Abstract
The Central Brain Tumor Registry of the United States (CBTRUS), in collaboration with the Centers for Disease Control and Prevention and the National Cancer Institute, is the largest population-based registry focused exclusively on primary brain and other central nervous system (CNS) tumors in the United States (US) and represents the entire US population. This report contains the most up-to-date population-based data on primary brain tumors available and supersedes all previous reports in terms of completeness and accuracy. All rates are age-adjusted using the 2000 US standard population and presented per 100,000 population. The average annual age-adjusted incidence rate (AAAIR) of all malignant and non-malignant brain and other CNS tumors was 24.71 per 100,000 population (malignant AAAIR=7.02 and non-malignant AAAIR=17.69). This overall rate was higher in females compared to males (27.62 versus 21.60 per 100,000) and non-Hispanic persons compared to Hispanic persons (25.09 versus 22.95 per 100,000). The most commonly occurring malignant brain and other CNS histopathology was glioblastoma (14.2% of all tumors and 50.1% of all malignant tumors), and the most common non-malignant histopathology was meningioma (39.7% of all tumors and 55.4% of all non-malignant tumors). Glioblastoma was more common in males, and meningiomas were more common in females. In children and adolescents (ages 0-19 years), the incidence rate of all primary brain and other CNS tumors was 6.20 per 100,000 population. An estimated 93,470 new cases of malignant and non-malignant brain and other CNS tumors are expected to be diagnosed in the US population in 2022 (26,670 malignant and 66,806 non-malignant). There were 84,264 deaths attributed to malignant brain and other CNS tumors between 2015 and 2019. This represents an average annual mortality rate of 4.41 per 100,000 population and an average of 16,853 deaths per year. The five-year relative survival rate following diagnosis of a malignant brain and other CNS tumor was 35.7%, while for non-malignant brain and other CNS tumors the five-year relative survival rate was 91.8%.
EXECUTIVE SUMMARY
The Central Brain Tumor Registry of the United States (CBTRUS), in collaboration with the Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI), is the largest population-based registry focused exclusively on primary brain and other central nervous system (CNS) tumors in the United States (US) and represents the entire US population. The CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019 contains the most up-to-date population-based data on primary brain tumors available through the surveillance system in the United States and supersedes all previous reports in terms of completeness and accuracy, thereby providing a current comprehensive source for the descriptive epidemiology of these tumors. All rates are age-adjusted using the 2000 US standard population and presented per 100,000 population.
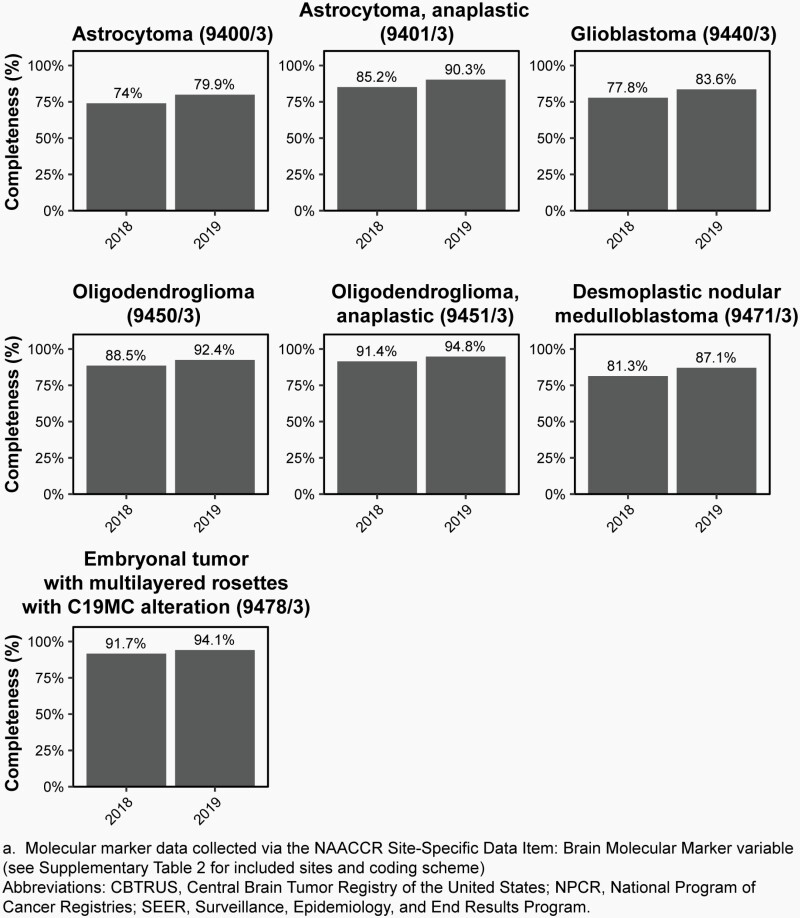
New to the CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019: This is the first CBTRUS report to present incidence rates for selected molecularly-defined brain and other CNS tumor histopathologies for diagnoses in 2018-2019. Completeness of data on selected brain molecular markers (BMM) has improved from 2018 to 2019.
Incidence
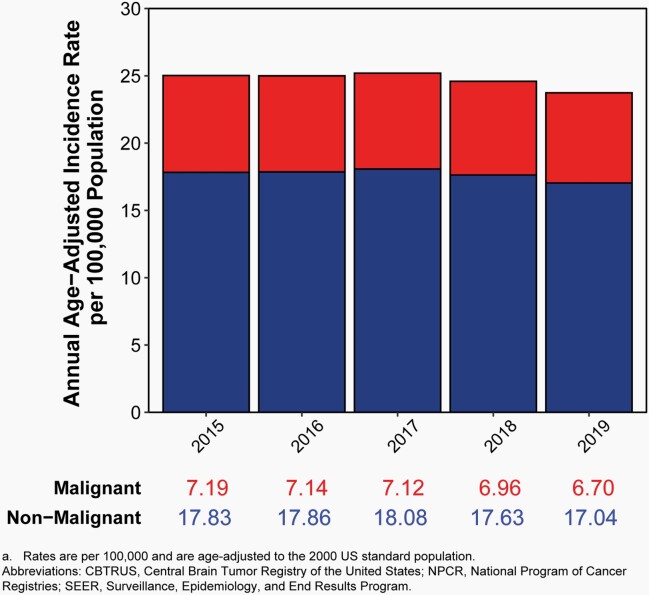
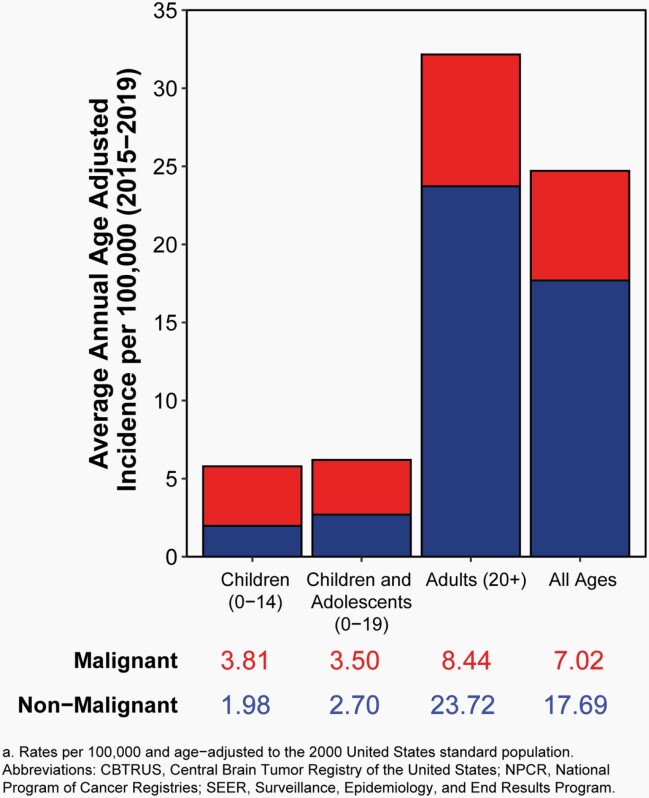
The average annual age-adjusted incidence rate (AAAIR) of all malignant and non-malignant brain and other CNS tumors was 24.71 per 100,000 population between 2015 and 2019. The AAAIR of malignant brain and other CNS tumors was 7.02 per 100,000 population, and the AAAIR of non-malignant brain and other CNS tumors was 17.69 per 100,000 population.
There have been no substantial changes in incidence of malignant brain tumors, with the exception of a slight, but significant, increase in the youngest age group (0-14 years).
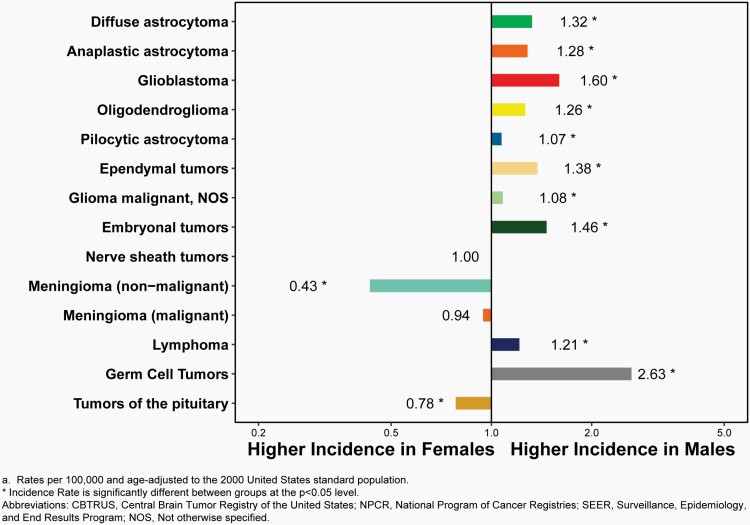
The overall incidence rate was higher in females compared to males (27.62 versus 21.60 per 100,000) and non-Hispanic persons (of any race) compared to Hispanic persons (25.09 versus 22.95 per 100,000).
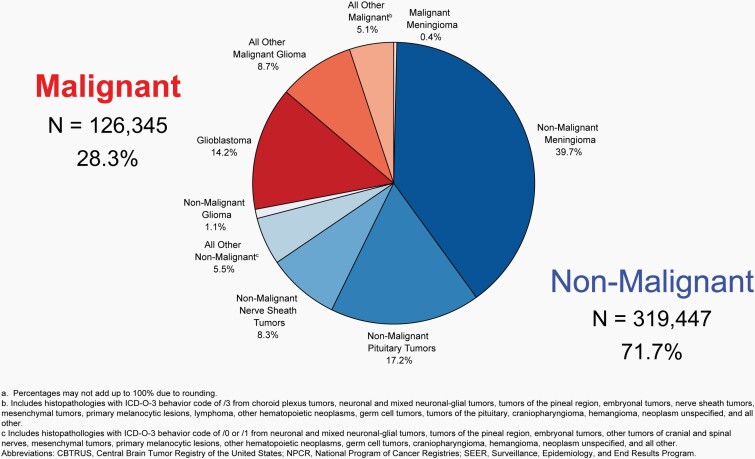
Approximately 28.3% of all brain and other CNS tumors were malignant and 71.7% were non-malignant, which makes non-malignant tumors more than twice as common as malignant tumors for the first time.
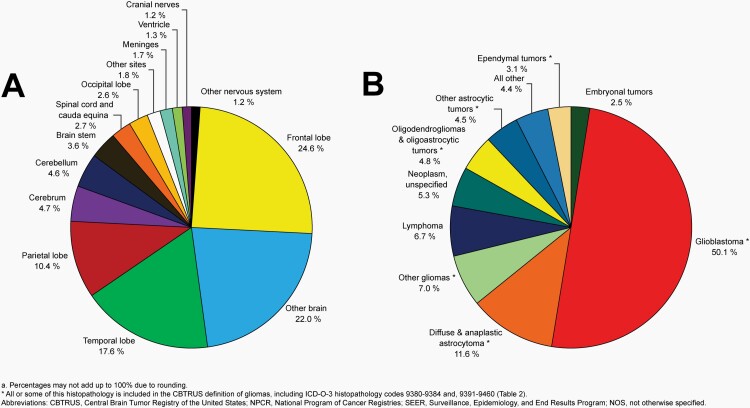
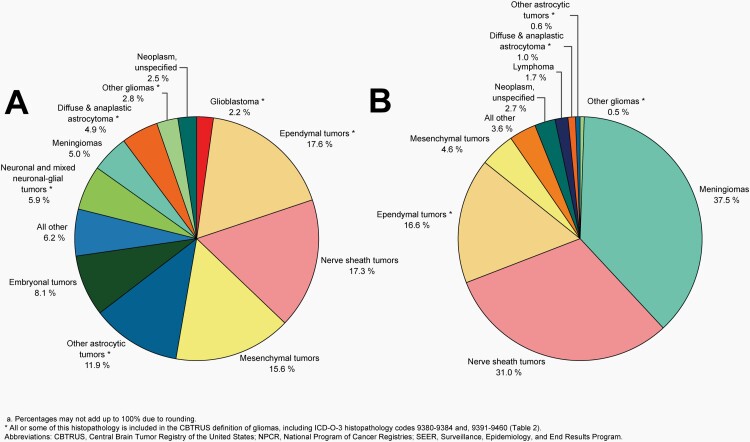
The most commonly occurring malignant brain and other CNS tumor histopathology was glioblastoma (14.2% of all tumors and 50.1% of all malignant tumors), and the most common non-malignant histopathology was meningioma (39.7% of all tumors and 55.4% of all non-malignant tumors). Glioblastoma was more common in males, and meningiomas were more common in females.
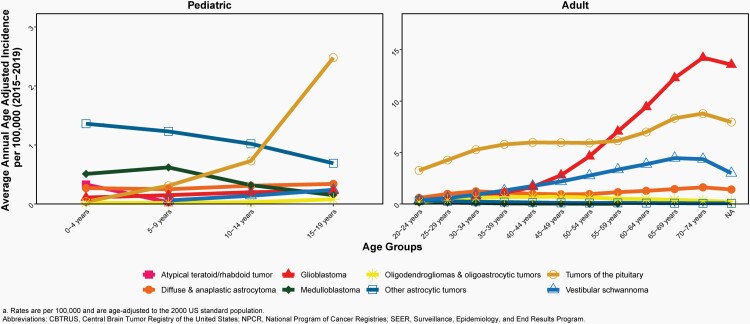
In children and adolescents (ages 0-19 years), the AAAIR of malignant and non-malignant brain and other CNS tumors was 6.20 per 100,000 population between 2015 and 2019.
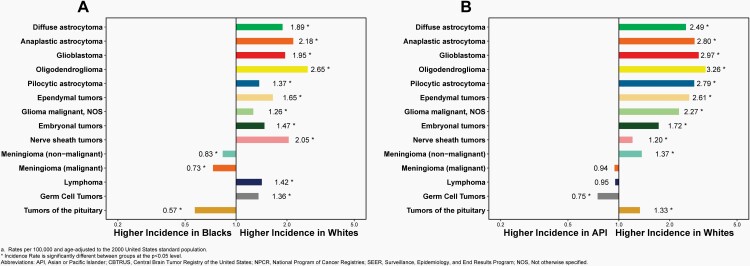
In children and adolescents (ages 0-19 years), incidence was higher in females compared to males (6.29 versus 6.10 per 100,000), White persons compared to Black persons (6.39 versus 4.89 per 100,000), and non-Hispanic persons compared to Hispanic persons (6.44 versus 5.47 per 100,000).
An estimated 93,470 new cases of malignant and non-malignant brain and other CNS tumors are expected to be diagnosed in the United States in 2022. This includes an expected 26,670 malignant and 66,800 non-malignant tumors.
Mortality
There were 84,264 deaths attributed to malignant brain and other CNS tumors between 2015 and 2019. This represents an average annual mortality rate of 4.41 per 100,000 population and an average of 16,853 deaths per year caused by malignant brain and other CNS tumors.
Survival
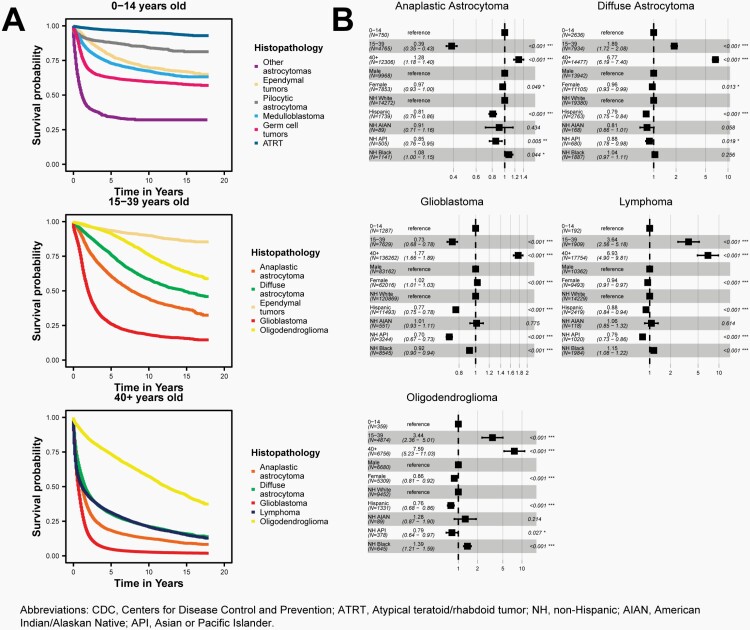
The five-year relative survival rate following diagnosis of a malignant brain and other CNS tumor was 35.7%. Survival following diagnosis with a malignant brain and other CNS tumor was highest in persons ages 0-14 years (75.1%) and ages 15-39 years (71.7%) as compared to those ages 40+ years (21.0%).
The five-year relative survival rate following diagnosis of a non-malignant brain and other CNS tumor was 91.8%. Survival following diagnosis with a non-malignant brain and other CNS tumor was highest in persons ages 15-39 years (98.3%) and ages 0-14 years (97.6%) as compared to those ages 40+ years (90.3%).
Introduction
The objective of the CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019 is to provide a comprehensive summary of the current descriptive epidemiology of primary brain and other CNS tumors in the US population. Primary brain and other CNS tumors include those tumors that originate from the tissues of the brain or CNS. CBTRUS obtained the latest available population-based data on all the reported newly diagnosed primary brain and other CNS tumors from the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR), and the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) Program for diagnosis years 2015-2019. Incidence counts and rates of primary malignant and non-malignant brain and other CNS tumors are presented by histopathology, sex, age, race, Hispanic ethnicity, and geographic location. Mortality rates calculated using the National Center for Health Statistics’ (NCHS) National Vital Statistics System (NVSS) data from 2015-2019, and relative survival rates, median survival, and adjusted hazard ratios for selected malignant and non-malignant histopathologies calculated using NPCR data for the period 2001-2018 (2004-2018 for non-malignant tumors) are also presented.
Background
CBTRUS is a unique professional research organization that focuses exclusively on providing high-quality statistical data on the population-based incidence of primary brain and other CNS tumors in the United States (for more information on CBTRUS see: http://www.cbtrus.org/about/).1 CBTRUS was incorporated as a nonprofit 501(c)(3) in 1992 following a study conducted by the American Brain Tumor Association (ABTA) to determine the feasibility of a population-based central registry focused on all reported primary brain and other CNS tumors in the United States.
This report represents the thirtieth (30 th ) anniversary of CBTRUS and the twenty-fifth (25 th ) statistical report published by CBTRUS. For this eleventh (11th) report published as a Supplement to Neuro-Oncology, the official journal of the Society for Neuro-Oncology (http://www.soc-neuro-onc.org), CBTRUS continues its past efforts to provide the most up-to-date population-based incidence rates for all reported newly-diagnosed primary brain and other CNS tumors by behavior (malignant and non-malignant), histopathology, age, sex, race, Hispanic ethnicity, selected (BMM), and geographic location. These data have been organized by clinically relevant histopathology groupings that reflect the 2016 World Health Organization (WHO) Classification of Tumours of the Central Nervous System, including selected molecularly-defined histopathologies beginning in diagnosis year 2018.2,3 These data provide important information for allocation and planning of specialty healthcare services such as clinical trials, disease prevention and control programs, and research activities. These data may also stimulate research into the causes of this group of diseases, which often result in significant morbidity and mortality.
CBTRUS is currently the only population-based site-specific registry in the United States that works in partnership with a public cancer surveillance organization, the CDC’s NPCR, and from which data are directly received through the NPCR Cancer Surveillance System (NPCR-CSS) Submission Specifications mechanism4 under a special agreement. Collection of central (state) cancer data was mandated in 1992 by Public Law 102-515, the Cancer Registries Amendment Act.5 This mandate was expanded to include non-malignant CNS tumors with the 2002 passage of Public Law 107–260, starting January 1, 2004.6 CBTRUS combines the NPCR data with data from the NCI’s SEER Program,7 which was established for national cancer surveillance in the early 1970s. All data from NPCR and SEER originate from tumor registrars who adhere to the Uniform Data Standards (UDS) for malignant and non-malignant brain and other CNS tumors as directed by the North American Association of Central Cancer Registries (NAACCR) (http://www.naaccr.org). Along with the UDS, there are quality control checks and a system for rating each central cancer registry (CCR) to ensure that these data are as accurate and complete as possible. As a surveillance partner, CBTRUS reports high-quality data on brain and other CNS tumors with histopathological specificity useful to the communities it serves.
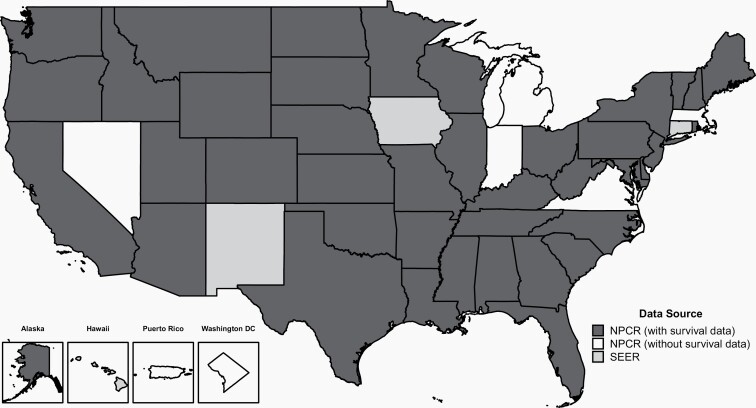
The CBTRUS database is comprised of the largest histopathology-specific aggregation of population-based data limited to the incidence and survival of primary brain and other CNS tumors in the United States, and it is likely the largest histopathology-specific aggregation of primary brain and other CNS tumor cases in the world. The CBTRUS database now includes both survival data from 42 CCRs and incidence data from all 52 CCRs in the United States and Puerto Rico (excluding Nevada cases from diagnosis years 2018-2019). Aggregate information on all cancers from all 52 CCRs (excluding Nevada cases from diagnosis years 2018-2019) in the United States, including primary brain and other CNS tumors, is available from the United States Cancer Statistics (USCS).8
Anatomic Location of Tumor Sites
Various terms are used to describe the regions of the brain and other CNS. The specific sites used in this report are based on the topography codes found in the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) and are broadly based on the categories and site codes defined in the SEER Site/Histology Validation List.9 CBTRUS groups ICD-O-3 sites C71.8 (Overlapping lesion of the brain) and C71.9 (Brain, Not Otherwise Specified [NOS]) into Other brain and C72.8 (Overlapping lesion of brain and CNS) and C72.9 (Nervous system, NOS) into Other nervous system for display in figures. This report also presents counts and incidence for specific sites separately in its tables. See Table 1 for the CBTRUS primary site groupings.
Table 1.
Central Brain Tumor Registry of the United States (CBTRUS), Brain and Other Central Nervous System Tumor Site Groupings
| Site | ICD-O-3a Site Code |
|---|---|
| Olfactory tumors of the nasal cavityb | C30.0 |
| Meninges (cerebral & spinal) | C70.0-C70.9 |
| Cerebral meninges | C70.0 |
| Spinal meninges | C70.1 |
| Meninges, NOS | C70.9 |
| Cerebrum | C71.0 |
| Frontal lobe of brain | C71.1 |
| Temporal lobe of brain | C71.2 |
| Parietal lobe of brain | C71.3 |
| Occipital lobe of brain | C71.4 |
| Ventricle | C71.5 |
| Cerebellum | C71.6 |
| Brain stem | C71.7 |
| Other brainc | C71.8-C71.9 |
| Overlapping lesion of brain | C71.8 |
| Brain, NOS | C71.9 |
| Spinal cord and cauda equine | C72.0-C72.1 |
| Spinal cord | C72.0 |
| Cauda equine | C72.1 |
| Cranial nerves | C72.2-C72.5 |
| Olfactory nerve | C72.2 |
| Optic nerve | C72.3 |
| Acoustic nerve | C72.4 |
| Cranial nerve, NOS | C72.5 |
| Other nervous systemc | C72.8-C72.9 |
| Overlapping lesion of brain and central nervous system | C72.8 |
| Nervous system, NOS | C72.9 |
| Pituitary and craniopharyngeal duct | C75.1-C75.2 |
| Pituitary gland | C75.1 |
| Craniopharyngeal duct | C75.2 |
| Pineal gland | C75.3 |
aInternational Classification of Diseases for Oncology, 3rd Edition, 2000. World Health Organization, Geneva, Switzerland.
bICD-O-3 histopathology codes 9522-9523 only.
cThese ICD-O-3 codes are combined for analysis in figures and tables presented in this report.
Abbreviations: NOS, not otherwise specified.
Classification by Histopathology
There are over 100 distinct types of primary CNS tumors, referred to as ‘histopathologies’, each with its own spectrum of clinical presentations, treatments, and outcomes. These histopathologies are reviewed periodically by neuropathologists and published by the World Health Organization (WHO) in Classification Reports known as “Blue Books.” Blue Books are published for all cancer sites by the WHO and utilize the ICD-O-3 for assignment of histopathology, behavior, and site codes. CBTRUS is using Histopathology Groupings according to 2016 WHO Classification of Tumours of the Central Nervous System.
The ICD-O-3 codes in this current CBTRUS grouping10 (Table 2) may include morphology codes that were not previously reported to CBTRUS.11 Gliomas are tumors that arise from glial or precursor cells and include glioblastoma, astrocytoma, oligodendroglioma, ependymoma, oligoastrocytoma (mixed glioma), and a few rare histopathologies. As there is no standard definition for gliomas, CBTRUS defines gliomas as ICD-O-3 histopathology codes 9380-9384 and 9391-9460 as starred in Table 2. It is also important to note that the statistics for lymphomas and hematopoietic neoplasms contained in this report refer only to those lymphomas and hematopoietic neoplasms that arise in the brain and other CNS ICD-O-3 topography codes.
Table 2.
Central Brain Tumor Registry of the United States (CBTRUS), 2021 Brain and Other Central Nervous System Tumor Histopathology Groupings (Based on 2016 WHO Classification)
| Histopathology | ICD-O-3a Histopathology Codesb | ICD-O-3a Histopathology and Behavior Codeb | |
|---|---|---|---|
| Malignant | Non-Malignant | ||
| Diffuse Astrocytic and Oligodendroglial Tumors | |||
| Diffuse astrocytoma* | 9381, 9400, 9410, 9411, 9420, 9442/1 | 9381/3, 9400/3, 9410/3, 9411/3, 9420/3 | 9442/1 |
| Anaplastic astrocytoma* | 9401 | 9401/3 | None |
| Glioblastoma* | 9440, 9441, 9442/3, 9445c | 9440/3, 9441/3, 9442/3, 9445/3 | None |
| Oligodendroglioma* | 9450 | 9450/3 | None |
| Anaplastic oligodendroglioma* | 9451, 9460 | 9451/3, 9460/3 | None |
| Oligoastrocytic tumors* | 9382 | 9382/3 | None |
| Other Astrocytic Tumors | |||
| Pilocytic astrocytoma* | 9421, 9425c | 9421/1d, 9425/3 | None |
| Unique astrocytoma variants* | 9384, 9424, 9431c | 9424/3 | 9384/1, 9431/1 |
| Ependymal tumors* | 9383, 9391 (excluding site C75.1 for behavior/1), 9392- 9394, 9396c | 9391/3, 9392/3, 9393/3, 9396/3 | 9383/1, 9391/1 (excluding site C75.1), 9394/1 |
| Other Gliomas | |||
| Glioma malignant, NOS* | 9380, 9385c | 9380/3, 9385/3 | None |
| Other neuroepithelial tumors* | 9423, 9430, 9444 | 9423/3, 9430/3 | 9444/1 |
| Neuronal and Mixed Neuronal-Glial Tumors* | 8680, 8681, 8690, 8693, 9412, 9413, 9490, 9492 (excluding site C75.1), 9493, 9505, 9506, 9509c, 9522 (site C30.0 only), 9523 (site C30.0 only) | 8680/3, 8693/3, 9490/3, 9505/3, 9509/3, 9522/3 (site C30.0 only), 9523/3 (site C30.0 only) | 8680/0,1, 8681/1, 8690/1, 8693/1, 9412/1, 9413/0, 9442/1, 9490/0, 9492/0 (excluding site C75.1), 9493/0, 9505/0,1, 9506/1, 9509/1 |
| Choroid Plexus Tumors | 9390 | 9390/3 | 9390/0,1 |
| Tumors of the Pineal Region | 9360, 9361, 9362, 9395c | 9362/3, 9395/3 | 9360/1, 9361/1 |
| Embryonal Tumors | 8963, 9364, 9470-9478c, 9480, 9500, 9501/3, 9502/3, 9508 | 8963/3, 9364/3, 9470/3, 9471/3, 9472/3, 9473/3, 9474/3, 9475/3, 9476/3, 9477/3, 9478/3, 9480/3, 9500/3, 9501/3, 9502/3, 9508/3 | None |
| Medulloblastoma | 9470-9472,9474-9478 | 9470/3, 9471/3, 9472/3,9474/3, 9475/3, 9476/3, 9477/3, 9478/3, | None |
| Atypical teratoid/rhabdoid tumor | 9508 | 9508/3 | None |
| Other embryonal tumorse | 8963, 9364, 9473, 9480, 9500, 9501, 9502 | 8963/3, 9364/3, 9473/3, 9480/3, 9500/3, 9501/3, 9502/3 | None |
| Tumors of Cranial and Paraspinal Nerves | |||
| Nerve sheath tumors | 9540, 9541, 9550, 9560, 9561, 9570, 9571 | 9540/3, 9560/3, 9561/3, 9571/3 | 9540/0,1, 9541/0, 9550/0, 9560/0,1, 9570/0, 9571/0 |
| Other tumors of cranial and paraspinal nerves | 9562, 9563 | None | 9562/0, 9563/0 |
| Tumors of Meninges | |||
| Meningioma | 9530-9535, 9537-9539 | 9530/3, 9538/3, 9539/3 | 9530/0,1, 9531/0, 9532/0, 9533/0, 9534/0, 9535/0, 9537/0, 9538/1, 9539/1 |
| Mesenchymal tumors | 8324, 8710, 8711, 8800-8806, 8810, 8811, 8815, 8821, 8824, 8825, 8830, 8831, 8835, 8836, 8840, 8850-8854, 8857, 8861, 8870, 8880, 8890, 8897, 8900-8902, 8910, 8912, 8920, 8921, 8935, 8990, 9040, 9120, 9125, 9130, 9131, 9136, 9150, 9161, 9170, 9180, 9210, 9220, 9231, 9240, 9241, 9243, 9260, 9370-9373 | 8710/3, 8711/3, 8800/3, 8801/3, 8802/3, 8803/3, 8804/3, 8805/3, 8806/3, 8810/3, 8811/3, 8815/3c, 8825/3, 8830/3, 8840/3, 8850/3, 8851/3, 8852/3, 8853/3, 8854/3, 8857/3, 8890/3, 8900/3, 8901/3, 8902/3, 8910/3, 8912/3, 8920/3, 8921/3, 8935/3, 8990/3, 9040/3, 9120/3, 9130/3, 9150/3, 9170/3, 9180/3, 9220/3, 9231/3, 9240/3, 9243/3, 9260/3, 9370/3, 9371/3, 9372/3 | 8324/0, 8711/0, 8800/0, 8810/0, 8811/0, 8815/0,1c, 8821/1, 8824/0,1, 8825/0,1, 8830/0,1, 8831/0, 8835/1, 8836/1, 8840/0, 8850/0,1, 8851/0, 8852/0, 8854/0, 8857/0, 8861/0, 8870/0, 8880/0, 8890/0,1, 8897/1, 8900/0, 8920/1, 8935/0,1, 8990/0,1, 9040/0, 9120/0, 9125/0, 9130/0,1, 9131/0, 9136/1, 9150/0,1, 9161/0,1, 9170/0, 9180/0, 9210/0, 9220/0, 9241/0, 9373/0 |
| Primary melanocytic lesions | 8720, 8728, 8770 | 8720/3, 8728/3, 8770/3 | 8728/0,1, 8770/0 |
| Other neoplasms related to the meninges | None | None | None |
| Lymphomas and Hematopoietic Neoplasms | |||
| Lymphoma | 9590, 9591, 9596, 9650-9655, 9659, 9661-9665, 9667, 9670, 9671, 9673, 9675, 9680, 9684, 9687, 9688, 9690, 9691, 9695, 9698, 9699, 9701, 9702, 9705, 9712, 9714, 9715, 9719, 9724, 9727-9729, 9735, 9737, 9738, 9750, 9751, 9755, 9756, 9811-9819, 9823, 9826, 9827, 9831, 9832, 9837, 9861, 9866, 9930, 9965, 9966, 9967, 9970, 9971, 9975 | 9590/3, 9591/3, 9596/3, 9650/3, 9651/3, 9652/3, 9653/3, 9654/3, 9655/3, 9659/3, 9661/3, 9662/3, 9663/3, 9664/3, 9665/3, 9667/3, 9670/3, 9671/3, 9673/3, 9675/3, 9680/3, 9684/3, 9687/3, 9688/3, 9690/3, 9691/3, 9695/3, 9698/3, 9699/3, 9701/3, 9702/3, 9705/3, 9712/3, 9714/3, 9715/3, 9719/3, 9724/3, 9727/3, 9728/3, 9729/3, 9735/3, 9737/3, 9738/3, 9750/3, 9751/3, 9755/3, 9756/3, 9811/3, 9812/3, 9813/3, 9814/3, 9815/3, 9816/3, 9817/3, 9818/3, 9819/3, 9823/3, 9826/3, 9827/3, 9831/3, 9837/3, 9861/3, 9866/3, 9930/3, 9965/3, 9966/3, 9967/3, 9971/3, 9975/3 | 9750/1, 9751/1, 9766/1, 9970/1 |
| Other hematopoietic neoplasms | 9731, 9733, 9734, 9740, 9741, 9749, 9752-9754, 9757-9758, 9759, 9760, 9766, 9860, | 9731/3, 9733/3, 9734/3, 9740/3, 9741/3, 9749/3, 9753/3, 9754/3, 9756/3, 9757/3, 9758/3, 9759/3, 9760/3, 9766/3, 9823/3, 9826/3, 9827/3, 9832/3, 9860/3, | 9740/1, 9752/1, 9753/1, 9766/1 |
| Germ Cell Tumors | 8440, 9060, 9061, 9064, 9065, 9070-9072, 9080-9083, 9084/3, 9085, 9100, 9101 | 8440/3, 9060/3, 9061/3, 9064/3, 9065/3, 9070/3, 9071/3, 9072/3, 9080/3, 9081/3, 9082/3, 9083/3, 9084/3, 9085/3, 9100/3, 9101/3 | 8440/0, 9080/0,1 |
| Tumors of Sellar Region | |||
| Tumors of the pituitary | 8040 (site C75.1 only), 8140 (site C75.1 only), 8146 (site C75.1 only), 8246, 8260 (site C75.1 only), 8270-8272, 8280, 8281, 8290, 8300, 8310, 8323, 9391/1 (site C75.1 only), 9432c (site C75.1 only), 9492 (site C75.1 only), 9580, 9582 | 8140/3, 8246/3, 8260/3, 8270/3, 8272/3, 8280/3, 8281/3, 8290/3, 8300/3, 8310/3, 8323/3, 9580/3 | 8040/0,1, 8140/0,1, 8146/0, 8260/0, 8270/0, 8271/0, 8272/0, 8280/0, 8281/0, 8290/0, 8300/0, 8310/0, 8323/0, 9391/1 (site C75.1 only), 9432/1, 9492/0 (site C75.1 only), 9580/0, 9582/0 |
| Craniopharyngioma | 9350-9352 | None | 9350/1, 9351/1, 9352/1 |
| Unclassified Tumors | |||
| Hemangioma | 9121-9123, 9133, 9140 | 9133/3, 9140/3 | 9121/0, 9122/0, 9123/0, 9133/1 |
| Neoplasm, unspecified | 8000-8005, 8010, 8020, 8021 | 8000/3, 8001/3, 8002/3, 8003/3, 8004/3, 8005/3, 8010/3, 8020/3, 8021/3 | 8000/0,1, 8001/0,1, 8005/0, 8010/0 |
| All other | 8320, 8452, 8713, 8896, 8963, 8980, 9084/0, 9173, 9363, 9503 | 8320/3, 8452/3, 8896/3, 8980/3, 9503/3 | 8452/1, 8713/0, 9084/0, 9173/0, 9363/0 |
aInternational Classification of Diseases for Oncology, 3rd Edition, 2000. World Health Organization, Geneva, Switzerland.
bSee the CBTRUS website for additional information about the specific histopathology codes included in each group: http://www.cbtrus.org.
cAdded starting with diagnosis year 2018.
dThis histopathologically is re-coded from behavior /1 to /3 and included in estimates for malignant brain and other central nervous system tumors by cancer surveillance organizations. Please see the following for more information: Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020;22(2):298-300. doi: 10.1093/neuonc/noz202.
eIncludes tumors formerly classified as primitive neuroectodermal tumors of the central nervous system (PNET).
* All or some of this histopathology is included in the CBTRUS definition of gliomas, including ICD-O-3 histopathology codes 9380-9384, 9391-9460.
Abbreviations: WHO, World Health Organization; NOS, not otherwise specified.
This report also utilizes the International Classification of Childhood Cancer (ICCC) grouping system for pediatric brain and other CNS tumors. ICCC categories for this report were generated using the SEER Main and Extended Classification for ICCC Recode ICD-O-3/WHO 200812 based on the ICCC, Third edition13,14 and 2007 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues15 (See Supplementary Table 1 for more information on this classification scheme). The ICCC was developed to provide a standard classification of childhood tumors for comparing incidence and survival across global geographic regions and time periods.
Classification by Behavior
Primary brain and other CNS tumors can be broadly classified as non-malignant (ICD-O-3 behavior codes of /0 for benign and /1 for uncertain) and malignant (ICD-O-3 behavior code of /3) (Table 2). Collection of central (state) cancer data was mandated in 1992 by Public Law 102-515 for all primary malignant tumors (ICD-O-3 behavior code of /3) (Table 2), the Cancer Registries Amendment Act.16 This mandate was expanded to include non-malignant brain and other CNS tumors (ICD-O-3 behavior code of /0 and /1) with the 2002 passage of Public Law 107–260, starting January 1, 2004.6
Classification by Brain Molecular Markers
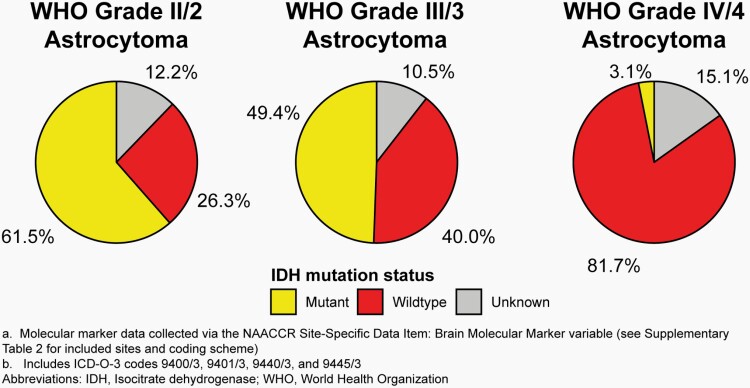
Primary brain and other CNS tumors are a highly heterogeneous group of diseases, and characterization of unique tumor histopathologies within this group has been refined over time. The development of technologies for characterizing DNA sequence, RNA abundance as a measure of gene activity, and biochemical alterations that affect gene expression such as DNA methylation have led to the discovery of several factors (known as ‘biomarkers’) that can be used to more accurately classify these tumors than histopathologic appearance alone. See Table 3 for a brief overview of selected biomarkers for primary brain and other CNS tumors and for discussion of pediatric biomarkers specifically. With the increased recognition of the value of biomarkers for specific brain tumor histopathologies in classification, the WHO Classification of Tumours of the Central Nervous System included biomarkers in its 2016 revision. However, implementing the collection of these markers in cancer registration is multi-faceted and includes an ongoing educational and training component.
Table 3.
Summary of Biomarkers Identified for Primary Brain and Other Central Nervous System Tumors and Collection Status in Central Cancer Registries
| Gene or Marker | Histopathology | Outcome | Collected by US Cancer Registry System |
|---|---|---|---|
| Large deletions (missing parts of the chromosome) in the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q) | Glioma (especially oligodendroglial tumors)1-5 | Improved response to chemotherapy and radiation, and increased survival. | Yes, collected as Site-specific factor 5 (2011-2017), Site-specific factor 6 (2011-2017), Site-specific data item: Chromosome 19q Status (2018+), Site-specific data item: Chromosome 1p Status (2018+). |
| Protein-truncating mutation in isocitrate dehydrogenase 1 (IDH1) or in isocitrate dehydrogenase 2 (IDH2) | Glioma (especially low grade astrocytomas and oligodendroglial tumors)4-6 | Increased survival time. | Yes, began in collection year 2018 (January 1), Site-specific data item: Brain Molecular Markers (2018+). |
| Loss of function mutation in alpha thalassemia/mental retardation syndrome X‐linked (ATRX) | Glioma (especially IDH-mutated glioma)4,7,8 | Increased survival time. | No |
| Methylation of the promoter of O-6-methylguanine-DNA methyltransferase (MGMT) | Glioblastoma9-11 | Limits ability of the tumor cells to repair DNA damage caused by chemotherapy and radiation; results in increased survival time. | Yes, collected as Site-specific factor 4 (2011-2017) and Site-specific data item: MGMT (2018+). |
| Glioma-CpG island methylator phenotype (G-CIMP), Genome-wide DNA methylation | Glioblastoma5,12 | Significantly increased survival time. | No |
| Amplification of epidermal growth factor receptor (EGFR) | Glioblastoma5,13 | Activates the RTK/RAS/PI3K pathway, leading to increased proliferation. Associated with poorer survival. | No |
| Mutation of promotor of Telomerase reverse transcriptase (TERT) | Glioma (oligodendroglial tumors and IDH-wildtype glioblastoma)5,14,15 | Facilitates increased telomere lengthening, and decreases survival in IDH-wildtype glioma. | No |
| Mutation or fusion of B-Raf (BRAF) | Glioma (particularly pediatric lower grade glioma)16 | Activates the RAS/MAPK pathway. Fusion leads to improved survival. | No |
| WNT-activated medulloblastoma | Medulloblastoma17-20 | Low prevalence of metastatic disease. Highest five-year survival. |
Yes, began in collection year 2018 (January 1), collected via new ICD-O-3 code. |
| SHH-activated and TP53-mutant medulloblastoma | Medulloblastoma17-21 | Occur primary in older children, very poor prognosis. | Yes, began in collection year 2018 (January 1), collected via new ICD-O-3 code. |
| SHH-activated and TP53-wildtype medulloblastoma | Medulloblastoma17-21 | Most common in adolescents and young children, good prognosis. | Yes, began in collection year 2018 (January 1), collected via Site-specific data item: Brain Molecular Markers (2018+). |
| non-WNT/non-SHH, Group 3 medulloblastoma subtype (also known as Group C) | Medulloblastoma17-20 | Increased prevalence of metastatic disease. Poorest five-year survival. | Yes, began in collection year 2018 (January 1), combined with group 4 and collected via new ICD-O-3 code. |
| non-WNT/non-SHH, Group 4 medulloblastoma subtype (also known as Group D) | Medulloblastoma17-20 | Increased prevalence of metastatic disease. Moderate five-year survival. | Yes, began in collection year 2018 (January 1), combined with group 3 and collected via new ICD-O-3 code. |
| C19MC amplification and presence of multilayered rosettes | Embryonal tumor22,23 | Highly aggressive, with average survival of 12 months after diagnosis. | Yes, began in collection year 2018 (January 1), collected via Site-specific data item: Brain Molecular Markers (2018+). |
References
1. Cairncross JG, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl. Cancer Inst. 1998; 90(19):1473-1479.
2. Vogelbaum MA, et al. Phase II trial of pre-irradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: long term results of RTOG BR0131. J. Neurooncol. 2015; 124(3):413-420.
3. van den Bent MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013; 31(3):344-350.
4. The Cancer Genome Atlas Research Network, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. New Engl. J. Med. 2015; 372(26):2481-2498.
5. Ceccarelli M, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016; 164(3):550-563.
6. Yan H, et al. IDH1 and IDH2 mutations in gliomas. New Engl. J. Med. 2009; 360(8):765-773.
7. Jiao Y, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012; 3(7):709-722.
8. Wiestler B, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013; 126(3):443-451.
9. Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl. J. Med. 2005; 352(10):997-1003.
10. Stupp R, et al. Chemoradiotherapy in malignant glioma: standard of care and future directions. J. Clin. Oncol. 2007; 25(26):4127-4136.
11. Hegi ME, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008; 26(25):4189-4199.
12. Noushmehr H, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010; 17(5):510-522.
13. Maire CL, Ligon KL. Molecular pathologic diagnosis of epidermal growth factor receptor. Neuro Oncol. 2014; 16 Suppl 8:viii1-6.
14. Arita H, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013; 126(2):267-276.
15. Eckel-Passow JE, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. New Engl. J. Med. 2015; 372(26):2499-2508.
16. Hawkins C, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin. Cancer Res. 2011; 17(14):4790-4798.
17. Kool M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012; 123(4):473-484.
18. Northcott PA, et al. Molecular subgroups of medulloblastoma. Expert Rev. Neurother. 2012; 12(7):871-884.
19. Northcott PA, et al. Medulloblastomics: the end of the beginning. Nat. Rev. Cancer. 2012; 12(12):818-834.
20. Northcott PA, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017; 547(7663):311-317.
21. Zhukova N, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013; 31(23):2927-2935.
22. Ceccom J, et al. Embryonal tumor with multilayered rosettes: diagnostic tools update and review of the literature. Clin. Neuropathol. 2014; 33(1):15-22.
23. Korshunov A, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol. 2014; 128(2):279-289.
As of 2011, SEER registries began collecting information on three validated biomarkers for primary brain and other CNS tumors as Site-Specific Factors (SSF): promoter methylation status of O-6-Methylguanine-DNA Methyltransferase (MGMT) (SSF 4), deletion of 1p (SSF 5), and deletion of 19q (SSF 6).17 Starting with diagnosis year 2018, the broad US cancer registry system began collecting information on multiple brain and other CNS markers, including isocitrate dehydrogenase 1/2 (IDH1/2) mutation, 1p/19q codeletion, medulloblastoma molecular subtypes, and all biomarkers found in 2016 WHO classification using the variable BMM (please see Supplementary Table 2 for an overview of applicable histopathologies and coding scheme). Additional molecularly-defined histopathologies from 2016 WHO were added using their new ICD-O-3 codes for which collection also began in 2018 (See Supplementary Table 3 for an overview of codes added in 2018). These data were available to CBTRUS for the first time with the 2021 NPCR and SEER data releases. As such these data are for the 2018 and 2019 diagnosis years only. CBTRUS evaluated the completeness of these markers in their first year (2018) of collection (please see Iorgulescu et al.18), and completeness of BMM for 2018 and 2019 is shown in Figure 3. CBTRUS is actively working to have all biomarkers included in the 2021 WHO classification included in cancer collection practices.
Fig. 3.
Completeness of the Brain Molecular Marker Variablea by Year at Diagnosis for Selected Histopathologies by ICD-O-3 Code, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2018-2019
Classification by WHO Grade
Unlike other types of cancer which are staged according to the American Joint Commission of Cancer (AJCC) schema, primary brain and other CNS tumors are not staged. They are classified according to the WHO Classification of Tumours of the Central Nervous System which assigns a grade (grade I through grade IV assigned prior to 2021 WHO Classification) based on predicted clinical behavior. The WHO classification scheme was first released in 2000,19 and though it was updated in 200720 and 2016,2 these updated schema were not fully implemented by US CCRs until diagnosis year 2019 or reporting year 2022. Updates made in 2007 and 2016 may affect diagnostic practices used in characterization of individual tumors included in this report. Significant changes were made to grading nomenclature and criteria in the 2021 fifth edition of the WHO Classification of Tumours of the Central Nervous System which are not yet reflected in the characterization of tumors included in this report. As of the 2021 WHO classification, grade is clinically reported using Arabic numerals, but for the purpose of reporting grade for cases collected under prior WHO Classification versions, CBTRUS continues to use Roman numerals.
The WHO grading assignments are recorded by cancer registrars as Collaborative Stage Site-Specific Factor (CS SSF)1 - WHO Grade Classification as directed in the AJCC, Eighth Edition, Chapter 72 on Brain and Spinal Cord21 (cases diagnosed from 2011-2017), Site-Specific Data Items (SSDI) Grade Pathological (cases diagnosed in 2018 or later), and SSDI Grade Clinical (cases diagnosed in 2018 or later). SSF variables were a required component of cancer registry data collection for brain and other CNS tumors beginning in 2004 for SEER registries, and beginning in 2011 for NPCR registries, and were collected through 2017 at which point they were replaced with SSDI. Completeness of these variables have improved significantly over time.17,22
Completeness of this variable is defined as having a value equal to WHO grade I, II, III, or IV. Cases where WHO grade is marked as ‘not applicable’ or ‘not documented’ are considered incomplete. It is not possible to conclusively determine WHO grade, which is based on the appearance of tumor cells, when a tumor is radiographically-confirmed only. Some tumor types (including tumors of the pituitary and lymphomas) are often not assigned a WHO grade. This information may also be assigned but not included in the pathology report.
Brain Tumor Definition Differences
Currently, NPCR, SEER, and NAACCR report primary brain and other CNS tumors differently from CBTRUS. The definition of primary brain and other CNS tumors used by these organizations in their published incidence and mortality statistics includes tumors located in the following sites with their ICD-O-3 site codes in parentheses: brain, meninges, and other CNS tumors (C70.0-9, C71.0-9, and C72.0-9), but excludes lymphoma and leukemia histopathologies (ICD-O-3 histopathology codes 9590-9989) from all brain and other CNS sites.23 In contrast, CBTRUS reports data on all tumor morphologies located within the Consensus Conference site definition including lymphoma and other hematopoietic histopathologies, tumors of the pituitary, and olfactory tumors of the nasal cavity (C30.0 [9522-9523]).11 Additionally, CBTRUS reports data on primary brain and other CNS tumors irrespective of behavior, whereas many reporting organizations may only publish rates for malignant brain and other CNS tumors due to the original mandate that focused only on malignant tumors, sometimes using the term “cancer” to broadly identify these tumors in their reports. These differences in definition therefore influence the direct comparison of published rates.
CBTRUS is currently engaged in ongoing collaboration with other cancer registry reporting groups, including SEER, to harmonize brain tumor reporting definitions. Therefore, it is likely that these reporting differences will cease to exist in the future.
Pilocytic astrocytoma is clinically considered and classified as a grade I, non-malignant (ICD-O-3 behavior code of /1) tumor by the WHO guidelines for brain and other CNS tumors.2 For the purposes of cancer registration, these tumors have historically been reported as malignant (ICD-O-3 behavior code of /3) tumors both in the United States and by the International Agency for Research on Cancer and International Association of Cancer Registries.24,25 Classification of these tumors as malignant has been followed by CBTRUS in its reporting unless otherwise stated. This practice does not correlate with their clinical classification (WHO Classification) and presents a challenge to correctly report population-based incidence and survival patterns associated with these tumors. Please see recent publications for additional discussion of the effect of this classification on cancer incidence and survival reporting.26,27
In the United States, cancer registries and surveillance groups only collect data on primary CNS tumors (meaning tumors that originate within the brain and spinal cord) and do not collect data on tumors that metastasize to the brain or spinal cord from other primary sites. As a result, only primary brain and other CNS tumors are included in this report.
TECHNICAL NOTES
Data Collection
CBTRUS does not collect data directly from patients’ medical records. Registration of individual cases (tumors) is conducted by cancer registrars at the institution where diagnosis and/or treatment occur and is then transmitted to the CCR, which further transmits this information to NPCR and/or SEER. Some CCRs also send their data to SEER; data from those CCRs are taken from the NPCR file to eliminate duplicate cases. As noted, data for CBTRUS analyses come from the NPCR and SEER programs. By law, all primary malignant and non-malignant CNS tumors are reportable diseases and CCRs play an essential role in the collection process. Brain and other CNS tumors are reported using the site definition described in Public Law 107-260.6 These data are population-based and represent a comprehensive documentation of all reported cancers diagnosed within a geographic region for the years included in this report.
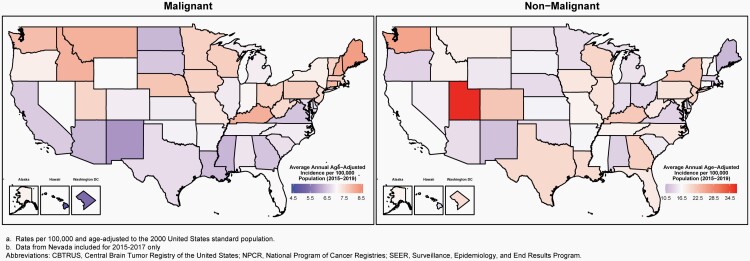
CBTRUS obtained de-identified incidence data from 52 CCRs (48 NPCR and 4 SEER) that include cases of malignant and non-malignant (benign and uncertain behaviors) primary brain and other CNS tumors. The population-based CCRs include 50 state registries, the District of Columbia, and Puerto Rico (Figure 1). Data were requested for all reported primary malignant and non-malignant tumors that were newly diagnosed from 2015 to 2019 at any of the following ICD-O-3 anatomic sites: brain, meninges, spinal cord, cranial nerves, and other parts of the CNS, pituitary and pineal glands, and olfactory tumors of the nasal cavity (ICD-O-3 site code C30.0 and histopathology codes 9522-9523 only) (Table 1).10
Fig. 1.
