Abstract
Objective
To inform clinicians of the first known case of a live born diagnosed with syndromic partial trisomy 15 and maternal uniparental disomy 15 resulting from a mosaic embryo transfer (MET). We believe that this case will highlight the need for standardized practice guidelines to address the potential risk of MET and the importance of prenatal follow-up after a pregnancy is achieved from a MET.
Design
Case report.
Setting
In vitro fertilization with preimplantation genetic testing for aneuploidy (PGT-A) and MET was completed at a fertility clinic in Canada. Postnatal testing and diagnosis were performed at the Medical Genetics Department of a hospital in Canada.
Patient(s)
A newborn male with a diagnosis of partial trisomy 15 and uniparental disomy (UPD) 15.
Intervention(s)
Mosaic embryo transfer after PGT-A was performed. Diagnostic testing performed after birth included a karyotype, fluorescence in situ hybridization analysis, chromosomal microarray, and microsatellite UPD testing.
Main Outcome Measure(s)
Confirmed nonmosaic partial aneuploidy of trisomy 15 and UPD15 in a symptomatic newborn conceived from MET.
Result(s)
Singleton pregnancy was achieved after a double embryo transfer involving 1 embryo diagnosed by PGT-A with high-level mosaic trisomy 15 and high-level mosaic deletion on chromosome 20 (mos(del(20)(q11.23-qter)). Routine prenatal screening and detailed fetal ultrasound did not identify any concerns. Postnatal genetic investigations, triggered by feeding difficulties in the newborn period, diagnosed the proband with maternal UPD15 and a supernumerary marker chromosome composed of 2 noncontiguous regions of chromosome 15. This karyotype is likely resulting from incomplete trisomy rescue occurring on the paternal chromosome 15.
Conclusion(s)
This case highlights the need for better guidelines and management of pregnancies achieved after MET.
Key Words: mosaic embryo transfer, PGT-A, aneuploidy, trisomy 15, uniparental disomy 15, trisomy rescue
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-22-00037
Introduction
Preimplantation genetic testing for aneuploidy (PGT-A) is used in addition to morphology-based grading to improve in vitro fertilization (IVF) transfer outcomes. Current PGT-A platforms, in addition to classifying embryos as euploid (normal chromosomal complement) or aneuploid (monosomic or trisomic), have the ability to detect mosaicism (a mixture of cells with different karyotypes) for complete or segmental chromosomal aneuploidy at levels between 20% and 80% (1). When no euploid embryos are available for transfer, mosaic embryos can be considered. It has been speculated that mosaic embryo transfers (METs) carry a potential risk for a chromosomally abnormal pregnancy and the birth of an affected child with intellectual disability and/or congenital anomalies. To help mitigate this risk, several groups have proposed scoring systems to prioritize mosaic embryos for transfer on the basis of the mosaicism level and the specific chromosomes involved (2, 3, 4). Published guidelines recommend cytogenetic prenatal diagnosis in cases of MET and uniparental disomy (UPD) testing when an imprinted chromosome is involved (5, 6). These recommendations are increasingly being questioned since a large study on outcomes of METs revealed only chromosomally normal fetuses in over 200 pregnancies (7, 8). To date, there have been 2 isolated reports of MET leading to the birth of a child where a postnatal blood test was able to identify the presence of an abnormal cell line. One reported a nonsyndromic liveborn with very low-level mosaicism (9), and the other reported a child with a diagnosis of 15q duplication syndrome after a euploid embryo transfer, which was later reanalyzed and found to be high-level mosaic for the same duplication (10). To our knowledge, our report provides the first documented live birth with a nonmosaic partial aneuploidy and UPD related to the initial mosaic PGT-A result.
