Highlights
-
•
The aroma types of fermented milk produced by L. bulgaricus were divided into milky-type, cheesy-type, fermented-type and miscellaneous-type.
-
•
The flavor fingerprints of different aroma types were established by GC-IMS.
-
•
Acetaldehyde, 2,3-butanedione, acetic acid, butanoic acid, hexanoic acid and δ-decalactone of different aroma types were determined by Flavoromics.
Abbreviations: L. bulgaricus, Lactobacillus delbrueckii subsp. bulgaricus; HS-GC-IMS, headspace-gas chromatography-ion mobility spectrometry; GC–MS, gas chromatography-mass spectrometry; GC-O-MS, gas chromatography-olfactometry-mass spectrometry; OSME, odor-specific magnitude estimation; OAV, the odor activity value; PCA, principal component analysis; QDA, quantitative descriptive analysis; RI, retention index; AR, aroma recombinant
Keywords: Lactobacillus delbrueckii subsp. bulgaricus, Fermented milk, Aroma types, Flavoromics, Key aroma-active compounds
Abstract
The aroma of the fermented milk produced by twenty-eight Lactobacillus delbrueckii subsp. bulgaricus strains was evaluated via quantitative descriptive analysis. According to the sensory analysis results, the fermented milks were grouped into milky-type, cheesy-type, fermented-type and miscellaneous-type. The representative samples of cheese-type and fermented-type were analyzed by headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) and flavoromics. A total of 95 volatile compounds were identified and particularly, 12 aroma-active compounds were detected by using gas chromatography-olfactometry-mass spectrometry (GC-O-MS). Among the different aroma types, 2,3-butanedione, δ-decalactone, acetaldehyde, butanoic acid, acetic acid and hexanoic acid were finally screened out as the key aroma-active compounds by quantitative and odor activity value (OAV) analysis combined with aroma recombination, omission and addition experiments. These findings were valuable in developing specific fermented milk products with different aroma profiles.
1. Introduction
Yogurt is a popular dairy product and is usually produced using the combination of L. bulgaricus and Streptococcus thermophilus (Kaneko et al., 2014). Because of its unique flavors and health-improving functions, such as providing vitamins D and calcium (Varghese et al., 2021), regulating gut microbiota, and enhancing immunity (Adam et al., 2005), yogurt is favored by the consumers worldwide. Flavor is an essential factor in yogurt quality, affecting consumers’ acceptance (Cheng, 2010). There are many kinds of yogurts in the market, for example, plain yogurt, fruity yogurt, cheesy yogurt, etc. The flavor diversity was achieved by adding spices or juice in yogurt processing (Routray & Mishra, 2011). However, these strategies could not meet the requirement of consumers for additive-free products (Chen et al., 2017). Currently, the homogenization or similarity of yogurt flavor has become the bottleneck restricting the development of the yogurt industry (Zhao et al., 2018). The main reason for this problem is the starter cultures used, which were shared by most of the yogurt producers. Therefore, yogurt starter strains with specific aroma and flavor characteristics should be further explored, which is significant to avoid the similarity of yogurt flavors without adding essences.
It was believed that acetaldehyde, acetone, 2,3-butanedione, and 3-hydroxy-2-butanedione were essential flavor compounds in yogurt (Liu et al., 2016). Some studies reported that the yields of acetaldehyde (Liu et al., 2016) and 2,3-butanedione (Tian et al., 2020) were regarded as important indexes for screening aroma-producing strains. However, there was no consistent conclusion about the effects of these compounds on the aroma of yogurt. Chinese liquor (Baijiu), which enjoyed a long history, could be divided into several aroma types such as Luzhou-flavor, Maotai-flavor, etc. (He et al., 2020). The typing methods of baijiu aroma may be used as a reference to classify the aroma types of yogurts according to the sensory analysis. The volatile compounds in yogurts of different aroma types could be analyzed by gas chromatography-mass spectrometry (GC–MS) to determine the key flavor compounds. Then, the key flavor compounds could be selected as the indicators for screening strains with specific aroma and flavor, which might be an effective way to solve the homogenization problem in yogurt flavor.
In recent decades, some new technologies such as gas chromatography-ion mobility spectrometry (GC-IMS) have emerged in food flavor research. GC-IMS has the advantages of high sensitivity, simple sample preparation, and short analysis time (Wang et al., 2020). However, GC-IMS could not provide a reliable qualitative determination as GC–MS. Therefore, the combination of GC–MS and GC-IMS could be an excellent choice to provide a comprehensive analysis of volatile components in yogurts. So far, >90 volatile compounds have been identified in yogurts. However, not all of these compounds could be detected by human olfactory receptors and contribute to the aroma of yogurt. The aroma-active compounds could produce the odor perception in the human brain (Dunkel et al., 2014). Recently, sensomics or flavoromics has been applied to identify the key aroma-active compounds in dairy products (Chen et al., 2021). The flavor of milk fan (Tian et al., 2019), kurut (Wang et al., 2020) and cheeses (Majcher et al., 2018) was investigated by flavoromics.
In this study, to type the aroma of fermented milk produced by L. bulgaricus, the sensory properties of fermented milk produced by 28 L. bulgaricus strains were analyzed and the types of aromas were classified based on sensory analysis; The fingerprints and the key aroma-active compounds of specific aroma types were investigated by GC-IMS and flavoromics approach.
2. Materials and methods
2.1. Chemicals
The retention index (RI) of the volatile compounds was calculated using n-ketones C4 ∼ C9 (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China) as external references in GC-IMS analysis. For GC–MS analysis, n-alkane standards (C7 ∼ C30) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2-Octanol as internal standard (IS) was purchased from Dr. Ehrenstorfer (GmbH, Augsburg, Germany). The standards used to establish the standard curves including acetaldehyde, 2-pentanone, 2,3-butanedione, 2-heptanone, 3-hydroxy-2-butanone, 2-nonanone, acetic acid, benzaldehyde, butanoic acid, hexanoic acid, octanoic acid, and δ-decalactone were purchased from Sigma-Aldrich (St. Louis, MO, USA). All of the chemicals were of chromatographic grade, and the purity was>98%.
2.2. Strains
Starter cultures for dairy products were collected from a local market in Tsingtao, Shandong Province, China. The starter cultures were homogenized in 0.9% NaCl solution. Dilutions up to 10-4 ∼ 10-6 were prepared, and 100 μL aliquots from the 10-4 to 10-6dillutions were spread onto MRS agar plates (Qingdao Hopo Bio-Technology Co., Ltd, China) and incubated at 37 ℃ under anaerobic conditions. Colonies were subjected to the catalase test and gram staining. Catalase-negative, gram-positive and rod-shaped colonies were purified two or three times in MRS agar plates (Hajimohammadi Farimani et al., 2016). Pure colonies were identified by 16S rRNA gene sequencing in Sangon Biotech (Shanghai, China) Co., Ltd. A total of 28 L. delbrueckii subsp. bulgaricus strains were finally isolated and kept at −80 ℃. The information on these L. bulgaricus strains is shown in Table S1. The strains were subcultured three times using a milk medium (Qingdao Hopo Bio-Technology Co., Ltd, China) for 18 h at 37 ℃ before use.
2.3. Preparation of fermented milk
Sterilized whole milk containing 3.0% protein (wt/vol) and 3.7% fat (wt/vol) was provided by a dairy company (Beijing Sanyuan Foods Co., Ltd), and 6% sucrose (wt/vol) was added. Then, the raw milk was pasteurized at 95 ℃ for 5 min and cooled quickly to 42 ℃. L. bulgaricus was inoculated into the raw milk with an inoculum size of 0.5% (vol/vol) and fermented at 42 ℃. When the titratable acidity (TA) reached above 60 ˚T, the fermentation process was ended immediately by an ice bath. The measurement of TA was conducted following ISO/TS 11869:2012 (ISO/IDF, 2012). Then, quantitative descriptive and instrumental analysis were performed after refrigerating the fermented milk at 4 ℃ for 24 h. Each sample was fermented by L. bulgaricus strain according to the above manufacturing method twice.
2.4. Quantitative descriptive analysis (QDA)
According to the method given by ISO 8586: 2012 (ISO, 2012), thirty students and staffs of the College of Food Science and Engineering of the Ocean University of China were invited as panel candidates, and they have been trained in relevant courses and have experience in sensory analysis. Thirty candidate panelists were trained for one month (1 h/ day) to familiarize, identify and describe the odorants commonly found in dairy products. Ten panelists (two males and eight females; average age of 25 years old) were selected based on availability and sensory perception abilities. Meanwhile, these ten panelists were retrained and asked to sniff the standard reference and grade the intensities of different aroma attributes. Besides, the training and selection of panelists and the formal descriptive sensory analysis were performed in a sensory laboratory of the College of Food Science and Engineering of Ocean University of China where the room temperature was controlled at 20 ℃.
