Highlights
-
•
Fermentation decreased pH and Aw and increased ANN, TBARS, TVB-N, and BAs in grass carp flesh.
-
•
Fermentation with Lactobacillus plantarum and flavourzyme could improve physicochemical properties of fermented grass carp.
-
•
Fermentation of Lactobacillus plantarum and flavourzyme could contribute to fermented grass carp with safety properties.
Keywords: Grass carp, Fermentation, Flavourzyme, Lactobacillus plantarum, Biogenic amines
Abstract
The present study aimed to investigate the change in physicochemical and safety properties of grass carp during fermentation with flavourzyme and Lactobacillus plantarum (FLF). The natural fermentation (NF) and fermentation with Lactobacillus plantarum (LF) samples were used as control. The results showed that with increasing fermentation time, the pH and water activity (Aw) in each fermented grass carp sample gradually decreased, while the a-amino nitrogen (ANN), thiobarbituric acid reactive substance (TBARS), total volatile base nitrogen (TVB-N), biogenic amines (BAs), and harmful microbial gradually increased. Besides, compared with NF samples at each fermentation time, significantly lower pH, Aw, TBARS, TVB-N, BAs, and harmful microbial presented in LF and FLF samples. However, FLF samples have a higher AAN content than that of NF and LF samples during fermentation. Overall, the fermentation with Lactobacillus plantarum and flavourzyme could contribute to fermented grass carp products with better physicochemical and safety properties.
1. Introduction
Grass carp (Ctenopharyngodon idellus), one of the four largest freshwater fish in China, is widely cultivated by farmers in Southeast Asia because of its fast growth and high nutritional value (Yang et al., 2020). In recent years, the aquaculture production of grass carp has increased significantly in China, from 4.222 million tons in 2015 to 5.571 million tons in 2020 (FAO, 2021). However, limited by its perishable characteristics, most grass carp are mainly sold as living bodies and primary processed products at lower price. The low processing utilization rate and added value of grass carp have restricted the development of the grass carp breeding and processing industry to some extent. How to effectively improve the added value and utilization rate of grass carp is currently the focus top of industrial producers and researchers.
Fish fermentation is a traditional food processing and preservation technique, which can not only effectively improve the processing efficiency and added value of freshwater fish but also give higher nutritional value and unique flavor to related fermented fish products. Fermented fish products are very popular with consumers and are mainly made from salt, fresh fish flesh, and corn rice flour by sealed fermentation for a certain period. Currently, according to variations in the fermentation methods, the fermentation method mainly includes natural fermentation (Zhao et al., 2021), inoculation fermentation with single or multiple dominant bacteria (Gao et al., 2016, Gao et al., 2016), and fermentation with exogenous enzymes (Li et al., 2014, Yang et al., 2020a, Zhang et al., 2017). Among these fermentation methods, natural fermentation has many disadvantages, such as being greatly affected by natural conditions, long fermentation cycles, difficulty to control fermentation conditions, high variability of product quality, and low safety, which is difficult to achieve large-scale industrial production. Inoculation fermentation is a commonly used method in fish processing, which can promote the formation of a better flavor of fish products by selecting a good starter for fish fermentation (Zhao et al., 2021). The main microbial starter used for fish fermentation is lactic acid bacteria, staphylococcus, and saccharomyces, such as Lactobacillus plantarum, Pediococcus pentosus, Lactobacillus paracasei, Staphylococcus xylosus, and Saccharomyces cerevisiae, etc, which can reduce the pH and play an important role in improving the safety and flavor of the fish product (Yang et al., 2020a, Zeng et al., 2016, Zhao et al., 2021). However, studies have found that low enzyme activity in lactic acid bacteria affects the maturation cycle of fermented products to a certain extent (Yang, Xia, Zhang, Xu, & Jiang, 2016). Fermentation with exogenous enzymes is a novel method to improve the quality of fermented fish products and shorten the fermentation time. Relative studies reported that the addition of exogenous enzymes can promote the decomposition of protein in meat during fermentation, thereby improving the nutritional and flavor quality of relevant fermented meat products (Yang et al., 2020a). Flavourzyme, a kind of exogenous enzyme with both endopeptidase and exopeptidase activity, which has been reported that can promote the decomposition of proteins, thus improving the nutritional and flavor quality of related-meat products (Li et al., 2014, Yang et al., 2020a, Zhang et al., 2017). Nevertheless, to our knowledge, there are few studies on fish product fermentation with exogenous enzymes. Moreover, no study has been conducted on the effects of flavourzyme on fermented products under fermentation with lactic acid bacteria, especially its safety and physicochemical characteristics.
Therefore, to investigate the effect of lactic acid bacteria and exogenous enzymes on physicochemical and safety properties of fermented grass carp, the dorsal meat of grass carp was used as the research object, and the Lactobacillus plantarum and flavourzyme were selected as the starter. The change in pH, water activity, α-amino nitrogen, total volatile base nitrogen content, harmful microbial, and biogenic amines of grass carp meat during fermentation with Lactobacillus plantarum and flavourzyme were monitored. Natural fermentation (NF) and fermentation with Lactobacillus plantarum (LF) were used as control. This study may provide some useful information for the improvement of fermentation methods and production of the high-quality fermented fish products.
2. Materials and methods
2.1. Materials
Twenty fresh grass carp (Ctenopharyngodon idellus, 2.5–3 kg/fish) were purchased from a local aquatic products market (Shanghai, China). All fish were killed, decapitated, descaled, and gutted, followed by a quick collection of dorsal meat. All the dorsal meat were cut into 3 cm × 2 cm × 2 cm cubes. Lactobacillus plantarum (No. 192567) was purchased from Beijing Beina Tronlink Biotechnology Research Institute (Beijing, China). Flavourzyme (enzyme activity 30,000 U/g) was purchased from Beijing Solebo Technology Co., ltd (Beijing, China). Toxic biogenic amines standards, histamine hydrochloride (purity 98 %), phenylethylamine hydrochloride (purity 98.6 %), tyramine hydrochloride (purity 99.1 %), putrescine hydrochloride (purity 99.8 %), cadaverine hydrochloride (purity 99.7 %), tryptamine hydrochloride (purity 98 %), spermine hydrochloride (purity 98.8 %), spermidine hydrochloride (purity 99.9 %), and octopamine hydrochloride (purity 99.3 %) were purchased from Sartorius Stedim Biotech GmbH (Goettingen, Germany). All the other chemical regents were of analytical grade.
