Abstract
Background
Frail older adults and caregivers need support from their home care teams in making difficult housing decisions, such as whether to remain at home, with or without assistance, or move into residential care. However, home care teams are often understaffed and busy, and shared decision-making training is costly. Nevertheless, overall awareness of shared decision-making is increasing. We hypothesized that distributing a decision aid could be sufficient for providing decision support without the addition of shared decision-making training for home care teams.
Objective
We evaluated the effectiveness of adding web-based training and workshops for care teams in interprofessional shared decision-making to passive dissemination of a decision guide on the proportion of frail older adults or caregivers of cognitively-impaired frail older adults reporting active roles in housing decision-making.
Methods
We conducted a stepped-wedge cluster randomized trial with home care teams in 9 health centers in Quebec, Canada. Participants were frail older adults or caregivers of cognitively impaired frail older adults facing housing decisions and receiving care from the home care team at one of the participating health centers. The intervention consisted of a 1.5-hour web-based tutorial for the home care teams plus a 3.5-hour interactive workshop in interprofessional shared decision-making using a decision guide that was designed to support frail older adults and caregivers in making housing decisions. The control was passive dissemination of the decision guide. The primary outcome was an active role in decision-making among frail older adults and caregivers, measured using the Control Preferences Scale. Secondary outcomes included decisional conflict and perceptions of how much care teams involved frail older adults and caregivers in decision-making. We performed an intention-to-treat analysis.
Results
A total of 311 frail older adults were included in the analysis, including 208 (66.9%) women, with a mean age of 81.2 (SD 7.5) years. Among 339 caregivers of cognitively-impaired frail older adults, 239 (70.5%) were female and their mean age was 66.4 (SD 11.7) years. The intervention increased the proportion of frail older adults reporting an active role in decision-making by 3.3% (95% CI –5.8% to 12.4%, P=.47) and the proportion of caregivers of cognitively-impaired frail older adults by 6.1% (95% CI -11.2% to 23.4%, P=.49). There was no significant impact on the secondary outcomes. However, the mean score for the frail older adults’ perception of how much health professionals involved them in decision-making increased by 5.4 (95% CI −0.6 to 11.4, P=.07) and the proportion of caregivers who reported decisional conflict decreased by 7.5% (95% CI −16.5% to 1.6%, P=.10).
Conclusions
Although it slightly reduced decisional conflict for caregivers, shared decision-making training did not equip home care teams significantly better than provision of a decision aid for involving frail older adults and their caregivers in decision-making.
Trial Registration
ClinicalTrials.gov NCT02592525; https://clinicaltrials.gov/show/NCT02592525
Keywords: shared decision-making, home care, nursing homes, patient engagement
Introduction
Aging is associated with a higher risk of developing disabilities that can lead to loss of autonomy [1,2]. When frail older adults start to lose autonomy, one of the most difficult decisions they face is whether to remain at home, with or without assistance, or move into residential care [3]. When these older adults have cognitive impairment, caregivers may have to make the decision instead, often with little support [4]. Making this difficult decision [5] can lead to stress, decisional conflict, and regret [6].
Shared decision-making (SDM) is a process whereby health professional, patients, and their caregivers work together to make health care choices based on the best evidence and what matters most to patients [7]. SDM tools, such as decision guides, are associated with better decision quality and decision-making processes without damaging patient or health system outcomes [8]. Decision guides can increase the involvement of frail older adults and caregivers in decisions about their care while improving agreement between them and their home care teams [9].
In previous work, an interprofessional SDM (IP-SDM) training program for home care teams with a decision guide increased by 12% (compared to usual care) the proportion of caregivers who reported being active in making housing decisions for frail older adults with cognitive impairment [10]. However, other studies have shown that educational interventions may make little difference to the actual practice of SDM with older adults with cognitive impairment and their surrogate decision makers [11]. In addition, given that home care teams are already very busy, and overall awareness of SDM is increasing [12], passive dissemination of decision guides alone could be enough to increase patient engagement in decision-making [13]. However, the effectiveness of decision guides alone, compared to their use as part of a multifaceted intervention, is unknown.
The aim of this study was to evaluate the effect of adding a blended web-based and in-person training program in IP-SDM for home care teams to passive dissemination of a decision guide on the proportion of frail older adults or caregivers reporting an active role in making housing decisions, compared with passive dissemination of the decision guide alone. We hypothesized that the addition of a training program in IP-SDM to the passive dissemination of a decision guide would increase the proportion of frail older adults or caregivers reporting an active role in the decision-making process.
Methods
Ethics Approval
We reported this trial following the extension of Consolidated Standards of Reporting Trials (CONSORT) for stepped-wedge cluster randomized trials [14]. The trial was registered at ClinicalTrials.gov (NCT02592525) and the protocol was published [15]. Ethics committee review approval has been obtained from the Multicenter Ethics Committee of Centre intégré de santé et de services sociaux de Laval (2015-2016/01-01-E).
Study Design
We conducted a cross-sectional, stepped-wedge cluster randomized trial (the Inter Professional Shared Decision-Making-Stepped Wedge Study) from November 2014 to December 2018 with home care teams at health centers in Quebec, Canada. We chose cluster randomization because the intervention was delivered at the health-center level, precluding individual randomization. A stepped-wedge design was chosen to facilitate recruitment, as all health centers would ultimately receive the intervention [16]. This design also offers more statistical power than a traditional parallel cluster study when there are large cluster-level effects (or intracluster correlations) [17]. Health centers were randomly allocated to 1 of 4 intervention start times (sequences), with 5 data collection periods (Figure 1).
Figure 1.
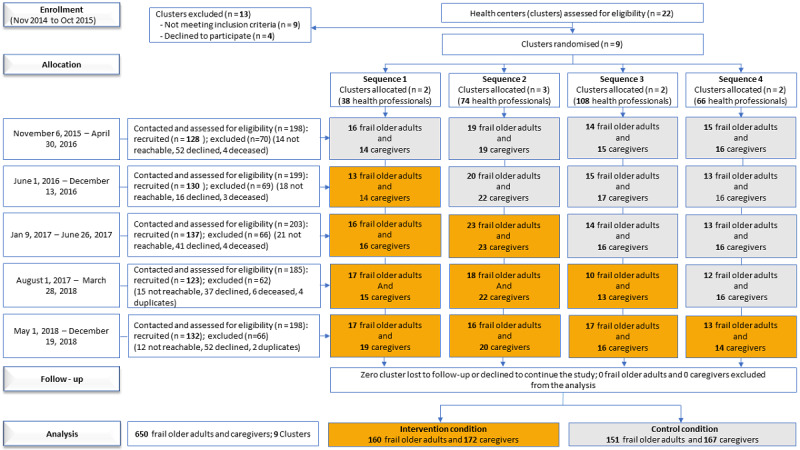
Flow chart of trial by allocated sequence and period_updated.