Referring to the ISO 5492: 2008 (ISO, 2008), five sensory descriptors including milky, creamy, cheesy, buttery and fermented were selected, and the definition and references of these five descriptors is shown in Table S2. A continuous 9-point scale, ranging from 0 (absence of the attribute) to 9 (very high intensity of the attribute), was used to measure aroma intensity. The scale of intensity for each reference was set as 7 points. Ten grams of fermented milk samples were weighed in disposable taste glass and coded with random three-digit codes at approximately 10 ℃. Each fermented milk sample was measured in triplicate. The panel evaluated 10 ∼ 12 samples during each 120-min session, and 16 total sessions were held to evaluate the 28 samples in three replicates. The panelists could have a rest after they evaluated 4 ∼ 5 samples. The data of sensory analysis were collected manually. To ensure the accuracy and reliability of the sensory evaluation, the professionals engaged in the R&D of dairy starters from the company Beijing Doit Biotechnology Co., Ltd were commissioned to guide and supervise our work of sensory analysis.
2.5. HS-GC-IMS analysis
One gram of fermented milk was placed into a 25-mL headspace glass sampling vial, which was incubated at 55 ℃ for 20 min at 500 rpm/min. After the incubation, 500 µL of headspace was automatically sucked and transferred into the GC-IMS instrument (FlavorSpec, GAS, Germany). A WAT-Wax capillary column (15 m × 0.53 mm) was chosen for volatile separation at isothermal 60 ℃. Nitrogen (purity ≥ 99.999%) was used as the carrier gas, the flow rate of which was set as follows: 2 mL/min (0 to 2 min), 2 ∼ 10 mL/min (2 to 10 min), 10 ∼ 100 mL/min (10 to 15 min), 100 ∼ 150 mL/min (15 to 20 min), 150 mL/min (20 to 30 min). The draft tube was under 45 ℃, where nitrogen was used as a drift gas at 150 mL/min flow. The data was processed by LAV (Laboratory Analytical Viewer) and GC × IMS Library Search. The retention index (RI) of volatile compounds was calculated using n-ketones C4 ∼ C9 as external references for the identification of compounds. Each fermented milk sample was analyzed in triplicate.
2.6. GC–MS analysis
HS-GC–MS was used to analyze the volatile compositions of the fermented milk samples. Ten grams of samples were put into a 40-mL glass vial with a silicon septum and 2 μL solution of 2-octanol (GmbH, Augsburg, Germany) with 0.328 mg/L were added as an internal standard. Then, the glass vial was equilibrated at 55 ℃ for 20 min and the SPME fiber coated with 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) was headed to the headspace of the vial for volatile extraction at 55 ℃ for 40 min. After extraction, the fiber was inserted into the injector port of GC–MS and desorbed for 6 min at 250 ℃ in splitless mode. The GC–MS analysis was operated using an Agilent 8890 GC/7000D MSD according to Tian et al., 2020, Dan et al., 2017 with minor modifications. The volatile compounds were determined on a capillary column Agilent HP-INNOWax column (60 m × 0.25 mm, 0.25 μm, Agilent Technologies) and a capillary column Agilent HP-5MS column (30 m × 0.25 mm, 0.25 μm, Agilent Technologies). For the HP-INNOWax column, the oven temperature was held at 35 ℃ for 3 min firstly, then increased to 120 ℃ at a rate of 4 ℃/min and then increased to 190 ℃ at 5 ℃/min, and finally increased to 230 ℃ at 10 ℃/min and held for 6 min. For the HP-5MS column, the oven temperature was maintained at 35 ℃ for 3 min firstly, and increased to 100 ℃ at a rate of 4 ℃/min and held for 2 min, then increased to 150 ℃ at 5 ℃/min, and finally increased to 250 ℃ at 10 ℃/min and held for 10 min. The volatile compounds were identified by MS and retention index (RI). The RI was calculated with a series of n-alkanes (C7 ∼ C30).
2.7. GC-O-MS analysis
The aroma-active compounds were located using Agilent 7890B-5977B GC/MSD equipped with an olfactory detection part (Gerstel ODP-2, Mülheim an der Ruhr, Germany). The GC effluent was set to be separated equally between the mass detector and the sniffer in a 1:1 ratio. A capillary column Agilent HP-INNOWax column (60 m × 0.25 mm, 0.25 μm) was chosen to separate the volatile compounds and the operating conditions were the same as the description in GC–MS analysis. The odor-specific magnitude estimation (OSME) analysis was conducted to evaluate the odor intensities of volatile compounds by ten well-trained panelists. The procedure of selection and training of panelists was the same as the description in session 2.4 Quantitative descriptive analysis (QDA). The time of the onset and end of the aroma-active compounds, the perceived odor characteristic, and aroma intensity (AI) were recorded. AI was marked using the 6-point scale from 0 (none detected) to 5 (extremely strong) (Tian et al., 2020). The GC-O-MS analysis was performed in triplicate by each panelist. The mean values of the AI marked by ten panelists were considered the final value of AI. To avoid potential loss of aroma-active compounds, the compound would be recognized as aroma-active compound when the AI value of one volatile compound was higher than zero. In addition to MS and RI, the odor descriptions of compounds and corresponding standard compounds were used for further identification of aroma-active compounds.
2.8. Quantitative and OAV analysis
The aroma-active compounds were quantified by establishing standard curves with GC–MS in SIM mode (Tan et al., 2021). To obtain a matrix similar to fermented milk, an aroma-blank matrix was prepared according to Tian et al. (Tian et al., 2020) with minor modification. Firstly, 3% (wt/vol) milk protein concentrate powder (MPC 80, Ningxia Cezanne dairy industry Co., Ltd., Yinchuan, China), 3.6% (wt/vol) sunflower seed oil (COFCO Corporation, Beijing, China), water, 1% (wt/vol) modified starch and 0.1% (wt/vol) pectin were mixed with a blender (2000 rpm/min, 55 ℃ for 30 min). The mixture was homogenized under 20 MPa and pasteurized at 95 ℃ for 5 min. Finally, the pH of the mixture was adjusted to 4.5 with lactate buffer solution. Ten grams of the mixture were added into a 40 mL glass vial with a silicon septum and 2-octanol was added as described above. The concentration gradient of each compound was set according to the semiquantitative results, and the standard curve of no<6 points was established. All calibration curves were replicated in triplicate. The calibration equations were listed in Table S6, where y represented the peak area ratio (peak area of standard compound / peak area of internal standard), and x represented the concentration ratio (concentration of standard compound /concentration of internal standard). The odor activity value (OAV) was calculated as the ratio of the concentration of compounds to its odor threshold. The odor thresholds of these compounds were collected from the information available in the literature (van Gemert, 2011). Each fermented milk sample was analyzed in triplicate.
2.9. Aroma recombination
To evaluate and compare the actual contribution of each aroma-active compound to the aroma profile of fermented milk samples, all of the aroma-active compounds were prepared at concentrations the same as their occurrence in the original samples in the aroma-blank mixture. After equilibrating at room temperature for 2 h, the aroma recombinant (AR) was finally obtained. Quantitative descriptive analysis (QDA) with ten well-trained panelists, as described in session 2.4, was used to evaluate the sensory scores of the recombination models and their corresponding samples.
2.10. Omission tests
To determine the compounds that influence the overall aroma profile of fermented milk samples, omission models based on the recombinant model were prepared by removing a single compound or a group of compounds from the recombination models. A total of 15 omission models and one recombination model were analyzed by a triangle test. Triangle tests were performed by randomly arranging one omission model and two recombination models for ten well-trained panelists to test whether or not there were some differences between the recombination models and the omission models and select the different one. If at least eight panelists recognized the omission models, it meant that this compound or this group of compounds omitted from the recombination model was or were significantly important to the overall aroma of samples (p < 0.05). Similarly, if there were nine panelists, it meant that this compound or this group of compounds was or were highly significantly important (p < 0.01), and 10 panelists meant very highly significantly important (p < 0.001) (Yang et al., 2019). Each test was conducted in triplicate.
2.11. Aroma addition experiments
Aroma addition experiments were operated to further verify and explore the effects of the key aroma compounds on the intensities of aroma attributes. All aroma-active compounds were mixed in the aroma-blank mixture at the same concentrations as their occurrence in the original sample of L6-11 to obtain aroma recombinant (AR). Then, the key aroma compounds including acetaldehyde, 2,3-butanedione, acetic acid, butanoic acid, hexanoic acid, and δ-decalactone revealed by omission test, were added to the aroma recombinant L6-11 at concentrations similar to those in the sample of L6-11. The intensity changes in aroma attributes were quantified by ten well-trained panelists as described in session 2.4 Quantitative descriptive analysis (Tian et al., 2019). The aroma addition experiments were performed in triplicate for every key aroma compound.
