Abstract
Objective
To determine the incidence and risk factors for intrauterine adhesions (IUAs) after minimally invasive and open myomectomy and hysteroscopic myomectomy (HM).
Design
Retrospective cohort study.
Setting
University-affiliated fertility center.
Patient(s)
Patients aged ≥18 years undergoing robotic-assisted or conventional laparoscopic minimally invasive myomectomy, abdominal myomectomy, or HM between January 2007 and January 2017. Only patients who underwent uterine cavity evaluation within 12 months of surgery via hysteroscopy or hysterosalpingography were included. Patients were excluded if they had a history of IUA before myomectomy.
Intervention(s)
Not applicable.
Main Outcome Measure(s)
The primary outcomes of this study were the presence and severity of IUA. The secondary outcomes were the identification of risk factors for IUA formation. The severity of IUAs was scored by 2 investigators using a previously published grading system by March et al.
Result(s)
Of 1,315 patients who underwent myomectomy, 173 (13.2%) met the inclusion criteria. Intrauterine adhesions were identified in 9.3% of all patients, 75.0% of which were classified as minimal. The incidence of IUA did not vary by modality: 8.6%, minimally invasive myomectomy; 7.8%, abdominal myomectomy; and 11.8%, HM. There were no differences in incidence of IUA by the number or size of fibroids removed. Of patients with IUA, 87.5% had submucosal fibroids resected compared with 58.6% without IUA.
Conclusion(s)
The incidence of postoperative IUA in women undergoing myomectomy of any modality is relatively low (9.3%) and does not vary by modality alone. Most IUAs are of minimal degree. The presence of submucosal fibroids is associated with an increased risk of IUA in all modalities.
Key Words: Intrauterine adhesions, myomectomy, robotic surgery, laparoscopy, hysteroscopy
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-22-00065
Uterine fibroids are the most common gynecologic tumor, with a prevalence of 70%–80% by the age of 50 years (1). In reproductive-age women, fibroids are estimated to be causal in 1%–2% of infertility diagnoses (2). Fibroids distorting the uterine cavity carry the highest risk of infertility and pregnancy loss (3). It has been postulated that this is due to aberrations in endometrial receptivity and the hormonal milieu of the endometrium (4). Although asymptomatic noncavity-distorting intramural fibroids do not seem to affect fertility, data suggest an adverse effect on the live birth rates in women with infertility undergoing in vitro fertilization (5).
Multiple treatment options exist for uterine fibroids, including medical management, radiofrequency ablation, uterine artery embolization, myomectomy, and hysterectomy. Myomectomy is the fertility-sparing intervention of choice in patients who have not completed childbearing. The modality of myomectomy is often dictated by the location, number, and size of fibroids as well as physician skill level with varying degrees of recovery time and complications rate reported (6, 7). Regardless of approach, myomectomy is not without reproductive risks.
Intrauterine adhesions (IUAs) may develop because of endometrial injury during myomectomy and negatively impact reproductive function. However, the incidence and severity of IUA after myomectomy are poorly understood. The mechanism of IUA formation is still largely unknown and likely multifactorial. Trauma to the basalis layer of the endometrium is commonly considered the inciting event in the formation of IUA (8, 9). Hysteroscopic myomectomy (HM) is clearly associated with the formation of IUA, with monopolar energy and multiple opposing fibroids associated with a higher risk of IUA formation (10, 11, 12, 13). However, few studies have reported on how laparoscopic or abdominal approaches affect the presence of postoperative IUA (10, 11, 12). Moreover, no studies to date have directly compared the incidence of IUA after all different myomectomy techniques. This type of information is useful in the setting of a patient-centered operative planning and counseling.
We reviewed our 10-year experience in patients who have undergone myomectomy followed by hysteroscopy or hysterosalpingography (HSG) to evaluate for the presence of IUAs. Our goal was to characterize the incidence and severity of postoperative IUA formation for each surgical modality. Furthermore, we explored patient and surgical level variables associated with IUA formation.
