Highlights
-
•
Various factors lead to gut microbiota dysbiosis and related diseases.
-
•
Marine polysaccharides can regulate dysbiosis as a potential prebiotic.
-
•
Regulation of intestinal barrier, gut microbiota and metabolites are key pathways.
Keywords: Marine polysaccharides, Gut microbiota, Dysbiosis, Prebiotic, Regulatory effects
Abstract
The gut microbiota dysbiosis is a state which the physiological combinations of flora are transformed into pathological combinations caused by factors such as diets, pollution, and drugs. Increasing evidence shows that dysbiosis is closely related to many diseases. With the continuous development and utilization of marine resources, marine polysaccharides have been found to regulate dysbiosis in many studies. In this review, we introduce the types of dysbiosis and the degree of it caused by different factors. We highlight the regulating effects of marine polysaccharides on dysbiosis as a potential prebiotic. The mechanisms of marine polysaccharides to regulate dysbiosis including protection of intestinal barrier, regulatory effect on gut microbiota, alteration for related metabolites, and some other possible mechanisms were summarized. And we aim to provide some references for the high-value utilization of marine polysaccharides and new targets for the treatment of gut microbiota dysbiosis by this review.
1. Introduction
The human microbiome is a group of microorganisms that colonize the host and exist in the skin, nasal and oral cavity, respiratory tract, gastrointestinal tract, and genitourinary system. Among them, the intestinal microecology is the largest microecological system in human body, where more than 104 species of microorganisms colonize, and these microbial communities are referred to gut microbiota (GM), which mainly bacteria, but also fungi, viruses, archaea and eukaryotes (Anwar, Iftikhar, Muzaffar, Almatroudi, Allemailem, Navaid, & Khurshid, 2021). The gut microbiome contains at least 150 times as many genes as human genome, so the gut microbiota can be considered as the “second genome” which influence the host’s health (Ait Chait et al., 2021, Qin et al., 2010, Xu et al., 2013). According to the relationship with the host, gut microbiota can be divided into commensal bacteria, conditional pathogenic bacteria, and pathogenic bacteria. These three types of bacteria maintain the normal proportion form a physiological combination of the flora when the body is in a stable state. Different gut microbiota has their specific functional characteristics, such as metabolic, protective, structural and neural functions, and their distribution in the gastrointestinal system also determines the functional characteristics of various parts of the gastrointestinal tract (Adak & Khan, 2019). Healthy human stomach is normally inhabited by five major phyla via Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria and Proteobacteria (Nardone & Compare, 2015). For small intestine, Firmicutes and Actinobacteria are the predominant phyla in the duodenum. The jejunum mostly supports the growth of Gram-positive aerobes and facultative anaerobes including Lactobacilli, Enterococci and Streptococci (El Aidy, van den Bogert, & Kleerebezem, 2015). The distal part of ileum close to the ileocecal valve is populated with anaerobes and Gram-negative organisms similar to the colon. In the large intestine, the bacterial is mainly dominated by Firmicutes and Bacteroidetes (Eckburg et al., 2005). The ratio between the bacterial species Bacteroidetes and Firmicutes has been shown to play an important role in health and disease (Mariat et al., 2009). The gut microbiota correct quantitative and qualitative structure (referred to as the eubiosis state) supports homeostasis of the whole organism. And it is now known that the gut microbiota it is not only involved in the processes related to digestion and absorption of substances alimentary, but also plays a role in substance metabolism, immunomodulation, heavy metals excretion in host life activities (Chi et al., 2019, Wang et al., 2021, Yeoh et al., 2021).
However, environmental pollution, diet, drugs and other factors can affect the microbiota composition, function and diversity (Caglayan et al., 2020, Hou et al., 2022, Jandhyala et al., 2015, Kucuk and Gulcin, 2016). Dysbiosis, an alteration in the composition of the microbiota associated with a disease state, has been linked with poor diet and numerous chronic diseases (Weiss & Hennet, 2017). Conversion of physiological combinations of flora to pathological combinations might lead to dysbiosis which is characterized by a decrease in beneficial bacteria, overgrowth of pathogenic bacteria, increase in substances that induce intestinal inflammation and disturbances in intestinal metabolism (Ait Chait et al., 2021, Hu and Zhou, 2011). With the cross-fertilization of modern biotechnology, growing evidence has revealed that gut microbiota not only plays a role in the gastrointestinal tract but also acts as a transmitter of information between the gut and other organs. This leads to the concept of bidirectional communication such as the gut-brain axis (Kaur et al., 2021), the gut-liver axis (Albillos, De Gottardi, & Rescigno, 2020) and the gut-kidney axis (Hua et al., 2022). So the results of gut microbiota dysbiosis may be a series of diseases, such as colon cancer, non-alcoholic fatty liver disease, hypertension, and other disease have been widely reported in epidemiological research (Fan and Pedersen, 2021, Palmu et al., 2021, Tang et al., 2019). Recent studies have reported that the abundance of gut microbiota affects the expression of anti-inflammatory factors and thus the severity of disease in patients with COVID-19. In the context of a global epidemic, it is important to advance research on gut microbiota dysbiosis (Yeoh et al., 2021). Gut microbiota regulation through different methods including fecal microbiota transplants (FMT), probiotics or prebiotic have drawn a lot of attention (Ait Chait et al., 2021). Especially, growing evidence from biology to clinic reported the regulatory effects of probiotics and prebiotics on gut microbiota and related diseases (Sanders, Merenstein, Reid, Gibson, & Rastall, 2019). Compared with the low survival rate of live bacteria delivered by probiotics and problems such as immune rejection of FMT, prebiotic have its unique advantages.
There are large resources that have the potential to become prebiotics in nature. Marine organisms grow in special environments and contain a variety of functional active substances such as polysaccharides, peptides, carotenoids, minerals, polyphenols and vitamins (Li, Xue, Zhang, & Wang, 2020). Marine polysaccharides (MPs) that have a wide variety and sources, possess various active functions such as anti-tumor (Ouyang et al., 2021), anti-coagulation (Ustyuzhanina et al., 2020), regulation of fat (Wei et al., 2022), lipid (Liu et al., 2021) and immunity (Casas-Arrojo, Decara, De Los Angeles Arrojo-Agudo, Perez-Manriquez, & Abdala-Diaz, 2021). Based on its rich functional features, MPs may regulate dysbiosis via certain mechanisms as potential prebiotics. However, the structure of marine polysaccharides is complex, and its specific regulation of gut microbiota dysbiosis is not fully clear. Furthermore, the overall development and research efforts are still insufficient, and the added value of products is low. Hence, we review the gut microbiota dysbiosis and the causes of this occurrence. The prebiotic characteristics of MPs and regulation mechanisms of it on gut microbiota dysbiosis were also summarized. This review will provide comprehensive new insights into the development of marine polysaccharides as prebiotics and their functional products to achieve intestinal microecological regulation, as well as improve marine livings utilization and added value.
2. Gut microbiota dysbiosis and its influencing factors
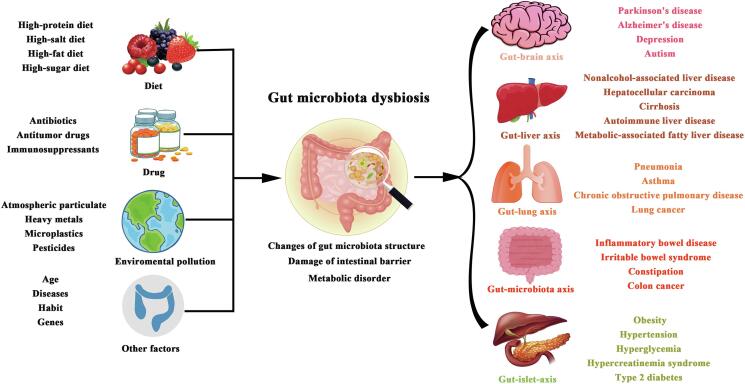
Rich genomic sequences of gut microbiota determine species diversity. Therefore, the related research about gut microbiota usually links the microbiota to human health through microbiota diversity analysis. Based on high-throughput technology, more than 90 % of gut microbiota belong to Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria (Lopez-Santamarina et al., 2020). The composition of gut microbiota varies among individuals due to geographical, genetic and lifestyle, but in general, the overall composition remains at a normal level (Xu et al., 2013). Alteration of number, composition, location, or functional activity of gut microbiota will bring imbalance in intestinal microecology which is called “dysbiosis”. In general, dysbiosis can be classified as (1) imbalance of gut microbiota proportion (it is the most common description of dysbiosis, presenting characteristics such as decrease of symbionts, increase of pathobionts and a loss of microbiota diversity); (2) bacterial translocation (there exist the concept called “leaky gut” which means that gut microbiota is transferred from original location to other organs and tissues); (3) dysfunctional activity of bacteria (the cause may be a decrease in the expression of the relevant functional genes in the gut microbiota induce inability to maintain the original intestinal microecological balance) (Ait Chait et al., 2021, Kumar Singh et al., 2019, Liu and Sun, 2014). Many factors that lead to dysbiosis, and different factors alter the composition of the flora in varied degrees (Fig. 1). Overall, many factors including environment, diet and drug usage are closely related to gut microbiota dysbiosis (Fig. 2).
Fig. 1.
Causes of gut microbiota dysbiosis and related diseases caused by it.
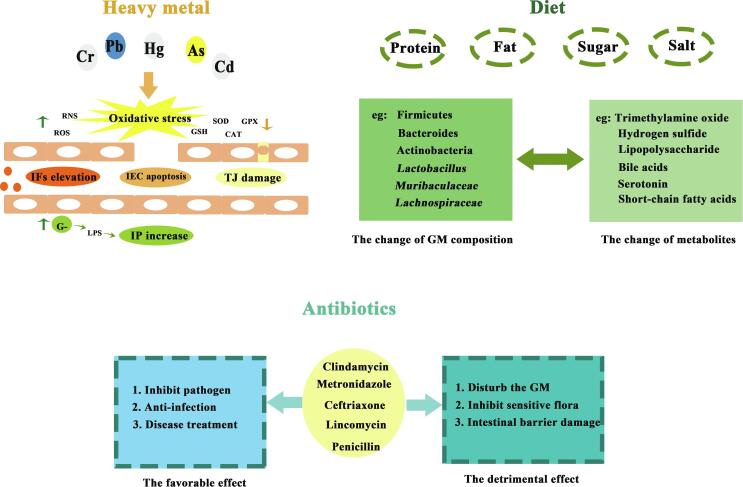
Fig. 2.
