Abstract
Hypertension‐related death is the leading cause of mortality worldwide, making blood pressure (BP) control an important issue. Salt substitute is a non‐pharmaceutical strategy to improve hypertension control. The goal of this study was to evaluate the effect of salt substitute on BP and cardiovascular disease. The authors searched the Cochrane Library and PubMed databases through March 2022, and assessed the risk‐of‐bias for included studies by the Cochrane risk‐of‐bias tool. Twenty‐three randomized controlled trials with 32073 patients were included in our systematic review. A meta‐analysis with random effects was performed to analyze the effects of salt substitute on systolic and diastolic BP, 24‐h urinary sodium and potassium, and cardiovascular and all‐cause mortality. In the random‐effects model, participants consuming salt substitute showed significant reduction in systolic BP (mean difference (MD) −4.80 mmHg, 95% confidence interval (CI) −6.12 to −3.48, P < 0.0001) and diastolic BP (MD −1.48 mmHg, 95% CI −2.06 to −0.90, P < 0.0001) compared with participants consuming normal salt. In the urine electrolyte analysis, the salt substitute group had significant reduction in 24‐h urine sodium (MD −22.96 mmol/24‐h, P = 0.0001) and significant elevation in 24‐h urine potassium (MD 14.41 mmol/24‐h, P < 0.0001). Of the five studies with mortality outcome data, salt substitute significantly reduced all‐cause mortality (hazard ratio 0.88, P = 0.0003). In conclusion, our analyses showed that salt substitute has a strong effect on lowering BP and reducing all‐cause mortality. By modifying the daily diet with salt substitute, the authors can improve BP control by using this non‐pharmaceutical management.
Keywords: Salt substitute, Blood pressure, Hypertension, Cardiovascular outcomes, Meta‐analysis
1. INTRODUCTION
Hypertension is a serious medical concern worldwide. It is well‐established that hypertension is one of the most leading causes of premature morbidity and mortality globally. In 2019, the prevalence of hypertension among adults aged 30–79 years was 33.1%, or nearly 1.25 billion people. 1 Poor blood pressure (BP) control can have sequelae of cardiovascular disease as well as fatal events such as ischemic heart disease and stroke, which were both among the top 10 global causes of death in 2019. 2 Therefore, BP control is a high priority clinically, and exerts a large burden on the healthcare system.
Among the multiple factors that affect BP control, dietary sodium intake is a substantial factor in individuals with or without hypertension. The relationship between a salt‐restricted diet and improved BP control has been widely recognized and confirmed by several studies, 3 , 4 and has attracted recent attention. There is also a linear dose‐response relationship between reduced sodium consumption and change in systolic BP. 5 Normally, diet is the main source of sodium, including the addition of salt while cooking or eating processed food. The World Health Organization (WHO) recommends reducing sodium consumption to < 2 g/day (equivalent to 5 g/day salt) in adults to improve health. 6 However, one study also reported that daily salt consumption levels in most countries greatly exceeded the WHO recommendations. 7 A recent Global Burden of Disease study reported that excessive intake of sodium was the leading dietary risk factor for mortality, accounting for 3.20 million deaths globally in 2017, 8 and argued that there is an urgent need to reformulate strategies to reduce sodium intake.
Salt substitutes replace sodium chloride with other substances, such as potassium chloride. Although decreased sodium intake is a recognized approach to reduce BP, the impact of salt substitutes on BP is still poorly understood and lacks data from systematic reviews. Moreover, dietary habits and cooking methods in Asian and Western countries are quite different, which may contribute to variations in mean sodium consumption. In addition, body mass index can also affect BP when consuming a high‐salt diet. 9 Nevertheless, the susceptibility to change in BP due to equal amounts of sodium intake (dose‐response effect) between Asian and Western countries remains unclear.
Therefore, the aim of this study was to investigate the effect of salt substitutes on BP and cardiovascular disease, and to compare the heterogeneity of these effects among countries/regions. By age and region, the proportion of all cardiovascular deaths attributed to sodium intake > 2.0 g per day was highest in East Asia and Southeast Asia, and this trend was apparent in adults whether they were older or younger than 70 years old. 5 This indicates that improved control of BP is an important issue for people living in these regions. Salt substitution is a non‐pharmaceutical and low‐cost intervention strategy to control BP, thereby decreasing the prevalence of cardiovascular disease. Unlike salt restriction, salt substitute is a more practical and enduring strategy to reduce sodium consumption and can result in more satisfactory adherence. Through the present rigorous review of relevant studies, the effectiveness of salt substitutes as an antihypertensive strategy can be comprehensively investigated.
2. METHODS
2.1. Protocol and registration
We conducted this systematic review and meta‐analysis by adhering to the Preferred Reporting Items for Systemic Review and Meta‐Analysis Statement (PRISMA) 2020 guidelines (Tables S1 and S2). 10 Due to the COVID‐19 pandemic, the International Prospective Register of Systematic Reviews (PROSPERO) failed to provide us with a registration number.
2.2. Eligibility criteria
2.2.1. Types of studies
Randomized controlled trials (RCT) or the followed‐up observational study of the original RCT satisfied the eligibility criteria. Cohort studies, retrospective studies, or case reports were excluded.
2.2.2. Types of participants
We did not exclude studies with patients of any specific age range, sex or baseline health status.
2.2.3. Types of intervention
We included trials investigating the effects of salt substitute, which contains a lower proportion of sodium in salt. Studies which had multiple interventions besides salt substitute were also eligible if salt substitute was one of the experimental factors.
2.2.4. Types of outcomes
We included studies investigating the effect of salt substitute on either peripheral blood pressure, urinary excretion of sodium and potassium from spot urine or 24‐h urine samples, or the mortality of cardiovascular diseases. Studies that did not report the outcome of interest mentioned above were excluded.
