Highlights
-
•
E-nose and GC–MS could distinguish the different pumpkin based on aroma profiles and volatile compounds.
-
•
It’s the first time to study the key volatile compound associated with ‘taro-like’ aroma of pumpkin fruit.
-
•
2-Acetyl-1-pyrroline is the key contributor to the ‘taro-like’ aroma of pumpkin fruit.
Keywords: Pumpkin, ‘taro-like’ aroma, Volatile compounds, E-nose, GC–MS, GC-O, 2-Acetyl-1-pyrroline
Abstract
‘Taro-like’ aroma is a pleasant flavor and value-added trait in pumpkin species imparted by unknown key volatile compounds. In this study, we used the electronic nose (E-nose), gas chromatography-mass spectrometry (GC–MS), and GC-Olfactometry (GC-O) to study the aroma profile, volatile compounds, and key contributors, respectively. By E-nose and GC–MS, we found significant differences in the aroma profiles and volatile compounds between fruits from five samples with/without ‘taro-like’ aroma. According to the analysis of differential volatile compounds obtained from GC–MS and the GC-O analysis of the sample with ‘taro-like’ aroma, we found that 2-acetyl-1-pyrroline representing the ‘taro’ odor was only identified in the sample with ‘taro-like’ aroma. Therefore, we conclude that 2-acetyl-1-pyrroline is the key contributor to the 'taro-like' aroma. Moreover, the relationship between 2-acetyl-1-pyrroline and ‘taro-like’ aroma was further verified via other pumpkin samples. Our results provide a theoretical basis for understanding the aroma characteristics of pumpkin fruit.
Introduction
Vegetable quality includes appearance and intrinsic quality, with aroma being the core of intrinsic quality for good flavor (Tieman et al., 2017, Vogel et al., 2010). Moreover, aroma, an important value-added trait imparted by volatile compounds, and their absence can significantly change consumption preference (Vogel et al., 2010). The research on the aroma of vegetables will consolidate the theoretical basis for the quality of vegetables. Therefore, it is of great significance to carry out identification of volatile compounds behind the different aroma of vegetables.
Pumpkin is a widely cultivated and consumed vegetable worldwide. In recent years, the quality of pumpkin fruit has been attracting increasing attention and is being studied extensively (Abbas et al., 2020, Torkova et al., 2018). However, the aroma quality of pumpkin has largely been ignored due to its complexity and the lack of germplasm resources with unique aroma, thereby making aroma an unfeasible trait for most breeding programs. Currently, for the study of aroma, technologies include electronic nose (E-nose), gas chromatography-mass spectrometry (GC–MS), GC-Olfactometry (GC-O), etc., which can comprehensively evaluate the aroma and identify the volatile compounds (Huang et al., 2019, Niu et al., 2019, Wu et al., 2021). To date, few studies have reported on the volatile compounds of pumpkin and its processed products (Bowman and Barringer, 2012, Leffingwell et al., 2015, Poehlmann and Schieberle, 2013, Zhou et al., 2017). Poehlmann and Schieberle (2013) found 47 odor-active compounds in Styrian Pumpkin Seed Oil, among which 2-acetyl-1-pyrroline showed the highest flavor dilution (FD) factors. Furthermore, previous studies have also found different germplasms with unique key volatile compounds. Forty volatile compounds were identified in the Cucurbita moschata pulp by solid phase microextraction (SPME) – GC–MS, of which eucalyptol, ethanol, and 2-heptanol were the most important compounds (Zhou, Mi, Hu, & Zhang, 2017). However, 2-propanol and dimethyl disulfide showed significant quantities in the Cucurbita pepo pulp via dynamic headspace GC–MS analyses (Leffingwell, Alford, & Leffingwell, 2015). Thus, the composition and contents of volatile compounds, along with the key volatile compounds varied greatly due to the diverse tested samples and aromatic traits.
A new pumpkin cultivar possessing ‘taro-like’ aroma is attracting increasing attention, since most commercial varieties lack this rare aroma. Thus, this aroma is a potential value-added trait for pumpkin quality. However, the use of this aroma is rare in pumpkin caused by the insufficient studies to date. For the in-depth understanding of this aroma, the overall profile of volatile compounds, key volatile compounds, needs to be identified. To the best of our knowledge, very few reports are available on the ‘taro-like’ aroma. The major volatile compounds in taro corms were palmitic acid and linoleic acid, and both 2-acetyl-1-pyrroline and 1-pyrroline were identified in taro volatile compounds (Wong, Chong, & Chee, 1998).
For a comprehensive analysis of the ‘taro-like’ aroma, it is essential to explore the volatile compounds behind this aroma, which is also important for the subsequent pumpkin quality breeding program, especially flavor breeding. In this study, we used fruits from five samples with and without ‘taro-like’ aroma. First, we performed a comparative analysis of the aroma profile using the E-nose. Second, we identified the composition and content of volatile compounds via GC–MS, and then we screened the differential volatile compounds for the key candidate volatile compounds responsible for the ‘taro-like’ aroma. Moreover, we applied GC-O to further determine the key aroma contributor, and verified its relationship with the ‘taro-like’ aroma using other pumpkin samples. Our study could enable the systematic analysis of ‘taro-like’ aroma, obtain the key aroma contributor, and provide a foundation to explore the metabolic basis of the ‘taro-like’ aroma.
Materials and methods
Plant materials
Five types of C. moschata samples, including NO. 44 with the ‘taro-like’ aroma and four other samples (NO. 45, NO. 301, NO. 326, and NO. 335) without the ‘taro-like’ aroma, were used for the E-nose and GC–MS analyses (Fig. S1). NO. 44 was used for the GC-O analysis. NO. 44 is an important germplasm obtained by the 13th generation selfing, and its origin is widely used in the fragrance-focused breeding of pumpkin in China. An additional 12 kinds of C. moschata samples were selected to verify the relationship between the key volatile compounds and the ‘taro-like’ aroma. The aroma characteristics of all the 17 samples of pumpkin were evaluated by a panel of 10 professional researchers (five male and five female breeders for vegetables), which helped to correctly judge the aroma characteristics with or without ‘taro-like’ aroma. All the samples were inbred lines with stable traits and these plants were grown under the same cultivation conditions in the fields of Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China. Mature fruits (45 days after pollination) were harvested for the E-nose, GC–MS, GC-O, and verification analyses. The fruits were sliced and snap frozen in liquid nitrogen and then maintained at −80 °C. Then, the samples were freeze-dried in a vacuum and ground to a fine powder.
Chemicals
C7-C40 saturated alkane mixture, hexanal, (E)-2-hexenal, 1-hexanol, 1-octen-3-ol, β-myrcene, octanal, (E,E)-2,4-heptadienal, benzeneacetaldehyde, nonanal, (E,Z)-2,6-nonadienal, 2,6,6-trimethyl-2-cyclohexene-1,4-dione, decanal, α-ionone, and β-ionone were purchased from Sigma-Aldrich Company ltd., USA. Benzaldehyde and benzyl alcohol were from Aladdin Bio-Chem Technology Co., ltd, Shanghai, China. 2-acetyl-1-pyrroline was purchased from TRC (Canada).
