Abstract
Aims/Introduction
To evaluate the association between ideal cardiovascular health metrics (ICVHM) and incident type 2 diabetes mellitus among Iranian men and women.
Materials and Methods
The study population included 7,488 Iranian adults aged ≥20 years (4,236 women) free from diabetes at baseline. The ICVHM was defined according to the American Heart Association's 2020 impact goals. The multivariable Cox proportional hazards regression analysis was used to calculate the hazard ratios (HRs) for ICVHM both as continuous and categorical variables.
Results
Over the median of 9.1 years of follow‐up, we identified 922 new cases of type 2 diabetes mellitus (526 women). Body mass index <30 kg/m2, untreated systolic/diastolic blood pressure <120/80 mmHg in both sexes, and physical activity ≥1,500 MET min/week (only among men) were significantly associated with a lower risk of type 2 diabetes mellitus. Each additional unit in the ICVHM was associated with a 21 and 15% lower risk of type 2 diabetes mellitus in men and women, respectively (P‐values <0.05). Compared with participants having poor cardiovascular health, the HR for type 2 diabetes mellitus risk was 0.69 (95% confidence interval [CI] 0.56–0.85) and 0.35 (95% CI 0.21–0.59) for men with intermediate and ideal CVHM, respectively. The corresponding values for women were 0.79 (95% CI 0.65–0.97) and 0.30 (95% CI 0.15–0.60), respectively. In a subpopulation with nutritional data (n = 2,236), ideal and intermediate nutritional status was associated with 83 and 77% lower risk of type 2 diabetes mellitus only among women (P‐values <0.05).
Conclusion
We found a strong inverse association between having higher global ICVHM with incident type 2 diabetes mellitus; which is mainly attributable to normal blood pressure, normal body weight, and intensive physical activity (only for men).
Keywords: American Heart Association, Ideal cardiovascular health metrics, Type 2 diabetes mellitus
Short abstract
Type 2 diabetes mellitus persists as a world epidemic, which is associated with increased risk of mortality and morbidity, particularly from cardiovascular diseases. Adherence to the American Heart Association cardiovascular health metrics, including body mass index, smoking status, physical activity, diet, total cholesterol, fasting plasma glucose, and blood pressure, is associated with a lower risk of cardiovascular disease and all‐cause mortality. In the current study, we examined the impact of sex on the association between ideal cardiovascular health metrics and the risk of type 2 diabetes mellitus. We found a strong inverse association between having higher global ideal cardiovascular health metrics with incident type 2 diabetes mellitus; the issue is mainly attributable to normal blood pressure, normal bodyweight and intensive physical activity (only for men).
INTRODUCTION
Type 2 diabetes mellitus persists as a world epidemic, which is associated with an increased risk of mortality and morbidity, particularly from cardiovascular diseases (CVDs) 1 , 2 , 3 . Diabetes was the ninth leading cause of mortality in 2019, estimated to have caused directly 1.5 million deaths globally 4 . The Middle East and North Africa region has the second‐highest increasing prevalence rate of type 2 diabetes mellitus in the world (87%), where it is estimated that 95 million of the population will be living with diabetes in 2030 5 . In Iran, the prevalence of diabetes based on a 2011 national survey was 9.90% in men and 12.86% in women, and the corresponding values in the 2016 survey were 10.01 and 11.55%, respectively 4 .
In January 2010, based on evidence from randomized clinical trials, the American Heart Association (AHA) proposed seven components as cardiovascular health metrics (CVHM), including four behavioral (body mass index [BMI], smoking, physical activity, and diet), and three biological factors (total cholesterol [TC], fasting plasma glucose [FPG], and blood pressure [BP]) 6 . According to the Iranian national data, the prevalence of achieving ideal CVHM (ICVHM) is relatively low in the general population 7 , 8 , with <4% of Iranian adults meeting six or more ICVHM 8 . Adherence to the AHA Life's Simple 7 has been associated with a lower risk of CVD and all‐cause mortality 9 , 10 . Most recently, an association between ICVHM and the development of type 2 diabetes mellitus has been described in a few studies mainly carried out among the US population, reporting that higher AHA‐defined ICVHM is accompanied by a lower risk of type 2 diabetes mellitus 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ; showing that type 2 diabetes mellitus risk was 30–60% and 71–89% lower in those with two or three and four or more ICVHM compared with participants who had zero or one ICVHM, respectively 16 , 17 , 18 .
To the best of our knowledge, the association of ICVHM with type 2 diabetes mellitus incidence in the high‐risk population of the Middle Eastern region has not been examined. Previous cohort studies did not examine the impact of sex on the association between ICVHM and the risk of type 2 diabetes mellitus. Hence, the objective of the present study was to investigate the sex‐specific association of ICVHM defined as the AHA's recommendation at baseline on incident type 2 diabetes mellitus in an Iranian population during approximately one decade of follow‐up in a population‐based cohort study.
MATERIALS AND METHODS
Study population
The Tehran Lipid and Glucose Study (TLGS) is a community‐based prospective cohort study carried out on an urban population in Tehran (Appendix S1). The design and methodology of the TLGS have been reported elsewhere 19 , 20 . For the present investigation, we included 9,997 participants aged ≥20 years who entered the third phase of the TLGS (as baseline). Exclusions included those with diagnosed type 2 diabetes mellitus at enrollment (671 undiagnosed type 2 diabetes mellitus and 438 on antidiabetic medication), missing data on baseline variables, including TC, high‐density lipoprotein cholesterol (HDL‐C), triglycerides (TG), FPG, 2‐h post‐challenge plasma glucose (2 h‐PCG), systolic BP, diastolic BP, smoking status, education levels, and marital status (n = 800), and follow‐up information on type 2 diabetes mellitus status to 20 March 2018 (n = 600), leaving us 7,488 individuals (3,252 men, 4,236 women). The nutrition information is available for a subsample of TLGS; thus, ICVHM based on all six components were recalculated for 2,236 individuals with available diet information in a secondary analysis (Figure 1).
Figure 1.
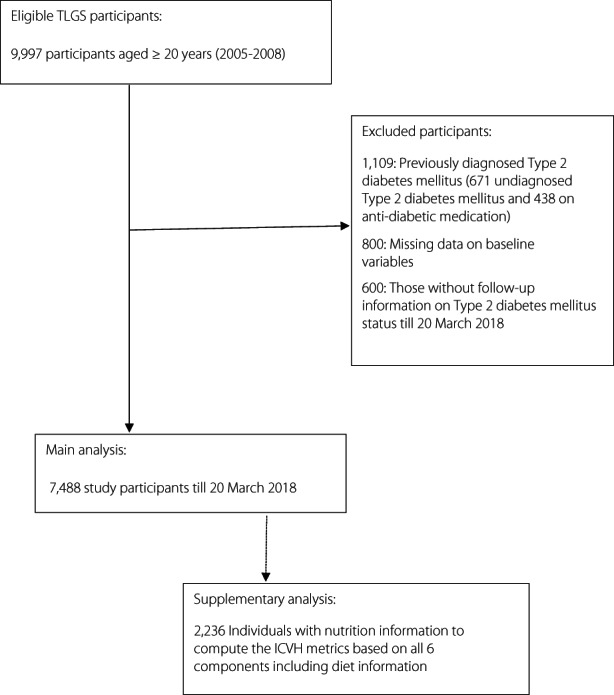
Flow diagram of the study participants. ICVH, ideal cardiovascular health metrics; T2DM, type 2 diabetes mellitus; TLGS, Tehran Lipids and Glucose Study.
