Abstract
Global warming and precipitation extremes (drought or increased precipitation) strongly affect plant primary production and thereby terrestrial ecosystem functioning. Recent syntheses show that combined effects of warming and precipitation extremes on plant biomass are generally additive, while individual experiments often show interactive effects, indicating that combined effects are more negative or positive than expected based on the effects of single factors. Here, we examined whether variation in biomass responses to single and combined effects of warming and precipitation extremes can be explained by plant growth form and community type. We performed a meta-analysis of 37 studies, which experimentally crossed warming and precipitation treatments, to test whether biomass responses to combined effects of warming and precipitation extremes depended on plant woodiness and community type (monocultures versus mixtures). Our results confirmed that the effects of warming and precipitation extremes were overall additive. However, combined effects of warming and drought on above- and belowground biomass were less negative in woody- than in herbaceous plant systems and more negative in plant mixtures than in monocultures. We further show that drought effects on plant biomass were more negative in greenhouse- than in field studies, suggesting that greenhouse experiments may overstate drought effects in the field. Our results highlight the importance of plant system characteristics to better understand plant responses to climate change.
Keywords: climate warming, precipitation increase, precipitation decrease, global change experiments, aboveground plant biomass, belowground plant biomass
1. Introduction
Anthropogenic climate change is affecting terrestrial ecosystems worldwide [1–3]. As primary producers, plants form the base of both above- and belowground food webs [4,5] and as such play a central role in carbon cycling and overall ecosystem function. Our ability to predict ecosystem responses to climate change therefore strongly depends on how above- and belowground plant biomass are affected [6,7]. Recent syntheses have indicated that climate warming and precipitation extremes (precipitation decreases, hereafter called ‘droughts’, or precipitation increases) are among the most pressing forms of anthropogenic climate change influencing the performance of terrestrial plants [8–10]. Moreover, different climate change factors can interactively affect plant performance, by either amplifying or dampening each other's positive or negative effect on plant biomass (also called multiplicative or nonlinear effects; [8,11,12]). However, in contrast with single effects of warming and precipitation extremes, relatively little is known about how combined effects of warming and precipitation extremes affect plant performance above- and belowground, and in particular, which factors may underlie the magnitude of such combined effects.
In terrestrial ecosystems, precipitation extremes are typically known to affect plant performance [9]. Recent meta-analyses indicated that summer droughts decrease terrestrial plant performance across habitats and that impacts of drought are stronger than the impacts of other predicted global change effects [10,13]. By contrast, precipitation increases, as well as warming, typically enhance plant performance [8,10,13]. While multi-factorial global change experiments have shown that warming and precipitation extremes can interactively affect plant performance [14,15], several meta-analyses detected additive combined effects (warming + precipitation extremes) rather than interactive combined effects (warming × precipitation extremes) [8,10,13]. However, these meta-analyses quantified overall interaction effect sizes based on datasets in which studies that separately examined the effects of warming or precipitation extremes were pooled with studies that examined both single and combined effects of warming and precipitation extremes [10,13]. Such pooling of studies with different experimental designs possibly could have obscured the detection of interactive effects. Moreover, to date, meta-analyses have mainly used abiotic variables (e.g. climatic conditions) to explain variation in (combined) effects of warming and precipitation extremes on terrestrial plant performance [8,10,13]. However, in addition to such abiotic effects, biotic characteristics of the study system may further help to explain the variation of single and combined effect sizes of warming and precipitation extremes on plant performance.
Plant responses to drought and warming may depend on adaptive strategies to overcome water shortages and thermal stress that are related to plant woodiness [16,17]. For instance, due to failures of the hydraulic system, droughts can have long-lasting negative impacts on woody plants and reduce their survival, but such impacts may not be immediately reflected in their biomass responses to drought [3,18]. By contrast, herbaceous plants often show immediate biomass responses to drought but may also recover quickly after the drought period is over [19,20]. These responses to drought may vary between plant shoots and roots given the differences in resource economics between these compartments, as well as among plant species, for example owing to variation in symbiotic relationships with soil microorganisms that help to maintain water uptake under dry conditions [21–23]. Moreover, root responses to warming for both woody plants and herbaceous plants could differ from shoot responses given that temperature buffering in soils is often higher than in air [24–26]. However, it is likely that warming effects on plant roots may be magnified in soils with limited water availability [27], as drought has consistently been shown to alter root growth and root resource uptake from the soil [28–31]. In this respect, it is also important to consider that plant responses to drought may also differ between field and greenhouse studies. For instance, plants in field conditions may be better able to show plastic responses, such as an increase in rooting depth, whereas plants grown in pots in greenhouse studies may be limited in their ability to exhibit trait plasticity to overcome abiotic stresses [32]. Moreover, compared to plants growing in the field, plants growing in pots may be affected by the limited capacity of their respective soils to buffer abiotic changes (e.g. increased temperatures) [33].
Plant monocultures and diverse plant communities are also likely to differ in their responses to warming and precipitation extremes, given that plant diversity often mediates negative environmental impacts and generally enhances plant community productivity [34,35]. Warming, for example, increased aboveground biomass in diverse plant communities compared to monocultures, although such an effect was not found for belowground biomass [36]. Moreover, communities with a high diversity of plant species typically are better able to maintain community productivity during drought events than low-diversity communities [37–39]. Such differences in drought resistance between low- and high-diversity communities may occur owing to stronger drought-ameliorating effects of belowground mutualists in diverse plant communities [40], as well as owing to an increasing probability of the presence of species with effective responses against drought in diverse communities compared to monocultures [41,42].
