Abstract
Background
This study explored the relationship between integrase strand transfer inhibitor (INSTI)-based anti-retroviral agents and weight gain over time, and the risk factors for weight gain in Korean people living with human immunodeficiency virus (PLWH).
Materials and Methods
The study was conducted retrospectively in PLWHs 18 years of age or older who took one of three INSTI-based single-tablet regimens (STRs) (tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat [TDF/F/EVG/c], tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat [TAF/F/EVG/c], and abacavir/lamivudine/dolutegravir [ABC/3TC/DTG]) for more than 2 years at three university-affiliated hospitals in South Korea from May 2014 to December 2020. Analysis was performed in the treatment-naïve and treatment-experienced groups, respectively.
Results
Individual INSTI-based STRs were associated with weight gain at the 24-month follow up in both treatment-naïve (n = 179) and treatment-experienced (n = 290) groups. Body mass index (BMI) categories changed over time for TAF/F/EVG/c and ABC/3TC/DTG, with significant increases in the rates of overweight and obesity in treatment-naïve patients, whereas there was no change for TDF/F/EVG/c. TAF/F/EVG/c significantly increased total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) compared to other regimens over 24 months. In the treatment-naïve group, a baseline CD4+ T cell count <100 cells/mm3, human immunodeficiency virus (HIV) viral load (VL) ≥100,000 copies/mL, no physical exercise, and TAF/F/EVG/c (vs. TDF/F/EVF/c) were risk factors for ≥10% weight gain. In the treatment-experienced group, age <45 years, BMI <25 kg/m2, and no physical exercise were risk factors for ≥5% weight gain.
Conclusion
INSTI-based STR continued to increase body weight at the 24-month follow up in treated and untreated Korean PLWH. Exercise, together with demographic-, HIV-, and anti-retroviral therapy-related factors, influenced weight gain. Therefore, when prescribing an INSTI-based STR, weight gain and metabolic changes should be closely monitored in PLWH with these risk factors.
Keywords: Human immunodeficiency virus, Anti-retroviral agents, Integrase inhibitor, Weight gain, Lipid
Introduction
Since the introduction of combined antiretroviral therapy (ART) in 1996, the morbidity and mortality rates of people living with human immunodeficiency virus (PLWH) have decreased dramatically [1,2]. However, PLWH must take ART for the rest of their lives. Recent guidelines recommend integrase strand transfer inhibitor (INSTI)-based regimens as first-line treatments for PLWH [3,4] due to their superior antiviral efficacy and tolerability, and low drug ??drug interaction rates compared to other regimens [5,6]. Furthermore, most treatment-experienced PLWH have switched to INSTI-based single-tablet antiretroviral regimen (STR) due to the low pill burden and good safety [7].
Weight gain has been reported in PLWH after the initiation of ART [8,9]. Possible mechanisms for ART-associated weight gain include a return to good health, especially in those with advanced human immunodeficiency virus (HIV), reflected in weight returning to the pre-illness baseline [8]. Although weight gain may indicate a positive response to ART in PLWH, excess weight gain and obesity increase the risk of associated comorbidities, such as diabetes, metabolic diseases, and cardiovascular disease (CVD) [10]. For each 1.0 kg/m2 gain in body mass index (BMI) after ART initiation, there is a 12% increased risk of diabetes irrespective of pre-ART BMI and an 18 – 20% increased risk of CVD in the normal pre-ART BMI group [11]. Factors associated with weight gain among PLWH include both demographic and HIV-specific characteristics, with greater weight gain observed in black people, women, and those with high pre-treatment HIV-1 viral load (VL) or low CD4+ T cell counts before treatment [8,12]. Several recent studies have reported greater weight gain and increased prevalence of overweight and obesity among patients receiving INSTI-based regimens for initial therapy in comparison to protease inhibitor (PI)- and non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens [8,13,14]. In particular, there is accumulating evidence that newer INSTI, such as dolutegravir (DTG) and bictegravir (BIC), are more closely associated with weight gain than other classes of antiretrovirals [12].
The prevalence rates of obesity and related metabolic complication show racial differences. There is limited information on the effects of ART on weight gain in Asian PLWH [15,16]. In treatment-naïve Asian PLWH, ART-associated weight gain was reported to continue to increase for 5 years following initiation of treatment [16]. In that study, a combination of DTG and tenofovir alafenamide (TAF) was associated with the greatest weight gain. In virally suppressed Asian PLWH, short-term weight gain was observed after switching from a PI- or NNRTI-based regimen to tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat (TAF/F/EVG/c) [15]. However, none of these studies focused on Korean PLWH.
Therefore, this study investigated changes in weight and lipid profile over time in Korean treatment-naïve and treatment-experienced PLWH receiving INSTI-based treatment. In addition, the risk factors for weight gain in these populations were analyzed.
Materials and Methods
1. Study design and participants
This study was conducted retrospectively in PLWH 18 years and older using INSTI-based STR for more than 2 years at three university hospitals from May 2014 to December 2020.
The study included both treatment-naïve and treatment-experienced groups. The treatment-naïve group had no previous history of ART exposure, while the treatment-experienced group patients were virologically suppressed (VL <200 copies/mL) at or prior to switching to INSTI-based STR.
The participants who stopped INSTI-based STR or switched to another regimen within 6 months of initiation, and participants who died or were lost to follow-up within 24 months of switching were excluded. Pregnant women, non-Korean nationals, and patients with virological failure (defined as failure to suppress and sustain plasma HIV-1 VL <200 copies/mL) were also excluded.
2. Ethics statement
This study was approved by the Institutional Review Board of Chonnam National University Hospital (IRB no. CNUH-2020-022). A waiver of the requirement for consent was granted given the retrospective nature of the clinical analyses.
