Abstract
Purpose of Review
This review summarizes current understanding of the role of denosumab, an inhibitor of receptor activator of nuclear kappa-B ligand (RANKL), in the management of 3 skeletal neoplasms: giant cell tumors, aneurysmal bone cysts, and fibrous dysplasia.
Recent Findings
A growing body of literature supports denosumab use in giant cell tumors, a neoplasm in which RANKL plays a clear pathogenic role. Comparatively less data is available in aneurysmal bone cysts and fibrous dysplasia; however, the pathogenic similarity of these disorders to giant cell tumors, as well as encouraging preliminary data, suggests denosumab may be useful. Denosumab’s inhibitory effects on bone turnover are fully reversible after drag discontinuation. This raises important unanswered questions for clinical management, including potential risks of tumor recurrence and bone turnover rebound.
Summary
Denosumab is a promising potential treatment for skeletal neoplasms. However, its clinical use is impacted by ongoing safety concerns related to postdiscontinuation rebound, particularly in children. There is a critical need to understand denosumab treatment and discontinuation effects on tumor recurrence and to develop strategies for long-term treatment in patients who cannot be managed surgically.
Keywords: RANKL, Metabolic bone diseases, Skeletal neoplasms, McCune-Albright syndrome
Introduction
Despite recent advances in understanding the pathogenesis of skeletal neoplasms, management remains challenging, in part due to a lack of targeted bone-altering therapies. Denosumab is a monoclonal antibody to receptor activator of nuclear kappa-B ligand (RANKL) that has specific, potent, and reversible effects on bone resorption. These unique properties have allowed denosumab to emerge as a potentially useful therapy for skeletal disorders that are mediated through the RANKL pathway. This review discusses the current state of denosumab use in 3 skeletal neoplasms: giant cell tumors (GCTs), aneurysmal bone cysts (ABCs), and fibrous dysplasia (FD). These distinct pathological entities are primarily treated surgically; however, they share striking similarities in the approach to adjuvant medical management, including the risks and benefits of denosumab.
Role of RANKL and OPG in Skeletal Metabolism
Skeletal homeostasis hinges on the harmonization between bone formation and resorption. In bone remodeling, tight coupling of osteoclast and osteoblast activity is necessary to maintain homeostasis and repair skeletal microdamage [1]. Bone growth in children occurs through bone modeling, where site-specific uncoupling of bone formation and resorption broadens and sculpts growing bone [2]. Skeletal neoplasms disrupt these processes, leading to discrete areas of local bone destruction. For this reason, targeting the bone remodeling cycle with antiresorptive medications is a common therapeutic strategy.
RANKL is a protein expressed by osteogenic cells that induces osteoclast differentiation by binding to its receptor RANK on osteoclast precursors [3]. RANKL is present in several transmembrane-bound and soluble isoforms, all of which participate in osteoclastogenesis [4]. The interaction between RANK and RANKL is inhibited by osteoprotegerin (OPG), a soluble, nonsignaling glycoprotein also expressed by osteogenic cells, which acts as an endogenous decoy receptor [5]. The balance between RANKL and OPG is critical to maintaining skeletal homeostasis, and disruption of this balance has been implicated in multiple disease processes, including skeletal neoplasms [6].
Denosumab
Denosumab is a fully human monoclonal antibody to the RANKL IgG2 immunoglobulin isotype [7, 8]. It binds RANKL with high affinity and specificity, mimicking OPG and leading to rapid and potent inhibition of bone resorption. Like other monoclonal antibodies, the pharmacokinetics of denosumab demonstrates dose-dependent, nonlinear elimination, which informs the dosing regimens for its two commercially available formulations. In adults given 60 mg subcutaneously, serum concentrations decline with a half-life around 30 days; when 60 mg doses are given at 6-month intervals, minimal drug accumulation occurs, and bone turnover markers increase toward baseline between doses [9]. This regimen forms the basis of the low-dose Prolia© formulation, approved by both the Food and Drug Administration and European Medicines Agency to treat adults with osteoporosis [8]. Doses above 60 mg lead to dose-dependent drug accumulation, and in adults given 120 mg monthly, denosumab serum concentration reaches steady state after 4–5 doses [10]. This results in continuous suppression of bone turnover, with no expected increase between doses. This regimen forms the basis of the high-dose Xgeva© formulation, approved for the treatment of adults with bone metastases and adults and skeletally mature adolescents with GCTs [7].
Denosumab received regulatory approval for the treatment of osteoporosis and bone metastases based on several phase 3 randomized controlled studies. The seminal FREEDOM trial included 3933 women with postmenopausal osteoporosis treated with the low-dose formulation and was the first to show decreased fractures compared to placebo [11•]. An open-label extension arm demonstrated continued gains in BMD and a low fracture incidence with up to 10 years of treatment [12]. This beneficial effect on fracture rate was replicated in phase 3 studies of patients receiving sex steroid deprivation therapy [13, 14]. Phase 3 studies of high-dose denosumab in patients with prostate (n=950) and breast cancer (n=1026) showed delayed time to first skeletal-related events compared to zoledronate [15].
Postdiscontinuation Effects
The relatively short half-fife of denosumab leads to a reversal of therapeutic effects after drag discontinuation. This represents a key difference from bisphosphonates, which unlike denosumab, incorporate into hydroxyapatite leading to long terminal half-lives and sustained therapeutic effects [16]. Studies of low-dose denosumab in postmenopausal women demonstrated a postdiscontinuation increase in bone turnover markers to 60% above baseline, which returned to pretreatment levels over 2 years along with a reversal in BMD gains [17]. The mechanism of rebound bone turnover is not fully understood but likely relates to upregulation of osteoclast-promoting factors and/or changes in RANKL and OPG production. The rebound effect thus appears to be more prominent in patients who have relatively more suppressed bone turnover during treatment, which may be associated with 1) higher-dose therapy, 2) longer duration of treatment, and/or 3) higher baseline bone turnover [18].
