Highlights
-
•
Pulsed electric field (PEF) technology enables the extraction of food pigments at lower temperatures.
-
•
PEF process intensification may reduce the extraction yield depending on the plant matrix.
-
•
Coupling PEF with other emerging technologies is a smart strategy to extract natural pigments.
-
•
The application of PEF technology in natural food pigment extraction still requires further studies.
Keywords: Non-thermal processing, Anthocyanins, Betalains, Carotenoids, Chlorophylls
Abstract
Coloring compounds are widely applied to manufacturing foods and beverages. The worldwide food market is replacing artificial colorants with natural alternatives, given the increased consumer demand for natural products. However, these substitutes are still an issue due to their high production cost and low chemical and physical stability. Furthermore, natural pigments are highly sensitive to processes applied in conventional extraction techniques, such as thermal, mechanical, and chemical stresses. In this regard, pulsed electric field (PEF) technology has emerged as a promising non-thermal alternative for recovering and producing natural colorings from food matrices. Its action mechanism on cell structures through the electroporation effect is a smart alternative to overcoming the challenging issues associated with producing natural colorants. In this scenario, this review provides an overview of the PEF assisted extraction of natural pigments and colorants, such as anthocyanins (red-blue-purple), betalains (red), carotenoids (yellow-orange-red), and chlorophylls (green) from plant sources. Moreover, the potential and limitations of this emerging technology to integrate the extraction process of natural colorants were discussed.
1. Introduction
Natural food colorants comprise a class of additives widely applied in the food industry. Their functionality is associated with the aggregation, restoration, or color enhancement of foods and beverages (Damodaran, Parkin, & Fennema, 2010). Therefore, they are widely used to standardize the color of food products, improving their appearance and sensory acceptability (Martins, Roriz, Morales, Barros, & Ferreira, 2016). Also, the increased demand for natural products makes plant and animal colorants stand out as one of the fastest-growing markets globally. As a result, the expected market growth for this sector from 2018 to 2024 is 11% (Wood, 2019).
The current changes in the world consumption pattern are directly associated with this search for natural products. Nowadays, consumers have greater access to nutritional information, mainly due to the emergence of internet technology. Thus, they became more critical of their food choices (Aadil et al., 2018). In this regard, modern consumers from different countries have intensified the search for natural and sustainable products that positively impact human health and support greater longevity and quality of life (Martins et al., 2016). Natural food colorants follow this trend because natural pigments are linked to beneficial health effects. In addition, they are recognized for their activity in preventing chronic non-communicable diseases (NCDs), which are not transmissible directly from one person to another. NCDs, such as heart diseases, diabetes, autoimmune diseases, chronic kidney disease, osteoporosis, cancers, Alzheimer's disease, and Parkinson's disease, are responsible for almost 70% of all deaths worldwide (Ribeiro & Veloso, 2021). Accordingly, implementing natural colorants to replace artificial ones is currently-one of the main concerns of the food industry. As a result, food manufacturers have been pressed to reformulate their products to remove synthetic colorings (Chung, Rojanasasithara, Mutilangi, & McClements, 2017). According to the U.S. Food and Drug Administration (FDA), a color additive is “any substance that imparts color to a food, drug, cosmetic, or the human body”. Currently, the FDA approves nine synthetic colorants for use in foods and beverages. However, a mixture of different synthetic colorings is commonly used in food products to achieve determined color shades, increasing the range of artificial colors in many products (Shea, 2020).
Despite recognized beneficial health effects of natural food colorants and pigments, the application of natural colorings in foods and beverages presents more technological drawbacks when compared to artificial colorants. For example, natural colorants present higher manufacturing costs, lower coloring power, uniformity, and chemical and physical stability (Damodaran et al., 2010). Additionally, the chemical structure of natural pigments presents considerable sensitivity to light, metals, oxygen, and reducing or oxidizing agents. Furthermore, the natural colorants have high instability in some water activity, pH, and temperature ranges (Aadil et al., 2018, Chung et al., 2017, Ribeiro and Veloso, 2021). The extraction process of these sensitive coloring compounds to produce natural colorants is complex. Moreover, separating these compounds from plant matrices is also challenging due to the composition of plant tissue that presents, in addition to the coloring compound, other non-polar constituents, such as fibers, carbohydrates, and proteins (Parniakov et al., 2015).
The extraction process of coloring compounds is conventionally carried out using three main techniques: maceration, Soxhlet, and hydrodistillation (Ngamwonglumlert, Devahastin, & Chiewchan, 2017). These extraction techniques solubilize the pigments in organic solvents, such as hexane, ethanol, acetone, and methanol (Parniakov et al., 2015). Furthermore, thermal, mechanical, and enzymatic methods are coupled to the extraction process to increase the mass transfer of intracellular components. However, these methods promote deleterious effects on cell integrity to enhance the contact area between the solvent and the compound of interest (Luengo, Martínez, Bordetas, Álvarez, & Raso, 2015). Furthermore, high temperatures, intense shear stresses, and longer holding times contribute to an expressive increase in the coloring compound degradation, reducing their extraction yield (Goettel, Eing, Gusbeth, Straessner, & Frey, 2013).
Non-thermal processing technologies have been widely studied to extract natural food colorants and pigments. These technologies employ lower temperatures and small amounts of solvent, increasing the chemical and physical stability of the colorants, energy efficiency, and extraction yield. Furthermore, by not using heat as the primary agent for the extraction processes, non-thermal technologies enable better conservation of thermosensitive components such as pigments (Barba et al., 2015, Barba et al., 2016, Redondo et al., 2018). The leading innovative non-thermal technologies that stand out in the market are high-intensity ultrasound (HIUS), pulsed electric field (PEF), pulsed light (PL), high hydrostatic pressure (HHP), and cold plasma (CP) (Nowacka et al., 2021). PEF technology, in turn, is a promising treatment of short duration, which provides high-intensity pulsed electric fields from a high current flow (Z.-H. Zhang, Wang, Zeng, Han, & Brennan, 2019). These high-intensity pulsed electric fields cause electroporation of cell membranes. This phenomenon destabilizes the cell's bilipid layer, making it more permeable and facilitating the extraction of intracellular compounds (Guerrero-Beltrán & Welti-Chanes, 2016).
By increasing the permeability of the cells, PEF enables a higher mass transfer of intracellular components (Barba et al., 2016). Thus, this emerging technology decreases the need for high temperatures and amounts of solvent, reducing environmental impacts and enhancing the energy efficiency of the processes. Moreover, it promotes minimal changes when it comes to the nutritional and sensory aspects of the product due to the low temperature and holding time of the extraction (Martínez et al., 2020, Moreira et al., 2019, Picart-Palmade et al., 2019, Priyadarshini et al., 2019, Redondo et al., 2018). However, for the integration of PEF on a large industrial scale, more studies to optimize the process conditions and parameters are still needed (Arshad et al., 2020).
Many studies about the application of PEF technology in the extraction process of natural food pigments and colorants are found in the literature (Aadil et al., 2018, Gachovska et al., 2010, Luengo et al., 2016, Medina-Meza and Barbosa-Cánovas, 2015, Nowacka et al., 2019, Pataro et al., 2020, Puértolas et al., 2013, Putranto et al., 2014, Siddeeg et al., 2019, Zhou et al., 2015). New extraction process designs based on PEF technology have been evaluated due to the high demand for natural food colorings in the world food market, besides the drawbacks of conventional extraction processes. In this scope, this review provides an overview of the PEF assisted extraction of natural pigments and colorants from plant matrices. Moreover, the potential and limitations of this emerging technology to integrate the extraction process of natural colorants were discussed.
2. Natural food pigments and colorants
Color is one of the main aspects of developing food products (Sun, Xin, & Alper, 2021). The color characteristics of the product affect its visual appearance, influencing consumers' perception of its flavor and quality (Martinez, Rando, Agante, & Abreu, 2021). This influence is justified by the ability of colors to refer to pre-developed concepts by consumers. They have perceptions that trigger positive or negative sensations depending on the tonality, brightness, and intensity of the food color (Luo et al., 2019, Spence, 2018).
The color influence on the sensory perception of consumers begins with food packaging. The packing of the same food product using two distinct color packages indicated that the color directly influenced consumers' perception of the product (Luo et al., 2019). Red-colored packaging was associated with a tastier product, while green packaging was associated with a healthy product by consumers. Thus, there is a close relationship between color and sensory perception and the purchase intention of a product.
Therefore, the color of the product needs to maintain its stability during the shelf life established for the food product. Furthermore, the changes observed in the color of the product during its processing, storage, packaging, and distribution stages must be overcome (Martins et al., 2016). Thereby, colorants are usually applied to food products to perform aggregating, intensifying, or restoring the color of their sensory profile (Ngamwonglumlert et al., 2017).
Colorants are additives divided into three categories according to their source: natural (animal, vegetable, or mineral), synthetic identical to the natural, or artificial (Damodaran et al., 2010). All types of colorants present chromophores in their structure. These chromophores are functional groups capable of absorbing energy from radiation, exciting their electrons at a higher energy level. Thus, when the chromophores' electrons return to their ground state, they release energy at a specific wavelength, setting the product color (Aadil et al., 2018, Martins et al., 2016, Selvaraj et al., 2021).
The natural colorants stand out among the three colorants categories due to the current changes in world consumption patterns. These patterns reflect that consumers are more critical and aware of the products they consume. As a result, they are increasingly looking for foods that promote beneficial effects on their health (Aadil et al., 2018, Martins et al., 2016). Natural colorants follow this trend as they present commercial appeal associated with their biological functionality (antimicrobial and antioxidant activity) and action in preventing NCDs. However, natural food colorants still present technological limitations that make complex and expensive their integration into food development. These colorants are highly sensitive to temperature, pH, water activity, light, reducing or oxidizing agents, metals, and oxygen (Aadil et al., 2018, Chung et al., 2017, Ngamwonglumlert et al., 2017, Ribeiro and Veloso, 2021). Additionally, natural colorants present a lower spectrum and color varieties than artificial ones. They also are more expensive due to the low energy efficiency of the extraction processes (Martins et al., 2016).
