 mmol/L Na2CO3, the absorbance was taken at 405 nm. The α-glucosidase inhibition (%) was determined as follows: [A1-(A2-A3)]/A1*100%, where A2 is the absorbance of the samples in the above measurement;A1 is the absorbance of the measurement in which the samples were replaced by PBS;A3 is the absorbance of the measurement in which the enzyme and pNPG were replaced by PBS.
2.8.3. Pancreatic lipase inhibition
The pancreatic lipase inhibitory activity was measured according to the method of Zhu et al. (2014). A buffer containing 13 mmol/L Tris, 1.3 mmol/L CaCl2 and 150 mmol/L NaCl was used to prepare 4-MUO and lipase. Briefly, 25 μL of samples and 50 μL of 4-MUO (0.1 mmol/L) were added to a 96-well microplate, respectively. Then, the lipase (50 U/mL, 25 μL) was added to initiate the enzyme reaction. After the 96-well microplate was incubated at 25 °C for 30 min, the enzyme reaction was stopped by adding sodium citrate (0.1 mol/L, pH 4.2, 100 μL). Finally, the fluorescence intensity was measured (excitation, 355 nm; emission, 460 nm). The pancreatic lipase inhibition (%) was calculated as follows: [1-(A2-A1)/(A4-A3)]*100%, where A2 is the fluorescence of the samples in the above measurement; A1 is the fluorescence of the measurement in which the enzyme was replaced by buffer; A3 is the absorbance of the measurement in which both the samples and enzyme were replaced by buffer; A4 is the absorbance of the measurement in which the samples were replaced by buffer.
2.9. Statistical analysis
All measurements were conducted in triplicates and the results were presented as the mean ± SD. Statistical analysis were conducted by one-way ANOVA of SPSS 19.0 software, and p < 0.05 indicated statistical significance. Pearson correlation was used to analyze the correlation between variables.
3. Results and discussion
3.1. Total phenolic contents
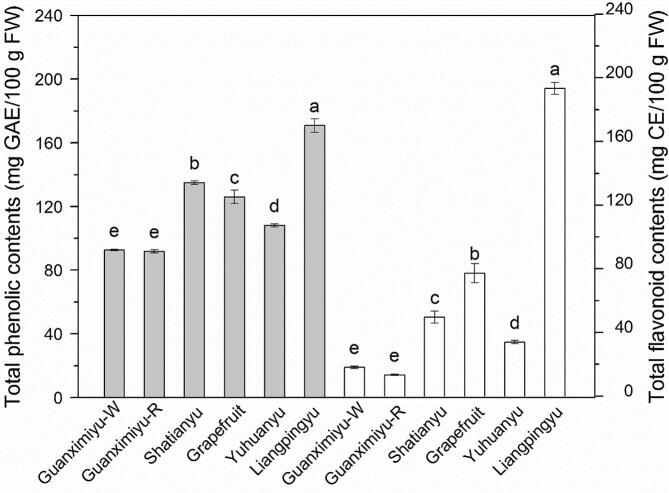
The TPC of the grapefruit and pomelo cultivars are presented in Fig. 1. The TPC ranged from 91.8 (Guanximiyu-R) to 170.9 (Liangpingyu) mg GAE/100 g FW with the coefficient of variation (CV) of 24.9% in the determined pomelo. Liangpingyu had the highest TPC, followed by Shatianyu, grapefruit and Yuhuanyu (p < 0.05). Guanximiyu-W and Guanximiyu-R had the lowest TPC (p > 0.05). The pomelo and grapefruit cultivars in the present study showed higher TPC than pomelo cultivars in Thailand (51.9–94.9 mg GAE/100 g FW) (Mäkynen et al., 2013). This is partly due to different methods used for phenolics extraction, together with the different cultivars tested. The TPC of Liangpingyu was higher than those of cherry (Wolfe et al., 2008) and the majority of litchi cultivars (Zhang et al., 2013) (101.5–170.5 mg GAE/100 g FW). Furthermore, the determined pomelo and grapefruit cultivars showed higher TPC than pineapple, banana, peach, lemon, pear and orange (70.6–94.3 mg GAE/100 g FW) (Wolfe et al., 2008). Therefore, pomelo, especially cultivars like Liangpingyu and Shatianyu, are excellent dietary sources of phenolics.
Fig. 1.
TPC and TFC of different pomelo and grapefruit cultivars. Bars with no letters in common are significantly different (p < 0.05).
