Highlights
-
•
Blue light and salicylic acid combination delayed fruit water loss and decay.
-
•
BL + SA treatment maintained the sensory and nutritional qualities of strawberries.
-
•
BL + SA treatment preserved strawberry bioactive components and antioxidant capacity.
Keywords: Strawberry, Blue light, Salicylic acid, Fruit quality, Postharvest
Abstract
Strawberry is a high economic and nutritional value fruit, but marketing is limited by a short postharvest life. The objective of this work is to assess the influence of blue light (BL) and salicylic acid (SA, 2 mM) on strawberry postharvest quality during cold storage. The results showed that the combination of BL and SA noticeably delayed weight loss, prevented decay, improved fruit skin brightness, and increased soluble protein. Strawberries treated with BL + SA had lower total soluble solids and titratable acidity contents among treatments but had no significant change during the entire storage. Additionally, contents of total flavonoids, phenolics, anthocyanins and proanthocyanidins, activities of superoxide dismutase (SOD) and ascorbate peroxidase (APX) and total antioxidant capacities in BL + SA-treated fruit were kept at stable levels throughout the entire storage. Collectively, these findings suggest that BL + SA treatment exhibits a high potential in maintaining postharvest fruit quality of strawberry fruit.
Introduction
Fresh fruit have been paid much attention by consumers and markets, due to their desirable flavors, delicious taste, abundant nutrients, and health-promoting effects. However, postharvest loss and waste across the supply chain of fruit can be more than 30 % before reaching the consumer tables, especially in developing and underdeveloped countries (Chen et al., 2021). The growing evidence indicates that intrinsic physiological senescence and fungal spoilage are the main elicitors among the causes of fruit losses (Tian, Qin, & Li, 2013). The use of synthetic chemicals is a simple and inexpensive way in controlling postharvest quality of the fresh produce, but it raises public concerns regarding the safety of food and environment and has currently limited by legislation and regulation in some countries. Accordingly, there is indeed a need for developing environmentally sustainable and consumer-friendly strategies as alternatives to synthetic chemicals to minimize the amount of postharvest losses and extent the shelf- life of fresh produce.
Light quality has remarkable potential in improving quality and prolonging storage period of fruit and vegetables at postharvest. Several studies on the application of UV radiation have demonstrated their ability to decrease the postharvest decay. However, UV radiation is more harmful at genetic level and more dangerous to the operator compared to visible light (Ramirez, Restrepo, Perez, & Jimenez, 2019). In recent advances, light-emitting diode (LED) technology provides the possibility for understanding the influence of visible light on fresh produce through modification of the light spectral composition (Zhang, Zhang, Xu, Mujumdar, & Guo, 2021). It has been found that blue light is positively involved in biosynthesis of fruit quality-related metabolites and disease resistance during postharvest storage, which results in an increasing application in the food industry (Kahramanoğlu et al., 2020). For instance, blue light can cause the decline of citrus postharvest decay incidence by increasing octanal and scoparone concentration (Ballester and Lafuente, 2017, Liao et al., 2013). Alongside, blue light is beneficial for pigment synthesis in postharvest fruit (Shi et al., 2014, Zhang et al., 2015).
Salicylic acid (SA) is recognized as a hormone-like endogenous substance, which participates in regulating a large amount of metabolic and physiological processes regarding plant growth, development, and stress response (Hayat, Hayat, Irfan, & Ahmad, 2010). Moreover, SA is a very simple phenolic compound and can be used as a natural and safe alternative chemical to control postharvest quality of horticultural crops (Asghari & Aghdam, 2010). Increasing reports have suggested that SA can induce plant resistance against disease development and chilling injury by enhancing related-gene expression and antioxidant capacities during fruit storage, and maintain or improve other quality attributes, such as sensory properties (flavor, appearance, texture), and nutritional compounds (García-Pastor et al., 2020, Haider et al., 2020).
Cultivated strawberry is of commercial and economic significance and mostly consumed fresh as well as in processed forms such as jams, wines, jellies, and juices. However, fresh strawberries have a high degree of perishability and a very short shelf-life mainly owing to their high susceptibility to mechanical injury, microbiological decay, water loss, and physiological disorder, which hinders their market sale. To date, multiple postharvest techniques have been investigated to preserve strawberry quality including blue light irradiation and salicylic acid application, but the combination effect of BL with SA have not been studied. Given the application and safety of BL and SA in postharvest preservation of fresh produce, this work aimed to estimate the impact of BL and SA combination on strawberry quality during cold storage, which may provide a supplementary postharvest treatment for strawberry as well as other fresh produce.
Materials and methods
Main chemicals
The main chemicals used in this study included Coomassie brilliant blue G250 (AR, Solarbio, Beijing, China), bovine serum albumin (AR, Solarbio, Beijing, China), ascorbic acid (AR, Sinopharm Chemical Reagent Co. Ltd, Shanghai, China), acetone (AR, Xilong Scientific, Guangdong, China), quercetin (standard products, Solarbio, Beijing, China), gallic acid (standard products, Solarbio, Beijing, China), 4-dimethylaminocinnamaldehyde (standard products, Sigma, MO, USA), Procyanidin B2 (standard products, Sigma, MO, USA), 1,1-Diphenyl-2-picryl-hydrazyl (BR, TCI, Tokyo, Japan).