Clinical and laboratory measurements
Study participants were interviewed, and demographic data and information about family history of diabetes (FH‐DM), history of CVD, medication history, education levels, marital status and smoking habits were collected using a standard questionnaire. Weight was measured to the nearest 100 g with light clothing and shoes removed. Height was measured in a standing position using a tape measure, while shoulders were in normal alignment. A blood sample including FPG, 2 h‐PCG, TG, TC, and HDL‐C was taken between 07.00 hours and 09.00 hours from all study participants after 12–14 h of overnight fasting. All the blood analyses were carried out at the TLGS research laboratory on the same day of blood collection 21 .
Variable definition
History of CVD was defined as previous ischemic heart disease or cerebrovascular accidents. Education levels were classified into three groups: <6 years (reference), 6–12 years, and ≥12 years of education. Marital status was categorized as single (reference), married or widowed/divorced. Type 2 diabetes mellitus was defined as having FPG ≥126 mg/dL and 2 h‐PCG ≥200 mg/dL or the use of antidiabetic medications. For the current study, the AHA's 2020 Impact Goals, which defined three CVHM categories – ‘ideal’, ‘intermediate’ and ‘poor’ – was used 6 . In the present study, FPG was regarded as a confounder rather than a component of CVHM. Therefore, the CVHM was computed using six components, including diet (Appendix S2). To calculate the total score for ICVHM, we recoded each metric as a binary variable (assuming a value of 1 point for the ‘ideal’ category vs 0 points for the ‘intermediate’ or ‘poor’ categories). The global ICVHM score was achieved by summing up the newly defined binary variables, and was categorized as ‘ideal’ if the total ICVHM number was four or more, ‘intermediate’ if the number was two or three, and ‘poor’ if the total ICVHM was zero or one 16 .
Statistical analysis
Baseline characteristics of the study population are described as the mean ± standard deviation values for continuous variables, and frequencies (%) for categorical variables. For covariates with a skewed distribution (e.g., TG), the median (interquartile range [IQR]) was also reported. Comparison of the baseline characteristics across global ICVH categories was carried out using the anova test for normally distributed continuous variables, the χ2‐test for categorical variables, and the Kruskal–Wallis test for skewed and ordered variables.
Among all the CVHM components, physical activity (P‐value = 0.008) and BP categories (P‐value = 0.04) had significant interactions with sex in the multivariable model; therefore, the analyses were carried out in men and women separately. The multivariable Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and their 95% confidence intervals (CIs) for the incidence of type 2 diabetes mellitus (Appendix S3) in different categories of CVHM (poor category as the reference), as well as per 1‐score increment of ICVHM.
A sequential modeling method was used to estimate the HRs with 95% CIs of different ICVHM for the development of type 2 diabetes mellitus: model 1 was adjusted for age; model 2 was further adjusted for clinical predictors, including education levels, marital status, FH‐DM, and history of CVD; and model 3 was further adjusted with laboratory measurements including baseline FPG, and TG/HDL‐C ratio. Well‐known predictors were selected from The 2022 American Diabetes Association guideline 22 and other important articles in this field 12 , 16 , 18 . All analyses were conducted using Stata version 14 SE (StataCorp, College Station, TX, USA), and a two‐tailed P < 0.05 was considered significant.
RESULTS
The baseline characteristics of study participants stratified by categories of global ICVHM in each sex are presented in Table 1. Compared with participants in the ideal category, in both men and women, those in the poor category tended to be older and less educated, but with higher BMI, FPG, TG, TC, and blood pressure measures. Baseline characteristics of responders (study participants) and non‐responders (those with missing data or lost to follow‐up) are shown in Table S1.
Table 1.
Baseline characteristics by global ideal cardiovascular health metric categories † and sex
| Overall (n = 7,488) | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Poor (n = 911) | Intermediate (n = 1,780) | Ideal (n = 561) | P‐value | Poor (n = 1,324) | Intermediate (n = 2,483) | Ideal (n = 429) | P‐value | ||
| Age (years) | 42.07 ± 14.7 | 47.75 ± 14.6 | 42.75 ± 15.2 | 35.39 ± 13.8 | <0.001 | 51.54 ± 13.1 | 37.92 ± 12.3 | 30.79 ± 9.0 | <0.001 |
| BMI (kg/m2) | 27.17 ± 4.7 | 28.82 ± 3.7 | 26.49 ± 3.9 | 23.16 ± 3.5 | <0.001 | 30.70 ± 4.3 | 26.81 ± 4.8 | 22.82 ± 3.1 | <0.001 |
| SBP (mmHg) | 112.88 ± 17.2 | 126.08 ± 16.0 | 115.58 ± 15.4 | 105.30 ± 10.0 | <0.001 | 122.72 ± 18.4 | 104.94 ± 13.4 | 99.24 ± 9.2 | <0.001 |