2.3. Data sources and search strategy
For study selection, the online databases of PubMed and the Cochrane Library were reviewed until March 2022. The search terms were defined as (salt substitute OR salt substitution OR low sodium substitute OR low sodium salt OR high potassium substitute OR potassium‐enriched salt OR smart salt OR mineral salt OR salt replacement) AND (blood pressure OR hypertension OR cardiovascular disease).
After removing duplicate studies, two reviewers (Y.C.T. and Y.P.T.) independently screened titles and abstracts. Full text was retrieved for further assessment. The reviewers independently assessed the review papers according to the inclusion and exclusion criteria.
2.4. Data extraction and quality evaluation
Three authors independently reviewed the review papers for data extraction. Each review paper was reviewed by at least two authors for quality assessment. The extracted components from eligible review papers were as follows: (1) the first author's name with year of publication, (2) study design, (3) country of the study, (4) number of participants in the intervention and control groups, (5) age and sex of the participants, (6) duration of follow‐up, (7) components of salt substitute, (8) underlying diseases with hypertension, diabetes mellitus, cardiovascular disease, or stroke, (9) BP, and (10) 24‐h urine or spot urine levels of sodium and potassium. For the evaluation of study quality, the Cochrane risk‐of‐bias tool for randomized trials (RoB) was implemented for assessment of study quality by reviewers. Discrepancies were resolved through discussion with a fourth reviewer (HMC).
2.5. Data synthesis and analysis
The primary outcome was the mean difference with 95% confidence intervals between the salt substitute group and the control group for both systolic and diastolic BP. The retrieved data were processed using Review Manager (Version 5.4, Cochrane Collaboration) from the Cochrane Collaboration. Statistical significance was set at P < 0.05. Meta‐analysis with a random‐effects model was employed to integrate between‐group mean differences of change in SBP, DBP, urinary sodium and potassium levels, and the most‐adjusted hazard ratios (HRs) of cardiovascular death and all‐cause mortality, using RevMan 5.4. Subgroup analysis was performed according to age, BP status, baseline SBP, year of publication, region, sample size, and study follow‐up period.
The heterogeneity among the included studies was evaluated using the Cochrane Q‐test, with a significance level of 0.05, and I2 statistics, where an I2 > 60% was considered highly heterogenous. 11 For outcomes integrated from more than eight studies, publication bias was assessed by producing a funnel plot and conducting Egger's test with the Comprehensive Meta‐Analysis software package (version 2.2.064; Biostat, Englewood, NJ, USA) to examine the symmetry of the funnel plot. 12
3. RESULTS
3.1. Literature search and selection
The initial search retrieved 1632 review papers. We first excluded 376 duplicate studies, and then screened the remaining 1256 studies based on titles and abstracts to exclude review papers on non‐relevant topics. From this step, an additional 1176 review papers were excluded. Two more review papers were added based on recommendations from experienced senior authors and the reference lists of the included studies, because these studies were eligible for our research topic. After further reviewing the 82 potentially relevant review papers, we identified 23 studies that met the inclusion criteria for further systematic review and meta‐analysis. 9 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 A diagram of the literature screening process is shown in Figure 1.
FIGURE 1.
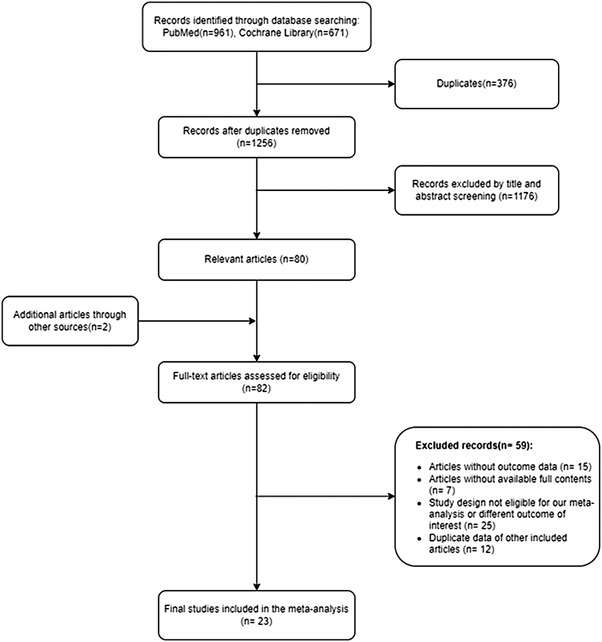
PRISMA flow diagram
3.2. Characteristics
Detailed characteristics of the included RCTs are shown in Table 1. A total of 32073 participants were included: 52.8% were male and 47.2% were female. The review papers were published between 1988 and 2022 and the main clinical medical condition was hypertension. Among these studies, 10 were conducted in China, two in the United Kingdom, one in Taiwan, one in Japan, one in Korea, one in Brazil, one in Finland, one in South Africa, one in the Netherlands, one in Italy, one in India, one in Peru, and one in Norway. The intervention in these trials all included salt substitute that adjusted the proportion of electrolytes to reduce the proportion of sodium in salt. The most common replacement for sodium chloride was potassium chloride (KCl). The follow‐up duration ranged from 4 weeks to 10 years.
TABLE 1.
