Abstract
Background
Allergic rhinitis (AR) management requires a coordinated effort from healthcare providers and patients. Pharmacists are key members of these integrated care pathways resolving medication‐related problems, optimizing regimens, improving adherence and recommending therapies while establishing liaisons between patients and physicians.
Methods
Allergic Rhinitis and its Impact on Asthma (ARIA) first published a reference document on the pharmacist's role in allergic rhinitis management in 2004. Several guidelines were developed over the past 20 years improving the care of allergic rhinitis patients through an evidence‐based, integrated care approach.
Results
This ARIA/EAACI/FIP Position Paper is based on the latest ARIA in the Pharmacy guidelines and provides: (a) a structured approach to pharmacists identifying people with AR and/or allergic conjunctivitis as well as those at risk of poor disease control; (b) an evidence‐based clinical decision support tool for optimising the management of allergic rhinitis in the community pharmacy; and (c) a framework of referral to the physician.
Conclusion
This document is not intended to be a mandatory standard of care but is provided as a basis for pharmacists and their staff to develop relevant local standards of care for their patients, within their local practice environment. Pharmacy care varies between countries, and the guide should be adapted to the local situation.
Keywords: allergic rhinitis, community pharmacy, pharmacist
1. INTRODUCTION
Allergic rhinitis (AR) is the most common form of non‐infectious rhinitis and is one of the most prevalent chronic diseases. 1 , 2 , 3 , 4
Cardinal symptoms of AR include rhinorrhoea, nasal obstruction, sneezing and nasal itching. In some cases, these symptoms are spontaneously reversible, while, in others, they can be controlled by adequate treatment. 5
Symptoms associated with AR have a significant impact on work 6 and school productivity, sleep 7 and social interactions. There is an association between symptoms of AR and decreased general health‐related quality of life. 8 , 9
Allergic conjunctivitis and asthma are common multimorbidities experienced by patients with AR. 10
Most AR patients choose to manage their condition via self‐medication with non‐prescription medicines. 11 , 12 , 13 The use of these medicines for self‐medication may be appropriate or inappropriate. 14 Community pharmacists play a critical role assisting in the management of AR and advising on the appropriate self‐medication. 15 , 16
Pharmacists may also assist in the identification of patients who are inappropriately self‐medicating resulting in a suboptimal treatment of their condition. They can also, if necessary, refer these patients for medical assessment. 17
The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines, which were first released 20 years ago and are continually being updated with the latest evidence, provide a guide to the latest evidence‐based integrated care approach to the management of AR. 18
2. STEP 1: DIFFERENTIAL DIAGNOSIS OF ALLERGIC RHINITIS IN THE COMMUNITY PHARMACY
Pharmacists play an important role in confirming an AR diagnosis: some patients purchasing AR medicines in the pharmacy will have a diagnosis of AR by a physician, others will have an appropriate self‐diagnosis of AR, and the remainder no diagnosis or an incorrect one 18 (Table 1).
AR symptoms may be similar to those of several conditions and confused with a viral infection such as the common cold/acute rhinosinusitis (including COVID‐19) or chronic rhinosinusitis 19 , 20 , 21 (Figure 1).
The presence of nasal itching, rhinorrhoea, sneezing and eye symptoms is often consistent with allergic rhinitis. A mild‐to‐moderate loss of smell (hyposmia) may be present in the most severe patients, and can be sudden, severe and sometimes isolated in COVID‐19 patients 22 (Figure 1).
TABLE 1.
Questions to help identify allergic rhinitis
| What is your main symptom? (Check for rhinorrhoea, sneezing, itchy nose, nasal congestion, loss of smell, watery or itchy eyes.) |
| How long have you had these symptoms? |
| Do you have the symptoms all the time or do they come and go? |
| Are you aware of anything that seems to bring the symptoms on, such as being outdoors, pollen seasons, contact with animals, something you handle at work or at home? |
| Has a doctor ever diagnosed hay fever, allergic rhinitis or asthma? |
| Is your nasal discharge clear and watery? (Purulent discharge suggests infection.) |
| Are you experiencing any wheezing or shortness of breath? (“Yes” may indicate asthma.) |
| Do you have an earache or any pain in your face? (“Yes” may indicate otitis media or rhinosinusitis.) |
FIGURE 1.
