Abstract
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in patients with rheumatoid arthritis (RA). Some studies have reported a decrease in CVD in patients with RA using hydroxychloroquine (HCQ). Most of these have had fewer participants and have analyzed only composite outcomes. We aimed to identify the association between the use of HCQ in patients with RA and the incidence of major adverse cardiac events (MACEs), cerebral infarction, and AMI.
Methods
This was a retrospective observational study using the TriNetX Diamond Network. Propensity score matching (PSM) was used to equilibrate the cohorts. The dependent variables in our study were MACE, cerebral infarction, and AMI.
Results
A total of 2,261,643 patients with RA were identified. Approximately 6% had been prescribed HCQ. Of those prescribed HCQ, 80% (112,743) were females, while of those not prescribed HCQ, 72.5% (1,536,937) were females. HCQ was associated with lower rates of MACE (HR 0.827, 95%CI 0.8,0.86), cerebral infarction (HR 0.824, 95% CI 0.78,0.87), and AMI (HR 0.9, 95% CI 0.85,0.96). These associations were not seen in patients taking biologics. HCQ was associated with lower MACE in all other subgroups.
Conclusion
In conclusion, HCQ was slightly beneficial in decreasing MACE and cerebral infarction in patients with RA. These associations were significantly lower in patients taking methotrexate or biologics. Although there was a significant decrease in the risk of AMI in all patients with RA, these results were not replicated in subgroup analyses, and there was an apparent increased risk of AMI with the use of HCQ in patients using biologics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10557-022-07387-z.
Keywords: Hydroxychloroquine, Rheumatoid Arthritis, Myocardial Infarction, Stroke, Cardiovascular Disease
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality in patients with rheumatoid arthritis (RA) [1, 2]. The relative risk of myocardial infarction in patients with RA is 2.0 (95% CI 1.232–3.29) [2]. Although some studies have shown an increased prevalence of CVD risk factors in patients with RA, the higher incidence of cardiovascular events is not fully explained by these risk factors alone [3]. CVD mortality in patients with RA is approximately 50% higher than in the general population [1], and inflammation has been postulated as the main driver for this increased risk [4, 5]. This is supported by high levels of cytokines in their plasma [6] and the decrease in CVD seen with anti-TNF and methotrexate [7–9]. RA has been associated with peripheral insulin resistance [10], dyslipidemia [11], endothelial dysfunction [12, 13], increased levels of fibrinogen, Von Willebrand factor, d-dimer, tissue plasminogen antigen [14], and platelet reactivity [15, 16], all of which can promote the development of CVD.
Hydroxychloroquine (HCQ) has been associated with decreased cardiovascular death in patients with RA, likely because of its beneficial effects on lipids, glucose, coagulation, inflammation, and endothelial function [17, 18]. Most studies before ours have had a smaller number of participants and have analyzed only composite outcomes (AMI, stroke, TIA, PAD) [17, 18]. This study aimed to determine the association between the use of HCQ in patients with RA and the odds of presenting AMI, CAD, and cerebral infarction by analyzing data from a large database of patients in the US healthcare system.
Materials and Methods
Data Source
We used the TriNetX Diamond Network, which provided access to third-party data from electronic medical records of community-based primary and specialty care, medical claims from claims clearinghouses (including inpatient, outpatient, specialty, PCP, and ancillary care settings), and pharmacy claims. This data is harmonized and curated by TriNetX every 3 months. Overall, the dataset represents 92 sites, 212 million patients, and 99% of U.S. health plans since 2014.
The TriNetX platform classifies diagnoses in ICD-10 codes. For health care organizations that provide data in ICD-9, the TriNetX platform transforms that data into ICD-10. More information regarding this dataset and the TriNeTx platform can be found at www.trinetx.com.
Study Design and Population
We performed a retrospective observational cohort study using the TriNetX Diamond Network, which provided access to third-party longitudinal data from 92 sites, 212 million patients, and 99% of US health plans. The data for this study was collected and analyzed in August 2022.