Materials and methods
Study Population and Design
All patients (n = 1,315) aged 18–50 years old undergoing robotic-assisted laparoscopic myomectomy (RM), traditional laparoscopic myomectomy (LM), abdominal myomectomy (AM), or HM between January 1, 2007, and January 1, 2017 at the Center for Infertility and Reproductive Surgery at Brigham and Women’s Hospital were reviewed for this retrospective cohort study. Patients were excluded if they did not undergo postoperative hysteroscopy or HSG within 12 months of their first myomectomy (n = 1,134). Women were also excluded if they underwent a combined myomectomy procedure (i.e., LM and HM) (n = 8) to be able to isolate the mode of myomectomy to subsequent IUA formation. None of the patients were known to have prior IUAs or congenital uterine anomalies. A total of 173 patients met these criteria and were included for analysis.
Outcomes and Definition of Study Groups
The primary outcomes were the presence and severity of IUAs on follow-up hysteroscopy or HSG. The decision for hysteroscopy vs. HSG was made at the discretion of the treating physician taking into account insurance, patient preference, and clinical scenario. Intrauterine adhesion severity was scored by 2 investigators (P.B., K.K.) as “minimal,” “moderate,” or “severe” using a method previously described by March et al. (14). A third investigator (A.G.) was designated a priori as a tie-breaker for discrepancies in scoring; however, no scoring discrepancies were encountered. Owing to the similarity in the surgical approach, RM and LM were combined into a minimally invasive surgery category (MIS) for analysis. Patients undergoing AM and HM were considered individually.
Surgical Techniques
In our practice, operative hysteroscopy is performed in an operating room under conscious sedation using a 22- or 26-Fr rigid hysteroscope paired with a telescope featuring a 12° direction of view. A fluid management system to maintain constant distention pressure between 80 and 100 mm Hg (Hysteromat II, Karl Storz) is used. When using monopolar electrosurgery, distention was provided with 1.5% glycine. Normal saline is used as distention media for bipolar electrosurgery. Laparoscopic myomectomies and RMs are performed in a technique previously described in a publication by our group (6). Abdominal myomectomy is performed in the dorsal lithotomy position via a Pfannenstiel skin incision. Diluted vasopressin (20 units in 60 mL of injectable saline) and/or a Penrose drain placed around the uterine cervix as a tourniquet are routinely used for hemostasis at the surgeon’s discretion. The smallest number of serosal incisions necessary to enucleate the fibroids is used. If the cavity is entered, the poliglecaprone 25 suture is used to oversew the endometrial defect, taking care to avoid having suture within the endometrium. The remaining myometrium is routinely closed in multiple layers using polyglactin 910 suture with an imbricating serosal layer.
Postoperative evaluation of the uterine cavity after myomectomy is not currently considered standard of care. However, a second look is recommended by the clinicians of our center when concern for residual pathology exists or when an assisted reproductive procedure is planned in the short term.
Second-Look Diagnostic Hysteroscopy or HSG
Our office diagnostic hysteroscopy technique, performed between cycle days 5 and 12, a minimum of 3 months after myomectomy, employs a 3.1-mm flexible video hysteroscope (Olympus HYF-XP, Olympus Surgical Technologies America), with normal saline solution as the distending medium. Hysterosalpingography is performed under fluoroscopy in a radiology suite using a large Cohen Acorn intrauterine cannula (Jarit) or an intrauterine balloon catheter, with water-based contrast dye.
Statistical Analysis
Parametric and nonparametric statistics were used to examine differences between groups. Given the small number of events encountered, Fischer’s exact test was used to compare differences between groups. Statistical significance was denoted by a P value of <.05. Given the small sample size and hypothesis-generating nature of the study, no adjustment for multiplicity of outcomes was performed. Data analysis was performed using STATA SE version 16 (StataCorp LP).
Ethical Approval
This study was approved by the Brigham and Women’s Institutional Review Board (study protocol number 2017P000047).
