Highlights
-
•
Changes of terpenoids in SGP were identified by widely targeted metabolomics.
-
•
88 terpenoids compounds including 30 types of ginsenosides were changed in SGP.
-
•
Conversion mechanism of ginsenosides during commercial sterilization was elucidated.
-
•
Ginsenoside ST3 was detected and F4, Rg3, and Rg5 were found in fresh ginseng pulp.
Keywords: Ginseng, Widely targeted metabolomics, Terpenoids, Ginsenosides
Abstract
Terpenoids such as ginsenosides are the most important phytochemicals and functional components in ginseng. Commercial sterilizing with high temperature and high pressure is also one of the common methods of ginseng food processing. However, the changes of terpenoids in fresh ginsengs commercially sterilized are unclear. In this study, fresh ginseng pulp (FGP) was commercially sterilized at 121℃ for 30 min, and terpenoid compounds were analyzed by widely targeted metabolomics based on UPLC-ESI-MS/MS system. The commercial sterilization induced the changes of 88 terpenoid compounds including 30 types of ginsenosides, and many minor ginsenoside Rh4, Rg6, Rk2, F4, Rs3, Rk3, Rk1, Rg5, Rg3, Rg4 were remarkably increased in fresh ginseng pulp. Importantly, the ginsenoside ST3 was detected and F4, Rg3, and Rg5 were also found in fresh ginseng pulp. Commercial sterilizing at 121℃ for 30 min will remarkably affect the species and number of ginsenosides in ginseng food.
Introduction
Ginseng (Panax ginseng C.A. Meyer), as one of the most popular traditional Chinese medicine in the world, is utilized as a dietary supplement (Riaz, Rahman, Zia-Ul-Haq, Jaffar, & Manea, 2019) and functional food with various health maintaining and pharmacological functions such as anti-cancer, antioxidant and anti-aging in China, Korea, and other regions of East Asia (Chung, Kim, Seguin, Jun, & Kim, 2012). Ginsengs contain a variety of compounds, including ginsenosides, organic acids, amino acids, polysaccharides and volatile oil (Qi, Wang, & Yuan, 2011). The main effective component of ginseng is generally considered as ginsenosides, also known chemically as ginseng saponins, which are responsible for ginseng's medicinal properties. Ginsenosides have been found and identified for more than 180 species, and they are regarded as the most important bioactive components in the ginseng (Zhang, Zhong, Li, & Zhang, 2020). Ginsenosides can be divided into four subtypes as protopanaxadiol (PPD), protopanaxatriol (PPT), oleanolic acid, and octillol considering that ginsenosides have a typical four-ring hydrophobic steroid-like structure with sugar moieties linked at the C-3, C-6, or C-20 position. The naturally occurring saponins exhibit a wide range of polarity and hydrophobicity due to the diversity of their chemical structures, which results in their distinctive biological functions (Zhang et al., 2020, Zhao et al., 2021). Different ginsenoside monomers have different functions including anti-inflammation, anti-cancer, anti-aging, anti-diabetic and cardiovascular protective activities (Gao et al., 2020). In general, minor ginsenosides have better pharmaceutical activity than major ginsenosides, which have smaller sizes, better bioavailability as well as permeability across the cell membrane (Xu, Fang & Chen, 2003).
As the main functional component of ginseng, the content and variety of ginsenosides become an important index to measure the quality of ginseng food. In the processing of ginseng, the process conditions have a great impact on the ginsenosides (Christensen, 2009), including heating (Kim et al., 2000), acid treatment, and fermentation (Bae, Han, Kim, & Kim, 2004). Many malonyl-ginsenosides occur in fresh ginseng, which are converted into other ginsenosides by demalonylation, decarboxylation, deglycosylation and dehydration when fresh ginseng is processed into red ginseng (Jung, Lee, & Paik, 2017). For example, malonyl-ginsenosides Rb1, Rc, Rb2 and Rd would be converted to ginsenosides Rb1, Rc, Rb2, Rd and generate malonic acid through hydrolysis (Chen, Balan & Popovich, 2020). The protopanaxadiol type ginsenoside Rb1, Rb2 and Rb3 would be converted to Rg3 and the protopanaxatriol type ginsenoside Re would be hydrolyzed to remove the glucose at the C-20 position to generate Rg2 in heat processing (Yao et al., 2021). Therefore, it is of vital importance to evaluate the ginseng product quality to master the changes of ginsenosides during the processing. Recently, adding fresh ginseng as a supplement in many foods is becoming increasingly popular (Chung, Lim, Ahn, Jeong & Kim, 2016), but the effect of process conditions especially the commercial sterilization (121 °C, 30 min) on ginsenosides during ginseng processing is still unclear.
Metabolomics includes the qualitative, quantitative, and dynamic study of endogenous molecules in organisms, organs, tissues, or cells under environmental conditions, and is usually used to analyze changes in food components in different processing methods. Widely targeted metabolomics was successfully employed to analyze the influence of withering on the formation of metabolites related to the taste of white tea (Chen et al., 2020) and the dynamic changes in non-volatile and volatile metabolites during green tea processing (Wang et al., 2021) as well as comparison of flavonoids and phenylpropanoids compounds in Chinese water chestnut treated with different processing methods (Hui et al., 2020). The composition of ginseng is complex, there are more than three hundred varieties of ginsenosides alone, and it is difficult to comprehensively analyze connections between each other at different processing methods. The use of widely targeted metabolomics can systematically and comprehensively analyze the changes of the ginsenosides in the ginseng for commercial sterilization treatment. In this study, widely targeted metabolomics based on was used to analyze the changes in terpenoids of commercially sterilized ginseng pulp (121℃, 30 min), and the terpenoids compounds difference between fresh and commercially sterilized ginseng pulp as well as the conversion of ginsenosides were systematically observed.
