Abstract
Persistent antigenic stimulation results in loss of effector function or physical deletion of antigen-specific CD8 T cells. This T-cell state is called T-cell exhaustion and occurs during chronic infection and cancer. Antigen-specific CD8 T cells during T-cell exhaustion express the inhibitory receptor PD-1, the expression of which plays a major role in T-cell dysfunction. PD-1 blockade re-invigorates CD8 T-cell immunity and has been proven effective against many different types of human cancer. To further improve the efficacy of PD-1-targeted immunotherapy in cancer patients, a better understanding of T-cell exhaustion is required. Recent studies have revealed that antigen-specific CD8 T cells during T-cell exhaustion are heterogeneous and have also uncovered the detailed mechanisms for PD-1-targeted immunotherapy. Here, we review the CD8 T-cell subsets that arise during T-cell exhaustion, the lineage relationship among these individual subsets and the role of each subset in PD-1 blockade. Also, we discuss potential strategies to enhance the efficacy of PD-1-targeted immunotherapy.
Keywords: cancer, chronic infection, immune checkpoint inhibitors, T-cell exhaustion, T-cell heterogeneity
T cell heterogeneity during T cell exhaustion
Graphical Abstract
Graphical Abstract.
Introduction
CD8 T cells are known as cytotoxic T lymphocytes (CTLs) and play an important role in not only controlling infection with intracellular pathogens but also anti-cancer immunity (1–5). After antigen stimulation, naive CD8 T cells undergo clonal expansion, acquire cytotoxic function and differentiate into effector T cells that kill target cells (1, 4). During acute infection, effector CD8 T cells eliminate pathogens and further differentiate into long-lived memory T cells that can quickly respond to re-exposure to the same pathogens (1, 4). This process in which effector T cells differentiate into memory CD8 T cells occurs in the absence of antigen, and the generated memory CD8 T cells persist in an antigen-independent manner. On the other hand, during chronic infection, CD8 T cells fail to clear pathogens, and the fate of these CD8 T cells stimulated with persistent antigens is totally different from that during acute infection (2, 5).
By the late 1980s, it was known that immunocompetent adult mice chronically infected with lymphocytic choriomeningitis virus (LCMV) had no detectable levels of CTLs measured by the chromium-release assay (6–9). However, it was unclear whether the loss of CTLs during chronic LCMV infection was due to deletion of virus-specific CD8 T cells or due to impaired function of these cells because the chromium-release assay can only detect functional CD8 T cells that possess cytotoxic activity.
In 1993, Moskophidis et al. published a paper to address this important issue about the relationship between virus persistence and virus-specific CD8 T cells (10). To count the number of virus-specific CD8 T cells without using the chromium-release assay, TCR-transgenic CD8 T cells that recognize an LCMV CD8 T-cell epitope were adoptively transferred into recipient mice. The authors found that the population of the transferred TCR-transgenic CD8 T cells expanded after chronic LCMV infection, but then rapidly declined and were physically deleted within 1 month after infection (10). On the basis of this observation, the paper concluded that deletion of LCMV-specific CD8 T cells was responsible for the loss of CTLs during chronic LCMV infection and proposed physical deletion of antigen-specific CD8 T cells as the mechanism of T-cell exhaustion (10). Although this concept was supported by other studies (11–13), subsequent research revealed that the T-cell deletion was not the solo mechanism of T-cell exhaustion.
