Abstract
Background:
Laboratory and medication data in electronic health records create opportunities for clinical decision support (CDS) tools to improve medication dosing, laboratory monitoring, and detection of side effects. This systematic review evaluates the effectiveness of such tools in preventing medication-related harm.
Methods:
We followed the Laboratory Medicine Best Practice (LMBP) initiative’s A-6 methodology. Searches of 6 bibliographic databases retrieved 8508 abstracts. Fifteen articles examined the effect of CDS tools on (a) appropriate dose or medication (n = 5), (b) laboratory monitoring (n = 4), (c) compliance with guidelines (n = 2), and (d) adverse drug events (n = 5). We conducted meta-analyses by using random-effects modeling.
Results:
We found moderate and consistent evidence that CDS tools applied at medication ordering or dispensing can increase prescriptions of appropriate medications or dosages [6 results, pooled risk ratio (RR), 1.48; 95% CI, 1.27–1.74]. CDS tools also improve receipt of recommended laboratory monitoring and appropriate treatment in response to abnormal test results (6 results, pooled RR, 1.40; 95% CI, 1.05–1.87). The evidence that CDS tools reduced adverse drug events was inconsistent (5 results, pooled RR, 0.69; 95% CI, 0.46–1.03).
Conclusions:
The findings support the practice of healthcare systems with the technological capability incorporating test-based CDS tools into their computerized physician ordering systems to (a) identify and flag prescription orders of inappropriate dose or medications at the time of ordering or dispensing and (b) alert providers to missing laboratory tests for medication monitoring or results that warrant a change in treatment. More research is needed to determine the ability of these tools to prevent adverse drug events.
BACKGROUND
Medication management is complex, and medication errors are a leading cause of preventable harm to patients. Thus, medication management is a particularly appropriate target for patient safety interventions. Ambulatory and hospitalized patient populations in the US each experience more than 500000 serious adverse drug events each year, and many or most of the adverse events are considered preventable (1–3).
With electronic health records (EHRs)15 now in use at essentially all healthcare organizations in the US, there are unprecedented opportunities to use the data within them to improve medication safety. An elegant example of this functionality is the use of clinical decision support (CDS) tools that monitor data elements in the EHR to automatically detect situations that place patients at risk of harm and allow more timely intervention (4, 5). Using electronic algorithms to address medication safety was one of the earliest applications of CDS tools (6–8).
Laboratory testing plays a prominent role in monitoring the appropriate and safe use of medications. The ability to link laboratory test results with specific medications creates the opportunity to improve the appropriate choice and dosing of medications (8–12), compliance with laboratory monitoring (13–15), and earlier and more reliable detection of side effects (16) and toxicity (13, 17, 18). This systematic review evaluates the effectiveness of CDS tools that link laboratory and medication data to prevent adverse drug events and to improve clinical outcomes.
METHODS
We followed the A-6 methodology, developed by the Centers for Disease Control and Prevention’s (CDC’s) Laboratory Medicine Best Practices (LMBP) initiative, for the evaluation of quality improvement practices (19–20). This approach is a validated method designed to transparently evaluate studies of practice effectiveness. The overarching goal of the review was to assess whether the use of laboratory-related CDS reduces the risk that patients will experience an adverse event or reduces the harm to patients who experience such an event. We accepted the definition of adverse events used by the individual studies to assess the evidence. To build a chain of evidence, the review examined evidence regarding the following specific questions on intermediate outcomes, health outcomes, and harms:
How accurately do the tools identify errors or patients at risk of an adverse event?
Are opportunities to avoid harm increased by the use of laboratory-related CDS tools?
Is the risk of adverse events or of severe adverse events reduced by use of the tools?
Is the severity of harms reduced among patients who experience an adverse drug event?
Does the implementation of laboratory-related CDS tools result in patients receiving unnecessary or inappropriate treatment?
Does the implementation of laboratory-related CDS tools result in patients not receiving needed treatment?
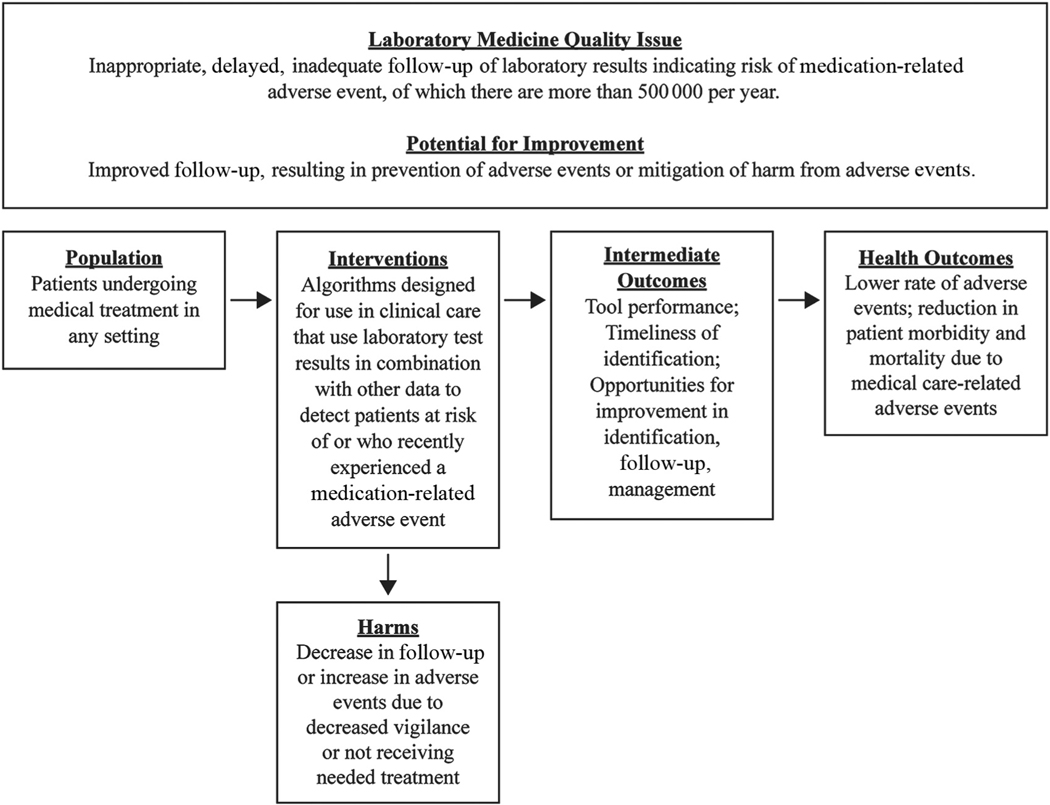
The review protocol was developed with the input of a panel of experts in clinical care, laboratory medicine, systematic review, informatics, and patient safety (see Appendix A in the Data Supplement that accompanies the online version of this article at http://www.jalm.org/content/vol3/issue6). The protocol is available from the corresponding author on request. The analytic framework for the review is shown in Fig. 1. The inclusion parameters of the review were as follows:
Fig. 1.
