Highlights
-
•
Roasting of germinated Sacha inchi improved the amino acids content.
-
•
Roasting decrease reducing sugars and phytosterols.
-
•
Roasting enhanced FAME levels in germinated seeds.
Keywords: Roasting, Germination, Sacha inchi, Essential amino acids, Maillard reaction
Abbreviations: BI, Browning index; FAME, Fatty acid methyl esters; FFA, Free fatty acid; GC, Gas chromatography; IS, Internal standards; PCA, Principal component analysis; TPC, Total phenolic compounds; UFA, Unsaturated fatty acids; HS-SPME, Autosampler headspace solid-phase microextraction; DVB/CAR/PDMS, Divinylbenzene/carboxen/polydimethylsiloxane
Abstract
This study examined the changes in metabolites together with the flavor profiles of germinated Sacha inchi seeds during roasting by using gas chromatography. The results indicated that roasting partially increased the browning index, amino acid levels, total phenolic content, and antioxidant capacity, but slightly decreased the levels of reducing sugars. Oxidized and rancid compounds were significantly decreased at a 180 °C roasting temperature. Pyrazine, furan, and pyrrole were Maillard reaction products that were increased at 180 °C of roasting. Roasting at 145 °C for 45 min after germination for 4 days was determined to be the optimal conditions for roasting germinated Sacha inchi seeds, which reduced the off-flavor and burned taste. The roasted germinated Sacha inchi seed contains higher amino acids than raw seed, which could be used as an alternative source for food products and supplements. In addition, the roasted germinated seeds at 4 days were recommended for food applications.
1. Introduction
Sacha inchi (Plukenetia volubilis Linneo) seed that contains oil (∼41 %) and protein (∼27 %) (Chirinos et al., 2013). Several commercial food products derived from Sacha inchi include oil, roasted seeds, protein powder, and flour. In addition, it has considerable economic value in the cosmetic and pharmaceutical industries. However, the increasing demand for protein-rich food products, supplements, beverages, and cosmetics may further spur the growth of the Sacha inchi industry. Germinated seeds of Sacha inchi represent an alternative source for food products, ingredients, and functional foods.
Germination has been used to enhance the nutritional quality of plant seeds. It also could help the novel bioactive compound formation, such as gamma-aminobutyric acid (GABA) (Shu, Frank, Shu, & Engel, 2008). Germinated Sacha inchi seeds contain a significant content of amino acids, antioxidant activity and total phenolic compounds (TPCs) (Keawkim, Lorjaroenphon, Vangnai, & Jom, 2021); however, the germination process results in an off-flavor that consumers may notice. Various processes may be used to alter the flavor during germination, such as steam treatment, radiation, and roasting.
Roasting is a widely used process to improve flavor, reduce unpleasant flavor, and extend the shelf life of legume seeds (Rizki, Kzaiber, Elharfi, Ennahli, & Hanine, 2015). Heating can change the physical properties and chemical composition via the Maillard reaction as well as enhancing antioxidant activity during roasting. However, excessive roasting can reduce the nutritional value and generate unpleasant flavors and toxic compounds. Monitoring the changes of metabolites and flavors during roasting may help to obtain the optimal roasting conditions.
A rapid, comprehensive technique is needed to evaluate the changes of metabolites and flavor compounds. Integrated metabolomics and flavoromics is an omics-based strategy that contributes to the fingerprinting of both volatile and non-volatile compounds. Moreover, only our previous publication has reported the changes of volatile and non-volatile metabolites during germination of Sacha inchi by using metabolomics coupled with flavoromics (Keawkim et al., 2021).
The nutritional quality of Sacha inchi seed could be enhanced during germination. However, the activity of lipoxygenase enzyme on the seed results in the formation of oxidation products such as aldehydes and alcohols. These products result in unpleasant flavor for consumers as they are usually characterized as beany, green, or rancid. Roasting may reduce off-flavor formation during germination, improve the flavor and remove undesirable volatiles produced in germinated Sacha inchi seeds through the Maillard reaction. In addition, roasted germinated Sacha inchi may contain higher amino acids and antioxidant activities than ungerminated Sacha inchi seed. The use of metabolomics and flavoromics will give a better understanding of the changes that occur during germination and roasting.
The effects of roasting on fatty acids, tocopherols, phytosterols, phenolic compounds, antioxidant activities (Štěrbová, Hlásná Čepková, Viehmannová, & Huansi, 2017) and anti-nutrients in Sacha inchi seeds has been reported. However, there are no studies described changes in flavors and the effect of roasting on germinated Sacha inchi. In the present study, we determined the physicochemical properties (color and browning index), metabolite and flavor changes, total phenolic contents (TPC), and antioxidant capacities of roasted germinated Sacha inchi seeds.
2. Materials and methods
2.1. Chemicals
The following chemicals and reagents were purchased from Sigma-Aldrich: internal standards (IS), reference standard (metabolomics and flavoromics), n-alkanes series (C6–C30), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2, 2-azobis (3-ethylbenzothialzoline-6- sulfonic acid) (ABTS), Folin–Ciocalteu reagents and gallic acid. All other chemical solvents were of analytical grade.
2.2. Seed sample preparation
Sacha inchi seeds were obtained from Kaeng Khro City, province of Chaiyaphum, Thailand, in 2020. Before roasting, germinated Sacha inchi seeds were prepared according to Keawkim et al. (2021). Germinated seeds were collected at 2 and 4 days. The samples were evaporated at 50 °C in a convection oven for 24 h. Raw and germinated Sacha inchi seeds were roasted at 100, 140, and 180 °C for 15, 30, and 45 min using a coffee roaster (1200 W, 220 V, 270 mm diameter, home coffee bean baker roaster non-stick) (Home Master, HJJXXKFJ010000, China), whereas the raw seed (ungerminated) was used as a control. After cooling, the seed coat was manually removed, finely ground into powder and kept at −20 °C.
2.3. Color and browning index
The color of the roasted germinated Sacha inchi powder exposed to different roasting conditions was measured using a colorimeter (UltraScan Pro, HunterLab, Reston, VA, USA). The browning index (BI) was measured using L* (whiteness/darkness), a* (redness/greenness), and b* (yellowness/blueness) values as described by Maskan (2001) (Maskan, 2001):
2.4. Untargeted metabolomics
Four fractions were done according to a previous study (Na Jom, Frank, & Engel, 2011) with slight modification. Briefly, lipid and polar extracts were sequentially extracted from 100 mg of sample powder, which were separately eluted with dichloromethane and 80:20 (v/v) of methanol and deionized water, respectively. After adding tetracosane (IS I) and 5α-Cholestane-3β-ol (IS II) solution into the 500 uL of lipid extract, the lipid extracted were left to be trans-esterified in methanol and consequently fractionated into fraction I and fraction II by solid-phase extraction. Phenyl-β-d-glucopyranoside (IS III), and p-chloro-l-phenylalanine (IS IV) were added into the polar extract. Then, the silylated derivatives of polar extracted for fraction III and fraction IV were done using selective hydrolysis. All four fractions were analysed by GC-FID. The GC condition was done according to Keawkim et al. (2021). Metabolite constituents were identified according to the reference standard comparison method and collected the peak area using the HP ChemStation A.06.03 program (Hewlett Packard). The concentrations of all metabolites were reported in term of the relative concentration (Keawkim et al., 2021).