TriNetX, LLC complies with the Health Insurance Portability and Accountability Act (HIPAA) and any additional data privacy regulations applicable to the contributing healthcare organizations. TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System to protect the healthcare data it has access to and meet the HIPAA Security Rule requirements. Any data displayed on the TriNetX Platform in aggregate form, or any patient-level data provided in a dataset generated by the TriNetX Platform, only contains de-identified data as per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
Inclusion and Exclusion Criteria
We included patients ≥ 18 years old with RA diagnosis defined as International Classification of Diseases, tenth Revision, Clinical Modification (ICD-10-CM), codes M05 (Rheumatoid Arthritis with Rheumatoid Factor) and M06 (other Rheumatoid Arthritis).
Our exposed cohort was defined as patients with a first prescription of HCQ recorded between January 1, 2016 and January 1, 2022, after any documented RA. We excluded all patients < 18 years old and any patients with a prescription of HCQ on or before December 31, 2015. The un-exposed cohort was defined by patients with a diagnosis of RA who did not have any recorded prescription of HCQ.
Four subgroups were analyzed: females, males, patients taking methotrexate (defined as the first instance of methotrexate prescription after an RA diagnosis that had not been on methotrexate on or before December 31, 2015), patients taking biologics (defined as the first instance of biologics prescription after an RA diagnosis that had not been on biologics on or before December 31, 2015). The medications considered as biologics were golimumab, anakinra, abatacept, tocilizumab, adalimumab, upadacitinib, etanercept, baricitinib, sarilumab, infliximab, infliximab, certolizumab pegol, and tofacitinib.
Dependent Variables
The dependent variables in our study were MACE (ICD-10 codes: I21, I22, I23, I24, I63, I65, I66), cerebral infarction (ICD-10 I63), and AMI (ICD-10 I21).
Data Collection
The TriNetX Diamond network was queried from January 2016 to January 2022. Outcomes were analyzed from 1 month to 5 years after the index event.
Statistical Analysis
All statistical analysis was performed using the TriNetX online platform. Baseline characteristics were compared using chi-square for categorical data and mean with standard deviation for continuous data.
Propensity score matching (PSM) was used to adjust for differences between cohorts. The TriNetX platform calculates propensity scores using logistic regression and then uses greedy nearest-neighbor matching algorithm to obtain matched cohorts with a caliper width of 0.1 pooled standard deviations. The covariates used in 1:1 PSM were age, sex, race, hypertensive diseases (ICD-10 I10 to I16), neoplasms (ICD-10 C00 to D49), diabetes mellitus (ICD-10 E08 to E13), atherosclerotic heart disease of native coronary artery (ICD-10 I25.1), chronic kidney disease (CKD) (ICD-10 N18), cerebral infarction (ICD-10 I63), acute myocardial infarction (ICD-10 I21), ischemic cardiomyopathy (ICD-10 I25.5), cardiovascular medications (including antilipemic agents, diuretics, beta-blockers, ace inhibitors, calcium channel blockers, angiotensin ii inhibitor, antiarrhythmics, antianginals, alpha-blockers), and methotrexate use.
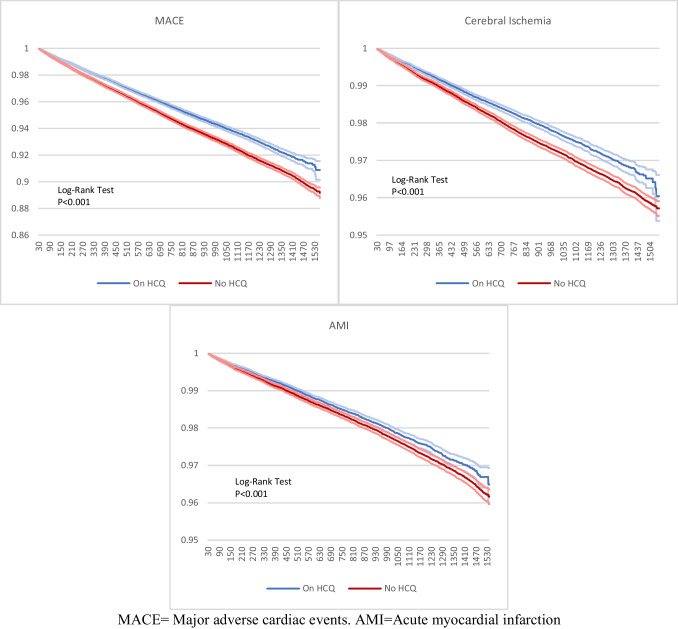
After PSM, hazard ratios (HRs) with 95% confidence intervals (CIs) and Kaplan Meier survival analysis were calculated. The TriNetX platform calculates HR and proportionality with R’s Survival package v3.2–3 and validates the numbers by comparing them with SAS version 9.4. For each analysis, patients with a history of the event were excluded. Statistical significance of the difference between cohorts in the Kaplan–Meier analysis was assessed with the log-rank test. A P value of < 0.05 was considered statistically significant.
