Highlights
-
•
Light physical activity in the daytime was associated with better sleep outcomes.
-
•
Any physical activity at night was associated with a decrease in total sleep time and sleep time window.
-
•
For better sleep health, daytime physical activity is recommended.
Keywords: Accelerometer, Exercise, Physical activity, Population-based study, Sleep health
Abstract
Background
Regular physical activity (PA) is an important behavior in improving sleep health. However, the short-term effects of PA on sleep are still controversial. This study aimed to verify the effect of different intensities of PA practiced in different periods of the day on the subsequent sleep night in a population-based cohort of young adults.
Methods
: Prospective analyses were conducted for PA performed during the day and its effect on the following sleep night using data from the 22-year follow-up of the 1993 Pelotas Birth Cohort in Brazil (mean age of participants = 22.6 years). Wrist-worn accelerometry was used to measure both PA and sleep parameters. Regarding intensity, we analyzed the sleep effect of light PA (LPA), moderate PA, and vigorous PA, stratified by sex. Sleep variables were sleep time window (STW; the difference between sleep onset and sleep end), total sleep time (TST; the sum of minutes classified as sleep in STW), and sleep percent (SP; SP = (TST/STW); expressed in percentage). We performed generalized estimating equations using Stata software.
Results
: The means of STW, TST, and SP were 443.6 min/day, 371.1 min/day, and 84%, respectively. Time spent in moderate PA and vigorous PA in the morning and afternoon was not associated with sleep variables. Among men, 10 min/day of morning LPA increased TST by 2.56 min/day. Among women, 10 min/day of morning LPA increased SP by 0.15 percentage points. Afternoon LPA also increased SP by 0.09 percentage points for women. Night PA seems to have an inverse effect on sleep variables for any intensity and both sexes.
Conclusion
: The effect of PA on sleep health is intrinsically related to the period of the day in which it is performed. The effect magnitude is different between sexes. For better sleep health, it is preferable that PA be performed during the day.
Graphical abstract
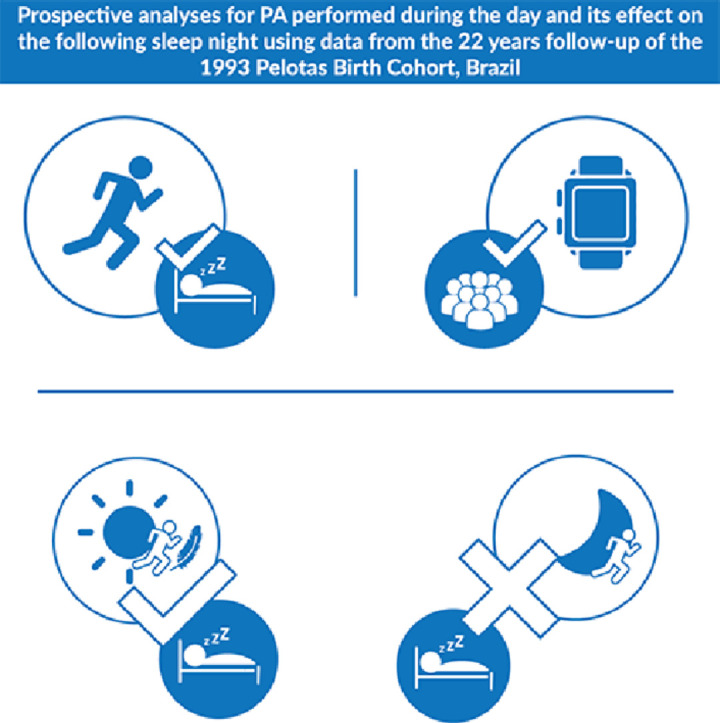
1. Introduction
Sleep has an important role in the metabolism and homeostasis of humans and consequently in general health.1,2 Poor sleep quality and inadequate duration have been associated with a higher risk of chronic diseases, such as hypertension,3, 4, 5 diabetes,3, 4, 5 and depression,5,6 as well as with mortality.7
Many behavioral aspects influence sleep, including physical activity (PA) levels. Studies have shown that PA may reduce the odds of both long and short sleep,5,8,9 improve sleep quality,10,11 and reduce the risk of insomnia and other sleep disorders.12,13 Some authors discuss differences in terms of the short-term effect on sleep health and the effect of the regular practice of PA on the same outcomes.14,15 While the effects of regular exercise are related to body composition changes, improvement of fitness levels, and heart rate variability, acute effects of exercise include central nervous system fatigue, increased body temperature, and the secretion of growth hormone and brain-derived neurotrophic factors.15,16 Furthermore, the majority of the literature about PA and sleep is based on cross-sectional associations, long-term associations (in cohort studies), or experimental studies, none of them with population-based samples.14,17,18 On the other hand, short-term associations remain unclear,18 probably because of the difficulty in measuring with questionnaires, the method frequently used in population-based studies. Another issue is the possible bidirectional relationship of this association, which highlights the importance of daily fluctuations.19,20 Sleep assessment involving multiple days for each individual may help to deal with these fluctuations, but sleep assessment has traditionally been measured only on a weekly basis.19,20
The association of PA and sleep seems to rely on the PA features such as intensity and time schedule.17,21 Although some authors have argued that PA performed at night has a sleep-disturbing effect, the evidence is still unclear.21 Some experimental studies have found that PA performed only during the daytime improves sleep outcomes, but these findings are limited by small samples and self-selection bias.22 A recent review of experimental studies did not find evidence that evening PA worsens sleep time.23 In addition, few studies have assessed different periods of the day and intensities of PA separately.21
Time spent on each intensity of PA occurring during specific periods of the day is difficult to measure in population-based studies, which usually depend on questionnaires based on recall periods of greater than 1 day. Furthermore, recent studies have shown that the effect of PA and 24-h movement patterns on sleep may be different between men and women, a finding that needs to be further explored with stratified analyses, for example.24,25 Accelerometry could be an alternative for measuring the effect of acute, free-living PA on the subsequent sleep night in population-based samples and provide some evidence regarding unanswered questions, including the relationship between sleep and PA intensity and the time of the day in which PA is performed.
Identifying the optimal time and intensity for performing PA in order to improve sleep parameters may help in the planning of population-level health actions. Hence, this study aimed to verify the effect of different intensities of PA performed during different periods of the day on the following sleep night in a population-based cohort of young adults.
2. Methods
2.1. Study design and sample
The present study is based on prospective analyses of PA practiced during the day and the following sleep night using data from the 22-year follow-up of the 1993 Pelotas Birth Cohort in Brazil (mean age = 22.6 years).26 This cohort participated in a multipurpose population-based study, and the 22-year follow-up gave special attention to precursors and risk factors for noncommunicable diseases.26 The eligibility criteria for the cohort included being born in one of 5 maternity hospitals in Pelotas and being the child of a mother who lived in an urban region of the city. The original cohort comprised 5249 child–mother pairs.27 For the 15-year follow-up, interviews took place in each participant's home. For the 18- and 22-year follow-ups, participants went to a research clinic for data collection. The response rate at the 22-year follow-up was 76.3% (3810 cohort members were assessed; 193 individuals had died). All follow-ups collected standardized information about sociodemographic and behavior characteristics, as well as health outcomes. More details about all cohort follow-up (including 22-year follow-up) can be found elsewhere.26, 27, 28
This study was approved by the Federal University of Pelotas Medical School. Ethical approval was obtained, and members of the cohort provided full written informed consent.
