Highlights
-
•
Volatile fingerprints of three Molixiang grapes were established using HS-GC-IMS.
-
•
GC-IMS coupled with PCA could distinguished the three Molixiang grapes well.
-
•
Sensory evaluation revealed significant (P ≤ 0.05) difference among the three samples.
-
•
E-2-octenal, styrene and benzaldehyde were potential geographical marker compounds.
Keywords: Table grapes, Volatile profiles, Gas chromatography-ion mobility spectrometry (GC-IMS), Geographical marker compounds
Abstract
In this study, the volatile fingerprints of GC-IMS showed great differences on the volatile profiles of Molixiang grapes collected from three different regions of China, which suggested that aroma of table grapes could be largely impacted by origin areas. Butyl lactate, E-2-octenal and Z-2-pentanol were mainly contained in MLX-A, the grapes sampled from Ningbo, China. High contents of p-cymene, styrene and γ-terpinene were observed in MLX-B grapes sampled from Beizhen, China. In addition, benzaldehyde and methyl benzoate were major contained in MLX-C grapes sampled from Zhangzhou, China. The PCA results revealed effective differentiation of samples from different geographical origin based on the information obtained from GC-IMS. Furthermore, sensory evaluation showed that the aroma characters of grapes from different geographical origin were significantly different (P ≤ 0.05). E-2-octenal, styrene and benzaldehyde might serve as the geographical marker compounds of origin area based on the results of GC-IMS analysis and sensory evaluation.
1. Introduction
Grapes are one of the most largely consumed fruit species worldwide with high nutritional qualities that have been employed in human diet since ancient times (Pezzuto, 2008). Grape fruit contains a good range of nutrient elements such as dietary fiber, vitamins and minerals, and also are rich source of bioactive phytochemicals including polyphenols, flavonoids, and anthocyanins that possess various health-promoting benefits (Sabra, Netticadan, & Wijekoon, 2021). A wide range of the grapes cultivar varieties have been developed for their different application forms during past centuries, such as directly consumed table grapes, wine grapes used for the production of wines or juice, and dried form known as raisin grapes (Samoticha, Jara-Palacios, Hernández-Hierro, Heredia, & Wojdyło, 2018). Till now, more than 8000 cultivars of Vitis vinifera grapes are grown worldwide for the purpose of commercial wine, raisin, and table grape production (Sabra et al., 2021). In addition to varietal difference, the quality, sensory properties and consumer acceptability of grapes as well as grape-based food products are significantly affected by geographical origin (Granato, Carrapeiro, Fogliano, & Ruth, 2016).
Table grapes are extensively planted and consumed worldwide due to their high nutritional values as well as unique sensory attributes. As reported previously, the global production of table grapes reached 22.7 million tons in 2017 and increased to 23.4 million metric tons in 2019–2020 (Anastasiou et al., 2018, Wu et al., 2018). In China, table grapes are one of the most favorable fruit and accounting for 80 % of total grape production predominantly, with the production of over 10 million tons (Wu et al., 2018). Hybrid cultivars (such as V. vinifera and V. labrusca) are mainly planted table grapes in China and other Asian countries due to their high sugar and lower acid levels as well as high disease resistance (Yang, Wang, Wu, Fang, & Li, 2011). Meanwhile, table grapes owning distinctive flavor have attracted strong interest among consumers and gained great popularity in most areas of China in recent years (Wu et al., 2019). For example, Hutai-8 grape variety (V. vinifera × V. labrusca) is widely cultivated in several provinces of China due to its strong strawberry-like odor as well as other advantages (Yao et al., 2021).
The grape sensory attributes and nutritional qualities are significantly influenced by various factors such as agronomical practices, field conditions and post-harvesting conditions, while genotype is considered as the determined factor leading to the variation (Hasanaliyeva et al., 2020, Wu et al., 2018). The comparison of phenolic compounds and antioxidant activity as well as aroma characterization among different table grape cultivars has been extensively reported up to now (Colombo et al., 2019, Xu et al., 2010). However, little information is available on variation of aroma compounds with specified table grape cultivar grown in different production region (Wu et al., 2018). In a recent study, the influence of terroir (the uniqueness of the growing place) on ‘Crimson’ table grapes has been investigated (Rodrigues et al., 2019). Regarding sensory evaluation, great differences (p ≤ 0.05) were observed for the same cultivar growing in two distinct vineyards, which suggested the importance of growing region on sensory attributes of table grapes (Rodrigues et al., 2019).
Molixiang grape, also known as Zuijinxiang grape and jasmine grape, is one of the most planted and consumed table grape cultivar in China with characteristics of jasmine-like aroma and no existence of seed in grape berries (Yang et al., 2021a). It has been planted in most grape producing areas of the country due to its high yield and nutritional quality (Yue et al., 2019). Beyond nutritional composition, aroma component is another important influential factor for table grape quality assessment, since aroma characteristic is the main indicator contributing to sensory attributes as well as consumer acceptance (Xi, Zha, He, Tian, & Jiang, 2020). Moreover, the geographical origin of the grapes was of great importance to producers and consumers (Margraf et al., 2016, Perestrelo et al., 2014), and geographical origin is a most applied indicator for quality assessment (Granato et al., 2016). For previous works, GC–MS and electronic nose were the main technologies used for flavor or volatile profile determination (Hanif et al., 2022, Song et al., 2020). In recent years, GC-IMS has served as an efficient and alternative technology for volatile detection, food classification and quality control due to its advantages of no sample pre-treatment and capability of combining with chemometric techniques for intuitive comparison of the differences in volatiles (Gu et al., 2021). The high sensitivity, detection speed and separation efficiency are also the distinct advantages of GC-IMS as compared to GC–MS (Wang et al., 2020a). In this work, GC-IMS coupled with principal component analysis (PCA) was employed as a new method for the geographical differentiation of Molixiang table grapes grown in China. Furthermore, sensory evaluation method was adapted to provide reliable data on sensory characteristics and consumer acceptability of the grapes. This work would provide an important tool for geographical region assurance and quality control of table grapes as well as other fruits.
2. Materials and methods
2.1. Plant material
Molixiang grape bunches were sampled from commercial vineyards located in, Ningbo, Zhejiang province, China (122°16′E and 30°33′N), Beizhen, Liaoning province, China (121°33′E and 41°19′N), and Zhangzhou, Fujian province, China (117°25′E and 23°42′N) in August 2021, which are three main Molixiang grapes producing areas in China. All bunches were collected at maturity stage and samples were named as MLX-A (Ningbo Molixiang), MLX-B (Beizhen Molixiang) and MLX-C (Zhangzhou Molixiang) respectively (Fig. 1). The fresh collected grape samples (Table 1) were transported on ice and 3.0 kg of each sample was stored at 4 °C until instrumental or sensory analysis, the remaining grapes (2.0 kg each) were packaged and stored at −20 °C for further use.