Here, we examine how combined experimental treatments of warming and drought or warming and increased precipitation affect terrestrial plant biomass both above- and belowground. We performed a meta-analysis using above- and belowground plant biomass data from studies in which warming- and precipitation changes were experimentally crossed, and associated the variation in single and combined effect sizes with two potential key biotic variables: plant woodiness and plant community type. We specifically test two hypotheses: (i) overall, combined effects of warming and precipitation extremes on above- and belowground plant biomass are additive (i.e. lack of amplifying and/or dampening interactive effects), although we expect that the effect sizes could be stronger for aboveground biomass than for belowground biomass and (ii) plant woodiness and plant community type (monoculture versus mixed) explain variation in both single and combined effects of warming and precipitation extremes on above- and belowground biomass. Finally, to understand whether variation in plant responses to warming and precipitation extremes in part may be determined by methodological approaches, we tested the hypothesis that (iii) single and combined effect size variability depend on whether a study was performed in the greenhouse or in the field.
2. Material and methods
(a) . Literature search and data collection
We performed a meta-analysis with data from two types of full-factorial experimental studies, which either examined above- and/or belowground plant biomass responses to experimental warming and drought or to experimental warming and increased precipitation. We report the meta-analyses of these two kinds of studies separately given the differences in their experimental design. We conducted a literature search in ISI Web of Science Core Collection on 11 January 2021 (cut-off date) using the following terms: (‘Temperature’ OR ‘Warming’ OR ‘Heat’) AND (‘Drought’ OR ‘Precipitation’ OR ‘Rainfall’ OR ‘Moisture’ OR ‘Flood*’) AND (‘Plant biomass’ OR ‘Plant product*’ OR ‘Shoot biomass’ OR ‘Root biomass’ OR ‘Aboveground biomass’ OR ‘Belowground biomass’ OR ‘Plant cover’) AND (‘Experiment*’ OR ‘Manipulat*’). The search was carried out for ‘All Fields’ in ISI Web of Science, which resulted in a total of 682 studies (with their title, abstract, author keywords and Keywords Plus) that were subsequently screened for experimental studies from which we could extract data. We first screened papers for the presence of experimental data on interactive effects of warming and precipitation extremes (drought or precipitation increase or both). Papers that met these criteria were then screened for the presence of above- and/or belowground plant biomass data. We were eventually able to extract biomass data from 37 independent, full-factorial experimental studies that measured aboveground and/or belowground plant biomass (also see PRISMA diagram in electronic supplementary material, figure S1). There were 27 studies experimentally applying warming and drought treatments and 19 studies experimentally applying warming and increased precipitation treatments in our meta-analysis. Some studies applied both drought and increased precipitation in their experiments (electronic supplementary material, data S1). Temperature increases in studies combining warming and drought ranged from +0.5°C to +10°C (average +4°C) and from +0.68°C to +11.4°C (average +3°C) in studies combining warming and increased precipitation. Experimental drought treatments (precipitation decreases) ranged from −92% to −5.54% (average −35.2%), while precipitation increases ranged from +15% to +203.4% (average +84.4%).
The data from each study were extracted from figures, tables or main text. We extracted mean, s.d. and sample sizes for above- and/or belowground plant biomass from all combinations of warming and precipitation extreme treatments in each study (electronic supplementary material, data S1). To extract the data from the figures, we either used Plot Digitizer [43] or ImageJ [44]. We obtained aboveground biomass (or shoot biomass) from 29 studies and belowground biomass from 17 studies across warming*drought and warming*increased precipitation interaction studies (electronic supplementary material, data S1).
(b) . Data analysis
We took a two-step approach in our meta-analysis. First, we estimated single effects of warming and precipitation extremes, and their interaction effects, on plant biomass. We performed this separately for above- and belowground plant biomass. Second, we used a set of moderator variables to examine what may explain the variation in effect sizes obtained in step 1.
We estimated the individual effects of warming and precipitation extremes on aboveground and belowground plant biomass using the Hedge's g effect size [45], with corrections for small sample sizes. The Hedge's g were calculated using the following formula:
where BiomassT is aboveground or belowground (mean) biomass in warming or precipitation regime treatment(s) and BiomassC is aboveground or belowground (mean) biomass in respective control treatments. S is the pooled s.d. and is estimated as follows:
where NT stands for the sample size of the given treatment and NC is the sample size of the respective control. and are variance of treatment and controls, respectively. J is the correction factor for small sample sizes and is calculated as
To estimate the interaction effect sizes, we also used Hedge's g with the following formula used in several previous studies [10,13,46,47]:
where BiomassAB is the (mean) aboveground or belowground biomass in the treatment combination of warming and drought or the treatment combination of warming and increased precipitation. BiomassA and BiomassB are (mean) aboveground or belowground biomass in warming and precipitation extreme treatments, respectively. BiomassC stands for (mean) aboveground or belowground biomass in control treatments. Si and Ji are the pooled standard deviations and correction term for sample bias, respectively, which were estimated as following.
and
where NC, NA, NB and NAB are sample sizes, and , , and are variance of control, warming, drought/increased precipitation, and combined treatments of warming and drought or warming and increased precipitation treatments, respectively. The variance of Hedge's g (interactive) was estimated using the following formula:
where d2 is the square of the weighted mean calculated as explained in Yue et al. [10].
We used random effects models for our meta-analysis, which allow flexibility in the variation of true effects from one study to the other [45]. Moreover, we always used study identity as a random intercept in all our meta-regression models to account for repeatability of single studies with multiple effect sizes. We used restricted maximum-likelihood estimators to obtain the effect size estimates and their variance owing to their efficiency in obtaining unbiased estimates [48]. The effect sizes were considered statistically significant when their 95% confidence intervals (CIs) did not overlap with zero. When the 95% CIs of interactive effect sizes overlapped with zero, combined effects were considered to be additive [47]. When not overlapping with zero, positive or negative interaction effects were considered to affect plant biomass more positively or negatively than expected based on the effects of single factors.
The total heterogeneity and its test statistics for each random effects model were further estimated to examine how heterogeneous Hedge's g's were for aboveground or belowground biomass across studies [49]. A significant total heterogeneity (p-value < 0.05) indicates a greater among-study variance than expected when accounting for the sampling error within the random effects models. If this is the case, it indicates that additional unexamined factors influenced effect sizes and its variance. We also tested for the effects of publication bias in the estimation of effect sizes using contour funnel plots (electronic supplementary material, figure S2). Visual inspection of funnel plots showed minimal publication bias in our meta-analysis for both studies that examined warming and drought effects and studies that examined warming and increased precipitation effects (electronic supplementary material, figure S2).
