Abstract
Aims/Introduction
Among colorectal cancer (CRC) patients, pre‐existing diabetes is suggested to influence poor prognosis, but the impact on adjuvant chemotherapy implementation is largely unknown. We aimed to compare the implementation rate of adjuvant chemotherapy between CRC patients with and without pre‐existing diabetes in a retrospective cohort study.
Materials and Methods
Colorectal cancer diagnosis information was obtained from the hospital‐based cancer registry of patients with stage III CRC who underwent curative surgery in 2013 in Japan (n = 6,344). Health claims data were used to identify diabetes and chemotherapy. We examined the association between diabetes and the implementation rate of adjuvant chemotherapy using a generalized linear model adjusted for age, sex, updated Charlson Comorbidity Index, hospital type and prefecture. Furthermore, we applied a mediation analysis to examine the extent to which postoperative complications mediated the association.
Results
Of the 6,344 patients, 1,266 (20.0%) had diabetes. The mean ages were 68.2 and 71.3 years for patients without and with diabetes, respectively. Compared with those without diabetes, patients with diabetes were less likely to receive adjuvant chemotherapy (crude rate 58.9 and 49.8%; adjusted percentage point difference 4.6; 95% confidence interval 1.7–7.5). The difference was evident for patients aged <80 years, and larger for platinum‐containing regimens than others. Mediation analysis showed that postoperative complications explained 9.1% of the inverse association between diabetes and adjuvant chemotherapy implementation.
Conclusions
We observed that patients with stage III CRC and diabetes are less likely to receive adjuvant chemotherapy than those without diabetes, and postoperative complications might partially account for the association.
Keywords: Adjuvant chemotherapy, Colorectal cancer, Diabetes
Preexisting diabetes may interfere with adjuvant chemotherapy for colorectal cancer. The interfering with the therapy is remarkable in platinating agent‐based regimens. Postoperative complications partially account for the association.

INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer deaths, and was the cause of approximately 880,000 deaths in 2018 worldwide 1 . For stage III CRC patients, micrometastases influence the exacerbation of poor prognosis; hence, adjuvant chemotherapy is recommended as per clinical practice guidelines 2 , 3 . Previous studies have shown an association between pre‐existing diabetes mellitus and CRC, with respect to both the CRC risk 4 and poor prognosis 5 , 6 . Additionally, a previous meta‐analysis showed that having diabetes mellitus as a comorbidity was associated with increased mortality in CRC patients 6 . Although the mechanisms underlying the influence of diabetes mellitus on mortality from CRC are still unclear, several studies have suggested that endogenous hyperinsulinemia and high concentrations of insulin‐like growth factor‐I facilitate carcinogenesis and metastases 7 , 8 , 9 , 10 , 11 . Furthermore, in patients with stage III colon cancer, those with pre‐existing diabetes mellitus were reportedly less likely to be treated with adjuvant chemotherapy than patients without diabetes mellitus 12 . Thus, pre‐existing diabetes mellitus might be responsible for the inadequate treatment of CRC. However, previous studies have not examined which regimen type has a lower implementation rate among CRC patients with pre‐existing diabetes mellitus, nor have background factors that explain this association been investigated.
For stage III CRC, implementation of adjuvant chemotherapy has been established as an indicator for the quality of cancer care 13 , 14 in Japan. Accordingly, we aimed to compare the status of adjuvant chemotherapy implementation for stage III CRC between patients with and without pre‐existing diabetes mellitus. The comparison of the implementation status was also assessed by the regimen type. Furthermore, we examined whether postoperative complications mediated the association between pre‐existing diabetes mellitus and the implementation of adjuvant chemotherapy. Our investigation would help to clarify the actual treatment for CRC patients with pre‐existing diabetes mellitus and improve the quality of CRC care.
MATERIALS AND METHODS
Study design
The present retrospective cohort study utilized data from 226 Designated Cancer Care Hospitals 13 . The Designated Cancer Care Hospitals provided Hospital‐based Cancer Registry 15 data, as well as health claims data based on the Diagnosis Procedure Combination/Per‐Diem Payment System (DPC) 16 , which was used to identify the presence or absence of diabetes mellitus, other comorbidities and adjuvant chemotherapy. The Hospital‐based Cancer Registry data provided information on CRC patients diagnosed with pathological stage III in 2013, and the dataset was linked to DPC data (October 2012–December 2014) using hospital and patient identification numbers.
For our analysis, we included patients diagnosed with pathological stage III CRC (n = 10,037) who received curative surgery of the colon or rectum (n = 9,375) and did not receive preoperative chemotherapy (n = 9,360). We excluded patients who were not linked to the DPC data before hospitalization to 8 weeks after surgery. Our final analytical sample included 6,344 patients (Figure 1).