3. Classification of INSTI-based STRs
Three INSTI-based STRs were included in this study: tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (TDF/F/EVG/c, Stribild®, Gilead Sciences, Foster City, CA, USA); TAF/F/EVG/c (Genvoya®, Gilead Sciences, Foster City, CA, USA); and abacavir/lamivudine/dolutegravir (ABC/3TC/DTG, Triumeq®, ViiV Healthcare Company, Brentford, UK).
4. Outcome measures
The primary outcome measures were the relationships among INSTI class, weight gain over time, and lipid profile changes in the treatment-naïve and treatment-experienced groups. The secondary objective was to determine whether an INSTI-based STR was an independent risk factor for weight gain.
5. Data collection and laboratory investigations
Data related to INSTI-based STR were collected from electronic medical records. Smoking, drinking, and exercise data were obtained from records of responses to questions asked during the initial assessment and follow-up counseling.
Baseline demographic and clinical data (values at initiation of INSTI) included sex, age, ART treatment period, CD4+ T cell count, HIV-1 VL, weight, height, smoking, alcohol consumption, physical exercise, and the fasting lipid profile. Physical exercise (aerobic and/or resistance) was defined as at least three times per week [17]. Baseline comorbidities including dyslipidemia, diabetes mellitus, hypertension, and CVD were also recorded. Dyslipidemia was defined as the presence of one or more of total cholesterol (TC) ≥240 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥160 mg/dL, high-density lipoprotein cholesterol (HDL-C) <40 mg/dL, and triglyceride (TG) ≥200 mg/dL or taking lipid-lowering medications [18].
Weight gain rate was defined as the ratio of the difference between the baseline weight and that at subsequent timepoints divided by the baseline weight. BMI was calculated as weight (kg) divided by the square of height (m2). Participants were divided into four groups according to BMI: underweight (<18.5 kg/m2), normal weight (18.5 – 24.9 kg/m2), overweight (25.0 – 29.9 kg/m2), and obese (≥30.0 kg/m2) [19].
Data on the CD4+ T cell count and HIV-1 VL, weight, and fasting lipids at 6, 12, 18, and 24 ± 1 months after initiation of INSTI were also collected. The analysis of fasting lipids encompassed TC, HDL-C, LDL-C, and TG. Absolute changes in the lipid profile at each measurement point were calculated relative to the baseline. Some data for measurement timepoints subsequent to the baseline were unavailable for some participants.
6. Statistical analysis
Data are presented separately for the treatment-naïve and treatment-experienced groups. The demographic and clinical characteristics of the participants are presented according to the INSTI-based regimens.
Demographic and clinical characteristics were obtained according to the INSTI-based regimens as median (interquartile range [IQR]), mean (standard deviation [SD]) or percentage with frequency values, as appropriate. Continuous variables were compared using analysis of variance (ANOVA) followed by Tukey’s post hoc test for normally distributed data. The normality of the data distribution was examined using the Kolmogorov–Smirnov test. Categorical variables were evaluated using Pearson’s chi-square test for differences between regimens. Repeated-measures ANOVA was used to compare weight gain relative to baseline among the 6-, 12-, 18-, and 24-month intervention timepoints. Participants with any missing data were excluded from analysis of repeated-measure ANOVA. Logistic regression analysis was performed to identify risk factors for weight gain. Significant weight gain was defined as corresponding to the upper quartile in each group. All tests of significance were two-tailed and P-values <0.05 were considered indicative of statistical significance. SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for data analyses.
Results
1. Demographic and clinical characteristics of the participants
During the study period, a total of 179 treatment-naïve patients and 290 treatment-experienced patients met the criteria for inclusion in this study. All participants were Korean. Table 1 shows the demographic and clinical characteristics of the two groups.
Table 1. Baseline demographic and clinical characteristics of the treatment-naïve (n = 179) and treatment-experienced (n = 290) groups.
| Variables | Treatment-naïve group (n = 179) | Treatment-experienced group (n = 290) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | TDF/F/EVG/c | TAF/F/EVG/c | ABC/3TC/DTG | P-value | Total | TDF/F/EVG/c | TAF/F/EVG/c | ABC/3TC/DTG | P-value | |||
