Key Points
Question
How does the efficacy of cefepime/enmetazobactam compare with piperacillin/tazobactam for the treatment of complicated urinary tract infection (UTI) or acute pyelonephritis?
Findings
In this randomized clinical trial that included 1034 patients, the proportion of patients infected with gram-negative pathogens who achieved clinical cure and microbiological eradication at the test-of-cure visit was 79.1% with cefepime/enmetazobactam compared with 58.9% with piperacillin/tazobactam, a difference that met the prespecified noninferiority margin of −10% as well as the prespecified criterion for superiority in favor of cefepime/enmetazobactam.
Meaning
Among patients with complicated UTI or acute pyelonephritis due to gram-negative pathogens, cefepime/enmetazobactam, compared with piperacillin/tazobactam, met criteria for noninferiority as well as superiority with respect to the primary efficacy outcome of clinical cure and microbiological eradication.
Abstract
Importance
Cefepime/enmetazobactam is a novel β-lactam/β-lactamase inhibitor combination and a potential empirical therapy for resistant gram-negative infections.
Objective
To evaluate whether cefepime/enmetazobactam was noninferior to piperacillin/tazobactam for the primary outcome of treatment efficacy in patients with complicated urinary tract infections (UTIs) or acute pyelonephritis.
Design, Setting, and Participants
A phase 3, randomized, double-blind, active-controlled, multicenter, noninferiority clinical trial conducted at 90 sites in Europe, North and Central America, South America, and South Africa. Recruitment occurred between September 24, 2018, and November 2, 2019. Final follow-up occurred November 26, 2019. Participants were adult patients aged 18 years or older with a clinical diagnosis of complicated UTI or acute pyelonephritis caused by gram-negative urinary pathogens.
Interventions
Eligible patients were randomized to receive either cefepime, 2 g/enmetazobactam, 0.5 g (n = 520), or piperacillin, 4 g/tazobactam, 0.5 g (n = 521), by 2-hour infusion every 8 hours for 7 days (up to 14 days in patients with a positive blood culture at baseline).
Main Outcomes and Measures
The primary outcome was the proportion of patients in the primary analysis set (patients who received any amount of study drug with a baseline gram-negative pathogen not resistant to either treatment and ≥105 colony-forming units [CFU]/mL in urine culture or the same pathogen present in concurrent blood and urine cultures) who achieved overall treatment success (defined as clinical cure combined with microbiological eradication [<103 CFU/mL in urine] of infection). Two-sided 95% CIs were computed using the stratified Newcombe method. The prespecified noninferiority margin was −10%. If noninferiority was established, a superiority comparison was also prespecified.
Results
Among 1041 patients randomized (mean age, 54.7 years; 573 women [55.0%]), 1034 (99.3%) received study drug and 995 (95.6%) completed the trial. Among the primary analysis set, the primary outcome occurred in 79.1% (273/345) of patients receiving cefepime/enmetazobactam compared with 58.9% (196/333) receiving piperacillin/tazobactam (between-group difference, 21.2% [95% CI, 14.3% to 27.9%]). Treatment-emergent adverse events occurred in 50.0% (258/516) of patients treated with cefepime/enmetazobactam and 44.0% (228/518) with piperacillin/tazobactam; most were mild to moderate in severity (89.9% vs 88.6%, respectively). A total of 1.7% (9/516) of participants who received cefepime/enmetazobactam and 0.8% (4/518) of those who received piperacillin/tazobactam did not complete the assigned therapy due to adverse events.
Conclusions and Relevance
Among patients with complicated UTI or acute pyelonephritis caused by gram-negative pathogens, cefepime/enmetazobactam, compared with piperacillin/tazobactam, met criteria for noninferiority as well as superiority with respect to the primary outcome of clinical cure and microbiological eradication. Further research is needed to determine the potential role for cefepime/enmetazobactam in the treatment of complicated UTI and pyelonephritis.
Trial Registration
ClinicalTrials.gov Identifier: NCT03687255
This randomized clinical trial compares the efficacy of cefepime/enmetazobactam vs piperacillin/tazobactam in achieving overall treatment success in patients with complicated urinary tract infections (UTIs) or acute pyelonephritis.
Introduction
The combination of the β-lactam piperacillin with the β-lactamase inhibitor tazobactam is used commonly to treat complicated urinary tract infections (UTIs) and other serious infections.1,2 However, an increasing prevalence of extended-spectrum β-lactamases, which cause resistance to most β-lactams except carbapenems, limits the therapeutic benefit of β-lactams.3,4 Prescribing piperacillin/tazobactam for infections that may be caused by extended-spectrum β-lactamase–producing bacteria may not be appropriate. New therapeutic options are needed.5 Combining β-lactams with novel β-lactamase inhibitors can restore antibacterial activity against β-lactam–resistant pathogens.
The fourth-generation cephalosporin cefepime has broad-spectrum activity against gram-negative pathogens and is used for treating UTIs, intra-abdominal infections, and pneumonia.6 In vitro, the novel β-lactamase inhibitor enmetazobactam (formerly AAI101) restored the activity of cefepime against β-lactamase–producing gram-negative pathogens, and was more potent than piperacillin/tazobactam against extended-spectrum β-lactamase producers.7 In prior studies, cefepime and enmetazobactam had similar pharmacokinetic profiles, with urinary excretion and similar half-lives.8,9 For registration of an investigational drug for complicated UTI, demonstration of noninferiority in an active-controlled study of this indication is required.10,11 Therefore, this randomized, phase 3 clinical trial was conducted to establish whether cefepime/enmetazobactam was noninferior to piperacillin/tazobactam with respect to efficacy and to evaluate adverse events in adult patients with complicated UTI or acute pyelonephritis.
Methods
Study Design
ALLIUM (A Phase 3, Randomized, Double-Blind, Multi-Center Study to Evaluate the Efficacy, Safety, and Tolerability of Cefepime-AAI101 Compared to Piperacillin/Tazobactam in the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis, in Adults) was a randomized, double-blind, active-controlled, multicenter, clinical trial designed to evaluate whether cefepime/enmetazobactam was noninferior to piperacillin/tazobactam for treatment efficacy over 7 to 14 days in adult patients with complicated UTI or acute pyelonephritis. The trial was conducted in accordance with the Declaration of Helsinki, applicable country laws and regulations, Good Clinical Practice guidelines, and US Food and Drug Administration and European Medicines Agency regulatory guidelines for complicated UTI trials.10,11 The study protocol (Supplement 1) and informed consent form were approved for each site by the respective competent institutional review board or independent ethics committees at each site. All participants provided written informed consent. Ninety sites in Europe (Belarus, Bulgaria, Croatia, Estonia, Georgia, Hungary, Latvia, Lithuania, Poland, Russia, Serbia, Slovakia, Spain, and Ukraine), North and Central America (Mexico and US), South America (Argentina and Peru), and South Africa enrolled patients. The statistical analysis plan is provided in Supplement 2.
