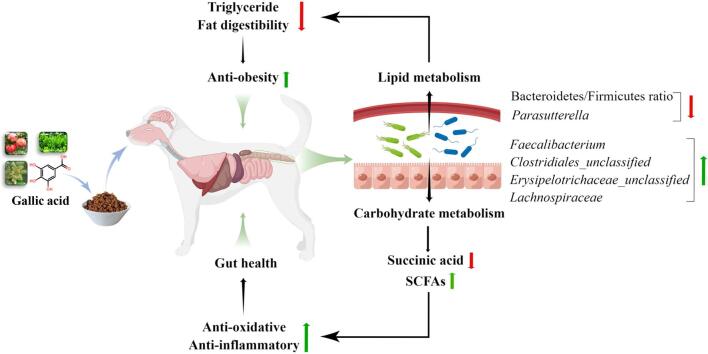
Graphical abstracts
Abbreviations: GA, gallic acid; GSH-Px, Glutathione peroxidase; SOD, Superoxide dismutase; CAT, Catalase; T-AOC, Total antioxidant capacity; MDA, Malondialdehyde; IgG, Immunoglobulin G; TNF-α, Tumor necrosis factor-alpha; IFN-γ, Interferon-gamma; IL-1β, Interleukin 1β; IL-8, Interleukin 8; SCFAs, Short-chain fatty acids; BCFAs, Branched-chain fatty acids; UPLC-Orbitrap-MS/MS, Ultra-performance liquid chromatography–Orbitrap–tandem mass spectrometry; BCS, Body condition score; FS, Fecal score; AAFCO, Association of American Feed Control Officials; PCA, Principal component analysis; QC, Quality control; RT, Retention time; RSD, Relative standard deviation; OPLS-DA, Orthogonal partial least squares discriminant analysis; RPT, Response permutation testing; VIP, Variable importance in the projection
Keywords: Gallic acid, Pet food, Dog, Nutrient digestibility, Gut microbiota, Short-chain fatty acids, Metabolomics
Highlights:
-
•
Long-term consumption of 0.02%∼0.08% GA had no negative effect on canine body condition.
-
•
GA intervention improved anti-oxidative and anti-inflammatory capacities.
-
•
0.08% GA regulated lipid metabolism in serum.
-
•
0.08% GA increased the relative abundance of SCFAs-producing bacteria.
-
•
0.08% GA regulated carbohydrate metabolism in fece.
Abstract
Gallic acid (GA) is a natural polyphenolic compound with many health benefits. To assess the potential risk of long-term consumption of GA to gut health, healthy dogs were fed a basal diet supplemented with GA (0%, 0.02%, 0.04%, and 0.08%) for 45 d, and fecal microbiota and metabolomics were evaluated. This study demonstrated that GA supplementation regulated serum lipid metabolism by reducing serum triglyceride, fat digestibility, and Bacteroidetes/Firmicutes ratio. In addition, the relative abundance of Parasutterella was significantly lower, and the SCFAs-producing bacteria were increased along with fecal acetate and total SCFAs contents accumulation in the 0.08% GA group. Metabolomics data further elucidated that 0.08% GA significantly affected carbohydrate metabolism by downregulating succinic acid in fece, thereby alleviating inflammation and oxidative stress. Overall, this study confirmed the beneficial effects of long-term consumption of GA on lipid metabolism and gut health, and the optimal level of GA supplementation was 0.08%.
1. Introduction
Polyphenolic compounds, ubiquitous in plant-derived foods, are an essential part of the human and animal diet. Recently, plant polyphenols have become increasingly popular worldwide as the consumption of polyphenols can reduce the risk of chronic disease, such as cardiovascular diseases, obesity, type-2 diabetes, and cancer (Cory et al., 2018, de Araújo et al., 2021). Polyphenols, due to their low bioavailability, reach the colon intactly (Mithul Aravind, Wichienchot, Tsao, Ramakrishnan, & Chakkaravarthi, 2021), so they interact with the hindgut microbiota, consequently, releasing metabolites (Plamada & Vodnar, 2022). In recent years, researchers have been paying increasing attention to the prebiotic role of dietary polyphenols by modulating gut microbiota and their metabolites (Ma & Chen, 2020). There is much evidence to support that polyphenols exert beneficial effects as prebiotic substrate by promoting the proliferation of beneficial bacteria and inhibiting pathogenic bacteria (Gowd, Karim, Shishir, Xie, & Chen, 2019), which leads to an increase in host-absorbable short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, together with a decrease in inflammation and obesity incidence (Nash et al., 2018). Interestingly, the canine and human gut microbiomes share highly similar gene content and dietary response mainly due to a long-term domestication process of dogs in the course of their evolution (Coelho et al., 2018). It appears that research on the canine gut microbiota could also be applied to humans.
Gallic acid (GA), 3,4,5-trihydroxybenzoic acid, is a natural polyphenolic compound ubiquitously found in fruits, vegetables, and medicinal plants (Yang et al., 2020). Studies showed that GA possessed many potential therapeutic properties including anti-oxidative, anti-inflammatory, anti-obesity, anti-cancer, and anti-microbial properties (Dludla et al., 2019, Yang et al., 2020). Our recent study found that short-term addition of 0.05% GA could alleviate environmental stress-induced oxidative stress, inflammatory response, and metabolic disorders by regulating gut microbiota in puppies (Yang et al., 2022). However, it should be noted that polyphenols combine with other compounds when consumed, thereby reducing nutritional value and limiting development as human health and animal husbandry products (Casanova-Martí et al., 2018). Moreover, a study on ruminants found that feeding pomegranate extract containing 16.9% GA equivalent reduced crude protein and fat digestibility of calves (Oliveira et al., 2010). To date, the potential risk of long-term consumption of GA to gut microbiota and metabolic profiles are unknown, as well as few studies focused on the effect of different levels of GA on the physiological function and digestion and absorption of nutrients in humans or others monogastric animals.
Therefore, we chose healthy beagle dogs as model animals to assess the potential risk of long-term consumption of GA to physiological function, nutrient digestibility, gut microbiota, and metabolic profiles. Here, we applied the 16S rRNA amplicon sequencing and ultra-performance liquid chromatography–Orbitrap–tandem mass spectrometry (UPLC-Orbitrap-MS/MS) to determine the effects of dietary supplementation with 0.02%, 0.04%, and 0.08% GA on fecal microbial composition and metabolic profiles. The results of this study will contribute to further understanding the beneficial effects of GA on gut health and determining an optimal dosage.
2. Materials and methods
All the experimental procedures applied in this study were reviewed and approved by the Experimental Animal Ethics Committee of South China Agricultural University (approval code 2021E028).