3.2. Total flavonoid contents
The TFC of the grapefruit and pomelo cultivars are presented in Fig. 1. The TFC ranged from 13.4 (Guanximiyu-R) to 193.3 (Liangpingyu) mg CE/100 g FW with the CV of 90.1%, indicating significant genotype differences in TFC among pomelo and grapefruit cultivars. Liangpingyu had the highest TFC, followed by grapefruit, Shatianyu and Yuhuanyu (p < 0.05). Guanximiyu-W and Guanximiyu-R had the lowest TFC (p > 0.05). In previous studies, the TFC of the pulp from different litchi, apple and strawberry varied from 39.4 to 129.8 (averaging 67 mg CE/100 g FW), 35.7–46.8, and 46.2–70.5 mg CE/100 g FW, respectively (Meyers et al., 2003, Wolfe et al., 2003, Zhang et al., 2013). The average content of the TFC in grapefruit and pomelo was 64.3 mg CE/100 g FW, which was higher than most of the fruits mentioned above.
3.3. Flavonoid compositions and contents
The compositions and contents of flavonoids in grapefruit and pomelo varieties were analyzed by UHPLC-ESI MS/MS. Fourteen flavonoids were detected in the determined grapefruit and pomelo. The MS, MS-MS data and retention time of the 14 flavonoid standards are presented in Table 1. The flavonoid compositions and contents are presented in Table 2. All the 14 flavonoids were detected in grapefruit, but narirutin and neohesperidin were not detected or below the detection limit in the 5 pomelo cultivars. In Guanximiyu-W and Guanximiyu-R, naringin was the most abundant flavonoid, followed by rhoifolin and cigranoside C, and the 3 compounds accounted for 91% and 86% of the detected flavonoids in Guanximiyu-W and Guanximiy-R, respectively. Melitidin was the largest amount of flavonoid in Shatianyu, followed by bergamjuicin, naringin, cigranoside B and cigranoside A, and the 5 compounds possessed 97% of the detected flavonoids in Shatainyu. Nevertheless, Rhoifolin was the most abundant flavonoid in Yuhuanyu, followed by naringin, and the 2 compounds took up 84% of the detected flavonoids in Yuhuanyu. Naringin was the most abundant flavonoid in Liangpingyu, followed by cigranoside C, melitidin, neoeriocitrin and rhoifolin, and the 5 compounds accounted for 98% of the detected flavonoids in Liangpingyu. Naringin was the largest amount of flavonoid in grapefruit, followed by narirutin, cigranoside C, neohesperidin, hesperidin and melitidin, and the 6 compounds possessed 98% of the detected flavonoids in grapefruit.
Table 1.
The retention time, MS and MS-MS data of flavonoid standards observed by UHPLC-ESI-MS/MS analysis.
| Compound | Retention time (min) | [M-H]− (m/z) | MS2 ions (m/z) |
|---|---|---|---|
| Cigranoside C | 10.97 | 595 | 567, 259, 577 |
| Isoquercitrin | 19.71 | 463 | 300, 271 |
| Neoeriocitrin | 21.53 | 595 | 459, 151, 576 |
| Narirutin | 24.58 | 579 | 271, 151, 295, 313 |
| Naringin | 26.47 | 579 | 459, 271, 235 |
| Cigranoside E | 27.65 | 883 | 619, 577, 659 |
| Bergamjuicin | 27.96 | 885 | 579, 621 |
| Rhoifolin | 28.13 | 577 | 269, 413 |
| Hesperidin | 28.53 | 609 | 301, 325, 242 |
| Neohesperidin | 30.48 | 609 | 301, 286, 343, 242 |
| Cigranoside A | 32.38 | 723 | 677, 659, 580, 621 |
| Cigranoside B | 34.87 | 723 | 677, 659, 580, 451 |
| Melitidin | 36.76 | 723 | 677, 579, 621, 661 |
| Cigranoside D | 37.28 | 721 | 268, 577, 619, 659 |
Table 2.
Flavonoid compositions of pomelo and grapefruit cultivars (μg/100 g FW). Values with no letters in common in each row are significantly different (p < 0.05). Tr: trace; nd: not detected.
