Abstract
Objectives:
The goal of this study was to examine how the administration and dosing of the anti-serotonergic medication cyproheptadine hydrochloride (HCl) affects involuntary muscle hypertonicity of the spastic and paretic hands of stroke survivors.
Materials and Methods:
A randomized, double-blinded, placebo-controlled longitudinal intervention study was performed as a component of a larger clinical trial. 94 stroke survivors with chronic, severe hand impairment, rated as levels 2 or 3 on the Chedoke-McMaster Stroke Assessment Stage of Hand (CMSA-H), were block randomized to groups receiving doses of cyproheptadine HCl or matched doses of placebo. Doses were increased from 4 mg BID to 8 mg TID over 3 weeks. Outcomes were assessed at baseline and after each of the three weeks of intervention. Primary outcome measure was grip termination time; other measures included muscle strength, spasticity, coactivation of the long finger flexors, and recording of potential adverse effects such as sleepiness and depression.
Results:
89 participants (receiving cyproheptadine HCl: 44, receiving placebo: 45) completed the study. The Cyproheptadine group displayed significant reduction in grip termination time, in comparison with the Placebo group (p<0.05). Significant change in the Cyproheptadine group (45% time reduction) was observed after only one week at the 4mg BID dosage. The effect was pronounced for those participants in the Cyproheptadine group with more severe hand impairment (CMSA-H level 2) at baseline. Conversely, no significant effect of Group * Session interaction was observed for spasticity (p=0.6) or coactivation (p=0.53). There were no significant changes in strength (p=0.234) or depression (p=0.441) during the trial.
Conclusions:
Use of cyproheptadine HCl was associated with a significant reduction in relaxation time of finger flexor muscles, without adversely affecting voluntary strength, although spasticity and coactivation were unchanged. Decreasing the duration of involuntary flexor activity can facilitate object release and repeated prehensile task performance.
Registration:
Clinical Trial number: NCT02418949
Keywords: Spasticity, Upper Extremity, Cyproheptadine, Chronic Stroke
Introduction
Hypertonicity is a common manifestation of sensorimotor system dysfunction following stroke. This phenomenon has been reported for extensors of the knee – rectus femoris (1), plantar flexors of the ankle – soleus (2), flexors of the elbow – biceps brachii (3), and flexors of the wrist – flexor carpi ulnaris (4). Hypertonicity in the long finger flexor muscles can present in multiple ways, including spasticity (5), excessive coactivation (6), and prolonged relaxation times (7). The hypertonicity can be substantial, with involuntary activation elicited by muscle stretch exceeding that produced by maximum voluntary contraction (MVC) (8).
The involuntary flexor activation often results in production of net flexion during attempted finger extension (8). This response may profoundly impact self-care and other activities of daily living by hindering the hand opening needed for grasp and release. The resulting fisted posture may interfere with basic hand hygiene, grooming and donning and doffing of garments. Thus, hypertonicity is a primary target for pharmacological interventions, such as botulinum toxin. Unfortunately, these agents may produce muscle paralysis, with a corresponding reduction in voluntary strength (9) for muscles already exhibiting substantial weakness. Deficits of 50% or greater in grip strength and finger flexion strength have been measured in stroke survivors with severe hand impairment (8, 10, 11), the population most likely to experience the greatest complications resulting from involuntary flexor activation.
While a great deal is unknown about the complex cellular and molecular mechanisms that regulate muscle tone in normal states and cause spasticity after upper motor neuron injury, spinal motoneuron hyperexcitability, arising from loss of supraspinal inhibition, is thought to be a primary contributor to the genesis of muscle hypertonicity (12). It is of note that monoaminergic neuromodulators, such as serotonin, have been found to play a significant role in modulating spinal motoneuron excitability in animal models and preparations. Intriguingly, the effects of the application of monoamines to spinal neurons, as described in the literature, are consistent with observed hand behaviors following stroke, including spasticity (an increased stretch reflex response), wind-up (increased muscle activity following subsequent trials), and prolonged termination times (time to de-activate muscle) (13, 14). The administration of neuromodulators, including serotonin, has been shown to raise motoneuron resting potential in vitro for neonatal rat preparations (15) and turtle preparations (16), thereby generating an increase in firing activity.
Thus, inhibiting the activity of key neuromodulators, for example, by the administration of pharmacological agents, may help to reduce involuntary muscle activation without limiting voluntary muscle activation. Administration of cyproheptadine hydrochloride (HCl), a histamine and serotonin 5-HT2 antagonist, has been reported to normalize muscle activation patterns and increase walking speed in people with spinal cord injury (SCI) whose gait was impeded by clonus (17). Another study found cyproheptadine to be an effective anti-spastic agent in individuals with SCI (18). In a pilot study in individuals with stroke, a single dose of 8mg of cyproheptadine HCl resulted in significant reduction in the time required to terminate activation of the long finger flexor muscles following MVC when compared to placebo, without significantly affecting grip strength (19).
The objective of the present study was to examine how the administration and dosing of cyproheptadine HCl would affect hypertonicity in stroke survivors with severe, chronic hand impairment. We hypothesized that the group receiving the cyproheptadine HCl would have a greater reduction in relaxation time, a measure of hypertonicity, for the finger flexors than the group receiving the placebo, without experiencing a reduction in finger flexion strength.
METHODS
As part of a longitudinal study examining the impact of a multimodal treatment paradigm on upper extremity function (clinical trial number: NCT02418949), stroke survivors received either increasing doses of cyproheptadine or a placebo over the first three weeks of the study, with no other intervention. Here, we focus on the first phase of the study to explicitly compare outcomes for the group of stroke survivors receiving daily doses of cyproheptadine HCL to those for the participants receiving placebo. Study participants were evaluated at baseline and after each of the three weeks of drug treatment.