2.1. Animals and diets
Twenty beagle dogs [average age 1.47 ± 0.02 years; average body weight (BW) = 13.77 ± 1.96 kg; average body condition score (BCS) = 5.65 ± 0.83] were randomly allotted to 1 of 4 dietary treatments (n = 5; 3 females and 2 males) according to their gender and BW. Gallic acid (GA, purity > 99%) in the present study was purchased from Wufeng Chicheng Biotech Co., Ltd (Yichang, China). Dietary treatments included: (1) basal diet group (CON group); (2) basal diet supplemented with 0.02% GA group (0.02% GA group); (3) basal diet supplemented 0.04% GA group (0.04% GA group); (4) basal diet supplemented with 0.08% GA group (0.08% GA group). The dosage ranges of GA supplements were determined based on our previous study (Yang et al., 2022). These diets met or exceeded the nutrient requirements of adult dogs recommended by the Association of American Feed Control Officials (AAFCO, 2017). The ingredients and nutrient levels of the experimental diets are presented in Table S1.
2.2. Experiment design
After at least one month of acclimation, dogs were randomly assigned to 4 experimental groups. The experiment period lasted for 45 days. All dogs were housed individually in the custom-made stainless steel metabolism cages (1.2 × 1.0 × 1.1 m kennels) under a constant temperature and humidity (23℃, 70%) with a 12 h light/dark cycle. A restricted diet of 130 g per dog was offered at each of the two daily meals at 8:00 am and 5:00 pm, respectively. All dogs were given fresh water ad libitum and socialized with each other or humans at least once a day. Serum biochemistry analysis, weighing, BCS (Laflamme, 1997), and fecal score (FS) (Middelbos, Fastinger, & Fahey, 2007) were performed before the morning feeding on d 0 (i.e., 1 day before the trial). During the trial, FS was adopted to assess the fecal form and consistency once every three days. In addition, the whole feces were collected on d 40 to 44. Next, the BW and BCS were recorded again on d 45 before the morning feeding, and fresh feces and blood samples were collected on d 45 for further detections.
2.3. Chemical analyses of diets and feces
To measure the apparent total tract digestibility of nutrients, four experimental feeds were collected once a week, and all diet and fece samples was kept in a freezer at −20℃ until analysis. The samples were oven-dried at 65℃ for 48 h to constant weight and finely ground to pass through a 1-mm mesh screen. Diet and fece samples were determined for dry matter and organic matter according to AOAC methods (Hortwitz & Latimer, 2007). Crude protein, total dietary fiber, and acid-hydrolyzed fat were carried out using AOAC with the help of a Kjeldahl method with semi-automatic Kjeldahl apparatus (VAPODEST 200, C. Gerhardt GmbH & Co. KG, Germany), an automatic fiber analyzer (FIBRETHERM FT12, C. Gerhardt GmbH & Co. KG, Germany), and a fatty analyzer (FT640, Grand Analytical Instrument Co., Ltd., Guangzhou, China). The GE was analyzed by an oxygen bomb calorimeter (IKA C 200, IKA (Guangzhou) Instrument Equipment Co., Ltd., Guangzhou, China). Feed amino acids were determined using a Hitachi L-8900 amino acid analyzer (Hitachi, Japan), and feed tryptophan was analyzed using a high-performance liquid chromatograph (Ultimate 3000, Thermo Fisher Scientific, USA). In addition, all fecal amino acids were detected using a UPLC-Orbitrap-MS/MS (Q-Exactive Focus, Thermo Fisher Scientific, USA). Finally, the digestibility of nutrients was calculated by referencing the following formula: Nutrient digestibility (%) = (Nutrient intake - Nutrient in feces) / Nutrient intake*100 (g/d, DM basis).
2.4. Serum biochemistry, antioxidant capacity, and inflammatory cytokines analyses
On d 45 after overnight fasting, blood samples were harvested. After centrifugation, the supernatants were collected as serum samples for further analysis. The obtained serum was used to measure blood biochemical parameters using an automatic biochemical analyzer (SMT-120VP, Chengdu Seamaty Technology Co., Ltd., Chengdu, China). The serum contents of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) were determined by commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). Serum immunoglobulin G (IgG), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin 1β (IL-1β), and interleukin 8 (IL-8) were detected using commercial canine ELISA kits (MEIMIAN, Jiangsu Meimian Industrial Co., Ltd., Jiangsu, China).
2.5. Fecal fermentation metabolites analysis
On d 45, fresh fecal pH was immediately measured after 10% fecal suspension (w/v) in ultrapure water with a portable pH meter (Starter 3100, Ohaus Instruments Co. Ltd., Shanghai, China). The fecal SCFAs and branched-chain fatty acids (BCFAs) concentrations were determined by the GCMS-QP2020 system (Shimadzu, Tokyo, Japan) with a DB-FFAP capillary column (30 m × 0.25 mm × 0.25 μm, Onlysci, China). The instrument parameters and sample processing procedures referred to our previous published papers (Yang et al., 2022). Fecal biogenic amine, lactate, indole, and 3-methylindole concentrations were measured by UPLC-Orbitrap-MS/MS (Q-Exactive Focus, Thermo Fisher Scientific, USA).
2.6. Fecal microbiota analysis
On d 45, the total microbial DNA in feces was extracted by the E.Z.N.A.® Stool DNA Kit (D4015, Omega, Inc., USA). The V3-V4 region of bacterial 16S rRNA gene was amplified using the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) with the barcode. PCR amplification was performed in a total volume of 25 μL reaction mixture containing 25 ng of template DNA, 12.5 μL PCR Premix, 2.5 μL of each primer. Cycling parameters were 98℃ for 30 s, followed by 32 cycles of 98℃ for 10 s, 54℃ for 30 s and 72℃ for 45 s with a final extension at 72℃ for 10 min. The PCR products of each sample were detected by 2% agarose gel electrophoresis, and then they were purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA), quantified by Qubit (Invitrogen, USA), and sequenced on NovaSeq PE250 platform at LC-Bio Technology Co., Ltd, Hang Zhou, Zhejiang Province, China. Reads were paired using FLASH. Quality filtering on the raw reads were performed to obtain the high-quality clean tags using the fqtrim (v0.94). Chimeric sequences were filtered using Vsearch software (v2.3.4). After dereplication using DADA2 in the QIIME2 software, the feature table and feature sequence were obtained. Then according to SILVA (release 138) classifier, feature abundance was normalized using relative abundance of each sample.
Venn diagram was drawn by the VennDiagrams in R software (v3.4.4). α-diversity (Observed_species, Shannon, Simpson, Chao1, and Goods_coverage) and β-diversity were calculated in QIIME2. The principal component analysis (PCA) was displayed using the vegen (2.5.4) and ggplot2 (3.2.0) in R software (v3.4.4). The nsegata-LEfSe software (094f447691f0) was used to perform LEfSe analysis.
2.7. Fecal and serum metabolomics analysis
Fecal and serum samples collected on d 45 were processed as described previously (Nuli et al., 2019, Yang et al., 2022) with minor modifications. We mixed 20 samples with equal volumes to obtain quality control (QC) samples. One QC sample was inserted into the queue for every 5 samples to ensure repeatability. The UPLC-Orbitrap-MS/MS system from Thermo Fisher Scientific (Q-Exactive Focus, USA) was served as an untargeted metabolomic approach and used to detect the fecal and serum metabolic profiles (Xin et al., 2018). The raw data were processed by Compound Discoverer 2.1 software (Thermo Fisher Scientific, USA) to produce a data matrix including retention time (RT), mass spectrometry (m/z), and peak intensity. Meanwhile, metabolic features with a relative standard deviation (RSD) >30% were excluded. Then we searched the mzCloud and mzVault libraries to identify metabolites from these data.