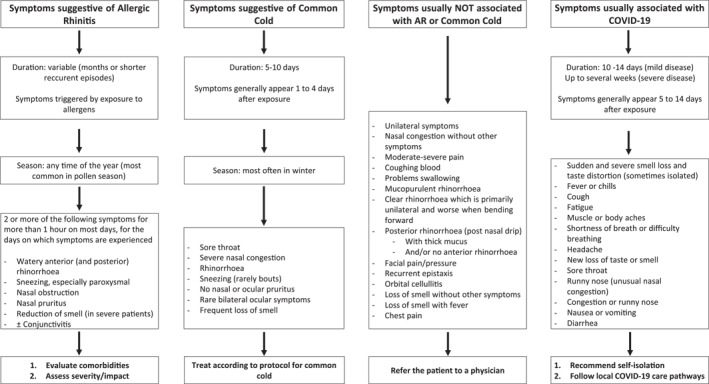
Recognising allergic rhinitis in the pharmacy. 1 For more information on which patients should be referred to a physician (and in what time frame), see Appendix III. Please refer to local guidelines for additional symptoms for referral
3. STEP 2a: ASSESSING COMMON AR COMORBIDITIES ‐ ALLERGIC CONJUNCTIVITIS
Eye symptoms are common in AR patients. However, they are not experienced by all AR patients. 23
The presence of conjunctivitis should always be assessed in patients with AR symptoms (Table 2 and Figure 2).
Importantly, conjunctivitis is not always caused by an allergen (e.g. chemical, irritant, bacterial, viral)
Photophobia (light sensitivity), eye burning, dry eyes and unilateral symptoms are unlikely to be associated with Allergic Conjunctivitis and need a physician evaluation. 24
TABLE 2.
Questions to help identify allergic conjunctivitis
| What is your main symptom? (Check for bilateral eye symptoms, eye itching, watery eyes, red eyes.) |
| Do you have allergic rhinitis? |
| Do your eyes burn? (“Yes” may indicate disease other than allergic rhinitis.) |
| Do your eyes burn? (“Yes” may indicate disease other than allergic rhinitis.) |
| Do you have photophobia? (“Yes” may indicate disease other than allergic rhinitis and the patient should be referred to a doctor.) |
FIGURE 2.
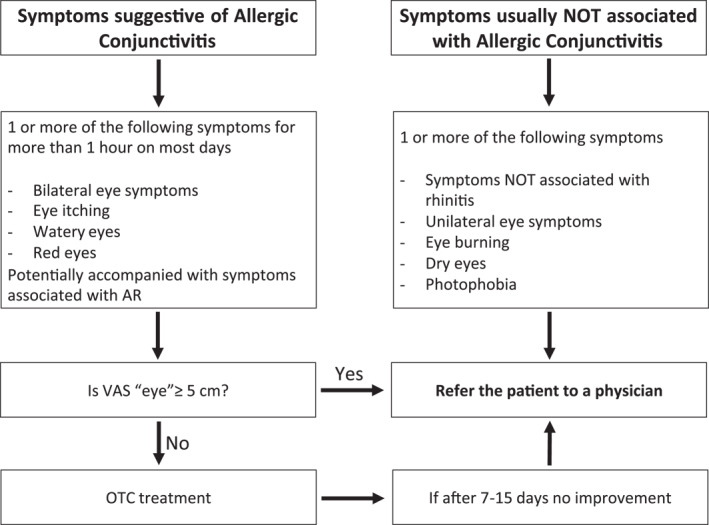
Screening of Allergic Conjunctivitis in the pharmacy 2
4. STEP 2b: ASSESSING COMMON AR COMORBIDITIES ‐ ASTHMA
AR and asthma often coexist, and asthma should always be evaluated in a patient presenting with allergic rhinitis symptoms 25 , 26 (Figure 3).
