Abstract
Acute myeloid leukemia (AML) is a heterogeneous disease caused by aberrant proliferation and/or differentiation of myeloid progenitors. However, only ∼65% of AML patients respond to induction chemotherapy and the overall survival rate for AML remains low (∼24% for 5-year survival). The conventional view suggests that ATP-binding cassette (ABC) transporters contribute to treatment failure due to their drug-effluxing capabilities. This might be overly simplistic. Some ABC transporters export endogenous substrates that have defined roles in normal hematopoietic progenitors. It is conceivable that these substances also provide an advantage to leukemic progenitors. This review will highlight how certain endogenous substrates impact normal hematopoietic cells and suggest that ABC transporters facilitate export of these substances to affect both normal hematopoietic and leukemic progenitors. For example, the ability to export certain endogenous ligands may facilitate leukemogenesis by modifying leukemic progenitor cell proliferation or survival. If so, the addition of ABC transporter inhibitors to traditional chemotherapy might improve therapeutic efficacy by not just increasing intracellular drug accumulation but also blocking the beneficial effects ABC transporter ligands have on cell survival.
1. HEMATOPOIESIS AND LEUKEMIA
1.1. Hematopoietic stem cells and ABC transporters
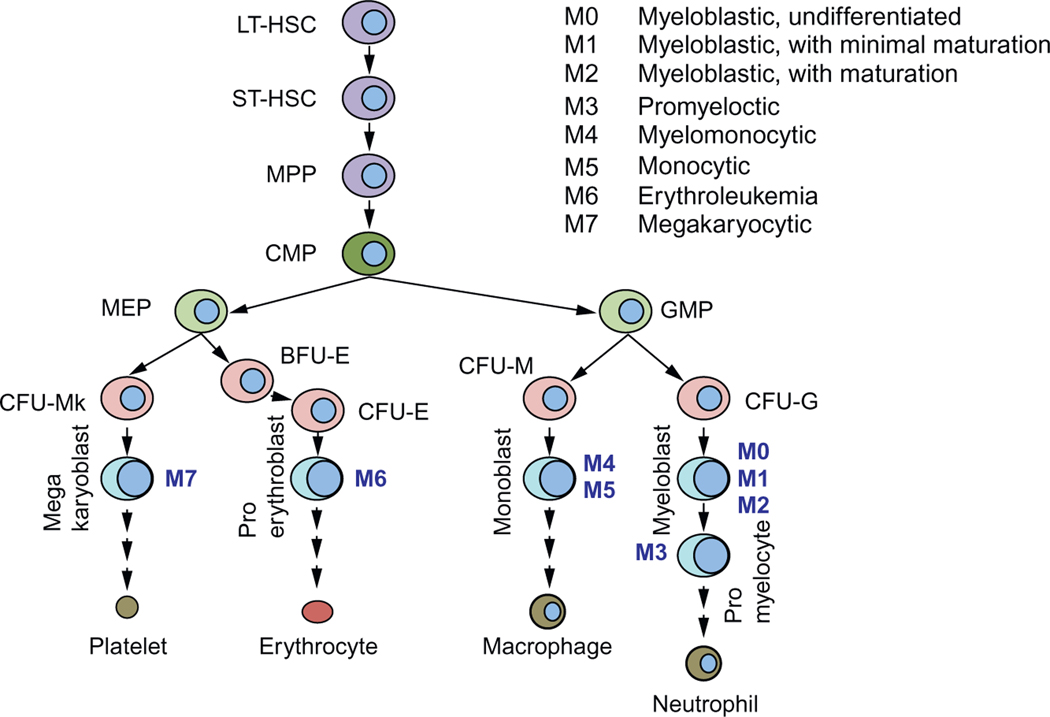
Hematopoietic stem cells (HSCs) have the potential to undergo self-renewal and differentiation into multiple lineage committed blood cells. HSCs are characterized by their ability to repopulate the bone marrow, and single HSC gives rise to all blood cell types in circulation (Osawa, Hanada, Hamada, & Nakauchi, 1996; Smith, Weissman, & Heimfeld, 1991). By transplanting genetically marked mouse bone marrow cells into lethally irradiated recipient mice, Till and McCulloch showed that each spleen colony-forming unit (CFU) originated from a single clonogenic cell (Becker, McCulloch, & Till, 1963). Subsequent studies have shown that HSCs and their progenitors can be identified and isolated using cell surface markers (Spangrude, Heimfeld, & Weissman, 1988). Long-term HSCs have an unlimited self-renewal capacity, whereas short-term HSCs have limited self-renewal ability. The HSC progeny gives rise to multipotent progenitors (MPPs), which then differentiate to lineage-restricted progenitors including the myeloid progenitors (Fig. 1). It is generally acknowledged that HSCs show properties such as quiescence, drug resistance (through the expression of several ATP-binding cassette (ABC) transporters), an active DNA-repair capacity, and resistance to apoptosis, which enable long life span. Murine HSCs are characterized by the absence of markers for committed blood lineage (Lin−) and expression of c-Kit and Sca-1 (Lin−Sca-1+c-Kit+, LSK). Additional cell surface markers can discriminate long- and short-term HSCs as well as MPPs. Common lymphoid progenitors (CLP) express IL-7R, whereas other committed progenitors do not. Oligopotent myeloid progenitors lose Sca-1 expression, and a combination of FcγR and CD34 expression identifies common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte/erythroid progenitors (MEPs).The hierarchy of myeloid progenitors was determined by a combination of morphology and surface markers that was identified in each progenitor population (Akashi, Traver, Miyamoto, & Weissman, 2000). For example, CMPs were capable of generating all myeloerythroid cells, whereas GMP generated only granulocyte/ macrophage (GM) cells and MEP formed only megakaryocyte/erythroid cells in their colonies.
Figure 1.
Hierarchy of hematopoiesis and leukemia in myeloid lineage. AML subtypes according to FAB classification (M0–M7) are indicated.
In humans, HSCs are identified by the cell surface markers, CD34 and CD38. Lineage-negative CD34+CD38− cells are enriched for progenitors capable of repopulating bone marrow of NOD–SCID mice with all lineages. Committed progenitors acquire the expression of CD38, and the human counterparts of CMP, GMP, and MEP were identified in the Lin−CD34+CD38+ population. Differentiation of progenitors into the myeloid lineage requires growth factors such as interleukin-3, GM-colony-stimulating factor, steel factor (c-Kit ligand), and erythropoietin as well as various transcription factors including runt-related transcription factor-1.
HSCs capacity to extrude toxins is likely related to high levels of ABC transporters that can protect cells from accumulation of endogenous and exogenous cytotoxic compounds (Table 1). In addition, the export of small molecules might serve a signaling function (e.g., leukotriene C4, LTC4). Side population (SP) cells are enriched for stem cells and are characterized by their ability to efflux the fluorescent dye, Hoechst 33342 (Goodell, Brose, Paradis, Conner, & Mulligan, 1996). ABCG2 was identified as the transporter responsible for Hoechst 33342 efflux which conferred the SP phenotype (Zhou et al., 2002). Absence of Abcg2 in hematopoietic cells did not affect normal unperturbed hematopoiesis. However, absence of Abcg2 rendered HSC markedly sensitive to chemotherapeutic agents that were Abcg2 substrates (e.g., mitoxantrone). Abcg2 may not protect all stem cells as embryonic stem cells or neural stem cells lack high Abcg2 expression (Ramalho-Santos, Yoon, Matsuzaki, Mulligan, & Melton, 2002).
Table 1.