Materials and methods
Sample preparation
5-year-old cultivated ginsengs were collected from Jingyu county (Jilin province, China). Fresh ginsengs of the same size and uniform thickness were selected, cleaned and then put into a homogenizer to make ginseng pulp. 50 g of ginseng pulp was taken as a sample. Sterilized ginseng pulp (SGP) was made from fresh ginseng pulp (FGP) sterilized at 121℃ for 30 min. Methanol, acetonitrile, and formic acid were HPLC grade (HPLC ≥ 98 %) and purchased from Merck (Germany). All other reagents used in this study were of analytical grade.
Sample extraction
After thawing the samples from the refrigerator at −80 °C, ginseng samples were mixed and then 50 mg was taken into centrifuge tubes. 600 μL 100 % methanol internal standard extract was added to centrifuge tubes and scrolled for 3 min. The supernatant was further filtered through a microporous filter membrane (0.22 μm) and stored in a sample flask for LC-MS/MS test after centrifuging at 16,000g, 4 °C for 10 min.
UPLC conditions
Chromatographic column, Agilent SB-C18 (1.8 μm, 2.1 mm*100 mm). The mobile phase was as follows: solvent A was consisted of ultra-pure water with 0.1 % formic acid added; solvent B was consisted of acetonitrile with 0.1 % formic acid added. Elution gradient: the proportion of phase B is 5 % within 0.00 min, and the proportion of phase B increases linearly to 95 % within 9.00 min and maintains at 95 % for 1 min as well as 10.00–11.10 min. The proportion of phase B decreased to 5 % and equilibrates at 5 % to 14 min. Flow velocity was 0.35 mL / min. The column temperature was 40 ℃ and the injection volume was 4 μL.
ESI-Q Trap-MS/MS
The LIT and triple quadrupole (QQQ) scans were obtained on a triple quadrupole linear ion trap mass spectrometer, AB4500 Q TRAP UPLC/MS/MS system with an ESI Turbo ion-spray interface which conducts in both positive and negative ion modes.
Statistical analysis
All experiments were performed three times and the analysis of variance was performed by SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Principal component analysis (PCA) was used to cluster samples based on the peak area of detected metabolites with R package models (https://www.r-project.org/).
Results
Changes or differences in the composition of metabolites between FGP and SGP
To evaluate the variation of the components between FGP and SGP, an untargeted metabolomics approach was carried out to analyze the different compounds of each sample. The overlay analysis of the QC-TIC diagram (Fig. 1A) and the sample multi-peak detection diagram (Fig. 1B) showed that the data had good repeatability and reliability in this study. Compounds in the fresh and sterilized ginseng samples were evaluated by LC-MS in MRM mode. A total of 869 changed compounds (87 organic acids, 21 lignin and coumarins, 118 phenolic acids, 68 flavonoids, 52 alkaloids, 88 terpenoids, 137 lipids, 61 nucleotides and derivatives, 118 amino acids and derivatives) changed in SGP were identified (Fig. 1C). A total of 88 changed components of terpenoids were characterized including 6 monoterpenoids, 1 diterpenoid, 16 triterpenes and 65 triterpene saponin, which are the most important functional components (Fig. 1D).
Fig. 1.
Total ion current of quality control samples (1A, B). Pie chart of the number of different types of all components (1C) in FGP vs SGP; Pie chart of the number of different types of terpenoids (1D) in FGP vs SGP.
Multivariate statistical analyses of the changed terpenoids compounds of FGP vs SGP
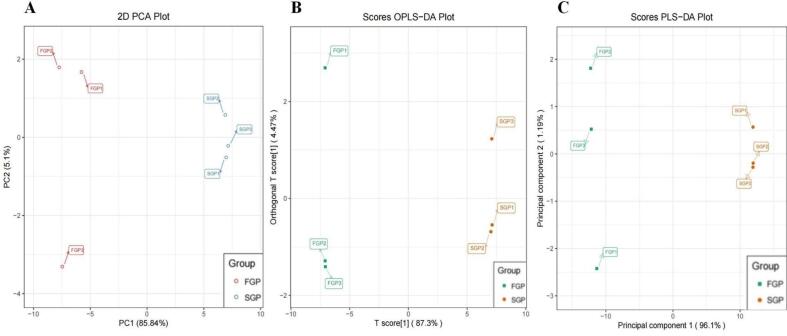
Fig. 3A showed the PCA score plot of ginseng based on all the changed terpenoid compounds, PC1 and PC2 were 5.1 % and 85.84 %, respectively. In the score plot of PCA, the FGP (fresh ginseng pulp) samples were clustered well and obviously distinguished from SGP samples, indicating that the terpenoids compounds in SGP (sterilized ginseng pulp) remarkably changed. PLS-DA (Fig. 2B) models was used to compare FGP and SGP (Q2 = 0.999, R2X = 0.0.961, R2Y = 0.999). In the OPLS-DA and PLS-DA models, FGP and SGP were prominently separated. As a multivariate statistical analysis method for supervising pattern recognition, OPLS-DA (Fig. 2C) is more sensitive to low correlation variables than other statistical methods (Martinez, Oliveira, Calvete, & Palencia, 2017). High predictability (Q2) and strong goodness of fit (R2X, R2Y) of the OPLS-DA models indicated the comparisons between FGP and SGP (Q2 = 0.990, R2X = 0.873, R2Y = 0.993).
Fig. 3.
Venn diagram 3A and volcano plot (3B) of differential terpenoids compounds in FGP vs SGP. Hierarchical cluster analysis of the terpenoids compound based on the normalized average abundance of the metabolomic profiles (3C) in FGP vs SGP. The heatmap was plotted using log2-(fold change) values from metabolomic dataset.