Figure 1.
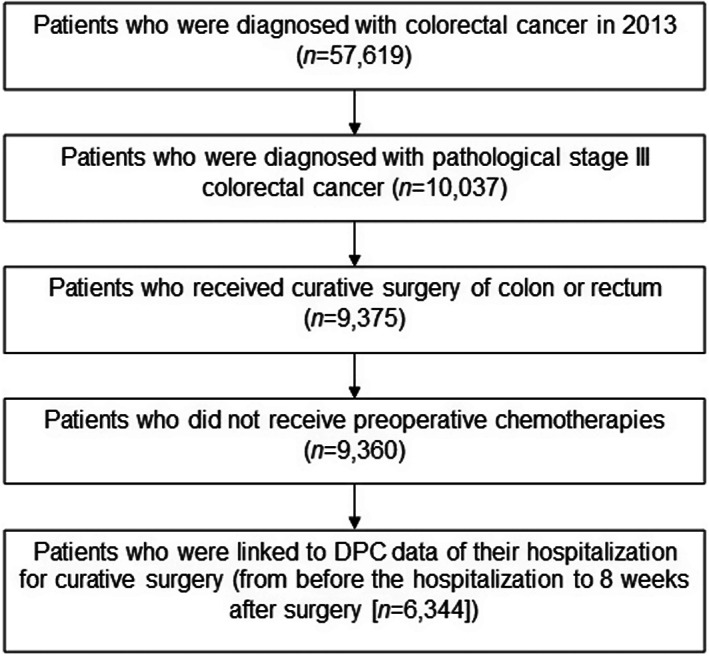
Flow chart of the study patients. DPC, Diagnosis Procedure Combination/Per‐Diem Payment.
Written informed consent was difficult to obtain, because some of the patients had died. Instead, we disclosed information regarding the current study, and gave the patient or the patient's agent an opportunity to refuse participation. The protocol of the current study was approved by the institutional review board of the National Cancer Center of Japan (approval No. 2018‐048), and was carried out according to the guidelines laid down in the Declaration of Helsinki.
Definition of diabetes mellitus
The DPC data carries information on disease codes based on the International Classification of Diseases, 10th Revision (ICD‐10). The disease codes are encoded in ‘main diagnosis,’ ‘admission‐precipitating diagnosis,’ ‘most resource‐consuming diagnosis,’ ‘second most resource‐consuming diagnosis,’ ‘comorbidities present at the time of admission’ and ‘conditions arising after admission’ 16 . Patients found to have a value of ‘E10x,’ ‘E11x,’ ‘E12x,’ ‘E13x’ or ‘E14x’ (code ICD‐10 indicating diabetes mellitus) in ‘admission‐precipitating diagnosis’ or ‘comorbidities present at the time of admission’ were identified as having diabetes mellitus. Patients who received antidiabetic agents (Table S1) during the period from the date of admission to the date of surgery were also considered to have diabetes mellitus. The diabetes mellitus diagnosis defined by the DPC data was validated in a previous study 17 , 18 .
Implementation rate of adjuvant chemotherapy
The indicator calculation method for the quality of cancer care was used to identify the implementation of adjuvant chemotherapy 14 . Specifically, we collected the data on whether patients received adjuvant chemotherapy for 8 weeks after surgery based on the procedure and drug codes of DPC data, and calculated the implementation rate.
Statistical analysis
The implementation rate of adjuvant chemotherapy was compared between patients with pre‐existing diabetes mellitus and those without diabetes mellitus, using a generalized linear model with normal distribution. In this model, we adjusted for age, sex, updated Charlson Comorbidity Index (CCI) 19 , type of hospital (Designated Cancer Care Hospitals or others) and prefectures where hospitals were located. To compute the CCI, we used diseases in the target items (‘admission‐precipitating diagnosis’ and ‘comorbidities present at the time of admission’) in the DPC data 19 . Diabetes and CRC were excluded from the CCI calculation. Furthermore, we carried out an age‐stratified analysis (<60, 60–69, 70–79, ≥80 years) and an analysis stratified by the absence or presence of additional non‐diabetes mellitus comorbidities (Table S2) 20 using diseases in the target items (‘admission‐precipitating diagnosis’ and ‘comorbidities present at the time of admission’) in the DPC data. We additionally carried out a comparison of the implementation rate of adjuvant chemotherapy between patients with an absence and with a presence of diabetes chronic complications (Table S3) 20 , among patients with pre‐existing diabetes mellitus. The presence of the complications was identified using the items in the DPC data, which were the same as those used in the stratified analysis of the non‐diabetes mellitus comorbidities.