ABC transporters and endogenous ligands
| Transporter | Ligand | Function |
|---|---|---|
|
| ||
| MRP1 (ABCC1) | LTC4, LTD4, LTE4 | Inflammatory response |
| GSH, GSSG, bilirubin, S1P | Redox | |
|
| ||
| MRP4 (ABCC4) | PGE1, PGE2, PGF2 | Cell migration |
| cAMP, cGMP, TXB2, LTB4, LTC4 | CFTR function modulation Inflammatory response |
|
|
| ||
| BCRP (ABCG2) | Flavonoids, porphyrins (PPIX) cGMP, folic acid | Survival under hypoxia |
1.2. Leukemic stem cells
Cancer stem cells are thought of as a rare group of cells that have self-renewal capacity coupled with the ability to produce tumors from a small number of initiating cells. The frequency of these cancer stem cells reportedly ranges from 0.0001% to 0.1% of the total cells; however, they may not be that rare (in some cases, e.g., myeloma; Huff & Matsui, 2008). Cancer stem cells were first identified in leukemia, but have also been identified in solid tumors such as brain, breast, cervix, and prostate (Dean, Fojo, & Bates, 2005). The leukemia-initiating cell population was identified and characterized 20 years ago (Bonnet & Dick, 1997; Lapidot et al., 1994). In acute myeloid leukemia (AML), the cell surface of leukemic stem cells (LSCs) resembles normal HSCs by both displaying the CD34+CD38− surface marker and possessing the ability to self-renew, proliferate, and differentiate. LSCs occur at an estimated frequency of 1 per 2×105 cells and produce AML when transplanted into NOD–SCID mice. In comparison, AML cells with the surface marker CD34+CD38+ have properties in common with more committed cells, i.e., limited self-renewal, and are nontumorigenic. These initial studies reported that regardless of the AML subtype (see Fig. 1), only CD34+CD38− AML cells produced leukemia in NOD–SCID recipient mice.
However, there are exceptions to the idea that only CD34+CD38− AML cells initiate leukemia. For instance, acute promyelocytic leukemia (French-American-British (FAB) class M3) is caused by an oncogenic PML–RARA fusion gene that is only detected in the CD34+CD38+ population. In this case, the CD34+CD38− cells were not tumorigenic. Moreover, recent studies using mouse leukemia models have suggested that the target cells for some oncogenes, such as MLL-AF9 and MOZ-TIF2, initiate AML in more committed progenitors such as the GMP (Huntly et al., 2004; Somervaille & Cleary, 2006). These LSCs resemble committed myeloid progenitors, but appear to have acquired the capacity for self-renewal, a property absent in their normal counterparts. Regardless of the origin, LSCs are self-renewing clonogenic cells that produce AML.
Self-renewal, increased proliferation, and altered differentiation are key features of leukemia cells. Notably, constitutive activation of pathways that drive proliferation is often observed in LSCs. For example, Wnt/β-catenin signaling is important in normal HSCs but is highly active in chronic myelogenous leukemia (CML) cells that are rapidly proliferating during blast crisis. PI3K activates signaling pathways such as Wnt and mTOR and is activated in AML. In contrast, PTEN, a PI3K antagonist that regulates self-renewal of normal HSC, is often downregulated in AML.
1.3. AML chemotherapy and ABC transporters
Chemotherapy is the mainstream treatment for AML; however, the development of drug resistance is a major obstacle in successfully achieving remission. As exporters of a variety of classes of chemotherapeutics, ABC transporters are considered to play a role in multidrug resistance in AML. For example, ABCB1/P-glycoprotein (P-gp) exports structurally diverse cancer chemotherapeutics including anthracyclines, one of the main chemotherapeutics used in the treatment of AML, and a marker for poor prognosis in AML (reviewed extensively in Shaffer et al., 2012). Because of its clinical relevance, P-gp inhibitors have been implemented in clinical trials to investigate if their addition to standard chemotherapeutic regimens improves clinical outcome. The results have been disappointing thus far and appear due to the nonspecificity of the inhibitors as well as the pharmacokinetic effects due to altered metabolism (Libby & Hromas, 2010). A widely accepted role for ABC transporters in AML chemotherapy is associated with their capacity to export drugs, thereby limiting leukemic cell drug exposure (Shaffer et al., 2012); however, other modes of resistance may involve intracellular drug sequestration.
Indeed, overexpression of ABCA3 increased lysosomal mass and lysosomal drug retention. This drug sequestration correlated with reduced cytotoxicity (Chapuy et al., 2009; Steinbach et al., 2006; Wulf et al., 2004). In this review, we focus on the potential role of some ABC transporters (in particular ABCC1, ABCC4, and ABCG2) in leukemia biology through their ability to modulate endogenous ligands.
2. ABC TRANSPORTERS THAT EXPORT REGULATORY MOLECULES
2.1. Cyclic nucleotides—cAMP
The cyclic nucleotide, cyclic adenosine monophosphate (cAMP), is generated by adenylate cyclase in response to extracellular ligands and mediates a broad range of cellular responses through activation of protein kinase A. Intracellular levels of cAMP are modulated by factors such as phosphodiesterases and export (Cheepala et al., 2013). However, cyclic nucleotides are also compartmentalized into domains in the plasma membrane (Li et al., 2007; Sinha et al., 2013). Cyclic AMP is produced from ATP when adenylate cyclase is activated by G protein-coupled receptors (GPCRs). In the bone marrow niche, several GPCRs including β-adrenergic receptors, prostaglandin E2 (PGE2) receptors, and the chemokine MIP-1α receptors play an important role in affecting hematopoietic cell cAMP levels. Cyclic nucleotide-regulated pathways are involved in hematopoietic progenitor cell (HPC) proliferation and differentiation. The bone marrow is densely innervated by β-adrenergic fibers, and PGE2 and MIP-1α are secreted by monocytes and macrophages. Therefore, it is not surprising that HPCs contain high concentrations of cAMP-dependent PKA (Kobsar et al., 2008). Activation of PKA mediates a cascade of signaling events including PI3K/Akt and mTOR pathways.
Interestingly, cAMP inhibits proliferation of the myeloid leukemia cell lines, human Mo7e, and the murine myeloid progenitor cell line, 32D (Hendrie & Broxmeyer, 1994; Lee, 1999). Furthermore, the stable cAMP analog, 8-chloroadenosine 3′,5′-cyclic monophosphate (8-CL-cAMP), inhibited the self-renewal capacity of blast progenitors from acute myeloblastic leukemia patients (Pinto et al., 1992). Stimulation of PKA inhibits HPC proliferation in HPCs from tumor patients (Kobsar et al., 2008). Thus, it appears, in myeloid leukemia cells, that elevation of cAMP and PKA activation inhibits myeloid leukemia proliferation, unless factors counterbalance this.
2.2. MRP4 and cAMP
Within the C subfamily of ABC transporters, 13 full-length multidrug-related proteins (MRPs; i.e., encode two membrane-spanning domains and two nucleotide-binding domains in one transcript) have been identified, together with the cystic fibrosis transmembrane conductance regulator (ABCC7) and the sulfonylurea receptors (ABCC8 and ABCC9). MRP4/ ABCC4 was the first ABC transporter shown to export nucleotide monophosphates (Schuetz et al., 1999), of which one of them (PMEA aka adefovir) resembled cAMP. Thus, it was not surprising that cAMP and cGMP were identified as endogenous substrates (Chen, Lee, & Kruh, 2001). Subsequent studies have identified other endogenous molecules such as ADP, eicosanoids, urate, bile acids, and conjugated steroid hormones as potential in vivo substrates (Ritter et al., 2005). Other ABCC family members MRP5 and 8 have also been shown to transport cAMP and cyclic guanosine monophosphate (cGMP), but these will not be discussed here as ABCC8 is discussed in the review by Nies et al. (Chapter 8).
MRP4 modulates the cAMP concentrations in cells in two ways: by affecting internal concentration by export and creating domains or gradients of cAMP concentration within the plasma membrane. In this context, MRP4 can reduce the local concentration of membrane by “flipping” cAMP from the membrane, to locally reduce its concentration (Li et al., 2007). MRP4 can also directly export cAMP as was originally shown by Kruh and colleagues (Chen et al., 2001). Distinguishing between MRP4 flippase and transport activity is not easily done.
