Highlights
-
•
β-glucan reduced 45 % and polyphenols increased 79 % after sprouting for 120 h.
-
•
Bread’s cell density and specific volume were the highest after sprouting for 72 h.
-
•
The starch digestibility was the lowest with oat flour sprouting for 72 h.
-
•
Two digestible fractions with different digestion rates was presented in the bread.
-
•
Sprouting for 72 h postponed t2start and reduced digestion rate by 7 % in the bread.
Keywords: Bread, Sprouted oat, β-glucan, Gluten, Starch digestion
Abstract
The aim of this study was to investigate the effect of sprouted oats substitutions on the in vitro digestibility of starch in the wheat bread. The physical and nutritional quality of wheat bread enriched with 20 % sprouted oat flour was compared. The polyphenols and γ-aminobutyric acid increased, while the content of starch and β-glucan in the mixed bread was gradually decreased. The specific volume of mixed bread reached the maximum with a 19.79 % reduction of area fraction and a 31.36 % increase cell density when sprouting for 72 h. Two digestible starch fractions with different digestion rates were observed from the LOS-CPS fitted starch digestograms. The microstructure revealed that large type A wheat starch was gelatinized after baking, whereas small type B wheat starch and oat starch were wrapped in protein-β-glucan complexes. This study suggests that properly sprouting has the potential to obtain nutritional bread with low starch digestibility.
1. Introduction
The prevalence of chronic diet-related metabolic diseases such as diabetes, obesity, and cardiovascular disease has increased significantly with people's increasing living standards and changing dietary patterns. Bread is one of baked and instant food that is accepted by most customers for its special flavor. Traditional bread processing is usually based on ordinary wheat flour while adding high sugar and oil ingredients such as sugar and butter. Thereby, ordinary wheat bread has a high glycemic index (GI) value. The starch of bread with high water content is prone to gelatinize at the high baking temperature, which causes a marked increase in the level of blood glucose and insulin immediately (Yuksel & Kayacier, 2022) and hence the bread is not suitable for patients with hyperglycemia and types 2 diabetes. In this regard, there is practical value in performing studies on starch in vitro digestion. Up to now, the majority of researchers adopt measures that adjust the ingredient ratio and optimize the process of bread to reduce the starch digestibility (Graça et al., 2021, Santos et al., 2021).
Coarse cereals possess abundant and rich nutrients, such as high-quality protein, dietary fiber, minerals, and vitamins (Fu, Zhang, Hu, Zhao, Tang, & Zou, 2020). Bread based on the pasting of wheat flour and coarse grain flour not only can enrich the diversity of baked food but also improve the GI value. Oats have recently attracted attention for their rich nutritional compositions, which have been considered to be a rich source of high-quality and various proteins with balanced amino acids following the human nutritional pattern (Gu, Qian, Sun, Ma, Tian, & Wang, 2022). It is also well established that oats contain large content of γ-aminobutyric acid (GABA) and polyphenols with physiological functions such as anti-oxidation and reducing cardiovascular and cerebrovascular diseases. Moreover, oats contain 8 % of soluble dietary fiber, which can prevent constipation and assist in lowering blood sugar (Roye et al., 2021). Oat β-glucan (OBG) could reduce the level of postprandial blood glucose and insulin response and maintain the stability of blood glucose (Kock et al., 2018). As the slow-release process of glucose, OBG could postpone the starch digestion. The addition of oat flour can affect the water absorption, dough formation time, and dough stability of oat-wheat mixed flour, which affects the texture properties of bread. Additionally, OBG increases the apparent viscosity and reduces the ductility and hardness of dough, resulting in the deterioration of bread quality. Sprouting changed the physical and chemical structure of grain and activates the endogenous enzymes in the process of germination. Moreover, sprouting improves the nutritional value of grains and reduces harmful or anti-nutritional factors (Aparicio-García, Martínez-Villaluenga, Frias, & Peñas, 2021). The emulsification, water absorption, and water solubility of sprouted oat flour are increased, and the flour and gelatinization attributes will shift. OBG will be degraded with the progression of the sprouting process, and thus the viscosity of OBG is reduced. Appropriate content of OBG can interact with water molecules to form a stable three-dimensional gel network structure under the action of multiple hydroxyl groups (Nguyen et al., 2022).
Currently, most of the researchers who explore the application of sprouted grains in the food industry, focus on the influence of formula, processing method, and active ingredients on the performance of products (Cardone et al., 2020, Suárez-Estrella et al., 2020). However, there are still few studies on the specific mechanism of sprouted grains affecting the starch digestibility of wheat bread. How to effectively regulate the starch digestion characteristics of bread and keep a better quality of the bread, is a critical issue to be solved in the food industry. It is important to achieve the desire for health and nutrition in the future staple food industry, as well the bread processing industry. As a result, this study intends to systematically explore how sprouted oat flour substitution influences the baking quality, in terms of the starch digestion and texture properties of wheat bread. It can be seen that this study is conducive to improving bread products with both low GI values and desirable textural attributes, which is in line with the healthy and nutritious terms in modern society.
2. Materials and methods
2.1. Materials
Wheat flour was purchased from Xinliang Grain and Oil Processing Co., ltd (Henan Province, China). Raw oats grain was provided by Yangufang Whole Grain Industry Development Co., ltd (Inner Mongolia, China). Instant dry yeast (Angel yeast Co., ltd, Hubei Province, China), butter, eggs, butter, sugar, and salt were purchased from a local trail store. Pancreatin and amyloglucosidase (200 U/ml) were purchased from Sigma-Aldrich Chemical. Co., ltd (St. Louis, MO, USA). Total starch (AA/AMG) assay kits were purchased from Megazyme International Co., ltd (Bray, Wicklow, Ireland). All other chemicals were analytical grade and used as received.
2.2. Preparation of sprouted oats flour
Oats with entire grains and maturity were screened, which were soaked in 2 % hypochlorous acid solution for 10 min and then immersed in 0.1 % H2O2 for 10 min for sterilization. After repeated washing with clean water, oat grains were soaked in pure water for another 12 h. Soaked oats were placed in the thermostatically-controlled germination chamber (Hengke, Shanghai, China) with the water circulating system to maintain air humidity at around 90 % (Aparicio-García et al., 2021). The sprouting was performed at 25 ℃ for 0, 24, 48, 72, 96, and 120 h, respectively. The sprouted oats were freeze-dried and milled with an HK-820 grinder (Xulang, Guangzhou, China) the sprouted oat flour was sieved by a 100-mesh sieve, the sieved powder was stored in sealed plastic bags at − 20° C for further analysis.