The PCA and orthogonal partial least squares discriminant analysis (OPLS-DA) were done with the SIMCA-P 14.1 software (Umetrics, Umea, Sweden). Response permutation testing (RPT) was performed to test the accuracy of OPLS-DA models. In addition, variable importance in the projection (VIP) was computed in the OPLS-DA model, and p-value was computed using an unpaired student’s t-test. The metabolites with VIP > 1 and p < 0.05 were deemed as the differential metabolites. The KEGG database was applied to functionally annotate these differential metabolites, which further were mapped to the KEGG pathway database using the MetaboAnalyst 5.0 (https://www.metaboanalyst.ca).
2.8. Statistical analysis
The experimental data (serum biochemistry, antioxidant capacity, inflammatory cytokines, BW, feed intake, nutrient digestibility, and fecal characteristics, and fermentation metabolites) were preprocessed using Microsoft Excel 2019, and then performed one-way ANOVA with Fisher’s LSD multiple comparison test using SPSS 26 software. Both linear and quadratic contrasts were applied to further elucidate the dose effect of GA in dogs. Kruskal-Wallis tests (p < 0.05), Wilcoxon tests (p < 0.05), and LDA score (LDA > 2) were used to perform LEfSe analysis. Graphs were drawn using GraphPad Prism 8.0 software. Mean values were based on 5 replicates per group, and data variability was expressed as the standard error of means (SEM). p < 0.05 was defined as statistical significance, and 0.05 < p < 0.10 was defined as a trend of difference. Spearman’s correlations and significance were calculated using the stats package in R software (v3.6.3) and clustering correlation heatmap with signs was performed using the OmicStudio tools at https://www.omicstudio.cn.
3. Results
3.1. Body condition, serum biochemistry, nutrient digestibility, and fecal fermentation metabolites
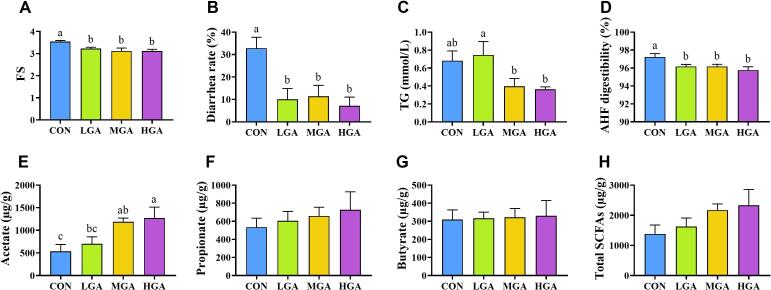
As shown in Fig. 1A and B, Dogs fed with 0.02%, 0.04%, and 0.08% GA had lower (p < 0.01) FS and diarrhea rate than the CON group. Moreover, the FS and diarrhea rate were linearly and quadratically reduced (p < 0.01) with GA supplementation. As shown in Table S2, dogs fed with GA had no effect on BCS, BW, feed intake, and fecal output (p > 0.05).
Fig. 1.
Effects of GA on FS, diarrhea rate, TG, AHF digestibility, and SCFAs in dogs. FS = fecal score; TG = triglyceride; AHF = acid-hydrolyzed fat; Total SCFAs = acetate + propionate + butyrate. Different letters indicate significant (p < 0.05) difference according to ANOVA with Fisher’s LSD multiple comparison test. CON = control; LGA = 0.02% GA; MGA = 0.04% GA; HGA = 0.08% GA.
The effects of GA supplementation on serum biochemical parameters are presented in Table S3. The serum biochemical parameters among 4 groups were within the normal reference range for all dogs on d 0. Nevertheless, the triglyceride (TG) levels were higher (p < 0.05) in the 0.02% and 0.08% GA groups than the CON group. On d 45, serum biochemistry profiles of dogs fed all 4 diets were within the normal reference range and did not differ (p > 0.05) among treatments, except for total bilirubin (TB) and TG. Supplementation with 0.08% GA increased (p < 0.05) TB level compared with the CON and 0.02% GA groups. The TG levels were decreased (p < 0.05) in the 0.04% and 0.08% GA groups compared with the 0.02% GA group (Fig. 1C). Both linear and quadratic increases (p < 0.05) were found in TB level with GA supplementation. TG, however, was linearly and quadratically decreased (p < 0.05) with the increase of GA dosages.
As presented in Fig. 1D, supplementation with 0.02%, 0.04%, and 0.08% GA reduced (p < 0.05) the digestibility of acid-hydrolyzed fat compared with the CON group. There were no differences (p > 0.05) in nutrient digestibility of dry matter, organic matter, gross energy, total dietary fiber, crude protein, and amino acids among the 4 dietary treatments (Table S4).
The effects of dietary GA supplementation on fecal fermentation metabolites are presented in Fig. 1E-H and Table S5. Fecal acetate concentration was elevated (p < 0.05) in the 0.04% and 0.08% GA groups compared with the CON group. Both linear and quadratic (p < 0.05) increases were observed in acetate with GA supplementation, and total SCFAs was linearly raised (p < 0.05) with the increase of GA dosages. Furthermore, fecal histamine concentration was linearly and quadratically increased (p < 0.05) with GA supplementation, and fecal tryptamine concentration displayed a linear increasing trend (p = 0.062) with increasing GA dosages. In addition, a changing trend (p = 0.099) in fecal putrescine concentration was found in the GA-treated groups.
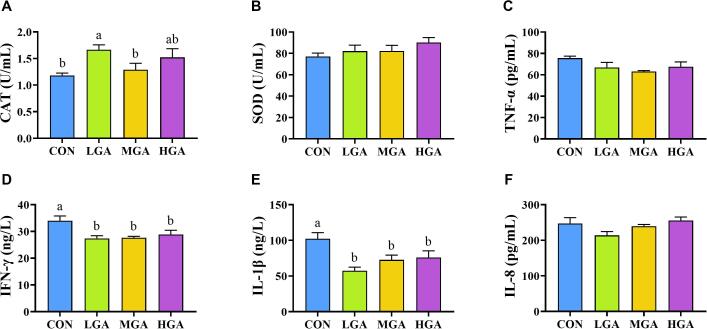
3.2. Serum antioxidant capacity and inflammatory cytokines
The effects of dietary GA supplementation on serum antioxidant capacity and inflammatory cytokines are presented in Fig. 2 and Table S6. The SOD activity exhibited a linear increasing trend (p = 0.069) with increasing GA dosages. Compared with the CON and 0.04% GA groups, 0.02% GA group had higher (p < 0.05) CAT activity. Moreover, TNF-α concentration was linearly and quadratically decreased (p < 0.05) as GA increased. Different levels of GA had lower (p < 0.05) IFN-γ concentration than the CON group, and IFN-γ concentration was linearly and quadratically decreased (p < 0.05) as GA increased. A decrease (p < 0.05) in IL-1β concentration was observed in dogs fed GA, and IL-1β concentration was decreased quadratically (p < 0.05) with GA supplementation. Dogs among 4 groups had a changing trend (p = 0.087) in IL-8 concentration. In addition, a quadratic regression relationship was found between the increase of GA dosages and IgG concentration.