Results
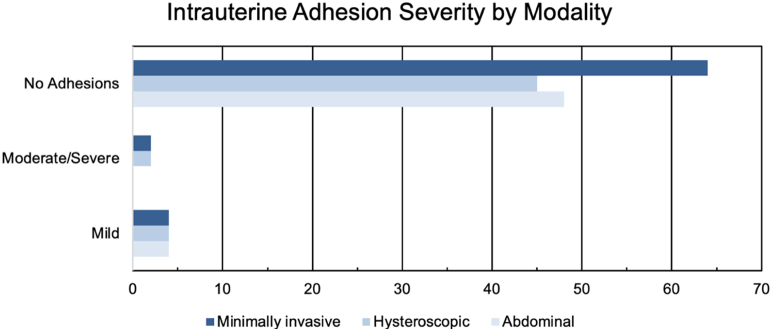
Between January 1, 2007, and January 1, 2017, a total of 173 women underwent either MIS (n = 70; RM, 63, and LM, 7), HM (n = 51), or AM (n = 52) followed by diagnostic hysteroscopy (n = 151) or HSG (n = 22) within 12 months of index myomectomy. The demographic characteristics of the study cohort are displayed in Table 1. In total, 16 patients (9.3%) were found to have postoperative IUAs: 8.6% in the MIS group, 11.8% in the HM group, and 7.8% in the AM group (P=.800) (Fig. 1). Patients with IUA tended to be younger (mean, 34.8 vs. 37.3 years; P=.056); however, there were no differences in gravidity, parity, indication for myomectomy, or history of prior uterine surgery between those with and without IUA (P>.05).
Table 1.
Patient characteristics.
| Variables | Intrauterine adhesions |
No intrauterine adhesions |
P value |
|---|---|---|---|
| N = 16 (9.3%) | N = 157 (90.7%) | ||
| Age, mean (SD) | 34.8 (5.5) | 37.3 (4.8) | .046 |
| Gravidity, median (IQR) | 0 (0-1.5) | 1 (0-1) | .240 |
| Parity, median (IQR) | 0 (0-0) | 0 (0-0) | .923 |
| Race, n (%) | .852 | ||
| White | 6 (37.5) | 65 (41.4) | |
| African American | 6 (37.5) | 43 (27.4) | |
| Asian | 2 (12.5) | 19 (12.1) | |
| Other/unknown | 2 (12.5) | 30 (19.1) | |
| Hispanic, n (%) | 0 (0) | 9 (5.7) | 1.000 |
| Indication for myomectomy, n (%)a | |||
| Recurrent pregnancy loss | 1 (6.3) | 9 (5.7) | 1.000 |
| Abnormal uterine bleeding | 8 (50.0) | 58 (36.9) | .418 |
| Bulk symptoms | 2 (12.5) | 20 (12.7) | 1.000 |
| Pelvic pain | 4 (25.0) | 20 (12.7) | .244 |
| Infertility | 8 (50.0) | 90 (57.3) | .605 |
| Other | 1 (6.3) | 7 (4.5) | .548 |
| Prior uterine surgery, n (%) | 7 (43.8) | 53 (34.9) | .585 |
Note: IQR = interquartile range; SD = standard deviation.
Patients may have more than 1 indication.
Figure 1.
Incidence of intrauterine adhesions by modality.
Surgical variables are displayed in Table 2. There were no differences in the presence of IUA by the fibroid number, diameter, or total specimen gross specimen weight (P>.05). Submucosal fibroids were more common in those with IUA than in those without (87.5 vs. 58.6%, P=.029). There were no differences in IUA with the addition of other intrauterine procedures (i.e., polypectomy, curettage, intrauterine device removal, or chromopertubation) (P=1.000). For patients undergoing MIS or AM, there were no differences in IUA by the number of layers used to close largest fibroid defect, use of barbed suture for closure of deepest layer, or incidental breeching of the endometrial cavity (P>.05). For all modalities, IUA did not differ by the use of postoperative IUA prevention strategies, such as intrauterine balloon and/or exogenous hormones (P>.05).
Table 2.
Surgical characteristics.