Characteristics of included clinical trials
| Study | References | Design of study | Country | Number of patients in intervention group (IG) | Number of patients in control group (CG) | Male proportion | Mean age (years) | Salt type of salt substitute | Salt type of control group | Baseline SBP a /DBP b in the intervention group (mmHg) | Follow‐up duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baek and coworkers (2015) | 13 | Preliminary randomized, double‐blind clinical trial | Korea | 18 | 20 | 36.8% |
IG:79.5 CG:80.0 |
85.03% NaCl c , 1495 ppm calcium, 2494 ppm potassium, 8190 ppm magnesium |
99.8% NaCl | 127.5/ 74 | 8 weeks |
| Barros and coworkers (2015) | 14 | Single‐blind randomized controlled trial | Brazil | 19 | 16 | 34.3% | 55.5 |
130 mg of sodium, 346 mg of potassium, 44 mcg of iodine (per gram salt) |
390 mg of sodium, 25 mcg of iodine (per gram salt) |
134.47/ 77.95 | 4 weeks |
| Bernabe‐Ortiz and coworkers (2020) | 15 | Stepped‐wedge cluster randomized trial | Peru | Not Applicable | Not Applicable | 49.6% | 43.3 |
75% NaCl, 25% KCl d |
Common salt | Not Applicable | 3 years |
| Chang and coworkers (2006) | 16 | Randomized trial | Taiwan | 768 | 1213 | 100% |
IG:74.8 CG:74.9 |
49% NaCl, 49% KCl, 2% other additives |
99.6% NaCl, 0.4% other additives |
131.3/ 71.2 | Average 31 months |
| Charlton and coworkers (2008) | 17 | A double‐blind randomized controlled trial | South Africa | 40 | 40 | 16.3% |
IG:61.8 CG:60.4 |
SOLO™ 41% less Na, 826% more K, 388% more Ca, 368% more Mg |
Standard commercial composition and 500 ml/d of artificially sweetened cool drink | 133.9/ 79.8 | 8 weeks |
| Che and coworkers (2022) | 18 | Prospective, multicenter, randomized, double‐blind study | China | 162 | 160 | 40.4% |
IG:62.96 CG:62.17 |
43% NaCl, 32% KCl, 25% other ingredients |
100% NaCl |
OBPM e :135.32/77.61 HBPM f :130.13/ 78.06 |
12 months |
| Geleijnse and coworkers (1994) | 19 | Randomized double blind placebo controlled trial | Netherlands | 49 | 51 | 51% |
IG:65.7 CG:67.1 |
41% NaCl, 41% KCl, 17% magnesium salts, 1% trace minerals |
100% NaCl | 158/ 89.8 | 24 weeks |
| Gilleran and coworkers (1996) | 20 | A randomized blind controlled parallel study | United Kingdom | 20 | 20 | 60% |
IG:62.5 CG:59.2 |
50% NaCl, 40% KCl, 10% MgSO₄ g |
100% NaCl | 163.2/ 91.2 | 9 months |
| Hu and coworkers (2018) | 21 | Randomized double–blind controlled trial | China | 297 | 296 | 47.4% |
Hypertensive patients IG:57.1 CG:57.6 Family members IG:45.5 CG:45.7 |
65% NaCl, 25% KCl, 10% MgSO₄ |
100% NaCl |
Hypertensive patients: 139.9/ 81.9 Family members: 124.1/ 75.9 |
12 months |
| Kawasaki and coworkers (1998) | 22 | Parallel controlled clinical trial | Japan | 21 | 20 | 48.8% |
IG:65.9 CG:65.8 |
22.9 g sodium, 10.11 g potassium, 1.24 g magnesium per 100 g mineral salt |
39 g sodium, 0.13 g potassium, 0.021 g magnesium per 100 g regular salt |
134.7/ 77.2 | 5 weeks |
| Li and coworkers (2007) | 23 | Double‐blind, randomized, controlled trial | China | 306 | 302 | 44.1% |
IG:59 CG:61 |
65% NaCl, 25% KCl, 10% MgSO4 |
100%NaCl | 159/ 93 | 12 months |
| Li and coworkers (2016) | 24 | A cluster‐randomized trial | China | 1294 | 1272 | 50% |
IG:55 CG:55 |
Reduced‐sodium, added‐potassium | Usual salt | Not mentioned | 18 months |
| Little and coworkers (2004) | 25 | Randomized controlled factorial trial | United Kingdom | 138 | 158 | 55.7% | 55 | Low sodium, high potassium salt | Normal salt | 154/ 94 | 6 months |
| Neal and coworkers (2021) | 26 | Open‐label, cluster‐randomized trial | China | 10505 | 10491 | 50.5% |
IG:65.2 CG:65.5 |
75%NaCl, 25%KCl |
100% NaCl | 153.8/ 89.1 | 5 years |
| Omvik and coworkers (1995) | 27 | Parallel, comparative design, randomized in a double‐blind manner | Norway | 20 | 20 | 67.5% |
IG:45.9 CG:42.7 |
57% NaCl, 28% KCI, 12% MgSO₄ |
Standard sodium chloride | 164/102 | 6 months |
| Sarkkinen and coworkers (2011) | 28 | A randomized, double‐blind, placebo‐controlled study | Finland | 22 | 23 | 51.1% |
IG:57 CG:54 |
50%NaCl, 25%KCl, 25% magnesium ammonium potassium chloride |
100%NaCl | 140/ 89 | 8 weeks |
| Sun and coworkers (2021) | 29 | An exploratory follow‐up of a randomized controlled trial | China | 209 | 219 | 49.1% |
IG:45.4 CG:46.8 |
65% NaCl, 25% KCl, 10% MgSO₄ |
100% NaCl | 154.6/ 92 | 10 years |
| Suppa and coworkers (1988) | 30 | Double blinded randomized controlled trial | Italy | 163 | 159 | 38.8% |
IG:47.1 CG:47.8 |
NaCl 25%, KCl 25%, Potassium citrate 15% |
100% NaCl | 149.2/ 93.5 | 4 weeks |
| Yang and coworkers (2018) | 31 | Single blind, randomized, controlled trial | China | 62 | 64 | 42.9% |
ISH h IG:67.8 CG:65.9 NISH i IG:67.3 CG:65.4 |
65% NaCl, 30% KCl, 5% calcium salts |
Normal salt |
ISH: 161/ 80.6 NISH: 159/ 85 |
6 months |
| Yu and coworkers (2021) | 32 |
Double‐blind randomized controlled trial |
India | 242 | 234 | 43.5% |
IG:61.5 CG:61.7 |
70% NaCl, 30% KCl |
100% NaCl | 132.8/ 83.7 | 3 months |
| Zhao and coworkers (2014) | 33 | A patient‐blinded randomized controlled trial | China | 141 | 141 | 41.1% |
IG:62.8 CG:63.5 |
65% NaCl, 25% KCl, 10% MgSO₄ |
100% NaCl | 176.1/ 103.2 | 3 months |
| Zhou and coworkers (2016) | 9 | A double‐blind, randomized controlled trial | China | 224 | 238 | 49.4% |
IG:45.63 CG:47.05 |
65% NaCl, 25% KCl, 10% MgSO₄ |
100% NaCl | 154.02/ 91.46 | 3 years |
| Zhou and coworkers (2009) | 34 | A single‐blind, randomized controlled trial | China | 119 | 129 | 44.8% |
Hypertensives IG:67.5 CG:65.7 Normotensives IG:68.1 CG:65.4 |
65% NaCl, 30% KCl, 5% calcium salts |
Normal salt |
Hypertensives: 159.7/ 83.3 Normotensives: 125/ 74.3 |
6 months |
Abbreviations: CG, control group; IG, intervention group.