Heavy metal, diet, antibiotics and dysbiosis. (Abbreviations: IFs, inflammatory factors; IEC, intestinal epithelial cells; TJ, tight junction; G-, gram negative bacteria; IP, intestinal permeability; GM, gut microbiota.
2.1. Environmental factor and gut microbiota dysbiosis
Environmental pollution is closely related to body health. Recently, the effect of heavy metals, atmospheric particulate matter, microplastics, pesticides, disrupting chemicals or xenobiotics and other pollutants on composition and function of microbiota and thereby inducing dysbiosis have been widely reported (Ding et al., 2021). Especially, heavy metal pollution is a common, wide-ranging problem that deserves attention. The cell damage, inflammatory response and the intestinal barrier integrity damage caused by oxidative stress considered to be one of the most critical mechanisms (Duan et al., 2020). And these damages may cause the bacterial translocation. Furthermore, the metabolic transformation of metals may result in alterations of gut microbiota. Overall, intestinal microecology is widely influenced by the above biological pathways.
The microbial composition of mice in varying degrees when exposed to various concentrations of lead, thereby promoted the production of intestinal inflammatory factors, decreased expression of colonic Muc-2 and intestinal tight junction genes, and increased intestinal permeability (Yu et al., 2021, Zhai et al., 2019). Low exposure of cadmium in drinking water resulted in a 26.8 % decrease in microbial community Alpha diversity, and the expression of tight junction proteins were also decreased (Liu et al., 2020). After administration of 50 ppm arsenic for 6 months, the necrosis of intestinal mucosal and intestinal gland, muscular thinning and other intestinal barrier damage was observed in mice. The abundance of gut microbiota was significantly reduced. The arsenic resistance genes carried by bacteria related to detoxification were decreased, and the metabolic composition was disordered (Li et al., 2021).
These studies have provided a deeper understanding of the toxic effects of heavy metal exposure on the intestinal tract, thereby promoting research related to the mechanisms regulating heavy metal-induced dysbiosis. At present, the research in this field mainly focuses on screening probiotics with potential to reduce toxicity and exploring the mechanism of toxicity reduction. It has been reported that probiotics alleviate heavy metal toxicity by adsorbing it, reducing oxidative stress and protecting intestinal barrier (Yu et al., 2016, Zhu et al., 2021). Zhai et al firstly confirmed that prebiotics also had the resistance to heavy metals and its protective effect mainly depended on the regulation of gut microbiota (Zhai et al., 2019). However, there are few studies on the detoxification pathway of prebiotics, and the specific mechanisms have not been clarified in detail. Studies concerning more in-depth exploration of the mechanism of prebiotic detoxification role will undoubtedly provide more reference in this field.
2.2. Diet and gut microbiota dysbiosis
With the improvement of living standards, people's daily diet structure also tends to be diversified. The effect of diet on gut microbiota via nutritional status has two sides. On one hand, undernutrition may relate to abnormal gut microbial population, which can perpetuate the vicious cycle of pathophysiology. On the other hand, overnutrition lead to obesity have adverse effect on the gut microbiota (Donovan, 2017). Moreover, the interaction between diet and gut microbiota has a certain effect on dysbiosis. Gut microbiota play an essential role in the digestion and absorption of food. Among them, Lactic acid bacteria (LAB) is a kind of common bacteria which can ferment sugars into lactic acid and to produce proteolytic enzymes (Chlebowska-Smigiel et al., 2019, Wasko et al., 2012), contributing a lot to human diet and long-term gut eubiosis (Chlebowska-Smigiel et al., 2017, Kieliszek et al., 2021). Nutrients in food such as dietary fibers, protein and lipid etc. play a various role in metabolic regulation. Therefore, the effect of food on gut microbiota differed by its ingredients. Even the same food ingredient has different effects on gut microbiota depend on its source and intake. For example, the plant proteins have been reported to promote growth of beneficial bacteria, whereas some animal proteins stimulate the growth of pathogenic bacteria (Rinninella et al., 2019).
Poor diets such as long-term high-fat diet (HFD), high-salt diet (HSD) and high-fat and high-sugar diet (HSFD) can significantly change the composition of gut microbiota and lead to varying degrees of dysbiosis. The appearance of dysbiosis is accompanied by changes in metabolites such as lipopolysaccharide (LPS), bile acids and serotonin were associated with metabolism-related diseases such as obesity and diabetes (Kumar Singh et al., 2019). HFD can affect the composition and metabolism of gut microbiota and induce intestinal inflammation. The unfavorable metabolites produced during fermentation process such as trimethylamine oxide and hydrogen sulfide may be one of the triggers for dysbiosis (Cai, Chen, Wu, Lin, & Liang, 2021). HFSD can reduce intestinal mucus secretion and alter gut microbiota composition with significant decrease of the genus Lactobacillus, Muribaculaceae, and Lachnospiraceae (Lee et al., 2021). HSD can induce increase of Firmicutes/Bacteroides (F/B) ratio and decrease of SCFA-producing bacteria (Hu et al., 2020). As we seen that poor diet is an important factor in causing dysbiosis, and dysbiosis continues to lead to disease. It was reported that the consumption of red and processed meat is strongly associated with a higher risk of colon cancer, which were closely related to their effect on gut microbiota (Song, Chan, & Sun, 2020). Meanwhile, disease is also the cause of dysbiosis, and people 's dietary intake will also change after dysbiosis. The first contact of neonates with microbes is provided by the maternal microbiota. Studies have shown that abnormal glucose metabolism in pregnant women with hyperglycemia will affect the phenotype of glucose metabolism in offspring, and changes in gut microbiota caused by abnormal glucose metabolism will also affect the intestinal flora of offspring (Xue et al., 2022). Moreover, mode of delivery, type of infant feeding and other perinatal factors can influence the establishment of the infant microbiota. It is precisely because of the interaction between diet-gut microbiota dysbiosis-disease, maintaining the balance of gut microbiota can be said to be an important balance between regulating normal diet and healthy body.
Reasonable adjustment of diet is the most simple and effective method to the dysbiosis caused by poor dietary structure. Fruits and vegetables are rich in dietary fiber, polysaccharides and polyphenols, which have the effect of increasing beneficial bacteria. In addition, the exploration and application of dietary supplements are still the focus of current field (Luo, Lin, Bordiga, Brennan, & Xu, 2021). Exploring more representative characteristic bacteria and related metabolic pathways that play a major role in dietary regulation can help to make up for some deficiency in this field.
2.3. Drug and gut microbiota dysbiosis
The effect of drugs on intestinal microecology is bidirectional. On the one hand, drugs can inhibit the growth and regulation of pathogenic bacteria; on the other hand, drugs can disturb the normal flora structure and cause gut microbiota dysbiosis (Liu & Sun, 2014). Antibiotics, which are widely used as antimicrobial agents, are shown to kill or inhibit sensitive flora in large quantities, simultaneously can cause intestinal barrier damage, and induce intestinal inflammatory substances (Fang, Hu, Nie, & Nie, 2019). Overall, antibiotics are vital to induce gut microbiota dysbiosis and often used to establish dysbiosis models. And gut microbiota dysbiosis caused by antibiotics is called antibiotic-associated diarrhea.
In view of various types of the effects of different antibiotics on gut microbiota are similar or different. Bacterial diversity and richness were decreased with the combination of clindamycin and metronidazole. At the phylum level, the abundance of Firmicutes, Bacteroidetes, and Proteobacteria were altered (Kanwal et al., 2018). The level of Proteobacteria and Bacteroidetes was decreased and the number of Firmicutes was increased after treatment with ceftriaxone (Li et al., 2019). Under lincomycin treatment, the number of Proteobacteria and Firmicutes were increased, but the Bacteroidetes were decreased, and the infiltration of inflammatory cells was found by histopathological observation of ileum (Bie, Duan, Meng, Guo, & Wang, 2021). It can be showed that antibiotics can inhibit pathogens, simultaneously can disturb the composition of normal flora. Therefore, it is of great value to seek substances that can replace antibiotics or alleviate dysbiosis caused by antibiotics.
Because of its excellent antibacterial activity, polysaccharides have potential as effective antibacterial agents in combination with antibiotics or may be considered as alternatives to antibiotics. MPs such as fucoidan from Sargassum tenerrimum, sulfated polysaccharides from marine red algae and chitosan proved to inhibit the growth of pathogenic microbes including Escherichia coli and Staphylococcus aureus. Therefore, its antibacterial properties were used to prepare antibacterial film and hydrogel for food preservation (Ashayerizadeh et al., 2020, Liu et al., 2019, Mohamed et al., 2022). Moreover, polysaccharide have the prebiotic potential in increasing the diversity of flora. However, due to the limitations of the molecular weight, structure and functional groups of polysaccharides and other factors, as well as the stability of bacteriostasis and the inhibitory species of polysaccharides, it’s still not completely clear when their inhibition mechanism is investigated.
2.4. Other factors and gut microbiota dysbiosis
In addition to the factors mentioned above, age, lifestyle, individual physiological characteristics, and many other reasons can also lead to dysbiosis of gut microbiota. The proportion of gut microbiota composition in the organism varies with age. Firmicutes account for a larger proportion in children and adults, while Bacteroidetes dominate in the elderly population (Xu et al., 2013). The number of Bifidobacterium decreased, while the ratio of Enterobacteriaceae and Enterococcus increased by age (Kumar Singh et al., 2019). Abnormal intestinal function such as long-term retention of intestinal contents caused by reduced intestinal motility and the lack of main immunoglobulins for resistant pathogens caused by immune dysfunction, may lead to excessive bacterial reproduction (Hu & Zhou, 2011).
Gut microbiota dysbiosis can cause a variety of diseases and diseases are also responsible for dysbiosis. It has been found that the composition of microbial communities altered by cerebral hemorrhage (CH) in mice. CH is accompanied by intestinal barrier damage such as gastrointestinal motility decreasing, mucosal muscular layer thinning and gland losing (Yu et al., 2021). Liver disease impairs bile secretion, thereby the body's inhibitory effect on foreign bacteria is weakened. And multiple organ failure will induce dysbiosis by changing the number of aerobic bacteria and the proportion of specific anaerobic bacteria (Liu & Sun, 2014). This indicates that the occurrence of disease often disturbs the balance of internal environment and has a corresponding effect on various organs and tissues. Meanwhile, the above physiological changes may lead to the release of many inflammatory factors, metabolic disorders and then cause alteration of gut microbiota.