Availability by Central Cancer Registry for SEER and NPCR Incidence (2015-2019) and Survival Data (2001-2018)
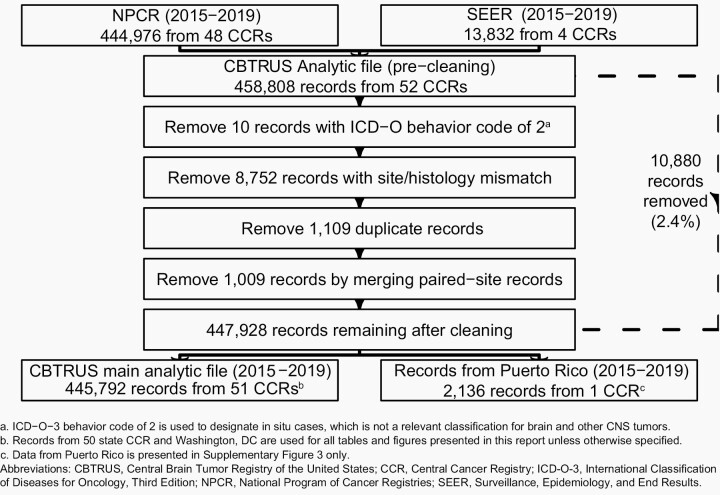
NPCR provided data on 444,976 primary brain and other CNS tumors diagnosed from 2015 to 2019 (Figure 2). An additional 13,832 case records for the period were obtained from SEER for primary brain and other CNS tumor case records from 2015 to 2019 for Connecticut, Hawaii, Iowa, and New Mexico only. These data were combined into a single dataset of 458,808 records for quality control. A total of 10,880 records (2.4%) were deleted from the final analytic dataset for one or more of the following reasons:
Fig. 2.
Overview of CBTRUS Data Edits Workflow, NPCR and SEER, 2015-2019
Records with ICD-O-3 behavior code of /2 (indicates in situ cases, which is not a relevant classification for brain and other CNS tumors).
Records with an invalid site/histopathology combination according to the CBTRUS histopathology grouping scheme.
Possible duplicate records that included a less accurate reporting source than microscopic confirmation, also referred to as histopathologic confirmation (e.g. radiographic versus microscopic confirmation), possible duplicate record for recurrent disease, or errors in time sequence of diagnosis.
Possible duplicate records for bilateral vestibular schwannoma or meningiomas that were merged to one paired-site record.
The final analytic dataset had 447,928 records, which included 445,792 records from the 50 state CCRs and the District of Columbia used in the analytic dataset, and an additional 2,136 records from Puerto Rico. Records from Puerto Rico are included only in a supplementary analysis (See Supplemental Material), and these cases are not included in the overall statistics presented in this report. Data were not available from Nevada for diagnosis years 2018 and 2019 due to data quality issues.
Age-adjusted incidence rates per 100,000 population for the entire United States for selected other cancers were obtained from the USCS, produced by the CDC and the NCI, for the purpose of comparison with brain and other CNS tumor incidence rates.8 This database includes both NPCR and SEER data and represents the entire US population.
De-identified survival data for malignant brain and other CNS tumors were obtained from NPCR for 42 CCRs for the years 2001 to 2018 and for non-malignant brain and other CNS tumors for the years 2004 to 2018. This dataset provides population-based information for 82% of the US population for the years 2001 to 2018 and is a subset of the data used for the incidence calculations presented in this report. Survival information is derived from both active and passive follow-up.
Mortality data used in this report are from the NVSS and include deaths where primary brain or other CNS tumor was listed as primary cause of death on the death certificate for individuals from all 50 states and the District of Columbia. These data were obtained from NVSS28 (includes death certification data for 100% of the US population) for malignant brain and other CNS tumors and comparison via SEER*Stat (for malignant brain tumors and comparison cancers). NVSS data are not collected through the cancer registration system. These data represent the primary cause of death listed on each individual death certificate, and as a result, deaths in persons with cancer may be recorded as non-cancer deaths.
Definitions
Measures in Surveillance Epidemiology
The CBTRUS Statistical Report presents the following population-based measures: incidence rates, mortality rates, observed survival (median survival time and hazard ratios), and relative survival rates (for more information on definitions of terms and measures used see: https://cbtrus.org/cbtrus-glossary/).
Variable Completeness in Cancer Registration
Obtaining the most accurate and complete cancer registration data possible is essential to generate accurate population-level statistics to guide public health planning. Agencies such as NAACCR and International Agency for Cancer Research (IACR) have developed stringent standards for evaluation of cancer registry data quality, and evaluate each specific registry by multiple metrics before including it in analytic datasets.29,30 While many measures of quality and completeness are assessed across all cancer sites, some variables are pertinent only to specific sites and/or histopathologies and require special care. In the case of primary brain and other CNS tumors, variables such as WHO grade are not relevant to certain histopathologies (e.g. many tumors of the pituitary) that are not assigned a WHO grade. Similarly, the BMM variable is applicable only to specific histopathologies. Variables like WHO grade or BMM may also not be expected to be found in the patient record for those who had their diagnosis confirmed via radiography as compared to histopathological examination. The 2022 CBTRUS Report evaluates the completeness of multiple variables, including: WHO grade (applicable to specific brain and other CNS sites and histopathologies only), BMM (applicable to specific histopathologies only), extent of surgical resection, and radiation treatment.
Statistical Methods
Statistical Software
Counts, means, medians, rates, ratios, proportions, and other relevant statistics were calculated using R 4.1.3 statistical software31 and/or SEER*Stat 8.4.0.32 Figures and tables were created in R 4.1.3 using the following packages: flextable, officer, orca, plotly, SEER2R, sf, survminer, tigris, and tidyverse.33-42 Rates are suppressed when counts are fewer than 16 within a cell but included in totals, except when data are suppressed from only one cell to prevent identification of the number in the suppressed cell. NOTE: reported percentages may not add up to 100% due to rounding.
Variable Definitions
CBTRUS presents statistics on the pediatric and adolescent age group 0-19 years as suggested by clinicians for clinical relevance. However, the 0-14 years age group is a standard age category for childhood cancer used by other cancer surveillance organizations and has been included in this report for consistency and comparison purposes.
Race categories in this report are all races: White, Black, American Indian/Alaskan Native (AIAN), and Asian/Pacific Islander (API). Other race, unspecified, and unknown race are included in statistics that are not race-specific. Hispanic ethnicity was defined using the NAACCR Hispanic Identification Algorithm, version 2, data element, which utilizes a combination of cancer registry data fields (Spanish/Hispanic Origin data element, birthplace, race, and surnames) to directly and indirectly classify cases as Hispanic or non-Hispanic.43
Estimation of Incidence Rates and Incidence Rate Ratios
Population data for each geographic region were obtained from the SEER program website44 for the purpose of rate calculation. All rates presented in this statistical report are age-adjusted. Crude incidence rates are calculated by dividing the total number of cases by the total population and cannot be compared to crude rates from other populations where the age distribution is different. Age-adjustment is a technique that is used to enable comparison between groups with different age distributions, such as rates between different states. Rates that have been age-adjusted are estimates of what the crude rate would be if the age distribution is equivalent to a standard population. Average annual age-adjusted incidence rates (AAAIR), average annual age-adjusted mortality rates (AAAMR), and 95% confidence intervals (95% CI) were estimated per 100,000 population based on five-year age groups and were standardized to the 2000 US standard population for consistency with other US reporting agencies.45
Incidence rate ratios (IRR) were generated based on these age-adjusted incidence rates. These IRR were used to compare groups, using the formulas described by Fay et al. to calculate p-values.46 Incidence rate ratios were considered statistically significantly different when the p-value was less than 0.05.
When comparing two rates to one another, it is important to consider whether they are truly different or whether the difference in the estimates may be due to random error. Two methods are used in this report for determining whether two values are ‘significantly different,’ meaning whether the evidence meets a level of strength (usually a 5% chance of error) where the difference can be assumed to not be due to random error. The first is the use of a 95% CI, which were calculated for all presented rates in this Report. A 95% CI is a range around an estimate, which, if sampling of the population were to be repeated, should contain the ‘true’ value for the population 95% of the time. If the CI of two estimates do not overlap, these values are considered significantly different with a less than 5% probability of happening by chance. The second method used is the calculation of p-values. A p-value is the probability of finding the observed or more extreme results by chance alone, and a p-value of <0.05 (or <5% chance of results being due to chance) is conventionally used as a cut-off for considering a value statistically significant. Therefore, a p-value <0.0001 could be interpreted as meaning the observed value (or a more extreme value) had a <0.01% chance of occurring by chance alone, and the difference can be considered statistically significant at the 0.01% level.
Estimation of Incidence Time Trends
Joinpoint 4.10.0.047 was used to estimate incidence time trends and generate annual percentage changes (APC) and 95% CI. Rather than calculating a single consistent slope of change over an entire period of time, Joinpoint allows for points where the slope of the trend can change during the time period (joinpoints). This method starts with a model that assumes one consistent trend over time, and tests whether the addition of these ‘joinpoints’ results in a model which has a fit that represents a statistically significant improvement over the model with no joinpoints. These models are tested through use of Monte Carlo permutations, e.g. the program repeats the same analysis multiple times using random samples to identify the ‘true’ proportion of times that a comparison is statistically significant. The models allowed for a maximum of three joinpoints (two for non-malignant tumors), a minimum of three observations from a joinpoint to either end of the time-period, and a minimum of three observations between joinpoints.48 The best fitting model is selected and may include anywhere from one to four trend periods depending on identified inflection points (maximum of three for non-malignant tumors) and number of years included in the model.
APC is the average percent change in incidence per year over the period included in the trend segment. Time trends analysis methods were used to estimate if the APC was significantly different from 0% (meaning no change in incidence from year to year). The 95% CI is a range around an estimate that, if sampling of the population were to be repeated, should contain the ‘true’ value for the population 95% of the time. If the 95% CI contains zero, one cannot be confident that the ‘true’ population APC value is significantly different from 0%. The joinpoint regression program fits a linear regression to annual incidence rates to test significance of changes overtime, with different trends lines connected at ‘joinpoints’ where there are changes in the direction of incidence trends. The best fitting model was determined through permutation tests, with a minimum of three observations required between two joinpoints, as well as a minimum of three observations required between a joinpoint and either end of the time-period.
Estimation of Expected Numbers of Brain and Other CNS Tumors in 2022 and 2023
Estimated numbers of expected primary malignant and non-malignant brain and other CNS tumors were calculated for 2022 and 2023. To project estimates of newly diagnosed brain and other CNS tumors in 2022 and 2023, age-adjusted annual brain tumor case counts were generated for 2000-2019 for malignant tumors, and 2006-2019 for non-malignant tumors (with the exception of CCR-specific estimates for Nevada, where diagnosis years 2018 and 2019 were not available due to data quality issues). These were generated by state, age, and histopathologic type. Joinpoint 4.10.0.047 was used to fit regression models to these case counts,49 which were used to predict numbers of cases in future years using the parameter from the selected models. Joinpoint regression allows for multiple lines to be fitted to incidence data across time, rather than assuming a consistent trend across the whole period. The points where these lines intersect are called ‘joinpoints’. The models allowed for a maximum of two joinpoints (one for non-malignant tumors), a minimum of three observations from a joinpoint to either end of the data, and a minimum of three observations between joinpoints.48 Modified Bayesian Information Criterion procedures included in Joinpoint were used to select the best fitting model. The overall totals presented are based on total malignant and non-malignant incidence, and the presented stratified rates may not add up to these totals.
Estimated numbers of cases are highly dependent on input data. Different patterns of incidence within strata can significantly affect the projected estimates, especially when the number of cases within a stratum is low. For CCR-specific projections, a model with no joinpoints was used to generate predictions as annual variability within some groups was extremely high. As a result, strata-specific estimates may not equal the total estimate presented. As these estimates are based on 14-20 years of observed data, projected totals may not be equal to average annual cases estimate from the last five years of data. Caution should be used when utilizing these estimates.
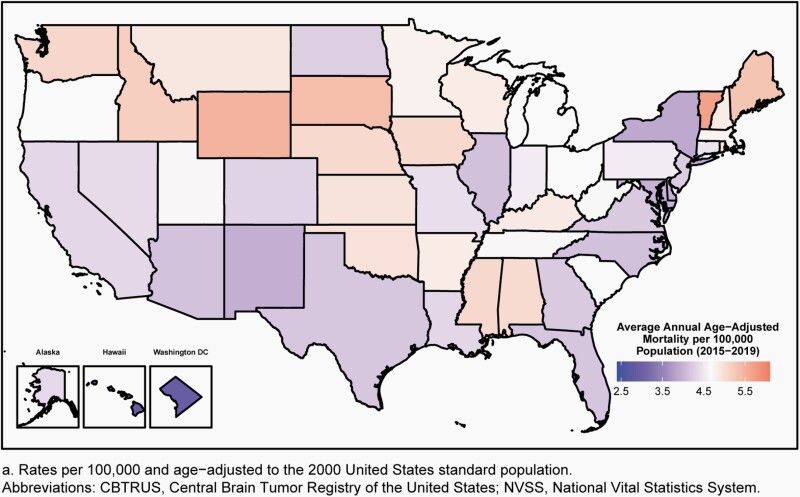
Estimation of Mortality Rates for Brain and Other CNS Tumors
Age-adjusted mortality rates for deaths resulting from all primary malignant brain and other CNS tumors were calculated using the mortality data available in SEER*Stat Online Database provided by NCHS from death certificates per 100,000 population.28 These data were available for 50 states and the District of Columbia only. In addition to the total age-adjusted rate for the United States, age-adjusted rates are presented by sex and state.
Survival Measures Used in This Report
Relative Survival Rates
Relative survival is a way of presenting survival patterns at a population level that is commonly used in cancer statistics reporting. This measure is presented as a percent of people living a period of time (e.g. five years after their diagnosis). Relative survival is calculated using observed survival (the percentage of people diagnosed with cancer that live to the period of time for which relative survival is calculated) and estimated survival (the percent of the general population of the same age that is expected to survive after being followed for that same period of time). This adjustment for estimated survival attempts to exclude deaths that would otherwise have occurred due to other causes. For example, if five-year relative survival for glioblastoma is 5%, that means that out of every 100 people diagnosed with glioblastoma, five will be living five years after diagnosis, excluding deaths attributed to other causes.
SEER*Stat 8.4.0 statistical software was used to estimate relative survival rates for primary malignant and non-malignant brain and other CNS tumor cases diagnosed between 2004-2018 in 42 NPCR CCRs. This software utilizes life-table (actuarial) methods to compute survival estimates and accounts for current follow-up. Second or later primary tumors, cases diagnosed at autopsy, cases in which race or sex is coded as other or unknown, and cases known to be alive but for whom follow-up time could not be calculated, were excluded from survival data analyses.
Observed Survival with Median Survival Times and Adjusted Hazard Ratios
Median survival time is another way of presenting survival patterns in a population. This measure is calculated using a method called a Kaplan-Meier estimator, which is used to estimate the proportion of individuals within a set that are alive at particular time points. The median survival time is the point at which exactly 50% of individuals have either died or been ‘censored’, meaning that their further survival status is unknown beyond a particular date.
Median survival time for all reported primary malignant brain and other CNS tumors diagnosed between 2001-2018 in 42 NPCR CCRs was calculated by histopathology using the Kaplan-Meier method in R 4.1.3 statistical software31 overall, as well as by three age groups (0-14 years old, 15-39 years old, and 40+ years old). Second or later primary tumors, cases diagnosed at autopsy, cases in which either race or sex is coded as other or unknown, and cases known to be alive but for whom follow-up time could not be calculated, were excluded from survival data analyses. NAACCR data item #1787, survival months presumed alive, was used to ascertain follow-up information.
The hazard ratio is a measure of how often an event (in this case, death) occurs in one group as compared to another group over time. A hazard ratio of one means that survival is equal in both groups, while a ratio of less than one means that survival is better in the comparison group than in the reference group. A ratio of greater than one means that survival is worse in the comparison group than in the reference group.
Cox proportional hazard models were used to test associations between demographic factors and overall survival by histopathology for malignant brain and other CNS tumors. All models were adjusted for age at diagnosis group (0-14 years [reference], 15-39 years, 40+ years), sex (male [reference], female), and race and ethnicity (White Non-Hispanic [reference], Black Non-Hispanic, AIAN Non-Hispanic, API Non-Hispanic, and Hispanic All Races). These models were used to estimate hazard ratios associated with each group and corresponding 95% CI and p-values. Adjusted estimates included all covariates (age at diagnosis, sex, race, and ethnicity) a priori, regardless of individual significance level. The proportional hazards assumption was tested separately by histopathology, and residuals were examined for all variables.
Data Interpretation
CBTRUS works diligently to support the broader surveillance efforts aimed at improving the collection and reporting of primary brain and other CNS tumors. CCR data provided to NPCR and SEER and, subsequently, to CBTRUS vary from year-to-year due to ongoing updates to cases from all cancer diagnosis years, as well as changes in collection and data refinement aimed to improve completeness and accuracy. Therefore, it is important to note that data from previous CBTRUS Reports cannot be compared to data in this current report, CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. This current report supersedes all previous reports in terms of coverage of the US population with the most up-to-date population-based information available, making these data the most accurate and timely to reference.
Several factors should be considered when interpreting the data presented in this report:
Incident counts of cases represent individual tumors and not persons. A single person could contribute multiple primary tumor cases to the data included in this report. The 445,792 tumors included in this report came from 439,916 individuals. Of these individuals, there were 5,439 individuals (1.2%) that contributed information on multiple tumors (two or more) to this report.
Data may be excluded from individual CCRs for specific years due to incomplete case ascertainment.
Random fluctuations in average annual rates are common, especially for rates based on small case counts. The CBTRUS policy to suppress data in cells with counts of fewer than 16 cases is consistent with the NPCR policy.
A 2007 policy change guiding the Veterans Health Administration (VHA) may have resulted in probable underreporting of cancer data—especially for males—to CCRs. Recent investigations suggest that underreporting for VHA facilities has diminished over time, and that the Veterans Affairs Central Cancer Registry (VACCR) now captures approximately 87-90% of cases.50,51 It is important to note that improved reporting to VACCR does not necessarily mean that reporting to the state CCR has improved. The VACCR does not submit data directly to NPCR or SEER.
Delays in reporting and late ascertainment are a reality and a known issue influencing registry completeness and, consequently, rate underestimations occur, especially for the most recent years.52,53,54 The SEER and NPCR programs allow for reporting delay of up to 22-23 months prior to public data release, but additional cases may still be discovered after that point. On average across all cancer sites, the submissions for the most recent diagnosis year are approximately 4% lower than the total number of cases that will eventually be submitted. This problem may be even more likely to occur in the reporting of non-malignant brain and other CNS tumors, where reporting often comes from non-hospital-based sources, such as free-standing clinics or outpatient facilities.
Type of diagnostic confirmation may also lead to increased reporting delay, with histopathologically-confirmed tumors being subject to less reporting delay than radiographically-confirmed tumors. In 2016, a study assessing the incidence of non-malignant brain and other CNS tumors corroborated the large variation in incidence between CCRs reported in this statistical report.55 The reasons for this variation remain inconclusive but what is consistently noted is the correlation between high incidence and high proportion of non-malignant cases collected without microscopic confirmation or surgery, in other words, clinically diagnosed cases of non-malignant brain tumors. At this current time, given the variation across CCRs, there is potential evidence of underreporting of non-malignant brain and other CNS tumors, the extent to which cannot be quantified at this time.55
Population estimates used for denominators affect incidence rates. CBTRUS has utilized population estimates based on the 2000 US Census for calculation of incidence and mortality rates in this report, as is standard practice in US cancer registry reporting.56,57
CBTRUS editing practices are reviewed, revised, and conducted yearly. These practices are aimed at refining the data for accuracy and clinical relevance and play a role in interpreting these report data. Exclusion of site and histopathology combinations considered invalid by the consulting neuropathologists who revised the CBTRUS site/histopathology validation list in 2021 may have the impact of underestimating the incidence of brain and other CNS tumors. Editing changes, such as the Multiple Primary and Histology Rules issued in 2007 and revised in 2018,58,59 also incorporate updates to the cancer registration coding rules that influence case ascertainment and data collection.23
Supplemental Data
CBTRUS has made supplemental additional figures and tables available. These materials are noted in the text as Supplementary Tables and Figures.
RESULTS
Incidence and Mortality in Comparison to Other Common Neoplasms in the United States
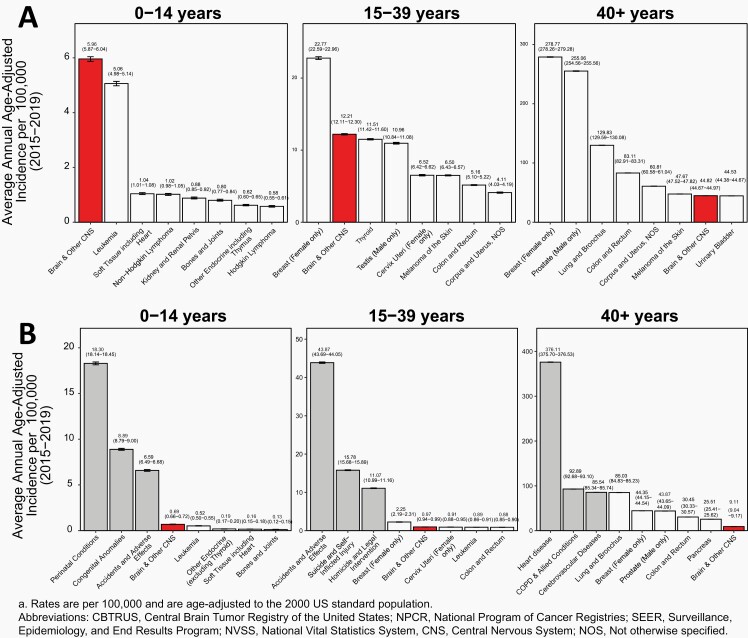
AAAIRs for primary brain and other CNS tumors (2015-2019) and a selection of common cancers (USCS, 2015-2019) in the United States are presented by age in Figure 4A for Children (ages 0-14 years), Adolescents and Young Adults (AYA) (ages 15-39 years), and Older Adults (ages 40+ years).
Fig. 4.
A) Average Annual Age-Adjusted Incidence Ratesa with 95% Confidence Intervals of All Primary Brain and Other Central Nervous System Tumors in Comparison to Top Eight Highest Incidence Cancers for Children Ages 0-14 Years, Adolescents and Young Adults Ages 15-39 Years, and Older Adults Ages 40+ Years, B) Average Annual Age-Adjusted Mortality Ratesa with 95% Confidence Intervals of All Primary Brain and Other Central Nervous System Tumors in Comparison to Top Five Causes of Cancer Death and Top Three Non-Cancer Causes of Death for Children Ages 0-14 Years, Adolescents and Young Adults Ages 15-39 Years, and Older Adults Ages 40+ Years, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019; and NVSS, 2015-2019
Brain and other CNS tumors (both malignant and non-malignant) were the most common tumor site in persons ages 0-14 years, with an AAAIR of 5.96 per 100,000 population.
Leukemia was the second most common tumor in persons ages 0-14 years, with an AAAIR of 5.06 per 100,000 population.
Brain and other CNS tumors (both malignant and non-malignant) among those ages 15-39 years had an AAAIR of 12.21 per 100,000 population. These tumors were the second most common tumor type in this age group.
Testicular cancer (males only) was the most common tumor type in males ages 15-39 years with an AAAIR of 10.96 per 100,000.
Breast cancer (females only) was the most common tumor type among those ages 15-39 years and 40+ years with AAAIRs of 22.77 and 278.77 per 100,000, respectively.
The second most common tumor type among those ages 40+ years was prostate cancer, which had an incidence rate of 255.06 per 100,000 (males only).
Brain and other CNS tumors (both malignant and non-malignant) were the seventh most common tumor type among persons age 40+ years with an AAAIR of 44.82 per 100,000 population.
AAAMR for primary malignant brain and other CNS tumors (2015-2019), a selection of common cancers, and the top three non-cancer causes of death in the United States are presented by age in Figure 4B.
The most common causes of death in persons ages 0-14 years were perinatal conditions (18.30 per 100,000).
Childhood brain and other CNS cancer, while rare, contributes substantially to cancer related mortality in children 0-14 years old. Malignant brain and other CNS tumors among persons ages 0-14 years had an AAAMR of 0.69 per 100,000 and were the fourth most common cause of death in this age group, and the most common cause of cancer death.
Accidents and adverse effects were the leading causes of death in persons ages 15-39 years (43.87 per 100,000).
Malignant brain and other CNS tumors among persons ages 15-39 years had an AAAMR of 0.97 per 100,000 and were the 11th most common cause of death in this age group and the second most common cause of cancer death, where their AAAMR was similar to that of leukemia (0.89 per 100,000).
Breast cancer (female only) was the most common cause of cancer death in this age group (2.25 per 100,000).
Heart disease was the largest contributor to mortality in persons ages 40+ years in the United States, with an AAAMR of 376.11 per 100,000 for major cardiovascular diseases.
Malignant brain and other CNS tumors among persons ages 40+ years had an AAAMR of 9.11 per 100,000 and were the 26th most common cause of death.
Lung and bronchus cancer was the most common cause of cancer death in those ages 40+ years (85.03 per 100,000).
Time Trends in Primary Brain and Other CNS Tumors
Time trends in cancer incidence rates are an important measure of the changing burden of cancer in a population over time. Many factors may lead to fluctuations in rates over time, and all of these must be considered when interpreting time trend results. When assessing trends in incidence over time it is critical to use the most recent data available, as delay in reporting may cause small fluctuations in incidence. Time trends analysis methods are used to estimate if the APC is significantly different from 0% (meaning no change in incidence from year to year). In addition to assessing statistical significance of changes in incidence over time, the size of this change must also be considered because with datasets as large as CBTRUS, miniscule fluctuations in incidence over time may be statistically significant, but not truly represent a large change in proportion of individuals over time.
Incidence rates of cancer overall and many specific cancer histopathologies have decreased over time.60 Overall, changes in incidence rates of all primary brain and other CNS tumors between 2000 and 2019 (limited to 2004 and 2019 for non-malignant tumors) have been small. As stated previously, there are many things that can affect incidence rates over time that are not related to ‘true’ changes in incidence of these tumors such as demographic changes, changes in histopathology classification, and changes in cancer registration procedures. The latter is especially applicable to the collection of non-malignant brain and other CNS tumors.
Malignant Brain and Other CNS Tumors
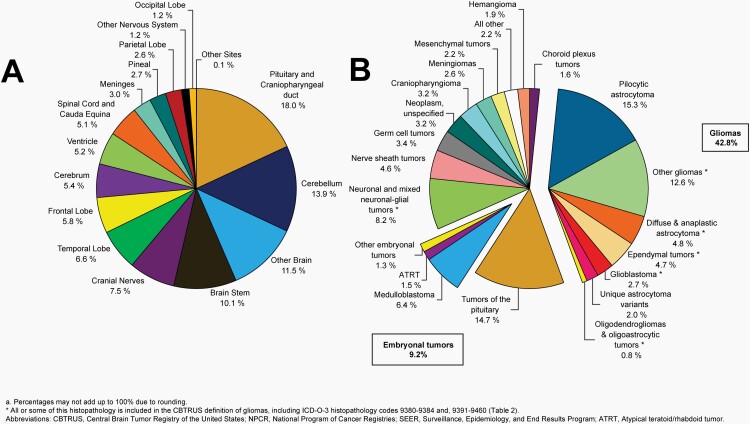
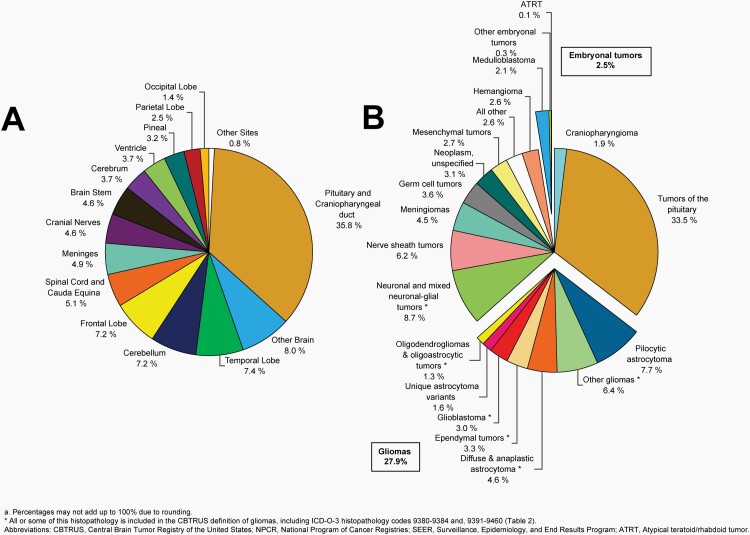
Please see Figure 5B for an overview of histopathologies included in all malignant brain and other CNS tumors.
Fig. 5.
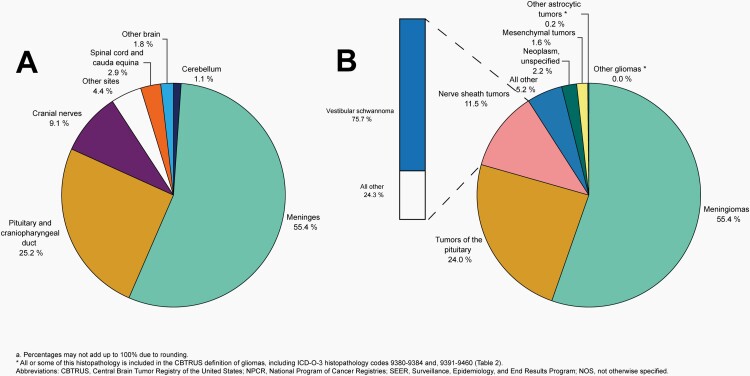
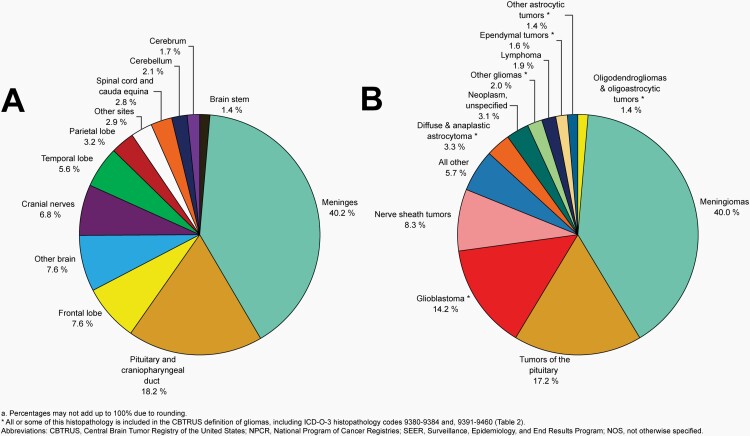
Distributiona of Malignant Primary Brain and Other Central Nervous System Tumors (Five-Year Total=126,345; Annual Average Cases=25,269) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics - NPCR and SEER, 2015-2019
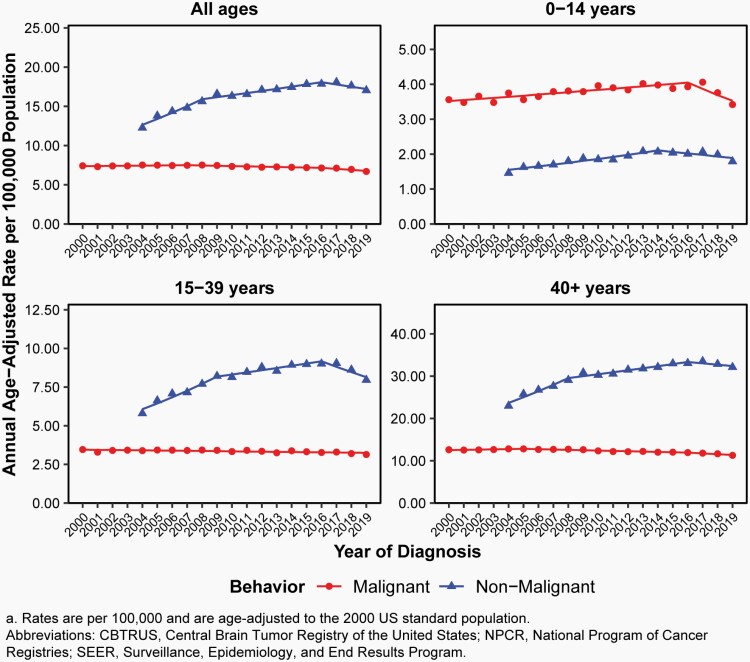
Overall, there have been no substantial changes in incidence of malignant brain tumors from 2000-2019.
From 2007-2016, there was a slight decrease in overall incidence (APC= -0.5% [95%CI: -0.7%, -0.3%], Figure 6, Supplementary Table 4).
There was a small but statistically significant increase in incidence in children (ages 0-14 years, APC=0.9% [95%CI: 0.6%, 1.2%]), a small but statistically significant decrease in AYA (ages 15-39 years, APC=-0.3% [95%CI: -0.5%, -0.2%]) from 2000-2016, and a small but statistically significant decrease in older adults from 2005-2016 (ages 40+ years, APC= -0.7% [95%CI: -0.8%, -0.5%] Figure 6, Supplementary Table 4).
Fig. 6.
Annual Age-Adjusted Incidence Ratesa of All Primary Brain and Other Central Nervous System Tumors and Incidence Trends by Behavior and Age Group at Diagnosis, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2000-2019 (varying)
Glioma
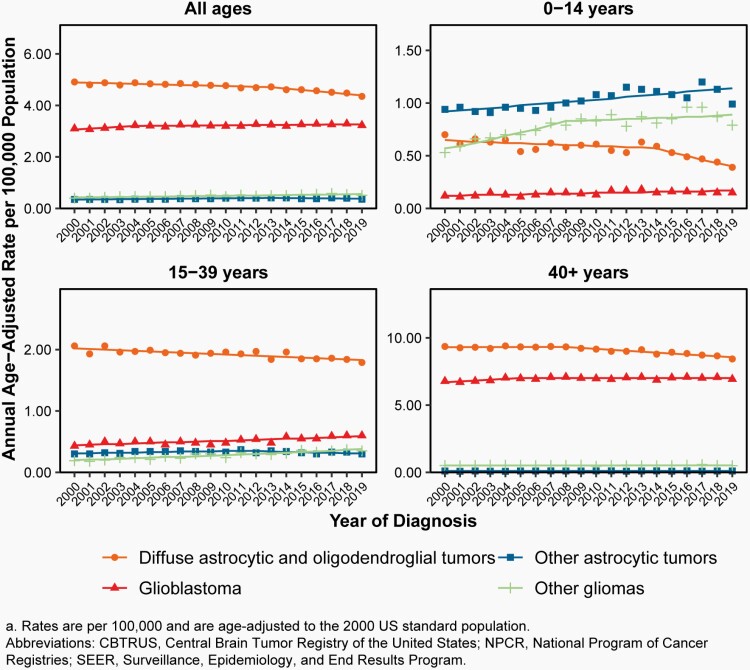
Please see Figure 7B for an overview of histopathologies included in in the broad category of glioma and Figure 8 for incidence trends of selected glioma histopathologies.
Fig. 7.
Distributiona of Primary Brain and Other Central Nervous System Gliomas (ICD-O-3 histopathology codes 9380-9384 and 9391-9460) (Five-Year Total=106,808; Annual Average Cases=21,362) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics - NPCR and SEER, 2015-2019
Fig. 8.
Annual Age-Adjusted Incidence Ratesa of Primary Brain and Other Central Nervous System Gliomas and Incidence Trends by Age Group at Diagnosis, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2000-2019
There was a slight increase in overall incidence of malignant gliomas (behavior codes /3 only) from 2000-2007 (APC=0.3% [95%CI: 0.0%, 0.6%]), followed by a small but significant decrease in incidence from 2007-2016 (APC= -0.4% [95%CI: -0.6%, -0.1%]) and 2016-2019 (APC= -2.1% [95%CI: -3.1%, -1.1%], Supplementary Table 4).
There was a significant increase in incidence in children (ages 0-14 years, APC=1.2% [95%CI: 0.8%, 1.5%]) from 2000-2016, and a significant decrease in incidence in AYA from 2014-2019 (ages 15-39 years, APC=-1.4% [95%CI: 1.2%, 4.0%], Supplementary Table 4).
Incidence in older adults (ages 40+ years) was relatively stable: there was a small but statistically significant decrease from 2007-2019 (APC= -0.7% [95%CI: -0.9%, -0.6%], Supplementary Table 4).
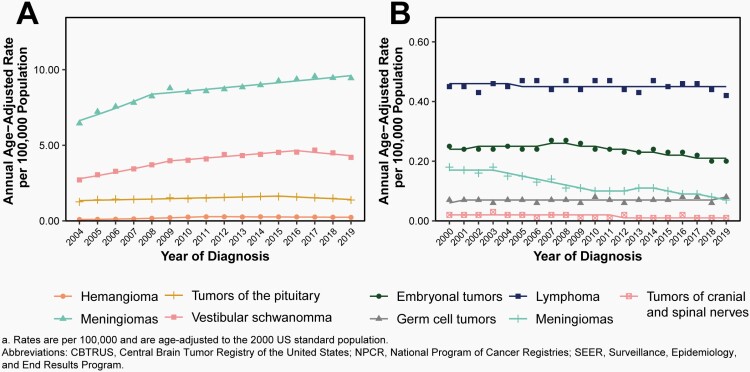
Malignant Meningioma
There was a significant decrease in incidence from 2000-2003 (APC= -1.2% [95%CI: -7.7%, 5.9%] and from 2014-2019 (APC= -6.9% [95%CI: -10.1%, -3.6%], Figure 9B, Supplementary Table 5).
Changes were made to diagnostic and grading criteria for meningioma in both the 2000 and 2007 revisions of the WHO classification, and gradual uptake of these classification changes may result in changing incidence of these tumors.
Fig. 9.
Annual Age-Adjusted Incidence Ratesa of Primary Brain and Other Central Nervous System Tumors and Incidence Trends by Histopathology for Selected A) Non-Malignant and B) Malignant Histopathologies, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2000-2019 (varying)
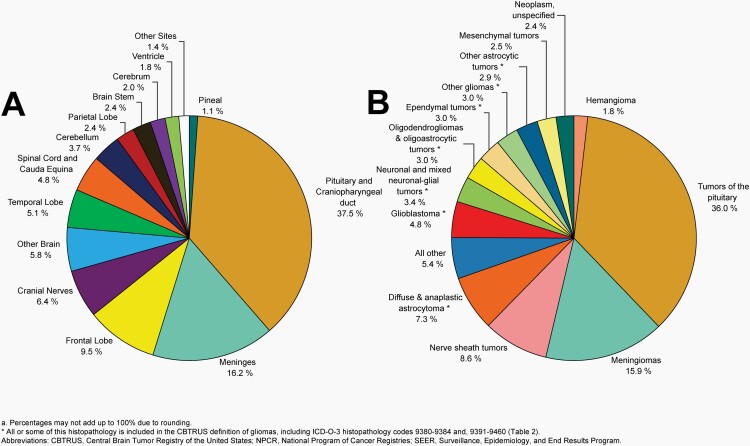
Non-Malignant Brain and Other CNS Tumors
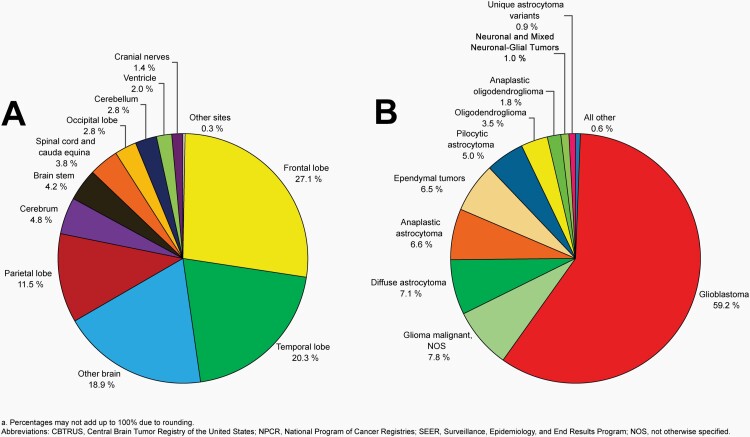
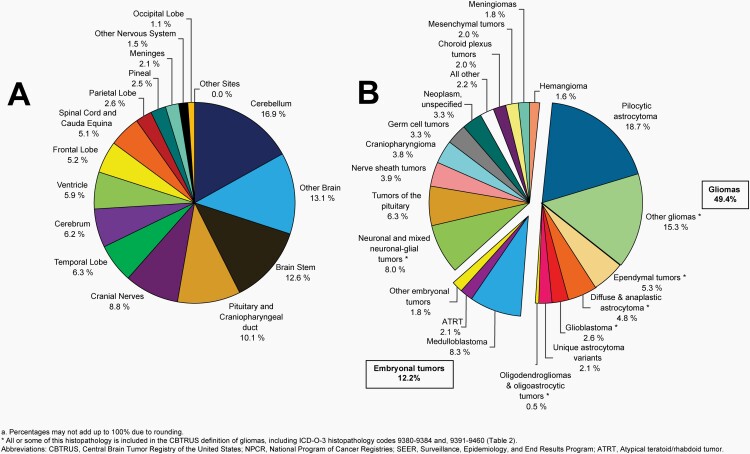
Please see Figure 10B for an overview of histopathologies included in all non-malignant brain and other CNS tumors.
Fig. 10.
Distributiona of All Non-Malignant Primary Brain and Other Central Nervous System Tumors (Five-Year Total=319,447; Annual Average Cases=63,887) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Overall, incidence of non-malignant brain tumors increased substantially after collection of these cases began by CCRs in 2004, likely attributed to improvements in collection with each collection year.
There was a significant increase in incidence of non-malignant brain tumors from 2004-2008 (APC=5.9% [95%CI: 3.8%, 8.1%]), and 2008-2016 (APC=1.6% [95% CI: 0.9%, 2.4%]). There was no significant change from 2016-2019 (Supplementary Table 6).
There was a small, but statistically significant, increase in incidence of these non-malignant brain tumors in children (2004-2014, APC=3.1% [95%CI: 2.2%, 4.0%]), in AYA (2004-2009, APC=6.2% [95%CI: 4.1%, 8.3%]), and in older adults (2004-2008, APC=5.7% [95%CI: 3.6%, 7.9%], Supplementary Table 4).
Incidence trends varied depending on diagnostic method. When analysis was limited to microscopically-confirmed tumors only, there was a small but significant increase in the incidence of non-malignant brain and other CNS tumors from 2004-2008 (APC=2.0% [95%CI: 0.4%, 3.6%]), followed by an insignificant decrease from 2008-2016 (APC=-0.3% [95% CI -0.9%, -0.3%]), and a significant decrease from 2016-2019 (APC= -2.5% [95% CI: -4.8%, -0.2%], Supplementary Table 7).
Radiographically-confirmed tumors experienced substantially higher increases in incidence. There was a statistically significant increase in incidence of radiographically-confirmed non-malignant tumors from 2004-2009 (APC=9.4% [95%CI: 7.1%, 11.8%]), with smaller but statistically significant increase from 2009-2016 (APC=2.9% [95%CI: 1.6%, 4.2%], Supplementary Table 7).
The increases in incidence in the non-malignant tumors are partially attributable to improved collection of radiographically-diagnosed cases as well as improvement in collection of non-malignant cases in general over time.
Non-Malignant Meningiomas
There was a significant increase of non-malignant meningiomas between 2004-2008 (APC=6.0% [95%CI: 3.7%, 8.4%]), followed by a smaller but statistically significant increase from 2008-2019 (APC=1.3% [95%CI: 0.9%, 1.7%], Figure 9A, Supplementary Table 6).
When analysis was limited to microscopically-confirmed cases, there was a slight significant decrease in incidence from 2008-2019 (APC= -0.7% [95% CI: -1.1%, -0.4%], Supplementary Table 7).
There was a significant increase in incidence of radiographically-diagnosed cases from 2004-2008 (APC=10.8% [95%CI: 7.3%, 14.5%]), and a smaller but still significant change from 2008-2019 (APC=2.6% [95%CI: 2.1%, 3.1%], Supplementary Table 7).
Non-Malignant Nerve Sheath Tumors and Vestibular Schwanomma
There was a small but significant increase in the incidence of non-malignant nerve sheath tumors between 2004-2016 (APC=1.8% [95%CI: 1.2%, 2.3%]) followed by a significant decrease from 2016-2019 (APC=-5.7% [95%CI: -9.6%, -1.6%], Supplementary Table 6)
When analysis was limited to microscopically-confirmed cases only, there was no significant change in incidence from 2004-2010 (Supplementary Table 7).
There was a significant increase in incidence of radiographically-diagnosed tumors between 2004-2007 (APC=9.1% [95%CI: 4.4%, 14.0%]) and 2007-2015 (APC=3.2% [95%CI: 2.2%, 4.1%]), followed by a significant decrease from 2015-2019 (APC=-4.0% [95%CI: -6.1%, -1.8%], Supplementary Table 7).
Non-Malignant Tumors of the Pituitary
There was a significant increase in non-malignant tumors of the pituitary from 2004-2009 (APC=7.3% [95%CI: 5.6%, 9.1%]), and a smaller but significant increase from 2009-2016 (APC=2.3% [95%CI: 1.2%, 3.3%], Figure 9A, Supplementary Table 6).
When analysis was limited to microscopically-confirmed tumors only, there was a significant increase (APC=4.4% [95%CI: 3.0%, 5.7%]) from 2004-2009, followed by a significant decrease from 2009-2016 (APC=-0.9% [95%CI: -1.8%, 0.0%], Supplementary Table 7).
There was a significant increase in incidence of radiographically-diagnosed tumors of the pituitary from 2004-2009 (APC=11.4% [95%CI: 8.0%, 14.9%]), and from 2009-2016 (APC= 4.8% [95% CI: 3.0%,6.8%], Supplementary Table 7).
Distributions and Incidence by Site, Behavior, and Histopathology
Counts and rates from the 445,792 brain and other CNS tumors (28.3% malignant, 126,345 cases; 71.7% non-malignant, 319,447 cases shown in Figure 11) reported during 2015-2019 overall and by sex for all ages are shown by site in Table 4 and by histopathology in Table 5. Counts and rates are shown by histopathology and behavior for selected histopathologies where there is a statistically sufficient number of cases to calculate rates.
Fig. 11.
Distributiona of All Primary Brain and Other Central Nervous System Tumors by Behavior (Five-Year Total=445,792; Annual Average Cases=87,427), CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Table 4.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals of All Brain and Other Central Nervous System Tumors by Sitec and Sex, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Site (ICD-O Topography Code) | Total | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | % of all tumors | Rate (95% CI) | 5-Year Total | Annual Average | % of all tumors | Rate (95% CI) | 5-Year Total | Annual Average | % of all tumors | Rate (95% CI) | |
| Olfactory tumors of the nasal cavity (C30.0)d | 735 | 147 | 0.2% | 0.04 (0.04-0.04) | 419 | 84 | 0.2% | 0.05 (0.04-0.05) | 316 | 63 | 0.1% | 0.03 (0.03-0.04) |
| Meninges (cerebral and spinal) (C70.0-C70.9) | 179,112 | 35,822 | 40.2% | 9.55 (9.50-9.59) | 48,792 | 9,758 | 26.5% | 5.69 (5.64-5.75) | 130,320 | 26,064 | 49.8% | 12.97 (12.90-13.04) |
| Cerebral meninges (C70.0) | 147,043 | 29,409 | 33.0% | 7.84 (7.80-7.88) | 40,373 | 8,075 | 21.9% | 4.71 (4.66-4.76) | 106,670 | 21,334 | 40.8% | 10.62 (10.56-10.69) |
| Spinal meninges (C70.1) | 7,591 | 1,518 | 1.7% | 0.40 (0.39-0.41) | 1,662 | 332 | 0.9% | 0.19 (0.18-0.20) | 5,929 | 1,186 | 2.3% | 0.58 (0.57-0.60) |
| Meninges, NOS (C70.9) | 24,478 | 4,896 | 5.5% | 1.31 (1.29-1.33) | 6,757 | 1,351 | 3.7% | 0.79 (0.77-0.81) | 17,721 | 3,544 | 6.8% | 1.77 (1.74-1.80) |
| Cerebrum (C71.0) | 7,460 | 1,492 | 1.7% | 0.43 (0.42-0.44) | 3,978 | 796 | 2.2% | 0.47 (0.46-0.49) | 3,482 | 696 | 1.3% | 0.39 (0.37-0.40) |
| Frontal, temporal, parietal, and occipital lobes of the brain (C71.1-C71.4) | 76,878 | 15,376 | 17.2% | 4.20 (4.17-4.23) | 43,063 | 8,613 | 23.4% | 4.96 (4.92-5.01) | 33,815 | 6,763 | 12.9% | 3.52 (3.49-3.56) |
| Frontal lobe (C71.1) | 33,973 | 6,795 | 7.6% | 1.88 (1.86-1.90) | 18,138 | 3,628 | 9.8% | 2.12 (2.09-2.15) | 15,835 | 3,167 | 6.1% | 1.67 (1.65-1.70) |
| Temporal lobe (C71.2) | 24,837 | 4,967 | 5.6% | 1.35 (1.33-1.36) | 14,876 | 2,975 | 8.1% | 1.70 (1.68-1.73) | 9,961 | 1,992 | 3.8% | 1.03 (1.01-1.06) |
| Parietal lobe (C71.3) | 14,252 | 2,850 | 3.2% | 0.76 (0.75-0.77) | 7,945 | 1,589 | 4.3% | 0.90 (0.88-0.92) | 6,307 | 1,261 | 2.4% | 0.64 (0.62-0.66) |
| Occipital lobe (C71.4) | 3,816 | 763 | 0.9% | 0.21 (0.20-0.21) | 2,104 | 421 | 1.1% | 0.24 (0.23-0.25) | 1,712 | 342 | 0.7% | 0.18 (0.17-0.18) |
| Ventricle (C71.5) | 4,109 | 822 | 0.9% | 0.25 (0.24-0.26) | 2,279 | 456 | 1.2% | 0.28 (0.27-0.29) | 1,830 | 366 | 0.7% | 0.22 (0.21-0.23) |
| Cerebellum (C71.6) | 9,291 | 1,858 | 2.1% | 0.58 (0.57-0.59) | 5,028 | 1,006 | 2.7% | 0.64 (0.62-0.65) | 4,263 | 853 | 1.6% | 0.52 (0.51-0.54) |
| Brain stem (C71.7) | 6,113 | 1,223 | 1.4% | 0.39 (0.38-0.40) | 3,284 | 657 | 1.8% | 0.42 (0.40-0.43) | 2,829 | 566 | 1.1% | 0.36 (0.35-0.38) |
| Other brain (C71.8-C71.9) | 33,701 | 6,740 | 7.6% | 1.84 (1.82-1.86) | 18,023 | 3,605 | 9.8% | 2.11 (2.08-2.15) | 15,678 | 3,136 | 6.0% | 1.61 (1.59-1.64) |
| Overlapping lesion of brain (C71.8) | 12,794 | 2,559 | 2.9% | 0.69 (0.68-0.70) | 7,210 | 1,442 | 3.9% | 0.83 (0.81-0.85) | 5,584 | 1,117 | 2.1% | 0.57 (0.55-0.58) |
| Brain, NOS (C71.9) | 20,907 | 4,181 | 4.7% | 1.16 (1.14-1.17) | 10,813 | 2,163 | 5.9% | 1.29 (1.26-1.31) | 10,094 | 2,019 | 3.9% | 1.04 (1.02-1.07) |
| Spinal cord and cauda equina (C72.0-C72.1) | 12,700 | 2,540 | 2.8% | 0.74 (0.73-0.76) | 6,761 | 1,352 | 3.7% | 0.81 (0.79-0.83) | 5,939 | 1,188 | 2.3% | 0.68 (0.66-0.70) |
| Spinal cord (C72.0) | 12,303 | 2,461 | 2.8% | 0.72 (0.71-0.73) | 6,560 | 1,312 | 3.6% | 0.79 (0.77-0.81) | 5,743 | 1,149 | 2.2% | 0.66 (0.64-0.68) |
| Cauda equina (C72.1) | 397 | 79 | 0.1% | 0.02 (0.02-0.03) | 201 | 40 | 0.1% | 0.02 (0.02-0.03) | 196 | 39 | 0.1% | 0.02 (0.02-0.03) |
| Cranial nerves (C72.2-C72.5) | 30,488 | 6,098 | 6.8% | 1.69 (1.67-1.71) | 14,307 | 2,861 | 7.8% | 1.65 (1.62-1.68) | 16,181 | 3,236 | 6.2% | 1.74 (1.71-1.76) |
| Olfactory nerve (C72.2) | 42 | 8 | 0.0% | 0.00 (0.00-0.00) | 17 | 3 | 0.0% | 0.00 (0.00-0.00) | 25 | 5 | 0.0% | 0.00 (0.00-0.00) |
| Optic nerve (C72.3) | 1,782 | 356 | 0.4% | 0.12 (0.12-0.13) | 859 | 172 | 0.5% | 0.12 (0.11-0.12) | 923 | 185 | 0.4% | 0.13 (0.12-0.13) |
| Acoustic nerve (C72.4) | 22,509 | 4,502 | 5.0% | 1.22 (1.21-1.24) | 10,581 | 2,116 | 5.7% | 1.20 (1.18-1.23) | 11,928 | 2,386 | 4.6% | 1.25 (1.23-1.27) |
| Cranial nerve, NOS (C72.5) | 6,155 | 1,231 | 1.4% | 0.34 (0.34-0.35) | 2,850 | 570 | 1.5% | 0.33 (0.32-0.34) | 3,305 | 661 | 1.3% | 0.36 (0.35-0.37) |
| Other nervous system (C72.8-C72.9) | 2,506 | 501 | 0.6% | 0.14 (0.13-0.15) | 1,266 | 253 | 0.7% | 0.15 (0.14-0.16) | 1,240 | 248 | 0.5% | 0.13 (0.13-0.14) |
| Overlapping lesion of brain & CNS (C72.8) | 348 | 70 | 0.1% | 0.02 (0.02-0.02) | 188 | 38 | 0.1% | 0.02 (0.02-0.03) | 160 | 32 | 0.1% | 0.02 (0.01-0.02) |
| Nervous system, NOS (C72.9) | 2,158 | 432 | 0.5% | 0.12 (0.12-0.13) | 1,078 | 216 | 0.6% | 0.13 (0.12-0.14) | 1,080 | 216 | 0.4% | 0.12 (0.11-0.12) |
| Pituitary and craniopharyngeal duct (C75.1- C75.2) | 81,005 | 16,201 | 18.2% | 4.74 (4.71-4.78) | 35,971 | 7,194 | 19.5% | 4.23 (4.18-4.27) | 45,034 | 9,007 | 17.2% | 5.35 (5.30-5.40) |
| Pituitary gland (C75.1) | 78,859 | 15,772 | 17.7% | 4.62 (4.58-4.65) | 34,845 | 6,969 | 18.9% | 4.09 (4.05-4.13) | 44,014 | 8,803 | 16.8% | 5.23 (5.18-5.28) |
| Craniopharyngeal duct (C75.2) | 2,146 | 429 | 0.5% | 0.13 (0.12-0.14) | 1,126 | 225 | 0.6% | 0.14 (0.13-0.15) | 1,020 | 204 | 0.4% | 0.12 (0.11-0.13) |
| Pineal (C75.3) | 1,694 | 339 | 0.4% | 0.11 (0.10-0.11) | 997 | 199 | 0.5% | 0.13 (0.12-0.14) | 697 | 139 | 0.3% | 0.09 (0.08-0.09) |
| TOTAL | 445,792 | 89,158 | 100.0% | 24.71 (24.63-24.78) | 184,168 | 36,834 | 100.0% | 21.60 (21.50-21.70) | 261,624 | 52,325 | 100.0% | 27.62 (27.51-27.73) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cThe sites referred to in this table are loosely based on the categories and site codes defined in the SEER site/histopathology validation list.
dICD-O-3 histopathology codes 9522-9523 only.
- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; US, United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, Not otherwise specified.
Table 5.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for All Brain and Other Central Nervous System Tumors by Major Histopathology Groupings, Histopathology, Behavior, and Sex, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | Total | Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | % of all tumors | Median Age | Rate (95% CI) | 5-Year Total |
Annual Average | % Malignantc | Rate (95% CI) | 5-Year Total | Annual Average | % Malignantc | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 84,012 | 16,802 | 18.8 | 63 | 4.50 (4.47-4.53) | 48,292 | 9,658 | 100.0 | 5.48 (5.43-5.53) | 35,720 | 7,144 | 100.0 | 3.64 (3.60-3.68) |
| Diffuse astrocytoma | 7,634 | 1,527 | 1.7 | 45 | 0.46 (0.45-0.47) | 4,270 | 854 | 99.9 | 0.52 (0.51-0.54) | 3,364 | 673 | 99.9 | 0.39 (0.38-0.41) |
| Anaplastic astrocytoma | 7,046 | 1,409 | 1.6 | 52 | 0.41 (0.40-0.42) | 3,847 | 769 | 100.0 | 0.46 (0.45-0.48) | 3,199 | 640 | 100.0 | 0.36 (0.35-0.37) |
| Glioblastoma | 63,258 | 12,652 | 14.2 | 65 | 3.26 (3.24-3.29) | 36,826 | 7,365 | 100.0 | 4.08 (4.04-4.12) | 26,432 | 5,286 | 100.0 | 2.55 (2.52-2.59) |
| Oligodendroglioma | 3,687 | 737 | 0.8 | 44 | 0.23 (0.22-0.24) | 2,037 | 407 | 100.0 | 0.26 (0.25-0.27) | 1,650 | 330 | 100.0 | 0.20 (0.19-0.22) |
| Anaplastic oligodendroglioma | 1,871 | 374 | 0.4 | 49 | 0.11 (0.11-0.12) | 1,038 | 208 | 99.8 | 0.13 (0.12-0.14) | 833 | 167 | 100.0 | 0.10 (0.09-0.10) |
| Oligoastrocytic tumors | 516 | 103 | 0.1 | 45 | 0.03 (0.03-0.03) | 274 | 55 | 100.0 | 0.03 (0.03-0.04) | 242 | 48 | 100.0 | 0.03 (0.03-0.03) |
| Other Astrocytic Tumors | 6,252 | 1,250 | 1.4 | 12 | 0.42 (0.41-0.43) | 3,293 | 659 | 93.3 | 0.44 (0.43-0.46) | 2,959 | 592 | 94.5 | 0.41 (0.39-0.42) |
| Pilocytic astrocytoma | 5,339 | 1,068 | 1.2 | 11 | 0.36 (0.35-0.37) | 2,805 | 561 | 100.0 | 0.38 (0.36-0.39) | 2,534 | 507 | 100.0 | 0.35 (0.34-0.36) |
| Unique astrocytoma variants | 913 | 183 | 0.2 | 17 | 0.06 (0.06-0.06) | 488 | 98 | 55.1 | 0.06 (0.06-0.07) | 425 | 85 | 61.9 | 0.06 (0.05-0.06) |
| Non-Malignant | 381 | 76 | 0.1 | -- | 0.03 (0.02-0.03) | 219 | 44 | -- | 0.03 (0.03-0.03) | 162 | 32 | -- | 0.02 (0.02-0.03) |
| Malignant | 532 | 106 | 0.1 | -- | 0.03 (0.03-0.04) | 269 | 54 | -- | 0.03 (0.03-0.04) | 263 | 53 | -- | 0.03 (0.03-0.04) |
| Ependymal Tumors | 6,911 | 1,382 | 1.6 | 45 | 0.42 (0.41-0.43) | 3,964 | 793 | 53.6 | 0.49 (0.47-0.50) | 2,947 | 589 | 60.6 | 0.35 (0.34-0.37) |
| Non-Malignant | 3,002 | 600 | 0.7 | -- | 0.18 (0.17-0.19) | 1,840 | 368 | -- | 0.22 (0.21-0.23) | 1,162 | 232 | -- | 0.14 (0.13-0.14) |
| Malignant | 3,909 | 782 | 0.9 | -- | 0.24 (0.23-0.25) | 2,124 | 425 | -- | 0.26 (0.25-0.28) | 1,785 | 357 | -- | 0.22 (0.21-0.23) |
| Other Gliomas | 8,854 | 1,771 | 2.0 | 37 | 0.55 (0.53-0.56) | 4,476 | 895 | 99.6 | 0.57 (0.55-0.59) | 4,378 | 876 | 99.5 | 0.53 (0.51-0.55) |
| Glioma malignant, NOS | 8,753 | 1,751 | 2.0 | 37 | 0.54 (0.53-0.55) | 4,437 | 887 | 100.0 | 0.56 (0.55-0.58) | 4,316 | 863 | 100.0 | 0.52 (0.51-0.54) |
| Other neuroepithelial tumors | 101 | 20 | 0.0 | 32 | 0.01 (0.01-0.01) | 39 | 8 | 48.7 | 0.00 (0.00-0.01) | 62 | 12 | 66.1 | 0.01 (0.01-0.01) |
| Non-Malignant | 41 | 8 | 0.0 | -- | 0.00 (0.00-0.00) | 20 | 4 | -- | 0.00 (0.00-0.00) | 21 | 4 | -- | 0.00 (0.00-0.00) |
| Malignant | 60 | 12 | 0.0 | -- | 0.00 (0.00-0.00) | 19 | 4 | -- | 0.00 (0.00-0.00) | 41 | 8 | -- | 0.01 (0.00-0.01) |
| Neuronal and Mixed Neuronal-Glial Tumors | 5,339 | 1,068 | 1.2 | 26 | 0.34 (0.33-0.35) | 2,911 | 582 | 18.2 | 0.37 (0.36-0.39) | 2,428 | 486 | 17.8 | 0.31 (0.30-0.32) |
| Non-Malignant | 4,379 | 876 | -- | -- | 0.29 (0.28-0.29) | 2,382 | 476 | -- | 0.31 (0.30-0.32) | 1,997 | 399 | -- | 0.26 (0.25-0.27) |
| Malignant | 960 | 192 | -- | -- | 0.06 (0.05-0.06) | 529 | 106 | -- | 0.06 (0.06-0.07) | 431 | 86 | -- | 0.05 (0.04-0.05) |
| Choroid Plexus Tumors | 827 | 165 | 0.2 | 20 | 0.05 (0.05-0.06) | 415 | 83 | 17.6 | 0.05 (0.05-0.06) | 412 | 82 | 11.7 | 0.05 (0.05-0.06) |
| Non-Malignant | 706 | 141 | -- | -- | 0.04 (0.04-0.05) | 342 | 68 | -- | 0.04 (0.04-0.05) | 364 | 73 | -- | 0.05 (0.04-0.05) |
| Malignant | 121 | 24 | -- | -- | 0.01 (0.01-0.01) | 73 | 15 | -- | 0.01 (0.01-0.01) | 48 | 10 | -- | 0.01 (0.00-0.01) |
| Tumors of the Pineal Region | 769 | 154 | 0.2 | 34 | 0.05 (0.04-0.05) | 322 | 64 | 69.6 | 0.04 (0.04-0.05) | 447 | 89 | 53.2 | 0.06 (0.05-0.06) |
| Non-Malignant | 307 | 61 | 0.1 | -- | 0.02 (0.02-0.02) | 98 | 20 | -- | 0.01 (0.01-0.01) | 209 | 42 | -- | 0.03 (0.02-0.03) |
| Malignant | 462 | 92 | 0.1 | -- | 0.03 (0.03-0.03) | 224 | 45 | -- | 0.03 (0.02-0.03) | 238 | 48 | -- | 0.03 (0.03-0.03) |
| Embryonal Tumors | 3,170 | 634 | 0.7 | 8 | 0.22 (0.21-0.22) | 1,909 | 382 | 100.0 | 0.26 (0.25-0.27) | 1,261 | 252 | 99.8 | 0.18 (0.17-0.19) |
| Tumors of Cranial and Paraspinal Nerves | 37,048 | 7,410 | 8.3 | 58 | 2.05 (2.03-2.07) | 17,785 | 3,557 | 0.6 | 2.05 (2.02-2.08) | 19,263 | 3,853 | 0.5 | 2.06 (2.03-2.09) |
| Nerve sheath tumors | 37,015 | 7,403 | 8.3 | 58 | 2.05 (2.03-2.07) | 17,764 | 3,553 | 0.6 | 2.05 (2.02-2.08) | 19,251 | 3,850 | 0.5 | 2.06 (2.03-2.09) |
| Non-Malignant | 36,804 | 7,361 | 8.3 | -- | 2.04 (2.02-2.06) | 17,658 | 3,532 | -- | 2.04 (2.01-2.07) | 19,146 | 3,829 | -- | 2.05 (2.02-2.08) |
| Malignant | 211 | 42 | 0.0 | -- | 0.01 (0.01-0.01) | 106 | 21 | -- | 0.01(0.01-0.02 | 105 | 21 | -- | 0.01 (0.01-0.01) |
| Other tumors of cranial and paraspinal nerves | 33 | 7 | 0.0 | 55 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 184,405 | 36,881 | 41.4 | 66 | 9.85 (9.81-9.90) | 51,371 | 10,274 | 2.3 | 6.00 (5.95-6.05) | 133,034 | 26,607 | 1.0 | 13.28 (13.21-13.36) |
| Meningiomas | 178,447 | 35,689 | 40.0 | 67 | 9.51 (9.46-9.55) | 48,335 | 9,667 | 1.5 | 5.64 (5.59-5.69) | 130,112 | 26,022 | 0.7 | 12.95 (12.87-13.02) |
| Non-Malignant | 176,832 | 35,366 | 39.7 | -- | 9.42 (9.38-9.47) | 47,602 | 9,520 | -- | 5.55 (5.50-5.60) | 129,230 | 25,846 | -- | 12.86 (12.79-12.93) |
| Malignant | 1,615 | 323 | 0.4 | -- | 0.09 (0.08-0.09) | 733 | 147 | -- | 0.08 (0.08-0.09) | 882 | 176 | -- | 0.09 (0.08-0.09) |
| Mesenchymal tumors | 5,815 | 1,163 | 1.3 | 51 | 0.34 (0.33-0.35) | 2,953 | 591 | 13.6 | 0.35 (0.34-0.37) | 2,862 | 572 | 12.7 | 0.33 (0.32-0.34) |
| Non-Malignant | 5,049 | 1,010 | 1.1 | -- | 0.30 (0.29-0.30) | 2,551 | 510 | -- | 0.30 (0.29-0.32) | 2,498 | 500 | -- | 0.29 (0.28-0.30) |
| Malignant | 766 | 153 | 0.2 | -- | 0.04 (0.04-0.05) | 402 | 80 | -- | 0.05 (0.04-0.05) | 364 | 73 | -- | 0.04 (0.04-0.05) |
| Primary melanocytic lesions | 143 | 29 | 0.0 | 61 | 0.01 (0.01-0.01) | 83 | 17 | 69.9 | 0.01 (0.01-0.01) | 60 | 12 | 51.7 | 0.01 (0.00-0.01) |
| Non-Malignant | 54 | 11 | 0.0 | -- | 0.00 (0.00-0.00) | 25 | 5 | -- | 0.00 (0.00-0.00) | 29 | 6 | -- | 0.00 (0.00-0.00) |
| Malignant | 89 | 18 | 0.0 | -- | 0.00 (0.00-0.01) | 58 | 12 | -- | 0.01 (0.01-0.01) | 31 | 6 | -- | 0.00 (0.00-0.00) |
| Lymphomas and Hematopoietic Neoplasms | 8,525 | 1,705 | 1.9 | 67 | 0.45 (0.44-0.46) | 4,352 | 870 | 99.9 | 0.49 (0.48-0.51) | 4,173 | 835 | 99.8 | 0.41 (0.39-0.42) |
| Lymphoma | 8,482 | 1,696 | 1.9 | 67 | 0.45 (0.44-0.46) | 4,328 | 866 | 99.9 | 0.49 (0.48-0.51) | 4,154 | 831 | 99.8 | 0.41 (0.39-0.42) |
| Other hematopoietic neoplasms | 43 | 9 | 0.0 | 59 | 0.00 (0.00-0.00) | 24 | 5 | 95.8 | 0.00 (0.00-0.00) | 19 | 4 | 94.7 | 0.00 (0.00-0.00) |
| Germ Cell Tumors | 1,280 | 256 | 0.3 | 15 | 0.09 (0.08-0.09) | 942 | 188 | 89.4 | 0.12 (0.12-0.13) | 338 | 68 | 79.3 | 0.05 (0.04-0.05) |
| Non-Malignant | 170 | 34 | 0.0 | -- | 0.01 (0.01-0.01) | 100 | 20 | -- | 0.01 (0.01-0.02) | 70 | 14 | -- | 0.01 (0.01-0.01) |
| Malignant | 1,110 | 222 | 0.2 | -- | 0.07 (0.07-0.08) | 842 | 168 | -- | 0.11 (0.10-0.12) | 268 | 54 | -- | 0.04 (0.03-0.04) |
| Tumors of Sellar Region | 79,996 | 15,999 | 17.9 | 51 | 4.69 (4.65-4.72) | 35,594 | 7,119 | 0.2 | 4.18 (4.14-4.23) | 44,402 | 8,880 | 0.1 | 5.28 (5.23-5.33) |
| Tumors of the pituitary | 76,863 | 15,373 | 17.2 | 51 | 4.50 (4.47-4.53) | 33,981 | 6,796 | 0.2 | 3.98 (3.94-4.03) | 42,882 | 8,576 | 0.1 | 5.10 (5.05-5.15) |
| Non-Malignant | 76,755 | 15,351 | 17.2 | -- | 4.49 (4.46-4.52) | 33,913 | 6,783 | -- | 3.98 (3.93-4.02) | 42,842 | 8,568 | -- | 5.09 (5.04-5.14) |
| Malignant | 108 | 22 | 0.0 | -- | 0.01 (0.00-0.01) | 68 | 14 | -- | 0.01 (0.01-0.01) | 40 | 8 | -- | 0.00 (0.00-0.01) |
| Craniopharyngioma | 3,133 | 627 | 0.7 | 45 | 0.19 (0.18-0.20) | 1,613 | 323 | 0.4 | 0.20 (0.19-0.21) | 1,520 | 304 | 0.1 | 0.18 (0.17-0.19) |
| Unclassified Tumors | 18,404 | 3,681 | 4.1 | 65 | 1.03 (1.02-1.05) | 8,542 | 1,708 | 38.5 | 1.05 (1.02-1.07) | 9,862 | 1,972 | 35.1 | 1.02 (1.00-1.04) |
| Hemangioma | 4,215 | 843 | 0.9 | 50 | 0.25 (0.24-0.26) | 1,956 | 391 | 0.0 | 0.24 (0.23-0.25) | 2,259 | 452 | 0.0 | 0.26 (0.25-0.27) |
| Neoplasm, unspecified | 13,647 | 2,729 | 3.1 | 70 | 0.75 (0.73-0.76) | 6,292 | 1,258 | 51.8 | 0.77 (0.75-0.79) | 7,355 | 1,471 | 46.7 | 0.73 (0.71-0.75) |
| Non-Malignant | 6,954 | 1,391 | 1.6 | -- | 0.39 (0.38-0.40) | 3,032 | 606 | -- | 0.37 (0.36-0.39) | 3,922 | 784 | -- | 0.41 (0.40-0.42) |
| Malignant | 6,693 | 1,339 | 1.5 | -- | 0.36 (0.35-0.36) | 3,260 | 652 | -- | 0.40 (0.38-0.41) | 3,433 | 687 | -- | 0.32 (0.31-0.33) |
| All other | 542 | 108 | 0.1 | 37.5 | 0.03 (0.03-0.04) | 294 | 59 | 9.2 | 0.04 (0.03-0.04) | 248 | 50 | 12.9 | 0.03 (0.03-0.03) |
| Non-Malignant | 483 | 97 | 0.1 | -- | 0.03 (0.03-0.04) | 267 | 53 | -- | 0.03 (0.03-0.04) | 216 | 43 | -- | 0.03 (0.02-0.03) |
| Malignant | 59 | 12 | 0.0 | -- | 0.00 (0.00-0.01) | 27 | 5 | -- | 0.00 (0.00-0.01) | 32 | 6 | -- | 0.00 (0.00-0.01) |
| TOTAL d | 445,792 | 89,158 | 100.0 | 61 | 24.71 (24.63-24.78) | 184,168 | 36,834 | 38.3 | 21.60 (21.50-21.70) | 261,624 | 52,325 | 21.4 | 27.62 (27.51-27.73) |
| Non-Malignant | 319,447 | 63,889 | 71.7 | -- | 17.69 (17.62-17.75) | 113,709 | 22,742 | -- | 13.36 (13.28-13.44) | 205,738 | 41,148 | -- | 21.68 (21.58-21.78) |
| Malignant | 126,345 | 25,269 | 28.3 | -- | 7.02 (6.98-7.06) | 70,459 | 14,092 | -- | 8.24 (8.18-8.30) | 55,886 | 11,177 | -- | 5.94 (5.89-5.99) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cAssigned behavior code of /3 (see Table 2).
dRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
Distribution of Tumors by Site and Histopathology
The distribution of brain and other CNS tumors by site is shown in Figure 12A and Table 4.
Fig. 12.
Distributiona of All Primary Brain and Other Central Nervous System Tumors (Malignant and Non-Malignant Combined; Five-Year Total=445,792; Annual Average Cases=89,158) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Overall, the most common tumor site was the meninges, representing 40.2% of all tumors.
Frontal (7.6%), temporal (5.6%), parietal (3.2%), and occipital lobes (0.9%) accounted for 17.2% of all tumors.
The cranial nerves (6.8%) and the spinal cord/cauda equina (2.8%) accounted for 9.7% of all tumors.
The pituitary and craniopharyngeal duct accounted for 18.2% of all tumors.
The distribution by brain and other CNS histopathologies is shown in Figure 12B and Table 5.
The most frequently reported histopathologies overall were meningiomas (40.0%), followed by tumors of the pituitary (17.2%) and glioblastoma (14.2%).
Tumors of the pituitary (17.2%) and nerve sheath tumors (8.3%) combined accounted for slightly more than one-fourth of all tumors (25.5%), the vast majority of which were non-malignant.
Distribution of Tumors by Site, Histopathology and Behavior
The distribution of malignant and non-malignant brain and other CNS tumors by site are shown in Figure 5A and Figure 10A, respectively.
For malignant tumors, frontal (24.6%), temporal (17.6%), parietal (10.4%), and occipital (2.6%) accounted for 55.2% of tumors (Figure 5A).
For non-malignant tumors, 55.4% of all tumors occurred in the meninges (Figure 10A).
The distribution of malignant and non-malignant brain and other CNS tumors by histopathology are shown in Figure 5B and Figure 10B, respectively, as well as in Table 5.
The most common of all malignant CNS tumor histopathologies was glioblastoma (50.1%, Figure 5B).
The most common of all non-malignant tumor histopathologies was meningioma (55.4%, Figure 10B).
The most common non-malignant nerve sheath tumor (based on multiple sites in the brain and CNS) was vestibular schwannoma (defined by histopathology code 9560, also formerly called acoustic neuromas) (75.7%, Table 6).
Table 6.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for Selected Non-Malignant Histopathologies by Sex, Age Group at Diagnosis, Race, Hispanic Ethnicity, and Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Group | Vestibular Schwannomac | Pituitary Adenomad | WHO Grade I Meningiomae | WHO Grade II Meningiomaf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Sex | ||||||||||||
| Male | 13,078 | 2,616 | 1.49 (1.47-1.52) | 29,868 | 5,974 | 3.49 (3.45-3.53) | 16,042 | 3,208 | 1.84 (1.81-1.87) | 4,050 | 810 | 0.46 (0.45-0.48) |
| Female | 14,795 | 2,959 | 1.56 (1.53-1.58) | 37,282 | 7,456 | 4.41 (4.36-4.45) | 43,389 | 8,678 | 4.47 (4.43-4.51) | 5,653 | 1,131 | 0.60 (0.58-0.61) |
| Age Groups | ||||||||||||
| 0-14 years | 213 | 43 | 0.07 (0.06-0.08) | 937 | 187 | 0.31 (0.29-0.33) | 108 | 22 | 0.04 (0.03-0.04) | 78 | 16 | 0.03 (0.02-0.03) |
| 15-39 years | 3,687 | 737 | 0.70 (0.68-0.72) | 19,150 | 3,830 | 3.56 (3.51-3.61) | 4,552 | 910 | 0.89 (0.87-0.92) | 1,063 | 213 | 0.20 (0.19-0.22) |
| 40-64 years | 14,399 | 2,880 | 2.59 (2.54-2.63) | 28,324 | 5,665 | 5.40 (5.34-5.47) | 27,964 | 5,593 | 5.03 (4.97-5.09) | 4,578 | 916 | 0.83 (0.80-0.85) |
| 65+ years | 9,574 | 1,915 | 3.74 (3.66-3.82) | 18,739 | 3,748 | 7.45 (7.34-7.56) | 26,807 | 5,361 | 10.73 (10.60-10.86) | 3,984 | 797 | 1.59 (1.54-1.64) |
| Race | ||||||||||||
| White | 23,866 | 4,773 | 1.62 (1.59-1.64) | 47,724 | 9,545 | 3.51 (3.48-3.55) | 47,661 | 9,532 | 3.15 (3.12-3.18) | 7,354 | 1,471 | 0.49 (0.48-0.51) |
| Black | 1,546 | 309 | 0.70 (0.67-0.74) | 13,520 | 2,704 | 6.24 (6.13-6.35) | 7,662 | 1,532 | 3.61 (3.53-3.69) | 1,547 | 309 | 0.71 (0.68-0.75) |
| American Indian/Alaska Native | 161 | 32 | 0.76 (0.64-0.89) | 614 | 123 | 2.90 (2.66-3.15) | 409 | 82 | 2.13 (1.92-2.35) | 57 | 11 | 0.30 (0.22-0.39) |
| Asian or Pacific Islander | 1,347 | 269 | 1.25 (1.18-1.32) | 2,883 | 577 | 2.71 (2.61-2.81) | 2,325 | 465 | 2.23 (2.13-2.32) | 507 | 101 | 0.49 (0.44-0.53) |
| Hispanic Ethnicity | ||||||||||||
| Non-Hispanic | 25,447 | 5,089 | 1.61 (1.59-1.63) | 55,782 | 11,156 | 3.82 (3.78-3.85) | 53,374 | 10,675 | 3.28 (3.25-3.31) | 8,731 | 1,746 | 0.55 (0.54-0.56) |
| Hispanic | 2,426 | 485 | 1.03 (0.99-1.07) | 11,368 | 2,274 | 4.51 (4.42-4.59) | 6,057 | 1,211 | 2.77 (2.69-2.84) | 972 | 194 | 0.43 (0.41-0.46) |
| TOTAL | 27,873 | 5,575 | 1.52 (1.50-1.54) | 67,150 | 13,430 | 3.91 (3.88-3.94) | 59,431 | 11,886 | 3.21 (3.18-3.24) | 9,703 | 1,941 | 0.53 (0.52-0.54) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cICD-O-3 histopathology code 9560/0 and ICD-O-3 topography code C72.4 and C72.5.
dICD-O-3 histopathology code 8272/0 and ICD-O-3 topography code C75.1.
eICD-O-3 histopathology codes 9530/0, 9531/0, 9532/0, 9533/0, 9534/0, and 9537/0. WHO grade may be reported according to 2007 or 2016 WHO classification depending on year of diagnosis, in which roman numerals are used to denote tumor grade.
fICD-O-3 histopathology codes 9530/1, 9531/1, 9532/1, 9533/1, 9534/1, 9537/1, 9538/1, and 9539/1. WHO grade may be reported according to 2007 or 2016 WHO classification depending on year of diagnosis, in which roman numerals are used to denote tumor grade.
- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; US, United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; WHO, World Health Organization; CI, confidence interval.
Distribution of Gliomas by Site and Histopathology
The broad category glioma (ICD-O-3 histopathology codes 9380–9384, 9391–9460 see Table 2 for more information) represented approximately 24% of all primary brain and other CNS tumors and 80.9% of malignant tumors. The distribution of gliomas by site and histopathology are shown in Figure 7A and Figure 7B, respectively.
The majority of gliomas occurred in the supra-tentorium (frontal, temporal, parietal, and occipital lobes combined) (61.8%). Only a very small proportion of gliomas occurred in areas of the CNS other than the brain.
Glioblastoma accounted for the majority of gliomas (59.2%).
Astrocytic tumors, including glioblastoma, accounted for 78% of all gliomas.
Incidence by Year and Behavior
Figure 13 presents the overall AAAIRs of all primary brain and other CNS tumors by year (2015-2019) and behavior. Incidence rates for all primary brain and other CNS tumors, 2015-2019, did not differ substantially by year (both overall and by behavior).
Fig. 13.
Annual Age-Adjusted Incidence Ratesa of All Primary Brain and Other Central Nervous System Tumors by Year and Behavior, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Incidence Rates by Major Histopathology Grouping, Specific Histopathology, and Behavior
AAAIRs overall by major histopathology grouping, specific histopathology, and behavior are shown in Table 5. Among CBTRUS major histopathology groupings, incidence rates were highest for tumors of the meninges (9.85 per 100,000 population), followed by tumors of the sellar region (4.69 per 100,000 population), diffuse astrocytic and oligodendroglial tumors (4.50 per 100,000 population), and tumors of the cranial and spinal nerves (2.05 per 100,000 population).
Among CBTRUS specific histopathology groupings, incidence rates were highest for meningiomas (9.51 per 100,000 population), tumors of the pituitary (4.5 per 100,000 population), glioblastoma (3.26 per 100,000 population), and nerve sheath tumors (2.05 per 100,000 population).
The majority of nerve sheath tumors were vestibular schwannoma (1.52 per 100,000, Table 6).
Of all vestibular schwannoma tumors, 62.5% were located in the acoustic nerve (Supplementary Figure 1).
For malignant tumors, the incidence rate was highest for glioblastoma (3.26 per 100,000 population), followed by glioma malignant, NOS (0.54 per 100,000), diffuse astrocytomas (0.46 per 100,000 population) and lymphomas (0.45 per 100,000 population).
For non-malignant tumors, the incidence rate was highest for non-malignant meningiomas (9.42 per 100,000 population), followed by non-malignant tumors of the pituitary (4.49 per 100,000 population).
Brain Molecular Marker Variable and Other Biomarkers
Biomarkers for Glioma
IDH mutation and 1/19q status
Gliomas, as the most common malignant primary brain and other CNS tumor type, have been subject to the greatest investigation. A recent review has described in detail the current state of glioma biomarker research.61 One of the earliest discoveries in glioma biomarkers was that oligodendroglioma often had large deletions (missing parts of the chromosome, also known as loss of heterozygosity) in the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q).62 In general, these deletions significantly predict positive response to chemotherapy and radiation treatment in oligodendroglioma and anaplastic oligodendroglioma.63-65 Mutations to the genes in IDH1 and in IDH2 have also been shown to be associated with improved prognosis in glioma.66-68 These mutations are common in lower grade gliomas (WHO grade II and WHO grade III), but are rare in glioblastoma.67 The combination of these two factors can be used to more accurately stratify glioma by prognosis than the previously utilized histopathological criteria,68,69 and have been incorporated into the definition of oligodendroglioma and astrocytoma in the 2016 update to the WHO classification.2
MGMT Methylation
Another alteration that is associated with improved survival in glioma is increased methylation (where methyl molecules are bonded to the DNA) of the promotor region of MGMT.70,71 MGMT is a DNA repair protein, and methylation of its promoter region effectively silences the gene and prevents transcription into RNA. It is assumed that the decreases in protein levels increase sensitivity to the alkylating chemotherapies (e.g. temozolomide) often used in the treatment of gliomas aimed to combat tumor growth through DNA damage.72 This alteration is common in glioblastoma and less common in lower grade glioma.
Other Biomarkers
Diffuse intrinsic pontine glioma (DIPG) is a name given to a group of aggressive tumors occuring in the pons that occurs primarily in children. In the 2016 WHO classification, these tumors are classified as diffuse midline glioma, H3 K27M-mutant (ICD-O-3 histopathology code 9385/3). These account for ~75% of brain stem tumors in children. Survival is very poor after diagnoses with these tumors. Due to the location of these tumors, they are often not biopsied and, therefore, have not been molecularly characterized to the extent of many other primary brain and other CNS tumor types. Recently, biopsy and autopsy protocols have allowed for collection of primary tumor samples that have been used for genomic profiling.73-75 These tumors have been found to be highly heterogeneous. Mutations in histone H3, Activin A receptor, type I (ACVR1), tumor protein p53 (TP53), platelet-derived growth factor receptor A (PDGFRA), phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA), and Myc (MYC) have been identified as characteristic of these tumors.74,76,77 A recent review has further summarized current developments in the genomics of DIPG.78
Biomarkers for Embryonal Tumors
Medulloblastoma Subtypes
Medulloblastoma is another tumor type that has been subject to significant molecular analysis. Using an analysis of gene expression (based on quantity of RNA transcribed from a gene), medulloblastoma was able to be subdivided into four distinct subtypes: wingless (WNT), sonic hedgehog (SHH), group 3 (also called group C), and group 4 (also called group D).79 These groups are associated with specific age groups, with SHH being most common in infants and adults, and all other groups being more common in childhood. Several review articles have elaborated on the details of these subgroups and their implications for diagnosis and treatment.80-82
Completeness of Molecular Markers
The BMM variable and molecularly-defined ICD-O-3 codes are specific to certain histopathologies (please see Supplementary Tables 2 and 3). Frequency of reported molecular markers for relevant histopathologies are shown in Table 7. Completeness of molecular marker reporting using BMM variable is shown in Figure 3.
Table 7.
Distribution of Brain Molecular Markers for Select Histopathologically-Confirmed Glioma and Embryonal Tumor Histopathologies, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2018-2019
| Histopathology | Frequency (%) |
|---|---|
| Diffuse Astrocytoma | |
| 9400/3: Diffuse astrocytoma, IDH-mutant a | 1,103 (42.7%) |
| 9400/3: Diffuse astrocytoma, IDH-wildtype a | 883 (34.2%) |
| 9400/3: Diffuse astrocytoma, IDH Status Unknown | 599 (23.2%) |
| Anaplastic Astrocytoma | |
| 9401/3: Anaplastic astrocytoma, IDH-mutant a | 1,159 (43.6%) |
| 9401/3: Anaplastic astrocytoma, IDH-wildtype a | 1,167 (43.9%) |
| 9401/3: Anaplastic astrocytoma, IDH Status Unknown | 331 (12.5%) |
| Glioblastoma | |
| 9440/3: Glioblastoma, IDH-wildtype a | 18,579 (76.8%) |
| 9440/3: Glioblastoma, IDH Status Unknown | 4,398 (18.2%) |
| 9441/3: Giant cell glioblastoma | 155 (0.6%) |
| 9442/3: Gliosarcoma | 475 (2%) |
| 9445/3: Glioblastoma, IDH-mutant b | 580 (2.4%) |
| Oligodendroglioma | |
| 9450/3: Oligodendroglioma, IDH-mutant and 1 p/19q co-deleted a | 1,227 (90.8%) |
| 9450/3: Oligodendroglioma, NOS | 124 (9.2%) |
| Anaplastic Oligodendroglioma | |
| 9451/3: Anaplastic oligodendroglioma, IDH-mutant and 1 p/19q co-deleted a | 653 (93.3%) |
| 9451/3: Oligodendroglioma, anaplastic | 47 (6.7%) |
| Medulloblastoma | |
| 9470/3: Medulloblastoma, NOS | 404 (48.5%) |
| 9471/3: Desmoplastic nodular medulloblastoma | 30 (3.6%) |
| 9471/3: Medulloblastoma, SHH-activated and TP53-wildtype a | 161 (19.3%) |
| 9472/3: Medullomyoblastoma | -- |
| 9474/3: Large cell medulloblastoma | 59 (7.1%) |
| 9475/3: Medulloblastoma, WNT-activated, NOS b | 29 (3.5%) |
| 9476/3: Medulloblastoma, SHH-activated and TP53-mutant b | -- |
| 9477/3: Medulloblastoma, non-WNT/non-SHH b | 134 (16.1%) |
aCollected in NAACCR Item #3816, Brain Molecular Markers.
bNew ICD-O-3 codes implemented in 2018.
-- Cases and rates are not presented when fewer than 16 cases were reported for the specific category.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; US, United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; NOS, not otherwise specified.
Among glioblastoma patients, 580 were coded as 9445/3, Glioblastoma IDH-mutant (2.4%), 18,579 were coded as 9440/3, Glioblastoma IDH-wildtype (76.8%), and 4,398 as 9440/3, Glioblastoma IDH Status Unknown (18.2%). Among those with unknown IDH status, 74 had a test ordered, but no results reported in patient chart (1.6%), while the remaining patients did not have IDH status documented in their patient record, or the information was miscoded/unknown (4,320, 98.2%).
Frequency of IDH-mutation reporting was high in diffuse astrocytoma (9400/3, 76.8%) and anaplastic astrocytoma (9401/3, 87.5%). Biomarker reporting was complete in 90.8% of oligodendroglioma coded as 9450/3 and 93.3% of anaplastic oligodendroglioma coded as 9451/3.
For medulloblastoma coded as 9471/3, 84.3% had complete biomarker reporting.
Completeness of biomarker reporting improved for all relevant ICD-O-3 codes from 2018 to 2019.
Frequency and Incidence of Molecularly-Defined Brain and Other CNS Tumor Histopathologies
Beginning in diagnosis year 2018, US cancer registry systems began collecting data on molecularly defined histologies introduced in the 2016 WHO classification of tumours of the CNS, including IDH-mutation and 1p/19q codeletion status for adult-type diffuse glioma, and medulloblastoma subtypes. Total cases of these histopathologies diagnosed in 2018-2019, age-adjusted incidence rates, median age of diagnosis, and distribution by sex and race/ethnicity are shown in Table 8.
Table 8.
Annual Age-Adjusted Incidence Ratesa, Median Age at Diagnosis, Sex, and Race/Ethnicity of Histopathologically-Confirmed Molecularly-Defined Brain and Other Central Nervous System Tumors by WHO Gradeb for Diagnosis Years 2018-2019, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2018-2019
| Tumor type | ICD-O-3 Histopathology Codes | WHO Grade | Total cases (2018-2019)c | Rate (95% CI) | Age (median, interquartile range) | Female (%) | Non-Hispanic White (%) | Non-Hispanic Black (%) | Hispanic (%) |
|---|---|---|---|---|---|---|---|---|---|
| Adult-type diffuse glioma | |||||||||
| IDH-mutant Astrocytoma (BMM 1, 3) |
9400/3, 9401/3, 9445/3 | All grades | 2,842 | 0.44 (0.43-0.46) | 36 (29-49) | 42.8 | 80.0 | 6.5 | 11.0 |
| IId | 845 | 0.14 (0.13-0.15) | 34 (28-44) | 41.4 | 79.3 | 6.3 | 10.8 | ||
| III | 947 | 0.15 (0.14-0.16) | 37 (29-48) | 42.9 | 80.9 | 5.9 | 11.2 | ||
| IV | 565 | 0.09 (0.08-0.09) | 39.5 (31-59) | 42.3 | 80.1 | 7.7 | 9.7 | ||
| IDH-wildtype Astrocytoma and Glioblastomad,e (BMM 2, 4, 5) |
9400/3, 9401/3, 9440/3 | All grades | 20,625 | 2.61 (2.57-2.64) | 65 (56-72) | 41.1 | 82.6 | 6.3 | 8.6 |
| II | 394 | 0.06 (0.05-0.06) | 54 (34.5-65) | 46.4 | 77.6 | 9.0 | 9.8 | ||
| III | 762 | 0.10 (0.10-0.11) | 59 (46-70) | 46.1 | 81.8 | 7.4 | 8.2 | ||
| IV | 14,773 | 1.85 (1.82-1.88) | 65 (56-72) | 40.2 | 82.8 | 6.2 | 8.6 | ||
| IDH-mutant & 1p/19q-codeleted Oligodendroglioma (BMM 6,7) |
All grades | 1,880 | 0.29 (0.28-0.31) | 45 (35-56) | 44.9 | 76.9 | 4.9 | 13.9 | |
| 9450/3, 9451/3 | II | 940 | 0.15 (0.14-0.16) | 42 (33-53) | 45.3 | 75.9 | 5.1 | 14.8 | |
| III | 640 | 0.10 (0.09-0.10) | 48 (37-58) | 43.8 | 77.9 | 4.8 | 13.2 | ||
| Medulloblastoma f | |||||||||
| SHH-activated & TP53-wildtype (BMM 8) | 9471/3 | All grades | 161 | 0.03 (0.02-0.03) | 20 (5-30) | 36.0 | 57.5 | 12.4 | 24.2 |
| SHH-activated & TP53-mutant | 9476/3 | All grades | < 16 cases | -- | -- | -- | -- | -- | -- |
| WNT-activated | 9475/3 | All grades | 29 | 0.01 (0.00-0.01) | 10 (7-12) | -- | 72.4 | -- | -- |
| Non-WNT/non-SHH | 9477/3 | All grades | 134 | 0.02 (0.02-0.03) | 8 (4-12) | 33.6 | 63.8 | 4.6 | 28.5 |
| Other tumor types | |||||||||
| Diffuse midline glioma, H3 K27M-mutant | 9385/3 | All grades | 331 | 0.06 (0.05-0.06) | 14 (7-31.5) | 54.7 | 56.9 | 12.5 | 24.4 |
| ETMR C19MC-altered (BMM 9) | 9478/3 | All grades | 27 | 0.00 (0.00-0.01) | 2 (1.5-3.5) | -- | -- | -- | -- |
| RELA-fusion ependymoma | 9396/3 | All grades | 18 | 0.00 (0.00-0.00) | 13 (4.25-17.25) | -- | -- | -- | -- |
aRates are per 100,000 and are age-adjusted to the 2000 US standard population.
bWHO grade is reported according to 2016 WHO classification, in which Roman numerals are used to denote tumor grade.
cExcludes cases with missing molecular classification data or that are not histopathologically-confirmed.
dAdult-type diffuse glioma cases reported as WHO grade I or “low-grade, NOS” were grouped with WHO grade II.
eIn WHO-CNS5, grading is denoted using Arabic numerals rather than roman numerals. In this 2021 revision, all IDH-wildtype adult-type diffuse astrocytic gliomas are classified as glioblastoma, IDH-wildtype, WHO CNS grade 4, without separate grades 2 or 3.
fBoth histopathologically-defined and new molecularly-defined ICD-O-3 codes for medulloblastomas were reported in the registry data; however, only a single ICD-O-3 diagnosis can be reported per case. As a result, the national incidence rates could not be estimated for the SHH-activated and TP53 mutant subtype.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified
The IDH-mutant astrocytoma subtype had incidence rate of 0.44 per 100,000 population, while IDH-wildtype astrocytoma subtype had an incidence rate of 2.61 per 100,000 population. Median age of diagnosis for these subtypes was 36 and 65 years, respectively.
When stratified by WHO grade, 61.5% of WHO grade II astrocytoma were IDH-mutant, while 49.4% and 3.1% of WHO grade III and IV astrocytoma were IDH-mutant (Figure 14).
The most common medulloblastoma subtype was SHH-activated & TP53-wildtype, which had an incidence rate of 0.03 per 100,000 population and a median age of diagnosis of 20 years.
Non-WNT/non-SHH medulloblastoma was the second most commonly occurring subtype, with an incidence rate of 0.02 per 100,000 and a median age of diagnosis of 8 years.
Incidence of the WNT-activated medulloblastoma subtype was 0.01 per 100,000 population, with a median age of diagnosis of 10 years. SHH-activated and TP53-mutant medulloblastoma subtypes were too rare to calculate incidence.
Molecular subtype data were missing for many medulloblastoma cases, but the completeness of these data is expected to increase in future years.
Embryonal tumors with multilayered rosettes, C19MC-altered had incidence rates of <0.01 per 100,000 population and a median age of diagnosis of 2 years.
Diffuse midline glioma, H3 K27M-mutant had an incidence rate of 0.06 per 100,000 population and a median age of diagnosis of 14 years.
Fig. 14.
Frequency of IDH Mutationa by WHO Grade for Selected Astrocytoma Histopathologiesb, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2018-2019
Distribution of Spinal Cord Tumors by Age
Although spinal cord tumors account for a relatively small percentage of brain and other CNS tumors, they result in significant morbidity. The most common histopathologies found in the spinal cord, spinal meninges, and cauda equina are presented in Figure 15 for both children (age 0-19 years, Figure 15A) and adults (ages 20+ years, Figure 15B).
Fig. 15.
Distributiona of Primary Spinal Cord, Spinal Meninges, and Cauda Equina Tumors by Histopathology in A) Children and Adolescents (Ages 0-19 Years, Five-Year Total=1,451; Annual Average Cases=290) and B) Adults (Ages 20+ Years, Five-Year Total=19,103; Annual Average Cases=3,821), CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
The predominant histopathology group for those ages 0-19 years was ependymal tumors (17.6%) followed by nerve sheath tumors (17.3%).
Meningiomas (37.5%) accounted for the largest proportion of spinal cord tumors among those ages 20 years and older.
Descriptive Summary of Meningiomas
Meningiomas were the most frequently reported brain and other CNS histopathology, accounting for 40% of tumors overall (Figure 12, Table 5).
Most meningiomas (82.0%) were located in the cerebral meninges, 4.2% were located in the spinal meninges, and approximately 13.7% did not have a specific meningeal site listed (Table 4).
Non-malignant meningiomas with ICD-O-3 behavior codes /0 (benign) or /1 (uncertain) accounted for 99.0% of reported meningiomas.
Of meningiomas with documented WHO grade (82.2%), 80.1% were WHO grade I, 18.3% were WHO grade II, and 1.5% were WHO grade III (Table 9).
Meningiomas were most common in adults ages 65 years and older, and one of the least common in children ages 0-14 years (Table 10, Supplementary Table 8).
Incidence of meningiomas increased with age, with a dramatic increase after age 65 years. Even among the population ages 85 years and older, these rates continued to be high (Supplementary Table 8).
Non-malignant meningiomas overall were 2.3 times more common in females compared to males. Incidence rate ratios were lowest between males and females in persons <20 years old (where incidence rates for males and females were approximately equal), and highest from 35-44 (Figure 16, Supplementary Figure 5).
Incidence of meningiomas was significantly higher in people who are Black compared to their White counterparts (Figure 17).
Ten-year relative survival for malignant meningiomas was 60%. Age had a large effect on survival after diagnosis with malignant meningioma: 10-year relative survival was 78% for the population ages 20-44 years, and 38.5% for ages 75+ years (Table 10, Supplementary Table 13).
Ten-year relative survival for non-malignant meningiomas was 83.4%. Age had a large effect on survival after diagnosis with non-malignant meningioma: 10-year relative survival was 93.2% in children 0–14, 95% in AYA 15-39, and 82.5% in adults 40+ years old (Table 11).
Site of meningioma affected survival after diagnosis with meningioma. For non-malignant meningiomas, 10-year relative survival was 83.2% for tumors in the cerebral meninges, but 94.8% for tumors in the spinal meninges (Supplementary Figure 6, Supplementary Table 12).
Survival was also higher in malignant meningiomas for spinal tumors, where 10-year relative survival was 71.1%, as compared to 59.9% for tumors in the cerebral meninges (Supplementary Figure 6, Supplementary Table 12).
Table 9.
Distribution of Histopathologically-Confirmed Brain and Other Central Nervous System Tumors by WHO Grade Completeness, Treatment Information Completeness, and Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | Number of Newly Diagnosed Tumors | Histopathologically-Confirmed (%)a | WHO Grade Completeness (%)b | Assigned WHO Gradec | Radiation Information Completenessd (%) | Surgical Extent of Resection Information Completenesse(%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete | Incomplete | Not Applicable | WHO Grade I | WHO Grade II | WHO Grade III | WHO Grade IV | |||||
| Diffuse Astrocytic and Oligodendroglial Tumors | 84,012 | 94.3% | 90.9% | 9.1% | 0.1% | 0.5% | 10.8% | 12.9% | 75.8% | 59.0% | 99.6% |
| Diffuse astrocytoma | 7,634 | 92.5% | 84.3% | 15.6% | 0.1% | 2.8% | 71.9% | 15.6% | 9.8% | 44.7% | 99.4% |
| Anaplastic astrocytoma | 7,046 | 99.3% | 94.1% | 5.9% | 0.1% | 0.2% | 2.2% | 89.6% | 8.0% | 69.1% | 99.6% |
| Glioblastoma | 63,258 | 93.6% | 91.1% | 8.8% | 0.1% | 0.2% | 0.2% | 0.7% | 98.9% | 60.6% | 99.7% |
| Oligodendroglioma | 3,687 | 96.9% | 92.4% | 7.6% | 0.0% | 1.4% | 89.2% | 7.7% | 1.7% | 38.2% | 99.5% |
| Anaplastic oligodendroglioma | 1,871 | 99.1% | 93.6% | 6.4% | 0.1% | 0.0% | 4.3% | 89.9% | 5.8% | 64.6% | 99.6% |
| Oligoastrocytic tumors | 516 | 97.3% | 91.2% | 8.8% | 0.0% | 1.5% | 43.0% | 46.3% | 9.2% | 61.8% | 99.8% |
| Other Astrocytic Tumors | 6,252 | 86.4% | 86.9% | 12.8% | 0.4% | 86.4% | 9.7% | 3.3% | 0.7% | 6.5% | 99.7% |
| Pilocytic astrocytoma | 5,339 | 87.9% | 87.2% | 12.4% | 0.4% | 95.3% | 3.7% | 0.6% | 0.4% | 4.9% | 99.8% |
| Unique astrocytoma variants | 913 | 77.5% | 84.5% | 15.4% | 0.1% | 25.8% | 50.5% | 21.2% | 2.5% | 15.8% | 99.4% |
| Malignant | 532 | 97.9% | 87.7% | 12.1% | 0.2% | 3.1% | 66.0% | 27.7% | 3.3% | 24.4% | 99.4% |
| Non-Malignant | 381 | 49.1% | 75.4% | 24.6% | 0.0% | 100.0% | 0.0% | 0.0% | 0.0% | 0.8% | 99.5% |
| Ependymal Tumors | 6,911 | 86.2% | 88.0% | 12.0% | 0.1% | 36.8% | 48.0% | 14.3% | 0.9% | 23.0% | 99.7% |
| Malignant | 3,909 | 93.4% | 89.5% | 10.4% | 0.1% | 2.6% | 73.4% | 22.7% | 1.3% | 34.2% | 99.9% |
| Non-Malignant | 3,002 | 76.9% | 85.5% | 14.4% | 0.0% | 93.6% | 5.9% | 0.2% | 0.3% | 7.8% | 99.5% |
| Other Gliomas | 8,854 | 41.7% | 51.9% | 47.1% | 1.0% | 10.3% | 22.8% | 19.5% | 47.3% | 26.4% | 99.3% |
| Glioma malignant, NOS | 8,753 | 41.1% | 51.7% | 47.3% | 1.0% | 10.3% | 21.7% | 19.6% | 48.4% | 26.3% | 99.3% |
| Other neuroepithelial tumors | 101 | 92.1% | 58.1% | 41.9% | 0.0% | 10.9% | 61.8% | 18.2% | 9.1% | 33.8% | 98.9% |
| Malignant | 60 | 98.3% | 44.1% | 55.9% | 0.0% | 14.8% | 29.6% | 37.0% | 18.5% | 43.5% | 98.3% |
| Non-Malignant | 41 | 82.9% | 82.4% | 17.6% | 0.0% | 7.1% | 92.9% | 0.0% | 0.0% | 17.9% | 100.0% |
| Neuronal and Mixed Neuronal-Glial Tumors | 5,339 | 91.7% | 65.7% | 19.5% | 14.8% | 82.8% | 13.5% | 2.8% | 0.9% | 12.9% | 99.6% |
| Malignant | 960 | 98.3% | 26.9% | 5.6% | 67.6% | 28.7% | 7.0% | 51.0% | 13.4% | 53.5% | 99.3% |
| Non-Malignant | 4,379 | 90.3% | 76.3% | 23.3% | 0.4% | 85.7% | 13.9% | 0.3% | 0.2% | 3.9% | 99.7% |
| Choroid Plexus Tumors | 827 | 87.4% | 76.8% | 23.1% | 0.1% | 64.8% | 20.9% | 13.6% | 0.7% | 3.9% | 99.4% |
| Malignant | 121 | 96.7% | 76.9% | 22.2% | 0.9% | 7.7% | 4.4% | 83.5% | 4.4% | 11.2% | 100.0% |
| Non-Malignant | 706 | 85.8% | 76.7% | 23.3% | 0.0% | 75.9% | 24.1% | 0.0% | 0.0% | 2.7% | 99.3% |
| Tumors of the Pineal Region | 769 | 79.2% | 42.1% | 0.0% | 57.9% | 0.0% | 100.0% | 0.0% | 0.0% | 39.1% | 99.5% |
| Malignant | 462 | 97.8% | 42.9% | 0.0% | 57.1% | 0.0% | 100.0% | 0.0% | 0.0% | 59.4% | 99.3% |
| Non-Malignant | 307 | 51.1% | 39.6% | 0.0% | 60.4% | --% | --% | --% | --% | 7.6% | 100.0% |
| Embryonal Tumors | 3,170 | 98.4% | 82.5% | 16.8% | 0.6% | 0.5% | 0.3% | 1.1% | 98.1% | 58.4% | 99.9% |
| Tumors of Cranial and Spinal Nerves | 37,048 | 48.1% | 43.8% | 56.2% | 0.0% | 99.4% | 0.3% | 0.1% | 0.2% | 14.2% | 99.4% |
| Nerve sheath tumors | 37,015 | 48.1% | 43.8% | 56.2% | 0.0% | 99.4% | 0.3% | 0.1% | 0.2% | 14.2% | 99.4% |
| Malignant | 211 | 83.9% | 22.6% | 77.4% | 0.0% | 57.5% | 12.5% | 20.0% | 10.0% | 32.2% | 98.3% |
| Non-Malignant | 36,804 | 47.9% | 44.0% | 56.0% | 0.0% | 99.6% | 0.3% | 0.0% | 0.1% | 14.1% | 99.4% |
| Other tumors of cranial and spinal nerves | 33 | 42.4% | 28.6% | 71.4% | 0.0% | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 100.0% |
| Tumors of Meninges | 184,405 | 37.4% | 80.5% | 19.5% | 0.1% | 79.9% | 18.0% | 2.0% | 0.1% | 6.1% | 99.6% |
| Meningiomas | 178,447 | 36.2% | 82.2% | 17.8% | 0.0% | 80.1% | 18.3% | 1.5% | 0.1% | 5.9% | 99.5% |
| Malignant | 1,615 | 78.1% | 88.1% | 11.9% | 0.0% | 20.4% | 15.0% | 63.6% | 1.0% | 34.4% | 98.5% |
| Non-Malignant | 176,832 | 35.8% | 82.1% | 17.9% | 0.0% | 81.4% | 18.4% | 0.2% | 0.1% | 5.6% | 99.6% |
| Mesenchymal tumors | 5,815 | 75.4% | 57.1% | 42.0% | 0.8% | 77.1% | 10.4% | 11.8% | 0.7% | 11.9% | 99.6% |
| Malignant | 766 | 96.3% | 42.8% | 53.2% | 4.1% | 11.3% | 14.4% | 70.2% | 4.1% | 47.5% | 99.5% |
| Non-Malignant | 5,049 | 72.3% | 60.1% | 39.7% | 0.2% | 86.6% | 9.8% | 3.3% | 0.2% | 6.3% | 99.6% |
| Primary melanocytic lesions | 143 | 88.1% | 11.9% | 83.3% | 4.8% | 60.0% | 13.3% | 6.7% | 20.0% | 35.0% | 99.2% |
| Malignant | 89 | 94.4% | 8.3% | 84.5% | 7.1% | 42.9% | 0.0% | 14.3% | 42.9% | 44.8% | 98.8% |
| Non-Malignant | 54 | 77.8% | 19.0% | 81.0% | 0.0% | 75.0% | 25.0% | 0.0% | 0.0% | 21.4% | 100.0% |
| Lymphomas and Hematopoietic Neoplasms | 8,525 | 94.9% | 1.9% | 97.3% | 0.8% | 84.3% | 1.3% | 4.6% | 9.8% | 16.5% | 99.0% |
| Lymphoma | 8,482 | 94.9% | 1.9% | 97.5% | 0.6% | 84.2% | 1.3% | 4.6% | 9.9% | 16.3% | 99.1% |
| Other hematopoietic neoplasms | 43 | 97.7% | 2.4% | 64.3% | 33.3% | 100.0% | 0.0% | 0.0% | 0.0% | 64.7% | 95.2% |
| Germ Cell Tumors | 1,280 | 86.6% | 8.8% | 43.4% | 47.8% | 18.8% | 7.8% | 7.8% | 65.6% | 53.5% | 99.0% |
| Malignant | 1,110 | 88.6% | 8.7% | 40.6% | 50.6% | 1.9% | 9.4% | 9.4% | 79.2% | 60.4% | 99.2% |
| Non-Malignant | 170 | 74.1% | 9.4% | 65.4% | 25.2% | 100.0% | 0.0% | 0.0% | 0.0% | 7.7% | 97.6% |
| Tumors of Sellar Region | 79,996 | 43.9% | 14.2% | 0.4% | 85.5% | 100.0% | 0.0% | 0.0% | 0.0% | 2.5% | 99.5% |
| Tumors of the pituitary | 76,863 | 42.3% | 11.8% | 0.0% | 88.2% | 100.0% | 0.0% | 0.0% | 0.0% | 1.8% | 99.5% |
| Malignant | 108 | 63.9% | 8.0% | 0.0% | 92.0% | -- | -- | -- | -- | 17.7% | 97.1% |
| Non-Malignant | 76,755 | 42.3% | 11.8% | 0.0% | 88.2% | 100.0% | 0.0% | 0.0% | 0.0% | 1.8% | 99.5% |
| Craniopharyngioma | 3,133 | 83.1% | 37.0% | 4.0% | 59.0% | 100.0% | 0.0% | 0.0% | 0.0% | 20.1% | 99.6% |
| Unclassified Tumors | 18,404 | 16.8% | 8.5% | 83.4% | 8.1% | 80.1% | 5.7% | 3.7% | 10.6% | 3.3% | 95.6% |
| Hemangioma | 4,215 | 28.3% | 8.7% | 91.0% | 0.3% | 97.1% | 2.9% | 0.0% | 0.0% | 1.4% | 98.7% |
| Neoplasm, unspecified | 13,647 | 11.9% | 8.1% | 77.1% | 14.8% | 68.1% | 7.8% | 7.8% | 16.4% | 4.1% | 92.7% |
| Malignant | 6,693 | 8.5% | 7.2% | 87.3% | 5.6% | 27.0% | 10.8% | 18.9% | 43.2% | 6.6% | 89.4% |
| Non-Malignant | 6,954 | 15.1% | 8.6% | 71.6% | 19.7% | 87.3% | 6.3% | 2.5% | 3.8% | 2.7% | 94.5% |
| All other | 542 | 50.2% | 9.9% | 87.5% | 2.6% | 65.4% | 7.7% | 0.0% | 26.9% | 3.3% | 99.3% |
| Malignant | 59 | 94.9% | 31.6% | 63.2% | 5.3% | 47.1% | 11.8% | 0.0% | 41.2% | 25.0% | 100.0% |
| Non-Malignant | 483 | 44.7% | 4.2% | 94.0% | 1.9% | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 99.1% |
| TOTAL | 445,792 | 53.4% | 65.7% | 19.1% | 15.2% | 40.4% | 14.1% | 7.7% | 37.7% | 17.9% | 99.5% |
| Malignant | 126,345 | 85.8% | 79.7% | 18.5% | 1.9% | 5.8% | 13.1% | 13.6% | 67.5% | 48.5% | 99.5% |
| Non-Malignant | 319,447 | 40.5% | 54.4% | 19.6% | 26.0% | 84.2% | 15.4% | 0.3% | 0.1% | 5.7% | 99.5% |
aHistopathologic confirmation includes tumors classified as diagnosis confirmed by positive histopathology, positive cytology, positive histopathology plus – positive immunophenotyping and/or positive genetic studies, or positive microscopic confirmation, method not specified.
bCompleteness is defined as having an assigned code that corresponds with a WHO grade as defined by the American Joint Commission on Cancer's Collaborative Staging schema, SSDI Clinical Grade (2018+ only) or SSDI Pathological Grade (2018+ only).
cGrade as recorded in the American Joint Commission on Cancer's Collaborative Staging schema, SSDI Clinical Grade (2018+ only) or SSDI Pathological Grade (2018+ only). WHO grade may be reported according to 2007 or 2016 WHO classification depending on year of diagnosis, in which roman numerals are used to denote tumor grade.
dRadiation is defined using a recoded variable based on NAACCR Item #1360 (http://datadictionary.naaccr.org/default.aspx?c=10#136). Completeness is defined as having a value other than 'none' or 'unknown.'
eSurgery is defined using a recoded variable based on NAACCR Item #1290 (http://datadictionary.naaccr.org/default.aspx?c=10#1290). Please see the SEER site-specific surgery codes for more information on coding for this variable. (https://seer.cancer.gov/archive/tools/SEER2003.surg.prim.site.codes.pdf). Completeness is defined as having a value other than 'unknown.'
