Abstract
Objective
To examine and further characterize the association between urinary levels of triclosan (TCS), a ubiquitous putative endocrine-disrupting chemical, and the risk of infertility.
Design
A retrospective cross-sectional study using the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey.
Setting
Not applicable.
Patient(s)
Female participants in the United States who completed the reproductive health questionnaire and provided urine samples for TCS level measurement from 2013 to 2016.
Intervention(s)
None.
Main Outcome Measure(s)
Rates of presumed infertility based on participants’ affirmative response to survey question RHQ074 (“Have you ever attempted to become pregnant over a period of at least a year without becoming pregnant?”).
Result(s)
A total of 11.7% of the overall female and 12.5% of the eligible study population met the criterion for presumed infertility. Creatinine-adjusted urinary TCS levels were significantly higher among those meeting the criterion for infertility compared with the levels among those who did not. On multivariable-adjusted analyses, individuals with undetectable levels of urinary TCS were 35% less likely to meet the specified infertility criterion compared with those with detectable TCS levels. The magnitude of association between TCS levels and infertility was strongest when comparing the lowest and highest quartiles. The directionality and magnitude of the relationship between TCS levels and infertility were maintained on age-restricted and weighted analyses; however, the associations did not retain statistical significance.
Conclusion(s)
In a nationally representative sample of women in the United States, an association between TCS exposure and inability to conceive over a period of 1 year is suggested by our analysis of the National Health and Nutrition Examination Survey data. The data infer a dose-response relationship.
Key Words: Triclosan, endocrine-disrupting chemical, infertility, environmental exposure
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-22-00063
Triclosan (TCS), 5-chloro-2-(2,4-dichlorophenoxy)phenol, is a synthetic, lipid-soluble compound that is commonly incorporated into a variety of personal care, household, veterinary, textile, pharmaceutical, and industrial products for its antimicrobial properties (1). Personal care products, including soaps, lotions, mouthwashes, shampoos, and toothpaste, are the most common sources of exposure. Triclosan concentrations in commonly used products are regulated at 0.1%–0.3% by the European Community Cosmetic Directive and the United States Food and Drug Agency in Europe and the United States, respectively (1, 2, 3, 4, 5). Significant amounts of TCS have been detected in wastewater treatment facilities, where the compound is incompletely filtered, as well as in bottled and tap water at concentrations up to 0.1 and 0.14 μg/L, respectively (1, 6, 7).
Because of its widespread use, particularly in industrialized countries, populations are continuously exposed to TCS through dermal mucosal absorption and ingestion (8, 9). Triclosan is retained in the body with a half-life of 21 hours (9). In a representative sample of participants aged ≥6 years, TCS was detected in the urine of nearly 75% of participants in the 2003–2004 National Health and Nutrition Examination Survey (NHANES) (10). Triclosan has also been detected in human breast milk (11, 12), plasma (13, 14), urine (10), brain (15), liver (15), and adipose tissue (15), correlating with consumer use patterns of the antimicrobial (1, 16). In several investigations, females tended to exhibit higher TCS concentrations than males, and the age group with the highest TCS levels tended to be in the 20s (7).
Biomonitoring of urinary environmental phenols is used to determine their prevalence in humans and the relevance of human exposure in public health. Triclosan is a polychlorinated phenoxy phenol that is similar to the structures of molecules known to affect the endocrine system, such as polychlorinated biphenyls and polybrominated diphenyl ethers (17, 18). Triclosan has been implicated as an endocrine disruptor via disruption of thyroid hormone homeostasis and via estrogen-mediated pathways (1, 19). For example, in studies exposing rats to various levels of oral TCS, TCS significantly decreased the level of total serum thyroxine (T4) in a dose-dependent manner (18, 19, 20, 21, 22). In an in vitro study of BG-1 ovarian cancer cells, TCS stimulated cancer cell growth by regulating cell cycle and apoptosis-related genes via an estrogen receptor–dependent pathway (23). In both in vitro and mouse breast cancer models, TCS exerted estrogenic effects via an estrogen reporter gene assay, altering the expression of ribonucleic acids and proteins in breast cancer cells (24). In rodent models, reproductive development and function are adversely affected by TCS via estrogen-mediated pathways, particularly affecting estrogen-induced age of onset of the vaginal opening and uterine weight and histology (19, 25, 26, 27).