| Compounds | Guanximiyu-W | Guanximiyu-R | Shatianyu | Yuhuanyu | Liangpingyu | Grapefruit |
|---|---|---|---|---|---|---|
| Cigranoside A | 55.1 ± 1.7 cd | 49.7 ± 4.2 cd | 1145 ± 85 a | 17.2 ± 0.9 d | 172 ± 2 b | 99.9 ± 3.1 c |
| Cigranoside B | 93.1 ± 2.4 d | 84.8 ± 3.3 d | 1773 ± 40 a | 19.4 ± 1 e | 321 ± 12 b | 168 ± 5 c |
| Cigranoside C | 800 ± 36 c | 544 ± 21 d | 188 ± 11 f | 404 ± 7 e | 3375 ± 113 a | 3165 ± 132 b |
| Cigranoside D | 83.8 ± 1.6 c | 75.7 ± 3.2 cd | 380 ± 12 a | 101 ± 6 b | 69.3 ± 3.9 d | 6.25 ± 0.11 e |
| Cigranoside E | 106 ± 2 b | 98.8 ± 4.7 c | 203 ± 5 a | 81.9 ± 1.9 d | 22.0 ± 0.8 e | 10.6 ± 0.9 f |
| Bergamjuicin | 191 ± 3 bc | 227 ± 6 b | 5148 ± 91 a | 10.2 ± 0.6 e | 69.8 ± 3.8 d | 142 ± 2 c |
| Neoeriocitrin | 185 ± 3 d | 82.9 ± 1.6 f | 104 ± 6 e | 481 ± 9 b | 1473 ± 15 a | 380 ± 10 c |
| Melitidin | 478 ± 19 c | 330 ± 17c | 23338 ± 874 a | 194 ± 7 c | 1745 ± 100 b | 1320 ± 17 b |
| Rhoifolin | 4023 ± 46 b | 1768 ± 37 c | 164 ± 6 f | 4135 ± 91 a | 1025 ± 37 d | 569 ± 29 e |
| Naringin | 6875 ± 72 c | 3613 ± 111 d | 2448 ± 100 d | 3245 ± 129 d | 30100 ± 885 b | 40430 ± 770 a |
| Hesperidin | 1.7 ± 0.2 b | 1.1 ± 0.1 b | 1.35 ± 0.05 b | 0.38 ± 0.01 b | 2.8 ± 0.2 b | 1670 ± 43 a |
| Neohesperidin | Tr | Tr | Tr | Tr | Tr | 2356 ± 107 |
| Narirutin | nd | nd | nd | nd | nd | 21973 ± 664 |
| Isoquercitrin | 15.9 ± 0.4 d | 12.1 ± 1 de | 9.88 ± 0.83 e | 109 ± 7 a | 23.6 ± 1.2 c | 35.5 ± 2.1 b |
| Sum | 12907 ± 82 d | 6887 ± 191 f | 34902 ± 1175 c | 8798 ± 105 e | 38398 ± 1672 b | 72325 ± 1554 a |
As firstly separated compounds from Shatianyu with new structures in our previous work (Deng et al., 2021), cigranoside C, D and E had never been reported before in pomelo and grapefruit. Their contents ranged from 188 (Shatianyu) to 3375 (Liangpingyu), 6.25 (grapefruit) to 380 (Shatianyu), 10.6 (grapefruit) to 203 (Shatianyu) μg/100 g FW, respectively. The contents of cigranoside C ranked second or third place in Liangpingyu, Guanximiyu-W, Guanximiyu-R and grapefruit among all the detected flavonoids. Although cigranoside F was also a new flavonoid isolated from Shatianyu, it was not detected in the pomelo and grapefruit cultivars. These might be attributed to the fact that cigranoside F was below the detection limit in Shatainyu or did not exist in other pomelo cultivars. Cigranoside A, cigranoside B, bergamjuicin and neoeriocitrin were previously found in traditional Chinese medicine, such as the pericarp of C. grandis huajuhong (cigranoside A and B) (Ma et al., 2018), the juice of C. bergamia bergamot (bergamjuicin) (Formisano et al., 2019) and the rhizomes of Drynaria fortunei (Kunze et Mett.) J. Sm (neoeriocitrin) (Yang et al., 2015). However, the 4 flavonoids had not been detected in pomelo pulp before, and their contents ranged from 17.2 (Yuhuanyu) to 1145 (Shatainyu), 19.4 (Yuhuanyu) to 1773 (Shatainyu), 10.2 (Yuhaunyu) to 5148 (Shatianyu) and 82.9 (Guanximiyu-R) to 1473 (Liangpingyu) μg/100 g FW, respectively. Melitidin and rhoifolin were previously detected in the juice of pomelo and grapefruit, but their contents were calculated based on naringin equivalent (Zhang et al., 2011). In the present study, melitidin and rhoifolin, with the contents ranging from 194 (Yuhuanyu) to 23,338 (Shatainyu) and 164 (Shatainyu) to 4135 (Yuhuanyu) μg/100 g FW, respectively, were determined by absolute quantification. Naringin was the predominant flavonoid in citrus fruits including sour orange, grapefruit and pomelo (Gattuso et al., 2007). Its contents ranged from 2448 (Shatianyu) to 40,430 (grapefruit) μg/100 g FW among the tested pomelo and grapefruit. This was comparable to the pomelo cultivars in Thailand (25–39 mg/100 g FW) (Chaiwong & Theppakorn, 2010). Hesperidin, neohesperidin and narirutin were mainly reported in grapefruit, sweet oranges, lemons, and limes (Gattuso et al., 2007, Khan and Dangles, 2014, Tocmo et al., 2020). These 3 flavonoids were mainly detected in grapefruit in the present study, with the contents of 1670, 2356 and 21,973 μg/100 g FW, respectively. These was comparable to the levels in grapefruit reported previously (Igual et al., 2013).