Participants and Eligibility
Study participants were frail older adults with loss of autonomy and caregivers of frail older adults with cognitive impairment who were recruited through the home care teams at the health centers. Home care teams were eligible if they (1) were involved in caring for frail older adults, (2) practiced in one of the health centers participating in the trial, and (3) were interprofessional (ie, involved more than 2 health professionals from different professions). Frail older adults were eligible if they (1) were aged ≥65 years; (2) were receiving care from one of the home care teams; (3) had made a decision about staying home or moving during the recruitment period; (4) were able to read, understand, and write French or English; and (5) were able to give informed consent. When frail older adults were cognitively impaired, their informal caregiver became the eligible participant. Caregivers were defined in this study as close relatives or friends and were eligible if they (1) were caring for a cognitively impaired older adult who was otherwise eligible; (2) were able to read, understand, and write French or English; and (3) provided informed consent to participate in the study. Frail older adults with cognitive impairment had been clinically evaluated by a health professional as no longer able to make decisions on their own.
Randomization
Health centers (clusters) were randomized to 1 of 4 sequences. Once participating home care teams had been identified, an independent biostatistician at the Ottawa Hospital Research Institute’s Methods Centre performed randomization using computer-generated numbers. Given the nature of the intervention, the investigators, project coordinator, and research assistants (RAs) collecting the data were not blinded. However, the allocation list was concealed from the research team for as long as possible and the RAs were asked not to discuss this information with any frail older adult or caregiver and not to refer to the intervention. Frail older adults and caregivers were blinded to the intervention.
Control
Before baseline data collection, we asked managers at all the enrolled health centers to distribute (ie, perform passive dissemination of) a decision guide for home care teams supporting frail older adults or caregivers in making housing decisions [4]. Dissemination of the decision guide was passive in the sense that although it was distributed in the health centers, we did not train the teams in how to use it. The decision guide, adapted from an online family decision support tool designed for the context of the home, had French and English versions [4,18]. It has the potential to help health professionals discuss with frail older adults or caregivers of cognitively impaired frail older adults the decision about the location of care [4,9,13].
Intervention
The intervention consisted of (1) a 1.5-hour web-based tutorial, based on the Ottawa Decision Support Tutorial, [19] that was completed individually by the health professionals in the participating home care teams at the cluster level, followed by (2) a 3.5-hour live interactive workshop. The web-based tutorial ensured that all participants arrived at the workshop with a similar knowledge of SDM concepts. The workshop included a lecture reviewing SDM concepts (especially the IP-SDM approach); a video demonstrating the approach in a home care team with a frail older adult making a housing decision [20]; training in using the decision guide [4]; and role play using the decision guide with feedback from facilitators [15,20]. The workshop, based on adult education principles [21], included decision-making about housing decisions with frail older adults, communication techniques, and, for frail older adults with cognitive impairment, strategies for fostering their participation or that of their caregivers in decision-making. All workshops were held at the health centers, had the same content, same materials, and same trainers, and were held as a single session [15]. All home care teams received the intervention at various time points. The decision guide distributed before the intervention was still available in sufficient quantities afterwards [15]. The digital format of the initial tutorial and the video were convenient and easily scalable to our 9 intervention sites and ensured that base elements of the training were standardized and identical. This is helpful in stepped-wedge trials, where control and intervention conditions are experienced at different times, there is implementation lag, and individuals are exposed to the intervention in different ways and locations. It also reduced time expenditure and costs, in contrast to in-person training, which had to be repeated at each crossover point [22]. However, our intervention overcame the disadvantages of web-based learning (mainly isolation) [23-25] with the in-person part of the training, which provided role play, feedback, and discussion opportunities for applying knowledge to skills and behavior [26].
Outcomes and Measurement
The primary outcome was the frail older adults’ or caregivers’ perception of the role they assumed in decision-making, as measured using a modified version of the Control Preferences Scale [27], a single question with five response categories: (1) “I made the decision,” (2) “I made the decision after seriously considering the health care professionals’ opinions,” (3) “the health care professionals and I shared the responsibility for the decision making,” (4) “the health care professionals made the decision after seriously considering my opinion,” and (5) “the health care professionals made the decision.” For sample size calculation and analysis, we dichotomized the primary outcome by collapsing categories 1, 2, and 3 into an “active” role and 4 and 5 into a “passive” role in decision-making.
Secondary outcomes assessed in frail older adults and caregivers were (1) their preferred option about whether the cognitively impaired older adult should stay at home or move to another location, and the actual decision made; (2) decisional conflict, assessed with the 16-item Decisional Conflict Scale [28,29]; (3) decision regret, assessed with the 5-item Decision Regret Scale [30]; and (4) perception of the extent to which health professionals involved them in decision-making, assessed with the Dyadic-OPTION scale, a 12-item instrument evaluating SDM behaviors during decision-making [31,32]. Other secondary outcomes included health-related quality of life, assessed only in the frail older adults with the 36 items of the Nottingham Health Profile [33-35], and burden of care, assessed only in the caregivers with the Zarit Burden Inventory scale [36-38].
Data Collection
Home care teams made lists of potentially eligible frail older patients. Trained RAs assigned to each health center contacted these patients or caregivers of frail older adults with cognitive impairment and asked if they wished to participate. The RAs then met all interested participants at their home or a place of their choice to complete informed consent and proceed with data collection. Data collection took place from November 2015 to December 2018. Due to practical constraints, some health centers started the intervention earlier or later than planned. The collected data included outcomes; the relationship between caregivers and frail older adults (when appropriate); and sociodemographic characteristics, including age, sex, and education, which were variables identified as predictors of our primary outcome, that is, that younger, female, well-educated (secondary school level or higher) people are more likely to take an active role in decisions about their health [27,39-41].
Sample Size
The sample size calculation was informed by preliminary data from another study [42]. We used the method developed by Hussey and Hughes [43] for stepped-wedge designs. We assumed an average of 8 frail older adults and 8 caregivers per health center in each data collection period and a time-independent intraclass correlation (ICC) of 0.05 [44]. To detect an absolute increase of 20% [45] in the primary outcome (ie, from 70% to 90%) with 80% power using a stepped-wedge design with 4 sequences and a 2-sided test at the 5% significance level, a total of 8 clusters (with a total of 320 caregivers) were required, [46] meaning 320 frail older adults and 320 caregivers of frail older adults with cognitive impairment. To account for potential loss to follow-up of clusters we recruited one more health center than planned.
Statistical Methods
We describe organizational settings and characteristics of the health professionals randomized to the trial and report the sociodemographics of the frail older adults and caregivers using frequencies and percentages, means and SD, or medians and IQR, as appropriate. We performed analyses with the intention-to-treat principle with the frail older adult or caregiver as the unit of analysis. The primary outcome was analyzed using a generalized linear mixed model (GLMM) with logit link. The prespecified primary analysis assumed a uniform within- and between-period correlation, adjusting for time effects (categorical) and specifying a random effect for cluster [43].
We performed secondary analyses by additionally adjusting for primary outcome predictors and for imbalanced baseline characteristics [47,48]. To explore the implications of bias due to misspecification of the correlation structure [49], we conducted analyses using 2 other correlation structures identified in the literature: nested exchangeable (specifying a random cluster effect and a random time by cluster interaction) [50,51] and exponential decay (an autoregressive between-period correlation) [52]. There are no guidelines for choosing the best-fitting covariance structure, so we used the pseudo–Akaike information criterion to select the best-fitting model and presented the results as sensitivity analyses. To estimate the absolute difference, as required by the CONSORT extension for stepped-wedge cluster randomized trials, [14] we applied GLMM using an identity link with the adaptive Gaussian–Hermite approximation to the likelihood maximum [53].