2.2. Preparation of samples
The fermented samples were prepared according to the method of Xu et al with some modifications as follows (Xu, Li, Xia, Zang, & Gao, 2019). The dorsal meat of grass carp were cured with salt (3 %, w/w) and sugar (2 %, w/w) at 4 °C for 24 h, followed by a maxing thoroughly with ground roasted corn (20 %, w/w). The resulting fish samples were divided into 3 batches and prepared with different starter cultures, including LF (1 % Lactobacillus plantarum) and FLF (a combination of 10 U/g flavourzyme and 1 % Lactobacillus plantarum) and a batch without any starter (natural fermentation, NF). Among them, the NF and LF samples were used as control. Then, the fish sample, corn, and starter cultures were completely mixed in a ceramic container and sealed tightly with water surrounded. These samples were incubated in an incubation chamber (KBF-240, Binder Inc, German) at 25 °C with 75 % humidity for 15 d. The samples were withdrawn and analyzed on 0, 5, 10, and 15 d, respectively. Finally, the corn flour in fermented samples were removed and obtained fish meat was kept at −80 °C for further analysis.
2.3. Determination of pH and water activity (Aw)
The pH was determined according to the method of Yang et al (2020). 2 g of samples were transferred into 18 mL of distilled water and homogenized for 1 min using an FM-200 homogenizer (Shanghai Fokker Equipment Co. ltd, Shanghai, China). Then, the homogeneous solution was centrifuged at 10000 × g for 10 min at 4 °C. The supernatant was collected, and the pH values were measured using a digital pH (Mettler Toledo FE28, Shanghai, China) at room temperature. The determination of Aw was conducted at 25 °C by a water activity tester (Decagon Devices, Pullman, USA). All tests were performed at least in triple.
2.4. Determination of α-amino nitrogen (AAN)
Determination of α-amino nitrogen was performed by formalin titration method with slight modification (Guidi & Abreu Gloria, 2015). A total of 5 g samples were homogenized with 100 mL of distilled water and subsequently centrifuged at 10000 × g for 1 min at 4 °C. The supernatant (20 mL) was diluted to 80 mL with distilled water, and then the combined supernatant was titrated with sodium hydroxide (NaOH, 0.05 mol/L) to the end pH of 8.2. 10 mL of formalin solution (37 %, v/v) was added and the mixture was titrated again with NaOH to the end pH of 9.2. The volume of NaOH consumed for the second titration was recorded as V2. The volume of NaOH consumed for the blank titration was recorded as V1. The AAN content was calculated by the following equation:
Where 0.014 is the molecular weight of nitrogen (g/mol).
2.5. Determination of thiobarbituric acid reactive substance (TBARS)
Determination of TBARS was performed according to the method of Gao et al. (2016). 1 g of samples were homogenized with 10 mL of cold trichloroacetic acid solution (7.5 %, w/v) for 1 min. After standing for 10 min at 4 °C, the homogeneous solution was filtered with neutral filter paper. Then, the filtrate (3 mL) and 2-thiobarbituric acid (5 mL, 0.02 mol/L) were mixed and reacted in a boiling water bath for 40 min. The absorbance of the reaction solution was measured at 532 nm. 1,1,3,3- tetraethoxypropane was used as the standard curve, and the TBARS value was expressed as mg MDA/kg sample.
2.6. Determination of total volatile base nitrogen content (TVB-N)
Determination of TVB-N was performed by the micro-diffusion method of Conway (Cobb, Alaniz, & Thompson, 1973).
2.7. Determination of harmful microbial
Determination of harmful microbial was performed according to the methods of Zeng et al. with slight modification (Zeng, Xia, Jiang, & Yang, 2013). The count of each microorganism was as follows: Enterobacteriaceae on Violet red bile glucose agar medium cultured at 37 °C for 24 h; Pseudomonas on CFC agar medium cultured at 25 °C for 48 h; Staphylococcus aureus on Baird-Parker plate cultured at 37 °C for 48 h. The number of harmful microbial was expressed as lg CFU/g.
2.8. Determination of biogenic amines
Determination of biogenic amines was performed according to the methods of Hu et al. (Hu, Xia, & Liu, 2007). 5 g of samples were homogenized with 20 mL perchloric acid (5 %, w/v) and centrifuged at 5000 r/min for 10 min. The mixture was filtered with neutral filter paper, and then the supernatant was collected in a 50 mL volumetric flask after two repetitions. The volume of supernatant was fixed to 50 mL with 5 % perchloric acid. The supernatant (10 mL) and sodium chloride (0.5 g) were mixed until completely dissolved, and then hexane was added to remove fat. After 10 min of ultrasound treatment, the upper organic phase of mixture was discarded after static stratification. The mixture (5 mL) was adjusted to pH 12 with cold potassium hydroxide solution (2 mol/L). Precolumn derivatization was performed. After blending with 1 mL of saturated sodiumbicarbonate (10 mg/mL) and 1 mL of dansyl chloride (10 mg/mL), the mixture was allowed to react at 60 °C for 20 min. Ammonia was added into reaction solution to stop derivatization, and then stand for 30 min in darkroom. The mixture was fixed to 5 mL acetonitrile. Finally, the supernatant was filtered with a 0.22 μm membrane for testing. The determination of biogenic amines was conducted using high-performance liquid chromatograph (W2690-2998, Waters Co., Milford, MA, USA). The main parameter was set as following: chromatographic column: Waters Symmetry C18 (250 mm length × 4.6 mm inner diameter × 5 μm thickness); detection wavelength: 254 nm; column temperature: 35°C; injection volume: 10 μL; flow rate: 1.0 mL/min. Gradient elution program was shown in Table 1. Standard BAs were used for the quantification of different BAs through the same derivatization and analysis methods.
Table 1.
