Abstract
Background
The proportion of antimicrobial-resistant Enterobacteriales as a causative pathogen of community-acquired acute pyelonephritis (APN) has been increasing. The aim of this study was to quantitatively evaluate the impact of antimicrobial resistance on medical costs and length of hospital stay for the treatment of APN.
Materials and Methods
A single-center retrospective cohort study was conducted between January 2018 and December 2019. All hospitalized patients aged ≥19 years who were diagnosed with community-acquired APN were recruited, and those diagnosed with Enterobacteriales as a causative pathogen were included. Log-linear regression analysis was performed to determine the risk factors for medical costs and length of hospital stay.
Results
A total of 241 patients participated in this study. Of these, 75 (31.1%) and 87 (36.1%) had extended-spectrum beta-lactamase (ESBL)-producing pathogens and ciprofloxacin-resistant pathogens as the causative pathogen, respectively. Based on the log-linear regression model, ESBL-producing Enterobacteriales is a causative pathogen that is, on average, 27.0%, or United States Dollar (USD) 1,211 (P = 0.026) more expensive than non-ESBL-producing Enterobacteriales. A patient who is a year older would incur USD 23 (P = 0.040) more, those having any structural problems in the urinary tract would incur USD 1,231 (P = 0.015) more, and those with a unit increase in the Pitt bacteremia score would incur USD 767 (P <0.001) more, with all other variables constant. Having a case in which ESBL-producing Enterobacteriales is a causative pathogen would explain staying 22.0% longer or 2 more days (P = 0.050) in the hospital than non-ESBL-producing Enterobacteriales. A patient who is 10 years older would, on average, would have to stay for half a day longer (P = 0.045). Any structural problems in the urinary tract explain a longer stay (2.4 days longer; P = 0.032), and moving from 0 to 5 on the Pitt bacteremia score would explain four more days (P = 0.038) in the hospital.
Conclusion
Patients with community-acquired APN with ESBL-producing Enterobacteriale as the causative pathogen would incur, on average, 27.0% higher medical costs and 22.0% longer hospitalization days than patients detected with non-ESBL-producing pathogens.
Keywords: Antimicrobial resistance, Acute pyelonephritis, Medical costs, Hospitalization
Introduction
Acquisition of antimicrobial resistance (AMR) erodes the effectiveness of antimicrobial therapy, and infections caused by antimicrobial-resistant bacteria result in poorer clinical outcomes, such as increased mortality, prolonged hospitalization, and increased medical costs [1,2]. The increase in antimicrobial-resistant Gram-negative Enterobacteriales is threatening human health [1]. According to the Global Antimicrobial Resistance and Use Surveillance System of the World Health Organization, the proportion of Escherichia coli and Klebsiella pneumoniae with ciprofloxacin resistance ranges from 8.4% to 92.9% and 4.1% to 79.4%, respectively [3]. Although the proportion of Enterobacteriales with AMR differs from country to country, an increasing trend has been observed in almost all regions [4,5]. In Korea, the resistance rate of E. coli to cefotaxime and fluoroquinolone increased from 10% in 2004 to 29.0% in 2013 and from 30.0% in 2004 to 42.0% in 2013 [6].
Acute pyelonephritis (APN) is a representative infectious disease caused by Gram-negative Enterobacteriales and is one of the most common community-acquired bacterial infections [7]. AMR increases not only the treatment failure rate but also medical costs and length of hospital stay among patients with APN [8,9]. A study in the US showed that APN caused by extended-spectrum beta-lactamase (ESBL)-producing E. coli and K. pneumoniae required higher medical costs United States Dollar [USD] 66,590 vs. USD 22,231) and length of hospital stay (11 days vs. 7 days) than that caused by non-ESBL-producing pathogens [9].
Similar to pathogens in other bacterial infectious diseases, the AMR rate of causative pathogens for APN, mostly Gram-negative Enterobacteriales, has been increasing in Korea [10]. As a result, for the treatment of APN in the past decade, the prescription of broad-spectrum antibiotics has increased significantly and the rate of clinical failure has increased [10,11]. However, the effect of AMR on APN, particularly its economic effects, has not yet been quantitatively evaluated in Korea. The aim of this study was to quantitatively evaluate the impact of AMR on medical costs and length of hospital stay for the treatment of APN.
Material and methods
1. Study setting and patient population
A retrospective cohort study was conducted at an 855-bed tertiary-care hospital in Korea between January 2018 and December 2019. All hospitalized patients aged ≥19 years whose primary discharge diagnosis codes were relevant to APN were screened: N10 (which includes acute infectious interstitial nephritis, acute pyelitis, and APN), N12 (which includes interstitial nephritis, pyelitis, and pyelonephritis), and N39.0 (which includes urinary tract infection [UTI], site not specified) codes from the International Classification of Diseases, Tenth Revision. Patients with community-acquired APN caused by Enterobacteriales were selected for this study. The inclusion criteria were as follows: (1) presence of fever (body temperature ≥37.8°C), (2) pyuria (≥5 - 9 white blood cells per high power field [HPF]), (3) Enterobacteriales (E. coli, Klebsiella spp., Proteus spp., Enterobacter spp., or Citrobacter spp.) through urine or blood cultures, and (4) clinical symptoms or signs relevant to APN judged by an infectious disease specialist. We excluded patients diagnosed with APN more than 48 h after admission, those transferred from other hospitals, those who had other reasons for fever and pyuria, and those with insufficient data. In addition, patients with prolonged hospitalization due to medical problems that were not associated with APN treatment were excluded.
2. Ethics statement
The study protocol was approved by the Institutional Review Board of Hanyang University Seoul Hospital (IRB number: 2020-05-030), and the requirement for written informed consent from the patients was waived because of the retrospective nature of the study.
3. Data collection and definitions
We collected microbiological data, demographic characteristics, underlying comorbidities, past history, initial clinical characteristics, treatment, and outcomes of the enrolled patients.