Despite a plethora of experimental data relating to TCS as a potent endocrine signal, evidence in humans is sparse and even conflicting. Similar to much of the early evidence on the toxicity of endocrine-disrupting chemicals, epidemiological studies on the endocrine-disrupting effects of TCS on human reproductive potential are inconsistent. Among 1,699 Canadian women in the Maternal-Infant Research on Environmental Chemicals study, TCS was associated with a longer time to pregnancy and reduced fecundity (fecundability odds ratio [OR], 0.84; 95% confidence interval [CI], 0.72–0.97) when comparing women in the highest quartile of urinary TCS concentrations (>72 μg/L) with those in lesser TCS quartiles (28). In a prospective cohort study of 648 women presenting to preconception care clinics, high TCS levels were associated with a 23% reduction in fecundability when compared with the lowest quartile, and there tended to be a dose-response pattern to this association (29). However, among 501 American couples in the Longitudinal Investigation of Fertility and the Environment Study, TCS was not associated with fecundity (fecundability OR, 1.01; 95% CI, 0.95–1.06) (30).
Recognition of the role of common environmental contaminants in the risk of reproductive compromise is essential for developing and implementing successful and efficient strategies for prevention. In this study, we aimed to further investigate the relationship between urinary TCS levels and female infertility in a representative national population sample of women of reproductive age in the United States.
Materials and methods
Study Population
The NHANES is a cross-sectional survey including health and nutritional data as well as detailed laboratory analyses administered by the National Center for Health Statistics of the Centers for Disease Control and Prevention (31). The NHANES uses a complex, multistage, probability sampling design to select participants that are representative of the civilian, noninstitutionalized United States population. The sampling design and survey methods have been described previously (31). Oversampling of certain population subgroups (persons aged ≥60 years as well as Hispanics and Blacks) is done to increase the reliability and precision of health status indicator estimates for these groups (32). The weighing of the NHANES analyses is used to account for the complex survey design (including oversampling), survey nonresponse, and poststratification adjustment to match total population counts from the Census Bureau (31). Neither institutional approvals nor funding were required because NHANES data are publicly accessible.
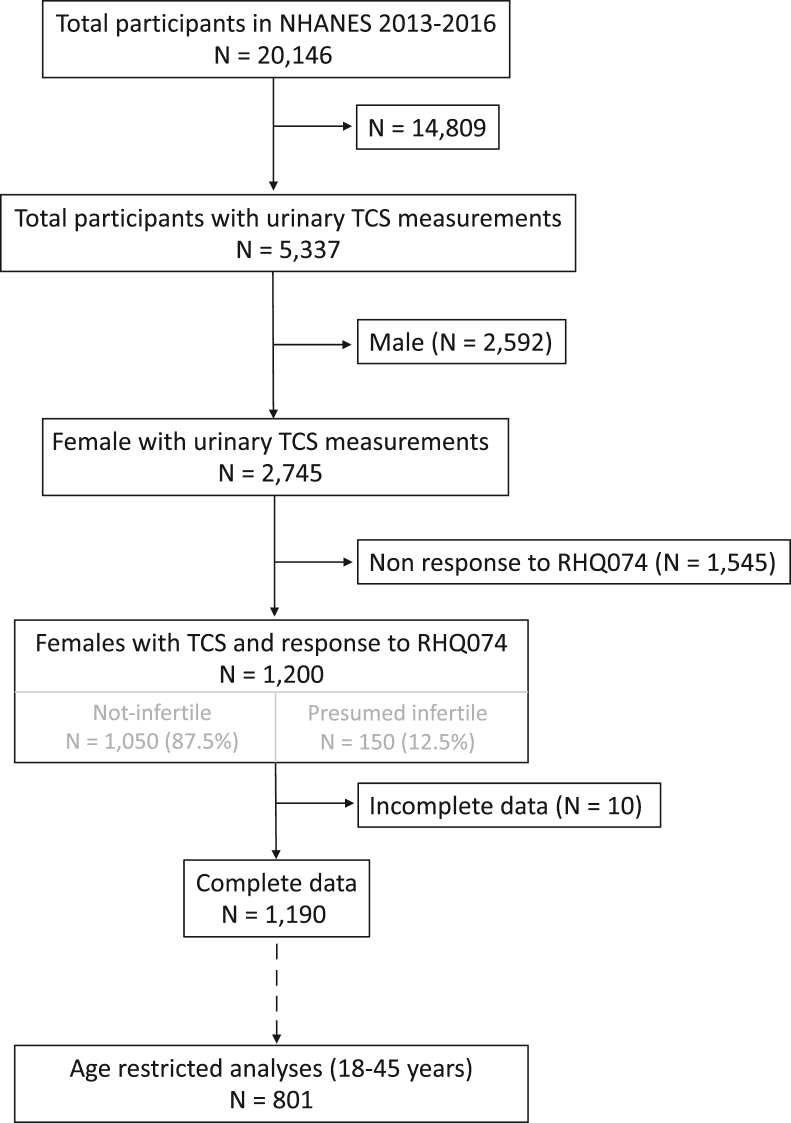
Figure 1 outlines the population sample selection for this study. The data for this study were extracted from 2 continuous NHANES cycles for 2013–2014 and 2015–2016, in which TCS levels were examined in a subset of enrollees. In the NHANES, urinary TCS levels were measured using solid-phase extraction coupled with high-phase liquid chromatography and tandem mass spectrometry, with the lower limit of assay detection being 1.7 μg/L. The TCS measurements were adjusted for urinary creatinine using the ratio of TCS to urinary creatinine (33). For this study, urinary TCS measures will refer to creatinine-adjusted values (expressed in ng/mg creatinine), as previously reported (33).