Plant materials and experimental design
Strawberries (Fragaria × ananassa Duch., cv. Benihoppe) were hand-harvested at red-ripe stage from a local commercial orchard in Chengdu, Sichuan Province, China, and immediately delivered to the laboratory. Uniform fruits without any damage were separated into two groups and respectively dipped into 2 mM SA solution and distilled water for two minutes. After immersion, the fruit were air-dried for approximately 30 min. Subsequently, fruits treated by SA and distilled water were respectively distributed into two group and put into a chamber installed light‐emitting diodes (LED) blue light (BL, 450 nm) and white light (WL) for WL + H2O, WL + SA, BL + H2O and BL + SA four treatments. The chamber was set at 8 °C, 85–90 % relative humidity, and 16 h of 100 μmol·m−2·s−1 light for 9 d. Fruits were sampled at three-day intervals during storage and rapidly frozen in liquid nitrogen, milled, and then stored at −80 °C. There were 3 replicates of 10 fruits per replicate in each treatment for each sampling date.
Water loss and decay incidence
The total weight of 10 strawberry fruits from each replicate was measured on each sampling day. The water loss was calculated as the percentage (%) of reduction in weight relative to the original weight. The fruit that showed rot, lesion, or visible fungal growth was considered as decay. The decay incidence was presented as the percentage of decay fruits with respect to the total number of fruits. The severity of decay was estimated on a 1–5 scale depending on the rotted area around the surface of strawberry fruit using the guideline established by Aghdam and Fard (2017).
Skin color, total soluble solids, and titratable acidity
The surface color of strawberries was evaluated by obtaining a*, b* and L* chromaticity values using a CR-400 chromometer (Konica Minolta, Japan). Color coordinates range from −a (greenness) to +a (redness), −b (blueness) to +b (yellowness), and L = 0 (black) to L = 100 (white). L* coordinate indicated the lightness. Total soluble solid (TSS) of strawberries was recorded via a digital pocket refractometer (PAL-1, Atago, Tokyo, Japan), and the results were presented as °Brix. The content of titratable acidity (TA) was measured by titrating the fruit extract against a solution of 0.1 mol L-1 sodium hydroxide (NaOH) and presented as citric acid percentage.
Soluble protein and ascorbic acid
The measurement of soluble protein content was achieved using Coomassie brilliant blue G250 (CBBG) staining (Li et al., 2010). Briefly, 0.5 g of finely ground fruit powder was homogenized in 5 mL of distilled water. A portion (1 mL) of upper phase was added 5 mL of CBBG dye after centrifugation, followed by a 2 min of rest. The absorbance of the mixture was then detected at the given wavelength (595 nm). The content of soluble protein was calculated using the standard protein (bovine serum albumin, BSA). One gram of sample was extracted with 10 mL of cold 5 % (w/v) trichloroacetic acid (TCA). The content of ascorbic acid (AsA) was assayed at 534 nm according to the procedure described by Jiang et al. (2018), and expressed as gram AsA per kilogram of fresh weight.
Total flavonoid, phenolic, anthocyanin and proanthocyanidin content
An amount of fruit sample was precisely weighed and mixed with 80 % acetone, followed by one-hour incubation at room temperature. The clear solution was collected by centrifugation for detection of total flavonoid and phenolic contents. Total flavonoid contents were assayed through aluminium chloride colorimetric approach (Sulaiman, Sajak, Ooi, & Seow, 2011). A calibration curve was constructed by using quercetin as the reference. The absorption peak of the reaction mixture was determined against 80 % acetone blank at 415 nm. Results were presented as milligram quercetin equivalent per kilogram of fresh weight. Folin–Ciocalteu method (Molan, Flanagan, Wei, & Moughan, 2009) was employed to quantify total phenolic contents. The absorption value of the solution was examined against 80 % acetone blank at 650 nm. Gallic acid was selected as the standard chemical for the construction of calibration curve. Results were expressed as gram gallic acid equivalents per kilogram of a sample on fresh weight basis.
The total anthocyanin content was detected using a modified pH differential method (Zhang, Hu et al., 2018). After 15 min incubation at room temperature of extracts diluted with 0.025 M KCl (pH 1.0), and 0.4 M NaAc (pH 4.5), the absorbance was read at 496 and 700 nm. The contents were expressed as gram of pelargonidin 3-glucoside per kilogram of fresh weight. Proanthocyanidins were analyzed by employing an improved 4-dimethylaminocinnamaldehyde (DMAC) method (Zhang, Hu et al., 2018). Fruit extract was stained with 0.1 % DMAC for about 15 min at room temperature. After that, the optical density of the solution was monitored at a wavelength of 640 nm. Procyanidin B2 was employed as the standard. The total proanthocyanidin levels were expressed as gram of procyanidin per kilogram of fresh weight.
Antioxidant enzymes
Frozen samples of approximately 0.5 g were quickly weighed and added to 5 mL of 100 mM ice-cold phosphate buffer (7. 0 pH), containing 1 mM EDTA and 4 % (w/v) of Polyclar AT (PVPP), as described by Loggini, Scartazza, Brugnoli, and Navari (1999). After spinning, the resulting supernatant was collected for determination of antioxidative enzymes. For assessment of superoxide dismutase (SOD) activity, the reaction system was comprised of 13 mM methionine, 75 μM NBT, 10 μM EDTA, and 2 μM riboflavin in 50 mM phosphate buffer (pH 7.8), and 100 μL of enzyme sample. SOD activity was detected by inhibition of the photochemical reduction of nitro blue tetrazolium (NBT) as monitored at 560 nm (Zhang, Li, et al., 2018). One unit of SOD activity was defined as the corresponding quantity of enzyme that caused a 50% inhibition of NBT reduction. For detection of ascorbate peroxidase (APX) activity, the assay mixture was consisted of 0.5 mM ascorbic acid and 0.1 mM EDTA in 50 mM phosphate buffer (pH 7.0), 100 μL of enzymatic solution and a final concentration of 0.1 mM hydrogen peroxide (H2O2). APX activity was determined by the decrease in absorbance of the reaction mixture at 290 nm as the oxidation of ascorbate to dehydroascorbate (Chongchatuporn, Ketsa, & van Doorn, 2013). One unit of APX activity was expressed as a decrease of 0.01 in absorbance per minute at room temperature.
