Abstract
Patients diagnosed with metastatic renal cell carcinoma (RCC) have ~12% chance for 5-year survival. The integrity of the extracellular matrix (ECM) that surrounds tumor cells influences their behavior and, when disturbed, it could facilitate local invasion and spread of tumor cells to distant sites. The interplay between von Hippel-Lindau/hypoxia inducible factor signaling axis and activated kinase networks results in aberrant ECM and tumor progression. Matrix metalloproteinases (MMPs) are proteolytic enzymes implicated in ECM remodeling, tumor angiogenesis, and immune cell infiltration. Understanding the cross-talk between kinase signaling and ECM proteolysis in RCC could provide insights into developing drugs that interfere specifically with the process of invasion. In this review, we discuss changes in the MMPs/ECM axis in RCC, prominent kinase signaling pathways implicated in MMPs induction, and comment on emerging extracellular regulatory networks that modulate MMPs activity.
Keywords: Renal cell carcinoma, Tyrosine kinase, Extracellular matrix, Tumor invasion, MMPs, TIMPs, Extracellular proteolysis
1. Introduction
Histologically, renal cell carcinoma (RCC) is classified into many different subtypes [1,2]. Together, the 3 most common, clear cell renal cell carcinoma (ccRCC), papillary renal cell carcinoma (PRCC) and chromophobe renal cell carcinoma (ChRCC), represent over >95% of renal cancers. Each subtype has a unique histological, genetic and molecular profile, and displays distinct clinical progression or response to treatment [3]. Nevertheless, management, prognosis and treatment options for all patients with RCC are guided by the tumor staging system (TNM) [4,5]. Patients with organ-confined RCC have the best prognosis (5-year survival rate, >90%). However, the ability of tumor cells to breakout from the primary tumor is detrimental for patient’s survival [6,7]. Regional tumor spread worsens prognosis and survival rates drop (5-year survival rate <70% for Stages I/II involving urinary collecting system, and Stage III), while for patients with recurrent or distant metastasis prognosis is poor (5-year survival rate ~10%, Stage IV) [4]. One hallmark of malignant tumors is the capability of cells to invade the extracellular matrix (ECM) initiating the process of metastasis.
2. MMPs and TIMPs expression in RCC
The ECM represents the key structural component of all tissues and organs. The ECM is a highly dynamic proteinaceous network that influences cell behavior by providing physical support and facilitating communication between tumor cells and the stroma [8]. Even though the composition of ECM varies across different RCC subtypes, uncontrolled matrix degradation and aberrant organization are commonly associated with tumor cell invasion and advanced malignancy [9]. The matrix metalloproteinases (MMPs) are a family of 24 zinc-dependent endopeptidases which are capable of digesting almost all components of the ECM [10]. Most MMPs are released from cells as zymogens and require extracellular activation to perform their proteolytic function. It is established that elevated levels of MMPs correlate with invasion, tumor angiogenesis, growth and resistance to treatments across many solid cancers [11]. To limit local tissue destruction and maintain matrix homeostasis, a family of 4 endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), functions to tightly control the proteolytic activity of all MMPs by blocking their active site [12,13].
Over the last twenty years studies have evaluated the predictive and prognostic value of MMPs expression and activity in patients with RCC. Gelatinases MMP2 and MMP9 levels have been studied the most; these 2 proteases predominantly degrade components of the basement membrane including collagens (IV, V, VII, X), elastin and fibronectin. They are required for tumor invasion, contributing to immune cell infiltration and tumor angiogenesis [10]. Uncontrolled MMPs activity creates an imbalance in MMPs/TIMPs ratio. This abnormal increase of net MMP activity is associated with extensive ECM degradation in many cancers. Using RT-PCR technology, the ratio of MMP2/TIMP2 was significantly increased in advanced RCC compared to normal tissue [14]. Separate studies, however, showed that individual levels of MMP2, MMP7, MMP9, MMP10, MMP14, TIMP1 and TIMP2 increase with TNM stage, particular in PRCC compared to ccRCC, and associate with poor prognosis and reduced survival [15-18].