For binary secondary outcomes, we conducted similar analyses. For continuous secondary outcomes, we used a linear mixed model, and summarized the intervention effects as mean differences. We obtained within-period intraclass correlation coefficients (WpICC), between-period intraclass correlation coefficients (BpICC), and cluster autocorrelation coefficients (CAC) for each outcome analyzed. We used α=.05 as the level of significance. All analyses were conducted using SAS (version 9.4, SAS Institute).
Results
Participants
Recruitment took place from November 2014 to December 2018. Interprofessional home care teams from 9 health centers with 281 health professionals participated in the study. Of 481 frail older adults contacted, 311 (64.6%) were recruited. Of 502 eligible caregivers contacted, 339 (67.5%) were recruited. There was no loss to follow-up of health centers, and no frail older adults, caregivers, or health centers were excluded (Figure 1). Sociodemographics of the frail older adults and caregivers were well balanced between allocated sequences (Multimedia Appendices 1 and 2).
Baseline Characteristics of Participants
Participating frail older adults were on average 81.2 (SD 7.5) years old; 66.9% (208/311) were female and 58.8% (183/311) had secondary education or higher. Baseline characteristics were well balanced between the intervention and control groups, except for education level (Table 1). Caregivers of frail older adults with cognitive impairment were on average 66.4 (SD 11.7) years old; 70.5% (239/339) were female and 87.3% (296/339) had secondary education or higher. Most caregivers (242/339, 71.4%) were retired or at home and 90.3% (306/339) were the child, spouse, or husband of the frail older adult. Among caregivers, baseline characteristics were well balanced between the intervention and control groups, except for age (Table 2).
Table 1.
Baseline characteristics of frail older adults without cognitive impairment (N=311).
| Characteristics | Control (n=151) | Intervention (n=160) | |||
| Age (years), mean (SD) | 81.6 (7.6) | 80.9 (7.4)a | |||
| Sex (female), n (%) | 101 (66.9) | 107 (66.9) | |||
| Education level, n (%) | |||||
|
|
Primary school | 44 (29.2) | 84 (52.5) | ||
|
|
Secondary school | 73 (48.3) | 51 (31.9) | ||
|
|
Postsecondary | 34 (22.5) | 25 (15.6) | ||
| Marital status, n (%) | |||||
|
|
Married/common-law partner | 45 (29.8) | 58 (36.3) | ||
|
|
Widowed | 72 (47.7) | 60 (37.5) | ||
|
|
Separated/divorced | 20 (13.3) | 25 (15.6) | ||
|
|
Single | 14 (9.2) | 17 (10.6) | ||
| Household income (CAD $)b, n (%) | |||||
|
|
Less than 30,000 | 83 (55.0) | 86 (53.8) | ||
|
|
30,000-59,999 | 34 (22.5) | 30 (18.8) | ||
|
|
60,000 and more | 4 (2.7) | 7 (4.4) | ||
|
|
I prefer not to answer/I do not know | 30 (19.9) | 37 (23.1) | ||
an=159
bA currency exchange rate of CAD $1=US $0.76 is applicable.
Table 2.
Baseline characteristics of caregivers of cognitively-impaired frail older adults (N=339).
| Characteristics | Control (n=167) | Intervention (n=172) | |||
| Age (years), mean (SD) | 64.2 (11.9) | 68.6 (11.2) | |||
| Sex (female), n (%) | 122 (73.1) | 117 (68.0) | |||
| Education level, n (%) | |||||
| Primary school | 19 (11.4) | 24 (14.0) | |||
| Secondary school | 63 (37.7) | 69 (40.1) | |||
| Postsecondary | 85 (50.9) | 79 (45.9) | |||
| Marital status, n (%) | |||||
|
|
Married/common-law partner | 129 (77.2) | 132 (76.7) | ||
|
|
Widowed | 7 (4.2) | 9 (5.2) | ||
|
|
Separated/divorced | 16 (9.6) | 18 (10.5) | ||
|
|
Single | 15 (9.0) | 13 (7.6) | ||
| Household income (CAD $)a, n (%) | |||||
|
|
Less than 30,000 | 37 (22.2) | 43 (25.0) | ||
|
|
30,000-59,999 | 54 (32.3) | 50 (29.1) | ||
|
|
60,000 or more | 51 (30.5) | 46 (26.7) | ||
|
|
I prefer not to answer/I do not know | 25 (15.0) | 33 (19.2) | ||
| Caregivers’ employment status, n (%) | |||||
|
|
Retired | 94 (56.3) | 114 (66.3) | ||
|
|
Employed | 56 (33.5) | 39 (22.7) | ||
|
|
At home (eg, unemployed/job seeker) | 17 (10.2) | 17 (9.9) | ||
|
|
Missing | 0 (0.0) | 2 (1.1) | ||
| Caregivers’ relationship to frail older adult, n (%) | |||||
|
|
Child | 94 (56.3) | 75 (43.6) | ||
|
|
Wife/husband or common-law partner | 59 (35.3) | 78 (45.3) | ||
|
|
Other (eg, family member or friend) | 14 (8.4) | 19 (11.1) | ||
aA currency exchange rate of CAD $1=US $0.76 is applicable.
Primary Outcomes
At baseline (period 1), when no health center had yet received the intervention, but they had been exposed to passive dissemination of the decision guide (ie, the control condition), 92% (59/64) of frail older adults and 83% (53/64) of caregivers of frail older adults with cognitive impairment already reported an active role in decision-making (Multimedia Appendices 3 and 4). In all, 92.1% (139/151) of frail older adults recruited under the control condition reported an active role in decision-making versus 94.3% (149/160) of frail older adults recruited under the intervention condition, for an absolute increase of 3.3% (95% CI –5.8% to 12.4%, P=.47) after accounting for the secular trend (Table 3). Similarly, 77.8% (130/167) of caregivers recruited under the control condition reported an active role in decision-making versus 80.8% (139/172) under the intervention condition, for an absolute increase of 6.1% (95% CI –11.8% to 23.4%, P=.49) (Table 4) after accounting for the secular trend. The ICC (WpICC) and the CAC were, respectively, 0.051 and 0.627 in the frail older adults and 0.045 and 0.493 in the caregivers of frail older adults with cognitive impairment (Multimedia Appendices 5 and 6).
Table 3.