In our second step, we used moderator analysis to test the effects of four variables (‘aboveground versus belowground’, ‘woody versus herbaceous’, ‘monoculture versus mixture’ and ‘field versus greenhouse’) on both additive and interactive effects of warming and precipitation extremes on plant biomass. The importance of each moderator for a given effect size from the experiments combining warming and drought or experiments combining warming and increased precipitation was estimated from the sum of Akaike weights [50]. All our analyses were carried out in R statistical software v. 4.1.0 [51], using the metafor package [52] for random effects models and multi-level meta-analysis. The sum of Akaike weights was estimated using the MuMin package [53].
3. Results
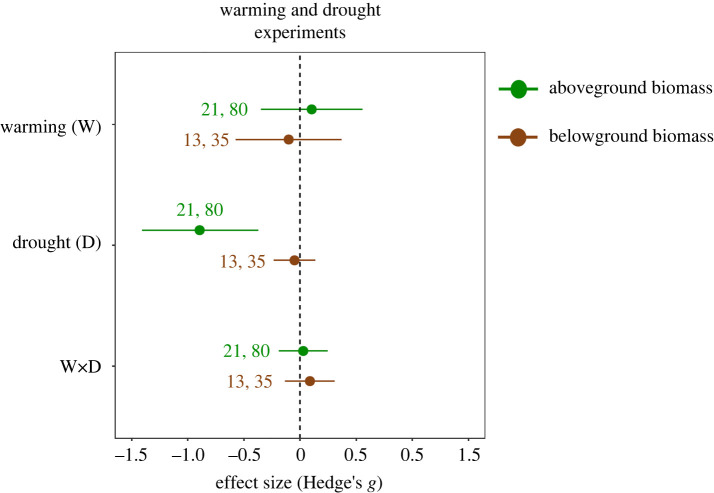
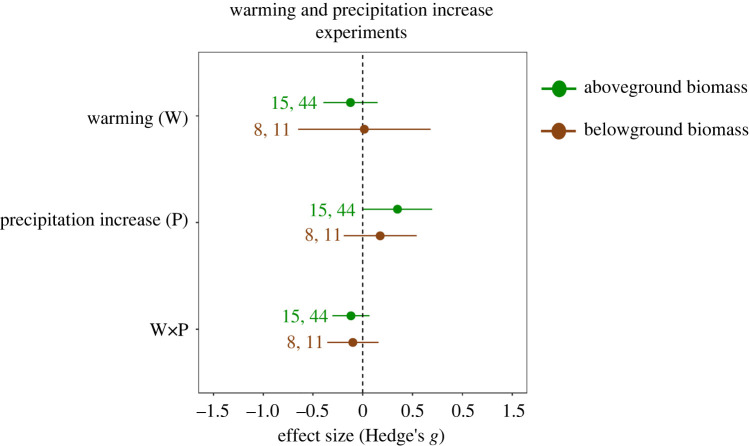
Drought reduced aboveground plant biomass (effect size (Hedge's g) = −0.89, 95% CI = −1.40, −0.37), but had no effect on belowground plant biomass (CI overlapping with zero, figure 1, details in table 1) despite significantly high heterogeneity among studies (table 1). In studies that combined warming and drought treatments, warming did not significantly affect above- or belowground biomass (CI overlapping with zero, figure 1, table 1). On average, combined effects of warming and drought on above- and belowground biomass did not differ from zero, indicating that individual effects of warming and drought were additive (figure 1, table 1). Increased precipitation slightly enhanced the aboveground biomass (effect size (Hedge's g) = 0.34, 95% CI = 0.0002, 0.69; figure 2, details in table 1) along with significantly higher heterogeneity among studies (table 1). Similar to studies examining warming and drought effects, warming did not significantly affect above- or belowground biomass in studies that combined warming and increased precipitation, and the interactive effect indicated that effects of warming and increased precipitation on above- and belowground were additive (CI overlapping with zero, figure 2, table 1).
Figure 1.
Mean effect sizes ± 95% CIs for experimental warming and drought effects on aboveground (upper effect sizes) and belowground (lower effect sizes) plant biomass. Warming and drought effects are significant when CIs do not overlap with zero. The values next to effect sizes stand for the number of studies and the number of unique cases, respectively. (Online version in colour.)
Table 1.
Effect size (Hedge's g) and standard errors (s.e.) of individual and interactive effects of warming and precipitation extremes (drought and increased precipitation) on aboveground and belowground plant biomass. Heterogenity test statistics Q, combined with respective degrees of freedom and p-value, are also provided. The effect of study identity as random intercept in our models is listed as their variance. Italicized effect sizes are statistically significant.
| effect size (s.e.) | CIs (95%) | test for heterogeneity (Q) | d.f. | p-value | variance component (study) | |
|---|---|---|---|---|---|---|
| aboveground biomass | ||||||
| warming (W) | 0.103 (0.223) | −0.347, 0.553 | 176.492 | 79 | <0.001 | 0.931 |
| drought (D) | −0.895 (0.263) | −1.407, −0.375 | 219.174 | 79 | <0.001 | 1.248 |
| W × D | 0.027 (0.110) | −0.188, 0.243 | 132.762 | 79 | <0.001 | 0.179 |
| warming (W) | −0.123 (0.138) | −0.395, 0.148 | 55.545 | 43 | 0.095 | 0.096 |
| precipitation increase (P) | 0.347(0.177) | <0.001, 0.695 | 61.848 | 43 | 0.031 | 0.258 |
| W × P | −0.119 (0.094) | −0.303, 0.065 | 65.983 | 43 | 0.013 | 0.042 |
| belowground biomass | ||||||
| warming (W) | −0.101 (0.240) | −0.571, 0.368 | 73.692 | 34 | <0.001 | 0.547 |
| drought (D) | −0.049 (0.094) | −0.233, 0.135 | 25.593 | 34 | 0.849 | 0.000 |
| W × D | 0.087 (0.111) | −0.132, 0.306 | 39.973 | 34 | 0.221 | 0.081 |
| warming (W) | 0.015 (0.338) | −0.647, 0.678 | 21.037 | 10 | 0.020 | 0.549 |
| precipitation increase (P) | 0.174 (0.186) | −0.189, 0.539 | 7.587 | 10 | 0.347 | 0.009 |
| W × P | −0.100 (0.130) | −0.356, 0.156 | 6.708 | 10 | 0.752 | 0.000 |
Figure 2.