Electronic nose analysis
We carried out the E-nose analysis using a PEN 3 E-nose device (Winmuster Airsense Analytics Inc., Schwerin, Germany). Briefly, 10 g powder of each sample was accurately weighed in 100 mL sealed glass vials, and equilibrated at room temperature for 1.5 h prior to analysis. The sampling parameters were set as: sampling time interval – 1 s, sensor automatic cleaning time – 100 s, sensor return to zero time – 3 s, analysis sampling time – 100 s, sample preparation time – 3 s, and sample intake flow rate – 190 mL/min. Each sample was analyzed five times. Data processing and linear discriminant analysis (LDA) were carried out using the Winmuster E-nose software. And the radar fingerprint chart of the aroma profile was performed using SPSS v17 (SPSS Inc., Chicago, IL, USA).
Extraction of volatile compounds
The volatile compounds were extracted via the HS-SPME technique. Three biological replicates derived from an individual plant were conducted. One gram sample was accurately placed in a 20 mL vial. Subsequently, internal standard of 1 μL 3-nonanone (0.04 μg/μL, chromatographic ethanol as solvent) was added into the vial. The vial was then sealed immediately and equilibrated for 2 min in the 70℃ water bath. A SPME fiber with Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS)-50/30 μm purchased from Supelco was used for extraction. The extraction was carried out at 70℃ for 35 min.
GC–MS analysis
We used a 7890B GC tograph with a 7000D triple quadrupole GC/MS (Agilent Technologies, USA) to carry out the analysis of volatile compounds. The desorption and GC–MS conditions were carried out according to the previous study (Qi et al., 2018).
Data processing and identification of volatile compounds
The raw GC–MS data was deconvoluted by the Masshunter Agilent Qualitative Analysis software (B.07.04, Agilent Technologies, USA). Next, the volatile compounds were identified using the previous reported methods (Ma et al., 2018, Qi et al., 2018). Seventeen available reference standards were selected for further identification. The relative content of the volatile compound was determined with a method related the peak areas of volatile compounds to that of the internal standard. The relative content was expressed as the means ± standard deviations (n = 3). To eliminate the invalid volatile compounds, all the identified volatile compounds were filtered using the following parameters: frequency values (occurs among >60% of replicates in one sample) and coefficient of variation (≤25%). Then, the unsupervised principal component analysis (PCA) and cluster analysis were performed to visualize the distinction among the different samples based on the above filtered compounds using the MetaboAnalyst tool (version 5.0, https://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml). Finally, to obtain the significantly different volatile compounds among the five samples, the one-way analysis of variance (ANOVA) and fold change (p < 0.01, FC ≥ 2) were applied based on the identified compounds using SPSS v17 (SPSS Inc., Chicago, IL, USA), and NO. 44 was selected as a reference. Moreover, the hierarchical cluster analysis (HCA) and Venn diagrams were analyzed using the final unique volatile compounds. HCA and Venn diagrams were established using MetaboAnalyst and UpSetR (Version 1.4.0), respectively.
GC-Olfactometry analysis
The key volatile compounds were identified with a GC–MS (QP2010, Shimadzu, Japan) coupled with an olfactory port OP 275 (GL Sciences, Japan) using the fruit of pumpkin sample NO. 44 with ‘taro-like’ aroma. The HS-SPME and GC–MS conditions were the same as described above. The experienced assessors from the Jiangnan University were employed for this experiment. First, the undiluted volatile compounds were analyzed to detect all odor impressions. Then, aroma extract dilution analysis (AEDA) was executed using the four stepwise dilution series 1:3, 1:9, 1:27, and 1:81 via split mode. The retention time, odor quality, and odor intensities of the volatile compounds were obtained. The odor intensities were evaluated on a scale of 0–3, in which 0, 1, 2, and 3 represented not detected, weak intensity, moderate intensity, and strong intensity, respectively. Aromatic volatile compounds with the highest dilution were selected as the key volatile compounds.
Results and discussion
A new pumpkin cultivar presents a unique ‘taro-like’ aroma. However, no previous report has systematically and comprehensively studied the volatile compounds of the ‘taro-like’ aroma till date in pumpkin. Therefore, to understand this aroma, we performed the combined aroma profile and volatile compounds analyses.
Aroma profile differences between samples using E-nose analysis
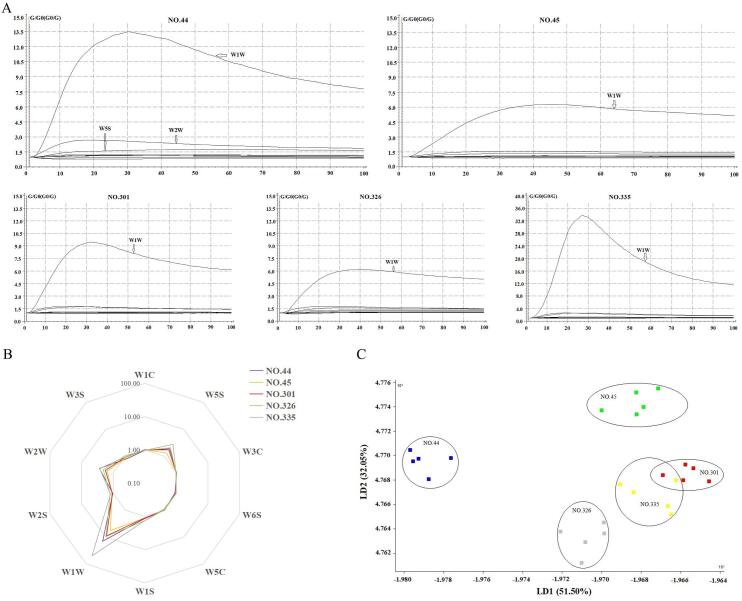
To accurately evaluate the aroma profile of the pumpkin, we used the E-nose, which is widely used in aroma recognition and differentiation, since it can acquire the overall profile of the volatile compounds but no detailed information of the composition or content of volatile compounds (Wu et al., 2021). Nevertheless, its application in the pumpkin has hardly been reported. A previous study found that E-nose could distinguish three different pumpkin species due to the differences of volatile compounds (Zhou, Mi, Hu, & Zhang, 2017). However, the authors did not show the response data of the E-nose sensors in detail. During E-nose analysis, the response value of each sensor with the changing time could be observed intuitively (Huang et al., 2019). In our study, the E-nose responses of different five samples displayed different response curves with all five samples reaching equilibrium within 80–100 s (Fig. 1A). The different odor intensities among five pumpkin samples were observed, because of the similar changes of signal variation trends except the intensity (Huang et al., 2019). For all five samples, the response values of W1W (sensitive to many terpenes and organosulfur compounds) significantly increased, and those of W5S (mainly sensitive to nitrous oxides) and W2W (sensitive to aromatics and organic sulfides) increased slightly, while those of the remaining sensors changed slightly. Moreover, for five samples, the signals of W2W and W5S increased to a high value in the first 20 s except for W5S of NO.44 with 30–40 s, and the signals of W1W increased to a high value in 25–50 s due to the different samples, which suggested that aromatics and organic sulfides, nitrous oxides, terpenes, organosulfur compounds may contribute more to the aroma profile of pumpkin. In the NO. 44, NO. 45, and NO. 326 samples, W1W displayed the highest response value, followed by W2W and W5S. In the NO. 301 and NO. 335 samples, the response value was high to low for W1W, W5S, and W2W, respectively. Moreover, among the above five different samples, W1W showed a higher response value. As per the radar chart shown in Fig. 1B, the response values of the sensors displayed differences among the five samples, which indicated that different aroma profiles were present in the five pumpkin samples. The response of six sensors suggested that pumpkin fruit may produce terpenes, organosulfur compounds, nitrous oxides, aromatics, organic sulfides, alcohols, and aliphatic compounds. We verified the results shown here using the identification and quantification of volatile compounds via GC–MS analysis.