Recently, a more comprehensive MMPs study was performed on RNA-seq data of the Cancer Genome Atlas, where the diagnostic and prognostic value of individual MMPs in fifteen different cancer types including ccRCC, PRCC and ChRCC was evaluated [19]. In general, for most MMPs, expression trends varied across different RCC subtypes compared to adjacent normal controls. Increased expression of MMP9 was observed in all 3 RCC subtypes, while MMP11 and MMP14 expression levels were predominantly higher in ccRCC and PRCC. In ChRCC, MMP13, MMP15, MMP24, MMP26 expression demonstrated the strongest up-regulation. MMPs expression was also significantly associated with overall survival in multiple tumor types. The majority of these significant relationships were in RCC, and for other tumor types only 1 to 3 MMPs were associated with overall survival [19]. These findings were in agreement with data from a recent proteomic analysis of cancer secretomes, suggesting that elevated levels of selected MMPs could be potential biomarkers for RCC progression [20].
Studies on the prognostic significance of MMPs or TIMPs mRNA levels remain undecided for RCC. Furthermore, research on MMPs or TIMPs circulating protein levels in serum or in urine is limited. In one such study, the MMP9/TIMP2 ratio was assessed by ELISA in the serum of patients with metastatic RCC treated with tyrosine kinase inhibitor sunitinib [21]. Although there were no significant differences in the individual levels of MMP9 and TIMP2, the MMP9/TIMP2 ratio was significantly higher at the time of progression compared to their baseline ratio; MMP/TIMP2 could potentially be used as a predictive biomarker for patients treated with sunitinib. An important limitation in all the studies mentioned is the absence of information on whether the MMPs measured were in fact proteolytically active, since only then are they capable of degrading the ECM and promote RCC invasion [22]. Indeed, collagenases MMP1 and MMP13 levels decreased with RCC tumor stage, however, their activity was significantly high and correlated with loss of collagen content [23]. Measuring tumor tissue MMPs activity or circulating levels of active MMPs using available fluorogenic MMP substrates and kinetic assays could be a significant predicting biomarker for RCC progression.
3. ECM and MMPs dysregulation has links to VHL and HIF pathways
ccRCC accounts for over 75% of all RCCs, the majority of which (>50%) demonstrate biallelic von Hippel-Lindau (VHL) gene defect leading to its inactivation [3,24]. Loss of function of the encoded tumor suppressor protein VHL leads to the stabilization of the hypoxia inducible factor alpha subunits (HIF1α/HIF2α) followed by transcriptional induction of HIF-responsive genes that promote tumor growth and angiogenesis.
In RCC, VHL-mediated regulation of the ECM occurs both in HIF-dependent and independent pathways [25,26]. These implicate VHL direct interaction with components of the ECM, including hydroxylated collagen IV and fibronectin, resulting in proper ECM assembly and suppressing of angiogenesis and tumor invasion [27,28]. In addition to components of ECM, VHL has been known to regulate cell-matrix and cell-cell adhesions in RCC. Loss of VHL results in down-regulation of cell junction proteins that would contribute to enhance tumor cell motility and invasion.
VHL is also implicated in elevated expression of MMPs in RCC. Highly invasive RCC cells lacking VHL demonstrated increased MMP2 levels and activity [26]. Proinvasive membrane type-1 matrix metalloproteinase (MT1-MMP or MMP14) is a transcriptional target of HIF-2α in VHL-null RCC [29]. MMP14 facilitates tumor cell invasiveness through increased degradation of type I collagen [29]. Degradation of ECM by MMP2, MMP9 or MMP14 would allow the release of cytokines, growth factors and proangiogenic fragments, including vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGFβ) shown to promote angiogenesis and invasion [30-32].