Figure 1.
Flowchart outlining population sample selection for the study (NHANES 2013–2016 data). NHANES = National Health and Nutrition Examination Survey; TCS = triclosan.
Statistical Methods
Participants’ affirmative response to the reproductive health survey question RHQ074—“Have you ever attempted to become pregnant over a period of at least a year without becoming pregnant?”—was deemed as presumed infertile. The response to question RHQ076“—Have you ever been to a doctor or other medical provider because you been unable to become pregnant?”—was examined for those presumed infertile. The relationship between urinary TCS levels and presumed infertility was examined with TCS data taken as a continuous variable as well as in quartiles. Participants with TCS levels lower than the assay limit of detection were grouped into the 1st quartile (Q1). Ranges of subsequent quartiles were as follows: 2nd quartile (Q2), 0.0064516–0.0443966; 3rd quartile, 0.0444444–0.2450000; and 4th quartile (Q4) 0.2466258–23.93235 ng/mg creatinine. Data distributions were analyzed for selection of appropriate statistical tests; parametric tests, such as Student’s t-test or ANOVA, and nonparametric tests, such as Mann U Whitney or Kruskal Wallis Rank Sum test, were used as applicable for comparing continuous data between groups by presumed infertility vs. not. Multivariable logistic regression analyses examined the association between urinary TCS and presumed infertility after adjusting for potential confounders using a stepwise forward model building approach. Urinary TCS levels in relation to various demographic factors (age at the time of survey completion, body mass index [BMI], and race and ethnicity [categorized as non-Hispanic Black, non-Hispanic White, Hispanic, and Other race]) were examined using spearman correlation and chi-square analyses as appropriate. Exposure to smoking was assessed on the basis of the participants’ affirmative response to question SMQ020—“Have you smoked at least 100 cigarettes in your entire life”, as well as a serum cotinine level of >3 ng/mL; relationships between smoking exposure with urinary TCS level and with infertility were examined. Covariates retained in the final model included age, BMI, race and ethnicity, and smoking exposure reflected in a cotinine level of >3 ng/mL (34, 35). Analyses comparing Q1 to Q2–Q4 were used to assess whether nondetectable levels of TCS were associated with lower odds of presumed infertility compared with any detectable level of TCS. Analyses comparing individual quartiles of TCS (Q2, 3rd quartile, and Q4) to Q1 were used to evaluate for a dose-response relationship.
Sensitivity analyses were conducted by restricting the population to participants who were deemed within the span of the reproductive age range (18–45 years) at the time of enrollment in NHANES and by weighted analyses using the NHANES sample to account for the oversampling of certain demographics (age and race/ethnicity) in the NHANES population, with an understanding that when variables employed in the calculation of sampling weights are also included in the statistical models (such as age, race, and ethnicity), weighted analyses can lessen the accuracy of effect estimates, as has been previously suggested (36, 37, 38). Cycle-specific subsample weights for participants with urinary phenol measurements (WTSA2YR) were divided by the number of cycles included in the analysis (2 cycles) to calculate multiyear sample weights.
We report the results of unweighted analyses in the body of this work (Table 2, Table 3 and Table 2, Table 3); weighted analyses (Supplementary Tables 1 and 3) and age-restricted analyses (Supplementary Tables 2–4) are presented as supplementary data. Continuous data of normal distribution are presented as mean ± SD, and skewed data are presented as median (interquartile range [IQR]), whereas categorical data are presented as a percentage. The magnitude of associations is presented as crude odds ratios and adjusted odds ratios (aORs) with a 95% CI. Stata V 16.1 (StataCorp LP, College Station, TX) was used for analyses, and a 2-tailed P value of <.05 was deemed statistically significant.