Fig. 2.
Multivariate statistical analyses via PCA (Fig. 2A), PLS-DA (Fig. 2B) and OPLS-DA (Fig. 2C) plot in FGP vs SGP.
Differential terpenoids compounds analysis of FGP vs SGP
The colors of the Venn diagrams indicated the final screening results, red was FGP and green was SGP (Fig. 3A). The colors of the scatter points demonstrated the final screening results, red represented components that were significantly increased; green represented components that were significantly decreased; grey represented components with no significant difference (Fig. 3B). To clarify the influence of sterilizing conditions on the components of fresh ginseng pulp, the key differences between the FGP and SGP were explored. To comprehensively observe the differences between FGP and SGP, all 46 components of terpenoids changed were screened according to the variable importance in projection (VIP) scores and fold change. The criteria for significant differences were a VIP score of ≥1 and a fold change of ≥2 or ≤0.5. The Venn diagram showed both common and unique components existed between the FGP and SGP samples. More specifically, 2 terpenoid compounds were unique in FGP and 15 terpenoid compounds were unique in SGP, while FGP vs SGP possessed 52 common substances. The volcano plot showed the changes in differential terpenoid compounds among the FGP and SGP samples. There were 46 differential components (12 increased and 34 decreased) between the FGP and SGP. Fig. 3C showed the heatmap of all changed terpenoid compounds in FGP and SGP. A total of 46 substances (VIP > 1.0 and p < 0.05) were selected and hierarchically clustered as the biomarkers for the comparisons. The changes in terpenoids compounds were two major variation trends: (i) some terpenoids compounds were decreased in SGP, including notoginsenoside R1, notoginsenoside Fc, ginsenoside Re, momordin Ic, vina-ginsenoside R1, yesanchinoside D, ginsenoside Rd, ginsenoside ST-3; (ii) the others were increased in SGP, including gypensapogenin F, ginsenoside F1, saponin v, ginsenoside Rg6, notoginsenoside R9, 20(S)-ginsenoside Rg3, ginsenoside Ro, ginsenoside Rk3, zingibroside R1, 20(S)-ginsenoside Rh1, ginsenoside Mc, Rb1, Rs3, Rc, Y, Rg2, Rk2, Rg5, Rk1, Rh4, Rg4, (20E)-ginsenoside F4.
Changed triterpenoid saponin in SGP
Ginsenoside Rh4, Rg6, notoginsenoside R9, medicagenic acid-3-O-glucosyl-(1,6)-glucosyl-(1,3)-glucoside, and ginsenoside Rk2 in SGP were increased by 134581.5, 23344.8, 17424.1, 15315.9, 11773.7 times, respectively, all above 10,000 times (Fig. 4A). (20E)-F4, ginsenoside Rs3, Rk3, Rc were increased by 5804.96, 3410.56, 1299.04, 1167.86 times respectively, all above 1000 times; Ginsenoside Rk1, Mc, Rg5, 20(S)-Rg3, Y, Rg4 were increased by 678.21, 438.33, 400.23, 240.26, 148.46, 136.25 times respectively, all above 100 times (Fig. 4B); Mogroside II-A1, mogroside Ie, 20(S)-Rh1, (24S)-pseudo-ginsenoside RT4, and pseudoginsenoside RT5 were increased by 58.74, 32.84, 25.62, 17.61,10.68, all above 10 times (Fig. 4C). Meanwhile, no significant difference was observed in some triterpenoid saponin, such as notoginsenoside Rb1, oleanonic acid, ginsenoside Rb3, Rb2, Rg1, Rf1, and their fold changes was 1.848–0.538 times (Table 1). In contrast, notoginsenoside M, ginsenoside Re, ginsenoside Rd, notoginsenoside N, ginsenoside ST-3, notoginsenoside Ft1, notoginsenoside R1, oleanolic acid-3-O-xylosyl(1 → 3)glucuronide, momordin Ic, quinquenoside R1, 6′'-Acetyl-ginsenoside Rd, yesanchinosides D, vina-ginsenoside R1 decreased significantly to 0.46, 0.41, 0.40, 0.39, 0.37, 0.33, 0.32, 0.31, 0.30, 0.22, 0.06, 0.02, 0.02 times after commercial sterilization respectively (Table 1). Notably, there is no ginsenoside Rg3 O-acetyl-O-glucoside-O-glucoside, notoginsenoside Fc in ginseng, indicating that these were converted into other saponins after commercial sterilization at 121℃ for 30 min (Table 1).
Fig. 4.
The significantly increased triterpenoid saponin in SGP. Rh4, ginsenoside Rh4; Rg6, ginsenoside Rg6; R9, notoginsenoside R9; Mg, medicagenic acid-3-O-glucosyl-(1,6)-glucosyl-(1,3)-glucoside; Rk2, ginsenoside Rk2; F4, (20E)-ginsenoside F4; Rs3, ginsenoside Rs3; Rk3, ginsenoside Rk3; Rc, ginsenoside Rc; Rk1, ginsenoside Rk1; Mc, ginsenoside Mc; Rg5, ginsenoside Rg5; Rg3, 20(S)-ginsenoside Rg3; Y, ginsenoside Y; Rg4, ginsenoside Rg4; II-A1, mogroside II-A1; Ie, mogroside Ie; Rh1, 20(S)-ginsenoside Rh1; Rt4, (24S)-pseudo-ginsenoside Rt4; Rt5, pseudoginsenoside Rt5; F1, ginsenoside F1; Rg2, ginsenoside Rg2; 3e,3-O-Rhamnosyl(1 → 2)glucosyl-2β,3β-dihydroxy-23-oxoolean-l2-en-28-oic acid-28-O-glucosyl ester; Sv, saponin V; R1, zingibroside R1; Ro, ginsenoside Ro; Rb1, ginsenoside Rb1; Nl, notoginsenoside l.