Analytic framework.
Population: Patients undergoing medical treatment in any type of healthcare setting.
Interventions: CDS tools designed for use in clinical care to detect patients who are at risk of or have experienced a medication-related adverse event that included 2 or more pieces of information, at least 1 of which was a laboratory test result.
Comparators: Patients managed without the use of medication-related CDS tools.
Outcomes: Timeliness of identification of patients at risk; predictive values, reduction in errors, or actions that may cause harm; rate of medication-related adverse events among patients at risk; morbidity or mortality in patients with a medication-related adverse event; and severity of morbidity in patients with a medication-related adverse event.
Timeframe: Studies published in English from January 1, 1990, through April 11, 2016.
Setting. Any patient care settings.
A professional librarian conducted literature searches in PubMed, Embase, Cochrane, Web of Science, PsychINFO, and CINAHL. The search terms captured relevant CDS tools, alerts, algorithms, CDS systems, errors, and patient safety (see Appendix B in the online Data Supplement). Citations were also identified by expert panel members and by manual searches of bibliographies of relevant studies. We sought unpublished studies through expert panelists and relevant professional organizations. Two scientists with experience in systematic review methodology independently evaluated each retrieved citation against predetermined criteria for inclusion. If they disagreed, a senior scientist reviewed the abstraction and decided if the citation fit the inclusion criteria and should be included. They excluded articles regarding CDS tools for the following reasons: (a) did not incorporate laboratory test results; (b) were letters, editorials, commentaries, or abstracts; (c) did not include data on the use of the tool in a patient population; (d) were about interventions that were not applied to patient care; (e) did not have a comparator; (f) did not assess an outcome of interest; or (g) did not have an appropriate study design (e.g., case reports or case series). This article reports on only CDS tools to address medication errors.
A public health scientist experienced in systematic review methodology abstracted data related to study characteristics, intervention components, outcomes, and results. A senior scientist reviewed each abstraction. Two senior reviewers independently appraised the quality of the included studies by using the A-6 quality appraisal tool (19). Studies that scored 4 or less out of 10 were excluded from analysis (19).
Evidence synthesis and meta-analysis
Evidence was synthesized by a key question for each intervention type and outcome. The strength of the evidence was rated as high, moderate, suggestive, or insufficient on the basis of the number of studies, the study ratings, and the consistency and magnitude of the effect size, as described by Christenson et al. (19). Briefly, a rating of high evidence requires ≥3 studies of good quality that found a substantial effect of the intervention; moderate evidence requires 2 studies of good quality with a substantial effect size or ≥3 good studies with moderate effect size; and suggestive evidence requires 1 good study with a substantial effect size, 2 good studies with a moderate effect size, or ≥3 fair studies of moderate effect size.
We conducted meta-analysis to determine pooled effect size when we had ≥3 studies of the same type of intervention and outcome. The χ2 test for heterogeneity was used to determine whether fixed effects modeling (homogeneous effects) or random-effects modeling (heterogeneous effects) were used for the meta-analysis.