Fig. 1.
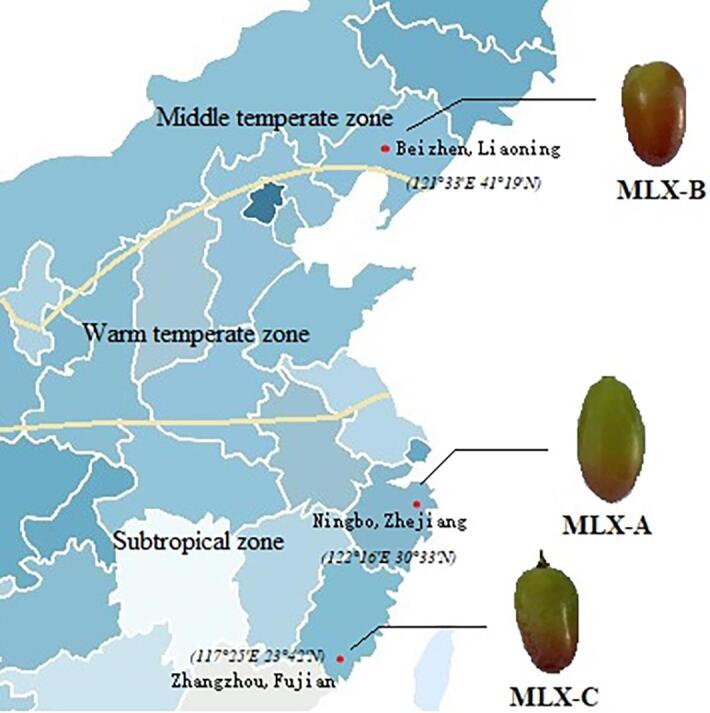
Distribution map of three Molixiang table grapes.
Table 1.
Plant material of Molixiang table grapes.
| Sample | Origin area | Vineyard | Weight (kg) |
|---|---|---|---|
| MLX-A | Ningbo, Zhejiang, China | Sanbei Maibira Fruit and Vegetable Farm | 1.5 |
| Laifu Agricultural Science and Technology Co. ltd | 1.5 | ||
| MLX-B | Beizhen, Liaoning, China | Baoxing Grape Production Cooperative | 1.5 |
| Changxing grape Production Cooperative | 1.5 | ||
| MLX-C | Zhangzhou, Fujian, China | Zhangpu County Yanjia Fruit Professional Cooperative | 1.5 |
| Ningde Kaiwen Agriculture Co., ltd | 1.5 |
2.2. Headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) analysis
Volatile organic compounds (VOCs) of Molixiang grapes were analyzed by GC-IMS method, which was composed of Agilent 490 gas chromatography (Agilent Technologies, Palo Alto, CA, USA) and IMS instrument (FlavourSpec®, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany) that equipped with a PAL3 Automatic sampler (CTC Analytics AG Company, Switzerland). Before GC-IMS analysis, each peeled grape sample (3.50 g) was transferred into a 20-mL headspace vial and incubated at oscillating heating mode (40 °C) with the speed of 500 rpm for 10 min. Then the headspace was injected by PAL3 sampler automatically with injection volume of 100 μL and injector temperature of 85 °C. The above injection method is slightly modified from the previous methods (Pu et al., 2020).
For GC detection, VOCs were separated by FS-SE-54-CB-1 capillary column (15 m × 0.53 mm, 1.0 μm film thickness) with column temperature fixed at 40 °C. High purity nitrogen (≥99.999 %) was used as carrier gas with initial flow rate of 2.0 mL/min for 2 min and increased to 20 mL/min within 8 min, and then increased to 100 mL/min within 10 min and maintained at 150 mL/min for 10 min. Nitrogen (≥99.999 % purity) was used as the drift gas with flow rate of 150 mL/min, volatiles were ionized in the IMS ionization chamber (300 MBq in positive ion mode) and ions were driven to the 9.8 cm drift tube with the nitrogen flow at temperature of 45 °C (Chen et al., 2021). The retention index (RI) of each volatile compound was calculated by Laboratory Analytical Viewer (LAV) using n-ketones C4-C9 (Sinopharm Chemical Reagent Beijing Co., ltd., Beijing, China) as external references. Identification of the volatile compound was based on the retention index (RI) and drift time (RIP relative) of standards in the GC-IMS Library. The Reporter plug-in and Gallery Plot plug-in were used to form the spectrogram and volatile fingerprints of grape samples. Principal component analysis (PCA) was performed using the Dynamic PCA plug-in to evaluate the regularity and difference among tested samples.
2.3. Sensory evaluation
The sensory evaluation of the three grape samples was performed using descriptive analysis. The sensory evaluation procedures were carried out according to Wang et al (2020b) with slight modification. Thirty-five healthy and non-smoking probable assessors were recruited from the students and staff members of the School of Perfume and Aroma Technology (Shanghai Institute of Technology, Shanghai, China). A panel of 10 well-trained panelists (five male and five female with age of 20–42) was selected for their familiarity with table grapes based on the triangle test. Before aroma evaluation, all panelists were trained about the characteristics of Molixiang table grapes and the sensory evaluation requirements (such as definition of quality attributes and the method of scoring) for more than 2 h a day and lasted a week to familiar with the descriptive terms of the grapes (Sato et al., 2021). Thereafter, vocabulary of sensory attributes was generated to obtain an aroma description of the grapes that covered the odor and aroma of tested samples (Zhang, Wang, Ding, Su, & Zhao, 2022). In addition, the panelists were trained to reach consensus on rating the intensity of the six defined odor/aroma attributes, including “green”, “sweet”, “fruity”, “woody”, “nutty”, and “pungent” that identified using reference compounds of hexanal, 5-methyl-2-furanmethanol, butyl acetate, p-cymene, benzaldehyde, and acetic acid respectively for attributes description (Feng, Huang, Crane, & Wang, 2018). Each sensory attribute was taken on a 10-point intensity scale (0–1, weaker; 2–3, weak; 4–5, middle; 6–7, strong; 8–9, stronger). To validate the reliability of the intensity scale, the recorded data of repeated panel performances was compared using different means of analysis of variance (ANOVA).
The sensory analysis was performed at room temperature under daylight with individual booths. Before sensory evaluation, the grape samples were peeled and presented in plastic cups labeled with randomly selected three-digit numbers. The assessors were asked to take three short sniffs to judge the aroma of the samples first and to rinse their mouth with pure water to minimize any residual effect (Mukhopadhyay, Majumdar, Goswami, & Mishra, 2013). Each sample was evaluated in triplicate and carefully scored after sensory judgment.