2.12. Statistical analysis
Data from the sensory analysis, quantitative analysis, and aroma recombination and addition analysis were evaluated by using an analysis of variance (ANOVA) with Duncan’s multiple comparison tests performed by IBM SPSS Statistics version 25. Here, p-values of < 0.05, <0.01 and < 0.001 were considered statistically significant and marked as *, ** and ***, respectively. Radar maps and PCA analysis for the results of sensory analysis and aroma classification were carried out using Origin 2019b (OriginLab, Northampton, United States). Heatmap analysis for concentrations of the six key aroma compounds was performed in MetaboAnalyst 5.0 (https://www.metaboanalyst.ca).
3. Results and discussion
3.1. Sensory analysis and aroma typing
The results of sensory analysis of the fermented milk produced by 28 L. bulgaricus are shown in Table 1. For the milky attribute, the samples of L6-12, L8-5, L9-2, L9-5, and L9-8 were the most scored with intensities of 3. The creamy attribute was only presented in the samples of L4-1-2, L6-11, L6-14, L8-4, L8-7, and L8-8, but it was weaker compared with cheesy, milky, and fermented attributes. For the cheesy attribute, L4-1-2 was the most scored with an intensity of 5, followed by L4-1–1, L4-2–3, L6-15, L8-6, L8-7, and L9-6 samples. For the fermented attribute, only the L6-11 sample was scored with an intensity higher than 5, followed by L9-5. Besides, the samples L6-12, L8-1 and L9-1 showed weak a buttery attribute. For quantitative descriptive analysis of the aroma of fermented milk, it is crucial to develop a sensory lexicon with definitions and scales. Some reports have shown some lexicons and references for aroma sensory evaluation of yogurt, such as grain-like, moldy, yeast, milk, sweaty, etc. (Brown and Chambers, 2015, Coggins et al., 2008). Considering the differences in dietary habits and cultures of different countries, we did not copy these lexicons completely. Instead, the lexicon including milky, creamy, cheesy, buttery, and fermented was finally determined according to the ISO 5492: 2008 (ISO, 2008). Twenty-eight samples had obvious differences in all five aroma attributes. The L4-1-2 showed a prominent cheesy aroma, and the sample of L6-11 showed the most outstanding fermented aroma. Interestingly, all of the 28 fermented milk samples showed lower scores in the milky, creamy, and buttery aroma. These phenomena indicated that different L. bulgaricus strains had varying metabolizing abilities to produce flavor compounds during milk fermentation, which provided a theoretical basis to establish screening methods of strains with specific sensory notes.
Table 1.
Mean scores (n = 10 panelists) for each aroma attributes and the results of aroma typing of fermented milk of L. bulgaricus.
| No. | Milky | Creamy | Cheesy | Buttery | Fermented | Flavor type |
|---|---|---|---|---|---|---|
| L4-1-1 | 1a | 01 | 4b2 | 0 | 0 | Cheesy-type |
| L4-1-2 | 1c | 1c | 5a | 0 | 3b | Cheesy-type |
| L4-2-3 | 0 | 0 | 4a | 0 | 1b | Cheesy-type |
| L4-2-8 | 0 | 0 | 2 | 0 | 0 | Cheesy-type |
| L5-1 | 0 | 0 | 3a | 0 | 2b | Cheesy-type |
| L6-10 | 0 | 0 | 3 | 0 | 0 | Cheesy-type |
| L6-11 | 1d | 2c | 3b | 0 | 6a | Fermented-type |
| L6-12 | 3b | 0 | 0 | 2c | 4a | Fermented-type |
| L6-14 | 0 | 1b | 0 | 0 | 3a | Fermented-type |
| L6-15 | 0 | 0 | 4a | 0 | 1b | Cheesy-type |
| L8-2 | 2a | 0 | 0 | 0 | 1b | Milky-type |
| L8-4 | 0 | 2c | 3b | 0 | 4a | Fermented-type |
| L8-5 | 3a | 0 | 0 | 0 | 1b | Milky-type |
| L8-6 | 0 | 0 | 4 | 0 | 0 | Cheesy-type |
| L8-7 | 0 | 2b | 4a | 1c | 0 | Cheesy-type |
| L8-8 | 0 | 2b | 3a | 0 | 0 | Cheesy-type |
| L9-1 | 0 | 0 | 3a | 2b | 0 | Cheesy-type |
| L9-2 | 3a | 0 | 3a | 0 | 0 | Miscellaneous-type |
| L9-3 | 2b | 0 | 0 | 0 | 4a | Fermented-type |
| L9-4 | 1b | 0 | 2a | 0 | 2a | Miscellaneous-type |
| L9-5 | 3b | 0 | 2c | 0 | 5a | Fermented-type |
| L9-6 | 2b | 0 | 4a | 0 | 0 | Cheesy-type |
| L9-7 | 2a | 0 | 0 | 0 | 2a | Miscellaneous-type |
| L9-8 | 3a | 0 | 0 | 0 | 2b | Milky-type |
| L9-9 | 2a | 0 | 1b | 0 | 1b | Milky-type |
| L9-11 | 0 | 0 | 3 | 0 | 0 | Cheesy-type |
| L9-12 | 0 | 0 | 2b | 0 | 4a | Fermented-type |
| L9-13 | 2b | 0 | 3a | 0 | 3a | Miscellaneous-type |
Note: 1The mean scores round to nearest integer. Attribute intensities were scored on a 0- to 9-point universal intensity scale where 0 indicates that the attribute is not perceived at all; 1 indicates doubt about the presence of the attribute; 2 indicates that the attribute is perceived but very slightly; 3 indicates that the attribute is clearly perceived, although it is slight; 4 indicates that the attribute is clearly perceived, but the intensity is much lower than reference; 5 indicates that the attribute is clearly perceived, but the intensity is lower than the reference; 6 indicates that the attribute is clearly perceived, but the intensity is slightly lower than the reference; 7 indicates that the intensity of the attribute is similar to the reference.; 8 indicates that the intensity of the attribute is higher than the reference; and 9 indicates that the intensity of attribute is much higher than the reference.
2Different superscript letters in the same row indicated significant differences (p < 0.05).
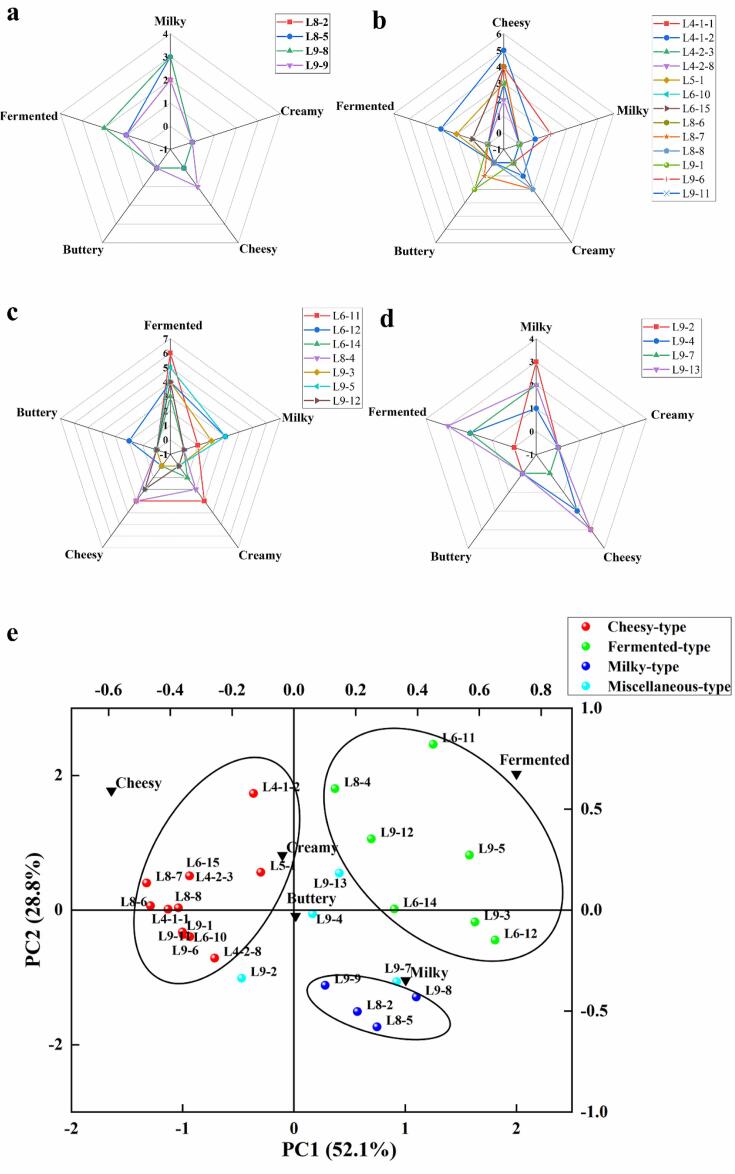
Referring to the classification methods of Chinese liquors, the rule of aroma typing in this study was set as follows: it would be assigned to the aroma type if a fermented milk sample had the highest score of a specific aroma attribute. Accordingly, the 28 fermented milk samples were divided into four aroma types: milky-type, cheesy-type, fermented-type, and miscellaneous-type, and the results are shown in Table 1. Miscellaneous type means that the scores of more than one attribute are the same and the highest. Four samples were grouped into milky-type, and 13, 7, and 4 fermented milk samples were classified into cheesy-type, fermented-type and miscellaneous-type, respectively. Radar maps of 4 different aroma types were structured according to the results of sensory analysis and are shown in Fig. 1. Among four fermented milk samples of the milky-type (Fig. 1a), L8-5 and L9-8 samples were the most scored with a milky intensity of 3. In cheesy-type (Fig. 1b), L4-1-2 showed the most robust cheesy aroma with an intensity of 5. Among seven fermented milk samples of fermented-type (Fig. 1c), L6-11 was the most scored with a fermented intensity of 6 followed by L9-5 with 5 points. As for the miscellaneous-type (Fig. 1d), L9-2 showed the same score for the milky and cheesy attribute, and L9-7 had the same score for the milky and fermented attribute. L9-4 and L9-13 were scored with the same intensity of cheesy and fermented attributes.