2.3. Preparation of sprouted oats dough and bread
Oat flour (20 g) (oats were sprouted for different durations), wheat flour (80 g), sugar (20 g), salt (1 g), yeast (1.5 g), whole egg liquid (10 g), and water were weighed. The amount of liquid was added to the mixer according to the optimal amount of water in the farinograph test. These materials were collected and blended into the rough dough in the dough mixer (Ashton SM500, USA) and then butter was added, blending continuously until the smooth dough surface was obtained. The dough was covered with plastic wrap and fermented at 30 ℃ for 1 h. Afterward, the fermented dough was shaped and exhausted (65 g / piece), and fermented for another 40 min at 30 ℃. Subsequently, the fermented dough was baked at 180 ℃ for 15 min. Finally, the fresh bread was cooled down at room temperature for 30 min before further analysis (Wang, Lao, Bao, Guan, & Li, 2021). Wheat flour substituted with 20 % sprouted oat flour for different time (0, 24, 48, 72, 96, 120 h) was named as WB + SO0, WB + SO24, WB + SO48, WB + SO72, WB + SO96, WB + SO120, respectively. WB represents wheat bread without substitution.
2.4. Chemical compositions of mixed bread flour
The total starch content of mixed dough was determined using the Megazyme (AA/AMG) Starch Analysis Kit (Wicklobre, Ireland). Protein, fat, and ash content was determined according to AACC 46–12.01, AACC 30–10.01and AACC 08–01.01, respectively. OBG was quantified using a 1.3:1.4 mixed-linkage Megazyme β-glucan kit under the manufacturer's instructions (Bray, Wicklow, Ireland). γ-aminobutyric acid (GABA) was determined by HPLC (Agilent, California, China) coupled with a diode array detector based on previously validated methods (Xie, Wang, Sun, Gu, & Yang, 2021). Total phenolic compounds were identified by the Folin–Ciocalteau reagent with modifications.
2.5. The determination of the structure of bread
Determination of the specific volume of bread: Fresh bread after cooling to room temperature was weighed, then the volume was measured by rapeseed substitution method. The specific volume was calculated according to the obtained mass and volume (S. Ding, Peng, Li, & Yang, 2019). P = V/m, where P is specific volume of bread, mL/g; V is bread volume (mL); M is bread quality (g).
Image analysis and visualization: Bread was cut into slices from the core area with a thickness of 1.5 cm. The photograph of the slice was scanned using a multi-functional scanner (HP M277, Hewlett–Packard, Palo Alto, CA, USA) in the RGB mode with 400 dpi resolution. The image (3 × 3 cm) process was conducted through Fiji Image J and Photoshop software. MATLAB R2010a was used for the determination and segmentation of bread characteristics (Puerta, Garzón, Rosell, Fiszman, Laguna, & Tárrega, 2021). Through the marking and statistics of images, the following indexes were obtained: Unit cell density (CD) and stomatal surface area fraction (AF) (Yu et al., 2019).
2.6. In vitro starch in bread digestion assay
The in vitro starch digestion assay was performed according to the sightly modified method (Li, Dhital, & Gidley, 2022). The bread crumbs were freeze-dried, ground, and sieved (100 mesh), then 50 mg of them were weighed and placed in a 50 mL centrifuge tube, and dispersed with 2 mL deionized water. Sodium acetate buffer (8 mL 0.2 M, pH 6.0) containing 0.33 mg trypsin and 16.7 μL amyloglucosidase was added to the centrifuge tube (37 ℃, 300 rpm). After reaction for 0, 5, 10, 15, 20, 30, 45, 60, 90, 120, 180, 240, and 300 min, reaction solution (0.1 mL) was mixed with 0.9 mL anhydrous ethanol to stop the digestion reaction. The released glucose concentration was measured by d-glucose (GOPOD format), and the final starch digestibility changed with time was acquired.
2.7. Sprouted oats bread digestion fitting to kinetics model
The logarithm of slope (LOS) and combination of parallel and sequential (CPS) fitting of starch digestion curve in bread can describe the starch digestion process in bread exhaustively (Wang et al., 2021). The starch digestion curve usually conforms to the first-order kinetic Eq. (1):
| (1) |
Where the parameter C(t) is the percentage of digested starch at time t, C∞ is the estimated maximum starch digestion percentage under the infinite extension of reaction time, C0 is the digestion percentage of starch at the beginning of the reaction, and k is the starch digestion rate constant.
Take the logarithm of Eq. (1) and analyze the digestion curve to further determine whether there are multiple digestion processes in the bread system:
| (2) |
However, in order to further determine the sequence of the digestion process of different starch components in the bread system, it is necessary to use the parallel sequence (CPS) model to continue to fit the starch digestion curve and distinguish the starch digestion patterns of multiple components. The digestion patterns can be further determined according to the CPS model:
| (3) |
Parameters C∞1 and C∞2 are the maximum starch digestibility at two different digestion stages, respectively, and C0 represents the starch digestibility at the beginning of the reaction. k1 and k2 are the rate constants of the two starch digestion components respectively, and t2start represents the starting time of the second stage of starch digestion. When t2start = 0, the parallel digestion mode is followed; t2start ≥ completion time of fast digestion fraction, indicating that sequential digestion mode is followed; When 0 < t2start < the completion time of the fast digestion part, the two digestion modes should be combined for analysis.
2.8. The microstructure evolution shift of the mixed bread in the digestion
The microstructure of wheat bread substituted by oat flour sprouted for different periods was performed when the digestion time was 0, 30, 60, and 90 min. In the end, the centrifuge tube was taken out and excessive anhydrous ethanol was added to terminate the digestion reaction. The supernatant was removed by centrifugation, and then the precipitation was repeatedly washed with anhydrous ethanol to be freeze-dried. The morphology of bread digest precipitate during the digestion process was inspected by using a Regulus 8100 scanning electron microscope (Hitachi, Japan) (Wilcox et al., 2021).
2.9. Statistical analysis
The means and standard deviations for all parameters were calculated in Excel 2020. Statistical analyses were conducted using IBM SPSS Statistics 26.0. The experimental data were determined by one-way ANOVA with Duncan’s test (P <0.05).
3. Results
3.1. Method summary
To study the effects of partial substitution of sprouted oats on the nutritional composition, texture characteristics and digestive characteristics of wheat bread. Wheat breads substituted with 20 % sprouted at different time (0, 24, 48, 72, 96, 120 h) was baked for further analysis. The determination of basic chemical composition was following the AACC methods. β-glucan, γ-aminobutyric acid and total phenolic compounds were determined to analysis the effect of sprouted oats on the active nutrients of bread. The textural structure and visualization of bread was obtained with the help of MATLAB R2010a and Fiji Image J software, which was used to describe the relationship among specific volume, cell density and area fraction. The follow-up study was about the effect of sprouted oat on the starch digestibility of bread, which is also the focus of this research. The in vitro starch digestion assay was performed by GOPOD format, then the logarithm of slope (LOS) and combination of parallel and sequential (CPS) was used to fit starch digestion curve. For more intuitive observation, the microstructure evolution shift of the mixed bread during the digestion was acquired by SEM.