Potential complications of postdiscontinuation rebound include hypercalcemia, which appears to be more common in children [18], and vertebral compression fractures, which have thus far been reported only in adults [19, 20]. Some evidence suggests that bisphosphonate treatment given around the time of denosumab discontinuation may partially mitigate this rebound effect [21]. However, there are no standardized clinical practices, and there is a critical need for studies investigating the safety of denosumab discontinuation.
The reversibility of denosumab’s therapeutic effects is an especially important consideration in skeletal neoplasms. First-line management for most neoplasms is surgical resection, and adjuvant bone-altering therapies can potentially improve surgical outcomes by decreasing tumor size or growth. However, the possibility of tumor “reactivation” during the rebound period raises questions about the risk of postoperative recurrence. Lesions that are large or located in potentially morbid locations may not be amenable to resection, necessitating long-term medical management.
Other Adverse Effects
Additional adverse effects of denosumab relate to inhibition of bone turnover. Osteonecrosis of the jaw (ONJ) is an uncommon but potentially serious complication of antiresorptive medications involving progressive bony destruction in the maxillofacial bones, often following invasive dental procedures. High-dose denosumab treatment is associated with a 1–5% risk of ONJ, which has been confirmed in studies of GCT [15, 22••, 23, 24]. Clinicians should be aware that the prevalence of ONJ may vary across disease states; for example, ONJ has been reported in 5% of one large cohort of FD patients treated with bisphosphonates, suggesting this population may be at higher risk [25].
Hypocalcemia may arise during antiresorptive therapy due to inhibition of osteoclast-mediated skeletal calcium release. In high-dose oncology studies, hypocalcemia was more frequent in patients receiving denosumab compared to zoledronate (12.5% versus 5.3%) [26], and there have been rare reports of fatal hypocalcemia postmarketing [7]. This risk appears to be increased in patients with renal failure and in those with higher baseline bone turnover [18].
Suppression of bone turnover also has the potential to affect bone growth in children, which relies on osteoclast activity to sculpt and widen bones [2]. Preclinical studies in juvenile animals given high-dose denosumab showed negative effects on growth and tooth eruption [7, 27]. However, case reports and small series in children have not demonstrated detrimental effects on dentition or bone shape with short-term denosumab treatment [28, 29].
While denosumab may have desirable therapeutic effects on pathologic bone, in patients with skeletal neoplasms, this is necessarily accompanied by reduced turnover and increased density in nonpathologic bone. Children and adolescents are at higher risk for the development of high bone mass due to vigorous modeling and remodeling rates during skeletal growth [30, 31].
Giant Cell Tumors
GCT is a primary bone neoplasm that largely affects patients in the third and fourth decades of life with a slight female predominance [32]. Histologically, GCTs are composed of neoplastic mesenchymal stromal cells and reactive osteoclast-like giant cells [33] (Fig. 1A & B). Proliferating primitive stromal cells of preosteoblast lineage highly express RANKL and thereby induce osteoclast formation and bone resorption via a RANKL-dependent mechanism [34]. GCT are characterized genetically by recurrent mutations (>90%) at the G34 position of H3F3A. These mutations occur exclusively in the stromal cells and are highly specific to GCT [35, 36].
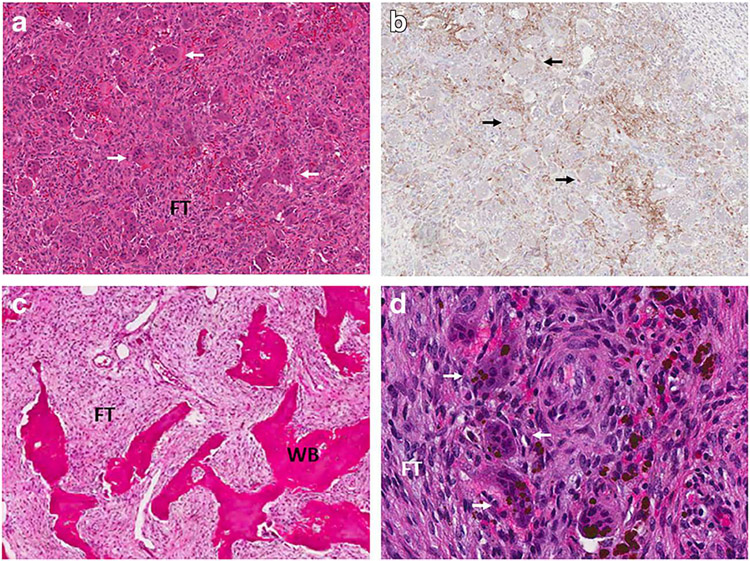
Fig. 1.
Representative histologic images. Upper panels show sections from a giant cell tumor. a Hematoxylin and eosin staining shows characteristic areas of fibrous tissue (FT) interspersed with osteoclast-like giant cells (white arrows). b RANKL immunostaining shows positivity in neoplastic stromal cells. Prominent giant cells are again visualized (black arrows). Lower panels show sections from a fibrous dysplasia lesion. c Hematoxylin and eosin staining shows characteristic areas of fibrous tissue (FT) interspersed with discontinuous trabeculae of abnormal woven bone (WB). d High-power view shows giant cells (white arrows) amidst a background of fibrous tissue (FT) comprised of neoplastic stromal cells
Although GCTs are typically benign, they are often locally aggressive causing significant bone destruction [37]. Radiographically, GCTs exhibit a classic “soap bubble appearance,” characterized by well-circumscribed, lobulated osteolytic lesions; the radiographic appearance is often assessed using Campanacci grade (I–III), which is based on the integrity of the tumor margins [38]. Rarely, a primary malignancy may arise in GCT, or lesions may undergo spontaneous or postradiation malignant transformation. Pulmonary metastases have also been reported [32].