| Variables | Intrauterine adhesions |
No intrauterine adhesions |
P value |
|---|---|---|---|
| N = 16 (9.3%) | N = 157 (90.7%) | ||
| Procedure type, n/N (%) | .800 | ||
| Abdominal | 4/52 (7.8) | 48/52 (92.3) | |
| Hysteroscopic | 6/51 (11.8) | 45/51 (88.2) | |
| Minimally invasive | 6/70 (8.6) | 64/70 (91.4) | |
| Other intrauterine procedures, n/N (%) | |||
| Polypectomy | 0/6 (0.0) | 4/45 (8.9) | 1.000 |
| D&C | 1/16 (6.3) | 18/157 (11.5) | 1.000 |
| IUD removal | 0/16 (0.0) | 1/157 (0.6) | 1.000 |
| Chromopertubation | 2/10 (20.0) | 22/112 (19.6) | 1.000 |
| Total fibroids removed, median (IQR) | 3.5 (1-14) | 3 (1-8) | .704 |
| Presence of submucosal fibroids, n (%) | 14 (87.5) | 89 (58.6) | .029 |
| Diameter of largest fibroid, median (IQR) | 5.0 (2.9-6.0) | 6 (4-9) | .096 |
| Postoperative adhesion preventiona, n (%) | 1 (6.3) | 4 (2.6) | .388 |
| Incidental adenomyosis, n (%) | 1 (6.3) | 2 (1.3) | .254 |
| Specimen weight (g), median (IQR) | 69.8 (11-250) | 165 (53.5-376.5) | .141 |
| For minimally invasive procedures and abdominal procedures | |||
| Preoperative GnRH agonist, n/N (%) | 0/10 (0.0) | 12/112 (7.6) | .596 |
| No. of layers of closure, median (IQR) | 4 (3-4) | 3 (3-4) | .070 |
| Barbed suture for deepest layer, n/N (%) | 2/10 (20.0) | 29/104 (27.9) | .726 |
| Cavity entry, n/N (%) | 7/10 (70.0) | 46/112 (41.1) | .100 |
| Intra-operative vasopressin, n/N (%) | 9/10 (90.0) | 98112 (87.5) | 1.000 |
Note: D&C = dilation and curettage; GnRH = gonadotropin-releasing hormone; IQR = interquartile range; IUD = intrauterine device; SD = standard deviation.
Hormone and/or intrauterine balloon.
Finally, there were no differences in the severity of IUA by the procedure type, total fibroids removed, presence of submucosal fibroids, combination of anterior and posterior fibroids, and total specimen weight (P>1.000) (Table 3).
Table 3.
Intrauterine adhesion severity.
| Variables | Intrauterine adhesion severity |
||
|---|---|---|---|
| Minimal |
Moderate to severe |
P value |
|
| N = 12 (75.0%) | N = 4 (25.0%) | ||
| Procedure type, n/N (%) | .604 | ||
| Abdominal myomectomy | 4/4 (100.0%) | 0 (0.0) | |
| Hysteroscopic myomectomy | 4/6 (66.7%) | 2/6 (33.3) | |
| Minimally invasive myomectomy | 4/6 (66.7%) | 2/6 (33.3) | |
| Total fibroids removed, median (IQR) | 6 (2-14) | 1.5 (1-2.5) | .073 |
| Presence of submucosal fibroids, n (%) | 11 (91.7) | 3 (75.0) | .450 |
| Combination of anterior and posterior fibroids, n (%) | 5 (41.7) | 1 (25.0) | 1.000 |
| Total specimen weight (g), median (IQR) | 93 (30-320) | 34.5 (9.4-109) | .322 |
Note: IQR = interquartile range.
Discussion
In a 10-year retrospective cohort of 1,315 patients undergoing either RM, LM, HM, or AM, the global incidence of postoperative IUAs diagnosed by postoperative hysteroscopy or HSG was 9.3%. There was no difference in the incidence of IUA by modality (8.6% in the MIS group, 11.8% in the HM group, and 7.8% in the AM group [P=.800]).
Uterine fibroids are a common feature of the human uterus, with a lifetime prevalence of up to 70% in white women and up to 80% in black women (15). Approximately 30% of women may experience abnormal uterine bleeding, pelvic pain, and infertility depending on fibroid location, size, and number (16, 17). The impact of fibroids on fertility remains controversial. These tumors are present in up to 27% of patients undergoing assisted reproduction and may be the only clearly identifiable cause of infertility in 1%–3% of infertile patients (18, 19, 20). As a result, patients are often counseled toward myomectomy to treat symptoms while maintaining reproductive ability or in the hopes of improving reproductive potential with assisted reproductive technologies, either in the setting of a difficult embryo transfer or with repeated in vitro fertilization failures without another identifiable cause.
The cornerstone of patient-centered surgery is an informed patient choice on the basis of the knowledge of risks and benefits of all treatment options. Intrauterine adhesions are a well-known complication of myomectomy, with potentially devastating effects on future reproductive potential. Regrettably, a surgeon’s ability to counsel patients on the risk of IUA after different myomectomy modalities is limited by the absence of comparative studies. A randomized trial to assess the risk of IUA after different myomectomy modalities makes little clinical sense currently because of the wealth of data supporting the utilization of the least invasive modality that is surgically feasible (personalized care). In the absence of a solid ethical basis to design a prospective study, our group performed a large, single-center retrospective study with stringent inclusion criteria and reliable, short-term, second-look evaluations of uterine cavities.