RESULTS
Search results
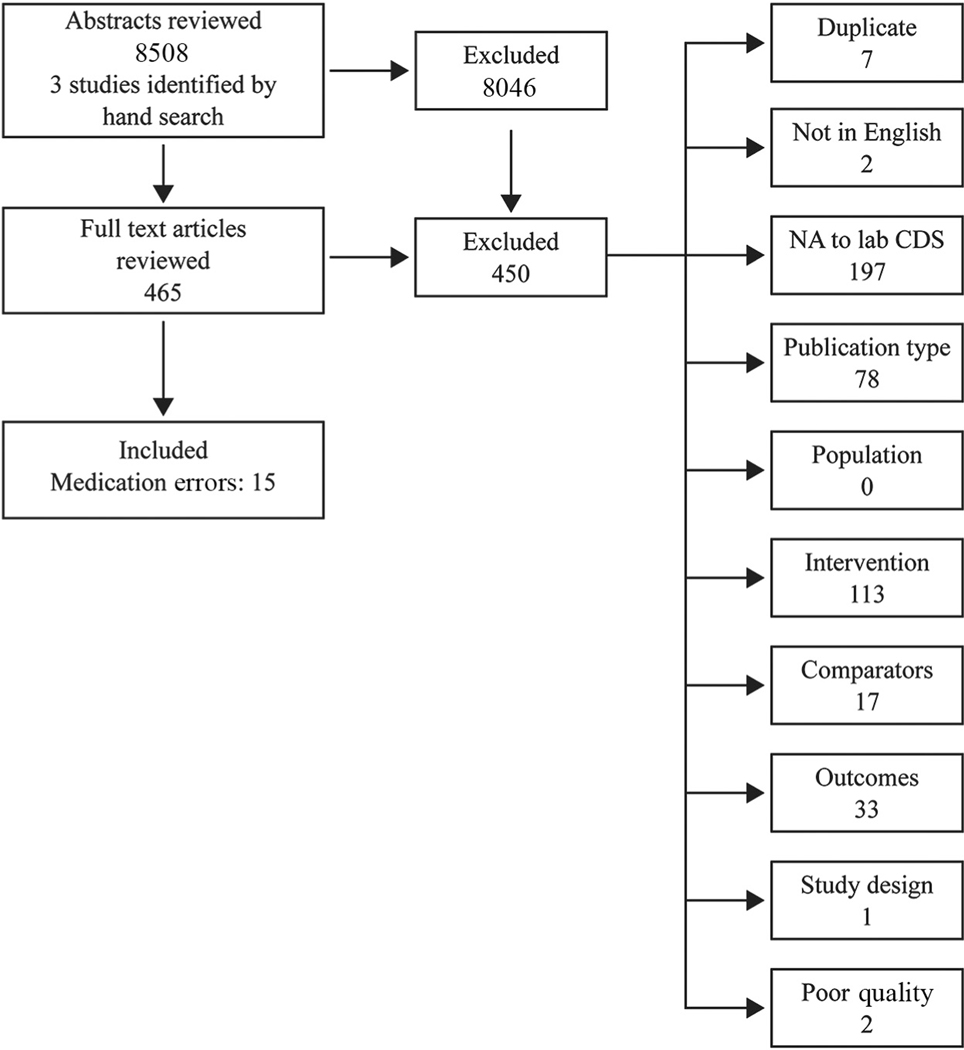
We retrieved 8508 abstracts and identified 3 studies from hand searches of bibliographies. Seventeen studies were included after full text review, but 2 (13, 18) were excluded because of poor study quality, leaving 15 studies for analysis (Fig. 2). These 15 papers examined the effect of CDS tools on 3 intermediate outcomes related to medication management: (a) exposure to inappropriate dose or medication (n = 5), (b) laboratory monitoring (n = 4), (c) compliance with other medication-related guidelines and recommendations (n = 2) and on the primary outcome of adverse drug events (n = 5).
Fig. 2.
Literature search results.
Table 1 and Fig. 3 summarize the evidence for each key question and intervention. The supplemental tables provide detailed information on the characteristics of the included studies (see Appendix C in the online Data Supplement) and the evidence tables for each key question (see Appendix D in the online Data Supplement).
Table 1.
Summary of body of evidence for the effect of CDS tools.
| Citation | RR (95% Cl) | Quality appraisal | Effect size rating | Consistencya | Strength of evidence |
|---|---|---|---|---|---|
| Increased likelihood of appropriate medication or dose | |||||
| Consistent | Moderate | ||||
| Awdlshu (2015) (9) | 1.89(1.45–2.47) | Good (9) | Moderate | ||
| Bhardwaja (2011) (24) | 1.31 (1.26–1.36) | Good (10) | Moderate | ||
| Chertow (2001) (27) | 1.71 (1.64–1.77) | Fair(7) | Moderate | ||
| Kazeml (2011) (23) | 1.49(1.40–1.59) | Fair(7) | Moderate | ||
| Selller (2009) (22): Residents | 1.43 (0.85–2.38) | Good (8) | Moderate | ||
| Selller (2009) (22): Senior physicians | 0.60(0.3–1.22) | Good (8) | Adverse | ||
| Meta-analysis | 1.48(1.27–1.74) | — | Moderate | Heterogeneous | |
| Increased use and receipt of recommended laboratory tests for monitoring medication safety | |||||
| Consistent | Moderate | ||||
| Galanter, K+ supplementation (2004) (10) | 1.73(1.61–1.85) | Fair(7) | Moderate | ||
| Galanter, dlgoxln concentrations (2004) (10) | 1.21 (1.16–1.26) | Fair(7) | Moderate | ||
| Hoch (2003) (14) | 1.04(1.03–1.05) | Good (8) | Minima | ||
| Matheny (2008) (15) | 1.24(0.71–2.15) | Good (10) | Moderate | ||
| Steele, recommended lab test ordered (2005) (25) | 1.44(1.27–1.63) | Good (9) | Moderate | ||
| Steele, Incorrect medication order stopped (2005) (25) | 1.97(1.26–3.08) | Good (9) | Moderate | ||
| Meta-analysis | 1.40(1.05–1.87) | — | Moderate | Heterogeneous | |
| Compliance with guidelines and recommendations | |||||
| Inconsistent | Insufficient | ||||
| Judge (2006) (26) | 1.11 (1.00–1.22) | Good (8) | Minima | ||
| Riggio (2009) (16) | 0.66(0.44–0.99) | Good (9) | Adverse | ||
| Reduction in adverse drug events | |||||
| Inconsistent | Moderate | ||||
| Evans (1998) (8) | 0.3(0.10–0.51) | Fair(7) | Substantial | ||
| Gurwltz (2008) (27) | 1.06(0.92–1.23) | Good (8) | Minima | ||
| Mullet (2001) (28) | 0.85(0.38–1.89) | Fair(7) | Minima | ||
| Rind (1994) (7) | 0.45 (0.22–0.94)b | Good (9) | Substantial | ||
| Steele (2005) (25) | 0.41 (0.10–1.77) | Good (9) | Substantial | ||
| Meta-analysis | 0.69 (0.46–1.03) | — | Moderate | Heterogeneous |
Consistency of the body of literature is a qualitative assessment of the direction of the effect. The body of literature Is considered consistent if the direction of the effect Is the same for all or almost all studies. The homogeneity of the studies In the meta-analyses Is a statistical test and was evaluated by the χ2 test for heterogeneity.