3.2. Chemical composition
To assess the nutrition of bread with 20 % sprouted oats addition, the chemical composition of bread is determined (Table 1). The addition of unsprouted oats increased the fat, β-glucan, ash, and polyphenol content of wheat bread while decreasing the starch content of mixed flour. However, the addition of sprouted oats increased the ash, protein, γ-aminobutyric acid and polyphenol content of wheat bread while decreasing the content of starch, fat and β-glucan of mixed flour. This is mainly because when oats were sprouting, the activity of various enzymes also increased sharply with the increase of sprouting time (J. Ding, Johnson, Chu, & Feng, 2019). Meanwhile, a variety of nutrients such as starch, fat, and β-glucan in oats were decomposed, allowing for sprouting and growth of oat. Starch is the most abundant and critical nutrient in oats, which reduced from 53.26 % to 49.63 %. Long-term sprouting significantly reduced the content of OBG (from 5.28 to 1.06 %) and fat (from 10.03 % to 9.36 %). As the extension of the sprouting time, the ash content in the mixed flour al also decreased from 2.40 % to 1.94 % gradually. Compared with pure wheat flour, the ash content of the mixed powder was significantly increased, which was mainly caused by large amounts of dietary fiber in oat flour (Sánchez-Pardo, Jiménez-García, & González-García, 2010). However, the content of γ-aminobutyric acid (GABA) and polyphenols increased gradually as sprouting time prolonged.
Table 1.
Effects of oat flour addition (for different sprouting times) on the chemical compositions of wheat bread.
| Starch (%, dw) | Protein (%, dw) | Total polyphenol (mg/g) | γ-aminobutyric acid | β-glucan (%, dw) | Fat (%, dw) | Ash (%, dw) | |
|---|---|---|---|---|---|---|---|
| WB | 53.26 ± 0.48e | 14.66 ± 0.34d | 0.60 ± 0.03a | 1.24 ± 0.04a | 0.84 ± 0.03a | 9.06 ± 0.11a | 1.33 ± 0.04a |
| WB + SO0 | 52.38 ± 0.22de | 12.86 ± 0.26a | 0.66 ± 0.02a | 3.56 ± 0.04a | 5.28 ± 0.08 g | 10.03 ± 0.12e | 2.40 ± 0.06e |
| WB + SO24 | 52.02 ± 0.28d | 13.22 ± 0.24ab | 0.72 ± 0.02b | 6.96 ± 0.06b | 4.86 ± 0.04f | 9.88 ± 0.08d | 2.32 ± 0.07de |
| WB + SO48 | 51.46 ± 0.33 cd | 13.56 ± 0.28b | 0.81 ± 0.04c | 9.48 ± 0.12c | 3.86 ± 0.07e | 9.76 ± 0.18 cd | 2.26 ± 0.06d |
| WB + SO72 | 51.08 ± 0.38c | 13.88 ± 0.16bc | 0.92 ± 0.03d | 11.76 ± 0.14d | 2.82 ± 0.06d | 9.62 ± 0.14c | 2.14 ± 0.08 cd |
| WB + SO96 | 50.32 ± 0.28b | 14.02 ± 0.12c | 0.94 ± 0.01de | 13.85 ± 0.13e | 1.23 ± 0.04c | 9.43 ± 0.13bc | 2.03 ± 0.06c |
| WB + SO120 | 49.63 ± 0.23a | 14.12 ± 0.14 cd | 0.96 ± 0.01e | 14.32 ± 0.10f | 1.06 ± 0.03b | 9.36 ± 0.16b | 1.94 ± 0.12b |
Note: Values are mean ± SD (n = 3). Values with different letters in the same column are significantly different (P < 0.05). WB represents the wheat bread, while WB + SO0-120 represents the breads with 0–120 h sprouting time of oats flour addition, respectively.
3.3. Textural analysis
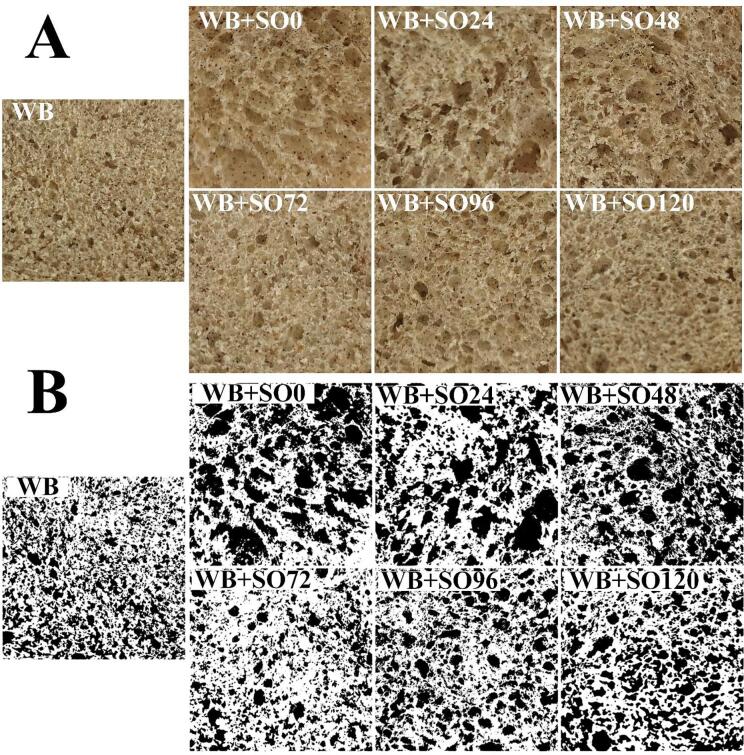
To further evaluate the palatability and appearance of bread, the texture properties were measured (Table 2). With the substitution of oat flour, the specific volume of wheat bread was significantly decreased from 4.02 to 2.52 mL/g (P < 0.05). The maximum specific volume value of bread with sprouted oat flour was 3.78 mL/g at the sprouting time of 72 h, which was closed to that of pure wheat bread. After spouting time over 72 h, the specific volume was undermined. The sprouted oats diluted the gluten, resulting in the failure to format the compact network structure of dough. With large pores and destruction of air-holding capacity, the specific volume of bread was reduced. Both the unit cell density and area fraction in the bread core are associated with the specific volume and hardness of bread. The distribution of pores is the result of the comprehensive action of various components of bread (Fig. 1). Pores in bread with superior quality are small and dense, and the distribution is homogenous. Otherwise, the pores in bread with deteriorated quality are large and sparse, and the distribution is uneven. The addition of oat flour decreased the number and the density of pores in bread but enlarged the average area fraction of pores, which means that proper sprouting treatment could improve the texture characteristics of mixed bread.
Table 2.