4. Tyrosine Kinase signaling and MMPs in RCC
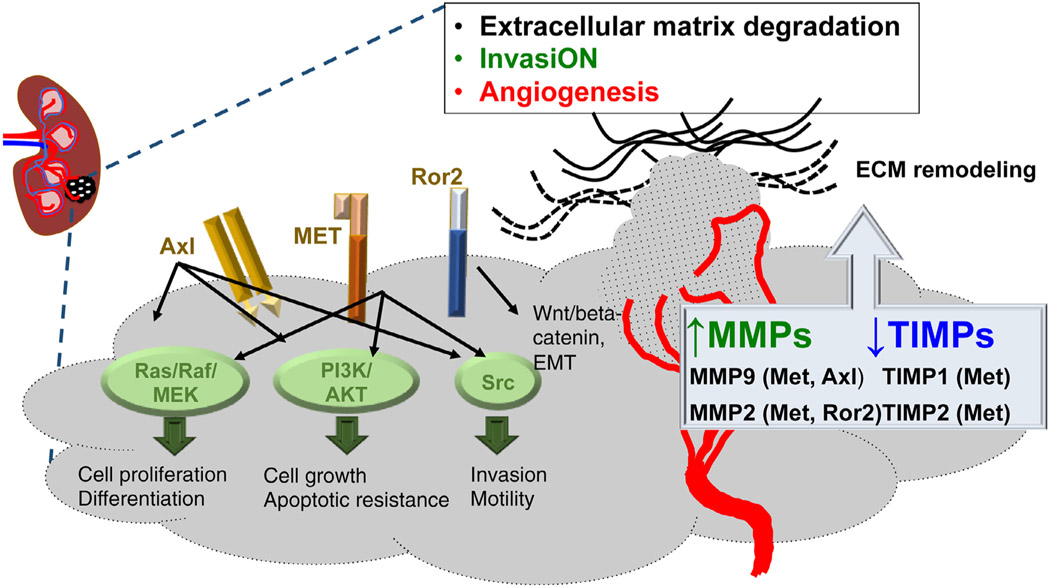
Tyrosine kinase signaling is critical in RCC pathogenesis. Mutations and overexpression of both receptor and nonreceptor tyrosine kinases contributes to the pathogenesis of RCCs and other cancers and serves as a target for therapy [33]. Receptor tyrosine kinases (RTKs) are enzymes that add phosphate groups to tyrosine residues on proteins implicated in signal transduction pathways. Regulation of tyrosine kinase activity is critical in events leading to oncogenesis, including cell cycle progression, differentiation, cellular orientation and motility, and regulation of cell death vs. survival signals. Targeted therapies, including approved nonselective tyrosine kinase inhibitors, demonstrated great benefit in patients with metastatic RCC. Interestingly, many of the targeted kinase pathways regulate the production and proinvasive and proangiogenic function of MMPs in RCC (Fig. 1).
Fig. 1.
Tyrosine kinase pathways in renal cell carcinoma linked to MMPs/TIMPs expression. MET, Axl, Src and Ror2 signaling networks regulate the expression of matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitor of metalloproteinases (TIMPs) in RCC. Changes in levels and activity of MMPs either due to loss of VHL or kinase signaling will impact on ECM remodeling, tumor invasion and metastasis. The capitalized part of ‘invasiON’ indicates that the process of invasion is switched on.
c-MET:
or hepatocyte growth factor receptor (HGFR) is a membrane receptor, with the hepatocyte growth factor (HGF) being the major ligand. In RCC, increased total MET and phospho-MET levels are associated with increased vascularization and poor outcome [34,35]. VHL−/− cells demonstrated enhanced invasiveness and branching morphogenesis in response to HGF, compared to VHL+/+ RCC cell lines. This correlated with up-regulation of MMP2 and MMP9 and down-regulation of MMPs inhibitors TIMP1 and TIMP2 [36]. Treatment with recombinant TIMPs blocked HGF-stimulated branching and invasion, demonstrating a concert between the loss of VHL, MET signaling, and matrix proteolysis [36]. These studies also demonstrated the important contribution of TIMPs in restricting MMPs activity in RCC.
Axl:
Expression of tyrosine kinase receptor AXL is associated with RCC angiogenesis, tumor metastasis and resistance to therapy [37,38]. Once activated, Axl is able to recruit PI3K/AKT/mTOR that regulates cell proliferation through the expression of NFκB. Furthermore, it acts over the RAS/RAF/ERK and PI3K/RAC pathways to regulate survival and cell motility. Axl-mediated activation of NFκB can induce MMP9 expression and hyperactivity, increasing cancer cell invasion and tumor progression [39].