Mean effect sizes ± 95% CIs for experimental warming and increased precipitation on aboveground (upper effect sizes) and belowground (lower effect sizes) plant biomass. Warming and increased precipitation effects are significant when CIs do not overlap with zero. The values next to effect sizes stand for the number of studies and the number of unique cases, respectively. (Online version in colour.)
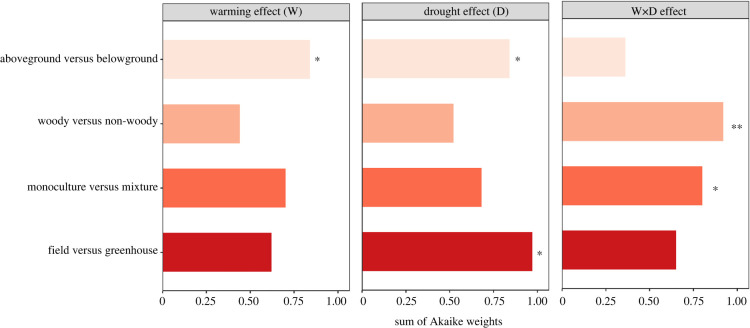
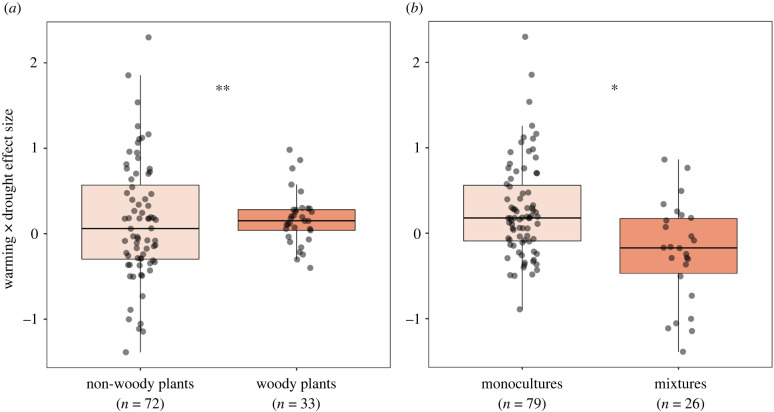
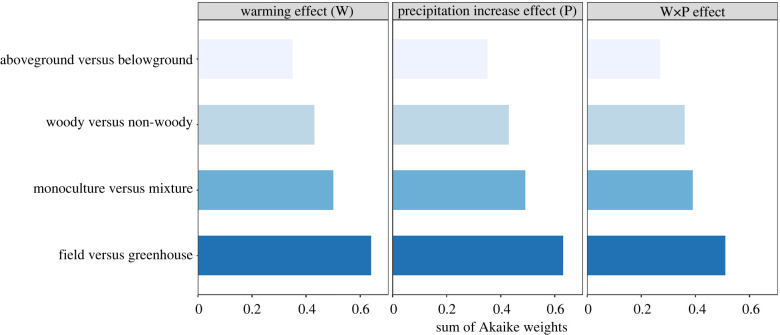
In studies combining warming and drought, warming effects depended most strongly on whether biomass responses were measured above- or belowground (figure 3), with more positive warming effects on aboveground than on belowground biomass, although differences between responses of aboveground and belowground biomass were small (electronic supplementary material, figure S3). Variation in plant biomass responses to drought was best explained by experimental type (greenhouse versus field), as impacts of drought on plant biomass were more negative in greenhouse studies than in field studies (figure 3; electronic supplementary material, figure S4). Moreover, drought affected aboveground biomass more negatively than belowground biomass (figure 3; electronic supplementary material, figure S4). The warming × drought interaction effect varied most between woody and herbaceous plants (figures 3 and 4). Warming and drought interactively affected herbaceous plants more negatively than woody plants (figure 4). Moreover, the warming × drought interaction effect size also differed between plant monocultures and mixtures (figures 3 and 4), as the interaction effect size was slightly more negative for plant mixtures than for plant monocultures (figure 4). Across experiments that combined warming and increased precipitation treatments, we found that experiment type (greenhouse versus field) consistently was the most important moderator in explaining the variation in all three effect sizes (figure 5), but these differences in plant responsiveness between greenhouse and field studies were nevertheless not significant (p-value > 0.05).
Figure 3.
Sum of Akaike weights of four moderator variables from multi-level meta-analytic models for biomass responses in experiments examining warming and drought effects. The higher the Akaike weights, the greater is the importance of the variable in explaining the variation of an effect size. The statistical significance of a given moderator variable is indicated by an asterisk, and when those without any asterisk sign are non-significant. Asterisks represent p-values < 0.05 (*) or p-values < 0.01 (**). (Online version in colour.)
Figure 4.
(a) Difference in the warming × drought interaction effect size between woody and herbaceous (non-woody) plant responses. (b) Difference in the warming × drought interaction effect size between plant monocultures and mixed plant communities. Positive or negative values indicate combined effects that are more positive or negative than expected based on single effects, respectively. Asterisks represent p-values < 0.05 (*) or p-values < 0.01 (**). Boxplots show the median effect size (horizontal line), first and third quartiles (rectangle), 1.5 × interquartile range (whiskers) and all effect sizes (as black dots). (Online version in colour.)