The standard treatment of GCT is surgical resection. The most definitive treatment, with the lowest recurrence rate, is en bloc removal with wide margins [38-40]. However, since most GCTs affect the metaepiphyseal region of long bones, such extensive resections often require endoprosthetic joint replacement or amputation. Limb salvage operations, such as intralesional excision and curettage, have better functional outcomes but are associated with high recurrence rates (27–65%), even with the addition of local adjuvants (12–27%) [38-43]. Still, some GCTs are deemed inoperable due to multiple foci or unfavorable location.
Denosumab is currently the only approved medical therapy for surgically unsalvageable GCTs. Approval was largely based on an open-label phase 2 trial of high-dose denosumab in adults and skeletally mature adolescents (n=526, median duration 32.3 months), where 80% of patients demonstrated clinical benefit, defined as pain reduction and improved function [22••]. Most patients also demonstrated radiographic improvement or stabilization. In patients with unresectable tumors, denosumab was effective for long-term control, with only 11% (28/262) experiencing disease progression. However, after treatment discontinuation, the risk of relapse increased, with disease progression in 26% (34/132) of patients. Therefore, studies are ongoing to evaluate a reduced dose (120 mg every 3 months) for long-term maintenance therapy (NCT03620149). Of note, in vitro studies demonstrate that denosumab effectively wipes out osteoclast-like giant cells from GCT tissue; however, stromal cells continue to proliferate, indicating that additional strategies are likely required to address these neoplastic cells [44].
Denosumab is also approved for surgically salvageable GCTs where resection is likely to result in severe morbidity. In the phase 2 study, a second cohort of patients with surgically salvageable tumors received a median 20.5 doses (IQR 15–43), including 6 adjuvant doses in patients who had surgery [22••]. In this cohort, 37% (90/248) did not end up requiring surgery, and of those who did, 44% (69/157) underwent a less morbid procedure than originally planned. However, 27% (42/157) of patients had disease recurrence postoperatively, which was much higher than the two previous interim analyses [45, 46]. Among patients who underwent curettage procedures, the recurrence rate was even higher at 34%. While there is a clear benefit of neoadjuvant denosumab for avoiding mutilating surgery, using it preoperatively for tumor downstaging remains highly controversial due to this potential increased risk of local recurrence.
A recent systematic review of patients who received neoadjuvant denosumab by Luengo-Alonso et al. reported a cumulative postoperative tumor recurrence rate of 9%, thereby dismissing the notion that neoadjuvant treatment resulted in an increased recurrence risk [47]. However, there were several crucial issues with methodology in this review. First, the follow-up times among the studies varied greatly and was not considered for inclusion; for example, the median follow-up time in the study by Goldschlager et al. was only 5 months which is inadequate since local recurrence commonly occurs up to 2 years after resection [48]. Second, there were discrepancies in the numbers of subjects per study, calling into question the accuracy of the recurrence rates. For example, the review reported a recurrence rate of 4% (2/25) in the study by Muller et al.; however, only 18 of the 25 patients received neoadjuvant denosumab, resulting in an actual recurrence rate of 11% [49]. Similarly, the review’s reported recurrence rate in the study by Borkowska et al. was 6% (2/35); however, only 17 of the 35 subjects in the study ultimately underwent surgery, resulting in an actual postoperative recurrence rate of 12% (2/17) total and 33% (2/6) among curettage procedures. Importantly, Luengo-Alonso et al.’s systematic review did not account for operation type, which is historically the most important factor for recurrence risk. Since the purpose of neoadjuvant denosumab is for tumor downsizing to allow for less morbid procedures, outcomes of en bloc resections, which already have a low recurrence rate without the addition of neoadjuvants, are not particularly relevant. This is reflected in a more recent study, which was not included in Luengo-Alonso et al.’s review, in which Rutkowski et al. evaluated postsurgical outcomes in 89 subjects who received neoadjuvant denosumab [50]. The overall recurrence rate was 21% (19/89), but among curettage procedures, the recurrence rate increased to 32% (16/50). These findings are similar to the recurrence rates reported in the initial phase 2 study [22••]. Both studies assert that their recurrence rates are similar to historical studies of isolated curettage, which range from 27 to 65%. However, a major limitation is the lack of control groups to directly compare outcomes.
Although there are no randomized control trials, a few studies have evaluated recurrence rates after intralesional resection between patients who received neoadjuvant denosumab and those who had surgery alone in large case–control studies. In a study by Chinder et al., patients underwent extensive curettage resection plus local phenol adjuvant treatment with or without neoadjuvant denosumab [51]. The inclusion criteria were more stringent than previous analyses: patients who had previous recurrence, prior surgery, radiotherapy, other medical therapy, adjuvant denosumab, or insufficient follow-up (<1 year) were excluded. The incidence of local recurrence was 43% (18/42) in patients who received neoadjuvant denosumab compared to only 19% (15/81) in patients who did not receive preoperative treatment over a mean 35-month follow-up. Multivariate analysis revealed that neoadjuvant denosumab was the only independent risk factor for recurrence. However, the group who received denosumab had higher Campanacci grade than the control group, which is an important limitation. Despite this, Chinder et al. provided the most homogenous group of patients reported to date, all of whom received the same surgical and adjuvant treatments performed by the same surgeon. An additional retrospective review by Agarwal et al. evaluated recurrence in a very heterogenous group of patients who underwent curettage with or without neoadjuvant denosumab [52]. This study attempted to match the groups for several factors including site, size, and previous recurrence. They also reported an increased incidence of recurrence in the denosumab group (44%, 11/25; median follow-up 27 months), compared to controls (21%, 7/34; median follow-up 60 months); however, the association between denosumab and recurrence was not statistically significant. Errani et al. reviewed 25 patients with GCT treated with curettage and denosumab with a median 42 month follow-up, reporting 60% (15/25) recurrence in patients treated with denosumab, compared to 16% (36/222) in patients treated with curettage alone [53]. Of note, patients in the denosumab group tended to have higher radiographic scores and were less likely to receive adjuvant phenol.