Case report
A 42-year–old woman and her male partner underwent 2 cycles of IVF after struggling with secondary infertility related to tubal factors and advanced maternal age. Preimplantation genetic testing for aneuploidy by next generation sequencing and PGTai (CooperGenomics proprietary algorithm) was performed on 8 embryos, which resulted in 2 high-level mosaic embryos and 6 aneuploid embryos after testing. After receiving the results, the clinic that the couple was being seen at changed ownership, and the case was referred to the new providers for consideration of a MET. Genetic counseling was provided to the couple before embryo transfer. This included a discussion regarding the technical and clinical difficulties in interpreting mosaic PGT-A results and the potential outcomes of mosaicism, which include a healthy infant, congenital anomalies, intellectual disability, fetal growth restriction, and other adverse perinatal outcomes. Given the involvement of chromosome 15, UPD risk was discussed. It was reviewed that data and information about MET are still being gathered and that our understanding will likely evolve over time; however, the research to date was certainly favorable for a healthy outcome should a pregnancy be established. Amniocentesis was reviewed as the best option for prenatal testing. The couple ultimately decided to transfer their 2 mosaic embryos at the same time. The transfer occurred 7 weeks after the pretest counseling. Written consent was obtained from the couple, and a consent form to publish was signed.
Of the 2 mosaic embryos transferred, 1 was reported as a high-level mosaic for trisomy 15 with a deletion on the long arm of chromosome 20 (del(20)(q11.23-qter) and the other was reported as a high-level mosaic for monosomy 21 and X. Sex results were disclosed for the second embryo to help with clinical management and was reported female (XX). Sex was not disclosed for the first mosaic embryo (mosaic T15 and del(20)(q11.23-qter)) because of laws against sex selection in Canada. Preimplantation genetic testing for aneuploidy classified them both as high-level mosaic, meaning 40%–80% of the cells tested were abnormal. Of note, this percentage represents the highest observed score for the embryo, and the scores for the individual anomalies were not provided by the testing company. A dating ultrasound performed at 7 weeks and 6 days showed 1 gestational sac. At this time, the couple was offered prenatal screening options and referred back to their midwife group with a summary indicating that the pregnancy resulted from the transfer of 2 mosaic embryos. An invasive prenatal diagnosis of the resulting pregnancy was not performed. Non-invasive prenatal screening was ordered by the couple’s midwife and showed no increased risk of viable aneuploidies. Ultrasound performed at 19 + 1 weeks gestation showed no fetal abnormalities. Ultrasounds performed between 32 and 39 weeks showed amniotic fluid at the lower limit of the normal range. Induction of labor was performed with the male proband born by vaginal delivery at 39 + 6 weeks, with a birth weight of 3255 g (27th percentile), length of 52 cm (67th percentile), and head circumference of 34.5 cm (37th percentile). There were no abnormalities of the placenta noted. A submucous cleft palate and patent foramen ovale were identified after delivery. The infant had a 5-week admission to the hospital after delivery because of difficulty staying awake for feeds, reflux, and oxygen desaturation related to airway issues, including laryngomalacia.