Figure 5.
Sum of Akaike weights of four moderator variables from multi-level meta-analytic models for biomass responses in experiments examining warming and increased precipitation effects. The higher the Akaike weights, the greater is the importance of the variable in explaining the variation of an effect size. (Online version in colour.)
4. Discussion
Determining terrestrial plant biomass responses to multiple global change factors is crucial to improve understanding of future plant communities and carbon dynamics in terrestrial ecosystems [6,54–56]. Towards this end, we performed a meta-analysis of experimental studies that examined the interactive effects of warming and precipitation extremes on plant biomass responses above- and belowground. In line with previous meta-analyses, our meta-analysis shows that drought negatively affected aboveground plant biomass, while increased precipitation had a slightly positive effect on aboveground plant biomass [10,13]. However, in contrast, a previous meta-analysis showed that above- and belowground biomass responses to both precipitation extremes are comparable [13], while in our analysis belowground biomass was not significantly affected by either of the precipitation extremes. It must be noted that our dataset included more measurements for above- than for belowground biomass (figure 1), and most of the strongly negative drought effect sizes came from studies that reported aboveground biomass responses (electronic supplementary material, data S2), but not belowground biomass responses. Therefore, the limited effect of drought on belowground biomass may at least in part be explained by a bias towards aboveground biomass measurements in warming and drought experiments. Experimental warming overall did not affect above- or belowground biomass, unlike what was found in previous meta-analyses [10,13]. Moreover, effects of precipitation extremes did not depend on interactions with warming, indicating that warming did not strengthen or weaken effects of increased precipitation or drought on plant biomass [10]. This was true despite the fact that we only included global change studies that tested warming and precipitation extremes interactively, as opposed to previous meta-analyses, which pooled single-effect and combined-effect studies [10,13]. Thus, although it included fewer studies than previous meta-analyses [10,13], our meta-analysis further confirms that warming and precipitation extremes on average exert additive effects on above- and belowground plant biomass.
Given that interactive effects of warming and precipitation extremes are found in individual studies (e.g. [14]), we examined whether specific biotic characteristics may explain variation in plant biomass responses to single or combined impacts. Indeed, our results show that the strength of interaction effects between warming and drought differs between woody and herbaceous plants as well as between plant monocultures and mixed plant communities. These results highlight that biotic contexts, such as plant growth form and plant community type, are important to consider when predicting plant biomass responses to combined effects of warming and drought [57,58].
While we did not find a significant interactive warming × drought effect on plant biomass across studies, we found that the interactive effect of warming and drought was more negative for herbaceous plants than for woody plants, indicating that herbaceous plants on average suffer more from drought under warm conditions than woody plant species do. Interestingly, the experimental systems in which interactive warming × drought effect sizes were most negative (Hedge's g lower than −1) were all mixed herbaceous communities that included grasses (electronic supplementary material, data S2). Possibly, warming further worsens drought effects on shallow-rooted, herbaceous plant species [19] and therefore may most negatively affect grass species [59,60], while woody plants may have overcome such adverse effects at least for a short duration owing to their greater ability to tolerate initial water shortages [28]. It should be noted that most studies in our meta-analysis examined drought responses for a limited amount of time, e.g. for a single growing season or shorter. However, under severe and prolonged drought, woody species will likely also show strong negative responses [3,61].
Differences between woody and non-woody species in their biomass responses may also be explained by underlying variation in life-history traits, e.g. those associated with the leaf economics spectrum [62]. We explored this possibility for responses among plant monocultures, by examining the correlations between values for specific leaf area (SLA) (extracted from the TRY database; [63]) and single factor and interaction effect sizes (see electronic supplementary material, figure S6). These analyses suggest that slow-growing plant species (i.e. species with low SLA values) tend to show a more positive response to interactive effects of warming and drought than fast-growing plant species (electronic supplementary material, figure S6), which is in line with the higher resistance against climate extremes associated with conservative traits [39,42]. However, these correlations can only be confirmed through examining a larger number of species. Species with low SLA values also tended to show more positive responses to increased precipitation (electronic supplementary material, figure S6), but the strong variation in responses among species prevents any conclusive interpretation of this relationship. Finally, it must be noted that variation in biomass responses among woody plant species could depend on their life stages, as younger plants, for example, respond more negatively to drought than mature individuals [64]. Moreover, as with increasing plant age differential responses in total biomass may become more difficult to detect, we suggest that examination of both biomass productivity and absolute biomass may yield a more complete understanding of global change impacts across plant systems. Therefore, including more information on drought treatments (i.e. length and severity), the study plant system (life stage and structural traits), and analysing various measures of biomass responses in future experimental and synthesis work may yield further insights into variation in drought responses among woody and herbaceous plants.
Our results also showed that the warming × drought effect size was more positive in plant monocultures than in mixed plant communities, indicating that, under warming, monocultures were less negatively affected by drought than plant mixtures. This is surprising, as plant diversity typically mediates negative drought impacts on biomass production [35,37–39], most importantly owing to the presence of a broader range of water-use strategies in diverse communities [39,42]. Moreover, high-diversity communities have been shown to benefit more from warming than monocultures [36], likely owing to positive plant diversity effects on soil water-holding capacity under warm conditions, as well as more strongly positive biomass responses to the warming-induced extension of the growing season [36]. This also would suggest that diverse communities may be less negatively affected by combined applications of drought and warming than monocultures, as drought effects are likely better mitigated. However, our results showed the opposite effect, although only subtly. Possibly, this effect may be driven by the same strongly negative effects of combined applications of drought and warming on mixed herbaceous communities that contained grasses, which also partly explained the difference in interactive global change effects between woody and herbaceous plant systems. Therefore, future studies should examine how functional community composition of plant communities relates to biomass responses to warming and precipitation extremes. Importantly, owing to the limited number of studies, we only differentiated between studies as monocultures and mixed plant communities, regardless of diversity level. A recent synthesis study indicated that biomass responses to global change such as warming are likely to depend on the number of plant species within a community [65]. Therefore, we do not draw any strong conclusions on how biomass responses to single or combined impacts of warming and drought change with increasing plant diversity. Nevertheless, our results encourage future studies to examine whether the interactive effects of warming and drought on plant biomass become more important along the plant diversity gradient.