Fig. 1.
The analyses of E-nose data: (A) The typical E-nose response curves of the five samples. (B) Radar fingerprint chart of the overall aroma profile in five different pumpkin samples. (C) Linear discriminant analysis (LDA) of the E-nose data for the different pumpkin samples.
We performed LDA to effectively distinguish between the samples via aroma characteristics based on the E-nose response, which would help us to understand the overall aroma profiles of the five different samples. The accumulative variance of the first two LD factors reached 83.55% (>80%) (Fig. 1C), which means the two LD factors cover the vast majority of the aroma information (Lan et al., 2021). LD1 and LD2 represented 51.50% and 32.05% of the total variance, respectively (Fig. 1C). Therefore, we used the LD1 as the main linear discriminant factor to distinguish the differences among the overall aroma profile. Along with LD1, we located the NO. 44 with the ‘taro-like’ aroma on the lower left side of the plot, while we found the other four samples without ‘taro-like’ aroma gathered in a different area on the right side. Moreover, good repeatability of the five replicates of each sample was also observed. Therefore, these results illuminated that the differing aroma profiles of five pumpkin samples could be effectively distinguished by E-nose. Although there was some overlap between the NO. 301 and NO. 335 samples, we observed some differences. In conclusion, these results illuminated that the overall aroma of different pumpkin fruits differed, and could be distinguished effectively by the E-nose.
Identification of volatile compounds and chemometric analysis
To obtain the different volatile compounds for the different aroma profiles mentioned above, we investigated the compositions and contents of the aromatic volatile compounds in the five samples using GC–MS. We identified a total of 53 volatile compounds in the five samples (Table 1). We identified 42, 39, 45, 45, and 40 volatile compounds in NO. 44, NO. 45, NO. 301, NO. 326, and NO. 335, respectively. We found that samples differed in their composition and content of the volatile compounds. Moreover, among the volatile compounds in the five samples, we found that 16, 16, 19, 19, and 17 were hydrocarbons; 9, 9, 12, 11, and 12 were aldehydes; 9, 7, 7, 6, and 6 were ketones; 5, 4, 5, 5, and 4 were alcohols; 1, 2, 1, 3, and 0 were esters; and 2, 1, 1, 1, and 1 were the other compounds (Table S1). In the previous study, alcohols, aldehydes, and hydrocarbons were the most important volatile compounds of pumpkin fruit (García-Parra, González-Cebrino, & Ramírez, 2020). However, in this study, the hydrocarbons, aldehydes, and ketones were the main volatile compounds of fruits from five samples (Table 1). Additionally, (E)-2-hexenal, benzyl alcohol, (E,Z)-3,6-nonadien-1-ol, 1,2,4-trimethyl-benzene, and 1,2,4-trimethyl-benzene showed the highest content in NO. 44, NO. 45, NO. 301, NO. 326, and NO. 335, respectively (Table 1), which differed from previous studies (Leffingwell et al., 2015, Zhou et al., 2017). The different identified volatile compounds might be due to the different tested samples, the high pressure thermal treatment, the condition of extraction of volatile compounds, GC–MS instrument, and the model of capillary column. For example, distinct volatile compounds were detected in different pumpkin seed oil (Poehlmann and Schieberle, 2013, Procida et al., 2013).
Table 1.
Volatile compounds identified in the fruits of five pumpkin samples.
| Volatiles | Retention Index (RI) |
Content (ng/g) |
||||||
|---|---|---|---|---|---|---|---|---|
| RI-practical | RI-NIST 14 Library a | RI-STD b | NO.44 | NO.45 | NO.301 | NO.326 | NO.335 | |
| Pyridine | 741 | 746 | 5.62 ± 0.62 | 7.40 ± 1.01 | 11.25 ± 0.82 | 12.65 ± 0.87 | 15.21 ± 3.80 | |
| (Z)-2-Penten-1-ol | 763 | 767 | 1.53 ± 0.29 | – | 2.11 ± 0.11 | 3.51 ± 0.33 | 2.65 ± 0.16 | |
| Toluene | 765 | 763 | 35.64 ± 3.33 | 20.49 ± 2.75 | 38.68 ± 9.79 | 31.46 ± 7.17 | 40.20 ± 27.88 | |
| Hexanal | 800 | 800 | 797 | 78.72 ± 6.85 | 7.09 ± 1.21 | 23.86 ± 0.46 | 11.55 ± 1.68 | 18.27 ± 1.11 |
| (E)-2-Hexenal | 852 | 854 | 852 | 103.17 ± 14.76 | 15.52 ± 3.75 | 75.85 ± 2.62 | 6.49 ± 1.18 | 81.08 ± 4.49 |
| 1-Hexanol | 865 | 868 | 864 | 58.47 ± 13.85 | 2.30 ± 0.36 | 11.00 ± 0.07 | 7.35 ± 0.77 | 11.18 ± 0.82 |
| 1,3-Dimethyl-benzene | 871 | 866 | 44.39 ± 8.02 | 25.14 ± 3.08 | 64.91 ± 8.12 | 44.03 ± 5.43 | – | |
| Nonane | 900 | 900 | 1.60 ± 0.38 | – | 2.61 ± 0.63 | 2.44 ± 0.12 | 6.68 ± 1.42 | |
| Heptanal | 901 | 901 | – | – | 2.29 ± 0.20 | 1.83 ± 0.42 | – | |
| Methional | 907 | 907 | 2.58 ± 1.12 | 6.50 ± 1.46 | 3.59 ± 0.17 | 2.93 ± 0.44 | 43.24 ± 4.35 | |