Table 1.
Fold changes of triterpenoid saponin in FGP vs SGP.
| Compound | VIP | p_value | FDR | Fold Change | Type |
|---|---|---|---|---|---|
| Notoginsenoside Rb1 | 1.27 | 0.00 | 0.00 | 1.85 | insig |
| Oleanonic acid | 1.11 | 0.02 | 0.04 | 1.84 | insig |
| Oleanolic acid-3-O-glucoside | 1.20 | 0.00 | 0.02 | 1.71 | insig |
| Notoginsenoside R2 | 1.23 | 0.00 | 0.02 | 1.70 | insig |
| Majoroside R2 | 1.16 | 0.06 | 0.12 | 1.52 | insig |
| Ursolic acid | 1.16 | 0.03 | 0.07 | 1.51 | insig |
| Majoroside R1 | 1.21 | 0.01 | 0.02 | 1.44 | insig |
| (23S)-3β-hydroxydammara-21-oic acid 21,23-lactone | 1.03 | 0.05 | 0.10 | 1.43 | insig |
| (20S)-2α,3β,12β,24 (S)-pentahydroxydammara-25-ene-20-O-β-d-glucopyranoside | 1.04 | 0.06 | 0.11 | 1.43 | insig |
| Mangiferolic acid | 1.11 | 0.08 | 0.14 | 1.42 | insig |
| 24,30-Dihydroxy-12(13)-enolupinol | 1.11 | 0.06 | 0.11 | 1.40 | insig |
| Betulinic acid | 1.18 | 0.01 | 0.03 | 1.38 | insig |
| Ginsenoside Rf | 1.16 | 0.01 | 0.03 | 1.26 | insig |
| Jasminoside C | 0.67 | 0.28 | 0.39 | 1.24 | insig |
| 10-O-[(E)-Caffeoyl]-geniposidic acid | 0.69 | 0.29 | 0.39 | 1.19 | insig |
| Camaldulenic acid | 0.70 | 0.28 | 0.39 | 1.16 | insig |
| Notoginsenoside R4 | 0.49 | 0.50 | 0.61 | 1.15 | insig |
| Ursonic acid | 0.76 | 0.26 | 0.36 | 1.11 | insig |
| Oleanolic acid-3-O-glucosyl(1 → 2)glucoside | 0.28 | 0.72 | 0.79 | 1.04 | insig |
| Ginsenoside F2 | 0.24 | 0.75 | 0.81 | 0.97 | insig |
| Gentiolactone | 0.73 | 0.30 | 0.40 | 0.93 | insig |
| Carnosol | 0.53 | 0.44 | 0.56 | 0.91 | insig |
| Chikusetsusaponin IVa | 0.94 | 0.10 | 0.17 | 0.88 | insig |
| Jasminoside N | 0.23 | 0.65 | 0.74 | 0.87 | insig |
| Genipin-1-O-(2′'-O-apiosyl)glucoside | 0.68 | 0.29 | 0.40 | 0.81 | insig |
| Calenduloside E | 0.82 | 0.20 | 0.29 | 0.81 | insig |
| Dehydrovomifoliol | 1.11 | 0.05 | 0.10 | 0.79 | insig |
| Ginsenoside Rb3 | 0.13 | 0.65 | 0.74 | 0.75 | insig |
| Notoginsenoside Fd | 0.94 | 0.08 | 0.15 | 0.72 | insig |
| Dioscin | 1.15 | 0.05 | 0.10 | 0.72 | insig |
| 2-Hydroxyoleanolic acid | 0.96 | 0.18 | 0.28 | 0.70 | insig |
| Ginsenoside Rb2 | 1.03 | 0.10 | 0.16 | 0.67 | insig |
| Ginsenoside Rg1 | 1.06 | 0.09 | 0.15 | 0.66 | insig |
| Sanchirhinoside A4 | 0.82 | 0.28 | 0.38 | 0.65 | insig |
| Notoginsenoside K | 1.09 | 0.10 | 0.17 | 0.58 | insig |
| Mogroside IVe | 1.16 | 0.01 | 0.03 | 0.57 | insig |
| Ginsenoside Rf1 | 1.17 | 0.05 | 0.10 | 0.55 | insig |
| Notoginsenoside Fe | 1.25 | 0.00 | 0.01 | 0.54 | insig |
| Compound | VIP | p_value | FDR | Fold Change | Type |
|---|---|---|---|---|---|
| Notoginsenoside M | 1.10 | 0.09 | 0.16 | 0.46 | down |
| Ginsenoside Re | 1.23 | 0.00 | 0.01 | 0.41 | down |
| Ginsenoside Rd | 1.21 | 0.00 | 0.01 | 0.40 | down |
| Notoginsenoside N | 1.17 | 0.10 | 0.16 | 0.39 | down |
| Ginsenoside ST-3 | 1.25 | 0.01 | 0.04 | 0.37 | down |
| Notoginsenoside Ft1 | 1.11 | 0.03 | 0.07 | 0.33 | down |
| Notoginsenoside R1 | 1.21 | 0.07 | 0.12 | 0.32 | down |
| Oleanolic acid-3-O-xylosyl(1→3)glucuronide | 1.28 | 0.00 | 0.00 | 0.31 | down |
| Momordin Ic | 1.27 | 0.00 | 0.00 | 0.30 | down |
| Quinquenoside R1 | 1.23 | 0.05 | 0.11 | 0.22 | down |
| 6′'-Acetyl-ginsenoside Rd | 1.27 | 0.01 | 0.04 | 0.06 | down |
| Yesanchinosides D | 1.27 | 0.01 | 0.02 | 0.02 | down |
| Vina-Ginsenoside R1 | 1.27 | 0.01 | 0.03 | 0.02 | down |
| Ginsenoside Rg3 O-acetyl-O-glucoside-O-glucoside | 1.28 | 0.01 | 0.03 | 0.00 | down |
| Notoginsenoside Fc | 1.28 | 0.03 | 0.06 | 0.00 | down |
Discussion
Ginsenosides are considered as the crucial indicator for evaluating the quality of ginseng, which exhibited various functions including anti-aging and anti-tumor activities (Chen, Balan & Popovich, 2020). Ginsenosides conversion mainly includes chemical, physical and microbial methods, among which physical methods, such as high temperature, are commonly used and studied (Cui, Wu, Zhao & Yin, 2016). Commercial sterilization at 121 °C for 30 min is an essential method for food processing, however, the effect of sterilizing on terpenoid compounds in ginseng is not completely investigated by now. In this study, fresh ginseng pulp (FGP) was commercially sterilized at 121℃ for 30 min, and terpenoid compounds were analyzed by widely targeted metabolomics based on UPLC-ESI-MS/MS system. We found the commercial sterilized induced the changes of 88 terpenoids compounds including 30 types of ginsenosides, and many minor ginsenoside Rh4, Rg6, Rk2, F4, Rs3, Rk3, Rk1, Rg5, Rg3, Rg4 are remarkably increased in fresh ginseng pulp, and a new ginsenoside ST3 was found in SGP. Interestingly, F4, Rg3, and Rg5 were also detected in fresh ginseng.