-- Percentages are not presented when category is not applicable.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; WHO, World Health Organization.
Table 10.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals of All Brain and Other Central Nervous System Tumors by Histopathology, and NCI Age at Diagnosis Groups, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | Childrenc (0-14) | AYAd (15-39) | Older Adults (40+) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 1,410 | 282 | 0.47 (0.44-0.49) | 9,819 | 1,964 | 1.84 (1.80-1.87) | 72,783 | 14,557 | 8.72 (8.65-8.78) |
| Diffuse astrocytoma | 627 | 125 | 0.21 (0.19-0.22) | 2,614 | 523 | 0.48 (0.46-0.50) | 4,393 | 879 | 0.56 (0.54-0.58) |
| Anaplastic astrocytoma | 222 | 44 | 0.07 (0.06-0.08) | 2,046 | 409 | 0.38 (0.36-0.39) | 4,778 | 956 | 0.60 (0.58-0.62) |
| Glioblastoma | 465 | 93 | 0.15 (0.14-0.17) | 3,038 | 608 | 0.58 (0.56-0.60) | 59,755 | 11,951 | 7.03 (6.97-7.09) |
| Oligodendroglioma | 64 | 13 | 0.02 (0.02-0.03) | 1,426 | 285 | 0.27 (0.25-0.28) | 2,197 | 439 | 0.31 (0.29-0.32) |
| Anaplastic oligodendroglioma | -- | -- | -- | 517 | 103 | 0.10 (0.09-0.11) | 1,345 | 269 | 0.18 (0.17-0.19) |
| Oligoastrocytic tumors | -- | -- | -- | 178 | 36 | 0.03 (0.03-0.04) | 315 | 63 | 0.04 (0.04-0.05) |
| Other Astrocytic Tumors | 3,658 | 732 | 1.21 (1.17-1.24) | 1,836 | 367 | 0.34 (0.32-0.35) | 758 | 152 | 0.10 (0.10-0.11) |
| Pilocytic astrocytoma | 3,283 | 657 | 1.08 (1.04-1.12) | 1,463 | 293 | 0.27 (0.26-0.28) | 593 | 119 | 0.08 (0.07-0.09) |
| Unique astrocytoma variants | 375 | 75 | 0.12 (0.11-0.14) | 373 | 75 | 0.07 (0.06-0.08) | 165 | 33 | 0.02 (0.02-0.03) |
| Non-Malignant | 232 | 46 | 0.08 (0.07-0.09) | 112 | 22 | 0.02 (0.02-0.02) | 37 | 7 | 0.01 (0.00-0.01) |
| Malignant | 143 | 29 | 0.05 (0.04-0.06) | 261 | 52 | 0.05 (0.04-0.05) | 128 | 26 | 0.02 (0.01-0.02) |
| Ependymal Tumors | 925 | 185 | 0.30 (0.28-0.32) | 1,924 | 385 | 0.36 (0.34-0.38) | 4,062 | 812 | 0.53 (0.51-0.54) |
| Non-Malignant | 112 | 22 | 0.04 (0.03-0.04) | 910 | 182 | 0.17 (0.16-0.18) | 1,980 | 396 | 0.26 (0.25-0.27) |
| Malignant | 813 | 163 | 0.27 (0.25-0.29) | 1,014 | 203 | 0.19 (0.18-0.20) | 2,082 | 416 | 0.27 (0.26-0.28) |
| Other Gliomas | 2,690 | 538 | 0.89 (0.85-0.92) | 1,925 | 385 | 0.35 (0.34-0.37) | 4,239 | 848 | 0.53 (0.52-0.55) |
| Glioma malignant, NOS | 2,668 | 534 | 0.88 (0.85-0.91) | 1,889 | 378 | 0.35 (0.33-0.36) | 4,196 | 839 | 0.53 (0.51-0.54) |
| Other neuroepithelial tumors | 22 | 4 | 0.01 (0.00-0.01) | 36 | 7 | 0.01 (0.00-0.01) | 43 | 9 | 0.01 (0.00-0.01) |
| Non-Malignant | -- | -- | -- | -- | -- | -- | 26 | 5 | 0.00 (0.00-0.01) |
| Malignant | -- | -- | -- | -- | -- | -- | 17 | 3 | 0.00 (0.00-0.00) |
| Neuronal and Mixed Neuronal-Glial Tumors | 1,411 | 282 | 0.47 (0.44-0.49) | 2,164 | 433 | 0.40 (0.38-0.42) | 1,764 | 353 | 0.23 (0.22-0.24) |
| Non-Malignant | 1,324 | 265 | 0.44 (0.41-0.46) | 1,973 | 395 | 0.36 (0.35-0.38) | 1,082 | 216 | 0.15 (0.14-0.16) |
| Malignant | 87 | 17 | 0.03 (0.02-0.04) | 191 | 38 | 0.04 (0.03-0.04) | 682 | 136 | 0.09 (0.08-0.09) |
| Choroid Plexus Tumors | 359 | 72 | 0.12 (0.11-0.13) | 210 | 42 | 0.04 (0.03-0.04) | 258 | 52 | 0.03 (0.03-0.04) |
| Non-Malignant | 261 | 52 | 0.09 (0.08-0.10) | -- | -- | -- | -- | -- | -- |
| Malignant | 98 | 20 | 0.03 (0.03-0.04) | -- | -- | ---- | -- | -- | ---- |
| Tumors of the Pineal Region | 143 | 29 | 0.05 (0.04-0.06) | 294 | 59 | 0.05 (0.05-0.06) | 332 | 66 | 0.04 (0.04-0.05) |
| Non-Malignant | 18 | 4 | 0.01 (0.00-0.01) | 117 | 23 | 0.02 (0.02-0.03) | 172 | 34 | 0.02 (0.02-0.03) |
| Malignant | 125 | 25 | 0.04 (0.03-0.05) | 177 | 35 | 0.03 (0.03-0.04) | 160 | 32 | 0.02 (0.02-0.02) |
| Embryonal Tumors | 2,144 | 429 | 0.71 (0.68-0.74) | 755 | 151 | 0.14 (0.13-0.15) | 271 | 54 | 0.04 (0.03-0.04) |
| Medulloblastoma | 1,464 | 293 | 0.48 (0.46-0.51) | 633 | 127 | 0.11 (0.11-0.12) | 155 | 31 | 0.02 (0.02-0.03) |
| Atypical teratoid/rhabdoid tumor | 365 | 73 | 0.12 (0.11-0.13) | 31 | 6 | 0.01 (0.00-0.01) | 16 | 3 | 0.00 (0.00-0.00) |
| All other embryonal | 315 | 63 | 0.10 (0.09-0.12) | 91 | 18 | 0.02 (0.01-0.02) | 100 | 20 | 0.01 (0.01-0.02) |
| Tumors of Cranial and Paraspinal Nerves | 686 | 137 | 0.23 (0.21-0.24) | 5,518 | 1,104 | 1.05 (1.02-1.07) | 30,844 | 6,169 | 3.79 (3.75-3.83) |
| Nerve sheath tumors | -- | -- | -- | -- | -- | -- | 30,818 | 6,164 | 3.79 (3.74-3.83) |
| Non-Malignant | -- | -- | -- | -- | -- | -- | 30,671 | 6,134 | 3.77 (3.73-3.81) |
| Malignant | -- | -- | -- | -- | -- | -- | 147 | 29 | 0.02 (0.02-0.02) |
| Other tumors of cranial and paraspinal nerves | -- | -- | -- | -- | -- | -- | 26 | 5 | 0.00 (0.00-0.00) |
| Tumors of Meninges | 680 | 136 | 0.22 (0.21-0.24) | 11,756 | 2,351 | 2.27 (2.23-2.32) | 171,969 | 34,394 | 20.91 (20.81-21.01) |
| Meningiomas | -- | -- | -- | 10,127 | 2,025 | 1.97 (1.93-2.01) | 168,002 | 33,600 | 20.41 (20.31-20.51) |
| Non-Malignant | -- | -- | -- | 10,015 | 2,003 | 1.95 (1.91-1.99) | 166,514 | 33,303 | 20.23 (20.13-20.33) |
| Malignant | -- | -- | -- | 112 | 22 | 0.02 (0.02-0.03) | 1,488 | 298 | 0.18 (0.17-0.19) |
| Mesenchymal tumors | 354 | 71 | 0.12 (0.10-0.13) | -- | -- | -- | 3,855 | 771 | 0.49 (0.47-0.50) |
| Non-Malignant | 285 | 57 | 0.09 (0.08-0.11) | -- | -- | -- | 3,324 | 665 | 0.42 (0.41-0.43) |
| Malignant | 69 | 14 | 0.02 (0.02-0.03) | -- | -- | -- | 531 | 106 | 0.07 (0.06-0.07) |
| Primary melanocytic lesions | -- | -- | -- | -- | -- | -- | 112 | 22 | 0.01 (0.01-0.02) |
| Non-Malignant | -- | -- | -- | -- | -- | -- | 44 | 9 | 0.01 (0.00-0.01) |
| Malignant | -- | -- | -- | -- | -- | -- | 68 | 14 | 0.01 (0.01-0.01) |
| Lymphomas and Hematopoietic Neoplasms | 89 | 18 | 0.03 (0.02-0.04) | 535 | 107 | 0.10 (0.09-0.11) | 7,901 | 1,580 | 0.94 (0.92-0.96) |
| Lymphoma | -- | -- | -- | -- | -- | -- | 7,862 | 1,572 | 0.94 (0.92-0.96) |
| Other hematopoietic neoplasms | -- | -- | -- | -- | -- | -- | 39 | 8 | 0.00 (0.00-0.01) |
| Germ Cell Tumors | 581 | 116 | 0.19 (0.18-0.21) | 625 | 125 | 0.11 (0.10-0.12) | 74 | 15 | 0.01 (0.01-0.01) |
| Non-Malignant | 82 | 16 | 0.03 (0.02-0.03) | 45 | 9 | 0.01 (0.01-0.01) | 43 | 9 | 0.01 (0.00-0.01) |
| Malignant | 499 | 100 | 0.16 (0.15-0.18) | 580 | 116 | 0.11 (0.10-0.11) | 31 | 6 | 0.00 (0.00-0.01) |
| Tumors of Sellar Region | 1,774 | 355 | 0.59 (0.56-0.61) | 23,663 | 4,733 | 4.39 (4.34-4.45) | 54,559 | 10,912 | 6.98 (6.91-7.04) |
| Tumors of the pituitary | 1,115 | 223 | 0.37 (0.35-0.39) | 22,980 | 4,596 | 4.26 (4.21-4.32) | 52,768 | 10,554 | 6.75 (6.69-6.81) |
| Non-Malignant | -- | -- | -- | -- | -- | -- | 52,678 | 10,536 | 6.74 (6.68-6.80) |
| Malignant | -- | -- | -- | -- | -- | -- | 90 | 18 | 0.01 (0.01-0.01) |
| Craniopharyngioma | 659 | 132 | 0.22 (0.20-0.24) | 683 | 137 | 0.13 (0.12-0.14) | 1,791 | 358 | 0.23 (0.21-0.24) |
| Unclassified Tumors | 1,010 | 202 | 0.33 (0.31-0.35) | 2,788 | 558 | 0.52 (0.50-0.54) | 14,606 | 2,921 | 1.80 (1.77-1.83) |
| Hemangioma | 287 | 57 | 0.09 (0.08-0.11) | 1,158 | 232 | 0.22 (0.20-0.23) | 2,770 | 554 | 0.36 (0.34-0.37) |
| Neoplasm, unspecified | 571 | 114 | 0.19 (0.17-0.20) | 1,503 | 301 | 0.28 (0.27-0.30) | 11,573 | 2,315 | 1.41 (1.38-1.44) |
| Non-Malignant | 409 | 82 | 0.14 (0.12-0.15) | 1,151 | 230 | 0.21 (0.20-0.23) | 5,394 | 1,079 | 0.66 (0.65-0.68) |
| Malignant | 162 | 32 | 0.05 (0.05-0.06) | 352 | 70 | 0.07 (0.06-0.07) | 6,179 | 1,236 | 0.75 (0.73-0.76) |
| All other | 152 | 30 | 0.05 (0.04-0.06) | 127 | 25 | 0.02 (0.02-0.03) | 263 | 53 | 0.03 (0.03-0.04) |
| Non-Malignant | 120 | 24 | 0.04 (0.03-0.05) | -- | -- | -- | -- | -- | -- |
| Malignant | 32 | 6 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- |
| TOTAL e | 17,560 | 3,512 | 5.79 (5.70-5.88) | 63,812 | 12,762 | 11.96 (11.87-12.06) | 364,420 | 72,884 | 44.65 (44.51-44.80) |
| Non-Malignant | 5,888 | 1,178 | 1.94 (1.89-1.99) | 46,363 | 9,273 | 8.71 (8.63-8.79) | 267,063 | 53,413 | 32.92 (32.79-33.05) |
| Malignant | 11,672 | 2,334 | 3.85 (3.78-3.92) | 17,449 | 3,490 | 3.25 (3.20-3.30) | 97,357 | 19,471 | 11.74 (11.66-11.81) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cChildren as defined by the National Cancer Institute, see: http://www.cancer.gov/researchandfunding/snapshots/pediatric.
dAdolescents and Young Adults (AYA), as defined by the National Cancer Institute, see: http://www.cancer.gov/cancertopics/aya.
eRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: AYA, Adolescents and Young Adults; CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
Fig. 16.
Incidence Rate Ratiosa by Sex (Males:Females) for Selected Primary Brain and Other Central Nervous System Tumor Histopathologies, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Fig. 17.
Incidence Rate Ratiosa by Race (A- Whites:Blacks and B- Whites:Asian Or Pacific Islanders [API]) for Selected Primary Brain and Other Central Nervous System Tumor Histopathologies, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Table 11.
One-, Five-, and Ten-Year Relative Survival Ratesa,b (RS) with 95% Confidence Intervals for Brain and Other Central Nervous System Tumors by Histopathology and Behavior, Overall, and by NCI Age Group at Diagnosis, CBTRUS Statistical Report: NPCR and SEER, 2001-2018 (varying)
| Histopathology | Age Groups (years) | All (2004-2018) | Malignant (2001-2018)c | Non-Malignant (2004-2018)d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ne | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) | Nf | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) | Ne | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) | ||
| Diffuse astrocytoma | 0-14g | 2,097 | 92.2 (91.0-93.3) | 82.3 (80.5-83.9) | 79.8 (77.8-81.7) | 2,627 | 91.9 (90.7-92.9) | 81.3 (79.7-82.8) | 79.2 (77.4-80.8) | -- | -- | -- | -- |
| 15-39h | 6,597 | 95.5 (95.0-96.0) | 78.1 (76.9-79.2) | 60.9 (59.3-62.4) | 7,918 | 95.0 (94.5-95.5) | 76.9 (75.8-77.9) | 59.2 (57.8-60.5) | -- | -- | -- | -- | |
| 40+ | 11,838 | 62.7 (61.7-63.6) | 33.4 (32.4-34.4) | 25.1 (24.1-26.1) | 14,446 | 61.0 (60.2-61.9) | 32.3 (31.5-33.2) | 23.8 (23.0-24.7) | -- | -- | -- | -- | |
| All ages | 20,532 | 76.3 (75.7-76.9) | 52.9 (52.1-53.6) | 42.4 (41.6-43.3) | 24,991 | 75.1 (74.5-75.6) | 51.8 (51.1-52.4) | 41.2 (40.5-41.9) | -- | -- | -- | -- | |
| Anaplastic astrocytoma | 0-14 | 656 | 66.9 (63.1-70.4) | 25.4 (21.9-29.1) | 19.8 (16.2-23.5) | 750 | 66.2 (62.7-69.5) | 25.1 (21.9-28.5) | 20.1 (17.0-23.5) | -- | -- | -- | -- |
| 15-39 | 4,058 | 92.5 (91.6-93.2) | 62.8 (61.1-64.5) | 46.2 (44.0-48.3) | 4,765 | 91.4 (90.6-92.2) | 61.3 (59.7-62.8) | 45.1 (43.2-46.9) | -- | -- | -- | -- | |
| 40+ | 10,321 | 57.2 (56.2-58.2) | 19.9 (19.1-20.8) | 14.0 (13.1-14.9) | 12,306 | 55.4 (54.5-56.3) | 19.4 (18.6-20.2) | 13.8 (13.1-14.6) | -- | -- | -- | -- | |
| All ages | 15,035 | 67.1 (66.3-67.9) | 31.7 (30.9-32.6) | 22.9 (22.1-23.8) | 17,821 | 65.5 (64.8-66.2) | 30.9 (30.1-31.6) | 22.5 (21.7-23.3) | -- | -- | -- | -- | |
| Glioblastoma | 0-14 | 1,102 | 57.1 (54.1-60.0) | 19.9 (17.4-22.5) | 16.6 (14.1-19.2) | 1,287 | 56.5 (53.7-59.2) | 20.9 (18.5-23.3) | 17.7 (15.4-20.0) | -- | -- | -- | -- |
| 15-39 | 6,467 | 76.8 (75.7-77.8) | 26.6 (25.4-27.8) | 18.6 (17.4-19.8) | 7,629 | 75.8 (74.8-76.7) | 26.4 (25.3-27.5) | 18.6 (17.5-19.7) | -- | -- | -- | -- | |
| 40+ | 117,076 | 40.6 (40.4-40.9) | 5.6 (5.5-5.8) | 3.4 (3.2-3.5) | 136,262 | 39.1 (38.8-39.3) | 5.3 (5.2-5.4) | 3.3 (3.1-3.4) | -- | -- | -- | -- | |
| All ages | 124,645 | 42.7 (42.4-43.0) | 6.9 (6.7-7.1) | 4.3 (4.2-4.5) | 145,178 | 41.2 (40.9-41.5) | 6.6 (6.5-6.8) | 4.3 (4.1-4.4) | -- | -- | -- | -- | |
| Oligodendroglioma | 0-14 | 272 | 97.4 (94.6-98.8) | 94.3 (90.6-96.5) | 92.2 (87.8-95.1) | 359 | 97.2 (94.8-98.5) | 94.3 (91.3-96.3) | 91.9 (88.1-94.5) | -- | -- | -- | -- |
| 15-39 | 3,934 | 98.6 (98.2-99.0) | 92.5 (91.5-93.4) | 78.5 (76.7-80.2) | 4,874 | 98.7 (98.3-99.0) | 92.2 (91.3-93.0) | 77.4 (75.9-78.9) | -- | -- | -- | -- | |
| 40+ | 5,445 | 92.5 (91.7-93.2) | 77.6 (76.3-78.9) | 64.0 (62.1-65.7) | 6,756 | 91.8 (91.1-92.5) | 76.6 (75.4-77.7) | 62.6 (61.0-64.1) | -- | -- | -- | -- | |
| All ages | 9,651 | 95.2 (94.7-95.6) | 84.2 (83.4-85.1) | 70.9 (69.6-72.1) | 11,989 | 94.8 (94.3-95.2) | 83.6 (82.8-84.3) | 69.7 (68.6-70.7) | -- | -- | -- | -- | |
| Anaplastic oligodendroglioma | 0-14 | -- | -- | -- | -- | 55 | 83.7 (71.0-91.2) | 59.8 (45.1-71.8) | 52.0 (36.7-65.2) | -- | -- | -- | -- |
| 15-39 | 1,269 | 95.9 (94.6-96.9) | 79.7 (77.0-82.0) | 63.4 (59.7-66.8) | 1,577 | 95.5 (94.3-96.4) | 77.7 (75.3-79.8) | 63.3 (60.2-66.1) | -- | -- | -- | -- | |
| 40+ | 2,998 | 85.9 (84.5-87.1) | 59.8 (57.7-61.8) | 46.4 (43.8-48.9) | 3,719 | 84.0 (82.7-85.2) | 55.9 (54.1-57.7) | 43.1 (40.9-45.1) | -- | -- | -- | -- | |
| All ages | 4,303 | 88.8 (87.8-89.7) | 65.7 (64.0-67.3) | 51.5 (49.4-53.6) | 5,351 | 87.4 (86.4-88.3) | 62.5 (61.0-63.9) | 49.3 (47.5-51.0) | -- | -- | -- | -- | |
| Oligoastrocytic tumors | 0-14 | 153 | 91.5 (85.7-95.0) | 80.6 (73.2-86.1) | 78.0 (70.2-84.0) | 194 | 90.7 (85.6-94.1) | 77.4 (70.8-82.8) | 75.5 (68.6-81.1) | -- | -- | -- | -- |
| 15-39 | 2,528 | 97.6 (96.9-98.1) | 81.0 (79.3-82.5) | 60.6 (58.3-62.9) | 3,088 | 97.3 (96.7-97.9) | 79.6 (78.1-81.0) | 59.4 (57.3-61.3) | -- | -- | -- | -- | |
| 40+ | 3,025 | 82.7 (81.3-84.1) | 54.9 (53.0-56.8) | 43.6 (41.4-45.7) | 3,659 | 81.9 (80.6-83.1) | 53.5 (51.8-55.2) | 42.0 (40.1-43.8) | -- | -- | -- | -- | |
| All ages | 5,706 | 89.6 (88.7-90.4) | 67.3 (66.0-68.5) | 52.1 (50.6-53.7) | 6,941 | 89.0 (88.2-89.8) | 65.9 (64.7-67.0) | 50.7 (49.4-52.1) | -- | -- | -- | -- | |
| Pilocytic astrocytoma | 0-14 | 8,156 | 99.0 (98.7-99.2) | 97.3 (96.8-97.6) | 95.9 (95.3-96.4) | 9,439 | 98.8 (98.6-99.0) | 97.0 (96.6-97.4) | 95.5 (95.0-96.0) | -- | -- | -- | -- |
| 15-39 | 3,973 | 98.5 (98.1-98.9) | 94.9 (94.0-95.6) | 93.0 (91.9-94.0) | 4,672 | 98.4 (98.0-98.7) | 94.7 (93.9-95.4) | 92.8 (91.8-93.6) | -- | -- | -- | -- | |
| 40+ | 1,348 | 92.1 (90.4-93.5) | 79.8 (77.1-82.3) | 76.9 (73.5-79.9) | 1,569 | 91.8 (90.2-93.1) | 78.9 (76.4-81.2) | 76.6 (73.6-79.3) | -- | -- | -- | -- | |
| All ages | 13,477 | 98.2 (97.9-98.4) | 94.8 (94.4-95.2) | 93.1 (92.5-93.7) | 15,680 | 98.0 (97.8-98.2) | 94.5 (94.1-94.9) | 92.9 (92.3-93.4) | -- | -- | -- | -- | |
| Unique astrocytoma variants | 0-14 | 995 | 97.5 (96.2-98.3) | 94.7 (92.9-96.0) | 91.9 (89.4-93.9) | 382 | 95.7 (93.1-97.4) | 88.1 (84.0-91.1) | 82.5 (77.1-86.7) | 661 | 98.2 (96.8-99.0) | 97.5 (95.9-98.5) | 96.0 (93.4-97.6) |
| 15-39 | 937 | 96.9 (95.5-97.9) | 86.5 (83.8-88.7) | 81.6 (78.2-84.6) | 718 | 97.1 (95.5-98.1) | 82.2 (78.9-85.1) | 77.3 (73.3-80.7) | 317 | 96.5 (93.7-98.1) | 93.9 (90.3-96.3) | 91.4 (86.4-94.5) | |
| 40+ | 349 | 83.3 (78.8-87.0) | 59.4 (53.3-64.9) | 52.6 (45.2-59.4) | 310 | 81.2 (76.2-85.2) | 53.4 (46.9-59.4) | 49.4 (42.1-56.3) | 75 | 91.1 (81.3-95.8) | 80.5 (68.1-88.5) | 65.5 (46.2-79.3) | |
| All ages | 2,281 | 95.1 (94.0-95.9) | 86.0 (84.3-87.5) | 81.8 (79.7-83.7) | 1,410 | 93.2 (91.7-94.5) | 77.6 (75.0-79.9) | 72.7 (69.7-75.4) | 1,053 | 97.2 (95.9-98.0) | 95.3 (93.6-96.5) | 92.6 (90.1-94.4) | |
| Ependymal tumors | 0-14 | 2,456 | 95.6 (94.7-96.3) | 80.4 (78.6-82.1) | 72.0 (69.7-74.2) | 2,597 | 94.6 (93.6-95.4) | 76.8 (75.0-78.5) | 67.3 (65.1-69.4) | 275 | 99.7 (97.0-100.0) | 97.5 (94.2-98.9) | 97.5 (94.2-98.9) |
| 15-39 | 5,002 | 98.3 (97.8-98.6) | 94.7 (93.9-95.4) | 91.7 (90.6-92.7) | 3,259 | 97.1 (96.5-97.7) | 91.1 (90.0-92.1) | 87.2 (85.8-88.5) | 2,230 | 99.5 (99.0-99.8) | 99.0 (98.3-99.5) | 97.7 (96.3-98.6) | |
| 40+ | 9,470 | 95.0 (94.5-95.5) | 91.0 (90.1-91.8) | 87.9 (86.6-89.1) | 5,834 | 93.2 (92.5-93.9) | 86.8 (85.6-87.9) | 83.0 (81.4-84.4) | 4,510 | 96.7 (96.0-97.3) | 95.2 (94.0-96.1) | 93.2 (91.2-94.8) | |
| All ages | 16,928 | 96.0 (95.7-96.4) | 90.5 (89.9-91.1) | 86.7 (85.8-87.5) | 11,690 | 94.6 (94.2-95.0) | 85.8 (85.0-86.5) | 80.7 (79.7-81.6) | 7,015 | 97.7 (97.3-98.1) | 96.5 (95.7-97.2) | 94.9 (93.6-96.0) | |
| Glioma malignant, NOS | 0-14 | 6,180 | 81.9 (80.9-82.8) | 69.5 (68.3-70.7) | 68.4 (67.1-69.6) | 7,201 | 81.1 (80.2-82.0) | 68.2 (67.1-69.3) | 67.1 (65.9-68.2) | -- | -- | -- | -- |
| 15-39 | 3,727 | 91.7 (90.8-92.6) | 79.1 (77.6-80.6) | 72.3 (70.4-74.1) | 4,269 | 91.2 (90.2-92.0) | 78.0 (76.6-79.3) | 70.7 (69.0-72.4) | -- | -- | -- | -- | |
| 40+ | 7,932 | 53.1 (51.9-54.2) | 35.9 (34.7-37.2) | 29.6 (28.2-31.0) | 9,355 | 51.4 (50.3-52.4) | 34.2 (33.1-35.3) | 28.1 (26.9-29.3) | -- | -- | -- | -- | |
| All ages | 17,839 | 71.3 (70.6-72.0) | 56.9 (56.1-57.7) | 52.5 (51.6-53.4) | 20,825 | 70.0 (69.3-70.6) | 55.3 (54.5-56.0) | 50.9 (50.1-51.7) | -- | -- | -- | -- | |
| Other neuroepithelial tumors | 0-14 | 65 | 96.9 (87.8-99.2) | 91.3 (80.1-96.4) | 91.3 (80.1-96.4) | 56 | 96.3 (85.9-99.1) | 89.9 (77.1-95.7) | 89.9 (77.1-95.7) | -- | -- | -- | -- |
| 15-39 | 88 | 96.6 (89.5-98.9) | 88.2 (78.1-93.9) | 84.7 (73.3-91.5) | 70 | 95.6 (86.8-98.6) | 87.0 (75.2-93.4) | 80.0 (65.8-88.8) | -- | -- | -- | -- | |
| 40+ | 105 | 72.7 (62.7-80.4) | 52.3 (40.7-62.6) | 41.8 (28.8-54.3) | 61 | 70.9 (57.2-81.0) | 42.3 (28.1-55.8) | 33.5 (19.5-48.1) | 55 | 76.8 (62.7-86.1) | 60.2 (43.8-73.2) | 53.4 (34.1-69.4) | |
| All ages | 258 | 87.0 (82.0-90.6) | 74.7 (68.2-80.1) | 69.6 (61.9-76.0) | 187 | 87.8 (81.9-91.9) | 73.4 (65.6-79.8) | 67.6 (58.8-75.0) | 96 | 86.9 (78.0-92.4) | 76.9 (65.8-84.8) | 73.9 (61.2-83.1) | |
| Neuronal and mixed neuronal-glial tumors | 0-14 | 3,264 | 98.8 (98.3-99.1) | 96.1 (95.3-96.8) | 95.3 (94.3-96.1) | 298 | 92.8 (89.1-95.2) | 81.4 (76.1-85.6) | 79.8 (74.3-84.3) | 3,024 | 99.3 (98.9-99.6) | 97.3 (96.5-97.8) | 96.5 (95.5-97.2) |
| 15-39 | 4,983 | 98.5 (98.1-98.8) | 95.5 (94.8-96.1) | 92.4 (91.3-93.4) | 590 | 94.7 (92.5-96.3) | 78.8 (75.0-82.1) | 70.4 (65.7-74.5) | 4,474 | 99.0 (98.6-99.3) | 97.6 (97.0-98.1) | 95.4 (94.3-96.2) | |
| 40+ | 3,987 | 93.4 (92.5-94.2) | 84.9 (83.3-86.2) | 80.1 (78.0-82.1) | 1,623 | 90.7 (89.0-92.1) | 76.9 (74.3-79.4) | 68.9 (65.3-72.2) | 2,575 | 94.6 (93.6-95.5) | 89.5 (87.8-91.0) | 86.0 (83.4-88.1) | |
| All ages | 12,234 | 96.9 (96.6-97.2) | 92.2 (91.6-92.8) | 89.2 (88.4-90.0) | 2,511 | 91.9 (90.7-93.0) | 77.9 (75.9-79.8) | 70.7 (68.1-73.1) | 10,073 | 98.0 (97.6-98.3) | 95.5 (94.9-95.9) | 93.3 (92.5-94.0) | |
| Choroid plexus tumors | 0-14 | 961 | 95.4 (93.8-96.6) | 89.9 (87.6-91.8) | 88.0 (85.4-90.2) | 283 | 86.1 (81.4-89.7) | 65.5 (59.2-71.1) | 60.2 (53.4-66.4) | 718 | 98.5 (97.1-99.2) | 97.5 (95.9-98.5) | 96.8 (94.8-98.1) |
| 15-39 | 586 | 98.2 (96.6-99.0) | 96.0 (93.7-97.5) | 92.5 (88.9-94.9) | -- | -- | -- | -- | 547 | 98.2 (96.6-99.1) | 97.2 (95.1-98.3) | 95.5 (92.2-97.4) | |
| 40+ | 676 | 89.7 (86.9-91.9) | 84.9 (81.0-88.0) | 81.6 (75.9-86.1) | -- | -- | -- | -- | 629 | 90.7 (87.8-92.9) | 86.5 (82.5-89.6) | 84.4 (78.4-88.9) | |
| All ages | 2,223 | 94.4 (93.3-95.3) | 90.0 (88.4-91.4) | 87.3 (85.2-89.1) | 379 | 85.8 (81.7-89.0) | 66.9 (61.5-71.7) | 58.2 (52.1-63.8) | 1,894 | 95.8 (94.7-96.7) | 93.9 (92.4-95.1) | 92.5 (90.3-94.1) | |
| Tumors of the pineal region | 0-14 | 378 | 88.9 (85.2-91.7) | 68.5 (63.1-73.3) | 63.1 (57.1-68.5) | 376 | 85.9 (81.9-89.1) | 62.4 (56.9-67.4) | 55.6 (49.7-61.1) | 62 | 98.4 (88.5-99.8) | 98.4 (88.5-99.8) | 98.4 (88.5-99.8) |
| 15-39 | 695 | 95.6 (93.7-96.9) | 86.4 (83.2-89.0) | 80.9 (76.8-84.4) | 417 | 93.4 (90.4-95.4) | 73.7 (68.5-78.2) | 63.4 (57.1-68.9) | 334 | 97.6 (95.2-98.8) | 97.3 (94.3-98.7) | 95.9 (91.6-98.0) | |
| 40+ | 754 | 90.6 (88.1-92.6) | 80.8 (76.9-84.0) | 72.8 (67.3-77.6) | 336 | 86.6 (82.2-89.9) | 70.0 (63.8-75.3) | 57.3 (49.4-64.4) | 453 | 92.6 (89.5-94.9) | 87.6 (82.8-91.1) | 83.0 (75.6-88.4) | |
| All ages | 1,827 | 92.2 (90.8-93.4) | 80.3 (78.0-82.3) | 73.9 (71.0-76.6) | 1,129 | 88.8 (86.8-90.6) | 68.7 (65.6-71.7) | 59.1 (55.3-62.6) | 849 | 95.1 (93.2-96.4) | 92.3 (89.6-94.3) | 89.5 (85.4-92.5) | |
| Embryonal tumors | 0-14 | 6,037 | 81.9 (80.9-82.9) | 64.0 (62.7-65.2) | 59.1 (57.7-60.5) | 7,197 | 81.5 (80.6-82.4) | 63.2 (62.0-64.4) | 58.4 (57.2-59.7) | -- | -- | -- | -- |
| 15-39 | 2,139 | 91.2 (89.9-92.4) | 71.4 (69.3-73.5) | 61.3 (58.8-63.8) | 2,599 | 90.6 (89.4-91.6) | 70.7 (68.8-72.6) | 61.0 (58.8-63.1) | -- | -- | -- | -- | |
| 40+ | 783 | 69.7 (66.2-72.8) | 45.4 (41.5-49.2) | 36.9 (32.6-41.2) | 919 | 69.7 (66.6-72.7) | 46.3 (42.7-49.8) | 37.2 (33.3-41.0) | -- | -- | -- | -- | |
| All ages | 8,959 | 83.1 (82.3-83.9) | 64.1 (63.0-65.2) | 57.7 (56.5-58.9) | 10,715 | 82.7 (82.0-83.4) | 63.6 (62.6-64.6) | 57.2 (56.2-58.3) | -- | -- | -- | -- | |
| Nerve sheath tumors | 0-14 | 2,253 | 99.8 (99.4-99.9) | 98.7 (98.1-99.2) | 97.9 (96.9-98.5) | -- | -- | -- | -- | 2,219 | 100.0 (0.0-100.0) | 99.1 (98.5-99.5) | 98.3 (97.4-98.9) |
| 15-39 | 13,341 | 99.3 (99.1-99.4) | 98.5 (98.2-98.7) | 97.7 (97.2-98.1) | -- | -- | -- | -- | 13,202 | 99.5 (99.4-99.6) | 98.8 (98.5-99.0) | 98.1 (97.6-98.5) | |
| 40+ | 71,631 | 99.2 (99.1-99.3) | 99.2 (99.1-99.3) | 99.2 (99.1-99.3) | 493 | 85.4 (81.8-88.4) | 76.2 (71.4-80.2) | 73.2 (67.0-78.5) | 71,267 | 99.3 (99.2-99.4) | 99.3 (99.2-99.4) | 99.3 (99.2-99.4) | |
| All ages | 87,225 | 99.2 (99.1-99.3) | 99.2 (99.1-99.3) | 99.2 (99.1-99.3) | 713 | 84.4 (81.4-87.0) | 74.2 (70.4-77.5) | 70.5 (65.8-74.7) | 86,688 | 99.3 (99.2-99.4) | 99.3 (99.2-99.4) | 99.3 (99.2-99.4) | |
| Other tumors of cranial and paraspinal nerves | 0-14 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 15-39 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 40+ | 53 | 97.5 (80.5-99.7) | 95.2 (75.2-99.1) | 89.0 (60.6-97.3) | -- | -- | -- | -- | 53 | 97.5 (80.5-99.7) | 95.2 (75.2-99.1) | 89.0 (60.6-97.3) | |
| All ages | 65 | 96.4 (85.0-99.2) | 92.8 (79.0-97.7) | 87.7 (66.2-95.9) | -- | -- | -- | -- | 65 | 96.4 (85.0-99.2) | 92.8 (79.0-97.7) | 87.7 (66.2-95.9) | |
| Meningiomas | 0-14 | 683 | 97.8 (96.3-98.7) | 95.6 (93.6-97.0) | 91.7 (88.6-94.0) | 59 | 89.8 (78.6-95.3) | 79.0 (65.9-87.5) | 73.7 (59.1-83.8) | 635 | 98.6 (97.2-99.3) | 96.8 (94.9-98.1) | 93.2 (90.1-95.3) |
| 15-39 | 23,524 | 98.8 (98.6-98.9) | 97.0 (96.7-97.2) | 94.7 (94.3-95.1) | 422 | 93.9 (91.0-95.8) | 84.2 (80.0-87.5) | 79.0 (74.0-83.1) | 23,200 | 98.8 (98.7-99.0) | 97.2 (96.9-97.4) | 95.0 (94.6-95.4) | |
| 40+ | 357,071 | 92.8 (92.7-92.9) | 87.3 (87.1-87.4) | 82.2 (81.9-82.5) | 4,644 | 83.2 (82.0-84.3) | 65.2 (63.4-66.8) | 57.9 (55.7-60.0) | 353,421 | 92.9 (92.8-93.0) | 87.5 (87.3-87.7) | 82.5 (82.2-82.8) | |
| All ages | 381,278 | 93.2 (93.1-93.3) | 87.9 (87.7-88.1) | 83.1 (82.8-83.4) | 5,125 | 84.2 (83.0-85.2) | 67.0 (65.4-68.6) | 60.0 (57.9-61.9) | 377,256 | 93.3 (93.2-93.4) | 88.2 (88.0-88.4) | 83.4 (83.1-83.7) | |
| Mesenchymal tumors | 0-14 | 1,270 | 97.9 (96.8-98.6) | 94.5 (92.8-95.7) | 92.3 (90.1-94.1) | 194 | 85.8 (79.9-90.0) | 69.0 (61.5-75.3) | 62.3 (54.0-69.5) | 1,110 | 99.4 (98.6-99.8) | 97.9 (96.6-98.7) | 96.3 (94.2-97.7) |
| 15-39 | 4,450 | 98.2 (97.7-98.5) | 95.9 (95.2-96.5) | 93.5 (92.4-94.4) | 541 | 92.3 (89.7-94.3) | 80.4 (76.4-83.7) | 71.9 (67.1-76.2) | 4,019 | 98.8 (98.4-99.1) | 97.5 (96.9-98.0) | 95.7 (94.6-96.5) | |
| 40+ | 10,687 | 94.3 (93.8-94.8) | 89.7 (88.8-90.5) | 84.5 (83.1-85.8) | 1,292 | 87.1 (85.0-88.9) | 69.1 (65.9-72.1) | 52.1 (48.0-56.0) | 9,596 | 95.2 (94.6-95.6) | 92.1 (91.2-92.9) | 88.2 (86.8-89.5) | |
| All ages | 16,407 | 95.7 (95.3-96.0) | 91.8 (91.2-92.3) | 87.6 (86.7-88.5) | 2,027 | 88.4 (86.8-89.8) | 72.3 (69.9-74.5) | 58.9 (55.9-61.8) | 14,725 | 96.5 (96.1-96.8) | 94.0 (93.4-94.6) | 90.9 (89.9-91.8) | |
| Primary melanocytic lesions | 0-14 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 15-39 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 40+ | 185 | 68.5 (60.7-75.0) | 43.2 (34.5-51.6) | 26.2 (16.3-37.2) | 130 | 60.5 (51.1-68.7) | 30.5 (21.7-39.8) | 16.3 (7.8-27.5) | 74 | 84.3 (72.6-91.3) | 59.1 (43.7-71.6) | 37.6 (19.2-56.0) | |
| All ages | 254 | 68.8 (62.3-74.3) | 46.0 (38.7-53.0) | 31.3 (23.0-39.9) | 182 | 58.4 (50.6-65.4) | 32.2 (24.6-40.0) | 20.6 (13.0-29.4) | 99 | 87.4 (78.2-92.9) | 66.5 (53.8-76.4) | 49.2 (33.9-62.9) | |
| Lymphoma | 0-14 | 171 | 91.0 (85.4-94.5) | 84.8 (78.1-89.6) | 79.2 (70.3-85.7) | 192 | 90.9 (85.8-94.3) | 84.8 (78.5-89.4) | 80.6 (73.0-86.3) | -- | -- | -- | -- |
| 15-39 | 1,528 | 67.3 (64.8-69.6) | 59.2 (56.6-61.8) | 54.9 (51.9-57.7) | 1,909 | 63.0 (60.7-65.1) | 54.1 (51.8-56.4) | 50.2 (47.6-52.7) | -- | -- | -- | -- | |
| 40+ | 15,196 | 53.9 (53.0-54.7) | 35.7 (34.8-36.6) | 27.4 (26.4-28.5) | 17,754 | 53.2 (52.5-54.0) | 34.4 (33.6-35.2) | 25.7 (24.8-26.6) | -- | -- | -- | -- | |
| All ages | 16,895 | 55.5 (54.7-56.3) | 38.5 (37.7-39.4) | 30.8 (29.8-31.8) | 19,855 | 54.6 (53.8-55.3) | 37.0 (36.2-37.7) | 28.9 (28.1-29.8) | -- | -- | -- | -- | |
| Other hematopoietic neoplasms | 0-14 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 15-39 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| 40+ | 148 | 82.3 (74.6-87.8) | 67.5 (57.6-75.5) | 63.0 (51.4-72.6) | 186 | 82.4 (75.7-87.4) | 65.0 (56.4-72.3) | 59.8 (49.9-68.4) | -- | -- | -- | -- | |
| All ages | 164 | 84.1 (77.0-89.1) | 67.9 (58.7-75.5) | 63.9 (53.1-72.8) | 209 | 84.4 (78.4-88.9) | 66.9 (58.9-73.6) | 62.4 (53.3-70.3) | -- | -- | -- | -- | |
| Germ cell tumors | 0-14 | 1,398 | 93.2 (91.7-94.5) | 89.2 (87.4-90.8) | 86.2 (83.9-88.2) | 1,420 | 92.7 (91.2-94.0) | 87.6 (85.6-89.3) | 83.8 (81.4-85.9) | 190 | 92.5 (87.5-95.6) | 91.2 (85.8-94.6) | 91.2 (85.8-94.6) |
| 15-39 | 1,500 | 95.3 (94.0-96.3) | 89.4 (87.6-91.0) | 87.3 (85.1-89.2) | 1,620 | 94.4 (93.1-95.4) | 88.4 (86.5-90.0) | 85.9 (83.8-87.8) | 127 | 99.3 (93.5-99.9) | 93.3 (86.2-96.8) | 91.0 (82.9-95.4) | |
| 40+ | 194 | 92.1 (86.8-95.3) | 83.5 (75.9-88.9) | 79.7 (70.9-86.1) | 88 | 81.4 (71.3-88.3) | 64.8 (52.7-74.6) | 61.4 (47.6-72.6) | 123 | 98.5 (90.1-99.8) | 92.9 (82.3-97.3) | 87.5 (75.2-94.0) | |
| All ages | 3,092 | 94.1 (93.2-94.9) | 89.0 (87.7-90.1) | 86.3 (84.8-87.8) | 3,128 | 93.2 (92.3-94.1) | 87.3 (86.0-88.5) | 84.3 (82.7-85.7) | 440 | 96.2 (93.7-97.7) | 92.2 (88.5-94.7) | 89.9 (85.5-93.0) | |
| Tumors of the pituitary | 0-14 | 2,430 | 99.8 (99.5-99.9) | 99.4 (98.9-99.7) | 99.2 (98.5-99.5) | -- | -- | -- | -- | 2,428 | 99.8 (99.5-99.9) | 99.4 (98.9-99.7) | 99.2 (98.5-99.5) |
| 15-39 | 53,372 | 99.7 (99.7-99.8) | 99.4 (99.3-99.5) | 98.8 (98.6-99.0) | -- | -- | -- | -- | 53,301 | 99.7 (99.7-99.8) | 99.4 (99.3-99.5) | 98.8 (98.6-99.0) | |
| 40+ | 116,677 | 97.5 (97.4-97.6) | 95.8 (95.6-96.0) | 93.4 (92.9-93.8) | 377 | 88.2 (84.2-91.3) | 79.3 (73.5-83.9) | 70.6 (62.6-77.2) | 116,373 | 97.5 (97.4-97.7) | 95.9 (95.6-96.1) | 93.4 (93.0-93.8) | |
| All ages | 172,479 | 98.2 (98.2-98.3) | 97.0 (96.8-97.1) | 95.2 (94.9-95.5) | 480 | 90.4 (87.1-92.9) | 81.6 (76.8-85.5) | 75.3 (68.9-80.6) | 172,102 | 98.3 (98.2-98.3) | 97.0 (96.9-97.2) | 95.3 (95.0-95.5) | |
| Craniopharyngioma | 0-14 | 1,843 | 98.7 (98.0-99.1) | 96.0 (94.9-96.9) | 92.6 (90.9-94.0) | -- | -- | -- | -- | 1,835 | 98.7 (98.1-99.2) | 96.1 (94.9-96.9) | 92.6 (90.9-94.0) |
| 15-39 | 1,891 | 96.2 (95.2-97.0) | 91.2 (89.7-92.5) | 87.3 (85.3-89.1) | -- | -- | -- | -- | 1,889 | 96.2 (95.2-97.0) | 91.3 (89.8-92.6) | 87.4 (85.4-89.2) | |
| 40+ | 4,265 | 88.9 (87.8-89.9) | 78.3 (76.7-79.8) | 69.2 (66.9-71.3) | -- | -- | -- | -- | 4,255 | 88.9 (87.9-89.9) | 78.4 (76.8-79.9) | 69.2 (66.9-71.4) | |
| All ages | 7,999 | 92.9 (92.3-93.5) | 85.6 (84.6-86.5) | 79.3 (78.0-80.6) | -- | -- | -- | -- | 7,979 | 92.9 (92.3-93.5) | 85.7 (84.7-86.6) | 79.3 (78.0-80.6) | |
| Hemangioma | 0-14 | 589 | 99.5 (98.3-99.9) | 98.5 (97.0-99.3) | 98.5 (97.0-99.3) | -- | -- | -- | -- | 589 | 99.5 (98.3-99.9) | 98.5 (97.0-99.3) | 98.5 (97.0-99.3) |
| 15-39 | 2,684 | 99.7 (99.3-99.9) | 98.9 (98.1-99.3) | 97.1 (95.7-98.1) | -- | -- | -- | -- | 2,678 | 99.7 (99.3-99.9) | 98.9 (98.2-99.3) | 97.2 (95.8-98.1) | |
| 40+ | 5,444 | 96.0 (95.4-96.6) | 92.1 (90.9-93.2) | 89.1 (87.0-91.0) | -- | -- | -- | -- | 5,438 | 96.1 (95.4-96.7) | 92.2 (90.9-93.3) | 89.2 (87.0-91.0) | |
| All ages | 8,717 | 97.4 (97.0-97.8) | 94.7 (93.9-95.4) | 92.5 (91.1-93.6) | -- | -- | -- | -- | 8,705 | 97.5 (97.0-97.8) | 94.8 (94.0-95.5) | 92.5 (91.2-93.7) | |
| Neoplasm, unspecified | 0-14 | 1,429 | 88.0 (86.2-89.6) | 85.0 (82.9-86.8) | 83.2 (80.9-85.2) | 391 | 64.5 (59.5-69.1) | 56.5 (51.3-61.4) | 53.2 (47.8-58.4) | 1,084 | 95.3 (93.8-96.4) | 94.0 (92.3-95.3) | 92.8 (90.8-94.5) |
| 15-39 | 4,246 | 93.5 (92.7-94.3) | 90.2 (89.2-91.2) | 88.1 (86.8-89.2) | 852 | 79.6 (76.7-82.2) | 68.4 (65.0-71.6) | 63.0 (59.2-66.6) | 3,560 | 96.1 (95.4-96.7) | 94.1 (93.2-94.9) | 92.3 (91.1-93.4) | |
| 40+ | 21,216 | 54.3 (53.6-55.0) | 45.3 (44.5-46.1) | 40.2 (39.2-41.2) | 9,675 | 27.4 (26.5-28.4) | 17.6 (16.7-18.5) | 15.1 (14.2-16.1) | 13,232 | 70.9 (70.1-71.7) | 62.0 (61.0-63.1) | 55.5 (54.1-56.8) | |
| All ages | 26,891 | 62.5 (61.9-63.2) | 54.8 (54.1-55.5) | 50.6 (49.7-51.4) | 10,918 | 33.0 (32.1-34.0) | 23.2 (22.3-24.1) | 20.5 (19.5-21.5) | 17,876 | 77.5 (76.9-78.2) | 70.6 (69.8-71.4) | 65.7 (64.6-66.7) | |
| All other | 0-14 | 402 | 89.0 (85.4-91.8) | 84.4 (80.3-87.8) | 84.4 (80.3-87.8) | 125 | 59.1 (49.9-67.3) | 39.4 (30.2-48.5) | 37.8 (28.4-47.1) | 295 | 99.4 (96.9-99.9) | 99.4 (96.9-99.9) | 99.4 (96.9-99.9) |
| 15-39 | 361 | 97.8 (95.6-98.9) | 94.1 (90.7-96.3) | 91.9 (87.4-94.9) | -- | -- | -- | -- | 332 | 98.9 (96.7-99.6) | 97.5 (94.5-98.9) | 95.2 (90.4-97.6) | |
| 40+ | 561 | 87.9 (84.4-90.6) | 84.8 (80.0-88.5) | 75.7 (68.5-81.5) | -- | -- | -- | -- | 537 | 89.2 (85.7-91.9) | 86.6 (81.3-90.5) | 77.5 (69.9-83.4) | |
| All ages | 1,324 | 91.0 (89.2-92.5) | 87.3 (84.8-89.4) | 83.3 (79.9-86.2) | 191 | 64.5 (57.1-70.9) | 42.8 (35.1-50.3) | 39.9 (31.9-47.8) | 1,164 | 94.6 (92.9-95.9) | 93.2 (90.7-95.0) | 88.8 (85.2-91.6) | |
| TOTAL i | 0-14 | 45,563 | 91.6 (91.3-91.8) | 83.1 (82.8-83.5) | 80.6 (80.2-81.0) | 35,490 | 87.4 (87.1-87.8) | 75.1 (74.6-75.6) | 71.9 (71.4-72.5) | 15,594 | 98.9 (98.8-99.1) | 97.6 (97.3-97.8) | 96.4 (96.0-96.8) |
| 15-39 | 154,227 | 97.0 (96.9-97.1) | 90.9 (90.7-91.1) | 86.8 (86.6-87.0) | 52,219 | 90.7 (90.5-91.0) | 71.7 (71.3-72.1) | 61.2 (60.6-61.7) | 110,555 | 99.2 (99.2-99.3) | 98.3 (98.2-98.4) | 97.1 (96.9-97.2) | |
| 40+ | 779,936 | 82.9 (82.9-83.0) | 72.5 (72.3-72.6) | 68.5 (68.4-68.7) | 231,959 | 49.2 (49.0-49.4) | 21.0 (20.8-21.2) | 16.7 (16.5-16.9) | 583,147 | 94.2 (94.1-94.3) | 90.3 (90.2-90.5) | 86.8 (86.5-87.0) | |
| All ages | 979,726 | 85.6 (85.5-85.7) | 76.0 (75.9-76.1) | 72.1 (72.0-72.3) | 319,668 | 60.3 (60.2-60.5) | 35.7 (35.5-35.9) | 30.5 (30.3-30.7) | 709,296 | 95.1 (95.0-95.2) | 91.8 (91.7-91.9) | 88.7 (88.6-88.9) |
aThe cohort analysis of survival rates was utilized for calculating the survival estimates presented in this table. Long-term cohort-based survival estimates reflect the survival experience of individuals diagnosed over the time period, and they may not necessarily reflect the long-term survival outlook of newly diagnosed cases.
bRates are an estimate of the percentage of patients alive at one, two, five, and ten years, respectively.
cAssigned behavior code of /3 (see Table 2).
dAssigned behavior code of /0 or /1 (see Table 2).
eTotal number of cases that occurred within the included NPCR and SEER registries between 2004 and 2018.
fTotal number of cases that occurred within the included NPCR and SEER registries between 2001 and 2018.
gChildren as defined by the National Cancer Institute, see: http://www.cancer.gov/researchandfunding/snapshots/pediatric.
hAdolescents and Young Adults (AYA), as defined by the National Cancer Institute, see: http://www.cancer.gov/cancertopics/aya.
iTotal includes histopathologies not listed in this table.