2.2. Accelerometry
As part of the 22-year follow-up of the 1993 Pelotas Birth Cohort, participants were invited to wear a triaxial accelerometer on the wrist Actigraph (GT3X; ActiGraph, Pensacola, FL, USA) for 6 days with a 24-h protocol. This protocol allowed an analysis of each participant's PA and sleep to be made throughout the week. At the end of the week, a staff member collected the device at the participant's house. Data were collected using a 5-s epoch and 60 Hz sampling frequency. The download was performed with Actilife software (Version 6.1; ActiGraph) and processed in R program with a GGIR package (Version 1.5-21; R Core Team, Vienna, Austria).29 This process checked for calibration error based on local gravity,30 abnormally high values and nonwear time, and vector magnitude was calculated using the Euclidian Norm minus 1g (ENMO) (Eq. (1)).
| Eq. (1) |
Sleep variables were obtained via the algorithm proposed by van Hees et al.,31 with automatic detection of sleep periods.32 The original work was found to be 83% accurate for sleep measures.31 Automatic detection assesses changes in the z-angle for each 5-min period31 and identifies values under the 10th percentile of movement in a complete day. The algorithm then identifies blocks of 30 min with this pattern of movement, with gaps lower than 60 min among them. Finally, only the longer block in a 24-h period (noon to noon), defined as the sleep time window (STW), is maintained.32 In our study, changes in the z-angle of 3° for each 5-min window were used. The sleep variables were STW (the difference between sleep onset and sleep end, expressed in minutes), total sleep time (TST; the total minutes classified as sleep after sleep onset), and sleep percent (SP; TST divided by STW, expressed as percent). In order to improve the quality of data, TST and STW of less than 2 h or more than 15 h, respectively, were excluded from the analysis. A total of 437 nights were excluded from the analysis due to these criteria.
The extracted PA variables for this study were minutes of light PA (LPA), moderate PA (MPA), and vigorous PA (VPA). LPA was defined as involving acceleration from 50 mg to 99 mg, MPA from 100 mg to 399 mg, and VPA 400 mg or more.33,34 While LPA and VPA were nonbouted variables, MPA was analyzed using a 5-min bout. The decision regarding the nonbouted variables was based on the fact that any bout criteria for VPA would be unable to capture activities having peaks of high intensity and short duration. Nonbouted LPA represented more routine activities, while a 5-min MPA bout represented more structured activities.
Daily PA occurring during the following periods was analyzed: morning (6:00 a.m. to 11:59 a.m.), afternoon (12:00 p.m. to 7:59 p.m.), night (8:00 p.m. to 11:59 p.m.), and entire day (6:00 a.m. to 11:59 p.m.). All PA variables occurring between 6:00 a.m. and 12:00 a.m. were analyzed, as the mean of sleep onset in our sample was 12:00 a.m. The subdivision of a day into morning, afternoon, and night periods took into account the cultural pattern of PA in Pelotas, Brazil.
All algorithms and cut-offs for PA and sleep were validated to triaxial accelerometers previously.31,34
2.3. Covariables
Considered as potential confounding factors, the following variables were included in the model: skin color (self-reported), wealth index, work status (none, only studies, only work, or both), having children less than 2 years of age (no, yes), screen time, percentage of fat mass, alcohol ingestion, current smoking (no, yes), illicit drug use, coffee ingestion, mental health, and using medication to sleep. The wealth index was obtained from a list of items using the first component of a principal component analyses divided into quintiles, according to standard strategies of wealth-related variables.35 Screen time was obtained by summing the time spent weekly on television, computer, and video games (divided into terciles). The percentage of fat mass was measured using air displacement plethysmography (BOD POD Composition System; COSMED, Albano Laziale, Italy). Alcohol ingestion was measured using the Alcohol Use Disorder Identification questionnaire, and a continuous score was used. The use of illicit drugs was measured using a confidential questionnaire, which asked participants about the use of a list of different drugs. We classified the use of illicit drugs as everyday, sometimes, and no use. Coffee ingestion in the last year was collected with a digital and self-reported food frequency questionnaire, which asked participants about frequency and amount.36 Using this information, coffee ingestion was calculated in grams per day. In order to measure mental health, a continuous-score, self-reporting questionnaire was used.37 Covariables were chosen based on a literature review; we included factors that might influence both PA timing and sleep. We did not include any variables that could potentially be in the path between PA and sleep (mediators) (Supplementary Fig. 1).
2.4. Data analysis
Our study included only participants who had at least 4 nights of sleep data, who had at least 16 valid h/day of accelerometry data (GGIR default), who were not working at night and who did not use medication for sleep, since the sleep medication could modify the sleep parameters. First, a comparison between the original cohort and the analytical sample was carried out (Supplementary Table 1). Percentages and a 95% confidence interval (95%CI) were used for categorical variables in the study. Means and 95%CIs were calculated for all outcomes for continuous variables. For PA variables, we calculated medians and interquartile ranges.
In order to test the effect of PA on sleep outcomes, we analyzed each 24-h period per participant. We used a generalized estimating equation to obtain regression coefficients (β). Due to the continuous nature of exposures and outcomes, we decided to work with the Gaussian family and identity link. The correlation structure for each association was based on the lower quasi-likelihood under the independence model criterion values of models. To improve the analysis, we used 10 min of PA instead of 1 min. Therefore, the change in sleep outcomes should be interpreted based on each increase of 10 min of PA. Supplementary Tables 2, 3, and 4 present changes in sleep outcomes for each 1 min of change in PA variables. The crude and adjusted regression coefficients (β) and respective 95%CIs for PA are presented for the following periods: morning, afternoon, night, and entire day.
All analyses were conducted using Stata (Version 12.1; StataCorp LP, College Station, TX, USA) and were stratified by sex. Significance was set at α = 0.05.
3. Results
We analyzed 2006 participants with valid accelerometer data for both PA and sleep (comprising 9687 cycles of 24 h) from a total of 3810 cohort members evaluated at the 22-year follow-up. Supplementary Table 1 shows a comparison between the original cohort (original sample born in 1993, n = 5249) and the analytic sample (with valid accelerometer data at the 22-year follow-up, n = 2006). The analyzed sample was similar to the original cohort, and no statistically significant difference was found according to sex (p = 0.383), maternal education (p = 0.077), family income (p = 0.863), or birth weight (p = 0.497).
Table 1 presents a description of the sample included in our analysis, as well as the means of the 3 outcomes related to the covariables. The majority of the analyzed participants were females (51.5%) and reported white skin color (61.0%). One-fifth had a job and were engaged in studies; 15.0% reported having children below 2 years of age. Regarding behaviors, 16.1% reported smoking, 13.6% used illicit drugs, and 20.1% reported harmful alcohol ingestion; 19.4% of the participants were classified as having common mental disorders. The means of STW, TST, and SP were 443.6 min (±67.2 min), 371.1 min (±58.5 min), and 84.0% (±5.8%), respectively.