Stroke survivors with severe hand impairment, rated as levels 2 or 3 on the Chedoke-McMaster Stroke Assessment Stage of Hand (CMSA-H) measure (20), were enrolled in the study. Participants were required to be in the chronic phase of recovery (> 6 months post-incident) and able to provide informed consent. Exclusion criteria included: presence of hemispatial neglect, apraxia, botulinum toxin injection in the upper extremity within the past 6 months, initiation of new anti-spasticity medication within the last 6 months, orthopedic impairments, or a history of seizure disorder. Participants were recruited through a registry, approved by the Northwestern University Institutional Review Board (IRB), of potential research participants that was maintained by the Shirley Ryan AbilityLab and Northwestern University Physical Therapy and Human Movement Sciences program, as well as through physician referrals, and posted flyers. All participants gave informed consent prior to enrollment in the study, in accordance with the policies of the Northwestern University IRB, which approved this study. The study conformed to the CONSORT Guidelines
In this double-blinded study, participants were randomly assigned to one of two groups: the Cyproheptadine group received incrementally increasing repeated doses of cyproheptadine HCl, taken orally, while the Placebo group received equivalent doses of a placebo in an identical pill form. The cyproheptadine HCl was crushed and added to an identical pill capsule to the placebo preparation to ensure total blinding of participant and study staff. Both preparations were obtained through study funds and were not sponsored by any specific company. Only the pharmacist and study statistician, neither of whom had contact with the participants, were aware of group assignment until the blind was broken at the conclusion of the study. A block group stratification of the randomization was performed to balance groups for severity of dysfunction based on baseline scores on the Fugl-Meyer Assessment of Motor Recovery after Stroke for the Upper Extremity (FMUE), a measure of upper limb motor impairment (21). Groups were stratified according to severe (FMUE < 20) or more moderate (FMUE ≥ 20) impairment level.
Dosage was gradually increased from 4 mg twice daily (morning and evening - BID) in the first week to 8 mg twice daily (morning and evening - BID) in the second week and 8 mg thrice daily (morning, noon, and evening - TID) in the third week. This third week dosage was guided by the recommended dosing on the approved labelling of cyproheptadine HCl and by study physicians. The rationale for gradually increasing dosage was to mitigate anticipated adverse effects of the cyproheptadine HCl. A previous study of the effectiveness of cyproheptadine HCL in treating spasticity in persons with spinal cord injury used similar dosing and titration methods (22). In cases where the participant perceived adverse effects to be intolerable following dosage increase, dosage was maintained at the level from the previous week; this accommodation was made only at the request of the participant and with the approval of both the principal investigator and medical monitor.
Outcome measures
Participants visited the laboratory at the Shirley Ryan AbilityLab for weekly evaluations and delivery of study medication at baseline and after weeks one, two, and three. Study medication was given to participants in a pill container, loaded by the study pharmacist, with instructions to return the empty pill container at the next visit. Study staff observed participants counting the pills each week to ensure correct dosages and participants self-administered medication orally. During each evaluation session, strength and hypertonicity of the hand were assessed using a customized jig (23), which allowed for movement of the fingers about the metacarpophalangeal (MCP) joints while maintaining fixed posture of the shoulder, arm, and wrist. Namely, the four fingers were placed inside a structure that could uniformly rotate the MCP joints under computerized control while simultaneously recording joint angle and torque. Surface electromyographic (EMG) electrodes (Bagnoli, Delsys, Natick MA) were placed over the flexor digitorum superficialis (FDS) and extensor digitorum communis (EDC) muscles.
The primary outcome measure, grip termination time was evaluated by having the participant create maximum isometric MCP flexion torque against the stationary fixture upon hearing a tone and relaxing when the tone ceased, in accordance with previous protocols (7). Grip termination time was defined as the time required for the magnitude of the rectified and filtered FDS EMG signal to return to resting level (within three standard deviations of the mean baseline value). Spasticity was quantified by the torque elicited by rotating the MCP joints into extension at 300°/s with the jig (5). Passive stiffness was removed from this torque by subtracting the torque recorded during a 10°/s movement to yield the spastic reflex torque. Finger flexion and extension strength were evaluated by having the participants produce maximum torque about the MCP joints of the four fingers isometrically against the stationary jig. Coactivation was quantified as the amount of FDS muscle activation during the voluntary maximum extension task. Additionally, grip force was measured with a handheld dynamometer (Jamar Technologies) in both the paretic and non-paretic hand. The reported residual grip strength was computed from the ratio of paretic grip strength/non-paretic grip strength.
Physical evaluations were performed weekly; these included measure of weight, symptom checklists, overall side effects or adverse events, and the Epworth Sleepiness Scale (ESS), a measure of overall sleepiness in which a higher score (maximum of 24 maximum) indicates greater drowsiness (24). The symptom checklist was developed based on the labelling of cyproheptadine HCl and its known side effects. The study physician met with each participant and administered the symptom checklist verbally; participants were also instructed to list any other adverse events they may have experienced during the week. At baseline and week 3, the Beck Depression Inventory Second Edition (BDI-II) was also completed to assess levels of depression (25); scores for this test range from 0–63, with a higher score indicating a greater level of depression. Serum liver panel tests were drawn at baseline and week 3. These data were reviewed by the study physician.
Statistical Analysis
The effects of Group (Cyproheptadine vs. Placebo) and Session on grip termination time were assessed using repeated measures ANCOVA with the covariates of FMUE and CMSA-H score at baseline. FMUE and CMSA-H were included as covariates to explore if initial impairment level had an effect on the outcome measures. Sessions had two levels, baseline and intent-to-treat, the latter indicating the value of the outcome measure at the initial evaluation for the maximum dosage for each participant. Post-hoc pairwise analyses within groups were performed, with specific focus on variables which exhibited any Group*Session interaction effects. An additional ANCOVA was performed to examine the effects of Group and Session on MCP flexion strength. Secondary analyses were performed on grip strength, spasticity, coactivation, ESS, and BDI-II using repeated measures ANCOVA to observe between-group differences. Data are reported as mean ± 95% confidence interval unless otherwise noted. The power based on the observed data on grip termination time was more than 70% at α=0.05 and all statistical analyses were performed on SPSS, version 28. Sample size was determined a priori to achieve power for the 4 groups observed in the main, multimodal clinical trial, not covered here. Power was greater for this initial phase as participants were segregated into two groups instead of four.