Effects of sprouted oat flour substation on the structure of wheat bread.
| Specific volume (mg/L) | CD (cell/cm2) | AF (%) | |
|---|---|---|---|
| WB | 4.02 ± 0.22 g | 72.44 ± 1.24 g | 24.02 ± 0.92a |
| WB + SO0 | 2.52 ± 0.12a | 45.92 ± 2.26a | 40.42 ± 0.66f |
| WB + SO24 | 2.74 ± 0.14b | 52.42 ± 2.22b | 36.02 ± 0.78e |
| WB + SO48 | 3.26 ± 0.11c | 58.62 ± 3.16d | 32.42 ± 0.45d |
| WB + SO72 | 3.78 ± 0.16f | 65.82 ± 1.56f | 27.88 ± 0.72b |
| WB + SO96 | 3.52 ± 0.14e | 60.32 ± 1.48e | 30.02 ± 0.42c |
| WB + SO120 | 3.34 ± 0.08d | 54.72 ± 1.62c | 35.34 ± 0.52e |
Note: Values are mean ± SD based on 9 technical measurements. Values with different letters in the same column are significantly different (P < 0.05). WB represents the wheat bread, while WB + SO0-120 represents the breads with 0–120 h sprouting time of oats flour addition, respectively. CD: cell density, AF: area fraction.
Fig. 1.
Cross-sectional pore images of bread substituted by oats sprouted for different duration. A: 2D images of inner crumbs of bread, B: The same central sections segmented with global thresholding concerning the porosity detected (3 × 3 cm). WB represents the wheat bread, while WB + SO0–120 represents the bread with sprouting flour for 0–120 h, respectively.
3.4. Digestion characteristics
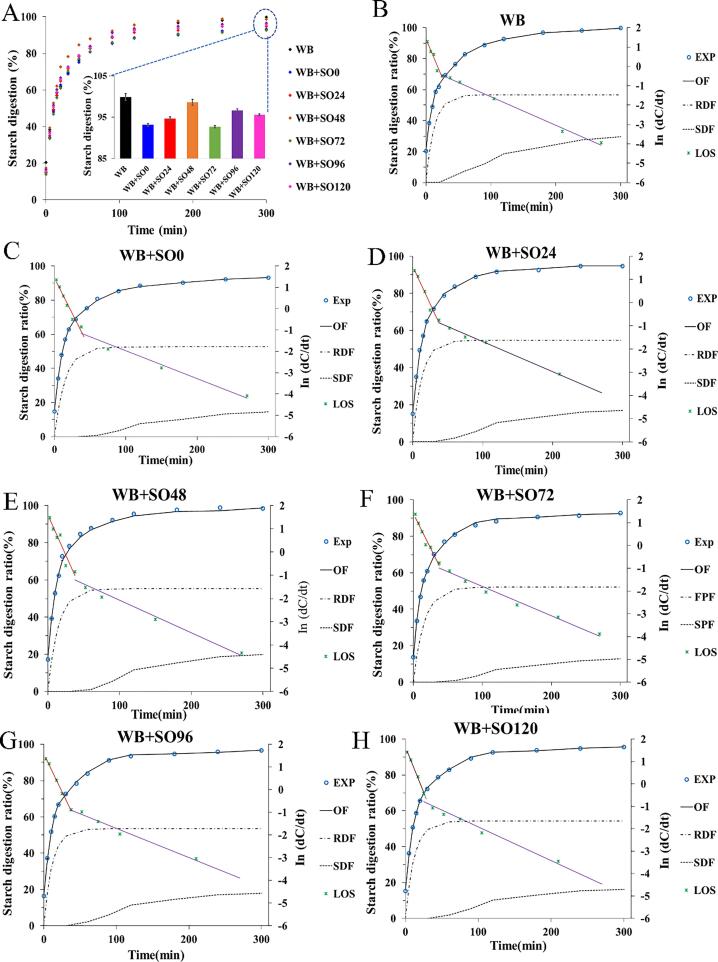
The in vitro starch digestibility of bread with oat flour sprouted for different time showed typical exponential increase trends (Fig. 2A). The starch in bread was massively consumed in the first 30 min, the digestibility of starch gradually slowed down and tended to be stable when the digestion time reached 120 min. The results showed that the digestion of mixed bread conformed to the first-order kinetic characteristics, and the maximum starch digestibility of mixed bread is more than 90 %. Compared with pure wheat bread, the starch digestibility of bread added to oat flour decreased by 7.28 %, which proved that the addition of oat flour reduced the starch digestibility of bread. To further quantify the dissimilarity of starch digestion of bread with oats sprouted at different time, LOS/CPS modeling was utilized to fit in vitro starch digestion curves of bread. The LOS curves presented two discontinuous stages, which indicated that there were different starch fractions with distinct digestion rate constants (Fig. 2 B-H). It was noteworthy that the starch digestion rate of bread reached the minimum value (92.22 %) with oat flour sprouted for 72 h. However, the starch digestion rate declined when the sprouting duration exceeded 72 h. The digestion fitted parameters are summarized in Table 3. As expected, the rate constant for the RDF (k1) was about 6 times higher than that for SDF (k2). The maximum digestion extent for the RDF (C∞1) was also much higher than that of the SDF(C∞2). Furthermore, the maximum digestion extent of RDF (C∞1) did not change significantly after adding different sprouted oats. However, the maximum digestion extent of SDF (C∞2) generally decreased compared with pure wheat bread, and the tendency was first decreased and then increased with the extension of sprouting time. The results suggested that proper sprouting treatment of oats can slow down the starch digestibility.
Fig. 2.
Digestograms for bread with the different sprouting time of oat flours (A) and their corresponding LOS/CPS kinetics model fittings (B–F). All CPS overall fittings have an R2 greater than 0.998. WB represents the wheat bread, while WB + SO0–120 represents the bread with sprouting flour for 0–120 h, respectively. Exp is experimental data. OF is the overall fit curve. RDF is the rapidly digestible fraction and SDF is the slowly digestible fraction. LOS is the logarithm of slope data.
Table 3.
Fitting parameters for the in vitro digestion of starch from bread with sprouted oat flour for different times by the combination of parallel and sequential kinetics models.
| k1 × 10-2 | k2 × 10-2 | C∞1 | C∞2 | t2start | |
|---|---|---|---|---|---|
| WB | 8.07 ± 0.16e | 1.48 ± 0.06e | 56.67 ± 0.05e | 31.03 ± 0.55 g | 25.75 ± 1.42a |
| WB + SO0 | 6.64 ± 0.11b | 1.31 ± 0.02b | 52.62 ± 0.02a | 15.68 ± 0.13b | 41.22 ± 0.32e |
| WB + SO24 | 7.48 ± 0.14d | 1.39 ± 0.02 cd | 54.68 ± 0.05c | 17.55 ± 0.18d | 38.24 ± 0.08d |
| WB + SO48 | 6.92 ± 0.12c | 1.41 ± 0.04 cd | 55.32 ± 0.04d | 20.56 ± 0.16f | 35.76 ± 0.17b |
| WB + SO72 | 6.03 ± 0.08a | 1.22 ± 0.02a | 52.22 ± 0.06a | 13.56 ± 0.15a | 44.36 ± 0.14f |
| WB + SO96 | 6.96 ± 0.08c | 1.43 ± 0.02d | 53.42 ± 0.04b | 18.36 ± 0.18e | 36.59 ± 0.64bc |
| WB + SO120 | 7.35 ± 0.13d | 1.37 ± 0.04c | 54.23 ± 0.06c | 16.56 ± 0.14c | 37.86 ± 0.25c |
Note: Values are mean ± SD (n = 9), WB represents the wheat bread, while WB + SO0-120 represents the bread with 0–120 h sprouting time of oats flour addition, respectively. Values with different letters in the same column are significantly different (P < 0.05).