Fig. 1.
Radar maps of flavor types of fermented milk by L. bulgaricus and the PCA bi-plot generated from the sensory analysis and aroma typing results. a. milky-type; b. cheesy-type; c. fermented-type; d. miscellaneous-type; e. the PCA bi-plot; samples of cheesy-type were shown in red color; samples of fermented-type were shown in green color; samples of milky-type were shown in blue color and samples of miscellaneous-type were shown in cyan color.
The PCA plot derived from the aroma characterization is shown in Fig. 1e, which indicates a pictorial relationship among fermented milk samples of four aroma types based on the intensity of the five aroma attributes. PCA could reduce the dimensionality of aroma data and intuitively characterize the prominent aroma features of fermented milk samples (Coggins et al., 2008). After PCA analysis, the fermented milk samples of four aroma types were differentiated on the plot. Samples of the milky-type were positively correlated with the milky attribute. Samples of the fermented-type were positively related to the fermented attribute and negatively correlated with the cheesy attribute. All of the 13 samples of the cheesy-type were positively correlated with the cheesy characteristic. Currently, no other study showed the viewpoints of aroma types of fermented milk, and the results of aroma typing in this study might offer suggestions to establish the aroma classification of fermented milk in the future to screen out novel strains with specific aroma features.
3.2. Identification of volatile compounds by GC-IMS and GC–MS
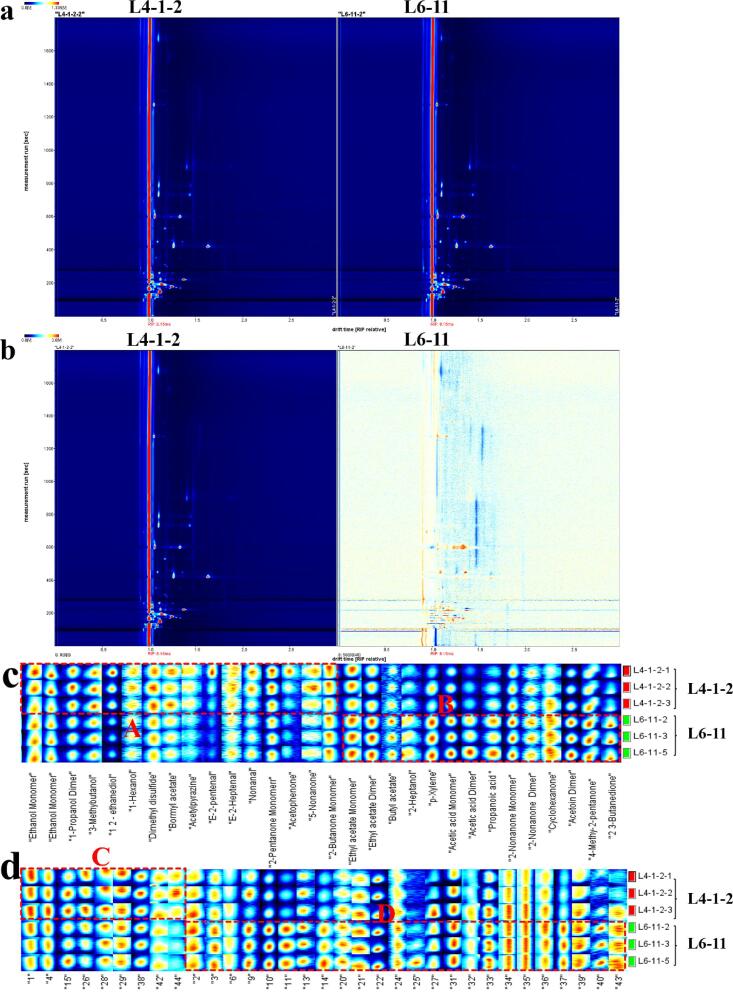
To elucidate the crucial compounds influencing the aroma typing, the volatile compounds of the two fermented milk samples were analyzed by GC-IMS, and the results are shown in Fig. 2. These two samples, including L4-1-2 (cheesy-type group) and L6-11 (fermented-type group), showed the highest scores in their corresponding sensory attributes. It was observed that all of the samples of the milky-type and miscellaneous-type groups showed lower scores in five aroma attributes (<3 points), so the samples of these two types were not selected for subsequent GC-IMS and flavoromics analysis. 2D topographic plots of volatile compounds in fermented milk samples showed no obvious difference in the composition of volatile components of the two samples (Fig. 2a). The sample of L4-1-2 was set as the background, and the differential plots were obtained by topographic plot deduction and are shown in Fig. 2b. There were lots of plots that showed differences in colors, which suggested that lots of compounds in the two samples differed in concentrations (Yang et al., 2021). To describe the differences of volatile compounds between the L4-1-2 of cheesy-type and the L6-11 of fermented-type, integral and qualitative analyses were operated on the GC-IMS spectra. A total of 48 volatile compounds, including 10 aldehydes, 5 esters, 13 ketones, 2 acids, 11 alcohols, 4 sulfides and 3 others, were identified (Table S3).
Fig. 2.
2D topographic plots of volatile compounds and the flavor fingerprints of two fermented milk samples selected. a. 2D-topographic plots of volatile compounds in two fermented milk samples selected. b. the differential plots of 2D-topographic plots of GC-IMS spectra of volatile compounds in two fermented milk samples selected (with the L4-1-2 as the background). c. The flavor fingerprints of two fermented milk samples selected. The areas of A and B were characteristic fingerprints of L4-1-2 and L6-11, respectively. d. The flavor fingerprints of unidentified plots in two fermented milk samples selected. The areas of C and D were characteristic fingerprints of L4-1-2 and L6-11, respectively.
To demonstrate the differences of different aroma types, the flavor fingerprints of fermented milk samples were structured and are shown in Fig. 2c. A total of 26 volatile compounds were identified as key compounds, including 9 ketones, 3 aldehydes, 6 alcohols, 2 acids, 3 esters, 1 sulfide and 3 other compounds. These 26 compounds were divided into area A and area B. Area A consisted of 15 volatile compounds including 5 alcohols, 1 sulfide, 3 aldehydes, 1 easter, 4 ketones, and 1 other compound. Dimethyl disulfide could provide an onion and cabbage flavor for yogurt (Tian et al., 2020), and its concentration in L4-1-2 was higher than that in L6-11, which might cause L4-1-2 had a higher score in cheesy attribute than L6-11. Besides, there were also 3 aldehydes in area A, which came from an amino acid by transamination. (E)-2-pentanal and nonanal could give fruit and green smell to yogurt (Engel et al., 2002, Wang et al., 2021). Area B was mainly composed of acids, esters, and ketones. Acetic acid could improve the sour smell of yogurt (Cheng, 2010). The concentration of acetic acid in L6-11 was higher than that in L4-1-2, which might be why the fermented score of L6-11 was higher than that of L4-1-2. 2-Nonanone, 3-hydroxy-2-butanone, and 2,3-butanedione, which offered a creamy aroma to yogurt (Cheng, 2010), and the concentrations of these compounds in L6-11 were higher than those in L4-1-2, which might account for the higher creamy score of L6-11 than that of L4-1-2. Besides, the intensities of 33 unidentified plots were significantly different (Fig. 2d). Area C consisted of 9 unidentified plots where these 9 plots in L4-1-2 showed higher intensities than those in L6-11. Moreover, area D included 24 unidentified plots and their intensities in L6-11 were higher than those in L4-1-2.