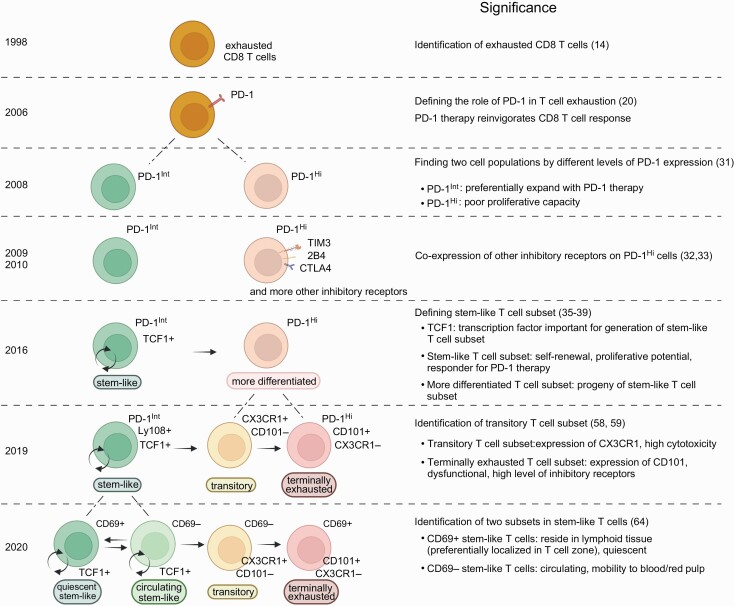
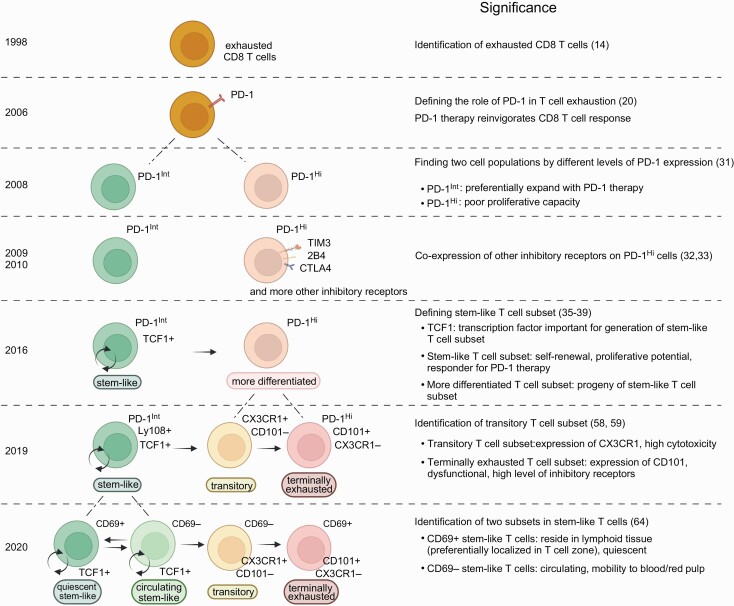
In 1998, Zajac et al. investigated non-transgenic endogenous virus-specific CD8 T-cell responses after chronic LCMV infection using MHC tetramers (14), the method of which was published by Altman et al. in 1996 (15). This study showed that two distinct mechanisms worked to silence anti-viral CD8 T-cell immunity during chronic LCMV infection. Similar to what was observed in the above study conducted by Moskophidis et al. (10), endogenous CD8 T cells specific for one of the dominant LCMV epitopes were deleted within approximately 1 month after infection (14). However, antigen-specific CD8 T cells recognizing another dominant epitope were maintained without reduction (Fig. 1). Importantly, these persisting LCMV-specific MHC-tetramer+ CD8 T cells had impaired cytotoxic activity as well as an impaired ability to produce cytokines upon stimulation (14), indicating that functional impairment of antigen-specific CD8 T cells is an additional mechanism of T-cell exhaustion. This dysfunctional phenotype was more pronounced without CD4 T-cell help and the severity of compromised function was associated with the prolonged presence of high levels of virus (14). This was the first reported evidence of the physical existence of dysfunctional antigen-specific CD8 T cells during chronic infection and these cells are now widely recognized as exhausted T cells (Fig. 1). Furthermore, this study opened novel possibilities to control chronic infection by using these dysfunctional T cells as therapeutic targets.
Fig. 1.
CD8 T-cell heterogeneity during T-cell exhaustion. Key findings about CD8 T-cell heterogeneity during T-cell exhaustion are shown in chronological order. Created with BioRender.com.
After the discovery of exhausted CD8 T cells (14), various approaches have been tested to re-activate these cells to control chronic infection (16–19). In 2006, a highly effective strategy was found to restore the function of exhausted CD8 T cells utilizing PD-1, an inhibitory receptor, in a mouse model of chronic LCMV infection (Fig. 1) (20). This work showed that PD-1 was up-regulated in exhausted CD8 T cells and the blockade of the PD-1 pathway enhanced the quantity and effector function of these cells, leading to better control of LCMV infection (20).
Importantly, this finding was quickly extended to not only other human chronic infections such as human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV) and hepatitis C virus (HCV), but also human cancer (21–25). Since the first U.S. Food and Drug Administration approval of an anti-PD-1 antibody (which blocks PD-1) for melanoma in 2014 (26), PD-1-targeted immunotherapy has been used and proven effective against many different types of human cancer (27, 28). However, not all cancer patients treated with PD-1 blockade show clinical benefit, and thus there is a critical need to improve the efficacy of this therapy. For this purpose, a better understanding of T-cell exhaustion and the mechanism of action of PD-1-targeted immunotherapy is necessary.