SBP: Systolic blood pressure.
DBP: Diastolic blood pressure.
NaCl: Sodium chloride.
KCl: Potassium chloride.
OBPM: Office blood pressure measurement.
HBPM: Home blood pressure measurement.
MgSO₄: Magnesium sulfate.
ISH: Isolated systolic hypertension.
NISH: Non‐isolated systolic hypertension.
3.3. Study quality
Assessment of the risk‐of‐bias was shown in Figures S1 and S2. The overall risk‐of‐bias was low for all included RCTs. Bias was appraised as low risk, unclear risk, high risk, which accounted for 58.8%, 27.1%, 14.1%, respectively.
3.4. Primary outcomes
Among the included studies, 21 trials reported changes in BP after intervention. Using a random‐effects model, the results of salt substitution on reducing systolic BP were significantly better than those of the control group (mean difference (MD) −4.80 mm Hg (95% confidence interval (CI) −6.12 to −3.48, P < 0.0001). Compared with the control group, diastolic BP also decreased significantly in the salt substitute group (MD −1.48 mm Hg, 95% CI −2.06 to −0.90, P < 0.0001). Comprehensive primary outcomes are shown in Figure 2.
FIGURE 2.
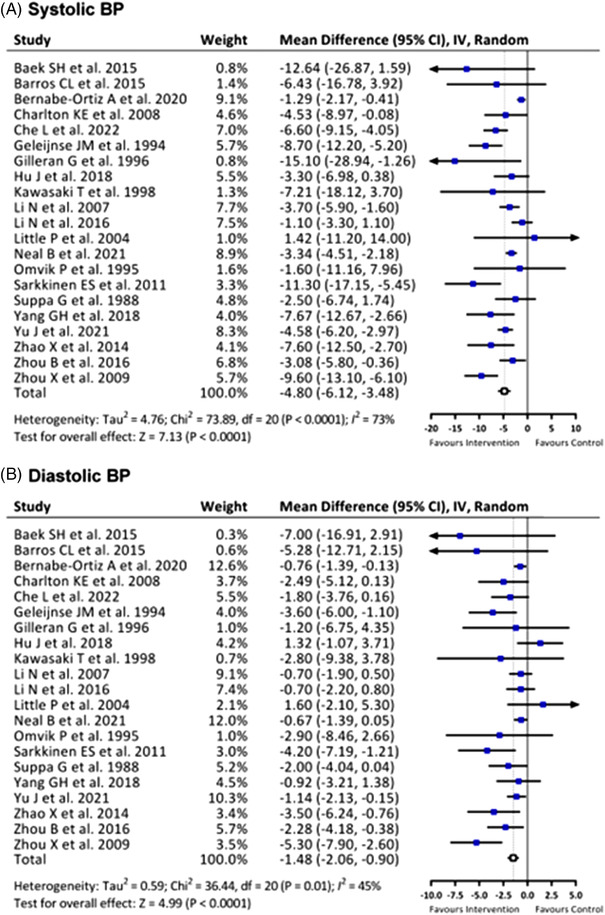
Effects of salt substitute on (A) systolic blood pressure (BP) and (B) diastolic BP
3.5. Secondary outcomes
3.5.1. Urinary excretion of sodium and potassium
Differences in urine sodium and potassium excretion and the urine Na/K ratio between the two groups are summarized in Figures 3 and S3. Although most of the review papers collected 24‐h urine samples, several review papers only obtained the first morning spot urine samples from participants. Compared with the control group, the intervention group showed significant reduction in 24‐h urine sodium excretion (MD −22.96 mmol/24‐h, 95% CI −34.67 to −11.26 mmol/24‐h, P = 0.0001); however, there was no significant difference in spot urine sodium concentration (MD −5.62 mmol/L, 95% CI −28.71 to 17.47 mmol/L, P = 0.63). For potassium excretion, the salt substitute group showed significant elevation in both 24‐h and spot urine potassium excretion (24‐h urine K: MD 14.41 mmol/24‐h, 95% confidence interval 10.26 to 18.56; P < 0.0001; spot urine K:7.51 mmol/L, 95% CI 4.31 to 10.71, P < 0.0001). In general, we found significant reduction in 24‐h urine Na/K ratio between the two groups (MD −0.63, 95% confidence interval −1.00 to −0.26, P = 0.0008), and the spot urine Na/K ratio was also significantly reduced in the salt substitute group (MD −1.07, 95% CI −1.72 to −0.42, P = 0.001). The results above were correspond with the phenomenon that salt substitute changed the dietary intake with more potassium but less sodium than normal salt for participants of these trials.
FIGURE 3.