Total antioxidant activity
The total antioxidant activities of analyzed samples were investigated by two assays. 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) assay was carried out through the protocol outlined by Brand-Williams, Cuvelier, and Berset (1995) with some modifications. The 0.2 mL of sample solution was added to 2.8 mL 60 μM DPPH, and then left the blend in the dark for a rest period of 30 min at room temperature. The optical density of the mixture was read at 517 nm. The scavenging activity was evaluated by the inhibition percentage of DPPH. The ferric reducing antioxidant power (FRAP) assay was performed using the approach of Benzie and Strain (1996). The 20 µL of sample extract was thoroughly blended with 1.8 mL of the fresh working FRAP reagent (300 mM pH 3.6 acetate buffer, 10 mM TPTZ and 20 mM FeCl3·6H2O in the volumetric ratio 10:1:1). The mixture was warmed at 37 °C for 30 min followed by recording the absorption value at 593 nm. The scavenging effect was expressed as mmol kg−1 fresh weight.
Data analysis
Experimental data were analyzed statistically through analysis of variance (ANOVA) with IBM SPSS Statistics 23.0. The differences between treatments were determined using Duncan's multiple range test at the significance level of P ≤ 0.05. The data were presented in the form of mean ± standard error (SE). A principal components analysis (PCA) was performed using Origin 2021 software (OriginLab Corporation, Northampton, MA, USA).
Results and discussion
Water loss, decay incidence and severity of decay
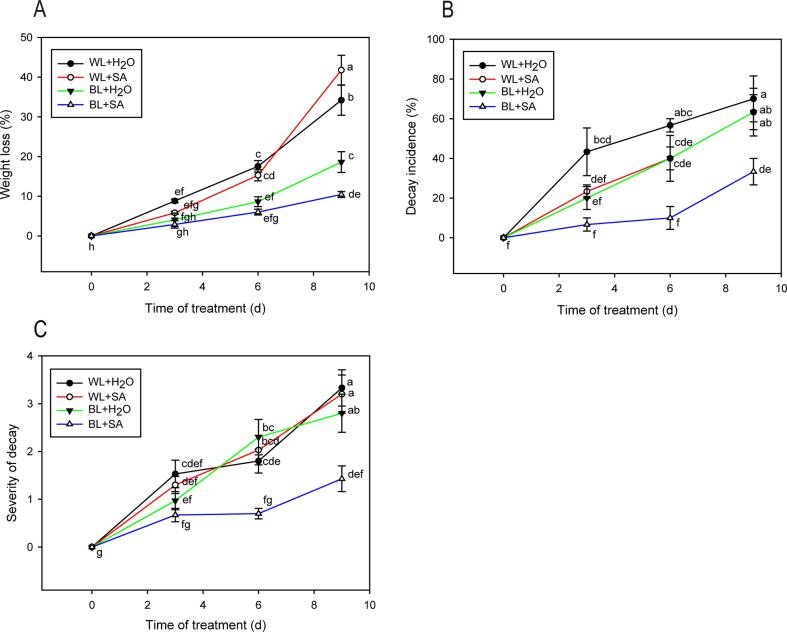
Postharvest water loss is one of key factors affecting the quality of fresh produce, which can initiate fruit shriveling and deterioration (Lufu, Ambaw, & Opara, 2020). Strawberry fruit is of high susceptibility for rapid water loss as a result of their extremely thin skin structure (Hernández-Muñoz, Almenar, Del Valle, Velez, & Gavara, 2008). Hence, minimization of water loss is crucial in optimizing the control strategy to ensure the extension of storage life of strawberries. Fig. 1A illustrated that all treated fruits showed a gradual loss of weight throughout storage. The weight loss of fruit irradiated with white light was significantly greater than that of fruit in blue light at day 6 and day 9. Furthermore, our results showed blue light combined with SA was the most effective technique for reducing the loss of fruit weight. After 9 d of fruit storage, weight loss in BL + SA was 10.44 %, compared with 18.62 % in BL + H2O, 34.20 % in WL + H2O and 41.78 % in WL + SA.
Fig. 1.
Weight loss (A), decay incidence (B) and severity of decay (C) of strawberry fruit either untreated or treated with BL and SA during storage. Data shown in the figure are presented as the mean ± SE of mean from three independent biological experiments (n = 3). The lowercase letters show no significant or significant difference based on the Duncan test (P ≤ 0.05).
As shown in Fig. 1B, the decay incidence of strawberries rose as the storage period progressed regardless of those treatments. Strawberries subjected to BL + SA maintained the lowest decay incidence, while WL + H2O-treated strawberries had the highest decay incidence during the entire storage. Although WL + SA and BL + H2O can delay the decay of strawberries, no significant difference was observed compared to WL + H2O treatment at day 9. Moreover, the BL + SA treatment remarkably reduced the severity of decay (Fig. 1C). Capacity of blue light and SA to inhibit the postharvest decay in harvested commodities has been widely reported. Blue light reduced citrus postharvest decay by inhibiting the fungal growth and inducing of defensive response (Lafuente and Alférez, 2015, Lafuente et al., 2021). SA application to susceptible horticultural crops enhanced pathogen resistance and delayed postharvest decay in a dosage-dependent manner (Asghari & Aghdam, 2010). Studies carried out by Babalar, Asghari, Talaei, and Khosroshahi (2007) on ‘Selva’ strawberries treated with different concentration of SA, showed that SA at 1 and 2 mM effectively inhibited fungal decay and retained overall quality, while 4 mM SA slightly damaged fruit. Here, strawberries treated with 2 mM SA strategy did not noticeably reduce the fruit decay in white light, probably due to the difference of sensitivity of strawberry varieties to SA.