2.5. Untargeted flavoromics
HS-SPME technique was carried out to extract the volatile compounds according described previous studied (Kim et al., 2021, Xu-Yan et al., 2012) with slight modification. Briefly, 3 g of sample (raw and roasted) and 1 µL of IS (1 µg/mL dodecanoic acid ethyl ester) were added to a 20 mL headspace vial. The samples were equilibrated at 70 °C for 10 min. The volatile components were collected on a 50/30 µm DVB/CAR/PDMS fiber at 70 °C for 30 min, then desorbed at 250 °C for 5 min. The volatile compounds in the samples were analyzed by using GC 6890 N coupled with a time-of-flight mass spectrometer (Leco Corp.). The GC condition and identification were done according to Keawkim et al. (2021). The concentrations of all metabolites were reported in term of the relative concentration.
2.6. TPC and antioxidant capacity
The sample extraction was done according to Atala, Vásquez, Speisky, Lissi, and López-Alarcón (2009) with slight modification. 0.1 mg/mL of sample in 75:25 v/v acetone and DI water solution was used to measure the TPC and antioxidant activity.
The phenolic compound was measured using the protocol expressed the previous study (Chirinos, Zorrilla, Aguilar-Galvez, Pedreschi, & Campos, 2016) with slight modification. Sample extract (50 µL), 7.5 % w/v Na2CO3 (125 µL), and Folin–Ciocalteu reagent (25 µL) were combined into a 96 well plate and reacted for 30 min. The sample absorbance at 755 nm was detected using a microplate reader (Tecan-Infinite M200 Pro). The TPC was calculated using the standard curve obtained with gallic acid. The results were represented as gallic acid equivalents (mg GAE/g sample).
Antioxidant activity in roasted germinated Sacha inchi was evaluated using DPPH and ABTS assays. The DPPH assay was conducted according to Brand-Williams, Cuvelier, and Berset (1995) with slight modification. The extracted solution (20 µL) was reacted with 0.1 mM DPPH (190 µL) for 30 min in a 96-well plate in the dark. The sample absorbance at 517 nm was measured using a microplate reader against a blank. According to the described previous study, with slight modification. A mixture of ABTS+ solution (1:1 vol ratio of 7 mmol/L ABTS solution and 2.45 mmol/L K2S2O8 solution) was left for 16 h in the dark. Diluted ABTS+ solution was combined with ethanol to obtain an absorbance of ∼ 0.7 ± 0.02 at a wavelength of 734 nm. Then, the ABTS+ solution (200 µL) and sample extract (10 µL) were incubated for 6 min in a 96-well plate and kept in the dark. The sample absorbance at 734 nm was measured using a microplate reader against a blank. Radical scavenging activity was used to define the antioxidant capacity in the sample using the following equation:
where A0 is the blank absorbance and As is the sample absorbance.
2.7. Statistical analysis
The relative concentration of metabolites and flavor compounds was exported for multivariate data analysis using XLSTAT base software, version 2021 (Addinsoft, New York, USA). Duncan's multiple range test at a significance level of p ≤ 0.05 was carried out to compare the means and the analysis was done using IBM SPSS Statistics Version 28.0 (Thaisoftup CO., Ltd., Thailand).
3. Results and discussion
3.1. Colors and browning index of roasted germinated Sacha inchi seeds
A significant (p < 0.05) difference in the color and BI of the non-roasted and roasted samples at different roasting temperatures and times is shown in Table 1. The increase in roasting temperatures and times caused a significant decrease in lightness (L*), but a significant increase in redness (a*) and yellowness (b*), and BI for all samples. However, the yellowness (b*) decreased at the highest roasting temperature. Some color components may have decomposed and were transformed under excessive roasting conditions (Kim et al., 2021). Non-enzymatic browning reactions primarily affect color change as a result of roasting (Sharma & Gujral, 2011). Compared with the ungerminated sample, the roasted germinated seed required a lower temperature and shorter roasting time to reach a similar BI. In particular, the germinated samples at 4 days exhibited more color changes than germinated seeds at 2 days. The germinated seeds contained higher residual substrates (free amino acids and reducing sugars) for color formation compared with the ungerminated seeds, which may have thermally enhanced the browning reaction (Kim et al., 2021).
Table 1.
Color and browning index of roasted ungerminated and germinated Sacha inchi powder at different temperatures and times.
| Samples (1) | L* | a* | b* | Browning index |
|---|---|---|---|---|
| raw (non-roasted) | 68.3 ± 0.40c | 2.17 ± 0.11l | 16.56 ± 0.50h | 29.53 ± 0.65kl |
| F100/15 | 68.77 ± 2.70cb | 2.30 ± 0.19l | 16.63 ± 1.50gh | 29.55 ± 1.72kl |
| F100/30 | 68.39 ± 1.21c | 2.68 ± 0.20l | 18.04 ± 1.20g | 32.89 ± 2.05k |
| F100/45 | 69.56 ± 1.12cb | 2.43 ± 0.13l | 17.48 ± 0.73fgh | 30.92 ± 0.66k |
| F140/15 | 69.59 ± 0.70cb | 3.23 ± 0.34k | 20.06 ± 0.46e | 36.74 ± 0.85j |
| F140/30 | 68.14 ± 0.68c | 5.20 ± 0.10ij | 21.87 ± 0.57d | 43.61 ± 1.16i |
| F140/45 | 67.91 ± 0.96c | 5.58 ± 0.36i | 23.34 ± 0.33bc | 47.36 ± 0.31h |
| F180/15 | 52.21 ± 2.85b | 10.99 ± 0.81bc | 18.00 ± 0.01fg | 57.00 ± 3.61g |
| F180/30 | 42.24 ± 0.75ij | 11.36 ± 0.28b | 14.96 ± 0.29i | 62.66 ± 1.56de |
| F180/45 | 41.31 ± 0.12j | 10.47 ± 0.40cd | 14.44 ± 0.09i | 60.84 ± 0.95ef |
| 2D (non-roasted) | 67.69 ± 0.89c | 1.25 ± 0.13m | 17.20 ± 0.20gh | 30.03 ± 0.67kl |
| 2D100/15 | 71.01 ± 1.27ab | 1.47 ± 0.56m | 17.38 ± 0.36fgh | 29.02 ± 1.64l |
| 2D100/30 | 68.81 ± 1.55b | 1.46 ± 0.23m | 17.54 ± 0.31fgh | 30.36 ± 0.97kl |
| 2D100/45 | 65.30 ± 0.61d | 2.21 ± 0.11l | 17.57 ± 0.25fgh | 33.19 ± 0.27k |
| 2D140/15 | 72.97 ± 0.93a | 2.46 ± 0.26l | 18.72 ± 1.01f | 31.52 ± 1.77kl |
| 2D140/30 | 69.03 ± 0.85b | 3.59 ± 0.22k | 21.33 ± 0.19e | 40.05 ± 0.58j |
| 2D140/45 | 67.80 ± 1.30c | 3.83 ± 0.03k | 20.18 ± 0.14e | 38.81 ± 1.02j |
| 2D180/15 | 62.16 ± 0.55e | 7.53 ± 0.20g | 24.48 ± 0.37ab | 57.99 ± 1.98fg |
| 2D180/30 | 49.21 ± 0.14h | 11.92 ± 0.17a | 21.28 ± 1.62e | 73.35 ± 5.83a |
| 2D180/45 | 43.76 ± 0.63i | 10.16 ± 0.33de | 14.20 ± 0.37i | 55.64 ± 1.98g |
| 4D (non-roasted) | 54.86 ± 0.65f | 3.63 ± 0.29k | 16.31 ± 0.36h | 39.48 ± 0.87j |
| 4D100/15 | 54.83 ± 0.82f | 3.75 ± 0.13k | 16.46 ± 0.26h | 40.07 ± 0.91j |
| 4D100/30 | 52.01 ± 3.61g | 4.97 ± 0.50j | 20.28 ± 1.93e | 55.36 ± 1.00g |
| 4D100/45 | 51.34 ± 0.17g | 6.49 ± 0.32h | 22.32 ± 0.45c | 65.04 ± 1.41cd |
| 4D140/15 | 61.32 ± 0.81e | 6.21 ± 0.09h | 23.45 ± 1.42bc | 54.67 ± 2.88g |
| 4D140/30 | 60.12 ± 0.70e | 7.18 ± 0.54g | 23.81 ± 0.12ab | 58.20 ± 0.78fg |
| 4D140/45 | 60.83 ± 1.49e | 8.80 ± 0.79f | 25.00 ± 0.83a | 62.60 ± 4.70de |
| 4D180/15 | 35.95 ± 2.22g | 6.51 ± 0.17e | 14.66 ± 0.70d | 43.14 ± 1.09c |
| 4D180/30 | 42.13 ± 0.85ij | 10.40 ± 0.47cd | 16.73 ± 0.20gh | 67.84 ± 2.05bc |
| 4D180/45 | 35.90 ± 0.65k | 10.58 ± 0.08cd | 13.73 ± 0.10i | 68.90 ± 1.06b |
Data are the means ± SD. Different letters in the same column are different with statistical significance (p < 0.05).