Different factors can lead to different degrees of gut microbiota dysbiosis with commonality and particularity. For example, the dysbiosis induced by heavy metals obviously leads to intestinal barrier damage because of oxidative stress, and the dysbiosis induced by diet is closely related to metabolic disorders. However, the dysbiosis induced by antibiotics shows obvious imbalance between beneficial and harmful bacteria. So, it is of great significance to clarify the factors leading to dysbiosis and targeted regulatory mechanism in promoting the research on gut microbiota and human health.
3. Prebiotic effects of marine polysaccharides
Marine polysaccharides (MPs) are a class of long-chain polymers derived from marine organisms and consist of multiple monosaccharide molecules linked by glycoside bonds. MPs can be divided into marine plant polysaccharides, marine animal polysaccharides and marine microbial polysaccharides according to their sources (Wang et al., 2018). Compared with terrestrial organisms’ polysaccharides, MPs contain a higher content of sulfate groups and exhibit multiple functional activities. MPs are widely used in many fields, such as in the food industries, it can be added as a basic component to develop functional foods or as a food additive to improve food characteristics. In the field of materials, it can be used to prepare electrospun fibers, hydrogels and nanocomposites. In the field of biomedicine, it is used in the development of marine drugs, the construction of targeted drug delivery system and the development of microecological agents (Angel et al., 2022, Gong et al., 2022, Muthukumar et al., 2021, Papon et al., 2022).
The action modes of MPs in organisms can be divided into two types, one is absorption after oral administration. In previous studies, it was believed that polysaccharides were not absorbed after oral administration due to their high molecular weight and hydrophilicity. However, recent studies have found that polysaccharides can be transported through paracellular pathway, transcellular pathways and M cell-mediated transport. Its absorption is affected by charge, molecular weight and spatial structure. It is generally believed that polysaccharides with positive charge and low molecular weight (Mw) are more easily absorbed (Zheng et al., 2022). Another action modes of MPs which is also an important way to play a role as prebiotic, is to rely on the biotransformation of GM to act in organisms.
Prebiotics are a kind of raw material that can selectively enhance the beneficial components of GM (Gurpilhares, Cinelli, Simas, Pessoa, & Sette, 2019). Its commodity utilization form is convenient, and it has strong resistance to the intestinal environment and has many beneficial effects such as against pathogens, improving intestinal function and immune regulation. There is growing evidence that prebiotics can effectively alleviate gut microbiota dysbiosis (Ait Chait et al., 2021, Markowiak and Slizewska, 2017, Sanders et al., 2019). To become a prebiotic need to meet three standards: (1) it’s not absorbed in the upper digestive tract; (2) it can promote the growth of beneficial bacteria; (3) its metabolites are beneficial to human health (Zheng, Chen, & Cheong, 2020). The traditional prebiotics with remarkable effects include fructooligosaccharides, galactooligosaccharides and inulin (Markowiak & Slizewska, 2017). With the advancement of research, new types of resources with prebiotic potential have emerged (Tang et al., 2022). In the exploration of marine probiotic resources, MPs from different sources show properties as high-quality prebiotics.
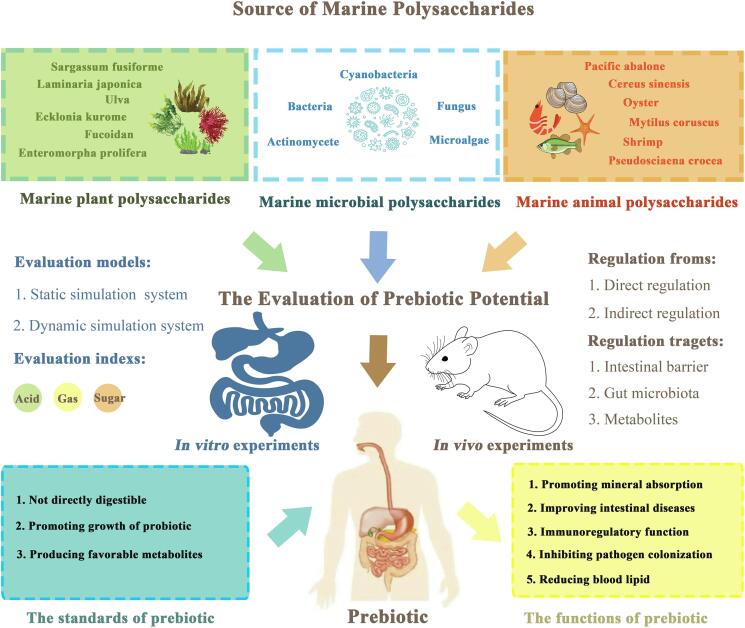
The evaluation of prebiotic potential of MPs is often combined with in vitro and in vivo experiments (Fig. 3). In vitro evaluation is mostly based on whether polysaccharides appear as prebiotics in simulated gastrointestinal digestion and fermentation models. The evaluation indexes mainly include the total sugar content, acid and gas production and the change of GM after in vitro fermentation. Polysaccharides from Sargassum fusiforme in vitro fermentation for 24 h, the utilization of carbohydrate was 53.17 %, and the contents of total SCFAs increased by 10.77 times. The diversity of gut microbiota was altered by a significant decrease in pH due to acid production. Compared with the control group, the number of beneficial bacteria including Bifidobacterium, Ruminococcaceae_UCG-014 and Lactobacillus were significantly increased in the polysaccharide group (Kong et al., 2021). Sulfated polysaccharides from Gracilaria Lemaneiformis were rarely degraded during digestion and 53.7 % of which were utilized by gut microbiota to produce large amounts of SCFAs during fermentation (Han et al., 2020). In vitro fermentation model, oyster polysaccharides promoted SCFAs production and up-regulated the abundance of beneficial bacteria such as Bacteroides and Prevotella (Ma, Jiang, & Zeng, 2021). Due to the differences in structural composition and Mw, MPs from different sources will specifically promote the growth of certain flora during in vitro fermentation, produce different metabolites, and show different fermentation characteristics (Lv et al., 2022). With the development of modern biological technology, qPCR and Fluorescent in situ hybridization (FISH) have been used to evaluate the potential of prebiotics in vitro, and the concept of prebiotic index has been introduced to explore the fermentation and prebiotic characteristics of MPs more comprehensively and deeply (de Medeiros et al., 2021, Lopez-Santamarina et al., 2022).
Fig. 3.
Prebiotic potential of marine polysaccharides.
In vivo studies, MPs rely on the degradation of gut microbiota and exert their prebiotic properties by directly and indirectly regulating the intestinal environment. Direct regulation is mainly achieved by regulating flora composition and metabolites, while indirect regulation is often associated with enhancing intestinal barrier, improving intestinal function and homeostasis. According to reports, after mice ingested keretan sulfate from shark cartilage, the overall flora structure changed, the abundance of beneficial bacteria increased significantly (Shang et al., 2016). For obese mice on a high-fat diet, oyster polysaccharides can ameliorate dysbiosis through modulated abnormal lipid metabolism, accelerated the production of SCFAs, regulated lipid metabolism in fat and liver, and enriched beneficial bacteria (Ma, Zhu, Ke, Jiang, & Zeng, 2022). An α-d-glucan from marine fungal has also been shown to alleviate colitis by repairing mucosal barriers and enrich immune-related and mucosal repair flora (Liu et al., 2020). These in vivo studies also further indicate that MPs can exert prebiotic properties not only limited to the regulation of the intestine, but also have a positive impact on various organs and internal environment of the whole body.
The structural composition and molecular weight of MPs is the important factor affecting the prebiotic properties. It has been reported that polysaccharides linked by (1 → 3)-linked-β-Glcp and (1 → 6)-linked-α-Galp backbones exhibit higher activity in intestinal barrier protection, and high-Mw β-glucan can better promote the maintenance of gastrointestinal homeostasis. Compared with medium-Mw and low-Mw polysaccharides, high-Mw alginate may produce more SCFAs, acetic acid and propionic acid due to its effect on each microbiota (Huang et al., 2022). This may also indicate that both high-Mw and low-Mw polysaccharides play an important role in the regulation of organism function. Low-Mw polysaccharides can better promote digestion and absorption, high-Mw polysaccharides also have excellent prebiotic potential. This may be because the structure of MPs is complex, and its function does not depend on a single effecting factor. More research on the structure–activity relationship and prebiotic potential to be further explored. In the process of exploring these potentialities, in order to make up for the lack of intestinal dynamics simulation in vitro evaluation (Lopez-Santamarina et al., 2020), gastrointestinal bionic system is being increasingly used to simulate the state of gastrointestinal digestion more realistically in vitro for exploring new prebiotic resources. For in vivo studies, scientists have also begun to focus on the intrinsic mechanisms by which prebiotics work. These works are undoubtedly promoting the high-value utilization of marine prebiotic resources.
4. Mechanisms of the regulatory effects of marine polysaccharides on gut microbiota dysbiosis
4.1. Regulatory effects of MPs on intestinal barrier
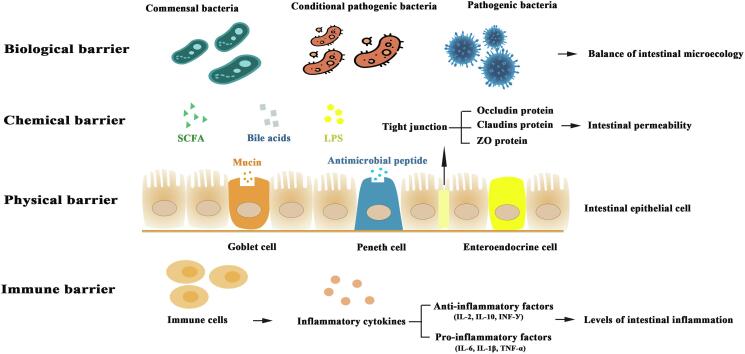
The intestinal barrier is an important structure to protect the body from external factors and assist the digestion and absorption of nutrients, which is of great significance to maintain intestinal homeostasis (Fig. 4). The intestinal barriers include physical barrier, chemical barrier, immune barrier, and biological barrier. The physical barrier is mainly composed of intestinal epithelial cells closely arranged by cell junctions, which constitute the first line of defense against the external environment. The chemical barrier consists of mucus secreted by intestinal epithelial cells, digestive juices, and antibacterial substances. The immune barrier is mainly composed of immune cells and immune factors. The immune system is activated to play a regulatory role when unfavorable factors invade the body. The biological barrier is composed of mucosal flora and intestinal flora, and the main flora of which such as Lactobacillus and Bifidobacterium, which contribute to maintain the intestinal microecological balance via limiting the colonization of pathogens (Ma et al., 2017, Qiu et al., 2020).