AR is a risk factor for the development of asthma. 27
In patients with asthma, AR may be associated with poor control of the disease. 25 , 28
FIGURE 3.
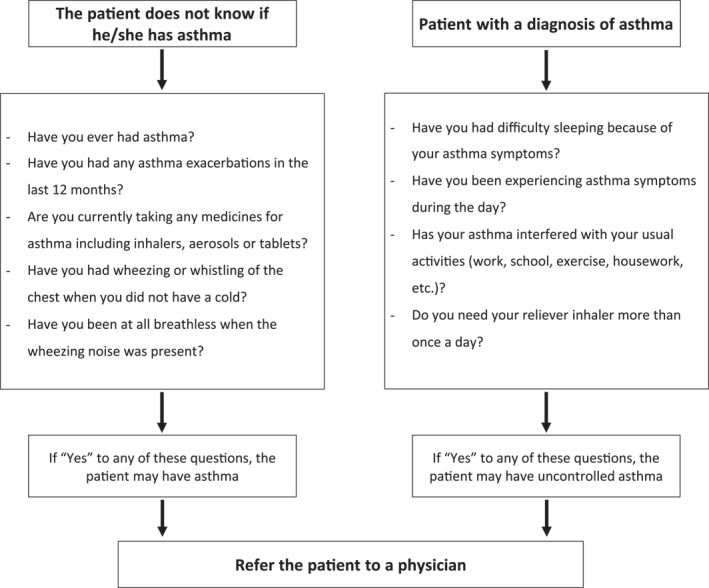
Screening of Asthma in AR patients in the pharmacy
5. STEP 3: ASSESSING THE SEVERITY OF ALLERGIC RHINITIS AND/OR ALLERGIC CONJUNCTIVITIS
The ARIA guidelines propose a classification of AR based on symptom control, quality of life, daily impact and duration. All of these can be combined into one question which relates to the degree to which the AR is bothersome 25 , 29 :
VAS “Nose” (0–10 cm): “How much are your nose symptoms bothering you today?”
VAS “Eyes” (0–10 cm): “How much are your eye symptoms bothering you today?”
Allergic rhinitis may be intermittent or persistent, but this does not influence the treatment to be recommended. The ARIA guidelines base treatment recommendations on the impact of symptoms on day‐to‐day living 5 , 25 , 30 (Figure 4).
FIGURE 4.
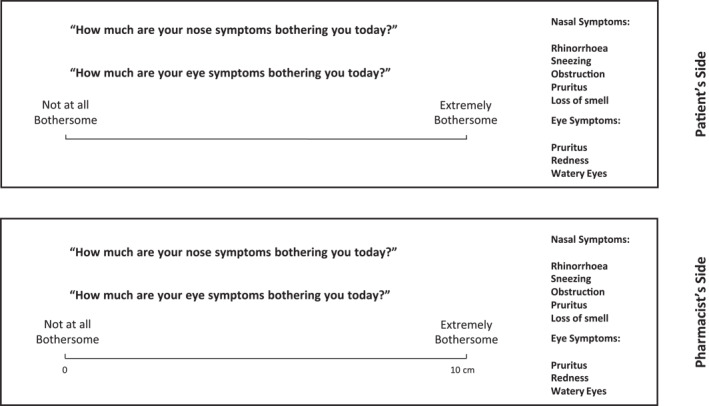
Determining the impact of Allergic Rhinitis and/or Allergic Conjunctivitis symptoms using VAS
6. STEP 4: TREATMENT OF ALLERGIC RHINITIS IN THE PHARMACY (FIGURE 5)
FIGURE 5.