We used mediation analysis to examine the mediating role of postoperative complications. The presence of postoperative complications, including ileus, sepsis, vascular complications, cardiac events, infectious diseases and acute renal failure 21 , was defined using the disease codes encoded in ‘conditions arising after admission’ in the DPC data. The total effect, direct effect (controlled direct effect + reference interaction), indirect effect (pure indirect effect + mediated interaction), and the ratios of direct and indirect effect to total effect were computed 22 , 23 using a command “med4way” 24 in Stata (StataCorp LLC, College Station, TX, USA). The direct effect showed the effect of diabetes mellitus on adjuvant chemotherapy implementation in the absence of postoperative complications, whereas the indirect effect represented the effect mediated through postoperative complications. To ensure the temporal order of the mediator (postoperative complications, prior) and the outcome (adjuvant chemotherapy implementation, posterior), we excluded 42 patients who could not be determined as having received adjuvant chemotherapy after the onset of postoperative complications (n = 6,302).
The comparison of the implementation rate of adjuvant chemotherapy was also examined according to the regimen type, which included platinum‐containing regimens or others (Table S4). Furthermore, the proportion of antiemetic drug therapy, administered to relieve the emesis of chemotherapy, was compared between patients with and without pre‐existing diabetes mellitus according to the regimen type. We defined ‘all antiemetic drug’ as administration of both dexamethasone and 5‐hydroxytryptamine 3 (5‐HT3) receptor antagonists, and examined the differences in the proportion of administration for ‘all antiemetic drugs’ and individual antiemetic drugs.
For the sensitivity analysis, we analyzed the data that excluded patients with renal disease (n = 57), mild liver disease (n = 249) and moderate or severe liver disease (n = 6) 20 according to the same items as the target for the CCI calculation (Table S5). For another sensitivity analysis, we changed the definition of diabetes as one based on the disease code only. All the statistical analyses were carried out using Stata version 15 (StataCorp LLC).
RESULTS
Among the 6,344 stage III CRC patients included in the present study, 1,266 had pre‐existing diabetes mellitus, whereas 5,078 did not have diabetes mellitus. The percentage of men was higher among patients with diabetes mellitus (61.9%) than among patients without diabetes mellitus (50.8%), and the mean (standard deviation [SD]) age was slightly higher in patients with diabetes mellitus (71.3, SD 9.7) than in those without diabetes mellitus (68.2, SD 12.0). The mean CCI did not differ between patients with diabetes mellitus (0.77, SD 1.60) and those without diabetes mellitus (0.81, SD1.78; Table 1).
Table 1.
Characteristics of stage III colorectal cancer patients according to the presence or absence of pre‐existing diabetes mellitus
| All | Without DM | With DM | |
|---|---|---|---|
| No. patients | 6,344 | 5,078 | 1,266 |
| Men (%) | 53.0 | 50.8 | 61.9 |
| Age in years, mean (SD) | 68.8 (11.6) | 68.2 (12.0) | 71.3 (9.7) |
| Type of hospital | |||
| Designated Cancer Care Hospitals (%) | 91.5 | 91.5 | 91.6 |
| Other hospitals (%) | 8.5 | 8.5 | 8.4 |
| Updated Charlson Comorbidity Index, mean (SD) | 0.80 (1.75) | 0.81 (1.78) | 0.77 (1.60) |
| Congestive heart failure (%) | 2.9 | 2.4 | 4.7 |
| Dementia (%) | 1.0 | 1.0 | 1.3 |
| Chronic pulmonary disease (%) | 2.8 | 2.9 | 2.2 |
| Rheumatologic disease (%) | 0.6 | 0.5 | 0.8 |
| Mild liver disease (%) | 3.9 | 3.8 | 4.6 |
| Diabetes with chronic complications (%) | 1.9 | 0 | 9.4 |
| Hemiplegia or paraplegia (%) | 0.03 | 0.04 | 0 |
| Renal disease (%) | 0.9 | 0.8 | 1.3 |
| Any malignancy, including leukemia and lymphoma (%) | 7.7 | 7.7 | 7.8 |
| Moderate or severe liver disease (%) | 0.09 | 0.10 | 0.08 |
| Metastatic solid tumor (%) | 7.7 | 8.1 | 6.1 |
| AIDS/HIV (%) | 0.06 | 0.06 | 0 |
AIDS/HIV, acquired immunodeficiency syndrome/human immunodeficiency virus; CRC, colorectal cancer; DM, diabetes mellitus; SD, standard deviation.