RESULTS
From April 2015 to May 2019, 108 stroke survivors with severe, chronic hand impairment were enrolled in the study. Of these enrolled participants, 10 did not qualify for the study and 2 chose not to participate. The remaining 94 participants were randomly assigned to either the Cyproheptadine or Placebo group (n=47 in each group). Two participants in the Cyproheptadine group chose to withdraw from the study for personal reasons while another participant withdrew due to an unrelated medical condition (rash). One participant in the Placebo group withdrew from the study due to an unrelated medical condition (Serious adverse event: seizure) while another subject chose to withdraw from the study for personal reasons. Thus, a total of 44 participants in the Cyproheptadine group and 45 participants in the Placebo group completed the study (Fig. 1). A total of 11 participants in the study did not reach the peak dose of 24mg/day due to concern about perceived side effects. Four of these 11 participants were taking the placebo; they took 16 mg/day for both weeks 2 and 3. Four participants in the Cyproheptadine group remained at the daily dose of 8mg/day for all 3 weeks, while three other participants in the Cyproheptadine group took 16mg/day for weeks 2 and 3. For the 89 individuals who completed the study, the groups did not differ at baseline in terms of age, time post-stroke, or FMUE (p>0.19) (Table 1).
Figure 1.
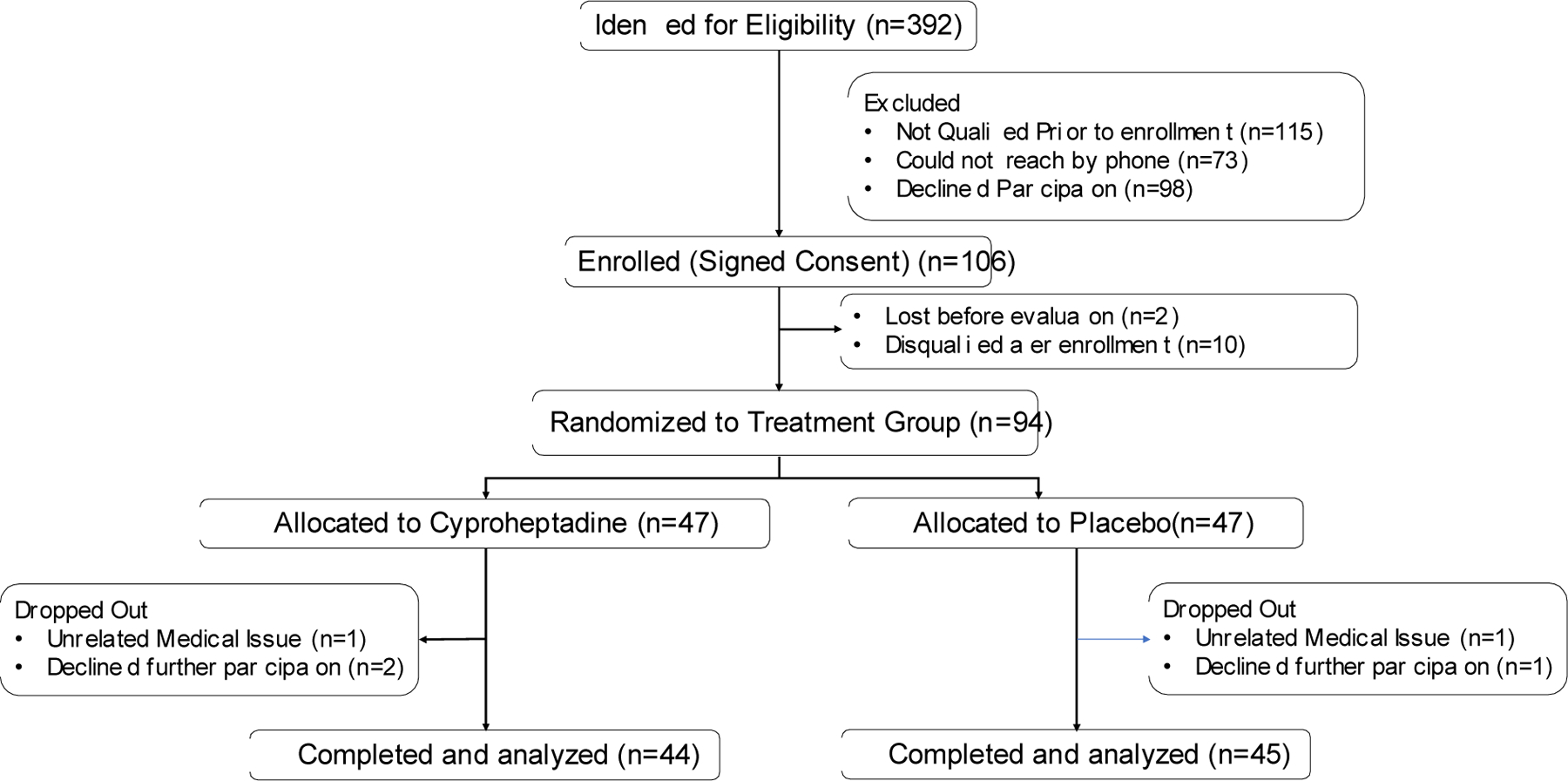
CONSORT diagram of participants. In this double-blind study, subjects were randomly assigned to either the Cyproheptadine or the Placebo group.
Table 1:
Summary of baseline characteristics (mean ± 95% CI) of participants for each subject group
| Baseline Characteristics | Drug (n=44) |
Placebo (n=45) |
|---|---|---|
| Age (yrs) | 59.6 ± 3.2 | 57.5 ± 3.0 |
| Time post-stroke (yrs) | 5.7 ± 1.4 | 7.2 ± 2.2 |
| Fugl-Meyer Upper Extremity | 15.4 ± 2.3 | 14.6 ± 1.7 |
| Gender (Female/Male) | 16 / 28 | 18 / 27 |
| Paretic hand (Right/Left) | 19 / 25 | 18 / 27 |
| CMSA-H Score (2/3) | 12 / 32 | 18 / 27 |
Administration of cyproheptadine HCl led to a substantial reduction in grip termination time that was maintained over the three-week trial period (Table 2, Fig. 2a). The repeated measures ANCOVA confirmed that the Group*Session interaction had a significant effect on the primary outcome measure of grip termination time (Wilk’s Lambda F=4.262, p-value=0.042) (Fig. 2b). The impact of the Group*Session interaction on grip termination time was not affected by the FMUE score (F= 0.271, p=0.604) and was moderately affected by CMSA-H score (F=2.976, p=0.088). Subjects in the Cyproheptadine group were able to relax FDS activity following MVC more quickly after just one week of taking the medication at the lowest dosage, 4mg BID (5.5±1.4 seconds at baseline to 2.9±1.4 seconds at week 1, F=5.666, p=0.02) (Fig. 2b). The impact in the Cyproheptadine group was especially large for participants with CMSA-H 2 (i.e., the more severely impaired individuals) compared to CMSA-H 3 (Fig. 2c). The grip termination time for this subset of subjects with CMSA-H 2 dropped from 8.6 ± 3.8 seconds at baseline to less than 3.1±3.7 seconds after week 1, and the grip termination time Group*Session interaction had a moderate effect (F=3.754, p=0.063). Subjects in the Cyproheptadine group who were rated CMSA-H 3 also experienced reduction in grip termination time after week 1, but the difference between Placebo and Cyproheptadine treatment group was not significant (F=0.110, p=0.741).