Overall, there is sufficient evidence to support that neoadjuvant denosumab prior to limb-salvage intralesional resection may be associated with increased risk of local recurrence. However, a large randomized clinical trial is still needed to confirm these findings; currently, there is at least one ongoing phase III trial of denosumab before curettage for giant cell tumor of bone [54]. Additional studies are also needed to determine the optimal treatment regimen prior to surgery.
Another concern regarding denosumab use in GCT is the potential risk of malignant transformation. In the initial phase 2 trial, malignant transformation occurred in 1% (5/526) of cases, which is lower that the historical incidence of 2% reported in the literature [22••]. In a recent literature review, Alagalili et al. reported 11 cases of sarcomatous transformation postdenosumab therapy in patients with GCT and no previous history of radiation [55]. However, the mechanism by which sarcomatous transformation of GCT occurs has not been elucidated, and there is currently no biological evidence to establish a causal relationship with denosumab.
Aneurysmal Bone Cysts
ABCs are benign, locally destructive skeletal neoplasms consisting of multiloculated cystic spaces filled with blood [56]. Histologically, the cystic spaces are separated by fibrous septa composed of dense fibroblast-like spindle cells, scattered osteoclast-like giant cells, and woven bone rimmed by osteoblasts [57]. Radiographically, lesions present as unilocular or multilocular cysts with thin, “eggshell” borders, with disruption of surrounding bony and soft tissue structures due to cyst expansion (Fig. 2). MRI may reveal fluid levels within the cyst, reflecting variably aged blood.
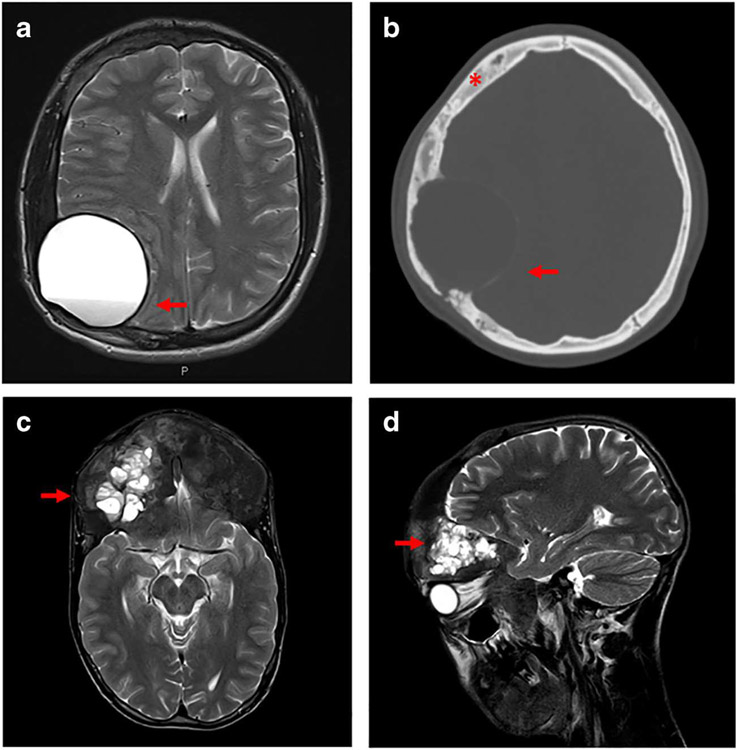
Fig. 2.
Representative radiographic images of secondary aneurysmal bone cysts (ABCs). Panels a and b show images from a patient with craniofacial fibrous dysplasia who developed a slow-growing, painless mass over several months. a MRI T2 sequence shows a large unilocular cyst with a visible fluid level (red arrow) and compression of surrounding brain tissue. b Corresponding CT scan reveals a thin “eggshell” border (red arrow), with no visible fluid levels. Note the presence of cranial fibrous dysplasia showing an expanded, “ground glass” homogeneity (red asterisk). Panels c and d show images from a patient with craniofacial fibrous dysplasia who presented with progressive unilateral proptosis over several months. MRI T2 sequence shows a multiloculated fluid-filled cyst in the left frontal area visible in axial (c) and sagittal (d) views
ABCs can affect any bone, but most frequently arise within the metaphyses of long bones and the posterior elements of vertebral bodies. Patients typically present with a combination of pain, swelling, and/or pathologic fracture, which may develop over a period of weeks to months. The majority of ABCs represent primary lesions; however, secondary ABCs may form within preexisting benign or malignant bone tumors, including GCT and FD [58-60]. Traditionally, ABCs were thought to develop as a reactive process due to local hemodynamic disturbances within the bone (such as arteriovenous fistula or venous thrombosis), leading to increased venous pressure, formation of dilated vascular spaces, and ultimately bony destruction [57]. However, more recent studies have identified chromosomal translocations involving USP6 gene on chromosome 17p.3 in up to 75% of primary ABCs, suggesting a true neoplastic etiology [61-64]. This finding is important for differentiating primary ABCs from secondary ABCs, which harbor different genetic aberrations, such as GNAS SNV in FD or H3F3 SNV in GCT.
The current standard of care for ABCs is curettage with local adjuvants (i.e., high-speed burr, argon beam, phenol, etc.), which has significantly decreased recurrence rates (7–12.5%) compared to curettage alone (59%) [65]. Although intralesional procedures have decreased morbidity compared to historic en bloc resections, vertebral ABCs and lesions in other critical locations remain a challenge. Like GCT and FD, RANK–RANKL signal activation is essential for ABC development and progression; neoplastic spindle cells in ABCs express high levels of RANKL resulting in osteoclast-like giant cell activation and osteolysis [66]. Due to the histopathological similarities between GCT and ABC, off-label denosumab therapy has been attempted.