Table 2.
Predictors of presumed infertility in National Health and Nutrition Examination Survey 2013–2016—results of unweighted multivariable logistic regression analysis (sample size = 1,190).
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| TCS quartiles | ||
| Q2-4 (detectable) | 1 (referent) | 1 (referent) |
| Q1 (undetectable) | 0.692 (0.460–1.041) | 0.654 (0.429–0.999)a |
| Age at survey | 1.035 (1.020–1.050)a | 1.034 (1.019–1.050)a |
| BMI (kg/m2) | 1.018 (0.998–1.039) | 1.013 (0.991–1.035) |
| Smoking exposure | ||
| Cotinine >3 (vs. <3) | 0.753 (0.500–1.351) | 0.671 (0.438–1.027) |
| Race | ||
| Non-Hispanic White | 1 (referent) | 1 (referent) |
| Hispanic | 0.537 (0.344–0.836)a | 0.398 (0.243–0.653) a |
| Non-Hispanic Black | 0.979 (0.656–1.462) | 0.652 (0.414–1.025) |
| Other race | 0.896 (0.554–1.450) | 0.645 (0.378–1.010) |
Note: The magnitude of association is presented as crude (unadjusted) and adjusted OR and 95% 95% CI. BMI = body mass index, CI = confidence interval; OR = odds ratio; TCS = triclosan.
P<.05.
Table 3.
Associations between individual quartiles of TCS and presumed infertility—results of unweighted multivariable logistic regression analysis, adjusting for age at survey, body mass index, smoking exposure (as determined by serum cotinine level), and race/ethnicity (sample size = 1,190 [Q1 = 333, Q2 = 260, Q3 = 298, Q4 = 299]).
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| TCS quartiles | ||
| Q1 | 1 (referent) | 1 (referent) |
| Q2 | 1.326 (0.795–2.213) | 1.528 (0.899–2.595) |
| Q3 | 1.381 (0.844–2.259) | 1.466 (0.883–2.432) |
| Q4 | 1.637 (1.014–2.644)a | 1.610 (0.983–2.638) |
Note: The magnitude of association is presented as crude (unadjusted) and adjusted OR and 95% CI. CI = confidence interval; OR = odds ratio; TCS = triclosan.
P<.05.
Results
From 2013–2016, 70.5% of the female NHANES participants with TCS level measurements and 71.9% of the eligible study population had detectable levels of urine TCS; 11.7% of the female NHANES participants and 12.5% of the eligible study population met the criterion for presumed infertile. Figure 1 outlines the study sample for this study.
Baseline demographic data of the population are presented in Table 1. Participants who met the specified criterion for presumed infertility were significantly older (P<.001) and had a higher BMI (P<.001) compared with those who did not meet this criterion (Table 1). A racial and ethnic differential in the proportion of women meeting the criterion for presumed infertility was also apparent (Table 1).
Table 1.
The National Health and Nutrition Examination Survey 2013–2016 female participant characteristics by fertility status (response to question RHQ074 “Have you ever attempted to become pregnant over a period of at least a year without becoming pregnant?”).
| Characteristics | Presumed infertile N = 150 |
Not infertile N = 1,050 |
P value |
|---|---|---|---|
| Age (y), mean ± SD | 42.55 ± 10.1 | 37.50 ± 12.59 | <.001 |
| BMI (kg/m2), mean ± SD | 30.71 ± 8.78 | 29.43 ± 7.98 | .073 |
| Smoker (%) | 28.00 | 30.10 | .600 |
| Serum cotinine >3 ng/mL (%) | 22.00 | 27.24 | .174 |
| Race/ethnicity (%) | |||
| Hispanic | 17.33 | 28.10 | .005 |
| Non-Hispanic White | 44.00 | 31.43 | .002 |
| Non-Hispanic Black | 24.00 | 24.38 | .919 |
| Other race | 14.67 | 16.10 | .655 |
| Sought fertility care? (%) | 59.33 | 0.86 | <.001 |
| TCS (ng/mg creatinine), median (IQR) | 0.065 (0.015–0.434) | 0.042 (0.000–0.223) | .032 |
Note: Continuous data are presented as mean (SD) or median (IQR) and categorical data are presented as percentage. BMI = body mass index; IQR = interquartile range; TCS = triclosan.