Recent studies revealed that multiple subsets among antigen-specific CD8 T cells exist during T-cell exhaustion and play different roles in PD-1-targeted immunotherapy (29, 30). In this review, we will summarize such cell subsets and discuss their features. Since studies on the heterogeneity of antigen-specific CD8 T cells during T-cell exhaustion are most advanced in the setting of chronic infection, we will focus here on T-cell subsets generated after chronic infection.
Discovery of a CD8 T-cell subset that responds to PD-1-targeted therapy during T-cell exhaustion
Although PD-1 blockade had been shown to restore CD8 T-cell responses during chronic infection (20), it was unclear whether PD-1-expressing antigen-specific CD8 T cells uniformly responded to the therapy or whether the T cells that proliferated after PD-1 blockade arose from a subpopulation of antigen-specific CD8 T cells.
One of the earlier studies addressed this question and showed that antigen-specific CD8 T cells contained a cell population that preferentially responds to PD-1-targeted immunotherapy (31). This work found that, during chronic LCMV infection, antigen-specific CD8 T cells that expressed intermediate levels of PD-1 (PD-1Int) were better rescued by PD-1 blockade compared to counterparts with high levels of PD-1 expression (PD-1Hi) (Fig. 1) (31). Subsequent studies further characterized these PD-1Int and PD-1Hi cell populations by examining other inhibitory receptors (32, 33). PD-1Hi CD8 T cells expressed higher levels of multiple inhibitory receptors such as 2B4, LAG-3, CD160 and TIM3 than did PD-1Int CD8 T cells in mice chronically infected with LCMV (Fig. 1) (32, 33). Co-expression of multiple inhibitory receptors on PD-1Hi CD8 T cells was associated with more severe dysfunction regarding proliferation and production of cytokines (32, 33). In addition to inhibitory receptors, Paley et al. demonstrated qualitative differences in antigen-specific CD8 T cells marked by expression of transcription factors T-bet and Eomes (34). T-betHi EomesLow CD8 T cells were relatively functional and expressed intermediate levels of PD-1 whereas T-betLow EomesHi CD8 T cells showed a dysfunctional phenotype with high PD-1 expression and were the progeny of T-betHi EomesLow CD8 T cells (34). Thus, these earlier studies clearly established that the antigen-specific CD8 T cells generated during chronic infection were functionally and phenotypically heterogeneous.
In 2016, multiple groups reported more detailed characteristics of antigen-specific CD8 T-cell heterogeneity during chronic infection (35–39). They found two distinct antigen-specific CD8 T-cell subsets that can be identified by TIM3 and TCF1 expression: TCF1+ Tim3– and TCF1– Tim3+ cell subsets (Fig. 1). The TCF1+ Tim3– cell subset had the ability to self-renew as well as differentiate into the other subset (TCF1– Tim3+), and was essential for maintaining the size of the antigen-specific CD8 T-cell population during chronic infection (Fig. 1) (35–39). Thus, preventing generation of this subset by knocking out TCF1 showed a striking loss of antigen-specific CD8 T cells over time after infection (35, 37, 38).
PD-1 was expressed on both subsets but its expression on the TCF1+ TIM3– subset was lower than that on the TCF1– Tim3+ subset. PD-1 blockade induced substantial proliferation of the TCF1+ Tim3– cell subset and increased the differentiation of this subset into the TCF1– Tim3+ subset. On the other hand, PD-1 blockade had minimal effect on the TCF1– Tim3+ subset because this subset exhibited a more severe dysfunctional phenotype characterized by high expression of multiple inhibitory receptors, impaired proliferative potential and poor cytokine production (Fig. 1).
Therefore, these studies demonstrated that the TCF1+ Tim3– cell subset plays an essential role in antigen-specific CD8 T-cell responses as well as PD-1-targeted immunotherapy during chronic infection (35–39). After identification of the TCF1+ Tim3– cell subset in a mouse model of chronic infection, a similar subset was found in virus-specific CD8 T cells during chronic human viral infection (38, 40–42). Although tumor-infiltrating CD8 T cells contain not only tumor-specific T cells but also other antigen-specific T cells recognizing non-tumor epitopes, for example, virus-specific T cells, earlier studies observed the TCF1+ Tim3– cell subset in human cancer (43–48). Furthermore, more recent studies demonstrated the presence of the TCF1+ Tim3– cell subset in tumor-specific CD8 T cells obtained from tumor-infiltrating lymphocytes (49, 50).