Skin color
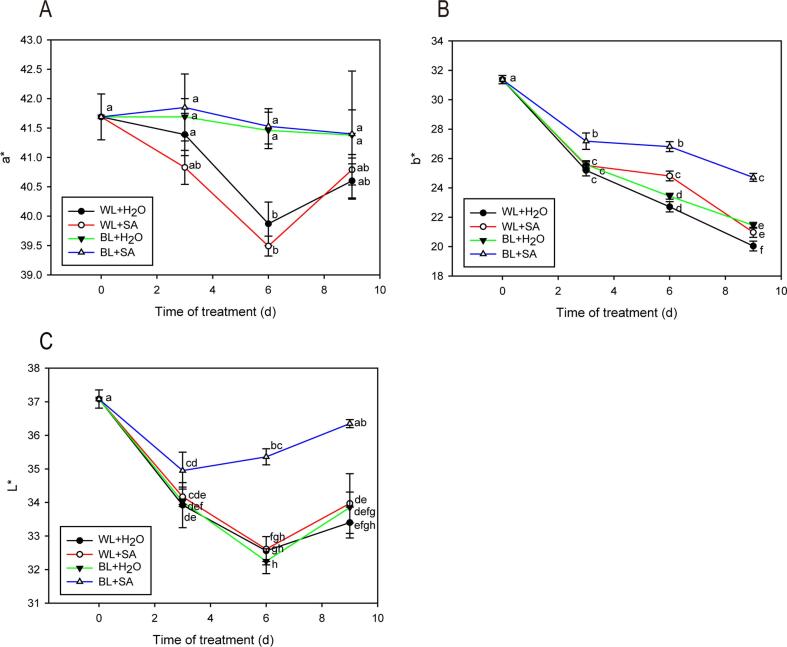
Appearance color is one of the most crucial parameters affecting consumers’ preferences and sensory perceptions of fresh produce. The change trend of a* value was similar in BL-treated fruits (BL + H2O and BL + SA) or WL-treated fruits (WL + H2O and WL + SA), indicating that light quality probably had a greater effect on a* value. The a* value showed no significant change in BL + H2O and BL + SA treatment during the storage, whereas WL + H2O and WL + SA had a significant decrease at the storage day 6. However, all treatments did not affect a* value eventually (Fig. 2A). The b* value displayed an overall decreasing trend. After 9 days of the storage, b* value in BL + SA was higher than BL + H2O, WL + SA and WL + H2O (Fig. 2B). The decrease of L* value is visualized by brightness loss, an indicator of browning in fresh-cut fruit (Gonzalez-Aguilar et al., 2008). Clearly, BL + SA treatment achieved a lower lightness loss than other treatment during the entire storage, due to its higher L* value (Fig. 2C).
Fig. 2.
Skin color of strawberry fruit either untreated or treated with BL and SA during storage. a*, redness (A), b*, yellowness (B), L*, brightness (C). Data shown in the figure are presented as the mean ± SE of mean from three independent biological experiments (n = 3). The lowercase letters show no significant or significant difference based on the Duncan test (P ≤ 0.05).
Total soluble solids, titratable acidity, soluble protein, and ascorbic acid
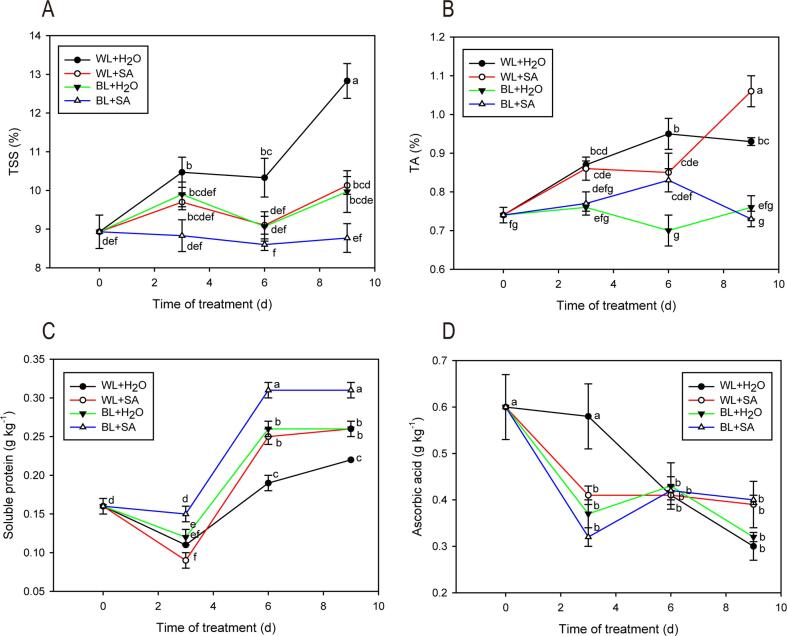
TSS is a pivotal internal quality attribute, and it can be employed to assess fruit maturity. At the end of the experiment, the TSS content in WL + H2O treatment (12.83%) was significantly higher than other treatments. The TSS content in WL + SA, BL + H2O and BL + SA treatments had no significant change throughout the storage period (Fig. 3A). These findings indicated that BL and especially SA did not significantly affect the TSS accumulation during the storage, in line with the investigation by Shafiee, Taghavi, and Babalar (2010) who observed that SA pre- and postharvest treatments did not change the TSS of strawberry fruit.