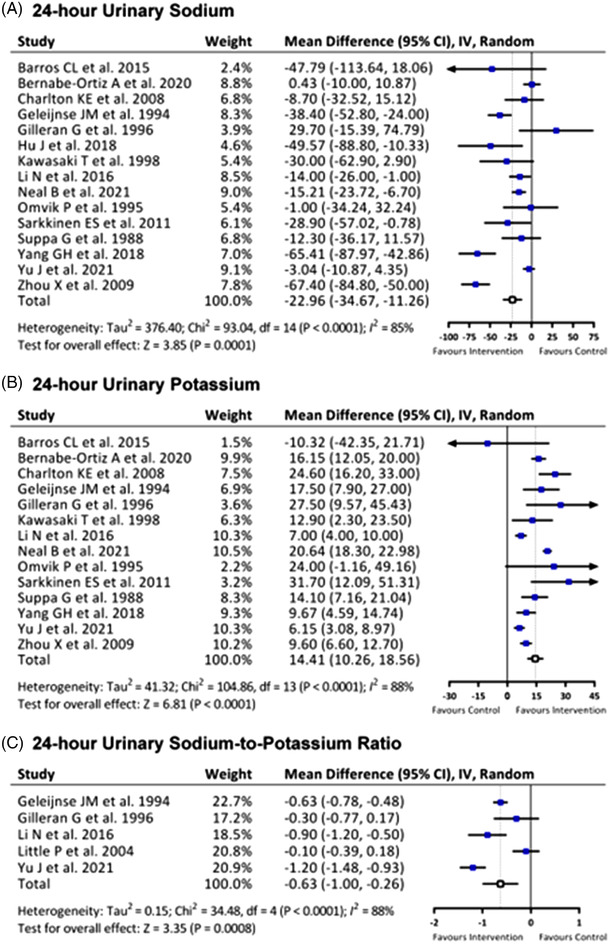
Effects of salt substitute on (A) 24‐h urinary sodium, (B) 24‐h urinary potassium, and (C) 24‐h urinary sodium‐to‐potassium ratio
3.5.2. Mortality
Five review papers reported the prevalence of mortality. As shown in Figure 4, compared with the control group, the salt substitute group showed significant reduction in all‐cause death (HR 0.88, 95% CI 0.82 to 0.94; P = 0.0003). Replacing normal salt with salt substitute also trended toward reducing cardiovascular death, but it did not achieve statistical significance (HR 0.72, 95% CI 0.52 to 1.00, P = 0.05).
FIGURE 4.
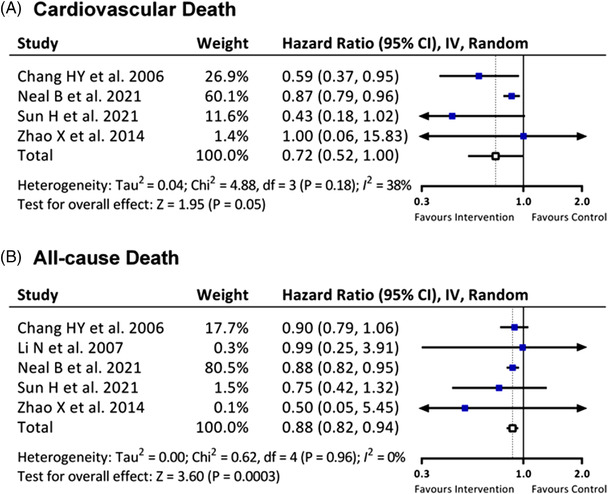
Effects of salt substitute on (A) cardiovascular death and (B) all‐cause death
3.6. Adverse effect of hyperkalemia
Because the proportion of potassium in the salt substitute is usually higher than that in the normal salt, it is a substantial issue whether consuming salt substitute causes hyperkalemia or not. Five included studies provided the data of follow‐up serum potassium level. As shown in Figure 5, using salt substitute increased the serum potassium by .1 mmol/L, but it did not achieve significant elevation in serum potassium level (MD 0.10, 95% CI −0.02 to 0.23, P = 0.10).
FIGURE 5.
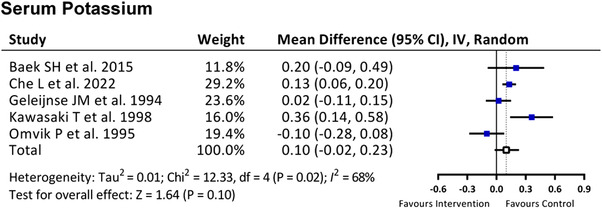
Effect of salt substitute on serum potassium level
3.7. Assessment of publication bias
The Egger's regression test showed a significant asymmetry in the funnel plot for systolic and diastolic BPs (P < 0.05, Table S3). Nevertheless, their effect sizes were not affected by potential publication bias through the trim and fill analysis (adjusted pooled MD −4.65, 95% CI −5.97 to −3.34 for systolic BP; MD −0.95, 95% CI −1.60 to −0.30 for diastolic BP; Figure S4).
3.8. Subgroup analysis
As shown in Figure 6, to thoroughly assess the effect of taking salt substitute on both systolic BP and diastolic BP in different subgroups, we further examined the primary outcome by subgroup analysis based on several factors that may have affected long‐term BP control, such as sample size, mean baseline systolic BP level, mean age of participants, and duration of follow‐up.
FIGURE 6.
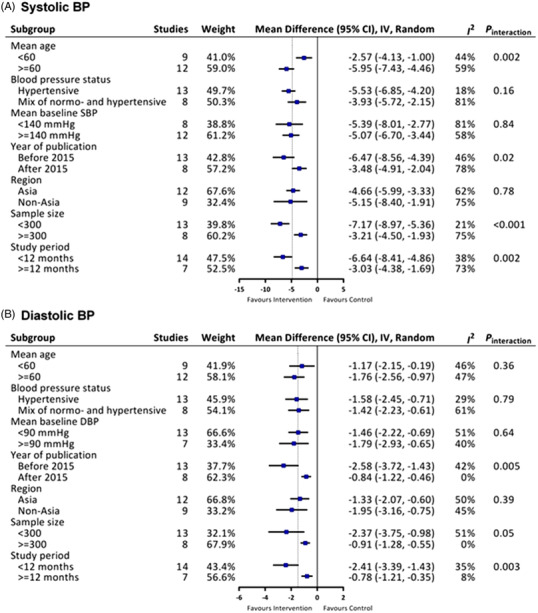
Subgroup analyses for the effects of salt substitute on (A) systolic BP and (B) diastolic BP
In general, subgroup analyses did not show effect modification by the above study characteristics, with the exception that salt substitute had a more pronounced effect on systolic BP in elderly populations aged >= 60 years, and there was a higher reduction in systolic BP in studies with a smaller sample size (< 300 participants).