TFC of the tested pomelo and grapefruit determined by calculating the monomer flavonoid contents was quite different from those measured by an SBC assay. Using the former method, grapefruit showed the highest TFC among these pomelo and grapefruit, almost twice that of Shatianyu and Liangpingyu. However, using the SBC assay, Liangpingyu showed the highest TFC, which was 3.8 and 2.5 times that of Shatianyu and grapefruit, respectively. The reason for the inconsistence between the two results might be that there were other undetected flavonoids among pomelo and grapefruit cultivars besides the 14 flavonoids detected in the present study, which could be inferred from the liquid chromatograms of these pomelo and grapefruit cultivars (supplementary Fig. S1).
3.4. Antioxidant activity
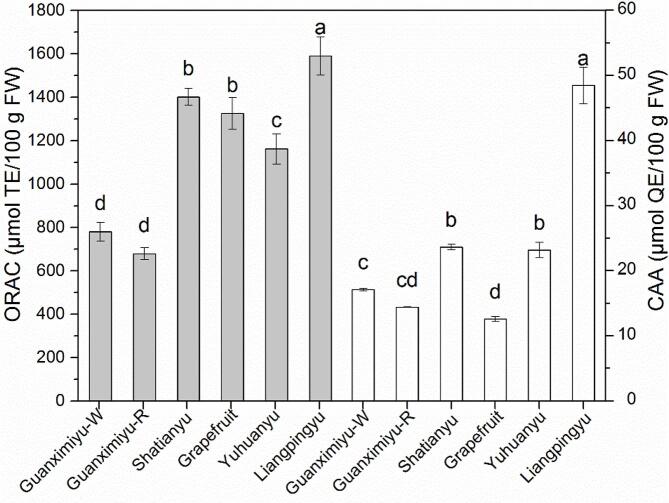
The antioxidant activity of the pomelo and grapefruit cultivars evaluated by ORAC and CAA are presented in Fig. 2. The ORAC values of these pomelo and grapefruit ranged from 678.9 to 1590.2 μmol TE/100 g FW with a CV of 31.1%, indicating significant genotype differences in the ORAC activity among pomelo and grapefruit cultivars. Liangpingyu showed the highest ORAC activity (p < 0.05), followed by Shatianyu and grapefruit. The ORAC values of the latter two were higher than that of Yuhuanyu (p < 0.05). Guanximiyu-W and Guanximiyu-R presented the lowest ORAC activity (p > 0.05) among the determined cultivars. Highly significant correlations were observed between the ORAC activity and phenolic contents of the tested pomelo and grapefruits, and the correlative coefficient r was as high as 0.93 (p < 0.01). Although the ORAC activities of pomelo and grapefruit are lower than that of some citrus fruits, such as orange and lemon (2887 and 1848 μmol TE/100 g FW, respectively) (Wolfe et al., 2008), they are comparable to those of pear, nectarine, watermelon, avocado, kiwifruit, mango, pineapple and banana (565–1759 μmol TE/100 g FW) (Wolfe et al., 2008).
Fig. 2.
ORAC and CAA values of different pomelo and grapefruit cultivars. Bars with no letters in common are significantly different (p < 0.05).