Fig. 3.
Total soluble solid (A), titratable acidity (B), soluble protein (C), and ascorbic acid (D) contents of strawberry fruit either untreated or treated with BL and SA during storage. Data shown in the figure are presented as the mean ± SE of mean from three independent biological experiments (n = 3). The lowercase letters show no significant or significant difference based on the Duncan test (P ≤ 0.05).
TA content of fruit under BL + H2O and BL + SA treatments had insignificant change throughout the entire storage, while the level of TA in WL + SA and WL + H2O remarkably increased and peaked at 1.06 % and 0.93 % at day 9, respectively (Fig. 3B).
The soluble protein content of the strawberries in each treatment exhibited an overall rising trend during the storage. Eventually, the soluble protein content in fruit treated BL + SA was noticeably higher than BL + H2O, WL + SA and WL + H2O, and the content in BL + H2O and WL + SA was significantly higher than WL + H2O (Fig. 3C). It was suggested that BL and SA contributed to soluble protein production. Increasing evidence demonstrates that many horticultural crops in blue light accumulate more protein, compared to other light qualities (Bian, Yang, & Liu, 2015).
Ascorbic acid is a natural antioxidant that is good for human health and rich in strawberry fruit. The prolonged storage always negatively affect the AsA content in fruit (Bose, Howlader, Jia, Wang, & Yin, 2019). Here, the content of AsA in all treated fruit showed an overall decreasing trend. In WL + H2O-treated fruit, the AsA level had a slight reduction within initial 3 d of storage, and then dropped sharply in the subsequent storage days. In other three treatments, the AsA content had a rapid drop firstly, and then showed an increase, followed by a decrease. No noticeable difference in AsA level was detected by statistical analysis among four treatments at the end of storage (Fig. 3D). It has been reported that BL treatment played a variable role in AsA accumulation (Loi et al., 2019, Ma et al., 2014, Zushi et al., 2020). In strawberries, BL treatment seemed not to have remarkable impact on AsA content (Xu et al., 2014), which was line with our results. In addition, Our SA treatment (2 mM) did not show the noticeably positive effect on AsA content, as reported by other studies (El-Mogy et al., 2019, Shafiee et al., 2010), but El-Mogy et al. (2019) indicated that SA in a high concentration can reduce ascorbic acid loss during storage.
Total flavonoid, phenolic, anthocyanin and proanthocyanin content
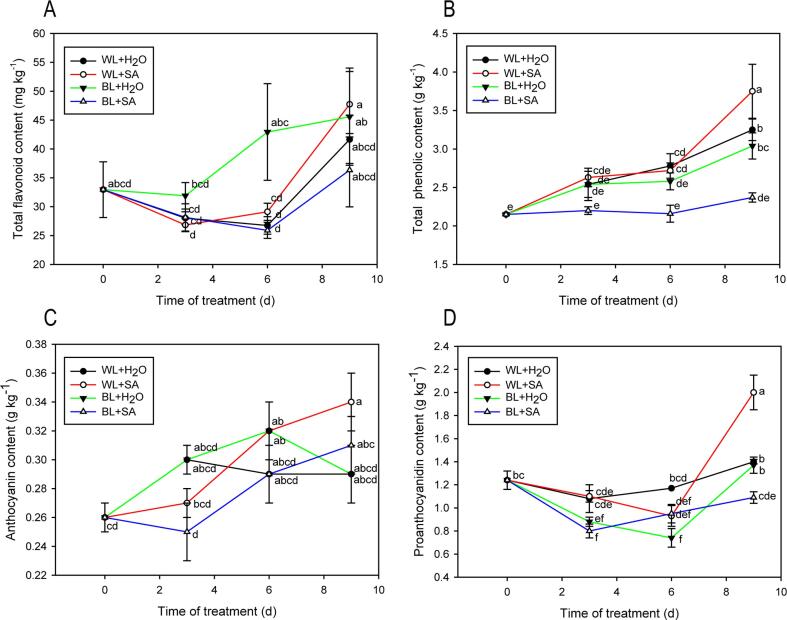
As shown in Fig. 4A, the total flavonoids in all treatments exhibited a downward trend firstly, but then had a sharply upward trend. However, there was no significant difference amongst all treatments after storage.
Fig. 4.
Total flavonoid (A), phenolics (B), anthocyanin (C), and proanthocyanin (D) contents of strawberry fruit either untreated or treated with BL and SA during storage. Data shown in the figure are presented as the mean ± SE of mean from three independent biological experiments (n = 3). The lowercase letters show no significant or significant difference based on the Duncan test (P ≤ 0.05).
The total phenolic content in WL + H2O, WL + SA and BL + H2O-treated fruit showed progressive increase and that in BL + SA had no significant change during entire storage. Eventually, the content of the total phenolics in WL + SA-treated fruit was significantly higher than WL + H2O, BL + H2O and BL + SA-treated fruit (Fig. 4B). It has been reported that blue light or salicylic acid have a positive role in increasing total phenolics (García-Pastor et al., 2020, Pérez-Ambrocio et al., 2018). However, our results found effect of combined BL and SA seemed to have an overall negative effect on total phenolic content compared to the WL + H2O.