Absence of MRP4 profoundly affects how cells migrate. For example, fibroblasts lacking MRP4 have both elevated membrane and intracellular cAMP and exhibit an enhanced rate of migration (Sinha et al., 2013). It is unknown if the migration of leukemic or hemapoietic cells is impacted in a similar way. Furthermore, how cAMP in the membrane versus intracellular cAMP affects proliferation and differentiation of normal hematopoietic and leukemia cells is unknown (Copsel et al., 2011).
2.3. Prostaglandins
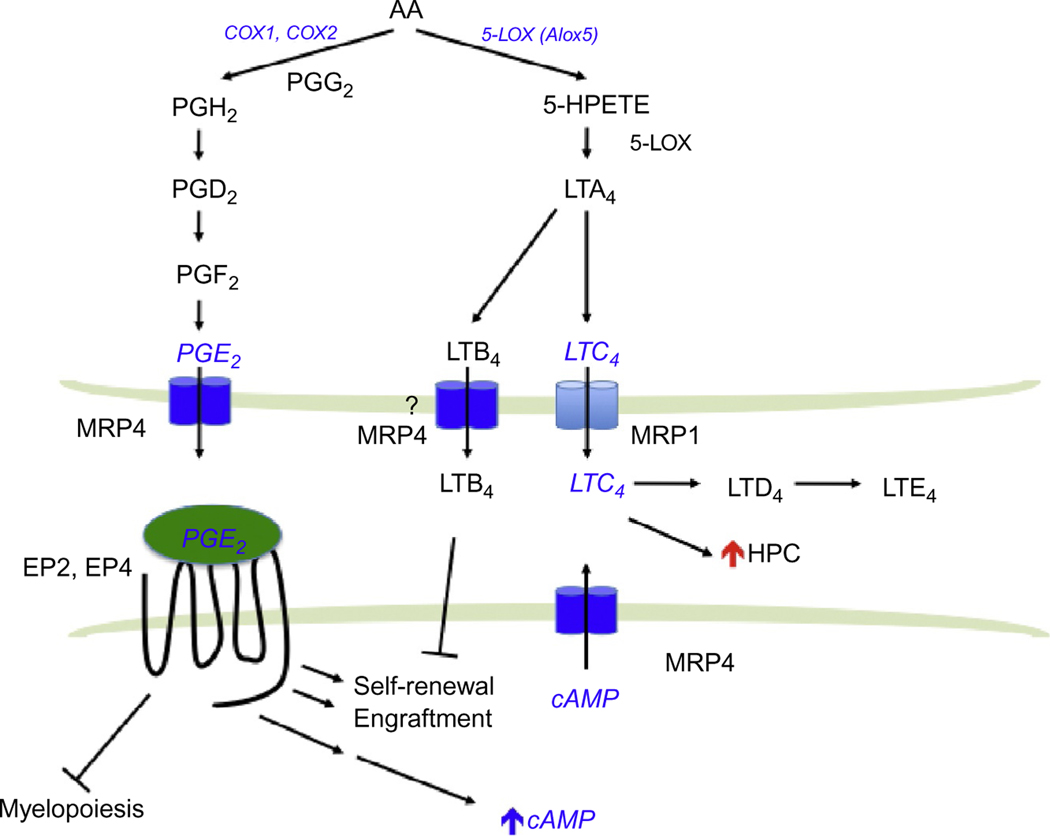
Prostaglandins are potent lipids derived from phospholipase-released arachidonic acid (AA) that are involved in numerous homeostatic biological functions and inflammation. Prostaglandins are synthesized de novo by prostaglandin H synthase (PGHS; referred to as COX for cyclooxygenase) when cells are activated by various external stimuli. PGHS exists as two isoforms referred to as PGHS-1 (COX-1) and PGHS-2 (COX-2; Fig. 2). In general, COX-1 is the enzyme responsible for basal, constitutive prostaglandins synthesis, whereas COX-2 is important in various inflammatory and “induced” settings. Prostaglandins are formed and “released” by most cells to act as autocrine- or paracrine-signaling molecules. In many cases, they initiate their effects via interaction with extracellular GPCRs to affect downstream signaling pathways (Breyer & Breyer, 2000).
Figure 2.
Eicosanoid biosynthesis pathways. Prostaglandins and leukotrienes are synthesized from a common precursor, arachidonic acid (AA). Molecules implicated in leukemia are highlighted in blue.
2.4. Prostaglandin and HSCs
PGE2 is the most abundant eicosanoid and has a variety of salutary effects in hematopoietic cells. In 1974, PGE2 was shown to regulate myeloid progenitor differentiation (Feher & Gidali, 1974). In the bone marrow, myeloid cells, as well as osteoclasts in particular, contain a high PGE2 synthetic capacity. Osteoclasts are an important component of the HSC niche and PGE2 released from these cells may regulate HSC and HPC function. PGE2 specifically binds to EP1–4 receptors, which are coupled to their respective G proteins to mediate distinct downstream pathways (Sugimoto & Narumiya, 2007). While EP3 receptor activation inhibits adenylate cyclase, EP2 and EP4 receptors activate it, which results in cAMP elevation. PGE2 dose-dependently inhibits growth of human and colony-forming units granulocyte/macrophage (CFU-GM) in vitro (Pelus, Broxmeyer, Kurland, & Moore, 1979; Pelus, Broxmeyer, & Moore, 1981) and myelopoiesis in vivo (Gentile, Byer, & Pelus, 1983) but stimulates erythroid and multilineage progenitor cells (Lu, Pelus, & Broxmeyer, 1984; Lu et al., 1987). Short-term ex vivo treatment of bone marrow cells with PGE2 increases the proportion of mouse colony-forming units spleen (CFU-S) (Feher & Gidali, 1974). In humans, PGE2 treatment increases the proportion of cycling CFU-GM from quiescent cells that appear to be stem cells. This effect is critically dependent on timing, duration of exposure, and concentration (Pelus, 1982).
Signal transduction after PGE2 engages in EP receptor can be either positive or negative, depending upon cell type. Positive signal transduction receptors include EP1, which triggers Ca2+ mobilization, and EP2 and EP4, which stimulate cAMP production. EP3 reduces cAMP concentration. PGE2 also enhances survival, proliferation, and homing of HSCs. Brief exposures to dmPGE2, a long-acting derivative of PGE2, ex vivo increase HSC frequency in murine bone marrow cells and enhance recovery of hematopoiesis in zebrafish following sublethal irradiation (Hoggatt, Singh, Sampath, & Pelus, 2009; Northet al., 2007). Based on these promising preclinical studies, dmPGE2 is being investigated in phase II clinical trials for patients with leukemia receiving umbilical cord blood transplantation to expand and improve engraftment of HSCs (Cutler et al., 2013; Goessling et al., 2011).
Hematopoietic progenitors show a competitive advantage in repopulation assays after a brief dmPGE2 treatment, due to an increase in the homing, survival, and proliferation of HSCs (Hoggatt et al., 2009). The increased homing capacity of HSCs was due to enhanced expression of the chemokine receptor, CXCR4. CXCR4 is a receptor for the chemoattractant stromal cell-derived factor-1α (SDF-1α)/CXCL12, which has a key role in the trafficking and homing of HSCs and HPCs to the bone marrow niche. The CXCL12/ CXCR4 axis plays an important role in progression of various tumors as CXCR4 increases metastasis of tumor cells into various organs, and CXCL12 can support the survival and proliferation of tumor cells. Notably, expression of CXCR4 is a poor prognostic marker in AML.