Calculated from presented data.
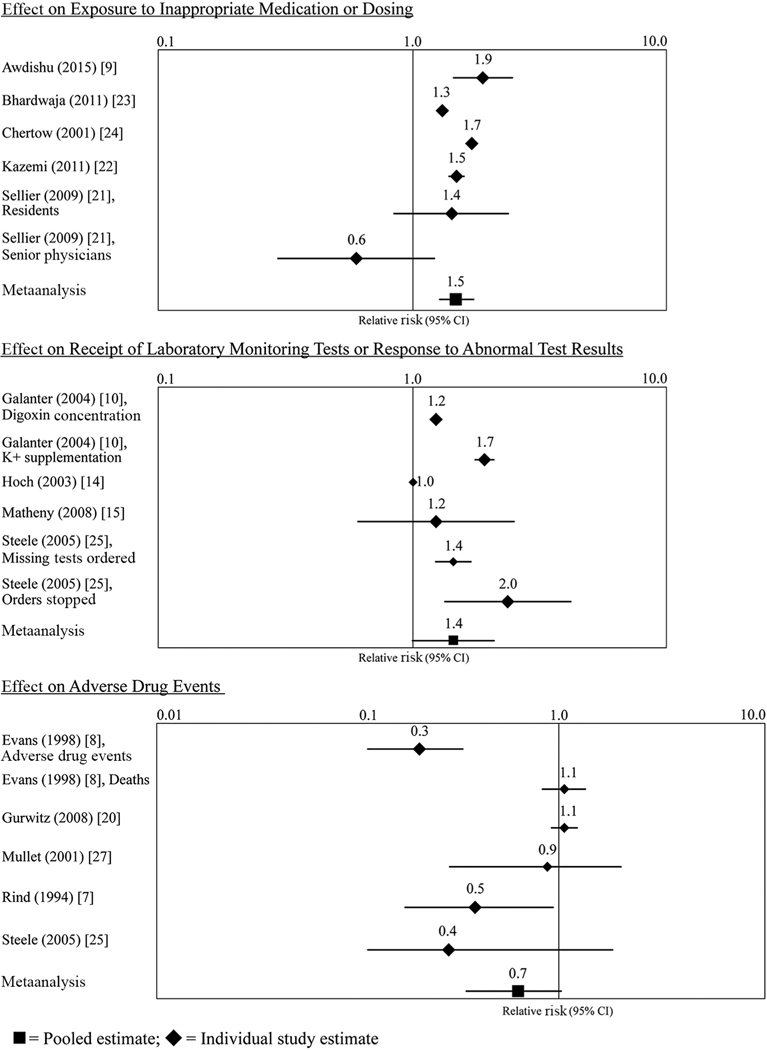
Fig. 3.
Effect of clinical support tools on key outcomes.
Effect on inappropriate dosing or medication
CDS tools designed to reduce prescriptions for inappropriate dosing or medication were embedded in computerized physician order entry (CPOE) systems (9, 21–23) or in pharmacy databases (24). Five studies (9, 21–24) provided moderate evidence that the use of CDS tools can reduce patient exposure to inappropriate medications or dosages (Table 1).
Four studies examined CDS tools embedded within CPOEs. A cluster randomized trial conducted in hospital and ambulatory settings found that prospective alerts increased the proportion of appropriate medication adjustment for patients with renal insufficiency almost 2-fold (OR, 1.89; 95% CI, 1.45–2.47) (9). Kazemi et al. (23) reported that antibiotic and anticonvulsant dosing errors were reduced significantly after implementation of CPOE with CDS, from 55% without CPOE to 53% with CPOE and to 34% with CPOE and CDS [calculated risk ratio (RR) for appropriate dosing: 1.49; 95% CI: 1.40–1.59]. Selliers et al. (22) reported that during the periods when a CDS tool was active, resident surgeons tended to write more appropriate prescriptions (RR, 1.43; 95% CI, 0.85–2.38), but senior physicians tended to write fewer appropriate prescriptions (RR, 0.6; 95% CI, 0.3–1.22). The modification of the effect by physician experience was statistically significant. Chertow et al. (21) used a CDS tool embedded in the hospital computing system to implement a dosing algorithm for inpatients with renal insufficiency. The intervention improved the proportion of medication orders that were both the right dose and frequency (calculated RR, 1.71; 95% CI, 1.64–1.77).
The fifth study (24) examined a tool targeted at pharmacists instead of physicians. The investigators conducted a randomized, controlled trial of a pharmacy alert designed to decrease errors in drug selection or dosing for 15 drugs frequently prescribed to patients with renal insufficiency. Prescriptions with inappropriate dosing were significantly less common in the intervention group than in the usual care group (33% vs 49%; P < 0.001). Patients treated during the intervention period were 31% more likely to receive the correct dose (calculated RR, 1.31; 95% CI, 1.26–1.36).
We conducted a meta-analysis of these 5 studies (9, 21–24). The χ2 test for heterogeneity indicated that the effect sizes were heterogeneous (P < 0.0001), so random-effects modeling was used to allow variation in the magnitude of the effect. The pooled effect size for the likelihood that new prescriptions were for an appropriate medication and dose was 1.48 (95% CI, 1.27–1.74) (Fig. 3). The body of evidence that CDS tools can reduce medication errors was rated as moderate and consistent.