Effect of the intervention on primary and secondary outcomes for frail older adults without cognitive impairment.
| Outcomes | Values | Absolute scale effect size | Relative scale effect size | ||||||||||
|
|
Control (n=151) | Intervention (n=160) | Proportion differencea/mean difference (95% CI) | P value | Odds ratio (95% CI)b | P value | |||||||
| Primary outcome, n (%) | |||||||||||||
|
|
Role assumed (active)c | 139 (92.1) | 149 (94.3) | 3.3 (–5.8 to 12.4) | .47 | 1.70 (0.28 to 10.4) | .56 | ||||||
| Secondary outcomes | |||||||||||||
|
|
Preferred housing option (stay at home),d n (%) | 100 (66.7) | 97 (60.6) | –9.4 (–27.0 to 8.2) | .29 | 0.65 (0.24 to 1.75) | .39 | ||||||
|
|
Housing decision made (stay at home),d n (%) | 41 (27.3) | 61 (38.1) | 3.3 (–14.1 to 20.7) | .71 | 1.16 (0.28 to 4.85) | .84 | ||||||
|
|
Decisional conflict (yes; scale ≥37.5), n (%) | 28 (18.5) | 20 (12.5) | –2.2 (–15.3 to 10.8) | .73 | 0.87 (0.20 to 3.74) | .85 | ||||||
|
|
Decisional regret (yes; scale >0), n (%) | 107 (70.9) | 108 (67.5) | –13.9 (–31.3 to 3.6) | .12 | 0.50 (0.12 to 2.11) | .34 | ||||||
|
|
Involvement in decision-making (Dyadic-OPTION),e mean (SD) | 65.8 (19.4) | 67.9 (17.2) | 5.8 (–0.5 to 12.1)f | .07 | N/Ag | N/A | ||||||
|
|
Quality of life (0-100),h mean (SD) | 72.9 (23.8) | 75.1 (22.3) | –2.1 (–10.0 to 5.9)g | .61 | N/A | N/A | ||||||
aGeneralized linear mixed model with logit link function including intervention as a binary variable, a fixed effect (categorical) for time, and specifying a random effect for cluster.
bLinear mixed model with dichotomous dependent variables to handle convergence issues and reported risk differences, which can be interpreted as a difference of proportions (dependent dichotomous variables coded 1/0) [54-56].
cn=149 and n=158 for the control and intervention groups, respectively.
dn=150 and n=159 for the control and intervention groups, respectively.
eAssessed on a continuous scale ranging from 0 to 100.
fLinear mixed model including intervention as binary variable, a fixed effect (categorical) for time, and specifying a random effect for cluster.
gN/A: not applicable.
hAssessed on a continuous scale ranging from 0 to 100.
Table 4.
Effect of the intervention on primary and secondary outcomes for caregivers of cognitively-impaired frail older adults (primary analysis).
| Outcomes | Outcome frequency | Absolute scale effect size | Relative scale effect size | ||||||||||||
|
|
Control (n=167) | Intervention (n=172) | Proportional differencea/mean difference (95% CI) | P value | Odds ratio (95% CI)b | P value | |||||||||
| Primary outcome, n (%) | |||||||||||||||
|
|
Role assumed (active) | 130 (77.8) | 139 (80.8) | 6.1 (–11.2 to 23.4) | .49 | 1.30 (0.66 to 2.55) | .45 | ||||||||
| Secondary outcomes | |||||||||||||||
|
|
Preferred housing option (stay at home), n (%) | 82 (49.1) | 83 (48.3) | –2.7 (–19.4 to 14.1) | .76 | 0.89 (0.41 to 1.95) | .77 | ||||||||
|
|
Housing decision made (stay at home), n (%) | 27 (16.2) | 36 (20.9) | 2.6 (–10.0 to 15.2) | .69 | 1.10 (0.46 to 2.62) | .83 | ||||||||
|
|
Decisional conflict (yes: scale ≥37.5), n (%) | 23 (13.8) | 19 (11.1) | –7.5 (–16.5 to 1.6) | .10 | 0.46 (0.19 to 1.11) | .08 | ||||||||
|
|
Decisional regret (yes: scale >0), n (%) | 117 (70.1) | 124 (72.1) | 1.7 (–15.0 to 18.3) | .84 | 1.03 (0.32 to 3.31) | .96 | ||||||||
|
|
Involvement in decision making (Dyadic-OPTION),c mean (SD) | 69.3 (17.6) | 69.4 (19.8) | 1.2 (–5.2 to 7.6)d | .72 | N/Ae | N/A | ||||||||
|
|
Burden of caref (0-88), mean (SD) | 34.6 (17.2) | 31.3 (16.5) | –1.1 (–6.2 to 4.0)d | .66 | N/A | N/A | ||||||||
aGeneralized linear mixed model with adaptive Gaussian–Hermite approximation to the likelihood maximum using an identity link, including intervention as binary variable, a fixed effect (categorical) for time, and specifying a random effect for cluster.
bGeneralized linear mixed model with logit link function, including intervention as binary variable, a fixed effect (categorical) for time, and specifying a random effect for cluster.
cAssessed on a continuous scale ranging from 0 to 100.
dLinear mixed model including intervention as binary variable, a fixed effect (categorical) for time, and specifying a random effect for cluster.
eN/A: not applicable.
fAssessed on continuous scale ranging from 0 to 88.
Secondary Outcomes
The intervention had no statistically significant effect on any secondary outcomes among the frail older adults or caregivers. Frail older adults’ perception of the extent to which health professionals involved them in decision-making scored an average of 67 of 100 with a (nonsignificant) mean increase of 5.4 (95% CI –0.6 to 11.4; P=.07). For caregivers, there was a nonsignificant effect on decisional conflict: 13.8% (23/167) in the control group versus 11% (19/172) in the intervention group, for an absolute decrease of 7.5% (95% CI –16.5% to 1.6%, P=.10) (Tables 3 and 4).
Discussion
Principal Findings
This study evaluated the effectiveness of adding training in IP-SDM for home care teams to the passive dissemination of a decision guide on the proportion of frail older adults, or caregivers of frail older adults with cognitive impairment, who reported taking an active role in making a housing decision. In this pragmatic trial, we observed a nonsignificant increase in the proportion of participants reporting an active role in decision-making. We observed no significant effect on any secondary outcomes. However, for frail older adults, there was an absolute (nonsignificant) increase in the extent to which health professionals involved them in decision-making and an absolute (nonsignificant) decrease in decisional conflict among caregivers. These results lead us to make the following observations.
Interpretation and Comparison With Prior Work
First, the nonsignificant increase observed in the primary outcome in both categories of participants (frail older adults and caregivers) may be explained by the fact that at baseline, the control group scored higher than expected. In our control condition, all clusters had been exposed to passive dissemination of the decision guide. In the trial that informed our sample size calculation (caregivers only), where the control group received usual care (ie, without the decision guide), fewer participants reported playing an active role at baseline and there was more room for improvement [10]. In the earlier trial, caregivers were also younger, and other studies confirm that younger people want a more active role in decision-making [57]. Both trials were pragmatic, and the loss of efficacy in a real clinical practice setting was to be expected. Interestingly, in both trials with caregivers of frail older adults with cognitive impairment, regardless of the decision-making role they assumed at baseline, an active decision-making role postintervention seemed to reach a similar threshold and go no further: in the first study, 79.6% took an active role postintervention [10], compared to 80.8% (139/172) in the current study. This suggests that among caregivers there is a natural ceiling to the expectation or desire to be active in decision-making on behalf of frail older adults. This threshold could be linked to discomfort with the role of being an active proxy decision-maker for more difficult and preference-sensitive decisions. At these times, it may be less stressful to surrender responsibility for decision-making to the clinician [58].