Participants
Eligible participants were 18 years or older with complicated UTI defined as having at least 2 of the following new or worsening symptoms: (1) dysuria, increased urinary frequency, or urinary urgency; (2) fever (oral/tympanic temperature ≥38 °C [≥100.4 °F] or rectal temperature ≥38.3 °C [≥100.9 °F]) observed by a clinician within 24 hours of screening; (3) lower abdominal or pelvic pain; (4) suprapubic tenderness on physical examination; or (5) nausea or vomiting 24 hours or less of screening. Participants also had to have at least 1 of the following: (1) male patients with urinary retention; (2) intermittent bladder catheterization or presence of an indwelling bladder catheter; (3) obstructive uropathy scheduled for medical or surgical relief during intravenous study therapy and before end of therapy; (4) voiding disturbance resulting in at least 100-mL residual urine; or (5) azotemia or acute pyelonephritis (defined also by flank pain with onset ≤7 days prior to randomization or costovertebral angle tenderness on physical examination) caused by a gram-negative uropathogen (≥105 colony-forming units [CFU]/mL in urine) that required hospitalization and treatment with at least 7 days of intravenous antibiotics, and was associated with pyuria (white blood cell count >10/μL in unspun urine or ≥10 cells/high-power field in spun urine sediment, or urinalysis/dipstick analysis positive for leukocyte esterase). Race and ethnicity were collected by self-report using fixed categories, consistent with regulatory requirements.12
Randomization, Stratification, and Masking
Patients were randomized by computer-generated random number using a centralized Interactive Response Technology system. Patients were randomized in a balanced 1:1 ratio (no blocks were used) into 1 of the 2 treatment groups, stratified by type of infection (acute pyelonephritis, complicated UTI with removable source of infection [eg, Foley catheter], or complicated UTI without removable source of infection but with other risk factors for adverse outcomes [eg, anatomical abnormality, neurogenic bladder, azotemia]), prior antibiotic therapy (short-acting antibiotic for up to 24 hours during the previous 72 hours before the qualifying baseline pathogen was obtained vs no prior antibiotic therapy), and region (Baltics, Eastern Europe, Latin America, South Africa, US, and Western Europe). The study drug was prepared by an unblinded pharmacist and administered by staff unaware of participant group assignment.
Procedures
Patients received a 2-hour infusion of either cefepime, 2 g/enmetazobactam, 0.5 g, or piperacillin, 4 g/tazobactam, 0.5 g, every 8 hours for 7 days (up to 14 days in patients with a positive blood culture at baseline).
Patients with moderate kidney impairment at baseline received a dose of cefepime, 1 g/enmetazobactam, 0.25 g, by 2-hour infusion every 8 hours starting at day 1. Piperacillin/tazobactam did not require dose adjustment.1
Consistent with European Medicines Agency guidance, participants were not changed to oral therapy.11 Signs and symptoms were monitored using a daily symptom assessment questionnaire. Clinical outcomes were assessed at day 3 of treatment, end of treatment, day 14 (7 [±2] days after end of treatment, ie, the test of cure visit), day 21 (14 [±2] days after end of treatment, ie, the late follow-up visit), and at time of early termination. Urine samples were obtained at screening, prior to study drug administration (baseline), and at all other assessment visits. Blood samples were collected at screening, prior to study drug administration, and at subsequent visits if clinically indicated or if the previous culture was positive.
Urine samples were collected by clean-catch midstream or from a newly inserted Foley catheter (bag specimens not permitted), bladder needle aspiration, suprapubic catheter, nephrostomy tube, or ureter aspiration. Up to 2-g negative bacterial isolates per urine culture (at ≥105 CFU/mL) were considered as qualifying baseline pathogens and sent to the central laboratory for identification confirmation, susceptibility testing, and genotyping. Urine was considered contaminated if 3 microorganisms or more were present unless 1 grew in a concurrently obtained blood culture.
Enterobacterales baseline pathogens with minimum inhibitory concentration of 1 μg/mL or more for ceftazidime, ceftriaxone, cefepime, meropenem, or cefepime/enmetazobactam were genotyped for β-lactamases including class A extended-spectrum β-lactamase and Klebsiella pneumoniae carbapenemase, class B metallo-β-lactamases, class C AmpC, and class D OXA-type β-lactamases, as described.13
Analysis Populations
The primary analysis set included all patients who received at least 1 dose of study drug and had a gram-negative baseline pathogen in urine at 105 CFU/mL or more or the same pathogen present in both blood and urine cultures that was not resistant to either cefepime/enmetazobactam (minimum inhibitory concentration ≤8 μg/mL) or piperacillin/tazobactam (minimum inhibitory concentration ≤64 μg/mL). Patients without exclusion based on susceptibility results were also analyzed, which included those in the primary analysis set in addition to those with a missing minimum inhibitory concentration determination and those with resistance to either cefepime/enmetazobactam (minimum inhibitory concentration ≥16 μg/mL) or piperacillin/tazobactam (minimum inhibitory concentration ≥128 μg/mL). Other study populations are defined in eTable 1 in Supplement 3.
Primary Outcome
The primary outcome was the proportion of patients in the primary analysis set who achieved a composite outcome of complete resolution of the baseline signs and symptoms present at screening (clinical cure) and reduction of qualifying baseline pathogen to less than 103 CFU/mL in urine (microbiological eradication) at day 14. Clinical and microbiological outcomes are defined in eTable 2 in Supplement 3. Participants missing data or lost to follow-up were categorized as failures in the primary analysis set.
Secondary Outcomes
Secondary outcomes presented here include the composite outcome and separate outcomes of clinical cure and microbiological eradication in the primary analysis set at day 3 of treatment, end of treatment, and at day 21; the clinical cure and microbiological eradication in the primary analysis set at day 14; the composite outcome and separate outcomes of clinical cure and microbiological eradication in patients without exclusion due to susceptibility results at day 3 of treatment, end of treatment, day 14, and day 21; the composite outcome of clinical cure and microbiological eradication in all patients receiving at least 1 dose of study drug at day 14; and the composite outcome of clinical cure and microbiological eradication in the prespecified subgroups of the primary analysis set at day 14 were age (<65, 65-<75, ≥75 years), sex, baseline creatinine clearance (severe impairment <30 mL/min/1.73 m2; moderate, 30-59 mL/min/1.73 m2; mild, 60-89 mL/min/1.73 m2; or normal ≥90 mL/min/1.73 m2 [to convert to mL/s/m2, multiply by 0.0137]), type of infection (see stratification above), prior antibiotic therapy (as above), region (as above), baseline Charlson Comorbidity Index score (<3 or ≥3), bacteremia at baseline, race (Black, White, or other [not identified]), country category (US or non-US), baseline diabetes, and extended-spectrum β-lactamase–producing Enterobacterales baseline pathogen. Other prespecified outcomes not presented in this article are listed in eTable 3 in Supplement 3.