F, ungerminated seed; 2D, germination time for 2 days; 4D, germination time for 4 days.
3.2. Relative quantitative analysis of identified metabolites
The dynamic changes in the roasted, germinated Sacha inchi seeds were analyzed for metabolites at different roasting temperatures and times. The 65 metabolites identified represent 65 % of all the compounds identified and were classified into four fractions. Fraction, I contained 12 FAMEs and 2 hydrocarbons, fraction II contained 9 FFAs, 2 fatty alcohols, and 5 phytosterols, fraction III contained 3 organic sugars and 3 sugar alcohols, and fraction IV contained 20 amino acids and 4 organic acids.
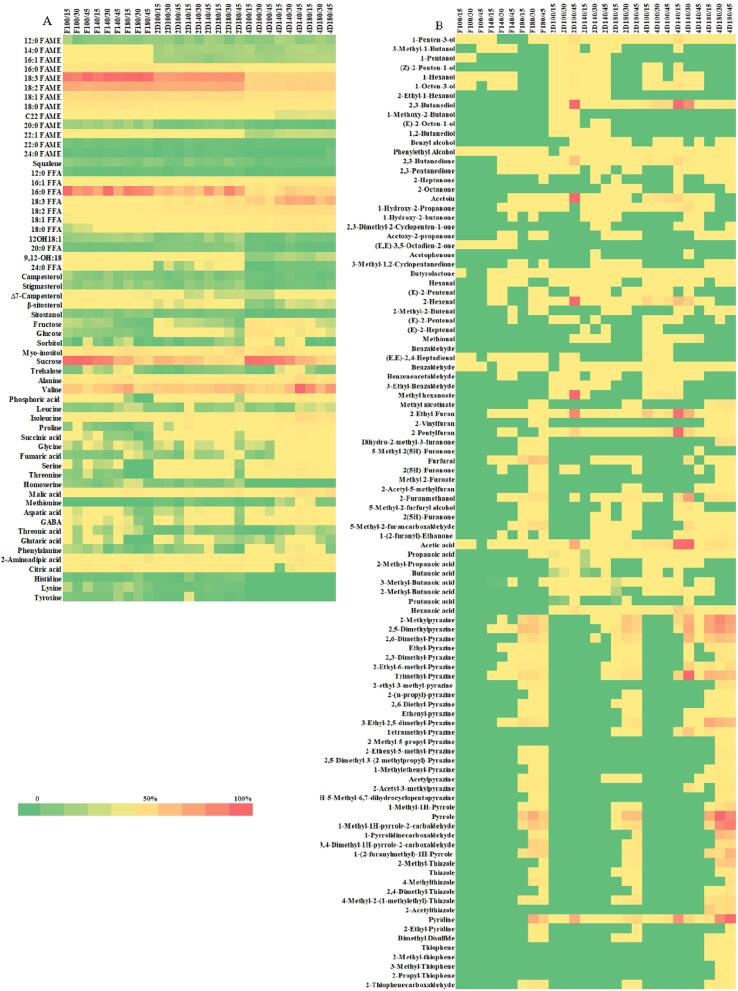
The relative content of metabolites (Fig. 1A) and flavors (Fig. 1B) were displayed in a heat plot, which describes the changes in the identified compounds during roasting. The results indicated that roasting did not affect the fatty acid profiles of either ungerminated or germinated Sacha inchi seeds, which is consistent with other studies of these seeds (Chirinos et al., 2013, Chirinos et al., 2016). This may result from the seed coat of the seeds acting as a shield to protect from oxidation of the unsaturated fatty acids (UFA). In addition, the natural antioxidant compounds, such as polyphenols and tocopherols in Sacha inchi seed tissues, may protect against UFA oxidation (Belcadi-Haloui, Zekhnini, El-Alem, & Hatimi, 2018). However, roasting of germinated Sacha inchi resulted in a total fatty acid content lower than that of roasted ungerminated seeds, which may be related to energy storage in the form of lipids (Perren & Escher, 2013). A previous study reported that the fatty acid content decreased during germination (Keawkim et al., 2021). Among the FAMEs, α-linolenic acid was the most prominent component during roasting, followed by linoleic, oleic, palmitic, and stearic acid. The same order of these fatty acids was also previously reported (Chirinos et al., 2016). The highest levels were found in germinated Sacha inchi seeds at a 180 °C roasting temperature. In particular, the FAME levels of roasted, germinated seeds at 2 days increased to 16, 4.2, 3.2, 2.6, and 1.9 % for palmitic, stearic, α-linolenic, linoleic, and oleic acid, respectively, compared with the control sample. The increase in FAME levels in the germinated seeds during roasting may result from water removal from the intact seed during roasting, which results in increased porosity of the plant cellular compartments by the dehydration process. This enhances the intracellular distribution of the compounds that occur in the oil. (Perren & Escher, 1999). However, the highest FAMEs were detected in ungerminated seeds compared with roasted germinated seeds, which may be related to storing energy in the form of lipid (Perren & Escher, 2013).
Fig. 1.
Heat plots of metabolites (A) and flavors (B) in roasted, germinated Sacha inchi seeds at different roasting temperatures and times. FAME, fatty acid methyl esters; FFA, free fatty acid; GABA, gamma-amino butyric acid; F, ungerminated seed and D, germination day.