Anthocyanin content presented overall increasing trend regardless of the given treatments. After the designated storage period, anthocyanin content only in WL + SA-treated fruit was significantly higher than the initial stage. However, there was no remarkable difference among four treatments after the storage (Fig. 4C). Light, especially blue light can give rise to the distinctive accumulation of anthocyanins before fruit ripening is complete. In this study, no significant difference between WL and BL treatments was found (Fig. 4C). This result was in line with the report on blueberries. It has been speculated that anthocyanin content is difficult to increase after fruit have fully ripened (Wang et al., 2020, Zhang et al., 2018).
As shown in Fig. 4D, the proanthocyanin content of WL + SA-treated fruit was eventually higher than other treatments and initial storage, while no significant difference was detected in other treatments compared to the initial storage, but that of WL + H2O and BL + H2O-treated fruit accumulated more than BL + SA.
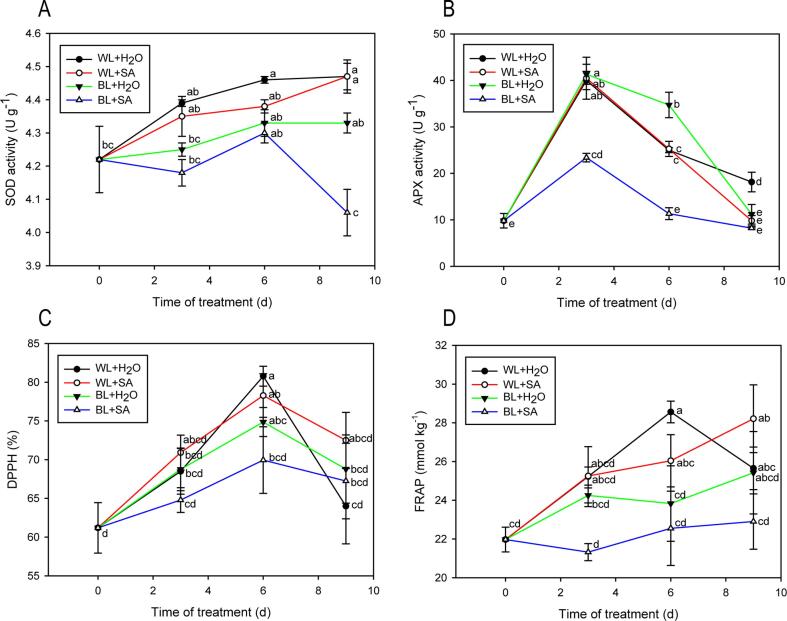
SOD, APX, and total antioxidant activities
SOD activity showed an overall increasing trend except BL + SA treatment (Fig. 5A). APX activity increased and peaked at the third day of storage, and then declined sharply in four treatments (Fig. 5B). Additionally, the activities of SOD and APX in BL + SA-treated fruit kept at the lowest level and almost had no significant change during the entire storage (Fig. 5A, B). The total antioxidant activity was evaluated by DPPH and FRAP parameters. The DPPH in all samples rose sharply within the initial 6 d of storage, and then descended in the following storage days. At the end of 9 d of storage, no prominent difference was observed among four treatments (Fig. 5C). The FRAP in all samples presented an overall increasing trend. Such an increase did not cause significant differences in antioxidant capacities before and after storage except for WL + SA. However, the FRAP in fruit treated with WL + SA had no significant difference from WL + H2O and BL + H2O after storage (Fig. 5D). Clearly, the total antioxidant capacity of BL + SA treatment was almost at the lowest level, but did not significantly change throughout the storage, which was agreement with the results of antioxidant enzymes and bioactive compounds (flavonoids, phenolics, anthocyanins and proanthocyanins), suggesting that BL + SA-treated fruit may be exposed to less oxidative stress.
Fig. 5.
SOD activity (A), APX activity (B), DPPH (C), and FRAP (D) of strawberry fruit either untreated or treated with BL and SA during storage. Data shown in the figure are presented as the mean ± SE of mean from three independent biological experiments (n = 3). The lowercase letters show no significant or significant difference based on the Duncan test (P ≤ 0.05).
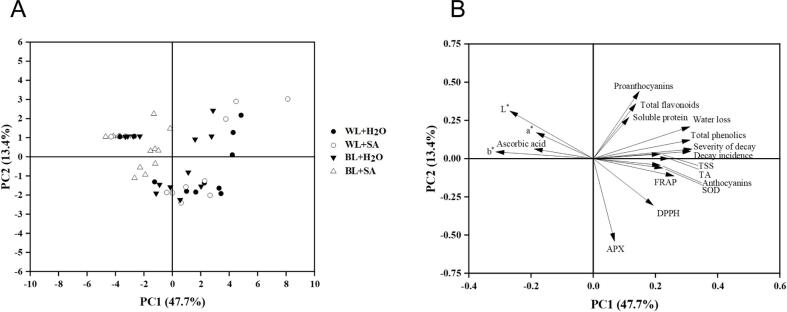
PCA analysis
PCA was conducted to obtain a comprehensive overview of variation in the quality of strawberry under different postharvest treatments. The first two principal components (PCs) were observed to explained 61.1% of the total variance. PC1 and PC2 accounted for 47.7% and 13.4% of the total variance, respectively. The samples subjected to BL + SA treatment can be distinguished from other treatment samples along PC1 and loaded in the negative direction of PC1, but there was no significant separation among samples submitted to the WL + H2O, WL + SA and BL + H2O treatments (Fig. 6A). Additionally, PC1 was positively correlated with most of the quality parameters including decay incidence, severity of decay, water loss etc., as well as negatively correlated with fruit skin color (a*, b* and L*) and ascorbic acid (Fig. 6B). This finding indicated that BL + SA treatment could maintain the fruit appearance and internal quality.
Fig. 6.