2.4. Statistical analysis
The HS-GC-IMS data was processed using Laboratory Analytical Viewer (LAV, G.A.S., Dortmund, Germany) with three plug-ins and GC × IMS Library Search (NIST database and IMS database). Topographic plots and fingerprints of volatile compounds were established by plug-ins of Reporter and Gallery Plot (G.A.S., Dortmund, Germany). Principal component analysis (PCA) was performed using the Dynamic PCA plug-in (G.A.S., Dortmund, Germany) to evaluate the regularity and difference among tested samples. The sensory evaluation data were statistically analyzed by SPSS 20.0 (SPSS Inc., USA) software using Analysis of variance (ANOVA) with a significant difference level of p ≤ 0.05. The sensory evaluation profile was made by Origin Pro 2021 (OriginLab Corporation, Northampton, MA, USA). All the measurements were performed in triplicate.
3. Results and discussion
3.1. HS-GC-IMS topographic plots of three different Molixiang grapes
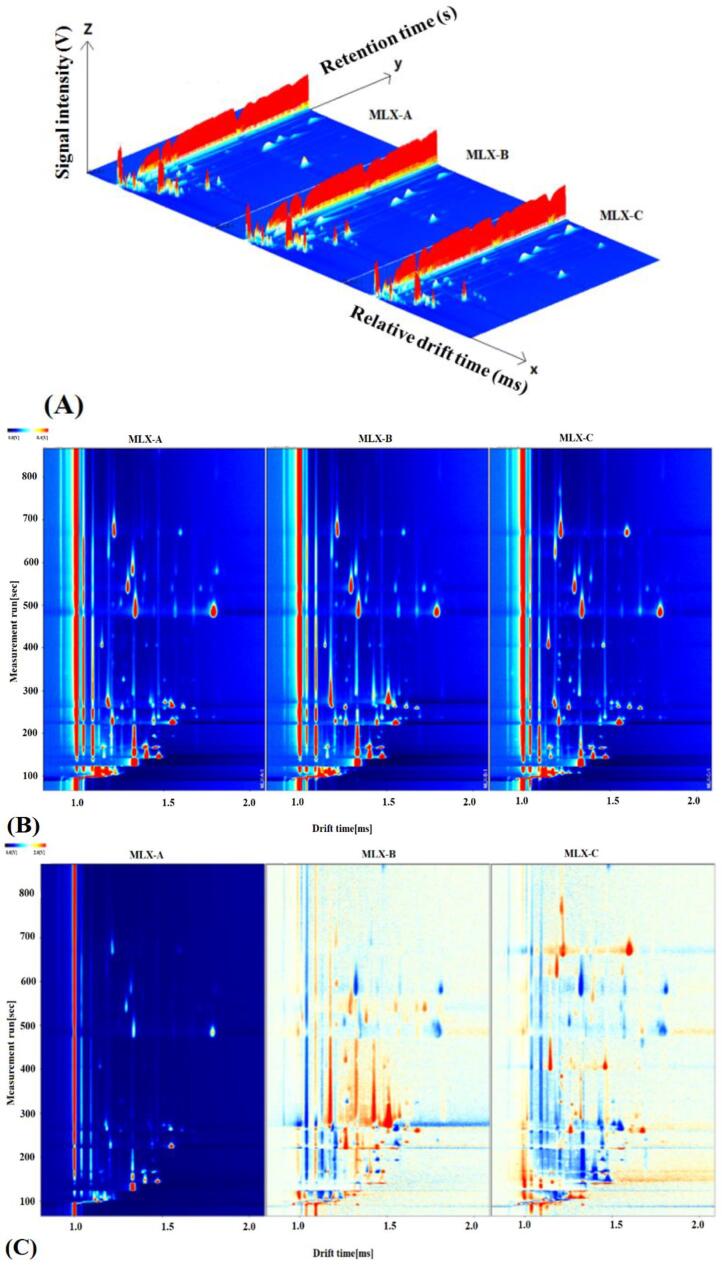
To explore the difference in volatile compounds of Molixiang grapes that caused by different geographical origin, the GC-IMS profiles of three grape samples (MLX-A, MLX-B and MLX-C) were obtained (Fig. 2). As shown in Fig. 2A, the GC-IMS analysis of grape volatiles resulted in a 3D-topographic plot using the Reporter plug-in, where X, Y, and Z-axes represent the ion migration time (DT) for identification, the retention time (RT) for GC separation, and the ion peak intensity for quantification (Li et al., 2019). The 2D-topographic spectra of volatile compounds in Molixiang grape samples with different geographical origins were shown in Fig. 2B. For IMS analysis, the capillary column separated VOCs entered the ionization reaction region individually to generate molecular ion groups for secondary separation under the migration region, and each volatile compound would be detected due to its different migration rates caused by collision with the drifting gas (Gu et al, 2021). That is, volatiles of grape samples could be identified qualitatively based on the differences of ion migration time and ion peak intensity of each separated compound. In Fig. 2A and 2B, the background of the GC-IMS spectra was blue, and the red vertical line at abscissa 1.0 was reactive ion peak (RIP) after normalization. Each point on both sides of the RIP peak represented a volatile compound and the color reflected the concentration of the compound, with white color represents lower concentration and red means higher concentration. As reported elsewhere, the darker color indicating the higher concentration of the volatile compound (Chen et al., 2021).
Fig. 2.
GC-IMS analysis of three different Molixiang grapes. (A) 3D-topographic plots; (B) 2D-topographic plots; (C) The difference comparison topographic plots.
In Fig. 2B, all the grape samples are rich in volatile compounds and most of the peak signals were observed in ranges of retention time 100 to 700 s and drift time 1.0 to 1.8 ms. To compare the aroma differences among the tested grape samples more conveniently, the topographical plot of MLX-A was taken as the reference and the topographical plots of MLX-B and MLX-C were deducted from the reference (Fig. 2C). The white color after deduction means the same concentration of a volatile compound in the two samples, while red dot indicates higher concentration of a volatile compound than that in reference and blue color implies lower concentration of a compound as compared to that in reference (Li et al., 2019). As shown in Fig. 2C, quite a lot red dots as well as blue dots were revealed in both MLX-B and MLX-C samples, which demonstrated significant difference in molecule structure and concentration of volatile compounds among three grape samples.