| DBP (mmHg) | 73.00 ± 10.3 | 81.41 ± 9.5 | 74.27 ± 9.3 | 68.09 ± 7.5 | <0.001 | 78.00 ± 10.0 | 68.85 ± 8.7 | 64.81 ± 7.7 | <0.001 |
| FPG (mg/dL) | 88.51 ± 8.8 | 92.13 ± 9.1 | 89.51 ± 8.4 | 86.17 ± 6.9 | <0.001 | 91.40 ± 9.8 | 86.41 ± 8.0 | 82.88 ± 6.2 | <0.001 |
| 2 h‐PCG | 101.01 ± 27.6 | 107.82 ± 30.3 | 98.09 ± 27.2 | 86.27 ± 21.7 | <0.001 | 114.08 ± 29.0 | 98.97 ± 24.7 | 86.91 ± 18.4 | <0.001 |
| Total cholesterol (mg/dL) | 187.44 ± 39.2 | 206.29 ± 37.4 | 182.85 ± 35.7 | 161.80 ± 27.7 | <0.001 | 218.63 ± 37.4 | 177.75 ± 33.9 | 159.84 ± 24.6 | <0.001 |
| HDL cholesterol (mg/dL) | 42.08 ± 10.2 | 37.43 ± 7.8 | 38.04 ± 8.7 | 39.09 ± 8.6 | 0.001 | 44.97 ± 10.4 | 44.94 ± 10.3 | 47.09 ± 9.6 | <0.001 |
| Triglycerides (mg/dL) | 124 (86–179) | 170 (125–243) | 133 (95–187) | 100 (76–143) | <0.001 | 155.5 (114–210) | 103 (76–147) | 76 (61–102) | <0.001 |
| Physical activity (MET min/week) | 1061.1 (263.3–2619.7) | 469.7 (0–1250.3) | 1115.7 (148.8–3149.7) | 2875.7 (1369.4–6251.5) | <0.001 | 714.5 (166.7–1360.4) | 1208.6 (357.2–2500.6) | 2292.2 (1667.1–3721.1) | <0.001 |
| Education levels (years) | |||||||||
| <6 | 1,632 (21.79) | 192 (21.08) | 290 (16.29) | 66 (11.76) | <0.001 | 639 (48.26) | 424 (17.08) | 21 (4.90) | <0.001 |
| 6–12 | 4,285 (57.22) | 461 (50.60) | 1,030 (57.87) | 388 (69.16) | 573 (43.28) | 1,539 (61.98) | 294 (68.53) | ||
| >12 | 1,571 (20.98) | 258 (28.32) | 460 (25.84) | 107 (19.08) | 112 (8.46) | 520 (20.94) | 114 (26.57) | ||
| Marital status | |||||||||
| Single | 1,310 (17.49) | 80 (8.78) | 385 (21.63) | 233 (41.53) | <0.001 | 53 (4.00) | 444 (17.88) | 115 (26.81) | <0.001 |
| Married | 5,755 (76.86) | 822 (90.23) | 1,368 (76.85) | 319 (56.86) | 1,052 (79.46) | 1,894 (76.28) | 300 (69.93) | ||
| Widowed/divorced | 423 (5.65) | 9 (0.99) | 27 (1.52) | 9 (1.61) | 219 (16.54) | 145 (5.84) | 14 (3.26) | ||
| Family history of type 2 diabetes mellitus (yes) | 1,356 (18.11) | 148 (16.25) | 321 (18.03) | 111 (19.79) | 0.20 | 220 (16.62) | 473 (19.05) | 83 (19.35) | 0.15 |
| History of CVD (yes) | 342 (4.57) | 86 (9.44) | 88 (4.94) | 21 (3.74) | <0.001 | 100 (7.55) | 44 (1.77) | 3 (0.7) | <0.001 |
Values are mean ± standard deviation or n (%) for normally distributed covariates and median (interquartile range) for skewed (e.g., TG and physical activity) variables.
Defined according to the number of ideal metrics: 0–1 (poor), 2–3 (intermediate) and 4–5 (ideal).
2 h‐PCG, 2‐h post challenge plasma glucose; BMI, body mass index; CVD, cardiovascular disease; CVH, cardiovascular health; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglycerides; WC, waist circumference.
During a median 9.1‐year follow up (IQR 7.5–10.1 years) of 7,488 eligible participants (3,252 men and 4,236 women) of the TLGS, 922 new cases of type 2 diabetes mellitus were identified (413 on glucose‐lowering medications), including 526 women and 396 men. The annual crude incidence rate of type 2 diabetes mellitus in the men population across poor, intermediate and ideal CVHM was 25.9 (95% CI 22.4–29.9), 12.8 (95% CI 11.1–14.7) and 3.5 (95% CI 2.2–5.6) per 1,000 person‐years, respectively; the corresponding values for women were 26.2 (95% CI 23.3–29.4), 11.1 (95% CI 9.8–12.6) and 2.4 (95% CI 1.2–4.6) per 1,000 person‐years, respectively.
Tables 2 and 3 present the HRs (95% CI) of the intermediate and ideal status of each CVHM component (compared with poor) for incident type 2 diabetes mellitus in three models, for men and women, respectively. The intermediate and ideal status of the BMI metric for both sexes in both models 2 and 3 were associated with a lower risk of type 2 diabetes mellitus. Furthermore, for both sex groups, ideal TC and blood pressure were associated with a lesser risk for incident type 2 diabetes mellitus after adjustment for age, educational levels, marital status, FH‐DM and history of CVD (model 2); the association remained significant with additional adjustment of FPG and TG/HDL‐C ratio, except for TC (model 3). Ideal physical activity status was significantly associated with a lower risk of type 2 diabetes mellitus only among men (0.73, 95% CI 0.58–0.91 in model 2; 0.76, 95% CI 0.60–0.95 in model 3). Furthermore, men with two, three and four or more ICVHM had a lower risk for incident type 2 diabetes mellitus in all three models; the corresponding HRs for incident type 2 diabetes mellitus were 0.72 (95% CI 0.52–1.00), 0.58 (95% CI 0.40–0.85) and 0.33 (95% CI 0.19–0.59), respectively in model 3 (Table 2). Among women, those with two, three and four or more ICVHM had a lower risk for incident type 2 diabetes mellitus in both models 1 and 2; the HRs remained significant after further adjustment with FPG and TG/HDL‐C ratio only for those having three (0.71, 95% CI 0.51–1.00) and four or more (0.30, 95% CI 0.15–0.62) components of ICVHM (Table 3).
Table 2.