Compared with the control sample, the roasting process significantly increased free fatty acid (FFA) levels in both ungerminated and germinated seed samples. The FFA content exhibited a constant value at a 100 °C roasting temperature and different roasting times. However, it was increased at temperatures higher than 140 °C and continuously increased to a roasting temperature of 180 °C. The most remarkable changes in FFA levels were observed in the roasted ungerminated seeds, which increased to 297.1, 174.3, 36.5, 33.9, 11.2, and 5.2 %, respectively, for palmitic, palmitoleic, α-linolenic, oleic, linoleic, and stearic acid compared with the control sample. The higher FFA content resulting from roasting at high temperatures may be associated with lipase activity, in which heat degrades lipase activity that may be inactivated at high temperatures and long exposure time. Consequently, damaged cells that contain hydrolytic enzymes may provide increased FFA after roasting (Alamprese, Ratti, & Rossi, 2009). However, the highest FFA levels were observed in roasted, germinated Sacha inchi seeds after 4 days.
The different roasting conditions influenced the phytosterol contents. We detected campesterol, Δ7-campesterol, stigmasterol, β-sitosterol, and sitostanol in the roasted Sacha inchi seeds. The content of these compounds in roasted samples was increased compared with the control sample and the highest levels were observed in ungerminated seeds. Campesterol and Δ7-campesterol levels decreased with increasing roasting temperature and higher levels were observed at 100 °C and 140 °C at short times (15–30 min). Stigmasterol, β-sitosterol, and sitostanol content increased with the roasting temperature and higher levels were observed near the beginning (10 min). The previous study reported the changes of phytosterols in buckwheat during roasting and implied that the plant cells affect a decline of free and esterified sterols levels, leading to improve the bioavailability and detection rates. The high roasting temperature could result in partial degradation of free sterols (Dziedzic et al., 2016).
Roasting caused a marked drop in reducing sugar (fructose and glucose) content in all roasted samples compared with the control. This change confirmed that Maillard browning and/or caramelization occurred during heating. The reducing sugars and free amino acids are essential substrates for the browning reaction to produce a favorable flavor, color, and overall appearance of roasted grains and beans (Demnati, Pacheco, Martínez, & Sánchez, 2018). However, higher reducing sugar content was found in 4-day roasted, germinated seeds at temperatures of 100 °C and 140 °C. They were 4–5-fold higher compared with that of the roasted ungerminated seeds. This may be related to the initial amount of reducing sugars present before roasting, which was much higher in germinated versus ungerminated seeds (Kim et al., 2021). Disaccharides including sucrose and trehalose were decreased during the roasting process. Sucrose was present at the highest levels in germinated seeds after 4 days. It was not significantly changed at 100 °C, but significantly decreased at the highest temperature (180 °C) to 55 % at 45 min compared with the initial time (15 min). The decrease in sucrose content may be the result of hydrolysis into fructose and glucose during roasting (McDaniel, White, Dean, Sanders, & Davis, 2012). Interestingly, the remaining sugar levels in the roasted, germinated Sacha inchi were much higher compared with that in the roasted ungerminated seeds. Regarding sugar alcohol content, sorbitol and myo-inositol were not significantly altered during roasting at temperatures lower than 140 °C, but sharply decreased at 180 °C. Zhou, Geng, Deng, Huang, and Jinqiu (2019) also reported the changes of sugar alcohols during roasting of seasame seed, in which it was proposed that sugar alcohols may not be substrates for flavor formation. However, the germinated seeds at 4 days were higher in sugar alcohols compared with the other seed samples.
The results for amino acids indicated that roasting enhanced the amino acid content compared with the control samples. Eight essential amino acids were identified in the present study including isoleucine, leucine, lysine, methionine, phenylalanine, threonine, histidine, and valine, which were increased during roasting, but slightly decreased at 180 °C for long roasting times. The highest relative concentration of amino acids was found in seeds after 4 days of germination, except for histidine and lysine, which were present at higher concentrations in the ungerminated seeds. Isoleucine, methionine, and threonine levels were altered during roasting and reached nearly 90 %, followed by phenylalanine (73 %), valine (61 %), and leucine (35 %) at a roasting temperature of 140 °C for 45 min compared with the ungerminated seeds under the same conditions. In addition, other amino acids were also increased in the germinated seeds after 4 days at 140 °C for 45 min. The increase in the free amino acids due to roasting was determined in a previous study, which found that increased free amino acid levels were associated with decreased protein content. Certain proteins could be decomposed by heating, resulted in increasing of free amino acids (Gonçalves, Borges, Rosa, Coutinho, & Silva, 2012). Acid compounds (phosphoric, succinic, fumaric, malic, aspartic, gamma-aminobutyric, glutaric, and citric acid) were detected during roasting and most acids were increased concomitantly with increased roasting temperature and times, with the highest concentration at 140 °C for 45 min in the 4-day germinated sample.
3.3. Relative quantitative analysis of identified flavors
Flavoromics identified 102 (42 %) of over 300 compounds, which were characterized into 12 groups including 13 alcohols, 13 ketones, 12 aldehydes, 2 esters, 8 volatile acids, 14 furans, 20 pyrazines, 5 thiophenes, 6 pyrroles, 6 thiazoles, 2 pyridines, and 1 sulfide compound.
flavor compounds, including alcohols, ketones, lactone, acids, and aldehydes, were primarily produced from the autoxidation and degradation of lipids, which is responsible for undesirable oxidized and rancid odor. Alcohol content was increased from 100 °C to 140 °C, then significantly decreased at 180 °C. Most alcohol compounds were present in the germinated seed, which had the highest levels in 2-day germinated seeds at 100 °C for 45 min, in particular, 2,3-butanediol and 1-penten-3-ol. Only 46 % of the total alcohols were found in the ungerminated seeds, with the highest levels occurring at roasting temperatures of 140 °C for 45 min. Most alcohol compounds disappeared at the highest roasting temperature, except 1-penten-3-ol, benzyl alcohol, and phenyl ethyl alcohol. The remaining alcohols present at the highest temperature may be hydrophilic compounds that are associated with oil during the roasting process (Xu-Yan et al., 2012). Ketone compounds were higher in the germinated versus ungerminated seeds. Total ketones (46 %) were detected in the ungerminated seeds at a roasting temperature of 180 °C. Among 13 ketones, only 2,3-butanedione and acetoin were significantly altered and present at high concentrations in the 2-day germinated seeds at a roasting temperature of 100 °C for 45 min. The levels sharply decreased as the temperature increased. Twelve aldehydes were identified in the samples during roasting, whereas most appeared in the germinated seeds. In the ungerminated seeds, 67 % of the total aldehydes were observed at a roasting temperature above 140 °C and slightly increased during roasting. Among these aldehydes, 2,4-heptadienal was the major compound and the highest concentration was measured at 180 °C for 45 min in ungerminated seeds. In contrast, the germinated Sacha inchi seeds had the highest level of aldehydes at 100 °C at short roasting times, but significantly decreased as the temperature and time increased. 2-hexenal, an oxidation product from linolenic acid (Cao et al., 2014), was the primary aldehyde in the germinated seeds and exhibited decreased levels during the roasting process. Most aldehydes detected in the samples were oxidation products, such as hexanal, 2-pentenal, 2-butenal, and 2-heptenal. These yield an off-flavor effect and were decreased as temperature and time increased. Eight volatile acids were detected in the samples, which were primarily present the germinated seeds. Acetic acid is the primary acid compound found in roasted samples (Zhou et al., 2019), probably because it is a product of the Maillard reaction (Davídek, Devaud, Robert, & Blank, 2006). Here, acetic acid was present at the highest levels in 4-day germinated samples at 140 °C for 15–30 min higher compared with ungerminated seeds (approx. 383-fold under the same roasting conditions). However, it was reduced after roasting at 180 °C in the germinated samples. In addition, hexanoic acid was detected in germinated seeds and significantly increased in the 4-day germinated seeds, particularly at a roasting temperature of 140 °C for 15–30 min. However, it was absent in the ungerminated seeds. Ester compounds may provide a fruity and sweet aroma (Sun et al., 2018). Of these, methyl hexanoate, methyl nicotinate, butyrolactone, and γ‐caprolactone were detected only in raw sesame seeds. In the germinated Sacha inchi seeds used in this study, methyl hexanoate was observed, showed the highest levels at 100 °C for 45 min, and was not detected at 180 °C. Methyl nicotinate was found at 180 °C and increased with time in both ungerminated and germinated samples. The highest concentration was found in the 4-day germinated seeds.