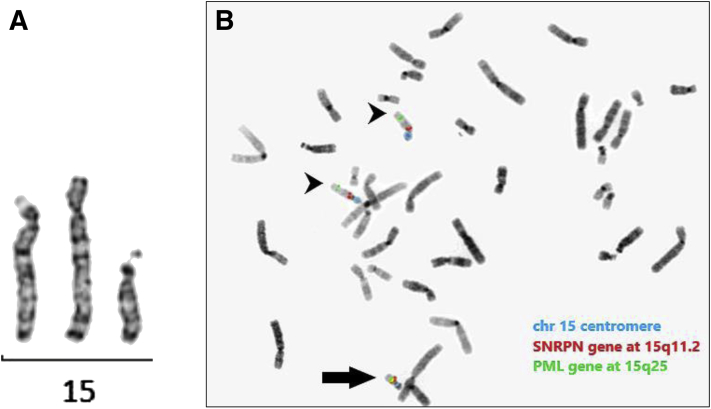
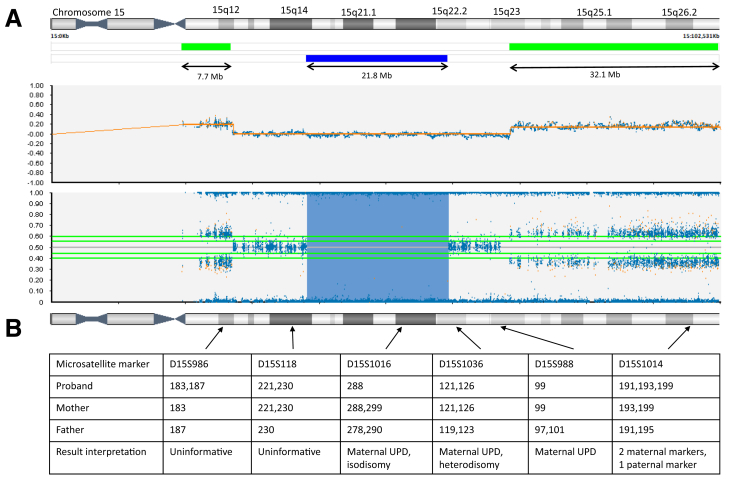
Karyotype analysis was performed in the newborn period and showed a supernumerary marker chromosome (SMC) derived from chromosome 15 in all 7 analyzed cells (Fig. 1A). The proband’s results reflected a male karyotype (XY), indicating that the conception was achieved from the high-level mosaic trisomy 15 embryo with a deletion on chromosome 20 and not the other mosaic embryo transferred, which was reported as female (XX) on PGT-A. Parental karyotypes showed normal results. Subsequent fluorescence in situ hybridization analysis with probes mapping to the SNRPN and PML genes (Vysis) at 15q11.2 and 15q24.1, respectively, showed the presence of 2 noncontiguous regions from chromosome 15 on the SMC, with a deletion of the interstitial region between the probes (Fig. 1B). Chromosomal microarray (CytoSNP-850K v1.2 BeadChip Illumina) further defined the regions forming the SMC as a 7.7 Mb centromeric region at 15q11.1q12 (hg19:20,071,673-27,741,737) and a 32.1 Mb telomeric region at 15q23q26.3 (hg19:70,334,218-102,461,162) (Fig. 2A). Chromosomal microarray showed no evidence of mosaicism. The final karyotype result was 47, XY,+del(15)(q12q23)dn. Chromosomal microarray also detected a 21.8 Mb region of homozygosity at 15q14q22.2 (hg19:39,159,590-60,995-837), suggestive of UPD15 (Fig. 2A). Maternal UPD15 was molecularly diagnosed with microsatellite markers (Fig. 2B). Therefore, the 2 intact chromosomes 15 present in the karyotype were of maternal origin, and the SMC was formed from the paternal copy of chromosome 15. The presence of maternal heterodisomic regions on chromosome 15 (shown by the presence of 2 different maternal markers, D15S1036 and D15S1014) indicated that trisomy 15 detected in the embryo originated in maternal meiosis (Fig. 2B).
Figure 1.
Cytogenetic analysis. (A) G-banded chromosome 15 showing partial trisomy 15 because of the supernumerary marker chromosome. (B) Metaphase showing the supernumerary marker chromosome positive for chromosome 15 fluorescence in situ hybridization probes (black arrow), in addition to 2 normal chromosomes 15 (black arrowheads): 15 centromere probe (blue signals), 15q11.2 SNRPN probe (red signals), 15q25 PML probe (green signals).
Figure 2.
Molecular analysis. (A) CytoSNP-850K array showing the profile of chromosome 15, with 2 noncontiguous gains (green bars) and a region of homozygosity (blue bar). Raw data are represented by log R ratio (upper) and B-allele frequency (lower). (B) Microsatellite markers analysis of the family trio showing confirmation of maternal uniparental disomy. Arrows indicate the position of microsatellite markers mapped to chromosome 15. UPD = uniparental disomy.