Fig. 4.
Functional role of the intestinal barrier.
When the intestinal barrier is damaged, the intestinal permeability, the opportunities for harmful bacteria to colonize and levels of LPS are increased, resulting in disruptions in the structure and distribution of the flora. Impaired intestinal barrier is often manifested as: inflammatory cell infiltration, intestinal villi shorter and disorder, mucosal edema, damage of epithelial cell and tight junction structure. Overall, MPs exhibit excellent potential in regulating gut microbiota dysbiosis via maintaining intestinal barrier integrity. On the one hand, it can reduce the occurrence of bacterial translocation. On the other hand, MPs can effectively prevent the development of gut microbiota into irreversible state (Bie et al., 2021, Kanwal et al., 2018, Yu et al., 2021, Zhai et al., 2019). It was reported that polysaccharides from Sargassum fusiforme has better regulatory effect up-regulate the genic expression of tight junction protein (Claudin-1 and ZO-1) and the main component of mucusin (Muc-2) in mice model of coliti than commonly used therapeutic drug 5- aminosalicylic acid (Han et al., 2021). Polysaccharides from Undaria pinnatifida regulated dysbiosis induced by HFD through improving colonic tissue damage and mucin structure, reducing colonic cell wall thickness and level of LPS (Jiang et al., 2021). Jellyfish polysaccharide exhibited effects on protecting epithelial goblet cells, improving intestinal epithelial integrity, permeability and reducing inflammatory cell infiltration (Cao et al., 2021). With the regulation of above three polysaccharides, the expression level of inflammatory factors had a favorable change compared with the model group, thereby reducing the inflammatory level of intestinal microecology.
Accumulating evidence show that the close association of MPs with the intestinal barrier. Protective effect of MPs on the integrity of the intestinal barrier is dependent on its own active function on the other hand, it can be indirectly regulated by modulating gut microbiota, inflammatory cytokines, and metabolites. Since the intestinal barrier is a complex and comprehensive system, most of the current studies focus on MPs macroscopic regulation, and there are few studies on their comprehensive and systematic evaluation. There exist a lot of questions to be further explored, including how MPs act on each barrier, what is the molecular recognition modes, and related specific pathway. In future studies, systematic evaluation of the regulatory effects of MPs targeting intestinal barrier has great promotion value in this field.
4.2. Regulatory effects of MPs on gut microbiota
Tens of thousands of bacteria in the gut mediate metabolism and immune system and other pathways that have important effects on body health (Table 1) (Payling et al., 2020). Firmicutes contain more transporters to transport carbohydrates to improve energy absorption. Bacteroidetes contains a variety of CAZymes and is the main SCFAs producing strain, which is beneficial to the degradation of polysaccharides and decrease of its abundance will increase the level of LPS in vivo and reduce the intestinal permeability. At present, many studies have shown that the proportion of Firmicutes in the gut microbiota of obese people and animals increases while that of Bacteroides decreases, hereby the change of F/B ratio is regarded as a manifestation of dysbiosis (Jiang et al., 2021). Actinobacteria can reverse the dysbiosis of gut microbiota caused by heavy metal exposure, via reducing toxicity and participating in lipid metabolism (Chen et al., 2020, Gokulan et al., 2018, Luo et al., 2021). Clostridiaceae and Proteobacteria are the main intestinal pathogens. The former is reported to be associated with various diseases such as cancer, and the latter is intended to be an indicator of gut microbiota dysbiosis (Fang et al., 2019, Wu et al., 2020). The functional role of gut microbiota is associated with the expression of related genes, including regulatory structural genes, immune cell transcription genes and metabolism-related genes. In the intestinal microecology, the presentation of microbial functional properties is based on the structure of the entire flora rather than on individual microorganisms. Therefore, Alpha diversity and Beta diversity are generally used to evaluate the intra-group and inter-group diversity in the flora analysis. LDA effect size (LEfse) analysis was used to determine the microorganisms that play an important role in each group. The correlation between microorganisms and other substances was analyzed by multi-omics analysis to explore the functional characteristics of it in the microecosystem.
Table 1.
Regulatory effects of marine polysaccharides toward on dysbiosis.
| Sources of MPs | Model | Significant Changes in Intestinal Barrier | Significant Changes in Gut Microbiota | Significant Changes in Metabolism |
|---|---|---|---|---|
| Gracilaria Lemaneiformis (Han, Ma et al., 2021) | Colitis BALB/c mice | Claudin-1, ZO-1, Muc-2 ↑ TNF-α, IL-6, IL-1β ↓ |
Firmicutes, Actinobacteria, Corynebacterium_1, Enterorhabdus↑ Bacteroidetes ↓ |
GPR43, Olfr78, GPR109A and acetic acid, propionic acid, butyric acid ↑ |
| Undaria pinnatifida (Jiang et al., 2021) | High fat diet BALB/c mice | Mucosa structure; IL-10 ↑ Thickness of colonic wall; LPS; TNF-α ↓ |
Bacteroidetes ↑ Firmicutes, Desulfovibrionales, Clostridia ↓ |
Acetate, propionate, butyrate ↑ TC, TG, LDL-c, MDA↓ |
| Jellyfish skin (Cao et al., 2021) | Colitis C57BL/6 mice | Goblet cells colonic epithelial permeability; ZO-1, occludin, Muc-2, E-cadherin, Tff3 ↑ Histological score, inflammatory cell infiltration; TNF-α, IL-6, IL-1β↓ |
Firmicutes, Proteobacteria, Actinobacteria, Erysipelotrichaceae, Bradyrhizobiaceae, Verrucomicrobiaceae ↑ Bacteroidetes↓ |
Pionate, butyrate, isobutyrate, valerate, isovalerate↑ |
| Ascophyllum nodosum (Wang et al., 2020) | Antibiotic treatment C57BL/6J mice | TNF-α, IL-1β, IL-6↓ IL-10↑ |
Verrucomicrobia, Ruminococcaceae_UCG_014, Akkermanisia↑ Proteobacteria Proteus Enterococcus↓ |
Acetic acid, propionic acid↑ |
| pacific abalone (Wu et al., 2020) | High fat diet BALB/c mice | — | F/B ratio↓ Prevotellaceae, Rikenellaceae, Flavobacteriacea↑ |
SOD, CAT, SCFAs↑ TG, TC↓ |
| oyster (Cai, Pan et al., 2021) | S180 tumour-bearing KM mice | Villus height, crypt depth, mucosa thickness, villus surface area and V/C ratio ↑ IL-1β, IL-6, TNF-α↓ |
F/B ratio, Verrucomicrobia, Odoribacter↑ Firmicutes, Bacteroidetes, Akkermansia, Prevotellaceae_UCG-001, Rikenellaceae_RC9_gut_group↓ |
To reverse the changes in nutritional and energy metabolism Acetic acid, propionic acid and n-butyric acid↑ |
| Cereus sinensis (Cui et al., 2021) | Antibiotic treatment C57BL/6 mice | Cecal villi were longer, thinner and relatively regular arrangement Inflammatory cell infiltration, edema of cecal tissue; IL-1β, IL-2, TNF-α↓ |
Phasecolarctobacterium, Bifidobacterium↑ Parabacteroides, Sutterella, Coprobacillus↓ |
Amino acid metabolism↑ Propionate, butyrate, acetate↑ |
* Abbreviations: Tight junction protein (TJs): Claudin-1, ZO-1, occludin; Muc-2, mucusin; LPS, lipopolysaccharide Inflammatory cytokines: TNF-α, IL-6, IL-1β, IL-10, IL-2; SCFA receptors: GPR43, Olfr78, GPR109A; Defective protein: E-cadherin, Tff3; Lipid metabolism related indicators: TC, TG, LDL-c; Oxidative stress correlation index: MDA, SOD, CAT; V/C ratio, villus height/crypt depth ratio; F/B ratio, Firmicutes/Bacteroides ratio.
MPs can alter the richness and diversity of gut microbiota and play a prebiotics role by increasing the abundance of probiotics and related functional bacteria (Zhu et al., 2021). In the mice model of dysbiosis induced by HFD, Enteromorpha clathrata polysaccharides, a kind of new prebiotic, can regulate microbial composition at phylum and genus levels, increase number of probiotics Prevotellaceae and enhance growth of butyrate-producing bacteria E.xylanophilum in the gut (Wei et al., 2021). Fucoidan can reverse antibiotic-induced gut microbiota dysbiosis by increasing Ruminococcaceae_UCG_014 and Akkermansiaand, and reducing harmful bacteria such as Proteus and Enterococcus (Wang et al., 2020). Sulfated polysaccharides from pacific abalone reduced the ratio of F/B that can be associated with obesity and increased the abundance of Prevotellaceae, Rikenellaceae and Flavobacteriacea (Wu et al., 2020). The study by Cui et al. on the prebiotic functions of MPs from Gelidium pacificum Okamura and Cereus sinensis showed that both of them could significantly change the composition of gut microbiota in mice, increased the abundance of SCFAs-producing bacteria and reduce potential harmful bacteria (Cui et al., 2020).
As research progresses, many studies are no longer confined to the analysis of structural changes in the gut microbiota, but rather to explore the targets of dysbiosis by finding the key flora that play a regulatory role. E. xylanophilum is reported to be negatively correlated with body weight and serum total cholesterol, with potential anti-obesity and regulatory dysbiosis (Wei et al., 2021). Expression of IL-1β and IL-6 was negatively correlated with Roseburia, while were positively correlated with Paraprevotella and Parasutterella (Wang et al., 2020). A.- muciniphila is a mucin degrading bacterium, which have the ability to reduce inflammation and improve intestinal barrier function, and upward adjustment of its abundance can be considered as regulation to dysbiosis (Lee et al., 2021). These studies have made up for the lack of pertinence in flora analysis. In the following studies on the mechanism of MPs regulating dysbiosis, specific pathways and gene expression of key bacteria that paly important regulatory roles can be explored, thereby providing more information for dietary intervention to alleviate dysbiosis.