3.9. Geographical location of the study area
As the main source of sodium comes from our diet, we are curious whether different eating habits and salt sensitivity of different ethnicity affect the effects of salt substitute on BP in different regions or not. Therefore, we analyzed the association between the BP reduction and geographical location in a subgroup analysis stratified by study region (Figure 6). However, we found that the effects of salt substitution on systolic BP and diastolic BP were comparable between Asian and non‐Asia countries. It didn't show significant difference between different geographical location of the study area.
4. DISCUSSION
4.1. Summary of main results
This systematic review for the effects of salt substitutes on BP and cardiovascular outcomes included 23 studies with 32073 participants. Primary outcome analyses showed that replacing common salt with a low‐sodium salt substitute when preparing food could reduce daily intake of sodium, and significantly lower BP and 24‐h sodium excretion. Furthermore, salt substitution significantly reduced all‐cause mortality by 12%.
4.2. Quality of the evidence
Most trials included were of moderate‐to‐high methodological quality. Insufficient explanation regarding the allocation concealment were the most common concerns for determining unclear or high risk‐of‐bias. Most of the evidence in this review was retrieved from trials with adequate blinding process. However, the first two largest RCT by Neal and coworkers (2021) 26 and Li and coworkers (2016) 24 were unblinded to the participants and caregivers, which may have led to performance bias and influence on outcomes. Barros and coworkers (2015) 14 was appraised as high risk‐of‐bias in random sequence generation due to non‐strict method of randomization.
The heterogeneity for systolic BP reduction from our analysis is very high. We believed this to be attributed to the following reasons. First, as mentioned above, the lack of blinding process can have an impact on blood pressure control. Besides, follow‐up duration and the numbers of recruited participants were varied in our included trials, and there were also different types of salt substitute among these trials. Furthermore, subgroup analysis of age demonstrated a lower heterogeneity and significant systolic BP reduction in the elderly patients (Figure 6). In summary, we suggested that the high heterogeneity of systolic BP reduction was partly related to the different characteristics of included studies.
Trials that sought to determine the effect of salt substitution on 24‐h urinary electrolyte excretion also encountered the common problem of unavailability of a portion of urinary data, mostly due to incomplete collection of urine samples or missing voids. This may have contributed to the attrition bias regarding our secondary outcome. We reviewed the previous studies for the feasibility of estimating 24‐h urine sodium excretion by spot urine sodium data. However, spot urinary measurements of sodium had been shown to either overestimate or underestimate the natriuresis occurring over the same 24‐h period. 35 Xu and coworkers found that using the most widely applied formulas such as Kawasaki method, INTERSALT method, and Tanaka method were all inadequate to estimate 24‐h urine sodium excretion by spot urine sodium at the Chinese population level. 36 As a result, for studies only provided spot urine samples, we directly performed the analysis by using spot urine sodium and potassium without estimating the 24‐h urine electrolyte level to avoid falsely assessing the sodium excretion. Although the spot urine electrolyte concentration cannot reflect the daily electrolyte excretion, it's worth noting that Xu and coworkers proposed that the spot urine Na/K ratio may be an alternative way to evaluate the daily urine N/K ratio. 36 This was compatible with the finding that the Na/K ratio were significantly lower in the salt substitute group either in 24‐h urine or spot urine data in our study.
4.3. Effect of salt reduction by means of using salt substitute on cardiovascular risk
Studies have shown that lower salt intake is associated with a reduced risk of cardiovascular disease. Several developed countries such as Finland and the UK have integrated salt intake reduction into health promotion policies, which has successfully contributed to decreasing the incidence of hypertension and deaths from ischemic heart disease and stroke. 37 According to the meta‐analysis published in 2021, a 5 mm Hg systolic BP reduction could lower the risk of major cardiovascular event by about 10%. 38 As a consequence, we estimated the cardiovascular risk rendered by salt substitute was around 9.6% based on the results of our meta‐analysis of SBP reduction 4.8 mm Hg. In conclusion, applying salt substitute in daily life to achieve better BP control is an effective tool for preventing cardiovascular disease.
Our findings address several unanswered questions regarding the role of salt substitute use and have important clinical implications. First, we provide the most complete documentation to‐date that suggests a significant decrease in BP in both hypertensive and non‐hypertensive individuals through salt substitution. This intervention could be considered a vital method for primary prevention of this highly prevalent disease. Second, although it is predictable that lowering BP by salt substitute could have an impact on hypertensive‐related cardiovascular outcomes, only a few studies have reported this potential benefit. Our study revealed that salt substitution contributed to 12% reduction in all‐cause mortality.
4.4. Application of salt substitute in Asian populations
The results of this study may have considerable impact on Asian populations for several reasons. First, Asian populations are influenced more by the salt consumption than Western populations. Previous studies have shown that Asian populations have a higher salt intake than Western populations due to dietary habits such as salted fermented foods. 39
Second, salt sensitivity is believed to play a role. This effect is associated with a variety of physiological, genetic and environmental factors which made salt‐sensitive population more likely to benefit from salt depletion than the salt‐resistant population. 40 Previous data have shown that Asian and African Americans had higher salt sensitivity than Caucasians due to genetic predisposition. 39 , 41 However, the result of our subgroup analysis revealed the amplitude of BP reduction in Asian group was not significantly higher than that in the non‐Asian group. We believed this phenomenon to be attributed to the nature that the participants who were recruited from specific geographical location in each trial may share similar genetic traits, which made it difficult to differentiate between salt‐sensitive and salt‐resistant individuals through genotype studies. Further real‐world research may be needed to investigate if Asian people could have better response in BP reduction to salt substitute than other races.