Exploratory Outcome
The microbiological outcome of recurrence, defined as isolation of the same baseline gram-negative pathogen from either urine culture or blood culture after eradication, in the primary analysis set at day 14 was an exploratory outcome.
Post Hoc Outcomes
Post hoc outcomes consisted of the proportion of patients with clinical cure in all patients who received at least 1 dose of study drug and in the subset of patients in the primary analysis set excluding baseline pathogens with an extended-spectrum β-lactamase genotype and/or a piperacillin/tazobactam minimum inhibitory concentration greater than 16 μg/mL.
Adverse Events
Adverse events were collected at each visit.
Sample Size Calculation
Investigators planned to randomize approximately 1040 patients, anticipating that approximately 811 would meet criteria for the primary analysis set, providing 90% power to demonstrate noninferiority of cefepime/enmetazobactam relative to piperacillin/tazobactam using a margin of 10% if an overall success rate of 74% was achieved in both groups. The US Food and Drug Administration considers a 10% noninferiority margin appropriate when evaluating a new antibacterial drug therapy against an accepted antibacterial drug for treatment of complicated UTI with a primary outcome of microbiological success and clinical resolution of baseline signs and symptoms 5 days or more after the end of treatment.10
Statistical Analyses
Interim analyses were not conducted. The stratified Newcombe 2-sided 95% CI for the proportional difference in overall treatment success between the 2 groups was performed for the primary and secondary end points and subgroups.14 The stratified Newcombe method was implemented based on the stratification factors used for randomization (type of infection, prior antibiotic therapy, and region). For the primary end point, noninferiority was concluded if the lower limit of the 2-sided 95% CI was greater than −10. If noninferiority was met, superiority without type I error α correction was defined if the treatment difference was positive and the lower bound of the 2-sided 95% CI was greater than zero.
Secondary end points were tested for superiority with statistical significance indicated if the lower bound of the 2-sided 95% CI was greater than zero. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Missing data and patients lost to follow-up were considered indeterminate, coded as failures in primary and secondary efficacy analyses, and included in the denominator for the overall success rate calculation. The analysis software was SAS version 9.3 (SAS Institute). Post hoc statistical analyses were performed using the χ2 test with statistical significance indicated when a 2-sided test resulted in P ≤ .05.
Results
Patient Disposition
A total of 1041 patients were randomized between September 24, 2018, and November 2, 2019, and 1034 received at least 1 dose of study drug (Figure 1). Characteristics of randomized patients are in Table 1. The median treatment duration was 8.0 days. In the cefepime/enmetazobactam group, 5.2% (27/516) of patients did not complete therapy compared with 4.1% (21/518) in the piperacillin/tazobactam group. The primary analysis set included 678 patients who received at least 1 dose of treatment and had a gram-negative bacterium that was not resistant to either treatment in urine at more than 105 CFU/mL or the same gram-negative pathogen in both urine and blood. Of these, 349 (51.5%) had acute pyelonephritis, 148 (21.8%) had a complicated UTI with a removable infection source, and 181 (26.7%) had a complicated UTI without a removable source of infection (eTable 4 in Supplement 3). Concurrent bacteremia was present in 11.0% (38/345) of patients receiving cefepime/enmetazobactam and 8.4% (28/333) of those receiving piperacillin/tazobactam. Infections with Enterobacterales occurred in 95.6% (648/678) of patients. The most common pathogen at baseline was Escherichia coli (76.4%; 518/678), followed by Klebsiella pneumoniae (9.7%; 66/678), Proteus mirabilis (5.6%; 38/678), Pseudomonas aeruginosa (3.5%; 24/678), and Enterobacter cloacae (1.5%; 10/678).
Figure 1. Patient Disposition and Analysis Populations.
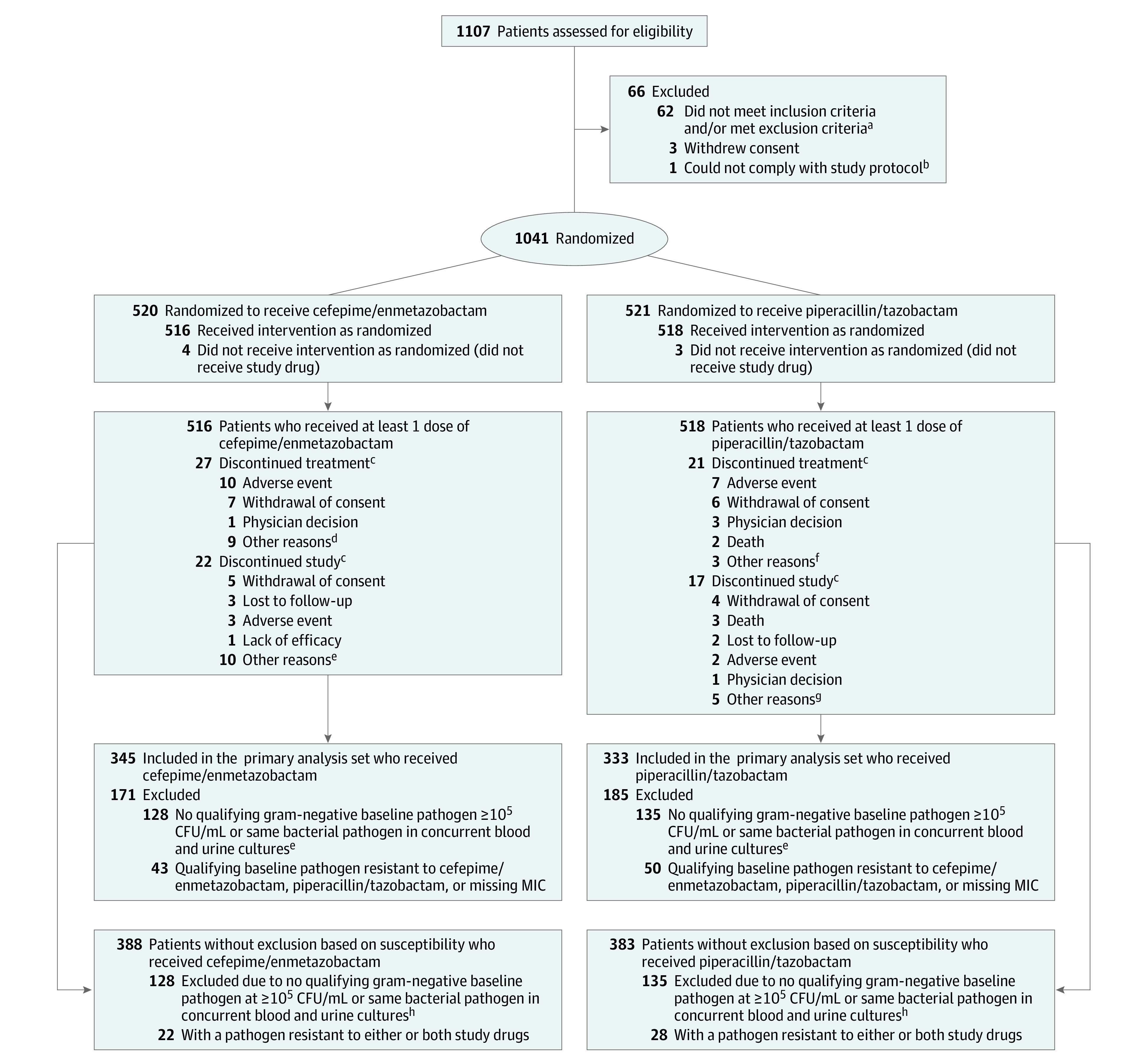
Analysis populations referenced in this study are described in the Methods section. Other study populations are defined in eTable 1 in Supplement 3.