3.5. Microstructure
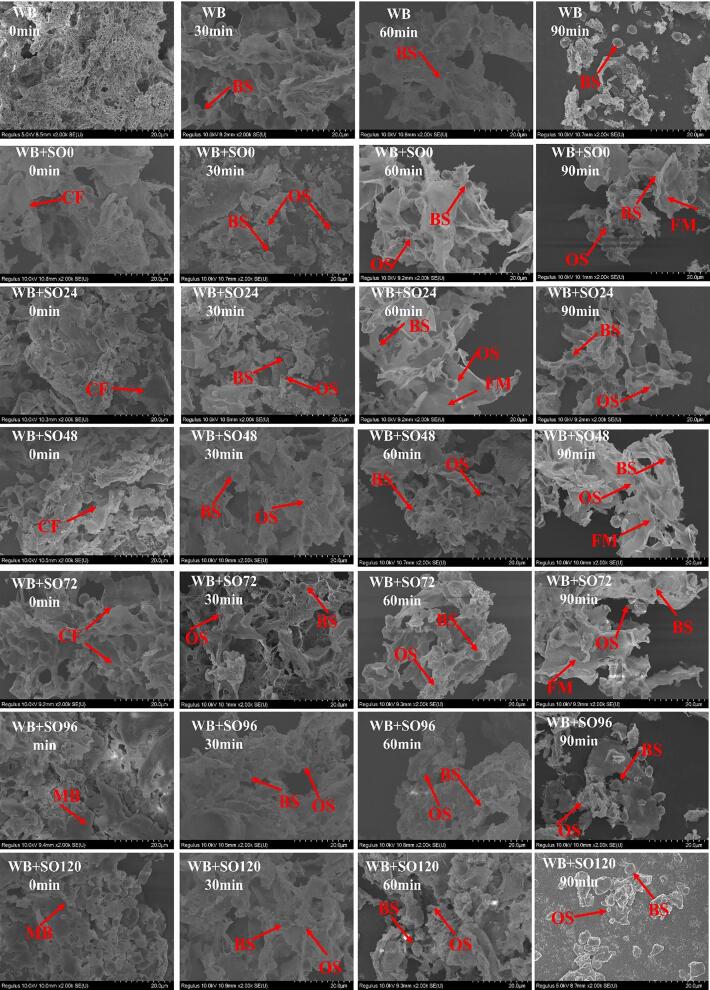
To further explore the digestion mechanism of bread with oats spouted for different times, the evolution of SEM microstructures during the digestion process is shown in Fig. 3. The three-dimensional network structure of gluten gel plays a critical role in the quality of bread. At the beginning of digestion, the wheat dough had a strong and continuous gluten network structure, which wrapped starch granules inside it. The distribution of the gel network was uniform and dense, and the overall structure of the dough is relatively stable. However, with the addition of oats flour, the gluten structure started to break and the protein became discontinuous. Moreover, some starch particles were gradually exposed, the cross-linking of gluten protein was sparse, and the pores became larger and rough. When the sprouting time was 72 h, the crosslinking compactness of gluten protein raised, and the network structure of gel tended to be continuous. Time from 96 to 120 h was the end phase of sprouting, and the basic nutrients in oats are consumed in large quantities. The combination of gluten protein and water was undermined, and the structure of gluten network deteriorated with the appearance of melting block (MB) structure. With the progress of digestion, the starch in bread was gradually degraded. When digestion time exceeded 30 min (around t2start), large holes appeared in the matrix and the continuous structure disappeared. Meanwhile, ungelatinized type B wheat starch and oat starch particles were gradually exposed. After 90 min of digestion, the fiber membrane (FB) structure appeared in the bread with sprouted oat flour caused by intertwining of OBG with protein, which proved that sprouting directly affected the digestion rate of bread.
Fig. 3.
SEM microstructure of bread at different digestion stages (0, 30, 60, and 90 min). WB + SO0-120 represents the bread with sprouted oat flour for 0–120 h, respectively. BS: type B wheat starch granules, OS: oat starch granules, CF: complex film, MB: melted block, FM: fiber membrane.
4. Discussion
4.1. Effect of sprouted oats flour on the chemical composition of wheat bread
Once the oats are sprouted, the external structure and internal quality of starch will change greatly. Starch in oats was gradually hydrolyzed with the progression of the sprouting process. Upon sprouting, the activity of α-amylase gradually increased, and more and more starch were decomposed and damaged. As β-glucanase activity is stimulated, OBG was decomposed into oligosaccharides containing several glycosyl groups. To promote the oat's growth, the proteins in oat grains were hydrolyzed into small molecular substances such as amino acids or amides under the action of protease. The contents of water-soluble and salt-soluble proteins in sprouted oats were increased, while the contents of alcohol-soluble proteins and alkaline proteins were decreased. This indicated that proteins were in a dynamic state of continuous synthesis and decomposition (Liu, Wang, Lu, Shu, Zhang, & Chen, 2022). Fat was regarded as one of the three major nutrients in oat. It is closely related to the processing quality of bread, although it is not as much as starch and protein. As the lipase activity in the aleurone layer was enhanced, the fat was hydrolyzed into glycerol and fatty acid under the action of lipase. In the sprouting process, both the epidermal and the external structure of oat grain was changed, so the content of ash in oat varied along with it. Polyphenols and GABA are mostly bound to cellulose or hemicellulose in cell wall by ester bonds, which could not be hydrolyzed by human digestive enzymes, resulting in low bioavailability. During the sprouting process, polyphenols and GABA were released due to the enzymatic degradation of cellulose (Randhir, Kwon, & Shetty, 2008). The protein was hydrolyzed to glutamic acid, providing sufficient substrate for glutamic acid decarboxylase, so the content of GABA increased. After sprouting, the activity of polyphenol oxidase was decreased, whereas the activity of anthracamide synthase was increased, which promoted the synthesis of polyphenols, and the contents of total polyphenols and free phenolic acids were significantly increased.