PCA analysis of postharvest quality during storage: (A) PCA score plot; (B) PCA loading plot.
Conclusion
It can be concluded from this study that blue light in combination with SA treatment was more effective in prolonging strawberry postharvest life by reducing weight loss, decay incidence and severity of decay, improving the skin color, increasing soluble protein content, as well as maintaining TSS and TA content. Moreover, BL + SA treatment maintained the non-enzymatic components (total flavonoid, phenolic, anthocyanin and proanthocyanidin), and activities of enzymatic antioxidants (SOD and APX), resulting in a stable antioxidant capacity during the entire storage. Hence, BL + SA postharvest treatment could be an environment-friendly method for preserving fruit quality and prolonging shelf-life.
CRediT authorship contribution statement
Yunting Zhang: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Shanlin Li: Formal analysis, Investigation, Methodology. Meiyi Deng: Software, Validation. Rui Gui: Software, Visualization. Yongqiang Liu: Investigation, Methodology. Xinpeng Chen: Software, Visualization. Yuanxiu Lin: Investigation. Mengyao Li: Visualization. Yan Wang: Software. Wen He: Methodology. Qing Chen: Software. Yong Zhang: Writing – review & editing. Ya Luo: Writing – review & editing. Xiaorong Wang: Writing – review & editing. Haoru Tang: Writing – review & editing, Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National College Students' Innovative Training Program (202110626013).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100384.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aghdam M.S., Fard J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria x anannasa cv. Selva) by enhancing GABA shunt activity. Food Chemistry. 2017;221:1650–1657. doi: 10.1016/j.foodchem.2016.10.123. [DOI] [PubMed] [Google Scholar]
- Asghari M., Aghdam M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends in Food Science & Technology. 2010;21(10):502–509. doi: 10.1016/j.tifs.2010.07.009. [DOI] [Google Scholar]
- Babalar M., Asghari M., Talaei A., Khosroshahi A. Effect of pre-and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chemistry. 2007;105(2):449–453. doi: 10.1016/j.foodchem.2007.03.021. [DOI] [Google Scholar]
- Ballester A.-R., Lafuente M.T. LED Blue Light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chemistry. 2017;218:575–583. doi: 10.1016/j.foodchem.2016.09.089. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bian Z.H., Yang Q.C., Liu W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. Journal of the Science of Food and Agriculture. 2015;95(5):869–877. doi: 10.1002/jsfa.6789. [DOI] [PubMed] [Google Scholar]
- Bose S.K., Howlader P., Jia X., Wang W., Yin H. Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via Abscisic acid signaling in strawberry. Food Chemistry. 2019;283:665–674. doi: 10.1016/j.foodchem.2019.01.060. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.-E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chen T., Ji D., Zhang Z., Li B., Qin G., Tian S. Advances and Strategies for Controlling the Quality and Safety of Postharvest Fruit. Engineering. 2021;7(8):1177–1184. doi: 10.1016/j.eng.2020.07.029. [DOI] [Google Scholar]
- Chongchatuporn U., Ketsa S., van Doorn W.G. Chilling injury in mango (Mangifera indica) fruit peel: Relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biology and Technology. 2013;86:409–417. doi: 10.1016/j.postharvbio.2013.07.023. [DOI] [Google Scholar]
- El-Mogy M.M., Ali M.R., Darwish O.S., Rogers H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. Journal of Berry Research. 2019;9(2):333–348. doi: 10.3233/JBR-180349. [DOI] [Google Scholar]
- García-Pastor M.E., Zapata P.J., Castillo S., Martínez-Romero D., Guillén F., Valero D., Serrano M. The effects of salicylic acid and its derivatives on increasing pomegranate fruit quality and bioactive compounds at harvest and during storage. Frontiers in Plant Science. 2020;11:668. doi: 10.3389/fpls.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Aguilar G.A., Celis J., Sotelo-Mundo R.R., De La Rosa L.A., Rodrigo-Garcia J., Alvarez-Parrilla E. Physiological and biochemical changes of different fresh-cut mango cultivars stored at 5° C. International Journal of Food Science & Technology. 2008;43(1):91–101. doi: 10.1111/j.1365-2621.2006.01394.x. [DOI] [Google Scholar]
- Haider S.-A., Ahmad S., Khan A.S., Anjum M.A., Nasir M., Naz S. Effects of salicylic acid on postharvest fruit quality of “Kinnow” mandarin under cold storage. Scientia Horticulturae. 2020;259 doi: 10.1016/j.scienta.2019.108843. [DOI] [Google Scholar]
- Hayat Q., Hayat S., Irfan M., Ahmad A. Effect of exogenous salicylic acid under changing environment: A review. Environmental and Experimental Botany. 2010;68(1):14–25. doi: 10.1016/j.envexpbot.2009.08.005. [DOI] [Google Scholar]
- Hernández-Muñoz P., Almenar E., Del Valle V., Velez D., Gavara R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria× ananassa) quality during refrigerated storage. Food Chemistry. 2008;110(2):428–435. doi: 10.1016/j.foodchem.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Jiang Z.-Y., Zhong Y., Zheng J., Ali M., Liu G.-D., Zheng X.-L. L-ascorbic acid metabolism in an ascorbate-rich kiwifruit (Actinidia. Eriantha Benth.) cv. ‘White’during postharvest. Plant Physiology and Biochemistry. 2018;124:20–28. doi: 10.1016/j.plaphy.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Kahramanoğlu İ., Nisar M.F., Chen C., Usanmaz S., Chen J., Wan C. Light: An alternative method for physical control of postharvest rotting caused by fungi of citrus fruit. Journal of Food Quality. 2020;2020 doi: 10.1155/2020/8821346. [DOI] [Google Scholar]