Discussion
To our knowledge, this report is the first documented case of a MET resulting in an adverse outcome of a live born diagnosed with partial trisomy and UPD in a likely nonmosaic form. This case highlights the challenges of predicting the outcome of METs and the need for standardized guidelines to provide continuous and personalized care for these couples throughout their pregnancy journey.
During this pregnancy, a chromosomal abnormality was not suspected prenatally because the repeated ultrasound investigations showed no clinically significant anomalies. The male patient was born with growth parameters in the normal range, submucous cleft palate, and patent foramen ovale, Feeding difficulties outside of what would be anticipated for a submucous cleft palate led to prolonged hospital admission. Genetic testing performed postnatally detected partial trisomy 15 in the form of an SMC and maternal UPD15. Through a process of elimination, because the other mosaic embryo transferred was reported female (XX) on PGT-A, male proband most likely resulted from the embryo with high-level mosaic trisomy 15 and chromosome 20 deletion (del(20)(q11.23-qter) PGT-A result. Large duplications of chromosome 15 material detected in this patient are predicted to cause significant health consequences. The presence of 3 copies of the 15q11.2q12 region encompassing the imprinted SNRPN locus, when the additional copy is of maternal origin, is associated with intellectual disability, ataxia, seizures, and behavioral problems (11). Overlapping duplications of the distal 15q23q26.3 region have been reported with neurodevelopmental phenotypes and variable congenital abnormalities, including overgrowth, renal anomalies, and dysmorphic features (12, 13, 14, 15). In addition, the large isodisomic region at 15q14-q22 may potentially be clinically relevant. Loss of heterozygosity present in this region resulted in a reduction to homozygosity for a large number of genes and mutations therein, which could have uncovered a yet to be diagnosed recessive condition.
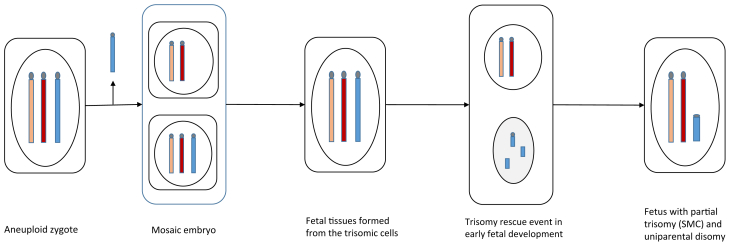
Karyotype analysis revealed a surprising finding of an SMC composed of 2 noncontiguous regions from chromosome 15. The formation of an SMC is a known consequence of an incomplete trisomy rescue. Trisomy rescue aims to eliminate the supernumerary chromosome from trisomic cells by separating and encapsulating the chromosome in a micronucleus, where it undergoes degradation (16). The degradation process within the micronucleus occurs through deoxyribonucleic acid double-strand breaks and chromosomal shattering, a process called chromothripsis. Generally, chromothripsis results in complete elimination of the encapsulated chromosome; however, when interrupted, it can result in partial losses and/or rearrangements of the excessive chromosome. This rearranged chromosome can then re-enter the nucleus in the form of an SMC (17). Additionally, when the trisomy rescue attempt involves the single chromosome of a given parental origin present in the cell, this event will also result in UPD of the same chromosome. It has been theorized that mosaic embryos are able to result in healthy euploid infants because of early rescue events, although, to our knowledge, this is the first case where we have evidence supporting this mechanism at work.