Widely targeted metabolomics analysis was utilized for massive metabolite profiling and comparative metabolomics of abundant plant species (Utpott, Rodrigues, de Oliveira Rios, Mercali, & Flôres, 2022). For example, an LC–MS/MS-based target metabolomics was used to investigate the 60 types of flavonoids and 11 types of anthraquinones in 40 cultivars of tartary buckwheat seeds from different regions (Wei et al., 2020). A total of 323 sensory trait-related metabolites were found when storage-related sensory characteristic variations of white penoy tea and the relationships with non-volatile components were studied using widely-targeted metabolomics (Fan, Huang, Tong, Guo & Gong, 2021). 536 different taste components were identified between two cultivars of loquat by widely targeted metabolomics (Zou, Wu, Shahid, He & Yang, 2020). Previous studies were mostly focused on the long-time steamed ginseng such as red ginseng and black ginseng by high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC–MS). Jin et al. found 3 types of ginsenosides Rg3, Rk2 and F2 in the whole ginseng by the HPLC (Jin et al., 2015). Likewise, Kwon used the HPLC method to analysis 8 types of ginsenosides in roasted ginseng (Yoon, Lee, & Kwon, 2010). Park detected 8 types of ginsenosides in fermented ginseng by using the liquid chromatograph coupled with triple quadrupole mass spectroscopy (Park, Seo, & Lee, 2018). Our study concentrated on the terpenoids compounds changed in the commercial sterilized ginseng pulp, especially to comprehensively analyze the changes in the triterpene saponins by the widely targeted metabolomics based on UPLC-ESI-MS/MS system which can detect more than 1600 terpenoid compounds. Notably, 88 changed terpenoid compounds including 30 types of ginsenosides in SGP were found in our study. Meanwhile, 11 ginsenosides including Rh4, Rg6, F4, Rg5 and Rg3 significantly increased in SGP (Fig. 4), while ginsenosides F4, Rg3, and Rg5 were not present in raw ginseng in the previous work (Kim et al., 2000), and 8 ginsenosides including Rb3, Rb2, Rg1, Rf1, Re, Rd decreased in SGP (Table 1). Notably, ginsenoside ST3 as a new type of dammarane ginsenoside was firstly detected in our study, which was never reported in previous studies. The results indicated that the widely target metabolomics based on LC–MS/MS is an effective method to analyze terpenoids compound change.
The changes of ginsenosides in ginsengs during heat processing have been widely studied (Piao, Huo, Kang, Mathiyalagan, & Wang, 2020). Ginsengs were usually processed into red and black ginseng for consumption. Fresh ginsengs were steamed the whole raw ginseng at 90–100℃ for 3 h, then dried by hot air, and dried in the sun to form red ginseng. Black ginseng was made by steaming the whole raw ginseng plant 9 times at 95-100℃ for 2–3 h and then dehydration (Kim et al., 2011). When the fresh ginsengs were steamed at 98 ℃ for 3 h (Kwak et al., 2017), the concentration of ginsenoside Re was slightly decreased. Kim (Kim et al., 2000) found Ginsenosides Rg5, F4 and Rg3 in the steamed ginseng at 120 °C for 2 h, but were not detected before steaming. Some ginsenosides (F4, Rg6, Rk3, Rh4, Rs3, and Rs4) do not exist or are rarely found in red ginseng (Park, Choi & Yokozawa, 2018). However, in this work, the ginsenosides Rh4, Rg6, F4, F1, Rc, Rk1, Rk2, Rk3, Rg5, Rg3 and Rg4 significantly increased (Fig. 4), the ginsenosides F2, Rb3, Rb2, Rg1, Rf1, Re, Rd, ST-3 decreased in SGP (Table 1), and interestingly F4, Rg6, Rk3, Rh4, Rs3 were detected in FGP. Ginsenoside Rh4 as a minor ginsenosides with a better anti-tumor effect (Duan et al., 2018) increased by 134581.5 times (Fig. 4A). Ginsenoside Rg3 as a minor ginsenoside increased by 240.2 times (Fig. 4C), which is known for its anticancer activities and has been developed into medicine and used in clinical treatments (Li et al., 2020). Rg5 and Rk1 increased by 400.2 and 678.2 times respectively (Fig. 4C), which showed potential anticancer activities (Elshafay et al., 2017). The minor ginsenosides Rh1 and Rg2 also increased by 25.6 (Fig. 4D) and 5.1 times (Fig. 4E) respectively. In summary, the commercial sterilization (121 °C for 30 min) can increase the content of minor ginsenosides in ginseng pulp.