Our results further showed that drought effects on plant biomass were significantly more negative in greenhouse studies than in field studies. Average relative reductions of water addition between control and drought-exposed experimental units indicated that treatment severity was stronger in greenhouse (42% reduction) than in field experiments (31% reduction; electronic supplementary material, data S1), although changes in water additions were not reported for all studies, which prevented us from performing any further analysis related to exact drought manipulations. Therefore, we suspect that more negative responses in greenhouse studies may in part be explained by differences in the strength of drought treatments between greenhouse and field experiments. However, the observed differences in plant responses to drought between greenhouse and field studies could also be partly explained by the limited ability of plants to express phenotypic plasticity (e.g. deeper root foraging) to overcome drought stress in (shallow) pot experiments, and by the limited capacity of potted soils to buffer environmental changes [32,33]. Experiment type (greenhouse or field) also consistently explained most of the variation in plant biomass responses to individual or combined applications of warming and increased precipitation, although these differences in effect sizes were not significant. Overall, this suggests that outcomes of studies on changes in water availability are to some extent affected by the type of experiment, indicating the need for carefully considering whether implemented treatments in greenhouse experiments resemble the conditions that plants experience under natural conditions[66,67].
In conclusion, our meta-analysis confirms that combined effects of warming and precipitation extremes on plant performance are overall additive and advance our current understanding of how plants' woodiness and community context could play an important role in explaining the combined effects of warming and drought on plant biomass. We suspect that these results are mostly valid at plant population and community levels as most studies included in our meta-analysis report biomass responses to warming and precipitation extremes at these two ecological scales. The clear additive effects of warming and both types of precipitation extremes on both above- and belowground plant biomass suggest that plants have distinct strategies to overcome these potential abiotic stresses. However, in our meta-analysis, we only examined the importance of a couple of plant biotic and experimental parameters of the examined plant systems that may help explain plant responses to climate change, while, for example, the incorporation of individual- and community-level shoot and root traits may further help to improve our general understanding of plant responses to drought [41]. Finally, our results suggest that precipitation treatments in greenhouse experiments are often stronger than in more realistically set-up field experiments. Therefore, to improve the predictability of plant responses to warming and precipitation extremes, future experiments should be focussed on examining plant responses to climate change across all important plant traits and carefully simulate climate change treatments.
Acknowledgements
We thank the organizers of Terrestrial Ecology Day at the Netherlands Institute of Ecology in 2019, where the ideas for this project were first discussed. We thank Ciska Veen and Marta Manrubia for their help with the data extraction and the discussion about the results.
Data accessibility
We provide the database with extracted data from previously published studies (electronic supplementary material, data S1) as well as the effect size data (electronic supplementary material, data S2) as electronic supplementary material [68].
Authors' contributions
R.A.W.: conceptualization, data curation, writing—original draft and writing—review and editing; J.R.D.L.: data curation and writing—review and editing; S.G.: data curation and writing—review and editing; S.E.H.: data curation and writing—review and editing; C.W.Q.: data curation and writing—review and editing; B.S.: data curation and writing—review and editing; K.S.: data curation and writing—review and editing; E.R.J.W.: data curation and writing—review and editing; Q.Y.: data curation and writing—review and editing; M.P.T.: conceptualization, data curation, formal analysis, methodology, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
M.P.T. acknowledges the support by the Swiss State Secretariat for Education, Research and lnnovation (SERI) under contract number M822.00029.
References
- 1.Ciais P, et al. 2005. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529-533. ( 10.1038/nature03972) [DOI] [PubMed] [Google Scholar]
- 2.Walther GR. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019-2024. ( 10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakur MP, Risch AC, van der Putten WH. 2022. Biotic responses to climate extremes in terrestrial ecosystems. iScience 25, 104559. ( 10.1016/j.isci.2022.104559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Putten WH, Vet LEM, Harvey JA, Wäckers FL. 2001. Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol. Evol. 16, 547-554. ( 10.1016/s0169-5347(01)02265-0) [DOI] [Google Scholar]
- 5.Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH. 2004. Ecological linkages between aboveground and belowground biota. Science 304, 1629-1633. ( 10.1126/science.1094875) [DOI] [PubMed] [Google Scholar]
- 6.Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin JM, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389-395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 7.Thuiller W. 2007. Biodiversity: climate change and the ecologist. Nature 448, 550-552. ( 10.1038/448550a) [DOI] [PubMed] [Google Scholar]
- 8.Wu ZT, Dijkstra P, Koch GW, Penuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob. Change Biol. 17, 927-942. ( 10.1111/j.1365-2486.2010.02302.x) [DOI] [Google Scholar]