In addition, PGE2 signaling through the EP4 receptor increases β-catenin signaling, suggesting synergistic cross talk between prostaglandin and Wnt pathways (Wang, Mann, & DuBois, 2004). This is consistent with the finding showing PGE2-mediated increases in cAMP associated with β-catenin stabilization in HSC promoting survival. The in vivo significance of the PGE2/Wnt interaction is that HSC survival is promoted (Goessling et al., 2009). cAMP elevation by forskolin phenocopied the increase in HSC proliferation as well as demonstrated the requirement for Wnt/ β-catenin. This PGE2 pathway might also be relevant in AML as downregulation of β-catenin by short hairpin RNA in both AML cell lines and AML blasts reduced proliferation of AML (Siapati et al., 2011).
2.5. MRP4 and prostaglandins
The understanding of how prostaglandins are extruded and taken up into cells is only a recent development because the conventional view was that these molecules entered and left cells by diffusion. Organic anion transporters mediate the uptake of prostaglandins (Schuster, 2002). The Borst lab was the first to demonstrate an ABC transporter (MRP4) actively export PGE1 and PGE2 (in the absence of additional cofactors, e.g., glutathione; Reid et al., 2003; Fig. 2). It is interesting that nonsteroidal anti-inflammatory drugs potently inhibit MRP4 and therefore the export of these proinflammatory prostaglandins. Cellular retention of PGE2 is related to MRP4 level with greater expression producing greater reductions in intracellular PGE2 concentrations (Reid et al., 2003). PGE2 export is blocked by inhibition or knockdown of MRP4 (Reid et al., 2003). Furthermore, MRP4 deficiency and RNA interference-mediated MRP4 knockdown significantly reduced extracellular PGE2. cAMP-dependent protein kinase activity and COX-2 expression are reduced in MRP4-deficient cells, suggesting that prostaglandin synthesis might be restrained along with a lack of prostaglandin transport (Lin et al., 2008). It is likely that MRP4-mediated export of PGE2 engages the EP receptors to modulate cAMP signaling and restrain prostaglandin synthesis. One could speculate that in the absence of MRP4, PGE2 levels in the bone marrow niche might be lower, producing reduced HSC function and/or homing. Furthermore, in HSC, MRP4 absence might also reduce PGE2 activation of EP2 and EP4, thereby reducing intracellular cAMP and producing a net reduction in HSC numbers.
2.6. Leukotrienes in hematopoietic cells
The leukotrienes are biosynthesized by oxygenation of AA by 5-lipoxygenase (5-LOX) and converted into the unstable intermediate LTA4 (Fig. 2). LTA4 is either enzymatically hydrolyzed to LTB4 or conjugated to glutathione forming the cysteinyl leukotriene LTC4. LTC4 is then converted to LTD4 and LTE4. Leukotrienes, like other eicosanoids, are mostly produced in myeloid cells (Lindgren & Edenius, 1993). 5-LOX is found in HSC (Bautz, Denzlinger, Kanz, & Mohle, 2001) and myeloid cells; however, loss of the Alox5 gene does not affect normal HSC function (Chen, Hu, Zhang, Peng, & Li, 2009). Notably, the leukotrienes LTB4, LTC4, andLTD4 increase the number of HSC both in mouse and in human (Braccioni et al., 2002; Elsas et al., 2008; Vore, Eling, Danilowicz, Tucker, & Luster, 1989). For LTB4, the increase in HSC is likely due to an increase in proliferation as well as a reduction in apoptosis (Chung et al., 2005).
In the BCR–ABL-mouse model of CML, 5-Lox/Alox5 is upregulated in LSCs. Interestingly, the increased 5-Lox activity is accompanied by an elevated plasma level of LTB4 (Chen et al., 2009). In a mouse model of CML, BCR–ABL failed to develop CML when bone marrow cells lacking Alox5 were used. The LSCs exhibited impaired differentiation, division, and displayed increased apoptosis (Chen et al., 2009). Accordingly, 5-LOX inhibitor, Zileuton, was more effective than the conventional therapeutic in prolonging the survival of CML mice. LTB4 has been implicated in reducing HSC self-renewal capacity and favoring differentiation (Chung et al., 2005); however, its precise role is not yet defined. Although it is not clear how Alox5 specifically regulates LSC, loss of Alox5 correlated with reduction of β-catenin expression in BCR–ABL-expressing hematopoietic cells. Furthermore, Alox5 function has been implicated in many signaling pathways including PI3K, which has an important role in AML.
2.7. MRP1 and leukotrienes
MRP1 was the second major drug transporter to be identified by the laboratories of Cole et al. (1992). MRP1 overexpression confers resistance to many drugs including anthracyclines and vinca alkaloids. MRP1 can act as a cotransporter with glutathione (GSH) of amphipathic organic anions, as well as an exporter of glutathione-, glucuronate-, or sulfate-conjugated drugs. Therefore, it is not surprising that the high-affinity endogenous substrate for MRP1 includes the GSH-conjugated leukotriene, LTC4. Using membrane vesicles prepared from MRP1 overexpressing HeLa cells, it was shown that MRP1 mediates ATP-dependent transport of LTC4, LTD4, and LTE4, with the highest affinity being for LTC4 (Leier et al., 1994).
A biological role for MRP1 as a LTC4 transporter in vivo was shown in the Mrp1-null mouse model (Wijnholds et al., 1997). Higher LTC4 concentrations were discovered in mast cells from Mrp1-null mice, but there was no obvious hematopoietic defect. Importantly, a dramatically attenuated LTC4-mediated inflammatory response was observed in mast cells from the Mrp1-null mice. These results showed that LTC4 export required MRP1 to mediate a normal mast cell inflammatory response. Accumulation of intracellular LTC4 resulted in increased LTB4 due to product inhibition of LTC4 synthase (Schultz, 2001). It is interesting to note that MRP4 has been shown to transport LTB4 in the presence of GSH (Rius, Hummel-Eisenbeiss, & Keppler, 2008). It is unknown if cross talk between MRP1 and MRP4 plays a role in inflammatory responses. However, because leukotriene biosynthesis is critical for LSC survival and self-renewal, LTC4 export by MRP1 might play a role in mediating signaling in leukemia cells (Fig. 2).
2.8. Porphyrin and ABCG2
One of the physiologically relevant substrates for ABCG2, pheophorbide a, a plant-derived chlorophyll metabolite, was identified using an Abcg2-null mouse model (Jonker et al., 2002). Although pheophorbide a is a chlorophyll metabolic breakdown product, it is a tetrapyrrole and structurally resembles endogenous porphyrins such as heme and protoporphyrin IX (PPIX). Heme is an essential cofactor to many proteins that regulate cell proliferation, death, and differentiation. Heme synthesis begins in the mitochondrial matrix with glycine and succinyl-CoA forming δ-aminolevulinic acid (ALA). Four enzymatic steps convert ALA to coproporphyrinogen III in the cytoplasm. The mitochondrial ABC transporter ABCB6 mediates the ATP-dependent import of coproporphyrinogen III back into the mitochondria where it is converted further to PPIX (Krishnamurthy et al., 2006; Lynch, Fukuda, Krishnamurthy, Du, & Schuetz, 2009). Iron is then enzymatically inserted into this tetrapyrrole to produce heme. Although heme is critical for multiple cell functions, its precursors, porphyrins, can be photoactivated to generate reactive oxygen species from molecular oxygen (see review by Ishikawa and colleagues, Chapter 7, Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor). In addition, PPIX can also induce cell death independent of photoactivation (Bednarz, ZawackaPankau, & Kowalska, 2007). The mechanism accounting for this nonphotoactivatable cell death is unknown but might be due to alterations in mitochondrial function. It is plausible that ABCG2 plays a role in modulating the excess intracellular PPIX to protect leukemic cells.