-- Rates were not presented for categories with 50 or fewer cases and were suppressed for rates where fewer than 16 cases were surviving within a category.
** Confidence interval could not be calculated.
Abbreviations: NCI, National Cancer Institute; CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
Descriptive Summary of Glioblastoma
Glioblastoma was the third most frequently reported CNS histopathology and the most common malignant tumor histopathology overall (Table 8).
Glioblastoma accounted for 14.2% of all primary brain and other CNS tumors and 50.1% of primary malignant brain tumors (Figure 12B and Figure 5B).
Glioblastoma was more common in older adults and was less common in children (Table 10, Figure 18); these tumors comprised approximately 2.7% of all brain and other CNS tumors reported among ages 0–19 years (Figure 19).
Incidence of glioblastoma increased with age, with rates highest in individuals ages 75 to 84 years (Supplementary Table 8).
Glioblastoma was 1.6 times more common in males than females (Figure 16).
Glioblastoma was 1.95 times higher among people who are White compared to people who are Black (Figure 17).
Relative survival estimates for glioblastoma were quite low; 6.9% of patients survived five years post-diagnosis. These survival estimates were somewhat higher for the small number of patients who were diagnosed under age 20 years (Table 11, Supplementary Table 13).
Fig. 18.
Age-Adjusted Incidence Ratesa of Brain and Other Central Nervous System Tumors by Selected Histopathologies and Age Group at Diagnosis A) Ages 0-19 Years and B) Ages 20+ Years CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Fig. 19.
Distributiona in Children and Adolescents (Ages 0-19 Years) of All Primary Brain and Central Nervous System Tumors (Five-Year Total=25,340; Annual Average Cases=5,068) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Descriptive Summary of Embryonal Tumors
Embryonal tumors were the most frequently reported brain and other CNS tumor histopathology in children ages 0-4 years, and the fourth most common tumor type overall in children and adolescents ages 0-19 years (Table 12, Figure 19).
Embryonal tumors accounted for 12.2% of all primary brain and other CNS tumors in children ages 0-14 years, 9.2% of tumors in children and adolescents ages 0-19 years, and 0.8% of tumors diagnosed overall (Figure 19B, Figure 20B, Table 8).
Embryonal tumors within the CBTRUS histopathologic grouping scheme included multiple different histopathologies: medulloblastoma, atypical teratoid/rhabdoid tumor (ATRT), and several other histopathologies (Table 2).
Incidence of medulloblastoma decreased with age. Incidence was 0.51 per 100,000 population, 0.62 per 100,000 population, 0.32 per 100,000 population, and 0.15 per 100,000 population in children age groups 0–4, 5–9, 10–14 years, and adolescents ages 15–19 years, respectively (Table 12).
Incidence of ATRT was 0.33 per 100,000 population and 0.03 per 100,000 population in children ages 0–4 and 5–9 years, respectively. There were too few of these cases in older age groups to report (Table 12).
Embryonal tumors were 1.46 times more common in males than females (Figure 16), and this sex difference was largest in medulloblastoma (Supplementary Figure 7).
Table 12.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for Children and Adolescents (Ages 0-19 Years), Brain and Other Central Nervous System Tumors by Histopathology and Age Group at Diagnosis, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | 0-19 Years | 0-4 Years | 5-9 Years | 10-14 Years | 15-19 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 2,102 | 420 | 0.51 (0.49-0.54) | 409 | 82 | 0.41 (0.38-0.46) | 430 | 86 | 0.42 (0.39-0.47) | 571 | 114 | 0.55 (0.51-0.60) | 692 | 138 | 0.66 (0.61-0.71) |
| Diffuse astrocytoma | 878 | 176 | 0.21 (0.20-0.23) | 222 | 44 | 0.22 (0.20-0.26) | 173 | 35 | 0.17 (0.15-0.20) | 232 | 46 | 0.22 (0.20-0.26) | 251 | 50 | 0.24 (0.21-0.27) |
| Anaplastic astrocytoma | 332 | 66 | 0.08 (0.07-0.09) | 51 | 10 | 0.05 (0.04-0.07) | 79 | 16 | 0.08 (0.06-0.10) | 92 | 18 | 0.09 (0.07-0.11) | 110 | 22 | 0.10 (0.09-0.13) |
| Glioblastoma | 695 | 139 | 0.17 (0.16-0.18) | 106 | 21 | 0.11 (0.09-0.13) | 150 | 30 | 0.15 (0.13-0.17) | 209 | 42 | 0.20 (0.18-0.23) | 230 | 46 | 0.22 (0.19-0.25) |
| Oligodendroglioma | 145 | 29 | 0.04 (0.03-0.04) | 17 | 3 | 0.02 (0.01-0.03) | 17 | 3 | 0.02 (0.01-0.03) | 30 | 6 | 0.03 (0.02-0.04) | 81 | 16 | 0.08 (0.06-0.10) |
| Anaplastic oligodendroglioma | 16 | 3 | 0.00 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Oligoastrocytic tumors | 36 | 7 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other Astrocytic Tumors | 4,382 | 876 | 1.08 (1.04-1.11) | 1,352 | 270 | 1.37 (1.29-1.44) | 1,247 | 249 | 1.23 (1.16-1.30) | 1,059 | 212 | 1.03 (0.96-1.09) | 724 | 145 | 0.69 (0.64-0.74) |
| Pilocytic astrocytoma | 3,885 | 777 | 0.95 (0.92-0.98) | 1,261 | 252 | 1.27 (1.20-1.35) | 1,121 | 224 | 1.11 (1.04-1.17) | 901 | 180 | 0.87 (0.82-0.93) | 602 | 120 | 0.57 (0.53-0.62) |
| Unique astrocytoma variants | 497 | 99 | 0.12 (0.11-0.13) | 91 | 18 | 0.09 (0.07-0.11) | 126 | 25 | 0.12 (0.10-0.15) | 158 | 32 | 0.15 (0.13-0.18) | 122 | 24 | 0.12 (0.10-0.14) |
| Malignant | 230 | 46 | 0.06 (0.05-0.06) | -- | -- | -- | 44 | 9 | 0.04 (0.03-0.06) | 87 | 17 | 0.08 (0.07-0.10) | -- | -- | -- |
| Non-Malignant | 267 | 53 | 0.07 (0.06-0.07) | -- | -- | -- | 82 | 16 | 0.08 (0.06-0.10) | 71 | 14 | 0.07 (0.05-0.09) | -- | -- | -- |
| Ependymal Tumors | 1,185 | 237 | 0.29 (0.27-0.31) | 451 | 90 | 0.46 (0.42-0.50) | 224 | 45 | 0.22 (0.19-0.25) | 250 | 50 | 0.24 (0.21-0.27) | 260 | 52 | 0.25 (0.22-0.28) |
| Malignant | 966 | 193 | 0.24 (0.22-0.25) | 432 | 86 | 0.44 (0.40-0.48) | 198 | 40 | 0.20 (0.17-0.22) | 183 | 37 | 0.18 (0.15-0.20) | 153 | 31 | 0.15 (0.12-0.17) |
| Non-Malignant | 219 | 44 | 0.05 (0.05-0.06) | 19 | 4 | 0.02 (0.01-0.03) | 26 | 5 | 0.03 (0.02-0.04) | 67 | 13 | 0.06 (0.05-0.08) | 107 | 21 | 0.10 (0.08-0.12) |
| Other Gliomas | 3,188 | 638 | 0.78 (0.76-0.81) | 902 | 180 | 0.91 (0.85-0.97) | 1,022 | 204 | 1.01 (0.95-1.07) | 766 | 153 | 0.74 (0.69-0.80) | 498 | 100 | 0.47 (0.43-0.52) |
| Glioma malignant, NOS | 3,160 | 632 | 0.78 (0.75-0.80) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other neuroepithelial tumors | 28 | 6 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 2,090 | 418 | 0.51 (0.49-0.53) | 375 | 75 | 0.38 (0.34-0.42) | 374 | 75 | 0.37 (0.33-0.41) | 662 | 132 | 0.64 (0.59-0.69) | 679 | 136 | 0.65 (0.60-0.70) |
| Malignant | 120 | 24 | 0.03 (0.02-0.04) | 36 | 7 | 0.04 (0.03-0.05) | 19 | 4 | 0.02 (0.01-0.03) | 32 | 6 | 0.03 (0.02-0.04) | 33 | 7 | 0.03 (0.02-0.04) |
| Non-Malignant | 1,970 | 394 | 0.48 (0.46-0.50) | 339 | 68 | 0.34 (0.31-0.38) | 355 | 71 | 0.35 (0.32-0.39) | 630 | 126 | 0.61 (0.56-0.66) | 646 | 129 | 0.61 (0.57-0.66) |
| Choroid Plexus Tumors | 407 | 81 | 0.10 (0.09-0.11) | 254 | 51 | 0.26 (0.23-0.29) | 47 | 9 | 0.05 (0.03-0.06) | 58 | 12 | 0.06 (0.04-0.07) | 48 | 10 | 0.05 (0.03-0.06) |
| Malignant | 98 | 20 | 0.02 (0.02-0.03) | 83 | 17 | 0.08 (0.07-0.10) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 309 | 62 | 0.08 (0.07-0.08) | 171 | 34 | 0.17 (0.15-0.20) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of the Pineal Region | 215 | 43 | 0.05 (0.05-0.06) | 55 | 11 | 0.06 (0.04-0.07) | 50 | 10 | 0.05 (0.04-0.07) | 38 | 8 | 0.04 (0.03-0.05) | 72 | 14 | 0.07 (0.05-0.09) |
| Malignant | 179 | 36 | 0.04 (0.04-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- | 54 | 11 | 0.05 (0.04-0.07) |
| Non-Malignant | 36 | 7 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- | 18 | 4 | 0.02 (0.01-0.03) |
| Embryonal Tumors | 2,338 | 468 | 0.58 (0.55-0.60) | 1,053 | 211 | 1.07 (1.00-1.13) | 715 | 143 | 0.71 (0.66-0.76) | 376 | 75 | 0.36 (0.33-0.40) | 194 | 39 | 0.18 (0.16-0.21) |
| Medulloblastoma | 1,626 | 325 | 0.40 (0.38-0.42) | 509 | 102 | 0.51 (0.47-0.56) | 623 | 125 | 0.62 (0.57-0.67) | 332 | 66 | 0.32 (0.29-0.36) | 162 | 32 | 0.15 (0.13-0.18) |
| Atypical teratoid/rhabdoid tumor | 372 | 74 | 0.09 (0.08-0.10) | 320 | 64 | 0.33 (0.29-0.36) | 32 | 6 | 0.03 (0.02-0.04) | -- | -- | -- | -- | -- | -- |
| All other embryonal | 340 | 68 | 0.08 (0.07-0.09) | 224 | 45 | 0.23 (0.20-0.26) | 60 | 12 | 0.06 (0.05-0.08) | -- | -- | -- | -- | -- | -- |
| Tumors of Cranial and Paraspinal Nerves | 1,173 | 235 | 0.29 (0.27-0.30) | 216 | 43 | 0.22 (0.19-0.25) | 182 | 36 | 0.18 (0.15-0.21) | 288 | 58 | 0.28 (0.25-0.31) | 487 | 97 | 0.46 (0.42-0.51) |
| Nerve sheath tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other tumors of cranial and paraspinal nerves | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 1,252 | 250 | 0.30 (0.29-0.32) | 231 | 46 | 0.24 (0.21-0.27) | 157 | 31 | 0.16 (0.13-0.18) | 292 | 58 | 0.28 (0.25-0.32) | 572 | 114 | 0.54 (0.50-0.59) |
| Meningiomas | 671 | 134 | 0.16 (0.15-0.18) | 59 | 12 | 0.06 (0.05-0.08) | 84 | 17 | 0.08 (0.07-0.10) | 175 | 35 | 0.17 (0.15-0.20) | 353 | 71 | 0.34 (0.30-0.37) |
| Malignant | 21 | 4 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 650 | 130 | 0.16 (0.15-0.17) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Mesenchymal tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Primary melanocytic lesions | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | 133 | 27 | 0.03 (0.03-0.04) | 23 | 5 | 0.02 (0.01-0.03) | 36 | 7 | 0.04 (0.02-0.05) | 30 | 6 | 0.03 (0.02-0.04) | 44 | 9 | 0.04 (0.03-0.06) |
| Lymphoma | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other hematopoietic neoplasms | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | 863 | 173 | 0.21 (0.20-0.23) | 106 | 21 | 0.11 (0.09-0.13) | 156 | 31 | 0.15 (0.13-0.18) | 319 | 64 | 0.31 (0.28-0.34) | 282 | 56 | 0.27 (0.24-0.30) |
| Malignant | 770 | 154 | 0.19 (0.18-0.20) | -- | -- | -- | 128 | 26 | 0.13 (0.11-0.15) | 302 | 60 | 0.29 (0.26-0.33) | -- | -- | -- |
| Non-Malignant | 93 | 19 | 0.02 (0.02-0.03) | -- | -- | -- | 28 | 6 | 0.03 (0.02-0.04) | 17 | 3 | 0.02 (0.01-0.03) | -- | -- | -- |
| Tumors of Sellar Region | 4,530 | 906 | 1.10 (1.07-1.13) | 186 | 37 | 0.19 (0.16-0.22) | 606 | 121 | 0.60 (0.55-0.65) | 982 | 196 | 0.95 (0.89-1.01) | 2,756 | 551 | 2.62 (2.53-2.72) |
| Tumors of the pituitary | 3,723 | 745 | 0.90 (0.87-0.93) | 44 | 9 | 0.04 (0.03-0.06) | 314 | 63 | 0.31 (0.28-0.35) | 757 | 151 | 0.73 (0.68-0.79) | 2,608 | 522 | 2.48 (2.39-2.58) |
| Craniopharyngioma | 807 | 161 | 0.20 (0.18-0.21) | 142 | 28 | 0.14 (0.12-0.17) | 292 | 58 | 0.29 (0.26-0.32) | 225 | 45 | 0.22 (0.19-0.25) | 148 | 30 | 0.14 (0.12-0.17) |
| Unclassified Tumors | 1,481 | 296 | 0.36 (0.34-0.38) | 336 | 67 | 0.34 (0.31-0.38) | 290 | 58 | 0.29 (0.25-0.32) | 384 | 77 | 0.37 (0.34-0.41) | 471 | 94 | 0.45 (0.41-0.49) |
| Hemangioma | 486 | 97 | 0.12 (0.11-0.13) | 73 | 15 | 0.07 (0.06-0.09) | 83 | 17 | 0.08 (0.07-0.10) | 131 | 26 | 0.13 (0.11-0.15) | 199 | 40 | 0.19 (0.16-0.22) |
| Neoplasm, unspecified | 811 | 162 | 0.20 (0.19-0.21) | 176 | 35 | 0.18 (0.15-0.21) | 169 | 34 | 0.17 (0.14-0.19) | 226 | 45 | 0.22 (0.19-0.25) | 240 | 48 | 0.23 (0.20-0.26) |
| Malignant | 213 | 43 | 0.05 (0.05-0.06) | 69 | 14 | 0.07 (0.05-0.09) | 49 | 10 | 0.05 (0.04-0.06) | 44 | 9 | 0.04 (0.03-0.06) | 51 | 10 | 0.05 (0.04-0.06) |
| Non-Malignant | 598 | 120 | 0.15 (0.13-0.16) | 107 | 21 | 0.11 (0.09-0.13) | 120 | 24 | 0.12 (0.10-0.14) | 182 | 36 | 0.18 (0.15-0.20) | 189 | 38 | 0.18 (0.16-0.21) |
| All other | 184 | 37 | 0.05 (0.04-0.05) | 87 | 17 | 0.09 (0.07-0.11) | 38 | 8 | 0.04 (0.03-0.05) | 27 | 5 | 0.03 (0.02-0.04) | 32 | 6 | 0.03 (0.02-0.04) |
| Malignant | 40 | 8 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 144 | 29 | 0.04 (0.03-0.04) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TOTAL c | 25,339 | 5,068 | 6.20 (6.12-6.27) | 5,949 | 1,190 | 6.03 (5.87-6.18) | 5,536 | 1,107 | 5.47 (5.33-5.62) | 6,075 | 1,215 | 5.88 (5.74-6.03) | 7,779 | 1,556 | 7.40 (7.24-7.57) |
| Malignant | 14,385 | 2,877 | 3.53 (3.47-3.59) | 4,439 | 888 | 4.49 (4.36-4.63) | 3,857 | 771 | 3.81 (3.69-3.93) | 3,376 | 675 | 3.27 (3.16-3.38) | 2,713 | 543 | 2.58 (2.49-2.68) |
| Non-Malignant | 10,954 | 2,191 | 2.67 (2.62-2.72) | 1,510 | 302 | 1.53 (1.46-1.61) | 1,679 | 336 | 1.66 (1.58-1.74) | 2,699 | 540 | 2.61 (2.52-2.71) | 5,066 | 1,013 | 4.82 (4.69-4.96) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; US, United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, Not otherwise specified.
Fig. 20.
Distributiona in Children (Ages 0-14 Years) of All Primary Brain and Central Nervous System Tumors (Five-Year Total=17,561; Annual Average Cases=3,512) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Distributions and Incidence by Age at Diagnosis
Incidence Rates by Age at Diagnosis
The overall AAAIR for 2015-2019 for all primary brain and other CNS tumors was 24.71 per 100,000 population (Table 5). The overall incidence rate was 5.79 per 100,000 population for children ages 0-14 years, 11.96 per 100,000 population for adolescents and young adults ages 15-39 years, and 44.65 per 100,000 population for adults ages 40+ years (Table 10). The overall incidence rates of tumors by behavior and age group (0-14 years, 0-19 years and 20+ years) are shown in Figure 21.
Fig. 21.
Average Annual Age-Adjusted Incidence Ratesa of All Primary Brain and Other Central Nervous System Tumors by Age Group at Diagnosis and Behavior, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Incidence Rates by Age at Diagnosis and Histopathology
The AAAIRs by age and histopathology at diagnosis are presented in Table 10, Table 12, andSupplementary Table 8, as well as in Figure 18A (Ages 0-19 Years) and Figure 18B (Ages 20+ Years).
The incidence rate for all brain and other CNS tumors was highest among ages 85+ years (90.05 per 100,000) and lowest among children and adolescents ages 0-19 years (6.2 per 100,000).
Incidence rates of pilocytic astrocytoma, germ cell tumors, and embryonal tumors were higher in the younger age groups and decreased with advancing age.
Incidence rates of meningiomas increased with age.
Incidence rates declined with increasing age for those ages 0-19 years for gliomas, choroid plexus tumors, and medulloblastomas.
Incidence rates of other astrocytic tumors and germ cell tumors were higher in the younger age groups and decreased with advancing age.
Median Age at Diagnosis
The median age at diagnosis for all primary brain and other CNS tumors was 61 years (Table 5).
The histopathology-specific median ages ranged from eight years for embryonal tumors to 70 years for neoplasm, unspecified.
Pilocytic astrocytomas, unique astrocytoma variants, neuronal and mixed neuronal-glial tumors, choroid plexus tumors, tumors of the pineal region, embryonal tumors, and germ cell tumors were histopathologies with younger median ages at diagnosis compared to other histopathologies.
The most commonly diagnosed histopathologies in older ages were glioblastoma, meningioma, and lymphoma (median age of 65, 67, and 67 years, respectively).
Distribution and Incidence Rates of Tumors by Site, Histopathology, and Age at Diagnosis
Distribution and Incidence Rates of Tumors by Site, Histopathology, and Age at Diagnosis in Children and Adolescents (Ages 0-19 Years)
Brain and other CNS tumors are the most common form of solid tumors in children, and they account for the majority of cancer mortality in this age-group. About 5.7% of the reported brain and other CNS tumors during 2015-2019 occurred in children and adolescents ages 0-19 years (Table 12). The distribution of brain and other CNS tumors for children and adolescents ages 0-19 years by site is shown in Figure 19A.
The largest percentages of tumors in childhood and adolescence were located in the pituitary and craniopharyngeal duct (18%).
Frontal, temporal, parietal, and occipital lobes of the brain accounted for 5.8%, 6.6%, 2.6%, and 1.2% of all brain and other CNS tumors in childhood and adolescence, respectively.
Cerebrum, ventricle, brain stem, and cerebellum tumors accounted for 5.4%, 5.2%, 10.1%, and 13.9% of all brain and other CNS tumors in childhood and adolescence, respectively.
The cranial nerves and the spinal cord and cauda equina accounted for 7.5% and 5.1% of all brain and other CNS tumors in childhood and adolescence, respectively.
Figure 19B presents the most common brain and other CNS histopathologies in children and adolescents ages 0-19 years.
For children and adolescents ages 0-19 years, pilocytic astrocytomas, other gliomas, and embryonal tumors accounted for 15.3%, 12.6%, and 9.2%, respectively.
Tumors of the pituitary were the most common nonglial and predominantly non-malignant histopathology and accounted for 14.7% of all tumors in this age group.
Gliomas accounted for approximately 44.6% of tumors in children and adolescents ages 0-19 years.
Medulloblastomas accounted for 69.5% of all embryonal tumors in this age group.
Distribution of Tumors by Site and Histopathology in Children (Ages 0-14 Years)
Approximately 3.9% of all reported tumors occurred in children ages 0-14 years. The distribution of brain and other CNS tumors for children ages 0-14 years by site is shown in Figure 20A.
Tumors of cerebellum (16.9%) comprised the largest proportion of tumors followed by tumors located in other brain (13.1%) and brain stem (12.6%).
Figure 20B presents the most common brain and other CNS histopathologies in children ages 0-14 years.
For children ages 0-14 years, pilocytic astrocytomas, other gliomas, and embryonal tumors accounted for 18.7%, 15.3%, and 12.2%, respectively.
Gliomas accounted for approximately 49.4% of tumors in children ages 0-14 years.
Of embryonal tumors, medulloblastomas, atypical teratoid rhabdoid tumors (ATRT), and all other embryonal tumors accounted for 68.3%, 17.2%, and 14.8%, respectively.
Distribution of Tumors by Site and Histopathology in Adolescents (Ages 15-19 Years)
About 1.7% of the reported brain and other CNS tumors during 2015-2019 occurred in adolescents ages 15-19 years for a total of 7,779 tumors diagnosed between 2015 and 2019 (Table 12). The distribution of these tumors by site is shown in Figure 22A.
Fig. 22.
Distributiona in Adolescents (Ages 15-19 Years) of All Primary Brain and Central Nervous System Tumors (Five-Year Total=7,779; Annual Average Cases=1,556) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
35.8% of these tumors were diagnosed in the pituitary and craniopharyngeal duct.
The frontal lobe, temporal lobe, occipital lobe, and parietal lobe accounted for 18.4% of tumors in this age group.
The distribution of brain and other CNS tumors in adolescents ages 15-19 years by histopathology is shown in Figure 22B.
The most common histopathology in adolescents was tumors of the pituitary (33.5%).
Gliomas accounted for approximately 27.9% of tumors in adolescents. Of these gliomas, the histopathology pilocytic astrocytoma accounted for 7.7% of all tumors in this age group.
Incidence Rates by Histopathology Defined by ICCC in Children and Adolescents (Ages 0-19 Years)
Supplementary Table 10 presents the CBTRUS brain and other CNS tumor data for children and adolescents used for this report according to the ICCC grouping system for pediatric cancers (See Supplementary Table 1 for more additional information on the ICCC classification scheme).
Distribution and Incidence Rates of Adolescent and Young Adult Primary Brain and Other CNS Tumors (Ages 15-39 Years)
There were 63,812 primary brain and other CNS tumors diagnosed in AYA between 2015 and 2019, representing 14.3% of all brain and other CNS tumors (Figure 23).
The overall incidence rate in this age group was 11.96 per 100,000 population (Table 10). Incidence of malignant tumors was 3.25 per 100,000, and incidence of non-malignant tumors was 8.71 per 100,000 (Table 10).
Tumors of the sellar region had the highest incidence (4.39 per 100,000 population), followed by tumors of the meninges (2.27 per 100,000 population) (Table 10).
The most common histopathology in AYA was tumors of the pituitary (4.26 per 100,000 population), followed by meningiomas (1.97 per 100,000 population) and nerve sheath tumors (1.04 per 100,000 population) (Table 10).
The majority of AYA brain and other CNS tumors occurred in the pituitary and craniopharyngeal duct (37.5%), followed by the meninges (16.2%) (Figure 23A).
Approximately 17.6% of tumors diagnosed in AYA were located within the frontal, temporal, parietal, and occipital lobes of the brain combined (Figure 23A).
Cerebrum, ventricle, cerebellum, and brain stem tumors combined accounted for about 9.9% of all AYA tumors (Figure 23A).
The predominately non-malignant tumors of the pituitary (36%), meningioma (15.9%), and nerve sheath (8.6%) represented over half of CNS tumors diagnosed in AYA (Figure 23B).
Gliomas accounted for approximately 24.8% of all brain and other CNS tumors in AYA, and about 82.4% of all malignant tumors (Figure 23B).
AYA had higher rates of relative survival than adults greater than 40 years old for all histopathologic types. Though one-year relative survival for most tumor types was higher for AYA than children, five- and ten-year survival were usually higher for children as compared to AYA (Table 11).
Fig. 23.
Distributiona in Adolescents and Young Adults (Ages 15-39 Years) of All Primary Brain and Other Central Nervous System Tumors (Five-Year Total=63,812; Annual Average Cases=12,762) by A) Site and B) Histopathology, CBTRUS Statistical Report: US Cancer Statistics - NPCR and SEER, 2015-2019
Distribution and Incidence Rates by CCR and Diagnostic Confirmation
The overall number of reported tumors is listed by CCR in Table 13. While most malignant tumors are diagnosed by histopathologic confirmation (where the patient receives surgery and diagnosis is confirmed on tissue by a pathologist), brain and other CNS tumors may also be diagnosed by radiographic confirmation only (where the tumor was visualized on MRI, CT, X-ray, or other imaging technology, but surgery was not performed). Please note, while five years of data are available for most included CCR, data were not available from Nevada for diagnosis years 2018 and 2019 due to data quality issues.
Table 13.
Five-Year Totala, Annual Average Totalb, Average Annual Age-Adjusted Incidence Ratesc with 95% Confidence Intervals, and Characteristics of All Brain and Other Central Nervous System Tumors by Central Cancer Registry, Behavior, and Diagnostic Confirmation, CBTRUS Statistical Report: US Cancer Statistics - NPCR and SEER, 2015-2019
| Central Cancer Registry | Total | Malignant | Non-Malignant | Average Annual 5-Year Populationd | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Histopatho- logically- Confirmed (%)e |
Radiographically-Confirmed (%)f | 5-Year Total | % Malignant | Histopatho- logically-Confirmed (%) |
Radiographically--Confirmed (%) | 5-Year Total | % Malignant | Histopatho- logically-Confirmed (%) |
Radiographically-Confirmed (%) | ||
| Alabama | 5,546 | 1,109 | 56.6 | 36.7 | 1,912 | 34.5 | 77.1 | 6.7 | 3,634 | 65.5 | 45.7 | 52.5 | 4,879,842 |
| Alaska | 880 | 176 | 47.8 | 48.8 | 267 | 30.3 | 81.7 | 13.1 | 613 | 69.7 | 33.1 | 64.3 | 738,443 |
| Arizona | 8,187 | 1,637 | 62.4 | 33.8 | 2,541 | 31 | 85 | 7.8 | 5,646 | 69 | 52.2 | 45.5 | 7,056,347 |
| Arkansas | 3,918 | 784 | 51.9 | 43.4 | 1,203 | 30.7 | 81.5 | 10.6 | 2,715 | 69.3 | 38.8 | 57.9 | 3,001,710 |
| California | 48,214 | 9,643 | 56 | 39.3 | 13,686 | 28.4 | 84.7 | 8.3 | 34,528 | 71.6 | 44.6 | 51.6 | 39,253,268 |
| Colorado | 8,558 | 1,712 | 47.3 | 49.9 | 2,060 | 24.1 | 83.4 | 11.8 | 6,498 | 75.9 | 35.9 | 62 | 5,614,247 |
| Connecticut | 5,143 | 1,029 | 63.9 | 33.2 | 1,656 | 32.2 | 89.6 | 7.1 | 3,487 | 67.8 | 51.7 | 45.6 | 3,576,860 |
| Delaware | 1,142 | 228 | 60.8 | 36.8 | 386 | 33.8 | 85.2 | 10.9 | 756 | 66.2 | 48.3 | 50 | 958,730 |
| District of Columbia | 851 | 170 | 50.4 | 46.3 | 180 | 21.1 | 85.6 | 7.2 | 671 | 78.8 | 41 | 56.8 | 694,814 |
| Florida | 32,991 | 6,598 | 51.1 | 45.2 | 9,048 | 27.4 | 83.8 | 10.5 | 23,943 | 72.6 | 38.8 | 58.3 | 20,914,084 |
| Georgia | 14,863 | 2,973 | 46.2 | 47.2 | 3,519 | 23.7 | 83.1 | 11 | 11,344 | 76.3 | 34.7 | 58.4 | 10,411,247 |
| Hawaii | 1,571 | 314 | 53 | 41.4 | 382 | 24.3 | 82.7 | 10.2 | 1,189 | 75.7 | 43.5 | 51.4 | 1,423,273 |
| Idaho | 2,374 | 475 | 57.6 | 39.8 | 750 | 31.6 | 83.3 | 13.1 | 1,624 | 68.4 | 45.8 | 52.1 | 1,719,482 |
| Illinois | 18,227 | 3,645 | 52.9 | 44.2 | 4,846 | 26.6 | 88 | 7.3 | 13,381 | 73.4 | 40.2 | 57.6 | 12,770,578 |
| Indiana | 7,782 | 1,556 | 54.1 | 41.9 | 2,603 | 33.5 | 84.2 | 9.6 | 5,179 | 66.6 | 39 | 58.1 | 6,668,180 |
| Iowa | 4,713 | 943 | 54.3 | 42.8 | 1,377 | 29.2 | 83.2 | 11.5 | 3,336 | 70.8 | 42.4 | 55.8 | 3,141,796 |
| Kansas | 3,641 | 728 | 53.7 | 43.4 | 1,100 | 30.2 | 88.4 | 8.4 | 2,541 | 69.8 | 38.7 | 58.6 | 2,911,994 |
| Kentucky | 7,374 | 1,475 | 46.6 | 46.4 | 2,002 | 27.1 | 80.1 | 11 | 5,372 | 72.8 | 34.1 | 59.6 | 4,452,328 |
| Louisiana | 6,620 | 1,324 | 53.2 | 41.4 | 1,625 | 24.6 | 86.1 | 9.8 | 4,995 | 75.4 | 42.5 | 51.7 | 4,668,950 |
| Maine | 1,574 | 315 | 69.4 | 27.3 | 689 | 43.8 | 84.2 | 10.3 | 885 | 56.2 | 58 | 40.5 | 1,336,616 |
| Maryland | 8,206 | 1,641 | 54.9 | 40.4 | 2,232 | 27.2 | 84.4 | 6.7 | 5,974 | 72.8 | 43.9 | 53 | 6,024,167 |
| Massachusetts | 8,250 | 1,650 | 67.4 | 28.2 | 2,972 | 36 | 87.8 | 6.6 | 5,278 | 64 | 55.9 | 40.4 | 6,853,785 |
| Michigan | 12,392 | 2,478 | 56.9 | 37.7 | 3,976 | 32.1 | 85 | 6.5 | 8,416 | 67.9 | 43.6 | 52.5 | 9,967,487 |
| Minnesota | 6,723 | 1,345 | 65.4 | 31.9 | 2,334 | 34.7 | 87 | 9.2 | 4,389 | 65.3 | 54 | 43.9 | 5,565,492 |
| Mississippi | 3,766 | 753 | 54.1 | 42.2 | 1,034 | 27.5 | 86.1 | 10.1 | 2,732 | 72.5 | 42 | 54.4 | 2,986,521 |
| Missouri | 8,544 | 1,709 | 51.1 | 44.2 | 2,539 | 29.7 | 83.8 | 8.6 | 6,005 | 70.3 | 37.2 | 59.3 | 6,108,928 |
| Montana | 1,593 | 319 | 53 | 42.6 | 501 | 31.4 | 80.4 | 14 | 1,092 | 68.6 | 40.4 | 55.8 | 1,051,887 |
| Nebraska | 2,243 | 449 | 57.8 | 39.1 | 805 | 35.9 | 84 | 10.8 | 1,438 | 64.1 | 43.1 | 54.9 | 1,914,725 |
| Nevadag | 2,190 | 730 | 54.5 | 40.8 | 687 | 31.4 | 82.7 | 8.3 | 1,503 | 68.6 | 41.6 | 55.7 | 2,358,182 |
| New Hampshire | 1,977 | 395 | 57.6 | 39.4 | 652 | 33 | 90.8 | 5.1 | 1,325 | 67 | 41.2 | 56.3 | 1,349,483 |
| New Jersey | 14,083 | 2,817 | 50.3 | 44.6 | 3,745 | 26.6 | 85.2 | 9.2 | 10,338 | 73.4 | 37.6 | 57.4 | 8,883,006 |
| New Mexico | 2,067 | 413 | 68.9 | 24.5 | 713 | 34.5 | 88.2 | 5.8 | 1,354 | 65.5 | 58.8 | 34.4 | 2,093,772 |
| New York | 32,321 | 6,464 | 50.3 | 46.2 | 7,906 | 24.5 | 85 | 10.2 | 24,415 | 75.5 | 39 | 57.8 | 19,578,958 |
| North Carolina | 14,618 | 2,924 | 52.4 | 44 | 3,947 | 27 | 85.9 | 9.2 | 10,671 | 73 | 40 | 56.9 | 10,273,504 |
| North Dakota | 841 | 168 | 46.4 | 50.8 | 258 | 30.7 | 83.3 | 11.6 | 583 | 69.3 | 30 | 68.1 | 758,438 |
| Ohio | 13,994 | 2,799 | 63.7 | 31.8 | 5,056 | 36.1 | 87.6 | 6.2 | 8,938 | 63.9 | 50.2 | 46.2 | 11,661,096 |
| Oklahoma | 4,567 | 913 | 58.1 | 37.8 | 1,511 | 33.1 | 82.1 | 9.3 | 3,056 | 66.9 | 46.2 | 51.8 | 3,935,285 |
| Oregon | 4,861 | 972 | 64.8 | 30.5 | 1,703 | 35 | 84.2 | 6.9 | 3,158 | 65 | 54.4 | 43.3 | 4,131,752 |
| Pennsylvania | 20,292 | 4,058 | 48.5 | 46.9 | 5,795 | 28.6 | 81.4 | 9.2 | 14,497 | 71.4 | 35.3 | 62 | 12,796,195 |
| Rhode Island | 1,144 | 229 | 67 | 28.6 | 452 | 39.5 | 87.4 | 6.6 | 692 | 60.5 | 53.6 | 42.9 | 1,057,750 |
| South Carolina | 6,463 | 1,293 | 54 | 41.8 | 1,990 | 30.8 | 85.6 | 8.5 | 4,473 | 69.2 | 40 | 56.6 | 5,027,109 |
| South Dakota | 1,082 | 216 | 41.2 | 55.5 | 312 | 28.8 | 75.3 | 17.6 | 770 | 71.2 | 27.4 | 70.8 | 871,720 |
| Tennessee | 9,174 | 1,835 | 50.8 | 45.9 | 2,632 | 28.7 | 85.6 | 8.8 | 6,542 | 71.3 | 36.8 | 60.8 | 6,713,977 |
| Texas | 37,517 | 7,503 | 47.4 | 47.1 | 9,593 | 25.6 | 80.6 | 12.8 | 27,924 | 74.4 | 36 | 58.9 | 28,256,995 |
| Utah | 6,114 | 1,223 | 39.1 | 59.5 | 1,092 | 17.9 | 84.2 | 12.7 | 5,022 | 82.1 | 29.3 | 69.7 | 3,097,989 |
| Vermont | 945 | 189 | 54.3 | 43.1 | 280 | 29.6 | 86.1 | 6.8 | 665 | 70.4 | 40.9 | 58.4 | 624,831 |
| Virginia | 9,257 | 1,851 | 60.2 | 35.9 | 3,010 | 32.5 | 85.8 | 6.1 | 6,247 | 67.5 | 47.9 | 50.2 | 8,464,705 |
| Washington | 13,542 | 2,708 | 43 | 52.2 | 3,191 | 23.6 | 80.7 | 11 | 10,351 | 76.4 | 31.4 | 64.9 | 7,407,203 |
| West Virginia | 2,834 | 567 | 49.2 | 46.1 | 821 | 29 | 86.4 | 8.7 | 2,013 | 71 | 34 | 61.4 | 1,819,133 |
| Wisconsin | 9,315 | 1,863 | 48.2 | 48.7 | 2,547 | 27.3 | 83.1 | 12.3 | 6,768 | 72.7 | 35 | 62.5 | 5,793,029 |
| Wyoming | 708 | 142 | 61.7 | 35.7 | 227 | 32.1 | 86.8 | 9.2 | 481 | 67.9 | 49.9 | 48.2 | 582,159 |
| TOTAL | 445,792 | 89,158 | 52.9 | 42.8 | 126,345 | 28.3 | 84.3 | 9.3 | 319,447 | 71.7 | 40.5 | 56 | 324,202,052 |
aWith the exception of Nevada, where total cases represent three years of diagnoses (2015, 2016, 2017).
bAnnual average cases are calculated by dividing the five-year total by five, with the exception of Nevada where annual average cases are obtained by dividing total by three.
cRates are per 100,000 and are age-adjusted to the 2000 US standard population.
dPopulation estimates were obtained from the United States Bureau of the Census available on the SEER program website.
eHistopathologic confirmation includes tumors classified as having diagnosis confirmed by: positive histopathology, positive cytology, positive histopathology plus – positive immunophenotyping and/or positive genetic studies, or positive microscopic confirmation, method not specified.
fRadiographic confirmation includes tumors classified as having diagnosis confirmed by Radiography and/or other imaging techniques without microscopic confirmation.
gData not available for diagnosis years 2018-2019.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category, or where the inclusion of the count and rate would allow for back-calculation of suppressed values. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
Approximately 71.7% of tumors were non-malignant, but there was variation by CCR (range: 56.2%-82.1%) (Table 13).
Overall, 52.9% of tumors were histopathologically-confirmed. A larger proportion of malignant tumors were histopathologically-confirmed (84.3%) compared to non-malignant tumors (40.5%) (Table 9).
A slight majority of non-malignant brain and other CNS tumors were radiographically-confirmed (56%).
The overall AAAIRs by age, behavior, and CCR are shown in Table 14, Supplementary Table 9, and Figure 20.
Table 14.
Five-Year Totala, Annual Average Totalb, Average Annual Age-Adjusted Incidence Ratesc with 95% Confidence Intervals for Brain and Other Central Nervous System Tumors by Age at Diagnosis, Behavior, and Central Cancer Registry, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Central Cancer Registry | All Ages | 0-19 Years | 20+ Years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Malignant | All | Malignant | All | Malignant | |||||||||||||
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Alabama | 5,546 | 1,109 | 19.92 (19.38-20.47) | 1,912 | 382 | 6.84 (6.53-7.16) | 323 | 65 | 5.27 (4.72-5.88) | 220 | 44 | 3.61 (3.15-4.12) | 5,223 | 1,045 | 25.81 (25.09-26.54) | 1,692 | 338 | 8.14 (7.74-8.55) |
| Alaska | 880 | 176 | 24.45 (22.79-26.21) | 267 | 53 | 7.17 (6.30-8.13) | 65 | 13 | 6.52 (5.03-8.31) | 38 | 8 | 3.75 (2.65-5.15) | 815 | 163 | 31.67 (29.42-34.04) | 229 | 46 | 8.55 (7.42-9.80) |
| Arizona | 8,187 | 1,637 | 20.46 (20.01-20.92) | 2,541 | 508 | 6.32 (6.07-6.58) | 514 | 103 | 5.62 (5.14-6.13) | 268 | 54 | 2.95 (2.60-3.32) | 7,673 | 1,535 | 26.43 (25.82-27.04) | 2,273 | 455 | 7.68 (7.36-8.02) |
| Arkansas | 3,918 | 784 | 23.04 (22.31-23.80) | 1,203 | 241 | 6.99 (6.59-7.41) | 216 | 43 | 5.51 (4.80-6.30) | 121 | 24 | 3.09 (2.56-3.69) | 3,702 | 740 | 30.10 (29.11-31.11) | 1,082 | 216 | 8.56 (8.05-9.11) |
| California | 48,214 | 9,643 | 23.00 (22.79-23.21) | 13,686 | 2,737 | 6.55 (6.44-6.67) | 2,648 | 530 | 5.26 (5.06-5.46) | 1,505 | 301 | 2.99 (2.84-3.15) | 45,566 | 9,113 | 30.14 (29.86-30.42) | 12,181 | 2,436 | 7.98 (7.84-8.13) |
| Colorado | 8,558 | 1,712 | 28.86 (28.24-29.49) | 2,060 | 412 | 6.91 (6.61-7.22) | 443 | 89 | 6.30 (5.72-6.91) | 219 | 44 | 3.12 (2.72-3.56) | 8,115 | 1,623 | 37.94 (37.10-38.79) | 1,841 | 368 | 8.43 (8.04-8.84) |
| Connecticut | 5,143 | 1,029 | 24.61 (23.92-25.32) | 1,656 | 331 | 7.93 (7.54-8.34) | 263 | 53 | 6.15 (5.43-6.94) | 155 | 31 | 3.71 (3.15-4.35) | 4,880 | 976 | 32.04 (31.11-32.99) | 1,501 | 300 | 9.63 (9.13-10.15) |
| District of Columbia | 851 | 170 | 25.00 (23.30-26.79) | 180 | 36 | 5.31 (4.54-6.18) | 42 | 8 | 5.79 (4.14-7.86) | 31 | 6 | 4.24 (2.86-6.05) | 809 | 162 | 32.72 (30.45-35.12) | 149 | 30 | 5.74 (4.83-6.77) |
| Delaware | 1,142 | 228 | 20.42 (19.20-21.70) | 386 | 77 | 6.83 (6.14-7.58) | 88 | 18 | 7.68 (6.16-9.47) | 51 | 10 | 4.46 (3.32-5.87) | 1,054 | 211 | 25.55 (23.96-27.22) | 335 | 67 | 7.79 (6.94-8.71) |
| Florida | 32,991 | 6,598 | 25.30 (25.02-25.59) | 9,048 | 1,810 | 7.09 (6.93-7.24) | 1,631 | 326 | 6.99 (6.66-7.34) | 900 | 180 | 3.87 (3.62-4.13) | 31,360 | 6,272 | 32.67 (32.29-33.05) | 8,148 | 1,630 | 8.38 (8.19-8.57) |
| Georgia | 14,863 | 2,973 | 27.29 (26.85-27.75) | 3,519 | 704 | 6.46 (6.24-6.68) | 975 | 195 | 6.97 (6.54-7.42) | 466 | 93 | 3.35 (3.06-3.67) | 13,888 | 2,778 | 35.47 (34.87-36.08) | 3,053 | 611 | 7.70 (7.43-7.99) |
| Hawaii | 1,571 | 314 | 18.82 (17.87-19.82) | 382 | 76 | 4.73 (4.25-5.24) | 60 | 12 | 3.62 (2.76-4.66) | 38 | 8 | 2.25 (1.59-3.09) | 1,511 | 302 | 24.94 (23.65-26.28) | 344 | 69 | 5.72 (5.11-6.38) |
| Iowa | 4,713 | 943 | 26.27 (25.50-27.06) | 1,377 | 275 | 7.62 (7.21-8.05) | 272 | 54 | 6.61 (5.85-7.45) | 149 | 30 | 3.64 (3.08-4.28) | 4,441 | 888 | 34.18 (33.14-35.24) | 1,228 | 246 | 9.22 (8.69-9.77) |
| Idaho | 2,374 | 475 | 25.37 (24.33-26.44) | 750 | 150 | 8.01 (7.43-8.62) | 126 | 25 | 5.16 (4.30-6.15) | 69 | 14 | 2.84 (2.21-3.59) | 2,248 | 450 | 33.50 (32.09-34.96) | 681 | 136 | 10.09 (9.32-10.90) |
| Illinois | 18,227 | 3,645 | 25.80 (25.42-26.19) | 4,846 | 969 | 6.88 (6.68-7.08) | 979 | 196 | 6.06 (5.69-6.45) | 543 | 109 | 3.38 (3.10-3.68) | 17,248 | 3,450 | 33.74 (33.23-34.26) | 4,303 | 861 | 8.28 (8.03-8.54) |
| Indiana | 7,782 | 1,556 | 21.22 (20.73-21.71) | 2,603 | 521 | 7.11 (6.83-7.40) | 503 | 101 | 5.71 (5.22-6.23) | 310 | 62 | 3.53 (3.15-3.95) | 7,279 | 1,456 | 27.45 (26.81-28.11) | 2,293 | 459 | 8.55 (8.19-8.92) |
| Kansas | 3,641 | 728 | 22.83 (22.07-23.60) | 1,100 | 220 | 6.90 (6.49-7.33) | 214 | 43 | 5.40 (4.70-6.18) | 123 | 25 | 3.10 (2.58-3.70) | 3,427 | 685 | 29.84 (28.81-30.88) | 977 | 195 | 8.43 (7.89-8.99) |
| Kentucky | 7,374 | 1,475 | 29.67 (28.98-30.38) | 2,002 | 400 | 8.05 (7.69-8.42) | 458 | 92 | 8.14 (7.41-8.92) | 250 | 50 | 4.45 (3.91-5.04) | 6,916 | 1,383 | 38.33 (37.41-39.27) | 1,752 | 350 | 9.49 (9.04-9.96) |
| Louisiana | 6,620 | 1,324 | 25.94 (25.31-26.59) | 1,625 | 325 | 6.38 (6.06-6.70) | 384 | 77 | 6.32 (5.70-6.98) | 215 | 43 | 3.51 (3.06-4.01) | 6,236 | 1,247 | 33.84 (32.98-34.71) | 1,410 | 282 | 7.53 (7.13-7.95) |
| Massachusetts | 8,250 | 1,650 | 21.01 (20.54-21.48) | 2,972 | 594 | 7.62 (7.34-7.91) | 450 | 90 | 5.65 (5.14-6.20) | 263 | 53 | 3.36 (2.97-3.80) | 7,800 | 1,560 | 27.19 (26.57-27.82) | 2,709 | 542 | 9.33 (8.97-9.70) |
| Maryland | 8,206 | 1,641 | 24.45 (23.91-25.00) | 2,232 | 446 | 6.73 (6.44-7.02) | 429 | 86 | 5.72 (5.19-6.29) | 237 | 47 | 3.17 (2.78-3.60) | 7,777 | 1,555 | 31.98 (31.26-32.72) | 1,995 | 399 | 8.16 (7.79-8.53) |
| Maine | 1,574 | 315 | 19.05 (18.05-20.08) | 689 | 138 | 8.28 (7.63-8.97) | 98 | 20 | 6.84 (5.55-8.35) | 68 | 14 | 4.83 (3.75-6.13) | 1,476 | 295 | 23.96 (22.67-25.29) | 621 | 124 | 9.66 (8.88-10.50) |
| Michigan | 12,392 | 2,478 | 21.51 (21.12-21.91) | 3,976 | 795 | 6.93 (6.71-7.16) | 591 | 118 | 4.82 (4.44-5.22) | 373 | 75 | 3.07 (2.76-3.40) | 11,801 | 2,360 | 28.22 (27.69-28.76) | 3,603 | 721 | 8.49 (8.20-8.78) |
| Minnesota | 6,723 | 1,345 | 21.73 (21.20-22.28) | 2,334 | 467 | 7.62 (7.30-7.94) | 459 | 92 | 6.38 (5.81-6.99) | 259 | 52 | 3.59 (3.17-4.05) | 6,264 | 1,253 | 27.91 (27.20-28.63) | 2,075 | 415 | 9.24 (8.83-9.66) |
| Missouri | 8,544 | 1,709 | 24.59 (24.06-25.14) | 2,539 | 508 | 7.35 (7.06-7.65) | 484 | 97 | 6.29 (5.75-6.88) | 308 | 62 | 4.01 (3.57-4.48) | 8,060 | 1,612 | 31.95 (31.24-32.68) | 2,231 | 446 | 8.69 (8.33-9.08) |
| Mississippi | 3,766 | 753 | 22.81 (22.06-23.57) | 1,034 | 207 | 6.31 (5.93-6.72) | 217 | 43 | 5.45 (4.75-6.22) | 133 | 27 | 3.35 (2.80-3.97) | 3,549 | 710 | 29.79 (28.79-30.81) | 901 | 180 | 7.51 (7.01-8.03) |
| Montana | 1,593 | 319 | 25.65 (24.34-27.02) | 501 | 100 | 8.02 (7.29-8.79) | 70 | 14 | 5.52 (4.30-6.97) | 41 | 8 | 3.23 (2.32-4.38) | 1,523 | 305 | 33.75 (31.99-35.59) | 460 | 92 | 9.94 (9.01-10.95) |
| North Carolina | 14,618 | 2,924 | 25.37 (24.95-25.79) | 3,947 | 789 | 6.92 (6.70-7.14) | 759 | 152 | 5.88 (5.47-6.31) | 456 | 91 | 3.56 (3.24-3.90) | 13,859 | 2,772 | 33.21 (32.65-33.78) | 3,491 | 698 | 8.27 (7.99-8.55) |
| North Dakota | 841 | 168 | 21.01 (19.57-22.54) | 258 | 52 | 6.32 (5.55-7.17) | 62 | 12 | 6.16 (4.72-7.90) | 36 | 7 | 3.51 (2.46-4.87) | 779 | 156 | 26.99 (25.05-29.03) | 222 | 44 | 7.46 (6.47-8.54) |
| Nebraska | 2,243 | 449 | 21.71 (20.80-22.66) | 805 | 161 | 7.76 (7.22-8.32) | 184 | 37 | 6.99 (6.01-8.07) | 105 | 21 | 3.96 (3.24-4.80) | 2,059 | 412 | 27.64 (26.42-28.90) | 700 | 140 | 9.28 (8.59-10.02) |
| New Hampshire | 1,977 | 395 | 24.28 (23.16-25.43) | 652 | 130 | 8.05 (7.41-8.74) | 107 | 21 | 7.08 (5.80-8.57) | 66 | 13 | 4.46 (3.44-5.68) | 1,870 | 374 | 31.19 (29.73-32.71) | 586 | 117 | 9.50 (8.71-10.34) |
| New Jersey | 14,083 | 2,817 | 27.89 (27.42-28.37) | 3,745 | 749 | 7.45 (7.21-7.70) | 756 | 151 | 6.93 (6.45-7.44) | 405 | 81 | 3.73 (3.38-4.12) | 13,327 | 2,665 | 36.32 (35.69-36.96) | 3,340 | 668 | 8.95 (8.64-9.27) |
| New Mexico | 2,067 | 413 | 17.45 (16.67-18.24) | 713 | 143 | 5.90 (5.46-6.37) | 121 | 24 | 4.47 (3.70-5.34) | 67 | 13 | 2.49 (1.93-3.16) | 1,946 | 389 | 22.67 (21.63-23.74) | 646 | 129 | 7.27 (6.70-7.88) |
| Nevadad | 2,190 | 730 | 22.75 (21.79-23.75) | 687 | 229 | 7.06 (6.53-7.63) | 133 | 44 | 6.02 (5.04-7.13) | 86 | 29 | 3.87 (3.09-4.78) | 2,057 | 656 | 29.48 (28.19-30.82) | 601 | 200 | 8.35 (7.68-9.06) |
| New York | 32,321 | 6,464 | 29.16 (28.83-29.49) | 7,906 | 1,581 | 7.20 (7.03-7.36) | 1,859 | 372 | 8.05 (7.69-8.42) | 907 | 181 | 3.94 (3.69-4.21) | 30,462 | 6,092 | 37.65 (37.22-38.09) | 6,999 | 1,400 | 8.51 (8.30-8.71) |
| Ohio | 13,994 | 2,799 | 21.06 (20.70-21.43) | 5,056 | 1,011 | 7.63 (7.41-7.85) | 1,016 | 203 | 6.95 (6.53-7.40) | 603 | 121 | 4.14 (3.82-4.49) | 12,978 | 2,596 | 26.74 (26.26-27.22) | 4,453 | 891 | 9.03 (8.76-9.31) |
| Oklahoma | 4,567 | 913 | 21.26 (20.64-21.90) | 1,511 | 302 | 6.98 (6.63-7.35) | 291 | 58 | 5.48 (4.87-6.15) | 178 | 36 | 3.34 (2.87-3.87) | 4,276 | 855 | 27.61 (26.77-28.48) | 1,333 | 267 | 8.45 (7.99-8.92) |
| Oregon | 4,861 | 972 | 20.49 (19.90-21.10) | 1,703 | 341 | 7.21 (6.86-7.57) | 284 | 57 | 5.87 (5.21-6.60) | 177 | 35 | 3.66 (3.14-4.24) | 4,577 | 915 | 26.37 (25.59-27.17) | 1,526 | 305 | 8.63 (8.19-9.09) |
| Pennsylvania | 20,292 | 4,058 | 26.53 (26.15-26.92) | 5,795 | 1,159 | 7.66 (7.45-7.87) | 992 | 198 | 6.56 (6.16-6.98) | 602 | 120 | 4.03 (3.72-4.37) | 19,300 | 3,860 | 34.57 (34.06-35.08) | 5,193 | 1,039 | 9.12 (8.86-9.38) |
| Rhode Island | 1,144 | 229 | 18.59 (17.48-19.75) | 452 | 90 | 7.30 (6.61-8.03) | 64 | 13 | 5.31 (4.08-6.79) | 40 | 8 | 3.41 (2.44-4.65) | 1,080 | 216 | 23.93 (22.47-25.47) | 412 | 82 | 8.86 (7.99-9.79) |
| South Carolina | 6,463 | 1,293 | 22.08 (21.53-22.65) | 1,990 | 398 | 6.78 (6.48-7.10) | 353 | 71 | 5.70 (5.12-6.32) | 205 | 41 | 3.32 (2.88-3.81) | 6,110 | 1,222 | 28.67 (27.93-29.43) | 1,785 | 357 | 8.18 (7.79-8.58) |
| South Dakota | 1,082 | 216 | 22.36 (20.99-23.79) | 312 | 62 | 6.48 (5.76-7.28) | 39 | 8 | 3.31 (2.35-4.52) | 25 | 5 | 2.10 (1.36-3.10) | 1,043 | 209 | 30.02 (28.15-31.98) | 287 | 57 | 8.25 (7.28-9.30) |
| Tennessee | 9,174 | 1,835 | 24.21 (23.70-24.72) | 2,632 | 526 | 6.96 (6.69-7.24) | 464 | 93 | 5.55 (5.06-6.08) | 276 | 55 | 3.30 (2.93-3.72) | 8,710 | 1,742 | 31.71 (31.03-32.40) | 2,356 | 471 | 8.43 (8.09-8.79) |
| Texas | 37,517 | 7,503 | 26.73 (26.46-27.01) | 9,593 | 1,919 | 6.74 (6.60-6.88) | 2,613 | 523 | 6.44 (6.19-6.69) | 1,380 | 276 | 3.39 (3.21-3.57) | 34,904 | 6,981 | 34.89 (34.52-35.27) | 8,213 | 1,643 | 8.09 (7.91-8.27) |
| Utah | 6,114 | 1,223 | 44.14 (43.03-45.28) | 1,092 | 218 | 7.55 (7.10-8.02) | 340 | 68 | 6.74 (6.04-7.50) | 171 | 34 | 3.35 (2.87-3.89) | 5,774 | 1,155 | 59.19 (57.65-60.76) | 921 | 184 | 9.23 (8.64-9.86) |
| Virginia | 9,257 | 1,851 | 19.73 (19.32-20.15) | 3,010 | 602 | 6.45 (6.22-6.69) | 541 | 108 | 5.17 (4.74-5.62) | 346 | 69 | 3.31 (2.97-3.68) | 8,716 | 1,743 | 25.59 (25.04-26.14) | 2,664 | 533 | 7.71 (7.42-8.02) |
| Vermont | 945 | 189 | 24.84 (23.18-26.59) | 280 | 56 | 7.55 (6.63-8.55) | 45 | 9 | 6.64 (4.83-8.90) | 24 | 5 | 3.74 (2.39-5.56) | 900 | 180 | 32.16 (29.97-34.47) | 256 | 51 | 9.08 (7.94-10.34) |
| Washington | 13,542 | 2,708 | 33.38 (32.80-33.96) | 3,191 | 638 | 7.88 (7.60-8.16) | 689 | 138 | 7.61 (7.05-8.20) | 387 | 77 | 4.24 (3.83-4.69) | 12,853 | 2,571 | 43.75 (42.97-44.53) | 2,804 | 561 | 9.34 (8.99-9.70) |
| Wisconsin | 9,315 | 1,863 | 28.00 (27.41-28.60) | 2,547 | 509 | 7.65 (7.35-7.97) | 440 | 88 | 6.12 (5.56-6.72) | 269 | 54 | 3.77 (3.33-4.25) | 8,875 | 1,775 | 36.80 (36.01-37.61) | 2,278 | 456 | 9.21 (8.83-9.61) |
| West Virginia | 2,834 | 567 | 25.90 (24.90-26.92) | 821 | 164 | 7.50 (6.97-8.06) | 149 | 30 | 7.19 (6.08-8.44) | 80 | 16 | 3.90 (3.09-4.85) | 2,685 | 537 | 33.42 (32.11-34.78) | 741 | 148 | 8.95 (8.28-9.65) |
| Wyoming | 708 | 142 | 22.02 (20.37-23.77) | 227 | 45 | 7.06 (6.14-8.08) | 36 | 7 | 4.80 (3.36-6.64) | 19 | 4 | 2.52 (1.52-3.93) | 672 | 134 | 28.95 (26.72-31.31) | 208 | 42 | 8.89 (7.68-10.24) |
| TOTAL | 445,792 | 89,158 | 24.71 (24.63-24.78) | 126,345 | 25,269 | 7.02 (6.98-7.06) | 25,339 | 5,068 | 6.20 (6.12-6.27) | 14,263 | 2,853 | 3.50 (3.44-3.56) | 420,453 | 84,091 | 32.15 (32.05-32.25) | 112,082 | 22,416 | 8.44 (8.39-8.49) |
With the exception of Nevada, where total cases represent three years of diagnoses (2015, 2016, 2017).