aThe inclusion and exclusion criteria are listed in the eBox in Supplement 3.
bIntravenous drug administration was not feasible in this patient and therefore the patient was not able to comply with the protocol.
cA patient could discontinue treatment but remain in the study, discontinue treatment but discontinue study at a later date, or discontinue treatment and discontinue study at the same time.
dThree patients refused continuation of study treatment; 1 patient did not have clinical improvement; 1 patient was deemed resistant to cefepime; 1 patient had an estimated glomerular filtration rate drop below 30 mL/min/1.73 m2 on day 2; 1 patient did not receive last dose on day 7 by error; in 1 patient treatment could not be continued due to lack of investigational product; and 1 patient discharged themself early.
eNine patients did not return to site for assessment but were contacted and 1 with whom contact was lost.
fTwo patients refused continuation of study treatment and 1 patient had a negative confirmation result of urine culture.
gFour patients did not return to site for assessment but were contacted and 1 patient had a negative confirmation result of urine culture.
hIncludes 10 patients who had a baseline culture that was considered contaminated and 16 patients with a baseline gram-positive pathogen only.
Table 1. Demographic and Baseline Characteristics of Patients Who Received at Least 1 Dose of Study Drug.
| No. (%) | ||
|---|---|---|
| Cefepime/enmetazobactam (n = 516) | Piperacillin/tazobactam (n = 518) | |
| Age, mean (SD), y | 55.0 (19.0) | 54.3 (19.1) |
| Age group, y | ||
| <65 | 312 (60.5) | 324 (62.5) |
| 65 to <75 | 126 (24.4) | 118 (22.8) |
| ≥75 | 78 (15.1) | 76 (14.7) |
| Sex | ||
| Male | 234 (45.3) | 232 (44.8) |
| Female | 282 (54.7) | 286 (55.2) |
| Race and ethnicity | ||
| Asian | 4 (0.8) | 1 (0.2) |
| Black | 1 (0.2) | 0 |
| White | 483 (93.6) | 488 (94.2) |
| Othera | 28 (5.4) | 29 (5.6) |
| Weight, mean (SD) [No.], kg | 76.1 (16.9) [515] | 75.3 (17.7) [517] |
| Height, mean (SD), cm | 168.5 (9.7) | 168.4 (9.1) |
| Body mass index, mean (SD) [No.]b | 26.8 (5.5) [515] | 26.5 (5.4) [517] |
| eGFR at baseline, mean (SD) [No.], mL/min/1.73 m2 | 72.9 (22.1) [490] | 72.38 (24.8) [486] |
| eGFR group at baseline, mL/min/1.73 m2 | ||
| Severe (<30) | 3 (0.6) | 6 (1.2) |
| Moderate (30-59) | 99 (19.2) | 113 (21.8) |
| Mild (60-89) | 280 (54.3) | 260 (50.2) |
| Normal (≥90) | 108 (20.9) | 107 (20.7) |
| Type of infection | ||
| Acute pyelonephritis | 251 (48.6) | 247 (47.7) |
| Complicated UTI with removable source of infectionc | 120 (23.3) | 127 (24.5) |
| Complicated UTI without removable source of infection but with other risk factors | 145 (28.1) | 144 (27.8) |
| Prior antibiotic therapy | ||
| Short-acting antibiotic up to 24 h | 43 (8.3) | 44 (8.5) |
| No prior antibiotic therapy | 473 (91.7) | 474 (91.5) |
| Region | ||
| Eastern Europe | 360 (69.8) | 363 (70.1) |
| Americas | 36 (7.0) | 38 (7.3) |
| Other countriesd | 120 (23.3) | 117 (22.6) |
| Country category | ||
| United States | 1 (0.2) | 0 |
| Non–United States | 515 (99.8) | 518 (100.0) |
| Charlson Comorbidity Index (CCI) score at baselinee | ||
| <3 | 311 (60.3) | 307 (59.3) |
| ≥3 | 202 (39.1) | 202 (39.0) |
| Presence of concurrent bacteremia at baseline | 41 (7.9) | 30 (5.8) |
| Diabetes at baseline | 79 (15.3) | 78 (15.1) |
| Enterobacterales baseline pathogen, extended-spectrum β-lactamase producing | 98 (19.0) | 89 (17.2) |
Abbreviations: eGFR, estimated glomerular filtration rate; UTI, urinary tract infection.
The “other” category indicates race and ethnicity were not identified.
Calculated as weight in kilograms divided by height in meters squared.
A total of 81 of 120 infection sources were removed from the cefepime/enmetazobactam group and 92 of 127 in the piperacillin/tazobactam group.
Countries include those from the Baltics, South Africa, and Western Europe.
The median CCI score at baseline was 2 for both the cefepime/enmetazobactam group (mean [SD], 2.2 [2.0]; range, 0-9) and the piperacillin/tazobactam group (mean [SD], 2.2 [2.2]; range, 0-15). For each decade over 40 years of age, a score of 1 was added to the CCI score. A score of 1 was given for any of the following: myocardial infarction (history, not electrocardiogram changes only), congestive heart failure, peripheral vascular disease (includes aortic aneurysm ≥6 cm), cerebrovascular disease (cerebrovascular accident with mild or no residual transient ischemic attack), dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease (without portal hypertension, includes chronic hepatitis), or diabetes without end-organ damage. A score of 2 was given for any of the following: hemiplegia, moderate or severe kidney disease, diabetes without end-organ damage (retinopathy, neuropathy, nephropathy, or brittle diabetes), tumor without metastases (excluded if >5 years from diagnosis), leukemia (acute or chronic), or lymphoma. A score of 3 was given for moderate or severe liver disease. A score of 6 was given for metastatic solid tumor or AIDS.