Gradient elution program for biogenic amine analysis.
| Time program/min | A:10 mmol/L Ammonium acetate/% | B: Acetonitrile/% |
|---|---|---|
| 0 | 40 | 60 |
| 5 | 40 | 60 |
| 7 | 50 | 50 |
| 8 | 40 | 60 |
| 10 | 30 | 70 |
| 15 | 20 | 80 |
| 20 | 20 | 80 |
| 25 | 30 | 70 |
| 30 | 40 | 60 |
2.9. Data analysis
All tests were performed in triplicate. The results were presented as the mean ± standard deviation (SD). Significance analysis (P < 0.05) was performed by one-way analysis of variance (ANOVA) and Duncan's multiple range tests using SPSS 21.0 software (IBM, Armonk, NY, USA). The graphs were drawn using Origin2019b software (Origin Lab Corporation, Northampton, USA).
3. Results and discussion
3.1. pH analysis
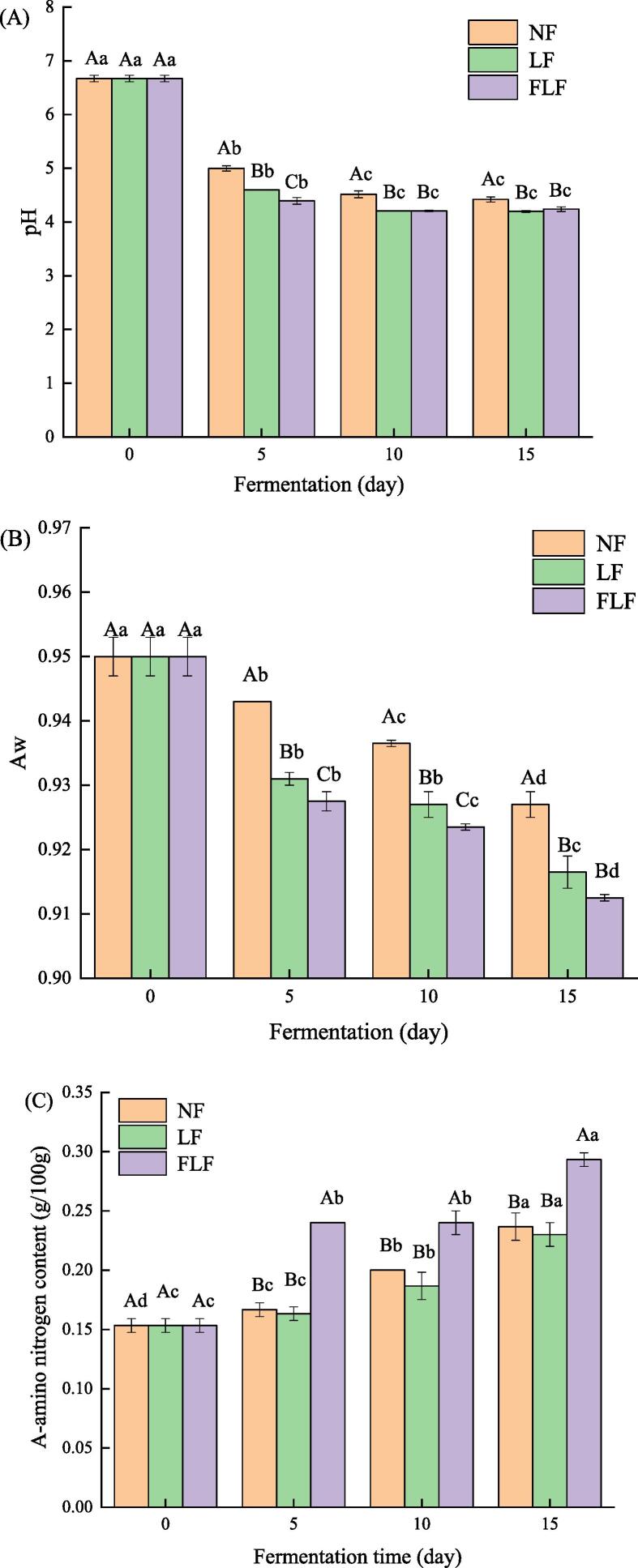
pH is one of the important factors affecting the safety of fermented products. Change in pH of grass carp samples with different fermentation conditions during fermentation were presented in Fig. 1A. As shown in Fig. 1A, the pH changes of all samples showed the same trend during fermentation, which decreased first and then tended to be stable as fermentation time increased. The initial pH value of the grass carp sample was 6.67 ± 0.06. After 5 days of fermentation, the pH changes of all samples decreased significantly. Notably, the pH value of FLF samples (4.39 ± 0.06) was significantly lower than that of NF samples (5.00 ± 0.05) and LF samples (4.60 ± 0.01), which indicated that the fermentation with Lactobacillus plantarum and flavourzyme would accelerate the pH reduction rate of grass carp samples at the early stage of fermentation. However, after 10 days of fermentation, the pH change of each sample tended to be stable. At 15 days of fermentation, the pH of NF, LF, and FLF fermented samples were 4.42 ± 0.05, 4.20 ± 0.02, and 4.24 ± 0.04, respectively. There was no significant change in pH between LF samples and FLF samples, suggesting that flavourzyme addition had little effect on the pH of grass carp samples at the late fermentation stage. Furthermore, the pH value of LF and FLF samples were significantly lower than that of NF samples at 15 days of fermentation, indicating that fermentation with Lactobacillus plantarum and fermentation with Lactobacillus plantarum and flavourzyme could be committed to better safety of fermented products.