4.2. Textural attributes of bread crumbs with oats flour sprouted for different times
The specific volume reflects the air retention of gluten network structure in bread, which plays an essential role in the quality of bread. The high content of OBG competed with the dough for moisture, which affected the secondary structure of gluten in the dough. The network structure of the dough was destroyed, which reduced the specific volume of bread. Moreover, too much starch in oats will hinder the formation of protein and starch complex in mixed dough and inhibit the volume of bread. It indicated that suitable OBG (sprouting for 72 h) is conducive to developing the gluten network and fermentation of pasting dough, so the specific volume of bread after baking was increased (Table 1). The crosslinking and aggregation of gluten improved the viscoelasticity and ductility of bread, enhancing the stability of protein network structure, which could maintain the CO2 produced by fermentation (Guo, Wang, Zhang, Wu, & Bao, 2021). Physicochemical properties of OBG in oats impacted the quality of bread. The incompatibility between high molecular weight OBG and bread components impeded the interaction among gluten molecules. Sprouting raised cell density and reduced their average area fraction, which suggested that spouting loosened the inner structure of bread. As the sprouting for 72 h, OBG developed a stable gluten protein network structure via noncovalent bonding (hydrogen bond and electrostatic interaction) (Ortiz de Erive, He, Wang, & Chen, 2020). Nevertheless, OBG has almost completely decomposed at the final phase of sprouting, and the texture of the bread became rough.
4.3. In vitro starch digestion of bread with oat flour sprouted for different times
The higher degree of maximum starch digestion was due to the gelatinization of starch particles during bread baking, which destroyed all the supramolecular structures of starch, including the crystalline structure and layered structure (Ozgolet, Yaman, Zeki Durak, & Karasu, 2022). Large number of polyphenols and OBG in oat could inhibit the activity of starch digestive enzymes (J. Xu et al., 2017). The components from LOS curves (Fig. 2 B-H) were referred to the rapidly digestible fraction (RDF) and the slowly digestible fraction (SDF).The CPS kinetic model showed that RDF and SDF were not started simultaneously, but shared an evident overlapping region. This suggested that the digestion of RDF and SDF followed the combination pattern of parallel and sequential digestion mode. This is different from the sequential starch digestion mode observed from the digestion of noodles, in which the outer starch is completely gelatinized and digested at a much higher rate than the relatively complete starch granules in the middle part (Fan, Guo, & Zhu, 2022). Due to starch was not completely gelatinized, the digestion rate slowed down. The addition of the oat flour lowered the starch digestion rate remarkably, which may be due to higher content of OBG combined with water, and the water required for starch hydration was deprived, or a high concentration of OBG made the enzyme more difficult to get close to gelatinized starch. On account of sprouting, OBG contributed to the formation of the protein gel network. The starch was wrapped into the system to form a protein-OBG-starch complex, which reduced the starch digestibility in the mixed matrix (Nguyen, Gilbert, Gidley, & Fox, 2020). In addition, mixed bread contained both wheat starch and oat starch, the biochemical reaction produced by oat sprouting affected the digestion rate of starch. Due to most OBG being degraded and the bread texture being destroyed, the limitation between starch and enzyme was diminished. Nevertheless, following the extension of sprouting, the polyphenols in oats were raised to partly inhibit the enzyme activity (Kan, Oliviero, Verkerk, Fogliano, & Capuano, 2020). These parameters in Table 3 are quite similar to that reported for the digestion of retrograded starches (Wang et al., 2021). In addition, the start time (t2start) of SDF heightened and then declined, which suggested the addition of oats flour mainly changed the digestion rate for the rapidly digestible starch fraction. This is possible because OBG induced variation of intermolecular interaction, which was related to the accessibility of digestive enzymes to the substrate.
4.4. Microstructure evolution during the in vitro starch digestion of the bread
Wheat starch is subdivided into type A starch (AS) and type B starch (BS), the particle size of type A starch and type B starch are 25–35 μm and 2–8 μm. Type A starch in wheat had higher amylose content and compared with wheat starch B, type A starch had a larger specific surface area and higher swelling potential. Therefore, type A wheat starch is more prone to gelatinization than type B wheat starch. Oat starch (OS) granules were smaller with polygonal shapes, the average particle size of which was 2–5 μm. The network structure wrapped starch granules may be formed by mixing fully gelatinized type A wheat starch particles with other bread components such as protein. The high content of OBG diluted wheat gluten, which disturbed the formation of the gluten protein gel network, and a complex film (CP) structure was constructed (Cleary, Andersson, & Brennan, 2007). Interestingly, the continuity of gluten protein increased with the extension of germination time which was owing to OBG being consumed during the sprouting process. OBG has good water absorption, which can bind the free water into the gel structure through capillary action to form a dense network structure. OBG precipitated the protein groups in gluten combined to form a uniform and dense protein network structure, which increases the elasticity and toughness of dough (S. Xu, Gong, Rafique, He, & Hu, 2021). It indicated that moderate sprouting could reduce or offset the weakening effect of OBG in the gel network structure of the mixed dough. In addition, with the passage of digestion time, wheat type B starch and oat starch particles with rough morphology were gradually exposed to the surface of gluten-OBG complex. This was mainly because oat starch granules were also gradually consumed during sprouting, the gelatinization process of which was also strictly restricted by OBG (Zhou, Dhital, Zhao, Ye, Chen, & Zhao, 2021). This can also explain the two different digestible fractions of starch observed in Fig. 3. Therefore, the fast digestion fraction (RDF) was the fully gelatinized type A wheat starch particles, while the slow digestion fraction (SDF) was composed of incomplete gelatinized or ungelatinized type B wheat starch and oat starch particles. Because the gelatinized starch lost its original ordered structure, the fully gelatinized starch digested faster than the natural starch (Gao et al., 2021). In addition, OBG participated in the network formed by gluten, resulting in the physical barrier that limited the contact between starch particles and amylase, and inhibited the digestion of the slow digestion fraction (SDF). On the other hand, sprouting increased the content of polyphenol in oats, which restrained the activity of amylase. According to the Fig. 2 and Table 3, the addition of sprouting oat flour mainly reduced the total digested starch by abating the slow digestion fraction. The results gathered indicated that adding B-type wheat starch particles or oat flour with appropriate sprouting time in the formula is promising to develop bread with slow digestion starch characteristics.