Table 1.
Distribution of sample according to variables used in study (Pelotas Birth cohort 1993, 22-year follow-up, Pelotas, Brazil).
| Sample |
Sleep time window (min) | Total sleep time (min) | Sleep percent (%) | ||
|---|---|---|---|---|---|
| Variable | n (%) | 95%CI | Mean (95%CI) | Mean (95%CI) | Mean (95%CI) |
| Sex | |||||
| Male | 972 (48.5) | 45.9–50.1 | 429.4 (425.3–433.4) | 356.4 (352.9–359.8) | 83.5 (83.1–83.9) |
| Female | 1034 (51.5) | 49.9–54.1 | 456.9 (452.8–461.0) | 385.0 (381.4–388.6) | 84.6 (84.2–84.9) |
| Skin color | |||||
| White | 1224 (61.0) | 58.9–63.2 | 444.9 (441.2–448.7) | 374.0 (370.8–377.3) | 84.4 (84.1–84.7) |
| Black | 339 (16.9) | 15.3–18.5 | 436.2 (429.2–443.1) | 361.9 (355.8–367.9) | 83.4 (82.8–84.0) |
| Brown | 359 (17.9) | 16.2–19.6 | 448.4 (441.2–455.5) | 372.2 (366.1–378.4) | 83.4 (82.8–84.0) |
| Other | 84 (4.2) | 3.3–5.1 | 432.8 (417.4–448.1) | 361.2 (347.9–374.5) | 83.8 (82.5–85.2) |
| Wealth index | |||||
| 1st quintile (poorest) | 389 (19.4) | 17.7–21.1 | 449.8 (443.1–456.5) | 372.2 (366.4–378.0) | 83.1 (82.5–83.7) |
| 2nd quintile | 411 (20.5) | 18.7–22.3 | 444.6 (438.1–451.1) | 369.7 (364.1–375.4) | 83.5 (83.0–84.1) |
| 3rd quintile | 429 (21.4) | 19.6–23.2 | 439.5 (433.1–445.8) | 367.3 (361.7–372.8) | 84.0 (83.4–84.5) |
| 4th quintile | 407 (20.3) | 18.5–22.1 | 441.5 (434.9–448.0) | 373.6 (367.9–379.3) | 84.9 (84.4–85.5) |
| 5th quintile (richest) | 370 (18.4) | 16.7–20.1 | 442.9 (436.1–449.8) | 373.2 (367.3–379.2) | 84.6 (84.0–85.3) |
| Work status | |||||
| None | 426 (21.2) | 19.4–23.0 | 457.1 (450.7–463.4) | 375.7 (370.2–381.3) | 82.6 (82.1–83.2) |
| Only studies | 262 (13.1) | 11.6–14.5 | 446.7 (438.6–454.8) | 376.8 (369.7–383.8) | 84.6 (83.9–85.3) |
| Only work | 908 (45.3) | 43.1–47.4 | 439.8 (435.5–444.2) | 367.4 (363.6–371.2) | 83.7 (83.5–84.2) |
| Both | 410 (20.4) | 18.7–22.2 | 435.7 (429.3–442.2) | 371.0 (365.3–376.6) | 85.5 (84.9–86.0) |
| Have children <2 years of age | |||||
| No | 1705 (85.0) | 83.4–86.6 | 444.2 (441.0–447.4) | 373.0 (370.2–375.7) | 84.3 (84.0–84.6) |
| Yes | 301 (15.0) | 13.4–16.6 | 440.1 (432.5–447.7) | 360.7 (354.1–367.2) | 82.5 (81.9–83.2) |
| Screen time (min) | |||||
| 1st tercile (lowest) | 790 (39.3) | 37.2–41.5 | 442.7 (438.0–447.4) | 370.6 (366.5–374.7) | 84.2 (83.7–84.6) |
| 2nd tercile | 637 (31.8) | 29.7–33.8 | 444.2 (439.0–449.4) | 371.1 (366.6–375.7) | 83.8 (83.4–84.3) |
| 3rd tercile (highest) | 579 (28.9) | 26.9–30.8 | 444.0 (438.5–449.5) | 371.8 (367.1–376.6) | 84.1 (83.6–84.5) |
| Current smoking | |||||
| No | 1683 (83.9) | 82.3–85.5 | 444.2 (440.9–447.4) | 372.5 (369.7–375.3) | 84.2 (83.9–84.5) |
| Yes | 323 (16.1) | 14.5–17.7 | 440.5 (433.1–447.8) | 363.8 (357.4–370.2) | 83.1 (82.4–83.7) |
| Harmful alcohol ingestiona | |||||
| No | 1603 (79.9) | 78.2–81.7 | 446.6 (443.3–450.0) | 374.2 (371.4–377.1) | 84.2 (83.9–84.4) |
| Yes | 403 (20.1) | 18.3–21.8 | 431.4 (424.9–437.9) | 358.8 (353.1–364.5) | 83.4 (83.0–84.1) |
| Illicit drug use | |||||
| No | 1733 (86.4) | 84.9–87.9 | 444.9 (441.7–448.0) | 372.9 (370.2–375.7) | 84.2 (83.9–84.5) |
| Yes | 273 (13.6) | 12.1–15.1 | 435.2 (427.2–443.1) | 359.7 (352.8–366.6) | 83.0 (82.4–83.7) |
| Common mental disorderb | |||||
| No | 1616 (80.6) | 78.8–82.3 | 444.8 (441.5–448.0) | 372.1 (369.3–375.0) | 84.0 (83.7–84.3) |
| Yes | 390 (19.4) | 17.7–21.2 | 438.6 (432.0–445.3) | 366.9 (361.1–372.7) | 84.0 (83.4–84.6) |
| Coffee ingestion (g/day) | |||||
| 1st tercile (lowest) | 699 (34.8) | 32.8–36.9 | 441.4 (436.4–446.3) | 368.8 (364.5–373.2) | 83.9 (83.5–84.4) |
| 2nd tercile | 678 (33.8) | 31.7–35.9 | 447.8 (442.7–442.7) | 374.3 (369.9–378.7) | 83.9 (83.4–84.3) |
| 3rd tercile (highest) | 629 (31.4) | 29.3–33.4 | 441.4 (436.2–436.2) | 370.3 (365.7–374.8) | 84.3 (83.8–84.8) |
| Fat mass (%) | |||||
| 1st tercile (lowest) | 718 (35.7) | 33.7–37.9 | 435.0 (430.1–440.9) | 361.2 (357.0–365.5) | 83.5 (83.0–83.9) |
| 2nd tercile | 629 (31.4) | 29.3–33.4 | 445.9 (440.7–451.2) | 374.0 (369.4–378.5) | 84.2 (83.8–84.7) |
| 3rd tercile (highest) | 659 (32.9) | 30.8–34.9 | 450.6 (445.5–455.7) | 379.2 (374.8–383.6) | 84.5 (84.0–84.9) |
| Total | 2006 (100.0) | 443.6 (440.6–446.5) | 371.1 (368.6–373.7) | 84.0 (83.8–84.3) | |
Note: Mean age of the sample was 22.6 years.