Urinary TCS levels were significantly higher among the presumed infertile group compared with those not meeting this infertility criterion (median, 0.065 [IQR, 0.015–0.434] vs. 0.042 [IQR, 0.000–0.223] ng/mg creatinine; P = .032) (Table 1). Urinary TCS levels were unrelated to BMI (r = −0.006; P = .839) or age in years at the time of survey completion (r = 0.039; P = .118). Urinary TCS levels were significantly lower among those acknowledging smoking of >100 cigarettes in their lifetime (median, 0.039 [IQR, 0.000–0.169] vs. 0.046 [IQR, 0.000–0.315] ng/mg creatinine; P = .020) and in those with objective evidence of tobacco exposure reflected in cotinine levels of >3 ng/mL (median, 0.039 [IQR, 0–0.130] vs. 0.048 [IQR, 0–0.343] ng/mg creatinine; P<.001).
A racial differential in urinary TCS levels was observed; self-identified Black women had significantly lower levels of urinary TCS than non-Black women (median, 0.033 [IQR, 0–0.122] vs. 0.047 [IQR, 0–0.298] ng/mg creatinine; P = .017).
Results of unweighted multivariable logistic regression analyses examining a relationship between urinary TCS levels (in quartiles) with a likelihood for infertility are presented in Tables 2 and 3. Women with undetectable levels of TCS (Q1) had a 35% lesser likelihood of meeting specified criterion for infertility after adjusting for age, BMI, race, and ethnicity than women with detectable urinary TCS levels (Q2–Q4) (P = .049) (Table 2). The magnitude of association between TCS and presumed infertility was stronger with increasing TCS quartiles (Table 3). Women in the highest quartile of TCS (Q4) had a 64% greater likelihood of meeting specified criterion for infertility compared with women with undetectable urinary TCS levels (P = .044) (Table 3). The directionality and magnitude of the association persisted on multivariable analysis, albeit with mitigation of statistical significance (aOR = 1.610; 95% CI, 0.983–2.638; P = .059) (Table 3).
As stated in the methods, the NHANES sample weighted analyses aimed at accounting for the differential probabilities of participant selection and nonresponse rates given the oversampling of certain demographics on the basis of age and race/ethnicity in the NHANES population. The directionality and magnitude of association between undetectable TCS levels and reduced risk for infertility in weighted and age-restricted sensitivity analyses were consistent with the results of unweighted analyses, albeit with mitigation of statistical significance (Supplemental Tables 1, 2, and 3, available online). Results of age-restricted multivariable logistic regression analyses among individual quartiles suggested a higher magnitude of association between Q4 and presumed infertility (Supplementary Table 4).
Discussion
In a large representative population of the United States, we have identified a directionality of association between TCS exposure and the probability of infertility. Women with undetectable levels of TCS had significantly lower odds of meeting the specified criterion for infertility after adjusting for age at the time of the survey, BMI, smoking exposure, and race/ethnicity (aOR = 0.65; 95% CI, 0.429–0.999). These results may reflect an underestimation of the difference given that women who self-identified as not having presumed infertility may have undiagnosed infertility if they had not attempted to conceive. The directionality and magnitude of this association persisted in sensitivity analyses, albeit with a loss of statistical significance that most likely reflects attenuation in study power. Some have cautioned regarding the validity of weighted analyses in situations when the variables employed in the calculation of sampling weights (such as age, race, and ethnicity in the NHANES sample weights) are also included in statistical models (such as in our analyses); lowering in the accuracy of effect estimates can result from such overcorrection, as is evident in our weighted analyses (Supplementary Tables 1 and 3), and in such situations, the unweighted analytic approach is preferred (36, 37, 38).
The magnitude of association between individual TCS quartiles and presumed infertility increased in a dose-dependent pattern. The association between presumed infertility and TCS was most pronounced among participants in the highest quartile (Q4) of TCS (Table 3 and Supplementary Table 4), although underpowered for individual groups. Overall, our findings are aligned with those of prior studies (N = 1,699 and N = 648) that reported high urinary TCS levels to be associated with a longer time to pregnancy and a 16%–23% reduction in fecundability (28, 29). However, our observations stand in contrast with the findings reported by the investigators of the Longitudinal Investigation of Fertility and the Environment Study (N = 501), wherein fecundability was unrelated to TCS (OR, 1.01; 95% CI, 0.95–1.06) (30).