Second, we observed high staff turnover during the study. In a postintervention follow-up, we found that of the 281 health professionals who received the intervention, less than half remained, possibly due to a major restructuring of the Quebec health care system occurring at the time [46]. High staff turnover was identified as one of the main barriers to engaging in IP-SDM [59]. Thus, many participants were being cared for by staff who had not been exposed to the intervention, likely contributing to its ineffectiveness. Repeating the intervention with replacement staff could have remedied this [60]. Periodic reminders [61] and postintervention coaching could have increased the long-term effects of the intervention and fidelity to it [62]. Changing clinical, organizational, and policy-making environments can have major impacts on pragmatic trials such as ours.
Finally, the health professionals were under severe time constraints. Caregivers may have felt they should not take up too much time talking about their preferences and values, although this was suggested by the decision guide [63]. In addition, the home care teams may have felt that SDM as presented in the training would be too time-consuming, even though they may, in fact, have already been collaborating with patients and their caregivers in decision-making [7]. The perception of SDM as an issue related to the quantity of time needs to shift to a perception that SDM is rather an issue related to the quality of time [63]. Our results could be interpreted as showing that in this context, with overworked staff and high turnover, the decision aid was the most appropriate and practical intervention for increasing client involvement in decision-making.
A strength of this trial was that it was pragmatic, according to the pragmatic–explanatory continuum indicator summary (PRECIS-2) [64]. Pragmatic trials are more applicable to real clinical practice [65] and increase external validity [66]. Second, no health center was lost to follow-up, reducing selection bias and indicating that the study was relevant to its participants. Decision support for housing decisions was clearly already of great interest even before the COVID-19 pandemic and its catastrophic consequences for long-term care residents made housing decisions a policy priority [67]. Third, all analyses gave similar results, demonstrating their consistency (Multimedia Appendices 7, 8, 9, and 10).
Limitations
This study had a number of limitations. First, we assumed our sample size would give us enough power to detect a 20% increase in our primary outcome, but the increase was 6.1% (not statistically significant). This lack of power may also explain why our study failed to detect a significant difference between the study groups, given the large CIs around their point estimates [10]. Second, identifying and recruiting participants after randomization may have increased the risk of selection bias, which would have caused under- or overestimation of the effect. However, the fact that characteristics were overall well balanced between groups indicates that this bias was minimal; we also adjusted for imbalanced variables to mitigate their influence on the estimate. Third, health professionals may have selected compliant participants, thereby inducing selection bias [68]. However, this limitation would have affected both the intervention and control groups. Fourth, the decision guide was distributed to all health professionals in the workshop. A question in our survey as to whether older adults and caregivers had been shown the decision guide should have provided us with a pseudofidelity variable regarding its use with patients [15], but due to a high level of missing data for this question, we could not include this as an outcome. It may be possible that there was a lack of fidelity to the implementation of the intervention. In this pragmatic trial, we were not able to be present at the consultations to assess this. A future mixed methods or qualitative study could provide this information and help us to better see the impact of the intervention. Finally, at the cluster level, the intervention may not be applicable in every setting, since home care services are organized differently from one jurisdiction to another [10]. At the individual level, however, the results of this study are generalizable to frail older adults and caregivers of frail older adults with cognitive impairment with similar characteristics facing housing decisions.
Conclusions
Adding IP-SDM training to passive dissemination of a decision guide for home care teams was not sufficient to induce frail older adults or caregivers of cognitively-impaired frail older adults to take a more active role in housing decisions. Baseline involvement in decision-making was already high, suggesting that home care teams are already practicing a form of collaborative decision-making with their clients. When home care teams are overworked and understaffed, providing them with high-quality practical tools may be the best way to support them in involving their clients in decision-making. Further research could explore more effective dissemination of decision guides, a new SDM focus on time quality instead of time quantity, and how to adapt SDM interventions to crisis situations (eg, pandemics), when staff are absent or turnover is especially high.
Acknowledgments
We thank Guy Lacroix, Sophie Desroches, Serge Dumont, Kimberly D Fraser, Stéphane Turcotte, Henriette Bourassa, Lise Roy, Geneviève Painchaud Guérard, Sylvie-Marianne Rhugenda, and Sabrina Guay-Bélanger for their involvement in the realization of the trial. We also thank the managers and home care teams of Centre intégré de santé et de services sociaux (CISSS) Abitibi-Témiscamingue, CISSS Montérégie-Ouest, Centre intégré universitaire de santé et de services sociaux (CIUSSS) Estrie, CISSS Laurentides, CISSS Laval, CISSS Outaouais, CISSS Montérégie-Est, CISSS Montérégie-Centre, and CIUSSS de l’Ouest-de-l’île-de-Montréal who participated in this study for the difficult and useful work they are doing every day for the well-being of older adults and their family caregivers. We thank Dr. Henian Chen, professor of biostatistics at the College of Public Health, University of South Florida, USA, and Yuanyuan Lu, his PhD student in biostatistics. Finally, we thank Louisa Blair for her editorial support.
Abbreviations
- BpICC
between-period intraclass correlation coefficient
- CAC
cluster autocorrelation coefficient
- GLMM
generalized linear mixed models
- ICC
intraclass correlation
- IP-SDM
interprofessional shared decision-making
- RA
research assistant
- SDM
shared decision-making
- WpICC
within-period intraclass correlation coefficients
Characteristic of frail elders by allocated sequence.
Characteristic of caregivers of cognitively-impaired frail elders by allocated sequence.
Marginal frequencies of the primary outcome by period and cluster for frail elderly without cognitive impairment.
Marginal frequencies of the primary outcome by period and cluster for caregivers of cognitively-impaired frail elderly.
Intraclass correlation coefficient (ICC) and cluster autocorrelation coefficient for primary and secondary outcomes among frail elders without cognitive impairment.
Intraclass correlation coefficient (ICC) and cluster autocorrelation coefficient for primary and secondary outcomes among caregivers of cognitively-impaired frail elders.
Effect of the intervention on primary and secondary outcomes for frail elders without cognitive impairment (secondary analyses).
Effect of the intervention on primary and secondary outcomes for caregivers of cognitively-impaired frail elders (secondary analyses).
Effect of the intervention on primary and secondary outcomes for frail elders without cognitive impairment using a model based on autoregressive between-period correlation (sensitivity analyses).
Effect of the intervention on primary and secondary outcomes for caregivers of cognitively-impaired frail elders using a model based on uniform between-period correlation (sensitivity analyses).
CONSORT eHEALTH Checklist (V 1.6.1).
Footnotes
Conflicts of Interest: DS holds a University of Ottawa research chair in knowledge translation to patients. The authors have no further conflcits of interest to declare.