Hazard ratios of cardiovascular health metrics for type 2 diabetes for men †
| n/N | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | ||
| Smoking status | |||||||
| Ideal | 122/1,158 | 1.09 (0.87–1.37) | 0.44 | 1.08 (0.86–1.36) | 0.51 | 1.17 (0.93–1.47) | 0.18 |
| Intermediate | 68/416 | 1.16 (0.88–1.53) | 0.29 | 1.14 (0.86–1.51) | 0.35 | 0.97 (0.74–1.29) | 0.86 |
| Poor | 206/1,678 | 1.00 | 1.00 | 1.00 | |||
| Body mass index | |||||||
| Ideal | 66/1,153 | 0.24 (0.18–0.32) | <0.001 | 0.25 (0.18–0.34) | <0.001 | 0.38 (0.28–0.52) | <0.001 |
| Intermediate | 204/1,500 | 0.55 (0.44–0.69) | <0.001 | 0.56 (0.45–0.70) | <0.001 | 0.62 (0.49–0.77) | <0.001 |
| Poor | 126/599 | 1.00 | 1.00 | 1.00 | |||
| Physical activity | |||||||
| Ideal | 155/1,332 | 0.79 (0.63–0.98) | 0.03 | 0.73 (0.58–0.91) | 0.006 | 0.76 (0.60–0.95) | 0.02 |
| Intermediate | 75/617 | 0.84 (0.64–1.10) | 0.21 | 0.85 (0.64–1.11) | 0.24 | 0.77 (0.59–1.02) | 0.07 |
| Poor | 166/1,303 | 1.00 | 1.00 | 1.00 | |||
| Total cholesterol | |||||||
| Ideal | 202/2,094 | 0.57 (0.42–0.78) | <0.001 | 0.58 (0.43–0.79) | 0.001 | 0.95 (0.68–1.33) | 0.77 |
| Intermediate | 143/883 | 0.82 (0.60–1.13) | 0.23 | 0.82 (0.60–1.14) | 0.24 | 1.17 (0.83–1.63) | 0.37 |
| Poor | 51/275 | 1.00 | 1.00 | 1.00 | |||
| Blood pressure | |||||||
| Ideal | 112/1,655 | 0.47 (0.35–0.63) | <0.001 | 0.47 (0.35–0.62) | <0.001 | 0.57 (0.43–0.77) | <0.001 |
| Intermediate | 188/1,164 | 0.96 (0.74–1.23) | 0.72 | 0.96 (0.75–1.24) | 0.76 | 0.96 (0.75–1.25) | 0.79 |
| Poor | 96/433 | 1.00 | 1.00 | 1.00 | |||
| No. CVH metrics | |||||||
| ≥4 | 17/561 | 0.17 (0.10–0.30) | <0.001 | 0.18 (0.10–0.31) | <0.001 | 0.33 (0.19–0.59) | <0.001 |
| 3 | 66/804 | 0.39 (0.27–0.56) | <0.001 | 0.39 (0.27–0.57) | <0.001 | 0.58 (0.40–0.85) | 0.005 |
| 2 | 127/976 | 0.56 (0.41–0.77) | <0.001 | 0.57 (0.41–0.78) | 0.001 | 0.72 (0.52–1.00) | 0.05 |
| 1 | 132/684 | 0.82 (0.60–1.12) | 0.21 | 0.82 (0.60–1.13) | 0.23 | 0.95 (0.69–1.31) | 0.75 |
| 0 | 54/227 | 1.00 | 1.00 | 1.00 | |||
Model 1: Adjusted for age.
Model 2: Model 1 + further adjusted for educational level, marital status, family history of diabetes and history of cardiovascular disease.
Model 3: Model 2 + further adjusted for baseline fasting plasma glucose, and triglycerides/high‐density lipoprotein cholesterol ratio.
The hazard ratios (HR) and 95% confidence intervals (CIs) of each metric were estimated in a separate Cox proportional hazards regression model.
Defined according to the number of ideal metrics: zero or one (poor), two or three (intermediate) and four or five (ideal).
CVH, cardiovascular health; n/N, number of cardiovascular disease/number of participants by the level of each metric.
Table 3.
Hazard ratios of cardiovascular health metrics for type 2 diabetes for women †
| n/N | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | ||
| Smoking status | |||||||
| Ideal | 28/308 | 0.93 (0.63–1.36) | 0.70 | 0.93 (0.64–1.37) | 0.72 | 1.05 (0.71–1.54) | 0.81 |
| Intermediate | 8/69 | 0.85 (0.42–1.70) | 0.64 | 0.86 (0.43–1.73) | 0.67 | 1.06 (0.52–2.13) | 0.88 |
| Poor | 490/3,859 | 1.00 | 1.00 | 1.00 | |||
| Body mass index | |||||||
| Ideal | 59/1,342 | 0.25 (0.19–0.34) | <0.001 | 0.26 (0.19–0.35) | <0.001 | 0.44 (0.33–0.60) | <0.001 |
| Intermediate | 188/1,677 | 0.50 (0.42–0.60) | <0.001 | 0.51 (0.42–0.62) | <0.001 | 0.68 (0.56–0.82) | <0.001 |
| Poor | 279/1,217 | 1.00 | 1.00 | 1.00 | |||
| Physical activity | |||||||
| Ideal | 225/1,739 | 1.05 (0.86–1.28) | 0.63 | 1.03 (0.84–1.25) | 0.80 | 1.20 (0.98–1.46) | 0.07 |
| Intermediate | 125/1,032 | 0.98 (0.78–1.24) | 0.89 | 1.00 (0.79–1.25) | 0.93 | 1.13 (0.90–1.42) | 0.31 |
| Poor | 176/1,465 | 1.00 | 1.00 | 1.00 | |||
| Total cholesterol | |||||||
| Ideal | 226/2,646 | 0.70 (0.53–0.92) | 0.01 | 0.70 (0.54–0.92) | 0.01 | 0.86 (0.65–1.13) | 0.27 |
| Intermediate | 213/1,134 | 1.09 (0.85–1.41) | 0.48 | 1.07 (0.83–1.38) | 0.6 | 1.08 (0.84–1.40) | 0.53 |
| Poor | 87/456 | 1.00 | 1.00 | 1.00 | |||
| Blood pressure | |||||||
| Ideal | 228/2,797 | 0.38 (0.29–0.49) | <0.001 | 0.38 (0.29–0.50) | <0.001 | 0.63 (0.48–0.82) | 0.001 |
| Intermediate | 190/1,065 | 0.67 (0.53–0.85) | 0.001 | 0.68 (0.53–0.86) | 0.002 | 0.86 (0.67–1.09) | 0.22 |
| Poor | 108/374 | 1.00 | 1.00 | 1.00 | |||
| No. CVH metrics | |||||||
| ≥4 | 9/429 | 0.12 (0.06–0.25) | <0.001 | 0.13 (0.06–0.25) | <0.001 | 0.30 (0.15–0.62) | 0.001 |
| 3 | 81/1,209 | 0.38 (0.27–0.52) | <0.001 | 0.38 (0.28–0.53) | <0.001 | 0.71 (0.51–1.00) | 0.04 |
| 2 | 157/1,274 | 0.59 (0.45–0.77) | <0.001 | 0.58 (0.45–0.76) | <0.001 | 0.90 (0.69–1.18) | 0.45 |
| 1 | 172/903 | 0.81 (0.63–1.03) | 0.08 | 0.79 (0.62–1.02) | 0.07 | 1.09 (0.85–1.40) | 0.48 |
| 0 | 107/421 | 1.00 | 1.00 | 1.00 | |||
Model 1: Adjusted for age.
Model 2: Model 1 + further adjusted for educational level, marital status, family history of diabetes and history of cardiovascular disease.
Model 3: Model 2 + further adjusted for baseline fasting plasma glucose, and triglycerides/high‐density lipoprotein cholesterol ratio.
The hazard ratio (HRs) and 95% confidence intervals (CIs) of each metric were estimated in a separate Cox proportional hazards regression model.
Defined according to the number of ideal metrics: 0–1 (poor), 2–3 (intermediate), and 4–5 (ideal).
CVH, cardiovascular health; n/N, number of cardiovascular disease/number of participants by the level of each metric.