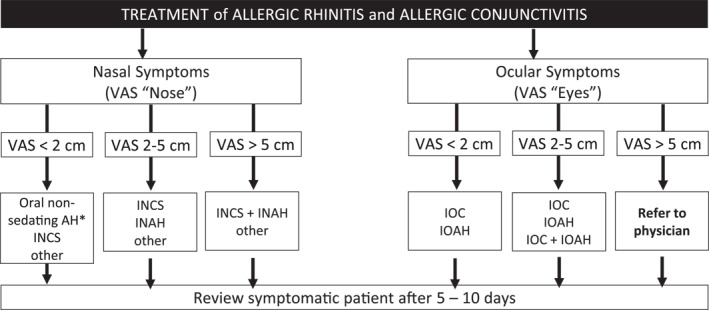
Treatment of allergic rhinitis in the pharmacy (adapted from 18 , 31 ). AH, antihistamine; INAH, intranasal antihistamine; INCS, intranasal corticosteroid; IOAH, intraocular antihistamine; IOC, intraocular cromone. *If nasal congestion is the main symptom; INCS if coexisting asthma. 32 This algorithm should be adapted to the regulations, needs, price of medications and cultural barriers of each country or region. VAS “Nose” (0–10 cm): “How much are your nose symptoms bothering you today?” VAS “Eyes” (0–10 cm): “How much are your eye symptoms bothering you today?”
By comparing AR control at the first dispensing of an OTC medication with the evolution of control during treatment, the algorithm can help both the pharmacist and the physician to optimise treatment (APPENDIX I and APPENDIX II).
The monitoring and self‐management of AR can be supported through the MASK‐air App 33 which can be downloaded for iPhone or Android (https://www.mask‐air.com/).
The cut‐off value for VAS “eye” is based on the results of the AR and the group's opinion, but has not been validated.
7. STEP 5: LONG‐TERM MONITORING AND PATIENT SUPPORT
It is critical that people with AR should be followed‐up over time, to ensure that their treatment is appropriate and to identify patients who require a referral to their physician (APPENDIX III). Follow‐up should occur 5–10 days post‐treatment initiation (Figure 6). 18 , 31
FIGURE 6.
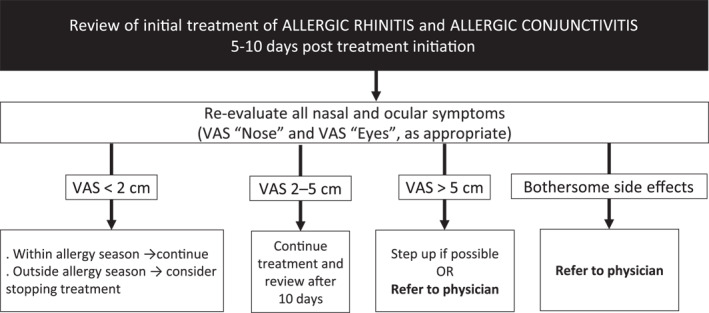
Follow‐up of AR treatment. AH, antihistamine; INAH, intranasal antihistamine; INCS, intranasal corticosteroid; IOAH, intraocular antihistamine; IOC, intraocular cromone. *If nasal congestion is the main symptom; INCS if coexisting asthma. This algorithm should be adapted to the regulations, needs, price of medications and cultural barriers of each country or region. VAS “Nose” (0–10 cm): “How much are your nose symptoms bothering you today?” VAS “Eyes” (0–10 cm): “How much are your eye symptoms bothering you today?”
In considering how to assist the patient in long‐term management, it is important to realise that AR is undertreated and underdiagnosed and that patient self‐selection is profound. 34 Patients often trivialise AR and do not realise the extent to which their AR presents a burden to their day‐to‐day living. Therefore, educating patients on recognising the impact of AR and counselling them on the goals they would like to achieve has been shown to be effective. 35 , 36 At this stage, unfortunately, there is much work to be done over time to ensure that patients remain adherent to their AR treatment 37 (Appendix IV).
The pharmacist should continue to suggest to the patient that the monitoring and self‐management of AR can be supported through the MASK‐air App 33 (which can be downloaded for iPhone or Android (https://www.mask‐air.com/)).