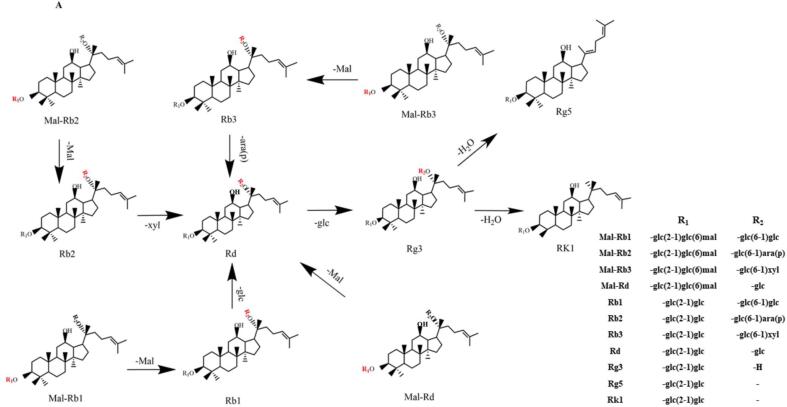
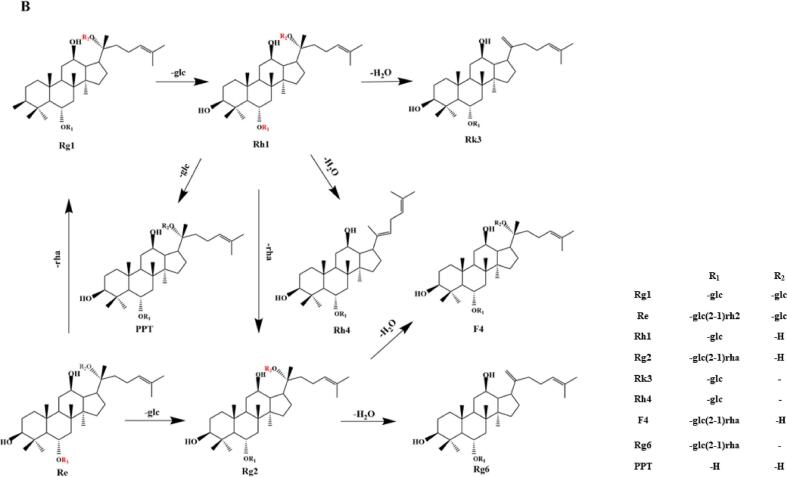
Commercial sterilization (120℃, 30 min) is a representative means of food processing to ensure food safety and improve the flavor of food. During the process of the heating ginsengs at high temperature, some major ginsenosides were converted to minor ginsenosides because temperature enhanced the chemical reaction such as dehydration, deglycosylation, and demalonylation (Xu et al., 2018). Some protopanaxadiol ginsenosides would be converted by demalonylation from their corresponding malonyl-ginsenoside (Chen, Balan & Popovich, 2020). In this study, malonyl-ginsenoside Rb1, Rb2, Rb3 and Rd would be converted to ginsenoside Rb1, Rb2, Rb3 and Rd by demalonylation and generate malonic acid through hydrolysis in our work (Fig. 5A). Rb1, Rb2 and Rb3 were converted to Rg3 by losing the glc-residue at C-20 (Fig. 5A). Besides, ginsenoside Rd was also formed by hydrolyzing the glc-, ara(p)-,or xyl-residue at C-20 of Rb1, Rb2, Rb3 (Fig. 5A). Furthermore, Rd was hydrolyzed to form the Rg3 and then Rg3 was further dehydrated at C-20 to generate Rg5 and Rk1 (Fig. 5A). These ginsenosides transformation above was consistent with that of steaming-induced red ginsengs, in which malonyl-ginsenoside Rb1, Rb2, ginsenoside Rb1 and Rb2 decreased, but Rg3, Rd and Rg5 increased (Xie et al., 2012). For protopanaxatriol type ginsenosides, some previous studies reported that the hydrolysis of the rhamnosyl residue at C-6 of protopanaxatriol ginsenoside occurred easily in the heating condition (Piao et al., 2020). In this study, the hydrolysis of the rhamnosyl residue at C-6 of ginsenoside Re formed ginsenoside Rg1, and the further hydrolysis of the glucosyl at C-20 of Rg1 produced Rh1 which then was converted to Rh4 and Rk3 through dehydration at C-20, and was also converted to PPT hydrolysis of the glucosyl at C-20 (Fig. 5B). S-type is the steric configuration of natural ginsenoside, and the steric configuration of the new ginsenoside would be changed after heat processing (Ryu, Yoon, & Lee, 2020). During the commercial sterilization, 20(S)-Rg2 in fresh ginseng was isomerized to 20(R)-Rg2 during the heating process (Yao et al., 2021). Moreover, ginsenoside Re was also hydrolyzed to remove the glucose at the C-20 position to generate 20(R)-Rg2 (Fig. 5B), and this pair of isomers were further converted to F4 and Rg6 respectively by losing one H2O (Fig. 5B), which were similar to the conversions of PPT type ginsenosides in the processing of steamed ginsengs (Wang et al., 2012). In addition, 20-glu-Rf was converted to Rf by losing the glc-residue at C-20 and then Rf was converted to Rg8 and Rg9 by hydrolyzation (Fig. 5C). The results suggested the commercial sterilization (121 °C for 30 min) induced some major ginsenosides to demalonylate, decarboxylate, deglycosylate, and dehydrate into minor ginsenosides in ginseng, which would remarkably improve the function of ginsengs.