Results
All results are summarized in Figs. 1 and 2 and Tables 1 and 2.
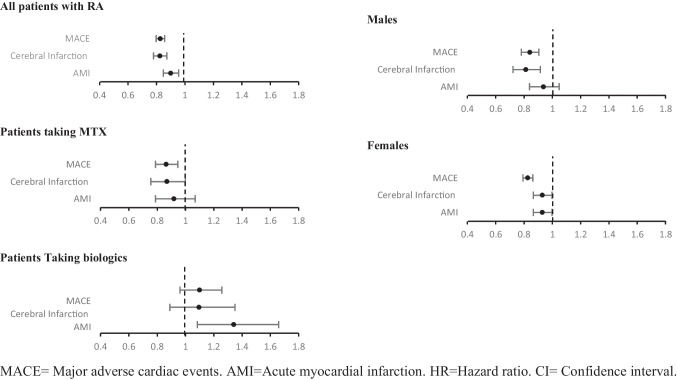
Fig. 1.
Forest plot representing outcomes/HR with 95%
Fig. 2.
Kaplan Meier analysis of all patients with RA
Table 1.
Cohort characteristics after propensity score matching in patients with RA with and without HCQ
| Results of PSM | |||||
|---|---|---|---|---|---|
| Hydroxychloroquine prescription | SD | ||||
| Yes | No | ||||
| Total number of patients | 140,857 | 140,857 | |||
| Age mean (SD) | 60.4 (14.6) | 60.4 (14.6) | |||
| N | Percentage | N | Percentage | ||
| Females | 112,743 | 80.04% | 112,783 | 80.07% | 0.00071067 |
| Males | 28,084 | 19.94% | 28,029 | 19.90% | 0.00097767 |
| Unknown race | 91,277 | 64.80% | 91,201 | 64.75% | 0.00112955 |
| White | 43,071 | 30.58% | 43,289 | 30.73% | 0.00335675 |
| Black or African American | 5892 | 4.18% | 5764 | 4.09% | 0.00456287 |
| Asian | 617 | 0.44% | 603 | 0.43% | 0.00151362 |
| Hypertensive diseases | 79,834 | 56.68% | 79,778 | 56.64% | 0.00080228 |
| Neoplasms | 49,780 | 35.34% | 49,722 | 35.30% | 0.0008615 |
| Diabetes mellitus | 33,794 | 23.99% | 33,646 | 23.89% | 0.00246234 |
| Atherosclerotic heart disease of native coronary artery | 18,824 | 13.36% | 18,655 | 13.24% | 0.0035328 |
| Chronic kidney disease (CKD) | 14,730 | 10.46% | 14,511 | 10.30% | 0.00509767 |
| Cerebral infarction | 4366 | 3.10% | 4212 | 2.99% | 0.00636312 |
| Acute myocardial infarction | 3220 | 2.29% | 2866 | 2.04% | 0.01728709 |
| Ischemic cardiomyopathy | 1210 | 0.86% | 1032 | 0.73% | 0.01422244 |
| Cardiovascular medications | 88,984 | 63.17% | 89,163 | 63.30% | 0.00263562 |
| Methotrexate | 36,388 | 25.83% | 36,273 | 25.75% | 0.00186616 |
SD standard difference, N number.
Table 2.