AUTHOR CONTRIBUTIONS
Olga Lourenco: Conceptualization; Equal, Methodology; Equal, Writing – original draft; Equal, Writing – review & editing; Equal, Biljana Cvetkovski: Writing – original draft; Equal, Writing – review & editing; Equal, Vicky Kritikos: Writing – original draft; Equal, Writing – review & editing; Equal, Rachel House: Writing – original draft; Equal, Writing – review & editing; Equal, Sophie Scheire: Writing – original draft; Equal, Writing – review & editing; Equal, Elisio M Costa: Writing – original draft; Equal, Writing – review & editing; Equal, Joao Fonseca: Writing – original draft; Equal, Writing – review & editing; Equal, Enrica Menditto: Writing – review & editing; Equal, Anna Bedbrook: Writing – review & editing; Equal, Slawomir Bialek: Writing – review & editing; Equal, Vitalis Briedis: Writing – review & editing; Equal, Koen Boussery: Writing – review & editing; Equal, Giorgio Walter Canonica: Writing – review & editing; Equal, Tari Haahtela: Writing – review & editing; Equal, Piotr Kuna: Writing – review & editing; Equal, Joaquim Mullol: Writing – review & editing; Equal, Valentina Orlando: Writing – review & editing; Equal, Bolesław Samoliński: Writing – review & editing; Equal, Dana Wallace: Writing – review & editing; Equal, Catherine Duggan: Writing – review & editing; Equal, Ema Paulino: Writing – review & editing; Equal, Goncalo Sousa Pinto: Writing – review & editing; Equal, Lars‐Ake Soderlund: Writing – review & editing; Equal, Jean Bousquet: Conceptualization; Equal, Supervision; Equal, Writing – review & editing; Equal, Sinthia Bosnic‐Anticevich: Conceptualization; Equal, Methodology; Equal, Supervision; Equal, Writing – original draft; Equal, Writing – review & editing; Equal.
CONFLICT OF INTEREST
Sinthia Bosnic‐Anticevich reports grants from TEVA, and personal fees from TEVA, AstraZeneca, Boehringer Ingelheim, GSK, Sanofi, and Mylan. João A. Fonseca reports personal fees from Viatris/Mylan. Piotr Kuna reports personal fees from Adamed, Berlin Chernie Menarini, Boehringer Ingelheim, Chiesi, AstraZeneca, Krka, Angellini, Novartis, Polpharma, Lekam, and GSK. Ema Paulino is the President of the Portuguese National Pharmacy Association and has been a Board member of the International Pharmaceutical Federation until October 2021. Biljana Svetkovski reports personal fees from GSK and Sanofi. Dana Wallace reports personal fees from Optinose, ALK, and Sanofi, and was a primary author on the JTFPP Rhinitis Practice Parameter Update 2020.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to express great appreciation to the Allergic Rhinitis and Its Impact on Asthma Working Group for valuable and constructive suggestions during the planning and development of this guide. We are particularly grateful for the assistance given by Adriano Raposo in figure editing. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Open access funding enabled and organized by Projekt DEAL.
Lourenço O, Cvetkovski B, Kritikos V, et al. Management of allergic rhinitis symptoms in the pharmacy Pocket guide 2022. Clin Transl Allergy. 2022;e12183. 10.1002/clt2.12183
ENDNOTES
In Figure 1, some of the items not associated with AR symptoms refer to rhinosinusitis and other diseases that need to be checked during differential diagnosis.
The cut‐off value for VAS “eye” is based on the results of the AR and on the group's opinion, but has not been validated. For determining VAS “eye”, see Figure 4.