Annual average cases are calculated by dividing the five-year total by five, with the exception of Nevada where annual average cases are obtained by dividing total by three.
Rates are per 100,000 and are age-adjusted to the 2000 US standard population.
Data not available for diagnosis years 2018-2019.
-- Counts are not presented when fewer than 16 cases were reported for the specific category, or where the inclusion of the count and rate would allow for back-calculation of suppressed values. The suppressed cases are included in the counts and rates for Totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
There was less variation by region for malignant tumor incidence rates (Figure 23A) compared to incidence rates for non-malignant tumors (Figure 23B). CCR and regional variations likely reflect differences in reporting and case ascertainment practices.
The overall AAAIRs of all tumors (malignant and non-malignant) for each individual CCR ranged from 17.45 to 44.14 per 100,000 population. Please see Supplementary Figure 2 for combined incidence of malignant and non-malignant tumors by CCR.
AAAIRs of all primary malignant tumors ranged from 4.73 to 8.28 per 100,000 population.
Among adults 20 years of age and older, CCR-specific incidence rates ranged from 5.74 to 10.09 per 100,000 population for malignant tumors.
In persons less than 20 years of age, incidence rates ranged from 2.09 to 4.83 per 100,000 population for malignant tumors.
Distribution by Histopathology, WHO Grade, Diagnostic Confirmation, and Treatment Completeness
The distribution of reported tumors with histopathologically-confirmed diagnosis from 2015 to 2019 is listed by histopathology and reported WHO grade in Table 9.
Overall, 65.7% of tumors had complete WHO grade information, but there was substantial variation by histopathology.
The histopathologic types with the highest WHO grade completeness were anaplastic astrocytoma (94.1%), anaplastic oligodendroglioma (93.6%), and oligodendroglioma (92.4%).
Distribution of Tumors in Puerto Rico
The distribution of brain and other CNS tumors diagnosed among residents of Puerto Rico by histopathology is shown in Supplementary Figure 3.
Approximately 40.2% of tumors were malignant and 59.8% were non-malignant. The most common histopathologies were non-malignant meningioma (27%), followed by glioblastoma (18.3%).
Distributions and Incidence by Sex
Distribution by Sex and Behavior
Overall, 41.3% of all tumors diagnosed between 2015 and 2019 occurred in males (184,168 tumors) and 58.7% in females (261,624 tumors) (Table 8).
Approximately 55.8% of the malignant tumors occurred in males (70,459 tumors between 2015 and 2019) and 44.2% in females (55,886 tumors between 2015 and 2019).
Approximately 35.6% of the non-malignant tumors occurred in males (113,709 tumors between 2015 and 2019) and 64.4% in females (205,738 tumors between 2015 and 2019).
Incidence Rates by Site and Sex
Incidence counts and average annual age-adjusted rates for brain and other CNS tumors by site and sex are shown in Table 7.
Incidence rates were highest for tumors located in the meninges (9.55 per 100,000 population) and lowest for olfactory tumors of the nasal cavity (0.04 per 100,000 population).
Incidence rates were higher in females than in males for tumors located in the cranial nerves, other nervous system, meninges, pituitary and craniopharyngeal duct, while males had higher incidence rates for tumors located in most other locations.
Incidence Rates by Sex and Histopathology
AAAIRs by sex and histopathology are shown in Table 8. Incidence rates for all primary brain and other CNS tumors combined were higher among females (27.62 per 100,000 population) than males (21.6 per 100,000 population).
The incidence rate of tumors of meninges was higher in females (13.28 per 100,000 population) than in males (6.0 per 100,000 population).
Incidence rate ratios (male:female) for selected histopathologies and histopathology groupings are shown in (Figure 15).
Incidence was higher in males for many histopathologies, such as germ cell tumors (p<0.0001), most glial tumors, lymphomas (p<0.0001), and embryonal tumors (p<0.0001).
In addition to non-malignant (p<0.0001) and malignant (p=0.2731) meningiomas, tumors of the pituitary (p<0.0001) were also more common in females than in males.
Incidence Rates by Sex and Histopathology in Children and Adolescents (Age 0-19 Years)
The incidence rates for brain and other CNS tumors in children and adolescents by histopathology and sex are shown in Table 15.
Table 15.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for Children and Adolescents (Ages 0-19 Years) by Histopathology and Sex, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | Total | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 2,102 | 420 | 0.51 (0.49-0.54) | 1,153 | 231 | 0.55 (0.52-0.58) | 949 | 190 | 0.47 (0.44-0.51) |
| Diffuse astrocytoma | 878 | 176 | 0.21 (0.20-0.23) | 475 | 95 | 0.23 (0.21-0.25) | 403 | 81 | 0.20 (0.18-0.22) |
| Anaplastic astrocytoma | 332 | 66 | 0.08 (0.07-0.09) | 188 | 38 | 0.09 (0.08-0.10) | 144 | 29 | 0.07 (0.06-0.08) |
| Glioblastoma | 695 | 139 | 0.17 (0.16-0.18) | 389 | 78 | 0.19 (0.17-0.21) | 306 | 61 | 0.15 (0.14-0.17) |
| Oligodendroglioma | 145 | 29 | 0.04 (0.03-0.04) | 71 | 14 | 0.03 (0.03-0.04) | 74 | 15 | 0.04 (0.03-0.05) |
| Anaplastic oligodendroglioma | 16 | 3 | 0.00 (0.00-0.01) | -- | -- | -- | -- | -- | -- |
| Oligoastrocytic tumors | 36 | 7 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- |
| Other Astrocytic Tumors | 4,382 | 876 | 1.08 (1.04-1.11) | 2,308 | 462 | 1.11 (1.06-1.15) | 2,074 | 415 | 1.04 (1.00-1.09) |
| Pilocytic astrocytoma | 3,885 | 777 | 0.95 (0.92-0.98) | 2,033 | 407 | 0.98 (0.93-1.02) | 1,852 | 370 | 0.93 (0.89-0.97) |
| Unique astrocytoma variants | 497 | 99 | 0.12 (0.11-0.13) | 275 | 55 | 0.13 (0.12-0.15) | 222 | 44 | 0.11 (0.10-0.13) |
| Malignant | 230 | 46 | 0.06 (0.05-0.06) | 114 | 23 | 0.05 (0.05-0.07) | 116 | 23 | 0.06 (0.05-0.07) |
| Non-Malignant | 267 | 53 | 0.07 (0.06-0.07) | 161 | 32 | 0.08 (0.07-0.09) | 106 | 21 | 0.05 (0.04-0.06) |
| Ependymal Tumors | 1,185 | 237 | 0.29 (0.27-0.31) | 683 | 137 | 0.33 (0.30-0.35) | 502 | 100 | 0.25 (0.23-0.27) |
| Malignant | 966 | 193 | 0.24 (0.22-0.25) | 548 | 110 | 0.26 (0.24-0.29) | 418 | 84 | 0.21 (0.19-0.23) |
| Non-Malignant | 219 | 44 | 0.05 (0.05-0.06) | 135 | 27 | 0.06 (0.05-0.08) | 84 | 17 | 0.04 (0.03-0.05) |
| Other Gliomas | 3,188 | 638 | 0.78 (0.76-0.81) | 1,577 | 315 | 0.76 (0.72-0.80) | 1,611 | 322 | 0.81 (0.77-0.85) |
| Glioma malignant, NOS | 3,160 | 632 | 0.78 (0.75-0.80) | -- | -- | -- | -- | -- | -- |
| Other neuroepithelial tumors | 28 | 6 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 2,090 | 418 | 0.51 (0.49-0.53) | 1,187 | 237 | 0.57 (0.54-0.60) | 903 | 181 | 0.45 (0.42-0.48) |
| Malignant | 120 | 24 | 0.03 (0.02-0.04) | 60 | 12 | 0.03 (0.02-0.04) | 60 | 12 | 0.03 (0.02-0.04) |
| Non-Malignant | 1,970 | 394 | 0.48 (0.46-0.50) | 1,127 | 225 | 0.54 (0.51-0.57) | 843 | 169 | 0.42 (0.39-0.45) |
| Choroid Plexus Tumors | 407 | 81 | 0.10 (0.09-0.11) | 235 | 47 | 0.11 (0.10-0.13) | 172 | 34 | 0.09 (0.07-0.10) |
| Malignant | 98 | 20 | 0.02 (0.02-0.03) | 63 | 13 | 0.03 (0.02-0.04) | 35 | 7 | 0.02 (0.01-0.02) |
| Non-Malignant | 309 | 62 | 0.08 (0.07-0.08) | 172 | 34 | 0.08 (0.07-0.10) | 137 | 27 | 0.07 (0.06-0.08) |
| Tumors of the Pineal Region | 215 | 43 | 0.05 (0.05-0.06) | 100 | 20 | 0.05 (0.04-0.06) | 115 | 23 | 0.06 (0.05-0.07) |
| Malignant | 179 | 36 | 0.04 (0.04-0.05) | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 36 | 7 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- |
| Embryonal Tumors | 2,338 | 468 | 0.58 (0.55-0.60) | 1,406 | 281 | 0.68 (0.64-0.71) | 932 | 186 | 0.47 (0.44-0.50) |
| Medulloblastoma | 1,626 | 325 | 0.40 (0.38-0.42) | 1,047 | 209 | 0.50 (0.47-0.54) | 579 | 116 | 0.29 (0.27-0.32) |
| Atypical teratoid/rhabdoid tumor | 372 | 74 | 0.09 (0.08-0.10) | 191 | 38 | 0.09 (0.08-0.11) | 181 | 36 | 0.09 (0.08-0.11) |
| All other embryonal | 340 | 68 | 0.08 (0.07-0.09) | 168 | 34 | 0.08 (0.07-0.09) | 172 | 34 | 0.09 (0.07-0.10) |
| Tumors of Cranial and Spinal Nerves | 1,173 | 235 | 0.29 (0.27-0.30) | 622 | 124 | 0.30 (0.27-0.32) | 551 | 110 | 0.27 (0.25-0.30) |
| Nerve sheath tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other tumors of cranial and spinal nerves | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 1,252 | 250 | 0.30 (0.29-0.32) | 610 | 122 | 0.29 (0.27-0.31) | 642 | 128 | 0.32 (0.30-0.35) |
| Meningiomas | 671 | 134 | 0.16 (0.15-0.18) | 313 | 63 | 0.15 (0.13-0.17) | 358 | 72 | 0.18 (0.16-0.20) |
| Malignant | 21 | 4 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 650 | 130 | 0.16 (0.15-0.17) | -- | -- | -- | -- | -- | -- |
| Mesenchymal tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Primary melanocytic lesions | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | 133 | 27 | 0.03 (0.03-0.04) | 75 | 15 | 0.04 (0.03-0.04) | 58 | 12 | 0.03 (0.02-0.04) |
| Lymphoma | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other hematopoietic neoplasms | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | 863 | 173 | 0.21 (0.20-0.23) | 595 | 119 | 0.28 (0.26-0.31) | 268 | 54 | 0.13 (0.12-0.15) |
| Malignant | 770 | 154 | 0.19 (0.18-0.20) | 536 | 107 | 0.26 (0.23-0.28) | 234 | 47 | 0.12 (0.10-0.13) |
| Non-Malignant | 93 | 19 | 0.02 (0.02-0.03) | 59 | 12 | 0.03 (0.02-0.04) | 34 | 7 | 0.02 (0.01-0.02) |
| Tumors of Sellar Region | 4,530 | 906 | 1.10 (1.07-1.13) | 1,406 | 281 | 0.67 (0.64-0.71) | 3,124 | 625 | 1.55 (1.49-1.60) |
| Tumors of the pituitary | 3,723 | 745 | 0.90 (0.87-0.93) | 954 | 191 | 0.45 (0.43-0.48) | 2,769 | 554 | 1.37 (1.32-1.42) |
| Craniopharyngioma | 807 | 161 | 0.20 (0.18-0.21) | 452 | 90 | 0.22 (0.20-0.24) | 355 | 71 | 0.18 (0.16-0.20) |
| Unclassified Tumors | 1,481 | 296 | 0.36 (0.34-0.38) | 779 | 156 | 0.37 (0.35-0.40) | 702 | 140 | 0.35 (0.33-0.38) |
| Hemangioma | 486 | 97 | 0.12 (0.11-0.13) | 260 | 52 | 0.12 (0.11-0.14) | 226 | 45 | 0.11 (0.10-0.13) |
| Neoplasm, unspecified | 811 | 162 | 0.20 (0.19-0.21) | 422 | 84 | 0.20 (0.18-0.22) | 389 | 78 | 0.19 (0.18-0.21) |
| Malignant | 213 | 43 | 0.05 (0.05-0.06) | 114 | 23 | 0.05 (0.05-0.07) | 99 | 20 | 0.05 (0.04-0.06) |
| Non-Malignant | 598 | 120 | 0.15 (0.13-0.16) | 308 | 62 | 0.15 (0.13-0.17) | 290 | 58 | 0.14 (0.13-0.16) |
| All other | 184 | 37 | 0.05 (0.04-0.05) | 97 | 19 | 0.05 (0.04-0.06) | 87 | 17 | 0.04 (0.03-0.05) |
| Malignant | 40 | 8 | 0.01 (0.01-0.01) | 19 | 4 | 0.01 (0.01-0.01) | 21 | 4 | 0.01 (0.01-0.02) |
| Non-Malignant | 144 | 29 | 0.04 (0.03-0.04) | 78 | 16 | 0.04 (0.03-0.05) | 66 | 13 | 0.03 (0.03-0.04) |
| TOTAL c | 25,339 | 5,068 | 6.20 (6.12-6.27) | 12,736 | 2,547 | 6.10 (6.00-6.21) | 12,603 | 2,521 | 6.29 (6.18-6.40) |
| Malignant | 14,385 | 2,877 | 3.53 (3.47-3.59) | 7,854 | 1,571 | 3.77 (3.69-3.85) | 6,531 | 1,306 | 3.28 (3.20-3.36) |
| Non-Malignant | 10,954 | 2,191 | 2.67 (2.62-2.72) | 4,882 | 976 | 2.33 (2.27-2.40) | 6,072 | 1,214 | 3.02 (2.94-3.09) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
AAAIRs were highest for other astrocytic tumors (1.08 per 100,000) and tumors of the sellar region (1.10 per 100,000). Among these tumors, the most common histopathologies were pilocytic astrocytoma (0.95 per 100,000) and tumors of the pituitary (0.90 per 100,000).
There were notable differences in incidence rates between males and females for neuronal and mixed neuronal-glial tumors, embryonal tumors, germ cell tumors, and tumors of the sellar region.
Incidence Rates by Race, Ethnicity, and Histopathology
Incidence rates by race and histopathology are shown in Table 16, and for children and adolescents ages 0-19 years in Table 17.
Table 16.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for All Brain and Other Central Nervous System Tumors by Histopathology and Racec, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | White | Black | American Indian/Alaska Native | Asian or Pacific Islander | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 74,935 | 14,987 | 4.92 (4.89-4.96) | 5,369 | 1,074 | 2.45 (2.38-2.52) | 439 | 88 | 2.15 (1.95-2.37) | 1,799 | 360 | 1.72 (1.64-1.80) |
| Diffuse astrocytoma | 6,581 | 1,316 | 0.50 (0.49-0.51) | 593 | 119 | 0.26 (0.24-0.29) | 48 | 10 | 0.21 (0.16-0.29) | 210 | 42 | 0.20 (0.17-0.23) |
| Anaplastic astrocytoma | 6,245 | 1,249 | 0.46 (0.44-0.47) | 462 | 92 | 0.21 (0.19-0.23) | 35 | 7 | 0.16 (0.11-0.22) | 174 | 35 | 0.16 (0.14-0.19) |
| Glioblastoma | 56,771 | 11,354 | 3.55 (3.52-3.58) | 3,979 | 796 | 1.82 (1.76-1.88) | 294 | 59 | 1.50 (1.33-1.70) | 1,240 | 248 | 1.20 (1.13-1.27) |
| Oligodendroglioma | 3,239 | 648 | 0.26 (0.25-0.27) | 210 | 42 | 0.10 (0.09-0.11) | 40 | 8 | 0.18 (0.13-0.24) | 88 | 18 | 0.08 (0.06-0.10) |
| Anaplastic oligodendroglioma | 1,638 | 328 | 0.12 (0.12-0.13) | 98 | 20 | 0.04 (0.04-0.05) | -- | -- | -- | 69 | 14 | 0.06 (0.05-0.08) |
| Oligoastrocytic tumors | 461 | 92 | 0.04 (0.03-0.04) | 27 | 5 | 0.01 (0.01-0.02) | -- | -- | -- | 18 | 4 | 0.02 (0.01-0.03) |
| Other Astrocytic Tumors | 4,994 | 999 | 0.45 (0.43-0.46) | 774 | 155 | 0.33 (0.31-0.35) | 60 | 12 | 0.23 (0.17-0.29) | 168 | 34 | 0.17 (0.15-0.20) |
| Pilocytic astrocytoma | 4,272 | 854 | 0.38 (0.37-0.40) | 659 | 132 | 0.28 (0.26-0.30) | -- | -- | -- | 131 | 26 | 0.14 (0.11-0.16) |
| Unique astrocytoma variants | 722 | 144 | 0.06 (0.06-0.07) | 115 | 23 | 0.05 (0.04-0.06) | -- | -- | -- | 37 | 7 | 0.04 (0.03-0.05) |
| Non-Malignant | 282 | 56 | 0.03 (0.02-0.03) | 69 | 14 | 0.03 (0.02-0.04) | -- | -- | -- | -- | -- | -- |
| Malignant | 440 | 88 | 0.04 (0.03-0.04) | 46 | 9 | 0.02 (0.01-0.03) | -- | -- | -- | -- | -- | -- |
| Ependymal Tumors | 5,851 | 1,170 | 0.45 (0.44-0.46) | 617 | 123 | 0.27 (0.25-0.30) | 54 | 11 | 0.24 (0.18-0.31) | 180 | 36 | 0.17 (0.15-0.20) |
| Non-Malignant | 2,610 | 522 | 0.20 (0.19-0.21) | 218 | 44 | 0.10 (0.09-0.11) | 27 | 5 | 0.12 (0.08-0.18) | 56 | 11 | 0.05 (0.04-0.07) |
| Malignant | 3,241 | 648 | 0.25 (0.25-0.26) | 399 | 80 | 0.17 (0.16-0.19) | 27 | 5 | 0.11 (0.07-0.17) | 124 | 25 | 0.12 (0.10-0.14) |
| Other Gliomas | 7,230 | 1,446 | 0.56 (0.55-0.58) | 1,009 | 202 | 0.45 (0.42-0.48) | 64 | 13 | 0.28 (0.21-0.37) | 243 | 49 | 0.25 (0.22-0.28) |
| Glioma malignant, NOS | 7,151 | 1,430 | 0.56 (0.54-0.57) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other neuroepithelial tumors | 79 | 16 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 34 | 7 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 45 | 9 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 4,344 | 869 | 0.36 (0.35-0.37) | 563 | 113 | 0.24 (0.22-0.27) | 39 | 8 | 0.16 (0.11-0.22) | 210 | 42 | 0.21 (0.18-0.24) |
| Non-Malignant | 3,527 | 705 | 0.30 (0.29-0.31) | 494 | 99 | 0.21 (0.19-0.23) | -- | -- | -- | -- | -- | -- |
| Malignant | 817 | 163 | 0.06 (0.06-0.06) | 69 | 14 | 0.03 (0.03-0.04) | -- | -- | -- | -- | -- | -- |
| Choroid Plexus Tumors | 681 | 136 | 0.06 (0.05-0.06) | 78 | 16 | 0.03 (0.03-0.04) | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 585 | 117 | 0.05 (0.04-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 96 | 19 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of the Pineal Region | 593 | 119 | 0.05 (0.04-0.05) | 114 | 23 | 0.05 (0.04-0.06) | -- | -- | -- | 30 | 6 | 0.03 (0.02-0.04) |
| Non-Malignant | 247 | 49 | 0.02 (0.02-0.02) | 35 | 7 | 0.02 (0.01-0.02) | -- | -- | -- | -- | -- | -- |
| Malignant | 346 | 69 | 0.03 (0.03-0.03) | 79 | 16 | 0.03 (0.03-0.04) | -- | -- | -- | -- | -- | -- |
| Embryonal Tumors | 2,515 | 503 | 0.23 (0.22-0.24) | 370 | 74 | 0.15 (0.14-0.17) | 36 | 7 | 0.14 (0.10-0.20) | 122 | 24 | 0.13 (0.11-0.16) |
| Tumors of Cranial and Spinal Nerves | 31,292 | 6,258 | 2.15 (2.13-2.17) | 2,316 | 463 | 1.05 (1.01-1.09) | 242 | 48 | 1.14 (1.00-1.30) | 1,918 | 384 | 1.78 (1.70-1.87) |
| Nerve sheath tumors | 31,263 | 6,253 | 2.15 (2.12-2.17) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 31,087 | 6,217 | 2.14 (2.11-2.16) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 176 | 35 | 0.01 (0.01-0.02) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other tumors of cranial and spinal nerves | 29 | 6 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 148,601 | 29,720 | 9.62 (9.57-9.67) | 23,645 | 4,729 | 11.43 (11.28-11.58) | 1,139 | 228 | 6.18 (5.81-6.57) | 7,002 | 1,400 | 7.01 (6.84-7.18) |
| Meningiomas | 143,767 | 28,753 | 9.27 (9.22-9.32) | 22,960 | 4,592 | 11.12 (10.97-11.27) | 1,096 | 219 | 5.98 (5.61-6.37) | 6,796 | 1,359 | 6.81 (6.65-6.98) |
| Non-Malignant | 142,513 | 28,503 | 9.19 (9.14-9.24) | 22,727 | 4,545 | 11.01 (10.86-11.16) | -- | -- | -- | 6,710 | 1,342 | 6.72 (6.56-6.89) |
| Malignant | 1,254 | 251 | 0.08 (0.08-0.09) | 233 | 47 | 0.11 (0.10-0.13) | -- | -- | -- | 86 | 17 | 0.09 (0.07-0.11) |
| Mesenchymal tumors | 4,712 | 942 | 0.35 (0.34-0.36) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 4,083 | 817 | 0.30 (0.29-0.31) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 629 | 126 | 0.05 (0.04-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Primary melanocytic lesions | 122 | 24 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 47 | 9 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 75 | 15 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | 7,117 | 1,423 | 0.45 (0.44-0.46) | 681 | 136 | 0.32 (0.29-0.34) | 56 | 11 | 0.30 (0.23-0.40) | 479 | 96 | 0.47 (0.43-0.51) |
| Lymphoma | 7,085 | 1,417 | 0.45 (0.43-0.46) | -- | -- | -- | 56 | 11 | 0.30 (0.23-0.40) | -- | -- | -- |
| Other hematopoietic neoplasms | 32 | 6 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | 962 | 192 | 0.09 (0.08-0.09) | 149 | 30 | 0.06 (0.05-0.07) | -- | -- | -- | 109 | 22 | 0.11 (0.09-0.14) |
| Non-Malignant | 131 | 26 | 0.01 (0.01-0.01) | 17 | 3 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- |
| Malignant | 831 | 166 | 0.07 (0.07-0.08) | 132 | 26 | 0.06 (0.05-0.07) | -- | -- | -- | -- | -- | -- |
| Tumors of Sellar Region | 56,950 | 11,390 | 4.22 (4.19-4.26) | 16,010 | 3,202 | 7.37 (7.26-7.49) | 748 | 150 | 3.51 (3.25-3.78) | 3,377 | 675 | 3.18 (3.07-3.29) |
| Tumors of the pituitary | 54,706 | 10,941 | 4.05 (4.01-4.09) | 15,393 | 3,079 | 7.10 (6.98-7.21) | 716 | 143 | 3.37 (3.11-3.63) | 3,238 | 648 | 3.04 (2.93-3.15) |
| Non-Malignant | 54,625 | 10,925 | 4.04 (4.01-4.08) | 15,372 | 3,074 | 7.09 (6.97-7.20) | -- | -- | -- | -- | -- | -- |
| Malignant | 81 | 16 | 0.01 (0.00-0.01) | 21 | 4 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- |
| Craniopharyngioma | 2,244 | 449 | 0.17 (0.16-0.18) | 617 | 123 | 0.27 (0.25-0.30) | 32 | 6 | 0.14 (0.10-0.20) | 139 | 28 | 0.14 (0.11-0.16) |
| Unclassified Tumors | 15,176 | 3,035 | 1.04 (1.02-1.06) | 2,008 | 402 | 0.97 (0.93-1.02) | 126 | 25 | 0.69 (0.57-0.83) | 589 | 118 | 0.60 (0.56-0.66) |
| Hemangioma | 3,435 | 687 | 0.26 (0.25-0.27) | 447 | 89 | 0.20 (0.19-0.22) | -- | -- | -- | 175 | 35 | 0.17 (0.14-0.19) |
| Neoplasm, unspecified | 11,309 | 2,262 | 0.75 (0.73-0.76) | 1,496 | 299 | 0.74 (0.70-0.78) | 90 | 18 | 0.53 (0.42-0.66) | 395 | 79 | 0.42 (0.38-0.46) |
| Non-Malignant | 5,566 | 1,113 | 0.38 (0.37-0.40) | 912 | 182 | 0.44 (0.41-0.47) | 46 | 9 | 0.26 (0.19-0.35) | 208 | 42 | 0.21 (0.18-0.24) |
| Malignant | 5,743 | 1,149 | 0.36 (0.35-0.37) | 584 | 117 | 0.30 (0.27-0.33) | 44 | 9 | 0.27 (0.19-0.37) | 187 | 37 | 0.20 (0.18-0.24) |
| All other | 432 | 86 | 0.03 (0.03-0.04) | 65 | 13 | 0.03 (0.02-0.04) | -- | -- | -- | 19 | 4 | 0.02 (0.01-0.03) |
| Non-Malignant | 391 | 78 | 0.03 (0.03-0.03) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 41 | 8 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TOTAL d | 361,241 | 72,248 | 24.65 (24.56-24.73) | 53,703 | 10,741 | 25.18 (24.96-25.40) | 3,033 | 607 | 15.15 (14.59-15.72) | 16,246 | 3,249 | 15.86 (15.61-16.11) |
| Malignant | 109,685 | 21,937 | 7.51 (7.47-7.56) | 9,759 | 1,952 | 4.43 (4.34-4.52) | 755 | 151 | 3.63 (3.36-3.91) | 3,398 | 680 | 3.34 (3.23-3.46) |
| Non-Malignant | 251,556 | 50,311 | 17.13 (17.06-17.20) | 43,944 | 8,789 | 20.75 (20.55-20.95) | 2,278 | 456 | 11.52 (11.03-12.03) | 12,848 | 2,570 | 12.51 (12.29-12.74) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cIndividuals with unknown race were excluded (N = 11,569).
dRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
Table 17.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for Children and Adolescents (Ages 0-19 Years), Brain and Other Central Nervous System Tumors by Histopathology and Racec, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | White | Black | American Indian/Alaska Native | Asian/Pacific Islander | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 1,677 | 335 | 0.54 (0.52-0.57) | 247 | 49 | 0.36 (0.32-0.41) | 19 | 4 | 0.25 (0.15-0.39) | 74 | 15 | 0.29 (0.23-0.36) |
| Diffuse astrocytoma | 706 | 141 | 0.23 (0.21-0.25) | 94 | 19 | 0.14 (0.11-0.17) | -- | -- | -- | 25 | 5 | 0.10 (0.06-0.14) |
| Anaplastic astrocytoma | 270 | 54 | 0.09 (0.08-0.10) | 38 | 8 | 0.06 (0.04-0.08) | -- | -- | -- | -- | -- | -- |
| Glioblastoma | 539 | 108 | 0.18 (0.16-0.19) | 93 | 19 | 0.14 (0.11-0.17) | -- | -- | -- | 29 | 6 | 0.11 (0.08-0.16) |
| Oligodendroglioma | 120 | 24 | 0.04 (0.03-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Anaplastic oligodendroglioma | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Oligoastrocytic tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other Astrocytic Tumors | 3,467 | 693 | 1.13 (1.10-1.17) | 558 | 112 | 0.82 (0.75-0.89) | 48 | 10 | 0.63 (0.46-0.83) | 111 | 22 | 0.43 (0.35-0.52) |
| Pilocytic astrocytoma | 3,091 | 618 | 1.01 (0.98-1.05) | 481 | 96 | 0.70 (0.64-0.77) | -- | -- | -- | 94 | 19 | 0.37 (0.30-0.45) |
| Unique astrocytoma variants | 376 | 75 | 0.12 (0.11-0.14) | 77 | 15 | 0.11 (0.09-0.14) | -- | -- | -- | 17 | 3 | 0.07 (0.04-0.11) |
| Malignant | 193 | 39 | 0.06 (0.05-0.07) | 51 | 10 | 0.07 (0.06-0.10) | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 183 | 37 | 0.06 (0.05-0.07) | 26 | 5 | 0.04 (0.03-0.06) | -- | -- | -- | -- | -- | -- |
| Ependymal Tumors | 938 | 188 | 0.31 (0.29-0.33) | 153 | 31 | 0.22 (0.19-0.26) | -- | -- | -- | 41 | 8 | 0.16 (0.11-0.22) |
| Malignant | 187 | 37 | 0.06 (0.05-0.07) | 16 | 3 | 0.02 (0.01-0.04) | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 751 | 150 | 0.24 (0.23-0.26) | 137 | 27 | 0.20 (0.17-0.24) | -- | -- | -- | -- | -- | -- |
| Other Gliomas | 2,439 | 488 | 0.80 (0.77-0.83) | 457 | 91 | 0.67 (0.61-0.74) | 29 | 6 | 0.38 (0.25-0.54) | 105 | 21 | 0.41 (0.33-0.49) |
| Glioma malignant, NOS | 2,418 | 484 | 0.79 (0.76-0.82) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other neuroepithelial tumors | 21 | 4 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 1,640 | 328 | 0.53 (0.51-0.56) | 277 | 55 | 0.41 (0.36-0.46) | 21 | 4 | 0.28 (0.17-0.42) | 82 | 16 | 0.32 (0.25-0.40) |
| Malignant | 1,540 | 308 | 0.50 (0.48-0.53) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 100 | 20 | 0.03 (0.03-0.04) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Choroid Plexus Tumors | 324 | 65 | 0.11 (0.09-0.12) | 48 | 10 | 0.07 (0.05-0.09) | -- | -- | -- | -- | -- | -- |
| Malignant | 249 | 50 | 0.08 (0.07-0.09) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 75 | 15 | 0.02 (0.02-0.03) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of the Pineal Region | 145 | 29 | 0.05 (0.04-0.06) | 51 | 10 | 0.07 (0.06-0.10) | -- | -- | -- | -- | -- | -- |
| Malignant | 29 | 6 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 116 | 23 | 0.04 (0.03-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Embryonal Tumors | 1,825 | 365 | 0.60 (0.57-0.63) | 284 | 57 | 0.41 (0.37-0.47) | 26 | 5 | 0.34 (0.22-0.49) | 106 | 21 | 0.41 (0.34-0.50) |
| Medulloblastoma | 1,289 | 258 | 0.42 (0.40-0.45) | 170 | 34 | 0.25 (0.21-0.29) | -- | -- | -- | 75 | 15 | 0.29 (0.23-0.36) |
| Atypical teratoid/rhabdoid tumor | 280 | 56 | 0.09 (0.08-0.10) | 55 | 11 | 0.08 (0.06-0.10) | -- | -- | -- | -- | -- | -- |
| All other embryonal | 256 | 51 | 0.08 (0.07-0.09) | 59 | 12 | 0.09 (0.07-0.11) | -- | -- | -- | -- | -- | -- |
| Tumors of Cranial and Spinal Nerves | 915 | 183 | 0.30 (0.28-0.32) | 130 | 26 | 0.19 (0.16-0.23) | -- | -- | -- | 43 | 9 | 0.17 (0.12-0.22) |
| Nerve sheath tumors | -- | -- | -- | 130 | 26 | 0.19 (0.16-0.23) | -- | -- | -- | 43 | 9 | 0.17 (0.12-0.22) |
| Other tumors of cranial and spinal nerves | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 974 | 195 | 0.32 (0.30-0.34) | 177 | 35 | 0.26 (0.22-0.30) | -- | -- | -- | 41 | 8 | 0.16 (0.11-0.22) |
| Meningiomas | 520 | 104 | 0.17 (0.15-0.18) | 103 | 21 | 0.15 (0.12-0.18) | -- | -- | -- | 17 | 3 | 0.07 (0.04-0.11) |
| Malignant | 502 | 100 | 0.16 (0.15-0.18) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 18 | 4 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Mesenchymal tumors | -- | -- | -- | 74 | 15 | 0.11 (0.09-0.14) | -- | -- | -- | -- | -- | -- |
| Primary melanocytic lesions | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | 95 | 19 | 0.03 (0.03-0.04) | 16 | 3 | 0.02 (0.01-0.04) | -- | -- | -- | -- | -- | -- |
| Lymphoma | 95 | 19 | 0.03 (0.03-0.04) | 16 | 3 | 0.02 (0.01-0.04) | -- | -- | -- | -- | -- | -- |
| Other hematopoietic neoplasms | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | 640 | 128 | 0.21 (0.19-0.22) | 101 | 20 | 0.15 (0.12-0.18) | -- | -- | -- | 81 | 16 | 0.31 (0.25-0.39) |
| Malignant | 68 | 14 | 0.02 (0.02-0.03) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 572 | 114 | 0.19 (0.17-0.20) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Sellar Region | 3,407 | 681 | 1.10 (1.06-1.13) | 657 | 131 | 0.96 (0.89-1.04) | 55 | 11 | 0.73 (0.55-0.95) | 149 | 30 | 0.58 (0.49-0.68) |
| Tumors of the pituitary | 2,820 | 564 | 0.90 (0.87-0.94) | 518 | 104 | 0.76 (0.70-0.83) | -- | -- | -- | 114 | 23 | 0.44 (0.36-0.53) |
| Malignant | 2,820 | 564 | 0.90 (0.87-0.94) | 518 | 104 | 0.76 (0.70-0.83) | -- | -- | -- | 114 | 23 | 0.44 (0.36-0.53) |
| Non-Malignant | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Craniopharyngioma | 587 | 117 | 0.19 (0.18-0.21) | 139 | 28 | 0.20 (0.17-0.24) | -- | -- | -- | 35 | 7 | 0.14 (0.09-0.19) |
| Unclassified Tumors | 1,170 | 234 | 0.38 (0.36-0.40) | 176 | 35 | 0.26 (0.22-0.30) | -- | -- | -- | 31 | 6 | 0.12 (0.08-0.17) |
| Hemangioma | 404 | 81 | 0.13 (0.12-0.14) | 42 | 8 | 0.06 (0.04-0.08) | -- | -- | -- | -- | -- | -- |
| Neoplasm, unspecified | 624 | 125 | 0.20 (0.19-0.22) | 104 | 21 | 0.15 (0.12-0.19) | -- | -- | -- | 16 | 3 | 0.06 (0.04-0.10) |
| Malignant | 469 | 94 | 0.15 (0.14-0.17) | 70 | 14 | 0.10 (0.08-0.13) | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 155 | 31 | 0.05 (0.04-0.06) | 34 | 7 | 0.05 (0.03-0.07) | -- | -- | -- | -- | -- | -- |
| All other | 142 | 28 | 0.05 (0.04-0.05) | 30 | 6 | 0.04 (0.03-0.06) | -- | -- | -- | -- | -- | -- |
| Malignant | 116 | 23 | 0.04 (0.03-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 26 | 5 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TOTAL d | 19,656 | 3,931 | 6.39 (6.30-6.48) | 3,332 | 666 | 4.89 (4.72-5.06) | 257 | 51 | 3.38 (2.98-3.82) | 894 | 179 | 3.47 (3.25-3.71) |
| Non-Malignant | 8,552 | 1,710 | 2.77 (2.71-2.82) | 1,473 | 295 | 2.16 (2.05-2.28) | 118 | 24 | 1.56 (1.29-1.87) | 367 | 73 | 1.42 (1.28-1.58) |
| Malignant | 11,104 | 2,221 | 3.63 (3.56-3.70) | 1,859 | 372 | 2.73 (2.60-2.85) | 139 | 28 | 1.82 (1.53-2.15) | 527 | 105 | 2.05 (1.88-2.23) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cIndividuals with unknown race were excluded (N = 1200).
dRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
Incidence rates for all primary brain and other CNS tumors combined were lower for people who are AIAN (15.15 per 100,000) compared to people who are White (24.65 per 100,000), Black (25.18 per 100,000), and API (15.86 per 100,000).
Incidence rates for non-malignant primary brain and other CNS tumors were highest in people who are Black (20.75 per 100,000) compared to people who are White (17.13 per 100,000), AIAN (11.52 per 100,000), and API (12.51 per 100,000).
Incidence rates for malignant primary brain and other CNS tumors were highest in people who are White (7.51 per 100,000) compared to people who are Black (4.43 per 100,000), AIAN (3.63 per 100,000), and API (3.34 per 100,000).
Incidence rate ratios (White:Black) for selected histopathologies are shown in Figure 17A.
Incidence rates for glioblastoma (p<0.0001), all other astrocytoma (p<0.0001), and nerve sheath tumors (p<0.0001) were approximately two times greater in people who are White than in people who are Black.
Incidence of oligodendroglioma was 2.65 times greater in people who are White than in people who are Black (p<0.0001).
Incidence rates for pilocytic astrocytoma (p<0.0001), ependymal tumors (p<0.0001), embryonal tumors (p<0.0001), lymphoma (p<0.0001), and germ cell tumors (p=0.0004) were also higher among the people who are White than people who are Black.
Incidence rates for non-malignant (p<0.0001) and malignant (p<0.0001) meningioma and tumors of the pituitary (p<0.0001) were higher among people who are Black than people who are White.
Incidence rate ratios (White:API) for selected histopathologies are shown in Figure 17B.
Incidence rates for glioblastoma (p<0.0001) were 2.97 times greater in people who are White than in people who are API.
Incidence of nerve sheath tumors (p<0.0001) was approximately 1.2 times higher in people who are White than in people who are API.
Germ cell tumors (p<0.0001) were 0.75 times greater among people who are API than people who are White.
Incidence rates by Hispanic ethnicity and histopathology are shown in Table 18, for children and adolescents ages 0-19 years in Table 19 and Supplementary Figure 4.