Furthermore, the importance of BP control is worthy of attention in Asians than in Western countries. One reason for this is that stroke, which is more highly related to hypertension than coronary artery disease, is more common in Asian countries than in Western countries. Another reason is that data from the Asia Pacific Cohort Studies Collaboration show that the association between BP and the risk of cardiovascular disease shows a stronger correlation in Asian populations than other populations such as Australia and New Zealand. 42 Besides, previous studies have also shown that awareness, treatment adherence, and risk factor reduction for hypertension are still lower in Asia than in Western countries, despite efforts made by health professionals. 43
Lastly, researchers have proposed that elderly people manifest a higher BP response to change in sodium intake. 44 , 45 , 46 It was compatible with our subgroup analysis that the systolic BP lowering effect was significantly stronger in the elderly populations aged more than 60 years. According to the World Population Prospects 2019 launched by the United Nations, 47 the countries in East and North‐east Asia have been faced with an unprecedented rate of population aging. Our study supports the implication that salt substitution is worthy of promotion and should attract more attention in Asian populations now and in the foreseeable future.
4.5. Strengths and limitations
To the best of our knowledge, this study is the largest meta‐analysis to investigate the impact of salt substitution on the hypertension control and major cardiovascular outcomes. This study showed that salt substitution could decrease systolic and diastolic BP, which would substantially ameliorate major cardiovascular outcomes. This indicates that salt substitutes can provide a similar benefit to salt reduction, but hardly change the taste of food. 48
Our study has several limitations: First, given that our systematic review included a variety of clinical trials, some undetected confounding factors may be present, especially different regimens of salt substitution and intervention durations. Second, although salt substitution is feasible for reducing hypertension, it may be harmful in patients with chronic kidney disease or tubular acidosis. Considering the significant increase in urinary potassium levels, patients with reduced glomerular filtration rates may be at risk of severe hyperkalemia under dietary interventions with a salt substitute. Participants in these trials excluded patients with renal insufficiency. Proper patient selection will be essential to further clinical implementation. Third, the enrolled patients in the included studies may differ from real‐world patients due to the lack of major comorbidities such as diabetes mellitus, renal failure, or cerebrovascular accident, which are known to be hypertension‐related diseases. Future real‐world implementation would provide stronger evidence of the benefit of salt substitution in patients with hypertension‐related comorbidities. Based on our systematic review, salt substitution should be promoted as an effective intervention to reduce the incidence of hypertension and hypertension‐related complications. Among Asian populations with salt‐sensitive genetic backgrounds and high dietary sodium intake, healthcare policies that encourage salt substitution could be effective tools to reduce hypertension and hypertension‐related complications.
5. CONCLUSIONS
In conclusion, our analyses showed that salt substitutes have a strong effect on lowering BP and reducing all‐cause mortality. By modifying the daily diet with salt substitutes, we can improve BP control by using this non‐pharmaceutical management method.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
Grants from the Ministry of Health and Welfare (MOHW104‐TDU‐B‐211‐113‐003, MOHW106‐TDU‐B‐211‐113001), an intramural grant from National Yang Ming Chiao Tung University (E107F‐M01‐0501), and Ministry of Science and Technology (MOST 106‐2314‐B‐075 ‐051 ‐MY3, MOST 109‐2314‐B‐010‐061 ‐, MOST 110‐2634‐F‐A49‐005). This work is particularly supported by “Yin Yen‐Liang Foundation Development and Construction Plan” of the School of Medicine, National Yang Ming Chiao Tung University.
Tsai Y‐C, Tsao Y‐P, Huang C‐J, et al. Effectiveness of salt substitute on cardiovascular outcomes: A systematic review and meta‐analysis. J Clin Hypertens. 2022;24:1147–1160. 10.1111/jch.14562
Yi‐Ching Tsai and Yen‐Po Tsao contributed equally.
REFERENCES
- 1. Zhou B, Carrillo‐Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet North Am Ed. 2021;398(10304):957‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH . Global health estimates: life expectancy and leading causes of death and disability. World Health Organization. 2020. [Google Scholar]
- 3. Graudal NA, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011;. 11:CD004022. [DOI] [PubMed] [Google Scholar]
- 4. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta‐analysis of randomised trials. BMJ. 2020;368:m315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624‐634. [DOI] [PubMed] [Google Scholar]
- 6. Organization WH. Guideline: sodium intake for adults and children. World Health Organization; 2012. [PubMed] [Google Scholar]
- 7. Thout SR, Santos JA, McKenzie B, et al. The science of salt: updating the evidence on global estimates of salt intake. J Clin Hypertens (Greenwich). 2019;21(6):710‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collaborators GBDRF . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou B, Webster J, Fu LY, et al. Intake of low sodium salt substitute for 3years attenuates the increase in blood pressure in a rural population of North China ‐ a randomized controlled trial. Int J Cardiol. 2016;215:377‐382. [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 12. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baek SH, Ahn JW, Lee H‐R, Cho SH, Kim J‐H. Anti‐hypertensive effect of a solar salt diet in elderly hypertensive patients: a preliminary randomized, double‐blind clinical trial. Korean J Health Promot. 2015;15(3):98‐107. [Google Scholar]
- 14. Barros CL, Sousa AL, Chinem BM, et al. Impact of light salt substitution for regular salt on blood pressure of hypertensive patients. Arq Bras Cardiol. 2015;104(2):128‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernabe‐Ortiz A, Sal YRVG, Ponce‐Lucero V, et al. Effect of salt substitution on community‐wide blood pressure and hypertension incidence. Nat Med. 2020;26(3):374‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang HY, Hu YW, Yue CS, et al. Effect of potassium‐enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83(6):1289‐1296. [DOI] [PubMed] [Google Scholar]