The CAA values of these pomelo and grapefruits ranged from 12.6 to 48.4 μmol QE/100 g FW with a CV of 56.7%, indicating that the CAA activity showed higher genotype differences than the ORAC activity among pomelo and grapefruit. Liangpingyu presented the highest CAA activity followed by Shatianyu and Yuhuanyu (p < 0.05). In comparison, the CAA value of the former was approximate twice that of the latter two. The CAA activity of grapefruit was lower than those of other determined cultivars except for Guanximiyu-R, which also showed similar activity to Guanximiyu-W. Correlation analysis showed that the Pearson coefficient r between the ORAC and CAA values of the tested pomelos and grapefruit was 0.66 (p = 0.15), indicating that the antioxidant activities reflected by these 2 methods were not completely consistent. Compared with ORAC, a chemical antioxidant determination method, CAA assay was conducted in a more physiological reaction system involving cells, which might give more indicative information for the in vivo activity of the tested samples. In addition to phenolic contents, the CAA activity of the samples is also influenced by their phenolic compositions since the uptake and metabolism of the antioxidants depended on their structures (Wolfe and Liu 2007). Our previous study found that naringin contributed the least to the CAA activity of phenolic extracts of Shatianyu pulp among the 11 isolated flavonoids (Deng et al., 2021). Coincidentally, the percentage of naringin in the TFC of grapefruit (52.3%) was higher than all the tested pomelo. These might explain why grapefruit showed the lowest CAA value despite its relatively high phenolic content. Liangpingyu showed higher CAA activity than cranberry, plum and cherry (27.4–47.9 μmol QE/100 g FW) (Wolfe et al., 2008), which were considered as good sources of natural antioxidants. Although Shatianyu and Yuhuanyu had much lower CAA values than Liangpingyu, they exhibited higher CAA activity than apple, red grape, kiwifruit, mango, pineapple and orange (13.7–21.9 μmol QE/100 g FW) (Wolfe et al., 2008). Moreover, the CAA activity of all tested pomelo and grapefruit was higher than lemon, peach, pear, cantaloupe and banana (3.2–12.3 μmol QE/100 g FW) (Wolfe et al., 2008). Therefore, pomelo, especially Liangpingyu and Shatianyu, are excellent sources of natural antioxidants.
3.5. Inhibitory activity to α-amylase, α-glucosidase
The inhibitory activity to α-amylase and α-glucosidase of different grapefruit and pomelo cultivars are presented in Table 3. The IC50 values of α-amylase and α-glucosidase inhibitory activity varied from 707.1 to 1788 and 1053 to 2514 mg fruit/mL, respectively. Shatianyu, grapefruit and Liangpingyu with proximate IC50 values showed higher α-amylase inhibitory activity than Guanximiyu-W, Guangximiyu-R and Yuhuanyu (p < 0.05). However, Yuhuanyu and Liangpingyu showed the highest inhibitory activity to α-glucosidase, followed by grapefruit, Guanximiyu-W and Shatianyu (p < 0.05), and Guanximiyu-R presented the lowest inhibitory activity to α-glucosidase (p < 0.05). IC50 values of commonly consumed fruits, such as sour cherry, strawberry, apple, banana, bilberry, peach and pomegranate, ranged from 18.18 to more than 200 mg fruit/mL against α-amylase and from 156.4 to 399.1 mg fruit/mL against α-glucosidase (Podsedek et al., 2014). Evidently, the tested pomelo and grapefruit presented much weaker inhibition to the saccharides hydrolyzing enzymes. Generally, flavonols and flavones showed stronger inhibition to these enzymes than flavanones and flavanols (Spínola et al., 2020, Sun et al., 2019), in which the hydroxylated 2,3-double bond transformed the near-planar molecular structure to a more non-planar and flexible stereochemical structure, forming steric hindrance to reduce the ability of flavonoids to bind with the enzymes (Sun, Warren and Gidley, 2019). The main flavonoids in pomelo and grapefruit analyzed in the present study were flavanones, while flavonols and flavonons were important components of many other fruits apart from flavanones (Balasuriya and Rupasinghe, 2012, Pérez-Navarro et al., 2021).
Table 3.
The inhibitory activities of different pomelo and grapefruit cultivars to α-amylase, α-glucosidase and pancreatic lipase. Values with no letters in common in each column are significantly different (p < 0.05).