References
- 1.Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017 Dec 11;5:335. doi: 10.3389/fpubh.2017.00335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murman D. The Impact of Age on Cognition. Semin Hear. 2015 Aug;36(3):111–21. doi: 10.1055/s-0035-1555115. https://europepmc.org/abstract/MED/27516712 .00674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy N, Dubé Roxanne, Després Carole, Freitas A, Légaré France. Choosing between staying at home or moving: A systematic review of factors influencing housing decisions among frail older adults. PLoS One. 2018;13(1):e0189266. doi: 10.1371/journal.pone.0189266. https://dx.plos.org/10.1371/journal.pone.0189266 .PONE-D-17-25696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvelink MM, Emond J, Menear M, Brière Nathalie, Freitas A, Boland L, Perez MMB, Blair L, Stacey D, Légaré France. Development of a decision guide to support the elderly in decision making about location of care: an iterative, user-centered design. Res Involv Engagem. 2016;2:26. doi: 10.1186/s40900-016-0040-0. https://researchinvolvement.biomedcentral.com/articles/10.1186/s40900-016-0040-0 .40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradshaw Siobhan Aine, Playford E Diane, Riazi Afsane. Living well in care homes: a systematic review of qualitative studies. Age Ageing. 2012 Jul;41(4):429–40. doi: 10.1093/ageing/afs069.afs069 [DOI] [PubMed] [Google Scholar]
- 6.McQueen P. The role of regret in medical decision-making. Ethic Theory Moral Prac. 2017 Nov 4;20(5):1051–1065. doi: 10.1007/s10677-017-9844-8. [DOI] [Google Scholar]
- 7.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, Edwards A, Barry M. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012 Oct;27(10):1361–7. doi: 10.1007/s11606-012-2077-6. https://europepmc.org/abstract/MED/22618581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stacey Dawn, Légaré France, Lewis Krystina, Barry Michael J, Bennett Carol L, Eden Karen B, Holmes-Rovner Margaret, Llewellyn-Thomas Hilary, Lyddiatt Anne, Thomson Richard, Trevena Lyndal. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017 Apr 12;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. https://europepmc.org/abstract/MED/28402085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey D, Légaré France, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017 Aug 15;318(7):657–658. doi: 10.1001/jama.2017.10289.2648613 [DOI] [PubMed] [Google Scholar]
- 10.Adekpedjou Rhéda, Stacey Dawn, Brière Nathalie, Freitas Adriana, Garvelink Mirjam M, Dogba Maman Joyce, Durand Pierre J, Desroches Sophie, Croteau Jordie, Rivest Louis-Paul, Légaré France. Engaging Caregivers in Health-Related Housing Decisions for Older Adults With Cognitive Impairment: A Cluster Randomized Trial. Gerontologist. 2020 Jul 15;60(5):947–957. doi: 10.1093/geront/gnz045. https://europepmc.org/abstract/MED/31095318 .5490227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ampe S, Sevenants A, Smets T, Declercq A, Van Audenhove C. Advance care planning for nursing home residents with dementia: Influence of 'we DECide' on policy and practice. Patient Educ Couns. 2017 Jan;100(1):139–146. doi: 10.1016/j.pec.2016.08.010.S0738-3991(16)30352-4 [DOI] [PubMed] [Google Scholar]
- 12.Govasli L, Solvoll B. Nurses' experiences of busyness in their daily work. Nurs Inq. 2020 Jul;27(3):e12350. doi: 10.1111/nin.12350. [DOI] [PubMed] [Google Scholar]
- 13.Giguère Anik, Légaré France, Grimshaw Jeremy, Turcotte Stéphane, Fiander Michelle, Grudniewicz Agnes, Makosso-Kallyth Sun, Wolf Fredric M, Farmer Anna P, Gagnon Marie-Pierre. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012 Oct 17;10:CD004398. doi: 10.1002/14651858.CD004398.pub3. https://europepmc.org/abstract/MED/23076904 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA, Dixon-Woods M, Aldcroft A, Doussau A, Grayling M, Kristunas C, Goldstein CE, Campbell MK, Girling A, Eldridge S, Campbell MJ, Lilford RJ, Weijer C, Forbes AB, Grimshaw JM. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. 2018 Nov 09;363:k1614. doi: 10.1136/bmj.k1614. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=30413417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Légaré France, Brière Nathalie, Stacey D, Lacroix G, Desroches S, Dumont S, Fraser KD, Rivest L, Durand PJ, Turcotte S, Taljaard M, Bourassa H, Roy L, Painchaud Guérard Geneviève. Implementing shared decision-making in interprofessional home care teams (the IPSDM-SW study): protocol for a stepped wedge cluster randomised trial. BMJ Open. 2016 Nov 24;6(11):e014023. doi: 10.1136/bmjopen-2016-014023. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=27884857 .bmjopen-2016-014023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015 Feb 06;350:h391. doi: 10.1136/bmj.h391. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=25662947 . [DOI] [PubMed] [Google Scholar]
- 17.Hemming Karla, Taljaard Monica. Reflection on modern methods: when is a stepped-wedge cluster randomized trial a good study design choice? Int J Epidemiol. 2020 Jun 01;49(3):1043–1052. doi: 10.1093/ije/dyaa077. https://europepmc.org/abstract/MED/32386407 .5835358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottawa Decision Support Tutorial. Ottawa Hospital Research Institute. [2022-08-31]. https://decisionaid.ohri.ca/ODST/index.php .
- 19.Boland L, Légaré France, Carley M, Graham ID, O'Connor Annette M, Lawson ML, Stacey D. Evaluation of a shared decision making educational program: The Ottawa Decision Support Tutorial. Patient Educ Couns. 2019 Feb;102(2):324–331. doi: 10.1016/j.pec.2018.09.008.S0738-3991(18)30709-2 [DOI] [PubMed] [Google Scholar]
- 20.Stacey D, Brière Nathalie, Robitaille H, Fraser K, Desroches S, Légaré France. A systematic process for creating and appraising clinical vignettes to illustrate interprofessional shared decision making. J Interprof Care. 2014 Sep;28(5):453–9. doi: 10.3109/13561820.2014.911157. [DOI] [PubMed] [Google Scholar]
- 21.Maltais P, Goulet F, Borduas F. Educational skills and knowledge needed and problems encountered by continuing medical education providers. J Contin Educ Health Prof. 2000;20(2):91–6. doi: 10.1002/chp.1340200205. [DOI] [PubMed] [Google Scholar]