Primary Outcome
The primary outcome of a composite of complete resolution of the baseline signs and symptoms present at screening (clinical cure) and reduction of the qualifying baseline pathogen to less than 103 CFU/mL in urine (microbiological eradication) at day 14 occurred in 273 of 345 patients (79.1%) in the cefepime/enmetazobactam group and 196 of 333 patients (58.9%) in the piperacillin/tazobactam group (difference, 21.2% [95% CI, 14.3%-27.9%]; Table 2), indicating that cefepime/enmetazobactam was noninferior to piperacillin/tazobactam. Cefepime/enmetazobactam also met the criterion for superiority compared with piperacillin/tazobactam (Table 2).
Table 2. Primary Outcome, Clinical Cure, and Microbial Eradication in the Primary Analysis Set.
| Response at visit | No. (%) | Treatment difference, % (95% CI)a | |
|---|---|---|---|
| Cefepime/enmetazobactam (n = 345) | Piperacillin/tazobactam (n = 333) | ||
| Day 14b | |||
| Overall successc | 273 (79.1) | 196 (58.9) | 21.2 (14.3 to 27.9) |
| Clinical cure | 319 (92.5) | 296 (88.9) | 3.5 (−1.0 to 8.0) |
| Microbiological eradication | 286 (82.9) | 216 (64.9) | 19.0 (12.3 to 25.4) |
| Day 3 of treatment | |||
| Overall success | 318 (92.2) | 293 (88.0) | 4.1 (−0.6 to 8.9) |
| Clinical cure | 18 (5.2) | 16 (4.8) | 0.5 (−3.1 to 4.0) |
| Improvementd | 317 (91.9) | 302 (90.7) | Not determined |
| Microbiological eradication | 323 (93.6) | 299 (89.8) | 3.8 (−0.6 to 8.3) |
| End of treatment | |||
| Overall success | 318 (92.2) | 311 (93.4) | −1.3 (−5.3 to 2.9) |
| Clinical cure | 323 (93.6) | 315 (94.6) | −1.1 (−4.8 to 2.7) |
| Microbiological eradication | 332 (96.2) | 322 (96.7) | −0.7 (−3.7 to 2.5) |
| Day 21e | |||
| Overall success | 236 (68.4) | 196 (58.9) | 10.7 (3.4 to 17.8) |
| Clinical cure | 299 (86.7) | 279 (83.8) | 2.8 (−2.7 to 8.3) |
| Microbiological eradication | 258 (74.8) | 221 (66.4) | 9.5 (2.6 to 16.3) |
Treatment difference was determined using the stratified Newcombe method.
The primary outcome was determined at day 14 (7 [±2 days] after end of treatment). There were 25 indeterminate responses (4.8%) (due to missing data) in the cefepime/enmetazobactam group and 30 (5.8%) in the piperacillin/tazobactam group (indeterminate responses were included in the denominator for calculating the overall outcome).
Overall success was determined as a composite of clinical cure (complete resolution of the baseline signs and symptoms present at screening) and microbiological eradication (<103 CFU/mL of qualifying baseline pathogen in urine).
Improvement was defined as lessening or incomplete resolution with no worsening of any other baseline clinical signs and symptoms, but continued intravenous therapy was warranted. This outcome category was only used at day 3 of treatment. The treatment difference was not determined for this outcome.
Day 21 occurred 14 (±2 days) after end of treatment.
Secondary Outcomes
Primary Analysis Set
There was no significant difference in the clinical cure rate between the 2 groups (319/345 [92.5%] for the cefepime/enmetazobactam group and 296/333 [88.9%] for the piperacillin/tazobactam group at day 14; treatment difference, 3.5% [95% CI, −1.0% to 8.0%]). The cefepime/enmetazobactam group had a significantly higher rate of microbiological eradication compared with piperacillin/tazobactam (82.9% [286/345] vs 64.9% [216/333]; treatment difference, 19.0% [95% CI, 12.3%-25.4%]) at day 14. The cefepime/enmetazobactam group had significantly better improvement in the composite outcome at day 21 (10.7% [95% CI, 3.4% to 17.8%]) but not at day 3 of treatment (4.1% [95% CI, −0.6% to 8.9%]) or at the end of treatment (−1.3% [95% CI, −5.3% to 2.9%]) (Table 2).
In the 20.9% (142/678) of patients with an extended-spectrum β-lactamase–producing baseline pathogen, 56 of 76 patients (73.7%) in the cefepime/enmetazobactam group and 34 of 66 patients (51.5%) in the piperacillin/tazobactam group achieved the composite outcome of clinical cure and microbiological eradication at day 14 (treatment difference, 30.2% [95% CI, 13.4%-45.1%]) (Figure 2).
Figure 2. Subgroup Analyses in the Primary Analysis Set.