There are a limited number of studies investigating denosumab in ABCs. Alhumaid et al. recently published a comprehensive review of 12 studies including a total 30 cases [67•]. The patient population was quite heterogenous, varying in terms of ABC location; primary, neoadjuvant, or adjuvant denosumab administration; surgical history; previous recurrence; and age. Most patients exhibited radiographic response (28/30), defined as ABC ossification and size stabilization, and clinical improvement (27/28). Of patients who completed or stopped denosumab, recurrence was reported in 21 % (5/24) of cases [68-70]. Two recurrences occurred in patients who received neoadjuvant denosumab followed by surgical resection, and in 3 denosumab was the primary treatment. Time until recurrence ranged from 15 to 24 months; however, the overall posttreatment follow-up period was highly variable and often unclear among the studies. For example, the average follow-up time in the Lange et al. series was only 3 months (range 2–4) [71], and in at least 16 of the reportedly stable cases, it was less than 2 years [67•]. Therefore, the true recurrence rate after denosumab treatment remains unclear and is likely higher than reported in this review. In an additional series by Durr et al. featuring 6 heterogeneous patients, recurrence occurred in 3 (50%) who received denosumab as adjuvant therapy after either curettage (N=2) or embolization (N=1) [72]. The remaining 3 patients exhibited clinical and radiographic resolution over a median 3-year follow-up; one received denosumab alone as primary treatment, and 2 received adjuvant denosumab after curettage.
Based on this limited literature, the utility of denosumab in ABC management is promising, especially in the setting of inoperable lesions; however many important questions remain, including the optimal treatment regimen, utility of neoadjuvant use for tumor downsizing, and long-term outcomes. The recurrence risk after denosumab treatment remains unclear. Of cumulative reported cases in the literature, recurrence occurred in 27% (8/30); however, this includes primary, neoadjuvant and adjuvant denosumab use of varying treatment regimens, ABCs in different locations, and patients with diverse surgical histories. Importantly, since ABCs predominantly affect young people, more research on the safety profile and long-term effects of denosumab in skeletally immature patients is also necessary [69, 71, 72].
Fibrous Dysplasia
FD is a rare disorder in which normal bone and marrow are replaced by fibro-osseous tissue, resulting in fractures, deformities, and disability [73]. Disease may affect one or multiple bones and may occur in association with extraskeletal features, including hyperpigmented macules and hyperfunctioning endocrinopathies (precocious puberty, hyperthyroidism, growth hormone excess, neonatal h y p e r c o r t i s o l i s m , F G F 2 3 - m e d i a t e d hypophosphatemia)(Fig. 3A & D). The combination of FD and extraskeletal features is termed McCune–Albright syndrome (MAS) [73]. FD/MAS arises due to somatic gain-of-function mutations in GNAS, which encodes the α-subunit of the Gs G-coupled protein receptor [74]. Constitutive receptor signaling impairs differentiation of skeletal stem cells, leading to the formation of discrete, expansile lesions. Histologically, FD presents quite similarly to GCT, with marrow fibrosis and prominent osteoclastogenesis, particularly in active lesions with high turnover and increased RANKL expression [75, 76](Fig. 1C & D). Radiographically, lesions have a typical “ground glass” appearance on computed tomography scans and X-rays [77]. Craniofacial lesions may lead to facial asymmetry and rarely functional deficits such as vision and hearing loss [78]. In the long bones, FD lesions are structurally unsound, leading to fractures, bowing deformities, and impaired ambulation [73]. Patients may also develop bone pain in a variety of locations [79, 80].
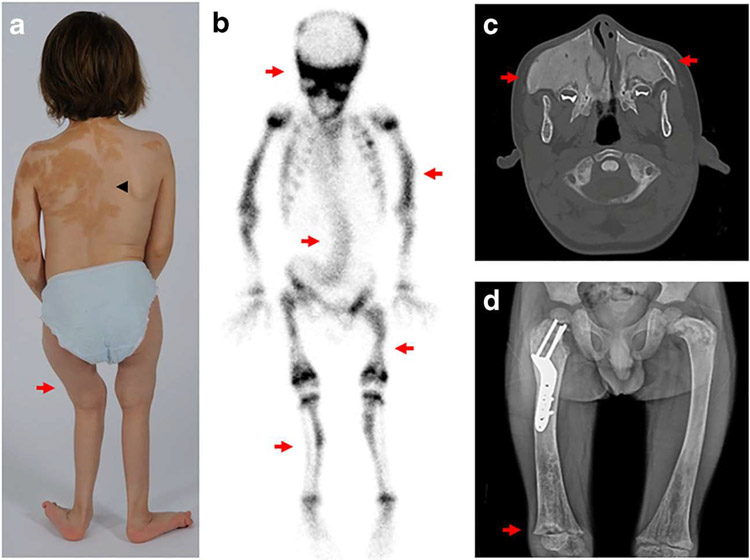
Fig. 3.