| 2-Acetyl-1-pyrroline | 921 | 922 | 920 | 46.44 ± 4.04 | – | – | – | – |
| 1-Methylethyl-benzene | 926 | 921 | 1.69 ± 0.41 | 1.06 ± 0.15 | 2.84 ± 0.28 | 2.91 ± 0.61 | 6.56 ± 1.44 | |
| Propyl-benzene | 956 | 953 | 8.03 ± 0.85 | 5.28 ± 0.98 | 13.16 ± 2.52 | 13.34 ± 3.02 | 27.96 ± 6.88 | |
| 4-Methyl-nonane | 961 | 961 | – | 0.66 ± 0.15 | 1.42 ± 0.26 | 1.07 ± 0.16 | 3.28 ± 0.63 | |
| Benzaldehyde | 965 | 962 | 966 | 77.60 ± 14.20 | 100.40 ± 22.48 | 117.53 ± 11.29 | 80.42 ± 13.48 | 136.38 ± 16.23 |
| 1-Octen-3-ol | 979 | 980 | 983 | 16.09 ± 3.58 | 13.78 ± 2.00 | 14.45 ± 0.56 | 8.71 ± 0.75 | 59.83 ± 2.17 |
| 6-Methyl-5-hepten-2-one | 983 | 986 | 4.66 ± 0.80 | 3.08 ± 0.69 | 4.78 ± 0.03 | 3.09 ± 0.12 | 6.42 ± 0.87 | |
| β-Myrcene | 990 | 991 | 985 | 1.47 ± 0.13 | – | – | – | – |
| 1,2,4-Trimethyl-benzene | 997 | 990 | 65.90 ± 14.47 | 56.53 ± 13.22 | 147.39 ± 17.75 | 150.64 ± 20.54 | 280.57 ± 33.77 | |
| Decane | 1000 | 1000 | 2.96 ± 0.55 | 2.07 ± 0.51 | 5.17 ± 0.90 | 6.30 ± 1.05 | 14.05 ± 0.31 | |
| Octanal | 1003 | 1003 | 1007 | 0.71 ± 0.05 | 0.80 ± 0.06 | 7.71 ± 0.24 | 2.30 ± 0.26 | 4.80 ± 0.22 |
| (E, E)-2,4-Heptadienal | 1010 | 1012 | 1014 | 1.39 ± 0.16 | 0.75 ± 0.01 | 4.18 ± 0.21 | 1.78 ± 0.13 | 3.77 ± 0.26 |
| Benzyl alcohol | 1036 | 1036 | 1041 | 87.34 ± 12.60 | 107.38 ± 2.63 | 119.46 ± 23.28 | 55.62 ± 5.50 | 120.44 ± 10.01 |
| 3,5,5-Trimethyl-3-cyclohexen-1-one | 1044 | 1044 | 2.30 ± 0.57 | 0.41 ± 0.10 | 1.17 ± 0.02 | – | 1.32 ± 0.13 | |
| Benzeneacetaldehyde | 1047 | 1045 | 1048 | 7.78 ± 1.03 | 28.03 ± 6.43 | 15.57 ± 0.87 | 10.75 ± 1.20 | 161.43 ± 14.51 |
| 1,4-Diethyl-benzene | 1050 | 1041 | 3.21 ± 0.73 | 3.22 ± 0.49 | 7.95 ± 1.67 | 8.65 ± 1.07 | 17.90 ± 1.64 | |
| 1-(1H-pyrrol-2-yl)-ethanone | 1059 | 1064 | 2.44 ± 0.18 | – | – | – | – | |
| 2-Methyl-decane | 1065 | 1064 | – | – | 1.68 ± 0.08 | 1.82 ± 0.26 | 4.74 ± 1.14 | |
| 2-Pyrrolidinone | 1067 | 1076 | 4.99 ± 1.02 | – | – | – | – | |
| p-Aminotoluene | 1068 | 1072 | – | – | 21.06 ± 3.08 | 11.30 ± 1.94 | 16.31 ± 3.40 | |
| (E,E)-3,5-Octadien-2-one | 1069 | 1073 | 10.68 ± 1.96 | 5.35 ± 0.33 | 10.76 ± 0.28 | 8.61 ± 0.40 | 15.20 ± 1.04 | |
| 1-Ethyl-2,4-dimethyl-benzene | 1081 | 1075 | 1.91 ± 0.07 | 2.46 ± 0.25 | 12.55 ± 2.99 | 13.89 ± 0.96 | 29.30 ± 5.75 | |
| Undecane | 1100 | 1100 | 23.85 ± 4.71 | 17.39 ± 2.52 | 23.93 ± 0.62 | 23.47 ± 2.25 | 32.50 ± 1.06 | |
| Nonanal | 1105 | 1104 | 1100 | 7.70 ± 0.15 | 8.38 ± 0.04 | 48.38 ± 1.80 | 18.41 ± 1.81 | 45.84 ± 0.41 |
| 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | 1145 | 1144 | 1152 | 2.39 ± 0.36 | 2.04 ± 0.10 | – | 2.63 ± 0.28 | 2.06 ± 0.02 |
| (E, Z)-2,6-Nonadienal | 1152 | 1155 | 1162 | 8.70 ± 1.86 | – | 47.23 ± 2.52 | – | 5.80 ± 0.46 |
| (E,Z)-3,6-Nonadien-1-ol | 1156 | 1156 | 9.12 ± 0.51 | 12.29 ± 2.73 | 155.66 ± 10.16 | 20.65 ± 2.14 | – | |
| Acetic acid, phenylmethyl ester | 1164 | 1164 | – | 0.89 ± 0.06 | – | 1.42 ± 0.12 | – | |
| Octanoic acid, ethyl ester | 1194 | 1196 | 4.06 ± 0.59 | 4.33 ± 0.88 | 6.88 ± 0.56 | 10.80 ± 1.11 | – | |
| Decanal | 1207 | 1206 | 1204 | 3.09 ± 0.18 | 4.21 ± 0.92 | 11.34 ± 0.27 | 4.56 ± 0.54 | 8.72 ± 0.80 |
| 2,6,6-Trimethyl-1-cyclohexene-1-acetaldehyde | 1263 | 1254 | – | – | 1.99 ± 0.13 | – | 0.84 ± 0.03 | |
| 3-Ethyl-undecane | 1265 | 1260 | 0.93 ± 0.06 | 0.85 ± 0.07 | 1.55 ± 0.38 | 1.43 ± 0.23 | – | |
| α-Ethylidene-benzeneacetaldehyde | 1273 | 1279 | – | – | – | 2.07 ± 0.26 | 30.40 ± 4.43 | |
| 5-Methyl-tridecane | 1353 | 1348 | 3.83 ± 0.11 | 5.46 ± 1.12 | 8.70 ± 0.64 | 4.73 ± 0.07 | 4.43 ± 0.81 | |
| 3-Methyl-tridecane | 1371 | 1371 | 36.62 ± 3.22 | 37.24 ± 3.69 | 44.42 ± 2.53 | 39.94 ± 1.38 | 37.28 ± 4.45 | |
| α-Ionone | 1428 | 1426 | 1428 | 2.33 ± 0.01 | – | 14.85 ± 1.55 | 3.56 ± 0.45 | – |
| (E)-6,10-Dimethyl-5,9-undecadien-2-one | 1449 | 1453 | 1.55 ± 0.25 | 2.59 ± 0.53 | 11.88 ± 0.97 | 3.02 ± 0.47 | 4.60 ± 0.56 | |
| β-Ionone | 1485 | 1486 | 1486 | 3.78 ± 0.01 | 1.94 ± 0.01 | 12.50 ± 1.61 | 6.51 ± 0.98 | 7.22 ± 0.77 |
| 4-Ethyl-tetradecane | 1545 | 1548 | 1.75 ± 0.28 | 4.43 ± 0.89 | 4.63 ± 0.31 | 3.33 ± 0.26 | 4.01 ± 0.52 | |
| 3-Methyl-pentadecane | 1571 | 1570 | 9.02 ± 0.57 | 16.07 ± 2.43 | 19.19 ± 1.09 | 14.28 ± 1.07 | 15.93 ± 1.64 | |
| 3-Methyl-heptadecane | 1772 | 1770 | – | 1.59 ± 0.27 | 2.84 ± 0.10 | 1.37 ± 0.31 | 1.37 ± 0.05 | |
| 6,10,14-Trimethyl-2-pentadecanone | 1842 | 1844 | – | 0.62 ± 0.02 | 1.37 ± 0.25 | – | – | |
| Linoleic acid ethyl ester | 2159 | 2162 | – | – | – | 4.34 ± 0.10 | – | |
aRI-NIST 14 Library: the published retention index of compounds in NIST 14 library.
bRI-STD: retention index of standard substance analyzed on the same column was calculated using the homologous series of n-alkanes.