Table 2:
Summary of outcome measures recorded at each testing session (Mean ± 95% CI) as compared to baseline.
| Outcome Measure | Cyproheptadine | Placebo | |||||
| Baseline | Intent-to-treat | Paired Sample Statistics | Baseline | Intent-to-treat | Paired Sample Statistics | Session * Group Effect | |
| Grip Termination Time (Sec) | 5.48 ± 1.41 | 2.65 ± 1.09 | p<0.001* | 5.63 ± 1.85 | 4.71 ± 1.67 | p=0.371 | p = 0.042* |
| CMSA-H Score 2 |
8.63 ± 3.75 | 3.77 ± 3.07 | p=0.023 | 6.57 ± 3.60 | 6.72 ± 3.28 | p=0.932 | p=0.063 |
| CMSA-H Score 3 |
4.30 ± 1.15 | 2.23 ± 0.97 | p=0.004 | 4.99 ± 1.96 | 3.37 ± 1.59 | p=0.204 | p=0.741 |
| MCP Flexion MVC (Nm) | 2.34 ± 0.4 | 2.31 ± 0.44 | p=0.672 | 1.94 ± 0.64 | 2.12 ± 0.44 | p=0.406 | p = 0.569 |
| Residual Grip Strength (Paretic Grip/Non-paretic Grip) | 0.13 ± 0.03 | 0.10 ± 0.03 | p=0.029 | 0.12 ± 0.03 | 0.12 ± 0.03 | p=0.679 | p = 0.234 |
| MCP Reflex Torque (Nm) | 0.66 ± 0.14 | 0.31 ± 0.06 | p=0.190 | 0.88 ± 0.18 | 0.80 ± 0.19 | p=0.187 | p = 0.600 |
| FDS Coactivation during Extension (%) | 0.31 ± 0.06 | 0.24 ± 0.05 | p=0.050 | 0.30 ± 0.07 | 0.24 ± 0.05 | p=0.115 | p = 0.530 |
| ESS (score) | 5.5 ± 1.0 | 5.74 ± 1.41 | p=0.824 | 7.6 ± 1.39 | 5.27 ± 1.47 | p<0.001* | p = 0.002* |
| BDI-II (score) | 7.45 ± 2.05 | 6.36 ± 1.81 | p=0.249 | 7.67 ± 1.97 | 4.72 ± 1.46 | p<0.001 | p = 0.441 |
Asterisk in the Session * Group Effect column indicates a significant effect of the interaction. Paired sample statistics are marked as significant if p<0.05 and the Session * Group interaction was also significant.
Figure 2.
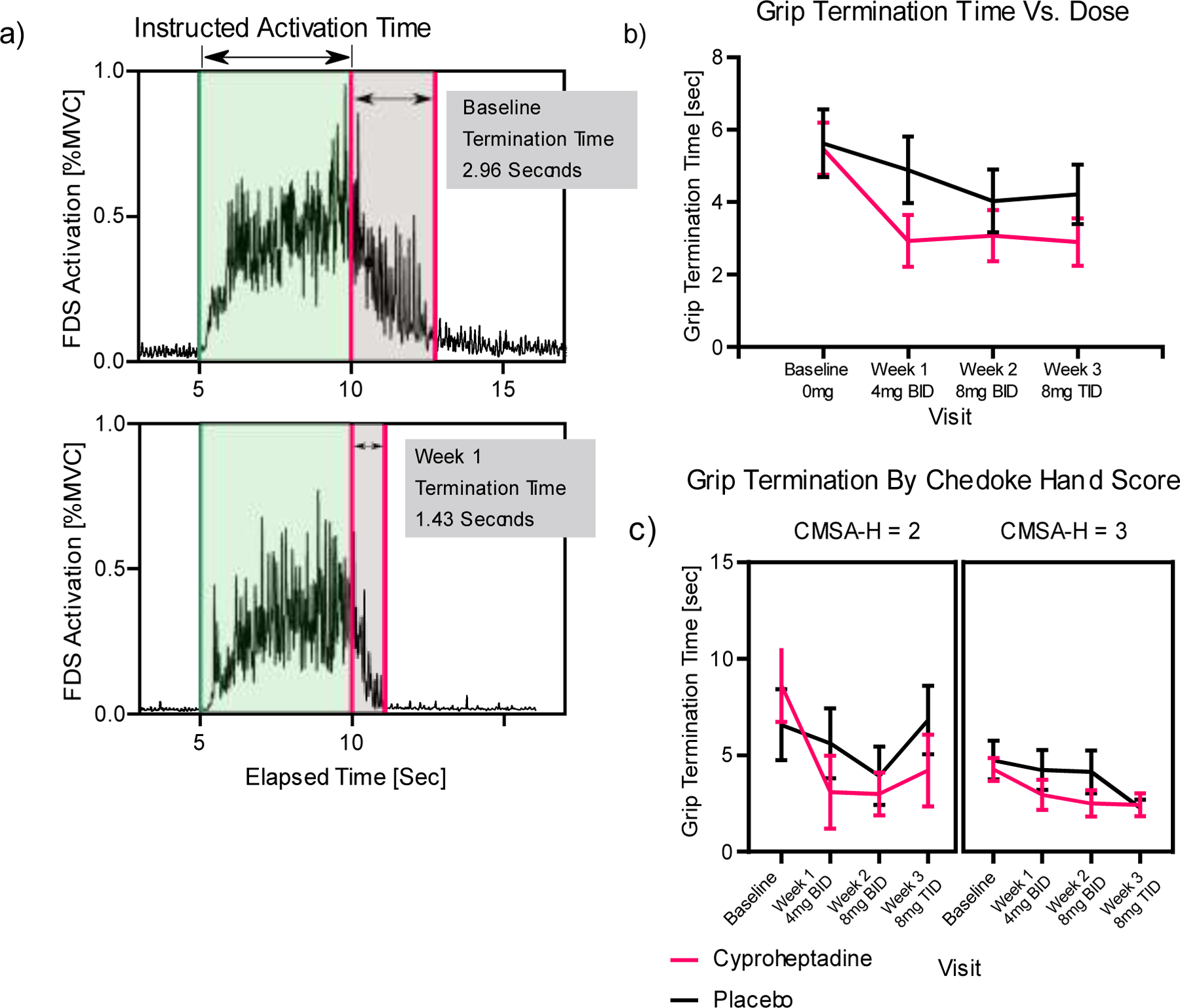
a) Representative EMG trace of participant in Cyproheptadine group who showed a decrease in grip termination time from baseline (top) to week 1 (bottom). The shaded green area denotes the instructed MVC activation time, as prompted by an auditory cue from a computer, while the shaded gray bar indi-cates the time for EMG activity to