Causative pathogens for APN were determined when organisms at ≥105 colony-forming units/mL were identified in urine culture (regardless of specimen type), and/or Enterobacteriales were isolated from blood cultures. ESBL positivity and ciprofloxacin susceptibility were determined using a semi-automated system (VITEK, bioMèrieux, Hazelwood, MO, USA or Microscan, Dade Behring, West Sacramento, CA, USA). The breakpoints of each compound were defined with reference to the Clinical and Laboratory Standards Institute [12], and the breakpoints of resistance (R) or intermediate (I) category were considered to indicate resistance. Based on the causative pathogen, we defined the groups as follows:
1) APN caused by ESBL-producing Enterobacteriales (ESBL+ group) vs. APN caused by non-ESBL-producing Enterobacteriales (ESBL- group)
2) APN caused by ciprofloxacin-resistant Enterobacteriales (CIP-R group) vs. APN caused by ciprofloxacin sensitive Enterobacteriales (CIP-S group)
Underlying comorbidities included components of the Charlson comorbidity index [13], bedridden status, and structural problems in the urinary tract. Structural problems in the urinary tract were defined if the case was associated with more than one of the following seven categories: intubated urinary tract, intermittent catheterization, neurogenic bladder, urolithiasis, urinary retention, polycystic kidney, and renal tumor. Past history that may have influenced the development of APN was recorded. Initial clinical characteristics included the Pitt bacteremia score [8], UTI symptoms, costovertebral tenderness, back pain, vomiting/diarrhea, hematuria (≥5 - 9 red blood cells per HPF), azotemia (serum blood urea nitrogen [BUN] ≥20 mg/dL and/or serum creatinine ≥1.4 mg/dL), and presence of bacteremia. We also collected data regarding antibiotic prescriptions for the treatment of APN. The initial antibiotic regimens were considered discordant if they did not include at least one antibiotic active against the causative organisms on in vitro susceptibility testing [14].
To assess outcomes, we recorded the length of hospital stay and clinical failure rate. Clinical failure was defined as death or recurrence of APN within 14 days of completing therapy. We excluded patients whose clinical status could not be evaluated 14 days after treatment. In addition, we recorded the Braden scale at admission and discharge to assess the performance status of the patients [15].
For the analysis of medical costs, the costs incurred during hospitalization were extracted from the hospital’s financial database. It consisted of consultation fee, hospitalization expenditures, meal, cost per medication, procedure or operation charge, laboratory examination charge, radiologic examination charge, etc. Non-reimbursed medical costs were excluded. All costs are in USD (1 USD ≒ 1,100 KRW).
Information regarding stay in a premium room during hospitalization was collected for analysis of risk factors for higher medical costs or longer length of stay.
4. Statistical analyses
The main outcome variables of interest, i.e., total medical costs and length of stay, are both continuous measures with positive skewness (skewness: 2.7 and 2.0, respectively). To deal with the skewed outcomes, we log transformed them and applied a linear regression. As much as log-linear models are widely used in health utilization and health expenditure literature, caution must be exercised when retransforming the conditional mean. We recovered the average marginal effects assuming that the regression error is homoscedastic and normally distributed [16]. We obtained standard errors that are robust to misspecifications.
We further adopted a more general approach by estimating the quantile of the conditional distribution (i.e., the relationship between the outcome and the control variables at different points in the conditional distribution), of which the median is its special case. Standard errors were obtained by bootstrapping 500 resamples.
Once the set of covariates was decided, univariate analysis was conducted to find significant variables that may explain the outcome variables using the rule-of-thumb with 0.25 as the cut-off (Supplementary Table 1). Then, standard stepwise selection was performed to decide the preliminary model based on the entry and removal cut-off of 0.15 and 0.2 level, respectively (Supplementary Table 2). The final set of control variables was decided after discussion among the investigators based on previous literature on APN [8].
For the analysis between patient groups according to the causative pathogen, categorical variables were analyzed using the Chi-square test or Fisher’s exact test, as appropriate, and continuous variables were analyzed using the Mann–Whitney U test or independent t-tests, as appropriate.
All statistical analyses were conducted using Stata/SE 16.1, for Windows (StataCorp LLC, College Station, TX, USA). Statistical significance was defined as a two-tailed P-value of <0.05.
Results
1. Comparison of clinical characteristics of community-acquired APN
A total of 241 patients with community-acquired APN caused by Enterobacteriales were recruited. Of these, E. coli was the most common pathogen, accounting for 90.0% of Enterobacteriales. The proportions of the ESBL+ and CIP-R groups were 31.1% (75/241) and 36.1% (87/241), respectively. There was no difference in the composition of uropathogens between the ESBL+ and ESBL- groups, as well as between the CIP-R and CIP-S groups (Table 1).
Table 1. Comparison of the causative pathogens.
| Pathogens | Total | Extended-spectrum beta-lactamase | Ciprofloxacin | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | P-value | Resistant | Sensitive | P-value | ||
| Escherichia coli (%) | 217 (90.0) | 70 (93.3) | 147 (88.6) | 0.212 | 80 (92.0) | 137 (89.0) | 0.441 |
| Klebsiella pneumoniae (%) | 13 (5.4) | 3 (4.0) | 10 (6.0) | 0.492 | 4 (4.6) | 9 (5.9) | 0.673 |
| Proteus spp. (%) | 7 (3.0) | 2 (2.7) | 5 (3.0) | 0.887 | 3 (3.4) | 4 (2.6) | 0.718 |
| Enterobacter spp. (%) | 3 (1.2) | 0 (0.0) | 3 (1.8) | 0.083 | 0 (0.0) | 3 (1.9) | 0.083 |
| Citrobacter spp. (%) | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0.319 | 0 (0.0) | 1 (0.6) | 0.319 |
| Total (%) | 241 (100.0) | 75 (100.0) | 166 (100.0) | 87 (100.0) | 154 (100.0) | ||
Table 2 shows clinical characteristics of patients with community-acquired APN. The mean age was not significantly different between the ESBL+ and ESBL- groups. The ESBL+ group showed higher Charlson’s comorbidity index (2.24 ± 2.35 vs. 1.55 ± 1.74, P = 0.026) and higher proportion of having past history of admission during 1 year prior to inclusion (41.3% vs. 23.5%, P = 0.008) than the ESBL-group, but there were no significant differences in the clinical features between the two groups.