Ror2:
Receptor tyrosine kinase-like orphan receptor 2 (Ror2) is linked to activation of canonical Wnt/beta-catenin signaling in ccRCC [40]. Ror2 appears to be a HIFα responsive gene following loss of VHL function, and its high expression correlates with aggressive and invasive RCC tumors. Studies have linked Ror2 levels with high expression and activity of MMP2 whereas subsequent down-regulation of Ror2 result in MMPs suppression [41]. Ror2 is currently considered an important therapeutic target in RCC.
c-Src:
Proto-oncogene c-Src nonreceptor tyrosine kinase is regularly recruited upon activation of receptor tyrosine kinase signaling (HGF/MET, EGFR, VEGFR, PDGFR, Axl) in RCC. c-Src tyrosine kinase is highly expressed in VHL+/+ RCC [42]. Xenografts with functional VHL were also sensitive to Src inhibitor dasatinib, whereas, in VHL-deficient RCC were resistant to the drug. In response to S1P stimulation (a platelet-derived lysophospholipid), c-Src has been shown to phosphorylate the cytoplasmic tail of MMP14, which is involved in endothelial and tumor cell migration [43,44]. This phosphorylation event is required for tumor-cell proliferation, for invasion through 3D collagen matrices, and for tumor growth in nude mice [43,44]. c-Src mediated phosphorylation of MMP14 could contribute to the aggressive phenotype of VHL+/+ RCC.
5. Latest discoveries on mechanisms of MMP2 regulation
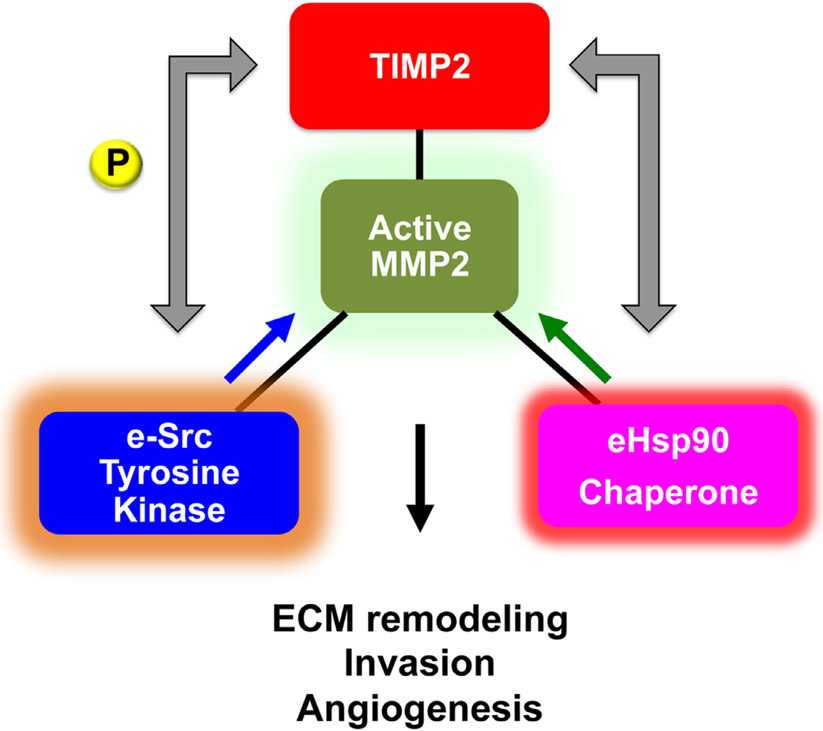
As mentioned earlier, changes in MMPs protein levels may not reflect exclusively the presence of active enzyme that would correlate with ECM proteolysis and tumor cell invasion. In recent years we, and others, have identified new mechanisms in MMP regulation, which are critical for ECM degradation, tumor invasiveness and metastasis. These include (a) the surprising extracellular role of c-Src tyrosine kinase-mediated phosphorylation of TIMP2 and (b) the stabilization and hyperactivation of MMP2 by secreted chaperone HSP90 (eHSP90) [45-47] (Fig. 2).
Fig. 2.
Extracellular regulation of MMP2 activity by secreted c-Src tyrosine kinase (e-Src) and molecular chaperone heat shock protein 90 (eHSP90). e-Src-mediated phosphorylation of TIMP2 facilitates the formation of TIMP2:MMP2 complex and enhances inhibition of active MMP2. Conversely, eHSP90 binding to MMP2 promotes stabilization of the complex and enhances active MMP2 proteolytic function.