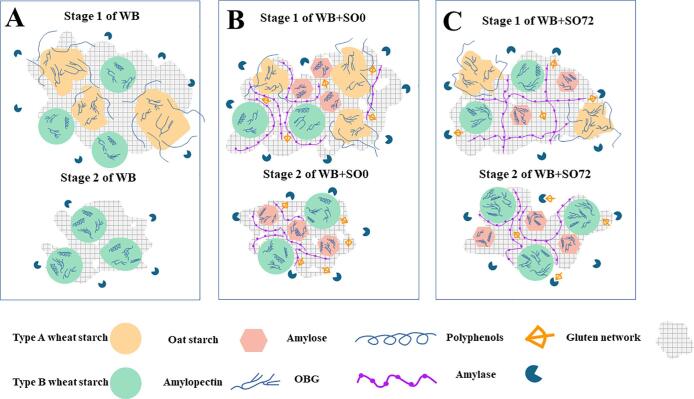
4.5. Proposed mechanism for the digestion of bread with sprouted oats flour
In summary, the digestive mechanism of wheat bread with oat flour spouted for different times was proposed in Fig. 4. In the rapid digestion stage, digestive enzymes mainly reacted with starch gelatinized wheat type A starch granules due to the loss of supramolecular structure. Meanwhile, pancreatin also gradually digested the protein covering type B wheat starch and oat starch granules. When type B wheat starch and oat starch granules were exposed to OBG-gluten complex, type B starch and oat starch granules began to dominate the digestion process, which belongs to the slow digestion stage (Srv et al., 2019). The CPS model fitted digestion diagram showed that when the second stage of digestion occurred, the first stage had not ended yet (Fig. 3 and Table 3), indicating that there was still fully gelatinized type A wheat starch in the system at the beginning of t2start. Therefore, the release of glucose molecules was due to the combined digestion of these two different digestible starch components. The starch digestibility of relatively complete type B wheat starch and oat starch granules was slow in the second digestion stage, so there are two-stage reactions with different digestion rates. Pure wheat starch contains only type A and type B wheat starch, so the digestion rate was fast and the duration was short, which was the reason for the high glycemic index of wheat bread (Wang et al., 2021). When unsprouted oat flour (WB + SO0) was added to wheat flour, due to the original complete structure of starch in oats and large amounts of OBG, the digestibility of starch in bread decreased significantly. With the extension of sprouting time (WB + SO72), the digestibility of bread starch further decreased. OBG participated in the gel network of wheat gluten protein, The film was constructed by starch-gluten-OBG complex, and the enzyme and substrate were physically hindered, which reduced the starch digestibility. In addition, the content of polyphenols in the system was increased and the activity of amylase was inhibited during oat sprouting. The starch and OBG were degraded extensively when sprouting time was further prolonged. However, the content of polyphenols increased significantly, which also slowed down the digestion of starch (Lucas-González, Ángel Pérez-Álvarez, Moscaritolo, Fernández-López, Sacchetti, & Viuda-Martos, 2021).
Fig. 4.
Proposed mechanism for the observed two stages of in vitro starch digestibility of bread substituted by wheat bread with sprouted oat flour for different sprouting times. A: wheat bread, B: wheat bread with unsprouted, C: wheat bread with sprouted oat flour for 72 h.
5. Conclusion
This is the first time that flours from spouted oat used as substitution in the wheat flour for breadmaking. The effect of spouted oat on nutrition composition, bread texture and in vitro starch digestibility was characterized in tandem, building a relationship between wheat and sprouted oat starch. Sprouted oat bread offers a more favorable nutrition profile than wheat bread due to its increasing effect on total polyphenol and γ-aminobutyric acid. β-glucan plays an important role in the texture properties, which maximized the specific volume of sprouted oat bread with the largest cell density and the minimum area fraction after 72 h sprouting. Interestingly, the digestion rate of the wheat bread with the substitution of 72 h spouted oats was reduced by 7.28 %. Through the LOS and CPS kinetic model fitting the digestion profiles, it was proved that there were two different digestive starch components in the mixed system, which were digested with a combination of parallel and sequential digestion patterns. From the evolution of bread SEM microstructures during the digestion process, it further suggested that starch components in the rapid digestion stage mainly involved the digestion of largely gelatinized type A wheat starch granules, while the slowly digestible starch fraction involved the digestion of type B wheat starch and oat starch granules exposed from the β-glucan-protein complex. Considering the nutritional value and low digestibility, wheat bread substituted by sprouted oats is likely to develop the product beneficial for the public health.
CRediT authorship contribution statement
Hongwei Cao: Writing – original draft, Investigation. Chong Wang: Writing – review & editing. Ranqing Li: Data curation. Xiao Guan: Funding acquisition, Supervision. Kai Huang: Visualization. Yu Zhang: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Young Elite Scientists Sponsorship Program by CAST (2021QNRC001); Major special projects of science and technology of Inner Mongolia Autonomous Region (2021SZD0017); Shanghai Sailing Program (20YF1433400); National Natural Science Foundation of China (32102140); the Domestic Science and Technology Cooperation Projects of Shanghai (21015801100).
References
- Aparicio-García N., Martínez-Villaluenga C., Frias J., Peñas E. Sprouted oat as a potential gluten-free ingredient with enhanced nutritional and bioactive properties. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.127972. [DOI] [PubMed] [Google Scholar]
- Cardone G., Grassi S., Scipioni A., Marti A. Bread-making performance of durum wheat as affected by sprouting. LWT. 2020;134 doi: 10.1016/j.lwt.2020.110021. [DOI] [Google Scholar]
- Cleary L.J., Andersson R., Brennan C.S. The behaviour and susceptibility to degradation of high and low molecular weight barley β-glucan in wheat bread during baking and in vitro digestion. Food Chemistry. 2007;102(3):889–897. doi: 10.1016/j.foodchem.2006.06.027. [DOI] [Google Scholar]
- Ding J., Johnson J., Chu Y.F., Feng H. Enhancement of γ-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound. Food Chemistry. 2019;283:239–247. doi: 10.1016/j.foodchem.2018.12.136. [DOI] [PubMed] [Google Scholar]
- Ding S., Peng B., Li Y., Yang J. Evaluation of specific volume, texture, thermal features, water mobility, and inhibitory effect of staling in wheat bread affected by maltitol. Food Chemistry. 2019;283:123–130. doi: 10.1016/j.foodchem.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Fan J.-X., Guo X.-N., Zhu K.-X. Impact of laccase-induced protein cross-linking on the in vitro starch digestion of black highland barley noodles. Food Hydrocolloids. 2022;124 doi: 10.1016/j.foodhyd.2021.107298. [DOI] [Google Scholar]
- Fu J., Zhang Y., Hu Y., Zhao G., Tang Y., Zou L. Concise review: Coarse cereals exert multiple beneficial effects on human health. Food Chemistry. 2020;325 doi: 10.1016/j.foodchem.2020.126761. [DOI] [PubMed] [Google Scholar]
- Gao J., Tan E.Y.N., Low S.H.L., Wang Y., Ying J., Dong Z., Zhou W. From bolus to digesta: How structural disintegration affects starch hydrolysis during oral-gastro-intestinal digestion of bread. Journal of Food Engineering. 2021;289 doi: 10.1016/j.jfoodeng.2020.110161. [DOI] [Google Scholar]