Representative clinical and radiographic images in fibrous dysplasia. a Photograph of a child shows typical skin hyperpigmentation (black arrowhead) and bowing of the lower extremities (red arrow). b Technetium-99 scintigraphy scan from the child in panel a shows diffuse tracer uptake in areas of fibrous dysplasia involving the skull, spine, and bilateral extremities (red arrows). c Axial computed tomography scan of the skull shows expansile maxillary lesions (red arrows) leading to compression of the nasal canal. Note the homogeneous, “ground glass” appearance of the bone. d Radiograph of the bilateral femurs shows extensive fibrous dysplasia involvement, with characteristic “ground glass” homogeneity and thin cortices. A surgical implant is visible in the right proximal femur. Note the rachitic growth plate reflecting FGF23-mediated hypophosphatemia (red arrow)
The mainstay of treatment in FD is surgery to repair and prevent fractures and deformities. Lesions are typically too extensive for complete resection, and techniques commonly used in other skeletal disorders (such as curettage and grafting) are frequently ineffective, leading to suboptimal outcomes [78, 81]. There are currently no effective, well-established medical treatments. Antiresorptive therapy with bisphosphonates has been advocated due to the increased bone turnover and osteoclastogenesis present in FD tissue. Some reports indicate that intravenous bisphosphonates may be helpful for bone pain; however, there is no evidence that bisphosphonates have direct effects on FD lesion progression or activity [30, 82-84]. It has been speculated that bisphosphonates may lack efficacy because their action requires incorporation into mineralizing matrix, which is greatly diminished in FD tissue [85]. Denosumab is therefore an intuitive potential treatment because it does not require matrix incorporation and, like in GCT, it can directly target ectopic osteoclasts. In a mouse model of FD, treatment with an anti-RANKL antibody prevented the formation of new lesions and promoted skeletal stem cell differentiation into functional osteoblasts, resulting in mineralized lamellar bone formation [86]. Similarly, the first FD patient treated with denosumab was a child with an aggressive femoral lesion, who demonstrated a dramatic decrease in lesion expansion and resolution of bone pain with a high-dose regimen [87]. A case series of 12 adults given various low-dose regimens (60 mg every 3–6 months over 13–30 months) reported improvement in pain and serum bone turnover markers [88]. Postdiscontinuation data was not included in this series; however, drug discontinuation in both the pediatric case and the mouse study led to bone turnover rebound above pretreatment levels, which in the child was associated with life-threatening hypercalcemia [87].
These data provide early but promising evidence that blocking the RANK/RANKL interaction in FD may have beneficial clinical effects and promote FD lesion mineralization. However, important questions remain regarding both safety and regimen particularly because effective management requires long-term treatment. Continuous denosumab therapy carries risks associated with bone-turnover suppression (particularly in growing children), while intermittent treatment carries the risk of rebound bone turnover and loss of therapeutic effects. Bisphosphonates have been proposed as a potential adjuvant treatment that may be used in conjunction with denosumab to maintain mineralization and prevent postdiscontinuation rebound in FD tissue [85]. However, there is no clinical data investigating the efficacy of this approach, which would lead to additional bone-turnover suppression and associated safety risks. An ongoing study (NCT03571191) will hopefully shed light on the clinical efficacy and histologic effects of denosumab treatment in FD, including postdiscontinuation rebound.
Conclusions and Future Directions
A growing body of literature supports a potential role for denosumab in the management of GCTs, ABCs, and FD; however, important questions remain regarding long-term safety and efficacy. In the absence of high-quality studies, it is imperative that clinicians think critically about treatment goals for each patient because individual factors weigh heavily in determining denosumab’s risks and benefits. These factors include potential surgical approaches, morbidity fiom recurrence, and risks of postdiscontinuation rebound, which may vary greatly between patients. It is essential to consider plans for chug discontinuation prior to starting denosumab and to discuss these options with the patient and multidisciplinary care team. Safety monitoring, such as regular dental exams and periodic bone density assessment, should be performed to mitigate risks and inform decision-making, particularly in children.
There is a critical need to develop strategies for long-term denosumab treatment in patients with inoperable skeletal neoplasms. While the high-dose formulation appears likely to have beneficial effects on tumor activity, this dose is not appropriate for long-term use due to potential skeletal toxicity. It is possible that long-term treatment with lower and/or less Sequent “maintenance” doses may balance the need for continued efficacy with the risks of bone-turnover suppression. Another possibility is repeated short courses of high-dose denosumab, with monitoring for rebound and increased tumor growth in between. Clinical studies investigating these alternate regimens are needed, including the potential role for adjuvant long-acting medications such as bisphosphonates. Given the preponderance of pediatric patients affected by skeletal neoplasms, it is essential that these future studies include children.
Funding
This work was supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research.
Footnotes
Conflict of Interest NIDCR receives funding from Amgen, Inc. for an investigator-sponsored study of denosumab treatment for fibrous dysplasia.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Seeman E Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(3):219–33. [DOI] [PubMed] [Google Scholar]
- 2.Rauch F Bone accrual in children: adding substance to surfaces. Pediatrics. 2007;119(Suppl 2):S137–40. [DOI] [PubMed] [Google Scholar]
- 3.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96(7):3540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda T, Kasai M, Utsuyama M, Hirokawa K. Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology. 2001;142(4):1419–26. [DOI] [PubMed] [Google Scholar]
- 5.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. [DOI] [PubMed] [Google Scholar]
- 6.Dougall WC, Chaisson M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev. 2006;25(4):541–9. [DOI] [PubMed] [Google Scholar]
- 7.Xgeva (denosumab) [package insert]. Thousand Oaks: Amgen, Inc; 2010. [Google Scholar]
- 8.Prolia (denosumab) [package insert]. Thousand Oaks: Amgen, Inc; 2010. [Google Scholar]
- 9.Sutjandra L, Rodriguez RD, Doshi S, Ma M, Peterson MC, Jang GR, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet. 2011;50(12):793–807. [DOI] [PubMed] [Google Scholar]
- 10.Gibiansky L, Sutjandra L, Doshi S, Zheng J, Sohn W, Peterson MC, et al. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin Pharmacokinet. 2012;51(4):247–60. [DOI] [PubMed] [Google Scholar]
- 11.•. Cummings SR, San Martin J, MR MC, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65 This seminal phase 3 controlled trial in patients with osteoporosis was the first to show a beneficial effect of denosumab on fracture rate.