Molecular testing in our patient showed the meiotic origin of trisomy 15 in the embryo. This indicates that an initially trisomic zygote underwent an early mitotic rescue event, which resulted in a mosaic embryo detected by PGT-A. This is thought to be a rather rare mechanism of formation of mosaic embryos; most diploid-aneuploid mosaics arise because of a mitotic error in a cell descended from a diploid conception (18). In our patient, a subsequent incomplete rescue event must then have occurred in the remaining trisomy 15 cells, leading to the formation of an SMC with UPD in the fetus (Fig. 3). Alternatively, the meiotic origin of trisomy 15 points to the possibility that the embryo was uniformly aneuploid and that the PGT-A provided an inaccurate result. Current PGT-A platforms estimate mosaic copy number on the basis of the sample profile being outside the established thresholds for a normal copy number but not reaching the threshold for uniform monosomy or trisomy (1). A recent review of embryos classified as a mosaic by PGT-A demonstrated that almost 1 of 3 were actually uniformly aneuploid, as shown by the evaluation of repeated trophectoderm biopsies. This may be true especially when aneuploidy is proven to be meiotic in origin (19, 20). The transfer of embryos with false-negative uniform aneuploidy is expected to lead to lower implantation success rates because these embryos are thought to have no reproductive potential (21). The reference laboratory used in this case reported a PGT-A detection rate of approximately 97%–98% and was not able to provide specific figures for their false-negative risk but had quoted it to be low. The reference laboratory uses the PGTai platform for all PGT-A, which reportedly improves accuracy and decreases the no-call rate. This further complicates MET cases and highlights the importance of timely genetic counseling before MET to help guide patients’ decision making when presented with mosaic PGT-A results.
Figure 3.
The proposed mechanism of the formation of the supernumerary marker chromosome (SMC) and uniparental disomy (UPD) in the fetal development involves an initial trisomic conceptus, with 2 maternal chromosome 15 (orange and red chromosome) and 1 paternal chromosome 15 (blue chromosome), with the loss of the paternal chromosome 15 from a proportion of cells resulting in a mosaic embryo, as detected by preimplantation genetic testing for aneuploidy. The presence of an SMC and matUPD15 in the blood of the proband suggests another incomplete trisomy rescue event later in the development. See the Discussion for a possible alternative mechanism explaining the presence of SMC and matUPD15.
In conclusion, this case of an infant boy diagnosed with nonmosaic chromosomal abnormalities related to the mosaicism detected in the embryo is, to our knowledge, the first case of a MET that resulted in an adverse outcome of a live born. This case highlights 3 key areas in support of standardized practice guidelines to address the potential risks of transfer of embryos with known mosaic chromosome abnormalities:
-
1.
Preimplantation genetic testing for aneuploidy results should become part of the prenatal record for an ongoing pregnancy, and the referral should indicate that a mosaic embryo was transferred.
-
2.
Prenatal testing options should be offered by a genetic counselor or other health care provider who is knowledgeable of current MET outcome data before a MET, and follow-up should be provided after a pregnancy is achieved. Testing for mosaicism prenatally is best achieved through amniocentesis because chorionic villus sampling may be complicated by confined placental mosaicism and may be falsely reassuring or alarming. Limitations of prenatal screening options, such as non-invasive prenatal screening, should be discussed because these tests are not designed to detect mosaicism and usually only screen for specific aneuploidies.
-
3.
A standardized approach is needed for prenatal UPD testing of embryos transferred with mosaicism for imprinted chromosomes because most PGT-A platforms do not detect or report UPD.
In addition to these recommendations after MET, the importance of pretest counseling before PGT-A should not be overlooked. There is currently no consensus between clinics regarding the reporting of mosaic results and the cut-off for mosaicism if it is reported (22). Each clinic is responsible for choosing the PGT-A platform they use, how mosaic results are received, the cut-off for mosaicism, and decisions around METs. Therefore, it is important for clinics to provide adequate and clinic-specific pretest counseling for patients so that patients are aware of the type of results they will receive and whether they would be allowed to transfer their mosaic embryos. The standardization of PGT-A results and further data from nonselection studies from different laboratory platforms may help guide clinical decisions and pretest counseling in the future. Standardization may be especially helpful when patients move their care to a new IVF provider after creating PGT-A tested embryo(s).