- Graça C., Raymundo A., Sousa I.D. Yoghurt and curd cheese addition to wheat bread dough: Impact on in vitro starch digestibility and estimated glycemic index. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.127887. [DOI] [PubMed] [Google Scholar]
- Gu Y., Qian X., Sun B., Ma S., Tian X., Wang X. Nutritional composition and physicochemical properties of oat flour sieving fractions with different particle size. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112757. [DOI] [Google Scholar]
- Guo J., Wang F., Zhang Z., Wu D., Bao J. Characterization of gluten proteins in different parts of wheat grain and their effects on the textural quality of steamed bread. Journal of Cereal Science. 2021;102 doi: 10.1016/j.jcs.2021.103368. [DOI] [Google Scholar]
- Kan L., Oliviero T., Verkerk R., Fogliano V., Capuano E. Interaction of bread and berry polyphenols affects starch digestibility and polyphenols bio-accessibility. Journal of Functional Foods. 2020;68 doi: 10.1016/j.jff.2020.103924. [DOI] [Google Scholar]
- Kock L.B., Brummer Y., Exley T., Rhymer C., Storsley J., Xie K.…Bordenave N. In vitro assessment of oat β-glucans nutritional properties: An inter-laboratory methodology evaluation. Carbohydrate Polymers. 2018;200:271–277. doi: 10.1016/j.carbpol.2018.07.082. [DOI] [PubMed] [Google Scholar]
- Li C., Dhital S., Gidley M.J. High-amylose wheat bread with reduced in vitro digestion rate and enhanced resistant starch content. Food Hydrocolloids. 2022;123 doi: 10.1016/j.foodhyd.2021.107181. [DOI] [Google Scholar]
- Liu S., Wang W., Lu H., Shu Q., Zhang Y., Chen Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends in Food Science & Technology. 2022;123:187–197. doi: 10.1016/j.tifs.2022.02.029. [DOI] [Google Scholar]
- Lucas-González R., Ángel Pérez-Álvarez J., Moscaritolo S., Fernández-López J., Sacchetti G., Viuda-Martos M. Evaluation of polyphenol bioaccessibility and kinetic of starch digestion of spaghetti with persimmon (Dyospyros kaki) flours coproducts during in vitro gastrointestinal digestion. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128142. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.L., Flanagan B.M., Tao K., Ni D., Gidley M.J., Fox G.P., Gilbert R.G. Effect of processing on the solubility and molecular size of oat β-glucan and consequences for starch digestibility of oat-fortified noodles. Food Chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131291. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.L., Gilbert R.G., Gidley M.J., Fox G.P. The contribution of β-glucan and starch fine structure to texture of oat-fortified wheat noodles. Food Chemistry. 2020;324 doi: 10.1016/j.foodchem.2020.126858. [DOI] [PubMed] [Google Scholar]
- Ortiz de Erive M., He F., Wang T., Chen G. Development of β-glucan enriched wheat bread using soluble oat fiber. Journal of Cereal Science. 2020;95 doi: 10.1016/j.jcs.2020.103051. [DOI] [Google Scholar]
- Ozgolet M., Yaman M., Zeki Durak M., Karasu S. The effect of five different sourdough on the formation of glyoxal and methylglyoxal in bread and influence of in vitro digestion. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131141. [DOI] [PubMed] [Google Scholar]
- Puerta P., Garzón R., Rosell C.M., Fiszman S., Laguna L., Tárrega A. Modifying gluten-free bread's structure using different baking conditions: Impact on oral processing and texture perception. LWT. 2021;140 doi: 10.1016/j.lwt.2020.110718. [DOI] [Google Scholar]
- Randhir R., Kwon Y.-I., Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovative Food Science & Emerging Technologies. 2008;9(3):355–364. doi: 10.1016/j.ifset.2007.10.004. [DOI] [Google Scholar]
- Roye C., Van Wayenbergh E., Henrion M., De Bondt Y., Chanvrier H., King R.…Courtin C.M. Extrusion-cooking affects oat bran physicochemical and nutrition-related properties and increases its β-glucan extractability. Journal of Cereal Science. 2021;102 doi: 10.1016/j.jcs.2021.103360. [DOI] [Google Scholar]
- Sánchez-Pardo M.E., Jiménez-García E., González-García I. Study about the addition of chemically modified starches (cross-linked cornstarches), dextrins, and oats fiber in baked pound cake. Journal of Biotechnology. 2010;150:316. doi: 10.1016/j.jbiotec.2010.09.298. [DOI] [Google Scholar]
- Santos F.G., Aguiar E.V., Rosell C.M., Capriles V.D. Potential of chickpea and psyllium in gluten-free breadmaking: Assessing bread's quality, sensory acceptability, and glycemic and satiety indexes. Food Hydrocolloids. 2021;113 doi: 10.1016/j.foodhyd.2020.106487. [DOI] [Google Scholar]
- Srv A., Mishra S., Hardacre A., Matia-Merino L., Goh K., Warren F., Monro J. Kernel structure in breads reduces in vitro starch digestion rate and estimated glycaemic potency only at high grain inclusion rates. Food Structure. 2019;21 doi: 10.1016/j.foostr.2019.100109. [DOI] [Google Scholar]
- Suárez-Estrella D., Cardone G., Buratti S., Pagani M.A., Marti A. Sprouting as a pre-processing for producing quinoa-enriched bread. Journal of Cereal Science. 2020;96 doi: 10.1016/j.jcs.2020.103111. [DOI] [Google Scholar]
- Wang X., Lao X., Bao Y., Guan X., Li C. Effect of whole quinoa flour substitution on the texture and in vitro starch digestibility of wheat bread. Food Hydrocolloids. 2021;119 doi: 10.1016/j.foodhyd.2021.106840. [DOI] [Google Scholar]
- Wilcox M.D., Cherry P., Chater P.I., Yang X., Zulali M., Okello E.J.…Pearson J.P. The effect of seaweed enriched bread on carbohydrate digestion and the release of glucose from food. Journal of Functional Foods. 2021;87 doi: 10.1016/j.jff.2021.104747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Wang P., Sun M., Gu Z., Yang R. Nitric oxide mediates γ-aminobutyric acid signaling to regulate phenolic compounds biosynthesis in soybean sprouts under NaCl stress. Food Bioscience. 2021;44 doi: 10.1016/j.fbio.2021.101356. [DOI] [Google Scholar]
- Xu J., Kuang Q., Wang K., Zhou S., Wang S., Liu X., Wang S. Insights into molecular structure and digestion rate of oat starch. Food Chemistry. 2017;220:25–30. doi: 10.1016/j.foodchem.2016.09.191. [DOI] [PubMed] [Google Scholar]
- Xu S., Gong Y., Rafique H., He T., Hu X. Effect of oat β-glucan addition on the staling properties of wheat-oat blended flour Chinese steamed bread. Bioactive Carbohydrates and Dietary Fibre. 2021;26 doi: 10.1016/j.bcdf.2021.100285. [DOI] [Google Scholar]
- Yu Y., Wang L., Qian H., Zhang H., Li Y., Wu G.…Rao Z. Effect of selected strains on physical and organoleptic properties of breads. Food Chemistry. 2019;276:547–553. doi: 10.1016/j.foodchem.2018.10.048. [DOI] [PubMed] [Google Scholar]
- Yuksel F., Kayacier A. Effects of addition of stale bread flour on the acrylamide, fatty acid composition, resistant starch content, and in vitro glycemic index in wheat chips production using response surface methodology. LWT. 2022;161 doi: 10.1016/j.lwt.2022.113354. [DOI] [Google Scholar]
- Zhou Y., Dhital S., Zhao C., Ye F., Chen J., Zhao G. Dietary fiber-gluten protein interaction in wheat flour dough: Analysis, consequences and proposed mechanisms. Food Hydrocolloids. 2021;111 doi: 10.1016/j.foodhyd.2020.106203. [DOI] [Google Scholar]