Fig. 5.
Transformation of ginsenosides during the commercial sterilization. (ara(p): α-l-arabinopyranosyl; glc: β-d-glucopyranosyl; xyl: β-d-xylopyranosyl; Mal: malonyl; Bu: trans-but-2-enoyl; rha, α-l-rhamnopyranosyl).
Conclusion
In this study, the impact of commercial sterilization (121℃, 30 min) on the changes of terpenoid compounds especially the ginsenosides transformation in fresh ginseng pulps were analyzed based on widely targeted metabolomics. A total of 88 terpenoid compounds including 30 types of ginsenosides, including many minor ginsenosides Rh4, Rg6, Rk2, F4, Rs3, Rk3, Rk1, Rg5, Rg3, Rg4 were remarkably increased in fresh ginseng pulps. Consequently, commercial sterilizing at 121℃ for 30 min remarkably affected the species and number of the ginsenosides, especially increase some minor ginsenosides content in ginseng pulps. The results of the present study provided a novel theoretical basis for researches of ginseng function and the development of ginsengs processing.
CRediT authorship contribution statement
Junshun Zhang: Conceptualization, Methodology, Investigation, Software, Investigation, Writing – original draft. Zhiyi Ai: Writing – review & editing, Software. Yue Hu: Software, Visualization. Yonghong Wang: Software, Visualization. Sitong Liu: Software, Visualization. Yongzhe Liu: Software, Visualization. Bo Nan: Writing – review & editing, Software, Supervision, Visualization. Yuhua Wang: Conceptualization, Writing – review & editing, Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Key R&D Program of Jilin Provincial Department of Science and Technology (20210204040YY).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100415.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bae E.-A., Han M.J., Kim E.-J., Kim D.-H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Archives of Pharmacal Research. 2004;27(1):61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- Chen Q., Shi J., Mu B., Chen Z., Dai W., Lin Z. Metabolomics combined with proteomics provides a novel interpretation of the changes in nonvolatile compounds during white tea processing. Food Chemistry. 2020;332 doi: 10.1016/j.foodchem.2020.127412. [DOI] [PubMed] [Google Scholar]
- Chen W., Balan P., Popovich D.G. Changes of ginsenoside composition in the creation of black ginseng leaf. Molecules. 2020;25(12):2809. doi: 10.3390/molecules25122809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Advances in Food and Nutrition Research. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- Chung I.-M., Kim J.-W., Seguin P., Jun Y.-M., Kim S.-H. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng CA Meyer): Effects of cultivation location, year, and storage period. Food Chemistry. 2012;130(1):73–83. doi: 10.1016/j.foodchem.2011.06.056. [DOI] [Google Scholar]
- Chung I.M., Lim J.J., Ahn M.S., Jeong H.N., Kim S.H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of korean ginseng (panax ginseng meyer) according to cultivation years. Journal of Ginseng Research. 2016;40(1):68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Wu S.Q., Zhao C.A., Yin C.R. Microbial conversion of major ginsenosides in ginseng total saponins by platycodon grandiflorum endophytes. Journal of Ginseng Research. 2016;40(4):366–374. doi: 10.1016/j.jgr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Wei B., Deng J., Mi Y., Dong Y., Zhu C., et al. The anti-tumor effect of ginsenoside Rh4 in MCF-7 breast cancer cells in vitro and in vivo. Biochemical and Biophysical Research Communications. 2018;499(3):482–487. doi: 10.1016/j.bbrc.2018.03.174. [DOI] [PubMed] [Google Scholar]
- Elshafay A., Ngo Xuan T., Salman S., Shaheen Y.S., Othman E.B., Elhady M.T., et al. Ginsenoside Rk1 bioactivity: A systematic review. Peerj. 2017;5:e3993. doi: 10.7717/peerj.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F.Y., Huang C.S., Tong Y.L., Guo H.W., Gong S.Y. Widely targeted metabolomics analysis of white peony teas with different storage time and association with sensory attributes. Food Chemistry. 2021;362(2) doi: 10.1016/j.foodchem.2021.130257. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li J., Wang J., Li X., Li J., Chu S., et al. Ginsenoside Rg1 prevent and treat inflammatory diseases: A review. International Immunopharmacology. 2020;87 doi: 10.1016/j.intimp.2020.106805. [DOI] [PubMed] [Google Scholar]
- Hui N.A., Hc D., Gl B., Ks B., Ms A., Zd A., et al. Comparison of flavonoids and phenylpropanoids compounds in chinese water chestnut processed with different methods. Food Chemistry. 2020;335 doi: 10.1016/j.foodchem.2020.127662. [DOI] [PubMed] [Google Scholar]
- Jin Y., Kim Y.-J., Jeon J.-N., Wang C., Min J.-W., Noh H.-Y., et al. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods for Human Nutrition. 2015;70(2):141–145. doi: 10.1007/s11130-015-0470-0. [DOI] [PubMed] [Google Scholar]
- Jung J., Lee N.-K., Paik H.-D. Bioconversion, health benefits, and application of ginseng and red ginseng in dairy products. Food Science and Biotechnology. 2017;26(5):1155–1168. doi: 10.1007/s10068-017-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Lee Y.C., Choi S.Y., Cho C.-W., Rho J., Lee K.-W. The changes of ginsenoside patterns in red ginseng processed by organic acid impregnation pretreatment. Journal of Ginseng Research. 2011;35(4):497–503. doi: 10.5142/jgr.2011.35.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., et al. Steaming of ginseng at high temperature enhances biological activity. Journal of Natural Products. 2000;63(12):1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- Li X., Chu S., Lin M., Gao Y., Liu Y., Yang S., et al. Anticancer property of ginsenoside Rh2 from ginseng. European Journal of Medicinal Chemistry. 2020;203 doi: 10.1016/j.ejmech.2020.112627. [DOI] [PubMed] [Google Scholar]