“–” Compounds were not detected in samples; or not fitted the filtrated parameters.
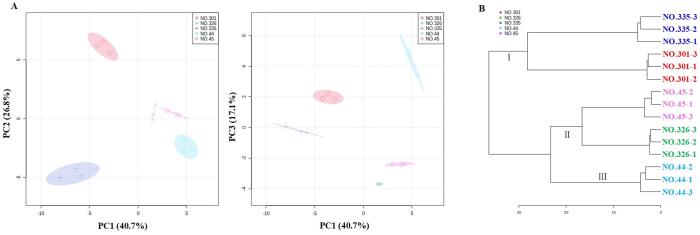
Then, we used the PCA and cluster analysis to obtain a preliminary overview of the differences and yet similarities between the five samples using the compounds listed in Table 1 (Fig. 2A). The first three principal components PC1, PC2, and PC3 explained 40.70%, 26.8%, and 17.10% of the variances, respectively. Generally, the PCs replaced the original dataset when they have >80% cumulated reliability of the original data (Ma et al., 2018). In this study, we observed an excellent separation of the five samples in Fig. 2A, and well separated NO. 44 and NO. 45, NO. 44 and NO. 301, NO. 44 and NO. 326, and NO. 44 and NO. 335 mainly based on PC2 and PC3, respectively, thus suggesting that the volatile compounds between these five samples were differentiated clearly due to the differences in their composition and contents. The cluster analysis indicated that we could divide the samples into three major categories (Fig. 2B). The category Ⅰ contained two samples, NO. 301 and NO. 335. The second major category was category Ⅱ including NO. 45 and NO. 326. Category III only displayed NO. 44 with the ‘taro-like’ aroma. Therefore, clear separation was observed based on the PCA and clustering results, which suggested that the GC–MS combined with chemometrics analysis was an accurate approach to distinguish the different pumpkin samples. Moreover, the cluster analysis result was highly consistent with the E-nose data and PCA result, and these GC–MS results helped in interpretation of these results for the E-nose data, with the differences of the overall aroma profile being caused by the different volatile compounds (Lan et al., 2021). The combined analyses of the E-nose and GC–MS data are essential for a better understanding of the aroma profile and volatile compounds (Huang et al., 2019, Lan et al., 2021, Niu et al., 2019).
Fig. 2.
Chemometric analyses for the GC–MS data. (A) Principal components analysis (PCA) of the volatile compounds obtained by GC–MS for different pumpkin samples; (B) The result of cluster analysis based on all identified volatile compounds from five pumpkin samples. Ⅰ– III: three major categories based on the volatile compounds.
Identification and verification of the key volatile compounds for the ‘taro-like’ aroma
The key to study aroma is to identify the crucial volatile compounds associated with it. Previous studies showed that only a few key odorants being involved in aroma perception, and the majority possibly not being key to a high odor impact (Huang et al., 2019). Thus, the identification of the key volatile compounds from the 42 volatile compounds of NO. 44 was crucial for evaluating the ‘taro-like’ aroma, which is commonly fulfilled by the GC-O and odor active value (OAV) methods (Niu et al., 2019, Poehlmann and Schieberle, 2013). OAV is defined as the ratio between the content of the compound and its odor threshold, which is vital for judging the role of a volatile compound for the aroma characteristic. Odor threshold refers to the minimum content of the volatile compound felt by the human senses in a certain medium. For an effective OAV analysis, the medium of the experimental sample should be similar to the medium of the reference threshold of the compound. For example, the calculation of OAV of volatile compound was performed in pumpkin seed oil based on the odor threshold from sunflower oil (Poehlmann & Schieberle, 2013). Most of the medium of the reported threshold of the compound is liquid, while the samples used in this study were freeze-dried powder. Thus, the results of the OAV analysis might not be accurate for this study. In this study, we screened and obtained the key candidate volatile compounds using the analysis of the differential volatile compounds between the five samples and the GC-O analysis of fruit from NO. 44.
Comparison of the differential volatile compounds
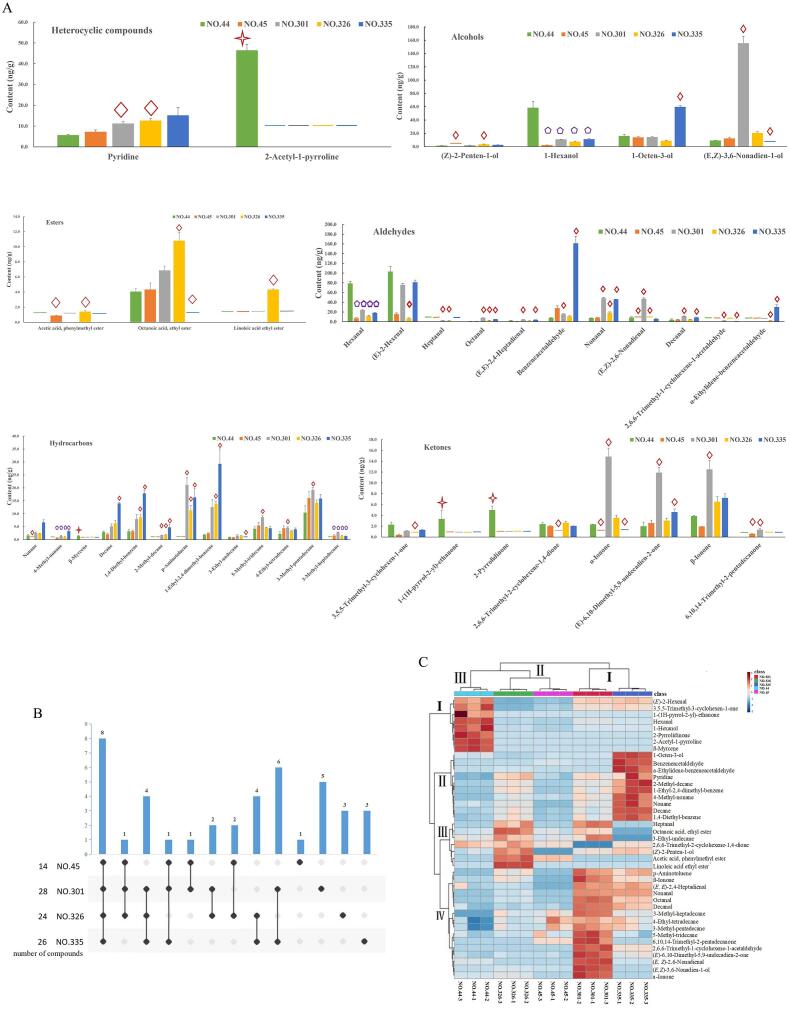
In previous studies, the contributors for the aroma profiles were obtained by the analysis of the differential volatile compounds (Chen et al., 2021, Qi et al., 2018). In our present study, we identified a total of 41 volatile compounds with significant differences (Fig. 3A). We detected 14, 28, 24, and 26 differential volatile compounds in the four different combinations, including NO. 44 vs NO. 45, NO. 44 vs NO. 301, NO. 44 vs NO. 326, and NO. 44 vs NO. 335, respectively (Fig. 3A and 3B). Since NO. 44 varies from the other four types of pumpkin samples due to their unique aroma characteristics, common differences of volatile compounds in different comparative combinations should be identified, which might help in the analysis of the ‘taro-like’ aroma. Therefore, to gain a more intuitive result of differential volatile compounds analysis, as illustrated by UpSet (Fig. 3B), we found that eight different volatile compounds, including 1-hexanol, hexanal, 2-acetyl-1-pyrroline, 4-methyl-nonane, β-myrcene, 3-methyl-heptadecane, 1-(1H-pyrrol-2-yl)-ethanone, and 2-pyrrolidinone, were the common differential volatile compounds in four comparison combinations (Fig. 3A and B). It was worth noting that the eight volatile compounds might affect the representation of the ‘taro-like’ aroma, and should be further identified by other methods.