Fig. 2.
Effects of GA on (A, B) antioxidant capacity and (C-F) inflammatory cytokines in dogs. Different letters indicate significant (p < 0.05) difference according to ANOVA with Fisher’s LSD multiple comparison test. CON = control; LGA = 0.02% GA; MGA = 0.04% GA; HGA = 0.08% GA.
3.3. Fecal microbiota
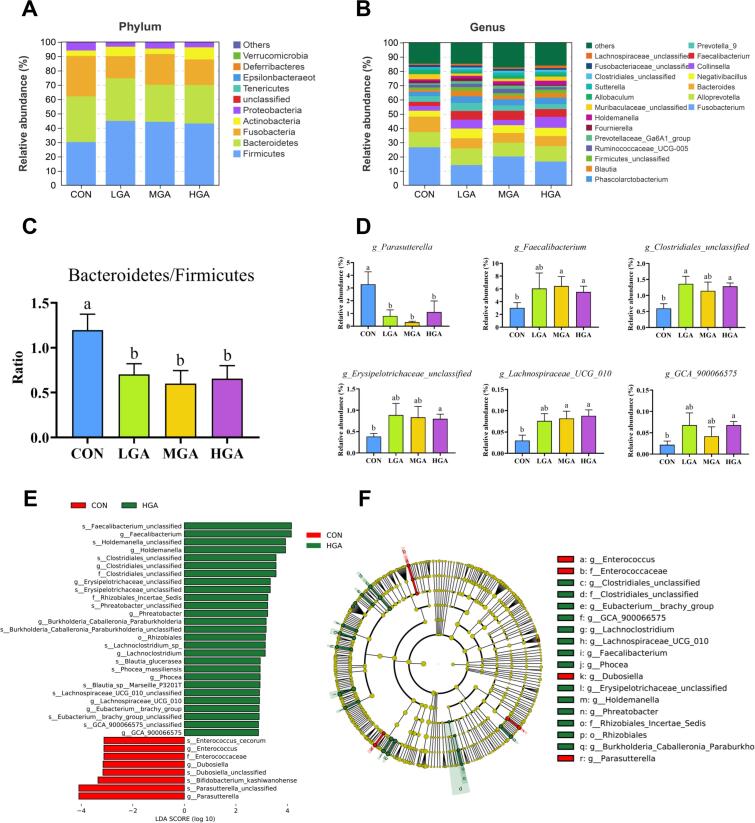
The effects of dietary GA supplementation on fecal microbiota are presented in Fig. 3. The Venn analysis identified 291 shared features among the 4 groups and 457, 484, 386, and 240 unique features in the CON, 0.02% GA, 0.04% GA, and 0.08% GA groups, respectively (Fig. S1). As presented in Table S7, there were no differences (p > 0.05) in Observed_species, Shannon, Simpson, Chao1, and Goods_coverage among the 4 dietary treatments. The PCA showed that there were similar microbial communities among the 4 groups (p > 0.05) (Fig. S2).
Fig. 3.
Relative abundance of fecal microbiota at the (A, C) phylum and (B, D) genus levels. (E, F) LEfSe analysis between the CON and HGA groups. Different letters indicate significant (p < 0.05) difference according to ANOVA with Fisher’s LSD multiple comparison test. CON = control; LGA = 0.02% GA; MGA = 0.04% GA; HGA = 0.08% GA.
At the phylum level, Firmicutes, Bacteroidetes, Fusobacteria, Actinobacteria, and Proteobacteria were the dominant bacteria, which accounted for >99% (Fig. 3A). In addition, the GA-treated groups had a significantly decreasing (p < 0.05) Bacteroidetes/Firmicutes (B/F) ratio compared with the CON group (Fig. 3C). At the genus level, the predominant genus consisted of Fusobacterium, Alloprevotella, Bacteroides, Negativibacillus, Collinsella, Faecalibacterium, Prevotella_9, Phascolarctobacterium, and Blautia (Fig. 3B). Among them, the GA-treated groups had a significantly decreasing (p < 0.05) Parasutterella, and 0.08% GA group had a significantly increasing (p < 0.05) Faecalibacterium, Clostridiales_unclassified, Erysipelotrichaceae_unclassified, Lachnospiraceae_UCG_010, and GCA_900066575 (Fig. 3D).
The LEfSe analysis was performed to identify microbial taxa that serve as biomarkers for the different groups. As shown in Fig. 3E and F, Parasutterella, Dubosiella, and Enterococcus remarkably enriched in the CON group, and Faecalibacterium, Holdemanella, Clostridiales_unclassified, Erysipelotrichaceae_unclassified, Phreatobacter, Burkholderia-Caballeronia-Paraburkholderia, Lachnoclostridium, Phocea, Lachnospiraceae_UCG_010, Eubacterium_brachy_group, and GCA_900066575 (i.e., Lachnospiraceae) remarkably enriched in the 0.08% GA group.
3.4. Fecal and serum metabolomics
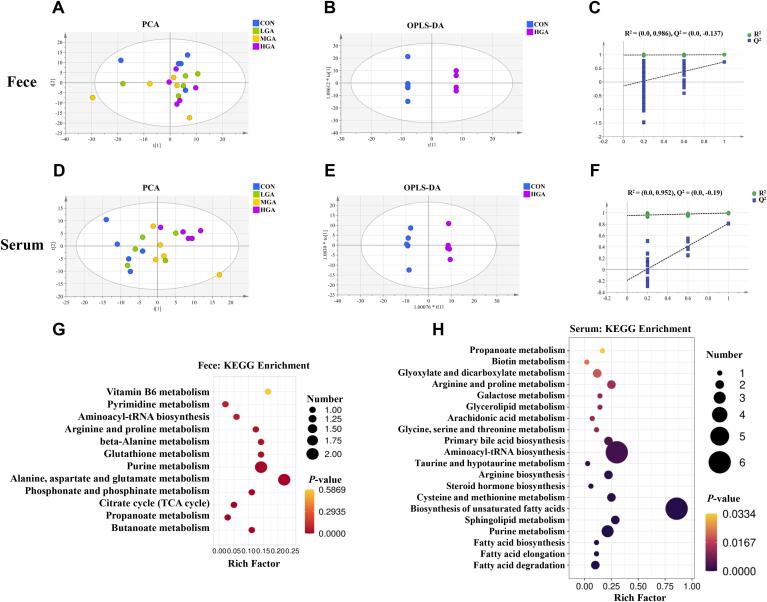
In this study, the fecal and serum metabolic profiles were detected using untargeted metabolomics techniques. We assessed the clustering of QC samples using PCA plot (Fig. S3). The PCA score plot displayed a tight clustering of QC samples, indicating stable analytical conditions and good repeatability during the detection. A total of 535 and 338 metabolites in feces and serum were detected. The PCA showed that a distinct separation existed in the fecal metabolites between the CON and 0.08% GA groups (Fig. 4A and D). The OPLS-DA model further distinguished the difference between the CON and 0.08% GA groups (Fig. 4B and E). The cumulative values of R2Y and Q2 were 0.999, 0.621 and 0.999, 0.669, which elucidated the stability and reliability of the model. In addition, the accuracy of the model was assessed using the RPT method. As shown in Fig. 4C and F, the values of R2 (0.986, 0.952) and Q2 (−0.137, −0.19) revealed the good accuracy of the OPLS-DA models.