Of the patients without diabetes mellitus, 2,993 (58.9%) received adjuvant chemotherapy, whereas 631 (49.8%) patients with diabetes mellitus received adjuvant chemotherapy. The difference in crude percentage points for adjuvant chemotherapy implementation rate was 9.1 (95% confidence interval [CI] 6.0–12.2). The difference was reduced after adjusting for age, sex, CCI, type of hospital, and prefecture (difference of percentage points 4.6, 95% CI 1.7–7.5; Table 2). In the age‐stratified analysis, the magnitudes of effect estimates tended to be greater in patients aged <80 years than in those aged ≥80 years (<60 years 7.7, 95% CI −0.4, 15.6; 60–69 years 8.9, 95% CI 3.7–14.2; 70–79 years 7.8, 95% CI 2.9–12.8); ≥80 years 0.2, 95% CI −5.1, 5.4; Table 2). When the model was adjusted additionally for postoperative complications, the differences became slightly smaller (all 3.9, 95% CI 1.0–6.8, <60 years 7.4, 95% CI −0.5, 15.4; 60–69 years 7.5, 95% CI 2.3–12.7; 70–79 years 7.2, 95% CI 2.2–12.2; ≥80 years −0.3, 95% CI −5.5, 4.9; Table 2). When we stratified by the absence or presence of additional non‐diabetes mellitus comorbidities, the difference was slightly smaller in patients without additional comorbidities (4.3, 95% CI 0.6–8.0, n = 4,212, for patients without additional comorbidities; 4.4, 95% CI −0.2, 9.1, n = 2,132, for patients with additional comorbidities; Table 2). When we made a comparison between the absence and presence of chronic complications of diabetes mellitus, we found that patients with diabetic chronic complications were less likely to receive adjuvant chemotherapy than those with pre‐existing diabetes mellitus without diabetic chronic complications (adjusted percentage point difference 10.2, 95% CI 1.4–18.9; Table S6).
Table 2.
Number of stage III colorectal cancer patients and percentage point difference of rate for receiving adjuvant chemotherapy between the presence and absence of pre‐existing diabetes mellitus
| Without DM (n = 5,078) | With DM (n = 1,266) | Difference in rate between patients without and with DM, percentage points (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Received/not received (n) | Rate of adjuvant chemotherapy (%) | Received/not received (n) | Rate of adjuvant chemotherapy (%) | Crude | Adjusted † | Adjusted ‡ | |
| All (n = 6,344) | 2,993/2,085 | 58.9 | 631/635 | 49.8 | 9.1 (6.0, 12.2) | 4.6 (1.7, 7.5) | 3.9 (1.0, 6.8) |
| Age group stratification | |||||||
| <60 years (n = 1,274) | 849/278 | 75.3 | 101/46 | 68.7 | 6.6 (−1.3, 14.5) | 7.7 (−0.4, 15.6) | 7.4 (−0.5, 15.4) |
| 60–69 years (n = 1,876) | 1,093/406 | 72.9 | 240/137 | 63.7 | 9.3 (3.9, 14.6) | 8.9 (3.7, 14.2) | 7.5 (2.3, 12.7) |
| 70–79 years (n = 2,110) | 917/687 | 57.2 | 252/254 | 49.8 | 7.4 (2.4, 12.4) | 7.8 (2.9, 12.8) | 7.2 (2.2, 12.2) |
| ≥80 years (n = 1,084) | 134/714 | 15.8 | 38/198 | 16.1 | −0.3 (−5.6, 5.0) | 0.2 (−5.1, 5.4) | −0.3 (−5.5, 4.9) |
| Stratification of non‐DM comorbidities | |||||||
| No any comorbidity (n = 4,212) | 2,130/1,297 | 62.2 | 423/362 | 53.9 | 8.3 (4.4, 12.1) | 4.3 (0.6, 8.0) | 3.3 (−0.3, 7.0) |
| Any comorbidity (n = 2,132) | 863/788 | 52.2 | 208/273 | 43.2 | 9.0 (4.0, 14.1) | 4.4 (−0.2, 9.1) | 4.1 (−0.6, 8.7) |
Adjusted for age (excluding analysis for age group stratification), sex, Charlson Comorbidity Index, type of hospital (Designated Cancer Care Hospitals or others) and prefecture where hospitals are located using the multilevel generalized linear model.
Additionally adjusted for the absence or presence of postoperative complications.
CI, confidence interval; CRC, colorectal cancer; DM, diabetes mellitus.