return to baseline (EMG amplitude within § 3SDs of baseline resting amplitude) the grip termination time. b) Grip termi-nation time over the course of the study for the group receiving cyproheptadine (light) and the group receiving matching pills of placebo (dark). c) Grip termination times vs. week for participants in CMSA-H 2 (left) and CMSA-H 3 (right) - cyproheptadine (light), placebo (dark). Only participants taking the listed doses are included in the plots. Error bars indicate standard error.
Neither Group nor Session nor their interaction was significantly associated with a change in finger strength, as measured by the maximum MCP flexion torque that could be produced with the four fingers (Cyproheptadine: 2.3±0.4 Nm at baseline to 2.3±0.3 Nm; Placebo: 1.9±0.6 Nm at baseline to 2.1±0.4 Nm) (Fig. 3a). The 95% confidence intervals for the change in both MCP flexion and extension torque included 0 for both groups at every week of the treatment. At baseline, only 6 of the participants could create any net extension torque. This value did not change throughout the three weeks of drug administration. There was no Group*Session effect for residual grip strength (F=1.439, p =0.234), although grip strength did decline significantly in the Cyproheptadine group (0.13±0.03 at baseline, 0.10±0.03 at intent-to-treat) while it stayed the same in the Placebo group (0.12±0.03 at baseline, 0.12±0.03 at intent-to-treat). No significant effects of Group or Session were seen with respect to flexor coactivation or spastic reflex torque (Table 2).
Figure 3.
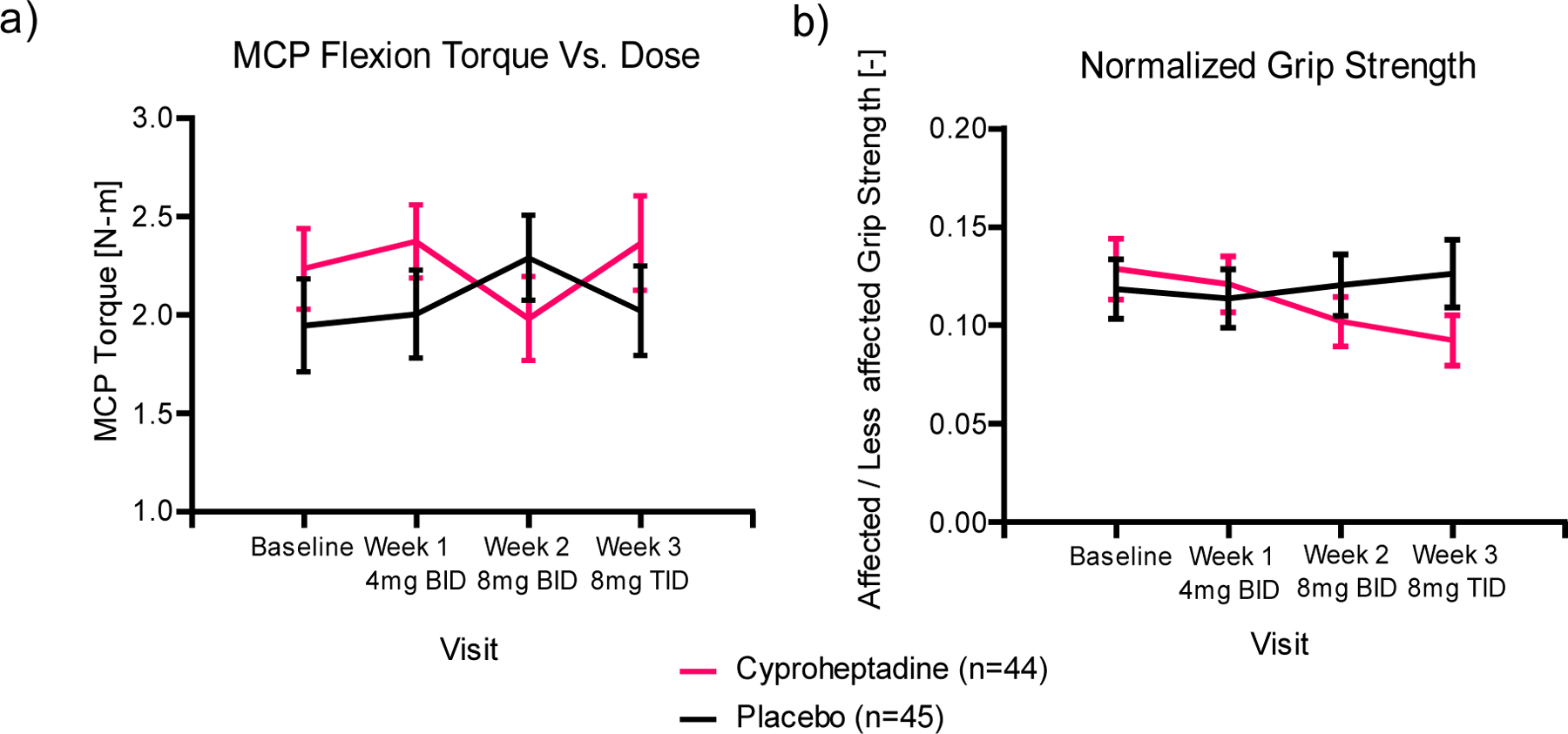
MCP flexion torque over the course of the study for the group receiving cyproheptadine (light) and the group receiving matching placebo pills (dark). Only those participants who reached the assigned dosages are included in this plot. a) MCP flexion torque vs. dose. b) Normalized grip strength vs. dose. Error bars indicate standard error.