Table 2. Comparison of clinical characteristics of community-acquired acute pyelonephritis.
| Characteristics | Total (n = 241) | Extended-spectrum beta-lactamase | Ciprofloxacin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 75) | Negative (n = 166) | P-value | Resistant (n = 87) | Sensitive (n = 154) | P-value | ||||
| Demographic data | |||||||||
| Age (years), mean ± SD | 65.25 ± 16.77 | 67.95 ± 17.69 | 64.04 ± 16.24 | 0.106 | 68.67 ± 15.61 | 63.32 ± 17.14 | 0.015 | ||
| Underlying co-morbidities | |||||||||
| Charlson’s comorbidity index, mean ± SD | 1.77 ± 1.97 | 2.24 ± 2.35 | 1.55 ± 1.74 | 0.026 | 2.07 ± 2.20 | 1.60 ± 1.81 | 0.091 | ||
| Diabetes mellitus (%) | 83 (34.4) | 28 (37.3) | 55 (33.1) | 0.532 | 33 (37.9) | 50 (32.5) | 0.399 | ||
| Hemiplegia (%) | 7 (2.9) | 0 (0.0) | 7 (4.2) | 0.008 | 2 (2.3) | 5 (3.3) | 0.661 | ||
| Cerebrovascular accident (%) | 27 (11.2) | 11 (14.7) | 16 (9.7) | 0.288 | 12 (13.8) | 15 (9.7) | 0.361 | ||
| Congestive heart failure (%) | 20 (8.3) | 14 (18.7) | 6 (3.6) | 0.002 | 12 (3.8) | 8 (5.2) | 0.393 | ||
| Connective tissue disease (%) | 31 (12.9) | 8 (10.7) | 23 (13.9) | 0.478 | 10 (11.5) | 21 (13.6) | 0.628 | ||
| Malignancy (%) | 40 (16.6) | 16 (21.3) | 24 (14.5) | 0.213 | 17 (19.5) | 23 (15.0) | 0.373 | ||
| Chronic pulmonary disease (%) | 7 (2.9) | 4 (5.3) | 3 (1.8) | 0.213 | 3 (3.5) | 4 (2.6) | 0.718 | ||
| Liver disease (%) | 11 (4.6) | 6 (8.0) | 5 (3.0) | 0.148 | 5 (5.8) | 6 (3.9) | 0.532 | ||
| Renal disease (%) | 29 (12.0) | 13 (17.3) | 16 (9.6) | 0.124 | 13 (14.9) | 16 (10.4) | 0.320 | ||
| Dementia (%) | 23 (9.5) | 10 (13.3) | 13 (7.8) | 0.221 | 12 (13.4) | 11 (7.1) | 0.121 | ||
| Bedridden state (%) | 29 (12.0) | 11 (14.7) | 18 (10.8) | 0.425 | 16 (18.4) | 13 (8.4) | 0.038 | ||
| Any structural problems on urinary tract (%) | 49 (20.3) | 15 (20.0) | 34 (20.5) | 0.932 | 20 (23.0) | 29 (18.8) | 0.453 | ||
| Intubated urinary tract (%) | 14 (5.8) | 7 (9.3) | 7 (4.2) | 0.173 | 9 (10.3) | 5 (3.3) | 0.050 | ||
| Intermittent catheterization (%) | 2 (0.8) | 1 (1.3) | 1 (0.6) | 0.618 | 1 (1.2) | 1 (0.7) | 0.705 | ||
| Benign prostatic hyperplasia (%) | 10/29 (34.5) | 6/13 (46.2) | 4/16 (25.0) | 0.257 | 4/15 (26.7) | 6/14 (42.9) | 0.380 | ||
| Neurogenic bladder (%) | 13 (5.4) | 3 (4.0) | 10 (6.0) | 0.492 | 4 (4.6) | 9 (5.8) | 0.673 | ||
| Urolithiasis (%) | 24 (10.0) | 8 (10.7) | 16 (9.6) | 0.810 | 11 (12.6) | 13 (8.4) | 0.322 | ||
| Urinary retention (%) | 5 (2.1) | 2 (2.7) | 3 (1.8) | 0.689 | 3 (3.5) | 2 (1.3) | 0.324 | ||
| Vesicoureteral reflux (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - | ||
| Vaginal wall prolapse (%) | 1/212 (0.5) | 1/62 (1.6) | 0/150 (0.0) | 0.321 | 1/72 (1.4) | 0/140 (0.0) | 0.321 | ||
| Polycystic kidney (%) | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0.319 | 0 (0.0) | 1 (0.7) | 0.319 | ||
| Renal tumor (%) | 4 (1.7) | 2 (2.7) | 2 (1.2) | 0.479 | 2 (2.3) | 2 (1.3) | 0.591 | ||
| Past history | |||||||||
| History of admission during 1 year prior to inclusion (%) | 70 (29.1) | 31 (41.3) | 39 (23.5) | 0.008 | 39 (44.8) | 31 (20.1) | <0.001 | ||
| History of antibiotic usage during 1 year prior to inclusion (%) | 82 (34.0) | 32 (42.7) | 50 (30.1) | 0.066 | 41 (47.1) | 41 (26.6) | 0.002 | ||
| History of urinary tract infection during 1 year prior to inclusion (%) | 35 (15.8) | 16 (21.3) | 22 (13.3) | 0.140 | 20 (23.0) | 18 (11.7) | 0.032 | ||
| Use of chemotherapeutic agents (%) | 13 (5.4) | 5 (6.7) | 8 (4.8) | 0.582 | 5 (5.8) | 8 (5.2) | 0.858 | ||
| Use of immunosuppressants (%) | 22 (9.1) | 6 (8.0) | 16 (9.6) | 0.675 | 7 (8.1) | 15 (9.7) | 0.655 | ||
| History of urinary catheterization during 1 month prior to inclusion (%) | 4 (1.7) | 2 (2.7) | 2 (1.2) | 0.479 | 2 (2.3) | 2 (1.3) | 0.591 | ||
| History of urinary tract operation during 3 months prior to inclusion (%) | 2 (0.8) | 1 (1.3) | 1 (0.6) | 0.618 | 1 (1.2) | 1 (0.7) | 0.705 | ||
| Clinical characteristics | |||||||||
| Pitt’s score, mean ± SD | 0.77 ± 1.01 | 0.73 ± 0.93 | 0.78 ± 1.04 | 0.712 | 0.85 ± 1.09 | 0.72 ± 0.95 | 0.356 | ||
| Urinary tract infection symptoms (%) | 155 (64.3) | 45 (60.0) | 110 (66.3) | 0.357 | 54 (62.1) | 101 (65.6) | 0.589 | ||
| Costovertebral angle tenderness (%) | 160 (66.4) | 46 (61.3) | 114 (68.7) | 0.276 | 49 (56.3) | 111 (72.1) | 0.016 | ||
| Back pain (%) | 62 (25.7) | 15 (20.0) | 47 (28.3) | 0.155 | 18 (20.7) | 44 (28.6) | 0.168 | ||
| Vomiting/diarrhea (%) | 64 (26.6) | 21 (28.0) | 43 (25.9) | 0.737 | 20 (23.0) | 44 (28.6) | 0.339 | ||
| Hematuria (%) | 124 (51.5) | 40 (53.3) | 84 (50.6) | 0.696 | 48 (55.2) | 76 (49.4) | 0.387 | ||
| Azotemia (%) | 99 (41.1) | 36 (48.0) | 63 (38.0) | 0.149 | 37 (42.5) | 62 (40.3) | 0.733 | ||
| Bacteremia (%) | 115/236 (48.7) | 36/75 (48.0) | 79/161 (49.1) | 0.879 | 41/85 (48.2) | 74/151 (49.0) | 0.910 | ||
| Treatment | |||||||||
| Discordance between initial antimicrobial regimen and the antimicrobial susceptibility of causative organisms (%) | 41 (17.2) | 40 (53.3) | 1 (0.6) | <0.001 | 26 (29.9) | 15 (9.9) | <0.001 | ||
| Duration of total antibiotics, days, mean ± SD | 19.40 ± 11.15 | 18.28 ± 9.70 | 19.90 ± 11.74 | 0.262 | 17.31 ± 8.26 | 20.58 ± 12.36 | 0.015 | ||
SD, standard deviation.