| N (%) | 179 (100.0) | 70 (39.1) | 55 (30.7) | 54 (30.2) | 290 (100.0) | 66 (22.8) | 136 (46.9) | 88 (30.3) | ||||
| Male gender, n (%) | 170 (95.0) | 66 (94.3) | 54 (98.2) | 50 (92.6) | 0.388 | 256 (88.3) | 55 (83.3) | 119 (87.5) | 82 (93.2) | 0.159 | ||
| Age (years), median (IQR) | 35 (26 - 48) | 32 (24 - 45) | 36 (28 - 56) | 37 (25 - 50) | 0.112 | 45 (33 - 55) | 43 (35 - 54) | 43 (32 - 53) | 50 (36 - 56) | 0.106 | ||
| ART treatment period (years), median (IQR) | - | - | - | - | - | 4 (2 - 8) | 4 (2 - 7)a | 4 (2 - 7)b | 6 (3 - 11)a,b | 0.002c | ||
| Weight (kg), median (IQR) | 66 (59 - 75) | 65 (59 - 74) | 68 (60 - 75) | 66 (57 - 78) | 0.268 | 66 (59 - 74) | 64 (57 - 74) | 66 (59 - 75) | 68 (59 - 74) | 0.374 | ||
| BMI (kg/m2), median (IQR) | 22.6 (20.6 - 5.0) | 22.3 (20.4 - 4.4) | 23.3 (21.3 - 5.1) | 22.7 (20.5 - 25.7) | 0.110 | 23.2 (21.4 - 5.2) | 22.6 (21.2 - 25.0) | 23.2 (21.1 - 4.9) | 23.4 (21.7 - 26.2) | 0.113 | ||
| BMI category, n (%) | 0.079 | 0.092 | ||||||||||
| Underweight | 13 (7.3) | 7 (10.0) | 1 (1.8) | 5 (9.3) | 9 (3.1) | 2 (3.0) | 4 (2.9) | 3 (3.4) | ||||
| Normal | 123 (68.7) | 51 (72.9) | 40 (72.7) | 32 (59.3) | 201 (69.3) | 48 (72.7) | 102 (75.0) | 51 (58.0) | ||||
| Overweight | 33 (18.4) | 12 (17.1) | 9 (16.4) | 12 (22.2) | 71 (24.5) | 15 (22.7) | 28 (20.6) | 28 (31.8) | ||||
| Obese | 10 (5.6) | - | 5 (9.1) | 5 (9.3) | 9 (3.1) | 1 (1.5) | 2 (1.5) | 6 (6.8) | ||||
| CD4+ T-cell count (cell/mm3) | ||||||||||||
| Median (IQR) | 280 (148 - 431) | 271a (168 - 520) | 217a,b (79 - 370) | 343b (236 - 458) | 0.003c | 568 (421 - 745) | 484a (363 - 686) | 575a (425 - 751) | 616 (478 - 800) | 0.031c | ||
| N (%) | 0.006c | 0.017c | ||||||||||
| <100 | 31 (17.3) | 9 (12.9) | 16 (29.1) | 6 (11.1) | 2 (0.7) | - | - | 2 (2.3) | ||||
| 100 to 200 | 28 (15.6) | 12 (17.1) | 10 (18.2) | 6 (11.1) | 6 (2.1) | - | 4 (2.9) | 2 (2.3) | ||||
| 200 to 349 | 50 (27.9) | 20 (28.6) | 14 (25.5) | 16 (29.6) | 32 (11.0) | 14 (21.2) | 12 (8.8) | 6 (6.8) | ||||
| 350 to 499 | 35 (19.6) | 8 (11.4) | 12 (21.8) | 15 (27.8) | 74 (25.5) | 20 (30.3) | 36 (26.5) | 18 (20.5) | ||||
| ≥500 | 35 (19.6) | 21 (30.0) | 3 (5.5) | 11 (20.4) | 176 (60.7) | 32 (48.5) | 84 (61.8) | 60 (68.2) | ||||
| HIV-1 VL (log10 copies/mL), median (IQR) | 4.4 (4.0 - 4.9) | 4.3 (3.9 - 4.9) | 4.8 (4.0 - 5.2) | 4.3 (4.0 - 4.7) | 0.512 | - | - | - | - | - | ||
| ≥100,000 copies/mL, n (%) | 39 (21.8) | 13 (18.6) | 20 (36.4) | 6 (11.1) | 0.004c | - | - | - | - | - | ||
| Smoking, n (%) | 88 (54.3) | 39 (56.5) | 27 (60.0) | 22 (45.8) | 0.348 | 29 (44.5) | 28 (42.4) | 60 (44.1) | 41 (46.6) | 0.870 | ||
| Alcohol consumption, n (%) | 77 (47.5) | 35 (50.7) | 19 (42.2) | 23 (47.9) | 0.672 | 131 (45.2) | 30 (45.5) | 62 (45.6) | 39 (44.3) | 0.981 | ||
| Physical exercise, n (%) | 49 (30.1) | 23 (32.9) | 11 (24.4) | 15 (31.2) | 0.616 | 128 (44.1) | 30 (45.5) | 59 (43.7) | 39 (44.3) | 0.973 | ||
| Comorbidities, n (%) | ||||||||||||
| Dyslipidemia | 14 (7.8) | 5 (7.1) | 2 (3.6) | 7 (13.0) | 0.186 | 83 (28.7) | 12 (18.5) | 30 (22.1) | 41 (46.6) | <0.001c | ||
| Diabetes mellitus | 19 (10.6) | 4 (5.7) | 7 (12.7) | 8 (14.8) | 0.219 | 39 (13.4) | 7 (10.6) | 17 (12.5) | 15 (17.0) | 0.463 | ||
| Hypertension | 14 (7.8) | 5 (7.1) | 4 (7.3) | 5 (9.3) | 0.895 | 41 (14.1) | 4 (6.1) | 15 (11.0) | 22 (25.0) | 0.001c | ||
| CVD | 5 (2.8) | - | 2 (3.6) | 3 (5.6) | 0.159 | 10 (3.4) | 1 (1.5) | 4 (2.9) | 5 (5.7) | 0.339 | ||
| Prior ART regimen, n (%) | - | <0.001c | ||||||||||
| NNRTI | - | - | - | - | 40 (13.8) | 19 (28.8) | 3 (2.2) | 18 (20.5) | ||||
| PI | - | - | - | - | 70 (24.1) | 34 (51.5) | 8 (5.9) | 28 (31.8) | ||||
| INSTI | - | - | - | - | 180 (62.1) | 13 (19.7) | 125 (91.9) | 42 (47.7) | ||||
a,bStatistically significant difference between groups determined by Turkey post hoc analysis (P <0.05).
cStatistically significant difference (P <0.05).
TDF/F/EVG/c, tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat; TAF/F/EVG/c, tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat; ABC/3TC/DTG abacavir/ lamivudine/ dolutegravir; IQR, interquartile range; ART, antiretroviral therapy; Kg, kilogram; BMI, body mass index; HIV, human immunodeficiency virus; VL, viral load; CVD, cardiovascular disease; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INSTI, Integrase strand-transfer inhibitor.
In treatment-naïve participants, 70 (39.1%) were started on a TDF/F/EVG/c regimen, 55 (30.7%) on TAF/F/EVG/c, and 54 (30.2%) on ABC/3TC/DTG. There were no differences in demographic and clinical characteristics among the three INSTI-based STR groups except a lower CD4+ T cell count (P = 0.003) and higher rate of HIV-1 VL ≥100,000 copies/mL (P = 0.004) in the TAF/F/EVG/c group.