For the Cyproheptadine group, the score on the Epworth Sleepiness Scale (ESS) did not significantly change (5.5±1.0 at baseline, 5.7±1.4 at intent-to-treat). The Placebo group, however, experienced a drop in ESS (7.6±1.4 at baseline, 5.3±1.5 at intent-to-treat) such that there was a significant Group*Session interaction (F = 9.807, p = 0.002). The BDI-II scores were not significantly affected by the Group*Session interaction (F=0.601, p=0.441, Table 2).
Adverse events encountered (frequencies listed in Table 3) were expected in accordance with the cyproheptadine HCl labelling. The adverse effects most often experienced by the Cyproheptadine group were dry mouth, low energy and motivation, constipation, weight gain (1.2kg in Cyproheptadine group, 0.5kg in Placebo, p = 0.221 between groups), and feeling restless or jittery. It should be noted that some participants experienced reductions in those symptoms while on the medication. Concurrently, subjects in the Placebo group reported some of those same adverse effects. Results of the serum liver panel tests showed no significant change from baseline to week 3 and raised no substantial medical concerns.
Table 3:
Summary of adverse effects (number of persons with worsened adverse effect from baseline | number of persons with improved adverse effect from baseline)
| ADVERSE EFFECT CHECKLIST | WEEK 3 WORSENED | IMPROVED FROM BASELINE |
|
|---|---|---|
| Cyproheptadine | Placebo | |
| DRY MOUTH | 14 | 1 | 5 | 7 |
| LOW ENERGY | 12 | 6 | 7 | 9 |
| LOW MOTIVATION | 11 | 4 | 4 | 0 |
| WEIGHT GAIN | 8 | 4 | 3 | 8 |
| CONSTIPATION | 9 | 3 | 4 | 5 |
| RESTLESS OR JITTERY | 8 | 4 | 4 | 2 |
| LIGHTHEADEDNESS | 8 | 6 | 5 | 5 |
| FREQUENT URINATION | 8 | 8 | 5 | 11 |
| SLOWNESS/TROUBLE GETTING MOVING | 8 | 10 | 3 | 14 |
| SLEEP PROBLEMS | 7 | 12 | 4 | 6 |
| DROOLING | 5 | 4 | 5 | 3 |
| SHAKING | 4 | 3 | 0 | 8 |
| ANXIETY | 4 | 10 | 1 | 4 |
| NAUSEA | 3 | 0 | 1 | 0 |
| MEMORY PROBLEMS | 3 | 5 | 1 | 10 |
| WEIGHT LOSS | 3 | 6 | 1 | 8 |
| DIFFICULTY URINATING | 2 | 2 | 3 | 1 |
| HEADACHES | 2 | 2 | 2 | 6 |
| DIFICULTY CONCENTRATING | 2 | 6 | 4 | 6 |
| BLURRED VISION | 2 | 11 | 2 | 6 |
| VOMITING | 1 | 1 | 0 | 0 |
| CHANGES IN BREASTS | 1 | 1 | 0 | 0 |
| PROBLEMS WITTH SEXUAL FUNCTION | 1 | 2 | 0 | 2 |
| MUSCLE STIFFNESS | 1 | 15 | 2 | 14 |
| CHANGES IN MENSES | 0 | 0 | 0 | 1 |
| HEART FLUTTERS | 0 | 1 | 1 | 0 |
| FALLS | 0 | 6 | 0 | 1 |
DISCUSSION
For certain muscle groups, such as the long finger flexors, stroke survivors present with a seemingly paradoxical combination of involuntary hyperexcitability of the motor units and voluntary hypoexcitability of these same motor units. Efforts to reduce the hyperexcitability with pharmacological agents such as botulinum toxin may exacerbate the limited capacity to voluntarily activate the desired motor units. Agents that target the monoaminergic neuromodulators rather than the synapses or neuromuscular junctions may be preferable in that they could potentially reduce involuntary excitation without limiting voluntary activation. Cyproheptadine HCl, a commercially available antihistamine drug that is most commonly marketed and used to treat upper respiratory symptoms associated with seasonal allergies and other allergic reactions, also has anti-serotonergic effects due to its ability to bind to the 5-HT serotonin receptors. Although cyproheptadine HCl has not been approved by the US Food and Drug Administration as an anti-spasticity agent, several studies have suggested its potential effectiveness for this purpose (18, 19).
In this study, repeated incrementally increasing dosing of cyproheptadine HCl or placebo was administered over 3 weeks. At the first post-initiation evaluation session, conducted after one week of dosing, the group receiving cyproheptadine exhibited significantly greater reduction in grip termination time, a measure of hypertonicity, than did the group receiving placebo. This reduction in grip termination time was maintained over the next two weeks for the Cyproheptadine group. In contrast, the Placebo group did not exhibit a significant reduction in grip termination time compared to baseline at any of the post-initiation evaluation sessions. Importantly, unlike the expected outcome when using botulinum toxin, finger flexion strength was not changed significantly across the three weeks during which cyproheptadine HCl was administered. Grip strength on the paretic side was reduced, but by only 3% of the value for the non-paretic side from the baseline to week 3 and the Group*Session interaction was not significant.