- 12.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–23. [DOI] [PubMed] [Google Scholar]
- 13.Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–82. [DOI] [PubMed] [Google Scholar]
- 15.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic arid for the treatment of bone metastases in patients with advanced breast cancer a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. [DOI] [PubMed] [Google Scholar]
- 16.Lewiecki EM. Bisphosphonates for the treatment of osteoporosis: insights for clinicians. Ther Adv Chronic Dis. 2010;1(3):115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–80. [DOI] [PubMed] [Google Scholar]
- 18.Boyce AM. Denosumab: an emerging therapy in pediatric Bone disorders. Curr Osteoporos Rep. 2017;15(4):283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–7. [DOI] [PubMed] [Google Scholar]
- 20.Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–8. [DOI] [PubMed] [Google Scholar]
- 21.Reid IR, Home AM, Mihov B, Gamble GD. Bone loss after denosumab: only partial protection with Zoledronate. Calcif Tissue Int. 2017;101(4):371–4. [DOI] [PubMed] [Google Scholar]
- 22.••. Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(12):1719–29 This phase 2 study of denosumab treatment in GCT showed long-term disease control for patients with unresectable and resectable tumours. Results suggested an increased risk of recurrence after drug discontinuation.
- 23.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metwally T, Burke A, Tsai JY, Collins MT, Boyce AM. Fibrous dysplasia and medication-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2016;74(10):1983–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25(3):440–6. [DOI] [PubMed] [Google Scholar]
- 27.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–23. [DOI] [PubMed] [Google Scholar]
- 28.Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. Safety and efficacy of denosumab in children with osteogenesis imperfect—a first prospective trial. J Musculoskelet Neuronal Interact 2016;16(1):24–32. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HD, Boyce AM, Tsai JY, Gafni RI, Farley FA, Kasa-Vubu JZ, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. 2014;99(3):891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, Riminucci M, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab. 2014;99(11):4133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res. 2008;23(10):1698–707. [DOI] [PubMed] [Google Scholar]
- 32.Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006;29(1):96–9. [DOI] [PubMed] [Google Scholar]
- 33.Zheng MH, Robbins P, Xu J, Huang L, Wood DJ, Papadimitriou JM. The histogenesis of giant cell tumour of bone: a model of interaction between neoplastic cells and osteoclasts. Histol Histopathol. 2001;16(1):297–307. [DOI] [PubMed] [Google Scholar]
- 34.Cowan RW, Singh G. Giant cell tumor of bone: a basic science perspective. Bone. 2013;52(1):238–46. [DOI] [PubMed] [Google Scholar]
- 35.Al-Ibraheemi A, Inwards CY, Zreik RT, Wenger DE, Jenkins SM, Carter JM, et al. Histologic spectrum of giant cell tumor (GCT) of bone in patients 18 years of age and below: a study of 63 patients. Am J Surg Pathol. 2016;40(12):1702–12. [DOI] [PubMed] [Google Scholar]
- 36.Noh BJ, Park YK. Giant cell tumor of bone: updated molecular pathogenesis and tumor biology. Hum Pathol. 2018;81:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Jaffe HL, Lichtenstein L, Portis RB. Giant cell tumor of bone: its pathologic appearance, grading, supposed variants and treatment. Arch Pathol. 1940;30:993–1031. [Google Scholar]
- 38.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106–14. [PubMed] [Google Scholar]
- 39.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235–42. [PubMed] [Google Scholar]
- 40.van der Heijden L, Dijkstra PDS, Blay JY, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer. 2017;77:75–83. [DOI] [PubMed] [Google Scholar]
- 41.Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90(5):1060–7. [DOI] [PubMed] [Google Scholar]
- 42.Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1–7. [DOI] [PubMed] [Google Scholar]
- 43.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134(9):969–78. [DOI] [PubMed] [Google Scholar]
- 44.Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am. 2014;96(15):e127. [DOI] [PubMed] [Google Scholar]
- 45.Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–8. [DOI] [PubMed] [Google Scholar]
- 46.Rutkowski P, Ferrari S, Grimer RJ, Stalley PD, Dijkstra SP, Pienkowski A, et al. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22(9):2860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J. Denosumab treatment for giant-cell tumor of bone: a systematic review of the literature. Arch Orthop Trauma Surg. 2019;139(10):1339–49. [DOI] [PubMed] [Google Scholar]
- 48.Goldschlager T, Dea N, Boyd M, Reynolds J, Patel S, Rhines LD, et al. Giant cell tumors of the spine: has denosumab changed the treatment paradigm? J Neurosurg Spine. 2015;22(5):526–33. [DOI] [PubMed] [Google Scholar]
- 49.Muller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016;14(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutkowski P, Gaston L, Borkowska A, Stacchiotti S, Gelderblom H, Baldi GG, et al. Denosumab treatment of inoperable or locally advanced giant cell tumor of bone—multicenter analysis outside clinical trial. Eur J Surg Oncol. 2018;44(9):1384–90. [DOI] [PubMed] [Google Scholar]
- 51.Chinder PS, Hindiskere S, Doddarangappa S, Pal U. Evaluation of local recurrence in giant-cell tumor of bone treated by neoadjuvant denosumab. Clin Orthop Surg. 2019;11(3):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal MG, Gundavda MK, Gupta R, Reddy R. Does denosumab change the giant cell tumor treatment strategy? Lessons learned from early experience. Clin Orthop Relat Res. 2018;476(9):1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, et al. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018;100(6):496–504. [DOI] [PubMed] [Google Scholar]
- 54.Urakawa H, Mizusawa J, Tanaka K, Eba J, Hiraga H, Kawai A, et al. A randomized phase III trial of denosumab before curettage for giant cell tumor of bone: Japan Clinical Oncology Group Study JCOG1610. Jpn J Clin Oncol. 2019;49(4):379–82. [DOI] [PubMed] [Google Scholar]