HSCs reside in a region of the bone marrow that has reduced oxygen level. The hypoxia-inducible factors (HIFs) are transcription factors, activated during hypoxia, that alter the metabolic pathways required for adaption to low oxygen environments (e.g., glycolytic enzymes). Hypoxia induces Abcg2 via HIFs (Krishnamurthy et al., 2004; Martin et al., 2008). The hematopoietic progenitors from Abcg2 KO mice exhibited reduced self-renewal of myeloid progenitors under hypoxia and chemical inhibition of ABCG2 in wild-type (WT) progenitors produced the same impairment in self-renewal. Conversely, overexpression of ABCG2 in a myeloid cell line (OCI-AML) resulted in increased hypoxic survival. The survival advantage conferred by ABCG2 was associated with its ability to export the heme precursor, PPIX (Krishnamurthy et al., 2004). During hypoxia, heme production is upregulated via increased ALAS and CPOX (Hofer, Wenger, Kramer, Ferreira, & Gassmann, 2003; Klinkenberg, Mennella, Luetkenhaus, & Zitomer, 2005; Vasconcelles et al., 2001). Therefore, ABCG2 may protect the hematopoietic and LSCs from cytotoxic PPIX over accumulation during hypoxia.
2.9. ABC transporters and AML
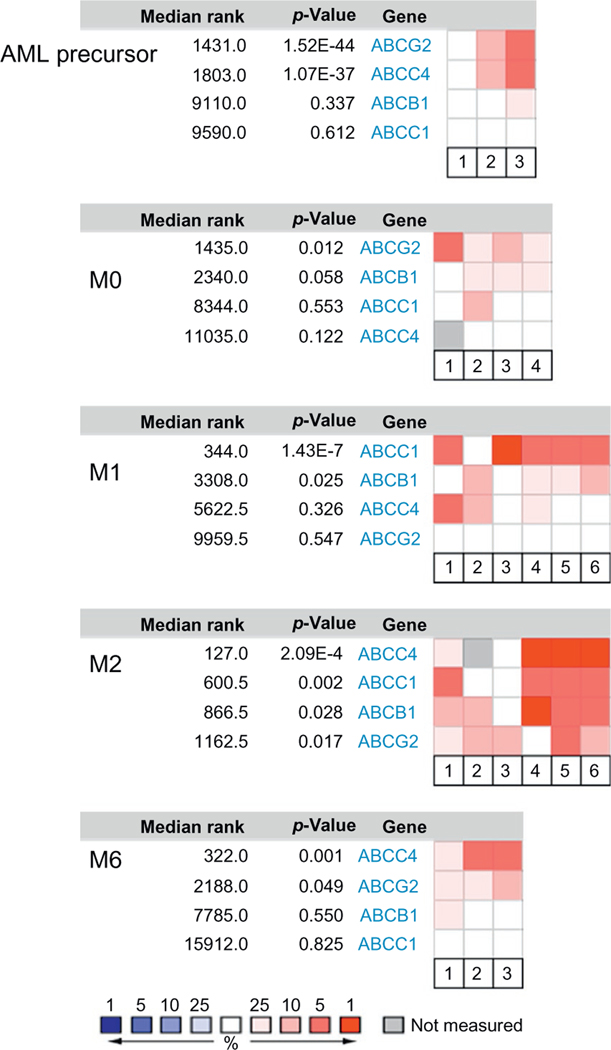
Much like normal hematopoiesis, AML blasts fall into a hierarchy, and depending on the progenitors stage, differentiation is arrested. AML can be divided into eight subtypes (M0–M7) according to the FAB classification based on the histopathology of the blasts (see Fig. 1). ABCG2 expression has been reported to correlate with an immature immunophenotype in normal hematopoietic cells, and its expression is downregulated upon differentiation of hematopoietic progenitors. One exception appears to be the erythroid-lineage which constitutively expresses ABCG2 (Raaijmakers, Van Den Bosch, Boezeman, De Witte, & Raymakers, 2002; Raaijmakers, van Emst, de Witte, Mensink, & Raymakers, 2002; Scharenberg, Harkey, & Torok-Storb, 2002; Zhouet al., 2002, 2001). We interrogated the Oncomine database (www.oncomine.org) to determine ABCG2 expression in various AML subtypes (Fig. 3). No significant expression was detected in myelocytic or monoblastic subtypes of AML (M3–M5). In contrast, ABCG2 was highly expressed in the myeloblastic leukemia subtypes (M0, without maturation) as well as in acute erythroleukemia (M6), a leukemia of red cell lineage. In pediatric AML M7, ABCG2 is highly expressed compared to other AML subtypes and a promoter unique to this subtype has been identified (Campbell et al., 2011).
Figure 3.
Gene expression pattern of MRP1, MRP4, and ABCG2 in human AML. Expression of MRP1, MRP4, and ABCG2 in adult AML patients was queried in Oncomine database for different FAB subtypes. Only the subtypes with significant expression of at least one of these transporters are shown. Red indicates high expression, blue indicates low expression, and gray indicates not measured. p-Value is for the median-ranked analysis.
Myelodysplastic syndrome (MDS) is a heterogeneous disorder with abnormal proliferation, morphology, and differentiation of myeloid progenitors and carries a high risk of transforming to AML. ABCC4 and ABCG2 were both highly expressed in this population considered to be “leukemic precursors” in two studies where one study included patients with MDS. Whether ABCC4 and ABCG2 play a role in a malignant transformation remains to be elucidated. Interestingly, there have been some rare, but well-documented, cases of MDS and AML that arose from therapy with the thiopurine, azathiopurine (Arnold, Ranson, & Abdalla, 1999). ABCC4 has been shown to transport thiopurine-derived nucleotides, and mice lacking Abcc4/Mrp4 displayed greater hematopoietic toxicity after thiopurine administration compared to the WT mice (Krishnamurthy et al., 2008). One could speculate that ABCC4 deficiency primes hematopoietic progenitors to DNA damage by thiopurines. This damage might be followed by an oncogenic second hit producing MDS or AML. Therefore, it is conceivable that patients with a single nucleotide polymorphism (SNP) in ABCC4 that alters its function might be more vulnerable to thiopurine-associated AML. Among several nonsynonymous SNPs found in ABCC4, 2269G>A (E757K) has been shown to result in a lower surface expression and results in a greater cellular accumulation of thiopurines (Krishnamurthy et al., 2008). This SNP is found at a high frequency in the Japanese population (>18%) as well as in Asians (∼4%). Indeed, this SNP was associated with greater hematopoietic toxicity in the Japanese IBD patients receiving thiopurine treatment (Ban et al., 2010). In addition, other ABCC4 non-synonymous SNPs (i.e., G187W and G487E) were shown to have reduced MRP4 function (Abla et al., 2008), although it is unknown if these are risk alleles for increased thiopurine toxicity.
Incomplete eradication of leukemic stems cells is considered to be one source of relapse in AML. Correlation between high ABCG2 expression and relapse has been reported in several studies. For example, a higher relapse rate was observed among one-third of the patients with high ABCG2 (Damiani et al., 2006). Higher ABCG2 expression was also associated with secondary AML and lower complete remission (van den Heuvel-Eibrink et al., 2007). In some AML patient samples, ABCG2 expression and function were higher at relapse compared to diagnosis (van der Kolk et al., 2002). A similar observation was made in 11 AML patient samples where five patients expressed higher ABCG2 at the time of relapse compared to diagnosis (Patel et al., 2013). In a childhood AML cohort, ABCG2 expression was higher at the time of relapse and the high ABCG2 expression correlated with worse overall survival. In addition, ABCG2 was ∼10-fold higher in patients who were refractory to treatment (Steinbach et al., 2002), and its expression was higher especially in CD34+CD38− leukemic progenitors (Ho, Hogge, & Ling, 2008). Unsupervised clustering based on gene expression profiles of samples from 170 AML patients resulted in six clusters with high ABCG2 expression (ranked 18th in the upregulated genes) found in a group with the poorest outcome (Wilson et al., 2006). However, ABCG2 expression did not predict complete remission (Uggla et al., 2005). These results imply that ABCG2 expression is probably higher in a rare LSC population that hastens relapse. Consistent with this is the finding that SP cells (high ABCG2-expressing cells) were more abundant in the bone marrow cells from de novo and refractory/relapsed adult AML patients than those from the normal or AML patients in remission (Huang et al., 2013).