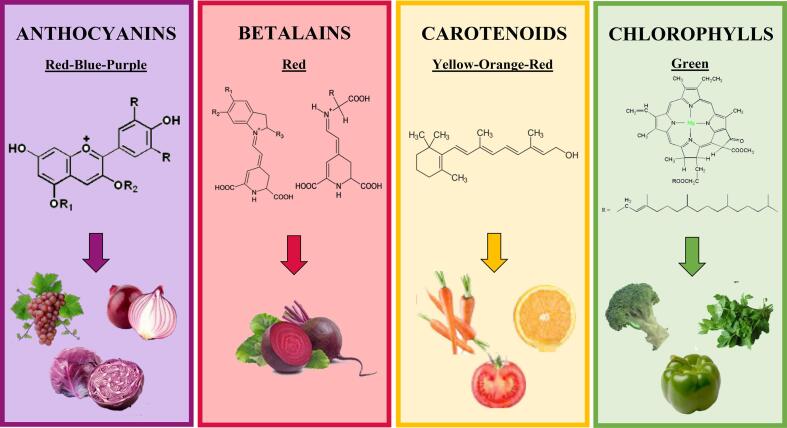
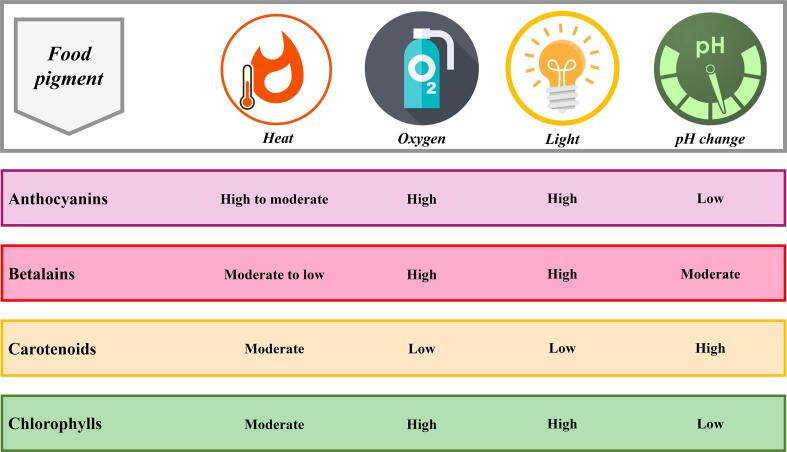
Fig. 1 presents the main pigments used to produce natural food colorants. They are derived from plant sources and divided into four main groups: anthocyanins (red-blue-purple), betalains (red), carotenoids (yellow-orange-red), and chlorophylls (green) (Rodriguez-Amaya, 2016). These compounds present different color stabilities when exposed to different environmental conditions. Fig. 2 shows the color stability of anthocyanins, betalains, carotenoids, and chlorophylls to heat, oxygen, light, and pH change. In this regard, the choice of extraction conditions needs to consider the characteristics of each pigment to minimize the degradation of the components (Ngamwonglumlert et al., 2017).
Fig. 1.
Main pigments used to produce natural food colorants: Anthocyanins, betalains, carotenoids, and chlorophylls.
Fig. 2.
Color stability of anthocyanins, betalains, carotenoids, and chlorophylls to heat, oxygen, light, and pH change. Adapted from Ngamwonglumlert et al. (2017).
Conventionally, natural colorants are extracted through solid–liquid extractions (SLE). In these processes, the plant tissues' solid particles migrate to a liquid phase by diffusion due to the concentration gradient difference. SLE extractions are usually performed with organic solvents that solubilize the pigments, such as ethanol, methanol, and hexane. Thus, separation and purification steps are carried out to eliminate the solvent of the extract and purify the extracted compounds after the extraction. The Soxhlet extraction is an example of SLE. In this exhaustive extraction technique, organic solvents are continuously cycled through the plant matrix to recover non-polar components by boiling and condensing the solvent. However, several disadvantages can be related to the Soxhlet extraction technique. For example, the extraction condition at the boiling point of the solvent for an extended period favors the thermal decomposition of target compounds (Aadil et al., 2018, Parniakov et al., 2015, Puértolas and Barba, 2016).
2.1. Anthocyanins
Anthocyanins comprise a group of pigments widely used in the food industry as natural colorants, whose color spectrum includes red, violet, and blue. These compounds belong to the flavonoid class and are composed of glycosides of polymethoxy and polyhydroxy derivatives of the flavylium ion or 2-phenylbenzopyrilium salts (Pascual-Teresa, 2014). Moreover, anthocyanins have in their structure a non-glycosylated base known as anthocyanidin. This non-glycosylated base, in turn, is usually associated with one or more molecules of sugars and other compounds derived from the reaction between sugars and acids present in the medium (Ribeiro & Veloso, 2021).
Furthermore, anthocyanidin is formed from double bonds conjugated between three carbon rings. This base is responsible for the coloring activity of the anthocyanins since it is the chromophore of their structure. Therefore, anthocyanins can be used as natural colorants in foods currently developed on the market, such as fermented milk, cream cheese, milkshakes, low pH drinks, pancakes, and omelets (Cortez, Luna-Vital, Margulis, & Gonzalez de Mejia, 2017).
Anthocyanins are found exclusively in vegetable sources like fruits and vegetables such as açaí, blackberry, fig, eggplant, strawberry, plum, jambolão, jabuticaba, cherry, acerola, raspberry, grape, apple, purple potato, and red cabbage (Kammerer, 2016). Also, the most abundant molecules belonging to the anthocyanin class are cyanidin, delphinidin, pelargonidin, malvidin, petunidin, and peonidin (Pascual-Teresa, 2014).
The nucleus of anthocyanins is 2-phenyl-benzopyrilium, also known as flavylium cation. It promotes a high structural variation for anthocyanin molecules due to the susceptibility of its aglycone fractions to acylation and glycosylation reactions (Pascual-Teresa, 2014). Therefore, due to this structural variation, anthocyanins stand out as one of the most diverse and widespread classes of pigments in the plant kingdom. More than 700 compounds have been isolated (Damodaran et al., 2010, Santos-Buelga et al., 2014). Besides that, the colors presented by these molecules depend on the pH of the medium they are in (Rodriguez-Amaya, 2016).
The red color is predominant at pH ranging from 1 to 2 because the flavylium ion is prevalent. The molecule shows a blue color from pH 2 to 4 due to the presence of the quinoidal base. Otherwise, a colorless profile is visualized in systems submitted to pH values from 4 to 6 due to the carbinol pseudobase. Finally, anthocyanins exposed to pH values higher than 6 show a pale yellow color due to the presence of chalcone in its structure (Delgado-Vargas et al., 2000, Ribeiro and Veloso, 2021, Rodriguez-Amaya, 2016, Rodriguez-Amaya, 2019).
In addition to pH, the stability of anthocyanin molecules is influenced by light, temperature, oxygen, presence of solvents, enzymes, sulfites, sugars, proteins, or other flavonoids (Ngamwonglumlert et al., 2017, Rodriguez-Amaya, 2016). Thereby, anthocyanins have a limited application as a food colorant and functional ingredient since their instability can change the sensory profile of the food (Cortez et al., 2017).
On the other hand, anthocyanins aggregate their functional properties to food matrices since these molecules are associated with preventive action against cardiovascular and neurological diseases (Pascual-Teresa, 2014). However, the literature on the subject is still limited. Thus, further studies about how anthocyanins promote beneficial effects on human health are needed.
2.2. Betalains
The beetroot is the primary source of betalains. However, they are also commonly found in swiss chards and cactus fruits. The betalains include the red/violet and/or yellow-orange color spectrum. Thereby, the main pigments associated with the production of colorants are betalains. The structure of these compounds is a product of the conjugation between betalamic acid and a primary or secondary amine. This pigment class is divided into two main groups: betacyanins (red/violet) and betaxanthins (orange-yellow). Both groups have betalamic acid as a precursor (Chandrasekhar et al., 2015, Damodaran et al., 2010, Delgado-Vargas et al., 2000).
Betanin is the main pigment in beetroot. It is the central molecule present in the class of betalains applied in the production of colorants. This compound has greater stability to pH variations than the anthocyanins, remaining stable in the pH range of 3 to 7. Betanins subjected to pH values lower than three change their color from red to blue-violet. On the other hand, pH values greater than 7 promote the alkaline hydrolysis of aldimine's bonds, degrading the molecule of betanin to cyclodopa-5-O-glycoside and betalamic acid (Ngamwonglumlert et al., 2017).
Betalains can also be thermally degraded as high temperatures induce oxidation, decarboxylation, and hydrolysis reactions of aldimine bonds in the molecule. These degradations promote drastic changes in its color (Ngamwonglumlert et al., 2017). Moreover, metallic ions can also favor the degradation of betalains since the presence of tin, aluminum, copper, and iron accelerates oxidation reactions. Therefore, the presence of these ions can contribute to changes of the color (Azeredo, 2009).
Betanin is more unstable than betalains, due to other intrinsic and extrinsic factors, which limits its application as a food colorant. These factors include chemical structure, additives, enzymes, water, oxygen, and light activity (Azeredo, 2009, Ribeiro and Veloso, 2021). Besides that, betanin has an earthy aftertaste due to the presence of geosmin and pyrazine in its extract. This fact also limits its use as a colorant (Rodriguez-Amaya, 2019). However, due to its high availability and coloring properties, betanin is one of the most important red food colorants. As with the other natural pigments, the intake of betalains can promote human health benefits. Studies have demonstrated the antioxidant, cardioprotective, antimicrobial, antiproliferative, anticancer, antidiabetic, and anti-inflammatory activity of betalains (Esatbeyoglu, Wagner, Schini-Kerth, & Rimbach, 2015).
2.3. Carotenoids
Carotenoids are pigments widely distributed in nature, more than 650 types have been identified, and approximately 100 have been found in food matrices. These pigments are tetraterpenes formed from the union of 8 isoprene molecules, which result in a long chain of double bonds. This structure is the chromophore of the carotenoids (Ribeiro & Veloso, 2021). The color spectrum of carotenoids depends on the quantity and distribution of double bonds and includes red, yellow, and orange (Rodriguez-Amaya, 2019). The main sources of carotenoids are squash, carrots, spinach, broccoli, sweet potatoes, papayas, and apricots. Unlike plants, animals are not capable of synthesizing carotenoids. However, they can absorb, modify, and deposit them in their tissues, such as the yellow in the egg yolk (Miller, Li, & Liu, 2014).
These pigments are divided into two main groups, carotenes and xanthophylls. The difference between carotenes and xanthophylls is the atoms that form their molecular structure. Carotenes present only carbon and hydrogen in their structure, while xanthophylls present carbon, hydrogen, and oxygen. Carotenoids are also classified into primary and secondary carotenoids according to their functionality. The primary carotenoids are vital molecules for plant photosynthesis. They are β-carotene, lutein, and zeaxanthin. The secondary ones are not associated directly with the plant survival mechanisms. They comprise α-carotene, lycopene, and astaxanthin (Ngamwonglumlert et al., 2017).
The extraction of carotenoids is usually carried out from their dissolution in organic solvents, considering their hydrophobic nature. Thus, tetrahydrofuran and petroleum ether are usually used to extract non-polar molecules, such as esterified xanthophylls and non-polar carotenes. On the other hand, the primary solvents used are acetone, ethyl acetate, and ethanol for more polar carotenoids, such as polar carotenes. The simultaneous extraction of these pigments is a complex process due to the different polarities of carotenoid molecules (Saini & Keum, 2018).
This class of fat-soluble pigments stands out mainly because they are precursors of vitamin A. This micronutrient is essential for maintaining the normal functions of the human body (Uenojo, Maróstica, & Pastore, 2007). Additionally, carotenoids are capable of stabilizing free radicals and scavenging singlet O2. Thus, they are highly effective as antioxidants, avoiding the oxidative processes in food. Among the main carotenoids in foods, the following stand out β-carotene, lycopene, β-cryptoxanthin, lutein, α-carotene, and zeaxanthin (Rodriguez-Amaya, 2016).
However, these compounds are unstable to extrinsic and intrinsic food factors and other pigments. Carotenoids have high pH stability and moderate heat stability, but present high sensitivity to oxygen and light. These factors can promote the oxidative degradation of pigments through the isomerization and oxidation processes of their molecules. These degradations promote color changes in the food (Ngamwonglumlert et al., 2017, Rodriguez-Amaya, 2019).