The TCF1+ TIM3– antigen-specific CD8 T cell subset during chronic infection is referred to by various names in published papers. Although the name ‘memory-like T cells’ is sometimes used in literature to describe TCF1+ TIM3– antigen-specific CD8 T cells (37, 51), the overall transcriptional and epigenetic signature of this subset is very different from that of memory T cells generated during acute infection (52–55). The name ‘progenitor of exhausted T cells’ or ‘precursor of exhausted T cells’ is also often used because this subset can give rise to TIM3+ T cells with an exhausted T-cell phenotype (5, 56). In addition to this differentiation capability into TIM3+ cells, the TCF1+ TIM3– antigen-specific CD8 T cells have one more important ability: self-renewal (35). Because the properties of self-renewal and generation of differentiated daughter cells are typical characteristics of stem cells, the TCF1+ TIM3– cell subset is also referred to as ‘stem-like T cells’ (2, 35). The stemness of the TCF1+ TIM3– T cell subset is a critical feature for maintaining the antigen-specific CD8 T-cell population during chronic infection. Therefore, in this review, this cell subset will henceforth be referred to as ‘stem-like’.
Another unique phenotype of stem-like CD8 T cells is expression of CXCR5 and their localization in secondary lymphoid tissues (35, 36). Because of a similar phenotype to follicular helper CD4 T cells, some papers refer to these cells as follicular CD8 T cells (36, 38, 40, 41, 57). However, quantitative analysis by microscopy showed that stem-like CD8 T cells were predominantly (over 50%) localized in the T-cell zone, whereas less than 30% of stem-like CD8 T cells were detected in the B-cell zone during chronic LCMV infection (35). Therefore, the name ‘follicular CD8 T cell’ does not appropriately represent the entire scope of stem-like CD8 T cells in LCMV-infected mice.
On the other hand, during HIV and SIV infection, virus-specific CXCR5+ CD8 T cells were detected more in the B-cell follicular zone than in the extrafollicular region (38, 40, 41, 57). This discrepancy between LCMV infection and HIV/SIV infection may be explained by differences in the cells that the virus targets for infection. LCMV rarely infects CD4 T cells, but CD4 T cells are the primary target of HIV and SIV. CXCR5+ CD8 T cells may migrate to the B-cell follicular zone to control HIV-infected or SIV-infected follicular helper CD4 T cells. Thus, stem-like CD8 T cells may change their localization in a manner dependent on antigen distribution, and it is important to investigate the localization of stem-like CD8 T cells in individual settings, for example, cancer and other chronic infections.
Identification of a transitory CD8 T-cell subset that has the ability to control infection and is generated from stem-like T cells during chronic infection
It was reported that TCF1– TIM3+ CD8 T cells generated from stem-like T cells can be divided into two subpopulations by the expression of the glycoprotein CD101 and the chemokine receptor CX3CR1: transitory T cells (CX3CR1+ CD101–) and terminally exhausted T cells (CX3CR1– CD101+) (Fig. 1) (58, 59). Transitory CD8 T cells were shown to be a proliferating cell subset generated from stem-like CD8 T cells. These transitory cells expressed high levels of effector molecules and had anti-viral functions to prevent excessive viral growth (58, 59). In contrast, CX3CR1– CD101+ terminally exhausted CD8 T cells showed impaired effector functions and rarely proliferated, and were progeny of transitory CD8 T cells (59).
There are at least two identified factors either one of which can result in the differentiation of stem-like CD8 T cells into CX3CR1+ transitory CD8 T cells. Zander et al. demonstrated that the generation of CX3CR1+ transitory CD8 T cells was dependent on IL-21 produced from antigen-specific CD4 T cells (58). These results are consistent with previous findings that IL-21 secreted by antigen-specific CD4 T cells improves effector function of antigen-specific CD8 T cells in controlling viral infection in chronically infected mice (60–63). In addition to CD4 T-cell help, another way to promote the generation of transitory CD8 T cells is anti-PD-1 therapy. PD-1 blockade substantially increased the number of CX3CR1+ transitory CD8 T cells even without CD4 T-cell involvement (58, 59). This indicates that the quantity of transitory CD8 T cells generated by PD-1 blockade is one of the key parameters for the success of this immunotherapy since these cells have the highest effector functions among antigen-specific CD8 T cells during T-cell exhaustion.
One more subset and lineage relationship during T-cell exhaustion
Beltra et al. further investigated antigen-specific CD8 T-cell responses during chronic viral infection (64). Since Ly108 is a surrogate marker of TCF1 (58, 65), stem-like T cells were distinguished from transitory and terminally exhausted T cells by Ly108 expression. The authors found that Ly108+ stem-like CD8 T cells could be divided into two subsets: CD69+ and CD69– stem-like T cells (Fig. 1) (64).