4.3. Regulatory effects of MPs on metabolites
There is a close interaction between MPs and gut microbiota. Indigestible polysaccharides provide an essential carbon source for the growth of gut microbiota, meanwhile polysaccharides were degraded via a variety of CAZymes encoded by gut microbiota to participate in metabolic regulation. CAZymes mainly include glycoside hydrolases (GHs), polysaccharide lyases (PLs), and carbohydrate esterases (CEs). There are differences in the number and composition ratio of CAZymes carried by different mrobiota, so that MPs will enrich specific microbiota during fermentation, thus affecting the overall microbiota structure (Zheng et al., 2020).Various metabolic transport modes of polysaccharides in vivo have been found, on the one hand, it can be fermented via the biotransformation of gut microbiota, such as Bacteroidetes, Clostridium and Phascolarctobacterium to produce SCFAs which are the main metabolites involved in improving metabolism (Fang et al., 2019).
SCFAs refer to saturated fatty acids containing one to six carbon atoms, such as acetic acid, propionic acid and butyric acid, which account for about 3:1:1 in the colon (Dalile, Van Oudenhove, Vervliet, & Verbeke, 2019). Butyric acid acts as the main energy source for intestinal epithelial cells, while other acids act on the organism through metabolic transport. SCFAs plays an important role in maintaining the structural function and integrity of the intestine. For physical barriers, SCFAs upregulates tight junction protein expression and reduces permeability. For biological barriers, SCFAs acts through reduce the pH value in the intestinal tract to play a certain inhibitory effect, and promote more SCFAs production when reach to certain pH. For chemical barrier, it can promote the expression of MUC2 genes and stimulate the release of defensins and antimicrobial peptides from Paneth cells to inhibit pathogenic bacteria. For immune barrier, SCFAs can act as histone deacetylase (HDAC) inhibitor, regulate apoptosis and related protein expression stability, as well as play an intestinal immunomodulatory role by activating cellular receptors such as G protein-coupled receptor (GPCR), Toll-like receptor (TLR) and Olfactory receptor 78 (OLFR78) to participate in regulating immune cell proliferation and inflammatory response. Different SCFAs have different activation of receptors and exhibit different binding affinity. Besides, SCFAs exhibits functional properties in immune regulation, energy metabolism regulation and brain activity regulation (Ma et al., 2022, Markowiak-Kopec and Slizewska, 2020, Poppe et al., 2021, Thomson et al., 2021, Wang et al., 2021). Increasing SCFAs content is a sign of the prebiotic properties of polysaccharides, which is of special significance to improve the symptoms of dysbiosis. The content of SCFAs could be increased by up-regulating SCFAs-producing bacteria or regulating the metabolic pathway. Oyster polysaccharides have been shown to increase the production of propionic acid and butyric acid in mice treated with 5-fluorouracil (Cai et al., 2021). The relative expression levels of GPR43, Olfr78 and GPR109A were increased by 0.72, 1.32 and 2.33 times respectively under the action of polysaccharides from Gracilaria Lemaneiformis, which effectively promoted the corresponding functions of SCFAs in the organism (Han et al., 2021).
In addition to the main metabolite SCFAs, secondary metabolites including hydrogen (H2), methane (CH4), carbon dioxide (CO2), hydrogen sulfide (H2S) and nitric oxide (NO) are also produced during the biotransformation of MPs. Studies have shown that these gases also play a role in intestinal function and immune regulation. Abnormal methane production has reported that is associated with the occurrence of some diseases and can be used as a marker for diagnosis and treatment. However, there are few studies on the regulation of these gases and dysbiosis, and the specific regulatory mechanism needs to be further explored (Song et al., 2021, Zhang et al., 2022).
On the other hand, MPs can act on gut microbiota to regulate its metabolism. It should be noted that the colonization of the gastrointestinal tract by gut microbiota is associated with production by no specific metabolites. These substances also support the breakdown of undigested food, are substrates for numerous biochemical pathways, and thus indirectly fulfill the functions of regulatory in the gastrointestinal tract (Debnath, Kumar, Kumar, Mehta, & Yadav, 2021). The key metabolites of gut microbiota include SCFAs, bile acids (BAs), amino acid, tryptamine and serotonin (Ghosh, Shanahan, & O'Toole, 2022). Among them, BAs can regulate lipid, glucose and energy metabolism by activating vitamins D3 receptor (VDR), farnesoid X receptor (FXR), Takeda Gprotein receptor (TGR5) and other related receptor, and its metabolism is closely related to metabolic syndrome such as obesity, type 2 diabetes and hyperlipidemia (Zhang et al., 2022). Recent studies have shown that regulating BAs metabolism helps to alleviate irradiation-induced intestinal damage (Guo, Da, Gao, Miao, Guo, Zhang, & Liu, 2022). The neurotransmitter 5-HT produced by the common tryptophan metabolic pathway in amino acid metabolism not only plays an important role in maintaining brain homeostasis, but also plays a role in emotion, intestinal motility regulation and intestinal permeability improvement. In addition to beneficial metabolites, the effect of MPs on adverse metabolites is also worthy of attention (Suganya & Koo, 2020). For example, the increase in the content of LPS will lead to an increase in intestinal permeability, disrupt the integrity of the intestinal barrier, and easily cause microbiota translocation to cause dysbiosis. It often mediates the inflammatory response by binding to the specific receptor TLR4. Mussel polysaccharidesα-d-glucan have been shown to inhibit LPS-TLR4-NF-κB pathway to reduce levels of inflammation in the body involved in improving nonalcoholic fatty liver disease (Wu et al., 2019, Zhang et al., 2022).
This shows that gut microbiota metabolites exhibit a role in the gut-islet axis, the gut-brain axis, and the gut-liver axis. Although the production of some relevant metabolites is closely associated with specific gut microbiota, there are corresponding studies showing that the increase of specific metabolites does not necessarily link to enrichment trend in the gut microbiota mediating their production. Therefore, it is affecting to elucidate the utilization sites and signal transduction of polysaccharides for gut microbiota with studies on the specific mechanisms of the overall metabolic network mediated by MPs in the biotransformation process on the dysbiosis (Cheng, Hu, Geng, & Nie, 2022).
4.4. Other potential regulatory effects of MPs
In addition to these main regulatory effects, there are still other regulatory pathways that have not been widely studied or are still unexplored. MPs exerting their own antioxidant, anti-inflammatory and anti-coagulant activities to mitigate the dysbiosis caused by external factors is certainly an important potential regulatory effect. Some studies have shown that in a model of hydrogen peroxide-induced intestinal epithelial cell injury, MPs can reduce oxidative stress levels and mitigate intestinal barrier damage by scavenging oxidative radicals, reducing lipid oxidation end products malondialdehyde (MDA), and normalizing the antioxidant enzyme system (Xiang et al., 2022). Moreover, some MPs have been reported to promote intestinal epithelial cell migration and proliferation, which have protective and repairing effects on the intestinal barrier (Qiu et al., 2020). In the case of increased intestinal inflammation, MPs can mediate TLR4 and NF-κB signaling pathway to down-regulate pro-inflammatory factors and up-regulate the expression of anti-inflammatory factors (Yao et al., 2022). And there exist study indicate that MPs inhibiting the expression of CC-chemokine 9 receptor (CCR9) and CC-chemokine ligand 25 (CCL-25) which is positively correlated with the inflammatory response, up-regulating the expression of transforming growth factor-β1 (TGF-β1) and clusters of differentiation 40 (CD40) which maintain the immune homeostasis to alleviate the inflammatory response (Lu et al., 2022). Furthermore, for the symptoms of dysbiosis associated with diseases, MPs can exert regulatory effects, synergistically enhance barrier function, regulate flora and metabolic transformation to alleviate the adverse effects of diseases. According to the study, polysaccharide from Laminaria japonica can regulate liver energy homeostasis to alleviate obesity-related nonalcoholic fatty liver disease (Zhang et al., 2021). Holothuria leucospilota polysaccharide can alleviate type 2 diabetes by regulating blood lipid and hormone levels (Zhao et al., 2020). Gloiopeltis furcata polysaccharide can remode mucin O-glycans to alleviate colitis (Pan et al., 2022). From these regulatory processes, it can be seen that the regulatory effect of MPs on dysbiosis is not independent, which is often interrelated, interactive, and finally plays an overall regulatory role. Therefore, in the future research, it is a meaningful direction to explore the overall regulation of MPs on dysbiosis and other potential mechanisms of action by multi-omics with the help of systems biology approach.
5. Conclusions and perspectives
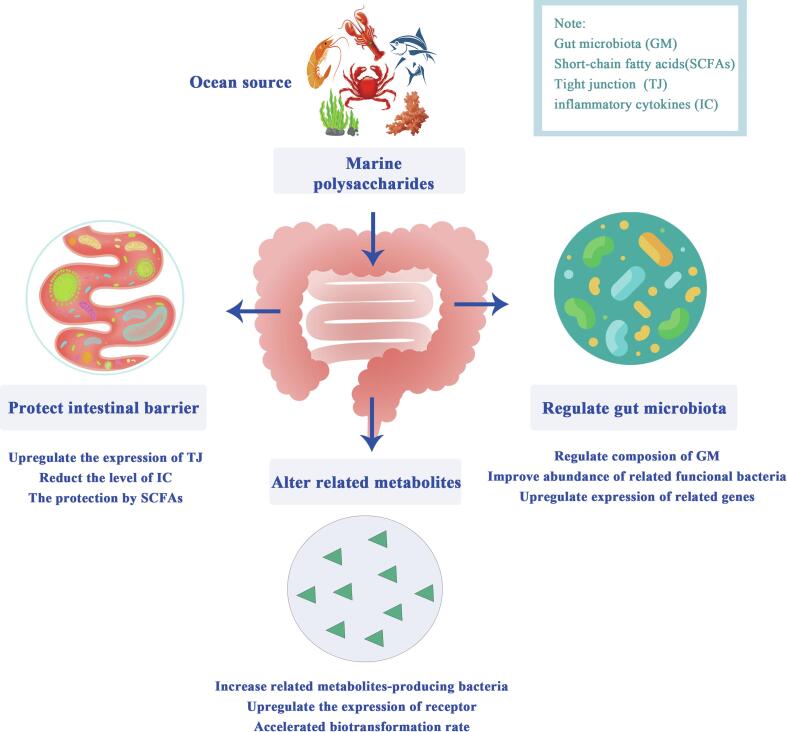
Gut microbiota dysbiosis is a common issue affecting human life. Studies have shown that dysbiosis are associated with many diseases and it's of great value to find some effective methods to rebalance dysbiosis. In this review, the regulatory effects on gut microbiota dysbiosis and potential prebiotic effect of MPs were summarized. MPs have the potential to regulate the gut microbiota dysbiosis directly and indirectly under different types or degrees of dysbiosis. On one hand, it can directly regulate the diversity and abundance of gut microbiota through increasing beneficial bacteria and decreasing pernicious bacteria. On the other hand, MPs indirectly alleviates gut microbiota dysbiosis by protecting the integrity of the intestinal barrier and being transformed into beneficial metabolites (Fig. 5).
