Abstract
The FCY2 gene of Saccharomyces cerevisiae encodes a purine-cytosine permease (PCP) that mediates the active transport of purines and cytosine. A structure-function model for this PCP has been recently proposed. In this study, we developed a plasmid-based system that generated a number of affinity-mutated alleles, enabling us to define new amino acids critical for permease function.
Design of a plasmid system for the selection of PCP affinity mutants.
In the yeast Saccharomyces cerevisiae, adenine (ADE), guanine (GUA), hypoxanthine (HYP), and cytosine (CYT) are taken up with the same affinity by a single proton symporter purine-CYT permease (PCP) encoded by the FCY2 gene (4, 9, 12, 13). Disruption of this gene abolishes active transport, despite the presence in the yeast genome of FCY21 and FCY22 (accession number X97346), which belong to the same gene family. Several mutants with modified uptake abilities have been selected in vivo (2, 4, 6), and their analysis has led to the generation of a functional model for this permease (7).
To test this model and to investigate further the structure-function relationship of the PCP, we isolated additional fcy2 affinity mutant alleles by various types of in vivo positive selection, making use of the broad specificity of this permease. PCP affinity mutants were isolated as previously described (4), by selecting cells able to overcome growth inhibition due to the outcompetition of two of the PCP substrates for entry into the cell. Two strains were constructed, RW123 (Δfcy2::HIS3 ade1-1 his3Δ200 trp1Δ [pPZ1-7 FCY2 TRP1]) and RW126 (Δfcy2::HIS3 ura29-15-30 his3Δ200 trp1Δ [pPZ1-7 FCY2 TRP1]), in which the FCY2 coding sequence was replaced with the HIS3 marker as previously described (1) and the wild-type FCY2 allele was carried on the pPZ1-7 single-copy plasmid (2). Both were used for selection with various pairs of substrates (Table 1). The presence of a plasmid-borne FCY2 allele made it easier to clone and sequence the selected mutations.
TABLE 1.
Selection of PCP affinity mutants
| Strain | Selective mediuma
|
Mutant phenotype | No. of independent selectionsb | No. of analyzed mutantsb | No. of plasmidic mutationsb | |
|---|---|---|---|---|---|---|
| Competing substrate (concn, μg · ml−1) | Limiting substrate (concn, μg · ml−1) | |||||
| RW123(pPZ1-7) | CYT (400) | ADE (3) | CYTr | 191 | 183 | 8 |
| 5MeC (200) | ADE (3) | 5MeCr | 50 | 65 | 0 | |
| RW126(pPZ1-7) | ADE (100) | CYT (3) | ADEr | 215 | 89 | 0 |
| HYP (100) | CYT (3) | HYPr | 145 | 73 | 0 | |
| GUA (60) | CYT (3) | GUAr | 110 | 14 | 0 | |
| 5MeC (200) | CYT (3) | 5MeCr | 110 | 81 | 50 | |
| Total | 821 | 505 | 58 | |||
Compositions of YNB selective media and phenotypes of selected mutants.
Number of isolated mutants and characterization of fcy2 mutations.
Due to its ade1-1 mutation, strain RW123 was auxotrophic for ADE and therefore required efficient ADE uptake by the PCP for growth. When this strain was plated on YNB medium containing a low concentration of ADE and a high concentration of another substrate of the PCP, the competition between the two bases prevented the uptake of sufficient quantities of ADE, leading to growth inhibition. This growth inhibition was achieved by adding ADE at 3 μg · ml−1 to the medium along with CYT at 400 μg · ml−1 or 5-methylcytosine (5MeC) at 200 μg · ml−1 as a competitor. The use of these two competitors defined two selection schemes, facilitating the selection of CYT-resistant (CYTr) and 5MeC-resistant (5MeCr) mutants, respectively.
Strain RW126, which carried the ura29-15-30 mutant allele, required an exogenous pyrimidine source, such as CYT, for growth. Thus, CYT at 3 μg · ml−1 was added to the medium as the limiting substrate and the competitor was ADE, HYP, GUA, or 5MeC. Competition conditions were achieved at the following concentrations: 150 μg · ml−1 for ADE, 150 μg · ml−1 for HYP, 60 μg · ml−1 for GUA, and 200 μg · ml−1 for 5MeC. Thus, based on the use of these four competitors, we were able to define four selection schemes, facilitating the selection of ADEr, HYPr, GUAr, and 5MeCr mutants, respectively.