A series of experiments including phenotypic and transcriptional analyses as well as adoptive transfer of each cell subset revealed that CD69+ stem-like CD8 T cells had features of quiescent progenitors and gave rise to CD69– stem-like T cells that further differentiated into transitory CD8 T cells (Fig. 1) (64). Importantly, CD69– stem-like CD8 T cells were able to de-differentiate into the progenitors (CD69+ stem-like T cells) (64). Since CD69 promotes lymphocyte retention in lymphoid tissue (66), CD69+ stem-like T cells were not detected in the blood and were predominantly localized in the white pulp of spleen (64). Such residency of CD69+ stem-like T cells was independently confirmed by Im et al. in parabiosis experiments (67). In contrast, a portion of CD69– stem-like T cells was localized in the blood-accessible splenic red pulp, and these cells were able to circulate in the blood (64).
Beltra et al. also showed that the formation of CD69– stem-like CD8 T cells as well as transitory CD8 T cells was promoted by CD4 T-cell help or PD-1 blockade (64). It is currently unclear which stem-like CD8 T cells will be the primary target of anti-PD-1 therapy to induce proliferation of antigen-specific CD8 T cells during chronic infection. However, it appears that at least CD69+ stem-like T cells can respond to PD-1 blockade, since PD-1 blockade increases antigen-specific CD8 T cells even in CD4 T-cell-depleted chronically infected mice in which CD69+ stem-like CD8 T cells are predominant (35, 59, 64).
Toward improving PD-1-targeted immunotherapy
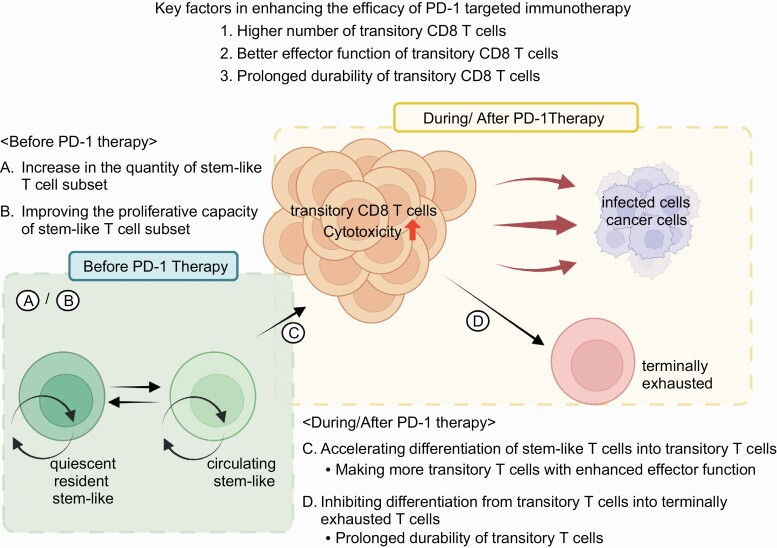
Stem-like CD8 T cells have been found among tumor-infiltrating lymphocytes and findings about the heterogeneity of antigen-specific CD8 T cells from chronic-infection studies can be applied to human cancer (43–48, 50). There are multiple possible ways to improve PD-1-targeted immunotherapy but we would like to focus here on the CD8 T cells. The key factors that should be manipulated to enhance the effectiveness of PD-1 blockade are the quantity, durability and functionality of transitory cells (Fig. 2) that are induced by the treatment since these are the main cells for targeting and destroying infected cells and tumor cells as discussed above.
Fig. 2.
Potential strategies to improve the efficacy of PD-1-targeted immunotherapy. Three key factors that should be manipulated to enhance the efficacy of PD-1 blockade are (1) number, (2) effector function and (3) durability. To manipulate these factors, four potential strategies are proposed. (A and B) Increase in the quantity (A) and/or improve the proliferative capacity (B) of the stem-like T-cell subset before PD-1 therapy. These should result in the generation of a higher number of transitory CD8 T cells after PD-1 blockade. (C) Accelerating the differentiation of stem-like T cells into transitory T cells during PD-1 blockade. This may be achieved by combination therapy and induces more transitory T cells with enhanced effector function. (D) Inhibiting differentiation from transitory T cells into terminally exhausted T cells during and/or after PD-1 therapy. This approach will prolong the durability of transitory T cells. Created with BioRender.com.