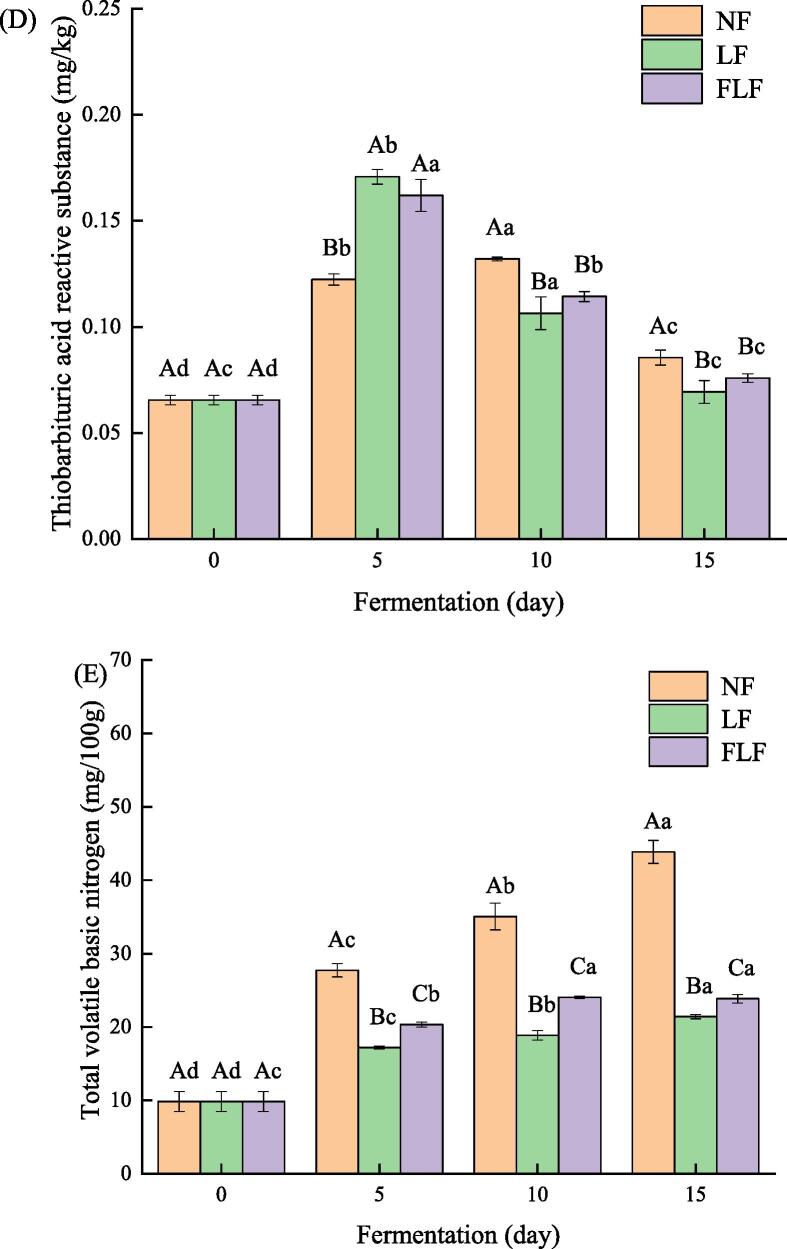
Fig. 1.
Chang in the pH(A), water activity(B), A-amino nitrogen content(C), thiobarbituric acid reactive substance(D), and total volatile basic nitrogen(E) of grass carp samples with different fermentation conditions during fermentation.
3.2. Aw analysis
The low Aw is considered to effectively inhibit the growth of microorganisms and ensure the storage stability of fermentation products. Change in the Aw of grass carp sample with different fermentation conditions during fermentation were shown in Fig 1B. As shown in Fig.1B, a relatively low Aw change was presented in all fermented samples because of the high relative humidity during the fermentation process. The initial value (0.950 ± 0.003) of the fermented sample was much lower than that of the fresh fish flesh due to salt curing pretreatment. Some studies indicated that salt curing can effectively reduce the Aw of fish products, thereby effectively inhibiting the spoilage of the fish products (Jittinandana et al., 2002, Wang et al., 2017). The Aw of all fermented samples were decreased gradually with increasing fermentation time, which may be related to the decline of pH during fermentation. Relevant studies have confirmed that the water-holding capacity of related meat products decreases significantly as the pH value decreased or is close to the isoelectric point value of protein (Capita et al., 2006, Huff-Lonergan and Lonergan, 2005). Besides, it was remarkable that FLF samples had the lowest Aw value during the whole fermentation process, followed by LF and NF samples. This phenomenon may be explained by enhanced protein hydrolysis caused by the inoculation of Lactobacillus plantarum and flavourzyme, thereby resulting in a further weakening of water holding capacity and reduction of Aw value. After 15 days of fermentation, the Aw of all fermented samples ranged from 0.913 ± 0.001 to 0.927 ± 0.007, with the lowest value (0.913 ± 0.001) being observed for FLF samples, indicating that fermentation with Lactobacillus plantarum and flavourzyme may contribute to fermented fish products with better storage stability.
3.3. AAN analysis
ANN is an important indicator in fermented products, which can not only reflect the degree of protein hydrolysis of fermented products but also reflect its fermentation degree to a certain extent. Higher ANN content could improve the umami taste of fermented products. Change in AAN content of grass carp samples with different fermentation conditions during fermentation were shown in Fig. 1C. As shown in Fig. 1C, the AAN content of all fermented samples increased gradually with the prolonging of fermentation time, indicating that proteins in fermented samples were continuously hydrolyzed during fermentation. This phenomenon might be contributing to the role of endogenous cathepsin and microbial protease in the fermentation process (Li et al., 2021). After 15 days of fermentation, the ANN content in the NF, FL, and FLF group samples increased from 0.153 ± 0.006 g/100 g to 0.237 ± 0.012 g/100 g, 0.230 ± 0.010 g/100 g, and 0.293 ± 0.006 g/100 g, respectively. Notably, the AAN content of the FLF sample was significantly higher than the other two groups at each fermentation time point, which indicated that fermentation with Lactobacillus plantarum and flavourzyme can promote the hydrolysis of protein and accelerate the maturation of fermented grass carp samples.