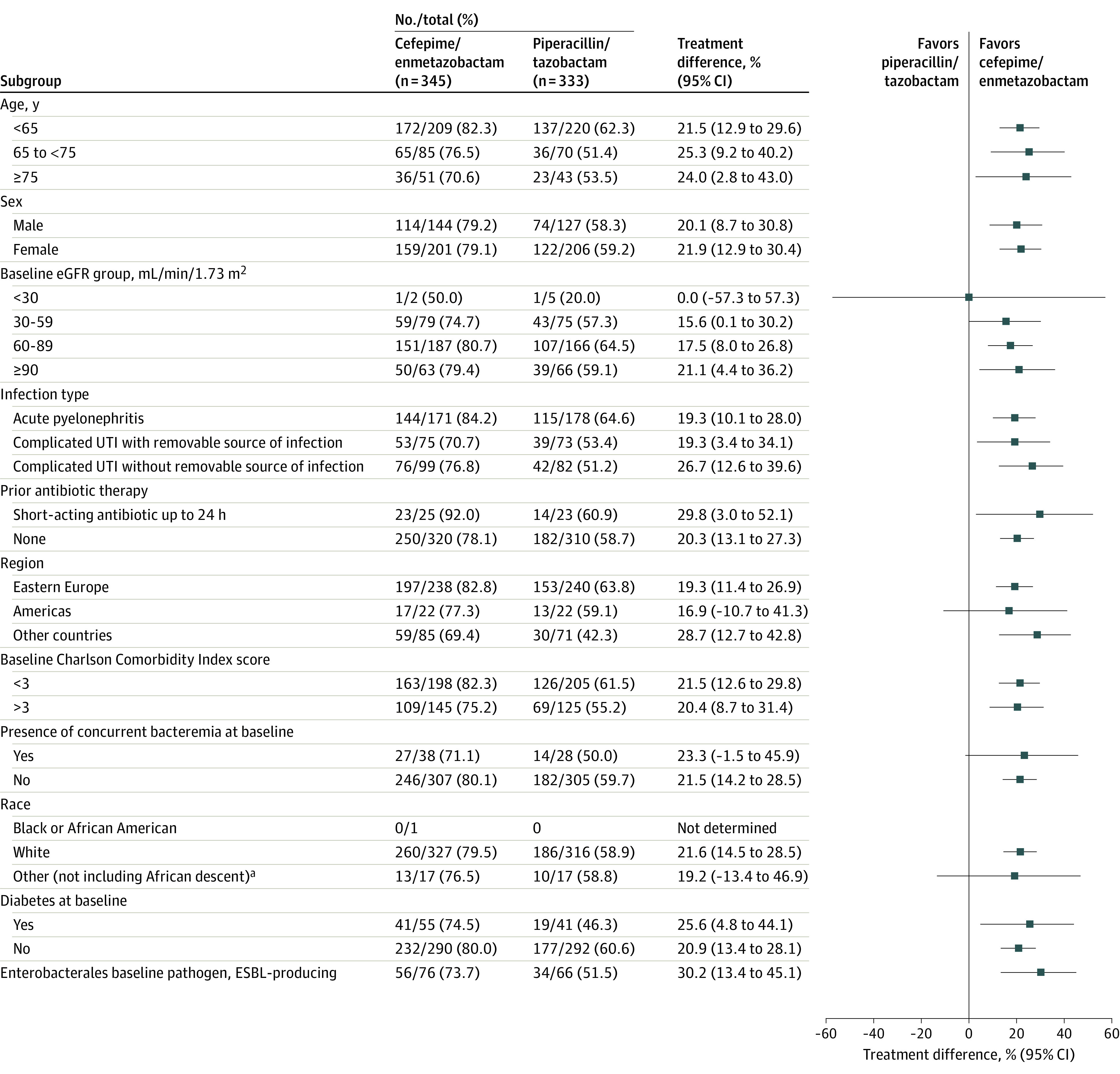
Treatment differences in the proportions of patients between the 2 treatment groups at day 14 were determined by the stratified Newcombe 2-sided 95% CIs. Treatment differences were not evaluated due to too low numbers for the Black race subgroup. eGFR indicates estimated glomerular filtration rate; ESBL, extended-spectrum β-lactamase; and UTI, urinary tract infection.
aThe “other” category indicates race was not identified.
Patients Without Exclusion Based on Susceptibility
In analyses that did not exclude patients either due to resistance to both study drugs or because they were missing a minimum inhibitory concentration determination, a statistically significant difference in the composite outcome of clinical cure and microbiological eradication was observed between the cefepime/enmetazobactam group (78.6%; 305/388) and the piperacillin/tazobactam group (58.7%; 225/383) at day 14 (treatment difference, 20.7% [95% CI, 14.1%-27.0%]; eTable 5 in Supplement 3). A significant difference in the microbiological eradication rate of the cefepime/enmetazobactam group (82.7%; 321/388) vs the piperacillin/tazobactam group (64.5%; 247/383) was also observed at day 14 (treatment difference, 19.0% [95% CI, 12.8%-25.0%]) (eTable 5 in Supplement 3).
Among patients who received at least 1 dose of drug, the composite outcome of clinical cure and microbiological eradication was significantly higher in the cefepime/enmetazobactam group (59.1%; 305/516) compared with the piperacillin/tazobactam group (43.4%; 225/518) at day 14 (treatment difference, 15.6% [95% CI, 9.5%-21.5%]). Of these, 24.8% (128/516) in the cefepime/enmetazobactam group and 26.1% (135/518) in the piperacillin/tazobactam group did not have a qualifying gram-negative baseline pathogen.
Exploratory Outcomes
In the primary analysis set, microbiological recurrence was lower in patients receiving cefepime/enmetazobactam (11.3%; 39/345) compared with piperacillin/tazobactam (29.4%; 98/333) at day 14.
Post Hoc Analyses
In patients who received at least 1 dose of drug, there was no significant difference in clinical cure in the cefepime/enmetazobactam group compared with the piperacillin/tazobactam group at day 14 (91.3% [471/516] vs 87.8% [455/518]; difference, 3.7% [95% CI, −0.06% to 7.5%]).
Among patients in the primary analysis set but excluding those with a piperacillin/tazobactam minimum inhibitory concentration greater than 16 μg/mL and/or an Enterobacterales pathogen with an extended-spectrum β-lactamase genotype, cefepime/enmetazobactam was significantly better than piperacillin/tazobactam (80.9% [212/262] vs 60.7% [156/257]; difference, 20.2% [95% CI, 12.4%-27.7%]).
Adverse Events
Among patients who received at least 1 dose of study drug, 258 of 516 patients (50.0%) in the cefepime/enmetazobactam group and 228 of 518 patients (44.0%) in the piperacillin/tazobactam group experienced treatment-emergent adverse events. Rates of drug-related treatment-emergent adverse events were 19.8% and 14.5%, respectively, and treatment-emergent serious adverse events were 4.3% and 3.7%, respectively (Table 3; eTable 6 in Supplement 3). The most common treatment-emergent adverse events were elevations of liver function parameters: alanine aminotransferase (11.4% vs 11.6%), aspartate aminotransferase (9.1% vs 8.9%), and blood bilirubin (5.8% vs 3.9%).
Table 3. Treatment-Emergent Adverse Events in Patients Who Received at Least 1 Dose of Study Drug.
| System organ class preferred terma | No. (%) | |
|---|---|---|
| Cefepime/enmetazobactam (n = 516) | Piperacillin/tazobactam (n = 518) | |
| Patients with any treatment-emergent adverse eventb | 258 (50.0) | 228 (44.0) |
| Investigations | 140 (27.1) | 135 (26.1) |
| Alanine aminotransferase increased | 59 (11.4) | 60 (11.6) |
| Aspartate aminotransferase increased | 47 (9.1) | 46 (8.9) |
| Blood bilirubin increased | 30 (5.8) | 20 (3.9) |
| Transaminases increased | 13 (2.5) | 19 (3.7) |
| Gastrointestinal disorders | 46 (8.9) | 49 (9.5) |
| Diarrhea | 21 (4.1) | 26 (5.0) |
| Infections and infestations | 44 (8.5) | 57 (11.0) |
| Urinary tract infection | 7 (1.4) | 11 (2.1) |
| Vascular disorders | 30 (5.8) | 12 (2.3) |
| Phlebitis | 14 (2.7) | 1 (0.2) |
| Nervous system disorders | 29 (5.6) | 16 (3.1) |
| Headache | 25 (4.8) | 12 (2.3) |
| Kidney and urinary disorders | 22 (4.3) | 19 (3.7) |
| Blood and lymphatic system disorders | 18 (3.5) | 18 (3.5) |
| Anemia | 13 (2.5) | 15 (2.9) |
| Metabolism and nutrition disorders | 15 (2.9) | 15 (2.9) |
| Hypokalemia | 11 (2.1) | 8 (1.5) |
| General disorders and administration site conditionsc | 13 (2.5) | 8 (1.5) |
| Skin and subcutaneous tissue disorders | 11 (2.1) | 4 (0.8) |
Coding was based on the Medical Dictionary for Regulatory Activities version 21.0.