How can we augment the number of transitory CD8 T cells after PD-1 therapy? One important way is to enhance the quantity of available stem-like CD8 T cells (Fig. 2A). Since the cells that proliferate and differentiate into transitory CD8 T cells after PD-1 blockade are primarily stem-like T cells (35, 59, 64), an increase in stem-like T cells should result in robust induction of transitory T cells after anti-PD-1 therapy.
In addition to the quantity, improving the quality of stem-like CD8 T cells, specifically their proliferative capacity, may result in a greater number of transitory T cells (Fig. 2B). The proliferative capacity of stem-like CD8 T cells is superior to that of transitory and terminally exhausted T cells but inferior to that of memory CD8 T cells generated during acute infection (64). If the proliferative capacity of stem-like CD8 T cells can be increased to the same extent as that of memory T cells, PD-1 blockade should yield more transitory CD8 T cells (Fig. 2B). To develop methods to improve the quantity and quality of stem-like CD8 T cells, further studies are required to gain a better understanding of the generation and maintenance of these cells.
In addition to manipulating stem-like CD8 T cells, the process of T-cell differentiation can be targeted to enhance the efficacy of PD-1 blockade. Differentiation of stem-like T cells into transitory T cells occurs in the steady state but PD-1 blockade accelerates this process, leading to the formation of a high number of transitory T cells (35, 59, 64). Further stimulation of this differentiation process during PD-1 blockade would be expected to increase the amount of transitory T cells even more (Fig. 2C). To this end, combination therapy may be a useful approach since a substantial increase in antigen-specific CD8 T cells was achieved after PD-1 combination therapy including IL-2, adoptive transfer of antigen-specific CD4 T cells, Treg cell depletion and blocking other inhibitory receptors (27, 68–72). Furthermore, it will be more ideal if such combination therapy improves the quality of transitory T cells compared with PD-1 blockade monotherapy (Fig. 2C). One of the essential qualitative features of transitory T cells is their effector function such as cytotoxic activity and cytokine production (58, 59, 64). Enhancing the effector function of transitory T cells may be beneficial for controlling tumor growth (Fig. 2C).
Another important quality is the durability of transitory T cells. Transitory T cells gradually lose their effector functions and differentiate into terminally exhausted T cells (59, 64). If combination therapy promotes the generation of more durable transitory T cells, the therapeutic effect would be improved. It is also possible to prolong the durability of transitory T cells by inhibiting their differentiation into terminally exhausted T cells (Fig. 2D). To establish the methods to improve the quality of transitory T cells, it will be important to elucidate the molecular and cellular mechanisms that regulate the differentiation of stem-like T cells into transitory T cells and terminally exhausted T cells.
Conclusions
It is now clear that antigen-specific CD8 T cells during chronic viral infection are heterogeneous and can be distinguished into at least four subsets: CD69+ quiescent stem-like T cells, CD69– circulating stem-like T cells, transitory T cells and terminally exhausted T cells. The lineage relationship among these subsets has been clarified and also great progress has been made in understanding how PD-1 blockade modulates each subset. In this review, we propose potential approaches that target antigen-specific CD8 T cells to improve the effect of PD-1 blockade on the basis of knowledge obtained from chronic infection studies. However, a further understanding of tumor-specific CD8 T cells may be needed to apply such approaches to cancer patients. As we discussed, individual pathogens target different types of cells and tissues, so the localization of pathogen-specific stem-like CD8 T cells can vary with different pathogens. Since the differences in the microenvironment between chronic infection and tumors are greater than those between individual chronic infections, the tumor microenvironment may influence the generation, differentiation, maintenance and localization of tumor-specific CD8 T cells, potentially resulting in unique features of T-cell exhaustion in cancer patients. Such tumor-specific features may represent new therapeutic targets that enhance PD-1 therapy. Thus, it will be crucial to further investigate the similarities and differences in antigen-specific CD8 T cells of chronic infection and tumors.
Acknowledgements
We thank Charles Perkins for his critical reading of the manuscript.
Contributor Information
Satomi Ando, Division of Infectious Diseases, Center for Inflammation and Tolerance, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH 45229, USA.
Koichi Araki, Division of Infectious Diseases, Center for Inflammation and Tolerance, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH 45229, USA.
Funding
National Institutes of Health (grant R01AI139675 to K.A.) and start-up funding from Cincinnati Children’s Hospital Medical Center (to K.A.).
Conflicts of interest statement: the authors declare no conflicts of interest.