Of the treatment-experienced participants, 66 (22.8%) switched to the TDF/F/EVG/c regimen, 136 (46.9%) to TAF/F/EVG/c, and 88 (30.3%) to ABC/3TC/DTG. The prior ART regimen was significantly different among the groups (P <0.001). PI was the most common prior regimen in the TDF/F/EVG/c group (51.5%), whereas INSTI was the most common prior regimen in the TAF/F/EVG/c (91.9%) and ABC/3TC/DTG (47.7%) groups. The prior ART treatment duration was longer in the ABC/3TC/DTG group than the other STR groups (P = 0.002). The TAF/F/EVG/c group had a significantly higher CD4+ T cell count than the TDF/F/EVG/c group (P = 0.031). The rates of dyslipidemia and hypertension were significantly higher for ABC/3TC/DTG than the other STRs (both P <0.001).
2. Weight gain
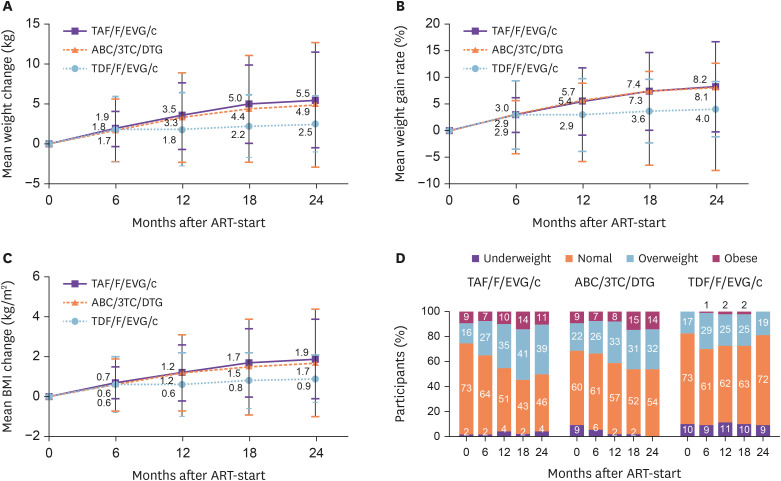
In the treatment-naïve group, mean weight gain was in the order TAF/F/EVG/c (5.5 kg [SD = 6.0]), ABC/3TC/DTG (4.9 kg [SD = 7.8]), and TDF/F/EVG/c (2.5 kg [SD = 3.5]) at 24 months, with mean weight gain rate of 8.2% (SD = 8.5), 8.1% (SD = 15.8), and 4.0% (SD = 5.2), respectively (Fig. 1A, 1B). Weight gain was observed from month 6 onward, and was higher than baseline at 12, 18, and 24 months after initiation of all three INSTI-based STRs (all P <0.001). In addition, no significant differences were observed in weight change between participants on DTG- and EVG-based ART (4.9 kg [SD = 7.8] vs. 3.9 kg [SD = 5.0], P = 0.428). Greater weight gain was observed in PLWH treated with TAF/F/EVG/c compared to TDF/F/ EVG/c (5.5 kg [SD = 6.0] vs. 2.5 kg [SD = 3.5], P = 0.021).
Figure 1. Changes in body weight, weight gain rate, body mass index (BMI), and BMI category distribution within 24 months after initiation of treatment with three fixed-dose combination drugs based on integrase strand transfer inhibitors compared to baseline in treatment-naïve patients. (A) Mean weight change, (B) mean weight gain rate, (C) mean BMI change, and (D) BMI category distribution.
TAF/F/EVG/c, tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat; ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; TDF/F/EVG/c, tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat; ART, antiretroviral therapy.
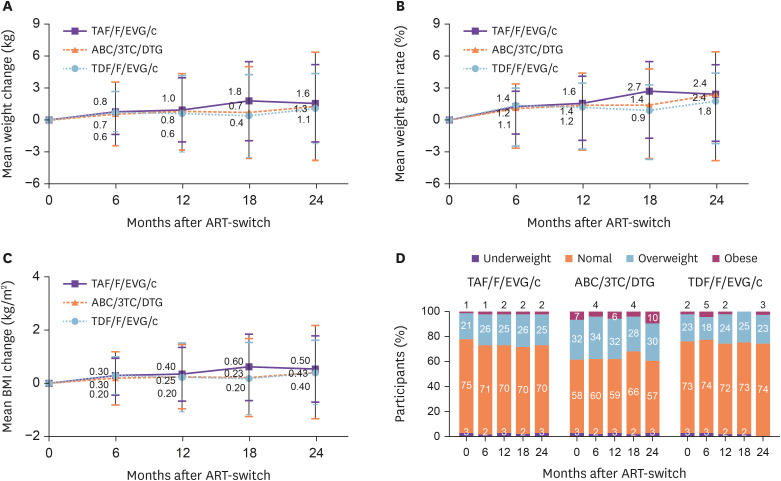
In the treatment-experienced group, mean weight gain at 24 months was 1.6 kg (SD = 3.6) for TAF/F/EVG/c, 1.3 kg (SD = 5.1) for ABC/3TC/DTG, and 1.1 kg (SD = 3.3) for TDF/F/EVG/c. Mean weight gain rates at 24 months were 2.4% (SD = 5.5) for TAF/F/EVG/c, 2.4% (SD = 7.8) for ABC/3TC/DTG, and 1.8% (SD = 4.9) for TDF/F/EVG/c (Fig. 2A, 2B). There were no significant differences in weight gain according to INSTI-based STR between the groups. Mean weight gain in the treatment group was 1.4 kg (SD = 4.1) at 24 months, significantly lower than that in the treatment-naïve group (4.3 kg [SD = 6.3]) (P <0.001).
Figure 2. Changes in body weight, weight gain rate body mass index (BMI), and BMI category distribution over 24 months of treatment with three fixed-dose regimens based on integrase strand transfer inhibitors compared to baseline in treatment-experienced patients. (A) Mean weight change, (B) mean weight gain rate, (C) mean BMI change, and (D) BMI category distribution.