Defined as adverse events occurring on or after the first dose of study drug. Only treatment-emergent adverse events occurring in more than 2% of patients in either treatment group are presented.
Include asthenia, chest discomfort, chest pain, feeling hot, infusion site extravasation, injection site inflammation, injection site thrombosis, edema, edema peripheral, pyrexia, sudden death, vessel puncture site bruise, vessel puncture site hematoma, vessel puncture site pain, and withdrawal syndrome.
One patient (0.2%) in the cefepime/enmetazobactam group and 3 patients (0.6%) in the piperacillin/tazobactam group experienced drug-related treatment-emergent serious adverse events. Nine patients (1.7%) in the cefepime/enmetazobactam group and 4 (0.8%) in the piperacillin/tazobactam group discontinued drug due to any treatment-emergent adverse events (eTable 7 in Supplement 3). Seven patients (0.7%) discontinued study drug due to drug-related treatment-emergent adverse events (5 patients [1.0%] receiving cefepime/enmetazobactam and 2 [0.4%] receiving piperacillin/tazobactam).
Three patients (0.6%) randomized to cefepime/enmetazobactam and 3 (0.6%) randomized to piperacillin/tazobactam died. No deaths were drug related. All deaths occurred during the follow-up period except for a case of septic shock in the piperacillin/tazobactam group.
Discussion
In adults with complicated UTI or acute pyelonephritis, cefepime/enmetazobactam was noninferior to piperacillin/tazobactam for the primary outcome of clinical and microbiological cure. Additionally, cefepime/enmetazobactam was superior to piperacillin/tazobactam for the primary outcome. These findings suggest that cefepime/enmetazobactam may be an appropriate empirical therapy for suspected gram-negative complicated UTI.
This study used a noninferiority design with a 10% noninferiority margin. The noninferiority comparison to a reproducibly effective and reliable drug is required by both the US Food and Drug Administration and the European Medicines Agency to register new antibiotics for complicated UTI.10,15 Of 6 antibiotics approved for complicated UTI using noninferiority designs since 2014, 2 studies used a noninferiority margin of 10% and 4 studies used a margin of 15%.16
Growing resistance rates have diminished the clinical utility of commonly used drugs for UTIs (eg, aminoglycosides, fluoroquinolones, β-lactams).4 The prevalence of extended-spectrum β-lactamases is increasing, and use of carbapenems to treat these infections has increased carbapenem resistance.3,4,17 Extended-spectrum β-lactamase–producing Enterobacterales are often resistant to antibiotic classes other than the β-lactams.18 New carbapenem-sparing therapies for infections caused by third-generation cephalosporin-resistant Enterobacterales are needed.19 In this trial, the prevalence of extended-spectrum β-lactamase genotypes was 20.9% in the primary analysis set and 24.6% in patients who were not excluded based on susceptibility. The efficacy of cefepime/enmetazobactam against pathogens with extended-spectrum β-lactamase genotypes is consistent with improved potency against extended-spectrum β-lactamases compared with piperacillin/tazobactam.7,13,20 These findings support future clinical studies to compare the efficacy of cefepime/enmetazobactam vs a carbapenem in patients with infections caused by extended-spectrum β-lactamases.
Empirical therapy for complicated UTI and other infections is selected based on the local epidemiology of antimicrobial resistance in conjunction with antimicrobial stewardship programs within individual hospitals. Patients receiving at least 1 dose of piperacillin/tazobactam had a clinical cure rate of 87.8% (455/518) and those in the primary analysis set had a cure rate of 88.9% (296/333) observed at day 14. In the TANGO I Trial of complicated UTI, piperacillin/tazobactam had a clinical cure rate of 86.3% (157/182) in the primary outcome population at day 14.21 In the ZEUS Trial of complicated UTI, the clinical cure rate was 91.6% (163/178) in the primary outcome population 5 days or more after the end of treatment.22 These findings support piperacillin/tazobactam as an empirical therapeutic agent for complicated UTI. However, it is not a preferred treatment for complicated UTI or other serious infections suspected or caused by extended-spectrum β-lactamase–producing Enterobacterales.23 Cefepime/enmetazobactam has the potential to treat patients at risk for extended-spectrum β-lactamase–producing infections. In contrast, other approved β-lactam/β-lactamase inhibitor combinations indicated for the treatment of complicated UTI (eg, ceftazidime/avibactam, meropenem/vaborbactam, and imipenem/cilastatin/relebactam) exhibit activity against carbapenem-resistant pathogens and should be reserved for carbapenem-resistant infections.
The rates of clinical cure among patients receiving at least 1 dose of study drug were not significantly different between the 2 treatment groups at day 14 (ie, 7 [±2] days after end of treatment). For drug registration, to support a conclusion of treatment benefit, regulations require a composite outcome that includes, in addition to clinical response, microbiological eradication (ie, <103 CFU/mL in urine), in part, because continued bacteriuria (>104 CFU/mL) represents a risk for relapse.10 Including microbiological eradication in the composite primary outcome facilitated an exploratory assessment of recurrence with the 2 drugs following treatment, which was lower in the cefepime/enmetazobactam group (11.3%; 39/345) compared with the piperacillin/tazobactam group (29.4%; 98/333).
Limitations
This study has several limitations. First, due to the European Medicines Agency regulatory requirements, oral stepdown antibiotic therapy was not used in this clinical trial. Second, a minimum of 7 days of intravenous antibiotics was required, which may not be generalizable to clinical practice. Third, 93.9% (971/1034) of participants were White and only 1 participant was Black, limiting generalizability of results. Fourth, the primary outcome was a composite end point that included microbiological eradication, which may not be commonly used or assessed in clinical practice.10,11 Fifth, 79.1% (536/678) of patients did not have an extended-spectrum β-lactamase–producing baseline pathogen, often obviating the need for treatment with a β-lactam/β-lactamase inhibitor combination.