References
- 1. Martin, M. D. and Badovinac, V. P. 2018. Defining memory CD8 T cell. Front. Immunol. 9:2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hashimoto, M., Kamphorst, A. O., Im, S. J.et al. . 2018. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med. 69:301. [DOI] [PubMed] [Google Scholar]
- 3. van der Leun, A. M., Thommen, D. S. and Schumacher, T. N. 2020. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat. Rev. Cancer 20:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reina-Campos, M., Scharping, N. E. and Goldrath, A. W. 2021. CD8(+) T cell metabolism in infection and cancer. Nat. Rev. Immunol. 21:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLane, L. M., Abdel-Hakeem, M. S. and Wherry, E. J. 2019. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37:457. [DOI] [PubMed] [Google Scholar]
- 6. Ahmed, R., Salmi, A., Butler, L. D.et al. . 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed, R., Simon, R. S., Matloubian, M.et al. . 1988. Genetic analysis of in vivo-selected viral variants causing chronic infection: importance of mutation in the L RNA segment of lymphocytic choriomeningitis virus. J. Virol. 62:3301. doi: 10.1128/JVI.62.9.3301-3308.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed, R. and Oldstone, M. B. 1988. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167:1719. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salvato, M., Borrow, P., Shimomaye, E. and Oldstone, M. B. 1991. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J. Virol. 65:1863. doi: 10.1128/JVI.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moskophidis, D., Lechner, F., Pircher, H. and Zinkernagel, R. M. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 11. Doherty, P. C. 1993. Immune exhaustion: driving virus-specific CD8+ T cells to death. Trends Microbiol. 1:207. doi: 10.1016/0966-842x(93)90133-c. [DOI] [PubMed] [Google Scholar]
- 12. Pantaleo, G., Soudeyns, H., Demarest, J. F.et al. . 1997. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc. Natl. Acad. Sci. U.S.A. 94:9848. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallimore, A., Glithero, A., Godkin, A.et al. . 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zajac, A. J., Blattman, J. N., Murali-Krishna, K.et al. . 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altman, J. D., Moss, P. A., Goulder, P. J.et al. . 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 16. Cramp, M. E., Rossol, S., Chokshi, S.et al. . 2000. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology 118:346. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 17. Blattman, J. N., Grayson, J. M., Wherry, E. J.et al. . 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9:540. [DOI] [PubMed] [Google Scholar]
- 18. Lu, W., Arraes, L. C., Ferreira, W. T. and Andrieu, J. M. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359. [DOI] [PubMed] [Google Scholar]
- 19. Wherry, E. J., Blattman, J. N. and Ahmed, R. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barber, D. L., Wherry, E. J., Masopust, D.et al. . 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682. [DOI] [PubMed] [Google Scholar]
- 21. Day, C. L., Kaufmann, D. E., Kiepiela, P.et al. . 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350. [DOI] [PubMed] [Google Scholar]
- 22. Radziewicz, H., Ibegbu, C. C., Fernandez, M. L.et al. . 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Velu, V., Titanji, K., Zhu, B.et al. . 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Topalian, S. L., Hodi, F. S., Brahmer, J. R.et al. . 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brahmer, J. R., Tykodi, S. S., Chow, L. Q.et al. . 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366:2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sunshine, J. and Taube, J. M. 2015. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 23:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribas, A. and Wolchok, J. D. 2018. Cancer immunotherapy using checkpoint blockade. Science 359:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaddepally, R. K., Kharel, P., Pandey, R.et al. . 2020. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 12:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chu, T. and Zehn, D. 2020. Charting the roadmap of T cell exhaustion. Immunity 52:724. doi: 10.1016/j.immuni.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 30. Im, S. J. and Ha, S. J. 2020. Re-defining T-cell exhaustion: subset, function, and regulation. Immune Netw. 20:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blackburn, S. D., Shin, H., Freeman, G. J. and Wherry, E. J. 2008. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl. Acad. Sci. U.S.A. 105:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blackburn, S. D., Shin, H., Haining, W. N.et al. . 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin, H. T., Anderson, A. C., Tan, W. G.et al. . 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 107:14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paley, M. A., Kroy, D. C., Odorizzi, P. M.et al. . 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Im, S. J., Hashimoto, M., Gerner, M. Y.et al. . 2016. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He, R., Hou, S., Liu, C.et al. . 2016. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 537:412. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 37. Utzschneider, D. T., Charmoy, M., Chennupati, V.et al. . 2016. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 45:415. [DOI] [PubMed] [Google Scholar]
- 38. Leong, Y. A., Chen, Y., Ong, H. S.et al. . 2016. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 17:1187. [DOI] [PubMed] [Google Scholar]