3.4. TBARS analysis
TBARS value is widely used to reflect the degree of lipid oxidation of meat and meat products (Jo & Ahn, 2000). An increase in TBARS indicates the production of secondary lipid oxidation products, and TBARS of qualified fish products should not be higher than 5 mg MDA/kg (Zeng et al., 2013). Change in TBARS value of grass carp samples with different fermentation conditions during fermentation were shown in Fig. 1D. As shown in Fig. 1D, the TBARS value of all samples increased first and then decreased gradually as fermentation time increased. The initial TBARS value of the sample was 0.066 ± 0.002 mg/kg. The TBARS value of NF (0.132 ± 0.001 mg/kg) reached the maximum value on the 10th day of fermentation, while the TBARS values of LF (0.171 ± 0.004 mg/kg) and FLF (0.162 ± 0.008 mg/kg) samples reached the maximum at 5 days of fermentation, indicating that inoculation of Lactobacillus plantarum accelerated the oxidation process of samples during sample fermentation. This phenomenon may be due to Lactobacillus plantarum being involved in the degradation of hydroperoxides into malondialdehyde at the early stage of fermentation, thus promoting the oxidation of fat in fermentation samples, which is consistent with the results reported by Gao et al. (2016). However, the decline in TBARS values of all samples at the end of the fermentation process may be explained by the degradation of aldehydes or further reactions with other compounds (e.g., amino acids and Maillard intermediates), which lead to the production of some other types of flavor compounds (Huang, Li, Huang, Li, & Sun, 2014). Furthermore, after 15 days of fermentation, the TBARS value of LF and FLF samples were significantly lower than that of NF samples, which indicated that the oxidation degree of fermentation with Lactobacillus plantarum and fermentation with Lactobacillus plantarum and flavourzyme were lower than that of natural fermentation at the end of the fermentation stage. Inoculation of Lactobacillus plantarum can significantly affect the lipid oxidation in the fermentation samples, and both fermentation methods can effectively inhibit the oxidation degree of fermented products.
3.5. TVB-N analysis
TVB-N is a common and effective method to analyze the spoilage degree of fish and fish products. The higher the content of TVB-N, the greater the degree of corruption. Change in the TVB-N value of grass carp samples with different fermentation conditions during fermentation were shown in Fig. 1E. As shown in Fig. 1E, the TVB-N value of all fermented samples increased continuously with the increase of fermentation time. However, after 10 days of fermentation, compared with NF samples, LF samples had a significantly lower TVB-N content, which may be due to the inoculation of Lactobacillus plantarum. Relative studies have reported that lactic acid and bacteriocin produced by Lactobacillus plantarum would neutralize volatile basic nitrogen in meat products, thereby inhibiting the accumulation of TVB-N content. Besides, inoculation of Lactobacillus plantarum may affect TVB-N production by inhibiting the growth of other pathogenic and psychrophilic bacteria during fermentation (Yin, Pan, & Jiang, 2002). Meanwhiles, after 15 days of fermentation, the TVB-N content of LF and FLF samples were 21.40 ± 0.27 mg/100 g and 23.85 ± 0.59 mg/100 g, respectively, which were lower than 25 mg/100 g, indicating that LF and FLF samples fermented for 15 days still maintain relatively good freshness and nutritional value.
3.6. Harmful microbial analysis
Enterobacter, Staphylococcus aureus and Pseudomonas are common pathogenic and spoilage bacteria in fermented fish products, which have an important influence on the safety of the products. Change in harmful microbial number in grass carp samples with different fermentation conditions during fermentation were shown in Table 2. The initial numbers of Enterobacter, Staphylococcus aureus and Pseudomonas were 2.36 ± 0.10, 3.25 ± 0.19, and 2.43 ± 0.38 lg CFU/g, respectively. The number of three harmful microbial changes in all samples showed the same trend during fermentation, which increased first and then decreased as fermentation time increased. The increase in harmful microbial numbers may be due to the abundance of nutrients in fish at the early stage of fermentation, which creates favorable conditions for the growth and reproduction of harmful microbial. However, the decrease in the number of harmful bacteria at the end of the fermentation, it may be related to the decrease of Aw, nutrient consumption, and accumulation of metabolites (Yang et al., 2021). Meanwhile, it was interesting to note that the number of three kinds of harmful microbial in LF and FLF samples increased at a lower rate than that in NF samples from the 5 days of fermentation, and the number of Enterobacter, Staphylococcus aureus, and Pseudomonas in LF and FLF samples were significantly lower than that in NF sample at the 15th day of fermentation, which indicated that Lactobacillus plantarum had a better inhibitory effect on harmful microbial. Nevertheless, there was no significant difference in harmful microbial numbers between LF and FLF samples at the end of fermentation, indicating that inoculation of flavourzyme had no effect on the growth of harmful microbial in fermentation samples.
Table 2.
Change in harmful microbial number in grass carp samples with different fermentation conditions during fermentation.
| Fermentation time (d) |
Enterobacter(lg CFU/g) |
Staphylococcus aureus (lg CFU/g) |
Pseudomonas(lg CFU/g) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| NF | LF | FLF | NF | LF | FLF | NF | LF | FLF | |
| 0 | 2.36 ± 0.10Ad | 2.36 ± 0.10Ad | 2.36 ± 0.10Ac | 3.25 ± 0.19Ad | 3.25 ± 0.19Ac | 3.25 ± 0.19Ac | 2.43 ± 0.38Ac | 2.43 ± 0.38Ac | 2.43 ± 0.38Ac |
| 5 | 6.88 ± 0.08Aa | 5.52 ± 0.11Bb | 5.61 ± 0.14Ba | 6.90 ± 0.04Ab | 6.18 ± 0.02Ba | 6.17 ± 0.04Ba | 6.42 ± 0.03Aa | 3.93 ± 0.08Cab | 4.26 ± 0.10Ba |
| 10 | 6.09 ± 0.01Ab | 5.95 ± 0.03Ba | 5.73 ± 0.08Ca | 7.90 ± 0.04Aa | 5.78 ± 0.16Cb | 6.27 ± 0.10Ba | 6.01 ± 0.02Aa | 4.26 ± 0.24Ba | 4.40 ± 0.17Ba |
| 15 | 5.24 ± 0.03Ac | 4.06 ± 0.02Bc | 4.05 ± 0.02Bb | 6.49 ± 0.13Ac | 5.59 ± 0.06Bb | 5.43 ± 0.10Bb | 4.38 ± 0.43Ab | 3.39 ± 0.36Bb | 3.39 ± 0.09Bb |
Note: Different uppercase letters (A–D) among the different treatments at the same time point indicate significant differences (p < 0.05), and different lowercase letters (a–d) among the different times for the same treatment indicate significant differences (p < 0.05).