In comparison, the mean age was higher in the CIP-R group than in the CIP-S group (68.67 ± 15.61 vs. 63.32 ± 17.14, P = 0.015). Charlson’s comorbidity index was similar between the CIP-S and CIP-R groups, while the proportion of patients who had a history of admission during 1 year prior to inclusion was higher in the CIP-R group than in the CIP-S group (44.8% vs. 20.1%, P <0.001). In addition, the proportion of patients with a history of antibiotic usage during 1 year prior to inclusion was higher in the CIP-R group than in the CIP-S group (47.1% vs. 26.6%, P = 0.002).
As for the treatment, the probability of discordance between the initial antimicrobial regimen and the antimicrobial susceptibility of causative organisms was higher in the ESBL+ group than in the ESBL- group (53.3% vs. 0.6%, P <0.001) and in the CIP-R group than in the CIP-S group (29.9% vs. 9.9%, P <0.001).
2. Comparison of outcomes of community-acquired APN
Table 3 shows outcomes of community-acquired APN. A patient in the ESBL+ group incurred higher medical costs compared to a patient in the ESBL- group (USD 3,730.2 vs. 3,119.3, P = 0.001). In detail, hospitalization expenditure (USD 1,331.2 vs. 1,099.0, P = 0.018), meal (USD 137.3 vs. 107.2, P = 0.008), cost of medication (USD 505.6 vs. 334.6, P <0.001), and procedure or operation charge (USD 376.5 vs. 271.2, P = 0.018) were higher in the ESBL+ group than in the ESBL- group. Similarly, total medical costs were higher in the CIP-R group compared to that of the CIP-S group (USD 3,730.2 vs. 3,119.3, P = 0.005). In detail, consultation fee (USD 141.7 vs. 113.0, P = 0.005), hospitalization expenditures (USD 1,360.7 vs. 1,067.5, P = 0.002), meals (USD 145.9 vs. 103.2, P = 0.005), cost of medication (495.6 vs. 346.3, P = 0.005), and procedure or operation charges (USD 326.2 vs. 275.0, P = 0.045) were higher in the CIP-R group than in the CIP-S group.
Table 3. Comparison of outcomes of community-acquired acute pyelonephritis.
| Outcomes | Total | Extended-spectrum beta-lactamase | Ciprofloxacin | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | P-value | Resistant | Sensitive | P-value | |||
| Medical costs, USD, median (IQR) | 3,350.3 (2,227.3 - 5,099.5) | 3,730.2 (2,928.9 - 5,692.4) | 3,119.3 (2,099.3 - 4,829.9) | 0.001 | 3,730.2 (2,524.4 - 5,937.7) | 3,119.3 (2,148.3 - 4,578.5) | 0.005 | |
| Consultation fee | 126.9 (100.2 - 179.8) | 139.9 (100.2 - 192.5) | 122.6 (98.7 - 179.7) | 0.212 | 141.7 (110.8 - 192.2) | 113.0 (98.4 - 1,715.9) | 0.005 | |
| Hospitalization expenditures | 1,194.3 (812.5 - 1,806.7) | 1,331.2 (959.6 - 2,054.7) | 1,099.0 (784.2 - 1,752.9) | 0.018 | 1,360.7 (1,003.5 - 2,406.9) | 1,067.5 (784.2 - 1,627.0) | 0.002 | |
| Meal | 117.4 (76.9 - 176.1) | 137.3 (89.9 - 208.9) | 107.2 (71.4 - 155.0) | 0.008 | 145.9 (87.9 - 202.1) | 103.2 (72.7 - 151.0) | 0.005 | |
| Cost for medication | 420.0 (238.2 - 656.4) | 505.6 (349.5 - 710.5) | 334.6 (222.1 - 622.8) | <0.001 | 495.6 (299.5 - 718.5) | 346.3 (230.3 - 610.2) | 0.005 | |
| Procedure or operation charge | 288.1 (159.3 - 707.3) | 376.5 (204.0 - 779.8) | 271.2 (143.5 - 667.1) | 0.018 | 326.2 (212.8 - 810.2) | 275.0 (143.5 - 667.1) | 0.045 | |
| Laboratory examination charge | 777.9 (505.1 - 1,088.7) | 827.8 (524.5 - 1,245.7) | 765.2 (487.6 - 1,057.3) | 0.489 | 802.7 (559.3 - 1,429.0) | 763.6 (459.8 - 1,043.4) | 0.350 | |
| Radiologic examination charge | 281.8 (150.5 - 516.1) | 290.9 (150.5 - 559.5) | 278.6 (150.5 - 484.0) | 0.489 | 288.7 (157.3 - 534.8) | 271.0 (150.5 - 510.6) | 0.229 | |
| Others | 18.2 (0 - 36.4) | 18.2 (0 - 36.4) | 18.2 (0 - 36.4) | 0.567 | 18.2 (0 - 28.6) | 18.2 (0 - 36.4) | 0.333 | |
| Length of hospital stay, median (IQR) | 9 (7 - 13) | 11 (8 - 14) | 8 (6 - 12) | <0.001 | 11 (7 - 14) | 8 (6 - 11) | <0.001 | |
| Clinical failure (%) | 14 (5.8) | 6 (8.0) | 8 (4.8) | 0.374 | 7 (8.1) | 7 (4.6) | 0.302 | |
| Change in Braden scale, mean ± SD | -1.01 ± 2.56 | -1.23 ± 2.84 | -0.91 ± 2.42 | 0.410 | -1.38 ± 3.10 | -0.81 ± 2.18 | 0.138 | |
USD, United States Dollar; IQR, interquartile range; SD, standard deviation.