- 55.Alaqaili SI, Abduljabbar AM, Altaho AJ, Khan AA, Alherabi JA. Malignant sarcomatous transformation of benign giant cell tumor of bone after treatment with denosumab therapy: a literature review of reported cases. Cureus. 2018;10(12):e3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muratori F, Mondanelli N, Rizzo AR, Beltrami G, Giannotti S, Capanna R, et al. Aneurysmal bone cyst: a review of management. Surg Technol Int 2019;35:325–35. [PubMed] [Google Scholar]
- 57.Lichtenstein L Aneurysmal bone cyst. A pathological entity commonly mistaken for giant-cell tumor and occasionally for hemangioma and osteogenic sarcoma. Cancer. 1950;3(2):279–89. [Google Scholar]
- 58.Buraczewski J, Dabska M. Pathogenesis of aneurysmal bone cyst. Relationship between the aneurysmal bone cyst and fibrous dysplasia of bone. Cancer. 1971;28(3):597–604. [DOI] [PubMed] [Google Scholar]
- 59.Martinez V, Sissons HA. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61(11):2291–304. [DOI] [PubMed] [Google Scholar]
- 60.Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Urtni KK. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921–31. [DOI] [PubMed] [Google Scholar]
- 61.Baumhoer D, Amary F, Flanagan AM. An update of molecular pathology of bone tumors. Lessons learned from investigating samples by next generation sequencing. Genes Chromosomes Cancer. 2019;58(2):88–99. [DOI] [PubMed] [Google Scholar]
- 62.Lau AW, Pringle LM, Quick L, Riquelme DN, Ye Y, Oliveira AM, et al. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem. 2010;285(47):37111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T, et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene. 2010;29(25):3619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol 2006;24(1):e1; author reply e2. [DOI] [PubMed] [Google Scholar]
- 65.Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9(4):435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelle DW, Ringler JW, Peacock JD, Kampfschulte K, Scholten DJ 2nd, Davis MM, et al. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res. 2014;164(2):139–48. [DOI] [PubMed] [Google Scholar]
- 67.•. Alhumaid I, Abu-Zaid A. Denosumab therapy in the management of aneurysmal bone cysts: a comprehensive literature review. Cureus. 2019;11(1):e3989. This review summarizes the most current clinical experience with denosumab treatment of ABCs.
- 68.Ntalos D, Priemel M, Schlickewei C, Thiesen DM, Rueger JM, Spiro AS. Therapeutic management of a substantial pelvic aneurysmatic bone cyst Including the off-label use of denosumab in a 35-year-old female patient. Case Rep Orthop. 2017;2017:9125493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurucu N, Akyuz C, Ergen FB, Yalcin B, Kosemehmetoglu K, Ayvaz M, et al. Denosumab treatment in aneurysmal bone cyst: evaluation of nine cases. Pediatr Blood Cancer. 2018;65(4). [DOI] [PubMed] [Google Scholar]
- 70.Pauli C, Fuchs B, Pfirrmann C, Bridge JA, Hofer S, Bode B. Response of an aggressive periosteal aneurysmal bone cyst (ABC) of the radius to denosumab therapy. World J Surg Oncol. 2014;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange T, Stehling C, Frohlich B, Klingenhofer M, Kunkel P, Schneppenheim R, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(6):1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durr HR, Grahneis F, Baur-Melnyk A, Knosel T, Birkenmaier C, Jansson V, et al. Aneurysmal bone cyst: results of an off label treatment with denosumab. BMC Musculoskelet Disord. 2019;20(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyce AM, Collins MT. Fibrous dysplasia/McCune–Albright syndrome: a rare, mosaic disease of Galphas activation. Endocr Rev. 2020;41(2):345–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N Engl J Med. 1991;325(24):1688–95. [DOI] [PubMed] [Google Scholar]
- 75.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187(2):249–58. [DOI] [PubMed] [Google Scholar]
- 76.de Castro LF, Burke AB, Wang HD, Tsai J, Florenzano P, Pan KS, et al. Activation of RANK/RANKL/OPG pathway is involved in the pathophysiology of fibrous dysplasia and associated with disease burden. J Bone Miner Res. 2019;34(2):290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, et al. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging. 2018;9(6):1035–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burke AB, Collins MT, Boyce AM. Fibrous dysplasia of bone: craniofacial and dental implications. Oral Dis. 2017;23(6):697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int 2008;19(1):57–63. [DOI] [PubMed] [Google Scholar]
- 80.Majoor BCJ, Traunmueller E, Maurer-Ertl W, Appelman-Dijkstra NM, Fink A, Liegl B, et al. Pain in fibrous dysplasia: relationship with anatomical and clinical features. Acta Orthop. 2019;90(4):401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stanton RP, Ippolito E, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;7 Suppl 1(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Florenzano P, Pan KS, Brown SM, Paul SM, Kushner H, Guthrie LC, et al. Age-related changes and effects of bisphosphonates on bone turnover and disease progression in fibrous dysplasia of Bone. J Bone Miner Res. 2019;34(4):653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88(10):4569–75. [DOI] [PubMed] [Google Scholar]
- 84.Majoor BC, Appelman-Dijkstra NM, Fiocco M, van de Sande MA, Dijkstra PS, Hamdy NA. Outcome of long-term bisphosphonate therapy in McCune–Albright syndrome and polyostotic fibrous dysplasia J Bone Miner Res. 2017;32(2):264–76. [DOI] [PubMed] [Google Scholar]
- 85.Collins MT, de Castro LF, Boyce AM. Denosumab for fibrous dysplasia: promising, but questions remain. J Clin Endocrinol Metab. 2020;105(11):e4179–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmisano B, Spica E, Remoli C, Labella R, Di Filippo A, Donsante S, et al. RANKL inhibition in fibrous dysplasia ofbone: a preclinical study in a mouse model of the human disease. J Bone Miner Res. 2019;34(12):2171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27(7):1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majoor BCJ, Papapoulos SE, Dijkstra PDS, Fiocco M, Hamdy NAT, Appelman-Dijkstra NM. Denosumab in patients with fibrous dysplasia previously treated with bisphosphonates. J Clin Endocrinol Metab. 2019;104(12):6069–78. [DOI] [PubMed] [Google Scholar]