Table 18.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for All Brain and Other Central Nervous System Tumors by Histopathology, Hispanic Ethnicityc, and Race, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | Non-Hispanic | All Hispanic | White Hispanic | Black Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 76,233 | 15,247 | 4.67 (4.64-4.71) | 7,779 | 1,556 | 3.49 (3.40-3.57) | 7,125 | 1,425 | 3.52 (3.43-3.60) | 208 | 42 | 2.00 (1.72-2.32) |
| Diffuse astrocytoma | 6,753 | 1,351 | 0.49 (0.47-0.50) | 881 | 176 | 0.34 (0.31-0.36) | 795 | 159 | 0.34 (0.31-0.36) | 23 | 5 | 0.16 (0.10-0.25) |
| Anaplastic astrocytoma | 6,363 | 1,273 | 0.44 (0.43-0.45) | 683 | 137 | 0.27 (0.25-0.29) | 616 | 123 | 0.27 (0.25-0.29) | 24 | 5 | 0.18 (0.11-0.28) |
| Glioblastoma | 57,829 | 11,566 | 3.36 (3.33-3.39) | 5,429 | 1,086 | 2.59 (2.52-2.66) | 4,999 | 1,000 | 2.61 (2.54-2.69) | 148 | 30 | 1.58 (1.31-1.87) |
| Oligodendroglioma | 3,209 | 642 | 0.24 (0.24-0.25) | 478 | 96 | 0.18 (0.16-0.19) | 431 | 86 | 0.18 (0.16-0.20) | -- | -- | -- |
| Anaplastic oligodendroglioma | 1,617 | 323 | 0.12 (0.11-0.12) | 254 | 51 | 0.10 (0.09-0.11) | 233 | 47 | 0.10 (0.09-0.11) | -- | -- | -- |
| Oligoastrocytic tumors | 462 | 92 | 0.03 (0.03-0.04) | 54 | 11 | 0.02 (0.02-0.03) | 51 | 10 | 0.02 (0.02-0.03) | -- | -- | -- |
| Other Astrocytic Tumors | 5,231 | 1,046 | 0.46 (0.45-0.47) | 1,021 | 204 | 0.30 (0.28-0.32) | 907 | 181 | 0.30 (0.28-0.32) | 26 | 5 | 0.15 (0.09-0.23) |
| Pilocytic astrocytoma | 4,494 | 899 | 0.40 (0.39-0.41) | 845 | 169 | 0.25 (0.23-0.26) | 748 | 150 | 0.25 (0.23-0.26) | 21 | 4 | 0.12 (0.07-0.20) |
| Unique astrocytoma variants | 737 | 147 | 0.06 (0.06-0.07) | 176 | 35 | 0.06 (0.05-0.07) | 159 | 32 | 0.06 (0.05-0.07) | -- | -- | -- |
| Non-Malignant | 301 | 60 | 0.03 (0.02-0.03) | 80 | 16 | 0.02 (0.02-0.03) | 71 | 14 | 0.02 (0.02-0.03) | -- | -- | -- |
| Malignant | 436 | 87 | 0.04 (0.03-0.04) | 96 | 19 | 0.03 (0.03-0.04) | 88 | 18 | 0.03 (0.03-0.04) | -- | -- | -- |
| Ependymal Tumors | 5,893 | 1,179 | 0.43 (0.42-0.44) | 1,018 | 204 | 0.36 (0.34-0.39) | 912 | 182 | 0.36 (0.34-0.39) | 27 | 5 | 0.18 (0.11-0.28) |
| Non-Malignant | 2,615 | 523 | 0.19 (0.18-0.19) | 387 | 77 | 0.15 (0.13-0.16) | 339 | 68 | 0.14 (0.13-0.16) | -- | -- | -- |
| Malignant | 3,278 | 656 | 0.25 (0.24-0.25) | 631 | 126 | 0.22 (0.20-0.24) | 573 | 115 | 0.22 (0.20-0.24) | -- | -- | -- |
| Other Gliomas | 7,598 | 1,520 | 0.57 (0.56-0.59) | 1,256 | 251 | 0.45 (0.43-0.48) | 1,097 | 219 | 0.44 (0.41-0.47) | 57 | 11 | 0.43 (0.31-0.58) |
| Glioma malignant, NOS | 7,519 | 1,504 | 0.57 (0.55-0.58) | 1,234 | 247 | 0.44 (0.42-0.47) | 1,077 | 215 | 0.43 (0.41-0.46) | 57 | 11 | 0.43 (0.31-0.58) |
| Other neuroepithelial tumors | 79 | 16 | 0.01 (0.00-0.01) | 22 | 4 | 0.01 (0.00-0.01) | 20 | 4 | 0.01 (0.00-0.01) | -- | -- | -- |
| Non-Malignant | 34 | 7 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 45 | 9 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 4,558 | 912 | 0.37 (0.35-0.38) | 781 | 156 | 0.25 (0.23-0.27) | 688 | 138 | 0.25 (0.23-0.27) | 31 | 6 | 0.19 (0.12-0.27) |
| Non-Malignant | 3,729 | 746 | 0.31 (0.30-0.32) | 650 | 130 | 0.20 (0.19-0.22) | 569 | 114 | 0.20 (0.18-0.22) | -- | -- | -- |
| Malignant | 829 | 166 | 0.06 (0.05-0.06) | 131 | 26 | 0.05 (0.04-0.06) | 119 | 24 | 0.05 (0.04-0.06) | -- | -- | -- |
| Choroid Plexus Tumors | 665 | 133 | 0.05 (0.05-0.06) | 162 | 32 | 0.05 (0.04-0.06) | 143 | 29 | 0.05 (0.04-0.06) | -- | -- | -- |
| Non-Malignant | 578 | 116 | 0.05 (0.04-0.05) | 128 | 26 | 0.04 (0.04-0.05) | 114 | 23 | 0.04 (0.03-0.05) | -- | -- | -- |
| Malignant | 87 | 17 | 0.01 (0.01-0.01) | 34 | 7 | 0.01 (0.01-0.01) | 29 | 6 | 0.01 (0.01-0.01) | -- | -- | -- |
| Tumors of the Pineal Region | 659 | 132 | 0.05 (0.05-0.06) | 110 | 22 | 0.04 (0.03-0.05) | 100 | 20 | 0.04 (0.03-0.05) | -- | -- | -- |
| Non-Malignant | 272 | 54 | 0.02 (0.02-0.02) | 35 | 7 | 0.01 (0.01-0.02) | 31 | 6 | 0.01 (0.01-0.02) | -- | -- | -- |
| Malignant | 387 | 77 | 0.03 (0.03-0.03) | 75 | 15 | 0.02 (0.02-0.03) | 69 | 14 | 0.03 (0.02-0.03) | -- | -- | -- |
| Embryonal Tumors | 2,453 | 491 | 0.22 (0.21-0.23) | 717 | 143 | 0.21 (0.19-0.23) | 651 | 130 | 0.21 (0.20-0.23) | 22 | 4 | 0.10 (0.06-0.16) |
| Tumors of Cranial and Spinal Nerves | 33,525 | 6,705 | 2.15 (2.13-2.18) | 3,523 | 705 | 1.45 (1.40-1.51) | 3,120 | 624 | 1.42 (1.37-1.48) | 96 | 19 | 0.83 (0.66-1.03) |
| Nerve sheath tumors | 33,495 | 6,699 | 2.15 (2.13-2.18) | 3,520 | 704 | 1.45 (1.40-1.50) | -- | -- | -- | 96 | 19 | 0.83 (0.66-1.03) |
| Non-Malignant | 33,329 | 6,666 | 2.14 (2.12-2.16) | 3,475 | 695 | 1.43 (1.39-1.49) | -- | -- | -- | -- | -- | -- |
| Malignant | 166 | 33 | 0.01 (0.01-0.01) | 45 | 9 | 0.02 (0.01-0.02) | -- | -- | -- | -- | -- | -- |
| Other tumors of cranial and spinal nerves | 30 | 6 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 165,351 | 33,070 | 9.97 (9.92-10.02) | 19,054 | 3,811 | 9.33 (9.20-9.47) | 17,215 | 3,443 | 9.25 (9.10-9.39) | 544 | 109 | 5.98 (5.45-6.54) |
| Meningiomas | 160,230 | 32,046 | 9.62 (9.57-9.66) | 18,217 | 3,643 | 9.02 (8.88-9.15) | 16,462 | 3,292 | 8.93 (8.79-9.07) | 532 | 106 | 5.88 (5.35-6.43) |
| Non-Malignant | 158,843 | 31,769 | 9.53 (9.48-9.58) | 17,989 | 3,598 | 8.90 (8.77-9.04) | 16,250 | 3,250 | 8.81 (8.67-8.96) | -- | -- | -- |
| Malignant | 1,387 | 277 | 0.08 (0.08-0.09) | 228 | 46 | 0.11 (0.10-0.13) | 212 | 42 | 0.11 (0.10-0.13) | -- | -- | -- |
| Mesenchymal tumors | 4,989 | 998 | 0.35 (0.34-0.36) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 4,351 | 870 | 0.30 (0.29-0.31) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 638 | 128 | 0.04 (0.04-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Primary melanocytic lesions | 132 | 26 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 50 | 10 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 82 | 16 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | 7,461 | 1,492 | 0.44 (0.43-0.45) | 1,064 | 213 | 0.51 (0.48-0.55) | 997 | 199 | 0.53 (0.49-0.56) | 17 | 3 | 0.18 (0.10-0.29) |
| Lymphoma | 7,427 | 1,485 | 0.44 (0.43-0.45) | 1,055 | 211 | 0.51 (0.48-0.54) | 989 | 198 | 0.52 (0.49-0.56) | 17 | 3 | 0.18 (0.10-0.29) |
| Other hematopoietic neoplasms | 34 | 7 | 0.00 (0.00-0.00) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | 997 | 199 | 0.09 (0.08-0.09) | 283 | 57 | 0.08 (0.07-0.09) | 251 | 50 | 0.08 (0.07-0.09) | -- | -- | -- |
| Non-Malignant | 137 | 27 | 0.01 (0.01-0.01) | 33 | 7 | 0.01 (0.01-0.02) | 30 | 6 | 0.01 (0.01-0.02) | -- | -- | -- |
| Malignant | 860 | 172 | 0.08 (0.07-0.08) | 250 | 50 | 0.07 (0.06-0.08) | 221 | 44 | 0.07 (0.06-0.08) | -- | -- | -- |
| Tumors of Sellar Region | 66,389 | 13,278 | 4.58 (4.55-4.62) | 13,607 | 2,721 | 5.33 (5.24-5.43) | 12,037 | 2,407 | 5.23 (5.14-5.33) | 486 | 97 | 3.85 (3.49-4.24) |
| Tumors of the pituitary | 63,773 | 12,755 | 4.39 (4.35-4.43) | 13,090 | 2,618 | 5.15 (5.06-5.24) | 11,579 | 2,316 | 5.05 (4.96-5.15) | 462 | 92 | 3.71 (3.35-4.09) |
| Non-Malignant | 63,682 | 12,736 | 4.38 (4.35-4.42) | 13,073 | 2,615 | 5.14 (5.05-5.23) | -- | -- | -- | 462 | 92 | 3.71 (3.35-4.09) |
| Malignant | 91 | 18 | 0.01 (0.00-0.01) | 17 | 3 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- |
| Craniopharyngioma | 2,616 | 523 | 0.19 (0.18-0.20) | 517 | 103 | 0.18 (0.17-0.20) | 458 | 92 | 0.18 (0.16-0.20) | 24 | 5 | 0.14 (0.09-0.22) |
| Unclassified Tumors | 15,998 | 3,200 | 1.03 (1.01-1.04) | 2,406 | 481 | 1.08 (1.03-1.13) | 2,159 | 432 | 1.07 (1.03-1.12) | 60 | 12 | 0.56 (0.41-0.74) |
| Hemangioma | 3,525 | 705 | 0.25 (0.24-0.26) | 690 | 138 | 0.26 (0.24-0.28) | 620 | 124 | 0.26 (0.24-0.29) | 22 | 4 | 0.16 (0.10-0.25) |
| Neoplasm, unspecified | 12,045 | 2,409 | 0.75 (0.73-0.76) | 1,602 | 320 | 0.77 (0.73-0.81) | 1,434 | 287 | 0.77 (0.72-0.81) | 37 | 7 | 0.39 (0.27-0.56) |
| Non-Malignant | 5,999 | 1,200 | 0.39 (0.38-0.40) | 955 | 191 | 0.42 (0.39-0.45) | 834 | 167 | 0.40 (0.38-0.43) | 26 | 5 | 0.29 (0.18-0.44) |
| Malignant | 6,046 | 1,209 | 0.36 (0.35-0.37) | 647 | 129 | 0.36 (0.33-0.39) | 600 | 120 | 0.36 (0.33-0.39) | -- | -- | -- |
| All other | 428 | 86 | 0.03 (0.03-0.04) | 114 | 23 | 0.04 (0.04-0.05) | 105 | 21 | 0.04 (0.04-0.05) | -- | -- | -- |
| Non-Malignant | 379 | 76 | 0.03 (0.02-0.03) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 49 | 10 | 0.00 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TOTAL d | 393,011 | 78,602 | 25.09 (25.01-25.17) | 52,781 | 10,556 | 22.95 (22.74-23.16) | 47,402 | 9,480 | 22.76 (22.55-22.98) | 1,591 | 318 | 14.55 (13.77-15.35) |
| Malignant | 112,404 | 22,481 | 7.25 (7.21-7.30) | 13,941 | 2,788 | 5.85 (5.75-5.96) | 12,699 | 2,540 | 5.91 (5.80-6.02) | 380 | 76 | 3.23 (2.87-3.61) |
| Non-Malignant d | 280,607 | 56,121 | 17.84 (17.77-17.90) | 38,840 | 7,768 | 17.09 (16.92-17.27) | 34,703 | 6,941 | 16.86 (16.67-17.04) | 1,211 | 242 | 11.32 (10.63-12.03) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cHispanic ethnicity is not mutually exclusive of race; Classified using the North American Association of Central Cancer Registries Hispanic Identification Algorithm, version 2 (NHIA v2).
dRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
Table 19.
Five-Year Total, Annual Average Totala, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for Children and Adolescents (Ages 0-19 Years), Brain and Other Central Nervous System Tumors by Histopathology, Hispanic Ethnicityc, and Race, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
| Histopathology | Non-Hispanic | All Hispanic | White Hispanic | Black Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Diffuse Astrocytic and Oligodendroglial Tumors | 1,730 | 346 | 0.56 (0.53-0.59) | 372 | 74 | 0.37 (0.33-0.41) | 333 | 67 | 0.37 (0.33-0.42) | -- | -- | -- |
| Diffuse astrocytoma | 736 | 147 | 0.24 (0.22-0.26) | 142 | 28 | 0.14 (0.12-0.17) | 125 | 25 | 0.14 (0.12-0.17) | -- | -- | -- |
| Anaplastic astrocytoma | 271 | 54 | 0.09 (0.08-0.10) | 61 | 12 | 0.06 (0.05-0.08) | 55 | 11 | 0.06 (0.05-0.08) | -- | -- | -- |
| Glioblastoma | 551 | 110 | 0.18 (0.16-0.19) | 144 | 29 | 0.14 (0.12-0.17) | 129 | 26 | 0.14 (0.12-0.17) | -- | -- | -- |
| Oligodendroglioma | 129 | 26 | 0.04 (0.03-0.05) | 16 | 3 | 0.02 (0.01-0.03) | 16 | 3 | 0.02 (0.01-0.03) | -- | -- | -- |
| Anaplastic oligodendroglioma | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Oligoastrocytic tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other Astrocytic Tumors | 3,611 | 722 | 1.18 (1.14-1.22) | 771 | 154 | 0.75 (0.70-0.81) | 689 | 138 | 0.76 (0.71-0.82) | 16 | 3 | 0.26 (0.15-0.42) |
| Pilocytic astrocytoma | 3,219 | 644 | 1.05 (1.02-1.09) | 666 | 133 | 0.65 (0.60-0.70) | 596 | 119 | 0.66 (0.61-0.71) | -- | -- | -- |
| Unique astrocytoma variants | 392 | 78 | 0.13 (0.12-0.14) | 105 | 21 | 0.10 (0.08-0.13) | 93 | 19 | 0.10 (0.08-0.13) | -- | -- | -- |
| Non-Malignant | 207 | 41 | 0.07 (0.06-0.08) | 60 | 12 | 0.06 (0.04-0.08) | 52 | 10 | 0.06 (0.04-0.08) | -- | -- | -- |
| Malignant | 185 | 37 | 0.06 (0.05-0.07) | 45 | 9 | 0.05 (0.03-0.06) | 41 | 8 | 0.05 (0.03-0.06) | -- | -- | -- |
| Ependymal Tumors | 912 | 182 | 0.30 (0.28-0.32) | 273 | 55 | 0.27 (0.24-0.30) | 248 | 50 | 0.28 (0.24-0.31) | -- | -- | -- |
| Non-Malignant | 170 | 34 | 0.05 (0.05-0.06) | 49 | 10 | 0.05 (0.04-0.07) | 43 | 9 | 0.05 (0.04-0.07) | -- | -- | -- |
| Malignant | 742 | 148 | 0.24 (0.23-0.26) | 224 | 45 | 0.22 (0.19-0.25) | 205 | 41 | 0.23 (0.20-0.26) | -- | -- | -- |
| Other Gliomas | 2,576 | 515 | 0.84 (0.81-0.88) | 612 | 122 | 0.60 (0.55-0.65) | 530 | 106 | 0.59 (0.54-0.64) | 31 | 6 | 0.48 (0.33-0.69) |
| Glioma malignant, NOS | 2,556 | 511 | 0.84 (0.81-0.87) | -- | -- | -- | -- | -- | -- | 31 | 6 | 0.48 (0.33-0.69) |
| Other neuroepithelial tumors | 20 | 4 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 1,711 | 342 | 0.55 (0.53-0.58) | 379 | 76 | 0.38 (0.34-0.42) | 336 | 67 | 0.38 (0.34-0.42) | 17 | 3 | 0.28 (0.16-0.45) |
| Non-Malignant | 1,618 | 324 | 0.52 (0.50-0.55) | 352 | 70 | 0.35 (0.31-0.39) | 312 | 62 | 0.35 (0.31-0.39) | 16 | 3 | 0.27 (0.15-0.43) |
| Malignant | 93 | 19 | 0.03 (0.02-0.04) | 27 | 5 | 0.03 (0.02-0.04) | 24 | 5 | 0.03 (0.02-0.04) | -- | -- | -- |
| Choroid Plexus Tumors | 310 | 62 | 0.10 (0.09-0.11) | 97 | 19 | 0.09 (0.08-0.11) | 85 | 17 | 0.09 (0.07-0.12) | -- | -- | -- |
| Non-Malignant | 240 | 48 | 0.08 (0.07-0.09) | 69 | 14 | 0.07 (0.05-0.09) | 62 | 12 | 0.07 (0.05-0.09) | -- | -- | -- |
| Malignant | 70 | 14 | 0.02 (0.02-0.03) | 28 | 6 | 0.03 (0.02-0.04) | 23 | 5 | 0.03 (0.02-0.04) | -- | -- | -- |
| Tumors of the Pineal Region | 171 | 34 | 0.06 (0.05-0.06) | 44 | 9 | 0.04 (0.03-0.06) | 37 | 7 | 0.04 (0.03-0.06) | -- | -- | -- |
| Non-Malignant | 28 | 6 | 0.01 (0.01-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 143 | 29 | 0.05 (0.04-0.05) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Embryonal Tumors | 1,814 | 363 | 0.60 (0.57-0.63) | 524 | 105 | 0.51 (0.46-0.55) | 475 | 95 | 0.52 (0.48-0.57) | 21 | 4 | 0.33 (0.20-0.50) |
| Medulloblastoma | 1,268 | 254 | 0.42 (0.40-0.44) | 358 | 72 | 0.35 (0.31-0.39) | 325 | 65 | 0.36 (0.32-0.40) | -- | -- | -- |
| Atypical teratoid/rhabdoid tumor | 291 | 58 | 0.10 (0.09-0.11) | 81 | 16 | 0.08 (0.06-0.10) | 76 | 15 | 0.08 (0.07-0.10) | -- | -- | -- |
| All other embryonal | 255 | 51 | 0.08 (0.07-0.09) | 85 | 17 | 0.08 (0.07-0.10) | 74 | 15 | 0.08 (0.06-0.10) | -- | -- | -- |
| Tumors of Cranial and Spinal Nerves | 928 | 186 | 0.30 (0.28-0.32) | 245 | 49 | 0.24 (0.21-0.28) | 208 | 42 | 0.23 (0.20-0.27) | -- | -- | -- |
| Nerve sheath tumors | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other tumors of cranial and spinal nerves | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Meninges | 954 | 191 | 0.31 (0.29-0.33) | 298 | 60 | 0.30 (0.27-0.33) | 274 | 55 | 0.31 (0.27-0.35) | -- | -- | -- |
| Meningiomas | 525 | 105 | 0.17 (0.15-0.18) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Malignant | 508 | 102 | 0.16 (0.15-0.18) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 17 | 3 | 0.01 (0.00-0.01) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Mesenchymal tumors | -- | -- | -- | 151 | 30 | 0.15 (0.13-0.18) | 140 | 28 | 0.16 (0.13-0.19) | -- | -- | -- |
| Non-Malignant | -- | -- | -- | 129 | 26 | 0.13 (0.11-0.15) | 118 | 24 | 0.13 (0.11-0.16) | -- | -- | -- |
| Malignant | -- | -- | -- | 22 | 4 | 0.02 (0.01-0.03) | 22 | 4 | 0.02 (0.02-0.04) | -- | -- | -- |
| Primary melanocytic lesions | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | 108 | 22 | 0.04 (0.03-0.04) | 25 | 5 | 0.02 (0.02-0.04) | 22 | 4 | 0.02 (0.02-0.04) | -- | -- | -- |
| Lymphoma | 108 | 22 | 0.04 (0.03-0.04) | 25 | 5 | 0.02 (0.02-0.04) | 22 | 4 | 0.02 (0.02-0.04) | -- | -- | -- |
| Other hematopoietic neoplasms | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | 658 | 132 | 0.21 (0.20-0.23) | 205 | 41 | 0.20 (0.18-0.23) | 185 | 37 | 0.21 (0.18-0.24) | -- | -- | -- |
| Non-Malignant | 78 | 16 | 0.03 (0.02-0.03) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 580 | 116 | 0.19 (0.17-0.20) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Tumors of Sellar Region | 3,226 | 645 | 1.03 (0.99-1.06) | 1,304 | 261 | 1.32 (1.25-1.40) | 1,145 | 229 | 1.31 (1.24-1.39) | 43 | 9 | 0.72 (0.52-0.97) |
| Tumors of the pituitary | 2,618 | 524 | 0.83 (0.80-0.86) | 1,105 | 221 | 1.13 (1.06-1.20) | 967 | 193 | 1.11 (1.04-1.19) | -- | -- | -- |
| Craniopharyngioma | 608 | 122 | 0.20 (0.18-0.22) | 199 | 40 | 0.20 (0.17-0.23) | 178 | 36 | 0.20 (0.17-0.23) | -- | -- | -- |
| Unclassified Tumors | 1,111 | 222 | 0.36 (0.34-0.38) | 370 | 74 | 0.37 (0.33-0.41) | 322 | 64 | 0.36 (0.32-0.40) | -- | -- | -- |
| Hemangioma | 368 | 74 | 0.12 (0.11-0.13) | 118 | 24 | 0.12 (0.10-0.14) | 106 | 21 | 0.12 (0.10-0.14) | -- | -- | -- |
| Neoplasm, unspecified | 610 | 122 | 0.20 (0.18-0.21) | 201 | 40 | 0.20 (0.17-0.23) | 169 | 34 | 0.19 (0.16-0.22) | -- | -- | -- |
| Non-Malignant | 443 | 89 | 0.14 (0.13-0.16) | 155 | 31 | 0.15 (0.13-0.18) | 129 | 26 | 0.15 (0.12-0.17) | -- | -- | -- |
| Malignant | 167 | 33 | 0.05 (0.05-0.06) | 46 | 9 | 0.05 (0.03-0.06) | 40 | 8 | 0.04 (0.03-0.06) | -- | -- | -- |
| All other | 133 | 27 | 0.04 (0.04-0.05) | 51 | 10 | 0.05 (0.04-0.07) | 47 | 9 | 0.05 (0.04-0.07) | -- | -- | -- |
| Non-Malignant | 98 | 20 | 0.03 (0.03-0.04) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Malignant | 35 | 7 | 0.01 (0.01-0.02) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TOTAL d | 19,820 | 3,964 | 6.44 (6.35-6.53) | 5,519 | 1,104 | 5.47 (5.33-5.62) | 4,889 | 978 | 5.48 (5.33-5.64) | 182 | 36 | 2.94 (2.53-3.40) |
| Non-Malignant | 8,379 | 1,676 | 2.70 (2.64-2.76 | 2,697 | 539 | 2.71 (2.61-2.81) | 2,373 | 475 | 2.69 (2.58-2.80) | 83 | 17 | 1.39 (1.10-1.72) |
| Malignant | 11,441 | 2,288 | 3.74 (3.67-3.81) | 2,822 | 564 | 2.76 (2.66-2.86) | 2,516 | 503 | 2.79 (2.68-2.90) | 99 | 20 | 1.55 (1.26-1.89) |
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cHispanic ethnicity is not mutually exclusive of race; Classified using the North American Association of Central Cancer Registries Hispanic Identification Algorithm, version 2 (NHIA v2).
dRefers to all brain tumors including histopathologies not presented in this table.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval; NOS, not otherwise specified.
The overall incidence rate for primary brain and other CNS tumors was 22.95 per 100,000 population among people who are Hispanic and 25.09 per 100,000 population among people who are non-Hispanic.
Lymphomas and other hematopoietic neoplasms and tumors of the sellar region were the only histopathologies that were higher in people who are Hispanic than in people who are non-Hispanic.
While there are several histopathologies where significant differences in incidence were observed by race and/or ethnicity, in most cases the actual difference in incidence rates is small and may not be biologically significant.
Incidence Rates by Histopathology and Race/Ethnicity in Children and Adolescents (Age 0-19 Years)
Table 17 shows incidence rates for brain and other CNS tumors by histopathology and race for children and adolescents ages 0-19 years. Incidence rates by histopathology and ethnicity for children and adolescents ages 0-19 years are shown in Table 19.
Incidence rates were highest among children and adolescents who are White (6.39 per 100,000) compared to children and adolescents who are Black (4.89 per 100,000 population), AIAN (3.38 per 100,000), or API (3.47 per 100,000).
Incidence rates were highest among children and adolescents who are non-Hispanic (6.44 per 100,000) compared to children and adolescents who are Hispanic (5.47 per 100,000).
Estimated Numbers of Expected Cases of All Primary Brain and Other CNS Tumors
Expected Cases by State
The estimated number of cases of all primary brain and other CNS tumors for 2022 and 2023 by State and Behavior are shown in Table 20. Overall total rates by states presented are based on total malignant and non-malignant incidence. Stratified rates may not add up to these totals. Estimated numbers of cases are highly dependent on input data. Different patterns of incidence within strata can substantively affect the projected estimates, and strata-specific estimates may not equal the total estimate presented. Caution should be used when utilizing these estimates.
Table 20.
Estimated Number of Casesa,b of Brain and Other Central Nervous System Tumors Overall and by Behavior by Central Cancer Registry for Diagnosis Years 2022 and 2023
| Central Cancer Registry | 2022 | 2023 | ||||
|---|---|---|---|---|---|---|
| Malignant | Non-Malignant | Total | Malignant | Non-Malignant | Totalc+ | |
| Alabama | 410 | 780 | 1,190 | 410 | 810 | 1,220 |
| Alaska | 60 | 140 | 200 | 60 | 140 | 200 |
| Arizona | 580 | 1,140 | 1,720 | 590 | 1,160 | 1,750 |
| Arkansas | 270 | 600 | 870 | 280 | 620 | 900 |
| California | 2,930 | 7,580 | 10,510 | 2,960 | 7,800 | 10,770 |
| Colorado | 460 | 1,420 | 1,870 | 460 | 1,460 | 1,920 |
| Connecticut | 340 | 760 | 1,090 | 340 | 780 | 1,120 |
| Delaware | 80 | 160 | 250 | 90 | 170 | 250 |
| District of Columbia | -- | -- | 210 | -- | -- | 220 |
| Florida | 1,910 | 5,140 | 7,050 | 1,930 | 5,270 | 7,200 |
| Georgia | 780 | 2,740 | 3,520 | 790 | 2,880 | 3,670 |
| Hawaii | 80 | 240 | 320 | 80 | 250 | 330 |
| Idaho | 160 | 380 | 540 | 160 | 400 | 560 |
| Illinois | 1,020 | 2,960 | 3,980 | 1,030 | 3,050 | 4,080 |
| Indiana | 550 | 1,060 | 1,610 | 560 | 1,070 | 1,630 |
| Iowa | 290 | 720 | 1,000 | 290 | 740 | 1,030 |
| Kansas | 230 | 570 | 800 | 230 | 590 | 820 |
| Kentucky | 450 | 1,180 | 1,630 | 460 | 1,210 | 1,670 |
| Louisiana | 340 | 1,180 | 1,520 | 350 | 1,220 | 1,570 |
| Maine | 140 | 180 | 320 | 140 | 180 | 320 |
| Maryland | 480 | 1,480 | 1,960 | 480 | 1,560 | 2,040 |
| Massachusetts | 600 | 1,050 | 1,660 | 610 | 1,080 | 1,690 |
| Michigan | 800 | 1,640 | 2,440 | 800 | 1,660 | 2,460 |
| Minnesota | 520 | 1,030 | 1,550 | 530 | 1,090 | 1,610 |
| Mississippi | 220 | 620 | 840 | 220 | 640 | 870 |
| Missouri | 540 | 1,260 | 1,790 | 540 | 1,290 | 1,830 |
| Montana | 110 | 240 | 350 | 110 | 250 | 360 |
| Nebraska | 170 | 310 | 480 | 170 | 310 | 490 |
| Nevadad | 260 | 590 | 850 | 270 | 610 | 880 |
| New Hampshire | 140 | 280 | 420 | 150 | 290 | 430 |
| New Jersey | 780 | 2,420 | 3,200 | 780 | 2,530 | 3,310 |
| New Mexico | 150 | 300 | 460 | 160 | 310 | 470 |
| New York | 1,590 | 5,250 | 6,830 | 1,590 | 5,400 | 6,990 |
| North Carolina | 880 | 2,480 | 3,360 | 890 | 2,580 | 3,470 |
| North Dakota | 60 | 130 | 180 | 60 | 130 | 190 |
| Ohio | 1,060 | 1,920 | 2,990 | 1,070 | 1,980 | 3,050 |
| Oklahoma | 310 | 750 | 1,070 | 310 | 780 | 1,100 |
| Oregon | 380 | 640 | 1,020 | 380 | 650 | 1,040 |
| Pennsylvania | 1,230 | 3,130 | 4,350 | 1,240 | 3,200 | 4,440 |
| Rhode Island | 90 | 120 | 200 | 90 | 120 | 200 |
| South Carolina | 450 | 1,030 | 1,480 | 460 | 1,070 | 1,520 |
| South Dakota | 70 | 190 | 260 | 70 | 200 | 270 |
| Tennessee | 580 | 1,450 | 2,030 | 590 | 1,480 | 2,070 |
| Texas | 2,080 | 6,550 | 8,630 | 2,110 | 6,820 | 8,930 |
| Utah | 250 | 1,390 | 1,640 | 250 | 1,490 | 1,740 |
| Vermont | 60 | 130 | 190 | 60 | 130 | 190 |
| Virginia | 680 | 1,360 | 2,040 | 690 | 1,400 | 2,090 |
| Washington | 700 | 2,420 | 3,130 | 720 | 2,530 | 3,240 |
| West Virginia | 160 | 440 | 600 | 160 | 460 | 620 |
| Wisconsin | 550 | 1,600 | 2,150 | 550 | 1,660 | 2,210 |
| Wyoming | 50 | 100 | 160 | 50 | 110 | 160 |
| United States | 26,670 | 66,800 | 93,470 | 26,940 | 67,440 | 94,390 |
aSource: Estimation based on CBTRUS, NPCR, and SEER 2000-2018 data for malignant tumors, and NPCR and SEER 2006-2018 data for non-malignant tumors.
bRounded to the nearest 10. Numbers may not add up due to rounding.
cTotal estimate is based on histopathology-specific estimate and may not add up to total by state.
dIncidence data not available for 2018-2019, these years are not used for estimation.
- Estimated number is less than 50. These cases are included in overall rates.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
The total number of new cases of primary brain and other CNS tumors for all 50 states and the District of Columbia in 2022 is estimated to be 93,470, with 26,670 malignant and 66,800 non-malignant cases.
For 2023, the estimate is 94,390 new cases of primary brain and other CNS tumors of which 26,940 and 67,440 are expected to be malignant and non-malignant, respectively.
Expected Cases by Histopathology and Age at Diagnosis
The estimated number of cases of all primary brain and other CNS tumors for 2022 and 2023 overall and by histopathology are shown in Table 21 and including by age groups in Supplementary Table 11.
Table 21.
Estimated Number of Casesa,b in the United States of Brain and Other Central Nervous System Tumors Overall and by Behavior and Histopathologyc for Diagnosis Years 2022 and 2023
| Histopathology | 2022 | 2023 | ||||
|---|---|---|---|---|---|---|
| Malignant | Non-Malignant | Total | Malignant | Non-Malignant | Total | |
| Diffuse Astrocytic and Oligodendroglial Tumors | -- | -- | 17,760 | -- | -- | 17,950 |
| Diffuse astrocytoma | -- | -- | 1,440 | -- | -- | 1,420 |
| Anaplastic astrocytoma | -- | -- | 1,090 | -- | -- | 1,020 |
| Glioblastoma | -- | -- | 14,190 | -- | -- | 14,490 |
| Oligodendroglioma | -- | -- | 660 | -- | -- | 650 |
| Anaplastic oligodendroglioma | -- | -- | 390 | -- | -- | 390 |
| Oligoastrocytic tumors | -- | -- | -- | -- | -- | -- |
| Other Astrocytic Tumors | 1,260 | 310 | 1,560 | 1,270 | 350 | 1,620 |
| Pilocytic astrocytoma | 1,130 | 220 | 1,340 | 1,130 | 260 | 1,400 |
| Unique astrocytoma variants | 130 | 90 | 220 | 130 | 90 | 230 |
| Ependymal Tumors | 710 | 680 | 1,390 | 700 | 690 | 1,390 |
| Other Gliomas | -- | -- | 1,990 | -- | -- | 2,030 |
| Glioma malignant, NOS | -- | 1,970 | -- | 2,010 | ||
| Other neuroepithelial tumors | -- | -- | -- | -- | -- | -- |
| Neuronal and Mixed Neuronal-Glial Tumors | 220 | 980 | 1,200 | 220 | 1,000 | 1,220 |
| Choroid Plexus Tumors | -- | -- | 170 | -- | -- | 170 |
| Tumors of the Pineal Region | 110 | 70 | 180 | 110 | 70 | 180 |
| Embryonal Tumors | -- | -- | 560 | -- | -- | 550 |
| Tumors of Cranial and Paraspinal Nerves | -- | -- | 6,380 | -- | -- | 6,190 |
| Nerve sheath tumors | -- | -- | 6,380 | -- | -- | 6,190 |
| Other tumors of cranial and paraspinal nerves | -- | -- | -- | -- | ||
| Tumors of Meninges | 470 | 41,600 | 42,060 | 460 | 42,710 | 43,170 |
| Meningiomas | 280 | 40,820 | 41,110 | 270 | 41,980 | 42,260 |
| Mesenchymal tumors | 160 | 760 | 920 | 160 | 710 | 870 |
| Primary melanocytic lesions | -- | -- | -- | -- | -- | -- |
| Lymphomas and Hematopoietic Neoplasms | -- | -- | 1,870 | -- | -- | 1,900 |
| Lymphoma | -- | -- | 1,860 | -- | -- | 1,890 |
| Other hematopoietic neoplasms | -- | -- | -- | -- | -- | -- |
| Germ Cell Tumors | -- | -- | 260 | -- | -- | 270 |
| Tumors of Sellar Region | -- | -- | 14,830 | -- | -- | 14,560 |
| Tumors of the pituitary | -- | -- | 14,170 | -- | -- | 13,900 |
| Craniopharyngioma | -- | -- | 650 | -- | -- | 660 |
| Unclassified Tumors | 1,430 | 1,830 | 3,260 | 1,440 | 1,730 | 3,180 |
| Hemangioma | -- | -- | 800 | -- | -- | 790 |
| Neoplasm, unspecified | 1,410 | 920 | 2,340 | 1,420 | 840 | 2,260 |
| All other | -- | -- | 130 | -- | -- | 130 |
| TOTAL | 26,670 | 66,800 | 93,470 | 26,940 | 67,440 | 94,390 |
aSource: Estimation based on CBTRUS, NPCR, and SEER 2000-2018 data for malignant tumors, and NPCR and SEER 2006-2018 data for non-malignant tumors.
bRounded to the nearest 10. Numbers may not add up due to rounding.
cTotal estimate is based on overall estimate. Histopathology-specific estimates may not add up to total.
- Estimated number is less than 50 or allows for back-calculation of a value less than 50. These cases are included in overall rates.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
Meningioma was the histopathology with the highest number of all estimated new cases, with 41,110 cases projected in 2022 and 42,260 cases projected in 2023.
Glioblastoma was the malignant histopathology with the highest number of cases, with 14,190 cases projected in 2022 and 14,490 cases projected in 2023.
For 2022 and 2023, the highest number of new cases is predicted in those age 65+ years, with 44,130 cases and 45,390 cases, respectively.
For 2022 and 2023, children ages 0-14 years are estimated to have 3,860 and 3,920 new cases of primary brain and other CNS tumors each year, respectively.
For 2022 and 2023, children and adolescents ages 0-19 years are estimated to have 5,220 and 5,230 new cases of primary brain and other CNS tumors each year, respectively.
Mortality Rates for Malignant Brain and Other CNS Tumors by State and Sex
AAAMR for primary malignant brain and other CNS tumors in the United States during 2015-2019 by state and sex are shown in Table 22 and Figure 25.
Table 22.
Five-Year Total, Average Annual Totala, and Average Annual Age-Adjusted Mortality Ratesb for Malignant Brain and Other Central Nervous System Cancer Overall and by State and Sex, CBTRUS Statistical Report: US Cancer Statistics – NCHS and NVSS, 2015-2019
| State | Total | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | 5-Year Total | Annual Average | Rate (95% CI) | |
| Alabama | 1,495 | 299 | 5.01 (4.76-5.28) | 818 | 164 | 6.01 (5.59-6.45) | 677 | 135 | 4.17 (3.86-4.51) |
| Alaska | 157 | 31 | 4.36 (3.67-5.15) | 86 | 17 | 4.48 (3.51-5.63) | 71 | 14 | 4.19 (3.23-5.33) |
| Arizona | 1,765 | 353 | 4.14 (3.94-4.34) | 484 | 97 | 5.79 (5.28-6.35) | 395 | 79 | 3.97 (3.58-4.40) |
| Arkansas | 879 | 176 | 4.84 (4.52-5.18) | 1,010 | 202 | 5.03 (4.72-5.36) | 755 | 151 | 3.33 (3.09-3.59) |
| California | 9,381 | 1,876 | 4.35 (4.26-4.44) | 5,343 | 1,069 | 5.33 (5.18-5.47) | 4,038 | 808 | 3.50 (3.39-3.61) |
| Colorado | 1,334 | 267 | 4.31 (4.08-4.56) | 744 | 149 | 5.07 (4.70-5.46) | 590 | 118 | 3.65 (3.36-3.97) |
| Connecticut | 997 | 199 | 4.47 (4.19-4.77) | 566 | 113 | 5.54 (5.08-6.03) | 431 | 86 | 3.62 (3.27-4.00) |
| Delaware | 248 | 50 | 3.98 (3.48-4.53) | 53 | 11 | 3.28 (2.45-4.32) | 50 | 10 | 2.60 (1.91-3.45) |
| District of Columbia | 103 | 21 | 2.90 (2.36-3.53) | 151 | 30 | 5.40 (4.54-6.37) | 97 | 19 | 2.80 (2.25-3.46) |
| Florida | 5,954 | 1,191 | 4.17 (4.06-4.28) | 3,370 | 674 | 5.09 (4.91-5.27) | 2,584 | 517 | 3.35 (3.21-3.49) |
| Georgia | 2,366 | 473 | 4.23 (4.06-4.41) | 1,305 | 261 | 5.15 (4.86-5.45) | 1,061 | 212 | 3.47 (3.26-3.69) |
| Hawaii | 252 | 50 | 2.90 (2.54-3.29) | 145 | 29 | 3.56 (2.99-4.21) | 107 | 21 | 2.28 (1.85-2.78) |
| Idaho | 509 | 102 | 5.17 (4.72-5.66) | 323 | 65 | 6.83 (6.09-7.65) | 186 | 37 | 3.64 (3.12-4.22) |
| Illinois | 3,066 | 613 | 4.13 (3.98-4.28) | 1,735 | 347 | 5.13 (4.88-5.38) | 1,331 | 266 | 3.31 (3.13-3.50) |
| Indiana | 1,763 | 353 | 4.55 (4.33-4.77) | 1,024 | 205 | 5.70 (5.35-6.08) | 739 | 148 | 3.56 (3.30-3.83) |
| Iowa | 962 | 192 | 5.05 (4.72-5.39) | 542 | 108 | 5.99 (5.48-6.54) | 420 | 84 | 4.22 (3.81-4.67) |
| Kansas | 828 | 166 | 4.93 (4.59-5.29) | 471 | 94 | 5.98 (5.43-6.56) | 357 | 71 | 4.01 (3.59-4.46) |
| Kentucky | 1,293 | 259 | 4.86 (4.59-5.14) | 715 | 143 | 5.79 (5.36-6.25) | 578 | 116 | 4.06 (3.73-4.42) |
| Louisiana | 1,159 | 232 | 4.33 (4.07-4.59) | 645 | 129 | 5.27 (4.86-5.71) | 514 | 103 | 3.53 (3.22-3.86) |
| Maine | 488 | 98 | 5.29 (4.80-5.81) | 291 | 58 | 6.71 (5.92-7.57) | 197 | 39 | 4.06 (3.47-4.72) |
| Maryland | 1,386 | 277 | 3.99 (3.78-4.21) | 765 | 153 | 4.86 (4.51-5.22) | 621 | 124 | 3.28 (3.02-3.56) |
| Massachusetts | 1,968 | 394 | 4.75 (4.54-4.97) | 1,109 | 222 | 5.90 (5.55-6.27) | 859 | 172 | 3.80 (3.55-4.08) |
| Michigan | 2,891 | 578 | 4.68 (4.51-4.87) | 1,625 | 325 | 5.73 (5.44-6.02) | 1,266 | 253 | 3.80 (3.59-4.03) |
| Minnesota | 1,548 | 310 | 4.77 (4.53-5.02) | 904 | 181 | 5.86 (5.48-6.27) | 644 | 129 | 3.82 (3.52-4.14) |
| Mississippi | 881 | 176 | 5.06 (4.73-5.42) | 463 | 93 | 5.90 (5.36-6.48) | 418 | 84 | 4.37 (3.95-4.83) |
| Missouri | 1,653 | 331 | 4.42 (4.21-4.65) | 928 | 186 | 5.40 (5.05-5.77) | 725 | 145 | 3.60 (3.33-3.88) |
| Montana | 332 | 66 | 4.89 (4.36-5.48) | 193 | 39 | 5.87 (5.04-6.80) | 139 | 28 | 3.99 (3.33-4.76) |
| Nebraska | 554 | 111 | 5.05 (4.63-5.50) | 317 | 63 | 6.06 (5.39-6.78) | 237 | 47 | 4.16 (3.63-4.75) |
| Nevada | 759 | 152 | 4.39 (4.08-4.73) | 424 | 85 | 5.12 (4.63-5.65) | 335 | 67 | 3.73 (3.33-4.16) |
| New Hampshire | 421 | 84 | 4.84 (4.37-5.36) | 240 | 48 | 5.88 (5.13-6.72) | 181 | 36 | 4.01 (3.41-4.68) |
| New Jersey | 2,279 | 456 | 4.24 (4.06-4.42) | 1,254 | 251 | 5.12 (4.83-5.42) | 1,025 | 205 | 3.50 (3.29-3.73) |
| New Mexico | 510 | 102 | 3.96 (3.61-4.33) | 281 | 56 | 4.62 (4.08-5.21) | 229 | 46 | 3.39 (2.95-3.89) |
| New York | 4,549 | 910 | 3.88 (3.77-4.00) | 2,509 | 502 | 4.70 (4.52-4.90) | 2,040 | 408 | 3.19 (3.05-3.34) |
| North Carolina | 2,502 | 500 | 4.13 (3.97-4.30) | 1,415 | 283 | 5.17 (4.90-5.46) | 1,087 | 217 | 3.28 (3.08-3.49) |
| North Dakota | 180 | 36 | 4.26 (3.64-4.95) | 104 | 21 | 5.09 (4.13-6.20) | 76 | 15 | 3.47 (2.71-4.38) |
| Ohio | 3,348 | 670 | 4.67 (4.50-4.83) | 1,878 | 376 | 5.70 (5.44-5.97) | 1,470 | 294 | 3.77 (3.57-3.98) |
| Oklahoma | 1,122 | 224 | 4.96 (4.67-5.27) | 608 | 122 | 5.86 (5.39-6.36) | 514 | 103 | 4.17 (3.81-4.56) |
| Oregon | 1,202 | 240 | 4.67 (4.40-4.95) | 707 | 141 | 5.74 (5.31-6.19) | 495 | 99 | 3.69 (3.36-4.05) |
| Pennsylvania | 3,740 | 748 | 4.55 (4.40-4.71) | 2,170 | 434 | 5.79 (5.54-6.04) | 1,570 | 314 | 3.51 (3.33-3.70) |
| Rhode Island | 327 | 65 | 4.93 (4.39-5.51) | 181 | 36 | 6.04 (5.17-7.03) | 146 | 29 | 4.00 (3.36-4.74) |
| South Carolina | 1,453 | 291 | 4.67 (4.43-4.93) | 795 | 159 | 5.57 (5.17-5.98) | 658 | 132 | 3.93 (3.62-4.25) |
| South Dakota | 276 | 55 | 5.36 (4.73-6.07) | 164 | 33 | 6.86 (5.82-8.03) | 112 | 22 | 4.08 (3.32-4.95) |
| Tennessee | 1,872 | 374 | 4.70 (4.49-4.93) | 1,056 | 211 | 5.76 (5.41-6.13) | 816 | 163 | 3.82 (3.55-4.10) |
| Texas | 5,934 | 1,187 | 4.18 (4.07-4.29) | 3,293 | 659 | 4.99 (4.82-5.17) | 2,641 | 528 | 3.48 (3.35-3.62) |
| Utah | 646 | 129 | 4.70 (4.34-5.08) | 389 | 78 | 5.90 (5.32-6.53) | 257 | 51 | 3.61 (3.18-4.09) |
| Vermont | 230 | 46 | 5.68 (4.93-6.51) | 126 | 25 | 6.66 (5.49-8.02) | 104 | 21 | 4.80 (3.87-5.90) |
| Virginia | 2,073 | 415 | 4.23 (4.04-4.42) | 1,160 | 232 | 5.19 (4.89-5.51) | 913 | 183 | 3.42 (3.20-3.66) |
| Washington | 2,152 | 430 | 5.08 (4.86-5.30) | 1,243 | 249 | 6.19 (5.84-6.55) | 909 | 182 | 4.07 (3.80-4.35) |
| West Virginia | 572 | 114 | 4.64 (4.25-5.06) | 320 | 64 | 5.55 (4.93-6.22) | 252 | 50 | 3.84 (3.36-4.38) |
| Wisconsin | 1,718 | 344 | 4.84 (4.61-5.08) | 1,001 | 200 | 5.97 (5.59-6.36) | 717 | 143 | 3.79 (3.51-4.10) |
| Wyoming | 187 | 37 | 5.47 (4.69-6.36) | 108 | 22 | 6.63 (5.39-8.08) | 79 | 16 | 4.42 (3.46-5.58) |
| TOTAL | 84,264 | 16,853 | 4.41 (4.38-4.44) | 47,551 | 9,510 | 5.38 (5.33-5.43) | 36,713 | 7,343 | 3.58 (3.54-3.61) |
aAnnual average deaths are calculated by dividing the five- year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
-- Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Abbreviations: CI, confidence interval; NCHS, National Center for Health Statistics; NVSS, National Vital Statistics System.
Fig. 25.
Average Annual Age-Adjusted Mortality Ratesa for Malignant Primary Brain and Other Central Nervous System Tumors by Central Cancer Registry, CBTRUS Statistical Report: NVSS, 2015-2019
The aggregate total number of observed deaths was 84,264, for an average annual age-adjusted mortality rate of 4.41 per 100,000 population.
There was considerable variation by individual state, which ranged from a low of 2.9 deaths per 100,000 population to a high of 5.68 deaths per 100,000 population. Rates may vary by state for multiple reasons, including demographic variation and procedures for deciding primary cause of death on a death certificate.
Males had a higher mortality rate for malignant brain and other CNS tumors than females in the US population, with 5.38 per 100,000 population as compared to 3.58 per 100,000 population.
Overall Survival and Relative Survival
Estimates of median survival in months by histopathology and age group for all individuals diagnosed with primary malignant brain and other CNS tumors irrespective of whether individuals received any treatment for their tumor are shown in Table 23. Survival curves for the most common histopathologies are shown by age group in Figure 26A.
Table 23.
Sixteen-Year Total Deaths and Median Survival in Months with 95% Confidence Intervals for Selected Primary Malignant Brain and Other Central Nervous System Tumor Histopathologies, CBTRUS Statistical Report: NPCR, 2001-201877
| Histopathology | N | Deaths | Median Survival (95% CI) |
|---|---|---|---|
| Diffuse astrocytoma | 25,047 | 13,802 | 61 (58-63) |
| Anaplastic astrocytoma | 17,821 | 12,453 | 20 (20-21) |
| Glioblastoma | 145,178 | 129,570 | 8 (8-8) |
| Oligodendroglioma | 11,989 | 3,722 | 199 (190-209) |
| Anaplastic oligodendroglioma | 5,351 | 2,532 | 103 (96-110) |
| Oligoastrocytic tumors | 6,941 | 3,503 | 113 (107-120) |
| Pilocytic astrocytoma | 15,813 | 1,197 | ** (**-**) |
| Unique astrocytoma variants | 2,463 | 420 | ** (**-**) |
| Ependymal tumors | 18,710 | 3,277 | ** (**-**) |
| Glioma malignant, NOS | 20,825 | 9,875 | 96 (87-106) |
| Other neuroepithelial tumors | 2,274 | 338 | ** (**-**) |
| Neuronal and mixed neuronal-glial tumors | 283 | 83 | ** (201-**) |
| Choroid plexus tumors | 12,602 | 1,559 | ** (**-**) |
| Tumors of the pineal region | 1,979 | 506 | ** (**-**) |
| Embryonal tumors | 10,715 | 4,194 | ** (**-**) |
| Nerve sheath tumors | 87,503 | 8,450 | ** (**-**) |
| Other tumors of cranial and spinal nerves | -- | -- | -- |
| Meningiomas | 382,599 | 107,072 | 185 (180-188) |
| Mesenchymal tumors | 16,764 | 2,943 | ** (**-**) |
| Primary melanocytic lesions | 281 | 174 | 28 (21-47) |
| Lymphoma | 19,855 | 13,519 | 16 (15-17) |
| Other hematopoietic neoplasms | 209 | 97 | 138 (98-**) |
| Germ cell tumors | 3,569 | 518 | ** (**-**) |
| Tumors of the pituitary | 172,615 | 20,558 | ** (**-**) |
| Craniopharyngioma | 8,005 | 1,673 | ** (**-**) |
| Hemangioma | 8,709 | 957 | ** (**-**) |
| Neoplasm, unspecified | 28,825 | 15,949 | 44 (41-48) |
| All other | 1,260 | 206 | ** (**-**) |
** Cannot be calculated due to median survival not being observed.-- Survival estimates are not presented when fewer than 100 cases were reported for the specific category.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NOS, not otherwise specified; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
Fig. 26.
A) Kaplan-Meier Survival Curves for the Five Most Common Histopathologies within Age Group at Diagnosis (Ages 0-14, 15-39, and 40+ Years) and B) Hazard Ratios And 95% Confidence Intervals for Sex, Age at Diagnosis, Race, and Ethnicity for the Five Most Common Histopathologies Overall, CBTRUS Statistical Report: NPCR 2001-2018
Fig. 24.
Average Annual Age-Adjusted Incidence Ratesa of Malignant and Non-Malignant Primary Brain and Other Central Nervous System Tumors by Central Cancer Registry, CBTRUS Statistical Report: US Cancer Statistics – NPCR and SEER, 2015-2019
Median survival was lowest for glioblastoma (8 months) and highest for oligodendroglioma (199 months, or approximately 16.6 years).
Median survival was not able to be estimated for pilocytic astrocytoma, unique astrocytoma variants, ependymal tumors, other neuroepithelial tumors, neuronal and mixed neuronal-glial tumors, choroid plexus tumors, tumors of the pineal region, embryonal tumors, nerve sheath tumors, other tumors of cranial and spinal nerves, germ cell tumors, tumors of the pituitary, craniopharyngioma, and hemangioma as >50% of individuals remained alive during the 15 year follow up period.
Many other published survival estimates (including many of those previously published by CBTRUS) incorporate treatment patterns which may explain differences between these population-level estimates and other published estimates.
Demographic factors such as age at diagnosis, sex, race, and ethnicity are known to have a significant effect on survival time after diagnosis in primary brain and other CNS tumors. Hazard ratios for the effect of age groups, sex, race, and ethnicity are shown in Table 24 for all individuals regardless of whether they received any treatment for their tumor. Hazard ratio estimates for demographic factors in the five most common histopathologies are shown by histopathology in Figure 26B.
Table 24.
Hazard Ratios for Death and 95% Confidence Intervals for Age Group at Diagnosis, Sex, Race, and Ethnicity for Selected Primary Malignant Brain and Other Central Nervous System Tumor Histopathologies, CBTRUS Statistical Report: NPCR, 2001-2018 (varying)
| Histopathology | N | Deaths | Age Groupsa | Sexb | Race & Ethnicityc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15-39 Yearsd | 40+ Years | Female | Black, Non-Hispanic | AIAN, Non-Hispanic | API, Non-Hispanic | Hispanic | ||||||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Diffuse astrocytoma | 25,047 | 13,802 | 1.89 (1.72-2.08) | <0.0001 | 6.77 (6.19-7.40) | <0.0001 | 0.96 (0.93-0.99) | 0.0132 | 1.04 (0.97-1.11) | 0.2563 | 0.81 (0.66-1.01) | 0.0583 | 0.88 (0.78-0.98) | 0.0194 | 0.79 (0.75-0.84) | <0.0001 |
| Anaplastic astrocytoma | 17,821 | 12,453 | 0.39 (0.35-0.43) | <0.0001 | 1.28 (1.18-1.40) | <0.0001 | 0.97 (0.93-1.00) | 0.0495 | 1.08 (1.00-1.15) | 0.0444 | 0.91 (0.71-1.16) | 0.4344 | 0.85 (0.76-0.95) | 0.0050 | 0.81 (0.76-0.86) | <0.0001 |
| Glioblastoma | 145,178 | 129,570 | 0.73 (0.68-0.78) | <0.0001 | 1.77 (1.66-1.89) | <0.0001 | 1.02 (1.01-1.03) | <0.0001 | 0.92 (0.90-0.94) | <0.0001 | 1.01 (0.93-1.11) | 0.7750 | 0.70 (0.67-0.73) | <0.0001 | 0.77 (0.75-0.78) | <0.0001 |
| Oligodendroglioma | 11,989 | 3,722 | 3.44 (2.36-5.01) | <0.0001 | 7.59 (5.23-11.03) | <0.0001 | 0.86 (0.81-0.92) | <0.0001 | 1.39 (1.21-1.59) | <0.0001 | 1.28 (0.87-1.90) | 0.2144 | 0.79 (0.64-0.97) | 0.0273 | 0.76 (0.68-0.86) | <0.0001 |
| Anaplastic oligodendroglioma | 5,351 | 2,532 | 0.74 (0.49-1.11) | 0.1410 | 1.57 (1.05-2.33) | 0.0261 | 0.89 (0.82-0.96) | 0.0031 | 1.24 (1.06-1.46) | 0.0089 | 0.91 (0.53-1.57) | 0.7405 | 0.78 (0.64-0.96) | 0.0192 | 0.79 (0.69-0.90) | 0.0007 |
| Oligoastrocytic tumors | 6,941 | 3,503 | 1.74 (1.29-2.35) | 0.0003 | 3.68 (2.74-4.94) | <0.0001 | 0.92 (0.86-0.98) | 0.0123 | 1.34 (1.17-1.53) | <0.0001 | 1.29 (0.87-1.89) | 0.2026 | 0.90 (0.74-1.09) | 0.2899 | 0.85 (0.75-0.95) | 0.0060 |
| Pilocytic astrocytoma | 15,813 | 1,197 | 1.81 (1.57-2.09) | <0.0001 | 8.32 (7.25-9.54) | <0.0001 | 0.85 (0.76-0.96) | 0.0064 | 1.35 (1.13-1.61) | 0.0008 | 1.25 (0.67-2.34) | 0.4786 | 0.85 (0.57-1.28) | 0.4398 | 1.09 (0.91-1.29) | 0.3409 |
| Unique astrocytoma variants | 2,463 | 420 | 2.13 (1.63-2.79) | <0.0001 | 7.94 (6.06-10.39) | <0.0001 | 0.78 (0.64-0.94) | 0.0106 | 0.84 (0.62-1.13) | 0.2410 | 1.06 (0.44-2.57) | 0.8927 | 1.10 (0.68-1.80) | 0.6919 | 0.83 (0.62-1.11) | 0.2007 |
| Ependymal tumors | 18,710 | 3,277 | 0.33 (0.29-0.37) | <0.0001 | 0.86 (0.79-0.94) | 0.0007 | 0.78 (0.73-0.84) | <0.0001 | 1.43 (1.27-1.60) | <0.0001 | 1.28 (0.86-1.90) | 0.2219 | 0.78 (0.61-0.99) | 0.0446 | 1.12 (1.01-1.24) | 0.0256 |
| Glioma malignant, NOS | 20,825 | 9,875 | 0.77 (0.71-0.83) | <0.0001 | 3.52 (3.35-3.69) | <0.0001 | 1.01 (0.97-1.05) | 0.7423 | 1.07 (1.00-1.14) | 0.0387 | 0.91 (0.69-1.20) | 0.5096 | 0.91 (0.81-1.02) | 0.1059 | 0.99 (0.93-1.06) | 0.8101 |
| Choroid plexus tumors | 2,274 | 338 | 0.55 (0.39-0.79) | 0.0011 | 2.41 (1.90-3.07) | <0.0001 | 0.89 (0.71-1.10) | 0.2744 | 1.74 (1.24-2.44) | 0.0012 | 2.03 (0.84-4.95) | 0.1171 | 1.38 (0.80-2.37) | 0.2458 | 0.93 (0.68-1.27) | 0.6420 |
| Other neuroepithelial tumors | 283 | 83 | 2.46 (0.80-7.53) | 0.1146 | 13.18 (4.75-36.56) | <0.0001 | 0.96 (0.60-1.52) | 0.8489 | 0.76 (0.34-1.70) | 0.5064 | ** (**-**) | ** | 0.83 (0.26-2.66) | 0.7502 | 0.85 (0.43-1.68) | 0.6362 |
| Neuronal and mixed neuronal-glial tumors | 12,602 | 1,559 | 1.43 (1.18-1.73) | 0.0002 | 6.04 (5.10-7.15) | <0.0001 | 0.78 (0.70-0.86) | <0.0001 | 1.49 (1.28-1.73) | <0.0001 | 1.80 (1.04-3.11) | 0.0357 | 1.09 (0.82-1.44) | 0.5538 | 1.15 (0.98-1.35) | 0.0826 |
| Tumors of the pineal region | 1,979 | 506 | 0.55 (0.43-0.70) | <0.0001 | 1.04 (0.83-1.30) | 0.7328 | 0.57 (0.47-0.68) | <0.0001 | 1.28 (1.02-1.61) | 0.0356 | 1.00 (0.41-2.43) | 0.9990 | 0.54 (0.25-1.14) | 0.1061 | 1.13 (0.88-1.45) | 0.3441 |
| Embryonal tumors | 10,715 | 4,194 | 0.83 (0.77-0.90) | <0.0001 | 1.96 (1.79-2.15) | <0.0001 | 1.00 (0.94-1.06) | 0.9695 | 1.20 (1.09-1.32) | 0.0002 | 0.71 (0.46-1.08) | 0.1053 | 1.02 (0.87-1.20) | 0.7888 | 0.96 (0.89-1.04) | 0.2917 |
| Nerve sheath tumors | 87,503 | 8,450 | 1.58 (1.19-2.10) | 0.0018 | 6.11 (4.66-8.01) | <0.0001 | 0.84 (0.81-0.88) | <0.0001 | 1.18 (1.08-1.29) | 0.0003 | 1.44 (1.12-1.86) | 0.0045 | 0.60 (0.53-0.69) | <0.0001 | 0.84 (0.77-0.91) | <0.0001 |
| Other tumors of cranial and spinal nerves | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Meningiomas | 382,599 | 107,072 | 0.80 (0.60-1.06) | 0.1178 | 5.75 (4.34-7.60) | <0.0001 | 0.70 (0.69-0.71) | <0.0001 | 1.03 (1.01-1.04) | 0.0080 | 0.80 (0.73-0.88) | <0.0001 | 0.63 (0.61-0.66) | <0.0001 | 0.71 (0.69-0.73) | <0.0001 |
| Mesenchymal tumors | 16,764 | 2,943 | 0.86 (0.68-1.08) | 0.1984 | 3.10 (2.52-3.82) | <0.0001 | 0.87 (0.81-0.94) | 0.0002 | 1.19 (1.06-1.34) | 0.0036 | 1.03 (0.71-1.50) | 0.8602 | 0.76 (0.60-0.96) | 0.0194 | 0.93 (0.82-1.05) | 0.2303 |
| Primary melanocytic lesions | 281 | 174 | 0.35 (0.18-0.67) | 0.0015 | 0.75 (0.43-1.30) | 0.3076 | 0.94 (0.69-1.28) | 0.6898 | 1.09 (0.56-2.15) | 0.7966 | ** (**-**) | ** | 0.98 (0.31-3.11) | 0.9780 | 1.00 (0.62-1.59) | 0.9860 |
| Lymphoma | 19,855 | 13,519 | 3.64 (2.56-5.18) | <0.0001 | 6.93 (4.90-9.81) | <0.0001 | 0.94 (0.91-0.97) | 0.0002 | 1.15 (1.08-1.22) | <0.0001 | 1.06 (0.85-1.32) | 0.6138 | 0.79 (0.73-0.86) | <0.0001 | 0.88 (0.84-0.94) | <0.0001 |
| Other hematopoietic neoplasms | 209 | 97 | ** (**-**) | ** | ** (**-**) | ** | 1.02 (0.68-1.54) | 0.9174 | 0.81 (0.49-1.35) | 0.4276 | 1.84 (0.44-7.65) | 0.4009 | 1.11 (0.40-3.08) | 0.8393 | 0.72 (0.37-1.38) | 0.3193 |
| Germ cell tumors | 3,569 | 518 | 1.00 (0.83-1.21) | 0.9997 | 2.08 (1.55-2.78) | <0.0001 | 1.30 (1.08-1.58) | 0.0069 | 0.87 (0.63-1.21) | 0.4191 | 1.59 (0.59-4.27) | 0.3558 | 1.03 (0.76-1.41) | 0.8313 | 1.08 (0.87-1.33) | 0.4991 |
| Tumors of the pituitary | 172,615 | 20,558 | 2.04 (1.30-3.22) | 0.0021 | 20.62 (13.15-32.33) | <0.0001 | 0.75 (0.73-0.77) | <0.0001 | 1.14 (1.10-1.18) | <0.0001 | 0.91 (0.76-1.08) | 0.2737 | 0.63 (0.58-0.69) | <0.0001 | 0.71 (0.68-0.75) | <0.0001 |
| Craniopharyngioma | 8,005 | 1,673 | 1.99 (1.58-2.50) | <0.0001 | 6.61 (5.43-8.05) | <0.0001 | 0.86 (0.78-0.95) | 0.0028 | 1.73 (1.55-1.93) | <0.0001 | 1.54 (0.94-2.53) | 0.0863 | 0.63 (0.46-0.85) | 0.0030 | 1.03 (0.88-1.20) | 0.7045 |
| Hemangioma | 8,709 | 957 | 1.49 (0.76-2.89) | 0.2436 | 11.15 (5.98-20.82) | <0.0001 | 0.65 (0.57-0.73) | <0.0001 | 1.45 (1.20-1.76) | 0.0001 | ** (**-**) | ** | 0.64 (0.44-0.95) | 0.0252 | 0.80 (0.65-0.99) | 0.0426 |
| Neoplasm, unspecified | 28,825 | 15,949 | 0.73 (0.63-0.85) | <0.0001 | 5.53 (4.87-6.29) | <0.0001 | 0.94 (0.91-0.97) | <0.0001 | 0.74 (0.70-0.78) | <0.0001 | 0.71 (0.57-0.88) | 0.0019 | 0.80 (0.72-0.89) | <0.0001 | 0.64 (0.61-0.68) | <0.0001 |
| All other | 1,260 | 206 | 1.53 (0.78-3.03) | 0.2182 | 9.22 (5.23-16.26) | <0.0001 | 0.67 (0.51-0.89) | 0.0057 | 1.11 (0.71-1.72) | 0.6452 | 3.36 (0.83-13.61) | 0.0901 | 0.49 (0.16-1.54) | 0.2223 | 0.95 (0.64-1.41) | 0.8008 |
Reference group is Children (<14 years) as defined by the National Cancer Institute, see: http://www.cancer.gov/researchandfunding/snapshots/pediatric.