- 17. Charlton KE, Steyn K, Levitt NS, et al. A food‐based dietary strategy lowers blood pressure in a low socio‐economic setting: a randomised study in South Africa. Public Health Nutr. 2008;11(12):1397‐1406. [DOI] [PubMed] [Google Scholar]
- 18. Che L, Song W, Zhang Y, Lu Y, Cheng Y, Jiang Y. A randomized, double‐blind clinical trial to evaluate the blood pressure lowing effect of low‐sodium salt substitution on middle‐aged and elderly hypertensive patients with different plasma renin concentrations. J Clin Hypertens (Greenwich). 2022;24(2):140‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geleijnse JM, Witteman JC, Bak AA, den Breeijen JH, Grobbee DE. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. BMJ. 1994;309(6952):436‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilleran G, O'Leary M, Bartlett WA, Vinall H, Jones AF, Dodson PM. Effects of dietary sodium substitution with potassium and magnesium in hypertensive type II diabetics: a randomised blind controlled parallel study. J Hum Hypertens. 1996;10(8):517‐521. [PubMed] [Google Scholar]
- 21. Hu J, Zhao L, Thompson B, Zhang Y, Wu Y. Effects of salt substitute on home blood pressure differs according to age and degree of blood pressure in hypertensive patients and their families. Clin Exp Hypertens. 2018;40(7):664‐672. [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki T, Itoh K, Kawasaki M. Reduction in blood pressure with a sodium‐reduced, potassium‐ and magnesium‐enriched mineral salt in subjects with mild essential hypertension. Hypertens Res. 1998;21(4):235‐243. [DOI] [PubMed] [Google Scholar]
- 23. China Salt Substitute Study Collaborative G . Salt substitution: a low‐cost strategy for blood pressure control among rural Chinese. A randomized, controlled trial. J Hypertens. 2007;25(10):2011‐2018. [DOI] [PubMed] [Google Scholar]
- 24. Li N, Yan LL, Niu W, et al. The effects of a community‐based sodium reduction program in rural china ‐ a cluster‐randomized trial. PLoS One. 2016;11(12):e0166620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ. 2004;328(7447):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385(12):1067‐1077. [DOI] [PubMed] [Google Scholar]
- 27. Omvik P, Myking OL. Unchanged central hemodynamics after six months of moderate sodium restriction with or without potassium supplement in essential hypertension. Blood Press. 1995;4(1):32‐41. [DOI] [PubMed] [Google Scholar]
- 28. Sarkkinen ES, Kastarinen MJ, Niskanen TH, et al. Feasibility and antihypertensive effect of replacing regular salt with mineral salt ‐rich in magnesium and potassium‐ in subjects with mildly elevated blood pressure. Nutr J. 2011;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun H, Ma B, Wu X, Wang H, Zhou B. Long‐term effect of salt substitute on all‐cause and cardiovascular disease mortality: an exploratory follow‐up of a randomized controlled trial. Front Cardiovasc Med. 2021;8:645902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suppa G, Pollavini G, Alberti D, Savonitto S. Effects of a low‐sodium high‐potassium salt in hypertensive patients treated with metoprolol: a multicentre study. J Hypertens. 1988;6(10):787‐790. [PubMed] [Google Scholar]
- 31. Yang GH, Zhou X, Ji WJ, et al. Effects of a low salt diet on isolated systolic hypertension: a community‐based population study. Medicine (Baltimore). 2018;97(14):e0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu J, Thout SR, Li Q, et al. Effects of a reduced‐sodium added‐potassium salt substitute on blood pressure in rural Indian hypertensive patients: a randomized, double‐blind, controlled trial. Am J Clin Nutr. 2021;114(1):185‐193. [DOI] [PubMed] [Google Scholar]
- 33. Zhao X, Yin X, Li X, et al. Using a low‐sodium, high‐potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient‐blinded randomized controlled trial. PLoS One. 2014;9(10):e110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou X, Liu JX, Shi R, et al. Compound ion salt, a novel low‐sodium salt substitute: from animal study to community‐based population trial. Am J Hypertens. 2009;22(9):934‐942. [DOI] [PubMed] [Google Scholar]
- 35. Naser AM, He FJ, Rahman M, Campbell NRC. Spot urine formulas to estimate 24‐hour urinary sodium excretion alter the dietary sodium and blood pressure relationship. Hypertension. 2021;77(6):2127‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Du X, Bai Y, et al. Assessment and validation of spot urine in estimating the 24‐h urinary sodium, potassium, and sodium/potassium ratio in Chinese adults. J Hum Hypertens. 2020;34(2):184‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He FJ, Pombo‐Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ Open. 2014;4(4):e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blood Pressure Lowering Treatment Trialists Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant‐level data meta‐analysis. Lancet. 2021;397(10285):1625‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kario K, Chen CH, Park S, et al. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension. 2018;71(3):375‐382. [DOI] [PubMed] [Google Scholar]
- 40. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(3 Pt 2):481‐490. [DOI] [PubMed] [Google Scholar]
- 41. Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26(7):521‐525. [DOI] [PubMed] [Google Scholar]
- 42. Arima H, Murakami Y, Lam TH, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia‐Pacific region. Hypertension. 2012;59(6):1118‐1123. [DOI] [PubMed] [Google Scholar]
- 43. Kim YD, Jung YH, Saposnik G. Traditional risk factors for stroke in East Asia. J Stroke. 2016;18(3):273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 Pt 2):II127‐134. [DOI] [PubMed] [Google Scholar]
- 45. Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18(1):67‐71. [DOI] [PubMed] [Google Scholar]
- 46. De Wardener HE, MacGregor GA. Sodium and blood pressure. Curr Opin Cardiol. 2002;17(4):360‐367. [DOI] [PubMed] [Google Scholar]
- 47. Nations U, World Population Prospects 2019. In: Department of Economic and Social Affairs PD, ed2019: https://population.un.org/wpp/
- 48. Liu Y, Chu H, Peng K, et al. Factors associated with the use of a salt substitute in rural China. JAMA Network Open. 2021;4(12):e213774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