REFERENCES
- 1. Mims JW. Epidemiology of allergic rhinitis. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S18‐S20. 10.1002/alr.21385 [DOI] [PubMed] [Google Scholar]
- 2. Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia‐Pacific adult surveys. Allergy Asthma Proc. 2012;33(Suppl 1):S113‐S141. 10.2500/aap.2012.33.3603 [DOI] [PubMed] [Google Scholar]
- 3. Baptist AP, Nyenhuis S. Rhinitis in the elderly. Immunol Allergy Clin. 2016;36(2):343‐357. 10.1016/j.iac.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider‐diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16(1):133. 10.1186/s12887-016-0673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its impact on Asthma (ARIA) guidelines‐2016 revision. J Allergy Clin Immunol. 2017;140(4):950‐958. [DOI] [PubMed] [Google Scholar]
- 6. Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of Rhinitis on work productivity: a systematic Review. J Allergy Clin Immunol Pract. 2018;6(4):1274‐1286. e1279. 10.1016/j.jaip.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 7. Munoz‐Cano R, Ribo P, Araujo G, Giralt E, Sanchez‐Lopez J, Valero A. Severity of allergic rhinitis impacts sleep and anxiety: results from a large Spanish cohort. Clin Transl Allergy. 2018;8(1):23. 10.1186/s13601-018-0212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoehle LP, Speth MM, Phillips KM, et al. Association between symptoms of allergic rhinitis with decreased general health‐related quality of life. Am J Rhinol Allergy. 2017;31(4):235‐239. 10.2500/ajra.2017.31.4444 [DOI] [PubMed] [Google Scholar]
- 9. Meltzer EO. Allergic Rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin. 2016;36(2):235‐248. 10.1016/j.iac.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 10. Bousquet J, Devillier P, Anto JM, et al. Daily allergic multimorbidity in rhinitis using mobile technology: A novel concept of the MASK study. Allergy. 2018;73(8):1622‐1631. [DOI] [PubMed] [Google Scholar]
- 11. Cvetkovski B, Kritikos V, Yan K, Bosnic‐Anticevich S. Tell me about your hay fever: a qualitative investigation of allergic rhinitis management from the perspective of the patient. NPJ Prim Care Respir Med. 2018;28(1):3. 10.1038/s41533-018-0071-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan R, Cvetkovski B, Kritikos V, et al. The burden of Rhinitis and the impact of medication management within the community pharmacy setting. J Allergy Clin Immunol Pract. 2018;6(5):1717‐1725. 10.1016/j.jaip.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 13. Tan R, Cvetkovski B, Kritikos V, et al. Management of allergic rhinitis in the community pharmacy: identifying the reasons behind medication self‐selection. Pharm Pract (Granada). 2018;16(3):1332. 10.18549/pharmpract.2018.03.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams A, Scadding G. Is reliance on self‐medication and pharmacy care adequate for rhinitis patients? Int J Clin Pract. 2009;63(1):98‐104. 10.1111/j.1742-1241.2008.01944.x [DOI] [PubMed] [Google Scholar]
- 15. Kuehl BL, Abdulnour S, O'Dell M, Kyle TK. Understanding the role of the healthcare professional in patient self‐management of allergic rhinitis. SAGE Open Med. 2015;3:2050312115595822. 10.1177/2050312115595822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cvetkovski B, Tan R, Kritikos V, et al. A patient‐centric analysis to identify key influences in allergic rhinitis management. NPJ Prim Care Respir Med. 2018;28(1):34. 10.1038/s41533-018-0100-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosnic‐Anticevich S, Kritikos V, Carter V, et al. Lack of asthma and rhinitis control in general practitioner‐managed patients prescribed fixed‐dose combination therapy in Australia. J Asthma. 2018;55(6):684‐694. 10.1080/02770903.2017.1353611 [DOI] [PubMed] [Google Scholar]
- 18. Bosnic‐Anticevich S, Costa E, Menditto E, et al. ARIA pharmacy 2018 “Allergic rhinitis care pathways for community pharmacy”: AIRWAYS ICPs initiative (European innovation partnership on active and healthy Ageing, DG CONNECT and DG sante) POLLAR (impact of Air POLLution on Asthma and Rhinitis) GARD demonstration project. Allergy. 2019;74(7):1219‐1236. [DOI] [PubMed] [Google Scholar]