3. KINASES IMPACT TRANSPORTER LOCATION AND FUNCTION
3.1. Kinases and ABC transporters
Phosphorylation regulates cellular processes by modulating protein function, localization, half-life, and protein–protein interaction. Protein kinases mediate the addition of phosphate groups onto their target proteins at tyrosine, threonine, or serine residues. Because of the wide range of target proteins, kinases are involved in the modulation of many cellular functions. ABC transporters are no exception where LC/MS analyses have identified bona fide phosphorylation of many of the ABC transporter family members (Stolarczyk, Reiling, & Paumi, 2011). In cancer cells including AML, kinases are often constitutively active and confer survival advantage. Therefore, specific kinase inhibitors targeted against these survival pathways, especially tyrosine kinase inhibitors (TKIs), have been widely employed in AML therapy.
Many TKIs, such as imatinib and nilotinib, have been shown to be substrates and/or inhibitors for ABC transporters (reviewed in Shukla, Chen, & Ambudkar, 2012). The mode of interaction of these was not known until recently. By using structure–activity relationships combined with mutagenesis, Shukla and colleagues showed that key residues of P-gp/ABCB1 bound nilotinib (a BCR–ABL kinase inhibitor), in the substrate-binding pocket rather than at the nucleotide-binding domain (Shukla et al., 2014). In addition, several serine/threonine kinases have been shown to modulate ABC transporter cellular localization and/or function. Because the relationship between ABC transporters and TKIs has been discussed in details elsewhere (Brozik et al., 2011; Shukla et al., 2012), we will focus on serine/threonine kinases as potential modulators of ABC transporter function.
3.2. Serine/threonine kinases Pim-1 and Akt affect ABCG2 location
The proto-oncogene pim-1 encodes a serine/threonine protein kinase with a wide array of substrates and regulates such processes as cell cycle and apoptosis. Recent studies suggest that it might regulate ABCG2, which is relevant because Pim-1 is highly expressed in AML (Chen, Redkar, Taverna, Cortes, & Gandhi, 2011). Pim-1L interacts directly with and phosphorylates ABCG2 at T362, which resides in the linker region between the nucleotide-binding domain and the membrane-spanning domain (Xie et al., 2008). Phosphorylation of ABCG2 at T362 increases the transport capability, and the coexpression of ABCG2 and Pim-1L, but not phosphorylation-incompetent ABCG2 (T362A), increased drug resistance. The importance of T362 phosphorylation was confirmed by the ABCG2 mutant harboring a T362D substitution to mimic the phosphorylation. This mutant ABCG2 showed increased drug resistance independent of Pim-1L activity. The phosphorylation of T362 regulates membrane localization as well as a homodimer formation, resulting in an increase in ABCG2 transport function. The Pim-1 kinase inhibitor SGI-1776 reduced ABCG2 activity through reduction in its surface expression. Thus, one therapeutic advantage of the Pim-1 kinase inhibitor is that it modulates ABCG2 function by affecting its cellular location.
Another kinase appears to also regulate ABCG2 intracellular location. The serine/threonine kinase, Akt, is activated in AML. Loss of Akt in mice produced a phenotype in the bone marrow compartment that suggested ABCG2 function was lost. In contrast, exogenous expression of Akt increased ABCG2 transport phenotype (Mogi et al., 2003). Furthermore, chemical inhibition of PI3K by LY294002 reduced ABCG2 transport activity. It is unknown how PI3K/Akt phosphorylation affects translocation or retention of ABCG2 at the plasma membrane (Mogi et al., 2003; Takada, Suzuki, Gotoh, & Sugiyama, 2005). In the BCR–ABL-expressing leukemia cell line, K562, inhibition of the TKI, imatinib, reduced ABCG2 protein levels (Nakanishi, Shiozawa, Hassel, & Ross, 2006). Although it is still unclear how Akt alters ABCG2 localization, it is likely that it or another kinase mediates phosphorylation of ABCG2.
3.3. Casein kinase 2 modulates MRP1 function
Casein kinase 2 (CK2) is a ubiquitously expressed and constitutively active serine/threonine protein kinase, which regulates numerous cellular processes such as cell growth, proliferation, and survival owing to its large number of substrates (Meggio & Pinna, 2003). CK2 is comprised of two catalytic subunits (α/α or α/α′) and two regulatory β subunits. CK2 expression is upregulated in many cancers, including AML. One of the substrates for CK2α is MRP1 and an increase in its transport function upon phosphorylation has been reported (Stolarczyk et al., 2012). The direct phosphorylation at Thr249 by CK2α increases doxorubicin efflux by ABCC1/MRP1. The Thr249 is located in the cytoplasmic loop between N-terminal extension (MSD1), which is unique to MRPs and the ABC core domains. In adult AML patients, high expression CK2α correlated with lower disease and overall survival compared to patients with low CK2α expression (Kim et al., 2007). CK2 selective inhibitors, tetrabromobenzotriazole or apigenin, induced cell death in primary AML blasts with a preference for cells with high CK2α expression. Thus, CK2α is a viable therapeutic target for AML and its inhibition will have an added benefit of inactivating MRP1.
4. FUTURE PERSPECTIVE
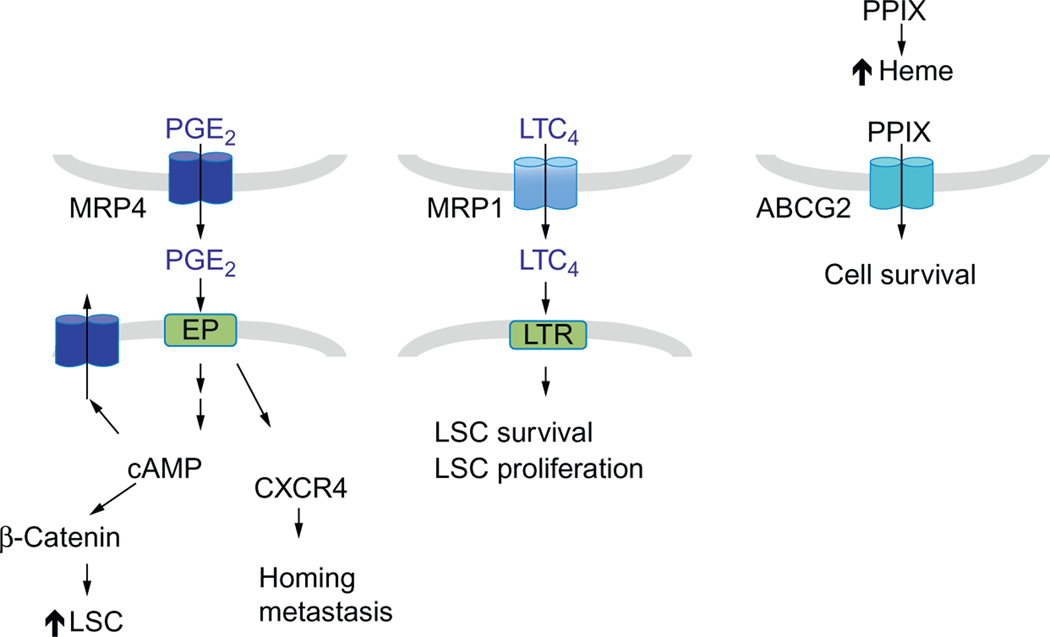
Efficient treatment of AML depends on elucidating the signaling pathways that provide LSCs with a survival advantage and unlimited self-renewal capacity. The conventional view functionally circumscribes ABC transporters as proteins that simply reduce treatment efficacy by either extruding chemotherapeutic drugs or sequestrating drugs within organelles such as lysosomes. However, recent discoveries of roles for ABC transporter ligands in HSCs suggest that export of these molecules, by ABC transporters, might have undefined salutary roles in leukemia (Fig. 4). The ligands for ABC transporters discussed here such as cAMP, PGE2, LTC4, and PPIX affect normal hematopoietic cell survival, differentiation, and/or self-renewal. It is unknown if these ligands have an analogous in LSCs. The future challenge is to discover these roles. For example, because exogenous leukotrienes maintain the LSC pool, it is conceivable that MRP1 might contribute to LSC function by supplying LTC4 to its receptor. Moreover, MRP4 might contribute to increased proliferation and metastatic capacity of leukemic cells, by extruding PGE2 enabling, it to affect (via its receptors) tumorigenicity in an autocrine or paracrine manner. ABCG2 may have a protective role for LSC by modulating the levels of cytotoxic porphyrins in the hypoxic niche or under increased demand for heme production. Some of these ABC transporters are regulated in part by serine/threonine protein kinases that are also upregulated in AML. Specific inhibitors for these kinases might be a viable addition to AML therapy because they might not only interfere with these serine/threonine kinases but also render ABC transporters nonfunctional, thus altering pathways important for LSC proliferation and survival.