Patients without diabetes mellitus were less likely to have postoperative complications than those with diabetes mellitus, and patients without postoperative complications were more likely to receive adjuvant chemotherapy than those with postoperative complications (Table S7). In our mediation analysis, the effect of pre‐existing diabetes mellitus accounted for most of the total effect (total effect 5.1, 95% CI 2.3–7.9, direct effect 4.6, 95% CI 1.7–7.5, indirect effect 0.5, 95% CI 0.2–1.0; Table 3). The mediation of postoperative complications explained 9.1% of the difference in the implementation of adjuvant chemotherapy between patients without and with diabetes mellitus (Table 3).
Table 3.
Results of the mediation analysis (n = 6,302)
| Effects | Percentage point difference (95% CI) | Percentage of TE, % (95% CI) |
|---|---|---|
| Total effect | 5.1 (2.3, 7.9) | 100 |
| Direct effect † | 4.6 (1.7, 7.5) | 90.9 (72.7, 97.2) |
| Indirect effect ‡ | 0.5 (0.2, 1.0) | 9.1 (2.8, 27.3) |
Adjusted for age, sex, Charlson Comorbidity Index, type of hospital (Designated Cancer Care Hospitals or others) and prefecture where hospitals are located.
Direct effect: the effect of diabetes on adjuvant chemotherapy implementation in the absence of postoperative complications.
Indirect effect: the effect of diabetes on adjuvant chemotherapy implementation mediated through postoperative complications.
CI, confidence interval; TE, total effect.
The adjusted percentage point difference in the implementation rate of platinum‐containing regimens was larger than that in other regimens (platinum‐containing regimens 4.2, 95% CI 2.0–6.5; other regimens 0.4, 95% CI −2.5, 3.3; Table 4). Furthermore, almost all of the patients who received platinum‐containing regimens were administered antiemetic drugs; whereas patients who received other regimens were not, regardless of whether they had pre‐existing diabetes mellitus or not. The difference in antiemetic administration between patients without and with diabetes mellitus among patients who received adjuvant chemotherapy was 4.5 (95% CI 0.6–8.4) for all the regimens, being reflected by the difference in the implementation rate of platinum‐containing regimens. The difference became smaller when we stratified by regimen types. The trend was similar among the type of antiemetic drugs used (Table S8).
Table 4.
Number of stage III colorectal cancer patients and difference in rate of receiving adjuvant chemotherapy between the presence and absence of pre‐existing diabetes mellitus per regimen type
| Regimen types | Without DM (n = 5,078) | With DM (n = 1,266) | Adjusted † difference in rate between patients without and with DM, percentage points (95% CI) | ||
|---|---|---|---|---|---|
| Received (n) | Rate of adjuvant chemotherapy (%) | Received (n) | Rate of adjuvant chemotherapy (%) | ||
| Platinum‐containing regimens ‡ | 1,188 | 23.4 | 208 | 16.4 | 4.2 (2.0, 6.5) |
| Other regimens § | 1,805 | 35.5 | 423 | 33.4 | 0.4 (−2.5, 3.3) |
Adjusted for age, sex, Charlson Comorbidity Index, type of hospital (Designated Cancer Care Hospitals or others) and prefecture where hospitals are located using the generalized linear model.
Platinum‐containing regimens include FOLFOX (folinic acid, fluorouracil, and oxaliplatin), CapeOX (capecitabine and oxaliplatin), UFT + LV + L‐OHP (tegafur/uracil, leucovorin, and oxaliplatin) and S‐1 + Plt (tegafur/gimeracil/oteracil and platinating agent).
Other regimens include UFT + LV (tegafur/uracil and leucovorin), 5FU + LV (fluorouracil and leucovorin), Cape (capecitabine), S‐1 (tegafur/gimeracil/oteracil) and FOLFIRI (folic acid, fluorouracil, and irinotecan).
CI, confidence interval; CRC, colorectal cancer; DM, diabetes mellitus.
When we excluded patients with renal or liver disease, the difference in the implementation rate of adjuvant chemotherapy between patients without and with diabetes mellitus was generally the same or slightly attenuated (4.3, 95% CI 1.3–7.2). In this sensitivity analysis, the proportion mediated through postoperative complications on the association between diabetes mellitus and adjuvant chemotherapy implementation was 12.6%. Additionally, when we defined patients with diabetes based on the disease code only (patients with diabetes: n = 1,038), the results were similar.
DISCUSSION
We observed that the implementation rate of adjuvant chemotherapy was 4.6 percentage points lower in patients with pre‐existing diabetes mellitus than in those without diabetes mellitus, after adjusting for potential confounders. The present results suggested that pre‐existing diabetes mellitus in patients with stage III CRC was associated with a lower implementation rate of adjuvant chemotherapy, especially for the platinum‐containing regimens. Our findings highlighted the need for heightened awareness of CRC care for patients with pre‐existing diabetes mellitus, to improve the implementation rate.