| Cultivars | Enzyme inhibition IC50 (mg of fresh fruit/mL) |
||
|---|---|---|---|
| α-amylase inhibition | α-glucosidase inhibition | pancreatic lipase inhibition | |
| Guanximiyu-W | 1558 ± 33b | 1274 ± 18b | 23.7 ± 2.1 a |
| Guanximiyu-R | 1788 ± 208b | 2514 ± 72 d | 58.1 ± 1.4b |
| Shatianyu | 707.1 ± 11.2 a | 1988 ± 10c | 240 ± 15c |
| Yuhuanyu | 1693 ± 99.3b | 1058 ± 62 a | 72.6 ± 6.3b |
| Liangpingyu | 798.3 ± 39.6 a | 1053 ± 73 a | 12.8 ± 0.3 a |
| Grapefruit | 877.8 ± 49.9 a | 1255 ± 37b | 11.4 ± 1.1 a |
3.6. Inhibitory activity to pancreatic lipase
The pancreatic lipase inhibitory activity of different pomelo and grapefruit cultivars are presented in Table 3. The IC50 values of pancreatic lipase inhibitory activity varied from 11.4 to 240 mg fruit/mL. Grapefruit, Liangpingyu and Guanximiyu-W presented the highest pancreatic lipase inhibitory activity, followed by Guanximiyu-R and Yuhuanyu (p < 0.05). Shatianyu showed the lowest inhibitory activity to pancreatic lipase, and its IC50 value was 10 to 21 times that of the former 3 cultivars (p < 0.05). In the tested grapefruit and pomelo, strong correlations were observed between the IC50 values of pancreatic lipase inhibitory activity and the content of cigranoside A (r = 0.92, p < 0.01), cigranoside B (r = 0.91, p < 0.05), cigranoside D (r = 0.97, p < 0.01), cigranoside E (r = 0.89, p < 0.05), bergamjuicin (r = 0.95, p < 0.01) and melitidin (r = 0.94, p < 0.01), respectively. These above-mentioned flavonoids all had a 3-hydroxy-3-methylglutaryl (HMG) substitution at 7-O-neohesperidoside of the A ring, indicating that the presence of HMG moiety in the structure of flavonoids might weaken their inhibitory activity to pancreatic lipase. The lowest inhibitory activity to pancreatic lipase of Shatianyu might be attributed to its highest content of HMG substituting flavonoids, which was 13 to 75 times higher than those of other pomelo and grapefruit cultivars. Huang et al. (2020) revealed that hesperidin could interact with pancreatic lipase through hydrogen bonds and van der Waals forces to change the secondary structure of pancreatic lipase, making itself the key pancreatic lipase inhibitor in citrus peel extracts. The highest content of hesperidin in grapefruit might explain its strongest pancreatic lipase inhibitory activity among the tested pomelo and grapefruit cultivars. Grapefruit, Liangpingyu and Guanximiyu-W showed comparable pancreatic lipase inhibitory activity with blackberry, strawberry, cherry, plum and apple (5.7–14 mg fruit/mL) and stronger inhibition of pancreatic lipase than pear, peach, banana and mandarine (30–135 mg fruit/mL) (Podsedek et al., 2014). Therefore, grapefruit and some pomelo cultivars were effective inhibitors of pancreatic lipase and had anti-obesity potential.
4. Conclusion
Significant varietal differences were observed in flavonoid profiles and in vitro bioactivity among different pomelo and grapefruit cultivars. Liangpingyu, Shatianyu and grapefruit had higher phenolic and flavonoid contents than other 3 pomelo cultivars. Fourteen flavonoid compounds were identified in pomelo and grapefruits. Naringin was the major flavonoid in grapefruit, Guanximiyu-W, Guanximiyu-R, and Liangpingyu, while melitidin and rhoifolin was the predominant flavonoid in Shatianyu and Yhuanyu, respectively. Cigranoside C, D and E were firstly quantified in pomelo and grapefruit since they were isolated from Shatianyu as new compounds. Furthermore, cigranoside C was one of the main flavonoid compounds in Liangpingyu, Guanximiyu-W, Guanximiyu-R and grapefruit, ranking second or third place among all the detected flavonoids. Cigranoside A, cigranoside B, bergamjuicin and neoeriocitrin were also firstly quantified in pomelo and grapefruit pulp. The total contents of the 7 flavonoids ranged from 1114.7 (Yuhuanyu) to 8941 (Shatainyu) μg/100 g FW, accounting for 5.5% to 25.6% of the total contents of 14 detected flavonoids. Pomelo and grapefruit possessed strong antioxidant activity, especially CAA activity. Despite their weak α-amylase and α-glucosidase inhibitory activity, the determined pomelo and grapefruit cultivars presented strong inhibition of pancreatic lipase, especially grapefruit, Liangpingyu and Guanximiyu-W, which showed lower IC50 values than many commonly consumed fruits. Therefore, pomelo and grapefruit are good daily sources of flavonoids and possess anti-obesity potential.