- 19. Wise SK, Lin SY, Toskala E, et al. International consensus statement on Allergy and rhinology: allergic Rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108‐352. [DOI] [PubMed] [Google Scholar]
- 20. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on Rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 21. Carter A, Dattani N, Hannan SA. Chronic rhinosinusitis. BMJ (Clinical Res ed). 2019;364:l131. 10.1136/bmj.l131 [DOI] [PubMed] [Google Scholar]
- 22. Hagemann J, Onorato GL, Jutel M, et al. Differentiation of COVID‐19 Signs and Symptoms from Allergic Rhinitis and Common Cold: an ARIA‐EAACI‐GA(2) LEN Consensus; 2021. [DOI] [PMC free article] [PubMed]
- 23. Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population‐based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482‐488. 10.1097/aci.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 24. Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol. 2020;124(2):118‐134. 10.1016/j.anai.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 25. Bousquet J, Hellings PW, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2019;143(3):864‐879. [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Cardenas V, Armour C, Benrimoj SI, Martinez‐Martinez F, Rotta I, Fernandez‐Llimos F. Pharmacists' interventions on clinical asthma outcomes: a systematic review. Eur Respir J. 2016;47(4):1134‐1143. 10.1183/13993003.01497-2015 [DOI] [PubMed] [Google Scholar]
- 27. Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population‐based study. Lancet. 2008;372(9643):1049‐1057. 10.1016/s0140-6736(08)61446-4 [DOI] [PubMed] [Google Scholar]
- 28. Bousquet JJ, Schunemann HJ, Togias A, et al. Next‐generation ARIA care pathways for rhinitis and asthma: a model for multimorbid chronic diseases. Clin Transl Allergy. 2019;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bousquet J, Arnavielhe S, Bedbrook A, et al. The Allergic Rhinitis and its Impact on Asthma (ARIA) score of allergic rhinitis using mobile technology correlates with quality of life: the MASK study. Allergy. 2018;73(2):505‐510. 10.1111/all.13307 [DOI] [PubMed] [Google Scholar]
- 30. Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007;62(4):367‐372. 10.1111/j.1398-9995.2007.01276.x [DOI] [PubMed] [Google Scholar]
- 31. Lourenco O, Bosnic‐Anticevich S, Costa E, et al. Managing Allergic Rhinitis in the pharmacy: an ARIA guide for implementation in practice. Pharmacy. 2020;8(2):85. 10.3390/pharmacy8020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Juel‐Berg N, Darling P, Bolvig J, et al. Intranasal corticosteroids compared with oral antihistamines in allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2017;31(1):19‐28. 10.2500/ajra.2016.30.4397 [DOI] [PubMed] [Google Scholar]
- 33. Bedard A, Basagana X, Anto JM, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: the MASK study. J Allergy Clin Immunol. 2019;144(1):135‐143. e136. [DOI] [PubMed] [Google Scholar]
- 34. Price DB, Smith PK, Harvey RJ, et al. Real‐life treatment of rhinitis in Australia: a historical cohort study of prescription and over‐the‐counter therapies for patients with and without additional respiratory disease. Pragmatic Observational Res. 2018;9:43‐54. 10.2147/por.s153266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milosavljevic A, Aspden T, Harrison J. Community pharmacist‐led interventions and their impact on patients' medication adherence and other health outcomes: a systematic review. Int J Pharm Pract. 2018;26(5):387‐397. 10.1111/ijpp.12462 [DOI] [PubMed] [Google Scholar]
- 36. Todorova A, Tsvetkova A, Mihaylova S, Andreevska K, Kondova A, Arnaoudova M. The impact of pharmaceutical care on improving the quality of life in patients with allergic rhinitis. CBU Int Conf Proc. 2017;5(0):6‐1027. 10.12955/cbup.v5.1064 [DOI] [Google Scholar]
- 37. Menditto E, Costa E, Midao L, et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK Study. Clin Exp Allergy. 2019;49(4):442‐460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