5.1. c-Src-mediated phosphorylation of TIMP2
Traditionally, nonreceptor tyrosine kinase c-Src is known to associate with the plasma membrane following its activation. More recently, active c-Src localization to endosomal membranes was linked to secretion of exosomes containing c-Src by human colon cancer cells [48]. Although not shown yet for renal cancer, enrichment of c-Src in extracellular vesicles (EV) was also demonstrated in other malignancies including prostate cancer [49,50]. We showed that c-Src secretion results in tyrosine phosphorylation of TIMP2, the endogenous MMP2 inhibitor [47]. This modification is essential for binding of TIMP2 to both zymogen proMMP2 and active MMP2 enzyme. Lack of TIMP2-Y90 phosphorylation abolishes TIMP2 complex with proMMP2 and consequently proMMP2 could not be processed and activated by membrane bound MMP14. Conversely, TIMP2-Y90 phosphorylation enhanced TIMP2 inhibitory function towards active MMP2. Similar mechanisms of c-Src kinase-mediated phosphorylation of extracellular substrates may occur in RCC. This may also be particularly pronounced in VHL+/+ cancers where c-Src levels and activity are elevated.
5.2. Extracellular HSP90 chaperones MMP2
Following exosomal release, heat shock protein 90 (eHSP90) binds and stabilizes active enzyme MMP2 outside the cell increasing the net active MMP2 proteolytic pool [46]. HSP90 is a highly abundant, 90kDa homodimeric molecular chaperone essential for the folding, activation, maturation and stability of hundreds of intracellular proteins referred as ‘clients’ [51]. Many of these clients are known tumor promoting proteins participating in the molecular pathogenesis of RCC including EGFR, AKT, MET, PDGFR, VEGFR and HIFs [52]. Extracellular HSP90 (eHSP90) interaction with MMP2 promotes motility and invasion of breast, prostate and fibrosarcoma tumor cells in vitro and metastasis in vivo, rendering eHSP90 an important for cancer therapy [53]. Studies from our lab have also shown that TIMP2 is part of an extracellular molecular switch that regulates eHSP90 chaperone function and modulates the formation and activity of eHSP90:MMP2 complex [45]. Although investigations are under way, we can perhaps expect a key role for eHSP90 particularly in VHL−/− RCC, where MMP2 stability would be critical in promoting and invasiveness and tumor angiogenesis.
6. Conclusions
Antiangiogenic therapies have significantly prolonged survival for patients with metastatic disease. However, current TKI therapies result in the development of drug resistance, particularly following use of antiangiogenic regimens. In recent years, understanding the involvement of the immune system in the course of kidney cancer has also led to the development of effective immune-based therapies. Although immunotherapy has become the current standard of care in advanced RCC, response rates are limited and additional therapeutic agents are needed. MMPs were chosen as targets for cancer therapy due to their inherent primary role in extracellular matrix remodeling and for cleavage of its components. It is now believed that our limited understanding on extracellular regulatory mechanisms of MMPs is a contributing factor toward our inability to potently inhibit MMPs proinvasive and proangiogenic function. Assessing changes in both levels and proteolytic function of active MMPs in the tumor stroma could be more meaningful in predicting ECM remodeling and RCC metastatic potential. Critical understanding of the role of extracellular kinases and chaperones in regulating MMPs activity and ECM degradation is essential for developing new or improving current targeted anticancer therapies in RCC.
Footnotes
This is part of the Seminar series guest edited by Drs. Singer and Bratslavsky and supervised by Dr. Maranchie.