As shown in Table 4, a 1‐point increase in the number of global ICVHM in both men and women decreased the risk of incident type 2 diabetes mellitus by 31% in model 2. Even after further adjustment in model 3, the suggestive lower risk was still found (HR 0.79, 95% CI 0.72–0.87 in men; 0.85, 95% CI 0.77–0.93 in women). The HRs of the behavioral and biological CVHM for incident type 2 diabetes mellitus showed that each additional unit in the number of ICVHM decreased the risk in model 2 among both sexes; whereas after further adjustment with FPG and TG/HDL‐C, the behavioral cardiovascular health among women did not have a significant association.
Table 4.
Hazard ratios of ideal cardiovascular health (per one additional unit) for type 2 diabetes †
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Men | ||||||
| Global cardiovascular health | 0.69 (0.63–0.75) | <0.001 | 0.69 (0.63–0.75) | <0.001 | 0.79 (0.72–0.87) | <0.001 |
| Behavioral cardiovascular health | 0.70 (0.62–0.80) | <0.001 | 0.69 (0.61–0.79) | <0.001 | 0.82 (0.72–0.94) | 0.004 |
| Biological cardiovascular health | 0.59 (0.51–0.68) | <0.001 | 0.59 (0.51–0.68) | <0.001 | 0.72 (0.62–0.84) | <0.001 |
| Women | ||||||
| Global cardiovascular health | 0.69 (0.63–0.76) | <0.001 | 0.69 (0.63–0.76) | <0.001 | 0.85 (0.77–0.93) | 0.001 |
| Behavioral cardiovascular health | 0.75 (0.65–0.86) | <0.001 | 0.74 (0.65–0.86) | <0.001 | 0.92 (0.80–1.06) | 0.28 |
| Biological cardiovascular health | 0.60 (0.52–0.68) | <0.001 | 0.60 (0.53–0.69) | <0.001 | 0.76 (0.66–0.87) | <0.001 |
Model 1: Adjusted for age.
Model 2: Model 1 + further adjusted for educational level, marital status, family history of diabetes and history of cardiovascular disease.
Model 3: Model 2 + further adjusted for baseline fasting plasma glucose, and triglycerides/high‐density lipoprotein cholesterol ratio.
Behavioral cardiovascular health (body mass index, smoking and physical activity). Biological cardiovascular health (total cholesterol and blood pressure).
Defined according to the number of ideal metrics: zero or one (poor), two or three (intermediate) four or five (ideal).
CVH, cardiovascular health; HR, hazard ratio.
The multivariable association between categories of global CVHM (poor, intermediate, and ideal) and incident type 2 diabetes mellitus in model 3 for men and women is shown in Figure 2 . Intermediate and ideal categories of CVHM (compared with poor) were associated with a lower risk of type 2 diabetes mellitus . We also observed that higher FPG, TG/HDL‐C ratio levels, and having a positive FH‐DM significantly increased the risk of type 2 diabetes mellitus in both sexes, whereas, among men, higher age and being married or widow/divorced (compared with being single) were also associated with an increased risk of type 2 diabetes mellitus .
Figure 2.
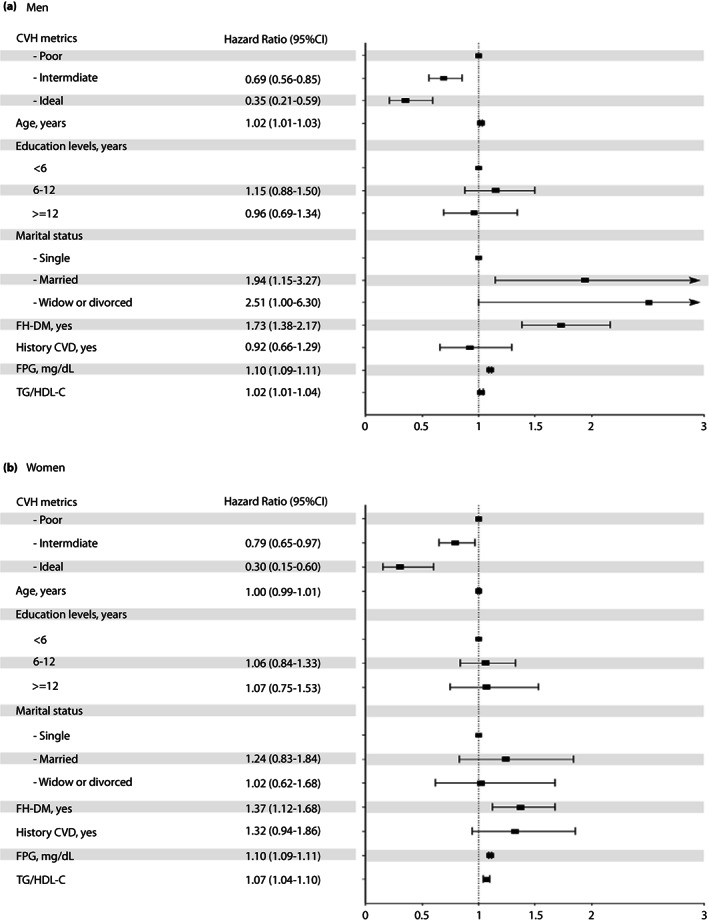
The multivariable association between categories of ideal cardiovascular health metrics (poor, intermediate and ideal) and incident type 2 diabetes mellitus for men and women. CVD, cardiovascular disease; FH‐DM, family history of diabetes; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; TG, triglycerides.
To show the robustness of the present findings, all of the analysis above were repeated in a subgroup of the TLGS population with dietary information (n = 2,236), among whom we found 228 (10.2%) type 2 diabetes mellitus events. As shown in Table S2, the results among men were generally in line with our main findings. For those with four or more ICVHM, a significant association with incident type 2 diabetes mellitus was observed even in model 3 (HR 0.27, 95% CI 0.08–0.84). Furthermore, intermediate and ideal nutrition status, among women, decreased the risk of incident type 2 diabetes mellitus by 77 and 83%, respectively, in model 2. Even after further adjustment in model 3, the suggestive lower risk was still found. After including diet information as another CVHM component, the results remained in line with the main analysis in both sexes.
Similar to the main analysis, as a sensitivity analysis, after excluding 342 participants with a history of CVD, the results essentially remained unchanged among both sexes (Table S3).
DISCUSSION
To the best of our knowledge, the current study represents the first prospective community‐based study to evaluate the sex‐specific association between ICVHM and the incidence of type 2 diabetes mellitus among a Middle Eastern population. The present study showed strong associations between BMI <30 kg/m2 categories and ideal BP, with a lower risk of type 2 diabetes mellitus in both sexes. However, among other CVHM components, the physical activity ≥1,500 MET min/week category was associated with a lower risk of incident type 2 diabetes mellitus, solely among men. Furthermore, the AHA's definition of CVHM, which consists of five components, showed that the risk of type 2 diabetes mellitus decreased by 21 and 15% for each additional global ICVHM after adjustment for well‐known type 2 diabetes mellitus risk factors, including FPG and TG/HDL‐C, in men and women, respectively. In both sexes, having intermediate and ideal categories of global CVHM were associated with at least 20 and 60% lower risk of incident type 2 diabetes mellitus, respectively.