Fig. 3.
The analyses of the differential volatile compounds among the five pumpkin samples: (A) The contents of differential volatile compounds in five different samples. The diamond, pentagon, and cross star markers represent the differential volatile compounds. Cross star marker represents the volatile compounds only detected in NO. 44. Pentagon marker represents the common differential volatile compounds in the four combinations. (B) Venn diagrams of differential volatile compounds in different pumpkin samples. The number on the left represents the number of differential volatile compounds with NO. 44 as the reference. The dot chart below represents the number of differential volatile compounds in the different samples. (C) HCA analysis of different volatile compounds in the five pumpkin samples. The red and blue colors annotate higher and lower abundance, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We used HCA to visualize the difference in volatile compounds among the five samples. As shown in Fig. 3C, three main groups were successfully clustered: NO. 301 and NO. 335 were included in a group Ⅰ; NO. 326 and NO. 45 were contained in group Ⅱ; while group III only contained NO. 44. Thus, the significant changes of the volatile compounds occurred in NO. 301 and NO. 335, rather than in NO. 326 and NO. 45, as per the contents changes of compounds presented in HCA, which was consistent with the cluster analysis and PCA results, thus suggesting the analysis method and data processing in this study were reliable.
Additionally, we divided the trend of the content of volatile compounds into four categories (Fig. 3C). Category Ⅰ had eight compounds, including (E)-2-hexenal, hexanal, 1-hexanol, 3,5,5-trimethyl-3-cyclohexen-1-one, 2-pyrrolidinone, 2-acetyl-1-pyrroline, β-myrcene, and 1-(1H-pyrrol-2-yl)-ethanone, and these showed higher levels in the NO.44, as compared with four other samples (Fig. 3C). Among the eight compounds, hexanal, 1-hexanol, 2-pyrrolidinone, 2-acetyl-1-pyrroline, 1-(1H-pyrrol-2-yl)-ethanone, and β-myrcene were the common differential volatile compounds mentioned above, which helped further narrow down the identification range of the key contributor for the ‘taro-like’ aroma. According to the previous reports, 1-hexanol and hexanal were found to present grassy and green odor, respectively (Poehlmann and Schieberle, 2013, Selli et al., 2018). Notably, we specifically detected four differential volatile compounds, including 2-acetyl-1-pyrroline, β-myrcene, 1-(1H-pyrrol-2-yl)-ethanone, and 2-pyrrolidinone, in the NO. 44 (Fig. 3A and Table 1), which might be the important volatile compounds contributing to the ‘taro-like’ aroma. This is the first report of the detection of 2-acetyl-1-pyrroline in the pumpkin fruit rather than in the pumpkin seed oil. Moreover, β-myrcene, 1-(1H-pyrrol-2-yl)-ethanone, and 2-pyrrolidinone have the high odor threshold (Gemert, 2011), which might have caused the lower OAV value (<1) in our present study. However, 2-acetyl-1-pyrroline represents a low odor threshold value according to the previous reports (Chen et al., 2013, Jinakot and Jirapakkul, 2019, Poehlmann and Schieberle, 2013) and, therefore, can be detected by the human nose at very low concentrations, thereby possibly being vital for the ‘taro-like’ aroma. Although the medium of the volatile compounds was different, OAV can provide a reference for this study.
GC-O analysis
We performed the GC-O analysis using fruit from NO. 44 to characterize the key volatile compounds associated with the ‘taro-like’ aroma. Different analytical methods in combination with GC-O, like direct intensity, AEDA, and frequency detection were used (Plutowska & Wardencki, 2008). AEDA with serial dilutions of the aroma extract is one of the most common methods for screening the key contributors to aroma (Chen et al., 2013, Poehlmann and Schieberle, 2013). In our present study, we screened the key volatile compounds via AEDA analysis combined with the odor intensities. We performed a five stepwise dilution series as described previously with modification (Matsui, Guth, & Grosch, 1998). We detected a total of 20 odors in the original extract (Table S2), and also listed the odor intensities and volatile compounds. However, we detected fewer odors due to the increase in dilution. We detected 18, 14, 7, and 4 odors in 1:3, 1:9, 1:27, and 1:81 dilutions, respectively (Table S2), and identified the key volatile compounds for the aroma by the last odor that could still be smelt with the highest dilution (1:81). At the highest dilution, we identified a total of four odors, namely taro, cucumber, raw wheat, and mint flavor, respectively (Table 2). Additionally, the taro odor consistently showed high intensity in different dilution tests. Moreover, the taro odor had the highest intensity with the scale of 2 in the highest dilution, followed by cucumber, raw wheat, and mint odors on the scale of 1. According to the retention time and MS results, the corresponding volatile compounds for taro, cucumber, and raw wheat was 2-acetyl-1-pyrroline, (E,Z)-2,6-nonadienal and 2,6,6-Trimethyl-2- cyclohexene-1,4-dione, respectively. The cucumber odor of (E,Z)-2,6-nonadienal has been reported in previous paper (Buescher & Buescher, 2001). And 2-acetyl-1-pyrroline showed the highest FD factor with the ‘roasted’, ‘popcorn-like’ odor in pumpkin seed oil (Poehlmann & Schieberle, 2013). However, we could not accurately identify the volatile compounds for the mint flavor. Moreover, we also detected (E, Z)-2,6-nonadienal in NO. 301 and NO. 335, while we found 2,6,6-Trimethyl-2-cyclohexene-1,4-dione in NO. 45, NO. 326, and NO. 335, thus indicating that it was not an important contributor to the ‘taro-like’ aroma. Additionally, the four stepwise dilution series experiments used in our study (the highest dilution was 1:81), provided sufficient results for identifying the key contributor to the ‘taro-like’ aroma.
Table 2.