Effect on laboratory monitoring of medication safety
Four studies (10, 14, 15, 25) examined the effect of CDS tools to improve laboratory monitoring of medication concentrations or potential side effects. The evidence was consistent and moderate. Matheny et al. (15) conducted a cluster randomized trial of the efficacy of an EHR reminder on the completion of overdue or missing serum creatinine tests. The tool did not improve the rate of completed tests (RR, 1.24; 95% CI, 0.71–2.15). Hoch et al. (14) examined the effect of an electronic alert to monitor the potassium concentrations of patients on diuretics. The tool had a significant but modest effect (RR, 1.04; 95% CI, 1.03–1.05). Steele (25) tested the effect of CPOE-embedded CDS on clinicians stopping orders, based on laboratory values and ordering missing laboratory tests. The RR for the stopping inappropriate medications based on laboratory values was 1.97 (95% CI, 1.26 –3.08); the RR for ordering missing laboratory tests was 1.44 (95% CI, 1.27–1.63). Galanter et al. (10) examined whether alerts regarding missing laboratory tests or abnormal laboratory test results among patients on digoxin resulted in an order for the recommended test or a treatment change, such as electrolyte supplementation, to address the abnormal laboratory value. The implementation group more rapidly checked for missing test results (P < 0.001) and ordered missing tests for digoxin concentrations within 24 h (OR, 1.21; 95% CI, 1.16 –1.26). The implementation group also more often ordered electrolyte supplementation for newly reported hypokalemia (calculated OR, 1.73; 95% CI, 1.61–1.85) and hypomagnesemia (P < 0.0001) within 24 h of an asynchronous alert. Supplementation for existing but untreated hypomagnesemia and hypokalemia was not significantly different between the control and intervention groups.
The meta-analytic summary of these studies used a random-effects model (χ2 test for heterogeneity, P value <0.0001). The pooled RR was 1.40 (95% CI, 1.05–1.87).
Effect on compliance with other recommendations
Two studies (16, 26) examined physician compliance with other recommendations related to medication safety. The evidence on these outcomes was inconsistent and insufficient. Judge et al. (26) examined the effect on physicians’ actions of the CDS tool created by Gurwitz et al. (27), which generated many types of alerts related to medication management. They found that physicians on the intervention units were more likely to take an appropriate action in response to the clinical situation than those on control units (RR, 1.11; 95% CI, 1.00–1.22). Riggio et al. (16) examined the effect of alerts of potential heparin-induced thrombocytopenia on the time to discontinuation of heparin and initiation of an alternative method of anticoagulation. They found that the time between platelet concentrations reaching the criteria for thrombocytopenia and the discontinuation of heparin was significantly longer after implementation of the tool (HR, 0.66; 95% CI, 0.44–0.99). We considered the interventions and outcomes examined in these studies too disparate for valid meta-analysis.
Effect on medication-related adverse events
Five studies (7, 8, 25, 27, 28) looked at the ability of CDS tools to reduce adverse drug events. Gurwitz (27) tested the ability of a decision tool combined with a CPOE to identify preventable drug-related adverse events in a cluster randomized trial. They found no difference between the intervention and control groups (RR, 1.06; 95% CI, 0.92– 1.23). Evans (8) tested a decision support system to help physicians determine antibiotic therapy. Antiinfective agent-related adverse events were more frequent during the preintervention period (0.025 events per patient) than the intervention period (0.007 events per patient) (RR, calculated, 0.30; 95% CI, 0.10–0.84; P = 0.018). There was no significant effect on overall mortality (RR, 1.07; 95% CI, 0.84–1.35), possibly because of lack of power to detect this outcome. Mullett et al. (28) adapted this antiinfective tool for use in a pediatric population. They found a small, nonsignificant difference in drug-related adverse events during the intervention period (RR, 0.85; 95% CI, 0.38–1.89). There was also a 36% decrease in the rate of subtherapeutic risk days and a 28% decrease in excessive dose days.
Rind (7) examined the effect of alerts regarding rising creatinine concentrations on the risk of patient renal impairment. Creatinine monitoring was improved and renal impairment reduced (RR, 0.45; 95% CI, 0.22–0.94) during the intervention periods compared to the control periods. Steele (25) tested the effect of a CDS combined with CPOE and reported that it did not reduce the proportion of potential adverse drug events in the postintervention period compared to preintervention periods, but the study was likely underpowered to detect such events (calculated RR, 0.41; 95% CI, 0.10–1.77).
The effect of CDS tools on the prevention of adverse drug events was heterogeneous (χ2 test for heterogeneity, P < 0.0001). With random-effects meta-analysis, the pooled estimate was RR, 0.69 (95% CI, 0.46–1.03). Although the body of evidence met the A-6 criteria for moderate evidence, the effect estimates were inconsistent in direction and magnitude and the meta-analysis confidence intervals included 1.00. The body of evidence was judged too inconsistent to support a recommendation on this outcome.
DISCUSSION
The advent of the EHR has provided the opportunity to use digital information to improve patient safety. CDS resources that combine patient-specific data with appropriate guidelines are an excellent example of this functionality. Both the Office of the National Coordinator and the National Academy of Medicine have endorsed efforts to improve the safety of healthcare by use of health informatics resources, both for diagnosis and for management (29–31).
Systematic review findings/implications
Healthcare systems with the technological capability should incorporate into their computer prescription ordering systems CDS tools that integrate laboratory test data. Specifically, these tools should
Identify and flag prescription orders for which laboratory data indicate a potentially inappropriate dose or medication at the point of order or dispensing. Consistent evidence of moderate strength indicates that such tools, when applied at the point of ordering or dispensing, can reduce the number of patients exposed to inappropriate medications or dose.
Alert providers when prescribed medication requiring laboratory monitoring has missing test results or when a patient has abnormal laboratory medication monitoring test results requiring a change in treatment. Consistent evidence of moderate strength indicates that such CDS tools improve receipt of laboratory monitoring of medication concentrations and potential side effects and the likelihood of appropriate response to abnormal results.