TAF/F/EVG/c, tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat; ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; TDF/F/EVG/c, tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat; ART, antiretroviral therapy.
3. Body mass index category
In the treatment-naïve group, the BMI change at 24 months was 1.9 kg/m2 (SD = 2.0) for TAF/F/EVG/c, 1.7 kg/m2 (SD = 2.7) for ABC/3TC/DTG, and 0.9 kg/m2 (SD = 1.2) for TDF/F/EVG/c (Fig. 1C). BMI categories changed over time for TAF/F/EVG/c and ABC/3TC/DTG, with significant increases seen in the overweight and obesity rates. During the 24-month follow-up, the rate of normal BMI decreased from 72.7% to 46.4%, and that of overweight increased from 16.4% to 39.3%, for TAF/F/EVG/c (P = 0.051). The rate of normal BMI decreased from 59.3% to 53.7%, and that of overweight increased from 22.2% to 31.7%, for ABC/3TC/DTG (P = 0.021) (Fig. 1D).
In the treatment-experienced group, the BMI change at 24 months was 0.5 kg/m2 (SD = 1.2) for TAF/F/EVG/c, 0.4 kg/m2 (SD = 1.7) for ABC/3TC/DTG, and 0.4 kg/m2 (SD = 1.2) for TDF/F/EVG/c (Fig. 2C). There was no change in BMI category distribution until 24 months after initiating the three INSTI-based STRs in the treatment group (Fig. 2D).
4. Changes in lipid profile
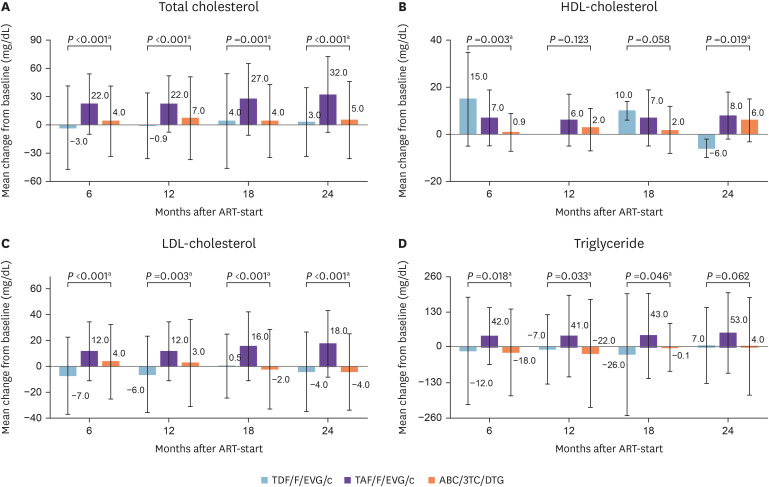
Lipid profiles are not shown separately because there were no differences between the treatment-naïve and treatment-experienced groups. The changes in lipid profile were compared between baseline and subsequent measurement timepoints. The mean changes in TC (31.6 mg/dL, SD = 40.2, P <0.001) and LDL-C (18.1 mg/dL, SD = 26.0, P <0.001) were higher for TAF/F/EVG/c compared to the other regimens at 24 months (Fig. 3). For TAF/F/EVG/c, the lipid profile was significantly different from baseline at 6 months, and tended to remain elevated until 24 months. On the other hand, LDL-C decreased over time in the ABC/3TC/DTG group.
Figure 3. Changes in lipid profile over 24 months of treatment with three integrase strand transfer inhibitor-based fixed-dose regimens compared to baseline.
aStatistically significant difference (P <0.05).
HDL, high-density lipoprotein; mo, month; LDL, low-density lipoprotein; TDF/F/EVG/c, tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat; TAF/F/EVG/c, tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat; ABC/3TC/DTG, abacavir/lamivudine/dolutegravir.
5. Risk factors for weight gain
To determine the factors associated with weight gain, we compared participants who gained weight after 24 months with those who did not (Supplementary Table 1). The upper quartile of weight gain was > 10% the weight gain rate in the treatment-naïve group and 5% in the treatment-experienced group at 24 months.
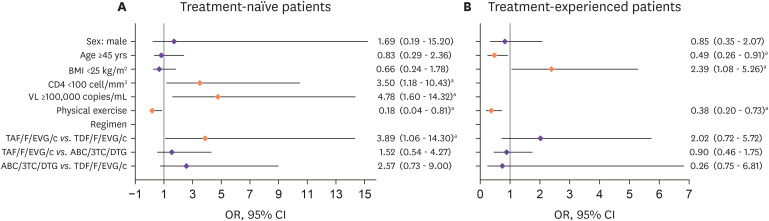
In the treatment-naïve group, CD4+ T cell count <100 cells/mm3 (odds ratio [OR]: 3.50, 95% confidence interval [CI]: 1.18 - 10.43, P = 0.024), HIV-1 VL ≥100,000 copies/mL (OR: 4.78, 95% CI: 1.60 – 14.32, P = 0.005), and being on the TAF/F/EVG/c instead of TDF/F/EVG/c regimen (OR: 3.89, 95% CI: 1.06 – 14.30, P = 0.041) were associated with ≥10% weight gain. Physical exercise (OR: 0.18, 95% CI: 0.04 – 0.81, P = 0.026) was a protective factor against weight gain (Fig. 4A).
Figure 4. Risk factors for weight gain in treatment-naïve and treatment-experienced individuals treated with integrase strand transfer inhibitors.
aStatistically significant difference (P <0.05).
BMI, body mass index, VL, viral load; TAF/F/EVG/c, tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat; TDF/F/EVG/c, tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat; ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; OR, odd ratio; CI, confidence interval.