References
- [1].Linehan WM. Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res 2012;22: 2089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 2015;48: 166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 2018;23:313–26 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Choueiri TK. Prognostic factors in patients with renal cell carcinoma. In: Richie JP, Atkins MB, editor. UpToDate. [Google Scholar]
- [5].Williamson SR, Taneja K, Cheng L. Renal cell carcinoma staging: pitfalls, challenges, and updates. Histopathology 2019;74:18–30. [DOI] [PubMed] [Google Scholar]
- [6].Cao C, Bi X, Liang J, Li L, Zhang H, Xiao Z, et al. Long-term survival and prognostic factors for locally advanced renal cell carcinoma with renal vein tumor thrombus. BMC Cancer 2019;19:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ha US, Lee KW, Jung JH, Byun SS, Kwak C, Chung J, et al. Renal capsular invasion is a prognostic biomarker in localized clear cell renal cell carcinoma. Sci Rep 2018;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010;123:4195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lohi J, Leivo I, Oivula J, Lehto VP, Virtanen I. Extracellular matrix in renal cell carcinomas. Histol Histopathol 1998;13:785–96. [DOI] [PubMed] [Google Scholar]
- [10].Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 2010;20:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Quintero-Fabian S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argaez V, Lara-Riegos J, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol 2019;9:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 2010;1803:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer 2017;17:38–53. [DOI] [PubMed] [Google Scholar]
- [14].Kugler A, Hemmerlein B, Thelen P, Kallerhoff M, Radzun HJ, Ringert RH. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol 1998;160:1914–8. [PubMed] [Google Scholar]
- [15].Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res 2001;7: 3113–9. [PubMed] [Google Scholar]
- [16].Lu H, Yang Z, Zhang H, Gan M, Zhou T, Wang S. The expression and clinical significance of matrix metalloproteinase 7 and tissue inhibitor of matrix metalloproteinases 2 in clear cell renal cell carcinoma. Exp Ther Med 2013;5:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miyata Y, Iwata T, Maruta S, Kanda S, Nishikido M, Koga S, et al. Expression of matrix metalloproteinase-10 in renal cell carcinoma and its prognostic role. Eur Urol 2007;52:791–7. [DOI] [PubMed] [Google Scholar]
- [18].Qiao ZK, Li YL, Lu HT, Wang KL, Xu WH. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in renal cell carcinoma. World J Surg Oncol 2013;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gobin E, Bagwell K, Wagner J, Mysona D, Sandirasegarane S, Smith N, et al. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019;19:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Robinson JL, Feizi A, Uhlen M, Nielsen J. A systematic investigation of the malignant functions and diagnostic potential of the cancer secretome. Cell Rep 2019;26:2622–35 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miyake H, Nishikawa M, Tei H, Furukawa J, Harada K, Fujisawa M. Significance of circulating matrix metalloproteinase-9 to tissue inhibitor of metalloproteinases-2 ratio as a predictor of disease progression in patients with metastatic renal cell carcinoma receiving sunitinib. Urol Oncol 2014;32:584–8. [DOI] [PubMed] [Google Scholar]
- [22].Remillard TC, Bratslavsky G, Jensen-Taubman S, Stetler-Stevenson WG, Bourboulia D. Molecular mechanisms of tissue inhibitor of metalloproteinase 2 in the tumor microenvironment. Mol Cell Therapies 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mlynarczyk G, Kudelski J, Darewicz B, Bruczko-Goralewska M, Romanowicz L. Suppressed expression but not activity of collagenases MMP-1 and MMP-13 in human renal carcinoma. Pathobiology 2019;86:201–7. [DOI] [PubMed] [Google Scholar]
- [24].Shen C, Kaelin WG Jr.. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol 2013;23:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jonasch E, Futreal PA, Davis IJ, Bailey ST, Kim WY, Brugarolas J, et al. State of the science: an update on renal cell carcinoma. Mol Cancer Res 2012;10:859–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kurban G, Hudon V, Duplan E, Ohh M, Pause A. Characterization of a von Hippel Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res 2006;66:1313–9. [DOI] [PubMed] [Google Scholar]
- [27].Kurban G, Duplan E, Ramlal N, Hudon V, Sado Y, Ninomiya Y, et al. Collagen matrix assembly is driven by the interaction of von Hippel-Lindau tumor suppressor protein with hydroxylated collagen IV alpha 2. Oncogene 2008;27:1004–12. [DOI] [PubMed] [Google Scholar]
- [28].Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN, et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell 1998;1:959–68. [DOI] [PubMed] [Google Scholar]