The volatile compounds in the two fermented milk samples, L4-1-2 and L6-11, were then investigated by GC–MS. The qualitative results of GC–MS are shown in Table S4. A total of 67 volatile compounds, including 9 aldehydes, 17 ketones, 13 organic acids, 12 alcohols, 3 lactones, 6 sulfides, 2 terpenes, and 5 other compounds, were identified. Comparing and integrating the qualitative results of GC-IMS and GC–MS, 48 volatile compounds were identified by GC-IMS, and 67 volatile compounds were identified by GC–MS. A total of 95 volatile compounds were identified via GC-IMS and GC–MS, and 21 volatile compounds were identified by both GC–MS and GC-IMS. It is worth noting that although GC–MS identified more compounds than GC-IMS, some compounds, including ethyl acetate, butyl acetate, small acid, ketones and alcohols were only identified by GC-IMS, due to its high sensitivity (Wang et al., 2020). Besides, there were 33 unidentified plots in GC-IMS spectra, which suggested that GC-IMS could not provide precise qualitative abilities as GC–MS. Therefore, the combination of GC-IMS and GC–MS could comprehensively analyze the volatile compounds in fermented milk.
3.3. Identification of aroma-active compounds in fermented milk by GC-O-MS
Although 95 volatile compounds were identified by GC-IMS and GC–MS, it could not confirm which compounds had aroma activities. Therefore, the aroma-active compounds were investigated by GC-O-MS analysis. Meanwhile, OMSE (odor-specific magnitude estimation) was performed to quantify the aroma-active compounds responsible for the aroma perception based on the aroma intensity (AI). Generally, higher AI values mean a more intense aroma and are important to the aroma of foods (Tan et al., 2021).
A total of 12 aroma-active compounds, including 5 ketones, 4 acids, 2 aldehydes and 1 lactone, were perceived and identified in samples L4-1-2 and L6-11 by comparing their MS, RI, and odor descriptions with authentic standards (Table 2). The RI, identification methods, and odor descriptions of the 12 aroma-active compounds are also shown in Table S5. In L4-1-2 and L6-11 were revealed 10 and 9 aroma-active compounds, respectively. From Table 2, 7 aroma-active were perceived in every fermented milk sample, among which 2,3-butanedione (1.38–1.85), butanoic acid (2.25–3.25), hexanoic acid (1.75–1.88), and δ-decalactone (2.75–3.25) presented relatively high AI values, and were therefore considered to be primary contributors to aroma of fermented milk. Ketones are usually produced by β-oxidation in the fermentation process. A total of 5 ketones were perceived in two fermented milk samples selected and 2,3-butanedione had the highest aroma intensities, followed by 2-nonanone. 2-Pentanone and 3-hydroxy-2-butanone had lower aroma intensities (AI < 1) and fewer contributions to the aroma of fermented milk. Some reports showed that 2,3-butanedione could provide a creamy aroma to fermented milk (Liu et al., 2016), and it was perceived at similar intensities between the two samples selected. Two aldehydes, including acetaldehyde and benzaldehyde, showed lower aroma intensities compared to other aroma-active compounds, which suggested that aldehydes had little influence on the flavor of fermented milk. In addition, four organic acids, including acetic acid, butanoic acid, hexanoic acid, and octanoic acid, were regarded as the aroma-active compounds. Acetic acid, butanoic acid, and hexanoic acid could give strong sour, cheesy and rancid smells (Cheng, 2010, Jing, 2020). Acetic acid, butanoic acid, and hexanoic acid were all screened out as aroma-active compounds in L4-1-2 and L6-11. Octanoic acid was only perceived in L6-11. Butanoic acid, hexanoic acid, and octanoic acid had higher aroma intensities (AI > 1), which indicated that these three acids played important contributions to the overall aroma of fermented milk. Meanwhile, acetic acid, hexanoic acid, and octanoic acid showed higher AIs in L6-11 than that in L4-1-2, and butanoic acid showed higher AIs in L4-1-2 than that of L6-11, which could explain the stronger fermented aroma of L6-11 and stronger cheesy aroma of L4-1-2. Besides, δ-decalactone which is produced mainly by hydrolysis and further esterification of hydroxy fatty acid triglycerides, showed creamy or coconut flavor (Drake et al., 2001, Schlutt et al., 2007) and was perceived at similar intensities between the two samples selected with the highest aroma intensity (AI > 2), which suggested that this compound played an indispensable role in the aroma of fermented milk samples. Ott et al. (Ott et al., 1997) analyzed aroma-active compounds in yogurt by GC-O, and 20 aroma compositions were perceived. However, only 11 aroma-active compounds were identified in their study, which differed from our study's results. This difference might be because that they adopted combined static and dynamic headspace and preparative simultaneous distillation–extraction under vacuum to extract the extraction aroma compositions which might be different from headspace solid microextraction in terms of extraction efficiency (Cheng, 2010).
Table 2.
The aroma intensities of aroma-active compounds in two fermented milk samples obtained by GC-O-MS analysis and their concentrations and OAV values.
| No. | Compound | Aroma Intensity (AI)a |
Concentrations / (ug/kg)b |
OAV |
|||
|---|---|---|---|---|---|---|---|
| L4-1-2 | L6-11 | L4-1-2 | L6-11 | L4-1-2 | L6-11 | ||
| 1 | Acetaldehyde | 0.8 ± 0.27 | 0.6 ± 0.22 | 3156.15 ± 151.38 a | 2675.76 ± 212.21 a | 50.91 | 43.16 |
| 2 | 2-Pentanone | 0.625 ± 0.25 | ─ | 218.09 ± 6.89 a | 186.03 ± 9.49b | < 1 | < 1 |
| 3 | 2,3-Butanedione | 1.85 ± 0.25 | 1.38 ± 0.48 | 115.17 ± 14.39b | 226.11 ± 16.78 a | 2.30 | 4.52 |
| 4 | 3-Hydroxy-2-butanone | ─ | 0.88 ± 0.48 | 105.90 ± 4.36 a | 103.81 ± 6.05 a | < 1 | < 1 |
| 5 | 2-Heptanone | 0.75 ± 0.29 | 1.13 ± 0.25 | 31862.57 ± 2845.62b | 49953.26 ± 1397.26 a | 3.98 | 6.24 |
| 6 | 2-Nonanone | 1.50 ± 0.58 | ─ | 15.60 ± 0.49b | 20.44 ± 2.97 a | < 1 | < 1 |
| 7 | Acetic acid | 0.80 ± 0.27 | 0.9 ± 0.22 | 38745.29 ± 923.60b | 60792.16 ± 3051.58a | 1.19 | 1.88 |
| 8 | Benzaldehyde | 0.25 ± 0.50 | ─ | 1.66 ± 0.29 a | 1.30 ± 0.25 a | < 1 | < 1 |
| 9 | Butanoic acid | 3.25 ± 0.50 | 2.25 ± 0.5 | 5136.69 ± 17.58 a | 3288.03 ± 374.17b | 1.06 | 1.66 |
| 10 | Hexanoic acid | 1.75 ± 0.50 | 1.88 ± 0.63 | 1039.66 ± 76.68b | 1409.02 ± 198.12 a | 15.99 | 21.68 |
| 11 | Octanoic acid | ─ | 1.88 ± 0.25 | 245.55 ± 18.55b | 519.37 ± 52.53 a | < 1 | < 1 |
| 12 | δ-Decalactone | 3.25 ± 0.50 | 2.75 ± 0.5 | 24.24 ± 2.82b | 32.66 ± 2.07 a | 9.70 | 13.06 |
Note: aThe aroma intensity was obtained by GC-O-MS, and the aroma intensities of compounds were reported as the mean ± standard deviation (SD).
b The concentrations of compounds were reported as the mean ± standard deviation (SD), and the values with different letters (a tod) in a row are significantly different using Duncan’s multiple comparison tests (P < 0.05).
3.4. Quantitation of aroma-active compounds in fermented milk
The differences in AI values of aroma-active compounds were mainly due to the concentration differences. Therefore, quantitative analysis was performed to further evaluate the contributions of these aroma-active compounds. The quantitative results of these 12 aroma-active compounds are shown in Table 2. It was found that acetaldehyde, 3-hydroxy-2-butanone, acetic acid, butanoic acid, and hexanoic acid were present in relatively high concentrations (>1mg/kg). Conversely, 2-pentanone, 2,3-butanedione, heptanone, 2-nonanone, benzaldehyde, octanoic acid, and δ-decalactone presented lower concentrations (<1mg/kg).