2.4. Chlorophylls
Chlorophylls are the pigments responsible for the green color of plants, algae, and bacteria. They are widely distributed in nature, found in plant chloroplasts generally associated with carotenoids, lipids, and lipoproteins. Moreover, chlorophylls are responsible for capturing energy for the process of photosynthesis, playing a vital role in plants (Damodaran et al., 2010).
The chlorophyll structure is formed by four pyrrole nitrogen rings linked to a central magnesium ion and a fifth ring containing a phytol tail. The phytol is responsible for the hydrophobicity of the molecule (Nagini, Palitti, & Natarajan, 2015). Chlorophyll has a high sensitivity to pH variations, remains stable over a range of 3.5 to 5.0, and is moderately sensitive to heat (Ngamwonglumlert et al., 2017). In an acidic medium, there is a substitution of the magnesium ion by two hydrogen atoms in the chlorophyll structure that leads to the development of a brown color resulting from the formation of pheophytin in the system (Ribeiro & Veloso, 2021).
Despite the problems associated with the sensitivity of chlorophylls to intrinsic and extrinsic food factors, they present crucial biological functions. Among these, the antimutagenic and anticarcinogenic activities that prevent chronic diseases (Yilmaz & Gökmen, 2016).
3. PEF technology
3.1. General aspects
PEF technology is a non-thermal, minimally invasive, and environment-friendly technique that has been widely used to treat biological tissues and biomaterials with several applications in food, biotechnology, medicine, and environmental technologies (Guerrero-Beltrán and Welti-Chanes, 2016, Vorobiev and Lebovka, 2017). Among its main applications in food systems, recovery of phytochemical compounds from plant matrices, microbial and enzymatic inactivation, dehydration and freezing, the concentration of bioactive compounds, and improvement of physicochemical, rheological, and structural properties of food products stand out (Barba et al., 2016, Li and Farid, 2016, Moreira et al., 2019, Redondo et al., 2018, Saldaña et al., 2021, Zhang et al., 2019).
The application of the PEF in extraction processes has been studied due to the electroporation ability to increase the mass transfer of intracellular components of the plant matrix (Barba et al., 2015, Goettel et al., 2013, Saldaña et al., 2021, Zhang et al., 2019). Furthermore, the treatment with an electric field destabilizes the bilipid layer of the cells, changing its permeability and facilitating the contact between the solvent and the target intracellular components. Thus, there is an increase in the yield of the extraction process (Guerrero-Beltrán & Welti-Chanes, 2016).
In this sense, integrating the PEF into the extraction process positively affects the extraction due to its ability to increase the mass transfer of intracellular components (Barba et al., 2016). Furthermore, this non-thermal technology reduces the temperature and concentration of solvent used in the extraction process as it facilitates accessibility to targeted intracellular components (Moreira et al., 2019). Thereby, reducing the use of natural resources and solvents is possible. Thus, this emerging technology presents the principles of green chemistry, as its application provides a positive social and environmental impact (Martínez et al., 2020, Picart-Palmade et al., 2019, Priyadarshini et al., 2019, Redondo et al., 2018).
In addition to increasing the yield of the extraction process, the PEF technology promotes minimal changes to the sensory and nutritional properties of the products (Moreira et al., 2019). This fact is directly associated with the low temperatures used during the process, around 30–40 °C. These corroborate reducing the harmful effects of thermosensitive compounds in the food matrix (Parniakov et al., 2015, Redondo et al., 2018). On the other hand, some negative aspects are also reported in the application of this emerging technology, such as free radical formation, possible leaching of bioactive compounds, and enzymatic degradation of recovered pigments (Barba et al., 2015, Zhang et al., 2011).
The 2030 schedule for the sustainable development of the global production system expected intensification of the search for sustainable technologies that present a high energy efficiency and promote minimal damage to the sensory and nutritional properties of food (Li and Farid, 2016, Picart-Palmade et al., 2019, Zhang et al., 2019). Therefore, the PEF stands out as a promising technology for several food science and technology sectors.
The use of milder process conditions during the extraction of coloring compounds is essential to maintain their chemical and physical stability. However, conventional extraction techniques, such as the Soxhlet, hydrodistillation, heat reflux, and boiling, apply medium and high temperatures during the extraction processing (Chalermchat et al., 2004, Li et al., 2006). The heat used by these processes contributes to the degradation of thermosensitive compounds present in the plant matrices. Therefore, it contributes to a lower extraction yield (Moreira et al., 2019). In this regard, the PEF is a promising extraction technology since it uses lower process temperatures, which favors the preservation of phytochemical compounds (Goettel et al., 2013).
On the other hand, the PEF technology also has some drawbacks that limit its integration in extraction processes. According to Picart-Palmade et al. (2019), the main PEF disadvantage is the high cost of the equipment, which implies a high initial investment. However, the operating cost is low, offsetting the initial investment (Arshad et al., 2020, Martínez et al., 2020). Another disadvantage concerns its effectiveness, which depends on the conductivity of the environment in which it is inserted. High conductivity values reduce the effectiveness and specificity of the electroporation phenomenon (Martínez et al., 2020).
Furthermore, applying the PEF technology in processing promotes a temperature gradient in the samples that varies according to their conductivity. Foods with higher conductivity have many conductive ions that converts electrical energy into heat during PEF treatment. Thereby, the treatments need to be thermally controlled to avoid further degradation of the thermosensitive components of the plant matrix (Samaranayake, Sastry, & Zhang, 2005). Therefore, cooling coils are commonly integrated to avoid excessive heating of the system and changes in the nutritional and sensory characteristics of the products (Ramos, Teixeira, Stringheta, Chaves, & Gomes, 2006).
Moreover, most foods treated by PEF technology are homogeneous liquids. The application of the technology in heterogeneous solid or liquid materials incorporates air into the product. The air incorporation promotes the phenomenon of dielectric rupture. This rupture occurs due to an electrical discharge between the two electrodes in the treatment chamber. This phenomenon catalyzes undesirable reactions in the food and can cause explosions in the equipment due to the spark formation (Ramos et al., 2006).
3.2. PEF system
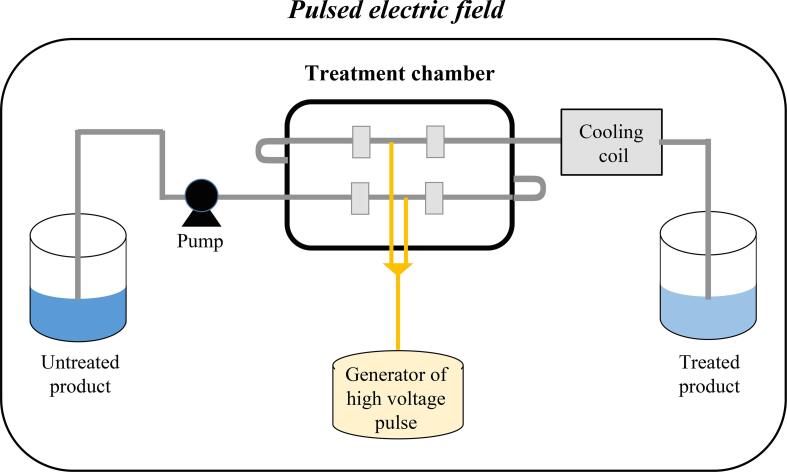
The equipment used in the PEF treatments presents a high-voltage pulse generator, treatment chamber, cooling coil (not common to all equipment), and an external control and monitoring system (Ramos et al., 2006). Fig. 3 presents the basic components of the PEF system. The treatments occur by the discharge of repetitive energy pulses to the food matrix, ranging from 20 to 80 kV/cm (high intensity) or from 0.1 to 0.3 kV/cm (low intensity) for a short time (less than 1 s) (Kumari et al., 2018, Saldaña et al., 2021).
Fig. 3.
Basic components of the pulsed electric field system.
This process is performed by inserting the food into the treatment chamber between two electrodes. The electrodes promote a differential of electric potential in the system due to the conductive properties of the food matrix (Redondo et al., 2018). The intensity of the treatment depends on the electrical flow applied to the product and the distance between both electrodes and their respective formats (Kovačić et al., 2021). The smaller the camera diameter and the distance between the electrodes, the greater the uniformity and intensity of the electric fields generated (Kumar, Barrett, Delwiche, & Stroeve, 2009). The operating parameters of PEF treatments are holding time or total treatment time, polarity, electric field strength, pulse repetition frequency, pulse specific energy, pulse shape, pulse width, and pulse number (Raso et al., 2016).
The electric pulse generator converts the electrical energy from the source into pulsed electric fields. This energy is stored in capacitors to further be directed to the treatment chamber (Kandušer, Belič, Čorović, & Škrjanc, 2017). These generated pulses can present several forms depending on the field strength and treatment time. The main waveforms are square, oscillatory, and exponential (Binoti et al., 2012). The generator has a relatively simple system, formed by a source, resistors, capacitor bank, oscilloscope, and other components common to electrical systems (Ramos et al., 2006). However, there are different types of PEF generators. Moreover, they can differ regarding their configuration. For example, closing switches are demanded generators with capacitive storage. In contrast, opening switches are required for generators with inductive storage (Mohammed & Eiss, 2012). In this regard, food manufacturers or researchers need to receive technical support from a PEF technology expert or company to acquire suitable machinery or equipment to meet the process requirements.
An external monitoring system controls the process parameters that guide the generation of high-intensity pulses. This system controls the strength of the electric field (kV/cm), pulse frequency (Hz), pulse duration (µ seconds), and wave shape. These parameters are defined according to the treated food, considering their respective densities, viscosities, specific heats, and electrical conductivities (Kovačić et al., 2021). In general, these properties are thermal dependent. Thus, the parameters used in electric field technology must be estimated according to the temperature used during the treatment (Raso et al., 2016).
The treatment chamber is the structure where the food or plant matrix will be subjected to high-intensity electrical pulses. In this chamber, at least two electrodes are present. They are supported in parallel by non-conductive materials (Barba et al., 2016). These are also placed inside the chamber to hold the food matrix properly. One of these electrodes has an associated high voltage, while the other has zero potential. Furthermore, the chamber structure presents voltage probes, a temperature measurement system, air removal equipment (which aims to reduce the phenomenon of dielectric breakdown), and a refrigeration system (Raso et al., 2016).
Moreover, the PEF treatment can be performed in batch or continuous chambers, depending on the treatment to be applied. Batch PEF-treatment chambers provide uniform treatments that are widely applied on a laboratory scale. This chamber enables a more uniform treatment since there is no continuous raw material input. For continuous PEF-treatment chambers, raw material input is continuous in the chamber, making it challenging to uniform the phenomenon of electroporation and heating. However, continuous chambers are required on an industrial scale (Binoti et al., 2012, Kovačić et al., 2021).
3.3. Extraction mechanism
The PEF extraction mechanism is based on applying low-intensity electric fields (0.5–5 kV/cm) to biological tissues. Thus, there is an accumulation of charges on the surface of cell membranes that reorient the electrical charges or dipoles of the lipid molecules present in their structure. Thereby, the membrane potential or membrane voltage of the bilipid layer is increased, promoting a weakening of the plasma membrane, and decreasing the selectivity for the entry of extracellular ionic components. The intensity of this potential depends on parameters such as electric field amplitude, sample conductivity, cell radius and shape, and the angle between the direction of the field lines and normal to the membrane (Kovačić et al., 2021).