3.2. Fingerprint analysis of volatile compounds in different Molixaing grapes
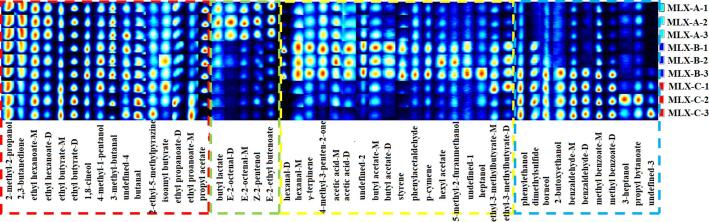
To further identify the differences of volatile compound profile among three Molixiang grapes, all the volatile compounds identified in the GC-IMS spectra were selected to generate the volatile fingerprints using the Gallery Plot plug-in (Fig. 3). As shown in Fig. 3, each row in the gallery plot revealed the entire signal peak of a grape sample and each column showed the signal intensity of the same compound presented in different grape samples. In the whole fingerprint spectrum, a total of 50 compounds were qualitatively detected (Fig. 3). Among these volatiles, 36 compounds were identified base on the searching results of GC-IMS Library and NIST database, including 12 esters, 10 alcohols, 6 aldehydes, 2 ketones, and other volatiles such as terpenes and acids (Table 2). It is a notable phenomenon that some compounds could produce two peak signals, which were caused by the monomer (M) and dimer (D) form of a compound (Fig. 3 and Table 2). In ionization region, the formation of dimers or polymers is related to the high proton affinity of the volatile compound and would result in the variation of drift time as compared to monomer form, therefore multiple signals of an individual compound would probably be observed in GC-IMS (Chen et al, 2021).
Fig. 3.
Fingerprints of volatile compounds in Molixiang grapes collected from different geographical origins.
Table 2.
GC-IMS global area set integration parameters obtained from three Molixiang grape samples.
| No. | Compound | Flavor description | MWa | RIb | RTc[s] | DT d [ms] | Type | Peak Intensity [mV] |
||
|---|---|---|---|---|---|---|---|---|---|---|
| MLX-A | MLX-B | MLX-C | ||||||||
| 1 | Z-2-pentenol | green, cherry, fruity | 86.1 | 759.6 | 195.869 | 1.4475 | 637 | – | 43 | |
| 2 | E-2-octenal | green, cucumber, fatty | 126.2 | 1053.2 | 585.557 | 1.8117 | Dimers | 893 | – | – |
| 3 | E-2-octenal | 126.2 | 1058.1 | 596.503 | 1.3277 | Monomer | 766 | 21 | – | |
| 4 | E-2-ethyl butenoate | musty, pungent, onion | 114.1 | 844.4 | 267.926 | 1.547 | 659 | 61 | 76 | |
| 5 | butyl lactate | green, fruity, lactonic, winey | 146.2 | 1018.2 | 511.152 | 1.2545 | 837 | 216 | – | |
| 6 | benzaldehyde | sweet, almond, nutty | 106.1 | 964.7 | 415.071 | 1.144 | Monomer | 19 | 20 | 608 |
| 7 | benzaldehyde | 106.1 | 960.4 | 408.404 | 1.4639 | Dimers | – | – | 591 | |
| 8 | dimethylsulfide | onion, sweet, corn, green | 62.1 | 513.5 | 93.821 | 0.9667 | – | 363 | 602 | |
| 9 | propyl bytanoate | fruity, sweet, apricot, pineapple | 130.2 | 912.5 | 342.006 | 1.2707 | 16 | – | 317 | |
| 10 | 2-butoxyethanol | camphor, pine, earthy, woody | 136.2 | 945.2 | 385.605 | 1.2077 | – | – | 493 | |
| 11 | 3-heptanol | green, herbal | 116.2 | 897.8 | 324.399 | 1.6752 | – | – | 320 | |
| 12 | phenylethanol | fresh, sweet, vanilla, woody | 122.2 | 1073.7 | 631.847 | 1.1835 | 120 | 208 | 637 | |
| 13 | methyl benzoate | wintergreen, almond, cherry | 136.1 | 1093 | 676.211 | 1.5984 | Dimers | – | – | 588 |
| 14 | methyl benzoate | 136.1 | 1097.3 | 686.087 | 1.2216 | Monomer | 29 | 30 | 589 | |
| 15 | borneol | pine, woody, camphor | 154.3 | 1134.8 | 772.523 | 1.2115 | – | 160 | 573 | |
| 16 | ethyl hexanoate | sweet, fruity, pineapple, green | 144.2 | 1010.7 | 496.255 | 1.3346 | Monomer | 1219 | 1080 | 1076 |
| 17 | ethyl hexanoate | 144.2 | 1006.5 | 488.137 | 1.3359 | Dimers | 1082 | 429 | 373 | |
| 18 | propyl acetate | celery fruity, raspberry pear | 102.1 | 688.6 | 152.239 | 1.4766 | 693 | 687 | 618 | |
| 19 | butanal | pungent, musty, green, bready | 72.1 | 553.3 | 105.849 | 1.118 | 544 | 587 | 796 | |
| 20 | 2-methyl-2-propanol | camphor | 74.1 | 533.1 | 99.744 | 1.1341 | 1316 | 1113 | 1267 | |
| 21 | 2–3-butanedione | butter, creamy, caramel, sweet | 86.1 | 548.4 | 104.352 | 1.1733 | 1436 | 878 | 275 | |
| 22 | ethyl propanoate | sweet, fruity, g rape, pineapple | 102.1 | 716.5 | 167.087 | 1.4643 | Dimers | 598 | 535 | 713 |
| 23 | ethyl propanoate | 102.1 | 718.9 | 168.562 | 1.1477 | Monomer | 436 | 322 | 708 | |
| 24 | isoamyl butyrate | fruity, green, sweet | 158.2 | 1044.8 | 567.064 | 1.3922 | 812 | 833 | 741 | |
| 25 | ethyl butyrate | fruity, pineapple, sweet | 116.2 | 800.5 | 228.349 | 1.5495 | Dimers | 686 | 637 | 973 |
| 26 | ethyl butyrate | 116.2 | 806.6 | 233.536 | 1.205 | Monomer | 654 | 327 | 989 | |
| 27 | 1,8-cineol | minty, herbal, eucalyptus | 154.3 | 1037.4 | 551.054 | 1.295 | 543 | 793 | 531 | |
| 28 | 4-methyl-1-pentanol | nutty | 102.2 | 839.4 | 263.091 | 1.6124 | 642 | 822 | 713 | |
| 29 | 3-methyl-butanal | peach, fruity, green, nutty | 86.1 | 685.5 | 150.829 | 1.4033 | 732 | 724 | 513 | |
| 30 | 2-ethyl-5-methylpyrazine | nutty, grassy | 122.2 | 1007 | 489.05 | 1.6726 | 603 | 619 | ||
| 31 | hexenal | sweet, almond, fruity, green | 98.1 | 861.9 | 285.178 | 1.1747 | Monomer | 253 | 987 | 193 |
| 32 | hexanal | 98.1 | 860.5 | 283.795 | 1.511 | Dimers | – | 323 | – | |
| 33 | ethyl 3-methylbutyrate | fruity, sweet, apple, pineapple | 130.2 | 841.3 | 264.882 | 1.263 | Monomer | 22 | 363 | 127 |
| 34 | ethyl 3-methylbutyrate | 130.2 | 838.1 | 261.837 | 1.6736 | Dimers | – | 375 | 128 | |
| 35 | 4-methyl-3-penten-2-one | pungent, earthy, vegetable, potato | 98.1 | 807.6 | 234.438 | 1.4459 | 307 | 967 | – | |
| 36 | butyl acetate | sweet, ripe banana, fruity | 116.2 | 814.7 | 240.527 | 1.2361 | Monomer | – | 783 | 96 |