This case raises concern that pregnancies achieved after the transfer of embryos with abnormal, in this case—mosaic, results obtained by PGT-A need additional prenatal testing options tailored to the type of mosaic aneuploidy identified. This case illustrates an example when UPD testing in pregnancy would have helped with management. Given that prenatal care providers might not be the best individuals to provide information on the management of pregnancies achieved from MET and that patients might not remember all of the information given at their pretransfer genetic counseling consult, referral to genetic counseling services is advisable after pregnancy is achieved. A MET consent form or handout might also be useful for patients to refer back to after they successfully achieve a pregnancy. Further reports of similar cases will help inform the prenatal testing algorithms, guidelines, and practices related to METs.
We hope that this case study does not discourage clinics from transferring mosaic embryos but instead highlights the importance of genetic counseling and prenatal follow-up after pregnancy is achieved using a mosaic embryo.
Acknowledgment
The authors thank Jill Mellon for proofreading the manuscript.
Footnotes
K.S.-B. has nothing to disclose. E.S. has nothing to disclose. O.Z. has nothing to disclose. J.M. has nothing to disclose. D.S. has nothing to disclose. M.V. has nothing to disclose. J.R. has nothing to disclose. M.S. has nothing to disclose.
Supported by the Pacific Centre for Reproductive Medicine for publication.
References
- 1.Ruttanajit T., Chanchamroen S., Cram D.S., Sawakwongpra K., Suksalak W., Leng X., et al. Detection and quantitation of chromosomal mosaicism in human blastocysts using copy number variation sequencing. Prenat Diagn. 2016;36:154–162. doi: 10.1002/pd.4759. [DOI] [PubMed] [Google Scholar]
- 2.Preimplantation Genetic Diagnosis International Society PGDIS position statement on the transfer of mosaic embryos 2021. https://pgdis.org/docs/PositionStatement.pdf Available at:
- 3.Grati F.R., Gallazzi G., Branca L., Maggi F., Simoni G., Yaron Y. An evidence-based scoring system for prioritizing mosaic aneuploid embryos following preimplantation genetic screening. Reprod Biomed Online. 2018;36:442–449. doi: 10.1016/j.rbmo.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Virtual Academy of Genetics COGEN position statement on chromosomal mosaicism detected in preimplantation blastocyst biopsies. https://ivf-worldwide.com/cogen/oep/publications/cogen-position-statement-on-chromosomal-mosaicism-detected-in-preimplantation-blastocyst-biopsies.html Available at.
- 5.Del Gaudio D., Shinawi M., Astbury C., Tayeh M.K., Deak K.L., Raca G., et al. Diagnostic testing for uniparental disomy: a points to consider statement from the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2020;22:1133–1141. doi: 10.1038/s41436-020-0782-9. [DOI] [PubMed] [Google Scholar]
- 6.Practice Committee and Genetic Counseling Professional Group (GCPG) of the American Society for Reproductive Medicine Clinical management of mosaic results from preimplantation genetic testing for aneuploidy (PGT-A) of blastocysts: a committee opinion. Fertil Steril. 2020;114:246–254. doi: 10.1016/j.fertnstert.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Viotti M., McCoy R.C., Griffin D.K., Spinella F., Greco E., Madjunkov M., et al. Let the data do the talking: the need to consider mosaicism during embryo selection. Fertil Steril. 2021;116:1212–1219. doi: 10.1016/j.fertnstert.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besser A.G., Mounts E.L., Grifo J.A. Evidence-based management of preimplantation chromosomal mosaicism: lessons from the clinic. Fertil Steril. 2021;116:1220–1224. doi: 10.1016/j.fertnstert.2021.07.1182. [DOI] [PubMed] [Google Scholar]