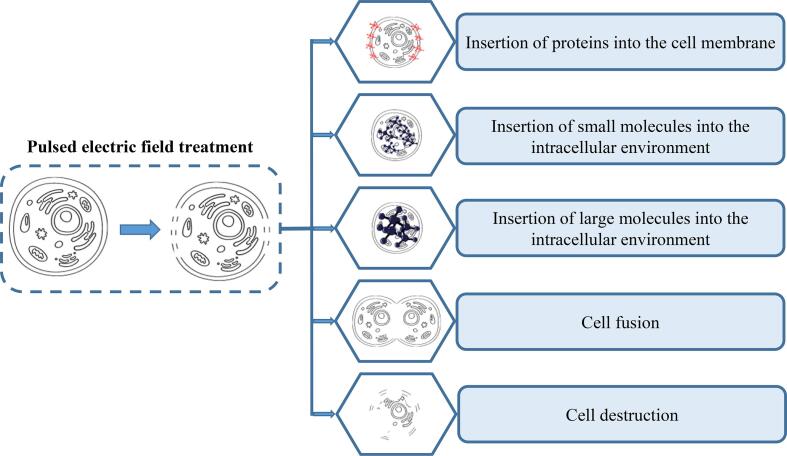
This membrane potential forms pores in the cell structure, changing its semi-permeability by reaching critical values. This phenomenon, called electroporation, can occur permanently or reversibly depending on the intensity of the applied electric field. The cells cannot return to their original semi-permeability after being hit by a specific field value (Kandušer et al., 2017). Electroporation can promote deleterious effects on cell integrity, such as cell fusion, protein aggregation to the plasma membrane, insertion of micro and macromolecules, and destruction of cell structure (Kandušer & Miklavčič, 2008). Fig. 4 presents the PEF effects on cell structure.
Fig. 4.
Effects of pulsed electric field on cell structure.
Electroporation promoted by the PEF in plant tissue comprises a dynamic and non-instantaneous process whose mechanism is divided into some phases . The first one occurs at approximately 10 ns and consists of polarization and charging of cell membranes, which promotes the initial formation of pores and temporary destabilization of the membrane structure. The second phase consists of the entire period of contact of the sample with electrical pulses (less than 1 s). In this step, the pores are expanded and aggregated. The third and final electroporation step comprises restructuring the cell membrane when it tries to return to its original semi-permeable structure (Pataro, Ferrari, & Donsì, 2011).
The formation of pores in the cell structure accelerates the mass transfer rate of its intracellular components by altering the permeability of the plasma membrane. Electroporation under critical conditions can promote the disintegration of membrane structures. Potentials in the range of 2 V at 4 °C, 1 V at 20 °C, and 500 mV at 30–40 °C have disintegrated the lipid-protein membranes of plant tissue (Pataro, Ferrari, & Donsì, 2011, Vorobiev and Lebovka, 2017).
The uniformity and intensity of the electroporation phenomenon are important to assess the ability of the PEF to increase mass transfer in plant tissue. The parameters for the evaluation include the intensity of the pulsed electric field, pulse type, number of pulses, treatment time, and components present in the plant matrix (Kandušer et al., 2017).
3.4. Green chemistry and sustainability principles
Many studies have pointed out PEF technology as a smart alternative for designing products and processes able to reduce or even eliminate the generation and use of hazardous substances (Basak & Annapure, 2022). Indeed, PEF assisted extraction processes meet all green chemistry concepts and sustainability principles. PEF is a green extraction process that enables the use of environmentally friendly solvents to extract natural colorants, avoiding the contamination of foods and beverages with toxic solvents (Barba et al., 2015). Regarding sustainability principles, PEF assisted extraction processes are more energy-efficient compared to conventional techniques. The short treatment time observed in PEF-based processes is one of the main characteristics of this innovative technology, which favors energy savings (Zia et al., 2022). However, there are scarce life cycle assessment studies investigating the environmental impacts of PEF technology on different manufacturing sectors, including extraction processes of bioactive compounds (Aganovic & Smetana, 2022). In this regard, deeper discussions concerning the sustainability and green chemistry principles as well as life cycle assessment studies for the production of natural food colorants are missing.
4. PEF assisted extraction of food pigments
PEF technology has been evaluated as a promising technique for increasing mass transfer during extraction process due to its deleterious effects on cell integrity (Ngamwonglumlert et al., 2017). The increase in the mass transfer is an effect of electroporation since the application of electric fields (0.5–5 kV/cm) forms pores in the cellular structure of plant tissue. Thus, electroporation alters the permeability of the plasma membrane, facilitating the mass transfer of pigments present in the system (Kovačić et al., 2021).
Exponential growth in the number of studies about the integration of the PEF in the extraction of pigments occurred in the last decades due to the capacity of this technology to facilitate the solvent diffusion to the intracellular medium (Gachovska et al., 2010, Luengo et al., 2016, Medina-Meza et al., 2016, Nowacka et al., 2019, Pataro et al., 2020, Puértolas et al., 2013, Putranto et al., 2014, Siddeeg et al., 2019, Zhou et al., 2015). Table 1 presents some interesting studies concerning the PEF assisted extraction of food pigments and colorants from plant matrices. These studies were selected because they aimed to increase energy efficiency and extraction yield and obtain stable bioactive compounds.
Table 1.
PEF assisted extraction of natural food pigments and colorings from plant matrices.
| Food pigment | Plant matrix | PEF process parameters | Major findings | References |
|---|---|---|---|---|
| Anthocyanins | Purple potatoes (Solanum tuberosum) |
|
Regardless of extraction temperature (10 - 40°C) or solvent (water and ethanol) used for extraction of anthocyanins, PEF-treated samples had greater extraction yield compared to those untreated. PEF treatment increased the anthocyanins recovery yield and reduced the extraction temperature besides eliminating the use of organic solvents. | (Puértolas et al., 2013) |
| Anthocyanins | Blueberry (Vaccinium sect. Cyanococcus) |
|
PEF treatment increased anthocyanin extraction yield and reduced the temperature and extraction time. High electric field strength (>20 kV/cm) and pulse number (>10 pulses) reduced anthocyanin extraction yield. PEF treatments were more efficient compared to high-intensity ultrasound treatments. | (Zhou et al., 2015) |
| Anthocyanins | Grape (Vitis vinifera L.) |
|
The PEF process intensification increased anthocyanin extraction. PEF treatment was more efficient for anthocyanin extraction than high-intensity ultrasound (up to 22%) and high voltage electrical discharges (up to 55%) treatments. | (Barba et al., 2015) |
| Anthocyanins | Plum peel (Prunus domestica var. Casselman) and grape peel (Vitis vinifera L.) |
|
The 25-mm diameter PEF chamber showed best results than the 7-mm diameter. The use of a PEF chamber of a larger diameter allowed the application of a higher residence time and a higher number of pulses, which increased the anthocyanin extraction yield. However, this treatment promoted a high degradation of ascorbic acid. | (Medina-Meza et al., 2016) |
| Betanins | Beetroot (Beta vulgaris L.) |
|
The electric field strength of 4.38 kV/cm and specific energy input of 4.10 kJ/kg allowed the extraction of 329% more betanin than the control treatment. | (Nowacka et al., 2019) |
| Betanins | Beetroot (Beta vulgaris L.) |
|
The betanin extraction yield was affected by the applied electric field strength, temperature, and pH of the liquid medium. However, the pressure and number of pulses used in the process did not significantly influence the betanin recovery. The pH 3.5 and temperatures in the range of 30 - 40 °C contributed to the highest yields. | (López et al., 2009) |
| Betanins | Beetroot (Beta vulgaris L.) |
|
PEF treatments in the range of ms and μs were effective for the disintegration of red beet cells enhancing betanin aqueous extraction. However, PEF treatments in the range of μs were more efficient for improving betanin extraction in terms of total specific energy consumption. | (Luengo et al., 2016) |
| Betanins | Red prickly pear (Opuntia stricta Haw.) |
|
PEF treatment was more profitable compared to ultrasound treatment due to lower energy consumption. Furthermore, scanning electron microscopy images revealed the ability of PEF treatment to induce cell wall permeabilization without disintegrating the cell tissue facilitating the selective recovery of the valuable intracellular compounds. | (Koubaa et al., 2016) |
| Carotenoids | Date fruit (Phoenix dactylifera L., Sukkari) |
|
The increase in the electric field strength promoted a positive effect on the total carotenoid content of the extracts from 2.9 ± 0.1 to 6.1 ± 0.1 µg/mL. | (Siddeeg et al., 2019) |
| Carotenoids | Tomato (Solanum Lycopersicon) |
|
PEF treatment increased the extraction yield of carotenoids, especially lycopene, using acetone or ethyl lactate in the proportion 1:40 g/mL as solvents. | (Pataro et al., 2020) |
| Carotenoids | Canary tomato (Solanum lycopersicum) peel |
|
The cell membrane electroporation of tomato peels increased the extraction of carotenoids. PEF treatment reduced the extraction time and the proportion of hexane applied as a solvent without affecting the lycopene, lutein, and β-carotene extraction yield. | (Luengo et al., 2014) |
| Carotenoids | Tomato (Lycopersicon eculentum L.) |
|
Previous application of a pulsed electric field treatment at 0.50 kV/cm and 1 kJ/kg before the process of steam bleaching at 60°C significantly increased the carotenoid extraction yield. | (Pataro et al., 2018) |
| Chlorophylls | Custard apple (Annona squamosa) leaves |
|
Higher energy PEF treatments were promising non-thermal treatments for improving the extraction yield of flavanols, such as rutin. However, they reduced the chlorophyll content in the extracts. | (Shiekh et al., 2021) |
| Chlorophylls | Spinach (Spinacia oleracea L.) |
|
PEF treatments inhibited the degradation of pigments, especially for the recovery of chlorophylls under different process temperatures (20, 35 and 45 °C). | (Zhang, Wang, Zeng, Han, & Wang, 2017) |
The main advantage of PEF assisted extraction is altering the cell permeability, promoting minimal thermal degradation. Thus, favoring the retention of thermosensitive compounds (Barba et al., 2015, Goettel et al., 2013, Saldaña et al., 2021, Zhang et al., 2019). Furthermore, Ngamwonglumlert et al. (2017) indicated PEF as a promising technology to assist the extraction of betalains since this class of pigments has a high sensitivity to heat. Nowacka et al. (2019) evaluated the effects of electric field strength (4.38 and 6.25 kV/cm), energy input (0–12.5 kJ/kg), and the number of pulses (10–30) on the mass transfer rate of betalains from beetroot (Beta vulgaris L.). The PEF application (4.38 kV/cm at 4.10 kJ/kg) in the extraction increased the yield of betanin by 329% and that of vulgaxanthin by 244% compared to the untreated sample.
The integration of the PEF in the anthocyanin extraction process also showed positive results (Gachovska et al., 2010). The PEF application (2.5 kV/cm, 50 pulses, and 15 μs) increased the extraction yield of total anthocyanins in red cabbage (Brassica oleracea var. capitata f. rubra) by 2.15 times. The pretreatment by applying a pulsed electric field did not accelerate the pigment degradation process due to heat or light.