Fig. 5.
Mechanisms of the regulatory effects of marine polysaccharides on gut microbiota dysbiosis.
From MPs have great potential to be prebiotics as described in this article. But high-added-value utilization of MPs is still in its infancy. The structure–activity relationship of MPs is still unclear for its complex composition and structure. What’s more, it is difficult to obtain uniform active components of MPs due to the complex and time-consuming purification process. More studies needed to focus on optimization of the purification process, structure–activity relationship and prebiotic activity evaluation of polysaccharide to promote its high-added-value utilization. There exist a lot of questions needed to be explored in the following studies. In terms of regulating gut microbiota dysbiosis, if MPs do not meet the criteria to be prebiotic, whether and how they still play a role is a point. Moreover, given the dysbiosis caused by different factors, whether there are differences in the regulation of polysaccharides is vital. Future research on MPs and human health may include (1) multi-omics (metabolomics, proteomics, metagenomics or ecogenomics) analysis of the changes in the gut microbiota after consumption of MPs; (2) in-depth studies on the functional role, action model and concrete mechanism of MPs in balancing gut microbiota and maintaining host health. (3) targeted explorations about key factors and targets (related gut microbiota and metabolites, the marker of dysbiosis, genetic markers) in the regulation of MPs and their potential as therapeutics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported in part by Key-Area Research and Development Program of Guangdong Province (2020B1111030004), National Key Research and Development Project (2020YFD0901101, 2019YFD0902005), Zhanjiang Ocean Youth Talent Innovation Project (2021E05019), the Science and Technology Program of Zhanjiang City (2019A01015), the Science and Technology Development Program of Shenzhen City, China (JCYJ20170818111335796) and the Innovative Team Program of High Education of Guangdong Province (2021KCXTD021).
References
- Adak A., Khan M.R. An insight into gut microbiota and its functionalities. Cellular and Molecular Life Sciences. 2019;76(2):473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Chait Y., Mottawea W., Tompkins T.A., Hammami R. Nutritional and therapeutic approaches for protecting human gut microbiota from psychotropic treatments. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2021;108 doi: 10.1016/j.pnpbp.2020.110182. [DOI] [PubMed] [Google Scholar]
- Albillos A., De Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. Journal of Hepatology. 2020;72(3):558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Angel N., Li S.N., Yan F., Kong L.Y. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends in Food Science & Technology. 2022;120:308–324. doi: 10.1016/J.TIFS.2022.01.003. [DOI] [Google Scholar]
- Anwar, H., Iftikhar, A., Muzaffar, H., Almatroudi, A., Allemailem, K. S., Navaid, S., . . . Khurshid, M. (2021). Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. BioMed Research Internationa, 2021. [DOI] [PMC free article] [PubMed]
- Ashayerizadeh O., Dastar B., Pourashouri P. Study of antioxidant and antibacterial activities of depolymerized fucoidans extracted from Sargassum tenerrimum. International Journal of Biological Macromolecules. 2020;151:1259–1266. doi: 10.1016/j.ijbiomac.2019.10.172. [DOI] [PubMed] [Google Scholar]
- Bie N., Duan S., Meng M., Guo M., Wang C. Regulatory effect of non-starch polysaccharides from purple sweet potato on intestinal microbiota of mice with antibiotic-associated diarrhea. Food Function. 2021;12(12):5563–5575. doi: 10.1039/d0fo03465g. [DOI] [PubMed] [Google Scholar]
- Caglayan C., Taslimi P., Turk C., Gulcin I., Kandemir F., Demir Y., Beydemir S. Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environmental Science Pollution Research. 2020;27(10):10607–10616. doi: 10.1007/s11356-020-07611-z. [DOI] [PubMed] [Google Scholar]
- Cai J., Chen Z., Wu W., Lin Q., Liang Y. High animal protein diet and gut microbiota in human health. Critical Reviews in Food Science and Nutrition. 2021;62(22):6225–6237. doi: 10.1080/10408398.2021.1898336. [DOI] [PubMed] [Google Scholar]
- Cai B., Pan J., Chen H., Chen X., Ye Z., Yuan H., Sun H., Wan P. Oyster polysaccharides ameliorate intestinal mucositis and improve metabolism in 5-fluorouracil-treated S180 tumour-bearing mice. Carbohydrate Polymers. 2021;256 doi: 10.1016/j.carbpol.2020.117545. [DOI] [PubMed] [Google Scholar]
- Cao Y., Gao J., Zhang L., Qin N., Zhu B., Xia X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food & Function. 2021;12(20):10121–10135. doi: 10.1039/d1fo02001c. [DOI] [PubMed] [Google Scholar]
- Casas-Arrojo V., Decara J., De Los Angeles Arrojo-Agudo M., Perez-Manriquez C., Abdala-Diaz R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nageli. Biomolecules. 2021;11(4):488. doi: 10.3390/biom11040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Liu B., Wang X., Chen K., Zhang K., Zhang L.…Wang M. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. International Journal of Biological Macromolecules. 2020;158:740–749. doi: 10.1016/j.ijbiomac.2020.04.201. [DOI] [PubMed] [Google Scholar]
- Cheng J., Hu J., Geng F., Nie S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Science and Human Wellness. 2022;11(5):1101–1110. [Google Scholar]
- Chi L., Xue J., Tu P., Lai Y., Ru H., Lu K. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Archives of Toxicology. 2019;93(1):25–35. doi: 10.1007/s00204-018-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowska-Smigiel A., Gniewosz M., Kieliszek M., Bzducha-Wrobel A. The Effect of Pullulan on the Growth and Acidifying Activity of Selected Stool Microflora of Human. Current Pharmaceutical Biotechnology. 2017;18(2):121–126. doi: 10.2174/1389201017666161229154324. [DOI] [PubMed] [Google Scholar]
- Chlebowska-Smigiel A., Kycia K., Neffe-Skocinska K., Kieliszek M., Gniewosz M., Kolozyn-Krajewska D. Effect of Pullulan on Physicochemical, Microbiological, and Sensory Quality of Yogurts. Current Pharmaceutical Biotechnology. 2019;20(6):489–496. doi: 10.2174/1389201020666190416151129. [DOI] [PubMed] [Google Scholar]
- Cui M.X., Wang Y., Elango J., Wu J.W., Liu K.H., Jin Y.Z. Cereus sinensis Polysaccharide Alleviates Antibiotic-Associated Diarrhea Based on Modulating the Gut Microbiota in C57BL/6 Mice. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.751992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M., Zhang M., Wu J., Han P., Lv M., Ling D., Liu K. Marine polysaccharides from Gelidium pacificum Okamura and Cereus sinensis reveal prebiotic functions. International Journal of Biological Macromolecules. 2020;164:4381–4390. doi: 10.1016/j.ijbiomac.2020.08.255. [DOI] [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nature Reviews Gastroenterology & Hepatology. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- de Medeiros V.P.B., de Souza E.L., de Albuquerque T.M.R., Sassi C.F.D., Lima M.D., Sivieri K.…Magnani M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Research. 2021;60 [Google Scholar]
- Debnath N., Kumar R., Kumar A., Mehta P.K., Yadav A.K. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnology & Genetic Engineering Reviews. 2021;37(2):105–153. doi: 10.1080/02648725.2021.1989847. [DOI] [PubMed] [Google Scholar]
- Ding H., Zhou T., Wang J., Liu Y., Xu P., Xu A. Research progress on the effects of typical environmental pollutants on gut microbiota and their underlying mechanisms. Asian Journal of Ecotoxicology. 2021;16(02):34–49. in Chinese. [Google Scholar]
- Donovan S.M. Introduction to the special focus issue on the impact of diet on gut microbiota composition and function and future opportunities for nutritional modulation of the gut microbiome to improve human health. Gut Microbes. 2017;8(2):75–81. doi: 10.1080/19490976.2017.1299309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Yu L., Tian F., Zhai Q., Fan L., Chen W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Science of the Total Environment. 2020;742 doi: 10.1016/j.scitotenv.2020.140429. [DOI] [PubMed] [Google Scholar]
- Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M.…Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidy S., van den Bogert B., Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Current Opinion in Biotechnology. 2015;32:14–20. doi: 10.1016/j.copbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Fang Q., Hu J., Nie Q., Nie S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends in Food Science & Technology. 2019;92:65–70. [Google Scholar]
- Ghosh, T. S., Shanahan, F., & O'Toole, P. W. The gut microbiome as a modulator of healthy ageing. Gastroenterology & hepatology, 19(9), 565-584. [DOI] [PMC free article] [PubMed]
- Gokulan K., Arnold M.G., Jensen J., Vanlandingham M., Twaddle N., Doerge D.R., Cerniglia C.E., Khare S. Exposure to Arsenite in CD-1 Mice during Juvenile and Adult Stages: Effects on Intestinal Microbiota and Gut-Associated Immune Status. mBio. 2018;9(4) doi: 10.1128/mBio.01418-18. e01418-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H., Li W., Sun J., Jia L., Guan Q., Guo Y., Wang Y. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. International Journal of Biological Macromolecules. 2022;211:711–728. doi: 10.1016/j.ijbiomac.2022.05.087. [DOI] [PubMed] [Google Scholar]
- Guo, L., Da, F., Gao, Q., Miao, X., Guo, J., Zhang, W., … Liu, J. (2022). Irradiation-induced intestinal injury is associated with disorders of bile acids metabolism. International Journal of Radiation Oncology Biology Physics. doi: 10.1016/j.ijrobp.2022.08.007. [DOI] [PubMed]
- Gurpilhares D.B., Cinelli L.P., Simas N.K., Pessoa A., Jr., Sette L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chemistry. 2019;280:175–186. doi: 10.1016/j.foodchem.2018.12.023. [DOI] [PubMed] [Google Scholar]