During roasting, new flavor compounds are produced by the Maillard reaction and thermal degradation of polar compounds. Forty-nine compounds were detected and categorized into five groups, including pyrazines (40.8 %), furans (30.6 %), pyrroles (12.2 %), thiazoles (12.2 %), and pyridines (4 %). During the roasting process, pyrazines were the most abundant compounds noticed in Sacha inchi. The results indicated that pyrazines developed after a roasting temperature of at least 140 °C, were significantly enhanced at 180 °C, but were absent at low temperatures for both ungerminated and germinated seeds. The highest pyrazine content was observed at the high roasting temperature and it was suggested in previous studies that pyrazines are strongly affected and accumulate during roasting (Xu-Yan et al., 2012). Pyrazine products, which are responsible for the roasted aroma and flavor, are primarily generated by the Maillard reaction (Liu et al., 2011). Among 20 pyrazines, 2-methyl pyrazine, 2,5-dimethyl-pyrazine, and 2,6-dimethyl-pyrazine were the predominant volatiles and were the major contributors to the sweet and roasted flavors of sesame seed oil (Zhou et al., 2019). The total pyrazine content was significantly increased after germination for 4 days, approximately 3-fold compared with the ungerminated seeds at 180 °C for 30 min. After germination, the amount of reducing sugar residue and free amino acids as substrates for the Maillard reaction were increased, which could enhance pyrazine formation during the roasting process (Kim et al., 2021). Fifteen furans were identified following roasting of the Sacha inchi seeds. The ungerminated seeds contained furan compounds at a temperature higher than 140 °C. Furfural and 5-methyl-2-furancarboxaldehyde were abundant and detected at the highest concentration in ungerminated samples at a roasting temperature of 180 °C for 30 min, but were reduced after a longer time. In the germinated samples, other furan compounds were present at high concentrations after germinating for 4 days. 2-ethyl furan, 2-pentyl furan, and 2-furanmethanol were observed in the 4-day germinated seed at a roasting temperature of 140 °C for 15 and 30 min. However, the total furan content of the seed after germinating for 4 days was higher (3.5-fold) compared with the ungerminated seed. Previous studies indicated that 2-ethyl furan and 2-pentyl furan are produced by lipid degradation of linoleic acid. 2-ethyl furan provides a strong burned, sweet, coffee-like flavor, whereas 2-pentyl furan yields a buttery flavor. In addition, furfural and furfuryl alcohol are produced from the Maillard reaction during heating (Farag, Khattab, Shamma, & Afifi, 2021). Other N-heterocycles, including the pyrrole and pyridine classes, were detected at a roasting temperature higher than 140 °C, and impart a roasted and/or smoky flavor. These compounds did not appear at low roasting temperatures, except pyridine was found under all roasting conditions and detected only in germinated seeds. Most compounds exhibited their highest levels in 4-day germinated seeds, which increased concomitantly with temperature and time. Pyrrole, 1-methyl-1-pyrrole-2-carbaldehyde, and pyridine are major compounds in the roasted germinated samples. In roasted sesame seeds, pyridine also contributed significantly to flavor (Nakamura, Nishimura, Masuda, & Mihara, 1989). In addition, at the highest roasting temperature, sulfur-containing compounds, including 6 thiazole and 5 thiophene compounds, were released, but did not appear at temperatures below 140 °C. The 4-day germinated seeds had the highest levels of thiazole and thiophene compounds, particularly 2-acetylthiazole, which were observed only in the 4-day germinated seeds. Furthermore, thiophenes were detected only in 4-day germinated seeds, except for 2-thiophenecarboxaldehyde, which was detected in both roasted ungerminated and germinated seeds. 2-thiophenecarboxaldehyde content was observed in ungerminated seeds at higher levels compared with 4-day germinated seeds by approximately 2.5-fold. 2-acetylthiazole and 2-thiophene carboxaldehyde are associated to the meat-like flavor attributes. An increase in these heterocyclic compounds at high temperatures was also observed in previous studies, in which they formed at higher temperatures by thermal degradation of sulfur-containing amino acids and carbonyl compounds (Chen et al., 2018). Dimethyl disulfide is a product from the thermally-induced Strecker degradation of methionine, which results in a burned flavor. This was detected at the highest roasting temperature and more was present in the roasted 4-day germinated seed (Pripis-Nicolau, Revel, Bertrand, & Maujean, 2000).
3.4. Multivariate analysis of metabolomics-flavoromics
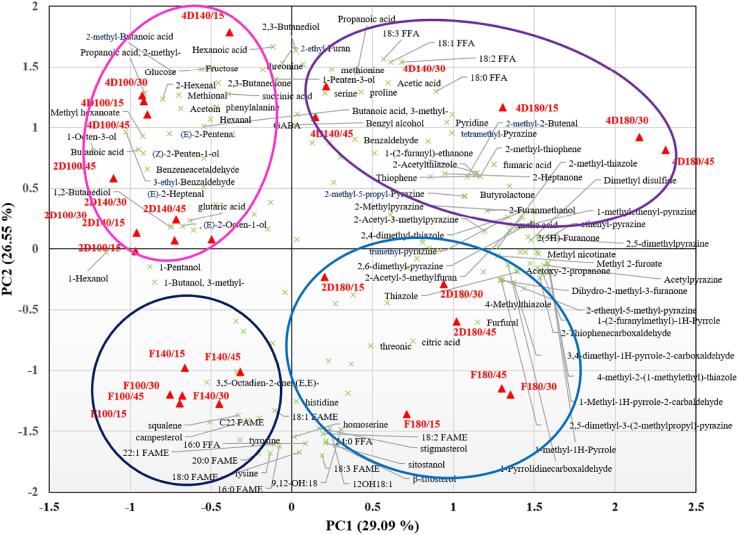