The present sex‐stratified analysis showed that normal‐ and overweight had a 56–62% and a 32–38% lower risk of type 2 diabetes mellitus, respectively, compared with their obese counterparts. In line with the present study, a few prospective studies have shown that having an intermediate and ideal BMI is found to be associated with an approximately 45 and 75% reduced risk of incident type 2 diabetes mellitus, respectively 16 , 17 . The increasing prevalence of overweight and obesity, especially in developing countries, is a serious health concern worldwide 23 . According to the Iranian national survey in 2016, approximately 59.3% of adults suffer from overweight/obesity, of which 53.6% of them are men and 64.7% of them are women 24 . As reported by Esteghamati et al. 25 the population attributable fraction of obesity and overweight for the prevalence of type 2 diabetes mellitus was 33.8 and 10.3%, respectively, during 2005–2011. As the prevalence of obesity increases, the prevalence of type 2 diabetes mellitus has also increased 26 . According to the published meta‐analysis of 18 prospective cohort studies (I 2 = 88%), the risk of developing type 2 diabetes mellitus among overweight and obese individuals was three‐ and seven fold higher than those with normal weight, respectively 27 ; posing significant economic challenges to the healthcare system. In a systematic review, Yusefzadeh et al. 28 reported that obesity accounts for 31.8 and 68.1% of direct and indirect health system costs compared with people with normal weight, respectively. As being overweight/obese plays a dominant role in the increasing burden of type 2 diabetes mellitus (medical or economic), individual or population healthcare interventions are crucial. As recommended by the Diabetes Prevention Program 29 , a 7% reduction in bodyweight decreased the risk of incident type 2 diabetes mellitus by 58% within a 3‐year follow‐up period.
We also found that high physical activity (≥1,500 MET mins/week), compared with low physical activity (<600 MET min/week), decreased the risk of type 2 diabetes mellitus by >20% only among men. In the meta‐analysis based on the harmonized data from nine cohort studies, Cloostrmans et al. 30 calculated a summary HR of 1.23 (95% CI 1.09–1.39) for low versus high physical activity in the development of type 2 diabetes mellitus. In another study, a high level of total physical activity was associated with a 35% lower risk of type 2 diabetes mellitus 31 . Despite the present results, the meta‐analysis of 10 prospective observational studies showed that moderate‐intensity physical activity decreased the risk of type 2 diabetes mellitus by 42% among women before adjustment for BMI 32 . The results of the present study showed that, unlike men, among women, physical activity ≥600 MET min/week was not associated with a lower risk of incident type 2 diabetes mellitus. This might be related to residual confounding, such as behavioral covariates (e.g., alcohol intake, sleeping disorders), sedentary activities (e.g., hours of watching television) or psychopathological factors (e.g., depression, stress, anxiety) 31 , 32 , 33 . In the present study, differences in the baseline physical activity levels between the two groups of poor and ideal physical activity were more prominent in men (median 469 (IQR 1,250.3) vs 2,875.7 (IQR 4,882.0) MET min/week) than women (median 714.5 (IQR 1,193.3) vs 2,292.2 (IQR 2054.0) MET min/week). Furthermore, the amount of baseline physical activity in individuals with ideal physical activity was lower in women than in men (P‐value <0.001). Esteghamati et al. 34 in a national survey of Iranians, reported that the level of vigorous activities is higher in men, as they engage in heavier physical activity at work, whereas women are often involved in household tasks, if both working and leisure physical activity time is taken into account for total physical activity level. Therefore, the lower variations in physical activity among women might lead to a non‐significant association between physical activity and the risk of type 2 diabetes mellitus. A similar sex difference in physical activity levels and type 2 diabetes mellitus was reported in Mexican Americans 35 .
Together with existing evidence, having BP <120/80 mmHg among non‐hypertensive individuals was associated with a minimum 43 and 37% lower risk of type 2 diabetes mellitus for men and women, respectively, even after further adjustment with FPG and TG/HDL‐C. These data were consistent with previous studies that showed normal BP was associated with a >50% lower risk of type 2 diabetes mellitus 16 , 17 , 18 . Furthermore, a meta‐analysis of 19 randomized trials showed that each 5mmHg reduction in the level of systolic BP decreased the risk of incident type 2 diabetes mellitus by 11% 36 . Hence, because of the high prevalence of high BP, especially among Iranians 37 , encouraging lifestyle changes, including reducing salt intake, which is more than two fold higher than the World Health Organization recommended level 38 , might be potentially associated with a lower risk of type 2 diabetes mellitus.
In the present study, ideal TC level (<200 mg/dL without medication) was associated with a lower risk of type 2 diabetes mellitus in both men and women, but the association was no more significant after further adjustment with FPG and TG/HDL‐C. However, TG/HDL‐C, as a surrogate for insulin resistance 39 , had a significant association with incident type 2 diabetes mellitus in both sexes.
Regarding smoking status, the findings follow previous studies showing that there was no strong association between smoking behavior and incident diabetes mellitus 12 , 17 , 18 . However, Joseph et al. 16 found a significantly lower risk of incident diabetes for ideal smoking status (relative risk 0.75, 95% CI 0.63–0.89). Recent evidence regarding the association between smoking status and the risk of diabetes is inconclusive. A meta‐analysis of 88 prospective studies (I 2 = 68.6%) showed that current smoking was associated with a 37% increased risk of diabetes compared with non‐smokers. Furthermore, this meta‐analysis, using 10 prospective studies (I 2 = 82.3%), showed that compared with never smokers, the risk of diabetes was increased by 54% for new quitters who had stopped smoking for <5 years 40 .
According to the present findings, the risk of diabetes for men and women decreased by 21 and 15% for each additional baseline ICVHM after further adjustment for well‐known type 2 diabetes mellitus risk factors, respectively. Similarly, in the Jackson Heart Study cohort, a 1‐unit increase in baseline ICVHM was associated with a 17% diabetes mellitus risk reduction 12 . Furthermore, a cohort study among the Kailuan population of China using a 12‐point CVHM score reported a 16 and a 19% reduced risk of diabetes for men and women for each additional baseline ICVHM, respectively 15 .
The present findings showed that across categories of CVHM, intermediate and ideal categories are associated with a 31% and 65% reduced risk of type 2 diabetes mellitus for men, and 21 and 70% for women, respectively. The Multi‐Ethnic Study of Atherosclerosis (MESA) and the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohorts showed that compared with participants with poor ICVH status, those with intermediate and ideal CVH status had a 30–34% and 71–75% lower risk of incident diabetes, respectively 16 , 17 . Additionally, the present results showed that having three and four or more ICVHM is associated with a 42 and 67% reduced risk of type 2 diabetes mellitus for men and 29 and 70% for women, respectively. In the Jackson Heart Study, having three or more ICVHM lowered the risk of incident type 2 diabetes mellitus by 37% compared with participants who had one or no ICVHM 12 .