The most potent odorants in pumpkin fruit of NO.44 sample.
| Odor quality | Odor intensity | Volatile compounds | Identification |
|---|---|---|---|
| taro | 2 | 2-acetyl-1-pyrroline | RT, MS |
| cucumber | 1 | (E, Z)-2,6-nonadienal | RT, MS |
| raw wheat | 1 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | RT, MS |
| mint | 1 | – |
The confirmation and verification of key volatile compounds for ‘taro-like’ aroma
According to the analysis of differential volatile compounds among five samples and GC-O analysis, we found that 2-acetyl-1-pyrroline representing the ‘taro’ odor was only identified in NO. 44. Thus, we concluded that 2-acetyl-1-pyrroline might be the key contributor to the ‘taro-like’ aroma of the pumpkin fruit. To validate 2-acetyl-1-pyrroline and better understand the relationship between 2-acetyl-1-pyrroline and the ‘taro-like’ aroma, we used 12 additional kinds of pumpkin samples. 2-acetyl-1-pyrroline was also only found in two samples with ‘taro-like’ aroma, while lacking in the ten samples without the ‘taro-like’ aroma (Fig. S2). Therefore, our results indicated that omitting 2-acetyl-1-pyrroline could possibly cause the lack of the ‘taro-like’ aroma in pumpkin. 2-acetyl-1-pyrroline is the key volatile of rice (Hinge, Patil, & Nadaf, 2016), which is also found in coconut (Dumhai et al., 2019), mung bean (Attar, Hinge, Zanan, Adhav, & Nadaf, 2017), muskmelon (Pang et al., 2012), cucumber (Yundaeng, Somta, Tangphatsornruang, Chankaew, & Srinives, 2015), winter melon (Ruangnam et al., 2017), sorghum (Yundaeng, Somta, Tangphatsornruang, Wongpornchai, & Srinives, 2013), etc. 2-acetyl-1-pyrroline was also detected in taro (Wong, Chong, & Chee, 1998). It has an attractive aroma which can be described as ‘nutty’, ‘roasted’, ‘popcorn-like’, and ‘pandan-like’. More importantly, Lin et al. (2014) reported that a rice mutant SA0420 exhibited an agreeable ‘taro-like’ aroma in its leaves and grains. They found that the SA0420 exhibited a prominently higher 2-acetyl-1-pyrroline level in its leaves and grains than in the non-aromatic rice samples. Therefore, we proposed that the 2-acetyl-1-pyrroline was the key contributor imparting the ‘taro-like’ aroma in the pumpkin fruit.
Conclusion
In this study, we used the E-nose, GC–MS, and GC-O to study the aroma profile, volatile compounds, and key contributors of the ‘taro-like’ aroma, respectively. We found significant differences in aroma profiles and volatile compounds between the fruits from five samples with/without the ‘taro-like’ aroma using the E-nose and GC–MS, respectively. We suggest that 2-acetyl-1-pyrroline is the key contributor to the ‘taro-like’ aroma of pumpkin fruit by analyzing the differential volatile compounds and GC-O. Moreover, we further confirmed the relationship between 2-acetyl-1-pyrroline and the ‘taro-like’ aroma by analyzing 12 other kinds of pumpkin samples. Thus, the results consolidate a theoretical basis for understanding the aroma characteristics of the pumpkin fruit.
CRediT authorship contribution statement
LI Junxing: Conceptualization, Methodology, Data curation, Writing – original draft. Miao Aiqing: Methodology. ZHAO Gangjun: Investigation. Liu Xiaoxi: Investigation. Wu Haibin: Investigation. Luo Jianning: Investigation. Gong Hao: Investigation. Zheng Xiaoming: Investigation. Deng Liting: Visualization. Ma Chengying: Supervision, Methodology, Software, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the funding support from Key-Area Research and Development Program of Guangdong Province (2020B020220003), Agricultural competitive industry discipline team building project of Guangdong Academy of Agricultural Sciences (202103TD, 202114TD), the Laboratory of Lingnan Modern Agriculture Project (NZ2021008), the Science and Technology Program of Guangdong Province (2021A1515011187, 2020A0505020006, 2019A050520002, 2017B030314111), National Modern Agricultural Technology System Construction Project (CARS-23-G-50), Construction of Modern Agricultural Industry Technology System Innovation Team of Guangdong Province (2021KJ110).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100435.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
The fruits of different five materials
Supplementary figure 2.
The Total ion chromatogram for the detection of 2-acetyl-1-pyrroline in additional 12 kinds of pumpkin samples
References
- Abbas H.M.K., Huang H.X., Yang Y.F., Xie Y.H., Zou J.F., Xue S.D.…Zhong Y.J. Characterization of starch in Cucurbita moschata germplasms throughout fruit development. Journal of Agricultural and Food Chemistry. 2020;68(36):9690–9696. doi: 10.1021/acs.jafc.0c03181. [DOI] [PubMed] [Google Scholar]
- Attar U., Hinge V., Zanan R., Adhav R., Nadaf A. Identification of aroma volatiles and understanding 2-acetyl-1-pyrroline biosynthetic mechanism in aromatic mung bean (Vigna radiata (L.) Wilczek) Physiology and Molecular Biology of Plants. 2017;23(2):443–451. doi: 10.1007/s12298-017-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T., Barringer S. Analysis of factors affecting volatile compound formation in roasted pumpkin seeds with Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) and sensory analysis. Journal of Food Science. 2012;71:C51–C60. doi: 10.1111/j.1750-3841.2011.02465.x. [DOI] [PubMed] [Google Scholar]
- Buescher R.H., Buescher R.W. Production and stability of (E, Z)-2, 6-nonadienal, the major flavor volatile of cucumbers. Journal of Food Science. 2001;66:357–361. doi: 10.1111/j.1365-2621.2001.tb11346.x. [DOI] [Google Scholar]
- Chen S., Wang D., Xu Y. Characterization of odor-active compounds in sweet-type Chinese rice wine by aroma extract dilution analysis with special emphasis on sotolon. Journal of Agricultural and Food Chemistry. 2013;61:9712–9718. doi: 10.1021/jf402867m. [DOI] [PubMed] [Google Scholar]
- Chen W., Qi D.D., Wang W.W., Miao A.Q., Ma C.Y. GC-MS analysis combined with sensory analysis revealed the various aroma characteristics of black tea resulted from different grafting rootstocks. Journal of Food Science. 2021;86(3):813–823. doi: 10.1111/1750-3841.15612. [DOI] [PubMed] [Google Scholar]
- Dumhai R., Wanchana S., Saensuk C., Choowongkomon K., Mahatheeranont S., Kraithong T.…Arikit S. Discovery of a novel CnAMADH2 allele associated with higher levels of 2- acetyl-1-pyrroline (2AP) in yellow dwarf coconut (Cocos nucifera L.) Scientia Horticulturae. 2019;243:490–497. [Google Scholar]
- García-Parra A., González-Cebrino F., Ramírez R. Volatile compounds of a pumpkin (Cucurbita moschata) purée processed by high pressure thermal processing. Journal of the Science of Food and Agriculture. 2020;100:4449–4456. doi: 10.1002/jsfa.10485. [DOI] [PubMed] [Google Scholar]