Outcomes
| On HCQ | Not on HCQ | ||||
|---|---|---|---|---|---|
| N | Outcome | N | Outcome | HR (95% CI) | |
| All patients with RA | |||||
| MACE | 127,139 | 4844 | 126,456 | 7470 | 0.827(0.798–0.858) |
| Cerebral Infarction | 136,115 | 2040 | 135,815 | 3188 | 0.824(0.779–0.872) |
| AMI | 137,280 | 1838 | 137,323 | 2701 | 0.9(0.848–0.956) |
| Patients taking MTX | |||||
| MACE | 27,154 | 848 | 27,398 | 1084 | 0.864(0.789–0.945) |
| Cerebral Infarction | 28,865 | 353 | 28,965 | 449 | 0.869(0.755–0.999) |
| AMI | 29,089 | 305 | 29,221 | 368 | 0.919(0.789–1.069) |
| Patients taking biologics | |||||
| MACE | 13,805 | 413 | 13,991 | 468 | 1.099(0.962–1.256) |
| Cerebral Infarction | 14,642 | 168 | 14,744 | 189 | 1.095(0.889–1.35) |
| AMI | 14,728 | 179 | 14,838 | 166 | 1.341(1.084–1.659) |
| Males | |||||
| MACE | 24,500 | 1225 | 24,287 | 1805 | 0.841(0.781—0.904) |
| Cerebral Infarction | 26,980 | 456 | 26,972 | 713 | 0.812(0.721—0.914) |
| AMI | 26,930 | 542 | 26,914 | 736 | 0.937(0.838—1.048) |
| Females | |||||
| MACE | 102,611 | 3619 | 102,261 | 5652 | 0.826(0.792—0.862) |
| Cerebral Infarction | 109,106 | 1584 | 108,903 | 2503 | 0.929 (0.865—0.998) |
| AMI | 110,320 | 1296 | 110,333 | 1854 | 0.929(0.865—0.998) |
MACE major adverse cardiac events, AMI acute myocardial infarction, HR hazard ratio, CI confidence interval.
Patient Characteristics
A total of 2,261,643 patients with RA were identified. Patients (6.2%) (140,857) had been prescribed HCQ. Of those prescribed HCQ, 80% (112,743) were females, while of those not prescribed HCQ, 72.5% (1,536,937) were females. The median age was 60.4 ± 14.6 and 62.6 ± 15.1 for patients that received HCQ and those who did not, respectively.
After PSM, each cohort had 140,857 patients, and all covariates were equilibrated with a SD of < 0.1. Baseline characteristics after PSM can be seen in Table 1. All the subgroups were matched with PSM with SD < 0.1 for all the covariates. The characteristics of each subgroup before and after PSM alongside propensity score density graphs can be seen in the supplementary material.
Outcomes
Major Cardiovascular Events
Among patients with RA, MACE was 17% lower in patients with HCQ (HR 0.827, 95%CI 0.8, 0.86). Similar results were seen in subgroup analysis, with 13% lower in those taking methotrexate (HR 0.864, 95%CI 0.8, 0.95), 16% in males (HR 0.841, 95% CI 0.78, 0.9), and 17% in females (HR 0.826, 95% CI 0.79, 0.86). There was no significant association in patients taking biologics (HR 1.099, 95% CI 0.96, 1.3).
Cerebral Infarction
Cerebral infarction was lower in patients using HCQ across all groups except for those on biologics (HR 1.095, 95% CI 0.89, 1.35): 18% lower in all patients with RA (HR 0.824, 95% CI 0.78, 0.87) and 16% lower in males (HR 0.812, 95% CI 0.72, 0.91). Although statistically significant, the upper CI for HR in females and patients on methotrexate was 0.99 making the interpretation of these results more difficult.
Acute Myocardial Infarction
Patients with RA receiving HCQ had 10% less AMI than those without (HR 0.9, 95% CI 0.85, 0.96). Females also had a statistically significant association with AMI (HR 0.929, 95% CI 0.865, 0.998). In patients taking biologics, they were 34% higher in the group with HCQ (HR 1.341, 95% CI 1.084, 1.659).