- 39. Wu, T., Ji, Y., Moseman, E. A.et al. . 2016. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1:eaai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miles, B., Miller, S. M., Folkvord, J. M.et al. . 2016. Follicular regulatory CD8 T cells impair the germinal center response in SIV and ex vivo HIV infection. PLoS Pathog. 12:e1005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petrovas, C., Ferrando-Martinez, S., Gerner, M. Y.et al. . 2017. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci. Transl. Med. 9:eaag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang, H., Li, L., Han, J.et al. . 2017. CXCR5(+) CD8(+) T cells indirectly offer B cell help and are inversely correlated with viral load in chronic hepatitis B infection. DNA Cell Biol. 36:321. doi: 10.1089/dna.2016.3571. [DOI] [PubMed] [Google Scholar]
- 43. Brummelman, J., Mazza, E. M. C., Alvisi, G.et al. . 2018. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J. Exp. Med. 215:2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gettinger, S. N., Choi, J., Mani, N.et al. . 2018. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat. Commun. 9:3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sade-Feldman, M., Yizhak, K., Bjorgaard, S. L.et al. . 2018. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siddiqui, I., Schaeuble, K., Chennupati, V.et al. . 2019. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50:195. [DOI] [PubMed] [Google Scholar]
- 47. Miller, B. C., Sen, D. R., Al Abosy, R.et al. . 2019. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jansen, C. S., Prokhnevska, N., Master, V. A.et al. . 2019. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oliveira, G., Stromhaug, K., Klaeger, S.et al. . 2021. Phenotype, specificity and avidity of antitumour CD8(+) T cells in melanoma. Nature 596:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eberhardt, C. S., Kissick, H. T., Patel, M. R.et al. . 2021. Functional HPV-specific PD-1(+) stem-like CD8 T cells in head and neck cancer. Nature 597:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Snell, L. M., MacLeod, B. L., Law, J. C.et al. . 2018. CD8(+) T cell priming in established chronic viral infection preferentially directs differentiation of memory-like cells for sustained immunity. Immunity 49:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ghoneim, H. E., Fan, Y., Moustaki, A.et al. . 2017. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jadhav, R. R., Im, S. J., Hu, B.et al. . 2019. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc. Natl. Acad. Sci. U.S.A. 116:14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abdel-Hakeem, M. S., Manne, S., Beltra, J. C.et al. . 2021. Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation. Nat. Immunol. 22:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yates, K. B., Tonnerre, P., Martin, G. E.et al. . 2021. Epigenetic scars of CD8(+) T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. 22:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kallies, A., Zehn, D. and Utzschneider, D. T. 2020. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 20:128. [DOI] [PubMed] [Google Scholar]
- 57. Mylvaganam, G. H., Rios, D., Abdelaal, H. M.et al. . 2017. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc. Natl. Acad. Sci. U.S.A. 114:1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zander, R., Schauder, D., Xin, G.et al. . 2019. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity 51:1028. doi: 10.1016/j.immuni.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hudson, W. H., Gensheimer, J., Hashimoto, M.et al. . 2019. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity 51:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Elsaesser, H., Sauer, K. and Brooks, D. G. 2009. IL-21 is required to control chronic viral infection. Science 324:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Frohlich, A., Kisielow, J., Schmitz, I.et al. . 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576. [DOI] [PubMed] [Google Scholar]
- 62. Yi, J. S., Du, M. and Zajac, A. J. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xin, G., Schauder, D. M., Lainez, B.et al. . 2015. A critical role of IL-21-induced BATF in sustaining CD8-T-cell-mediated chronic viral control. Cell Rep. 13:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beltra, J. C., Manne, S., Abdel-Hakeem, M. S.et al. . 2020. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen, Z., Ji, Z., Ngiow, S. F.et al. . 2019. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shiow, L. R., Rosen, D. B., Brdickova, N.et al. . 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540. [DOI] [PubMed] [Google Scholar]
- 67. Im, S. J., Konieczny, B. T., Hudson, W. H.et al. . 2020. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc. Natl. Acad. Sci. U.S.A. 117:4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aubert, R. D., Kamphorst, A. O., Sarkar, S.et al. . 2011. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 108:21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. West, E. E., Jin, H. T., Rasheed, A. U.et al. . 2013. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123:2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Penaloza-MacMaster, P., Kamphorst, A. O., Wieland, A.et al. . 2014. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 211:1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Galon, J. and Bruni, D. 2019. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18:197. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 72. Ren, Z., Zhang, A., Sun, Z.et al. . 2022. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. J. Clin. Invest. 132:e153604. [DOI] [PMC free article] [PubMed] [Google Scholar]