In the treatment-experienced group, BMI <25 kg/m2 (OR: 2.39, 95% CI: 1.08 – 5.26, P = 0.031) was associated with ≥5% weight gain. Age ≥45 years (OR: 0.49, 95% CI: 0.26 – 0.91, P = 0.023) and physical exercise (OR: 0.38, 95% CI: 0.20 – 0.73, P = 0.004) were negatively related to ≥5% weight gain (Fig. 4B).
Discussion
During the 24-month follow-up, continuous increases in weight gain and weight gain rate were observed in both treatment-naïve and treatment-experienced Korean PLWH taking three INSTI-based STRs. The proportions of obese and overweight increased over time in the treatment-naïve group, especially for TAF/F/EVG/c and ABC/3TC/DTG. The TC, LDL-C, and TG levels were higher after 6 months in the TAF/F/EVG/c group compared to the other groups and remained elevated until 24 months. Risk factors for weight gain were multifactorial, and included demographic, HIV-related, and ART-related factors, i.e., low CD4 counts, high VL, TAF/F/EVG/c (vs. TDF/F/EVF/c), and lack of physical exercise in the treatment-naïve group, and younger age, lower BMI, and lack of physical exercise in the treatment-experienced group. To the best of our knowledge, this is the first study to investigate the weight gain and lipid profile changes in Korean PLWH undergoing ART. Risk factors for weight gain in these populations were also evaluated.
In the treatment-naïve group, there were no significant differences in weight gain according to the three INSTI-based STRs, although PLWH treated with TAF/F/EVG/c and ABC/3TC/DTG tended to gain more weight at 2 years than those treated with TDF/F/EVG/c. Weight gain is common after ART but appears to be greater in patients receiving INSTIs [12]. A recent analysis of data pooled from eight phase 3 randomized-controlled trials showed that patients treated with DTG gained significantly more weight than those treated with EVG [8]. Participants taking DTG and EVG showed average weight gain of 4.07 and 2.72 kg at 96 weeks, respectively [8]. However, those studies included a limited number of Asian patients. In a recent study of Asian PLWH, those started on DTG- and EVG-based ART gained an average of 3.2 and 2.2 kg at 2 years, respectively [16]. In the present study, Korean PLWH started on DTG- and EVG-based ART gained an average of 4.9 kg (8.1%) and 3.9 kg (6.0%) at 2 years, respectively. The differences between the DTG- and EVG-based ART groups in this study were not significant, consistent with previous observations of Asian populations [16]. However, the differences in weight gain between the two studies may be due to racial differences and small sample sizes.
TAF and TDF are both prodrugs of tenofovir and have similar efficacy. TAF has recently replaced TDF as it has less effect on markers of renal and bone toxicity. However, TAF has unfavorable effects on weight gain and dyslipidemia [8,20]. In the present, there was a difference in weight gain according to whether TDF or TAF was the background therapy for EVG/c; total weight gain and the weight gain rate were higher for TAF/F/EVG/c than TDF/F/EVF/c in the treatment-naïve group. However, the baseline characteristics of the TAF/F/EVG/c group in this study showed lower CD4 and higher HIV-1 viral load, which were known risk factors for weight gain in other studies [8,12]. Although multivariate analysis showed that TAF as backbone therapy was an independent risk factor for ≥10% weight gain in the treatment-naïve group, these baseline characteristics may have influenced the study results.
Long-term use of ART is related to metabolic complications such as dyslipidemia [21]. ART can induce raised levels of TC, LDL-C, and TG, and has variable effects on HDL-C levels [22]. In a large cross-sectional study, the prevalence of hypercholesterolemia, hypertriglyceridemia, and low HDL-cholesterol was 10 – 27%, 23 – 40%, and 19 – 27%, respectively [22]. In this study, the incidence of dyslipidemia before initiation of or switch to INSTI was 11% and 30% in treatment-naïve and treatment-experienced patients, respectively, suggesting an increased incidence of dyslipidemia in the ART-experienced group. The occurrence of dyslipidemia reportedly differs according to ART regimen. Regimens containing drugs of both the PI and NNRTI classes had the highest prevalence of dyslipidemia [23]. By contrast, another cohort study revealed that dyslipidemia was less common with INSTI than with boosted PI. Among INSTIs, dyslipidemia was more common with EVG/c and raltegravir (RAL) compared with DTG [24]. In this study, the changes in lipid profile in the TAF/F/EVG/c group were significantly greater than those in the TDF/F/EVG/c and ABC/3TC/DTG groups, except for HDL-C. TAF/F/EVG/c was associated with significant increases in TC, LDL-C, HDL-C, and TG from 6 months after initiation compared to baseline; all of these markers tended to remain elevated, consistent with observations in virologically suppressed HIV-positive patients in Taiwan [15]. Prior ART regimen in this study differed among three INSTI-based STRs in treatment-experienced group. PI and NNRTI were 80% of prior regimen in the TDF/F/EVG/c group, whereas INSTI was 92% of prior regimen in the TAF/F/EVG/c groups. Notably, the prior ART regimen for most of patients (88%) who switched to TAF/F/EVG/c was TDF/F/EVG/c. These finding suggest that close monitoring of the lipid profile is necessary in in the group using TAF as a backbone therapy.