The total length of hospital stay was longer in the ESBL+ group than in the ESBL- group (11 vs. 8 days, P <0.001), as well as in the CIP-R group than in the CIP-S group (11 vs. 8 days, P <0.001). No significant differences were observed in the proportion of clinical failure and change in Braden scale between the ESBL+ and ESBL- groups and the CIP-R and CIP-S groups, respectively.
3. Risk factors for higher medical costs and longer length of stay using the log-linear regression model
The results in Tables 4 and 5 display the recovered average marginal effect of each statistically significant variable on total medical costs and length of stay, respectively. Note that the coefficients are the estimates obtained from the log-linear regression model (i.e., the outcome variable is ln [total cost] and ln [length of stay]). Therefore, the coefficient estimates should not be interpreted as a marginal effect on the total costs or length of stay but as semi-elasticity. In the last column, we report the recovered average marginal effect of a unit increase in a control variable on the level outcome (i.e., total costs and length of stay), adjusting for all other factors.
Table 4. Risk factors for higher medical costs using a log-linear regression model.
| Parameters | Coefficient estimate | Standard error | P-value | Average marginal effect |
|---|---|---|---|---|
| ESBL-producing Enterobacteriales as a causative pathogen | 0.273 | 0.122 | 0.026 | 1,210.52 |
| Ciprofloxacin-resistance Enterobacteriales as a causative pathogen | -0.024 | 0.082 | 0.771 | -106.51 |
| Age | 0.005 | 0.003 | 0.040 | 23.18 |
| Female sex | 0.086 | 0.123 | 0.486 | 381.29 |
| Charlson’s comorbidity index | -0.011 | 0.018 | 0.517 | -50.62 |
| Bedridden status | -0.284 | 0.130 | 0.030 | -1,262.58 |
| Any structural problem in urinary tract | 0.277 | 0.113 | 0.015 | 1,230.72 |
| History of admission during 1 year prior to inclusion | -0.014 | 0.115 | 0.903 | -62.83 |
| History of antibiotic usage during 1 year prior to inclusion | -0.222 | 0.092 | 0.017 | -987.19 |
| History of urinary tract infection during 1 year prior to inclusion | 0.004 | 0.096 | 0.965 | 18.71 |
| Use of chemotherapeutic agents | 0.185 | 0.148 | 0.213 | 819.60 |
| Use of immunosuppressants | 0.015 | 0.101 | 0.885 | 64.46 |
| History of urinary catheterization during 1 month prior to inclusion | 0.366 | 0.244 | 0.134 | 1,625.40 |
| History of urinary tract operation during 3 months prior to inclusion | -0.384 | 0.190 | 0.044 | -1,705.39 |
| Pitt score | 0.173 | 0.037 | <0.001 | 766.71 |
| Discordance between antibiotic susceptibility of the causative pathogen and initial antibiotic regimen | -0.044 | 0.123 | 0.721 | -194.40 |
| Initial Braden scale | -0.054 | 0.013 | <0.001 | -240.16 |
| Stayed in a premium room at least for a day during hospitalization | 0.040 | 0.104 | 0.703 | 176.44 |
| Constant | 15.678 | 0.371 | <0.001 | ‒ |
“Coefficient estimate” column displays the estimate from log-linear regression (semi-elasticity) and “average marginal effect” column reports the average marginal effect of a unit increase in the control variable on medical costs. The coefficient of the constant term in linear regression is estimated to capture the intercept; thus, its average marginal effect is omitted.
ESBL, extended-spectrum-beta-lactamase.
Table 5. Risk factors for longer length of stay using a log-linear regression model.
| Parameters | Coefficient estimate | Standard error | P-value | Average marginal effect |
|---|---|---|---|---|
| ESBL-producing Enterobacteriales as a causative pathogen | 0.217 | 0.108 | 0.045 | 2.30 |
| Ciprofloxacin-resistance Enterobacteriales as a causative pathogen | 0.023 | 0.086 | 0.789 | 0.24 |
| Age | 0.005 | 0.002 | 0.045 | 0.05 |
| Female sex | 0.142 | 0.104 | 0.171 | 1.51 |
| Charlson’s comorbidity index | -0.009 | 0.016 | 0.591 | -0.09 |
| Bedridden status | -0.077 | 0.151 | 0.613 | -0.82 |
| Any structural problem in urinary tract | 0.224 | 0.104 | 0.032 | 2.38 |
| History of admission during 1 year prior to inclusion | 0.046 | 0.120 | 0.703 | 0.49 |
| History of antibiotic usage during 1 year prior to inclusion | -0.113 | 0.094 | 0.230 | -1.20 |
| History of urinary tract infection during 1 year prior to inclusion | -0.006 | 0.090 | 0.951 | -0.06 |
| Use of chemotherapeutic agents | 0.156 | 0.134 | 0.245 | 1.66 |
| Use of immunosuppressants | 0.047 | 0.084 | 0.574 | 0.50 |
| History of urinary catheterization during 1 month prior to inclusion | 0.378 | 0.231 | 0.102 | 4.02 |
| History of urinary tract operation during 3 months prior to inclusion | -0.110 | 0.154 | 0.475 | -1.17 |
| Pitt score | 0.076 | 0.036 | 0.038 | 0.80 |
| Discordance between antibiotic susceptibility of the causative pathogen and initial antibiotic regimen | 0.038 | 0.105 | 0.714 | 0.41 |
| Initial Braden scale | -0.009 | 0.017 | 0.598 | -0.10 |
| Stayed in a premium room at least for a day during hospitalization | -0.029 | 0.098 | 0.765 | -0.31 |
| Constant | 1.829 | 0.489 | <0.001 | ‒ |
“Coefficient estimate” column displays the estimate from log-linear regression (semi-elasticity) and “average marginal effect” column reports the average marginal effect of a unit increase in the control variable on length of stay. The coefficient of the constant term in linear regression is estimated to capture the intercept; thus, its average marginal effect is omitted.