- 22.Copas AJ, Lewis JJ, Thompson JA, Davey C, Baio G, Hargreaves JR. Designing a stepped wedge trial: three main designs, carry-over effects and randomisation approaches. Trials. 2015 Aug 17;16:352. doi: 10.1186/s13063-015-0842-7. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-015-0842-7 .10.1186/s13063-015-0842-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Bayer I, Gewurtz R, Larivière Nadine, Letts L. Comparing web-based and in-person educational workshops for canadian occupational therapists and understanding their learning experiences: mixed methods study. JMIR Med Educ. 2022 Jan 04;8(1):e31634. doi: 10.2196/31634.v8i1e31634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rottenberg S, Williams A. Web-based delivery of the caregiving essentials course for informal caregivers of older adults in ontario: mixed methods evaluation study. JMIR Aging. 2021 Jun 15;4(2):e25671. doi: 10.2196/25671. https://aging.jmir.org/2021/2/e25671/ v4i2e25671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Mansfield J, Muff A, Meschiany G, Lev-Ari S. Adequacy of web-based activities as a substitute for in-person activities for older persons during the covid-19 pandemic: survey study. J Med Internet Res. 2021 Jan 22;23(1):e25848. doi: 10.2196/25848. https://www.jmir.org/2021/1/e25848/ v23i1e25848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger F, Hacke C, Potthoff J, Scheibler F, Rueffer JU, Kuch C, Wehkamp K. The effect of a scalable online training module for shared decision making based on flawed video examples - a randomized controlled trial. Patient Educ Couns. 2021 Jul;104(7):1568–1574. doi: 10.1016/j.pec.2020.11.033.S0738-3991(20)30657-1 [DOI] [PubMed] [Google Scholar]
- 27.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992 Sep;45(9):941–50. doi: 10.1016/0895-4356(92)90110-9.0895-4356(92)90110-9 [DOI] [PubMed] [Google Scholar]
- 28.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 29.Legaré F, Graham ID, O’Connor AM, Dolan JG, Bélanger-Ducharme F. Prise de décision partagée : traduction et validation d'une échelle de confort décisionnel du médecin. Pédagogie Médicale. 2008 Aug 01;4(4):216–222. doi: 10.1051/pmed:2003031. https://www.pedagogie-medicale.org/articles/pmed/pdf/2003/04/pmed20034p216.pdf . [DOI] [Google Scholar]
- 30.Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281–92. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 31.Melbourne E, Sinclair K, Durand M, Légaré France, Elwyn G. Developing a dyadic OPTION scale to measure perceptions of shared decision making. Patient Educ Couns. 2010 Feb;78(2):177–83. doi: 10.1016/j.pec.2009.07.009.S0738-3991(09)00265-1 [DOI] [PubMed] [Google Scholar]
- 32.Melbourne E, Roberts S, Durand M, Newcombe R, Légaré France, Elwyn G. Dyadic OPTION: Measuring perceptions of shared decision-making in practice. Patient Educ Couns. 2011 Apr;83(1):55–7. doi: 10.1016/j.pec.2010.04.019.S0738-3991(10)00190-4 [DOI] [PubMed] [Google Scholar]
- 33.Bucquet D, Condon S, Ritchie K. The French version of the Nottingham Health Profile. A comparison of items weights with those of the source version. Soc Sci Med. 1990;30(7):829–35. doi: 10.1016/0277-9536(90)90207-9. [DOI] [PubMed] [Google Scholar]
- 34.Sharples LD, Todd CJ, Caine N, Tait S. Measurement properties of the Nottingham Health Profile and Short Form 36 health status measures in a population sample of elderly people living at home: results from ELPHS. Br J Health Psychol. 2000;5(3):217–233. doi: 10.1348/135910700168874. [DOI] [Google Scholar]
- 35.Faria CDCM, Teixeira-Salmela LF, Nascimento VB, Costa AP, Brito NDP, Rodrigues-De-Paula F. Comparisons between the Nottingham Health Profile and the Short Form-36 for assessing the quality of life of community-dwelling elderly. Rev Bras Fisioter. 2011 Oct;15(5):399–405. doi: 10.1590/s1413-35552011005000023.S1413-35552011005000023 [DOI] [PubMed] [Google Scholar]
- 36.Zarit S, Orr NK, Zarit JM. The hidden victims of Alzheimer's disease: Families under stress. New York, NY: NYU Press; 1985. [Google Scholar]
- 37.Seng Boon Kheng, Luo Nan, Ng Wai Yee, Lim June, Chionh Hui Ling, Goh Jenny, Yap Philip. Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Ann Acad Med Singap. 2010 Oct;39(10):758–63. [PubMed] [Google Scholar]
- 38.Hébert R, Bravo G, Girouard D. Fidélité de la traduction française de trois instruments d'évaluation des aidants naturels de malades déments. Can J Aging. 2010 Nov 29;12(3):324–337. doi: 10.1017/s0714980800013726. [DOI] [Google Scholar]
- 39.Sepucha K, Mulley AG. A perspective on the patient's role in treatment decisions. Med Care Res Rev. 2009 Feb;66(1 Suppl):53S–74S. doi: 10.1177/1077558708325511.1077558708325511 [DOI] [PubMed] [Google Scholar]
- 40.Elkin EB, Kim SH, Casper ES, Kissane DW, Schrag D. Desire for information and involvement in treatment decisions: elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol. 2007 Nov 20;25(33):5275–80. doi: 10.1200/JCO.2007.11.1922.25/33/5275 [DOI] [PubMed] [Google Scholar]
- 41.Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp KG. Physician, patient, and contextual factors affecting treatment decisions in older adults with cancer and models of decision making: a literature review. Oncol Nurs Forum. 2012 Jan;39(1):E70–83. doi: 10.1188/12.ONF.E70-E83. https://europepmc.org/abstract/MED/22201670 .X90151107806H032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Légaré France, Brière Nathalie, Stacey D, Bourassa H, Desroches S, Dumont S, Fraser K, Freitas A, Rivest L, Roy L. Improving decision making on location of care with the frail elderly and their caregivers (the DOLCE study): study protocol for a cluster randomized controlled trial. Trials. 2015 Feb 12;16:50. doi: 10.1186/s13063-015-0567-7. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-015-0567-7 .10.1186/s13063-015-0567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007 Feb;28(2):182–91. doi: 10.1016/j.cct.2006.05.007.S1551-7144(06)00063-2 [DOI] [PubMed] [Google Scholar]
- 44.Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clin Trials. 2005;2(2):99–107. doi: 10.1191/1740774505cn071oa. [DOI] [PubMed] [Google Scholar]
- 45.Légaré France, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012 Sep 18;184(13):E726–34. doi: 10.1503/cmaj.120568. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=22847969 .cmaj.120568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O-7.2 Act to Modify the Organization and Governance of the Health and Social Services Network, in Particular by Abolishing the Regional Agencies. Gouvernement du Québec. 2015. [2022-09-02]. https://www.legisquebec.gouv.qc.ca/en/document/cs/O-7.2 .