The Center for Infertility and Reproductive Surgery of Brigham and Women’s Hospital, where all surgeries in this study were performed, is staffed by reproductive endocrinologists and infertility subspecialists trained as advanced reproductive surgeons. A surprising finding of our study was the high percentage of patients who did not undergo a second-look intrauterine evaluation at our center within a year of the myomectomy. Indeed, of 1,315 patients, only 173 (13.2%) were eligible for inclusion. The fact that almost 87% of our postmyomectomy patients did not meet our stringent follow-up inclusion criteria may be due in part to the fact that we are a national referral center. After surgery, many of our patients return to their referring gynecologists and infertility specialists for fertility care. As explained earlier, we decided to only include our own second-look evaluations in this analysis, because of the standardized adhesion scoring criteria used. As a result, all patients who were referred to us for surgery only and had second-look endometrial cavity evaluation with an outside provider were not captured by this study.
Some of our patients and clinicians prefer an ultrasound-based evaluation of the endometrial cavity and tubal patency with sonohysterogram postoperatively. This ultrasound-based imaging is operator dependent and performed by an outside group at our institution. Thus, sonohysterograms did not allow for precise and independent objective scoring of IUA in this study, and these patients were excluded. Another possible reason for the low study inclusion rate is that many of our postmyomectomy patients were able to conceive either spontaneously or with first-line fertility treatments, such as intrauterine insemination. Without the need for assisted reproductive technologies, patients do not need a postmyomectomy uterine evaluation (unless the surgeon has a specific surgical concern). The possibility that our patients failed to follow-up for fertility treatment due to lack of access to care is less realistic, given the Massachusetts state mandate that commercial insurances cover the cost of assisted reproduction for infertile couples.
The observed high rate of attrition from myomectomy to a documented second-look evaluation highlights the objective challenges of such a retrospective study and possibly explains why we still lack good comparative studies on the incidence of IUA after different types of myomectomies. A multicenter study could provide a larger data set but would likely result in greater surgical technique variability. Because minimally invasive myomectomy techniques are considered advanced surgical techniques, limiting the data set to a high-volume, single center with a stable group of operators offers some degree of standardization in quality and technique. We believe that this is a strength of this study because it is increasingly rare to find reproductive medicine programs performing RM, LM, AM, and HM at high volume.
Our study also features some evident limitations. First, the unexpectedly low study size and, thus, low absolute numbers of those with IUA in all treatment groups made it statistically impossible to perform some of the comparisons we had originally planned. For example, we were not able to study the impact of the size, number, and depth of penetration of fibroids on the incidence of postoperative adhesions. Published adhesion rates after HM have been described in the range of 7.5%– 20% (10, 21); likewise, the rare studies investigating IUA after open myomectomy and minimally invasive myomectomy were 19%–25.1% and 21%, respectively (13, 22, 23). The lower rate of adhesions found in our surgical patient population may reflect technical excellence in a high-volume subspecialized reproductive surgery practice, or could have occurred by chance, owing to the low percentage of cases with second-look information. Finally, we were unable to use the International Federation of Gynaecology and Obstetrics fibroid staging system given the retrospective nature of this data. We believe the International Federation of Gynaecology and Obstetrics staging would have enhanced generalizability of our data for patient counseling.
In summary, on the basis of the 10-year experience of a single, large academic center’s with multimodality myomectomy for patients desiring future childbearing, we report an incidence of postoperative IUAs of 9.3%, which did not vary significantly by the surgical modality used. Furthermore, aside from the presence of submucosal fibroids, there were no readily identifiable risk factors associated with IUA formation.
Footnotes
A.R.G. is a consultant for Medicaroid, Inc., and Lumenis, Inc. P.B. has nothing to disclose. K.W.K. has nothing to disclose. E.U. has nothing to disclose. E.H. has nothing to disclose.
P.B. and K.W.K. should be considered similar in author order.