- Martinez F., Oliveira J.A., Calvete E.O., Palencia P. Influence of growth medium on yield, quality indexes and SPAD values in strawberry plants. Scientia Horticulturae. 2017;217:17–27. doi: 10.1016/j.scienta.2017.01.024. [DOI] [Google Scholar]
- Park C.H., Choi J.S., Yokozawa T. Increase in the hydroxyl radical-scavenging activity of panax ginseng and ginsenosides by heat-processing. Drug Discoveries & Therapeutics. 2018;12(3):114–121. doi: 10.5582/ddt.2018.01010. [DOI] [PubMed] [Google Scholar]
- Park S.E., Seo S.H., Lee K.I., et al. Metabolite profiling of fermented ginseng extracts by gas chromatography mass spectrometry. Journal of Ginseng Research. 2018;42(1):57–67. doi: 10.1016/j.jgr.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X.M., Huo Y., Kang J.P., Mathiyalagan R., Wang Y.P. Diversity of ginsenoside profiles produced by various processing technologies. Molecules. 2020;25(19) doi: 10.3390/molecules25194390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L.-W., Wang C.-Z., Yuan C.-S. Isolation and analysis of ginseng: Advances and challenges. Natural Product Reports. 2011;28(3):467–495. doi: 10.1039/C0NP00057D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz M., Rahman N.U., Zia-Ul-Haq M., Jaffar H., Manea R. Ginseng: A dietary supplement as immune-modulator in various diseases. Trends in Food Science & Technology. 2019;83:12–30. doi: 10.1016/j.tifs.2018.11.008. [DOI] [Google Scholar]
- Ryu J., Yoon J., Lee Y.W. Kinetic study of the thermal conversion of ginsenosides using lumped groups in steaming, hydrothermal reactions, and co 2 -assisted hydrothermal reactions. The Journal of Supercritical Fluids. 2020;167 doi: 10.1016/j.supflu.2020.105041. [DOI] [Google Scholar]
- Utpott M., Rodrigues E., de Oliveira Rios A., Mercali G.D., Flôres S.H. Metabolomics: An analytical technique for food processing evaluation. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130685. [DOI] [PubMed] [Google Scholar]
- Wang D., Liao P.Y., Zhu H.T., Chen K.K., Min X., Zhang Y.J., et al. The processing of panax notoginseng and the transformation of its saponin components. Food Chemistry. 2012;132(4):1808–1813. doi: 10.1016/j.foodchem.2011.12.010. [DOI] [Google Scholar]
- Wang, H., Hua, J., Yu, Q., Li, J., Wang, J., Deng, Y., Yuan, H., & Jiang, Y. (2021). Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chemistry, 363, 130131–130131. https://doi.org/10.1016/j.foodchem.2021.130131. [DOI] [PubMed]
- Wei Y.A., Yong S.B., Gd C., Gq B., Ys A., Ym A., et al. Liquid chromatography–mass spectrometry-based metabolomics analysis of flavonoids and anthraquinones in fagopyrum tataricum l. gaertn. (tartary buckwheat) seeds to trace morphological variations. Food Chemistry. 2020;331(127354) doi: 10.1016/j.foodchem.2020.127354. [DOI] [PubMed] [Google Scholar]
- Xie Y.Y., Luo D., Cheng Y.J., Ma J.F., Wang Y.M., Liang Q.L., et al. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from panax ginseng by means of hplc-esi-ms/msn-based multicomponent quantification fingerprint. Journal of Agricultural and Food Chemistry. 2012;60(33):8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. Journal of Ethnopharmacology. 2003;84(2–3):187–192. doi: 10.1016/S0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Xu X.-F., Gao Y., Xu S.-Y., Liu H., Xue X., Zhang Y., et al. Remarkable impact of steam temperature on ginsenosides transformation from fresh ginseng to red ginseng. Journal of Ginseng Research. 2018;42(3):277–287. doi: 10.1016/j.jgr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F., Li X., Sun J., Cao X., Liu M., Li Y., et al. Thermal transformation of polar into less-polar ginsenosides through demalonylation and deglycosylation in extracts from ginseng pulp. Scientific Reports. 2021;11:13–15. doi: 10.1038/s41598-021-81079-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.-R., Lee G.-D., Kwon J.-H. Monitoring of roasting-induced changes in ginsenoside composition of ginseng (Panax ginseng CA Meyer) Food Science and Biotechnology. 2010;19(1):151–157. doi: 10.1007/s10068-010-0021-2. [DOI] [Google Scholar]
- Zhang T., Zhong S., Li T., Zhang J. Saponins as modulators of nuclear receptors. Critical Reviews in Food Science and Nutrition. 2020;60(1):94–107. doi: 10.1080/10408398.2018.1514580. [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhong S., Hou L., Wang Y., Xing X., Guan T., et al. Computational and experimental characterization of estrogenic activities of 20 (S, R)-protopanaxadiol and 20 (S, R)-protopanaxatriol. Journal of Ginseng Research. 2020;44(5):690–696. doi: 10.1016/j.jgr.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhang T., Liang Y., Zou H., Zhang J. Inhibitory activities of 20 (R, S)-protopanaxatriol against epidermal growth factor receptor tyrosine kinase. Food and Chemical Toxicology. 2021;155 doi: 10.1016/j.fct.2021.112411. [DOI] [PubMed] [Google Scholar]
- Zou S., Wu J., Shahid M.Q., He Y., Yang X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chemistry. 2020;323 doi: 10.1016/j.foodchem.2020.126822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.