Discussion
HCQ was associated with lower rates of MACE and cerebral infarction across all subgroups, except for those on biologics. It is important to point out that the upper 95% CI of the HR for cerebral infarction was 0.999 in females and patients on methotrexate, making difficult the interpretation of these results. Although there was a significant association with lower AMI in all patients with RA, this was not reproduced in subgroup analysis. Patients on biologics had an increased risk of AMI with HCQ. Our work is in line with recent studies that have reported a positive association between the use of HCQ in patients with RA and CVD [17, 18] and contrasts those results from Lane et al., who performed a large retrospective study and found that long-term HCQ use was associated with increased risk of cardiovascular mortality when compared to sulfasalazine (HR 1.65 95%CI 1.12–2.44) and did not find a significant association with AMI, all-cause mortality, and stroke [19] and with the work of D’Andrea et al., who showed no difference in AMI and stroke between HCQ and methotrexate [20]. Although these results show that HCQ has similar or poorer cardiovascular outcomes compared to sulfasalazine and methotrexate, our study has the benefit of comparing the use of HCQ versus no HCQ in different subgroups of patients, including those taking other disease-modifying antirheumatic drugs (DMARDs).
There are several mechanisms by which HCQ may decrease the risk of atherosclerosis in patients with RA. Most of its action is likely due to its effects on lipids, glucose, coagulation, inflammation, and endothelial function. HCQ modulates the ATM (ataxia-telangiectasia mutated) protein kinase pathway with subsequent decreased JNK (Jun-N- terminal kinase) and LPL activity in macrophages [21]. JNK decreases adiponectin [22], and its inhibition increases insulin sensitivity [23] and decreases atherosclerosis [24, 25]. Although some experimental studies have failed to prove changes in serum cholesterol levels [21, 25], pooled data analysis from a meta-analysis showed significant changes in total cholesterol (− 13.1 mg/dL 95%CI − 20.9 to − 5.3), LDL (− 12.3 mg/dL 95%CI − 20.2 to − 4.6), triglycerides (− 12.5 mg/dL 95%CI − 28.9 to 3.9), and HDL (+ 1.6 mg/dL 95%CI − 0.96 to 4.3) [26]. Its effects on glucose control are evidenced by decreased incidence of diabetes mellitus (HR 0.59 95%CI 0.49–0.7) [26], improved insulin sensitivity in obese populations[27], and better glycemic control [28]. These are likely due to inhibition of insulin degradation by decreased leukocyte lysosomal enzymes, alpha-glucosidase, and hexosaminidase-A [29] and increased insulin receptor affinity[30] and insulin secretion [31].
HCQ inhibits platelet aggregation by acting in the arachidonic acid cascade via inhibition of lysosomal phospholipase A and C [32, 33]. On the vasculature, it stimulates the release of nitric oxide in endothelial cells inhibiting endothelial cell proliferation [34]. HCQ may decrease inflammation by lowering the release of TNF-alpha, blocking its conversion to mature protein from its precursor and diminishing the release of IL-1 and IL-6 as well as their mRNA levels [35]. The fact that the associations were weaker in patients taking other DMARDs supports the theory that CVD in patients with RA may be mediated, at least in part, by inflammation. This is supported by the CANTOS trial, where the use of canakinumab (a therapeutic monoclonal antibody targeting interleukin-1β) led to a significantly lower rate of recurrent cardiovascular events than placebo, independently of lipid levels [36]. The use of HCQ in patients taking other methotrexate was associated with lower MACE. There was no association between patients taking biologics and MACE or cerebral infarction. Interestingly, this group of patients had higher AMI with HCQ. These results are of particular importance, considering that the use of HCQ alone in patients with RA is only moderately effective and may be better used in conjunction with other DMARDs[37].
Considering the high percentage of females in our study, subgroups of females and males were analyzed. There was a slightly lower risk of MACE in both groups. There was no statistically significant association with AMI in males. In females, there was a lower risk of AMI and cerebral infarction; however, the upper limit of CI interval was close to one, making the interpretation of these results difficult. This contrasts with the work by Hung et al., who reported decreased incidence of CAD in female patients with RA on HCQ (HR 0.3, P < 0.01) but did not find a significant association in male patients [18]. This could have been due to the low number of males included after PSM. Our study has the advantage of a larger population (approximately 27,000 versus 49 males), which may have allowed us to find more differences.
It is important to remember that HCQ can have significant side effects, mainly arrhythmias and heart failure [38], and its safety profile has recently been put into consideration given the widespread use of the medication at the early times of the COVID-19 pandemic [39].