Fig. 4.
Effects of dietary GA supplementation on fecal and serum metabolic profiles of dogs. (A-C, G) Fecal PCA, OPLS-DA, RPT, and KEGG metabolic pathways enrichment analysis. (D-F, H) Serum PCA, OPLS-DA, RPT, and KEGG metabolic pathways enrichment analysis. The color of the point was p-value, and the redder, the more significant enrichment. The size of the spot represented the number of different metabolites enriched. CON = control; LGA = 0.02% GA; MGA = 0.04% GA; HGA = 0.08% GA. n = 5.
Differential fecal metabolites between the CON and 0.08% GA groups were screened out using the standard of VIP > 1 (168 metabolites) and p < 0.05 (38 metabolites), and a total of 37 differential metabolites were confirmed (Table S8). By using KEGG enrichment analysis, these differential metabolites were mapped into the specific pathways to identify the effects of 0.08% GA on the metabolic pathways in dogs. As shown in Fig. 4G and Table S9, 0.08% GA supplementation significantly changed 9 metabolic pathways, in which carbohydrate metabolism (butanoate metabolism, propanoate metabolism, and TCA cycle), amino acid metabolism (alanine, aspartate, and glutamate metabolism, arginine and proline metabolism, glutathione metabolism, beta-alanine metabolism, and phosphonate and phosphinate metabolism), and nucleotide metabolism (purine metabolism) were the predominantly involved metabolic pathways. Differential metabolites influencing these metabolic pathways were succinic acid, l-asparagine, spermidine, ciliatine, and adenosine.
Similarly, a total of 73 differential serum metabolites were confirmed (Table S10). As shown in Fig. 4H and Table S11, 0.08% GA supplementation significantly changed 20 metabolic pathways, in which lipid metabolism (fatty acid degradation, fatty acid elongation, fatty acid biosynthesis, sphingolipid metabolism, biosynthesis of unsaturated fatty acids, steroid hormone biosynthesis, primary bile acid biosynthesis, arachidonic acid metabolism, and glycerolipid metabolism), nucleotide metabolism (purine metabolism, cysteine and methionine metabolism, and glycine, serine and threonine metabolism), amino acid metabolism (arginine biosynthesis, taurine and hypotaurine metabolism, and arginine and proline metabolism), carbohydrate metabolism (galactose metabolism, glyoxylate and dicarboxylate metabolism, and propanoate metabolism), translation (aminoacyl-trna biosynthesis), and metabolism of cofactors and vitamins (biotin metabolism).
3.5. Spearman’s correlation analysis
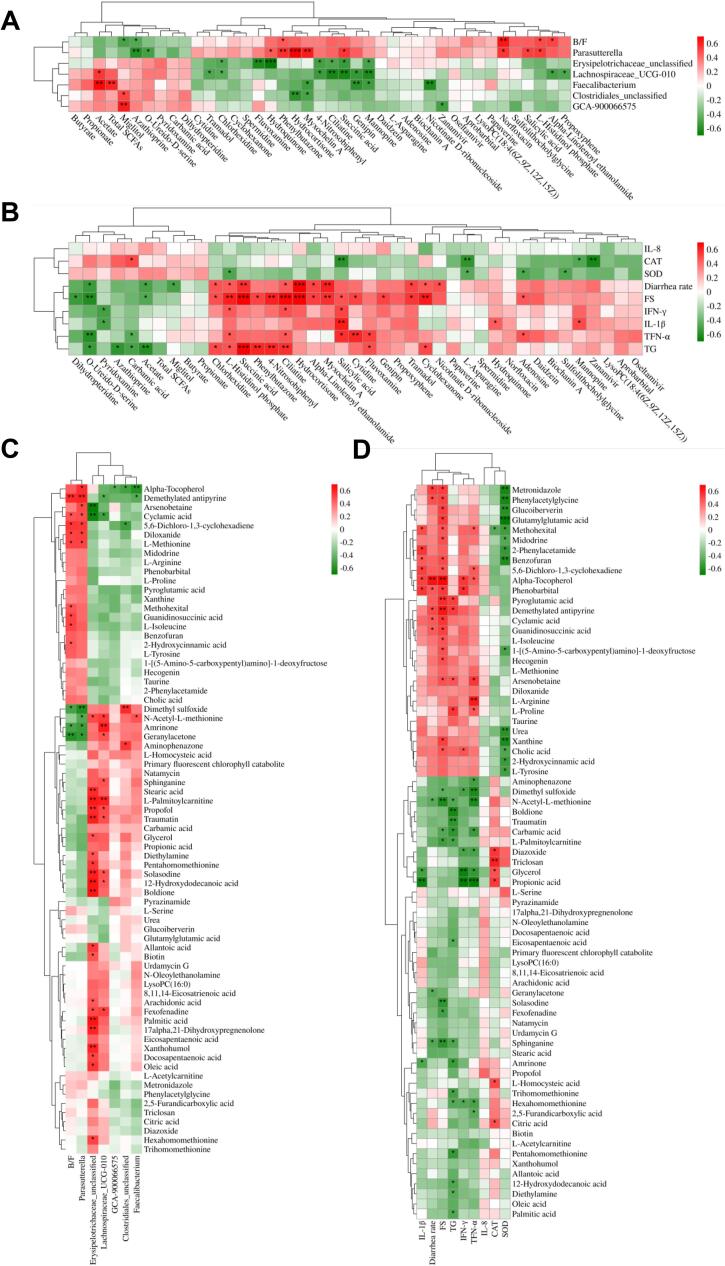
To further clarify the association between gut microbiota and metabolic changes, we performed Spearman’s correlation analysis on the differential fecal microbiota and fecal/serum metabolites, and the differential fecal/serum metabolites and serum/fecal parameters. The correlation is considered as statistically significant with correlation coefficient |r| > 0.6 and p < 0.01 in this study (Table S12-15). The results are visualized as heatmaps in Fig. 5. As shown in Fig. 5A, Erysipelotrichaceae_unclassified was negatively associated with hydroquinone and fluvoxamine. Parasutterella was positively associated with hydrocortisone and myxochelin A. Faecalibacterium was positively associated with acetate and total SCFAs, but negatively associated with nicotinate d-ribonucleoside. Lachnospiraceae_UCG-010 was negatively associated with ciliatine, succinic acid, and mannopine. GCA-900066575 was positively associated with miglitol. B/F ratio was positively associated with Norfloxacin. As shown in Fig. 5B, diarrhea rate and FS were positively associated with hydrocortisone, succinic acid, ciliatine, cyclohexanone, l-histidinol phosphate, myxochelin A, and 4-nitrosobiphenyl, but negatively associated with O-ureido-d-serine. The TG was positively associated with succinic acid and phenylbutazone. The IL-1β was positively associated with salicylic acid. The CAT was negatively associated with zanamivir. In addition, as shown in Fig. 5C, Erysipelotrichaceae_unclassified was positively associated with 12-hydroxydodecanoic acid, boldione, palmitic acid, l-palmitoylcarnitine, traumatin, and solasodine, but negatively associated with arsenobetaine and cyclamic acid. Lachnospiraceae_UCG-010 was positively associated with amrinone and l-palmitoylcarnitine. Parasutterella was negatively associated with dimethyl sulfoxide. As shown in Fig. 5D, TNF-α was positively associated with l-arginine, but TNF-α, IFN-γ, and IL-1β were negatively associated with propionic acid, glycerol, dimethyl sulfoxide, and N-acetyl-l-methionine. The CAT was positively associated with triclosan, but SOD was negatively associated with glutamylglutamic acid, phenylacetylglycine, metronidazole, xanthine, and benzofuran. The FS and diarrhea rate were positively associated with alpha-tocopherol and demethylated antipyrine, but negatively associated with N-acetyl-l-methionine. The TG was negatively associated with boldione.