Eight points or more in Alcohol Use Disorder Identification indicates harmful alcohol ingestion.
Common disorder was defined according to self-reporting questionnaire-20 score (≥8 points for women and ≥6 points for men).
Abbreviation: 95%CI = 95% confidence interval.
Table 2 shows medians of LPA, MPA, and VPA according to period of day and sex. During the 24-h period, the median for LPA in both sexes was around 150 min. Men spent 22.9 min in MPA and 5.1 min in VPA, while for women these values were 13.3 min and 3.0 min, respectively. There were no relevant differences by sex in the medians for morning PA and night PA. In the afternoon, women spent half as much time in MPA compared to men (10.7 min for men and 5.9 min for women).
Table 2.
Description of physical activity according to periods of day and sex (Pelotas Birth Cohort 1993, 22-year follow-up, Pelotas, Brazil) (median (interquartile interval)).
| Men | Women | |
|---|---|---|
| Morning | ||
| LPA (min) | 35.6 (15.5–61.6) | 35.1 (21.0–50.3) |
| MPA (min) | 0.0 (0.0–8.8) | 0.0 (0.0–5.1) |
| VPA (min) | 0.7 (0.1–1.8) | 0.3 (0.1–1.0) |
| Afternoon | ||
| LPA (min) | 77.8 (58.6–94.6) | 78.8 (64.0–95.5) |
| MPA (min) | 10.7 (4.1–24.4) | 5.9 (0.0–14.6) |
| VPA (min) | 2.6 (1.3–5.1) | 1.5 (0.7–3.0) |
| Night | ||
| LPA (min) | 28.1 (20.4–44.7) | 30.4 (22.3–39.1) |
| MPA (min) | 0.0 (0.0–4.7) | 0.0 (0.0–0.0) |
| VPA (min) | 0.6 (0.3–1.4) | 0.3 (0.2–0.8) |
| Entire day | ||
| LPA (min) | 147.4 (109.8–182.3) | 145.9 (119.8–178.3) |
| MPA (min) | 22.9 (9.0–42.0) | 13.3 (4.9–26.0) |
| VPA (min) | 5.1 (2.6–10.3) | 3.0 (1.4–6.5) |
Abbreviations: LPA = light physical activity (nonbouted); MPA = moderate physical activity (5-min bout); VPA = vigorous physical activity (nonbouted).
Table 3 presents results of STW according to sex. After adjustments, higher PA levels during the morning and the afternoon were not associated with STW for either sex. PA practiced at night showed an inverse association with STW in the following sleep night. Among men, each 10 min of LPA, MPA, and VPA at night decreased the STW by 14.44 min (95%CI: –16.56 to –12.33), 5.01 min (95%CI: –7.49 to –2.53), and 12.03 min (95%CI: –18.22 to –5.84), respectively. Among women, STW decreased by 13.60 min (95%CI: –15.79 to –11.42), 10.37 min (95%CI: –14.70 to –6.03), and 20.31 min (95%CI: –33.25 to –7.37) for LPA, MPA, and VPA, respectively. During the entire day, each 10 min of LPA and MPA decreased STW by 1.14 min (95%CI: –1.84 to –0.43) and 1.33 min (95%CI: –2.62 to –0.05), respectively, for women only.
Table 3.
Crude and adjusted association of physical activity and sleep time window according to sex (Pelotas Birth Cohort 1993, 22-year follow-up, Pelotas, Brazil).
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Crude |
Adjusted |
Crude |
Adjusted |
|||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | |
| Morning | ||||||||
| LPA | 0.03 (–1.03 to 1.11) | 0.948 | 0.82 (–0.31 to 1.96) | 0.153 | –0.79 (–2.03 to 0.45) | 0.211 | –0.55 (–1.82 to 0.72) | 0.399 |
| MPA | –0.71 (–2.40 to 0.98) | 0.412 | –0.60 (–2.29 to 1.10) | 0.491 | –0.55 (–3.27 to 2.16) | 0.691 | –0.64 (–3.36 to 2.09) | 0.647 |
| VPA | 1.53 (–4.63 to 7.70) | 0.627 | 1.73 (–4.45 to 7.91) | 0.584 | 2.48 (–6.03 to 11.00) | 0.568 | 2.36 (–6.17 to 10.88) | 0.588 |
| Afternoon | ||||||||
| LPA | 0.19 (–0.92 to 1.30) | 0.735 | 0.56 (–0.58 to 1.69) | 0.336 | –0.34 (–1.43 to 0.74) | 0.533 | –0.19 (–1.30 to 0.91) | 0.731 |
| MPA | –0.29 (–1.42 to 0.84) | 0.618 | –0.45 (–1.59 to 0.70) | 0.442 | –0.51 (–2.39 to 1.38) | 0.597 | –0.64 (–2.55 to 1.26) | 0.509 |
| VPA | 0.39 (–3.72 to 4.50) | 0.852 | 0.01 (–0.41 to 0.42) | 0.987 | 2.39 (–3.42 to 8.20) | 0.419 | 2.43 (–3.40 to 8.25) | 0.414 |
| Night | ||||||||
| LPA | –14.44 (–16.56 to –12.33) | <0.001 | –14.44 (–16.56 to –12.33) | <0.001 | –13.57 (–15.73 to –11.40) | <0.001 | –13.60 (–15.79 to –11.42) | <0.001 |
| MPA | –4.70 (–7.17 to –2.23) | <0.001 | –5.01 (–7.49 to –2.53) | <0.001 | –10.30 (–14.63 to –5.99) | <0.001 | –10.37 (–14.70 to –6.03) | <0.001 |
| VPA | –11.56 (–17.75 to –5.37) | <0.001 | –12.03 (–18.22 to –5.84) | <0.001 | –19.80 (–38.74 to –6.85) | <0.001 | –20.31 (–33.25 to –7.37) | <0.001 |
| Entire day | ||||||||
| LPA | –0.73 (–1.33 to –0.14) | 0.015 | –0.52 (–1.14 to 0.10) | 0.103 | –1.19 (–1.88 to –0.50) | 0.001 | –1.14 (–1.84 to –0.43) | 0.002 |
| MPA | –0.64 (–1.35 to 0.07) | 0.078 | –0.73 (–1.45 to –0.01) | 0.049 | –1.22 (–2.48 to 0.05) | 0.059 | –1.33 (–2.62 to –0.05) | 0.042 |
| VPA | –1.55 (–4.11 to 1.00) | 0.233 | –1.76 (–4.34 to 0.81) | 0.179 | –0.19 (–3.99 to 3.61) | 0.923 | –0.25 (–4.08 to 3.57) | 0.898 |
Notes: The changes in outcomes are relative to an increase of 10 min of PA. Adjustment to skin color, wealth index, study/work status, having children <2 years old, screen time, mental health, % fat mass, alcohol use, current smoking, illicit drug use, medication to sleep, and coffee ingestion.
Abbreviations: 95%CI = 95% confidence interval; LPA = light physical activity (nonbouted); MPA = moderate physical activity (5-min bout); VPA = vigorous physical activity (nonbouted).