Cyproheptadine HCl may have affected grip termination delays by disrupting serotonin’s impact on persistent inward currents (PICs). PICs, intrinsic properties of motoneurons, act as amplifiers of synaptic inputs from other neurons and facilitate sustained firing of the motor units following synaptic input (26–28), sometimes for multiple seconds without the need for further synaptic input (28). Elevated levels of serotonin have been shown to increase the duration of evoked muscle activations (29), possibly due to the role of serotonin in promoting PICs in the motoneuron (30). It has been hypothesized that serotonin levels are increased in stroke survivors, as suggested by manifestations such as hyperhidrosis (31, 32). This situation is exacerbated by the lack of GABAergic inhibition after stroke (33–35), as GABA inhibition decreases PIC activity. The introduction of cyproheptadine HCl may reduce the influence of serotonin by competitively binding to motoneuronal 5-HT2 receptors, thereby reducing the probability of PIC generation.
In contrast, other manifestations of hypertonicity, such as spasticity and coactivation, were not significantly impacted by the introduction of cyproheptadine HCl, perhaps because these manifestations involve different underlying mechanisms. For example, spasticity may arise from the elevated resting potential of motoneurons (12), while excessive coactivation may be driven by reduced reciprocal inhibition following stroke (36). Indeed, it has been previously observed that spasticity, excessive coactivation, and increased grip termination time were not significantly correlated with each other at baseline in this cohort (8). Multiple interventions may be needed to target these different manifestations of hypertonicity.
Reducing the extent of involuntary flexor activity alone has significant functional implications. Efficient prehensile action requires a sequence of actions: hand opening to position the digits, grip force production of appropriate magnitude and direction to secure the object, and digit extension followed by hand relaxation to release the object (37). Prolonged activity in the long finger flexors, as seen in stroke survivors (7), can impact all 3 actions. Sustained flexor activity following the grasp phase interferes with digit extension and thus object release, a fundamental component of functional hand use during activities of daily living (38, 39). The resulting delays in release may become extremely frustrating, contributing to disuse of the entire upper extremity. Prolonged finger flexor activation can even extend into the next prehensile action, thereby interfering with hand opening and generation of properly directed grasping forces. We have observed that repeated attempts at finger extension, for example, can result in worsening performance due to sustained involuntary flexor activity (5).
While oral administration greatly facilitates drug delivery, it does increase the potential for adverse side effects. In this study, we did not see any significant increases in levels of depression, as measured by the BDI-II, or drowsiness, as measured by the ESS. It should be noted, however, that there was a difference between the groups in ESS due to the Placebo group experiencing a reduction in the ESS score (indicating less sleepiness) over the three weeks It is not surprising that dry mouth and low energy and motivation were commonly reported symptoms for the Cyproheptadine group, as these adverse effects are well documented for antihistamine and anti-serotonergic agents. Additionally, 7 individuals receiving cyproheptadine HCl chose not to receive the full dose because of perceived intolerable adverse effects; however, four individuals receiving placebo also declined to take the full dose. It is possible that the number or physical size of the pills (capsules) may have impacted these decisions.
It is encouraging that the favorable effects of cyproheptadine HCl on grip termination time were seen after only one week of use and on the lowest dose. The reduced grip termination time was largely maintained after that first week, thereby suggesting that the lowest dose used in this study (4mg BID) may be sufficient to achieve the desired effects. The manufacturer-suggested dosage of cyproheptadine HCl is based on its effects as an antihistamine agent, so it is promising that the anti-serotonergic effects are seen at a lower dosage. Using a lower dose may also mitigate some of the undesirable adverse effects. The 7 subjects in the Cyproheptadine group who remained on lower doses of medication in weeks 2 and/or 3 showed a 4% decrease in grip strength relative to the non-paretic hand from baseline at week 2 and only a 2% decrease from baseline at week 3.
Limitations
All stroke survivors who participated in this study were in the chronic phase of recovery. The effect of cyproheptadine HCl on neuromuscular hypertonicity and strength of the hand in stroke survivors during the acute or sub-acute phase of recovery may differ, and this question may warrant further study. Additionally, spastic reflexes of the thumb muscles were not examined, and would be an intriguing next step for this research in light of the functional importance of the thumb for grasping and pinching tasks. It is also possible that different dosing schedules would impact outcomes. The half-life for cyproheptadine HCl is roughly 8 hours and peak plasma concentrations tend to occur 3–4 hours after oral administration (40). Timing of drug administration was chosen here to aid in compliance, but an alternate dose scheduling may have led to further improvements. Finally, we did not investigate longer-term outcomes or the impact of extended use of cyproheptadine HCl.
Acknowledgements
We would like to thank Trai “Rosie” Zoldan for her incredible assistance as the Research Pharmacist for this study. We would also like to thank Kathy Pacholski for her invaluable participant recruitment skills.
Grant support:
This study was supported by the National Institutes of Health: 5R01HD075813
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Derek Kamper, UNC/NC State Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, North Carolina, USA; Closed-Loop Engineering for Advanced Rehabilitation Research Core, North Carolina State University, Raleigh, North Carolina, USA; Department of Physical Medicine & Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA;.
Alexander Barry, Shirley Ryan AbilityLab, Arms + Hands Lab, Chicago, IL, USA.
Naveen Bansal, Marquette University, Department of Mathematical and Statistical Sciences, Milwaukee, WI, USA.
Mary Ellen Stoykov, Shirley Ryan AbilityLab, Arms + Hands Lab, Chicago, IL, USA; Department of Physical Medicine & Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Kristen Triandafilou, Shirley Ryan AbilityLab, Arms + Hands Lab, Chicago, IL, USA.
Lynn Vidakovic, Shirley Ryan AbilityLab, Chicago, IL, USA; Department of Physical Medicine & Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
NaJin Seo, Medical University of South Carolina, Rehabilitation Sciences, Charleston, SC, USA.