- Lafuente M.T., Alférez F. Effect of LED Blue Light on Penicillium digitatum and Penicillium italicum Strains. Photochemistry and Photobiology. 2015;91(6):1412–1421. doi: 10.1111/php.12519. [DOI] [PubMed] [Google Scholar]
- Lafuente M.T., Romero P., Ballester A.-R. Coordinated activation of the metabolic pathways induced by LED blue light in citrus fruit. Food Chemistry. 2021;341 doi: 10.1016/j.foodchem.2020.128050. [DOI] [PubMed] [Google Scholar]
- Li D., Li C., Sun H., Wang W., Liu L., Zhang Y. Effects of drought on soluble protein content and protective enzyme system in cotton leaves. Frontiers of Agriculture in China. 2010;4(1):56–62. doi: 10.1007/s11703-010-0102-2. [DOI] [Google Scholar]
- Liao H.-L., Alferez F., Burns J.K. Assessment of blue light treatments on citrus postharvest diseases. Postharvest Biology and Technology. 2013;81:81–88. doi: 10.1016/j.postharvbio.2013.02.019. [DOI] [Google Scholar]
- Loggini, Scartazza, Brugnoli, Navari I. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiology. 1999;119(3):1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M., Liuzzi V.C., Fanelli F., De Leonardis S., Creanza T.M., Ancona N.…Mulè G. Effect of different light-emitting diode (LED) irradiation on the shelf life and phytonutrient content of broccoli (Brassica oleracea L. var. italica) Food Chemistry. 2019;283:206–214. doi: 10.1016/j.foodchem.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Lufu R., Ambaw A., Opara U.L. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Scientia Horticulturae. 2020;272 doi: 10.1016/j.scienta.2020.109519. [DOI] [Google Scholar]
- Ma G., Zhang L., Setiawan C.K., Yamawaki K., Asai T., Nishikawa F.…Kato M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biology and Technology. 2014;94:97–103. doi: 10.1016/j.postharvbio.2014.03.010. [DOI] [Google Scholar]
- Molan A.L., Flanagan J., Wei W., Moughan P. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chemistry. 2009;114(3):829–835. doi: 10.1016/j.foodchem.2008.10.028. [DOI] [Google Scholar]
- Pérez-Ambrocio A., Guerrero-Beltrán J., Aparicio-Fernández X., Ávila-Sosa R., Hernández-Carranza P., Cid-Pérez S., Ochoa-Velasco C. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biology and Technology. 2018;135:19–26. doi: 10.1016/j.postharvbio.2017.08.023. [DOI] [Google Scholar]
- Ramirez R., Restrepo L., Perez C., Jimenez A. Physical, chemical and processing postharvest technologies in strawberry. Strawberry: Pre-and post-harvest management techniques for higher fruit quality. 2019:1–16. [Google Scholar]
- Shafiee M., Taghavi T., Babalar M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Scientia Horticulturae. 2010;124(1):40–45. doi: 10.1016/j.scienta.2009.12.004. [DOI] [Google Scholar]
- Shi L., Cao S., Chen W., Yang Z. Blue light induced anthocyanin accumulation and expression of associated genes in Chinese bayberry fruit. Scientia Horticulturae. 2014;179:98–102. doi: 10.1016/j.scienta.2014.09.022. [DOI] [Google Scholar]
- Sulaiman S.F., Sajak A.A.B., Ooi K.L., Seow E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. Journal of Food Composition and Analysis. 2011;24(4–5):506–515. doi: 10.1016/j.jfca.2011.01.020. [DOI] [Google Scholar]
- Tian S., Qin G., Li B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Molecular Biology. 2013;82(6):593–602. doi: 10.1007/s11103-013-0035-2. [DOI] [PubMed] [Google Scholar]
- Wang Y.-W., Ludwig H.D., Scherm H., van Iersel M.W., Nambeesan S.U. Blue light does not affect fruit quality or disease development on ripe blueberry fruit during postharvest cold storage. Horticulturae. 2020;6(4) doi: 10.3390/horticulturae6040059. [DOI] [Google Scholar]
- Xu F., Shi L., Chen W., Cao S., Su X., Yang Z. Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Scientia Horticulturae. 2014;175:181–186. doi: 10.1016/j.scienta.2014.06.012. [DOI] [Google Scholar]
- Zhang Y., Hu W., Peng X., Sun B., Wang X., Tang H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. Journal of Photochemistry and Photobiology B: Biology. 2018;186:225–231. doi: 10.1016/j.jphotobiol.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Y., He Y., Hu W., Zhang Y., Wang X., Tang H. Identification of NADPH oxidase family members associated with cold stress in strawberry. Febs Open Bio. 2018;8(4):593–605. doi: 10.1002/2211-5463.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ma G., Yamawaki K., Ikoma Y., Matsumoto H., Yoshioka T.…Kato M. Effect of blue LED light intensity on carotenoid accumulation in citrus juice sacs. Journal of Plant Physiology. 2015;188:58–63. doi: 10.1016/j.jplph.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang M., Xu B., Mujumdar A.S., Guo Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Comprehensive Reviews in Food Science and Food Safety. 2021 doi: 10.1111/1541-4337.12887. [DOI] [PubMed] [Google Scholar]
- Zushi K., Suehara C., Shirai M. Effect of light intensity and wavelengths on ascorbic acid content and the antioxidant system in tomato fruit grown in vitro. Scientia Horticulturae. 2020;274 doi: 10.1016/j.scienta.2020.109673. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.