ESBL, extended-spectrum-beta-lactamase.
Holding all other factors constant, the treatment cost of APN for a case in which ESBL-producing Enterobacteriales is a causative pathogen would be, on average, 27.0%, or USD 1,211 (P = 0.026) more expensive than non-ESBL-producing pathogen. A patient who is a year older would incur USD 23.18 (P = 0.040) more, having any structural problems in the urinary tract would incur USD 1,231.72 (P = 0.015) more, and a unit increase in the Pitt bacteremia score would incur USD 766.71 (P <0.001) higher costs, all other things constant. However, the total costs were lower if one had had a history of antibiotic usage during 1 year prior to inclusion (USD 987.19 lower; P = 0.017) or had a history of urinary tract operation during the 3 months prior to inclusion (USD 1,705.39 lower, P = 0.04). A point higher on the Braden scale at admission was associated with lowering of costs by USD 240.16 (P <0.001).
Adjusting for other factors, having a case in which ESBL-producing Enterobacteriales is a causative pathogen would explain staying 22.0% longer or 2 more days (P = 0.050) in the hospital than non-ESBL-producing Enterobacteriales. A patient who is 10 years older would, on average, would have to stay for half a day longer (P = 0.045). Any structural problems in the urinary tract explain a longer stay (2.4 days longer; P = 0.032), and moving from 0 to 5 on the Pitt bacteremia score would explain four more days (P = 0.038) in the hospital.
4. Risk factors for higher medical costs and longer length of stay using the quantile regression model
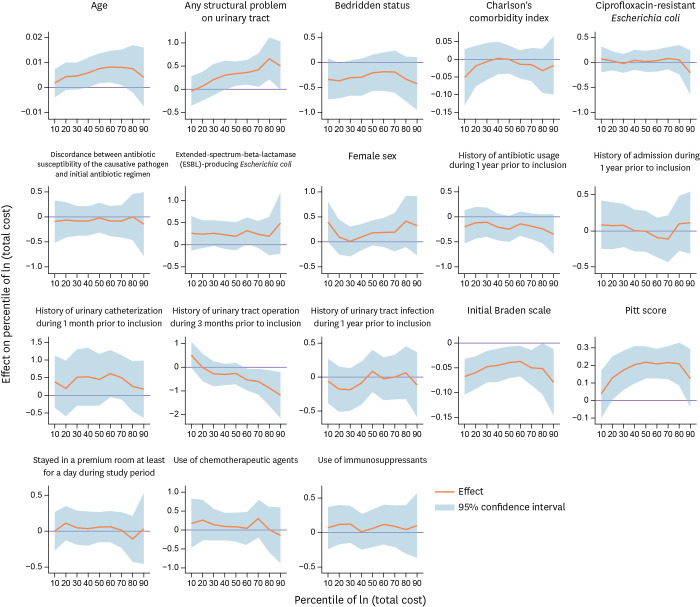
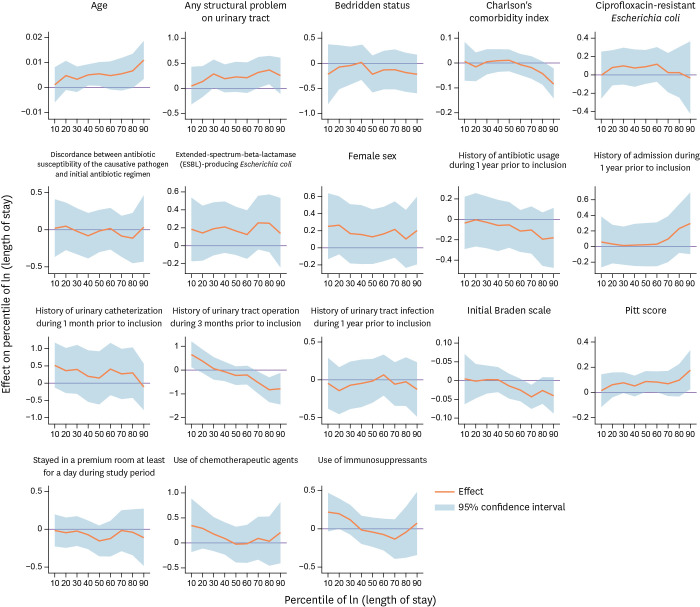
Figures 1 and 2 present the point estimates and 95% confidence intervals (bands) from quantile regression. The corresponding point estimates and standard errors can be found in Supplementary Tables 3 and 4, respectively, in which we report the conditional quantile at 0.1 through 0.9 (0.1 quantile and 10th percentile are synonymous). Note that the outcome is in log-scale; thus, the coefficient estimate can be interpreted as semi-elasticities. We do not recover the average marginal effect because the semi-elasticity serves the purpose of presenting the impact of regressors on the conditional distribution of the outcomes. For example, if the coefficient estimate is 0.02, then a one-unit change in that variable is associated with a proportionate increase of 0.02 or 2.0%.
Figure 1. The quantile regression results for total cost (in log-scale).
Figure 2. The quantile regression results for length of stay (in log-scale).
Figure 1 presents the quantile regression results for the total costs (in log scale). This shows that age has a positive relationship with incurring higher total costs at the 50th or 60th percentile but not at the tails. That is, at the median or at the 60th percentile, being 1 year older is associated with 0.8% (P = 0.030) higher treatment expenditures. In the higher quantiles, the point estimates suggest that having any structural problems in the urinary tract is associated with 31.0 – 50.0% higher medical costs. Although having ESBL-producing Enterobacteriales as a causative pathogen is, on average, associated with 27.0% higher medical costs (Table 4), the quantile regression results suggest that the main impact was made in the 60th percentile, which incurred 27% higher costs. Similarly, this suggests that history of urinary tract operation during the 3 months prior to inclusion is associated with significantly lower (50.0 - 116.0%) medical costs in the higher percentiles. The initial Braden scale at the time of admission or the Pitt bacteremia score seemed to affect the outcome distribution mostly throughout the quantiles.
Figure 2 presents the quantile regression results for the length of stay (in log scale). While having any structural problem in the urinary tract was associated with a 22.0% longer stay in the hospital on average (Table 5), and the impact was significant in the upper quantiles (32.0 - 36.0% longer in the 70th and 80th percentiles). History of having a urinary tract operation during the 3 months prior to inclusion was associated with a shorter length of stay in the upper quantiles (54.0 – 78.0% shorter), but with a longer length of stay in the lowest quantile (65.0% longer). A one-unit increase in the Braden scale at the time of admission explains a 4.3% shorter stay at the 70th percentile.