3.7. Bas analysis
Biogenic amines, a group of low molecular nitrogen organic compounds with biological activity, can be divided into three categories according to the substitution of hydrogen atoms by alkyl or aromatic groups in their ammonia molecules, which are referred to aliphatic (e.g., putrescine, cadaverine, spermidine, spermine, etc), aromatic (e.g., phenylethylamine, tyramine, etc) and heterocyclic biogenic amines (e.g., tryptamine, histamine, etc) (Jain & Verma, 2018). Excessive consumption of biogenic amines, particularly histamine and tyramine, could cause serious health hazards, such as blood pressure imbalance, headache, breathing difficulties, and vomiting (Shalaby, 1996). Therefore, the biogenic amine is regarded as an important evaluation indicator for microbial pollution and quality deterioration in fermented foods (Tasic et al., 2012). Change in BA content of grass carp samples with different fermentation conditions during fermentation were shown in Table 3. As shown in Table 3, 8 kinds of BAs were detected in the fermented samples, including phenethylamine, putrescine, cadaverine, histamine, octopamine, tyramine, spermidine, and spermine. In the whole fermentation process, cadaverine, histamine, octopamine, tyramine and spermidine were the main BAs. The content of histamine and tyramine changes in all samples showed the same trend during fermentation. With increasing fermentation time, the content of histamine increased first and then decreased, while the content of tyramine increased gradually. The contents of other BAs fluctuated in the fermentation process and their variation trends were significantly different, which may be caused by the unstable decomposition of some biogenic amines. Some studies have reported that putrescine, spermidine, and spermine are usually formed by a multi-step reaction in meat products (Benkerroum, 2016, Min-Ki et al., 2009). Histamine and tyramine are considered to be the most toxic bioamines in fermented foods (Shalaby, 1996). After 15 days of fermentation, the content of histamine and tyramine in NF samples was significantly higher than that in the other two samples. It was noteworthy that the contents of histamine and tyramine in LF and FLF samples were relatively lower than NF samples at the end stage of fermentation, which was suspected to be related to the lower number of Enterobacter and Pseudomonas (Table 2). Studies have confirmed that Enterobacter and Pseudomonas have high decarboxylase activity, which can decarboxylate free amino acids in meat products and is closely related to the formation of biogenic amines (Bover-Cid, Miguelez-Arrizado, & Vidal-Carou, 2001). Besides, at the later stage of fermentation, the total bioamine content in each fermentation sample increased significantly from the initial value of 22.16 ± 0.13 mg/kg to 31.94 ± 0.75, 24.33 ± 0.31 and 25.53 ± 0.04 mg/kg, respectively, which was much lower than 1000 mg/kg (Standard for biogenic amine limits, Food and Drug Administration). Moreover, compared with the NF samples, total biogenic amines of LF and FLF samples were decreased by 29.94 % and 25.11 %, respectively. Significantly lower biogenic amine content presented in LF and FLF samples, which indicated that fermentation with Lactobacillus plantarum and flavourzyme was effective method to control the formation of biogenic amines in fermented samples.
Table 3.
Change in biogenic amines content of grass carp samples with different fermentation conditions during fermentation.
| Sample | Fermentation time(d) | Biogenic amines content(mg/kg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenethylamine | Putrescine | Cadaverine | Histamine | Octopamine | Tyramine | Spermidine | Spermine | Total content | ||
| NF | 0 | 0.39 ± 0.00Ad | 0.83 ± 0.06Ac | 2.98 ± 0.06Ab | 3.19 ± 0.00Ac | 4.46 ± 0.10Ab | 3.39 ± 0.10Ac | 5.11 ± 0.06Aab | 1.82 ± 0.00Ad | 22.16 ± 0.13Ad |
| 5 | 1.08 ± 0.02Ac | 1.97 ± 0.07Ab | 4.81 ± 0.07Aa | 3.91 ± 0.02Ab | 4.00 ± 0.05Ac | 3.75 ± 0.12Ab | 4.70 ± 0.07Bb | 2.20 ± 0.00Ac | 26.42 ± 0.10Ac | |
| 10 | 3.16 ± 0.37Ab | 3.07 ± 0.15Aa | 5.23 ± 0.49Aa | 4.93 ± 0.28Aa | 8.24 ± 0.20Aa | 3.96 ± 0.34Ab | 4.80 ± 0.03Cab | 2.62 ± 0.00Ab | 36.00 ± 0.53Aa | |
| 15 | 6.10 ± 0.14Aa | 1.15 ± 0.41ABc | 3.25 ± 0.13Aa | 3.98 ± 0.31Ab | 4.59 ± 0.20Cb | 4.38 ± 0.01Aa | 5.46 ± 0.73Aa | 3.03 ± 0.01Aa | 31.94 ± 0.75Ab | |
| LF | 0 | 0.39 ± 0.00Aa | 0.83 ± 0.06Aa | 2.98 ± 0.06Ab | 3.19 ± 0.00Ab | 4.46 ± 0.10Ab | 3.39 ± 0.10Aa | 5.11 ± 0.06Aab | 1.82 ± 0.00Ab | 22.16 ± 0.13Ab |
| 5 | 0.38 ± 0.09Ba | 0.88 ± 0.11Ba | 3.37 ± 0.12Ba | 3.23 ± 0.11Bb | 4.23 ± 0.78Ab | 3.39 ± 0.22Aa | 5.03 ± 0.29Aab | 1.79 ± 0.13Bb | 22.30 ± 1.39Bb | |
| 10 | 0.60 ± 0.45Ba | 0.98 ± 0.23Ba | 2.98 ± 0.01Bb | 3.67 ± 0.08Ba | 3.64 ± 0.12Cb | 3.48 ± 0.31Aa | 4.96 ± 0.05Bab | 1.82 ± 0.17Cb | 22.13 ± 1.05Bb | |
| 15 | 0.60 ± 0.28Ba | 0.82 ± 0.05Ba | 3.03 ± 0.01Ab | 3.22 ± 0.05Bb | 5.96 ± 0.29Ba | 3.61 ± 0.01Aa | 4.79 ± 0.06Ab | 2.30 ± 0.11Ba | 24.33 ± 0.31Ba | |
| FLF | 0 | 0.39 ± 0.00Ab | 0.83 ± 0.06Ab | 2.98 ± 0.06Aa | 3.19 ± 0.00Ab | 4.46 ± 0.10Ab | 3.39 ± 0.10Ab | 5.11 ± 0.06Aa | 1.82 ± 0.00Ab | 22.16 ± 0.13Ab |
| 5 | 0.35 ± 0.01Bb | 1.06 ± 0.11Ba | 3.10 ± 0.18Ca | 3.40 ± 0.19Bab | 4.29 ± 0.34Ab | 3.50 ± 0.66Ab | 4.92 ± 0.10ABbc | 1.93 ± 0.13ABb | 22.55 ± 1.63Bb | |
| 10 | 0.33 ± 0.00Bb | 0.81 ± 0.02Bb | 2.97 ± 0.04Ba | 3.66 ± 0.43Ba | 4.81 ± 0.80Bb | 3.88 ± 0.23Aab | 5.04 ± 0.01Aab | 2.29 ± 0.09Ba | 23.80 ± 0.40Bb | |
| 15 | 0.53 ± 0.07Ba | 0.87 ± 0.01Bb | 3.02 ± 0.03Aa | 3.16 ± 0.02Bb | 6.58 ± 0.09Aa | 4.53 ± 0.02Aa | 4.89 ± 0.07Aa | 1.94 ± 0.04Cb | 25.53 ± 0.04Ba | |
Note: Different uppercase letters (A–D) among the different treatments at the same time point indicate significant differences (p < 0.05), and different lowercase letters (a–d) among the different times for the same treatment indicate significant differences (p < 0.05).