Conclusions
Among patients with complicated UTI or acute pyelonephritis caused by gram-negative pathogens, cefepime/enmetazobactam, compared with piperacillin/tazobactam, met criteria for noninferiority as well as superiority with respect to the primary outcome of clinical cure and microbiological eradication. Further research is needed to determine the potential role for cefepime/enmetazobactam in the treatment of complicated UTI and pyelonephritis.
Trial Protocol
Statistical Analysis Plan
eTable 1. Study Populations Analyzed in the Phase 3 Study
eTable 2. Description of Clinical and Microbiological Outcomes Used in the Phase 3 Study
eTable 3. Listing of All Secondary Outcomes Assessed in the Phase 3 Study Not Presented in This Report
eTable 4. Demographic and Baseline Characteristics of Patients in the Primary Analysis Set
eTable 5. Overall Success, Clinical Cure and Microbiological Eradication in Patients Without Exclusion Based on Susceptibility
eTable 6. Overview of Adverse Events in All Patients Receiving at Least One Dose of Study Drug
eTable 7. Listing of Adverse Events Leading to Discontinuation of Study Drug in All Patients Receiving at Least One Dose of Study Drug
eBox. Exclusion and Inclusion Criteria Used Among 1107 Patients Assessed for Study Eligibility
Data Sharing Statement
References
- 1.Pfizer . ZOSYN full prescribing information. Accessed May 17, 2021. https://labeling.pfizer.com/showlabeling.aspx?format=PDF&id=1177
- 2.Magill SS, Edwards JR, Beldavs ZG, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi: 10.1001/jama.2014.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, National Center for Emerging Zoonotic and Infectious Diseases, Division of Healthcare Quality Promotion, Antibiotic Resistance Coordination and Strategy Unit . Antibiotic Resistance Threats in the United States. Centers for Disease Control and Prevention; 2019. doi: 10.15620/cdc:82532 [DOI] [Google Scholar]
- 4.Castanheira M, Deshpande LM, Mendes RE, Canton R, Sader HS, Jones RN. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY Antimicrobial Surveillance Program. Open Forum Infect Dis. 2019;6(suppl 1):S23-S33. doi: 10.1093/ofid/ofy347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayden MK, Won SY. Carbapenem-sparing therapy for extended-spectrum β-lactamase-producing E coli and Klebsiella pneumoniae bloodstream infection: the search continues. JAMA. 2018;320(10):979-981. doi: 10.1001/jama.2018.12565 [DOI] [PubMed] [Google Scholar]
- 6.Endimiani A, Perez F, Bonomo RA. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6(6):805-824. doi: 10.1586/14787210.6.6.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belley A, Morrissey I, Hawser S, Kothari N, Knechtle P. Third-generation cephalosporin resistance in clinical isolates of Enterobacterales collected between 2016-2018 from USA and Europe: genotypic analysis of β-lactamases and comparative in vitro activity of cefepime/enmetazobactam. J Glob Antimicrob Resist. 2021;25:93-101. doi: 10.1016/j.jgar.2021.02.031 [DOI] [PubMed] [Google Scholar]
- 8.Carmeli Y, Knechtle P, Hardenberg J, Knecht M. A randomized phase 2 study of cefepime combined with the novel extended spectrum β-lactamase inhibitor enmetazobactam in hospitalized adults with complicated urinary tract infections (cUTI) including acute pyelonephritis (AP). Open Forum Infect Dis. 2019;6(suppl 2):S539. doi: 10.1093/ofid/ofz360.1341 [DOI] [Google Scholar]
- 9.Motta P, English S, Barth P. Human mass balance study of enmetazobactam using 14C analyzed by AMS: abstract 4429. Paper presented at: 30th European Congress of Clinical Microbiology and Infectious Diseases; April 18-21, 2020; Paris, France. [Google Scholar]
- 10.US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Complicated urinary tract infections: developing drugs for treatment: guidance for industry. June 2018. Accessed September 10, 2018. https://www.fda.gov/media/71313/download
- 11.European Medicines Agency . Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. December 19, 2018. Accessed July 21, 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf
- 12.Food and Drug Administration . Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug administration staff. October 26, 2016. Accessed April 15, 2022. https://www.fda.gov/media/75453/download
- 13.Morrissey I, Magnet S, Hawser S, Shapiro S, Knechtle P. In vitro activity of cefepime-enmetazobactam against gram-negative isolates collected from United States and European hospitals during 2014-2015. Antimicrob Agents Chemother. 2019;63(7):e00514-19. doi: 10.1128/AAC.00514-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873-890. doi: [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency . Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. May 19, 2022. Accessed July 18, 2022. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf
- 16.Portsmouth S, Bass A, Echols R, Tillotson G. Heterogeneity of recent phase 3 complicated urinary tract infection clinical trials. Open Forum Infect Dis. 2021;8(3):ofab045. doi: 10.1093/ofid/ofab045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob Agents Chemother. 2013;57(10):5131-5133. doi: 10.1128/AAC.00607-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castanheira M, Simner PJ, Bradford PA. Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob Resist. 2021;3(3):dlab092. doi: 10.1093/jacamr/dlab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Global Priority List of Antibiotic-Resistant Bacteria To Guide Research: Discovery and Development of New Antibiotics. WHO Press; 2017. [Google Scholar]
- 20.Papp-Wallace KM, Bethel CR, Caillon J, et al. Beyond piperacillin-tazobactam: cefepime and AAI101 as a potent β-lactam-β-lactamase inhibitor combination. Antimicrob Agents Chemother. 2019;63(5):e00105-19. doi: 10.1128/AAC.00105-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319(8):788-799. doi: 10.1001/jama.2018.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye KS, Rice LB, Dane AL, et al. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, a phase 2/3 randomized trial. Clin Infect Dis. 2019;69(12):2045-2056. doi: 10.1093/cid/ciz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. IDSA guidance on the treatment of antimicrobial-resistant gram-negative infections: version 1.0. March 7, 2022. Accessed April 19, 2022. https://www.idsociety.org/practice-guideline/amr-guidance/# [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Study Populations Analyzed in the Phase 3 Study
eTable 2. Description of Clinical and Microbiological Outcomes Used in the Phase 3 Study
eTable 3. Listing of All Secondary Outcomes Assessed in the Phase 3 Study Not Presented in This Report
eTable 4. Demographic and Baseline Characteristics of Patients in the Primary Analysis Set
eTable 5. Overall Success, Clinical Cure and Microbiological Eradication in Patients Without Exclusion Based on Susceptibility
eTable 6. Overview of Adverse Events in All Patients Receiving at Least One Dose of Study Drug
eTable 7. Listing of Adverse Events Leading to Discontinuation of Study Drug in All Patients Receiving at Least One Dose of Study Drug
eBox. Exclusion and Inclusion Criteria Used Among 1107 Patients Assessed for Study Eligibility
Data Sharing Statement