| 37 | butyl acetate | 116.2 | 810.1 | 236.555 | 1.6144 | Dimers | – | 721 | – | |
| 38 | heptanol | musty, herbal, green, woody | 116.2 | 961.3 | 409.746 | 1.3991 | – | 430 | 27 | |
| 39 | γ -terpinene | oily, woody, lemon, herbal | 136.2 | 1058.4 | 597.27 | 1.2126 | 252 | 903 | 209 | |
| 40 | styrene | sweet, floral, plastic | 104.2 | 871.6 | 295.266 | 1.4268 | – | 637 | – | |
| 41 | acetic acid | pungent, fruity | 60.1 | 567.3 | 110.076 | 1.1526 | Monomer | 93 | 879 | – |
| 42 | acetic acid | 60.1 | 567.4 | 110.096 | 1.1521 | Dimers | 362 | 963 | 217 | |
| 43 | p-cymene | citrus,rancid, woody, green pepper | 134.2 | 1033.6 | 543.085 | 1.7133 | 30 | 601 | 31 | |
| 44 | phenylacetaldehyde | green, clover, honey, sweet | 120.2 | 1034.8 | 545.662 | 1.5517 | 56 | 622 | – | |
| 45 | 5-methyl-2-furanmethanol | sweet, caramel | 112.1 | 970.1 | 423.773 | 1.2651 | – | 533 | 20 | |
| 46 | hexyl acetate | fruity, green, sweet | 144.2 | 1022.2 | 519.408 | 1.3769 | 21 | 531 | – | |
a-MW means the molecule weight of the volatiles.
b-RI means the retention index of the volatiles on an FS-SE-54-CB-1 capillary column.
c-RT means the retention time of the volatiles on GC-IMS.
d-DT means the drift time of the volatiles on GC-IMS.
There were 12 identified volatile compounds, including 2-methyl-2-propanol, 2-3-butanedione, ethyl hexanoate (M and D), ethyl butyrate (M and D), 1,8-cineol, 4-methyl-1-pentanol, 3-methyl-butanal, butanal, 2-ethyl-5-methylpyrazine, isoamyl butyrate, ethyl propanoate (M and D), and propyl acetate had been detected in all grape samples from three geographical regions with little difference in concentration as labeled with red rectangle in Fig. 3. Further, 4 identified compounds (i.e., butyl lactate, E-2-octenal, Z-2-pentanol and E-2-ethyl butenoate) were major presented in MLX-A samples and revealed in low concentration or could not be detected in MLX-B and MLX-C samples (labeled with green rectangle in Fig. 3). It was observed that 12 identified compounds were dominant volatiles in MLX-B samples as labeled with yellow rectangle in Fig. 3. In addition, several volatiles (i.e., phenylacetaldehyde, dimethyl sulfide, borneol, 2-butoxyethanol, benzaldehyde, methyl benzoate, 3-heptanol, and propyl bytanoate) were mainly accumulated in MLX-C grape samples (labeled with blue rectangle in Fig. 3). The obtained information suggested the possibility to distinguish Molixiang grapes from different geographical regions of China based on the volatile profile characterized by GC-IMS. As reported previously, GC-IMS has been successfully used in discrimination of various food products through volatile compound analysis (Wang et al., 2020a).
Esters and terpenes are important volatile compounds that contribute to the fruity/floral characters of table grape berries (Yang et al., 2011), which were observed in all the detected grape samples (Fig. 3). Aldehydes and alcohols possess green leafy aroma characters (Liu et al., 2022), while the peak intensity of each volatile compound was significantly different (Fig. 3 and Table 2). As reported elsewhere, high concentration of aldehydes would result in strong nutty and fatty aroma and sometimes also present a rancid odor while turn to green and pleasant odor with the decreased concentration (Zhang et al., 2020), therefore the aroma of three Molixiang grapes would be varied due to the different concentration of contained aldehydes. Concretely, the detected aldehydes in MLX-A were rich in concentration and abundant in types (such as E-2-octenal, hexanal and phenylacetaldehyde), while the types of detected aldehydes were less abundant though the concentration of benzaldehyde was relatively high in MLX-C grapes (Fig. 3). MLX-A grapes contained large number of alcohols such as Z-2-pentenol, 2-methyl-2-propanol, and 4-methyl-1-pentanol that could mainly provide green note (Fig. 3 and Table 2). For MLX-B sample, high contents of p-cymene, styrene, and γ-terpinene were observed (Fig. 3), which would probably contribute to the woody aroma of the grapes (Table 2). As for MLX-C, benzaldehyde was identified as predominated volatile compound that contained, which would result in sweet and nutty aroma of the sample (Fig. 3 and Table 2), though the types of detected aldehydes were relatively few.
Yao et al (2021) found a total of 84 free aroma compounds in Hutai-8 table grapes using HS-SPME-GC–MS. Among them, esters and aldehydes were the main volatile compounds and the concentration of acids was least. This conclusion is consistent with our findings. Acetic acid was the only one type of acids in our study. Wu et al (2019) screened 35 volatiles as the differential compounds in grape berry of three aroma types (Strawberry, Fox and Muscat) and found monoterpenes are the primary aromatic compounds responsible for the aroma using GC–MS. Since GC–MS has detected approximately 70 monoterpenes in table grapes and wine, the number of volatile compounds detected by GC-IMS in this study (36 of 50 detected were identified) is considerable, and in agreement with Dunlevy et al study that monoterpene alcohols and monoterpene aldehydes were main detections, further indicating that GC-IMS is suitable for the study of flavor substances in grapes (Dunlevy et al., 2009). In previous studies, a combination of instrumental analysis and sensory evaluation is necessary. Therefore, requirements for sensory evaluation are still needed in subsequent experiments for further determining the sensory perception of the table grapes.