The results for TST are presented in Table 4. For men only, each 10 min of morning LPA increased TST by 2.56 min (95%CI: 1.57 to 3.56). Afternoon PA was not associated with TST for either sex. Night PA was associated with lower TST. For men, each 10 min of LPA, MPA, and VPA decreased TST by 11.09 min (95%CI: –12.81 to –9.38), 4.28 min (95%CI: –6.33 to –2.22), and 9.31 min (95%CI: –14.44 to –4.19) min, respectively. The associations among women were in the same direction, but presented higher magnitudes. For women, 10 min of LPA, MPA, and VPA decreased TST by 10.81 min (95%CI: –12.56 to –9.05), 8.38 min (95%CI: –12.04 to –4.73), and 15.25 min (95%CI: –26.14 to –4.36), respectively. Evaluating the whole day, MPA decreased TST by 0.80 min (95%CI: –1.40 to –0.19) for men and 1.16 min (95%CI: –2.24 to –0.08) for women.
Table 4.
Crude and adjusted association of physical activity and total sleep time according to sex (Pelotas Birth Cohort 1993, 22-year follow-up, Pelotas, Brazil).
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Crude |
Adjusted |
Crude |
Adjusted |
|||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | |
| Morning | ||||||||
| LPA | 1.84 (0.89 to 2.79) | <0.001 | 2.56 (1.57 to 3.56) | <0.001 | 0.02 (–1.02 to 1.07) | 0.964 | 0.24 (–0.84 to 1.31) | 0.667 |
| MPA | –1.08 (–2.47 to 0.32) | 0.129 | –0.81 (–2.22 to 0.60) | 0.258 | –0.08 (–2.37 to 2.21) | 0.943 | –0.23 (–2.53 to 2.06) | 0.842 |
| VPA | 0.18 (–4.93 to 5.29) | 0.944 | 0.87 (–4.26 to 6.00) | 0.740 | 2.98 (–4.21 to 10.18) | 0.417 | 2.32 (–4.85 to 9.50) | 0.526 |
| Afternoon | ||||||||
| LPA | 0.23 (–0.64 to 1.10) | 0.605 | 0.59 (–0.31 to 1.49) | 0.200 | –0.25 (–1.16 to 0.67) | 0.598 | 0.26 (–0.67 to 1.20) | 0.582 |
| MPA | –0.73 (–1.67 to 0.20) | 0.125 | –0.66 (–1.61 to 0.29) | 0.172 | –0.54 (–2.13 to 1.05) | 0.506 | –0.82 (–2.43 to 0.78) | 0.313 |
| VPA | –0.27 (–3.67 to 3.13) | 0.875 | –0.03 (–3.45 to 3.39) | 0.985 | 1.59 (–3.32 to 6.50) | 0.527 | 1.37 (–3.53 to 6.27) | 0.583 |
| Night | ||||||||
| LPA | –11.66 (–13.42 to –9.91) | <0.001 | –11.09 (–12.81 to –9.38) | <0.001 | –11.49 (–13.22 to –9.76) | <0.001 | –10.81 (–12.56 to –9.05) | <0.001 |
| MPA | –4.28 (–6.32 to –2.24) | <0.001 | –4.28 (–6.33 to –2.22) | <0.001 | –8.01 (–11.74 to –4.44) | <0.001 | –8.38 (–12.04 to –4.73) | <0.001 |
| VPA | –9.40 (–14.52 to –4.28) | <0.001 | –9.31 (–14.44 to –4.19) | <0.001 | –14.11 (–25.05 to –3.17) | 0.011 | –15.25 (–26.14 to –4.36) | 0.006 |
| Entire day | ||||||||
| LPA | –0.36 (–0.85 to 0.14) | 0.159 | –0.14 (–0.66 to 0.37) | 0.585 | –0.67 (–1.26 to –0.08) | 0.026 | –0.50 (–1.10 to 0.10) | 0.102 |
| MPA | –0.86 (–1.44 to –0.27) | 0.004 | –0.80 (–1.40 to –0.19) | 0.010 | –0.95 (–2.02 to 0.12) | 0.082 | –1.16 (–2.24 to –0.08) | 0.036 |
| VPA | –1.68 (–3.80 to 0.44) | 0.119 | –1.48 (–3.61 to 0.65) | 0.174 | 0.06 (–3.16 to 3.27) | 0.972 | –0.27 (–3.49 to 2.95) | 0.868 |
Notes: The changes in outcomes are relative to an increase of 10 min of PA. Adjustment to skin color, wealth index, study/work status, having children <2 years old, screen time, mental health, % fat mass, alcohol use, current smoking, illicit drug use, medication to sleep, and coffee ingestion.
Abbreviations: 95%CI = 95% confidence interval; LPA = light physical activity (nonbouted); MPA = moderate physical activity (5-min bout); VPA = vigorous physical activity (nonbouted).
Table 5 shows the effect of PA on SP according to sex. For women, each 10 min of morning LPA increased SP by 0.15% (95%CI: 0.07%–0.23%), and each 10 min of afternoon LPA increased SP by 0.09% (95%CI: 0.02%–0.16%). Night PA was not associated with SP for either sex. For the entire day, LPA increased SP among women by 0.07% (95%CI: 0.03%–0.11%).
Table 5.
Crude and adjusted association of physical activity and sleep percent according to sex (Pelotas Birth Cohort 1993, 22-year follow-up, Pelotas, Brazil).
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Crude |
Adjusted |
Crude |
Adjusted |
|||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | |
| Morning | ||||||||
| LPA | 0.07 (–0.00 to 0.14) | 0.056 | 0.07 (–0.01 to 0.14) | 0.070 | 0.15 (0.07 to 0.23) | <0.001 | 0.15 (0.07 to 0.23) | <0.001 |
| MPA | –0.09 (–0.19 to 0.01) | 0.069 | –0.07 (–0.17 to 0.03) | 0.168 | 0.08 (–0.10 to 0.24) | 0.386 | 0.06 (–0.1 to 0.23) | 0.477 |
| VPA | –0.14 (–0.49 to 0.22) | 0.458 | –0.09 (–0.46 to 0.28) | 0.636 | 0.24 (–0.23 to 0.72) | 0.319 | 0.20 (–0.27 to 0.68) | 0.400 |
| Afternoon | ||||||||
| LPA | 0.01 (–0.05 to 0.08) | 0.703 | 0.01 (–0.01 to 0.01) | 0.760 | 0.01 (–0.06 to 0.08) | 0.763 | 0.09 (0.02 to 0.16) | 0.012 |
| MPA | –0.09 (–0.16 to –0.02) | 0.007 | –0.07 (–0.14 to 0.01) | 0.038 | –0.01 (–0.12 to 0.11) | 0.915 | –0.04 (–0.16 to 0.08) | 0.492 |
| VPA | –0.15 (–0.38 to 0.08) | 0.205 | –0.09 (–0.33 to 0.14) | 0.445 | –0.06 (–0.43 to 0.30) | 0.741 | –0.11 (–0.47 to 0.24) | 0.531 |
| Night | ||||||||
| LPA | 0.11 (–0.03 to 0.25) | 0.134 | 0.11 (–0.03 to 0.26) | 0.122 | 0.01 (–0.11 to 0.14) | 0.825 | 0.07 (–0.06 to 0.19) | 0.311 |
| MPA | –0.03 (–0.17 to 0.12) | 0.701 | –0.01 (–0.16 to 0.13) | 0.852 | 0.18 (–0.06 to 0.42) | 0.141 | 0.16 (–0.07 to 0.40) | 0.174 |
| VPA | 0.04 (–0.32 to 0.40) | 0.834 | 0.06 (–0.29 to 0.42) | 0.726 | 0.39 (–0.32 to 1.11) | 0.282 | 0.37 (–0.35 to 1.08) | 0.318 |
| Entire day | ||||||||
| LPA | 0.03 (–0.01 to 0.07) | 0.097 | 0.03 (–0.01 to 0.07) | 0.110 | 0.03 (–0.01 to 0.07) | 0.129 | 0.07 (0.03 to 0.11) | <0.001 |
| MPA | –0.06 (–0.11 to –0.02) | 0.005 | –0.05 (–0.09 to –0.01) | 0.037 | 0.03 (–0.05 to 0.11) | 0.423 | 0.01 (–0.07 to 0.09) | 0.809 |
| VPA | –0.11 (–0.26 to 0.04) | 0.138 | –0.07 (–0.22 to 0.08) | 0.367 | 0.08 (–0.16 to 0.32) | 0.522 | 0.02 (–0.21 to 0.26) | 0.864 |
Notes: The changes in outcomes are relative to increase of 10 min of PA. Adjustment for skin color wealth index, study/work status, having children <2 years old, screen time, mental health, % fat mass, alcohol use, current smoking, illicit drug use, medication to sleep and coffee ingestion.