Fig. 2 shows the PCA biplot and distribution of all identified metabolites and flavors at different temperatures and times. The ungerminated and germinated Sacha inchi seeds were separated based on roasting temperature and times. PC1 separated the roasted ungerminated and 4-day germinated seeds and the metabolites of 4-day germinated seeds at a roasting temperature higher than 140 °C for long roasting times (30 and 45 min) compared with the lowest temperature (100 °C) at early times (15 min) (Fig. S1). The flavors from the highest temperature at longer roasting times (180 °C at 30, and 45 min) were clearly isolated from 100 °C and 140 °C temperatures and early roasting times (15 min) (Fig. S2). The PCA biplots of the combined metabolites and flavors showed four distinct clusters along with the PC (Fig. 2). The first two PCs contained 55.64 % of the total variation, whereas PC1 and PC2 encompassed 29.09 % and 26.55 % of the total variance, respectively. Along with PC1, the high roasting temperature for the Sacha inchi samples was clustered on the right side, whereas samples roasted at a low temperature were clustered on the left side. Pyrazine, furan, pyrrole, thiazole, and thiophene compounds were strongly influenced by the right side of PC1, whereas FAMEs, free fatty acids, alcohols, aldehydes, and volatile acids were closely located on the left side of PC1. The four clusters, including cluster I, consisted of six treatments, including roasted ungerminated seeds at 100 °C for 15, 30, and 45 min (F100/15, F100/30 and F100/45) and 140 °C for 15, 30, and 45 min (F140/15, F140/30 and F140/45), which included FAMEs and free fatty acids. Cluster II consisted of six treatments of roasted ungerminated seeds, including 180 °C for 15, 30, and 45 min (F180/15, F180/30, and F180/45) and roasted 2-day germinated seeds, including 180 °C for 15, 30, and 45 min (2D180/15, 2D180/30 and 2D180/45). They contained FAMEs, free fatty acids, and furan, pyrazines, and thiazole. Cluster III consisted of five treatments of roasted 4-day germinated seeds at 140 °C for 15, 30, and 45 min (4D140/15, 4D140/30 and 4D140/45), and 180 °C for 15, 30, and 45 min (4D180/15, 4D180/30 and 4D180/45). It contained free fatty acids, amino acids, volatile acids, pyrazines, thiophene, and thiazole. Cluster V consisted of 10 treatments of roasted two-day germinated seeds at 100 °C for 15, 30, and 45 min (2D100/15, 2D100/30 and 2D100/45), 140 °C for 15, 30, and 45 min (2D140/15, 2D140/30 and 2D140/45), and roasted 4-day germinated seeds at 100 °C for 15, 30 and 45 min (4D100/15, 4D100/30 and 4D100/45) and 140 °C for 15 min (4D140/15), which were comprised of sugars, amino acids, alcohols, and aldehydes. FAMEs were primarily detected in ungerminated seeds, particularly at roasting temperatures below 140 °C. The number of amino acids (40 %) and sugars (57 %) were contained in germinated Sacha inchi, especially the samples from 4-day germination at a roasting temperature of 140 °C. In addition, at the highest roasting temperature, many pyrazines, furans, thiophenes, pyrroles, and thiazole compounds were released from the roasted ungerminated and germinated Sacha inchi seeds.
Fig. 2.
Biplot of PCA from metabolites and flavor compounds in Sacha inchi samples at different roasting temperatures and times (p ≤ 0.05). FAME, fatty acid methyl esters; FFA, free fatty acid; GABA, gamma-amino butyric acid; F, ungerminated seed and D, germination day.
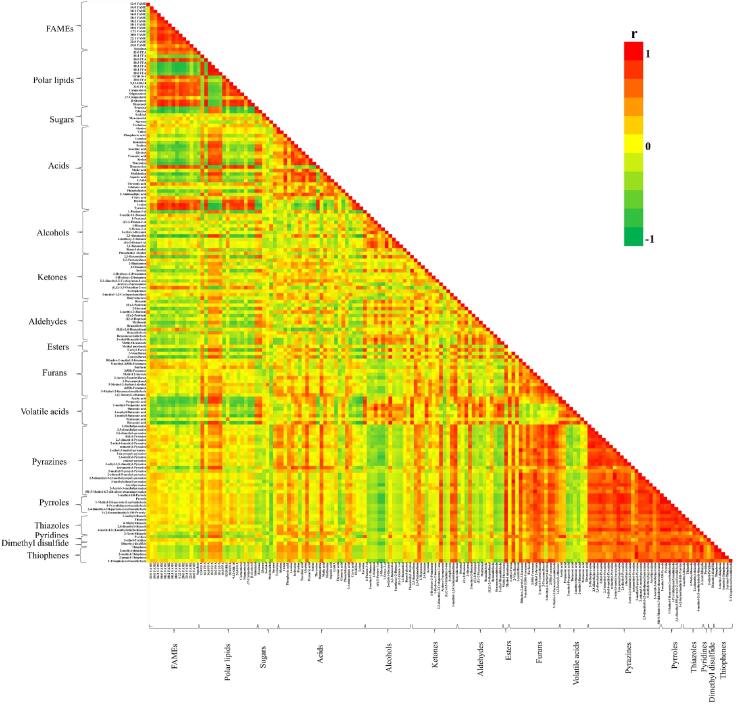
The pairwise correlation analysis between metabolites and flavor compounds resulting from Sacha inchi roasting at different temperatures and times is presented in Fig. 3 and the compounds in each class was specified in Table S1. The roasting process had a significantly effect on the color and flavor compounds of Sacha inchi. The component of alcohols, ketones, aldehydes, volatile acids and furans, negatively correlated with FAME compounds, especially 1-penten-3-ol, 2,3-butanediol, 2,3-butanedione, 2-ethyl-furan, 2-pentyl-furan, acetic acid, propanoic acid, 2-methyl-butanoic acid, and 2-methyl-hexanoic acid, and strongly (r < −0.7) with 16:0 FAME, 18:3 FAME, 18:2 FAME, and 18:0 FAME. However, these flavor components positively correlated with free fatty acids, especially 2,3-butanediol, 2-ethyl-furan, 2-pentyl-furan, acetic acid, propanoic acid, and hexanoic acid, and strongly (r > 0.7) with 16:1 FFA, 18:3 FFA, 18:2 FFA, and 18:1 FFA. In previous studies, free fatty acids and triglycerides were precursors in the autoxidation of lipids, primarily resulting in aldehydes, ketones, acids, alcohols, and furans. Pyrazine, pyrrole, thiazole, furan, thiophene, and pyridine compounds correlated negatively with sugars (fructose, glucose, and sucrose), but positively with acids. Among the amino acids, alanine, valine, leucine, proline, and glycine exhibited the highest correlation with these flavors. The Maillard reaction or/and caramelization and thermal degradation are important synthetic pathways that lead to flavor formation during roasting. The chemical interaction between reducing sugars and amino acids generate various flavor compounds, including pyrazines, pyrroles, thiophenes, thiazoles, and furans (Hwang, Hartman, Rosen, & Ho, 1993).
Fig. 3.
The lower triangular heat map represents the pairwise correlation analysis between metabolites and flavor compounds at different roasting temperatures and times. Each square represents a Spearman's rank correlation coefficient at a significance level of p ≤ 0.05. Orange-red, strong positive correlation (r > 0.7); green, strong negative correlation (r < −0.7). FAMEs, fatty acid methyl esters; FFA, free fatty acid; F, ungerminated seed and D, germination day.
The germinated seeds of Sacha inchi contained a high reducing sugar and amino acid content, especially after germinating for 4 days. We found that a higher level of essential amino acids and reducing sugars were present at roasting conditions of 140 °C for 45 min, which produced a roasted aroma, but a lower off-flavor and burned taste. Therefore, these conditions should be applied in the processing of plant protein-based products. In addition, changes in metabolites and flavors during roasting may be associated with biological activity.