Figure 4.
Models depicting possible roles for ABC transporters in promoting AML through transport of endogenous ligands. MRP4 effluxes PGE2, which acts on EP receptors to increase LSC and metastasis. MRP4 also modulates cAMP levels. LTC4 effluxed by MRP1 can act on CysLTR to promote LSC survival and proliferation. Heme biosynthesis is upregulated in highly proliferating cells. ABCG2 might alleviate toxic effect of excess PPIX when heme production is inadequate.
ACKNOWLEDGMENTS
This work was supported by NIH Grants 2R01 GM60904, P30 CA21745, and CA21865 and by the American Lebanese Syrian Associated Charities (ALSAC). We thank Dr. J.-P. Gillet for his insightful and helpful comments.
ABBREVIATIONS
- ABC
ATP-binding cassette
- ALA
δ-aminolevulinic acid
- AML
acute myeloid leukemia
- CK
casein kinase
- CML
chronic myelogenous leukemia
- CMP
common myeloid progenitor
- COX
cyclooxygenase
- GMP
granulocyte-macrophage progenitor
- GPCR
G protein-coupled receptor
- HIF
hypoxia-inducible factor
- HPC
hematopoietic progenitor cell
- HSC
hematopoietic stem cell
- IBD
inflammatory bowel disease
- LSC
leukemic stem cell
- LSK
Lin −Sca-1+c-Kit+
- LTC4
leukotriene C4
- MDS
myelodysplastic syndrome
- MEP
megakaryocyte/erythroid progenitor
- MPP
multipotent progenitors
- MRP
multidrug-related protein
- PGE2
prostaglandin E2
- PPIX
protoporphyrin IX
- SNP
single nucleotide polymorphism
REFERENCES
- Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, et al. (2008). The human multidrug resistance protein 4 (MRP4, ABCC4): Functional analysis of a highly polymorphic gene. The Journal of Pharmacology and Experimental Therapeutics, 325, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, & Weissman IL. (2000). A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature, 404, 193–197. [DOI] [PubMed] [Google Scholar]
- Arnold JA, Ranson SA, & Abdalla SH. (1999). Azathioprine-associated acute myeloid leukaemia with trilineage dysplasia and complex karyotype: A case report and review of the literature. Clinical and Laboratory Haematology, 21, 289–292. [DOI] [PubMed] [Google Scholar]
- Ban H, Andoh A, Imaeda H, Kobori A, Bamba S, Tsujikawa T, et al. (2010). The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. Journal of Gastroenterology, 45, 1014–1021. [DOI] [PubMed] [Google Scholar]
- Bautz F, Denzlinger C, Kanz L, & Mohle R. (2001). Chemotaxis and transendothelial migration of CD34(+) hematopoietic progenitor cells induced by the inflammatory mediator leukotriene D4 are mediated by the 7-transmembrane receptor CysLT1. Blood, 97, 3433–3440. [DOI] [PubMed] [Google Scholar]
- Becker AJ, McCulloch CE, & Till JE. (1963). Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature, 197, 452–454. [DOI] [PubMed] [Google Scholar]
- Bednarz N, Zawacka-Pankau J, & Kowalska A. (2007). Protoporphyrin IX induces apoptosis in HeLa cells prior to photodynamic treatment. Pharmacological Reports, 59, 474–479. [PubMed] [Google Scholar]
- Bonnet D, & Dick JE. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine, 3, 730–737. [DOI] [PubMed] [Google Scholar]
- Braccioni F, Dorman SC, O’Byrne PM, Inman MD, Denburg JA, Parameswaran K, et al. (2002). The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. The Journal of Allergy and Clinical Immunology, 110, 96–101. [DOI] [PubMed] [Google Scholar]
- Breyer MD, & Breyer RM. (2000). Prostaglandin E receptors and the kidney. American Journal of Physiology. Renal Physiology, 279, F12–F23. [DOI] [PubMed] [Google Scholar]
- Brozik A, Hegedus C, Erdei Z, Hegedus T, Ozvegy-Laczka C, Szakacs G, et al. (2011). Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: Substrates, chemosensitizers or inducers of acquired multidrug resistance? Expert Opinion on Drug Metabolism & Toxicology, 7, 623–642. [DOI] [PubMed] [Google Scholar]
- Campbell PK, Zong Y, Yang S, Zhou S, Rubnitz JE, & Sorrentino BP. (2011). Identification of a novel, tissue-specific ABCG2 promoter expressed in pediatric acute megakaryoblastic leukemia. Leukemia Research, 35, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy B, Panse M, Radunski U, Koch R, Wenzel D, Inagaki N, et al. (2009). ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug. Haematologica, 94, 1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepala S, Hulot JS, Morgan JA, Sassi Y, Zhang W, Naren AP, et al. (2013). Cyclic nucleotide compartmentalization: Contributions of phosphodiesterases and ATP-binding cassette transporters. Annual Review of Pharmacology and Toxicology, 53, 231–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Zhang H, Peng C, & Li S. (2009). Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nature Genetics, 41, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZS, Lee K, & Kruh GD. (2001). Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. The Journal of Biological Chemistry, 276, 33747–33754. [DOI] [PubMed] [Google Scholar]
- Chen LS, Redkar S, Taverna P, Cortes JE, & Gandhi V. (2011). Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia. Blood, 118, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JW, Kim GY, Mun YC, Ahn JY, Seong CM, & Kim JH. (2005). Leukotriene B4 pathway regulates the fate of the hematopoietic stem cells. Experimental & Molecular Medicine, 37, 45–50. [DOI] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. (1992). Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science, 258, 1650–1654. [DOI] [PubMed] [Google Scholar]
- Copsel S, Garcia C, Diez F, Vermeulem M, Baldi A, Bianciotti LG, et al. (2011). Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. The Journal of Biological Chemistry, 286, 6979–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, et al. (2013). Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood, 122, 3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani D, Tiribelli M, Calistri E, Geromin A, Chiarvesio A, Michelutti A, et al. (2006). The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica, 91, 825–828. [PubMed] [Google Scholar]
- Dean M, Fojo T, & Bates S. (2005). Tumour stem cells and drug resistance. Nature Reviews. Cancer, 5, 275–284. [DOI] [PubMed] [Google Scholar]
- Elsas PX, Queto T, Mendonca-Sales SC, Elsas MI, Kanaoka Y, & Lam BK. (2008). Cysteinyl leukotrienes mediate the enhancing effects of indomethacin and aspirin on eosinophil production in murine bone marrow cultures. British Journal of Pharmacology, 153, 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher I, & Gidali J. (1974). Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature, 247, 550–551. [DOI] [PubMed] [Google Scholar]
- Gentile P, Byer D, & Pelus LM. (1983). In vivo modulation of murine myelopoiesis following intravenous administration of prostaglandin E2. Blood, 62, 1100–1107. [PubMed] [Google Scholar]
- Goessling W, Allen RS, Guan X, Jin P, Uchida N, Dovey M, et al. (2011). Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell, 8, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. (2009). Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell, 136, 1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, & Mulligan RC. (1996). Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. The Journal of Experimental Medicine, 183, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie PC, & Broxmeyer HE. (1994). Myeloid cell proliferation stimulated by steel factor is pertussis toxin sensitive and enhanced by cholera toxin. International Journal of Immunopharmacology, 16, 547–560. [DOI] [PubMed] [Google Scholar]
- Ho MM, Hogge DE, & Ling V. (2008). MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Experimental Hematology, 36, 433–442. [DOI] [PubMed] [Google Scholar]