A previous study among stage III colon cancer patients who were diagnosed between 1993 and 1999 in the USA reported that the difference in the implementation rate of adjuvant chemotherapy was 2.4 percentage points (60.7% for patients without diabetes and 58.3% for patients with diabetes) 12 . The difference shown in the present results was larger in magnitude; this heterogeneity might have been caused by a difference in era, ethnic group or cancer site of interest (our study also included rectum cancer). Pre‐existing diabetes mellitus might influence CRC prognosis by lowering the adjuvant chemotherapy implementation rate. However, we did not consider the prognosis of the patients in our analyses. A comparison of the prognosis after adjuvant chemotherapy between patients with and without diabetes mellitus is recommended in future studies.
Previous studies have suggested that postoperative complications, which might hinder the initiation of adjuvant chemotherapy, are relatively common in cancer patients with pre‐existing diabetes mellitus 25 . Thus, we speculated that postoperative complications might result in a partial mediation of the association of comorbid diabetes mellitus with a reduction in the implementation rate of adjuvant chemotherapy. The mediation analysis estimated that the effects mediated by postoperative complications accounted for approximately just 9% of the total effects. The remaining 91% of the association might be explained by patient characteristics, such as poor glycemic control, severity of the diabetes mellitus, the presence of additional comorbidities and socioeconomic status 26 . The present findings from the mediation analysis suggested a limited mediating role of postoperative complications in the association between diabetes and implementation rate of adjuvant chemotherapy. Therefore, optimal glycemic control might increase the implementation rate of adjuvant chemotherapy, through mainly mechanisms other than the prevention of postoperative complications. In addition, the present results showed that with respect to the severity of the diabetes mellitus, the patients who experienced diabetic chronic complications showed a larger reduction in the implementation rate than those without the complications.
We compared the regimen types to explain the reduction in the implementation rate among diabetes mellitus patients. In previous randomized controlled trials, it was shown that CapeOX and FOLFOX (oxaliplatin combination therapies) for patients with stage III CRC were associated with an improvement of prognosis compared with 5FU + LV (fluorouracil and leucovorin) 27 , 28 . However, the platinum‐containing regimens are a risk for adverse reactions, such as neuropathy, nephropathy and heart failure, as a result of the platinating agent. In the present study, the difference was larger in platinum‐containing regimens than in other regimens. Because diabetes mellitus has similar risks that could possibly exacerbate the adverse reactions by the platinating agents, pre‐existing diabetes mellitus might be a barrier to receiving platinum‐containing regimens, even if the regimens could improve the prognosis of CRC patients. To prevent the poor general condition due to diabetes mellitus, incorporating the diabetologist's support in the clinical team might be beneficial.
The age‐stratified analysis showed significant differences in the implementation rate of adjuvant chemotherapy between patients without and with diabetes mellitus among those aged <80 years. However, in patients aged >80 years, there was little difference. Previous studies suggested that older patients with CRC received adjuvant chemotherapy less frequently 29 , 30 . Therefore, the higher the age, the greater the number of reasons (e.g., poor general condition caused by aging) that might obstruct the chemotherapy and attenuate the influence of preexisting diabetes mellitus.
Alternatively, the low implementation rate of adjuvant chemotherapy among diabetes mellitus patients might be explained by the higher prevalence of comorbid kidney diseases in cancer patients with diabetes mellitus. According to a report on adjuvant chemotherapy for CRC care in 2013 31 , the reasons for not administering adjuvant chemotherapy were the presence of kidney disorders, liver disorders, other comorbidities, poor general conditions, older age, multiple cancers and postoperative complications. However, given that we found similar results when excluding patients with renal and liver diseases, the residual confounding by these illnesses is unlikely to explain the association. Furthermore, the difference was marginally significant when we excluded patients with any other non‐diabetes mellitus comorbidities, suggesting an association of pre‐existing diabetes mellitus itself with a reduction of the implementation rate of adjuvant chemotherapy. Other reasons could not be controlled; therefore, further studies regarding these reasons are required.