- 9.Kahraman S., Cetinkaya M., Yuksel B., Yesil M., Pirkevi Cetinkaya C. The birth of a baby with mosaicism resulting from a known mosaic embryo transfer: a case report. Hum Reprod. 2020;35:727–733. doi: 10.1093/humrep/dez309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mounts E.L., Zhu S.O., Sanderson R.K., Coates A., Hesla J.S. Mosaic embryo diagnosis correlated with abnormal 15q duplication syndrome in offspring. Fertil Steril. 2019;112:e241–e242. [Google Scholar]
- 11.Lusk L., Vogel-Farley V., DiStefano C., Jeste S. In: GeneReviews. Adam M.P., Ardinger H.H., Pagon R.A., et al., editors. Seattle (Washington): University of Washington; Seattle: 1993. Maternal 15q duplication syndrome. [PubMed] [Google Scholar]
- 12.O’Connor R., Al-Murrani A., Aftimos S., Asquith P., Mazzaschi R., Eyrolle-Guignot D., et al. Pure duplication of the distal long arm of chromosome 15 with Ebstein anomaly and clavicular anomaly. Case Rep Genet. 2011;2011:898706. doi: 10.1155/2011/898706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim E.Y., Kim Y.K., Kim M.K., Jung J.M., Jeon G.W., Kim H.R., et al. A case of de novo duplication of 15q24-q26.3. Korean J Pediatr. 2011;54:267–271. doi: 10.3345/kjp.2011.54.6.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatton-Brown K., Pilz D.T., Orstavik K.H., Patton M., Barber J.C.K., Collinson M.N., et al. 15q overgrowth syndrome: a newly recognized phenotype associated with overgrowth, learning difficulties, characteristic facial appearance, renal anomalies and increased dosage of distal chromosome 15q. Am J Med Genet A. 2009;149A:147–154. doi: 10.1002/ajmg.a.32534. [DOI] [PubMed] [Google Scholar]
- 15.Burada F., Streata I., Ungureanu A., Ruican D., Nagy R., Serban-Sosoi S., et al. Prenatal diagnosis of a pure 15q distal trisomy derived from a maternal pericentric inversion: a case report. Exp Ther Med. 2021;21:304. doi: 10.3892/etm.2021.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsubara K., Yanagida K., Nagai T., Kagami M., Fukami M. De novo small supernumerary marker chromosomes arising from partial trisomy rescue. Front Genet. 2020;11:132. doi: 10.3389/fgene.2020.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtas N.E., Xumerle L., Leonardelli L., Delledonne M., Brusco A., Chrzanowska K., et al. Small supernumerary marker chromosomes: a legacy of trisomy rescue? Hum Mutat. 2019;40:193–200. doi: 10.1002/humu.23683. [DOI] [PubMed] [Google Scholar]
- 18.McCoy R.C. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–463. doi: 10.1016/j.tig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin D., Xu J., Treff N.R. Preimplantation genetic testing for aneuploidy: a review of published blastocyst reanalysis concordance data. Prenat Diagn. 2021;41:545–553. doi: 10.1002/pd.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handyside A.H., McCollin A., Summers M.C., Ottolini C.S. Copy number analysis of meiotic and postzygotic mitotic aneuploidies in trophectoderm cells biopsied at the blastocyst stage and arrested embryos. Prenat Diagn. 2021;41:525–535. doi: 10.1002/pd.5816. [DOI] [PubMed] [Google Scholar]
- 21.Treff N.R., Marin D. The “mosaic” embryo: misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil Steril. 2021;116:1205–1211. doi: 10.1016/j.fertnstert.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Kim T.G., Neblett M.F., Shandley L.M., Omurtag K., Hipp H.S., Kawwass J.F. National mosaic embryo transfer practices: a survey. Am J Obstet Gynecol. 2018;219 doi: 10.1016/j.ajog.2018.09.030. 602.e1–7. [DOI] [PubMed] [Google Scholar]