3.3. PCA analysis of Molixiang grapes from different regions
In order to evaluate the regularity and difference among aroma profiles of tested grape samples, principal component analysis (PCA) technique was used due to its distinctive advantage in classifying samples based on multivariate data analysis (Yang et al., 2021b). The GC-IMS obtained information (such as peak position and peak intensity) of detected volatile compounds in Molixiang samples were analyzed using the Dynamic PCA plug-in, which was used to ascertain the differences among tested grape samples. The total contribution ratio of the first two principal components reached 84 % (PC1 accounting for 53 % and PC2 accounting for 31 % of cumulative variance contribution) and was higher than total ratio of 60 % (Fig. 4), which was suggested sufficient to characterize similarities between different samples (Guo, Zhao, Ma, Wang, & Wang, 2022). As shown in Fig. 4, grape samples from the same geographical origin were close to each other based on the PCA distribution map, with MLX-A, MLX-B, and MLX-C samples represented by light blue, dark blue and red dots respectively. However, grape samples from different geographical origins were separately distributed in the distribution map (Fig. 4). MLX-A samples were clustered in the bottom left area and MLX-B were clustered in the upper left area, while MLX-C samples were located in the right (Fig. 4). Consequently, GC-IMS coupled with PCA presented good efficiency for classifying Molixiang grapes produced from different geographical regions of China.
Fig. 4.
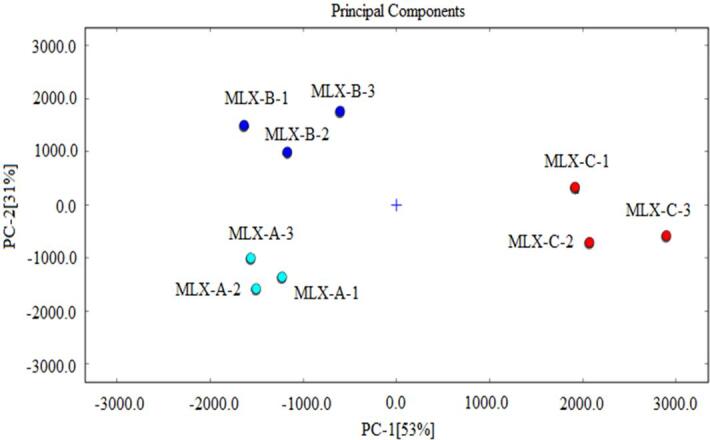
PCA analysis of Molixiang grape samples.
The variations of volatile profile among Molixiang grapes might attribute to their location difference in longitude, latitude, temperature zone and so on. MLX-A was collected from Ningbo, China (122°16′E and 30°33′N) and MLX-C was collected from Zhangzhou, China (117°25′E and 23°42′N), which were both located at subtropical zone (Fig. 1). However, Ningbo is slightly north and more closely to warm temperate zone with a relatively mild climate, the aroma volatiles of MLX-A was quite different from MLX-C grapes (Fig. 3 and Fig. 4). As shown in Table 2, high content of aldehydes (including E-2-octenal and 3-methyl butanal) and alcohols (Z-2-pentenol and 2-methyl-2-propanol) were observed in MLX-A as compared to MLX-C, suggesting grapes collected from Ningbo are more preferred in biosynthesis and accumulation of these volatile compounds. High content of these compounds might enhance the green note of MLX-A due to their aroma characteristics (Table 2). As for MLX-B grapes, 34 aroma volatile compounds were detected by the GC-IMS (Fig. 3 and Table 2), which was the most abundant among the three tested samples. Beizhen (121°33′E and 41°19′N) is located at middle temperature zone of China that has the least annual precipitation among the three grape production areas, the mild water deficit might enhance the aroma volatiles biosynthesis of grapes as reported previously (des Gachons et al., 2005).
3.4. Sensory evaluation
As shown in Fig. 5, the sensory evaluation results showed that the six sensory descriptions of the three Molixiang grape samples were significantly different, among which four descriptions (i.e., green, woody, pungent and sweet) revealed extremely significant difference (P ≤ 0.01), followed by nutty and fruity notes (P ≤ 0.05). The obtained information indicated that Molixiang grapes collected from three different regions would possess different aroma characteristics. MLX-A obtained the highest score in pungent and green aroma while revealed the lowest score in woody and sweet aroma (Fig. 5). Green note is often associated with alcohols and aldehydes, which possess low thresholds and therefore can play important role in the overall flavor of samples even at low concentrations (Yang et al., 2021b). The more contained concentrations of alcohols and aldehydes in MLX-A (Fig. 3) were consistent with the more remarkable green flavor properties of the grape sample (Fig. 4). As for MLX-B grapes, woody and fruity flavor was more dominated (P ≤ 0.05) as compared to MLX-A and MLX-C based on the sensory descriptive analysis (Fig. 5), which was probably associated with high concentration of terpenes (woody) and esters (fruity) contained in the grapes (Fig. 3 and Table 2). Combined with GC-IMS results, fruit flavor score was positively correlated with butyl acetate, hexyl acetate and ethyl-3-methylbutyrate identified in MLX-B. MLX-C grapes possessed significant nutty and sweet flavor characteristics as compared to other two grape samples (Fig. 5), which was mainly attributed to aldehydes that could provide nutty and fatty aroma and esters that could provide sweet aroma (Yang et al., 2009). As shown in Fig. 3, the concentration of benzaldehyde was relatively high in MLX-C although the lack of diversity in aldehydes volatiles was observed. It is noted that the aroma property of grapes could be impacted not only by the concentration of volatiles but also by odor threshold of each compound. Further, some un-identified volatiles might also play important role in the aroma contribution.
Fig. 5.
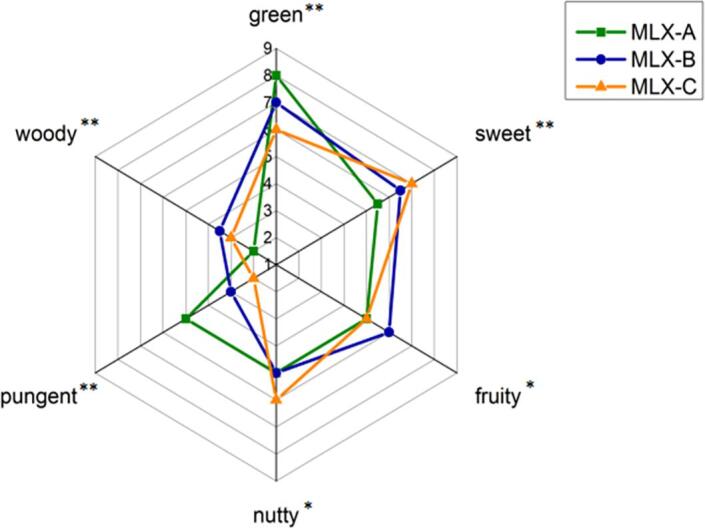
The sensory evaluation profiles of Molixiang grape samples (* P ≤ 0.05, ** P ≤ 0.01).