The contributions of aroma-active compounds are not only determined by their concentrations but also by their odor threshold values. Therefore, the OAV values of aroma-active compounds were calculated to further analyze the contributions of aroma-active compounds. The results are shown in Table 2. The OAV values of 7 aroma-active compounds were higher than 1, including acetaldehyde (OAV 43.16–50.91), 2,3-butanedione (OAV 2.30–4.52), 2-heptanone (OAV 3.98–6.24), acetic acid (OAV 1.19–1.88), butanoic acid (OAV 1.06–1.66), hexanoic acid (OAV 15.99–21.68) and δ-decalactone (OAV 9.70–13.06). They were regarded as contributors to the sample aroma based on the principle of OAVs. Among these seven compounds, 2,3-butanedione, butanoic acid, hexanoic acid, and δ-decalactone showed relatively high AI values, which were in accordance with the results of GC-O-MS analysis. Though δ-decalactone with a typical creamy aroma was present in very low concentration (24.24–32.66 ug/kg), it was regarded as an influential aroma contributor to fermented milk samples due to its extremely odor thresholds (0.0025 mg/kg). The results of GC-O-MS showed that the AI values of acetaldehyde were low in both two selected fermented milk samples, while its OAV value was the highest, which might be due to different mediums of GC-O-MS analysis and quantitative analysis. In GC-O-MS analysis, AI values were determined by panelists based on the threshold of acetaldehyde in the air (threshold value = 1 mg/m3), which was different from its threshold in the milk (threshold value = 0.062 mg/kg) (van Gemert, 2011, Wang et al., 2021). The OAV values of 2-pentanone, 3-hydroxy-2-butanone, 2-nonanone, benzaldehyde, and octanoic acid were<1, which meant that these five aroma-active compounds would play an auxiliary role in the overall aroma of fermented milk. At the same time, these five aroma-active compounds were perceived by GO-O with low AI values, indicating a high consistency between GC-O-MS and quantitative analysis. Thus, seven aroma-active compounds were provisionally regarded as key aroma compounds of the fermented milk sample selected.
3.5. Aroma recombination
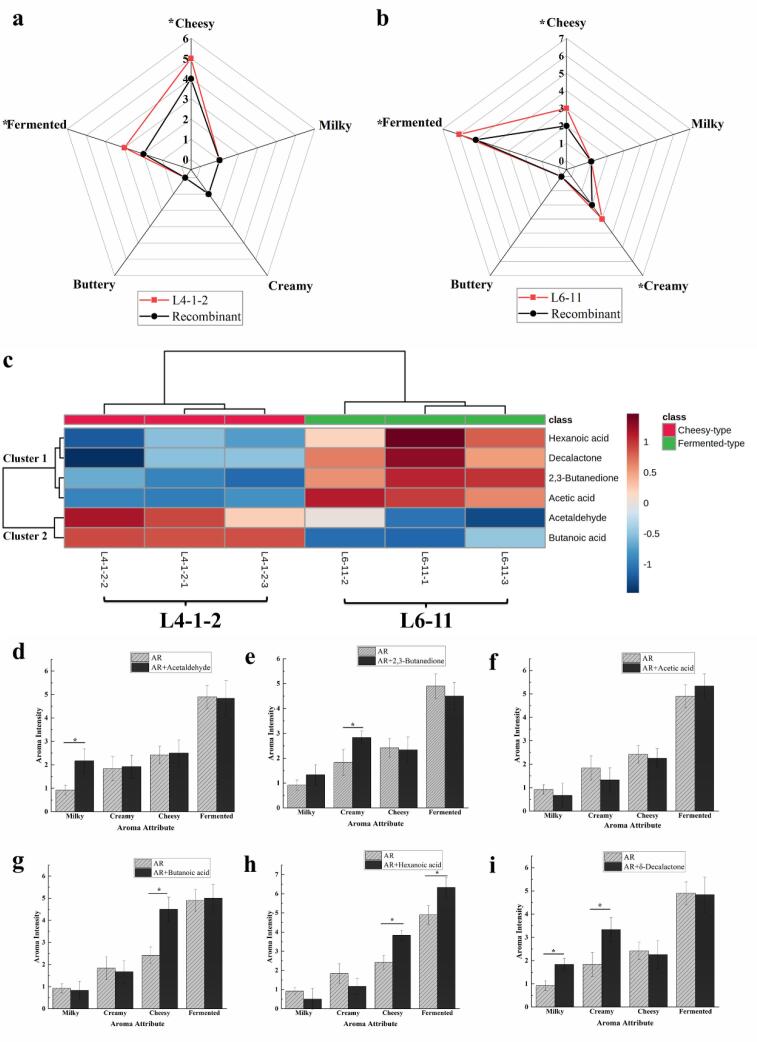
Though GC-O-MS and quantitative analysis were used to isolate and identify the aroma components from the food matrix, they could not reflect the real interactions among these aroma-active compounds. Therefore, aroma recombination was performed to verify qualitative and quantitative results of aroma-active compounds and to confirm the contribution of such compounds to the overall aroma. The results of aroma recombination analysis are shown in Fig. 3(a-b). All of the aroma-active compounds were prepared according to the concentrations in the original samples. The aroma of recombination models of L4-1-2 and L6-11 were shown good similarity with the original samples L4-1-2 and L6-11, respectively. However, there were still slight differences found in some aroma intensities, such as fermented, creamy, and cheesy. In aroma recombinant of L4-1-2 (Fig. 3a), the scores of fermented and cheesy attributes of aroma recombinant were significantly lower than those of L4-1-2 (p < 0.05). Meanwhile, the scores of fermented, creamy, and cheesy attributes in aroma recombinant of L6-11 (Fig. 3b) were significantly lower than those of L6-11 (p < 0.05). These differences were possibly caused by variable aroma release from different matrices. There were some gaps between the aroma-blank and the matrix of real fermented milk, which could influence the release of aroma compounds. Besides, it was reported that headspace solid microextraction showed poor extractability for the polar and semi-volatile compounds (Rigling et al., 2019), which could cause these differences.
Fig. 3.
Aroma profiles and the corresponding aroma recombinants of two fermented milk samples by L. bulgaricus of cheesy-type (a) and fermented-type (b). c. The heatmap analysis of the concentrations of key aroma-active compounds in two fermented milk samples selected. d-i, Aromatic effects of the additions of 6 key aroma compounds on the aroma attributes. d, acetaldehyde; e, 2,3-butanedione, f, acetic acid; g, butanoic acid; h, hexanoic acid; i, δ-decalactone. (* indicated significance at p < 0.05 by ANOVA with Duncan’s multiple comparison tests; AR, aroma recombinant).
3.6. Aroma omission test
To further verify the contributions of these 12 aroma-active compounds to the aroma profiles of fermented milk, omission tests were conducted using 15 models in which single compounds or a group of compounds were omitted. The recombined model of L6-11 was used as a guide, and omission models were evaluated by a triangle test. The statistical results are shown in Table 3. It could be seen that the omission of all ketones (model 1) showed a very highly significantly different aroma than that of the recombined model (p < 0.001), which indicated that ketones played essential roles in the overall aroma of fermented milk. The models omitting only 2,3-butanedione presented a highly significant difference (model 1–2, p < 0.01). The models omitting only 2-heptanone (model 1–3), 3-hydroxy-2-butanone (model 1–4), and 2-nonanone (model 1–5) showed no significant difference compared with the recombined model of L6-11, which suggested that only 2,3-butanedione responsible for creamy aroma played indispensable roles in the overall aroma of fermented milk among five ketone compounds. Besides, nine out of ten panelists recognized model 4 omitting δ-decalactone, which suggested δ-decalactone was another main contributor to the overall flavor of fermented milk. Meanwhile, δ-decalactone could confer coconut or creamy flavor. Nine panelists recognized model 2 lacking all aldehydes and model 2–1 omitting acetaldehyde. No panelist recognized the model omitting the benzaldehyde, which indicated that only acetaldehyde could play an indispensable role in the overall aroma of fermented milk. Acetaldehyde could give a rich milky aroma (Gaafar, 2010), which suggested that acetaldehyde might be the main contributor to the milky attribute of fermented milk samples. Furthermore, models 3 and 3–3, which lacked all acids showed very a highly significant difference (p < 0.001), and 8 and 9 panelists recognized models 3–1 lacking acetic acid and 3–2 lacking butanoic acid, respectively. Moreover, there was no significant difference between model 3–4 lacking octanoic acid and the recombined model of L6-11. Therefore, acetic acid, butanoic acid, and hexanoic acid were important aroma-active compounds. It is worth mentioning that acetic acid and butanoic acid were not found to have high OAV (OAV < 2), which could be explained why the current estimate of odor thresholds of these two compounds was not fully estimated (Bi et al., 2020). Considering the results of the aroma recombination and aroma omission test, the conclusion could be drawn that acetaldehyde, 2,3-butanedione, acetic acid, butanoic acid, hexanoic acid, and δ-decalactone were the key aroma compounds of the fermented milk by L. bulgaricus.