Fig. 5.
Heatmaps of Spearman’s correlation analysis between (A) differential genera and fecal metabolites, (B) differential fecal metabolites and serum/fecal parameters, (C) differential genera and serum metabolites, and (D) differential serum metabolites and serum/fecal parameters. Red and green boxes represent positive and negative correlations, respectively. B/F ratio = Bacteroidetes/Firmicutes ratio; FS = Fecal score; TG = triglyceride. The symbol (*) indicates a significant correlation (* p < 0.05, ** p < 0.01, and *** p < 0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The naturally-occurring polyphenolic compounds may serve as functional ingredients in the human and animal diets due to its beneficial effects. In the present study, we selected beagles as model animals to evaluate the effects of dietary supplementation with different levels of GA on physiological function, antioxidant capacity, inflammatory cytokines, nutrient digestibility, and fecal characteristics, fermentation metabolites, microbiota, and metabolome. As expected, all the 4 experimental diets had similar ingredient composition and nutrient levels, except for supplementation with 0.02%, 0.04%, and 0.08% GA. Briefly, dogs fed with GA had no effect on most serum biochemical parameters, body condition, feed intake, and fecal characteristics, while supplementation with GA influenced serum TB, TG, acid-hydrolyzed fat digestibility, diarrhea rate, and fecal SCFAs and enhanced anti-oxidative and anti-inflammatory capacities, as well as modulated fecal microbiota and metabolic profiles.
All serum biochemical parameters were within the normal reference range for all dogs in the 4 groups on d 0 and d 45, indicating all dogs remained healthy without any signs of gastrointestinal discomfort throughout the study. The accumulation of serum TB level is a common symptom of liver damage (Vítek & Ostrow, 2009). Although 0.08% GA significantly increased TB level (5.32 µmol/L) on d 45 and displayed a dose-dependent relationship, it's still in the safety range (0.0–15.0 µmol/L), indicating long-term feeding of 0.02%∼0.08% GA does not negatively affect liver function. Additionally, the 0.02% and 0.08% GA groups had higher serum TG levels than the CON group on d 0. However, after 45 days of GA supplementation, we found a surprise that 0.04% and 0.08% GA decreased serum TG levels with a dose-dependent relationship, indicating long-term feeding of GA has the potential to improve lipid metabolism and prevent obesity. Our data is consistent with previous studies in obese mice (Bak et al., 2013, Paraiso et al., 2019). Meanwhile, one interesting finding is that supplementation with 0.02%∼0.08% GA reduced the digestibility of acid-hydrolyzed fat, which is consistent with previous report that feeding pomegranate extract containing 16.9% GA equivalent reduced fat digestibility of calves (Oliveira et al., 2010). Additionally, serum metabolome further showed that 0.08% GA mainly influenced lipid metabolism. These results support the idea that GA has the potential of regulating lipid metabolism and fighting against obesity.
Gallic acid is an excellent astringent that exhibits anti-diarrheal effects (Sterneder et al., 2021). We previously demonstrated that dietary supplementation with 0.05% GA reduced FS and exhibited antidiarrheal activity against environmental stress-induced diarrhea in puppies (Yang et al., 2022). Similarly, this study found that the addition of 0.02%, 0.04%, and 0.08% GA remarkably reduced the FS and diarrhea rate of healthy dogs, among them, dogs fed with 0.08% GA had lowest diarrhea rate. Gut microbial-derived SCFAs are known to be able to modulate immune function and play important roles in diarrheal diseases (Gagné et al., 2013). Previous studies have found that GA supplementation can increase cecal or fecal acetate and total SCFAs concentrations (Masek et al., 2014, Yang et al., 2022). Similar results were obtained in this study, with higher GA dosages leading to higher acetate and total SCFAs concentrations, which may be one of the reasons why GA supplementation possesses the potential to alleviate diarrheal symptoms.
The formation of reactive oxygen species and subsequent generation of oxidative stress can lead to inflammatory response (Hussain, Tan, Yin, Blachier, Tossou, & Rahu, 2016). We previously found that 0.05% GA could alleviate oxidative damage and inflammatory response in stressful puppies (Yang et al., 2022). Researches in other animals have also yielded similar results (Pandurangan et al., 2015). The current study showed that dogs fed with 0.02% GA had the highest serum CAT activity, and 0.08% GA had the highest SOD activity. Our results are different from previous report, which found short-term feeding of 0.05% GA increased GSH-Px activity and decreased MDA content in stressful puppies, but had no effect on serum T-AOC and SOD activities (Yang et al., 2022). It is possible that differences in duration of feeding GA and physiological states could be responsible for these differential antioxidant pathways. Our results also found that 0.02%∼0.08% GA supplementation had robust anti-inflammatory role. These results seem to be consistent with other research suggesting that GA supplementation alleviated inflammatory response in different physiological states of animals (Pandurangan et al., 2015).
Gut microbiota variation may be a key mechanism resulting in intestinal inflammation (Yang et al., 2020). In accordance with the present results, Firmicutes, Bacteroidetes, Fusobacteria, Actinobacteria, and Proteobacteria are the five predominant bacterial phyla in the canine gastrointestinal tract (Pilla & Suchodolski, 2019). There were no differences in bacteria at the phylum level among groups, and no changes were observed for α- and β-diversity of fecal microbiota in dogs fed with GA, indicating long-term feeding of 0.02%∼0.08% GA had no obvious effect on gut microbial richness and diversity of healthy dogs. Nevertheless, one interesting finding was that 0.02%∼0.08% GA significantly decreased the B/F ratio. This result is in line with previous studies which had confirmed that dogs fed high-protein and low-carbohydrate diets, as an effective weight loss strategy, had a decrease in B/F ratio (Moinard et al., 2020), and dietary supplementation with green tea polyphenols reduced B/F ratio and intestinal inflammation in obese dogs (Li et al., 2020). Therefore, we further speculate that GA can improve lipid metabolism by regulating gut microbiota.