Abbreviations: 95%CI = 95% confidence interval; LPA = light physical activity (nonbouted); MPA = moderate physical activity (5-min bout); VPA = vigorous physical activity (nonbouted).
4. Discussion
This study assessed the short-term effect of PA on sleep outcomes in a population-based sample of young adults. For both sexes, PA practiced during the night led to a decrease in STW and TST, regardless of the intensity of this activity. For men, morning LPA was associated with higher TST, and among women, morning and afternoon LPA improved SP.
It is important to emphasize that, when considering the entire day, PA seemed to decrease STW, TST, and SP. However, depending on the period when PA was performed, we found different results. For PA performed at night, STW and TST decreased almost proportionally to the increase in the amount of time LPA and MPA was performed. For SP, we did not find this relationship since this variable is the ratio of both TST and STW. On the other hand, morning and afternoon LPA was associated with higher TST and SP.
The difference in the effect of PA according to the time of day when it was performed is cited in previous studies,17,22 yet almost all evidence comes from studies without population-based samples. For example, 2 previous meta-analyses included only randomized controlled exercise interventions with restricted samples.14,24 While causal evidence is clearer in this type of design, it is hard to extrapolate to the general population. Another issue regarding the comparability of our results is the limited number of studies using a similar approach in young adults, the use of objectively measured PA and sleep outcomes.22 Laboratory-based studies evaluating night exercise found an increase in heart rate variability, but this did not affect the cortisol curve or sleep measures such as duration or quality.15,38,39 Surveys carried out in high-income countries evaluating the perception of participants on the effect of day and night exercise on sleep showed that, in general, individuals recognized that any exercise was a behavior that improved sleep outcomes, irrespective of the period in which it was performed.40,41 A study carried out in the United States with telephone interviews found that morning PA increased the odds of good sleep quality by 88% and decreased the odds of waking unrefreshed by 44%.10 A study with college students in China, also with subjective measures, found that daytime PA decreases the odds of poor sleep quality by 21%, but no association was found with night exercise.42
There are some explanations for an inverse association between night PA and sleep in this study. One study with 20- to 30-year-old men tested the effect of exercise before sleep and identified that the onset of melatonin secretion started 30 min later in exercised compared to nonexercised individuals, delaying the circadian phase. The difference between the exercise and nonexercise groups was attenuated in the second day and disappeared in the third day.43 This result suggests that night PA may be harmful to sleep outcomes in the short term but not in the long term.43 Because we analyzed our study participants for only 1 week, we were not able to evaluate whether individuals who regularly practiced PA at night had normal sleep. The laboratory studies suggest the hypothesis that, with time, the negative effect of night PA is attenuated. This point needs to be considered in the interpretation of our findings.
Thermoregulation may also explain the effect of PA on sleep. PA increases body temperature, triggering the thermoregulation process.44 Some authors suggest that PA performed close to sleep time may not provide enough time for the body temperature to decrease, retarding sleep onset,44 but this evidence is not clear.22 Additionally, individuals who performed PA at night may be different from those who did so in the morning or afternoon. This may be related to work or study schedules and aspects of lifestyle. Additional studies exploring the influences of the period during the day in which PA is performed are needed to understand these differences.
Many studies have found an association between PA and mental health, chronic diseases, and mortality.45, 46, 47 These studies have found that any PA is better than none, and we recognize the importance of PA in promoting healthy living. In our study, PA performed at night seemed to decrease STW and TST but had no effect on SP. This indicates that the intensity of the PA and the period of the day in which it is carried out need to be considered in order to improve sleep health.
In the present study, LPA, but not MPA or VPA, was positively associated with sleep outcomes. Data (not presented) with bouted LPA showed no association with sleep outcomes. The nonbouted LPA measure used in this study may represent more general activities than those considered structured. The literature discusses the influence of bout length on PA final measures.48 Regarding MPA, we used a 5-min bout, as it traditionally tries to measure structured activities. Additional analyses with other bout criteria for MPA (nonbouted and 10-min bouts) did not show any associations either. The different bout criteria may influence PA constructs captured from an accelerometer and total time spent at each intensity.48 It is possible that, regarding sleep specifically, the amount of low-intensity daily activities is more important than the amount of time spent on activities of moderate or high intensity. In addition, most studies that found an association between moderate to VPA and sleep in adults used self-reported measures in which intensity was based on the perception of individuals.49, 50, 51 In a study with 54 young adults using the Fitbit Flex device, Mead et al.52 found that PA does not influence subsequent sleep; instead, nights with a longer than usual sleep duration were related to PA conducted during the next day. Furthermore, the number of people who spent time in MPA and VPA is very low in our sample. Thus, LPA seems to be the intensity level that really makes some difference at a population level. To improve sleep outcomes, interventions with a focus on total diurnal movement may be an option. Future research should explore other aspects of the association between PA and sleep, including different intensities of PA during the day, as well as fluctuations in its intensity. For example, if individuals with a pattern of LPA conducted during a given period of the day spend some time performing VPA, the VPA may have different effects on the subsequent sleep night. Unfortunately, this kind of analysis was not possible with our dataset.
There are other relevant aspects related the findings from our study. While some direct associations between LPA and sleep outcomes, as well as some inverse associations between night PA and sleep outcomes, were found, some of these comparisons were not statistically significant. Nevertheless, these comparisons can reveal possible pathways to further exploration. For example, we expected to find a statistically significant association between MPA and VPA performed during the day and sleep outcomes because previous experimental studies (in general, interventions with MPA and VPA) have shown that these types of exercise may increase slow wave sleep and decrease Stage 1 sleep, resulting in a more restorative night.15,17,44 This relationship seems to be direct and should be studied further. In addition, no association was found between any daytime PA and STW. Although our findings were not statistically significant, they are relevant because they show that diurnal PA is at least not harmful to sleep.