- Han R., Ma Y., Xiao J., You L., Pedisic S., Liao L. The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria Lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food and Chemical Toxicology. 2021;149 doi: 10.1016/j.fct.2021.112001. [DOI] [PubMed] [Google Scholar]
- Han R., Pang D., Wen L., You L., Huang R., Kulikouskaya V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. Journal of Functional Foods. 2020;64 [Google Scholar]
- Hou K., Wu Z., Chen X., Wang J., Zhang D., Xiao C.…Chen Z. Microbiota in health and diseases. Signal Transduction and Targeted Therapy. 2022;7(1):135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhou G. Study progress in common pathogenies of intestinal dysbateriosis. Medical Recapitulate. 2011;17(02):239–241. in Chinese. [Google Scholar]
- Hu L., Zhu S., Peng X., Li K., Peng W., Zhong Y.…Zhao B. High Salt Elicits Brain Inflammation and Cognitive Dysfunction, Accompanied by Alternations in the Gut Microbiota and Decreased SCFA Production. Journal of Alzheimers Ddisease. 2020;77(2):629–640. doi: 10.3233/JAD-200035. [DOI] [PubMed] [Google Scholar]
- Hua Q., Han Y., Zhao H., Zhang H., Yan B., Pei S.…Li D. Punicalagin alleviates renal injury via the gut-kidney axis in high-fat diet-induced diabetic mice. Food & Function. 2022;13(2):867–879. doi: 10.1039/d1fo03343c. [DOI] [PubMed] [Google Scholar]
- Huang Y., Chen H., Zhang K., Lu Y., Wu Q., Chen J., Chen Y. Extraction, purification, structural characterization, and gut microbiota relationship of polysaccharides: A review. International Journal of Biological Macromolecules. 2022;213:967–986. doi: 10.1016/j.ijbiomac.2022.06.049. [DOI] [PubMed] [Google Scholar]
- Jandhyala S., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D. Role of the normal gut microbiota. World Journal of Gastroenterology. 2015;21(29):8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Zheng W., Sun X., Jiang G., Wu S., Xu Y., Song S., Ai C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. International Journal of Biological Macromolecules. 2021;167:1587–1597. doi: 10.1016/j.ijbiomac.2020.11.116. [DOI] [PubMed] [Google Scholar]
- Kanwal S., Joseph T.P., Owusu L., Xiaomeng R., Meiqi L., Yi X. A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients. 2018;10(8):1003. doi: 10.3390/nu10081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Behl T., Bungau S., Kumar A., Uddin M.S., Mehta V.…Arora S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson's Disease. Current Neuropharmacology. 2021;19(2):233–247. doi: 10.2174/1570159X18666200606233050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M., Pobiega K., Piwowarek K., Kot A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules. 2021;26(7):1858. doi: 10.3390/molecules26071858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q.H., Zhang R.F., You L.J., Ma Y.X., Liao L., Pedisic S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. Food and Chemical Toxicology. 2021;151 doi: 10.1016/j.fct.2021.112145. [DOI] [PubMed] [Google Scholar]
- Kucuk M., Gulcin I. Purification and characterization of the carbonic anhydrase enzyme from Black Sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on enzyme activity. Environmental Toxicology and Pharmacology. 2016;44:134–139. doi: 10.1016/j.etap.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Kumar Singh A., Cabral C., Kumar R., Ganguly R., Kumar Rana H., Gupta A.…Pandey A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients. 2019;11(9):2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.H., Chen C.H., Hsu Y.Y., Chuang P.T., Shih M.K., Hsu W.H. Polysaccharides Obtained from Cordyceps militaris Alleviate Hyperglycemia by Regulating Gut Microbiota in Mice Fed a High-Fat/Sucrose Diet. Foods. 2021;10(8):1870. doi: 10.3390/foods10081870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Xue C., Zhang T., Wang Y. The interaction between dietary marine components and intestinal flora. Marine Life Science & Technology. 2020;2(2):161–171. [Google Scholar]
- Li D., Yang Y., Li Y., Li Z., Zhu X., Zeng X. Changes induced by chronic exposure to high arsenic concentrations in the intestine and its microenvironment. Toxicology. 2021;456 doi: 10.1016/j.tox.2021.152767. [DOI] [PubMed] [Google Scholar]
- Li N., Zhao Y., Chen Z., Wang B., Luo X., Wang Q. Dynamic effects of antibiotic-induced intestinal dysbacteriosis on intestinal mucosa barrier and liver function. Chinese Journal of Animal Nutrition. 2019;31(03):1278–1287. in Chinese. [Google Scholar]
- Liu Y., Li Y., Xia Y., Liu K., Ren L., Ji Y. The Dysbiosis of Gut Microbiota Caused by Low-Dose Cadmium Aggravate the Injury of Mice Liver through Increasing Intestinal Permeability. Microorganisms. 2020;8(2):211. doi: 10.3390/microorganisms8020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu W., Wang Y., Ma Y., Huang L., Zou C.…Liu G. Inhibitory Effect of Depolymerized Sulfated Galactans from Marine Red Algae on the Growth and Adhesion of Diarrheagenic Escherichia coli. Marine Drugs. 2019;17(12):694. doi: 10.3390/md17120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Sun T. Research progress of intestinal dysbacteriosis. Medical Recapitulate. 2014;20(03):468–471. in Chinese. [Google Scholar]
- Liu W., Tang S., Zhao Q., Zhang W., Li K., Yao W., Gao X. The α-D-glucan from marine fungus Phoma herbarum YS4108 ameliorated mice colitis by repairing mucosal barrier and maintaining intestinal homeostasis. International Journal of Biological Macromolecules. 2020;149:1180–1188. doi: 10.1016/j.ijbiomac.2020.01.303. [DOI] [PubMed] [Google Scholar]
- Liu X., Xi X., Jia A., Zhang M., Cui T., Bai X.…Liu C. A fucoidan from Sargassum fusiforme with novel structure and its regulatory effects on intestinal microbiota in high-fat diet-fed mice. Food Chemistry. 2021;358 doi: 10.1016/j.foodchem.2021.129908. [DOI] [PubMed] [Google Scholar]
- Lopez-Santamarina A., Cardelle-Cobas A., Mondragon A.D., Sinisterra-Loaiza L., Miranda J.M., Cepeda A. Evaluation of the potential prebiotic effect of Himanthalia elongata, an Atlantic brown seaweed, in an in vitro model of the human distal colon. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111156. [DOI] [PubMed] [Google Scholar]
- Lopez-Santamarina A., Miranda J.M., Mondragon A.D.C., Lamas A., Cardelle-Cobas A., Franco C.M., Cepeda A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules. 2020;25(4) doi: 10.3390/molecules25041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Liu Y., Tang S., Zhang W., Yu Q., Shi C., Cheong K. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chemistry- X. 2022;13 doi: 10.1016/j.fochx.2021.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Lin X., Bordiga M., Brennan C., Xu B. Manipulating effects of fruits and vegetables on gut microbiota – a critical review. International Journal of Food Science & Technology. 2021;56(5):2055–2067. [Google Scholar]
- Lv K., Yuan Q., Li H., Li T., Ma H., Gao C.…Zhao L. Chlorella pyrenoidosa Polysaccharides as a Prebiotic to Modulate Gut Microbiota: Physicochemical Properties and Fermentation Characteristics In Vitro. Foods. 2022;11(5):725. doi: 10.3390/foods11050725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Dai Z., Sun K. Intestinal Epithelial Cell Endoplasmic Reticulum Stress and Inflammatory Bowel Disease Pathogenesis: An Update Review. Frontiers in Immunology. 2017;8:1271. doi: 10.3389/fimmu.2017.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Jiang S., Zeng M. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Research International. 2021;149:11064. doi: 10.1016/j.foodres.2021.110646. [DOI] [PubMed] [Google Scholar]
- Ma J., Piao X., Mahfuz S., Long S., Wang J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Animal Nutrition. 2022;9:159–174. doi: 10.1016/j.aninu.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhu L., Ke H., Jiang S., Zeng M. Oyster (Crassostrea gigas) polysaccharide ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat diet fed mice. International Journal of Biological Macromolecules. 2022;216:916–926. doi: 10.1016/j.ijbiomac.2022.07.100. [DOI] [PubMed] [Google Scholar]
- Mariat D., Firmesse O., Levenez F., Guimaraes V.D., Sokol H., Dore J.…Furet J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiology. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak P., Slizewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Beneficial Microbes. 2017;11(2):135–149. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak-Kopec P., Slizewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrient. 2020;12(4):1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A.E., Elgammal W.E., Eid A.M., Dawaba A.M., Ibrahim A.G., Fouda A., Hassan S.M. Synthesis and characterization of new functionalized chitosan and its antimicrobial and in-vitro release behavior from topical gel. International Journal of Biological Macromolecules. 2022;207:242–253. doi: 10.1016/j.ijbiomac.2022.02.173. [DOI] [PubMed] [Google Scholar]
- Muthukumar J., Chidambaram R., Sukumaran S. Sulfated polysaccharides and its commercial applications in food industries-A review. Journal of Food Science and Technology-Mysore. 2021;58(7):2453–2466. doi: 10.1007/s13197-020-04837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone G., Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases. United European Gastroenterology Journal. 2015;3(3):255–260. doi: 10.1177/2050640614566846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y., Qiu Y., Liu Y., Zhu R., Chen Y., EI-Seedj H.R., Chen X., Zhao C. Cancer-fighting potentials of algal polysaccharides as nutraceuticals. Food Research International. 2021;147 doi: 10.1016/j.foodres.2021.110522. [DOI] [PubMed] [Google Scholar]
- Palmu J., Lahti L., Niiranen T. Targeting Gut Microbiota to Treat Hypertension: A Systematic Review. International Journal of Environmental Research and Public Health. 2021;18(3):1248. doi: 10.3390/ijerph18031248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Fu T., Cheng H., Mi J., Shang Q., Yu G. Polysaccharide from edible alga Gloiopeltis furcata attenuates intestinal mucosal damage by therapeutically remodeling the interactions between gut microbiota and mucin O-glycans. Carbohydrate Polymers. 2022;278 doi: 10.1016/j.carbpol.2021.118921. [DOI] [PubMed] [Google Scholar]