- [29].Petrella BL, Lohi J, Brinckerhoff CE. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene 2005;24:1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bostrom AK, Lindgren D, Johansson ME, Axelson H. Effects of TGF-beta signaling in clear cell renal cell carcinoma cells. Biochem Biophys Res Commun 2013;435:126–33. [DOI] [PubMed] [Google Scholar]
- [31].Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004;16:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu F, Chang K, Ma J, Qu Y, Xie H, Dai B, et al. The oncogenic role of COL23A1 in clear cell renal cell carcinoma. Sci Rep 2017;7:9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alonso-Gordoa T, Garcia-Bermejo ML, Grande E, Garrido P, Carrato A, Molina-Cerrillo J. Targeting tyrosine kinases in renal cell carcinoma: "New Bullets against Old Guys". Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 2013;24:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miyata Y, Kanetake H, Kanda S. Presence of phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res 2006;12:4876–81. [DOI] [PubMed] [Google Scholar]
- [36].Koochekpour S, Jeffers M, Wang PH, Gong C, Taylor GA, Roessler LM, et al. The von Hippel-Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor-induced invasion and branching morphogenesis in renal carcinoma cells. Mol Cell Biol 1999;19:5902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu H, Liu R, Ma B, Li X, Yen HY, Zhou Y, et al. Axl receptor tyrosine kinase is a potential therapeutic target in renal cell carcinoma. Br J Cancer 2015;113:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zucca LE, Morini Matushita MA, da Silva Oliveira RJ, Scapulatempo-Neto C, de Lima MA, Ribeiro GG, et al. Expression of tyrosine kinase receptor AXL is associated with worse outcome of metastatic renal cell carcinomas treated with sunitinib. Urol Oncol 2018;36:11 e3–e21. [DOI] [PubMed] [Google Scholar]
- [39].Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW. Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene 2008;27:4044–55. [DOI] [PubMed] [Google Scholar]
- [40].Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, et al. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 2009;28: 2513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rasmussen NR, Debebe Z, Wright TM, Brooks SA, Sendor AB, Brannon AR, et al. Expression of Ror2 mediates invasive phenotypes in renal cell carcinoma. PLoS One 2014;9:e116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suwaki N, Vanhecke E, Atkins KM, Graf M, Swabey K, Huang P, et al. A HIF-regulated VHL-PTP1B-Src signaling axis identifies a therapeutic target in renal cell carcinoma. Sci Transl Med 2011;3:85ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nyalendo C, Beaulieu E, Sartelet H, Michaud M, Fontaine N, Gingras D, et al. Impaired tyrosine phosphorylation of membrane type 1-matrix metalloproteinase reduces tumor cell proliferation in three-dimensional matrices and abrogates tumor growth in mice. Carcinogenesis 2008;29:1655–64. [DOI] [PubMed] [Google Scholar]
- [44].Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, et al. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J Biol Chem 2007;282:15690–9. [DOI] [PubMed] [Google Scholar]
- [45].Baker-Williams AJ, Hashmi F, Budzynski MA, Woodford MR, Gleicher S, Himanen SV, et al. Co-chaperones TIMP2 and AHA1 competitively regulate extracellular HSP90: client MMP2 activity and matrix proteolysis. Cell Rep 2019;28:1894–906 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol 2004;6:507–14. [DOI] [PubMed] [Google Scholar]
- [47].Sánchez-Pozo J, Baker-Williams AJ, Woodford MR, Bullard R, Wei B, Mollapour M, et al. Extracellular phosphorylation of TIMP-2 by secreted c-Src tyrosine kinase controls MMP-2 activity. iScience. 2018;1:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hikita T, Kuwahara A, Watanabe R, Miyata M, Oneyama C. Src in endosomal membranes promotes exosome secretion and tumor progression. Sci Rep 2019;9:3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, et al. c-Src, Insulin-like growth factor I receptor, G-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. J Cell Biochem 2017;118:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Di Noto G, Paolini L, Zendrini A, Radeghieri A, Caimi L, Ricotta D. C-src enriched serum microvesicles are generated in malignant plasma cell dyscrasia. PLoS One 2013;8:e70811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 2010;11:515–28. [DOI] [PubMed] [Google Scholar]
- [52].Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010;10: 537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wong DS, Jay DG. Emerging roles of extracellular Hsp90 in cancer. Adv Cancer Res 2016;129:141–63. [DOI] [PubMed] [Google Scholar]