This study has some limitations that should be highlighted. First, we used automatic detection for sleep variables. One review recommends using sleep logs to guide accelerometry sleep analysis in order to reduce classification errors.53 Although this procedure may improve final estimates, its application in large samples may reduce the adherence to the accelerometry protocol or increase the loss of information. Thus, we opted to use an automatic detection algorithm for sleep to balance data quality and logistical considerations.32 Second, the absence of chronotype classification is another important limitation. An individual's chronotype may influence that person's preferences in performing PA or in choosing sleep time. However, we believe that this limitation had a low impact on our results since we excluded those who worked or studied during the night. Third, because we only had participant data from 1 week, we could not evaluate whether the negative association of night PA and sleep remained after the participants adapted, as previous studies have suggested.43 Fourth, although we have examined the association of PA with the subsequent sleep night, it is possible that individuals with chronic sleep problems are less likely to carry out PA, resulting in reverse causality. This may be more likely with regard to LPA given that LPA does not necessarily include exercise but does include daily activities. Thus, individuals with poor sleep may have had more movement both at night and during the day. Finally, we analyzed around 40% of the original cohort (2006 out of 5249 individuals). However, there were no differences between the samples analyzed and original cohort regarding sociodemographic variables (Supplementary Table 1), which suggests that the analyzed individuals were similar to the original cohort members, and results are probably free of bias regarding this topic.
This study also has some notable strengths. First, our study was conducted using a population-based sample living in a middle-income country where studies on this topic are scarce. Those living in higher income countries potentially have different PA and sleep patterns, thus providing new insight into the sleep characteristics of this particular population. Furthermore, the use of objective measures of both PA and sleep make the results more robust, and analysis using daily accelerometry allowed us to verify important aspects of PA. Finally, our findings suggest that the magnitude and direction of the association between PA and sleep are related to the period when PA is performed.
5. Conclusion
Daytime LPA is important to sleep outcomes. PA practiced at night may be harmful to sleep in the short term. Therefore, for better sleep health, the practice of daytime PA should be recommended.
Acknowledgments
Acknowledgments
The authors thank the members of the Research Group GEPEA (Grupo de Estudos e Pesquisas em Acelerometria). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (Finance Code 001) and by the Science and Technology Department of the Brazilian Ministry of Health, with resources transferred through the Brazilian National Council for Scientific and Technological Development (CNPq) (400943/2013-1). Helen Gonçalves (305759/2017-5), Ana Menezes (302029/2017-6), Fernando Barros, and Fernando C. Wehrmeister (309236/2018-5) received funding through a CNPq research productivity grant. Andrea Wendt was funded by a CAPES PhD scholarship (Finance Code 001).
Authors’ contributions
AW, ICMS, and FCW conceived the study, performed analysis, and drafted the manuscript; HG, FB, and AM carried out critical analysis of the paper. All authors have read and approved the final version of the manuscript, and agree with the order of the presentation of authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.04.007.
Appendix. Supplementary materials
References
- 1.Copinschi G., Leproult R., Spiegel K. The important role of sleep in metabolism. Front Horm Res. 2014;42:59–72. doi: 10.1159/000358858. [DOI] [PubMed] [Google Scholar]
- 2.Shukla C., Basheer R. Metabolic signals in sleep regulation: Recent insights. Nat Sci Sleep. 2016;8:9–20. doi: 10.2147/NSS.S62365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buysse D.J. Sleep health: Can we define it? Does it matter. Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley D., Ancoli-Israel S., Britz P., Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Yoon H.S., Yang J.J., Song M., et al. Correlates of self-reported sleep duration in middle-aged and elderly Koreans: From the Health Examinees Study. PloS One. 2015;10 doi: 10.1371/journal.pone.0123510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J., Zhu G., Zhao Q., et al. Prevalence and risk factors of poor sleep quality among Chinese elderly in an urban community: Results from the Shanghai aging study. PLoS One. 2013;8:e81261. doi: 10.1371/journal.pone.0081261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin J., Jin X., Shan Z., et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Q.H., Li H., Zhang X.H., Zhang T., Cui J., Xu G.Z. Associations between sleep duration and physical activity and dietary behaviors in Chinese adolescents: results from the Youth Behavioral Risk Factor Surveys of 2015. Sleep Med. 2017;37:168–173. doi: 10.1016/j.sleep.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y., Yang L., Shen X., Zhai L., Fan C., Zhang D. Effect of leisure-time aerobic exercise and muscle strength activity on sleep duration: Results from the 2012 National Health Interview Survey. J Public Health. 2016;24:117–124. [Google Scholar]
- 10.Buman M.P., Phillips B.A., Youngstedt S.D., Kline C.E., Hirshkowitz M. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America Poll. Sleep Med. 2014;15:755–761. doi: 10.1016/j.sleep.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Chen L.J., Fox K.R., Sun W.J., Lo M.K., Ku P.W. Prospective associations between different categories of physical activity and insomnia in older adults. Int J Sport Psychol. 2014;45:173–186. [Google Scholar]
- 12.Inoue S., Yorifuji T., Sugiyama M., Ohta T., Ishikawa-Takata K., Doi H. Does habitual physical activity prevent insomnia? A cross-sectional and longitudinal study of elderly Japanese. J Aging Phys Act. 2013;21:119–139. doi: 10.1123/japa.21.2.119. [DOI] [PubMed] [Google Scholar]
- 13.Janson C., Lindberg E., Gislason T., Elmasry A., Boman G. Insomnia in men—a 10-year prospective population based study. Sleep. 2001;24:425–430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 14.Kelley G.A., Kelley K.S. Exercise and sleep: A systematic review of previous meta-analyses. J Evid Based Med. 2017;10:26–36. doi: 10.1111/jebm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida S., Shioda K., Morita Y., Kubota C., Ganeko M., Takeda N. Exercise effects on sleep physiology. Front Neurol. 2012;3:48. doi: 10.3389/fneur.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driver S., Taylor S.R. Sleep disturbances and exercise. Sports Med. 1996;21:1–6. doi: 10.2165/00007256-199621010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kredlow M.A., Capozzoli M.C., Hearon B.A., Calkins A.W., Otto M.W. The effects of physical activity on sleep: A meta-analytic review. J Behav Med. 2015;38:427–449. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 18.Kovacevic A., Mavros Y., Heisz J.J., Fiatarone Singh M.A. The effect of resistance exercise on sleep: A systematic review of randomized controlled trials. Sleep Med Rev. 2018;39:52–68. doi: 10.1016/j.smrv.