Shiekh, Olatunde, Zhang, Huda, and Benjakul (2021) examined the impact of electric field strength (2, 4, and 6 kV/cm) and specific energy input (45, 94, and 142 kJ/kg) on the recovery of chlorophylls from custard apple (Annona squamosa) leaves. They observed an adverse effect of the electric field strength and specific energy on the extraction yield of chlorophylls. The total chlorophyll content was lower in the custard apple leaf extract treated with PEF than in the non-treated samples. This low efficiency of PEF technology may be associated with the polarity of the solvent used (Ngamwonglumlert et al., 2017). Non-polar solvents have an almost negligible conductivity, so the electric field applied in the system is not uniformly transported throughout the plant matrix, affecting the efficiency of the process. Thus, this technology is more suitable for extracting betalains and anthocyanins. These are extracted with polar solvents, while carotenoids and chlorophylls are typically extracted with non-polar solvents. However, the extraction of total carotenoids by applying the pulsed electric field is promising. Luengo, Álvarez, and Raso (2014) evaluated the effects of this non-thermal technology integrated into the extraction process of carotenoids from canary tomato (Solanum lycopersicum) peel. The pretreatment of the peels with the PEF allowed the same extraction yield, decreasing the extraction time and the proportion of hexane used. Therefore, this technology increased the mass transfer of pigments during the extraction process and reduced the process intensity (temperature and amount of solvent). Furthermore, the PEF technology follows the principles of green chemistry, as its application reduces energy expenditure and waste of solvents associated with the process,contributing to a lower environmental impact (Aadil et al., 2018, Martínez et al., 2020, Moreira et al., 2019, Redondo et al., 2018).
Despite all the advantages explained throughout this topic, the PEF still presents factors limiting its integration in the manufacturing process of natural food colorants. Currently, few studies relate the economic and technological feasibility associated with applying the PEF on an industrial scale. Additionally, the high cost of equipment linked to PEF technology makes it difficult its compete with other technologies (Arshad et al., 2020, Picart-Palmade et al., 2019, Priyadarshini et al., 2019). On the other hand, PEF technology was successfully scaled up to produce potatoes and root vegetable-based products, such as potato chips.
Applying the PEF treatment in products with high conductivity can also promote their ohmic heating. For these products, the heat gradient is not correctly adjusted and, thus, electrical energy is converted to heat. This phenomenon occurs in products that present a high number of conductive ions. Thus, the thermosensitive pigments of the product are degraded due to temperature increase (Samaranayake et al., 2005). This effect is responsible for significantly reducing the extraction yields. However, the integration of cooling coils in the basic components of the PEF system can minimize the effects of ohmic heating and enable the control of the heat gradient during the process (Ramos et al., 2006).
Also, applying the PEF can promote dielectric breakdown in the system, degrading the pigments present in the medium. This phenomenon comprises an electrical discharge between the two electrodes in the treatment chamber. It occurs more in heterogeneous liquid and solid matrices. These tend to incorporate the air into the system (Ramos et al., 2006, Raso et al., 2016).
5. Optimization of PEF assisted extraction process conditions
The optimization of extraction processes assisted by PEF demands the implementation of structured methods and strategies to enhance the recovery of natural colorings and pigments from plant matrices. In this regard, the main process parameters related to PEF performance in the extraction process are electric field strength, specific energy input, pulse number, treatment time, and temperature (Arshad et al., 2020). These process parameters modulate the electroporation phenomenon in the plant cells impacting the release and diffusion of their phytochemical compound content into a liquid medium or solvent. Overall, intensifying the mentioned PEF process parameters, the electroporation intensifies, enhancing the extraction of the target compounds. However, depending on the plant matrix characteristics, such as cell size distribution between peel and pulp, the structure and chemical and physical stability of the natural colorant, as well as its relative location in the plant cell and ability to bind to the matrix, the intensification of the PEF extraction process parameters may reduce the extraction yield (Arruda et al., 2021). Indeed, the electric field strength demanded to promote electroporation in cell membranes may differ for each plant matrix. Donsì, Ferrari, Fruilo, and Pataro (2010) verified that the PEF treatment could exhibit different effects in fruit varieties of the same species. They compared the PEF impact on phenolic compound content in wines produced from two grape varieties, Piedirosso and Aglianico. The authors observed a mild effect of PEF on the extraction of polyphenols from Piedirosso grapes compared to Aglianico grapes. They explained the differences in terms of the degree of grape maturation or different biological structures.
The low energy demand of PEF treatments is one of the main advantages of the application of this technology in the food industry. This factor results in a lower cost for the PEF assisted processing and compensates for the high initial investment required by this innovative technology (Picart-Palmade et al., 2019, Priyadarshini et al., 2019). However, the process parameters must be evaluated for each plant raw material to establish suitable methods and protocols to determine the electric field strength and the optimal pulse duration to achieve high energy efficiency in the natural pigment extraction process (Arshad et al., 2020, Barba et al., 2015).
Zhou et al. (2015) evaluated the effects of field strength variation (5–30 kV/cm) on extraction of anthocyanins from blueberry. The best extraction condition was 20 kV/cm, 10 pulses, and 60% ethanol, also considering the economic aspects of the process. Additionally, these conditions reduced the extraction temperature and processing time. As discussed throughout this review and by Zhou et al. (2015), the pulsed electric field can reduce the intensity of extraction conditions (temperature and amount of solvent), increasing the mass transfer of pigments by changing the permeability of cells. Therefore, the PEF technology contributes to a more sustainable colorants production system (Luengo et al., 2014, Martínez et al., 2020, Moreira et al., 2019, Redondo et al., 2018).
The performance of this emerging technology to favor the extraction of food pigments using water as solvent was reported by Puértolas et al. (2013). They evaluated the effects of the PEF in integrating the extraction process of anthocyanins from purple-fleshed potato (Solanum tuberosum). In this study, the solid–liquid extraction was performed using water and ethanol (48% and 96%) at temperatures ranging from 10 to 40 °C and an extraction time from 60 to 480 min. The results were promising since the extraction yield (65.8 mg/100 g) applying an electric field and using water at 40 °C for 480 min was similar to that obtained for the untreated sample and extracted with 96% ethanol (63.9 mg/100 g). Furthermore, this study demonstrated the PEF performance to provide better extraction yields using water as a solvent since typically ethanol and methanol extracted 3–4 times more anthocyanins than water (Bridgers, Chinn, & Truong, 2010). Puértolas et al. (2013) demonstrated the advantages of PEF technology using a low temperature process condition and water as solvent without affecting the extraction process yield (Häckl & Kunz, 2018).
Luengo et al. (2014) also observed that for the canary tomatoes (Solanum lycopersicum) pre-treated with PEF there was a reduction from 45% to 30% of the percentage of hexane used in the extraction process of carotenoids without affecting the process yield.
In addition to amount of solvent, the extraction time is another relevant aspect of process optimization because its value is inversely proportional to the energy efficiency of the process. López, Puértolas, Condón, Raso, and Alvarez (2009) investigated the effects of electric field strength (0, 1, 3, 5, 7, and 9 kV/cm), specific energy (0, 0.02, 0.09, 0.24, 0.50, and 0.70 kJ /kg), and extraction time (5, 10, 30, 60, 120, 180, 240, 300, and 400 min) on the extraction of betanin from red beetroot. The extraction treatment using red beetroot pretreated at 5 pulses of 2 μs at 7 kV/cm and employing an extraction time of 300 min recovered about 90% of the total betanins. The result was promising since the time to extract betanin from the untreated beet was five times longer.
6. Industrial scale
The feasibility of implementing new technology in processes needs to consider its sustainability. This concept comprises economic analysis, and social and environmental aspects linked to system production. Therefore, a macro visualization of the integration of the PEF into the natural colorant extraction process is required to evaluate its effectiveness. Thus, it will be possible to increase the extraction yield by increasing the energy efficiency of the process (Li and Farid, 2016, Picart-Palmade et al., 2019, Zhang et al., 2019).
The current demands of the food market also include green technologies since with the determination of the Agenda 2030 for sustainability and development of the United Nations, the search for processes that support sustainable development worldwide has been intensifying (Picart-Palmade et al., 2019). The PEF stands out as an emerging and promising technology in the food market. However, the implementation of PEF technology on a large scale needs further studies. Furthermore, these need to maximize their energy efficiency, and process safety since the literature on the subject is still limited (Arshad et al., 2020).
The equipment used to apply PEF technology needs to generate electrical discharges or pulses with a high frequency and power to meet industrial standards and allow continuous and large-scale treatment (Puértolas & Barba, 2016). The equipment for the extraction process integrated into the PEF with a flow rate ranging from 1000 to 2000 kg/h needs has a power of 3 kW to allow the generation of pulses in the range of 20 to 30 kV at a frequency of 200–300 Hz. The equipment must also have a large-volume treatment chamber and one or several pulse generators to achieve the high process volume required by industrial standards (Barba et al., 2016). The treatment chamber also affects the effectiveness of the PEF (Medina-Meza et al., 2016). For example, a chamber with a larger diameter (25 mm) promoted a more efficient extraction of anthocyanins, flavonoids, and phenolic compounds than a smaller one (7 mm). This result was associated with a longer residence time of the sample in the treatment chamber and a greater number of pulses provided. Also, these conditions corroborated with a greater extraction yield. On the other hand, the equipment needs to support electrodes with large areas to generate a high current flow and pulse repetition frequencies, thus enabling higher mass flow in the system (Arshad et al., 2020).
The complexity of PEF technology machinery is reflected in its high price (Priyadarshini et al., 2019). Thus, implementing this technology on an industrial scale requires a high initial investment, which varies between € 75,000 and € 400,000 depending on the process energy needs and production scale (Puértolas & Barba, 2016).
Therefore, energy efficiency needs to promote a return on the initial investment in a short period for its application in industrial processes to be viable. Indeed, high efficient processes employing PEF have been reported in the literature. For example, Braakman (2003) evaluated the economic viability of the PEF assisted extraction of polyphenols from grape pomace. The results were promising since the expenses linked to electricity during the process were from 0.01 to 0.2 €/t considering € 0.1/kWh as a basis.
The low energy consumption of processes that apply PEF to increase the mass transfer of plant tissues makes this technology stand out from others. For example, Puértolas and Barba (2016) observed a lower energetic cost on the permeabilization of plant tissue of apple and potato by PEF technique (1–5 kJ/kg) in comparison tomechanical (20–40 kJ/kg), enzymatic (60–100 kJ/kg), and thermal (100 kJ/kg) methods. In addition, the high-energy efficiency of the PEF process helps offset its high initial investment, which allows this technology to be competitive in the market.
The feasibility of the implementation of PEF technology in food processing was investigated by Picart-Palmade et al. (2019). They attributed its ability to increase the mass transfer rate of pigments to the central positive aspect linked to this technology. Additionally, they verified its effect in reducing the extraction time and intensity of the process parameters, which contribute to an increase in extraction yield and a reduction in the degradation of thermolabile compounds. These factors contribute to a reduction in energy expenditure and the use of natural resources associated with extraction processes.