Basic chemical measurements, such as total acidity (TA), total soluble solids (TSS), TSS/TA value and polyphenols, were the most frequently used parameters to determine the quality of table grapes that could provide important information for quality measurement and control (Tyagi et al., 2020). However, there are still limitations to use these parameters for quality evaluation as they have shown poor correlation with sensory perceptions (Hampson et al., 2000, Rolle et al., 2013). Most notably, eating quality is difficult to measure objectively (Hampson et al., 2000). In this work, sensory analysis provided another useful method to assess preferences and differences among three Molixiang grapes. Interestingly, the sensory evaluation result was coincided with the GC-IMS analysis, suggesting that GC-IMS could be used as an efficient technology for authenticity determination and quality evaluation of table grapes.
4. Conclusion
Aroma character is an important factor for table grapes quality assessment that could be affected by different geographical origin. In the present work, volatile components and aroma characters of Molixiang table grapes planted in three different areas of China were analyzed using HS-GC-IMS and sensory evaluation method. The GC-IMS spectrogram revealed great differences among the three tested grape samples including MLX-A from Ningbo, MLX-B from Beizhen, and MLX-C from Zhangzhou. GC-IMS results revealed that a total of 50 compounds had been detected and 36 volatile compounds been identified in Molixiang grapes, including 12 esters, 10 alcohols, 6 aldehydes, 2 ketones, and several other volatiles. The PCA results indicated that differences in volatiles among the samples from different origin areas were evident. Therefore, GC-IMS combined with PCA was an efficient and alternative method for geographical differentiation of Molixiang table grapes. Furthermore, sensory analysis revealed that MLX-A had more green and pungent notes (P ≤ 0.05) that mainly attributed to the high contained concentration of E-2-octenal (not detected in MLX-B and MLX-C). MLX-B had more sweet and woody aroma (P ≤ 0.05), which probably due in large part to the high level presence of styrene (not detected in MLX-A and MLX-C grapes). MLX-C had relatively more pronounced nutty aroma (P ≤ 0.05) that potentially caused by benzaldehyde (not detected in MLX-A and MLX-B). Hence, these compounds could probably be used as geographical marker compounds to determine the origin of Molixiang table grapes. However, further investigation should be undertaken to explore the mechanism of variation in aroma volatiles accumulation under different geographical origins.
CRediT authorship contribution statement
Tao Feng: Methodology, Supervision, Funding acquisition, Project administration. Jiaqing Sun: Investigation, Formal analysis, Writing – review & editing. Shiqing Song: Investigation, Formal analysis. Huatian Wang: Supervision, Writing – review & editing. Lingyun Yao: Supervision, Conceptualization, Methodology, Writing – review & editing. Min Sun: Methodology, Supervision, Funding acquisition. Kai Wang: Methodology, Supervision. Da Chen: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Key Laboratory of cigarette flavoring Technology in Tobacco Industry (TX2018001) and Shanghai Gaofeng & Gaoyuan Project for University Academic Program Development (1021GN203004005).
References
- Anastasiou E., Balafoutis A., Darra N., Psiroukis V., Biniari A., Xanthopoulos G., Fountas S. Satellite and proximal sensing to estimate the yield and quality of table grapes. Agriculture. 2018;8(7):94. doi: 10.3390/agriculture8070094. [DOI] [Google Scholar]
- Chen Y., Li P., Liao L., Qin Y., Jiang L., Liu Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130055. [DOI] [PubMed] [Google Scholar]
- Colombo, F., Lorenzo, C, D., Regazzoni, L., Fumagalli, M., Sangiovanni, E., Peres de Sousa, L., Bavaresco, L., Tomasi, D., Bosso, A., Aldini, G., Restani, P., & Mario Dell'Agli, M. (2019). Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food & Function, 10(4), 1797-1807. doi: 10.1039/C8FO02175A. [DOI] [PubMed]
- Des Gachons C.P., Leeuwen C.V., Tominaga T., Soyer J.P., Gaudillère J.P., Dubourdieu D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. Journal of the Science of Food and Agriculture. 2005;85(1):73–85. doi: 10.1002/jsfa.1919. [DOI] [Google Scholar]
- Dunlevy J., Kalua C., Keyzers R., Boss P. In: Grapevine Molecular Physiology & Biotechnology. Roubelakis-Angelakis K.A., editor. Springer; Dordrecht: 2009. The production of flavour & aroma compounds in grape berries; pp. 293–340. [DOI] [Google Scholar]
- Feng S., Huang M., Crane J.H., Wang Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.) Journal of Food and Drug Analysis. 2018;26(2):497–503. doi: 10.1016/j.jfda.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D., Carrapeiro M.M.d., Fogliano V., Ruth S.M.v. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: A review. Trends in Food Science & Technology. 2016;52:31–48. doi: 10.1016/j.tifs.2016.03.013. [DOI] [Google Scholar]
- Gu S., Zhang J., Wang J., Wang X., Du D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. Trends in Analytical Chemistry. 2021;144 doi: 10.1016/j.trac.2021.116435. [DOI] [Google Scholar]
- Guo S., Zhao X., Ma Y., Wang Y., Wang D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130939. [DOI] [PubMed] [Google Scholar]
- Hanif M., Xie B., Wei S., Li J., Gao C., Wang R., Brestic M. Characterization of the volatile profile from six different varieties of Chinese chives by HS-SPME/GC–MS coupled with E. NOSE. Journal of King Saud University-Science. 2022;34(4) doi: 10.1016/j.jksus.2022.101971. [DOI] [Google Scholar]
- Hampson C.R., Quamme H.A., Hall J.W., MacDonald R.A., King M.C., Cliff M.A. Sensory evaluation as a selection tool in apple breeding. Euphytica. 2000;111(2):79–90. doi: 10.1023/A:1003769304778. [DOI] [Google Scholar]
- Hasanaliyeva G., Chatzidimitrou E., Wang J., Baranski M., Volakakis N., Seal C.…Rempelos L. Effects of production region, production systems and grape type/variety on nutritional quality parameters of table grapes; Results from a UK retail survey. Foods. 2020;9(12):1874. doi: 10.3390/foods9121874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Yang R., Zhang H., Wang S., Chen D., Lin S. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chemistry. 2019;290:32–39. doi: 10.1016/j.foodchem.2019.03.124. [DOI] [PubMed] [Google Scholar]