Selection of affinity mutants.
Five hundred five independent mutants obtained from 821 rounds of selection were retained for further analysis. Fifty-eight contained a mutation in the plasmid-borne FCY2 gene; 50 were selected by competition between ADE and CYT in strain RW123(pPZ1-7); and 8 were selected by competition between CYT and 5MeC in strain RW126(pPZ1-7). The remaining 447 mutants displayed chromosomal mutations. Four of the six selection systems used did not generate plasmid mutations (Table 1).
The sequences of the 58 plasmid-borne mutant fcy2 alleles defined eight classes of mutations (Table 2). Five of these classes corresponded to single missense mutations (paC-A, paC-B, and pcM-A to -C), and three corresponded to extensive nucleotide replacements resulting in several amino acid changes (paC-C, pcM-D, and pcM-E). We assessed substrate uptake in the mutants to determine the kinetic parameters of transport Vmax (maximal rate of uptake) and Ktapp (apparent Michaelis constant of transport) for the various substrates of the PCP (Table 2).
TABLE 2.
Amino acid substitutions and kinetic parameters (Ktapp and Vmax) of products of mutated FCY2 allelesa
| FCY2 allele | No. of selected alleles | Amino acid replacement(s) | Transport parameters
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADE
|
CYT
|
HYP
|
GUA
|
|||||||
| Ktapp | Vmax | Ktapp | Vmax | Ktapp | Vmax | Ktapp | Vmax | |||
| Wild type | 1.2 | 175 | 2.3 | 240 | 2.5 | 200 | 1.6 | 220 | ||
| paC-A | 6 | S369F | 2.8 | 305 | 26 | 780 | 14 | 115 | 12.5 | 110 |
| paC-B | 1 | T370N | 1.4 | 86 | 18 | 59 | 16.5 | 12 | 9.4 | 11 |
| pcM-A | 31 | N377I | 16 | 248 | 36.5 | 1,750 | 25 | 437 | 28.2 | 99 |
| pcM-B | 10 | N377T | 0.67 | 58 | 9 | 453 | 14.1 | 147 | 20.3 | 57 |
| pcM-C | 7 | G280A | 9.5 | 35 | 13.5 | 380 | 13.2 | 33 | 13.5 | 64 |
| paC-C | 1 | I371V, I375V, N377G, V478A | 2.8 | 250 | 25 | 605 | 23.2 | 65 | 10 | 27 |
| pcM-D | 1 | I371V, I375V, N377G | 3.1 | 288 | 30 | 735 | 17.6 | 48 | 28.8 | 57 |
| pcM-E | 1 | A335S, I371V, I375V, N377G, S441A, F447I | 2.9 | 253 | 27.7 | 495 | 15.8 | 38 | 70 | 107 |
paC-type mutated alleles are from CYTr mutants of strain RW123, and pcM-type alleles are from 5MeCr mutants of strain RW126. Ktapp values are apparent Michaelis constants of transport (micromolar). Vmax values are maximal rates of uptake expressed in picomoles of incorporated substrate per 107 cells per minute. Ktapp and Vmax values were determined as described by Brèthes et al. (3), with seven concentrations of radiolabeled substrate. Experiments were performed in duplicate.
Single missense mutations in the S369-to-N377 segment.
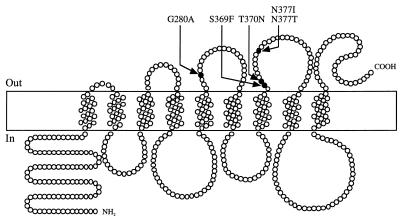
Four of the five single missense mutations affected a short peptide segment of the PCP, from serine 369 (S369) to asparagine 377 (N377), corresponding to a hydrophilic external loop of the PCP (7) (Fig. 1). Three of these mutations corresponded to amino acid substitutions not previously found (S369F, T370N, and N377T). For these three substitutions, the Ktapp for ADE was close to the value obtained with a wild-type strain but the Ktapp was significantly higher than that of the wild-type strain for CYT, HYP, and GUA.
FIG. 1.
Positions of selected amino acid substitutions in the PCP model proposed by Ferreira et al. (7).
In the T370N mutant, these changes were also accompanied by a general decrease in Vmax values (by a minimum factor of 2 for ADE and a maximum of 20 for GUA), suggesting that the amino acid substitution also slowed down the overall transport process. In contrast, the N377I substitution obtained by this approach and in a previous study (2) led to a large increase in all Ktapp values (by factors of 13, 16, 10, and 17 for ADE, CYT, HYP, and GUA, respectively) and the Vmax for CYT was also significantly higher than that of the wild type (about seven times as high).