We carried out a sensitivity analysis only among those with complete data on nutrition status, which yielded similar results to our main analysis for total ICVHM. This is partly due to the mediated effects of a healthy diet through intermediate and ideal cardiovascular risk factors, such as TC and BP. However, intermediate and ideal nutrition status decreased the risk of type 2 diabetes mellitus by >70% only among women, whereas for men, the association remained non‐significant. Recent evidence is inconsistent regarding the risk for type 2 diabetes mellitus related to nutrition status. Findings from the REGARDS cohort showed an inverse association between ideal dietary status and risk of type 2 diabetes mellitus (adjusted relative risk 0.81, 95% CI 0.68–0.97) 16 , whereas others did not have such an association 12 , 17 , 18 . A meta‐analysis showed that adherence to components of relatively similar diets to the AHA dietary recommendations, including the Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH), and Alternative Healthy Eating Index (AHEI), was associated with a 13–21% reduction in diabetes risk 41 . Maybe the AHA diet score does not consider multiple other dietary factors associated with reduced risk of type 2 diabetes mellitus, including dairy intake, glycemic index and glycemic load 42 , 43 . Previously, among a Tehranian population, each 100 g/day increase in milk intake was reported to be associated with 41% lower type 2 diabetes mellitus risk only in men 44 . Furthermore, a high intake of white rice (>250 g/day), with a high glycemic load among the Iranian population, is reported to confer a twofold increase in the risk of type 2 diabetes mellitus 45 . Higher intake of polyphenols, which are abundant in tea and coffee, and nut consumption, are also shown to reduce the type 2 diabetes mellitus risk 46 , 47 .
The strengths of the present analysis include the use of a large sample size, extended follow up, and information on a broad spectrum of biological and behavioral factors, with detailed information about various CVHM components and a comprehensive diagnosis of diabetes using FPG and 2 h‐PCG, to reduce the potential of misclassifications. Furthermore, we used the exact biological measurements for the definition of CVHM. However, the present study did have several important limitations. First, we used a single measure of cardiovascular health metrics at baseline, so this study could not account for changes in the metrics, as well as confounders, over the follow‐up time. Second, data for diet in the present study were only available for approximately one‐third of individuals, which could limit the accuracy of the findings on the association between diet and type 2 diabetes mellitus. Therefore, the results for diet should be interpreted with caution. Finally, the present study was carried out in the metropolitan area of Tehran, and therefore, we might not generalize it to the rural zone of the country.
The present investigation of a large community‐based sample of adults in the Middle East and North Africa region during approximately one decade of follow up carries potentially significant implications from a primary prevention standpoint. We found the association between global CVHM and incident type 2 diabetes mellitus is mainly driven by normal BP, having a non‐obese BMI and physical activity ≥1,500 MET min/week (only for men). In both sexes, a 1‐unit increase in the number of ICVHM decreased the risk of incident type 2 diabetes mellitus by >15%, and having an intermediate and ideal category of global CVH metrics was associated with at least 20 and 60% lower risk of incident type 2 diabetes mellitus, respectively, in both sexes. These findings imply the importance of implementing lifestyle interventions to optimize cardiovascular health behaviors at both individual and population levels. Individuals are encouraged to make lifestyle changes aiming to maintain a normal weight and blood pressure to prevent the increasing trend of diabetes.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: This study was approved by the institutional review board of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Informed consent: All participants provided written informed consent.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Appendix S1 | Tehran lipids and glucose study design.
Appendix S2 | Definition of cardiovascular health metrics.
Appendix S3 | Type 2 diabetes mellitus event date.
Appendix S4 | Abbreviations.
Table S1 | Baseline characteristics by responders and non‐responders.
Table S2 | HRs of cardiovascular health metrics for type 2 diabetes mellitus among those with nutrition data by gender*.
Table S3 | HRs of ideal cardiovascular health metrics for type 2 diabetes mellitus among those without a history of CVD by gender*.
ACKNOWLEDGMENT
We express appreciation to the participants of district 13, Tehran, for their enthusiastic support.
REFERENCES
- 1. An Y, Zhang P, Wang J, et al. Cardiovascular and all‐cause mortality over a 23‐year period among Chinese with newly diagnosed diabetes in the Da Qing IGT and diabetes study. Diabetes Care 2015; 38: 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor KS, Heneghan CJ, Farmer AJ, et al. All‐cause and cardiovascular mortality in middle‐aged people with type 2 diabetes compared with people without diabetes in a large UKprimary care database. Diabetes Care 2013; 36: 2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Preis SR, Hwang S‐J, Coady S, et al. Trends in all‐cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham heart study, 1950–2005. Circulation 2009; 119: 1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asgari S, Khalili D, Mehrabi Y, et al. Letter to the editor regarding “Nationwide prevalence of diabetes and prediabetes and associated risk factors among Iranian adults: analysis of data from PERSIAN cohort study”. Diabetes Ther 2021;13:217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atlas ID . IDF Atlas 10th Edition 2021, 2021; 4. Available from: https://diabetesatlas.org/atlas/tenth‐edition/. Accessed February 03, 2022
- 6. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010; 121: 586–613. [DOI] [PubMed] [Google Scholar]
- 7. Moghaddam MM, Mohebi R, Hosseini F, et al. Distribution of ideal cardiovascular health in a community‐based cohort of Middle East population. Ann Saudi Med 2014; 34: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahmani F, Asgari S, Khalili D, et al. National trends in cardiovascular health metrics among Iranian adults using results of three cross‐sectional STEPwise approaches to surveillance surveys. Sci Rep 2021; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramírez‐Vélez R, Saavedra JM, Lobelo F, et al. Ideal Cardiovascular Health and Incident Cardiovascular Disease Among Adults: A Systematic Review and Meta†analysis. Mayo Clin Proc 2018; 93:1589–1599. [DOI] [PubMed] [Google Scholar]