4. Conclusion
Fish fermentation is a traditional food processing and preservation techniques, which can not only effectively improve the processing efficiency and added value of freshwater fish but also give higher nutritional value and unique flavor to related fermented fish products. The effect of Lactobacillus plantarum and flavourzyme on physicochemical and safety properties of grass carp during fermentation were investigated. The natural fermentation and fermentation with Lactobacillus plantarum fermentation samples were as control. The results showed that the pH and Aw in fermented grass carp gradually decreased, while the ANN, TBAR, TVB-N, and harmful microbial and biogenic amines gradually increased with increasing fermentation time. Compared with NF samples at each fermentation stage, significant lower pH, Aw, TBARS, TVB-N, harmful microbial, and biogenic amines were presented in LF and FLF group samples. However, FLF group samples have an obviously higher AAN content than that of NF and LF group samples during fermentation. All these results indicated that fermentation with Lactobacillus plantarum and flavourzyme can accelerate the acid production in fish meat, weaken its water holding capacity, inhibit its oxidation degree, and promote a better flavor and safety quality for fermented samples. This study may be conducive to a better understanding of the change in physicochemical and safety properties of grass carp during fermentation with flavourzyme and Lactobacillus plantarum, and provide some useful information for the production of high-quality fermented fish products.
CRediT authorship contribution statement
Huiya Xu: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Naiyong Xiao: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Jiani Xu: Investigation. Quanyou Guo: Funding acquisition, Project administration, Resources. Wenzheng Shi: Funding acquisition, Project administration, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key R&D Program of China [grant numbers 2019YFD0902003], the Central Public-interest Scientific Institution Basal Research Fund, CAFS [NO. 2020TD68], and the Central Public-interest Scientific Institution Basal Research Fund, ECSFR, CAFS (NO. 2021M01).
Contributor Information
Quanyou Guo, Email: dhsguoqy@163.com.
Wenzheng Shi, Email: wzshi@shou.edu.cn.
References
- Benkerroum N. Biogenic amines in dairy products: Origin, incidence, and control means. Comprehensive Reviews in Food Science and Food Safety. 2016;15(4):801–826. doi: 10.1111/1541-4337.12212. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S., Miguelez-Arrizado J., Vidal-Carou M.C. Biogenic amine accumulation in ripened sausages affected by the addition of sodium sulphite. Meat Science. 2001;59(4):391–396. doi: 10.1016/S0309-1740(01)00091-2. [DOI] [PubMed] [Google Scholar]
- Capita R., Llorente-Marigomez S., Prieto M., Alonso-Calleja C. Microbiological profiles, pH, and titratable acidity of chorizo and salchichon (two Spanish dry fermented sausages) manufactured with ostrich, deer, or pork meat. Journal of Food Protection. 2006;69(5):1183–1189. doi: 10.4315/0362-028X-69.5.1183. [DOI] [PubMed] [Google Scholar]
- Cobb B.F., III, Alaniz I., Thompson C.A., Jr. Biochemical and microbial studies on shrimp: Volatile nitrogen and amino nitrogen analysis. Journal of Food Science. 1973;38(3):431–436. doi: 10.1111/j.1365-2621.1973.tb01447.x. [DOI] [Google Scholar]
- FAO . Food and Agriculture Organization of the United Nation; Rome: 2021. The state of world fisheries and aquaculture 2020. [Google Scholar]
- Gao P., Wang W., Jiang Q., Xu Y., Xia W. Effect of autochthonous starter cultures on the volatile flavour compounds of Chinese traditional fermented fish (Suan yu) International Journal of Food Science & Technology. 2016;51(7):1630–1637. doi: 10.1111/ijfs.13134. [DOI] [Google Scholar]
- Gao P., Wang W., Xia W., Xu Y., Jiang Q. Lipolysis and lipid oxidation caused by Staphylococcus xylosus 135 and Saccharomyces cerevisiae 31 isolated from Suan yu, a traditional Chinese low-salt fermented fish. International Journal of Food Science and Technology. 2016;51(2):419–426. doi: 10.1111/ijfs.12997. [DOI] [Google Scholar]
- Guidi L.R., Abreu Gloria M.B. Bioactive amines in soy sauce: Validation of method, occurrence and potential health effects (vol 133, pg 323, 2012) Food Chemistry. 2015;182:333. doi: 10.1016/j.foodchem.2015.02.057. [DOI] [PubMed] [Google Scholar]
- Hu Y., Xia W., Liu X. Changes in biogenic amines in fermented silver carp sausages inoculated with mixed starter cultures. Food Chemistry. 2007;104(1):188–195. doi: 10.1016/j.foodchem.2006.11.023. [DOI] [Google Scholar]
- Huang Y., Li H., Huang T., Li F., Sun J. Lipolysis and lipid oxidation during processing of Chinese traditional smoke-cured bacon. Food Chemistry. 2014;149:31–39. doi: 10.1016/j.foodchem.2013.10.081. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Science. 2005;71(1):194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Jain A., Verma K.K. Strategies in liquid chromatographic methods for the analysis of biogenic amines without and with derivatization. Trac-Trends in Analytical Chemistry. 2018;109:62–82. doi: 10.1016/j.trac.2018.10.001. [DOI] [Google Scholar]