Discussion
To the best of our knowledge, this is the first study in Korea to quantitatively analyze the impact of antimicrobial-resistant Enterobacteriales on the economic aspects of patients with APN. The quantitative evaluation of the risk factors, including AMR, on the medical costs and hospitalization duration for the treatment of APN is the strength of the present study compared to the previous studies dealing with the medical costs of APN.
As shown in our results, the ESBL+ and CIP-R groups required higher medical costs for the treatment of APN than their control groups. Given that the length of stay of the ESBL+ and CIP-R groups was longer than that of their control groups, it seems that higher medical costs might be closely associated with a longer length of stay. In fact, looking at the detailed categories of medical costs, the costs that increase according to hospitalization duration, such as hospitalization expenditure and meals, were significantly higher in the ESBL+ and CIP-R groups than in the control groups. Although not as important as costs associated with hospitalization duration, another category that contributes to increasing medical costs in the ESBL+ and CIP-R groups was the cost of medication. It is possible that the difference in antibiotic costs might be the main reason for the difference in the cost of medication between patients with APN with antimicrobial-resistant pathogens and those without. In Korea, for adults with normal kidney function, the daily cost of ertapenem, which is usually prescribed for APN caused by ESBL-producing Enterobacteriales in the study hospital, was USD 33.87, while the daily costs of cefotaxime and ciprofloxacin, which are usually prescribed for APN caused by non-ESBL-producing Enterobacteriales in the study hospital, were USD 18.50 and USD 32.16, respectively [17].
According to the log-linear regression model, if the causative pathogen is ESBL-producing Enterobacteriales, the estimated additional medical costs and hospitalization period for the treatment of APN were USD 1,211 and 2.3 days, respectively. Compared to the studies performed in the US, the estimated additional hospitalization period was similar, while the estimated additional medical costs were lower. A retrospective study in the US showed that antimicrobial-resistant organisms were associated with higher medical costs (USD 19,000) and longer length of hospital stay (2.74 days) for the treatment of UTI [18]. According to another retrospective study in the US, the additional medical costs and length of hospital stay for patients with UTI due to ESBL-producing Enterobacteriales were USD 3,658 and 2 days, respectively [19]. In comparison, the difference in additional medical costs between Korea and the UK seems not as significant as that between Korea and the US. According to a retrospective study in the UK, UTI caused by antimicrobial-resistant organisms was more likely to have increased hospital costs by USD 1,259 and length of stay by 1.12 days [20]. A reasonable explanation for such differences may be the difference in each country’s healthcare system. The healthcare system in Korea has a high coverage rate of the National Health Insurance System and relatively lower medical costs compared to that of other countries [21]. Because almost the entire population, including low-income families, receives a benefit from the healthcare system in Korea, the hospital entry barriers are low, so hospitalization is not difficult even for severe diseases. Further studies are necessary to determine the exact components that can explain the differences in additional medical costs among countries.
Other factors that increased the total medical costs and the length of hospital stay in patients with APN were older age, having any structural problems in the urinary tract, and an increase in the Pitt bacteremia score. The findings of the present study are concordant with those of the previous studies. A previous descriptive study using reimbursement data in Korea revealed that an increase in age was associated with prolonged hospitalization duration and higher medical costs in APN treatment [7]. Similarly, some retrospective studies showed that age ≥65 years was a risk factor for prolonged hospitalization in patients with APN [22,23]. A previous study in Korea found that structural problems in the urinary tract increased hospitalization stay by 2.7 - 3 days [24,25]. Furthermore, a prospective cohort study showed that a high Pitt score was associated with worse 30-day all-cause mortality in APN, suggesting that a high Pitt score might also be associated with prolonged hospitalization duration and higher medical costs [26].
Another finding in our study was that a history of antibiotic use during the 1 year prior to inclusion, a history of urinary tract operation during the 3 months prior to inclusion, and a higher Braden scale at admission decreased the total medical costs. Given that the Braden scale could reflect the performance status of the patients, the reverse association between the Braden scale and medical costs seems reasonable [15]. In contrast, a history of antibiotic usage or a history of urinary tract surgery has been considered as a factor associated with antimicrobial-resistant pathogens or worse outcomes [8,27]. A possible explanation for the findings in the present study is that patients who get sick regularly might go to the hospital earlier when they have symptoms than those who are not frequently sick, preventing the symptoms from getting worse [28]. In addition, if previous medical records, such as the result of bacterial culture exist, it might be helpful for the selection of appropriate initial antibiotic therapy and could lead to better clinical outcomes. Further research is required to clarify this.
There are several potential limitations of our study. First, only hospitalized patients from a large hospital were recruited. Given that the study hospital is a tertiary-care hospital, patients in study hospitals may have a greater number of underlying comorbidities and may present with more severe clinical symptoms than those in smaller hospitals. Therefore, the results cannot be generalized to the entire population. Second, the proportion of patients who underwent outpatient parenteral antibiotic therapy (OPAT) and its cost was not taken into account. If the OPAT ratio is low in a situation where a large portion of medical costs are hospitalization and meals, medical costs and length of stay are expected to increase. In fact, OPAT is not actively performed in most Korean hospitals including the study hospital, thanks to relatively low admission cost and national healthcare insurance system that covers ≥98.0% of population [21]. Third, the evaluation of antimicrobial resistance was confined only to ESBL production and ciprofloxacin resistance. In fact, we did not evaluate other important AMRs, such as aminoglycosides and trimethoprim/sulfamethoxazole. In addition, the effect of multidrug resistance was not analyzed. Fourth, using Korean data alone is certainly insufficient to shed light on the global AMR problem that is related to an increase in medical costs and length of hospital stay. Due to the differences in the healthcare system and medical costs in each country, our results cannot be generalized to other countries. Despite this limitation, our experience may be informative for other countries.
In conclusion, the community-acquired APN for a case in which ESBL-producing Enterobacteriales is a causative pathogen would explain, on average, 27.0% higher medical costs and 22.0% longer hospitalization days than in patients with APN caused by non-ESBL-producing Enterobacteriales.
Footnotes
Funding: This study was supported by a grant from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Korean government (MSIT) (Funding No. 2019M3E5D1A01066063). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest: No conflict of interest.
- Conceptulization: BK.