In conclusion, HCQ was slightly beneficial in decreasing MACE and cerebral infarction in patients with RA. These associations were significantly lower in patients taking methotrexate or biologics. Although there was a significant decrease in the risk of AMI in all patients with RA, these results were not replicated in subgroup analyses, and there was an apparent increased risk of AMI with the use of HCQ in patients using biologics. Considering that the safety profile of the use of HCQ in patients with RA has not been completely elucidated, more studies and, if possible, randomized controlled trials are required to prove the benefit of HCQ in patients with RA for the prevention of MACE, cerebral ischemia, and AMI.
Limitations
Several limitations in our study should be pointed out. We analyzed a large database containing information from electronic medical records, claiming clearing houses, and pharmacy claims and therefore could not verify the accuracy of reporting of diseases and medications. We could not account for severity of disease; we tried to mitigate this with subgroup analysis of patients taking other DMARDs and PSM. We could not account for medication duration, adherence, and dosage. Despite PSM, there might be other confounders that were not considered. Although most of the US healthcare data is included in the diamond network, it is possible that some patients had outcomes or medications reported in organizations not included in the database. In that case, the information could not have been analyzed.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
All the authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Andres Cordova Sanchez. The first draft of the manuscript was written by Andres Cordova Sanchez, Farzam Khokhar, and Danielle Olonoff. Revision and supervision of this study were performed by Robert Carhart.
Funding
The authors of the manuscript provided funding.
Data Availability
Data is available to all individuals and institutions with access to the TriNetX database.
Declarations
Ethics Approval
Ethics approval for this study was not required since all data was obtained from a de-identified database.
Informed Consent and Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Avina-Zubieta JA, Abrahamowicz M, De Vera MA, Choi HK, Sayre EC, Rahman MM, et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatol (Oxford) 2013;52(1):68–75. doi: 10.1093/rheumatology/kes353. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–1307. doi: 10.1161/01.CIR.0000054612.26458.B2. [DOI] [PubMed] [Google Scholar]
- 3.del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44(12):2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 5.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–1925. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright HL, Bucknall RC, Moots RJ, Edwards SW. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatol (Oxford) 2012;51(3):451–459. doi: 10.1093/rheumatology/ker338. [DOI] [PubMed] [Google Scholar]
- 7.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 8.Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL, et al. Tumor necrosis factor α inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66(3):355–363. doi: 10.1002/acr.22166. [DOI] [PubMed] [Google Scholar]
- 9.WG Dixon KD Watson M Lunt Hyrich KL Rheumatology Biologics Register Control Centre C, Silman AJ et al Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56(9):2905–12. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paolisso G, Valentini G, Giugliano D, Marrazzo G, Tirri R, Gallo M, et al. Evidence for peripheral impaired glucose handling in patients with connective tissue diseases. Metabolism. 1991;40(9):902–907. doi: 10.1016/0026-0495(91)90064-4. [DOI] [PubMed] [Google Scholar]
- 11.Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41(Suppl 2):97–101. doi: 10.2337/diab.41.2.S97. [DOI] [PubMed] [Google Scholar]
- 12.Wong M, Toh L, Wilson A, Rowley K, Karschimkus C, Prior D, et al. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48(1):81–89. doi: 10.1002/art.10748. [DOI] [PubMed] [Google Scholar]
- 13.Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106(17):2184–2187. doi: 10.1161/01.CIR.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 14.McEntegart A, Capell HA, Creran D, Rumley A, Woodward M, Lowe GD. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatol (Oxford) 2001;40(6):640–644. doi: 10.1093/rheumatology/40.6.640. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Wang NS, Yan CG, Li JH, Tang LQ. The significance of platelet activation in rheumatoid arthritis. Clin Rheumatol. 2007;26(5):768–771. doi: 10.1007/s10067-007-0550-0. [DOI] [PubMed] [Google Scholar]