In this review, we examined the utility of decision support tools that use laboratory data to improve medication safety. In total, 15 studies (7–10, 14–16, 21–28) provided information on the effectiveness of laboratory-based CDS tools to prevent medication errors or adverse drug events. Consistent evidence of moderate strength supports the utility of CDS tools, when implemented at the point of order or dispensing, to reduce patients’ exposure to inappropriate medications or dosing errors and that CDS tools that flag missing or abnormal laboratory monitoring test results can increase orders of recommended laboratory tests or instigate appropriate treatment changes. The evidence was insufficient and inconsistent to conclude that laboratory-based CDS tools influence physician compliance with medication-related guidelines. Importantly, there was insufficient evidence to conclude that CDS tools decrease adverse events. The evidence in this systematic review supports the practice of healthcare systems with the technological capability incorporating CDS tools into CPOE systems. We recognize that these tools have only been shown to affect practice metrics, not the ultimate outcome of fewer adverse events. The identified evidence was insufficient and inconsistent to conclude that laboratory-based CDS tools reduce adverse drug events: we identified only 4 studies, of which 3 had wide CIs. The absence of good evidence cannot be interpreted to mean that adverse event reduction will not occur with CDS. Further research in this area is needed. We did not find studies that investigated potential harms or unintended consequences of CDS tools.
This review is the most recent in a growing body of evidence that the value to patients of laboratory medicine relies on more than the delivery of accurate test results (32–36). Inappropriate omission or inclusion of laboratory tests, or the misapplication of results, have been shown to be major sources of patient harm (30). Only one (17) of the studies had an author identifiable as from a laboratory department, and it did not include authors from other departments. Laboratory physicians and scientists can systematically ensure that opportunities for reducing harm are appropriately prioritized and successfully implemented (37). In the authors’ opinion, the involvement of laboratory scientists will be critical to successful implementation of the recommended interventions.
The systematic review methodology has several strengths that limit the risk of bias in the finding. These strengths include a rigorous protocol with defined inclusion and exclusion criteria, transparent and systematic search criteria evaluated on their ability to identify known relevant citations, and a defined methodology for evaluating the identified evidence. There are some limitations to our review and findings, however. A variety of terms are used to describe the types of CDS tools examined here. Although our search strategy included multiple terms and captured all our known citations, we may have missed some articles. As noted above, the literature included relatively few studies that examined health outcomes or adverse drug events, limiting our ability to draw conclusions in this area. The small body of literature on the relevant questions and the varied outcomes reported required us to classify findings into broad outcomes groups. We could have inadvertently introduced bias through this grouping.
As with any systematic review, selective publication of positive studies could have led us to overestimate the effect of the evaluated interventions. We actively sought unpublished studies and searched conference abstracts and other gray literature to reduce the potential for publication bias. The results of the searches did not suggest publication bias accounted for our findings, however. We did not identify any unpublished studies, and we identified a full text article for each study identified by a conference abstract.
The sensitivity and specificity of the CDS tool obviously affects its impact on patient safety. Metzger et al. used a simulation tool to examine the effectiveness of CPOE systems to detect medication orders at high risk of causing a serious adverse drug event (38). Among the 62 hospitals studied, implemented CPOE systems detected only 53% of the orders that would have caused fatalities and 44% of orders that would have caused a serious adverse drug event. The highest performing hospitals, which detected 70%–80% of problematic medication orders, had implemented advanced CDS.
In addition, the design and implementation of CDS tools greatly affect their effectiveness in practice. Kawamoto et al. (39) conducted a systematic review of the literature and found that such tools improved practice 68% of the time. Four features independently predicted the effectiveness of a CDS tool:
Decision support is supplied automatically in the clinician work flow.
The CDS provides recommendations, not just assessments.
The support is provided when the decision is being made.
The CDS tool was computer based.
These features are also included in Bates et al.’s (40) “ten commandments” of CDS tools. In addition, their commandments stress the importance that the CDS be fast (<1 s to change screens), anticipate the needs of the clinician, be easy to use (with usability testing), present recommendations simply, and require little or no data input by the clinician. Bates et al. (40) also noted that it is easier to get physicians to change treatments or actions than to get them to stop an action or treatment with no replacement. Awdishu et al. (9) suggested that this tendency may explain why their CDS tool affected dose adjustment more strongly than discontinuation of medication and why alerts at the initial medication prescription were more successful than those for existing prescriptions.
The most critical feature for a successful CDS tool is that it be well integrated within the clinician’s work flow at decision-making. Several of the studies in our review stress the importance of presenting alerts during the process of ordering or dispensing medication to provide guidance and allow changes in real time (8–10, 22, 24). Evans et al. (8) also explicitly noted that their tool was designed to be easy to use and access and to save the clinician time. Their CDS tool retrieved information needed for a prescription in 3.5 s, compared to 14 min for a search by an infectious disease specialist. The tool achieved physician acceptance and dramatic improvements in clinical and financial outcomes, leading to requested installation in additional facilities within the healthcare system, which emphasizes the great importance of usability in design and implementation of such a tool (8).
The studies in this review noted other factors that contributed to the success or failure of their tools:
Collaboration with clinicians and leaders across departments, institutional support, and sponsorship by key stakeholders during tool development (24, 27).
Openness and response to feedback on operational problems (24).
Presentation of all relevant information on the same screen as that used to order treatment or take action (10).
Specific and immediately relevant alerts and recommendations (10, 26, 27).
Highly visible notices (15).
Ongoing decision support systems (27).