- 10. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012; 125: 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Climie RE, van Sloten TT, Périer M‐C, et al. Change in cardiovascular health and incident type 2 diabetes and impaired fasting glucose: the Whitehall II study. Diabetes Care 2019; 42: 1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Effoe VS, Carnethon MR, Echouffo‐Tcheugui JB, et al. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc 2017; 6: e005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu J, Li M, Xu Y, et al. Early life famine exposure, ideal cardiovascular health metrics, and risk of incident diabetes: findings from the 4C study. Diabetes Care 2020; 43: 1902–1909. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Shi J, Wang A, et al. Changes in ideal cardiovascular health status and risk of new‐onset type 2 diabetes: the Kailuan prospective study. Medicine 2016; 95: e4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. 3Liu X , Cui L, Wang A, et al. Cumulative exposure to ideal cardiovascular health and incident diabetes in a Chinese population: the Kailuan study. J Am Heart Assoc 2016; 5: e004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joseph JJ, Bennett A, Tcheugui JBE, et al. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for geographic and racial differences in stroke (REGARDS) study. Diabetologia 2019; 62: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph JJ, Echouffo‐Tcheugui JB, Carnethon MR, et al. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the multi‐ethnic study of atherosclerosis. Diabetologia 2016; 59: 1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fretts AM, Howard BV, McKnight B, et al. Life's simple 7 and incidence of diabetes among American Indians: the strong heart family study. Diabetes Care 2014; 37: 2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azizi F, Ghanbarian A, Momenan AA, et al. Prevention of non‐communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials 2009; 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azizi F, Zadeh‐Vakili A, Takyar M. Review of rationale, design, and initial findings: Tehran Lipid and Glucose Study. Int J Endocrinol Metab 2018; 16(4 Suppl): e84777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derakhshan A, Sardarinia M, Khalili D, et al. Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow‐up: Tehran Lipid and Glucose Study. PLoS One 2014; 9: e102563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Draznin B, Aroda VR, Bakris G, et al. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2022. Diabetes Care 2022; 45(Supplement_1): S17–S38. [DOI] [PubMed] [Google Scholar]
- 23. Collaboration NRF. Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19· 2 million participants. The Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Djalalinia S, Saeedi Moghaddam S, Sheidaei A, et al. Patterns of obesity and overweight in the Iranian population: findings of STEPs 2016. Front Endocrinol 2020; 11: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esteghamati A, Etemad K, Koohpayehzadeh J, et al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract 2014; 103: 319–327. [DOI] [PubMed] [Google Scholar]
- 26. Trasande L, Chatterjee S. The impact of obesity on health service utilization and costs in childhood. Obesity 2009; 17: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 27. Abdullah A, Peeters A, de Courten M, et al. The magnitude of association between overweight and obesity and the risk of diabetes: a meta‐analysis of prospective cohort studies. Diabetes Res Clin Pract 2010; 89: 309–319. [DOI] [PubMed] [Google Scholar]
- 28. Yusefzadeh H, Rashidi A, Rahimi B. Economic burden of obesity: a systematic review. Soc Health Behav 2019; 2: 7. [Google Scholar]
- 29. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, 2002. [DOI] [PMC free article] [PubMed]
- 30. Cloostermans L, Wendel‐Vos W, Doornbos G, et al. Independent and combined effects of physical activity and body mass index on the development of type 2 diabetes – a meta‐analysis of 9 prospective cohort studies. Int J Behav Nutr Phys Act 2015; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aune D, Norat T, Leitzmann M, et al. Physical activity and the risk of type 2 diabetes: a systematic review and dose–response meta‐analysis. Eur J Epidemiol 2015; 30: 529–542. [DOI] [PubMed] [Google Scholar]
- 32. Jeon CY, Lokken RP, Hu FB, et al. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007; 30: 744–752. [DOI] [PubMed] [Google Scholar]
- 33. Anothaisintawee T, Reutrakul S, Van Cauter E, et al. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta‐analysis. Sleep Med Rev 2016; 30: 11–24. [DOI] [PubMed] [Google Scholar]
- 34. Koohpayehzadeh J, Etemad K, Abbasi M, et al. Gender‐specific changes in physical activity pattern in Iran: national surveillance of risk factors of non‐communicable diseases (2007–2011). Int J Public Health 2014; 59: 231–241. [DOI] [PubMed] [Google Scholar]
- 35. Monterrosa AE, Haffner SM, Stern MP, et al. Sex difference in lifestyle factors predictive of diabetes in Mexican‐Americans. Diabetes Care 1995; 18: 448–456. [DOI] [PubMed] [Google Scholar]
- 36. Nazarzadeh M, Bidel Z, Canoy D, et al. Blood pressure lowering and risk of new‐onset type 2 diabetes: an individual participant data meta‐analysis. The Lancet 2021; 398: 1803–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asgari S, Khaloo P, Khalili D, et al. Status of hypertension in Tehran: potential impact of the ACC/AHA 2017 and JNC7 guidelines, 2012–2015. Sci Rep 2019; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rezaei S, Mahmoudi Z, Sheidaei A, et al. Salt intake among Iranian population: the first national report on salt intake in Iran. J Hypertens 2018; 36: 2380–2389. [DOI] [PubMed] [Google Scholar]
- 39. Kim‐Dorner S‐J, Deuster PA, Zeno SA, et al. Should triglycerides and the triglycerides to high‐density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism 2010; 59: 299–304. [DOI] [PubMed] [Google Scholar]
- 40. Pan A, Wang Y, Talaei M, et al. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta‐analysis of prospective studies. J Nutr 2017; 147: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 42. Aune D, Norat T, Romundstad P, et al. Dairy products and the risk of type 2 diabetes: a systematic review and dose‐response meta‐analysis of cohort studies. Am J Clin Nutr 2013; 98: 1066–1083. [DOI] [PubMed] [Google Scholar]
- 43. Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta‐analysis. Am J Clin Nutr 2014; 100: 218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moslehi N, Shab‐Bidar S, Mirmiran P, et al. Associations between dairy products consumption and risk of type 2 diabetes: Tehran Lipid and Glucose Study. Int J Food Sci Nutr 2015; 66: 692–699. [DOI] [PubMed] [Google Scholar]
- 45. Golozar A, Khalili D, Etemadi A, et al. White rice intake and incidence of type‐2 diabetes: analysis of two prospective cohort studies from Iran. BMC Public Health 2017; 17: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Esfandiar Z, Hosseini‐Esfahani F, Mirmiran P, et al. The association of dietary polyphenol intake with the risk of type 2 diabetes: Tehran Lipid and Glucose Study. Diabetes Metab Syndr Obes Targets Ther 2020; 13: 1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Asghari G, Ghorbani Z, Mirmiran P, et al. Nut consumption is associated with lower incidence of type 2 diabetes: the Tehran Lipid and Glucose Study. Diabetes Metab 2017; 43: 18–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | Tehran lipids and glucose study design.
Appendix S2 | Definition of cardiovascular health metrics.
Appendix S3 | Type 2 diabetes mellitus event date.
Appendix S4 | Abbreviations.
Table S1 | Baseline characteristics by responders and non‐responders.
Table S2 | HRs of cardiovascular health metrics for type 2 diabetes mellitus among those with nutrition data by gender*.
Table S3 | HRs of ideal cardiovascular health metrics for type 2 diabetes mellitus among those without a history of CVD by gender*.