Table 3.
Omission experiments from the recombination model.
| No. | Odorants omitted from the complete recombination | na | Significanceb |
|---|---|---|---|
| 1 | All ketones | 10 | *** |
| 1–1 | 2-Pentanone | 0 | ─ |
| 1–2 | 2,3- Butanedione | 9 | ** |
| 1–3 | 2- Heptanone | 0 | ─ |
| 1–4 | 3-Hydroxy-2-butanone | 6 | ─ |
| 1–5 | 2-Nonanone | 0 | ─ |
| 2 | All aldehydes | 9 | ** |
| 2–1 | Acetaldehyde | 9 | ** |
| 2–2 | Benzaldehyde | 0 | ─ |
| 3 | All acids | 10 | *** |
| 3–1 | Acetic acid | 8 | * |
| 3–2 | Butanoic acid | 9 | ** |
| 3–3 | Hexanoic acid | 10 | *** |
| 3–4 | Octanoic acid | 4 | ─ |
| 4 | δ-Decalactone | 9 | ** |
Note: a Number of panelists from 10 panelists who recognized the aroma difference by a triangle test.
b*: Significant (p < 0.05); **: highly significant (p < 0.01); ***: very highly significant (p < 0.001);
─:no significant (p > 0.05).
To reflect the composition differences of key aroma-active compounds in the fermented milk samples, the concentrations of the above-mentioned key aroma compounds were presented in the form of a heatmap, as shown in Fig. 3c. Six compounds were divided into two clusters based on the dendrogram. Cluster 1 consisted of four aroma-active compounds, including 2,3-butanedione, hexanoic acid, acetic acid, and δ-decalactone. 2,3-Butanedione could provide creamy flavor (Cheng, 2010), and its concentration in L6-11 was significantly higher than that in L4-1-2. The score of a creamy attribute of L6-11 was higher than that of L4-11, which suggested this compound might be the key aroma-active compound for the creamy attribute of fermented milk. It was worth noting that the threshold of 3-hydroxy-2-butanedione (threshold = 8 mg/kg) was much bigger than that of 2,3-butanedione (threshold = 0.05 mg/kg), and 2,3-butanedione could be irreversibly reduced to 3-hydroxy-2-butanedione by diacetyl reductase (Smid & Kleerebezem, 2014), thereby reducing the contribution of 2,3-butanedione to the creamy aroma of fermented milk. Therefore, the activity of diacetyl reductase should be considered when screening the strains with prominent creamy flavor. δ-Decalactone was proved to significantly contribute to a creamy odor (Chen et al., 2021, Schlutt et al., 2007). Similar to 2,3-butanedione, the concentration of δ-decalactone in L6-11 was significantly higher than that in L4-1-2, suggesting that δ-decalactone might also be one of the main contributors to the creamy attribute of fermented milk samples. Besides, hexanoic acid and acetic acid could show a rich sour smell. It could be concluded that acetic acid and hexanoic acid might be the main contributors to fermented attributes. Cluster 2 contained two compounds, including acetaldehyde and butanoic acid. Butanoic acid could offer cheese flavor, and the concentration in L4-1-2 was significantly higher than that of L6-11, which suggested that butanoic acid might be the main contributor to the cheesy attribute.
3.7. Effects of the addition of key aroma compounds on aroma attributes
To further explore and verify the effects of the above six aroma-active compounds on aroma attributes of fermented milk, additional experiments were performed, and the results are shown in Fig. 3. The addition of acetaldehyde could improve the aroma intensities of the milky attribute significantly (p < 0.05), which indicated that acetaldehyde was an important contributor to milky attributes. When adding the 2,3-butanedione (Fig. 3e) and δ-decalactone (Fig. 3i) into the AR, the creamy intensities showed significant improvements (p < 0.05), which could be concluded that these two compounds contributed to the creamy attribute. Meanwhile, the additions of δ-decalactone could significantly improve the intensities of the milky attribute, suggesting that acetaldehyde and δ-decalactone might have some important synergistic olfactory effects (Chen et al., 2021). When acetic acid was added to AR, the intensity of fermented attribute increased from 4.9 to 5.33. Still, there was no significant difference (p > 0.05), which indicated that acetic acid was not the main contributor to the fermented aroma. The addition of hexanoic acid not only improved the aroma intensity of the fermented aroma significantly but also enhanced the cheesy aroma, which could be concluded that hexanoic acid was the main contributor to the fermented attribute, and some synergistic olfactory effects also existed in hexanoic acid and butanoic acid. Besides, the addition of 2,3-butanedione (Fig. 3d) and hexanoic acid (Fig. 3h) could reduce the average aroma intensity of fermented attribute (from 4.9 to 4.5), milky attribute (from 0.92 to 0.5), and creamy attribute (from 1.83 to 1.19) to some extent, which suggested that there were some masking or inhibitory effects in aroma compounds (Picon et al., 2019).
In this study, the aroma types of fermented milk samples were based on a prominent aroma attribute of fermented milk samples. The score of cheesy attributes of L4-1-2 assigned to cheesy-type was higher than the other four aroma attributes, and butanoic acid was the main attributor of a cheesy attribute. Therefore, it could be concluded that the butanoic acid was the decisive aroma compound in cheesy-type. Similarly, hexanoic acid and acetic acid were the decisive aroma compounds in the fermented-type.
4. Conclusions
The aroma attributes of fermented milk samples by 28 L. bulgaricus were evaluated, and four aroma types, including milky-type, cheesy-type, fermented-type and miscellaneous-type, were obtained. A total of 95 volatile compounds in cheese-type and fermented-type were identified by GC-IMS and GC–MS, and 12 aroma-active compounds were selected by GC-O-MS. Finally, six aroma-active compounds were determined as the key ones including 2,3-butanedione, δ-decalactone, acetaldehyde, butanoic acid, acetic acid, and hexanoic acid. Butanoic acid was the decisive aroma compound for the cheesy-type, and hexanoic acid was the decisive aroma compound of fermented-type. In the future, these compounds could be used as indicators to screen the strains with the characteristics of cheesy-types or fermented-types.