Elliot Roth, Shirley Ryan AbilityLab, Arms + Hands Lab, Chicago, IL, USA; Department of Physical Medicine & Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
REFERENCES
- 1.Caty GD, Detrembleur C, Bleyenheuft C, et al. Effect of simultaneous botulinum toxin injections into several muscles on impairment, activity, participation, and quality of life among stroke patients presenting with a stiff knee gait. Stroke; a journal of cerebral circulation 2008;3910:2803–8. [DOI] [PubMed] [Google Scholar]
- 2.Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain 1994;117 (Pt 2):355–63. [DOI] [PubMed] [Google Scholar]
- 3.O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain 1996;119 ( Pt 5):1737–49. [DOI] [PubMed] [Google Scholar]
- 4.Marciniak C Poststroke hypertonicity: upper limb assessment and treatment. Top Stroke Rehabil 2011;183:179–94. [DOI] [PubMed] [Google Scholar]
- 5.Kamper DG, Rymer WZ. Quantitative features of the stretch response of extrinsic finger muscles in hemiparetic stroke. Muscle Nerve 2000;236:954–61. [DOI] [PubMed] [Google Scholar]
- 6.Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve 2001;245:673–81. [DOI] [PubMed] [Google Scholar]
- 7.Seo NJ, Rymer WZ, Kamper DG. Delays in grip initiation and termination in persons with stroke: effects of arm support and active muscle stretch exercise. J Neurophysiol 2009;1016:3108–15. [DOI] [PubMed] [Google Scholar]
- 8.Barry AJ, Kamper DG, Stoykov ME, et al. Characteristics of the severely impaired hand in survivors of stroke with chronic impairments. Top Stroke Rehabil 2021:1–11. [DOI] [PubMed]
- 9.Chen YT, Zhang C, Liu Y, et al. The Effects of Botulinum Toxin Injections on Spasticity and Motor Performance in Chronic Stroke with Spastic Hemiplegia. Toxins (Basel) 2020;128. [DOI] [PMC free article] [PubMed]
- 10.Hoffmann G, Conrad MO, Qiu D, et al. Contributions of voluntary activation deficits to hand weakness after stroke. Top Stroke Rehabil 2016;236:384–92. [DOI] [PubMed] [Google Scholar]
- 11.Seo NJ, Rymer WZ, Kamper DG. Altered digit force direction during pinch grip following stroke. Exp Brain Res 2010;2024:891–901. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Francisco GE, Rymer WZ. A New Definition of Poststroke Spasticity and the Interference of Spasticity With Motor Recovery From Acute to Chronic Stages. Neurorehabil Neural Repair 2021;357:601–10. [DOI] [PubMed] [Google Scholar]
- 13.Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 2000;2017:6734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JF, Paul KD, Lee RH, et al. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol 1996;752:620–8. [DOI] [PubMed] [Google Scholar]
- 15.Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol 1990;430:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornby TG, McDonagh JC, Reinking RM, et al. Effects of excitatory modulation on intrinsic properties of turtle motoneurons. J Neurophysiol 2002;881:86–97. [DOI] [PubMed] [Google Scholar]
- 17.Wainberg M, Barbeau H, editors. Modulatory action of cyproheptadine on the locomotor pattern of spastic paretic patients. Program No. 308.5. Neuroscience 1986 Abstracts; 1986.; Washington, D.C.: Society for Neuroscience. [Google Scholar]
- 18.Nance PW. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc 1994;173:150–6. [DOI] [PubMed] [Google Scholar]
- 19.Seo NJ, Fischer HW, Bogey RA, et al. Effect of a serotonin antagonist on delay in grip muscle relaxation for persons with chronic hemiparetic stroke. Clin Neurophysiol 2011;1224:796–802. [DOI] [PubMed] [Google Scholar]
- 20.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993;241:58–63. [DOI] [PubMed] [Google Scholar]
- 21.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine 1974;71:13–31. [PubMed] [Google Scholar]
- 22.Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry 1990;539:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann G, Kamper DG, Kahn JH, et al. Modulation of stretch reflexes of the finger flexors by sensory feedback from the proximal upper limb poststroke. J Neurophysiol 2009;1023:1420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;146:540–5. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. BDI-II, Beck depression inventory: manual. Boston: Harcourt Brace; 1996. vi, 38 p. p. [Google Scholar]
- 26.Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 2005;312:135–56. [DOI] [PubMed] [Google Scholar]
- 27.Revill AL, Fuglevand AJ. Effects of persistent inward currents, accommodation, and adaptation on motor unit behavior: a simulation study. J Neurophysiol 2011;1063:1467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 1998;802:572–82. [DOI] [PubMed] [Google Scholar]
- 29.Perrier JF, Tresch MC. Recruitment of motor neuronal persistent inward currents shapes withdrawal reflexes in the frog. J Physiol 2005;562Pt 2:507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrier JF, Rasmussen HB, Christensen RK, et al. Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des 2013;1924:4371–84. [DOI] [PubMed] [Google Scholar]
- 31.Benarroch EE. Thermoregulation: recent concepts and remaining questions. Neurology 2007;6912:1293–7. [DOI] [PubMed] [Google Scholar]
- 32.Korpelainen JT, Sotaniemi KA, Myllyla VV. Asymmetric sweating in stroke: a prospective quantitative study of patients with hemispheral brain infarction. Neurology 1993;436:1211–4. [DOI] [PubMed] [Google Scholar]
- 33.McPherson JG, Ellis MD, Heckman CJ, et al. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol 2008;1006:3236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YK, Yang EJ, Cho K, et al. Functional Recovery After Ischemic Stroke Is Associated With Reduced GABAergic Inhibition in the Cerebral Cortex: A GABA PET Study. Neurorehabil Neural Repair 2014;286:576–83. [DOI] [PubMed] [Google Scholar]
- 35.Hounsgaard J, Hultborn H, Jespersen B, et al. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 1988;405:345–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artieda J, Quesada P, Obeso JA. Reciprocal inhibition between forearm muscles in spastic hemiplegia. Neurology 1991;412 ( Pt 1):286–9. [DOI] [PubMed] [Google Scholar]
- 37.Flanagan JR, Tresilian JR. Grip-load force coupling: a general control strategy for transporting objects. J Exp Psychol Hum Percept Perform 1994;205:944–57. [DOI] [PubMed] [Google Scholar]
- 38.Lang CE, DeJong SL, Beebe JA. Recovery of thumb and finger extension and its relation to grasp performance after stroke. J Neurophysiol 2009;1021:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trombly CA, Quintana LA. The effects of exercise on finger extension of CVA patients. Am J Occup Ther 1983;373:195–202. [DOI] [PubMed] [Google Scholar]
- 40.Greaves MW. Antihistamines. Dermatol Clin 2001;191:53–62. [DOI] [PubMed] [Google Scholar]