- Gemert L.J.V. Oliemans Punter & Partners BV; The Netherlands: 2011. Odour Threshold values in water in odour thresholds. Compilations of odour threshold values in air, water and other media. [Google Scholar]
- Hinge V.R., Patil H.B., Nadaf A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice. 2016;9:1–22. doi: 10.1186/s12284-016-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.H., Zheng X., Chen Z.H., Zhang Y.Y., Du M., Dong X.P.…Zhu B.W. Fresh and grilled eel volatile fingerprinting by e-Nose, GC-O, GC–MS and GC×GC-QTOF combined with purge and trap and solvent-assisted flavor evaporation. Food Research International. 2019;115:32–43. doi: 10.1016/j.foodres.2018.07.056. [DOI] [PubMed] [Google Scholar]
- Jinakot, I., & Jirapakkul, W. (2019). Volatile aroma compounds in jasmine rice as affected by degree of milling. Journal of Nutritional Science and Vitaminology, 65, S231-S234. 10.3177/jnsv.65.S231. [DOI] [PubMed]
- Lan T., Gao C.X., Yuan Q.Y., Wang J.Q., Zhang H.X., Sun X.Y.…Ma T.T. Analysis of the aroma chemical composition of commonly planted Kiwifruit cultivars in China. Foods. 2021;10:1645. doi: 10.3390/foods10071645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffingwell J.C., Alford E.D., Leffingwell D. Identification of the volatile constituents of raw pumpkin (Cucurbita pepo L.) by dynamic headspace analyses. Leffingwell Reports. 2015;7:1–14. doi: 10.13140/RG.2.2.27813.29923. [DOI] [Google Scholar]
- Lin D., Chou S., Wang A., Wang Y., Kuo S., Lai C.…Wang C. A proteomic study of rice cultivar TNG67 and its high aroma mutant. Plant Science. 2014;214:20–28. doi: 10.1016/j.plantsci.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Ma C., Li J., Chen W., Wang W., Qi D., Pang S., Miao A. Study of the aroma formation and transformation during the manufacturing process of oolong tea by solid-phase micro-extraction and gas chromatography-mass spectrometry combined with chemometrics. Food Research International. 2018;108:413–422. doi: 10.1016/j.foodres.2018.03.052. [DOI] [PubMed] [Google Scholar]
- Matsui T., Guth H., Grosch W. A comparative study of potent odorants in peanut, hazelnut, and pumpkin seed oils on the basis of aroma extract dilution analysis (AEDA) and gas chromatography-olfactometry of headspace samples (GCOH) Lipd/Fett. 1998;100:51–56. [Google Scholar]
- Niu Y., Wang R., Xiao Z., Zhu J., Sun X., Wang P. Characterization of ester odorants of apple juice by gas chromatography-olfactometry, quantitative measurements, odour threshold, aroma intensity and electronic nose. Food Research International. 2019;120:92–101. doi: 10.1016/j.foodres.2019.01.064. [DOI] [PubMed] [Google Scholar]
- Pang, X., Guo, X., Qin, Z., Yao, Y., Hu, X., & Wu, J. (2012). Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. Journal of Agricultural and Food Chemistry, 60(17), 4179-4185. 10.1021/jf300149m. [DOI] [PubMed]
- Plutowska B., Wardencki W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages – A review. Food Chemistry. 2008;107:449–463. doi: 10.1016/j.foodchem.2007.08.058. [DOI] [Google Scholar]
- Poehlmann S., Schieberle P. Characterization of the aroma signature of Styrian pumpkin seed oil (Cucurbita pepo subsp. pepo var. Styriaca) by molecular sensory science. Journal of Agricultural and Food Chemistry. 2013;61:2933–2942. doi: 10.1021/jf400314j. [DOI] [PubMed] [Google Scholar]
- Procida G., Stancher B., Cateni F., Zacchigna M. Chemical composition and functional characterisation of commercial pumpkin seed oil. Journal of the Science of Food and Agriculture. 2013;93:1035–1041. doi: 10.1002/jsfa.5843. [DOI] [PubMed] [Google Scholar]
- Qi, D. D., Miao, A. Q., Cao, J. X., Wang, W. W., Chen, W., Pang, S., He, X. G., & Ma, C. Y. (2018). Study on the effects of rapid aging technology on the aroma quality of white tea using GC-MS combined with chemometrics: In comparison with natural aged and fresh white tea. Food Chemistry, 265, 189-199. 10.1016/j.foodchem.2018.05.080. [DOI] [PubMed]
- Ruangnam S., Wanchana S., Phoka N., Saeansuk C., Mahatheeranont S., de Hoop S.J.…Arikit S. A deletion of the gene encoding amino aldehyde dehydrogenase enhances the “pandan–like” aroma of winter melon (Benincasa hispida) and is a functional marker for the development of the aroma. Theoretical and Applied Genetics. 2017;130(12):2557–2565. doi: 10.1007/s00122-017-2976-3. [DOI] [PubMed] [Google Scholar]
- Selli S., Kelebek H., Kesen S., Sonmezdag A.S. GC-MS olfactometric and LC-DAD-ESI-MS/MS characterization of key odorants and phenolic compounds in black dry-salted olives. Journal of the Science of Food and Agriculture. 2018;98:4104–4111. doi: 10.1002/jsfa.8927. [DOI] [PubMed] [Google Scholar]
- Tieman D., Zhu G., Resende M.F.R., Jr., Lin T., Nguyen C., Bies D.…Klee H. A chemical genetic roadmap to improved tomato flavor. Science. 2017;355(6323):391–394. doi: 10.1126/science.aal1556. [DOI] [PubMed] [Google Scholar]
- Torkova A.A., Lisitskaya K.V., Filimonov I.S., Glazunova O.A., Kachalova G.S., Golubev V.N., Fedorova T.V. Physicochemical and functional properties of Cucurbita Maxima pumpkin pectin and commercial citrus and apple pectins: A comparative evaluation. PLoS One. 2018;13(9):1–24. doi: 10.1371/journal.pone.0204261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.T., Tieman D.M., Sims C.A., Odabasi A.Z., Clark D.G., Klee H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum) Journal of the Science of Food and Agriculture. 2010;90(13):2233–2240. doi: 10.1002/jsfa.4076. [DOI] [PubMed] [Google Scholar]
- Wong K.C., Chong F.N., Chee S.G. Volatile constituents of Taro (Colocasia esculents (L.) Schott) Journal of Essential Oil Research. 1998;10(1):93–95. doi: 10.1080/10412905.1998.9700849. [DOI] [Google Scholar]
- Wu S., Yang J., Dong H., Liu Q., Li X., Zeng X., Bai W. Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms. Food Chemistry. 2021;342(3) doi: 10.1016/j.foodchem.2020.128381. [DOI] [PubMed] [Google Scholar]
- Yundaeng C., Somta P., Tangphatsornruang S., Chankaew S., Srinives P. A single base substitution in BADH/AMADH is responsible for fragrance in cucumber (Cucumis sativus L.), and development of SNAP markers for the Fragrance. Theoretical and Applied Genetics. 2015;128:1881–1892. doi: 10.1007/s00122-015-2554-5. [DOI] [PubMed] [Google Scholar]
- Yundaeng C., Somta P., Tangphatsornruang S., Wongpornchai S., Srinives P. Gene discovery and functional marker development for fragrance in sorghum (Sorghum bicolor (L.) Moench) Theoretical and Applied Genetics. 2013;126(11):2897–2906. doi: 10.1007/s00122-013-2180-z. [DOI] [PubMed] [Google Scholar]
- Zhou C.L., Mi L., Hu X.Y., Zhu B.H. Evaluation of three pumpkin species : Correlation with physicochemical, antioxidant properties and classification using SPME-GC-MS and E-nose methods. Journal of Food Science and Technology. 2017;54:3118–3131. doi: 10.1007/s13197-017-2748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.