CRediT authorship contribution statement
Mei Deng: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Visualization. Lihong Dong: Methodology, Investigation, Data curation, Formal analysis. Xuchao Jia: Resources, Validation. Fei Huang: Methodology, Visualization. Jianwei Chi: Methodology, Formal analysis. Zafarullah Muhammad: Writing – review & editing. Qin Ma: Formal analysis. Dong Zhao: Formal analysis. Mingwei Zhang: Supervision, Project administration, Funding acquisition, Writing – review & editing. Ruifen Zhang: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Guangdong Special Support Program (2019BT02N112), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2021KJ117), the Special fund for Scientific Innovation Strategy-Construction of High-level Academy of Agriculture Science (R2020PY-JG011, 202108TD), and Guangzhou Science and Technology Planning Project (201903010051, 202103000055).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100368.
Contributor Information
Mingwei Zhang, Email: mwzhh@vip.tom.com.
Ruifen Zhang, Email: ruifenzhang@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Balasuriya N., Rupasinghe H.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chemistry. 2012;135(4):2320–2325. doi: 10.1016/j.foodchem.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Chaiwong S., Theppakorn T. Bioactive compounds and antioxidant capacity of pink pummelo (Citrus Grandis (L.) Osbeck) cv. “Thong Dee” in Thailand. Journal of the International Society for Southeast Asian Agricultural Sciences. 2010;16(2):10–16. [Google Scholar]
- Deng M., Jia X., Dong L., Liu L., Huang F., Chi J.…Zhang R. Structural elucidation of flavonoids from Shatianyu (Citrus grandis L. Osbeck) pulp and screening of key antioxidant components. Food Chemistry. 2021;366:130605. doi: 10.1016/j.foodchem.2021.130605. [DOI] [PubMed] [Google Scholar]
- Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry. 2002;50(10):3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Formisano C., Rigano D., Lopatriello A., Sirignano C., Ramaschi G., Arnoldi L.…Taglialatela-Scafati O. Detailed phytochemical characterization of bergamot polyphenolic fraction (BPF) by UPLC-DAD-MS and LC-NMR. Journal of Agricultural and Food Chemistry. 2019;67(11):3159–3167. doi: 10.1021/acs.jafc.8b06591. [DOI] [PubMed] [Google Scholar]
- Gattuso G., Barreca D., Gargiulli C., Leuzzi U., Caristi C. Flavonoid composition of Citrus juices. Molecules. 2007;12(8):1641–1673. doi: 10.3390/12081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Zhang Y., Shen S., Zhi Z., Cheng H., Chen S., Ye X. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chemistry. 2020;326:126785. doi: 10.1016/j.foodchem.2020.126785. [DOI] [PubMed] [Google Scholar]
- He X., Liu D., Liu R.H. Sodium borohydride/chloranil-based assay for quantifying total flavonoids. Journal of Agricultural and Food Chemistry. 2008;56(20):9337–9344. doi: 10.1021/jf070954+. [DOI] [PubMed] [Google Scholar]
- Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Prior R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. Journal of Agricultural and Food Chemistry. 2002;50(16):4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- Igual M., Garcia-Martinez E., Camacho M.M., Martínez-Navarrete N. Jam processing and storage effects on β-carotene and flavonoids content in grapefruit. Journal of Functional Foods. 2013;5(2):736–744. [Google Scholar]
- Khan M.K., Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. Journal of Food Composition and Analysis. 2014;33(1):85–104. [Google Scholar]
- Lu Y., Li D., Li L., Belwal T., Xu Y., Lin X.…Luo Z. Effects of elevated CO2 on pigment metabolism of postharvest mandarin fruit for degreening. Food Chemistry. 2020;318:126462. doi: 10.1016/j.foodchem.2020.126462. [DOI] [PubMed] [Google Scholar]
- Liu X., Luo F., Li P., She Y., Gao W. Investigation of the interaction for three Citrus flavonoids and α-amylase by surface plasmon resonance. Food Research International. 2017;97:1–6. doi: 10.1016/j.foodres.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Lin Y.S., Chen C.R., Wu W.H., Wen C.L., Chang C.I., Hou W.C. Anti-α-glucosidase and anti-dipeptidyl peptidase-IV activities of extracts and purified compounds from Vitis thunbergii var. taiwaniana. Journal of Agricultural and Food Chemistry. 2015;63(28):6393–6401. doi: 10.1021/acs.jafc.5b02069. [DOI] [PubMed] [Google Scholar]
- Liu R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. The American Journal of Clinical Nutrition. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Ma S.G., Wang R.B., Li W.R., Liu Y.B., Qu J., Li Y.…Yu S.S. New C-2 diastereomers of flavanone glycosides conjugated with 3-hydroxy-3-methylglutaric acid from the pericarp of Citrus grandis (L.) Osbeck. Bioorganic Chemistry. 2018;80:519–524. doi: 10.1016/j.bioorg.2018.06.024. [DOI] [PubMed] [Google Scholar]