CRediT authorship contribution statement
Ao Liu: Data curation, Methodology. Qiqi Liu: Data curation, Methodology. Yushan Bu: Data curation, Methodology. Haining Hao: Formal analysis. Tongjie Liu: Formal analysis. Pimin Gong: Visualization, Methodology, Writing – review & editing. Lanwei Zhang: Funding acquisition, Project administration, Writing – review & editing, Supervision. Chen Chen: Data curation, Methodology. Huaixiang Tian: Data curation, Methodology. Huaxi Yi: Funding acquisition, Project administration, Resources, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (31972051, 31771987) and Key Program of Natural Science Foundation of Shandong Province in China (No. ZR2020KC009).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100385.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adam A.C., Rubio-Texeira M., Polaina J. Lactose: The Milk Sugar from a Biotechnological Perspective. Critical Reviews in Food Science and Nutrition. 2005;44(7–8):553–557. doi: 10.1080/10408690490931411. [DOI] [PubMed] [Google Scholar]
- Bi S., Xu X., Luo D., Lao F., Pang X., Shen Q.…Wu J. Characterization of Key Aroma Compounds in Raw and Roasted Peas (Pisum sativum L.) by Application of Instrumental and Sensory Techniques. Journal of Agricultural and Food Chemistry. 2020;68(9):2718–2727. doi: 10.1021/acs.jafc.9b07711. [DOI] [PubMed] [Google Scholar]
- Brown M.D., Chambers D.H. Sensory Characteristics and Comparison of Commercial Plain Yogurts and 2 New Production Sample Options. Journal of Food Science. 2015;80(12):S2957–S2969. doi: 10.1111/1750-3841.13128. [DOI] [PubMed] [Google Scholar]
- Chen, C., Liu, Z., Yu, H., Xu, Z., & Tian, H. (2021). Flavoromic determination of lactones in cheddar cheese by GC-MS-olfactometry, aroma extract dilution analysis, aroma recombination and omission analysis. Food Chemistry, 368, 130736-130736. 10.1016/j.foodchem.2021.130736. [DOI] [PubMed]
- Chen C., Zhao S., Hao G., Yu H., Tian H., Zhao G. Role of lactic acid bacteria on the yogurt flavour: A review. International Journal of Food Properties. 2017;20(sup1):S316–S330. [Google Scholar]
- Cheng H. Volatile flavor compounds in yogurt: A review. Critical reviews in food science and nutrition. 2010;50(10):938–950. doi: 10.1080/10408390903044081. [DOI] [PubMed] [Google Scholar]
- Coggins P.C., Schilling M.W., Kumari S., Gerrard P.D. Development of a sensory lexicon for conventional milk yogurt in the United States. Journal of Sensory Studies. 2008;23(5):671–687. doi: 10.1111/j.1745-459X.2008.00179.x. [DOI] [Google Scholar]
- Dan T., Wang D., Jin R.L., Zhang H.P., Zhou T.T., Sung T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. Journal of Dairy Science. 2017;100(4):2488–2500. doi: 10.3168/jds.2016-11528. [DOI] [PubMed] [Google Scholar]
- Drake M.A., McIngvale S.C., Gerard P.D., Cadwallader K.R., Civille G.V. Development of a descriptive language for cheddar cheese. Journal of Food Science. 2001;66(9):1422–1427. doi: 10.1111/j.1365-2621.2001.tb15225.x. [DOI] [Google Scholar]
- Dunkel A., Steinhaus M., Kotthoff M., Nowak B., Krautwurst D., Schieberle P., Hofmann T. Nature's Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angewandte Chemie-International Edition. 2014;53(28):7124–7143. doi: 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- Engel E., Baty C., le Corre D., Souchon I., Martin N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. Journal of Agricultural and Food Chemistry. 2002;50(22):6459–6467. doi: 10.1021/jf025579u. [DOI] [PubMed] [Google Scholar]
- Gaafar A.M. Volatile flavour compounds of yoghurt. International Journal of Food Science and Technology. 2010;27(1):87–91. [Google Scholar]
- Hajimohammadi Farimani R., Najafi M.B.H., Bazzaz B.S.F., Edalatian M.R., Bahrami A.R., Belen Florez A., Mayo B. Identification, typing and functional characterization of dominant lactic acid bacteria strains from Iranian traditional yoghurt. European Food Research and Technology. 2016;242(4):517–526. doi: 10.1007/s00217-015-2562-3. [DOI] [Google Scholar]
- He Y., Liu Z., Qian M., Yu X., Xu Y., Chen S. Unraveling the chemosensory characteristics of strong-aroma type Baijiu from different regions using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and descriptive sensory analysis. Food Chemistry. 2020;331 doi: 10.1016/j.foodchem.2020.127335. [DOI] [PubMed] [Google Scholar]
- ISO. (2008). Sensory analysis - Vocabulary In, vol. ISO 5492: 2008). Geneva: International Organization for Standardization.
- ISO. (2012). Sensory analysis - General guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors In, vol. ISO 8586: 2012). Geneva: International Organization for Standardization.
- ISO/IDF. (2012). ISO-PDTS11869/IDF-DRM150:2012 Fermented Milks: Determination of Titratable Acidity — Potentiometric Method. In, vol. ISO/TS 11869-2012). Geneva: International Organization for Standardization.
- Jing H. Black garlic: Processing, composition change, and bioactivity. eFood. 2020;1(3):242–246. [Google Scholar]
- Kaneko D., Igarashi T., Aoyama K. Reduction of the off-flavor volatile generated by the yogurt starter culture including Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus in soymilk. Journal of Agricultural and Food Chemistry. 2014;62(7):1658–1663. doi: 10.1021/jf404567e. [DOI] [PubMed] [Google Scholar]
- Liu W., Yu J., Sun Z., Song Y., Wang X., Wang H.…Zhang H. Relationships between functional genes in Lactobacillus delbrueckii ssp bulgaricus isolates and phenotypic characteristics associated with fermentation time and flavor production in yogurt elucidated using multilocus sequence typing. Journal of Dairy Science. 2016;99(1):89–103. doi: 10.3168/jds.2015-10209. [DOI] [PubMed] [Google Scholar]
- Majcher M.A., Myszka K., Gracka A., Grygier A., Jelen H.H. Key Odorants of Lazur, a Polish Mold-Ripened Cheese. Journal of Agricultural and Food Chemistry. 2018;66(10):2443–2448. doi: 10.1021/acs.jafc.6b04911. [DOI] [PubMed] [Google Scholar]
- Ott A., Fay L.B., Chaintreau A. Determination and origin of the aroma impact compounds of yogurt flavor. Journal of Agricultural and Food Chemistry. 1997;45(3):850–858. [Google Scholar]
- Picon A., Lopez-Perez O., Torres E., Garde S., Nunez M. Contribution of autochthonous lactic acid bacteria to the typical flavour of raw goat milk cheeses. International Journal of Food Microbiology. 2019;299:8–22. doi: 10.1016/j.ijfoodmicro.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Rigling M., Fraatz M.A., Troegel S., Sun J., Zorn H., Zhang Y. Aroma Investigation of Chios Mastic Gum (Pistacia lentiscus Variety Chia) Using Headspace Gas Chromatography Combined with Olfactory Detection and Chiral Analysis. Journal of Agricultural and Food Chemistry. 2019;67(49):13420–13429. doi: 10.1021/acs.jafc.9b00143. [DOI] [PubMed] [Google Scholar]
- Routray W., Mishra H.N. Scientific and technical aspects of yogurt aroma and taste: A review. Comprehensive Reviews in Food Science and Food Safety. 2011;10(4):208–220. [Google Scholar]
- Schlutt B., Moran N., Schieberle P., Hofmann T. Sensory-directed identification of creaminess-enhancing volatiles and semivolatiles in full-fat cream. Journal of Agricultural and Food Chemistry. 2007;55(23):9634–9645. doi: 10.1021/jf0721545. [DOI] [PubMed] [Google Scholar]
- Smid E.J., Kleerebezem M. Production of aroma compounds in lactic fermentations. Annual Review of Food Science and Technology. 2014;5:313–326. doi: 10.1146/annurev-food-030713-092339. [DOI] [PubMed] [Google Scholar]
- Tan, F., Wang, P., Zhan, P., & Tian, H. (2021). Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chemistry, 366, 130604-130604. 10.1016/j.foodchem.2021.130604. [DOI] [PubMed]
- Tian H., Sun X., Yu H., Ai L., Chen C. Characterization of the key aroma compounds in Yunnan goat milk cake using a sensory-directed flavor analysis. Journal of Food Science. 2020;85(11):3981–3997. doi: 10.1111/1750-3841.15490. [DOI] [PubMed] [Google Scholar]
- Tian H., Xu X., Chen C., Yu H. Flavoromics approach to identifying the key aroma compounds in traditional Chinese milk fan. Journal of Dairy Science. 2019;102(11):9639–9650. doi: 10.3168/jds.2019-16796. [DOI] [PubMed] [Google Scholar]
- Tian H., Yu B., Yu H., Chen C. Evaluation of the synergistic olfactory effects of diacetyl, acetaldehyde, and acetoin in a yogurt matrix using odor threshold, aroma intensity, and electronic nose analyses. Journal of Dairy Science. 2020;103(9):7957–7967. doi: 10.3168/jds.2019-17495. [DOI] [PubMed] [Google Scholar]
- van Gemert, L. J. (2011). Odour thresholds: Compilations of odour threshold values in air, water and other media : Oliemans Punter.
- Varghese J.E., Balasubramanian B., Velayuthaprabhu S., Thirunavukkarasu V., Rengarajan R.L., Murugesh E.…Anand A.V. Therapeutic effects of vitamin D and cancer: An overview. Food Frontiers. 2021;2(4):417–425. [Google Scholar]
- Wang B., Wang J., Xu L., Zhang J., Ai N., Cao Y. Characterization of the key odorants in kurut with aroma recombination and omission studies. Journal of Dairy Science. 2020;103(5):4164–4173. doi: 10.3168/jds.2019-17521. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang Z., Xu L., Wang B., Zhang J., Li B.…Tan L. Key aroma compounds identified in Cheddar cheese with different ripening times by aroma extract dilution analysis, odor activity value, aroma recombination, and omission. Journal of Dairy Science. 2021;104(2):1576–1590. doi: 10.3168/jds.2020-18757. [DOI] [PubMed] [Google Scholar]
- Wang S., Chen H., Sun B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS) Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- Yang P., Song H., Wang L., Jing H. Characterization of key aroma-active compounds in black garlic by sensory-directed flavor analysis. Journal of Agricultural and Food Chemistry. 2019;67(28):7926–7934. doi: 10.1021/acs.jafc.9b03269. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang B., Fu Y., Shi Y., Chen F., Guan H.…Zhang N. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128880. [DOI] [PubMed] [Google Scholar]
- Zhao L., Feng R., Ren F., Mao X. Addition of buttermilk improves the flavor and volatile compound profiles of low-fat yogurt. LWT-Food Science and Technology. 2018;98:9–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.