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Irish L.A., Kline C.E., Rothenberger S.D., et al. A 24-hour approach to the study of health behaviors: Temporal relationships between waking health behaviors and sleep. Ann Behav Med. 2014;47:189–197. doi: 10.1007/s12160-013-9533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambiase M.J., Gabriel K.P., Kuller L.H., Matthews K.A. Temporal relationships between physical activity and sleep in older women. Med Sci Sports Exerc. 2013;45:2362–2368. doi: 10.1249/MSS.0b013e31829e4cea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline C.E. The bidirectional relationship between exercise and sleep: Implications for exercise adherence and sleep improvement. Am J Lifestyle Med. 2014;8:375–379. doi: 10.1177/1559827614544437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youngstedt S.D., Kline C.E. Epidemiology of exercise and sleep. Sleep Biol Rhythms. 2006;4:215–221. doi: 10.1111/j.1479-8425.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stutz J., Eiholzer R., Spengler C.M. Effects of evening exercise on sleep in healthy participants: A systematic review and meta-analysis. Sports Med. 2019;49:269–287. doi: 10.1007/s40279-018-1015-0. [DOI] [PubMed] [Google Scholar]
- 24.Park H., Suh B. Association between sleep quality and physical activity according to gender and shift work. J Sleep Res 2020;29:e12924. doi:10.1111/jsr.12924. [DOI] [PubMed]
- 25.Wennman H., Pietilä A., Rissanen H., et al. Gender, age and socioeconomic variation in 24-hour physical activity by wrist-worn accelerometers: The FinHealth 2017 Survey. Sci Rep. 2019;9:6534. doi: 10.1038/s41598-019-43007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonçalves H., Wehrmeister F.C., Assunção M.C.F., et al. Cohort profile update: The 1993 Pelotas (Brazil) birth cohort follow-up at 22 years. Int J Epidemiol. 2018;47 doi: 10.1093/ije/dyx249. 1389–90e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victora C.G., Hallal P.C., Araújo C.L., Menezes A.M., Wells J.C., Barros F.C. Cohort profile: The 1993 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2008;37:704–709. doi: 10.1093/ije/dym177. [DOI] [PubMed] [Google Scholar]
- 28.Gonçalves H., Assunção M.C., Wehrmeister F.C., et al. Cohort profile update: The 1993 Pelotas (Brazil) birth cohort follow-up visits in adolescence. Int J Epidemiol. 2014;43:1082–1088. doi: 10.1093/ije/dyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Hees VT, Fang Z, Zhao JH, Heywood J, Mirkes E, Sabia S. Raw accelerometer data analysis. Available at: https://cran.r-project.org/web/packages/GGIR/GGIR.pdf. [accessed 12.07.2019].
- 30.van Hees V.T., Fang Z., Langford J., et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. J Appl Physiol (1985) 2014;117:738–744. doi: 10.1152/japplphysiol.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Hees V.T., Sabia S., Anderson K.N., et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hees V.T., Sabia S., Jones S.E., et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8:12975. doi: 10.1038/s41598-018-31266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva I.C., van Hees V.T., Ramires V.V., et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43:1959–1968. doi: 10.1093/ije/dyu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildebrand M., van Hees V.T., Hansen B.H., Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 35.Demographic and Health Surveys (DHS). Wealth index construction. Available at: https://www.dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm. [accessed 17.12.2019].
- 36.Schneider B.C., Motta J.V., Muniz L.C., et al. Design of a digital and self-reported food frequency questionnaire to estimate food consumption in adolescents and young adults: Birth cohorts at Pelotas, Rio Grande do Sul, Brazil (Desenho de um questionário de frequência alimentar digital autoaplicado para avaliar o consumo alimentar de adolescentes e adultos jovens: coortes de nascimentos de Pelotas, Rio Grande do Sul) Rev Bras Epidemiol. 2016;19:419–432. doi: 10.1590/1980-5497201600020017. [in Brazilian] [DOI] [PubMed] [Google Scholar]
- 37.Mari J.J., Williams P. A validity study of a psychiatric screening questionnaire (SRQ-20) in primary care in the city of Sao Paulo. Br J Psychiatry. 1986;148:23–26. doi: 10.1192/bjp.148.1.23. [DOI] [PubMed] [Google Scholar]
- 38.Myllymäki T., Kyröläinen H., Savolainen K., et al. Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. J Sleep Res. 2011;20:146–153. doi: 10.1111/j.1365-2869.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 39.Uçar C., Özgöçer T., Yildiz S. Late-night exercise affects the autonomic nervous system activity but not the hypothalamo-pituitary-adrenal axis in the next morning. J Sports Med Phys Fitness. 2018;58:57–65. doi: 10.23736/S0022-4707.16.06766-9. [DOI] [PubMed] [Google Scholar]
- 40.Hasan J., Urponen H., Vuori I., Partinen M. Exercise habits and sleep in a middle-aged Finnish population. Acta Physiol Scand Suppl. 1988;574:33–35. [PubMed] [Google Scholar]
- 41.Vuori I., Urponen H., Hasan J., Partinen M. Epidemiology of exercise effects on sleep. Acta Physiol Scand Suppl. 1988;574:3–7. [PubMed] [Google Scholar]
- 42.Yu Q.C., Ma W.J., Zou Y.F., et al. Impact of evening exercise on college students' sleep quality (晚锻炼对大学生睡眠质量的影响) Zhonghua Yu Fang Yi Xue Za Zhi. 2013;47:542–546. [in Chinese] [PubMed] [Google Scholar]
- 43.Buxton O.M., Lee C.W., L'Hermite-Baleriaux M., Turek F.W., Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 44.Driver H.S., Taylor S.R. Exercise and sleep. Sleep Med Rev. 2000;4:387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 45.Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. The Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sallis J.F., Bull F., Guthold R., et al. Progress in physical activity over the Olympic quadrennium. The Lancet. 2016;388:1325–1336. doi: 10.1016/S0140-6736(16)30581-5. [DOI] [PubMed] [Google Scholar]
- 47.Schuch F.B., Vancampfort D., Firth J., et al. Physical activity and incident depression: A meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175:631–648. doi: 10.1176/appi.ajp.2018.17111194. [DOI] [PubMed] [Google Scholar]
- 48.Orme M., Wijndaele K., Sharp S.J., Westgate K., Ekelund U., Brage S. Combined influence of epoch length, cut-point and bout duration on accelerometry-derived physical activity. Int J Behav Nutr Phys Act. 2014;11:34. doi: 10.1186/1479-5868-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitano N., Tsunoda K., Kai Y., et al. Impact of leisure-time physical activity pattern on subjective sleep quality in Japanese workers: A 1-year follow-up study. Bull Phys Fitness Res Inst. 2017;115:15–22. [Google Scholar]
- 50.Paparrigopoulos T., Tzavara C., Theleritis C., Psarros C., Soldatos C., Tountas Y. Insomnia and its correlates in a representative sample of the Greek population. BMC Public Health. 2010;10:531. doi: 10.1186/1471-2458-10-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Štefan L., Krističević T., Sporiš G. The associations of self-reported physical fitness and physical activity with sleep quality in young adults: A population-based study. Ment Health Phys Act. 2018;14:131–135. [Google Scholar]
- 52.Mead M.P., Baron K., Sorby M., Irish L.A. Daily associations between sleep and physical activity. Int J Behav Med. 2019;26:562–568. doi: 10.1007/s12529-019-09810-6. [DOI] [PubMed] [Google Scholar]
- 53.Martin J.L., Hakim A.D. Wrist actigraphy. Chest. 2011;139:1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