- Mäkynen K., Jitsaardkul S., Tachasamran P., Sakai N., Puranachoti S., Nirojsinlapachai N.…Adisakwattana S. Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chemistry. 2013;139(1–4):735–743. doi: 10.1016/j.foodchem.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Meyers K.J., Watkins C.B., Pritts M.P., Liu R.H. Antioxidant and antiproliferative activities of strawberries. Journal of Agricultural and Food Chemistry. 2003;51(23):6887–6892. doi: 10.1021/jf034506n. [DOI] [PubMed] [Google Scholar]
- Pérez-Navarro J., Izquierdo-Caas P.M., Mena-Morales A., Martínez-Gascuea J., Gómez-Alonso J. Genotypic variation in phenolic composition of novel white grape genotypes (Vitis vinifera L.) Journal of Food Composition and Analysis. 2021;102:103987. [Google Scholar]
- Podsedek A., Majewska I., Redzynia M., Sosnowska D., Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. Journal of Agricultural and Food Chemistry. 2014;62(20):4610–4617. doi: 10.1021/jf5008264. [DOI] [PubMed] [Google Scholar]
- Spínola V., Llorent-Martínez E.J., Castilho P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein glycation. LWT - Food Science and Technology. 2020;118:108727. [Google Scholar]
- Salahuddin M.A.H., Ismail A., Kassim N.K., Hamid M., Ali M.S.M. Phenolic profiling and evaluation of in vitro antioxidant, α-glucosidase and α-amylase inhibitory activities of Lepisanthes fruticosa (Roxb) Leenh fruit extracts. Food Chemistry. 2020;331:127240. doi: 10.1016/j.foodchem.2020.127240. [DOI] [PubMed] [Google Scholar]
- Sun L., Warren F.J., Gidley M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends in Food Science & Technology. 2019;91:262–273. [Google Scholar]
- Sun Y., Tao W., Huang H., Ye X., Sun P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food chemistry. 2019;279:321–327. doi: 10.1016/j.foodchem.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Su, D., Ti, H., Zhang, R., Zhang, M., Wei, Z., Deng, Y., & Guo, J. (2014). Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chemistry, 158, 385–391. [DOI] [PubMed]
- Tocmo R., Pena-Fronteras J., Calumba K.F., Mendoza M., Johnson J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Comprehensive Reviews in Food Science and Food Safety. 2020;19(4):1969–2012. doi: 10.1111/1541-4337.12561. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou Q., Chen X.Y., Li X., Wang Y., Zhang J.L. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: Evaluation of anti-oxidation and α-glucosidase inhibition effect. Food Research International. 2017;100:312–324. doi: 10.1016/j.foodres.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Wolfe K.L., Kang X., He X., Dong M., Zhang Q., Liu R.H. Cellular antioxidant activity of common fruits. Journal of Agricultural and Food Chemistry. 2008;56(18):8418–8426. doi: 10.1021/jf801381y. [DOI] [PubMed] [Google Scholar]
- Wolfe K.L., Liu R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural and Food Chemistry. 2007;55(22):8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- Wolfe K., Wu X., Liu R.H. Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry. 2003;51(3):609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Xi W.P., Fang B., Zhao Q.Y., Jiao B.N., Zhou Z.Q. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chemistry. 2014;161(11):230–238. doi: 10.1016/j.foodchem.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Yang Z.Y., Kuboyama T., Kazuma K., Konno K., Tohda C. Active constituents from Drynaria fortunei Rhizomes on the attenuation of Aβ25–35-induced axonal atrophy. Journal of Natural Products. 2015;78(9):2297–2300. doi: 10.1021/acs.jnatprod.5b00290. [DOI] [PubMed] [Google Scholar]
- Zhu Y.T., Jia Y.W., Liu Y.M., Liang J., Ding L.S., Liao X. Lipase ligands in Nelumbo nucifera leaves and study of their binding mechanism. Journal of Agricultural and Food Chemistry. 2014;62(44):10679–10686. doi: 10.1021/jf503687e. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zeng Q., Deng Y., Zhang M., Wei Z., Zhang Y., Tang X. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chemistry. 2013;136(3–4):1169–1176. doi: 10.1016/j.foodchem.2012.09.085. [DOI] [PubMed] [Google Scholar]
- Zhang M., Duan C., Zang Y., Huang Z., Liu G. The flavonoid composition of flavedo and juice from the pummelo cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus paradisi) from China. Food Chemistry. 2011;129(4):1530–1536. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.