- Liu, P., Zheng, P., cheng Feng, L., Gong, Z., Zheng, L., Gao, S., Wang, X., Ye, F., Huang, J., &Liu, Z. (2022). Dynamic changes in the aroma profile of Qingzhuan tea during its manufacture, Food Chemistry, 375, 131847. doi: 10.1016/j.foodchem.2021.131847. [DOI] [PubMed]
- Margraf T., Santos É.N.T., Andrade E.F.d., Ruth S.M.v., Granato D. Effects of geographical origin, variety and farming system on the chemical markers and in vitro antioxidant capacity of Brazilian purple grape juices. Food Research International. 2016;82:145–155. doi: 10.1016/j.foodres.2016.02.003. [DOI] [Google Scholar]
- Mukhopadhyay S., Majumdar G.C., Goswami T.K., Mishra H.N. Fuzzy logic (similarity analysis) approach for sensory evaluation of chhana podo. LWT – Food Science and Technology. 2013;53(1):204–210. doi: 10.1016/j.lwt.2013.01.013. [DOI] [Google Scholar]
- Perestrelo R., Barros A.S., Rocha S.M., Câmara J.S. Establishment of the varietal profile of Vitis vinifera L. grape varieties from different geographical regions based on HS-SPME/GC–qMS combined with chemometric tools. Microchemical Journal. 2014;116:107–117. doi: 10.1016/j.microc.2014.04.010. [DOI] [Google Scholar]
- Pezzuto J.M. Grapes and human health: A perspective. Journal of Agricultural and Food Chemistry. 2008;56(16):6777–6784. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- Pu D., Duan W., Huang Y., Zhang Y., Sun B., Ren F.…Tang Y. Characterization of the key odorants contributing to retronasal olfaction during bread consumption. Food Chemistry. 2020;318 doi: 10.1016/j.foodchem.2020.126520. [DOI] [PubMed] [Google Scholar]
- Rodrigues S.R., Laranjo M., Coelho R., Martins P., Rato A.E., Vaz M.…Agulheiro-Santos A.C. Terroir influence on quality of ‘Crimson’ table grapes. Scientia Horticulturae. 2019;245:244–249. doi: 10.1016/j.scienta.2018.10.035. [DOI] [Google Scholar]
- Rolle L., Giacosa S., Gerbi V., Bertolino M., Novello V. Varietal comparison of the chemical, physical, and mechanical properties of five colored table grapes. International Journal of Food Properties. 2013;16(3):598–612. doi: 10.1080/10942912.2011.558231. [DOI] [Google Scholar]
- Sabra A., Netticadan T., Wijekoon C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoticha J., Jara-Palacios M.J., Hernández-Hierro J.M., Heredia F.J., Wojdyło A. Phenolic compounds and antioxidant activity of twelve grape cultivars measured by chemical and electrochemical methods. European Food Research and Technology. 2018;244(11):1933–1943. doi: 10.1007/s00217-018-3105-5. [DOI] [Google Scholar]
- Sato M., Ikram M.M.M., Pranamuda H., Agusta W., Putri S.P., Fukusaki E. Characterization of five Indonesian mangoes using gas chromatography–mass spectrometry-based metabolic profiling and sensory evaluation. Journal of Bioscience and Bioengineering. 2021;132(6):613–620. doi: 10.1016/j.jbiosc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- Song J., Chen Q., Bi J., Meng X., Wu X., Qiao Y., Lyu Y. GC/MS coupled with MOS e-nose and flash GC e-nose for volatile characterization of Chinese jujubes as affected by different drying methods. Food Chemistry. 2020;331 doi: 10.1016/j.foodchem.2020.127201. [DOI] [PubMed] [Google Scholar]
- Tyagi K., Maoz I., Vinokur Y., Rodov V., Lewinsohn E., Lichter A. Enhancement of table grape flavor by postharvest application of monoterpenes in modified atmosphere. Postharvest Biology and Technology. 2020;159 doi: 10.1016/j.postharvbio.2019.111018. [DOI] [Google Scholar]
- Wang S., Chen H., Sun B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS) Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xiao Q., Zhuang J., Feng T., Ho C.-T., Song S. Characterization of aroma-active compounds in four yeast extracts using instrumental and sensory techniques. Journal of Agricultural and Food Chemistry. 2020;68(1):267–278. doi: 10.1021/acs.jafc.9b06751. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang W., Duan S., Song S., Xu W., Zhang C.…Wang S. In-depth aroma and sensory profiling of unfamiliar table-grape cultivars. Molecules. 2018;23(7):1703. doi: 10.3390/molecules23071703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhang W., Yu W., Zhao L., Song S., Xu W.…Wang S. Study on the volatile composition of table grapes of three aroma types. LWT – Food Science and Technology. 2019;115 doi: 10.1016/j.lwt.2019.108450. [DOI] [Google Scholar]
- Xi X., Zha Q., He Y., Tian Y., Jiang A. Influence of cluster thinning and girdling on aroma composition in ‘Jumeigui’ table grape. Scientific Reports. 2020;10(1):6877. doi: 10.1038/s41598-020-63826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Zhang Y., Cao L., Lu J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chemistry. 2010;119(4):1557–1565. doi: 10.1016/j.foodchem.2009.09.042. [DOI] [Google Scholar]
- Yang C., Wang Y., Liang Z., Fan P., Wu B., Yang L.…Li S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC–MS. Food Chemistry. 2009;114(3):1106–1114. doi: 10.1016/j.foodchem.2008.10.061. [DOI] [Google Scholar]
- Yang C., Wang Y., Wu B., Fang J., Li S. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chemistry. 2011;128(4):823–830. doi: 10.1016/j.foodchem.2010.11.029. [DOI] [Google Scholar]
- Yang M., Luo Z., Gao S., Belwal T., Wang L., Qi M.…Li L. The chemical composition and potential role of epicuticular and intracuticular wax in four cultivars of table grapes. Postharvest Biology and Technology. 2021;173 doi: 10.1016/j.postharvbio.2020.111430. [DOI] [Google Scholar]
- Yang Y., Wang B., Fu Y., Shi Y., Chen F., Guan H.…Zhang N. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128880. [DOI] [PubMed] [Google Scholar]
- Yao H., Jin X., Feng M., Xu G., Zhang P., Fang Y.…Meng J. Evolution of volatile profile and aroma potential of table grape Hutai-8 during berry ripening. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110330. [DOI] [PubMed] [Google Scholar]
- Yue Q., Zhang C., Wang Q., Wang W., Wang J., Wu Y. Analysis on genetic diversity of 51 Grape germplasm resources. Ciência Rural. 2019;49(11) doi: 10.1590/0103-8478cr20190247. [DOI] [Google Scholar]
- Zhang C., Wang Y., Ding D., Su J., Zhao Z. Volatile profiles of Allium tenuissimum L. flower fried by four different oils, using SPME–GC–MS, and sensory evaluation coupled with partial least squares regression. Journal of Food Composition and Analysis. 2022;109 doi: 10.1016/j.jfca.2022.104461. [DOI] [Google Scholar]
- Zhang X., Dai Z., Fan X., Liu M., Ma J., Shang W.…Zhou Z. A study on volatile metabolites screening by HS-SPME-GC-MS and HS-GC-IMS for discrimination and characterization of white and yellowed rice. Cereal Chemistry. 2020;97(2):496–504. doi: 10.1002/cche.10264. [DOI] [Google Scholar]