Further analysis of bacteria at the genus level, we found that GA supplementation decreased the relative abundance of pro-inflammatory bacterium Parasutterella (Wang et al., 2021, Wu et al., 2022). Previous studies showed that the potentially beneficial bacteria Faecalibacterium, Clostridiales_unclassified, Erysipelotrichaceae, and Lachnospiraceae are linked to SCFAs production and was positively correlated with metabolic homeostasis improvement (Parada Venegas et al., 2019, van der Hee and Wells, 2021). In this study, compared with the CON group, 0.08% GA increased the relative abundances of Faecalibacterium, Clostridiales_unclassified, Erysipelotrichaceae_unclassified, Lachnospiraceae_UCG_010, and GCA_900066575 (Lachnospiraceae), revealing that the decreasing Parasutterella and increasing SCFAs-producing bacteria abundances induced by GA are important reasons for the relief of inflammation and diarrhea in this study. Meanwhile, the correlation analysis also further verified a positive association between Faecalibacterium and acetate/total SCFAs. Nevertheless, our previous findings in stressful puppies suggested that 0.05% GA increased the relative abundance of fecal Lactobacillus and decreased Escherichia–Shigella and Clostridium_sensu_stricto_1 (Yang et al., 2022). In all, these results suggest that different levels of GA have distinct regulatory effects on bacteria due to different physiological states and feeding duration in dogs.
Gut microbiota-derived metabolites are intimately related to host physiology, including nutritional status, metabolism, and stress response (Sekirov, Russell, Antunes, & Finlay, 2010). Thus, an untargeted metabolomics was performed to determine the effects of GA supplementation on fecal metabolic profiles. The PCA and OPLS-DA plots displayed a distinct separation between the CON and 0.08% GA groups. The KEGG enrichment analysis showed that 0.08% GA affected carbohydrate, amino acid, and nucleotide metabolism. Similar conclusion was obtained in previous study (Yang et al., 2022). Among them, Parasutterella is a producer of succinic acid (Ju, Kong, Stothard, & Willing, 2019) which is known for its immunomodulatory properties (Connors, Dawe, & Van Limbergen, 2019). It has been reported that reducing the accumulation of succinic acid may regulate inflammatory cytokine levels, thereby enhancing the immunity of animals (Geng et al., 2021, Liu et al., 2022). Our study found that dogs fed with 0.08% GA had a decreasing concentration of succinic acid in feces, thereby enhancing the immunity of dogs. Moreover, enrichment analysis further confirmed that the decreasing succinic acid affected the butanoate metabolism, propanoate metabolism, and TCA cycle. These results showed that GA could inhibit the proliferation of Parasutterella, which led to a reduction in the production of succinic acid in feces, thereby enhancing immunity and reducing diarrhea rate. Hydrocortisone (i.e., cortisol) is the main glucocorticoid secreted by the adrenal cortex and it is involved in the stress response (Adinoff, Junghanns, Kiefer, & Krishnan-Sarin, 2005). Similar to succinic acid, we also found that altered fecal bacteria were significantly associated with hydrocortisone which was positively associated with diarrhea rate. From the correlation analysis, we found multiple strong correlations between differential fecal microbiota and metabolites which affected diarrhea rate, antioxidant capacity, inflammatory response, and lipid metabolism, thereby improving gut health. However, the association between gut microbiota and metabolic changes still needs to be further explored and verified.
5. Conclusion
In conclusion, the current findings suggested that dietary supplementation with GA (0.02%, 0.04%, and 0.08%) had no negative effect on body condition. GA supplementation regulated serum lipid metabolism by reducing serum triglyceride, fat digestibility, and B/F ratio. Moreover, GA intervention decreased the relative abundance of Parasutterella, and increased that of SCFAs-producing bacteria (including Faecalibacterium, Clostridiales_unclassified, Erysipelotrichaceae_unclassified, and Lachnospiraceae) along with fecal acetate and total SCFAs contents accumulation in the 0.08% GA group. The KEGG enrichment analysis further elucidated that 0.08% GA mainly affected carbohydrate metabolism (butanoate metabolism, propanoate metabolism, and TCA cycle) by downregulating succinic acid in feces. Importantly, strong correlations between the altered fecal microbiota and metabolic profiles revealed that GA supplementation improved hindgut metabolic activity by modulating gut microbiota, thereby promoting gut health by enhancing antioxidant capacity and reducing inflammation and diarrhea rate. Overall, this study confirmed the beneficial effects of long-term consumption of GA on lipid metabolism and gut health, and the optimal level of GA supplementation was 0.08%.
CRediT authorship contribution statement
Kang Yang: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Shiyan Jian: Validation, Data curation. Dan Guo: Validation, Investigation. Chaoyu Wen: Validation. Zhongquan Xin: Validation. Limeng Zhang: Validation. Tao Kuang: Investigation. Jiawei Wen: Investigation. Yulong Yin: Conceptualization, Methodology, Writing – review & editing. Baichuan Deng: Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Key R&D Program of China (Grant No. 2021YFD1300400), National Natural Science Foundation of China (Grant Nos. 31790411, 32002186), Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010322), Guangzhou Basic and Applied Basic Research Foundation (Grant No. 202102020850), and Independent Research and Development Projects of Maoming Laboratory (Grant No. 2021ZZ003).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100377.
Contributor Information
Yulong Yin, Email: yinyulong@isa.ac.cn.
Baichuan Deng, Email: dengbaichuan@scau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- AAFCO (2017). Association of American Feed Control Officials (AAFCO). Oxford, IN.
- Adinoff B., Junghanns K., Kiefer F., Krishnan-Sarin S. Suppression of the HPA axis stress-response: Implications for relapse. Alcoholism-Clinical and Experimental Research. 2005;29(7):1351–1355. doi: 10.1097/01.alc.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak E.J., Kim J., Jang S., Woo G.H., Yoon H.G., Yoo Y.J., Cha J.H. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scandinavian Journal of Clinical & Laboratory Investigation. 2013;73(8):607–614. doi: 10.3109/00365513.2013.831470. [DOI] [PubMed] [Google Scholar]
- Casanova-Martí À., Serrano J., Portune K.J., Sanz Y., Blay M.T., Terra X.…Pinent M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food & Function. 2018;9(3):1672–1682. doi: 10.1039/C7FO02028G. [DOI] [PubMed] [Google Scholar]
- Coelho L.P., Kultima J.R., Costea P.I., Fournier C., Pan Y., Czarnecki-Maulden G.…Bork P. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome. 2018;6:72. doi: 10.1186/s40168-018-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J., Dawe N., Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2019;11(1):25. doi: 10.3390/nu11010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: A mini-review. Frontiers in Nutrition. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo F.F., de Paulo Farias D., Neri-Numa I.A., Pastore G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.127535. [DOI] [PubMed] [Google Scholar]
- Dludla P., Nkambule B., Jack B., Mkandla Z., Mutize T., Silvestri S.…Mazibuko-Mbeje S. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients. 2019;11(1):23. doi: 10.3390/nu11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné J.W., Wakshlag J.J., Simpson K.W., Dowd S.E., Latchman S., Brown D.A.…Fahey G.C. Effects of a synbiotic on fecal quality, short-chain fatty acid concentrations, and the microbiome of healthy sled dogs. BMC Veterinary Research. 2013;9:246. doi: 10.1186/1746-6148-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T., He F., Su S., Sun K., Zhao L., Zhao Y.…Sun H. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA, DHA, and succinic and palmitic acids, and enhance immunity of weaned piglets. Research in Veterinary Science. 2021;137:56–67. doi: 10.1016/j.rvsc.2021.04.011. [DOI] [PubMed] [Google Scholar]
- Gowd V., Karim N., Shishir M.R.I., Xie L., Chen W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends in Food Science & Technology. 2019;93:81–93. doi: 10.1016/j.tifs.2019.09.005. [DOI] [Google Scholar]
- Hortwitz, W., & Latimer, G. W. (2007). Official methods of analysis of AOAC international.: AOAC International: Gaithersburg, MD.
- Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Medicine and Cellular Longevity. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T., Kong J.Y., Stothard P., Willing B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME Journal. 2019;13(6):1520–1534. doi: 10.1038/s41396-019-0364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme D. Development and validation of a body condition score system for dogs. Canine Practice. 1997;22(4):10–15. doi: 10.2307/1592173. [DOI] [Google Scholar]
- Li Y., Rahman S.U., Huang Y., Zhang Y., Ming P., Zhu L.…Wu J. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. Journal of Nutritional Biochemistry. 2020;78 doi: 10.1016/j.jnutbio.2019.108324. [DOI] [PubMed] [Google Scholar]
- Liu Q., Li B., Li Y., Wei Y., Huang B., Liang J.…Tang R. Altered faecal microbiome and metabolome in IgG4-related sclerosing cholangitis and primary sclerosing cholangitis. Gut. 2022;71:899–909. doi: 10.1136/gutjnl-2020-323565. [DOI] [PubMed] [Google Scholar]
- Ma G., Chen Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. Journal of Functional Foods. 2020;66 doi: 10.1016/j.jff.2020.103829. [DOI] [Google Scholar]
- Masek T., Starcevic K., Mikulec Z. The influence of the addition of thymol, tannic acid or gallic acid to broiler diet on growth performance, serum malondialdehyde value and cecal fermentation. European Poultry Science. 2014;78:1–8. doi: 10.1399/eps.2014.64. [DOI] [Google Scholar]
- Middelbos, I. S., Fastinger, N. D., & Jr. Fahey, G. C. (2007). Evaluation of fermentable oligosaccharides in diets fed to dogs in comparison to fiber standards. Journal of Animal Science, 85(11), 3033-3044. 10.2527/jas.2007-0080. [DOI] [PubMed]
- Mithul Aravind S., Wichienchot S., Tsao R., Ramakrishnan S., Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Research International. 2021;142 doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- Moinard A., Payen C., Ouguerram K., André A., Hernandez J., Drut A.…Leray V. Effects of high-fat diet at two energetic levels on fecal microbiota, colonic barrier, and metabolic parameters in dogs. Frontiers in Veterinary Science. 2020;7 doi: 10.3389/fvets.2020.566282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash V., Ranadheera C.S., Georgousopoulou E.N., Mellor D.D., Panagiotakos D.B., McKune A.J.…Naumovski N. The effects of grape and red wine polyphenols on gut microbiota - a systematic review. Food Research International. 2018;113:277–287. doi: 10.1016/j.foodres.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Nuli R., Azhati J., Cai J., Kadeer A., Zhang B., Mohemaiti P. Metagenomics and faecal metabolomics integrative analysis towards the impaired glucose regulation and type 2 diabetes in uyghur-related omics. Journal of Diabetes Research. 2019;2019:2893041. doi: 10.1155/2019/2893041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R.A., Narciso C.D., Bisinotto R.S., Perdomo M.C., Ballou M.A., Dreher M., Santos J.E. Effects of feeding polyphenols from pomegranate extract on health, growth, nutrient digestion, and immunocompetence of calves. Journal of Dairy Science. 2010;93(9):4280–4291. doi: 10.3168/jds.2010-3314. [DOI] [PubMed] [Google Scholar]
- Pandurangan, A. K., Mohebali, N., Mohd. Esa, N., Looi, C. Y., Ismail, S., & Saadatdoust, Z. (2015). Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. International Immunopharmacology, 28(2), 1034-1043. 10.1016/j.intimp.2015.08.019. [DOI] [PubMed]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G.…Hermoso M.A. Short chain fatty acids (SCFAs)-Mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso A.F., Sousa J.N., Andrade J., Mangabeira E.S., Lelis D.F., de Paula A.…Santos S. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach. Life Sciences. 2019;237 doi: 10.1016/j.lfs.2019.116914. [DOI] [PubMed] [Google Scholar]
- Pilla R., Suchodolski J.S. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Frontiers in Veterinary Science. 2019;6:498. doi: 10.3389/fvets.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamada D., Vodnar D.C. Polyphenols-gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients. 2022;14(1):137. doi: 10.3390/nu14010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiological Reviews. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Sterneder S., Stoeger V., Dugulin C.A., Liszt K.I., Di Pizio A., Korntheuer K.…Somoza V. Astringent gallic acid in red wine regulates mechanisms of gastric acid secretion via activation of bitter taste sensing receptor TAS2R4. Journal of Agricultural and Food Chemistry. 2021;69(36):10550–10561. doi: 10.1021/acs.jafc.1c03061. [DOI] [PubMed] [Google Scholar]
- van der Hee B., Wells J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends in Microbiology. 2021;29(8):700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Vítek L., Ostrow J.D. Bilirubin chemistry and metabolism; Harmful and protective aspects. Current Pharmaceutical Design. 2009;15(25):2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tao H., Huang H., Xiao Y., Wu X., Li M.…Wu X. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food & Function. 2021;12(7):3142–3158. doi: 10.1039/D0FO03061A. [DOI] [PubMed] [Google Scholar]
- Wu Z., Cheng W., Wang Z., Feng S., Zou H., Tan X.…Wei H. Intestinal microbiota and serum metabolic profile responded to two nutritional different diets in mice. Frontiers in Nutrition. 2022;8 doi: 10.3389/fnut.2021.813757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z., Ma S., Ren D., Liu W., Han B., Zhang Y.…Deng B. UPLC-Orbitrap-MS/MS combined with chemometrics establishes variations in chemical components in green tea from Yunnan and Hunan origins. Food Chemistry. 2018;266:534–544. doi: 10.1016/j.foodchem.2018.06.056. [DOI] [PubMed] [Google Scholar]
- Yang K., Deng X., Jian S., Zhang M., Wen C., Xin Z.…Deng B. Gallic acid alleviates gut dysfunction and boosts immune and antioxidant activities in puppies under environmental stress based on microbiome-metabolomics analysis. Frontiers in Immunology. 2022;12 doi: 10.3389/fimmu.2021.813890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Zhang L., Liao P., Xiao Z., Zhang F., Sindaye D.…Deng B. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.580208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.