- Data curation: TC, BK.
- Formal analysis: TC, BK.
- Funding acquisition: BK.
- Investigation: TC, JA, YSK, HP, BK.
- Methodology: TC, JA, YSK, HP, BK.
- Project administration: BK.
- Resources: JA, BK.
- Software: TC, JA.
- Supervision: BK.
- Validation: TC, JA, YSK, HP, BK.
- Visualization: TC.
- Writing – original draft: TC, JA, BK.
- Writing – review & editing: BK.
SUPPLEMENTARY MATERIALS
Joint report of the result of univariate analysis
Stepwise selection results
Effects of variables on medical costs using a quantile regression model
Effects of variables on length of stay using a quantile regression model
References
- 1.Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012;27:128–142. doi: 10.3904/kjim.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J, Jr Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn BM. Alarming antimicrobial resistance trends emerge globally. JAMA. 2020;324:223. doi: 10.1001/jama.2020.11330. [DOI] [PubMed] [Google Scholar]
- 4.Carlet J, Jarlier V, Acar J, Debaere O, Dehaumont P, Grandbastien B, Le Coz P, Lina G, Pean Y, Rambaud C, Roblot F, Salomon J, Schlemmer B, Tattevin P, Vallet B. Trends in antibiotic consumption and resistance in France over 20 years: Large and continuous efforts but contrasting results. Open Forum Infect Dis. 2020;7:ofaa452. doi: 10.1093/ofid/ofaa452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013-2017. BMC Infect Dis. 2019;19:742. doi: 10.1186/s12879-019-4387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D, Ahn JY, Lee CH, Jang SJ, Lee H, Yong D, Jeong SH, Lee K. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in Gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: Analysis of Korean atimicrobial resistance monitoring system (KARMS) data from 2013 to 2015. Ann Lab Med. 2017;37:231–239. doi: 10.3343/alm.2017.37.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim B, Myung R, Kim J, Lee MJ, Pai H. Descriptive epidemiology of acute pyelonephritis in Korea, 2010-2014: Population-based study. J Korean Med Sci. 2018;33:e310. doi: 10.3346/jkms.2018.33.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim B, Kim J, Seo MR, Wie SH, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Ki M, Pai H. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013;41:603–612. doi: 10.1007/s15010-013-0441-z. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Suppl 2):S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 10.Kwon KT, Kim B, Ryu SY, Wie SH, Kim J, Jo HU, Park SY, Hong KW, Kim HI, Kim HA, Kim MH, Bae MH, Sohn YH, Kim J, Lee Y, Pai H. Changes in clinical characteristics of community-acquired acute pyelonephritis and antimicrobial resistance of uropathogenic Escherichia coli in South Korea in the past decade. Antibiotics (Basel) 2020;9:617. doi: 10.3390/antibiotics9090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim B, Myung R, Lee MJ, Kim J, Pai H. Trend of antibiotics usage for acute pyelonephritis in Korea based on national health insurance data 2010-2014. BMC Infect Dis. 2019;19:554. doi: 10.1186/s12879-019-4191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI) CLSI document M100-S15. 30th ed. Wayne, PA: CLSI; 2020. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Ki HK, Son JS, Lee SS, Kim YS, Jung SI, Kim SW, Chang HH, Ryu SY, Kwon KT, Lee H, Moon C, Shin SY Korean Network for Study of Infectious Diseases (KONSID) Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli . Int J Antimicrob Agents. 2010;36:284–287. doi: 10.1016/j.ijantimicag.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12:396–401. doi: 10.12788/jhm.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 17.Druginfo: Medicines detail search. [Accessed 18 December 2020]. Available at: https://www.druginfo.co.kr/
- 18.Neidell MJ, Cohen B, Furuya Y, Hill J, Jeon CY, Glied S, Larson EL. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis. 2012;55:807–815. doi: 10.1093/cid/cis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacVane SH, Tuttle LO, Nicolau DP. Impact of extended-spectrum β-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med. 2014;9:232–238. doi: 10.1002/jhm.2157. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen HQ, Nguyen NTQ, Hughes CM, O’Neill C. Trends and impact of antimicrobial resistance on older inpatients with urinary tract infections (UTIs): A national retrospective observational study. PLoS One. 2019;14:e0223409. doi: 10.1371/journal.pone.0223409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang UI, Kim HW, Noh YS, Wie SH. A comparison of the clinical characteristics of elderly and non-elderly women with community-onset, non-obstructive acute pyelonephritis. Korean J Intern Med. 2015;30:372–383. doi: 10.3904/kjim.2015.30.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efstathiou SP, Pefanis AV, Tsioulos DI, Zacharos ID, Tsiakou AG, Mitromaras AG, Mastorantonakis SE, Kanavaki SN, Mountokalakis TD. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med. 2003;163:1206–1212. doi: 10.1001/archinte.163.10.1206. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Seo MR, Kim SJ, Kim J, Wie SH, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother. 2017;49:22–30. doi: 10.3947/ic.2017.49.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha YE, Kang CI, Joo EJ, Park SY, Kang SJ, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. Clinical implications of healthcare-associated infection in patients with community-onset acute pyelonephritis. Scand J Infect Dis. 2011;43:587–595. doi: 10.3109/00365548.2011.572907. [DOI] [PubMed] [Google Scholar]
- 26.Horcajada JP, Shaw E, Padilla B, Pintado V, Calbo E, Benito N, Gamallo R, Gozalo M, Rodríguez-Baño J ITUBRAS group; Grupo de Estudio de Infección Hospitalaria (GEIH); Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect. 2013;19:962–968. doi: 10.1111/1469-0691.12089. [DOI] [PubMed] [Google Scholar]
- 27.Wie SH, Ki M, Kim J, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Clinical characteristics predicting early clinical failure after 72 h of antibiotic treatment in women with community-onset acute pyelonephritis: a prospective multicentre study. Clin Microbiol Infect. 2014;20:O721–O729. doi: 10.1111/1469-0691.12500. [DOI] [PubMed] [Google Scholar]
- 28.Ruger JP, Richter CJ, Spitznagel EL, Lewis LM. Analysis of costs, length of stay, and utilization of emergency department services by frequent users: implications for health policy. Acad Emerg Med. 2004;11:1311–1317. doi: 10.1197/j.aem.2004.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Joint report of the result of univariate analysis
Stepwise selection results
Effects of variables on medical costs using a quantile regression model
Effects of variables on length of stay using a quantile regression model