There is inconsistent evidence on how physicians’ experience and characteristics affect their response to CDS tool alerts. Awdishu et al. (9) found that residents were less likely to respond to alerts than more experienced physicians (9). They hypothesized that residents may become desensitized to the alerts because they place more orders than senior physicians and therefore experience more alerts. In contrast, Sellier et al. (22) found that residents were more likely than senior physicians to respond to alerts. In fact, senior physicians were more likely to take actions counter to those recommended during the intervention periods. They suggested that senior physicians may disagree with the recommendations or have judged that the benefit of their prescription outweighed the risk. Hoch et al. also found that younger physicians were more likely to respond to alerts, as were women physicians (14).
Approximately 25% of preventable medication-related admissions can be attributed to failures of appropriate monitoring (41). As Bates and Gawande (40) observed, “monitoring is inherently boring and is not performed well by humans.” The key contributor of the CDS tools examined here is their ability to compensate for this human deficiency by continuously monitoring for defined indications of a problem. Their usefulness relies on the comprehensiveness of the defined indicators and the willingness of healthcare professionals to consider the information provided by the tools. The usefulness of these tools may be optimized by laboratory involvement in the CDS design. Thoughtful design and technical content are critical to the success of CDS tools.
Supplementary Material
IMPACT STATEMENT.
Ambulatory and hospitalized patients in the US each experience >500000 serious adverse drug events yearly. The ability to link laboratory test results with specific medications creates the opportunity to improve the choice and dosing of medication and supports earlier detection of side effects and toxicity. This systematic review provides evidence supporting the practice of healthcare systems with the technological capacity incorporating laboratory test-based CDS tools into computerized physician ordering systems to (a) identify prescription orders of inappropriate dose or medications and (b) alert providers to missing laboratory tests for medication monitoring or results that warrant treatment changes.
Acknowledgments:
The authors appreciate the thoughtful insights offered by the following expert panel members: Dr. David West, University of Colorado; Dr. Hardeep Singh, Houston Veteran’s Administration Patient Safety Center of Inquiry and Baylor College of Medicine; Dr. Ranjjt Singh, State University of New York at Buffalo, Department of Family Medicine; and Dr. Meera Viswanathan, RTI-EPC Evidence-Based Practice Center, RTI International.
N. Whitehead, statistical analysis; L. Williams, financial support; S. Kennedy, administrative support; N. Ubaka-Blackmoore, statistical analysis.
Role of Sponsor:
The funding organizations played a direct role in the design of study, review and interpretation of data, preparation of manuscript, and final approval of manuscript.
Research Funding:
Contract number 200-2014-F-61251/006 from the Centers for Disease Control and Prevention.
Footnotes
Nonstandard abbreviations: EHR, electronic health record; CDS, clinical decision support; CDC, Centers for Disease Control and Prevention; LMBP, Laboratory Medicine Best Practices; CPOE, computerized physician order entry.
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the offiial position of the Centers for Disease Control and Prevention.
Definition: Laboratory-related CDS tool, consists of an algorithm that uses laboratory test results and other data to identify potential medication errors or medication-related patient harms and alert a healthcare team member to prompt a change in action to prevent or mediate the potential harm.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
Employment or Leadership: L. Williams, CDC Atlanta GA; S. Kennedy, RTI International; D. Classen, Pascal Metrics; J. Nichols, JALM, AACC; T. Lorey, JALM, AACC, Kaiser Permanente; P. Epner, Society to Improve Diagnosis in Medicine, Silicon BioDevices, Inc dba Xip Diagnostics.
Consultant or Advisory Role: P. Epner, Viewics, Inc.
Stock Ownership: P. Epner, Silicon BioDevices, Inc. dba Xip Diagnostics.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
REFERENCES
- 1.Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, et al. Adverse drug events in ambulatory care. N Engl J Med 2003;348:1556–64. [DOI] [PubMed] [Google Scholar]
- 2.Tache SV, Sonnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother 2011;45:977–89. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine Committee on Identifying and Preventing Medication Errors. Preventing medication errors. Wahington (DC): National Academies Press; 2007. p. 28. [Google Scholar]
- 4.Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care 2003;12 Suppl 2:ii39 – 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Classen DC, Resar R, Griffin F, Federico F, Frankel T, Kimmel N, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood) 2011;30: 581–9. [DOI] [PubMed] [Google Scholar]
- 6.Classen DC, Pestotnik SL, Evans RS, et al. Description of a computerized adverse drug event monitor using a hospital information system. Hosp Pharm 1992;27:774, 776 –9, 783. [PubMed] [Google Scholar]
- 7.Rind DM, Safran C, Phillips RS, Wang Q, Calkins DR, Delbanco TL, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med 1994;154:1511–7. [PubMed] [Google Scholar]
- 8.Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, Orme JF Jr, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998;338:232–8. [DOI] [PubMed] [Google Scholar]
- 9.Awdishu L, Coates CR, Lyddane A, et al. The impact of real-time alerting on appropriate prescribing in kidney disease: a cluster randomized controlled trial. J Am Med Inform Assoc 2016;23:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanter WL, Polikaitis A, DiDomenico RJ. A trial of automated safety alerts for inpatient digoxin use with computerized physician order entry. J Am Med Inform Assoc 2004;11:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manns B, Tonelli M, Culleton B, Faris P, McLaughlin K, Chin R, et al. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney dsease. Clin J Am Soc Nephrol 2012;7:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura Y, Yamaguchi T, Hasegawa H, Yoshihara K, Zhang Q, Mineno T, Takeda H. Alert system for inappropriate prescriptions relating to patients’ clinical condition. Methods Inf Med 2009;48:566–73. [DOI] [PubMed] [Google Scholar]