- 47.Ivers NM, Halperin IJ, Barnsley J, Grimshaw JM, Shah BR, Tu K, Upshur R, Zwarenstein M. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials. 2012 Aug 01;13:120. doi: 10.1186/1745-6215-13-120. https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-13-120 .1745-6215-13-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanderWeele Tyler J, Shpitser Ilya. A new criterion for confounder selection. Biometrics. 2011 Dec;67(4):1406–13. doi: 10.1111/j.1541-0420.2011.01619.x. https://europepmc.org/abstract/MED/21627630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemming K, Taljaard M, Forbes A. Analysis of cluster randomised stepped wedge trials with repeated cross-sectional samples. Trials. 2017 Mar 04;18(1):101. doi: 10.1186/s13063-017-1833-7. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-017-1833-7 .10.1186/s13063-017-1833-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girling AJ, Hemming K. Statistical efficiency and optimal design for stepped cluster studies under linear mixed effects models. Stat Med. 2016 Jun 15;35(13):2149–66. doi: 10.1002/sim.6850. https://europepmc.org/abstract/MED/26748662 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooper R, Teerenstra S, de Hoop E, Eldridge S. Sample size calculation for stepped wedge and other longitudinal cluster randomised trials. Stat Med. 2016 Nov 20;35(26):4718–4728. doi: 10.1002/sim.7028. [DOI] [PubMed] [Google Scholar]
- 52.Kasza J, Hemming K, Hooper R, Matthews J, Forbes A. Impact of non-uniform correlation structure on sample size and power in multiple-period cluster randomised trials. Stat Methods Med Res. 2019 Mar;28(3):703–716. doi: 10.1177/0962280217734981. [DOI] [PubMed] [Google Scholar]
- 53.Austin Peter C. Estimating multilevel logistic regression models when the number of clusters is low: a comparison of different statistical software procedures. Int J Biostat. 2010 Apr 22;6(1):Article 16. doi: 10.2202/1557-4679.1195. https://www.degruyter.com/document/doi/10.2202/1557-4679.1195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemming K, Ryan R, Gill P, Westerby P, Jolly K, Marshall T. Targeted case finding in the prevention of cardiovascular disease: a stepped wedge cluster randomised controlled trial. Br J Gen Pract. 2016 Oct;66(651):e758–67. doi: 10.3399/bjgp16X686629. https://bjgp.org/cgi/pmidlookup?view=long&pmid=27528707 .bjgp16X686629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leontjevas R, Gerritsen DL, Smalbrugge M, Teerenstra S, Vernooij-Dassen MJ, Koopmans RT. A structural multidisciplinary approach to depression management in nursing-home residents: a multicentre, stepped-wedge cluster-randomised trial. Lancet. 2013 Jun 29;381(9885):2255–64. doi: 10.1016/S0140-6736(13)60590-5.S0140-6736(13)60590-5 [DOI] [PubMed] [Google Scholar]
- 56.Hannan PJ, Murray DM. Gauss or Bernoulli? A Monte Carlo comparison of the performance of the linear mixed-model and the logistic mixed-model analyses in simulated community trials with a dichotomous outcome variable at the individual level. Eval Rev. 1996 Jun;20(3):338–52. doi: 10.1177/0193841X9602000306. [DOI] [PubMed] [Google Scholar]
- 57.Deber RB, Kraetschmer N, Urowitz S, Sharpe N. Do people want to be autonomous patients? Preferred roles in treatment decision-making in several patient populations. Health Expect. 2007 Sep;10(3):248–58. doi: 10.1111/j.1369-7625.2007.00441.x. doi: 10.1111/j.1369-7625.2007.00441.x.HEX441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pel-Littel RE, Snaterse M, Teppich NM, Buurman BM, van Etten-Jamaludin FS, van Weert JCM, Minkman MM, Scholte Op Reimer Wilma J M. Barriers and facilitators for shared decision making in older patients with multiple chronic conditions: a systematic review. BMC Geriatr. 2021 Feb 06;21(1):112. doi: 10.1186/s12877-021-02050-y. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-021-02050-y .10.1186/s12877-021-02050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Légaré France, Stacey D, Brière Nathalie, Fraser K, Desroches S, Dumont S, Sales A, Puma C, Aubé Denise. Healthcare providers' intentions to engage in an interprofessional approach to shared decision-making in home care programs: a mixed methods study. J Interprof Care. 2013 May;27(3):214–22. doi: 10.3109/13561820.2013.763777. https://europepmc.org/abstract/MED/23394265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuller C, Michie S, Savage J, McAteer J, Besser S, Charlett A, Hayward A, Cookson BD, Cooper BS, Duckworth G, Jeanes A, Roberts J, Teare L, Stone S. The Feedback Intervention Trial (FIT)--improving hand-hygiene compliance in UK healthcare workers: a stepped wedge cluster randomised controlled trial. PLoS One. 2012;7(10):e41617. doi: 10.1371/journal.pone.0041617. https://dx.plos.org/10.1371/journal.pone.0041617 .PONE-D-12-02454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson MJ, May CR. Promoting professional behaviour change in healthcare: what interventions work, and why? A theory-led overview of systematic reviews. BMJ Open. 2015 Sep 30;5(9):e008592. doi: 10.1136/bmjopen-2015-008592. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=26423853 .bmjopen-2015-008592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zomahoun HTV, Guénette Line, Grégoire Jean-Pierre, Lauzier S, Lawani AM, Ferdynus C, Huiart L, Moisan J. Effectiveness of motivational interviewing interventions on medication adherence in adults with chronic diseases: a systematic review and meta-analysis. Int J Epidemiol. 2017 Apr 01;46(2):589–602. doi: 10.1093/ije/dyw273.dyw273 [DOI] [PubMed] [Google Scholar]
- 63.Yahanda Alexander T, Mozersky Jessica. What's the role of time in shared decision making? AMA J Ethics. 2020 May 01;22(5):E416–422. doi: 10.1001/amajethics.2020.416. https://journalofethics.ama-assn.org/article/whats-role-time-shared-decision-making/2020-05 .amajethics.2020.416 [DOI] [PubMed] [Google Scholar]
- 64.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015 May 08;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 65.Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217–24. doi: 10.31887/DCNS.2011.13.2/npatsopoulos. https://europepmc.org/abstract/MED/21842619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lurie JD, Morgan TS. Pros and cons of pragmatic clinical trials. J Comp Eff Res. 2013 Jan;2(1):53–8. doi: 10.2217/cer.12.74. [DOI] [PubMed] [Google Scholar]
- 67.Linton SL, Leifheit KM, McGinty EE, Barry CL, Pollack CE. Association between housing insecurity, psychological distress, and self-rated health among US adults during the COVID-19 pandemic. JAMA Netw Open. 2021 Sep 01;4(9):e2127772. doi: 10.1001/jamanetworkopen.2021.27772. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2021.27772 .2784598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ertmann RK, Nicolaisdottir DR, Kragstrup J, Siersma V, Overbeck G, Wilson P, Lutterodt MC. Selection bias in general practice research: analysis in a cohort of pregnant Danish women. Scand J Prim Health Care. 2020 Dec;38(4):464–472. doi: 10.1080/02813432.2020.1847827. https://europepmc.org/abstract/MED/33242291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristic of frail elders by allocated sequence.
Characteristic of caregivers of cognitively-impaired frail elders by allocated sequence.
Marginal frequencies of the primary outcome by period and cluster for frail elderly without cognitive impairment.
Marginal frequencies of the primary outcome by period and cluster for caregivers of cognitively-impaired frail elderly.
Intraclass correlation coefficient (ICC) and cluster autocorrelation coefficient for primary and secondary outcomes among frail elders without cognitive impairment.
Intraclass correlation coefficient (ICC) and cluster autocorrelation coefficient for primary and secondary outcomes among caregivers of cognitively-impaired frail elders.
Effect of the intervention on primary and secondary outcomes for frail elders without cognitive impairment (secondary analyses).
Effect of the intervention on primary and secondary outcomes for caregivers of cognitively-impaired frail elders (secondary analyses).
Effect of the intervention on primary and secondary outcomes for frail elders without cognitive impairment using a model based on autoregressive between-period correlation (sensitivity analyses).
Effect of the intervention on primary and secondary outcomes for caregivers of cognitively-impaired frail elders using a model based on uniform between-period correlation (sensitivity analyses).
CONSORT eHEALTH Checklist (V 1.6.1).