The strength of the present study was that this was a nationwide survey that analyzed the details of regimen types, antiemetic drugs, the patient's age, diabetic chronic complications and other comorbidities. Despite the advantages, the present study also had some limitations. First, patients who were transferred to other hospitals for 8 weeks after surgery could not be included in our analysis, as we could not find out whether the patients received adjuvant chemotherapy at the new hospital. Second, the diabetes severity was not considered. The general health, which might interfere with chemotherapy, may be poorer depending on the seriousness of the diabetes mellitus. Third, the DPC data had some drawbacks as follows: (i) as the data were based on medical service fee requests, there was a lack of information regarding the clinical diagnosis; (ii) the data might have included erroneous calculations, billing omissions and coding errors. These errors may have led to an improper assessment of the diagnosis of diabetes mellitus. However, the diabetes mellitus diagnosis in the DPC data was validated 17 , 18 . Fourth, uncontrolled and residual confounding could not be completely ruled out. Thus, pre‐existing diabetes mellitus alone might not have been responsible for the observed difference in the implementation rate of adjuvant chemotherapy. Fifth, we could not estimate the effects of the pathological conditions themselves that caused diabetes on the administration of adjuvant chemotherapy owing to the small number of patients with the ICD‐10 code of E13x (diabetes induced by other diseases or drugs).
In conclusion, we found that pre‐existing diabetes mellitus was associated with a lower implementation rate of adjuvant chemotherapy, especially the platinum‐containing regimens, among stage III CRC patients. Approximately 9% of the association was explained by postoperative complications. From the viewpoint of equalization in the quality of CRC care, management by a clinical team that is aware that patients with pre‐existing diabetes mellitus have lower implementation rates of adjuvant chemotherapy might lead to a higher quality of cancer care and improved prognosis.
FUNDING
This research was funded by the AMED (grant number JP18ck0106370) from the Japan Agency for Medical Research and Development.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution (the institutional review board of the National Cancer Center of Japan, Approval No. 2018–048), and it conforms to the provisions of the Declaration of Helsinki.
Informed consent: Written informed consent was difficult to obtain, because some of the patients had died. Instead, we disclosed information regarding the current study, and gave the patient or the patient's agent an opportunity to refuse participation. The institutional review board of the National Cancer Center of Japan approved the use of the opt‐out approach for consent in the hospital.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1 | World Health Organization Anatomical Therapeutic Chemical codes of antidiabetic drugs for diabetes definition.
Table S2 | Comorbidities and International Classification of Diseases, 10th Revision codes for the stratified analysis according to the presence or absence of additional non‐diabetes comorbidities.
Table S3 | International Classification of Diseases, 10th Revision Codes for the stratified analysis by the presence or absence of chronic complications of diabetes.
Table S4 | Regimen types and included regimens.
Table S5 | Diseases and International Classification of Diseases, 10th Revision codes excluded from the sensitivity analysis.
Table S6 | Percentage point difference of rate for receiving adjuvant chemotherapy between the presence and absence of chronic complications of diabetes (n = 1,266)
Table S7 | Difference between diabetes and postoperative complications, and postoperative complications and adjuvant chemotherapy (n = 6,302).
Table S8 | Differences in antiemetic drugs, dexamethasone and 5‐hydroxytryptamine 3 receptor antagonist administration between the presence and absence of pre‐existing diabetes melliuts among patients who received adjuvant chemotherapy.
ACKNOWLEDGMENTS
We sincerely thank the all patients and staff members involved in the Hospital‐based Cancer Registry for their valuable contributions.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. International Agency for Research on Cancer . Colorectal Cancer: Globocan 2018. Cancer Today. Available from http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9‐Colorectum‐fact‐sheet.pdf. Accessed June 1, 2019. [Google Scholar]
- 2. Costas‐Chavarri A, Nandakumar G, Temin S, et al. Treatment of patients with early‐stage colorectal cancer: ASCO resource‐stratified guideline. J Glob Oncol 2019; 5: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018; 23: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta‐analysis. J Natl Cancer Inst 2005; 97: 1679–1687. [DOI] [PubMed] [Google Scholar]
- 5. Barone BB, Yeh HC, Snyder CF, et al. Long‐term all‐cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta‐analysis. JAMA 2008; 300: 2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein KB, Snyder CF, Barone BB, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta‐analysis. Dig Dis Sci 2010; 55: 1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannucci E. Insulin, insulin‐like growth factors and colon cancer: a review of the evidence. J Nutr 2001; 131: 3109S–3120S. [DOI] [PubMed] [Google Scholar]
- 8. Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin‐like growth factor‐I (IGF‐I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 2002; 94: 972–980. [DOI] [PubMed] [Google Scholar]