Dyslipidemia is a risk factor for CVD. The traditional risk factors for CVD include smoking, sedentary lifestyle, high blood pressure, high BMI, hypercholesterolemia, diabetes, unhealthy diet, and psychosocial stress [25,26]. The Data collection on Adverse Effects of Anti-HIV Drugs Study (DAD) equation, which was developed to evaluate the cardiovascular risk of HIV-infected patients, takes into account ART exposure [27]. The use of some ART agents is associated with an increased risk of CVD, including some ritonavir-boosted PIs (lopinavir, indinavir, darunavir), and nucleoside reverse transcriptase inhibitors (didanosine and ABC) [27,28]. Another ART agent, efavirenz, is associated with dyslipidemia, although it has not been shown to increase CVD risk [29]. In a post hoc analysis of atherosclerotic CVD risk profiles of TAF and TDF, lipid changes associated with TAF-based regimens did not substantively affect CVD risk compared to TDF at week 96 [20]. The effects of INSTIs on CVD in PLWH are controversial. In a retrospective cohort study, INSTI-based regimens were associated with a 21% decreased risk of incident CVD [30]. By contrast, in another study, RAL, EVG/c, and DTG were associated with a 2.5-fold higher incidence of CVD within the first 6 months of exposure in comparison to no INSTI exposure [31]. Further long-term studies in larger Korean PLWH populations are needed to evaluate the individual effects of INSTI-based ART exposure on CVD risk.
In this study, physical exercise was a protective factor against weight gain in both the treatment-naïve and treatment-experienced groups. These findings highlight the importance of exercise in ART-related weight gain in PLWH for the first time. Physical exercise has been shown to improve mental health, quality of life, immunity, and physical function in PLWH [32]. Furthermore, lifestyle modifications, including exercise, are necessary to control age-related comorbidities [17]. Performing progressive resistive exercise or a combination of resistive and aerobic exercise, at least three times per week for at least 6 weeks, led to improvements in cardiorespiratory fitness, strength, weight, and body composition among PLWH [17]. In the study, participants who performed physical exercise gained less weight, unlike those who did not exercise. This suggests that weight gain, which is an inevitable adverse effect of INSTI-based STRs, may be substantially modified by physical exercise. It is necessary to recommend and monitor regular exercise in PLWH receiving ART.
This study had several limitations. First, this was a retrospective study, and weight and lipid profile data were not available at all timepoints for all participants. Therefore, there may have been selection and information bias. Second, other factors that could affect weight change, such as diet, psychosocial stress, etc., were not included in the analysis. Third, smoking, alcohol consumption, and exercise data were collected qualitatively instead of quantitatively during counseling. Finally, the incidences of diabetes mellitus, metabolic disease, and CVD after the introduction of INSTI-based STR were not evaluated because of the small number of participants in the study. Further studies on the effects of weight gain and lipid profile changes after initiation or switching of INSTI-based regimens on the occurrence of comorbidities, such as diabetes mellitus, metabolic disease, and CVD, are needed. In addition, studies on weight gain and lipid profile change in recently introduced INSTI-based regimen are needed in the future. Despite these limitations, this study analyzed weight gain and changes in lipid profiles after initiation of INSTI-based STR, which is currently recommended for Korean PLWH. Furthermore, the importance of physical exercise for preventing weight gain in PLWH was highlighted. These results could be useful data for INSTI-based STR treatment of Korean PLWH.
In conclusion, INSTI-based STR was associated with weight gain at the 24-month follow-up in both treatment-naïve and treatment-experienced groups in Korea PLWH. Total weight gain and the weight gain rate tended to be higher in participants treated with TAF/F/EVG/c and ABC/3TC/DTG compared to TDF/F/EVG/c, in both groups. TAF/F/EVC/c was associated with higher levels of lipids, including TC, LDL-C, and TG. The risk factors for weight gain treatment-naïve and treatment-experienced were multifactorial in nature; demographic factors, HIV-related factors, and ART all exert an influence. When selecting antiretroviral drugs in PLWH, the characteristics thereof and demographic data of the patients should be taken into consideration. In addition, lifestyle modifications, such as physical exercise, to prevent weight gain and related comorbidities should be actively encouraged.
Footnotes
Funding: None.
Conflict of Interest: SIJ is an associate editor of Infection & Chemotherapy; however, she was not involved in the peer reviewer selection, evaluation, or decision-making for this article. Otherwise, no potential conflicts of interest relevant to this article are reported.
- Conceptualization: JK, EKC, UJK, SIJ.
- Data curation: JK, HJN, YJJ, HJL.
- Formal analysis: JK, EKC, UJK.
- Investigation: JK, HJN, YJJ, HJL.
- Methodology: JK, HHC, EKC, SIJ.
- Project administration: JK, HJN, YJJ, HJL.
- Resources: HHC, SWK, KHP, SIJ.
- Software: JK, EKC.
- Supervision: EKC, UJK, SIJ.
- Validation: JK, SEK, UJK, SJK, SIJ.
- Visualization: JK, SIJ.
- Writing - original draft: JK, EKC.
- Writing - review & editing: EKC, UJK, SIJ.