These four point mutations in the S369-to-N377 segment accounted for more than 80% of the selected clones, indicating the important role played by this short peptide segment in the permease activity. These new results and the I371, N374, I375, P376, and N377 substitutions previously studied (2, 6, 8) clearly show that this region is involved in maintaining the PCP in a functional configuration, probably playing an active part in the conformational changes needed for solute translocation. In particular, asparagine 377 is thought to play an essential role in the solute-binding site, which is consistent with our results, because more than 70% of the selected mutations (41 of 58) affected this residue.
Modification of the G280 residue.
Replacement of the glycine at position 280 with an alanine (G280A) resulted in a global increase in Ktapp values for all substrates (by a factor of six to seven) and in a similar decrease in Vmax values, except for CYT. These strong effects on global uptake parameters indicate that glycine 280 is involved in the transport process. Moreover, considering the weak structural change responsible for these strong effects (basically, replacement of a hydrogen atom with a methyl group), glycine 280 has to be strongly conserved for maintenance of PCP activity.
This residue is located in a hydrophilic external loop, adjacent to that containing the S369-to-N377 segment (7) (Fig. 1). It therefore seems likely that these two predicted loops are involved in the correct folding of the protein and/or the establishment of the active site. This is consistent with the results of a recent study (7) that identified residue S372, located in the same region as G280, as a potential intragenic suppressor of the N374I substitution.
Multimutated alleles.
Three plasmid mutants showed multiple missense mutations in the fcy2 sequence. The region mutated depended on the allele: 17 mutations led to four amino acid substitutions for the paC-C allele, 17 mutations led to three amino acid substitutions for pcM-D, and 37 mutations led to six amino acid substitutions for pcM-E. These mutated regions displayed sequences identical to that of FCY22, strongly suggesting that ectopic recombination had occurred between the plasmid-borne FCY2 allele and the FCY22 gene. The recombinant sequences were 100 to 400 bp in size, and all surrounded the region encoding the hydrophilic S369-to-N377 segment. The three alleles had three substitutions in common: I371V, I375V, and N377G. Previous site-directed mutagenesis experiments (2) showed that the mutant phenotype resulted principally from the N377G substitution. As the FCY21 gene encodes an asparagine residue at this position, we believe that recombination between this duplicated gene and FCY2 may well occur but cannot result in the mutant phenotype.
The effects on substrate uptake parameters were similar in the three mutants: ADE incorporation was not affected, the Ktapp for CYT was about 10 times higher than that in the wild type, that for HYP was 6 to 9 times higher, and that for GUA was also significantly higher. However, the increase in Ktapp for GUA depended on the size of the mutated sequence, from a sixfold increase for paC-C to an increase of more than 40-fold for the largest mutated allele, pcM-E. This size-dependent modification indicated that one or more of the additional amino acid replacements was responsible for further functional changes in GUA uptake, suggesting that other features of the structure-function relationship are important.
General considerations.
All of the mutations selected in this work and in previous studies affect hydrophilic segments predicted to be external or cytoplasmic; none affect hydrophobic regions. Consistent with recent studies of bacterial and fungal nucleobase transporter mutants with altered substrate specificity (5, 11), this strongly suggests that discrimination among the four substrates of the PCP probably occurs during the binding step, which is then followed by a common translocation process. Moreover, a global decrease in apparent affinity for CYT, GUA, and HYP was observed for all of the selected mutations, suggesting that the structural discriminants involved in ADE recognition differ from those for CYT, GUA, and HYP binding. Thus, every uptake modification for one of these three molecules should lead to a similar effect for the other two. This observation may account for the results of some of the screenings, involving affinity decoupling between CYT and GUA, or between CYT and HYP, in which PCP mutants were not isolated in our selection scheme.
Only about 10% of the mutants selected with this approach bore an expected mutant plasmid-borne fcy2 allele (58 of 505). Thus, most of the mutations selected corresponded to chromosomal loci. It would be of great interest to study these mutations, as they reflect a significant adaptive response to major substrate variations. For instance, some of these mutants may result from the functional modification of other transporters, as has been observed in similar studies: during selection for suppressors that restored potassium uptake in Δtrk1 Δtrk2 cells, unusual gain-of-function mutations were characterized in various transporters, such as amino acid permeases Bap2 and Hip1, glucose transporters Hxt1 and Hxt3, and glucose-galactose transporter Gal2 (10, 14).