Postulated Mechanisms of Action
Triclosan is thought to alter endometrial physiology in animal models and humans, potentially compromising the reproductive potential (39). Human endometrial cells treated with TCS in the presence and absence of progesterone were found to arrest endometrial stromal cells at the G2/M phase of the cell cycle (39). Triclosan was also found to increase gene expression and protein levels of decidualization markers, such as the insulin growth factor binding protein 1 and prolactin, amplifying the effect of progesterone (39). In a uterotrophic assay in 18-day-old female Wistar rats treated with regimens of TCS for 3 days (postnatal day 18–20), TCS decreased the perimetrium thickness with an exposure of 8.0 mg/kg/d. Still, there was no difference in parameters, including uterine weight, endometrial stroma, myometrium, and luminal epithelium, between groups exposed to various TCS concentrations (40). In the concomitant 2-generation model, TCS also decreased female sexual receptivity in the F0 and F1 generations and decreased growing follicle number in the F1 generation with exposure to 2.4 mg/kg/d (40). However, there was no significant effect of TCS on sexual receptivity, follicle number, antral follicle count, mating, fertility, postimplantation loss, gestation index percentage, live birth, and viability (P>.05) (40).
Additional studies suggest that TCS is associated with abnormal menstrual patterns. For example, in a prospective cohort study of 698 women presenting to preconception care clinics in the People’s Republic of China, high TCS levels were associated with increased risks of prolonged menstrual cycles (aOR = 2.08; 95% CI, 1.00–2.31) and abnormal menstruation (aOR= 1.47; 95% CI, 1.05–2.06), where normal menstruation was defined as a normal cycle duration between 21 and 35 days, duration of menstrual bleeding between 3 and 7 days, and self-reported normal amount of menstrual bleeding (26).
Several mammalian studies suggest that TCS may be associated with disturbed implantation. In a study of inseminated mice, the administration of 18 and 27 mg/animal/d of TCS or subthreshold doses of TCS in combination with bisphenol A in the preimplantation period caused disruption of blastocyst implantation and a reduced number of implantation sites, mimicking the effects of 17β-estradiol (41). Additionally, combined subthreshold doses of TCS and bisphenol A, which were individually ineffective, reduced the number of implantation sites and increased gestational length (41). In a study among 450 women undergoing 674 in vitro fertilization cycles in Poland, urinary levels of TCS were associated with decreased implantation rates (P = .03), although not significantly associated with metaphase II stage oocytes, embryo quality, fertilization rate (42).
Disparities in TCS Exposure and Infertility Burden
Racial and ethnic differences in TCS exposure and infertility burden are notable in this study. Urinary TCS levels were significantly lower in the non-Hispanic Black population. This observation may reflect differentials in exposure to environmental contaminants; alternative hypotheses, such as differences in tissue storage and excretion, also merit consideration. Women of Hispanic ethnicity were significantly less likely to report infertility, and non-Hispanic Whites were more likely to report infertility. These findings may reflect sociodemographic differences in infertility burden, disparities in access to reproductive care, and/or underreporting of infertility among certain racial/ethnic groups because of a cultural discomfort with infertility labels (43, 44, 45).
There are several limitations to this study. The retrospective nature and cross-sectional design are not suitable for ascertaining cause and effect relationships. Because of the methodological features of the NHANES, the data reflect participants’ demographic characteristics and laboratory values at the time of the survey and data collection rather than at the time of experienced delay in successful conception. To minimize the impact of this limitation, we repeated analyses among women of reproductive age (≥18 and ≤45 years old) who would be more likely to have experienced a delay in conceiving closer to the time of data collection. In addition, TCS measurements in spot urine samples may not represent intraindividual variability and long-term exposure. The rate of detectable levels of urine TCS in our study is relatively unchanged from 2003 (approximately 72% vs. 75%, respectively) (10), suggesting that exposure on a population level has remained relatively stable. Furthermore, we assumed that TCS exposure and, thus, urinary TCS levels are relatively steady in an individual over time. Despite the reasonably short half-life of TCS, this assumption was made because TCS is a ubiquitous compound to which humans are constantly exposed. Our presumption of infertility was based on participants’ responses in a self-reported questionnaire and is, therefore, subject to recall and response bias. Lastly, this study does not account for partners’ exposures to TCS and male factor contribution to difficulties with conception. Our rationale for presenting unweighted analyses is to avoid overadjustment given the syngamy of variables germane to the calculation of sample weights and relevant as covariates, an analytic constraint that merits deeper examination and wider recognition.