- Jittinandana S., Kenney P.B., Slider S.D., Kiser R.A. Effect of brine concentration and brining time on quality of smoked rainbow trout fillets. Journal of Food Science. 2002;67(6):2095–2099. doi: 10.1111/j.1365-2621.2002.tb09507.x. [DOI] [Google Scholar]
- Jo C., Ahn D.U. Volatiles and oxidative changes in irradiated pork sausage with different fatty acid composition and tocopherol content. Journal of Food Science. 2000;65(2):270–275. doi: 10.1111/j.1365-2621.2000.tb15992.x. [DOI] [Google Scholar]
- Li C., Zhang F., Yang Q., Meng J., He L., Deng L.…Zeng X. Microorganism and physiochemical characteristic of high-salt (Suan Yu), a traditional Chinese fermented fish. Journal of Aquatic Food Product Technology. 2021;30(8):916–931. doi: 10.1080/10498850.2021.1957049. [DOI] [Google Scholar]
- Li F., Yan Q., Zou Y., Huang M., Kang Z., Zhou G. Effect of flavourzyme on proteolysis, antioxidant capacity and sensory attributes of Chinese sausage. Meat Science. 2014;98(1):34–40. doi: 10.1016/j.meatsci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Min-Ki K., Jae-Hyung M., Han-Joon H. Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chemistry. 2009;116(1):87–95. doi: 10.1016/j.foodchem.2009.02.010. [DOI] [Google Scholar]
- Shalaby A.R. Significance of biogenic amines to food safety and human health. Food Research International. 1996;29(7):675–690. doi: 10.1016/S0963-9969(96)00066-X. [DOI] [Google Scholar]
- Tasic T., Ikonic P., Mandic A., Jokanovic M., Tomovic V., Savatic S., Petrovic L. Biogenic amines content in traditional dry fermented sausage Petrovska klobasa as possible indicator of good manufacturing practice. Food Control. 2012;23(1):107–112. doi: 10.1016/j.foodcont.2011.06.019. [DOI] [Google Scholar]
- Wang W., Xia W., Gao P., Xu Y., Jiang Q. Proteolysis during fermentation of Suanyu as a traditional fermented fish product of China. International Journal of Food Properties. 2017;20(Suppl. 1):S166–S176. doi: 10.1080/10942912.2017.1293089. [DOI] [Google Scholar]
- Xu Y., Li L., Xia w., Zang J., Gao P. The role of microbes in free fatty acids release and oxidation in fermented fish paste. LWT – Food Science and Technology. 2019;101:323–330. doi: 10.1016/j.lwt.2018.11.027. [DOI] [Google Scholar]
- Yang F., Xia W., Zhang X., Xu Y., Jiang Q. A comparison of endogenous and microbial proteolytic activities during fast fermentation of silver carp inoculated with Lactobacillus plantarum. Food Chemistry. 2016;207:86–92. doi: 10.1016/j.foodchem.2016.03.049. [DOI] [PubMed] [Google Scholar]
- Yang J., Jiang C., Bao R., Liu M., Lv J., Yang Z.…Lin X. Effects of flavourzyme addition on physicochemical properties, volatile compound components and microbial community succession of Suanzhayu. International Journal of Food Microbiology. 2020;334:108839. doi: 10.1016/j.ijfoodmicro.2020.108839. [DOI] [PubMed] [Google Scholar]
- Yang J., Lu j., Zhu Q., Tao Y., Zhu Q., Guo C.…Show P. Isolation and characterization of a novel Lactobacillus plantarum MMB-07 from traditional Suanyu for Acanthogobius hasta fermentation. Journal of Bioscience and Bioengineering. 2021;132(2):161–166. doi: 10.1016/j.jbiosc.2020.12.016. [DOI] [PubMed] [Google Scholar]
- Yang W., Shi W., Qu Y., Wang Z., Shen S., Tu L.…Wu H. Research on the quality changes of grass carp during brine salting. Food Science & Nutrition. 2020;8(6):2968–2983. doi: 10.1002/fsn3.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L.J., Pan C.L., Jiang S.T. Effect of lactic acid bacterial fermentation on the characteristics of minced mackerel. Journal of Food Science. 2002;67(2):786–792. doi: 10.1111/j.1365-2621.2002.tb10677.x. [DOI] [Google Scholar]
- Zeng X., Chen X., Zhang W. Characterization of the microbial flora from Suan Yu, a Chinese traditional low-salt fermented fish. Journal of Food Processing and Preservation. 2016;40(5):1093–1103. doi: 10.1111/jfpp.12690. [DOI] [Google Scholar]
- Zeng X., Xia W., Jiang Q., Yang F. Effect of autochthonous starter cultures on microbiological and physico-chemical characteristics of Suan yu, a traditional Chinese low salt fermented fish. Food Control. 2013;33(2):344–351. doi: 10.1016/j.foodcont.2013.03.001. [DOI] [Google Scholar]
- Zhang S., Zhang C., Qiao Y., Xing L., Kang D., Khan I.A., Zhou G. Effect of Flavourzyme on proteolysis, antioxidant activity and sensory qualities of Cantonese bacon. Food Chemistry. 2017;237:779–785. doi: 10.1016/j.foodchem.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Y., Li C., Li L., Yang X., Wu Y.…Zhao Y. Novel insight into physicochemical and flavor formation in naturally fermented tilapia sausage based on microbial metabolic network. Food Research International. 2021;141:110122. doi: 10.1016/j.foodres.2021.110122. [DOI] [PubMed] [Google Scholar]