- Papon N., Copp B.R., Courdavault V. Marine drugs: Biology, pipelines, current and future prospects for production. Biotechnology Advances. 2022;54 doi: 10.1016/j.biotechadv.2021.107871. [DOI] [PubMed] [Google Scholar]
- Payling L., Fraser K., Loveday S.M., Sims I., Roy N., McNabb W. The effects of carbohydrate structure on the composition and functionality of the human gut microbiota. Trends in Food Science & Technology. 2020;97:233–248. [Google Scholar]
- Poppe J., van Baarle L., Matteoli G., Verbeke K. How Microbial Food Fermentation Supports a Tolerant Gut. Molecular Nutrition & Food Research. 2021;65(5):e2000036. doi: 10.1002/mnfr.202000036. [DOI] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C.…Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–70. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Veeraperumal S., Lv J., Wu T., Zhang Z., Zeng Q.…Cheong K.L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydrate Polymers. 2020;246 doi: 10.1016/j.carbpol.2020.116626. [DOI] [PubMed] [Google Scholar]
- Rinninella E., Cintoni M., Raoul P., Lopetuso L.R., Scaldaferri F., Pulcini G.…Mele M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients. 2019;11(10) doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nature Reviews Gastroenterology Hepatology. 2019;16(10):605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- Shang Q., Li Q., Zhang M., Song G., Shi J., Jiang H.…Yu G. Dietary Keratan Sulfate from Shark Cartilage Modulates Gut Microbiota and Increases the Abundance of Lactobacillus spp. Marine Drugs. 2016;14(12):224. doi: 10.3390/md14120224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Chan A., Sun J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology. 2020;158(2):322–340. doi: 10.1053/j.gastro.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Wang Y., Huang L., Shen M., Yu Y., Yu Q.…Xie J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Research International. 2021;140 doi: 10.1016/j.foodres.2020.109858. [DOI] [PubMed] [Google Scholar]
- Suganya K., Koo B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. International Journal of Molecular Sciences. 2020;21(20):7551. doi: 10.3390/ijms21207551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Ding R., Sun J., Liu J., Kan J., Jin C. The impacts of natural polysaccharides on intestinal microbiota and immune responses - a review. Food Function. 2019;10(5):2290–2312. doi: 10.1039/c8fo01946k. [DOI] [PubMed] [Google Scholar]
- Tang N.Y., Wang X.M., Yang R., Liu Z.M., Liu Y.X., Tian J.J.…Li W. Extraction, isolation, structural characterization and prebiotic activity of cell wall polysaccharide from Kluyveromyces marxianus. Carbohydrate Polymers. 2022;289 doi: 10.1016/j.carbpol.2022.119457. [DOI] [PubMed] [Google Scholar]
- Thomson C., Garcia A.L., Edwards C.A. Interactions between dietary fibre and the gut microbiota. Proceedings of the Nutrition Society. 2021;80(4):398–408. doi: 10.1017/S0029665121002834. [DOI] [PubMed] [Google Scholar]
- Ustyuzhanina N.E., Bilan M.I., Dmitrenok A.S., Silchenko A.S., Grebnev B.B., Stonik V.A.…Usov A.I. Fucosylated Chondroitin Sulfates from the Sea Cucumbers Paracaudina chilensis and Holothuria hilla: Structures and Anticoagulant Activity. Marine Drugs. 2020;18(11):540. doi: 10.3390/md18110540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ai C., Wen C., Qin Y., Liu Z., Wang L.…Song S. Fucoidan isolated from Ascophyllum nodosum alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food & Function. 2020;11(6):5595–5606. doi: 10.1039/d0fo00668h. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang X., Jiang H., Cai C., Li G., Hao J., Yu G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydrate Polymers. 2018;195:601–612. doi: 10.1016/j.carbpol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhou J., Selma-Royo M., Simal-Gandara J., Collado M.C., Barba F.J. Potential benefits of high-added-value compounds from aquaculture and fish side streams on human gut microbiota. Trends in Food Science & Technology. 2021;112:484–494. [Google Scholar]
- Wasko A., Kieliszek M., Targonski Z. Purification and characterization of a proteinase from the probiotic Lactobacillus rhamnosus OXY. Preparative Biochemistry and Biotechnology. 2012;42(5):476–488. doi: 10.1080/10826068.2012.656869. [DOI] [PubMed] [Google Scholar]
- Wei B., Zhang B., Du A., Zhou Z., Lu D., Zhu Z.…Wang H. Saccharina japonica fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria. Carbohydrate Polymers. 2022;290 doi: 10.1016/j.carbpol.2022.119411. [DOI] [PubMed] [Google Scholar]
- Wei J., Zhao Y., Zhou C., Zhao Q., Zhong H., Zhu X.…Yu G. Dietary Polysaccharide from Enteromorpha clathrata Attenuates Obesity and Increases the Intestinal Abundance of Butyrate-Producing Bacterium, Eubacterium xylanophilum, in Mice Fed a High-Fat Diet. Polymers. 2021;13(19) doi: 10.3390/polym13193286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G.A., Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cellular and Molecular Life Sciences. 2017;74(16):2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Liu Y., Jiang P., Xu Y., Zheng W., Song S., Ai C. Effect of sulfate group on sulfated polysaccharides-induced improvement of metabolic syndrome and gut microbiota dysbiosis in high fat diet-fed mice. International Journal of Biological Macromolecules. 2020;164:2062–2072. doi: 10.1016/j.ijbiomac.2020.08.010. [DOI] [PubMed] [Google Scholar]
- Wu R., Ma L., Li P. Effect of Chondroitin Sulfate from Sturgeon on intestinal flora of mice with Colorectal Cancer. Food Science. 2020;41(15):216–223. in Chinese. [Google Scholar]
- Wu J., Shao H., Zhang J., Ying Y., Cheng Y., Zhao D.…Ling P. Mussel polysaccharide α -D-glucan (MP-A) protects against non-alcoholic fatty liver disease via maintaining the homeostasis of gut microbiota and regulating related gut-liver axis signaling pathways. International Journal of Biological Macromolecules. 2019;130(2019):68–78. doi: 10.1016/j.ijbiomac.2019.02.097. [DOI] [PubMed] [Google Scholar]
- Xiang X., Wang R., Chen H., Chen Y., Shen G., Liu S.…Chen L. Structural characterization of a novel marine polysaccharide from mussel and its antioxidant activity in RAW264.7 cells induced by H2O2. Food BioScience. 2022;47 [Google Scholar]
- Xu X., Xu P., Ma C., Tang J., Zhang X. Gut microbiota, host health, and polysaccharides. Biotechnology Advances. 2013;31(2):318–337. doi: 10.1016/j.biotechadv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Xue C., Xie Q., Zhang C., Hu Y., Song X., Jia Y.…Yuan H. Vertical transmission of the gut microbiota influences glucose metabolism in offspring of mice with hyperglycaemia in pregnancy. Microbiome. 2022;10(1):122. doi: 10.1186/s40168-022-01318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Liu M., Chen X., You L., Ma Y., Hileuskaya K. Effects of UV/H2O2 degradation and step gradient ethanol precipitation on Sargassum fusiforme polysaccharides: Physicochemical characterization and protective effects against intestinal epithelial injury. Food Research International. 2022;155 doi: 10.1016/j.foodres.2022.111093. [DOI] [PubMed] [Google Scholar]
- Yeoh Y., Zuo T., Lui G., Zhang F., Liu Q., Li A.Y.L.…Siew C.N. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Yu Y., Xiao Y., Tian F., Narbad A., Zhai Q., Chen W. Lead-induced gut injuries and the dietary protective strategies: A review. Journal of Functional Foods. 2021;83 [Google Scholar]
- Yu L., Zhai Q., Tian F., Liu X., Wang G., Zhao J.…Chen W. Potential of Lactobacillus plantarum CCFM639 in Protecting against Aluminum Toxicity Mediated by Intestinal Barrier Function and Oxidative Stress. Nutrients. 2016;8(12):783. doi: 10.3390/nu8120783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zhou G., Shao B. Gut Microbiota Dysbiosis Induced by Intracerebral Hemorrhage Aggravates Neuroinflammation in Mice. Frontiers In Microbiology. 2021;12 doi: 10.3389/fmicb.2021.647304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Wang J., Cen S., Zhao J., Zhang H., Tian F., Chen W. Modulation of the gut microbiota by a galactooligosaccharide protects against heavy metal lead accumulation in mice. Food & Function. 2019;10(6):3768–3781. doi: 10.1039/c9fo00587k. [DOI] [PubMed] [Google Scholar]
- Zhang D., Liu J., Cheng H., Wang H., Tan Y., Feng W., Peng C. Food Research International. 2022;31 doi: 10.1016/j.foodres.2022.111653. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang L., Zhao N., Hong Z., Cai B., Le Q.…He J. Soluble Polysaccharide Derived from Laminaria japonica Attenuates Obesity-Related Nonalcoholic Fatty Liver Disease Associated with Gut Microbiota Regulation. Marine Drugs. 2021;19(12):699. doi: 10.3390/md19120699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Liu Q., Cao J., Xu Y., Pei Z., Fan H.…Li C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food and Chemical Toxicology. 2020;135 doi: 10.1016/j.fct.2019.110886. [DOI] [PubMed] [Google Scholar]
- Zheng L., Chen X., Cheong K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. International Journal of Biological Macromolecules. 2020;151:344–354. doi: 10.1016/j.ijbiomac.2020.02.168. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Pan X., Luo L., Zhang Q., Huang X., Liu Y.…Zhang Y. Advances in oral absorption of polysaccharides: Mechanism, affecting factors, and improvement strategies. Carbohydrate Polymers. 2022;282 doi: 10.1016/j.carbpol.2022.119110. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Han Y., Ding Y., Zhu B., Song S., Xiao H. Health effects of dietary sulfated polysaccharides from seafoods and their interaction with gut microbiota. Comprehensive Reviews in Food Science and Food Safety. 2021;20(3):2882–2913. doi: 10.1111/1541-4337.12754. [DOI] [PubMed] [Google Scholar]
- Zhu J., Yu L., Shen X., Tian F., Zhao J., Zhang H., Chen W., Zhai Q. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. International Journal of Molecular Sciences. 2021;22(20):11045. doi: 10.3390/ijms222011045. [DOI] [PMC free article] [PubMed] [Google Scholar]