- 9. Wu Y, Yakar S, Zhao L, et al. Circulating insulin‐like growth factor‐I levels regulate colon cancer growth and metastasis. Cancer Res 2002; 62: 1030–1035. [PubMed] [Google Scholar]
- 10. Renehan AG, Zwahlen M, Minder C, et al. Insulin‐like growth factor (IGF)‐I, IGF binding protein‐3, and cancer risk: Systematic review and meta‐regression analysis. Lancet 2004; 363: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 11. Kasuga M, Ueki K, Tajima N, et al. Report of the Japan diabetes society/Japanese cancer association joint committee on diabetes and cancer. Cancer Sci 2013; 104: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gross CP, McAvay GJ, Guo Z, et al. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer 2007; 109: 2410–2419. [DOI] [PubMed] [Google Scholar]
- 13. Iwamoto M, Nakamura F, Higashi T. Monitoring and evaluating the quality of cancer care in Japan using administrative claims data. Cancer Sci 2016; 107: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higashi T, Nakamura F, Saruki N, et al. Establishing a quality measurement system for cancer care in Japan. Jpn J Clin Oncol 2013; 43: 225–232. [DOI] [PubMed] [Google Scholar]
- 15. Higashi T, Nakamura F, Shibata A, et al. The national database of hospital‐based cancer registries: a nationwide infrastructure to support evidence‐based cancer care and cancer control policy in Japan. Jpn J Clin Oncol 2014; 44: 2–8. [DOI] [PubMed] [Google Scholar]
- 16. Yasunaga H, Matsui H, Horiguchi H, et al. Clinical epidemiology and health services research using the diagnosis procedure combination database in Japan. Asian Pac J Dis Manag 2013; 7: 19–24. [Google Scholar]
- 17. Yamana H, Moriwaki M, Horiguchi H, et al. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 2017; 27: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanehara R, Goto A, Goto M, et al. Validation study on diabetes definitions using Japanese diagnosis procedure combination data among hospitalized patients. J Epidemiol 2021. 10.2188/jea.JE20210024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 20. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 21. Sugihara T, Yasunaga H, Horiguchi H, et al. Robot‐assisted versus other types of radical prostatectomy: population‐based safety and cost comparison in Japan, 2012‐2013. Cancer Sci 2014; 105: 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VanderWeele TJ. A unification of mediation and interaction: a 4‐way decomposition. Epidemiology 2014; 25: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue K, Yan Q, Arah OA, et al. Air pollution and adverse pregnancy and birth outcomes: Mediation analysis using metabolomic profiles. Curr Environ Health Rep 2020; 7: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Discacciati A, Bellavia A, Lee JJ, et al. Med4way: A Stata command to investigate mediating and interactive mechanisms using the four‐way effect decomposition. Int J Epidemiol 2019; 48: 15–20. [DOI] [PubMed] [Google Scholar]
- 25. Yap R, Wilkins S, Staples M, et al. The effect of diabetes on the perioperative outcomes of colorectal cancer surgery patients. PLoS One 2016; 11: e0167271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagamine Y, Kondo N, Yokobayashi K, et al. Socioeconomic disparity in the prevalence of objectively evaluated diabetes among older Japanese adults: JAGES cross‐sectional data in 2010. J Epidemiol 2019; 29: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andre T, Boni C, Mounedji‐Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–2351. [DOI] [PubMed] [Google Scholar]
- 28. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011; 29: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 29. Potosky AL, Harlan LC, Kaplan RS, et al. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol 2002; 20: 1192–1202. [DOI] [PubMed] [Google Scholar]
- 30. Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 2005; 294: 2703–2711. [DOI] [PubMed] [Google Scholar]
- 31. Center for Cancer Control and Information Services, National Cancer Center Japan . Development of a quality indicator and establishing quality measurement system for cancer care. Available from: https://www.ncc.go.jp/jp/cis/divisions/health_s/health_s/010/index.html Accessed October 19, 2020 (in Japanese).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | World Health Organization Anatomical Therapeutic Chemical codes of antidiabetic drugs for diabetes definition.
Table S2 | Comorbidities and International Classification of Diseases, 10th Revision codes for the stratified analysis according to the presence or absence of additional non‐diabetes comorbidities.
Table S3 | International Classification of Diseases, 10th Revision Codes for the stratified analysis by the presence or absence of chronic complications of diabetes.
Table S4 | Regimen types and included regimens.
Table S5 | Diseases and International Classification of Diseases, 10th Revision codes excluded from the sensitivity analysis.
Table S6 | Percentage point difference of rate for receiving adjuvant chemotherapy between the presence and absence of chronic complications of diabetes (n = 1,266)
Table S7 | Difference between diabetes and postoperative complications, and postoperative complications and adjuvant chemotherapy (n = 6,302).
Table S8 | Differences in antiemetic drugs, dexamethasone and 5‐hydroxytryptamine 3 receptor antagonist administration between the presence and absence of pre‐existing diabetes melliuts among patients who received adjuvant chemotherapy.
Data Availability Statement
Research data are not shared.