Despite these limitations, this study has several strengths. The data were extracted from a large national population-based representative survey over 4 years. We also adjusted for potential confounders; examined cotinine levels as an objective measure of tobacco exposure, and conducted unweighted, weighted, and age-restricted sensitivity analyses.
Conclusion
Our large population-based study indicated TCS exposure as a plausible risk factor for compromised fertility in women of reproductive age. The observed racial differential in TCS exposure merits further investigation to examine the sources of that exposure and the potential for racial differentials in metabolic clearance and the roles of injustice, poverty, neighborhood quality, housing quality, and nutritional status as plausible underpinnings.
DIALOG: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-22-00063
Footnotes
G.B. has nothing to disclose. J.K. has nothing to disclose. H.S.T. reports grants from National Institutes of Health and AbbVie (both unrelated to the topic of the manuscript) and is an unpaid board member of Environment and Human Health. L.P. reports grants from the National Institute of Aging (1RF1 AG057547-01 and NHLBI ID# R01 HL135089, both unrelated to the topic of the manuscript); consulting fees from Flo-Health, and royalties from Springer and Wolter-Kluwer.
Preliminary results from this study were presented in the statement of the ASRM in Baltimore, Maryland (2021).
Supplementary data
References
- 1.Dann A.B., Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 2.Sabaliunas D., Webb S.F., Hauk A., Jacob M., Eckhoff W.S. Environmental fate of triclosan in the River Aire Basin, UK. Water Res. 2003;37:3145–3154. doi: 10.1016/S0043-1354(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.F., Tian Y. Reproductive endocrine-disrupting effects of triclosan: population exposure, present evidence and potential mechanisms. Environ Pollut. 2015;206:195–201. doi: 10.1016/j.envpol.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Daughton C.G., Ternes T.A. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 1999;107(Suppl 6):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodricks J.V., Swenberg J.A., Borzelleca J.F., Maronpot R.R., Shipp A.M. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- 6.Li X., Ying G.G., Su H.C., Yang X.B., Wang L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int. 2010;36:557–562. doi: 10.1016/j.envint.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Weatherly L.M., Gosse J.A. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev. 2017;20:447–469. doi: 10.1080/10937404.2017.1399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss T., Howes D., Williams F.M. Percutaneous penetration and dermal metabolism of triclosan (2,4, 4'-trichloro-2'-hydroxydiphenyl ether) Food Chem Toxicol. 2000;38:361–370. doi: 10.1016/s0278-6915(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 9.Sandborgh-Englund G., Adolfsson-Erici M., Odham G., Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 10.Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adolfsson-Erici M., Pettersson M., Parkkonen J., Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–1489. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 12.Dayan A.D. Risk assessment of triclosan [Irgasan] in human breast milk. Food Chem Toxicol. 2007;45:125–129. doi: 10.1016/j.fct.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Allmyr M., Adolfsson-Erici M., McLachlan M.S., Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Hovander L., Malmberg T., Athanasiadou M., Athanassiadis I., Rahm S., Bergman A., et al. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch Environ Contam Toxicol. 2002;42:105–117. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- 15.Geens T., Neels H., Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere. 2012;87:796–802. doi: 10.1016/j.chemosphere.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Ying G.G., Zhao J.L., Chen Z.F., Lai H.J., Su H.C. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ Int. 2013;52:81–86. doi: 10.1016/j.envint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen N., Skirrow R.C., Osachoff H., Wigmore H., Clapson D.J., Gunderson M.P., et al. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006;80:217–227. doi: 10.1016/j.aquatox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Crofton K.M., Paul K.B., Devito M.J., Hedge J.M. Short-term in vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ Toxicol Pharmacol. 2007;24:194–197. doi: 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Stoker T.E., Gibson E.K., Zorrilla L.M. Triclosan exposure modulates estrogen-dependent responses in the female Wistar rat. Toxicol Sci. 2010;117:45–53. doi: 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- 20.Zorrilla L.M., Gibson E.K., Jeffay S.C., Crofton K.M., Setzer W.R., Cooper R.L., et al. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- 21.Paul K.B., Hedge J.M., Bansal R., Zoeller R.T., Peter R., DeVito M.J., et al. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300:31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao X.Y., Hua X., Xiong J.W., Zhu W.T., Zhang J., Chen L. Impact of triclosan on female reproduction through reducing thyroid hormones to suppress hypothalamic kisspeptin neurons in mice. Front Mol Neurosci. 2018;11:6–17. doi: 10.3389/fnmol.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J.Y., Yi B.R., Go R.E., Hwang K.A., Nam K.H., Choi K.C. Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ Toxicol Pharmacol. 2014;37:1264–1274. doi: 10.1016/j.etap.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.R., Hwang K.A., Nam K.H., Kim H.C., Choi K.C. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol. 2014;27:834–842. doi: 10.1021/tx5000156. [DOI] [PubMed] [Google Scholar]