The PEF technology is already available on the market to integrate industrial processes. The leading distribution companies are PurePulse (Netherlands), Pulsemaster (Germany), DTI/Elea (Germany), Scandinova (Sweden), and Steribeam (Wek-Tec, Germany) (Priyadarshini et al., 2019).
7. Remarks and current challenges
The main parameters that influence the solid–liquid extraction of pigments include the solvent polarity, the target pigment, extraction time, temperature, and frequency of agitation applied in the process (Saini & Keum, 2018). These conditions allow the solvent to cross the cell wall barriers, breaking its structure and penetrating inside the cells. Thus, these conditions enable the solvent interaction with the target pigment and its solubilization, allowing the subsequent pigment isolation (Martínez et al., 2020, Moreira et al., 2019, Redondo et al., 2018).
In addition to the cell wall, the pigments associated with other components present in the cell structure, such as proteins and acids, make the extraction process challenging. This factor is aggravated according to the increase in the complexity of the plant tissue since solvents have low selectivity in the dissolution of components present in the system. Therefore, the greater the amount of other non-polar constituents such as insoluble fibers, carbohydrates, and proteins in the vegetable tissue, the higher the compromising of the purity of the extraction process (Ngamwonglumlert et al., 2017, Parniakov et al., 2015).
Moreover, these physicochemical barriers constituted by plant tissue are responsible for limiting the mass transfer of intracellular components, such as pigments, difficulting their extraction. In this way, the PEF stands out for its ability to increase the mass transfer in the system with minimal degradation of the thermosensitive components. This technology also facilitates the contact of solvents with the compounds, allowing a significant increase in extraction yield (Barba et al., 2015, Barba et al., 2016, Kovačić et al., 2021, Redondo et al., 2018).
The PEF technology allows the intensity reduction of parameters applied during extraction, such as temperature and solvent concentration, facilitating solvent contact with target pigments (Martínez et al., 2020, Moreira et al., 2019, Puértolas et al., 2013, Redondo et al., 2018, Zhou et al., 2015). This reduction enables greater process energy efficiency and minimizes pigment degradation. In addition, these components have an expressive sensitivity to heat, pH, light, and oxygen (Ngamwonglumlert et al., 2017, Rodriguez-Amaya, 2019). Therefore, PEF technology has significantly increased pigment extraction yield, which is currently-one of the main limitations of producing natural colorants (Martins et al., 2016).
This high energy efficiency stands out as one of the main advantages of the PEF compared to other techniques usually integrated into the extraction of natural colorants, such as thermal, mechanical, and enzymatic methods (Picart-Palmade et al., 2019, Puértolas and Barba, 2016). These conventional techniques employ long extraction times and/or high mechanical or thermal energy, degrading the pigments and reducing the process energy efficiency (Luengo et al., 2015). On the other hand, the PEF technology promotes low thermal degradation of pigments. Furthermore, it employs a short extraction time, which varies according to the plant raw material (Barba et al., 2015, Redondo et al., 2018).
The extraction processes assisted by this innovative technology present high-energy efficiency due to low intensity, promoting less degradation of thermosensitive components. The PEF stands out as a green technology since its integration into the process significantly reduces the spending on natural resources (Arshad et al., 2020). This characteristic corroborates the competitiveness of this technology in the food market, as the industries are searching for ways to reduce the environmental impacts generated by production systems (Picart-Palmade et al., 2019).
Studies have been reported the effects of PEF technology in the extraction of anthocyanins, betalains, carotenoids, and chlorophylls (Gachovska et al., 2010, Luengo et al., 2016, Medina-Meza et al., 2016, Nowacka et al., 2019, Pataro et al., 2020, Puértolas et al., 2013, Putranto et al., 2014, Siddeeg et al., 2019, Zhou et al., 2015). This emerging technology has stood out for extracting betalains that present high sensitivity to heat. Also, PEF technology can protect the anthocyanins from thermal degradation and, thus, allow a higher extraction yield (Luengo et al., 2016, Nowacka et al., 2019). Moreover, satisfactory results have been observed for the extraction of anthocyanins. This pigment is sensitive to pH, and the PEF does not change this intrinsic factor (Gachovska et al., 2010, Medina-Meza et al., 2016, Puértolas et al., 2013, Siddeeg et al., 2019, Zhou et al., 2015). Furthermore, the polarity of the solvent used to extract anthocyanins and betalains favors the extraction process using PEF. Polar solvents facilitate the transport of the electric field throughout the extension of the plant matrix (Ngamwonglumlert et al., 2017). However, the efficiency of the PEF is not observed in the treatment of plant matrices in non-polar solvents since these solvents have an almost negligible conductivity. Thereby, the transmission of the electric field by food does not occur well, reducing the effects of this technology in increasing mass transfer. Thus, the effectiveness of PEF treatment in extracting non-polar carotenoids and chlorophylls is low due to the nature of the solvent applied during its extraction (Aadil et al., 2018, Ngamwonglumlert et al., 2017, Saini and Keum, 2018).
In addition to yield, the cost of operating and maintaining the equipment, sustainability, initial investment, and the process integration feasibility must be evaluated to employ this emerging technology on an industrial scale. These factors are critical as they define the competitiveness of this technology compared to other techniques currently applied to increase mass transfer (Arshad et al., 2020, Picart-Palmade et al., 2019, Priyadarshini et al., 2019). However, studies about the economic viability of the PEF technology on an industrial scale are limited. Thus, more studies to optimize the parameters and economically evaluate the application of PEF on a larger scale are necessary to maximize energy efficiency and ensure a safe investment in this technology (Arshad et al., 2020).
Although few studies have been dedicated to investigating the economic feasibility of the PEF technology, CA patent No. CA2666104C describes the improvement of the extraction of phytochemical compounds present in plant tissue through this technology. The plant matrix was first compressed and then subjected to treatment with the PEF. The application of the field was under fixed conditions (6 kV/cm, with a capacitance of 1 µF and 200 pulses for the discharge capacitors) to enable the extraction of its phytochemical components (Ngadi, Raghavan, & Gachovska, 2007).
The application of PEF technology in pigment extraction still requires further studies. In addition to allowing the optimization of extraction to produce a colorant with higher purity, yield, and stability, the researchers need to look at the effects of this emerging technology on the chemical structure of coloring compounds.
8. Future directions
Natural colorants are a future trend in the functional food market. These additives present antimicrobial and antioxidant effects that prevent NCDs (Ribeiro and Veloso, 2021, Wood, 2019). Moreover, PEF assisted extraction of natural colorants also stands out as a future trend since this is a non-thermal high-efficiency technology. This technology promotes low degradation of thermosensitive compounds and allows a high process yield (Picart-Palmade et al., 2019, Puértolas and Barba, 2016).
However, as reported in this review, new studies need to optimize processing conditions using PEF technology. Furthermore, studies about the scaling up the PEF processing to an industrial scale are needed. The high yield of the extraction process obtained on a laboratory scale and its low energy expenditure must be maintained at larger scales to offset the price associated with the PEF machinery (Arshad et al., 2020, Barba et al., 2015). On the other hand, PEF technology was successfully scaled up to produce potato and root vegetable chips. Indeed, potato processing is one of the biggest clients of PEF companies nowadays. PEF-treated potatoes present microscopic holes in their cell membranes through which sugars and amino acids are released before cooking. Electroporation reduces the formation of harmful acrylamides and lowers oil content after the frying step (Ostermeier, Hill, Dingis, Töpfl, & Jäger, 2021). In this case, PEF technology enhanced the food safety and technological properties of potato chips. Thereby, the PEF technology scaling up to industrial manufacturing for producing natural food colorants may result from the incentive arising from the consumer market's demand due to high-value-added PEF-produced food coloring products.
The association of the PEF with other technologies is an alternative that can increase the energy efficiency of the process. For example, ultrasound technology can be associated with the PEF in processing since it is a non-thermal technology that contributes to a lower degradation of thermosensitive compounds. This association may allow higher effects on the permeability of the cell structure, promoting an expressive increase in the mass transfer of the system and thus allowing an increase in extraction yield (Aadil et al., 2018, Manzoor et al., 2019, Wiktor et al., 2018, Wiktor et al., 2019).
A synergistic effect may affect the cell membrane structures through two physical phenomena. First, the PEF promotes electroporation, and the ultrasound provides sonoporation (Medina-Meza et al., 2016, Wiktor et al., 2019). Some studies indicate that the association of these technologies does not promote a deleterious effect on the thermosensitive components of plant matrices (Aadil et al., 2018, Manzoor et al., 2019, Medina-Meza et al., 2016, Medina-Meza et al., 2016). Therefore, the association of these two technologies is a future direction to achieve a higher extraction process energy efficiency.
Studies have already indicated that the synergistic effect of both technologies increased the extraction efficiency of natural colorants. For example, Manzoor et al. (2019) evaluated the impact of a treatment that combined the effects of a PEF and ultrasound in an extract of almonds (Prunus dulcis). The extract obtained by the association of the two technologies had a higher total content of anthocyanins than the control extract, and the one treated by the two isolated technologies. Aadil et al. (2018) reported the efficiency of the combined treatments. They compared the effects of these isolated and integrated innovative technologies on the total anthocyanins present in grapefruit juice (Citrus maxima). The use of integrated technologies maintained a higher content of anthocyanins in the juice (1.68 ± 0.09 mg/L) than the isolated treatments with ultrasound (1.47 ± 0.05 mg/L) or with PEF (1.58 ± 0.03 mg/L).
Therefore, the ability of integrated technologies to preserve the total amount of pigments present in the system has already been studied. However, the association of PEF to ultrasound technology for pigment extraction needs more investigation. This association can provide a greater extraction yield, allowing the feasibility of expanding these technologies to an industrial scale.
9. Conclusion
The integration of extraction techniques can reduce the energy required to extract natural pigments. Low energy extraction conditions, in turn, can better preserve the pigments sensitive to heat, pH, and light. Thus, the integrated techniques may promote an increase in energy efficiency and a reduction in the degradation of thermolabile compounds.
The integration of the PEF technology into the extraction process of anthocyanins, betalains, carotenoids, and chlorophylls stands out due to its action mechanism on plant matrices. As discussed throughout this review, PEF technology increases the mass transfer of the pigments through the phenomenon of electroporation, avoiding their thermal degradation. Therefore, a PEF assisted process provides a higher extraction yield, greater energy efficiency using shorter extraction times, lower amount of solvent, and lower temperature conditions. However, few studies report the effects of PEF on chlorophyll extraction, so more investigations are needed to verify its effects on the extraction of these pigments.
Further studies are also needed to optimize process parameters that apply PEF. These optimizations can maximize energy efficiency for later application on an industrial scale. In addition, the economic feasibility of the processes and the possibility of integrating the PEF with other technologies also need to be studied. These studies can indicate whether the high initial investment in implementing this innovative technology on a large scale will be economically advantageous.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was funded by the São Paulo Research Foundation - FAPESP (grant number #2020/11255-3).
References
- Aadil R.M., Zeng X.-A., Han Z., Sahar A., Khalil A.A., Rahman U.U.…Mehmood T. Combined effects of pulsed electric field and ultrasound on bioactive compounds and microbial quality of grapefruit juice. Journal of Food Processing and Preservation. 2018;42(2):e13507. [Google Scholar]
- Aganovic, K., & Smetana, S. (2022). Environmental Impact Assessment of Pulsed Electric Fields Technology for Food Processing. Food Engineering Series (pp. 521-539).