References
- 1.Baird D.D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Casini M.L., Rossi F., Agostini R., Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22:106–109. doi: 10.1080/09513590600604673. [DOI] [PubMed] [Google Scholar]
- 3.Buttram V.C., Jr., Reiter R.C. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 4.Rackow B.W., Taylor H.S. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rikhraj K., Tan J., Taskin O., Albert A.Y., Yong P., Bedaiwy M.A. The impact of noncavity-distorting intramural fibroids on live birth rate in in vitro fertilization cycles: a systematic review and meta-analysis. J Womens Health (Larchmt) 2020;29:210–219. doi: 10.1089/jwh.2019.7813. [DOI] [PubMed] [Google Scholar]
- 6.Jin C., Hu Y., Chen X.C., Zheng F.Y., Lin F., Zhou K., et al. Laparoscopic versus open myomectomy--a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14–21. doi: 10.1016/j.ejogrb.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Gargiulo A.R., Srouji S.S., Missmer S.A., Correia K.F., Vellinga T.T., Einarsson J.I. Robot-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy. Obstet Gynecol. 2012;120:284–291. doi: 10.1097/AOG.0b013e3182602c7d. [DOI] [PubMed] [Google Scholar]
- 8.Valle R.F., Sciarra J.J. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459–1470. doi: 10.1016/0002-9378(88)90382-1. [DOI] [PubMed] [Google Scholar]
- 9.Yang J.H., Chen M.J., Wu M.Y., Chao K.H., Ho H.N., Yang Y.S. Office hysteroscopic early lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil Steril. 2008;89:1254–1259. doi: 10.1016/j.fertnstert.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Touboul C., Fernandez H., Deffieux X., Berry R., Frydman R., Gervaise A. Uterine synechiae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertil Steril. 2009;92:1690–1693. doi: 10.1016/j.fertnstert.2008.08.108. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari S., Ganguly I., Agarwal P., Singh A., Gupta N. Effect of myomectomy on endometrial cavity: a prospective study of 51 cases. J Hum Reprod Sci. 2016;9:107–111. doi: 10.4103/0974-1208.183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lev-Toaff A.S., Karasick S., Toaff M.E. Hysterosalpingography before and after myomectomy: clinical value and imaging findings. AJR Am J Roentgenol. 1993;160:803–807. doi: 10.2214/ajr.160.4.8456668. [DOI] [PubMed] [Google Scholar]
- 13.Asgari Z., Hafizi L., Hosseini R., Javaheri A., Rastad H. Intrauterine synechiae after myomectomy; laparotomy versus laparoscopy: non-randomized interventional trial. Iran J Reprod Med. 2015;13:161–168. [PMC free article] [PubMed] [Google Scholar]
- 14.March C.M., Israel R., March A.D. Hysteroscopic management of intrauterine adhesions. Am J Obstet Gynecol. 1978;130:653–657. doi: 10.1016/0002-9378(78)90322-8. [DOI] [PubMed] [Google Scholar]
- 15.Giuliani E., As-Sanie S., Marsh E.E. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149:3–9. doi: 10.1002/ijgo.13102. [DOI] [PubMed] [Google Scholar]
- 16.Stewart E.A. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 17.Wallach E.E., Vlahos N.F. Uterine myomas: an overview of development, clinical features, and management. Obstet. Gynecol. 2004;104:393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 18.Ezzati M., Norian J.M., Segars J.H. Management of uterine fibroids in the patient pursuing assisted reproductive technologies. Womens Health (Lond) 2009;5:413–421. doi: 10.2217/whe.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook H., Ezzati M., Segars J.H., McCarthy K. The impact of uterine leiomyomas on reproductive outcomes. Miner Ginecol. 2010;62:225–236. [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X.C., Segars J.H. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012;39:521–533. doi: 10.1016/j.ogc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebbag L., Even M., Fay S., Naoura I., Revaux A., Carbonnel M., et al. Early second-look hysteroscopy: prevention and treatment of intrauterine post-surgical adhesions. Front Surg. 2019;6:50. doi: 10.3389/fsurg.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capmas P., Pourcelot A.G., Fernandez H. Are synechiae a complication of laparotomic myomectomy? Reprod Biomed Online. 2018;36:450–454. doi: 10.1016/j.rbmo.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Laganà A.S., Garzon S., Dababou S., Uccella S., Medvediev M., Pokrovenko D., et al. Prevalence of intrauterine adhesions after myomectomy: a prospective multicenter observational study. Gynecol Obstet Investig. 2022;87:62–69. doi: 10.1159/000522583. [DOI] [PubMed] [Google Scholar]