- 13.Morel P, Vandel B. Adverse drug reaction monitoring and the Internet: evaluation of the use of the Internet by French Pharmacovigilance Centres and a non-exhaustive survey of websites of interest for collecting information about adverse drug reaction. Therapie 1999;54:525–32. [PubMed] [Google Scholar]
- 14.Hoch I, Heymann AD, Kurman I, Valinsky LJ, Chodick G, Shalev V. Countrywide computer alerts to community physicians improve potassium testing in patients receiving diuretics. J Am Med Inf Assoc 2003;10:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matheny ME, Sequist TD, Seger AC, Fiskio JM, Sperling M, Bugbee D, et al. A randomized trial of electronic clinical reminders to improve medication laboratory monitoring. J Am Med Inform Assoc 2008;15:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riggio JM, Cooper MK, Leiby BE, Walenga JM, Merli GJ, Gottleib JE. Effectiveness of a clinical decision support system to identify heparin iduced thrombocytopenia. J Thromb Thrombolysis 2009;28:124–31. [DOI] [PubMed] [Google Scholar]
- 17.Flynn N, Dawnay A. A simple electronic alert for acute kidney injury. Ann Clin Biochem 2015;52(Pt 2):206–12. [DOI] [PubMed] [Google Scholar]
- 18.Mansour H, Dilkhush D, Lannigan J, Whalen KL. The impact of a computerized potassium alert on adverse drug events an pharmacists’ interventions. J Pharm Technol 2010;26:55–9. [Google Scholar]
- 19.Christenson RH, Snyder SR, Shaw CS, Derzon JH, Black RS, Mass D, et al. Laboratory medicine best practices: systematic evidence review and evaluation methods for quality improvement. Clin Chem 2011;57:816–25. [DOI] [PubMed] [Google Scholar]
- 20.Division of Laboratory Services Centers for Diseas Control and Prevention. Laboratory Medicine Best Practices (LMBP™) 2015. https://www.cdc.gov/labbestpractices/index.html (Accessed July 2017).
- 21.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA 2001;286:2839–44. [DOI] [PubMed] [Google Scholar]
- 22.Sellier E, Colombet I, Sabatier B, Breton G, Nies J, Zapletal E, et al. Effect of alerts for drug dosage adjustment in inpatients with renal insufficiency. J Am Med Inform Assoc 2009;16:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemi A, Ellenius J, Pourasghar F, Tofighi S, Salehi A, Amanati A, Fors UG. The effect of Computerized Physician Order Entry and decision support system on medication errors in the neonatal ward: experiences from an Iranian teaching hospital. J Med Syst 2011;35:25–37. [DOI] [PubMed] [Google Scholar]
- 24.Bhardwaja B, Carroll NM, Raebel MA, Chester EA, Korner EJ, Rocho BE, et al. Improving prescribing safety in patients with renal insufficiency in the ambulatory setting: the Drug Renal Alert Pharmacy (DRAP) program. Pharmacotherapy 2011;31:346–56. [DOI] [PubMed] [Google Scholar]
- 25.Steele AW, Eisert S, Witter J, Lyons P, Jones MA, Gabow P, Ortiz E. The effect of automated alerts on provider ordering behavior in an outpatient setting. PLoS Med 2005;2:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judge J, Field TS, DeFlorio M, Laprino J, Auger J, Rochon P, et al. Prescribers’ responses to alerts during medication ordering in the long term care setting. J Am Med Inform Assoc 2006;13:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, et al. Effect of computerized provider order entry with clinical decision suppor on adverse drug events in the long-term care setting. J Am Geriatr Soc 2008;56:2225–33. [DOI] [PubMed] [Google Scholar]
- 28.Mullett CJ, Evans RS, Christenson JC, Dean JM. Development and impact of a computerized pediatric antiinfective decision support program. Pediatrics 2001; 108:E75. [DOI] [PubMed] [Google Scholar]
- 29.Committee on Patient Safety and Health Information Technology; Institute of Medicine. Health IT and patient safety: building safer systems for better care. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 30.National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington (DC): National Academies Press; 2015. [Google Scholar]
- 31.The Office of the National Coordinator for Health Information Technology. Federal health IT strategic plan 2015–2020. Washington (DC): United States Department of Health and Human Services; 2015. [Google Scholar]
- 32.Buehler SS, Madison B, Snyder SR, Derzon JH, Cornish NE, Saubolle MA, et al. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 2016;29:59–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaRocco MT, Franek J, Leibach EK, Weissfeld AS, Kraft CS, Sautter RL, et al. Effectiveness of preanalytic practices on contamination and diagnostic accuracy of urine cultures: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 2016;29:105–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Layfield C, Rose J, Alford A, Snyder SR, Apple FS, Chowdhury FM, et al. Effectiveness of practices for improving the diagnostic accuracy of non ST elevation myocardial infarction in the emergency department: a laboratory medicine best practices systematic review. Clin Biochem 2015;48:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebow EB, Derzon JH, Fontanesi J, Favoretto AM, Baetz RA, Shaw C, et al. Effectiveness of automated notification and customer service call centers for timely and accurate reporting of critical values: a laboratory medicine best practices systematic review and meta-analysis. Clin Biochem 2012;45:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandhu PK, Musaad SM, Remaley AT, Buehler SS, Strider S, Derzon JH, et al. Lipoprotein biomarkers and risk of cardiovascular disease: a laboratory medicine best practices (LMBP) systematic review. J Appl Lab Med 2016; 1:214–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf 2013;22 Suppl 2: ii6 –ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzger J, Welebob E, Bates DW, et al. Mixed results in the safety performance of computerized physician order entry. Health Aff (Millwood) 2010;29:655–63. [DOI] [PubMed] [Google Scholar]
- 39.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med 2003;348: 2526–34. [DOI] [PubMed] [Google Scholar]
- 41.Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care 2003;12: 280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.