- 16.Mac Mullan PA, Peace AJ, Madigan AM, Tedesco AF, Kenny D, McCarthy GM. Platelet hyper-reactivity in active inflammatory arthritis is unique to the adenosine diphosphate pathway: a novel finding and potential therapeutic target. Rheumatol (Oxford) 2010;49(2):240–245. doi: 10.1093/rheumatology/kep377. [DOI] [PubMed] [Google Scholar]
- 17.Sharma TS, Wasko MC, Tang X, Vedamurthy D, Yan X, Cote J, Bili A. Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc. 2016;5(1):e002867. 10.1161/JAHA.115.002867 [DOI] [PMC free article] [PubMed]
- 18.Hung YM, Wang YH, Lin L, Wang PYP, Chiou JY, Wei JC. Hydroxychloroquine may be associated with reduced risk of coronary artery diseases in patients with rheumatoid arthritis: a nationwide population-based cohort study. Int J Clin Pract. 2018;72(5):e13095. doi: 10.1111/ijcp.13095. [DOI] [PubMed] [Google Scholar]
- 19.Lane JCE, Weaver J, Kostka K, Duarte-Salles T, Abrahao MTF, Alghoul H, et al. Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study. Lancet Rheumatol. 2020;2(11):e698–e711. doi: 10.1016/S2665-9913(20)30276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Andrea E, Desai RJ, He M, Glynn RJ, Lee H, Weinblatt ME, et al. Cardiovascular risks of hydroxychloroquine vs methotrexate in patients with rheumatoid arthritis. J Am Coll Cardiol. 2022;80(1):36–46. doi: 10.1016/j.jacc.2022.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, et al. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4(5):377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327(2):460–467. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10(10):1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 24.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306(5701):1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 25.Shukla AM, Bose C, Karaduta OK, Apostolov EO, Kaushal GP, Fahmi T, et al. Impact of hydroxychloroquine on atherosclerosis and vascular stiffness in the presence of chronic kidney disease. PLoS One. 2015;10(9):e0139226. doi: 10.1371/journal.pone.0139226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rempenault C, Combe B, Barnetche T, Gaujoux-Viala C, Lukas C, Morel J, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2018;77(1):98–103. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 27.Mercer E, Rekedal L, Garg R, Lu B, Massarotti EM, Solomon DH. Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthritis Res Ther. 2012;14(3):R135. doi: 10.1186/ar3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, Ceriello A, et al. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus A new job for an old drug? Ann Intern Med. 1990;112(9):678–81. doi: 10.7326/0003-4819-112-9-678. [DOI] [PubMed] [Google Scholar]
- 29.Blazar BR, Whitley CB, Kitabchi AE, Tsai MY, Santiago J, White N, et al. In vivo chloroquine-induced inhibition of insulin degradation in a diabetic patient with severe insulin resistance. Diabetes. 1984;33(12):1133–1137. doi: 10.2337/diab.33.12.1133. [DOI] [PubMed] [Google Scholar]
- 30.Sorimachi K, Okayasu T, Yasumura Y. Increase in insulin binding affinity by chloroquine in cultured rat hepatoma cells. Endocr Res. 1987;13(1):49–60. doi: 10.1080/07435808709023662. [DOI] [PubMed] [Google Scholar]
- 31.Asamoah KA, Robb DA, Furman BL. Chronic chloroquine treatment enhances insulin release in rats. Diabetes Res Clin Pract. 1990;9(3):273–278. doi: 10.1016/0168-8227(90)90056-Y. [DOI] [PubMed] [Google Scholar]
- 32.Achuthan S, Ahluwalia J, Shafiq N, Bhalla A, Pareek A, Chandurkar N, et al. Hydroxychloroquine's Efficacy as an antiplatelet agent study in healthy volunteers: a proof of concept study. J Cardiovasc Pharmacol Ther. 2015;20(2):174–180. doi: 10.1177/1074248414546324. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzawa Y, Hostetler KY. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980;255(11):5190–5194. doi: 10.1016/S0021-9258(19)70769-8. [DOI] [PubMed] [Google Scholar]
- 34.Ghigo D, Aldieri E, Todde R, Costamagna C, Garbarino G, Pescarmona G, et al. Chloroquine stimulates nitric oxide synthesis in murine, porcine, and human endothelial cells. J Clin Invest. 1998;102(3):595–605. doi: 10.1172/JCI1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang CH, Choi JH, Byun MS, Jue DM. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatol (Oxford) 2006;45(6):703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 37.Rempenault C, Combe B, Barnetche T, Gaujoux-Viala C, Lukas C, Morel J, et al. Clinical and structural efficacy of hydroxychloroquine in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 2020;72(1):36–40. doi: 10.1002/acr.23826. [DOI] [PubMed] [Google Scholar]
- 38.Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41(10):919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 39.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available to all individuals and institutions with access to the TriNetX database.