Acknowledgments
This research was supported by a grant from the French Ministère de l'Education Nationale, de la Recherche et de la Technologie (95-5-11550).
We thank Thierry Ferreira, Daniel Brèthes, and Jean Chevallier for help with the transport assays and for helpful comments on this work. We also thank Catherine Spehner for technical assistance.
REFERENCES
- 1.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in S. cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch J C, Sychrova H, Souciet J L, Jund R, Chevallier M R. Determination of a specific region of the purine-cytosine permease involved in the recognition of its substrates. Mol Microbiol. 1992;6:2989–2997. doi: 10.1111/j.1365-2958.1992.tb01757.x. [DOI] [PubMed] [Google Scholar]
- 3.Brèthes D, Chirio M C, Napias C, Chevallier M R, Lavie J L, Chevallier J. In vivo and in vitro studies of the purine-cytosine permease of Saccharomyces cerevisiae. Eur J Biochem. 1992;204:699–704. doi: 10.1111/j.1432-1033.1992.tb16684.x. [DOI] [PubMed] [Google Scholar]
- 4.Chevallier M R, Jund R, Lacroute F. Characterization of cytosine permeation in Saccharomyces cerevisiae. J Bacteriol. 1975;122:629–641. doi: 10.1128/jb.122.2.629-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diallinas G, Valdez J, Sophianopolou V, Rosa A, Scazzocchio C. Chimeric protein analysis reveals a region involved in function and specificity of purine transporters in the filamentous fungus Aspergillus nidulans conserved in bacteria, plants and metazoans. EMBO J. 1998;17:3827–3837. doi: 10.1093/emboj/17.14.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira T, Brèthes D, Pinson B, Napias C, Chevallier J. Functional analysis of mutated purine-cytosine permease from Saccharomyces cerevisiae. J Biol Chem. 1997;272:9697–9702. doi: 10.1074/jbc.272.15.9697. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira T, Chevallier J, Paumard P, Napias C, Brèthes D. Screening of an intragenic second-site suppressor of purine-cytosine permease from Saccharomyces cerevisiae. Eur J Biochem. 1999;260:22–30. doi: 10.1046/j.1432-1327.1999.00140.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira T, Napias C, Chevallier J, Brèthes D. Evidence for a dynamic role for proline 376 in the purine-cytosine permease of Saccharomyces cerevisiae. Eur J Biochem. 1999;263:57–64. doi: 10.1046/j.1432-1327.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins P, Chevallier M R, Jund R, Eddy A A. Use of plasmid vectors to show that the uracil and cytosine permeases of the yeast Saccharomyces cerevisiae are electrogenic proton symports. FEMS Microbiol Lett. 1988;49:173–177. [Google Scholar]
- 10.Liang H, Ko C H, Herman T, Gaber R F. Trinucleotide insertions, deletions, and point mutations in glucose transporters confer K+ uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:926–935. doi: 10.1128/mcb.18.2.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meintanis C, Karagouni A D, Diallinas G. Amino acid residues N450 and Q449 are critical for the uptake capacity and specificity of UapA, a prototype of a universally conserved nucleobase-ascorbate transporter family. Mol Membr Biol. 2000;17:47–58. doi: 10.1080/096876800294489. [DOI] [PubMed] [Google Scholar]
- 12.Pinson B, Pillois X, Brèthes D, Chevallier J, Napias C. In vivo phosphorylation of the purine/cytosine permease from the plasma membrane of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1996;239:439–444. doi: 10.1111/j.1432-1033.1996.0439u.x. [DOI] [PubMed] [Google Scholar]
- 13.Reichert U, Forêt M. Energy coupling in hypoxanthine transport of yeast. Potentiometric evidence of proton symport and potassium antiport. FEBS Lett. 1977;83:325–328. doi: 10.1016/0014-5793(77)81033-8. [DOI] [PubMed] [Google Scholar]
- 14.Wright M B, Ramos J, Gomez M J, Moulder C, Scherrer M, Munson G, Gaber R F. Potassium transport by amino acid permeases in Saccharomyces cerevisiae. J Biol Chem. 1997;272:13647–13652. doi: 10.1074/jbc.272.21.13647. [DOI] [PubMed] [Google Scholar]