3.5. Total phenolic content and antioxidant activity
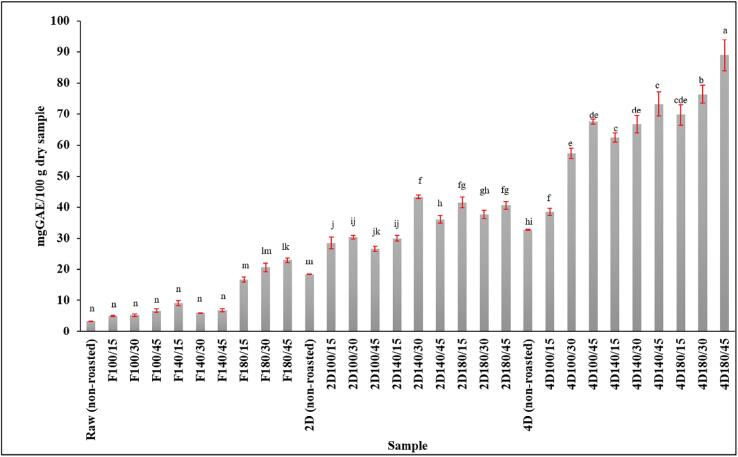
Roasting had a significant influence on TPC. TPC of roasted germinated Sacha inchi seeds are presented in Fig. 4. The results indicate that roasting enhances TPC in both ungerminated and germinated samples. Roasting did not significantly affect TPC at 100 °C and 140 °C, but resulted in significant changes at 180 °C in both ungerminated and germinated Sacha inchi seeds. Particularly in the ungerminated seeds, which displayed a TPC of 6.8-fold higher than the control samples. However, the roasted 4-day germinated Sacha inchi seeds contained higher TPC compared with the roasted 2-day germinated or ungerminated seeds. A similar trend was reported in roasted Sacha inchi kernel. A report suggested that roasting at 190 °C for 35 min positively increased TPC in Sacha inchi seeds (Štěrbová et al., 2017). An increase of TPC at the highest roasting temperature in all samples may be correlated with compounds that form through the Maillard reaction, react to the Folin-Ciocalteu reagent, and exhibit antioxidant properties. In addition, roasting may destroy plant cells, causing more bound phenolics to be released in the oil after roasting (Chirinos et al., 2016, Zou et al., 2018).
Fig. 4.
Total phenolic content in roasted, germinated Sacha inchi seeds at different roasting temperatures and times. Data are the means ± SD. Different letters in the same column are different with statistical significance (p < 0.05); F, ungerminated seed and D, germination day.
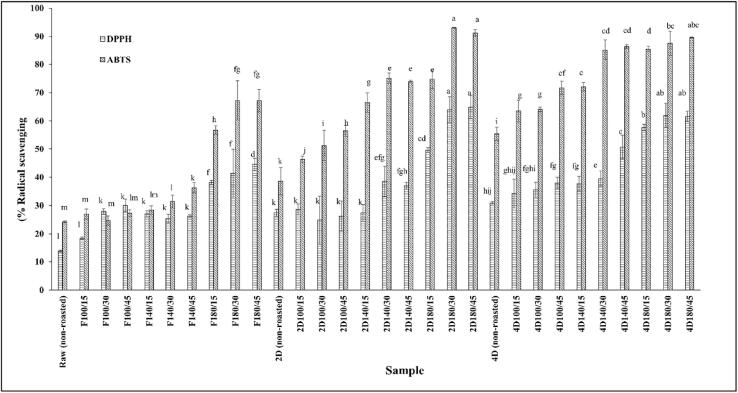
Several studies have reported that TPC positively correlates with antioxidant activity during roasting (Lemos et al., 2012, Štěrbová et al., 2017). The antioxidant activity of roasted, germinated Sacha inchi seeds at different roasting temperatures and times was measured using DPPH and ABTS assays (Fig. 5). The roasting temperature significantly improved the antioxidant activity compared with the control sample. The most remarkable changes in antioxidant activity were detected at 180 °C of roasting temperature. In the ungerminated Sacha inchi samples, antioxidant activity was higher compared with the control sample (approximately 3.2- and 2.7-fold for the DPPH and ABTS assay, respectively). However, the antioxidant activities of the roasted 4-day germinated seeds were higher compared to that of the roasted 2-day germinated seeds and the ungerminated seeds. The higher antioxidant activity in the germinated versus the ungerminated seeds has also been reported in roasted, germinated brown rice. The germinated brown rice contained higher residual phenolic compounds and Maillard reaction products, such as furans and pyrazines (Kim et al., 2021).
Fig. 5.
Antioxidant activity in roasted, germinated Sacha inchi seeds at different roasting temperatures and times. Data are the means ± SD. Different letters in the same column are different with statistical significance (p < 0.05); F, ungerminated seed and D, germination day.
4. Conclusions
The effect of roasting conditions on germinated Sacha inchi seeds was determined for metabolites and flavors using coupled metabolomics and flavoromics analyses. Browning formation, amino acid content, off-flavors, roasted flavors, and antioxidant capacities were increased during roasting; however, reducing sugars and phytosterols were decreased as roasting temperature increased. Roasting enhanced FAME levels in germinated seeds, but not significantly in ungerminated seeds. However, higher essential fatty acids were detected in ungerminated Sacha inchi seeds. Higher amino acids and antioxidant activities were observed in the germinated seeds. The better roasting conditions for a higher amino acid levels and antioxidant capacities should be 140 °C for 45 min combined with 4-day germination for Sacha inchi seeds, which may be incorporated into food products, ingredients, and functional foods.