- 9.Franklin J, Serra-Diaz JM, Syphard AD, Regan HM. 2016. Global change and terrestrial plant community dynamics. Proc. Natl Acad. Sci. USA 113, 3725-3734. ( 10.1073/pnas.1519911113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue K, Fornara DA, Yang W, Peng Y, Peng C, Liu Z, Wu F. 2017. Influence of multiple global change drivers on terrestrial carbon storage: additive effects are common. Ecol. Lett. 20, 663-672. ( 10.1111/ele.12767) [DOI] [PubMed] [Google Scholar]
- 11.Reich PB, Sendall KM, Stefanski A, Rich RL, Hobbie SE, Montgomery RA. 2018. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 562, 263-267. ( 10.1038/s41586-018-0582-4) [DOI] [PubMed] [Google Scholar]
- 12.Walther GR. 2003. Plants in a warmer world. Perspect. Plant Ecol. Evol. Syst. 6, 169-185. ( 10.1078/1433-8319-00076) [DOI] [Google Scholar]
- 13.Song J, et al. 2019. A meta-analysis of 1119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat. Ecol. Evol. 3, 1309-1320. ( 10.1038/s41559-019-0958-3) [DOI] [PubMed] [Google Scholar]
- 14.Hoeppner SS, Dukes JS. 2012. Interactive responses of old-field plant growth and composition to warming and precipitation. Glob. Change Biol. 18, 1754-1768. ( 10.1111/j.1365-2486.2011.02626.x) [DOI] [Google Scholar]
- 15.Zhu K, Chiariello NR, Tobeck T, Fukami T, Field CB. 2016. Nonlinear, interacting responses to climate limit grassland production under global change. Proc. Natl Acad. Sci. USA 113, 10 589-10 594. ( 10.1073/pnas.1606734113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debinski DM, Wickham H, Kindscher K, Caruthers JC, Germino M. 2010. Montane meadow change during drought varies with background hydrologic regime and plant functional group. Ecology 91, 1672-1681. ( 10.1890/09-0567.1) [DOI] [PubMed] [Google Scholar]
- 17.Hatfield JL, Prueger JH. 2015. Temperature extremes: effect on plant growth and development. Weather Clim. Extremes 10, 4-10. ( 10.1016/j.wace.2015.08.001) [DOI] [Google Scholar]
- 18.Choat B, Brodribb TJ, Brodersen CR, Duursma RA, Lopez R, Medlyn BE. 2018. Triggers of tree mortality under drought. Nature 558, 531-539. ( 10.1038/s41586-018-0240-x) [DOI] [PubMed] [Google Scholar]
- 19.Hoover DL, Knapp AK, Smith MD. 2014. Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95, 2646-2656. ( 10.1890/13-2186.1) [DOI] [Google Scholar]
- 20.Wilcox KR, et al. 2020. Rapid recovery of ecosystem function following extreme drought in a South African savanna grassland. Ecology 101, e02983. ( 10.1002/ecy.2983) [DOI] [PubMed] [Google Scholar]
- 21.Bergmann J, et al. 2020. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 6, eaba3756. ( 10.1126/sciadv.aba3756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigelt A, et al. 2021. An integrated framework of plant form and function: the belowground perspective. New Phytol. 232, 42-59. ( 10.1111/nph.17590) [DOI] [PubMed] [Google Scholar]
- 23.Revillini D, Gehring CA, Johnson NC. 2016. The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct. Ecol. 30, 1086-1098. ( 10.1111/1365-2435.12668) [DOI] [Google Scholar]
- 24.Lembrechts JJ, et al. 2020. SoilTemp: a global database of near-surface temperature. Glob. Chang. Biol. 26, 6616-6629. ( 10.1111/gcb.15123) [DOI] [PubMed] [Google Scholar]
- 25.Thakur MP. 2020. Climate warming and trophic mismatches in terrestrial ecosystems: the green–brown imbalance hypothesis. Biol. Lett. 16, 20190770. ( 10.1098/rsbl.2019.0770) [DOI] [Google Scholar]
- 26.Liu H, Wang H, Li N, Shao J, Zhou X, van Groenigen KJ, Thakur MP. 2022. Phenological mismatches between above- and belowground plant responses to climate warming. Nature Climate Change 12, 97-102. ( 10.1038/s41558-021-01244-x) [DOI] [Google Scholar]
- 27.Zhou L, et al. 2022. Global systematic review with meta-analysis shows that warming effects on terrestrial plant biomass allocation are influenced by precipitation and mycorrhizal association. Nat. Commun. 13, 4914. ( 10.1038/s41467-022-32671-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C. 2015. How tree roots respond to drought. Front. Plant. Sci. 6, 547. ( 10.3389/fpls.2015.00547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasibeder R, Fuchslueger L, Richter A, Bahn M. 2015. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 205, 1117-1127. ( 10.1111/nph.13146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, et al. 2019. Plants alter their vertical root distribution rather than biomass allocation in response to changing precipitation. Ecology 100, e02828. ( 10.1002/ecy.2828) [DOI] [PubMed] [Google Scholar]
- 31.Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30-50. ( 10.1111/j.1469-8137.2011.03952.x) [DOI] [PubMed] [Google Scholar]
- 32.Turner NC. 2019. Imposing and maintaining soil water deficits in drought studies in pots. Plant. Soil 439, 45-55. ( 10.1007/s11104-018-3893-1) [DOI] [Google Scholar]
- 33.Poorter H, Bühler J, van Dusschoten D, Climent J, Postma JA. 2012. Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 39, 839-850. ( 10.1071/FP12049) [DOI] [PubMed] [Google Scholar]
- 34.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Ann. Rev. Ecol. Evol. Syst. 45, 471-493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 35.Huang Y, et al. 2018. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 362, 80-83. ( 10.1126/science.aat6405) [DOI] [PubMed] [Google Scholar]
- 36.Cowles JM, Wragg PD, Wright AJ, Powers JS, Tilman D. 2016. Shifting grassland plant community structure drives positive interactive effects of warming and diversity on aboveground net primary productivity. Glob. Chang. Biol. 22, 741-749. ( 10.1111/gcb.13111) [DOI] [PubMed] [Google Scholar]
- 37.Wagg C, O'Brien MJ, Vogel A, Scherer-Lorenzen M, Eisenhauer N, Schmid B, Weigelt A. 2017. Plant diversity maintains long-term ecosystem productivity under frequent drought by increasing short-term variation. Ecology 98, 2952-2961. ( 10.1002/ecy.2003) [DOI] [PubMed] [Google Scholar]