- Arshad R.N., Abdul-Malek Z., Munir A., Buntat Z., Ahmad M.H., Jusoh Y.M.M.…Aadil R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends in Food Science & Technology. 2020;104:1–13. doi: 10.1016/j.tifs.2020.07.008. [DOI] [Google Scholar]
- Arruda H.S., Silva E.K., Peixoto Araujo N.M., Pereira G.A., Pastore G.M., Marostica Junior M.R. Anthocyanins recovered from agri-food by-products using innovative processes: Trends, challenges, and perspectives for their application in food systems. Molecules. 2021;26(9) doi: 10.3390/molecules26092632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeredo H.M.C. Betalains: Properties, sources, applications, and stability – a review. International Journal of Food Science & Technology. 2009;44(12):2365–2376. doi: 10.1111/j.1365-2621.2007.01668.x. [DOI] [Google Scholar]
- Barba F.J., Brianceau S., Turk M., Boussetta N., Vorobiev E. Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food and Bioprocess Technology. 2015;8(5):1139–1148. doi: 10.1007/s11947-015-1482-3. [DOI] [Google Scholar]
- Barba F.J., Grimi N., Vorobiev E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Engineering Reviews. 2015;7(1):45–62. doi: 10.1007/s12393-014-9095-6. [DOI] [Google Scholar]
- Barba F.J., Parniakov O., Pereira S.A., Wiktor A., Grimi N., Boussetta N.…Vorobiev E. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Research International. 2015;77:773–798. doi: 10.1016/j.foodres.2015.09.015. [DOI] [Google Scholar]
- Barba F.J., Zhu Z., Koubaa M., Sant'Ana A.S., Orlien V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends in Food Science & Technology. 2016;49:96–109. doi: 10.1016/j.tifs.2016.01.006. [DOI] [Google Scholar]
- Basak S., Annapure U.S. Trends in “green” and novel methods of pectin modification - A review. Carbohydrate Polymers. 2022;278 doi: 10.1016/j.carbpol.2021.118967. [DOI] [PubMed] [Google Scholar]
- Binoti M.L., Ramos A.M., Teixeira L.J.Q., Stringheta P.C., Minim V.R.D.P., Pirozi M.R. Campo elétrico pulsado. Ciência Rural. 2012;42(5):934–941. doi: 10.1590/s0103-84782012005000025. [DOI] [Google Scholar]
- Braakman L. Breaktrhough in pasteurization: Pulsed electric fields. Food Eng Ingr. 2003;28(3):34–38. [Google Scholar]
- Bridgers E.N., Chinn M.S., Truong V.-D. Extraction of anthocyanins from industrial purple-fleshed sweetpotatoes and enzymatic hydrolysis of residues for fermentable sugars. Industrial Crops and Products. 2010;32(3):613–620. doi: 10.1016/j.indcrop.2010.07.020. [DOI] [Google Scholar]
- Chalermchat Y., Fincan M., Dejmek P. Pulsed electric field treatment for solid–liquid extraction of red beetroot pigment: Mathematical modelling of mass transfer. Journal of Food Engineering. 2004;64(2):229–236. doi: 10.1016/j.jfoodeng.2003.10.002. [DOI] [Google Scholar]
- Chandrasekhar J., Sonika G., Madhusudhan M.C., Raghavarao K.S.M.S. Differential partitioning of betacyanins and betaxanthins employing aqueous two phase extraction. Journal of Food Engineering. 2015;144:156–163. doi: 10.1016/j.jfoodeng.2014.07.018. [DOI] [Google Scholar]
- Chung C., Rojanasasithara T., Mutilangi W., McClements D.J. Stability improvement of natural food colors: Impact of amino acid and peptide addition on anthocyanin stability in model beverages. Food Chemistry. 2017;218:277–284. doi: 10.1016/j.foodchem.2016.09.087. [DOI] [PubMed] [Google Scholar]
- Cortez R., Luna-Vital D.A., Margulis D., Gonzalez de Mejia E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Comprehensive Reviews in Food Science and Food Safety. 2017;16(1):180–198. doi: 10.1111/1541-4337.12244. [DOI] [PubMed] [Google Scholar]
- Damodaran, S., Parkin, K. I., & Fennema, O. R. (2010). Química de Alimentos de Fennema. (4 ed.). Porto Alegre.
- Delgado-Vargas F., Jiménez A.R., Paredes-López O. Natural pigments: Carotenoids, anthocyanins, and betalains — Characteristics, biosynthesis, processing, and stability. Critical Reviews in Food Science and Nutrition. 2000;40(3):173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Donsì F., Ferrari G., Fruilo M., Pataro G. Pulsed electric field-assisted vinification of aglianico and piedirosso grapes. Journal of Agricultural and Food Chemistry. 2010;58(22):11606–11615. doi: 10.1021/jf102065v. [DOI] [PubMed] [Google Scholar]
- Esatbeyoglu T., Wagner A.E., Schini-Kerth V.B., Rimbach G. Betanin—A food colorant with biological activity. Molecular Nutrition & Food Research. 2015;59(1):36–47. doi: 10.1002/mnfr.201400484. [DOI] [PubMed] [Google Scholar]
- Gachovska T., Cassada D., Subbiah J., Hanna M., Thippareddi H., Snow D. Enhanced anthocyanin extraction from red cabbage using pulsed electric field processing. Journal of Food Science. 2010;75(6) doi: 10.1111/j.1750-3841.2010.01699.x. E323 E329. [DOI] [PubMed] [Google Scholar]
- Goettel M., Eing C., Gusbeth C., Straessner R., Frey W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Research. 2013;2(4):401–408. doi: 10.1016/j.algal.2013.07.004. [DOI] [Google Scholar]
- Guerrero-Beltrán J.Á., Welti-Chanes J. In: Encyclopedia of Food and Health. Caballero B., Finglas P.M., Toldrá F., editors. Academic Press; Oxford: 2016. Pulsed Electric Fields; pp. 561–565. [Google Scholar]
- Häckl K., Kunz W. Some aspects of green solvents. Comptes Rendus Chimie. 2018;21(6):572–580. doi: 10.1016/j.crci.2018.03.010. [DOI] [Google Scholar]
- Kammerer D.R. In: Handbook on Natural Pigments in Food and Beverages. Carle R., Schweiggert R.M., editors. Woodhead Publishing; 2016. 3 - Anthocyanins; pp. 61–80. [Google Scholar]
- Kandušer M., Belič A., Čorović S., Škrjanc I. Modular Serial Flow Through device for pulsed electric field treatment of the liquid samples. Scientific Reports. 2017;7(1):8115. doi: 10.1038/s41598-017-08620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandušer M., Miklavčič D. Electrotechnologies for Extraction from Food Plants and Biomaterials. New York, NY; Springer, New York: 2008. Electroporation in Biological Cell and Tissue: An Overview; pp. 1–37. [Google Scholar]
- Koubaa M., Barba F.J., Grimi N., Mhemdi H., Koubaa W., Boussetta N., Vorobiev E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innovative Food Science & Emerging Technologies. 2016;37:336–344. doi: 10.1016/j.ifset.2016.04.015. [DOI] [Google Scholar]
- Kovačić Đ., Rupčić S., Kralik D., Jovičić D., Spajić R., Tišma M. Pulsed electric field: An emerging pretreatment technology in a biogas production. Waste Management. 2021;120:467–483. doi: 10.1016/j.wasman.2020.10.009. [DOI] [PubMed] [Google Scholar]
- Kumar P., Barrett D., Delwiche M., Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009;48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- Kumari B., Tiwari B.K., Hossain M.B., Brunton N.P., Rai D.K. Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food and Bioprocess Technology. 2018;11(2):223–241. doi: 10.1007/s11947-017-1961-9. [DOI] [Google Scholar]
- Li B.B., Smith B., Hossain M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Separation and Purification Technology. 2006;48(2):182–188. doi: 10.1016/j.seppur.2005.07.005. [DOI] [Google Scholar]
- Li X., Farid M. A review on recent development in non-conventional food sterilization technologies. Journal of Food Engineering. 2016;182:33–45. doi: 10.1016/j.jfoodeng.2016.02.026. [DOI] [Google Scholar]
- López N., Puértolas E., Condón S., Raso J., Alvarez I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. Journal of Food Engineering. 2009;90(1):60–66. doi: 10.1016/j.jfoodeng.2008.06.002. [DOI] [Google Scholar]
- Luengo E., Álvarez I., Raso J. Improving carotenoid extraction from tomato waste by pulsed electric fields. Frontiers in Nutrition. 2014;1:12. doi: 10.3389/fnut.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo E., Martínez J.M., Álvarez I., Raso J. Effects of millisecond and microsecond pulsed electric fields on red beet cell disintegration and extraction of betanines. Industrial Crops and Products. 2016;84:28–33. doi: 10.1016/j.indcrop.2016.01.016. [DOI] [Google Scholar]
- Luengo E., Martínez J.M., Bordetas A., Álvarez I., Raso J. Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innovative Food Science & Emerging Technologies. 2015;29:15–22. doi: 10.1016/j.ifset.2015.02.012. [DOI] [Google Scholar]
- Luo D., Yu L., Westland S., Mahon N. The influence of colour and image on consumer purchase intentions of convenience food. Journal of the International Colour Association. 2019 [Google Scholar]
- Manzoor M.F., Zeng X.-A., Rahaman A., Siddeeg A., Aadil R.M., Ahmed Z.…Niu D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. Journal of Food Science and Technology. 2019;56(5):2355–2364. doi: 10.1007/s13197-019-03627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez J.M., Delso C., Álvarez I., Raso J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Comprehensive Reviews in Food Science and Food Safety. 2020;19(2):530–552. doi: 10.1111/1541-4337.12512. [DOI] [PubMed] [Google Scholar]
- Martinez L.M., Rando B., Agante L., Abreu A.M. True colors: Consumers’ packaging choices depend on the color of retail environment. Journal of Retailing and Consumer Services. 2021;59 doi: 10.1016/j.jretconser.2020.102372. [DOI] [Google Scholar]
- Martins N., Roriz C.L., Morales P., Barros L., Ferreira I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends in Food Science & Technology. 2016;52:1–15. doi: 10.1016/j.tifs.2016.03.009. [DOI] [Google Scholar]
- Medina-Meza I.G., Barbosa-Cánovas G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. Journal of Food Engineering. 2015;166:268–275. doi: 10.1016/j.jfoodeng.2015.06.012. [DOI] [Google Scholar]
- Medina-Meza I.G., Boioli P., Barbosa-Cánovas G.V. Assessment of the effects of ultrasonics and pulsed electric fields on nutritional and rheological properties of raspberry and blueberry purees. Food and Bioprocess Technology. 2016;9(3):520–531. doi: 10.1007/s11947-015-1642-5. [DOI] [Google Scholar]
- Miller D.D., Li T., Liu R.H. In Reference Module in Biomedical Sciences: Elsevier. 2014. Antioxidants and Phytochemicals. [Google Scholar]
- Moreira S.A., Alexandre E.M.C., Pintado M., Saraiva J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Research International. 2019;115:177–190. doi: 10.1016/j.foodres.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Mohammed M., Eiss A. In Structure and Function of Food Engineering. 2012. Pulsed electric fields for food processing technology. [Google Scholar]
- Nagini S., Palitti F., Natarajan A.T. Chemopreventive potential of chlorophyllin: A review of the mechanisms of action and molecular targets. Nutrition and Cancer. 2015;67(2):203–211. doi: 10.1080/01635581.2015.990573. [DOI] [PubMed] [Google Scholar]
- Ngadi, M., Raghavan, V., & Gachovska, T. (2007). Pulsed electric field enhanced method of extraction. In M. University (Ed.), (Vol. CA2666104C).