Reference group is males.
Reference group is White non-Hispanic.
Adolescents and Young Adults (AYA), as defined by the National Cancer Institute, see: http://www.cancer.gov/cancertopics/aya.
** Cannot be calculated.
-- Survival estimates are not presented when fewer than 100 cases were reported for the specific category.
Abbreviations: AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; HR: Hazard Ratio; NOS, not otherwise specified; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
AYA (15-39 years) had better overall survival as compared to children ages 0-14 years old in almost half of the histopathologies evaluated. Children and AYA age groups had similar survival in germ cell tumors.
Older adults (40+ years old) had poorer survival than children (0-14 years) in nearly every histopathology with the exception of primary melanocytic lesions.
Females generally had better survival outcomes as compared to males with the exception of glioblastoma, glioma malignant, NOS, embryonal tumors, other hemopoietic neoplasms, and germ cell tumors.
Individuals who are Black, non-Hispanic had poorer survival outcomes as compared to individuals who are White, non-Hispanic with the exception of glioblastoma, unique astrocytoma variants, other neuroepithelial tumors, other hemopoietic neoplasms, germ cell tumors, and neoplasm unspecified.
Individuals who are AIAN, non-Hispanic had poorer survival as compared to individuals who are White, non-Hispanic in many histopathologies, though the small size of this population meant that many of these associations were non-significant.
Being an API, non-Hispanic individual was associated with improved survival in many histopathologies as compared to individuals who were White, Non-Hispanic, though many of these associations were non-significant.
Hispanic ethnicity was associated with improved survival in most histopathologies.
Many other published survival estimates (including many of those previously published by CBTRUS83-85) incorporate treatment patterns which may explain differences between these population-level estimates and other published estimates.
When interpreting these results, it is important to remember that these models do not incorporate important factors that affect survival such as treatment patterns, health insurance, or type of facility at which an individual received treatment, all of which may be associated with these demographic factors as well as overall survival.
Relative Survival Rates for Brain and Other CNS Tumors by Site and Behavior
Relative survival estimates by site and behavior are presented in Table 25 and Supplementary Table 12.
Table 25.
One-, Five-, and Ten-Year Relative Survival Ratesa,b with 95% Confidence Intervals for Brain and Other Central Nervous System Tumors by Site and Behavior, CBTRUS Statistical Report: NPCR, 2001-2018 (varying)
| Site (ICD-O Topography Code) | All (2004-2018) | Malignant (2001-2018)c | Non-Malignant (2004-2018)d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ne | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) | Ne | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) | Nf | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) | |
| Olfactory tumors of the nasal cavity (C30.0)g | 1,767 | 91.2 (89.6-92.5) | 79.8 (77.3-82.1) | 72.0 (68.5-75.3) | 1,793 | 92.5 (91.0-93.7) | 81.7 (79.3-83.8) | 73.2 (69.9-76.2) | -- | -- | -- | -- |
| Meninges (cerebral and spinal) (C70.0-C70.9) | 383,894 | 93.2 (93.1-93.3) | 87.9 (87.7-88.1) | 83.1 (82.8-83.4) | 6,024 | 83.7 (82.7-84.7) | 67.2 (65.8-68.7) | 60.1 (58.3-61.9) | 378,284 | 93.4 (93.3-93.5) | 88.3 (88.1-88.5) | 83.6 (83.3-83.8) |
| Cerebral meninges (C70.0) | 313,886 | 93.1 (93.0-93.2) | 87.8 (87.6-88.0) | 82.8 (82.5-83.1) | 4,351 | 84.7 (83.5-85.8) | 67.2 (65.5-68.9) | 59.9 (57.7-62.0) | 309,834 | 93.3 (93.2-93.4) | 88.1 (87.9-88.3) | 83.2 (82.9-83.5) |
| Spinal meninges (C70.1) | 17,249 | 97.5 (97.1-97.8) | 96.1 (95.4-96.7) | 94.0 (92.7-95.1) | 446 | 85.6 (81.8-88.7) | 75.3 (70.1-79.8) | 71.1 (64.5-76.8) | 16,827 | 97.8 (97.5-98.1) | 96.7 (96.0-97.3) | 94.8 (93.4-95.9) |
| Meninges, NOS (C70.9) | 52,759 | 92.0 (91.8-92.3) | 85.9 (85.4-86.3) | 81.1 (80.3-81.8) | 1,227 | 79.5 (77.0-81.8) | 64.2 (60.8-67.4) | 56.8 (52.7-60.7) | 51,623 | 92.3 (92.1-92.6) | 86.5 (86.0-86.9) | 81.8 (81.0-82.5) |
| Cerebrum (C71.0) | 17,763 | 58.0 (57.2-58.7) | 38.1 (37.3-38.9) | 34.3 (33.5-35.2) | 15,433 | 52.8 (52.0-53.6) | 29.7 (29.0-30.5) | 26.1 (25.2-26.9) | 3,142 | 89.0 (87.7-90.1) | 84.6 (83.0-86.2) | 80.5 (78.1-82.6) |
| Frontal, temporal, parietal, and occipital lobes of the brain (C71.1-C71.4) | 189,945 | 59.9 (59.7-60.2) | 31.9 (31.6-32.1) | 26.4 (26.2-26.7) | 174,036 | 57.9 (57.6-58.1) | 27.4 (27.2-27.6) | 21.5 (21.3-21.8) | 19,565 | 90.9 (90.4-91.3) | 86.9 (86.3-87.5) | 83.4 (82.5-84.2) |
| Frontal lobe (C71.1) | 81,943 | 62.2 (61.9-62.5) | 37.0 (36.7-37.4) | 30.5 (30.1-30.9) | 75,551 | 61.2 (60.9-61.6) | 34.3 (33.9-34.6) | 27.1 (26.7-27.5) | 7,927 | 89.3 (88.5-90.0) | 84.3 (83.3-85.3) | 79.8 (78.3-81.2) |
| Temporal lobe (C71.2) | 60,591 | 60.8 (60.4-61.2) | 29.1 (28.7-29.5) | 24.4 (24.0-24.9) | 54,708 | 58.0 (57.6-58.4) | 23.1 (22.7-23.5) | 17.8 (17.4-18.2) | 6,753 | 93.8 (93.1-94.4) | 91.0 (90.1-91.8) | 89.0 (87.8-90.1) |
| Parietal lobe (C71.3) | 37,208 | 54.0 (53.5-54.5) | 25.4 (24.9-25.9) | 20.9 (20.4-21.5) | 34,965 | 51.4 (50.9-52.0) | 20.7 (20.2-21.2) | 16.2 (15.8-16.7) | 3,407 | 88.5 (87.2-89.6) | 84.2 (82.6-85.7) | 80.2 (78.0-82.3) |
| Occipital lobe (C71.4) | 10,203 | 58.2 (57.2-59.1) | 30.1 (29.1-31.1) | 26.0 (24.9-27.1) | 8,812 | 53.9 (52.8-55.0) | 22.0 (21.1-23.0) | 17.9 (17.0-18.9) | 1,478 | 91.5 (89.8-92.9) | 88.5 (86.1-90.4) | 83.8 (80.4-86.6) |
| Ventricle (C71.5) | 10,613 | 86.4 (85.7-87.0) | 78.9 (78.0-79.8) | 74.9 (73.8-76.0) | 5,006 | 76.7 (75.5-77.9) | 64.0 (62.6-65.4) | 59.3 (57.7-60.9) | 6,041 | 94.5 (93.8-95.1) | 91.6 (90.6-92.4) | 88.7 (87.3-90.0) |
| Cerebellum (C71.6) | 23,174 | 88.4 (87.9-88.8) | 79.1 (78.5-79.7) | 75.5 (74.8-76.3) | 15,597 | 85.8 (85.3-86.4) | 72.8 (72.1-73.6) | 68.2 (67.3-69.0) | 9,167 | 94.9 (94.4-95.4) | 92.4 (91.6-93.2) | 90.4 (89.1-91.5) |
| Brain stem (C71.7) | 16,015 | 77.5 (76.8-78.1) | 62.2 (61.3-63.0) | 57.3 (56.3-58.2) | 13,608 | 72.5 (71.7-73.3) | 53.3 (52.4-54.2) | 48.3 (47.4-49.3) | 3,891 | 92.6 (91.7-93.5) | 88.4 (87.1-89.6) | 84.5 (82.5-86.3) |
| Other brain (C71.8-C71.9) | 77,468 | 53.2 (52.8-53.5) | 34.8 (34.4-35.1) | 30.7 (30.3-31.1) | 66,419 | 46.8 (46.4-47.2) | 25.5 (25.1-25.9) | 21.5 (21.1-21.8) | 14,869 | 85.7 (85.1-86.3) | 80.4 (79.6-81.2) | 76.1 (75.1-77.2) |
| Overlapping lesion of brain (C71.8) | 33,220 | 45.7 (45.2-46.3) | 21.4 (20.9-21.9) | 17.2 (16.8-17.8) | 32,458 | 44.1 (43.6-44.7) | 18.6 (18.1-19.0) | 14.3 (13.8-14.8) | 2,255 | 82.8 (81.0-84.4) | 77.3 (75.1-79.3) | 72.3 (69.6-74.9) |
| Brain, NOS (C71.9) | 44,248 | 58.8 (58.3-59.2) | 44.7 (44.2-45.2) | 40.9 (40.3-41.4) | 33,961 | 49.3 (48.7-49.8) | 32.1 (31.5-32.6) | 28.3 (27.7-28.8) | 12,614 | 86.3 (85.6-86.9) | 81.0 (80.1-81.8) | 76.8 (75.7-78.0) |
| Spinal cord and cauda equina (C72.0-C72.1) | 31,097 | 96.1 (95.8-96.3) | 93.0 (92.5-93.4) | 91.2 (90.6-91.8) | 10,029 | 89.9 (89.3-90.5) | 81.8 (80.9-82.7) | 78.0 (76.9-79.1) | 21,781 | 99.1 (98.9-99.2) | 98.5 (98.1-98.9) | 98.0 (97.2-98.6) |
| Spinal cord (C72.0) | 29,969 | 96.0 (95.8-96.3) | 92.9 (92.5-93.3) | 91.0 (90.3-91.6) | 9,794 | 89.9 (89.3-90.5) | 81.7 (80.8-82.6) | 77.9 (76.7-79.0) | 20,855 | 99.1 (98.9-99.2) | 98.6 (98.1-98.9) | 98.0 (97.1-98.6) |
| Cauda equina (C72.1) | 1,128 | 97.0 (95.6-98.0) | 94.8 (92.3-96.5) | 94.4 (91.7-96.3) | 235 | 89.8 (84.8-93.2) | 85.3 (78.8-90.0) | 83.5 (75.4-89.1) | 926 | 98.6 (97.2-99.3) | 97.6 (94.5-98.9) | 97.1 (93.8-98.7) |
| Cranial nerves (C72.2-C72.5) | 72,578 | 99.3 (99.2-99.4) | 99.3 (99.2-99.4) | 99.3 (99.2-99.4) | 4,386 | 97.3 (96.7-97.8) | 94.2 (93.3-94.9) | 93.1 (92.0-94.0) | 68,633 | 99.5 (99.4-99.6) | 99.5 (99.4-99.6) | 99.5 (99.4-99.6) |
| Olfactory nerve (C72.2) | 95 | 95.3 (87.2-98.3) | 93.2 (80.4-97.8) | 89.0 (72.3-95.8) | 34 | 91.7 (74.0-97.5) | 74.1 (51.7-87.3) | 66.7 (42.5-82.5) | 64 | 97.5 (84.8-99.6) | 97.5 (84.8-99.6) | 97.5 (84.8-99.6) |
| Optic nerve (C72.3) | 4,273 | 98.3 (97.8-98.7) | 96.0 (95.2-96.7) | 95.2 (94.2-96.1) | 3,931 | 98.0 (97.5-98.5) | 95.6 (94.8-96.3) | 94.8 (93.9-95.6) | 740 | 99.8 (88.8-100.0) | 97.8 (93.9-99.2) | 96.5 (91.0-98.6) |
| Acoustic nerve (C72.4) | 56,027 | 99.5 (99.4-99.6) | 99.5 (99.4-99.6) | 99.5 (99.4-99.6) | 148 | 94.3 (88.3-97.3) | 93.2 (86.4-96.7) | 93.0 (81.6-97.4) | 55,918 | 99.5 (99.4-99.6) | 99.5 (99.4-99.6) | 99.5 (99.4-99.6) |
| Cranial nerve, NOS (C72.5) | 12,183 | 98.9 (98.6-99.2) | 98.7 (98.3-99.0) | 98.7 (98.3-99.0) | 273 | 88.8 (84.0-92.2) | 75.8 (69.5-81.0) | 71.6 (63.4-78.2) | 11,911 | 99.3 (99.0-99.5) | 99.2 (98.8-99.5) | 99.2 (98.8-99.5) |
| Other nervous system (C72.8-C72.9) | 6,083 | 79.8 (78.7-80.8) | 72.3 (70.9-73.6) | 67.8 (66.0-69.4) | 3,075 | 63.7 (62.0-65.5) | 50.4 (48.3-52.4) | 44.3 (41.9-46.6) | 2,985 | 97.3 (96.6-98.0) | 94.7 (93.4-95.8) | 91.4 (89.3-93.1) |
| Overlapping lesion of brain & CNS (C72.8) | 786 | 74.8 (71.4-77.8) | 66.0 (62.0-69.7) | 60.7 (55.5-65.5) | 447 | 61.0 (56.1-65.5) | 45.4 (40.0-50.5) | 37.2 (31.0-43.3) | 346 | 95.1 (91.7-97.2) | 90.5 (85.5-93.9) | 87.4 (78.8-92.7) |
| Nervous system, NOS (C72.9) | 5,297 | 80.5 (79.4-81.6) | 73.2 (71.8-74.5) | 68.8 (67.0-70.5) | 2,628 | 64.2 (62.3-66.1) | 51.2 (49.0-53.4) | 45.4 (42.8-48.0) | 2,639 | 97.6 (96.8-98.2) | 95.2 (93.8-96.2) | 91.9 (89.8-93.7) |
| Pituitary and craniopharyngeal duct (C75.1- C75.2) | 183,633 | 97.9 (97.8-98.0) | 96.3 (96.2-96.5) | 94.3 (94.0-94.5) | 1,320 | 87.4 (85.3-89.2) | 76.3 (73.5-78.9) | 69.2 (65.6-72.4) | 182,453 | 98.0 (97.9-98.1) | 96.5 (96.3-96.7) | 94.5 (94.2-94.7) |
| Pituitary gland (C75.1) | 178,233 | 98.1 (98.0-98.2) | 96.7 (96.5-96.9) | 94.8 (94.5-95.1) | 1,295 | 87.6 (85.6-89.4) | 76.7 (73.9-79.3) | 69.5 (65.9-72.9) | 177,078 | 98.2 (98.1-98.2) | 96.9 (96.7-97.0) | 95.0 (94.7-95.3) |
| Craniopharyngeal duct (C75.2) | 5,400 | 92.4 (91.6-93.1) | 84.6 (83.4-85.8) | 77.6 (75.9-79.2) | -- | -- | -- | -- | 5,375 | 92.5 (91.7-93.2) | 84.8 (83.6-85.9) | 77.8 (76.1-79.4) |
| Pineal (C75.3) | 4,312 | 91.9 (91.0-92.7) | 82.7 (81.4-84.0) | 78.4 (76.7-80.0) | 2,942 | 90.0 (88.9-91.1) | 77.2 (75.4-78.8) | 71.6 (69.6-73.5) | 1,678 | 94.6 (93.2-95.6) | 91.3 (89.4-92.9) | 88.7 (85.9-90.9) |
aThe cohort analysis of survival rates was utilized for calculating the survival estimates presented in this table. Long-term cohort-based survival estimates reflect the survival experience of individuals diagnosed over the time period, and they may not necessarily reflect the long-term survival outlook of newly diagnosed cases.
bRates are an estimate of the percentage of patients alive at one, two, five, and ten years, respectively.
cAssigned behavior code of /3 (see Table 2).
dAssigned behavior code of /0 or /1 (see Table 2)
eTotal number of cases that occurred within the included NPCR and SEER registries between 2001 and 2018.
fTotal number of cases that occurred within the included NPCR and SEER registries between 2004 and 2018.
gICD-O-3 histopathology codes 9522-9523 only.
-- Rates were not presented for categories with 50 or fewer cases and were suppressed for rates where fewer than 16 cases were surviving within a category.
** Confidence interval could not be calculated.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NCI, National Cancer Institute; NOS, not otherwise specified; NPCR, National Program of Cancer Registries; RS, Relative Survival; SEER, Surveillance, Epidemiology, and End Results Program.
The highest overall five-year survival was for tumors occurring in the acoustic nerves (99.5%).
The lowest overall five-year survival was for tumors occurring in the overlapping lesion of the brain (21.4%).
The five-year survival for malignant tumors by site ranged from 18.6% (tumors in the overlapping lesion of the brain) to 95.6% (tumors in the optic nerves).
The five-year survival for non-malignant tumors ranged from 72.3% (tumors in the overlapping lesion of the brain) to 99.5% (tumors in the cranial nerves and acoustic nerve).
Relative Survival Rates for Brain and Other CNS Tumors by Histopathology, Behavior and Age Groups
Relative survival estimates for brain and other CNS tumors by histopathology, behavior, and age at diagnosis are shown in Table 11 and Supplementary Table 13.
There was large variation in survival estimates for all ages depending upon tumor histopathology; five-year survival rates were 99.2% for tumors of the pituitary and 6.9% for glioblastoma.
Survival generally decreased with older age at diagnosis; children and young adults generally had better survival outcomes for most histopathologies.
Among predominantly non-malignant histopathologies, five-year survival was lowest in primary melanocytic lesions which had five-year relative survival of 66.5%.
Among predominantly non-malignant histopathologies, five-year survival was highest in nerve sheath tumors which had five-year relative survival of 99.3%.
In general, relative survival in most histopathologies was higher in adolescents and young adults ages 15-39 years as compared to children and adults of all other ages.
Strengths and Limitations of Cancer Registry Data
CBTRUS, in collaboration with the CDC and NCI, is the largest population-based registry focused exclusively on primary brain and other CNS tumors in the United States and represents cases collected from the entire US population. The CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019 contains the most up-to-date population-based data on all primary brain tumor and other CNS tumors available through the cancer surveillance system in the United States.
Registration of individual cases is conducted by cancer registrars at the institution where diagnosis or treatment occurs and is then transmitted to the CCR, which further transmits this information to NPCR and/or SEER. CCRs, those contributing data to NPCR and to SEER, only report cases to the CDC and NCI for persons who are residents of that particular state, so duplicate records should not occur for persons who may have traveled across state lines for treatment. As a result, the CBTRUS dataset is a complete recording of all reported cases for the time period examined, 2015-2019, with minimal duplicates.
Currently, there is no publicly available data source for the collection of survival and outcomes data from all geographic regions in the United States via the cancer registry system. Survival data used for this report are collected by NPCR for 42 of the 51 CCRs in the United States—primarily through linkage with death certificate and other administrative records—and by SEER for the remaining CCRs—through active and passive methods—and the feasibility of these data for use in survival studies has been evaluated86,87 and shown to produce reliable and robust estimates of cancer survival. Use of passive follow-up with record linkage may result in overestimation of survival in some populations, such as those whose members are more likely to leave the state or country.
No mechanism currently exists for central pathology review of cases within the US cancer registry system, and histopathology code assignment at case registration is based on histopathology information contained in the patient’s medical record. The WHO Classification of Tumours of the Central Nervous System was revised in 1993, 2000, 2007, 2016, and 2021.2,19,20,88,89 As of 2018, the US cancer registry system uses the 2016 classification for data abstraction, but tumors included in this report may have been diagnosed using any of the available classifications prior to 2016 due to the variation in adoption of new standards by individual physicians and medical practices. As a result, histopathologies are reflective of the prevailing criteria for the histopathology at the time of case registration. This means that despite changes to the histopathology schema that may occur over time, it is not possible, without additional variables, to go back and reclassify tumors based on the new criteria. In addition to changes in histopathologic criteria over time, there is significant inter-rater variability in histopathological diagnosis of glioma.90,91 This also means that incomplete, incorrect, or alternatively stated diagnoses included in a pathology report or other medical record may result in an incorrect reporting of the details of an individual case. For example, an anaplastic oligodendroglioma recorded in a pathology record as oligodendroglioma WHO grade III may be incorrectly recorded as an oligodendroglioma when the accurate category is an anaplastic oligodendroglioma.
US cancer registration requires the reporting of cases that are confirmed by different types of diagnostic procedures, including both histopathologic confirmation (where surgery was performed and the diagnosis confirmed on a tissue specimen by a pathologist) and radiographic confirmation (where diagnosis was made based solely on imaging criteria, such as an MRI, CT scan, or X-ray). Only histopathologic confirmation allows certainty on the assignment of a specific histopathology as well as for an assignment of a WHO grade. Many tumors have unique characteristics that make them identifiable on imaging, and thereby qualify as a valid type of diagnostic procedure, but it is important to consider the decreased level of certainty of specifying the correct histopathology in these tumors.
CONCLUDING COMMENT
The CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019 comprehensively describes the most up to date (October 2022) population-based incidence, mortality, and relative survival of primary malignant and non-malignant brain and other CNS tumors collected and reported by central cancer registries covering the entire US population. This report aims to serve as a useful resource for researchers, clinicians, patients, and families. CBTRUS continually revises its reports to reflect the current collection and reporting practices of the broader surveillance community in which it works, while integrating the input it receives from the clinical and research communities, especially from neuropathologists, when possible. In this way, CBTRUS facilitates communication between the cancer surveillance and the brain tumor research and clinical communities and contributes meaningful insight into the descriptive epidemiology of all primary brain and other CNS tumors in the United States.92
CBTRUS Mission
CBTRUS is a not-for-profit corporation committed to providing a resource for gathering and disseminating current epidemiologic data on all primary brain and other central nervous system tumors, benign and malignant, for the purposes of accurately describing their incidence and survival patterns, evaluating diagnosis and treatment, facilitating etiologic studies, establishing awareness of the disease, and, ultimately, for the prevention of all brain tumors.
Disclaimer
CBTRUS is a not-for-profit corporation which gathers and disseminates epidemiologic data on primary brain and other central nervous system tumors to facilitate research and establish awareness of the disease. CBTRUS makes no representations or warranties, and gives no other assurances or guarantees, express or implied, with respect to the accuracy or completeness of the data presented. The information provided in this report is not intended to assist in the evaluation, diagnosis, or treatment of individual diseases. Persons with questions regarding individual diseases should contact their own physician to obtain medical assistance. The contents in this report are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or of the NCI.
Jill S. Barnholtz-Sloan, Ph.D. is a full-time paid employee of the NIH/NCI. Gino Cioffi, M.P.H. and Kristin A. Waite, Ph.D. are full-time contractors of the NIH/NCI.
Supplementary Material
ACKNOWLEDGMENTS
This report was prepared by the CBTRUS Co-Scientific Principal Investigator, Quinn T. Ostrom, Ph.D., M.P.H. and her research team from Duke University School of Medicine in collaboration with Co-Scientific Principal Investigator Jill S. Barnholtz-Sloan, Ph.D., her research staff affiliated with the NCI, DCEG, whose services were provided by DCEG, and CBTRUS President and Chief Mission Officer Carol Kruchko, collectively known as the CBTRUS Team. The CBTRUS data presented in this report were provided through an agreement with the CDC, NPCR. In addition, CBTRUS used data from the research data files of the NCI, SEER Program. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general.
We acknowledge the efforts of the tumor registrars at hospitals and treatment centers, the CCRs, and the staff from the NPCR and SEER programs, whose efforts to collect accurate and complete data have made this report possible. We are also grateful to our Consulting Neuropathologists, Drs. Janet Bruner, Roger McLendon, Tarik Tihan, and Daniel Brat, who answered our questions and provided feedback throughout the year, to our Board of Directors and Advisors, and especially Drs. Melissa Bondy, Elizabeth Claus, and Nancy Stroup and to Claudia Smits, L.L.M., Reda J. Wilson, M.P.H., C.T.R., and Mary Elizabeth O’Neil, M.P.H.
Glossary
Abbreviations
- AAAIR
Average Annual Age-Adjusted Incidence Rate
- AAAMR
Average Annual Age-Adjusted Mortality Rate
- ABTA
American Brain Tumor Association
- ACVR1
Activin A Receptor, Type I
- AIAN
American Indian/Alaskan Native
- AJCC
American Joint Commission on Cancer
- APC
Annual Percent Change
- API
Asian or Pacific Islander
- AYA
Adolescents and Young Adults
- ATRT
Atypical Teratoid/Rhabdoid Tumor
- CBTRUS
Central Brain Tumor Registry of the United States
- CCR
Central Cancer Registry
- CDC
Centers for Disease Control and Prevention
- CI
Confidence Interval
- CNS
Central Nervous System
- CS
Collaborative Staging
- DIPG
Diffuse Intrinsic Pontine Glioma
- IACR
International Agency for Cancer Research
- ICD-O-3
International Classification of Diseases for Oncology, Third Edition
- ICCC
International Classification of Childhood Cancer
- IDH1/2
Isocitrate Dehydrogenase 1/2
- IRR
Incidence Rate Ratio
- MGMT
O-6-Methylguanine-DNA Methyltransferase
- NAACCR
North American Association of Central Cancer Registries
- NCHS
National Center for Health Statistics
- NCI
National Cancer Institute
- NOS
Not Otherwise Specified
- NPCR
National Program of Cancer Registries
- NPCR-CSS
NPCR Cancer Surveillance System
- NVSS
National Vital Statistics System
- PDGFRA
Platelet-derived Growth Factor Receptor A
- PI3KCA
Phosphatidylinositol 3-Kinase Catalytic subunit Alpha
- SEER
Surveillance, Epidemiology, and End Results
- SHH
Sonic Hedgehog
- SSDI
Site-Specific Data Items
- SSF
Site-Specific Factors
- SSF 4
Promoter methylation status of O-6-Methylguanine-DNA Methyltransferase
- SSF 5
Deletion of the 1p
- SSF 6
Deletion of 19q
- TP53
Tumor Protein p53
- UDS
Uniform Data Standards
- US
United States
- USCS
United States Cancer Statistics
- VACCR
Veterans Affairs Central Cancer Registry
- VHA
Veterans Health Administration
- WHO
World Health Organization
- WNT
Wingless
Contributor Information
Quinn T Ostrom, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA; Duke Cancer Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Mackenzie Price, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Corey Neff, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Gino Cioffi, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA.
Kristin A Waite, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Jill S Barnholtz-Sloan, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, Maryland, USA; Center for Biomedical Informatics & Information Technology (CBIIT), National Cancer Institute, Bethesda, Maryland, USA.
The CBTRUS Scientific Team
Quinn T. Ostrom, Ph.D., M.P.H., CBTRUS Co-Scientific -Principal Investigator, Assistant Professor, The Preston Robert Tisch Brain Tumor Center at Duke University Medical Center and Department of Neurosurgery, Duke University School of Medicine, Durham, NC
Jill S. Barnholtz-Sloan, Ph.D., CBTRUS Co-Scientific Principal Investigator, Associate Director and Senior Investigator, Center for Biomedical Informatics & Information Technology (CBIIT) and Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD
Gino Cioffi, M.P.H., Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD
Corey Neff, M.P.H., Department of Neurosurgery, Duke University School of Medicine, Durham, NC
Mackenzie Price, M.P.H., Department of Neurosurgery, Duke University School of Medicine, Durham, NC
Kristin A. Waite, Ph.D., Trans Divisional Research Program (TDRP), Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD
The CBTRUS Consulting Neuropathologists
Daniel J. Brat, M.D., Ph.D., Professor and Chair, Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, IL
Janet M. Bruner, M.D., MD Anderson Cancer Center, Houston, TX (Retired)
Roger E. McLendon, M.D., Professor, The Preston Robert Tisch Brain Tumor Center at Duke University Medical Center and Department of Pathology, Duke University Medical Center, Durham, NC
Tarik Tihan, M.D., Ph.D., Professor, Neuropathology Division, Department of Pathology, School of Medicine, University of California San Francisco (UCSF), San Francisco, CA
The CBTRUS Board of Directors
Carol Kruchko, President & Chief Mission Officer, Central Brain Tumor Registry of the United States, Hinsdale, IL
Steven Brem, M.D., Professor, Penn Neuroscience Center-Neurosurgery, Perelman Center for Advanced Medicine, Philadelphia, PA
Donald Segal, J.D., Treasurer, Segal McCambridge Singer & Mahoney, Ltd., Chicago, IL
Elizabeth B. Claus, M.D., Ph.D., Professor, Departments of Biostatistics and Neurosurgery, Yale University, New Haven, CT, Attending Neurosurgeon, Brigham and Women’s Hospital, Boston, MA
Fred H. Hochberg, M.D., Visiting Scientist, Department of Neurosurgery, University of California at San Diego, San Diego, CA
Margaret Wrensch, Ph.D., Professor, Neuroepidemiology Division, Department of Neurological Surgery, School of Medicine, University of California-San Francisco, San Francisco, CA
Darell D. Bigner, M.D., Ph.D. (Emeritus Member), Edwin L. Jones, Jr. and Lucille Finch Jones Cancer Research Professor, Department of Pathology, Emeritus Director, The Preston Robert Tisch Brain Tumor Center, Chief, Preuss Laboratory for Brain Tumor Research, Duke, University Medical Center, Durham, NC
L. Lloyd Morgan (Emeritus Member), Patient Advocate, Berkeley, CA.
The CBTRUS Board of Advisors
Hoda Anton-Culver, Ph.D., Distinguished Professor, Department of Medicine; Principal Investigator, All of Us Precision Medicine Research Program; Director, Genetic Epidemiology Research Institute, University of California-Irvine, Irvine, CA
Melissa Bondy, Ph.D., Professor and Chair, Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA
Jennifer Cullen, Ph.D., Professor, School of Medicine, Case Western Reserve University, Cleveland, OH
Faith Davis, Ph.D., Professor Emeritus, School of Public Health, University of Alberta, Edmonton, Canada
Roberta McKean-Cowdin, Ph.D., Associate Professor, Department of Epidemiology, University of Southern California, Los Angeles, CA
Nancy Stroup, Ph.D., Epidemiologist (Retired).
John Villano, M.D., Ph.D., Professor, Division of Medical Oncology, University of Kentucky Markey Cancer Center, Lexington, KY
Funding
CBTRUS is honored to be included among the research awardees of the following organizations which have contributed to the analyses resulting from the CBTRUS database in 2022: the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056 Amendment/Modification No:00002, the American Brain Tumor Association, Novocure, the Musella Foundation for Brain Tumor Research & Information, Inc. The Sontag Foundation, National Brain Tumor Society, the Uncle Kory Foundation, the Pediatric Brain Tumor Foundation, and the Zelda Dorin Tetenbaum Memorial Fund as well as private and in kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or of the NCI.
Selected CBTRUS Scientific Publications
Cote DJ, et al. “Glioma incidence and survival variations by county-level socioeconomic measures.” Cancer. 2019 Oct 1;125(19):3390-3400. doi: 10.1002/cncr.32328. PMID: 31206646; PMCID: PMC6744292.
This analysis of glioma incidence and survival based on county-levels of SES identifies a significant association between both increased incidence and improved survival for individuals with glioma in higher SES counties.
Dong M, et al. “Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis.” Cancer Epidemiol Biomarkers Prev. 2020 Jul;29(7):1389-1397. doi: 10.1158/1055-9965.EPI-20-0036. PMID: 32349967.
This analysis uses a pan-cancer approach to interrogate sex differences in cancer incidence and survival, with a special focus on brain and other CNS tumors.
Iorgulescu JB, et al. “Molecular Biomarker-Defined Brain Tumors: Epidemiology, Validity, and Completeness in the United States.” Neuro Oncol. 2022 Apr 23:noac113. doi: 10.1093/neuonc/noac113. Epub ahead of print. PMID: 35460555.
This analysis investigated the completeness and validity of the novel brain molecular markers (BMM) site-specific data item after its first year of collection.
Kruchko C, et al. “Cancer collection efforts in the United States provide clinically relevant data on all primary brain and other CNS tumors.” Neurooncol Pract. 2019 Sep;6(5):330-339. doi: 10.1093/nop/npz029. PMID: 31555447; PMCID: PMC6753356.
A summary of cancer registration efforts and data sources in the United States.
Kruchko C, et al. “The CBTRUS story: providing accurate population-based statistics on brain and other central nervous system tumors for everyone.” Neuro Oncol. 2018 Feb 19;20(3):295-298. doi: 10.1093/neuonc/noy006. PMID: 29471448; PMCID: PMC5817957.
A summary of the history and mission of the Central Brain Tumor Registry of the United States.
Low JT, et al. “Primary brain and other central nervous system tumors in the United States (2014-2018): A summary of the CBTRUS statistical report for clinicians.” Neurooncol Pract. 2022 Feb 22;9(3):165-182. doi: 10.1093/nop/npac015. PMID: 35601966; PMCID: PMC9113389.
A special, condensed CBTRUS Statistical Report designed to be a streamlined and useful resource for practicing clinicians.
Ostrom QT, et al. “CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018.” Neuro Oncol. 2022 Sep 6;24(Suppl 3):iii1-iii38. doi: 10.1093/neuonc/noac161. PMID: 36066969; PMCID: PMC9447434.
This special report, funded by the Pediatric Brain Tumor Foundation, presents incidence and survival statistics for children 0-14 using histopathology groupings that were re-organized to be a more accurate representation of clinical behavior in pediatric brain tumors.
Ostrom QT, et al. “American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012.” Neuro Oncol. 2016 Jan;18 Suppl 1(Suppl 1):i1-i50. doi: 10.1093/neuonc/nov297. PMID: 26705298; PMCID: PMC4690545.
This special report, funded by the American Brain Tumor Association, presents incidence and survival statistics for adolescents and young adults (ages 15-39).
Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020 Feb 20;22(2):298-300. doi: 10.1093/neuonc/noz202. PMID: 31637436; PMCID: PMC7442407.
This letter describes the history of inclusion of pilocytic astrocytoma in cancer registry reporting, and the effect of varying behavior classification for these tumors on incidence and survival patterns.
Patil N, et al. “Epidemiology of Brainstem High-Grade Gliomas in Children and Adolescents in the United States, 2000-2017.” Neuro Oncol. 2020 Dec 21:noaa295. doi: 10.1093/neuonc/noaa295. PMID: 33346835.
This manuscript details the descriptive epidemiology, including incidence, survival and prevalence, for gliomas of the brain stem in children and adolescents.
Truitt G, et al. “Partnership for defining the impact of 12 selected rare CNS tumors: a report from the CBTRUS and the NCI-CONNECT.” J Neurooncol. 2019 Aug;144(1):53-63. doi: 10.1007/s11060-019-03215-x. PMID: 31209773.
This analysis, completed in collaboration with the National Cancer Institute’s NCI-CONNECT program, presents incidence, survival, and prevalence estimates for a selection of rare tumor histopathologies that are the focus of the NCI-CONNECT program.
Wang, G, et al. “Importance of the intersection of age and sex to understand variation in incidence and survival for primary malignant gliomas.” Neuro Oncol. 2022 Feb 1;24(2):302-310. doi: 10.1093/neuonc/noab199. PMID: 34387331; PMCID: PMC8804884.
This manuscript assesses the relationship between age and sex on primary malignant glioma incidence and survival.
Waite KA, et al. “Aligning the Central Brain Tumor Registry of the United States (CBTRUS) histology groupings with current definitions.” Neurooncol Pract. 2022 Mar 24;9(4):317-327. doi: 10.1093/nop/npac025. PMID: 35859542; PMCID: PMC9290890.
This manuscript traces the rationale for changes made to the CBTRUS histopathology grouping scheme in order to better align it with modern diagnostic criteria.
Zhang AS, et al. “Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010.” Neuro Oncol. 2017 May 1;19(5):726-735. doi: 10.1093/neuonc/now252. PMID: 28039365; PMCID: PMC5464453.
This analysis presents a novel statistical method for estimating complete prevalence from geographically-limited cancer survival statistics, and includes age-specific survival estimates for the most common brain and CNS tumor histopathologies.
References
- 1. Kruchko C, Ostrom QT, Gittleman H, Barnholtz-Sloan JS. The CBTRUS story: providing accurate population-based statistics on brain and other central nervous system tumors for everyone. Neuro Oncol. 2018; 20(3):295-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN OH, Wiestler OD, Cavanee WK, ed WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 3. Waite KA, Cioffi G, Kruchko C, et al. Aligning the Central Brain Tumor Registry of the United States (CBTRUS) histology groupings with current definitions. Neurooncol. Pract. 2022; 9(4):317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). National Program of Cancer Registries Cancer Surveillance System Rationale and Approach. 1999; http://www.cdc.gov/cancer/npcr/pdf/npcr_css.pdf. Accessed July 21, 2020.
- 5. Cancer Registries Amendment Act, 102nd Cong. § 515 (1992). https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3372.pdf. Accessed July 21, 2020.
- 6. Benign Brain Tumor Cancer Registries Amendment Act, 107th Cong. § 260 (2002). http://www.gpo.gov/fdsys/pkg/PLAW-107publ260/pdf/PLAW-107publ260.pdf. Accessed July 21, 2020.
- 7. National Cancer Institute. Overview of the SEER Program.http://seer.cancer.gov/about/overview.html. Accessed July 21, 2020.
- 8. Centers for Disease Control and Prevention National Center for Health Statistics. National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: U.S. Cancer Statistics Incidence Analytic Database – 2001-2018. United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Based on the November 2020 submission. 2021. [Google Scholar]
- 9. Surveillance Research Program - National Cancer Institute. ICD-0-3 SEER Site/Histology Validation List. 2019; https://seer.cancer.gov/icd-o-3/sitetype.icdo3.20190618.pdf. Accessed July 14, 2020.
- 10. Fritz A PC, Jack A, Shanmugaratnam K, Sobin L, Perkin DM, Whelan S ed International Classification of Diseases for Oncology, Third edition: World Health Organization; 2000. [Google Scholar]
- 11. McCarthy BJ, Surawicz T, Bruner JM, Kruchko C, Davis F. Consensus Conference on Brain Tumor Definition for registration. November 10, 2000. Neuro Oncol. 2002;4(2):134-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surveillance Epidemiology aERSP. ICCC Recode Third Edition ICD-O-3/IARC 2017.2017; https://seer.cancer.gov/iccc/iccc-iarc-2017.html. Accessed July 1, 2021.
- 13. International Incidence of Childhood Cancer, Volume III. Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 14. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005; 103(7):1457-1467. [DOI] [PubMed] [Google Scholar]
- 15. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition: World Health Organization; 2007. [Google Scholar]
- 16. Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009; 6(5):359-362. [DOI] [PubMed] [Google Scholar]
- 17. Ostrom QT, Gittleman H, Kruchko C, et al. Completeness of required site-specific factors for brain and CNS tumors in the Surveillance, Epidemiology and End Results (SEER) 18 database (2004-2012, varying). J. Neurooncol. 2016; 130(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iorgulescu JB, Sun C, Neff C, et al. Molecular Biomarker-Defined Brain Tumors: Epidemiology, Validity, and Completeness in the United States. Neuro Oncol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleihues P, Cavenee W, eds. Tumours of the nervous system: World Health Organization classification of tumours. Lyon, France: IARC Press; 2000. [Google Scholar]
- 20. Louis D, Wiestler O, Cavanee W, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 21. American Joint Committee on Cancer. Collaborative Stage Data Collection System Version 02.05. 2020; https://cancerstaging.org/cstage/schema/Pages/version0205.aspx. Accessed July 12, 2020.
- 22. Lym RL, Ostrom QT, Kruchko C, et al. Completeness and concordancy of WHO grade assignment for brain and central nervous system tumors in the United States, 2004-2011. J. Neurooncol. 2015; 123(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16Suppl 4:iv1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferlay J, Rous B. Histological groups. In: Bray F, Colombet M, Ferlay J, et al., eds. Cancer Incidence in Five Continents Volume XI 2019. [Google Scholar]
- 25. Surveillance Epidemiology and End Results (SEER) Program. Solid Tumor Rules: Non-Malignant CNS2019; https://seer.cancer.gov/tools/solidtumor/Non_Malignant_CNS_STM.pdf. Accessed July 21, 2020.
- 26. Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020; 22(2):298-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ostrom QT, Price M, Ryan K, et al. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States 2014-2018. Neuro Oncol. 2022;24(Suppl 3):iii1-iii38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2019) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2021. Underlying mortality data provided by NCHS (www.cdc.gov/nchs). 2021.
- 29. Standards for Cancer Registries Volume III: Standards for Completeness, Quality, Analysis, Management, Security and Confidentiality of Data. 2008; https://www.naaccr.org/wp-content/uploads/2016/11/Standards-for-Completeness-Quality-Analysis-Management-Security-and-Confidentiality-of-Data-August-2008PDF.pdf. Accessed July 21, 2020.
- 30. Bray F, Ferlay J. Data Comparability and Quality. Cancer Incidence in Five Continents, Vol. XI 2017; https://ci5.iarc.fr/CI5-XI/Pages/Chapter5.aspx. Accessed July 21, 2020. [Google Scholar]
- 31. R Core Team. R: A language and environment for statistical computing.2021; http://www.r-project.org/. Accessed April 20, 2021.
- 32. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.4.0.2022; www.seer.cancer.gov/seerstat. Accessed May 27, 2022.
- 33. Luo J. SEER2R: reading and writing SEER*STAT data files. R package version 1.0.2012; http://cran.r-project.org/package=SEER2R. Accessed July 21, 2020.
- 34. Gohel D, Hangler F, Sander L, et al. officer: Manipulation of Microsoft Word and PowerPoint Documents. 2020; https://cloud.r-project.org/web/packages/officer/index.html. Accessed July 21, 2020.
- 35. Gohel D, Fazilleau Q, Nazarov M, Robert T, Barrowman M, Yasumoto A. flextable: Functions for Tabular Reporting.2020; https://davidgohel.github.io/flextable/. Accessed July 21, 2020.
- 36. Hočevar T, Demšar J. Computation of Graphlet Orbits for Nodes and Edges in Sparse Graphs. J. Stat. Softw. 2016; 71(10):24. [Google Scholar]
- 37. Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. J. Open Source Softw. 2019; 4(43):1686. [Google Scholar]
- 38. Sievert C. Interactive Web-Based Data Visualization with R, plotly, and shiny.2020; https://plotly-r.com/. Accessed July 21, 2020.
- 39. Xie Y. knitr: A General-Purpose Package for Dynamic Report Generation in R. R package version 1.29.2020; https://yihui.org/knitr/. Accessed July 21, 2020.
- 40. Walker K, Rudis B. tigris: Load Census TIGER/Line Shapefiles.2020; https://github.com/walkerke/tigris. Accessed May 27, 2022.
- 41. Kassambara A, Kosinski M, Biecek P, Fabian S. survminer: Drawing Survival Curves using ‘ggplot2’. 2020; https://rpkgs.datanovia.com/survminer/index.html. Accessed May 27, 2022.
- 42. Pebesma E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018; 10(1):439–446. [Google Scholar]
- 43. NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. September 2012; https://www.naaccr.org/wp-content/uploads/2016/11/NHIA-v2.2.1.pdf. Accessed July 21, 2020.
- 44. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Populations - Total U.S. (1990-2019) - Linked To County Attributes - Total U.S., 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2020. 2020; http://seer.cancer.gov/popdata/.
- 45. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat. Methods Med. Res. 2006;15(6):547-569. http://www.ncbi.nlm.nih.gov/pubmed/17260923. [DOI] [PubMed] [Google Scholar]
- 46. Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006; 62(3):847-854. [DOI] [PubMed] [Google Scholar]
- 47. Joinpoint Regression Program, Version 4.10.0.0; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. 2022; https://surveillance.cancer.gov/joinpoint/. Accessed June 1, 2022.
- 48. Zhu L, Pickle LW, Ghosh K, et al. Predicting US- and state-level cancer counts for the current calendar year: Part II: evaluation of spatiotemporal projection methods for incidence. Cancer. 2012; 118(4):1100-1109. [DOI] [PubMed] [Google Scholar]
- 49. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000; 19(3):335-351. [DOI] [PubMed] [Google Scholar]
- 50. Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014; 120(9):1290-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil. Med. 2012; 177(6):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J. Natl. Cancer Inst. 2002;94(20):1537-1545. http://www.ncbi.nlm.nih.gov/pubmed/12381706. [DOI] [PubMed] [Google Scholar]
- 53. Midthune DN, Fay MP, Clegg LX, Feuer EJ. Modeling Reporting Delays and Reporting Corrections in Cancer Registry Data. J. Am. Stat. Assoc. 2005; 100(469):61-70. [Google Scholar]
- 54. Surveillance Epidemiology and End Results (SEER) Program. Cancer Incidence Rates Adjusted for Reporting Delay.2020; http://surveillance.cancer.gov/delay/. Accessed July 21, 2020.
- 55. Li XR, Kruchko C, Wu XC, et al. Are Benign and Borderline Brain Tumors Underreported? J. Registry. Manag. 2016; 43(4):187-194. [PubMed] [Google Scholar]
- 56. Anderson RN, Rosenberg HM.. Report of the Second Workshop on Age Adjustment Dec 1998. 0083-2073 (Print); 0083-2073. [PubMed] [Google Scholar]
- 57. Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl. Vital Stat. Rep. 1998; 47(3):1-16, 20. [PubMed] [Google Scholar]
- 58. L. D, Johnson C, Adams S, Negoita S. Solid Tumor Rules.2018; https://seer.cancer.gov/tools/solidtumor/. Accessed July 21, 2020.
- 59. Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards B.. The 2007 Multiple Primary and Histology Coding Rules. Bethesda, MD: National Cancer Institute;2007. [Google Scholar]
- 60. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022; 72(1):7-33. [DOI] [PubMed] [Google Scholar]
- 61. Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017; 14(7):434-452. [DOI] [PubMed] [Google Scholar]
- 62. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl. Cancer Inst. 1998; 90(19):1473-1479. [DOI] [PubMed] [Google Scholar]
- 63. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013; 31(3):337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vogelbaum MA, Hu C, Peereboom DM, et al. Phase II trial of pre-irradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: long term results of RTOG BR0131. J. Neurooncol. 2015; 124(3):413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013; 31(3):344-350. [DOI] [PubMed] [Google Scholar]
- 66. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. New Engl. J. Med. 2015; 372(26):2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. New Engl. J. Med. 2009; 360(8):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. The Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. New Engl. J. Med. 2015; 372(26):2481-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ceccarelli M, Barthel FP, Malta TM, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016; 164(3):550-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl. J. Med. 2005; 352(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 71. Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008; 26(25):4189-4199. [DOI] [PubMed] [Google Scholar]
- 72. Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J. Clin. Oncol. 2007; 25(26):4127-4136. [DOI] [PubMed] [Google Scholar]
- 73. Jones C, Baker SJ. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer. 2014; 14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014; 46(5):444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mackay A, Burford A, Carvalho D, et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell. 2017; 32(4):520-537 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoffman LM, DeWire M, Ryall S, et al. Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol. Commun. 2016; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grill J, Puget S, Andreiuolo F, Philippe C, MacConaill L, Kieran MW. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr. Blood Cancer. 2012; 58(4):489-491. [DOI] [PubMed] [Google Scholar]
- 78. Lapin DH, Tsoli M, Ziegler DS. Genomic Insights into Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2017; 7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012; 123(4):473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Northcott PA, Dubuc AM, Pfister S, Taylor MD. Molecular subgroups of medulloblastoma. Expert Rev. Neurother. 2012; 12(7):871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat. Rev. Cancer. 2012; 12(12):818-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017; 547(7663):311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018; 4(9):1254-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gittleman H, Ostrom QT, Stetson LC, et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neurooncol. Pract. 2019; 6(6):451-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J. Natl. Cancer Inst. Monographs. 2014; 2014(49):198-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilson RJ, O’Neil ME, Ntekop E, Zhang K, Ren Y. Coding completeness and quality of relative survival-related variables in the National Program of Cancer Registries Cancer Surveillance System, 1995-2008. J. Registry. Manag. 2014; 41(2):65-71; quiz 96-67. [PMC free article] [PubMed] [Google Scholar]
- 88. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993; 3(3):255-268. [DOI] [PubMed] [Google Scholar]
- 89. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010; 120(3):297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Aldape K, Simmons ML, Davis RL, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer. 2000; 88(10):2342-2349. [PubMed] [Google Scholar]
- 92. Kruchko C, Gittleman H, Ruhl J, et al. Cancer collection efforts in the United States provide clinically relevant data on all primary brain and other CNS tumors. Neurooncol. Pract. 2019; 6(5):330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.