- 38.Fry EL, Manning P, Allen DG, Hurst A, Everwand G, Rimmler M, Power SA. 2013. Plant functional group composition modifies the effects of precipitation change on grassland ecosystem function. PLoS ONE 8, e57027. ( 10.1371/journal.pone.0057027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnabel F, et al. 2021. Species richness stabilizes productivity via asynchrony and drought-tolerance diversity in a large-scale tree biodiversity experiment. Sci. Adv. 7, eabk1643. ( 10.1126/sciadv.abk1643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JA, Koch AM, Forsythe J, Johnson NC, Tilman D, Klironomos J. 2020. Resistance of soil biota and plant growth to disturbance increases with plant diversity. Ecol. Lett. 23, 119-128. ( 10.1111/ele.13408) [DOI] [PubMed] [Google Scholar]
- 41.Lozano YM, Aguilar-Trigueros CA, Flaig IC, Rillig MC. 2020. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 34, 2224-2235. ( 10.1111/1365-2435.13656) [DOI] [Google Scholar]
- 42.Craven D, et al. 2018. Multiple facets of biodiversity drive the diversity–stability relationship. Nat. Ecol. Evol. 2, 1579-1587. ( 10.1038/s41559-018-0647-7) [DOI] [PubMed] [Google Scholar]
- 43.Huwaldt JA, Steinhorst S. 2013. Plot digitizer 2.6.8. PlotDigitizer Software (https://plotdigitizer.com/)
- 44.Schneider CA , Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671-675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. 2021. Introduction to meta-analysis. New York, NY: John Wiley & Sons. [Google Scholar]
- 46.Gurevitch J, Morrow LL, Wallace A, Walsh JS. 1992. A meta-analysis of competition in field experiments. Am. Nat. 140, 539-572. ( 10.1086/285428) [DOI] [Google Scholar]
- 47.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304-1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 48.Viechtbauer W. 2005. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J. Educ. Behav. Stat. 30, 261-293. ( 10.3102/10769986030003261) [DOI] [Google Scholar]
- 49.Koricheva J, Gurevitch J, Mengersen K. 2013. Handbook of meta-analysis in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 50.Cinar O, Umbanhowar J, Hoeksema JD, Viechtbauer W. 2021. Using information-theoretic approaches for model selection in meta-analysis. Res. Synth. Methods 12, 537-556. ( 10.1002/jrsm.1489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R Core Development Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 52.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1-48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 53.Barton K, Barton MK. 2015. Package ‘mumin’. Version 1, 439.
- 54.Keenan TF, Williams CA. 2018. The terrestrial carbon sink. Annu. Rev. Environ. Res. 43, 219-243. ( 10.1146/annurev-environ-102017-030204) [DOI] [Google Scholar]
- 55.Dusenge ME, Duarte AG, Way DA. 2019. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 221, 32-49. ( 10.1111/nph.15283) [DOI] [PubMed] [Google Scholar]
- 56.Xu L, et al. 2021. Changes in global terrestrial live biomass over the 21st century. Sci. Adv. 7, eabe9829. ( 10.1126/sciadv.abe9829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volaire F. 2018. A unified framework of plant adaptive strategies to drought: crossing scales and disciplines. Glob. Change Biol. 24, 2929-2938. ( 10.1111/gcb.14062) [DOI] [PubMed] [Google Scholar]
- 58.Kannenberg SA, Guo JS, Novick KA, Anderegg WRL, Feng X, Kennedy D, Konings AG, Martínez-Vilalta J, Matheny AM. 2022. Opportunities, challenges and pitfalls in characterizing plant water-use strategies. Funct. Ecol. 36, 24-37. ( 10.1111/1365-2435.13945) [DOI] [Google Scholar]
- 59.Heitschmidt RK, Klement KD, Haferkamp MR. 2005. Interactive effects of drought and grazing on northern great plains rangelands. Rangeland Ecol. Manage. 58, 11-19. () [DOI] [Google Scholar]
- 60.Mackie KA, Zeiter M, Bloor JMG, Stampfli A. 2019. Plant functional groups mediate drought resistance and recovery in a multisite grassland experiment. J. Ecol. 107, 937-949. ( 10.1111/1365-2745.13102) [DOI] [Google Scholar]
- 61.Johnson DM, et al. 2018. Co-occurring woody species have diverse hydraulic strategies and mortality rates during an extreme drought. Plant Cell Environ. 41, 576-588. ( 10.1111/pce.13121) [DOI] [PubMed] [Google Scholar]
- 62.Wright IJ, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821-827. ( 10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 63.Kattge J, et al. 2020. TRY plant trait database – enhanced coverage and open access. Glob. Change Biol. 26, 119-188. ( 10.1111/gcb.14904) [DOI] [PubMed] [Google Scholar]
- 64.Cavender-Bares J, Bazzaz FA. 2000. Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia 124, 8-18. ( 10.1007/PL00008865) [DOI] [PubMed] [Google Scholar]
- 65.Shao J, et al. 2022. Warming effects on grassland productivity depend on plant diversity. Glob. Ecol. Biogeogr. 31, 588-598. ( 10.1111/geb.13441) [DOI] [Google Scholar]
- 66.Poorter H, Fiorani F, Pieruschka R, Wojciechowski T, van der Putten WH, Kleyer M, Schurr U, Postma J. 2016. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 212, 838-855. ( 10.1111/nph.14243) [DOI] [PubMed] [Google Scholar]
- 67.Kennedy AD. 1995. Simulated climate change: are passive greenhouses a valid microcosm for testing the biological effects of environmental perturbations? Glob. Change Biol. 1, 29-42. ( 10.1111/j.1365-2486.1995.tb00004.x) [DOI] [Google Scholar]
- 68.Wilschut RA, et al. 2022. Combined effects of warming and drought on plant biomass depend on plant woodiness and community type: a meta-analysis. Figshare. ( 10.6084/m9.figshare.c.6214736) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wilschut RA, et al. 2022. Combined effects of warming and drought on plant biomass depend on plant woodiness and community type: a meta-analysis. Figshare. ( 10.6084/m9.figshare.c.6214736) [DOI] [PMC free article] [PubMed]
Data Availability Statement
We provide the database with extracted data from previously published studies (electronic supplementary material, data S1) as well as the effect size data (electronic supplementary material, data S2) as electronic supplementary material [68].