CRediT authorship contribution statement
Kannika Keawkim: Investigation, Formal analysis, Writing – original draft. Kriskamol Na Jom: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the Faculty of Science and Technology of Huachiew Chalermprakiet University and Faculty of Agro-Industry, Kasetsart University for research support. Kasetsart University Research and Development Institute are acknowledged for English proofreading service and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100399.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alamprese C., Ratti S., Rossi M. Effects of roasting conditions on hazelnut characteristics in a two-step process. Journal of Food Engineering. 2009;95(2):272–279. doi: 10.1016/j.jfoodeng.2009.05.001. [DOI] [Google Scholar]
- Atala E., Vásquez L., Speisky H., Lissi E., López-Alarcón C. Ascorbic acid contribution to ORAC values in berry extracts: An evaluation by the ORAC-pyrogallol red methodology. Food Chemistry. 2009;113(1):331–335. doi: 10.1016/j.foodchem.2008.07.063. [DOI] [Google Scholar]
- Belcadi-Haloui R., Zekhnini A., El-Alem Y., Hatimi A. Effects of roasting temperature and time on the chemical composition of argan oil. International Journal of Food Science. 2018;2018:7683041. doi: 10.1155/2018/7683041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cao J., Zou X.-G., Deng L., Fan Y.-W., Li H., Li J., Deng Z.-Y. Analysis of nonpolar lipophilic aldehydes/ketones in oxidized edible oils using HPLC-QqQ-MS for the evaluation of their parent fatty acids. Food Research International. 2014;64:901–907. doi: 10.1016/j.foodres.2014.08.042. [DOI] [PubMed] [Google Scholar]
- Chen X., Yu J., Cui H., Xia S., Zhang X., Yang B. Effect of temperature on flavor compounds and sensory characteristics of Maillard reaction products derived from mushroom hydrolysate. Molecules (Basel, Switzerland) 2018;23(2) doi: 10.3390/molecules23020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos R., Zorrilla D., Aguilar-Galvez A., Pedreschi R., Campos D. Impact of roasting on fatty acids, tocopherols, phytosterols, and phenolic compounds present in Plukenetia huayllabambana seed. Journal of Chemistry. 2016;2016:1–10. doi: 10.1155/2016/6570935. [DOI] [Google Scholar]
- Chirinos R., Zuloeta G., Pedreschi R., Mignolet E., Larondelle Y., Campos D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chemistry. 2013;141(3):1732–1739. doi: 10.1016/j.foodchem.2013.04.078. [DOI] [PubMed] [Google Scholar]
- Davídek T., Devaud S., Robert F., Blank I. Sugar fragmentation in the Maillard reaction cascade: Isotope labeling studies on the formation of acetic acid by a hydrolytic β-dicarbonyl cleavage mechanism. Journal of Agricultural and Food Chemistry. 2006;54(18):6667–6676. doi: 10.1021/jf060667q. [DOI] [PubMed] [Google Scholar]
- Demnati D., Pacheco R., Martínez L., Sánchez S. Effect of roasting temperature and time on the chemical composition and oxidative stability of Argan (Argania Spinosa L.) oils. European Journal of Lipid Science and Technology. 2018;120(7):1700136. doi: 10.1002/ejlt.201700136. [DOI] [Google Scholar]
- Dziedzic K., Szwengiel A., Górecka D., Rudzińska M., Korczak J., Walkowiak J. The effect of processing on the phytosterol content in buckwheat groats and by-products. Journal of Cereal Science. 2016;69:25–31. doi: 10.1016/j.jcs.2016.02.003. [DOI] [Google Scholar]
- Farag M.A., Khattab A.R., Shamma S., Afifi S.M. Profiling of primary metabolites and volatile determinants in mahlab cherry (Prunus Mahaleb L.) seeds in the context of its different varieties and roasting as analyzed using chemometric tools. Foods (Basel, Switzerland) 2021;10(4):728. doi: 10.3390/foods10040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves B., Borges O., Rosa E., Coutinho J., Silva A.P. Effect of cooking on free amino acid and mineral profiles of sweet chestnut (Castanea sativa Mill.) Fruits. 2012;67(3):201–214. doi: 10.1051/fruits/2012013. [DOI] [Google Scholar]
- Hwang H.I., Hartman T.G., Rosen R.T., Ho C.T. Formation of pyrazines from the Maillard reaction of glucose and glutamine-amide-15N. Journal of Agricultural and Food Chemistry. 1993;41(11):2112–2115. doi: 10.1021/jf00035a054. [DOI] [Google Scholar]
- Keawkim K., Lorjaroenphon Y., Vangnai K., Jom K.N. Metabolite–flavor profile, phenolic content, and antioxidant activity changes in sacha inchi (Plukenetia Volubilis L.) seeds during germination. Foods. 2021;10(10) doi: 10.3390/foods10102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Han J.A., Lim S.T., Cho D.H. Effects of germination and roasting on physicochemical and sensory characteristics of brown rice for tea infusion. Food Chemistry. 2021;350 doi: 10.1016/j.foodchem.2021.129240. [DOI] [PubMed] [Google Scholar]
- Lemos M.R.B., de Almeida Siqueira E.M., Arruda S.F., Zambiazi R.C. The effect of roasting on the phenolic compounds and antioxidant potential of baru nuts [Dipteryx alata Vog.] Food Research International. 2012;48(2):592–597. doi: 10.1016/j.foodres.2012.05.027. [DOI] [Google Scholar]
- Liu X., Jin Q., Liu Y., Huang J., Wang X., Mao W., Wang S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. Journal of Food Science. 2011;76(3):C404–C412. doi: 10.1111/j.1750-3841.2011.02073.x. [DOI] [PubMed] [Google Scholar]
- Maskan M. Kinetics of colour change of kiwifruits during hot air and microwave drying. Journal of Food Engineering. 2001;48(2):169–175. doi: 10.1016/S0260-8774(00)00154-0. [DOI] [Google Scholar]
- McDaniel K.A., White B.L., Dean L.L., Sanders T.H., Davis J.P. Compositional and mechanical properties of peanuts roasted to equivalent colors using different time/temperature combinations. Journal of Food Science. 2012;77(12):C1293–C1299. doi: 10.1111/j.1750-3841.2012.02979.x. [DOI] [PubMed] [Google Scholar]
- Na Jom K., Frank T., Engel K.-H. A metabolite profiling approach to follow the sprouting process of mung beans (Vigna radiata) Metabolomics. 2011;7:102–117. doi: 10.1007/s11306-010-0236-5. [DOI] [Google Scholar]
- Nakamura S., Nishimura O., Masuda H., Mihara S. Identification of volatile flavor components of the oil from roasted sesame seeds. Agricultural and Biological Chemistry. 1989;53(7):1891–1899. doi: 10.1080/00021369.1989.10869549. [DOI] [Google Scholar]
- Perren R., Escher F. Investigation on the hot air roasting of nuts. The Manufacturing Confectioner. 1999;77:123–127. [Google Scholar]
- Perren R., Escher F.E. In: Improving the Safety and Quality of Nuts. Harris L.J., editor. Woodhead Publishing; 2013. 8 - Impact of roasting on nut quality; pp. 173–197. [Google Scholar]
- Pripis-Nicolau L., Revel G., Bertrand A., Maujean A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. Journal of Agricultural and Food Chemistry. 2000;48:3761–3766. doi: 10.1021/jf991024w. [DOI] [PubMed] [Google Scholar]
- Rizki H., Kzaiber F., Elharfi M., Ennahli S., Hanine H. Effects of roasting temperature and time on the physicochemical properties of sesame (Sesamum indicum L.) seeds. International Journal of Innovation and Applied Studies. 2015;11(1):148–155. [Google Scholar]
- Sharma P., Gujral H.S. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Research International. 2011;44(1):235–240. doi: 10.1016/j.foodres.2010.10.030. [DOI] [Google Scholar]
- Shu X.L., Frank T., Shu Q.Y., Engel K.H. Metabolite profiling of germinating rice seeds. Journal of Agricultural and Food Chemistry. 2008;56(24):11612–11620. doi: 10.1021/jf802671p. [DOI] [PubMed] [Google Scholar]
- Štěrbová L., Hlásná Čepková P., Viehmannová I., Huansi D.C. Effect of thermal processing on phenolic content, tocopherols and antioxidant activity of Sacha Inchi kernels. Journal of Food Processing and Preservation. 2017;41(2):e12848. [Google Scholar]
- Sun J., Li Q., Luo S., Zhang J., Huang M., Chen F.…Li H. Characterization of key aroma compounds in Meilanchun sesame flavor style baijiu by application of aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission/addition experiments. RSC Advances. 2018;8:23757–23767. doi: 10.1039/C8RA02727G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Yan D., Ping-Ping L., Fang W., Mu-lan J., Ying-Zhong Z., Guang-Ming L., Yuan-Di Z. The impact of processing on the profile of volatile compounds in sesame oil. European Journal of Lipid Science and Technology. 2012;114(3):277–286. doi: 10.1002/ejlt.201100059. [DOI] [Google Scholar]
- Zhou Q., Geng F., Deng Q., Huang F., Jinqiu W. Dynamic analysis of polar metabolites and volatile compounds in sesame seeds during roasting. Cereal Chemistry. 2019 doi: 10.1002/cche.10134. [DOI] [Google Scholar]
- Zou Y., Gao Y., He H., Yang T. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. LWT. 2018;90:246–253. doi: 10.1016/j.lwt.2017.12.038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.