SUPPLEMENTARY MATERIAL
Baseline demographic and clinical characteristics according to weight gain in the treatment-naïve (n = 179) and treatment- experienced (n = 290) groups
References
- 1.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11:492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services; [Accessed 20 January 2022]. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. [Google Scholar]
- 4.Ryom L, De Miguel R, Cotter AG, Podlekareva D, Beguelin C, Waalewijn H, Arribas JR, Mallon PWG, Marzolini C, Kirk O, Bamford A, Rauch A, Molina JM, Kowalska JD, Guaraldi G, Winston A, Boesecke C, Cinque P, Welch S, Collins S, Behrens GMN EACS Governing Board. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022 doi: 10.1111/hiv.13268. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly SG, Masters MC, Taiwo BO. Initial antiretroviral therapy in an integrase inhibitor era: Can we do better? Infect Dis Clin North Am. 2019;33:681–692. doi: 10.1016/j.idc.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messiaen P, Wensing AM, Fun A, Nijhuis M, Brusselaers N, Vandekerckhove L. Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis. PLoS One. 2013;8:e52562. doi: 10.1371/journal.pone.0052562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotte L, Ferry T, Pugliese P, Valantin MA, Allavena C, Cabié A, Poizot-Martin I, Rey D, Duvivier C, Cheret A, Dellamonica P, Pradat P, Parienti JJ Dat’AIDS study group. Effectiveness and tolerance of single tablet versus once daily multiple tablet regimens as first-line antiretroviral therapy - Results from a large french multicenter cohort study. PLoS One. 2017;12:e0170661. doi: 10.1371/journal.pone.0170661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, Brown TT, Rockstroh JK, Wei X, Carter CC, Zhong L, Brainard DM, Melbourne K, Das M, Stellbrink HJ, Post FA, Waters L, Koethe JR. Weight gain following initiation of antiretroviral therapy: Risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–1389. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP, Koethe JR, Sterling TR. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70:1267–1274. doi: 10.1093/cid/ciz407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013;29:435–440. doi: 10.1089/aid.2012.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, de Wit S, Smith CJ, d’Arminio Monforte A, Phillips A, Weber R, Lundgren J, Law MG D:A:D Study Group. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2016;17:255–268. doi: 10.1111/hiv.12294. [DOI] [PubMed] [Google Scholar]
- 12.Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33:10–19. doi: 10.1097/QCO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, Hulgan T, Raffanti S, Haas DW, Sterling TR, Koethe JR. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76:527–531. doi: 10.1097/QAI.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourgi K, Jenkins CA, Rebeiro PF, Palella F, Moore RD, Altoff KN, Gill J, Rabkin CS, Gange SJ, Horberg MA, Margolick J, Li J, Wong C, Willig A, Lima VD, Crane H, Thorne J, Silverberg M, Kirk G, Mathews WC, Sterling TR, Lake J, Koethe JR North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23:e25484. doi: 10.1002/jia2.25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo PH, Sun HY, Chuang YC, Wu PY, Liu WC, Hung CC. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Int J Infect Dis. 2020;92:71–77. doi: 10.1016/j.ijid.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Ando N, Nishijima T, Mizushima D, Inaba Y, Kawasaki Y, Kikuchi Y, Oka S, Gatanaga H. Long-term weight gain after initiating combination antiretroviral therapy in treatment-naïve Asian people living with human immunodeficiency virus. Int J Infect Dis. 2021;110:21–28. doi: 10.1016/j.ijid.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Ibeneme SC, Irem FO, Iloanusi NI, Ezuma AD, Ezenwankwo FE, Okere PC, Nnamani AO, Ezeofor SN, Dim NR, Fortwengel G. Impact of physical exercises on immune function, bone mineral density, and quality of life in people living with HIV/AIDS: a systematic review with meta-analysis. BMC Infect Dis. 2019;19:340. doi: 10.1186/s12879-019-3916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. Screening for lipid disorders in children and adolescents: Recommendation statement. Am Fam Physician. 2016;94 Online. [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–ixii. 1–253. [PubMed] [Google Scholar]
- 20.Huhn GD, Shamblaw DJ, Baril JG, Hsue PY, Mills BL, Nguyen-Cleary T, McCallister S, Das M. Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide versus tenofovir disoproxil fumarate. Open Forum Infect Dis. 2019;7:ofz472. doi: 10.1093/ofid/ofz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekolo CE, Nguena MB, Ewane L, Bekoule PS, Kollo B. The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health. 2014;14:236. doi: 10.1186/1471-2458-14-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunfeld C. Dyslipidemia and its treatment in HIV infection. Top HIV Med. 2010;18:112–118. [PMC free article] [PubMed] [Google Scholar]
- 23.Friis-Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, d’Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN, Lundgren JD DAD study group. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 24.The RESPOND Study Group. Incidence of dyslipidemia in people with HIV who are treated with integrase inhibitors versus other antiretroviral agents. AIDS. 2021;35:869–882. doi: 10.1097/QAD.0000000000002811. [DOI] [PubMed] [Google Scholar]
- 25.Maggi P, Di Biagio A, Rusconi S, Cicalini S, D’Abbraccio M, d’Ettorre G, Martinelli C, Nunnari G, Sighinolfi L, Spagnuolo V, Squillace N. Cardiovascular risk and dyslipidemia among persons living with HIV: a review. BMC Infect Dis. 2017;17:551. doi: 10.1186/s12879-017-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, Mack WJ, Cohen MH, Jacobson L, Gange SJ. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–1081. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]
- 27.Friis-Møller N, Thiébaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, Fontas E, Worm S, Kirk O, Phillips A, Sabin CA, Lundgren JD, Law MG DAD study group. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 28.Ryom L, Lundgren JD, El-Sadr W, Reiss P, Kirk O, Law M, Phillips A, Weber R, Fontas E, d’ Arminio Monforte A, De Wit S, Dabis F, Hatleberg CI, Sabin C, Mocroft A D:A:D study group. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV. 2018;5:e291–e300. doi: 10.1016/S2352-3018(18)30043-2. [DOI] [PubMed] [Google Scholar]
- 29.Sinxadi PZ, McIlleron HM, Dave JA, Smith PJ, Levitt NS, Haas DW, Maartens G. Plasma efavirenz concentrations are associated with lipid and glucose concentrations. Medicine (Baltimore) 2016;95:e2385. doi: 10.1097/MD.0000000000002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Halloran JA, Sahrmann J, Butler AM, Olsen MA, Powderly WG. Brief report: Integrase strand transfer inhibitors are associated with lower risk of incident cardiovascular disease in people living with HIV. J Acquir Immune Defic Syndr. 2020;84:396–399. doi: 10.1097/QAI.0000000000002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar S, Brown TT. CROI 2021: Metabolic and other complications of HIV infection or COVID-19. Top Antivir Med. 2021;29:328–333. [PMC free article] [PubMed] [Google Scholar]
- 32.Jaggers JR, Hand GA. Health benefits of exercise for people living with HIV: A review of the literature. Am J Lifestyle Med. 2014;10:184–192. doi: 10.1177/1559827614538750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline demographic and clinical characteristics according to weight gain in the treatment-naïve (n = 179) and treatment- experienced (n = 290) groups