- 25.Ahn K.C., Zhao B., Chen J., Cherednichenko G., Sanmarti E., Denison M.S., et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. 2008;116:1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H., Du G., Zhang W., Hu J., Wu D., Song L., et al. The in vitro estrogenic activities of triclosan and triclocarban. J Appl Toxicol. 2014;34:1060–1067. doi: 10.1002/jat.3012. [DOI] [PubMed] [Google Scholar]
- 27.Jung E.M., An B.S., Choi K.C., Jeung E.B. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol Lett. 2012;208:142–148. doi: 10.1016/j.toxlet.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Vélez M.P., Arbuckle T.E., Fraser W.D. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril. 2015;103:1011–1020 e2. doi: 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W., Zhou W., Huo X., Zhao S., Gan Y., Wang B., et al. Triclosan and female reproductive health: a preconceptional cohort study. Epidemiology. 2019;30(Suppl 1):S24–S31. doi: 10.1097/EDE.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 30.Smarr M.M., Sundaram R., Honda M., Kannan K., Louis G.M. Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples’ fecundity. Environ Health Perspect. 2017;125:730–736. doi: 10.1289/EHP189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/nchs/nhanes.htm. Accessed January 1, 2022.
- 32.Liu X., Tsilimingras D., Paul T.K. Prevalence and changes of untreated isolated systolic hypertension among non-Hispanic Black adults in the United States. Hypertens Res. 2014;37:685–691. doi: 10.1038/hr.2014.58. [DOI] [PubMed] [Google Scholar]
- 33.Lankester J., Patel C., Cullen M.R., Ley C., Parsonnet J. Urinary triclosan is associated with elevated body mass index in NHANES. PLoS One. 2013;8:e80057–e80064. doi: 10.1371/journal.pone.0080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benowitz N.L., Bernert J.T., Caraballo R.S., Holiday D.B., Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 35.Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13:1236. doi: 10.3390/ijerph13121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korn E.L., Graubard B.I. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver M.K., Lozoff B., Meeker J.D. Blood cadmium is elevated in iron deficient U.S. children: a cross-sectional study. Environ Health. 2013;12:117–126. doi: 10.1186/1476-069X-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis R.C., Meeker J.D. Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the United States National Health and Nutrition Examination Survey 2011-2012. Fertil Steril. 2015;103:172–178. doi: 10.1016/j.fertnstert.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forte M., Mita L., Cobellis L., Merafina V., Specchio R., Rossi S., et al. Triclosan and bisphenol a affect decidualization of human endometrial stromal cells. Mol Cell Endocrinol. 2016;422:74–83. doi: 10.1016/j.mce.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Montagnini B.G., Pernoncine K.V., Borges L.I., Costa N.O., Moreira E.G., Anselmo-Franci J.A., et al. Investigation of the potential effects of triclosan as an endocrine disruptor in female rats: uterotrophic assay and two-generation study. Toxicology. 2018;410:152–165. doi: 10.1016/j.tox.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Crawford B.R., Decatanzaro D. Disruption of blastocyst implantation by triclosan in mice: impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol. 2012;34:607–613. doi: 10.1016/j.reprotox.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Radwan P., Wielgomas B., Radwan M., Krasiński R., Klimowska A., Zajdel R., et al. Triclosan exposure and in vitro fertilization treatment outcomes in women undergoing in vitro fertilization. Environ Sci Pollut Res Int. 2021;28:12993–12999. doi: 10.1007/s11356-020-11287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker G., Castrillo M., Jackson R., Nachtigall R.D. Infertility among low-income Latinos. Fertil Steril. 2006;85:882–887. doi: 10.1016/j.fertnstert.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 44.Nachtigall R.D., Castrillo M., Shah N., Turner D., Harrington J., Jackson R. The challenge of providing infertility services to a low-income immigrant Latino population. Fertil Steril. 2009;92:116–123. doi: 10.1016/j.fertnstert.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inhorn M.C., Fakih M.H. Arab Americans, African Americans, and infertility: barriers to reproduction and medical care. Fertil Steril. 2006;85:844–852. doi: 10.1016/j.fertnstert.2005.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.