- Ngamwonglumlert L., Devahastin S., Chiewchan N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Critical Reviews in Food Science and Nutrition. 2017;57(15):3243–3259. doi: 10.1080/10408398.2015.1109498. [DOI] [PubMed] [Google Scholar]
- Nowacka M., Dadan M., Janowicz M., Wiktor A., Witrowa-Rajchert D., Mandal R.…Janiszewska-Turak E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Comprehensive Reviews in Food Science and Food Safety. 2021;20(5):5097–5144. doi: 10.1111/1541-4337.12824. [DOI] [PubMed] [Google Scholar]
- Nowacka M., Tappi S., Wiktor A., Rybak K., Miszczykowska A., Czyzewski J.…Tylewicz U. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods. 2019;8(7) doi: 10.3390/foods8070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermeier R., Hill K., Dingis A., Töpfl S., Jäger H. Influence of pulsed electric field (PEF) and ultrasound treatment on the frying behavior and quality of potato chips. Innovative Food Science & Emerging Technologies. 2021;67 doi: 10.1016/j.ifset.2020.102553. [DOI] [Google Scholar]
- Parniakov O., Barba F.J., Grimi N., Marchal L., Jubeau S., Lebovka N., Vorobiev E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innovative Food Science & Emerging Technologies. 2015;27:79–85. doi: 10.1016/j.ifset.2014.11.002. [DOI] [Google Scholar]
- Pascual-Teresa S. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Archives of Biochemistry and Biophysics. 2014;559:68–74. doi: 10.1016/j.abb.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Pataro G., Carullo D., Falcone M., Ferrari G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innovative Food Science & Emerging Technologies. 2020;63 doi: 10.1016/j.ifset.2020.102369. [DOI] [Google Scholar]
- Pataro G., Carullo D., Siddique A., Falcone M., Donsì F., Ferrari G. Improved extractability of carotenoids from tomato peels as side benefits of PEF treatment of tomato fruit for more energy-efficient steam-assisted peeling. Journal of Food Engineering. 2018;233 doi: 10.1016/j.jfoodeng.2018.03.029. [DOI] [Google Scholar]
- Pataro G., Ferrari G., Donsì F. Mass Transfer Enhancement by Means of Electroporation. Mass Transfer in Chemical Engineering Processes. In. Intechopen. 2011 doi: 10.5772/22386. [DOI] [Google Scholar]
- Picart-Palmade L., Cunault C., Chevalier-Lucia D., Belleville M.-P., Marchesseau S. Potentialities and limits of some non-thermal technologies to improve sustainability of food processing. Frontiers Nutrition. 2019;5(130) doi: 10.3389/fnut.2018.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshini A., Rajauria G., O'Donnell C.P., Tiwari B.K. Emerging food processing technologies and factors impacting their industrial adoption. Critical Reviews in Food Science and Nutrition. 2019;59(19):3082–3101. doi: 10.1080/10408398.2018.1483890. [DOI] [PubMed] [Google Scholar]
- Puértolas E., Barba F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food and Bioproducts Processing. 2016;100:172–184. doi: 10.1016/j.fbp.2016.06.020. [DOI] [Google Scholar]
- Puértolas E., Cregenzán O., Luengo E., Álvarez I., Raso J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chemistry. 2013;136(3):1330–1336. doi: 10.1016/j.foodchem.2012.09.080. [DOI] [PubMed] [Google Scholar]
- Putranto A., Argo B., Wijana S. Green pulsed electric field-assisted extraction method of total carotenoid carrot pulp using olive oil as solvent. Indonesian Green Technology Journal. 2014;3:1–9. [Google Scholar]
- Ramos, A. M., Teixeira, L. J. Q., Stringheta, P. C., Chaves, J. B. P., & Gomes, J. C. (2006). Aplicação de campos elétricos pulsados de alta intensidade na conservação de alimentos. Ceres (Vol. 53, p. 15). Viçosa: Universidade Federal de Viçosa.
- Raso J., Frey W., Ferrari G., Pataro G., Knorr D., Teissie J., Miklavčič D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innovative Food Science & Emerging Technologies. 2016;37:312–321. doi: 10.1016/j.ifset.2016.08.003. [DOI] [Google Scholar]
- Redondo D., Venturini M.E., Luengo E., Raso J., Arias E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innovative Food Science & Emerging Technologies. 2018;45:335–343. doi: 10.1016/j.ifset.2017.12.004. [DOI] [Google Scholar]
- Ribeiro J.S., Veloso C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocolloids. 2021;112 doi: 10.1016/j.foodhyd.2020.106374. [DOI] [Google Scholar]
- Rodriguez-Amaya D.B. Natural food pigments and colorants. Current Opinion in Food Science. 2016;7:20–26. doi: 10.1016/j.cofs.2015.08.004. [DOI] [Google Scholar]
- Rodriguez-Amaya D.B. Update on natural food pigments – A mini-review on carotenoids, anthocyanins, and betalains. Food Research International. 2019;124:200–205. doi: 10.1016/j.foodres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Saini R.K., Keum Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chemistry. 2018;240:90–103. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- Saldaña M.D.A., Silva E.K., Olmos Cornejo J.E., Orozco Lopez C.L. In: Comprehensive Foodomics. Cifuentes A., editor. Elsevier; Oxford: 2021. 2.52 - Green Processes in Foodomics: Biorefineries in the Food Industry; pp. 808–824. [Google Scholar]
- Samaranayake C.P., Sastry S.K., Zhang H. Pulsed ohmic heating–A novel technique for minimization of electrochemical reactions during processing. Journal of Food Science. 2005;70(8) doi: 10.1111/j.1365-2621.2005.tb11515.x. e460 e465. [DOI] [Google Scholar]
- Santos-Buelga C., Mateus N., De Freitas V. Anthocyanins. Plant Pigments and Beyond. Journal of Agricultural and Food Chemistry. 2014;62(29):6879–6884. doi: 10.1021/jf501950s. [DOI] [PubMed] [Google Scholar]
- Selvaraj V., Swarna Karthika T., Mansiya C., Alagar M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. Journal of Molecular Structure. 2021;1224 doi: 10.1016/j.molstruc.2020.129195. [DOI] [Google Scholar]
- Shea, T. (2020). Four incredibly harmful effects artificial dyes have on our health. Food Nerd. Retrieved from: https://www.foodnerdinc.com/blogs/food-for-thought/artificial-food-coloring-no-thank-you Accessed March, 14 2022.
- Shiekh K.A., Olatunde O.O., Zhang B., Huda N., Benjakul S. Pulsed electric field assisted process for extraction of bioactive compounds from custard apple (Annona squamosa) leaves. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129976. [DOI] [PubMed] [Google Scholar]
- Siddeeg A., Faisal Manzoor M., Haseeb Ahmad M., Ahmad N., Ahmed Z., Khan K.I.M.…Ammar A.F. Pulsed electric field-assisted ethanolic extraction of date palm fruits: Bioactive compounds, antioxidant activity and physicochemical properties. Processes. 2019;7(9) doi: 10.3390/pr7090585. [DOI] [Google Scholar]
- Spence C. Background colour & its impact on food perception & behaviour. Food Quality and Preference. 2018;68:156–166. doi: 10.1016/j.foodqual.2018.02.012. [DOI] [Google Scholar]
- Sun L., Xin F., Alper H.S. Bio-synthesis of food additives and colorants-a growing trend in future food. Biotechnology Advances. 2021;47 doi: 10.1016/j.biotechadv.2020.107694. [DOI] [PubMed] [Google Scholar]
- Uenojo M., Maróstica M., Jr, Pastore G. Carotenoids: Properties, applications and biotransformation in flavor compounds. Química Nova. 2007;30:616–622. [Google Scholar]
- Vorobiev E., Lebovka N. In: Handbook of Electroporation. Miklavčič D., editor. Springer International Publishing; Cham: 2017. Selective extraction of molecules from biomaterials by pulsed electric field treatment; pp. 655–670. [Google Scholar]
- Wiktor A., Dadan M., Nowacka M., Rybak K., Witrowa-Rajchert D. The impact of combination of pulsed electric field and ultrasound treatment on air drying kinetics and quality of carrot tissue. LWT. 2019;110:71–79. doi: 10.1016/j.lwt.2019.04.060. [DOI] [Google Scholar]
- Wiktor A., Gondek E., Jakubczyk E., Dadan M., Nowacka M., Rybak K., Witrowa-Rajchert D. Acoustic and mechanical properties of carrot tissue treated by pulsed electric field, ultrasound and combination of both. Journal of Food Engineering. 2018;238:12–21. doi: 10.1016/j.jfoodeng.2018.06.001. [DOI] [Google Scholar]
- Wood, L. (2019). $5 Billion Natural Dyes Market - Global Outlook and Forecasts 2019-2024. Retrieved from: https://www.prnewswire.com/news-releases/5-billion-natural-dyes-market---global-outlook-and-forecasts-2019-2024-300797306.html Accessed 20/10/2021 2021.
- Yilmaz C., Gökmen V. In: Encyclopedia of Food and Health. Caballero B., Finglas P.M., Toldrá F., editors. Academic Press; Oxford: 2016. Chlorophyll; pp. 37–41. [Google Scholar]
- Zhang S., Yang R., Zhao W., Liang Q., Zhang Z. The first ESR observation of radical species generated under pulsed electric fields processing. Lwt - Food Science and Technology. 2011;44:1233–1235. doi: 10.1016/j.lwt.2010.11.016. [DOI] [Google Scholar]
- Zhang Z.-H., Wang L.-H., Zeng X.-A., Han Z., Wang M.-S. Effect of pulsed electric fields (PEFs) on the pigments extracted from spinach (Spinacia oleracea L.) Innovative Food Science & Emerging Technologies. 2017;43:26–34. doi: 10.1016/j.ifset.2017.06.014. [DOI] [Google Scholar]
- Zhang Z.-H., Wang L.-H., Zeng X.-A., Han Z., Brennan C.S. Non-thermal technologies and its current and future application in the food industry: A review. International Journal of Food Science & Technology. 2019;54(1):1–13. doi: 10.1111/ijfs.13903. [DOI] [Google Scholar]
- Zhou Y., Zhao X., Huang H. Effects of pulsed electric fields on anthocyanin extraction yield of blueberry processing by-products. Journal of Food Processing and Preservation. 2015;39(6):1898–1904. doi: 10.1111/jfpp.12427. [DOI] [Google Scholar]
- Zia S., Khan M.R., Shabbir M.A., Aslam Maan A., Khan M.K.I., Nadeem M.…Aadil R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Reviews International. 2022;38(6):1166–1196. doi: 10.1080/87559129.2020.1772283. [DOI] [Google Scholar]