- Hofer T, Wenger RH, Kramer MF, Ferreira GC, & Gassmann M. (2003). Hypoxic up-regulation of erythroid 5-aminolevulinate synthase. Blood, 101, 348–350. [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, & Pelus LM. (2009). Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood, 113, 5444–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY, Li WJ, et al. (2013). Inactivation of PTEN increases ABCG2 expression and the side population through the PI3K/Akt pathway in adult acute leukemia. Cancer Letters, 336, 96–105. [DOI] [PubMed] [Google Scholar]
- Huff CA, & Matsui W. (2008). Multiple myeloma cancer stem cells. Journal of Clinical Oncology, 26, 2895–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N, et al. (2004). MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell, 6, 587–596. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, et al. (2002). The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proceedings of the National Academy of Sciences of the United States of America, 99, 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Eom JI, Cheong JW, Choi AJ, Lee JK, Yang WI, et al. (2007). Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clinical Cancer Research, 13, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Klinkenberg LG, Mennella TA, Luetkenhaus K, & Zitomer RS. (2005). Combinatorial repression of the hypoxic genes of Saccharomyces cerevisiae by DNA binding proteins Rox1 and Mot3. Eukaryotic Cell, 4, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobsar A, Heeg S, Krohne K, Opitz A, Walter U, Bock M, et al. (2008). Cyclic nucleotide-regulated proliferation and differentiation vary in human hematopoietic progenitor cells derived from healthy persons, tumor patients, and chronic myelocytic leukemia patients. Stem Cells and Development, 17, 81–91. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, et al. (2006). Identification of a mammalian mitochondrial porphyrin transporter. Nature, 443, 586–589. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, et al. (2004). The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. The Journal of Biological Chemistry, 279, 24218–24225. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Schwab M, Takenaka K, Nachagari D, Morgan J, Leslie M, et al. (2008). Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Research, 68, 4983–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature, 367, 645–648. [DOI] [PubMed] [Google Scholar]
- Lee AW. (1999). Synergistic activation of mitogen-activated protein kinase by cyclic AMP and myeloid growth factors opposes cyclic AMP’s growth-inhibitory effects. Blood, 93, 537–553. [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, & Keppler D. (1994). The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. The Journal of Biological Chemistry, 269, 27807–27810. [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, et al. (2007). Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell, 131, 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby E, & Hromas R. (2010). Dismounting the MDR horse. Blood, 116, 4037–4038. [DOI] [PubMed] [Google Scholar]
- Lin ZP, Zhu YL, Johnson DR, Rice KP, Nottoli T, Hains BC, et al. (2008). Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Molecular Pharmacology, 73, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren JA, & Edenius C. (1993). Transcellular biosynthesis of leukotrienes and lipoxins via leukotriene A4 transfer. Trends in Pharmacological Sciences, 14, 351–354. [DOI] [PubMed] [Google Scholar]
- Lu L, Pelus LM, & Broxmeyer HE. (1984). Modulation of the expression of HLA-DR (Ia) antigens and the proliferation of human erythroid (BFU-E) and multipotential (CFU-GEMM) progenitor cells byprostaglandin E. Experimental Hematology, 12, 741–748. [PubMed] [Google Scholar]
- Lu L, Pelus LM, Piacibello W, Moore MA, Hu W, & Broxmeyer HE. (1987). Prostaglandin E acts at two levels to enhance colony formation in vitro by erythroid (BFU-E) progenitor cells. Experimental Hematology, 15, 765–771. [PubMed] [Google Scholar]
- Lynch J, Fukuda Y, Krishnamurthy P, Du G, & Schuetz JD. (2009). Cell survival under stress is enhanced by a mitochondrial ATP-binding cassette transporter that regulates hemoproteins. Cancer Research, 69, 5560–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, et al. (2008). Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circulation Research, 102, 1075–1081. [DOI] [PubMed] [Google Scholar]
- Meggio F, & Pinna LA. (2003). One-thousand-and-one substrates of protein kinase CK2? FASEB Journal, 17, 349–368. [DOI] [PubMed] [Google Scholar]
- Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, et al. (2003). Akt signaling regulates side population cell phenotype via Bcrp1 translocation. The Journal of Biological Chemistry, 278, 39068–39075. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Shiozawa K, Hassel BA, & Ross DD. (2006). Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL-expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinib-induced reduction of BCRP expression. Blood, 108, 678–684. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. (2007). Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature, 447, 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, & Nakauchi H. (1996). Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science, 273, 242–245. [DOI] [PubMed] [Google Scholar]
- Patel C, Stenke L, Varma S, Lindberg ML, Bjorkholm M, Sjoberg J, et al. (2013). Multidrug resistance in relapsed acute myeloid leukemia: Evidence of biological heterogeneity. Cancer, 119, 3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM. (1982). Association between colony forming units-granulocyte macrophage expression of Ia-like (HLA-DR) antigen and control of granulocyte and macrophage production. A new role for prostaglandin E. The Journal of Clinical Investigation, 70, 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM, Broxmeyer HE, Kurland JI, & Moore MA. (1979). Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. The Journal of Experimental Medicine, 150, 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelus LM, Broxmeyer HE, & Moore MA. (1981). Regulation of human myelopoiesis by prostaglandin E and lactoferrin. Cell and Tissue Kinetics, 14, 515–526. [DOI] [PubMed] [Google Scholar]
- Pinto A, Aldinucci D, Gattei V, Zagonel V, Tortora G, Budillon A, et al. (1992). Inhibition of the self-renewal capacity of blast progenitors from acute myeloblastic leukemia patients by site-selective 8-chloroadenosine 30,50-cyclic monophosphate. Proceedings of the National Academy of Sciences of the United States of America, 89, 8884–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers HG, Van Den Bosch G, Boezeman J, De Witte T, & Raymakers RA. (2002). Single-cell image analysis to assess ABC-transporter-mediated efflux in highly purified hematopoietic progenitors. Cytometry, 49, 135–142. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, van Emst L, de Witte T, Mensink E, & Raymakers RA. (2002). Quantitative assessment of gene expression in highly purified hematopoietic cells using real-time reverse transcriptase polymerase chain reaction. Experimental Hematology, 30, 481–487. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, & Melton DA. (2002). “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science, 298, 597–600. [DOI] [PubMed] [Google Scholar]
- Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, et al. (2003). The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proceedings of the National Academy of Sciences of the United States of America, 100, 9244–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Kock K, & Kroemer HK. (2005). Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metabolism Reviews, 37, 253–278. [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, & Keppler D. (2008). ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4). The Journal of Pharmacology and Experimental Therapeutics, 324, 86–94. [DOI] [PubMed] [Google Scholar]
- Scharenberg CW, Harkey MA, & Torok-Storb B. (2002). The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood, 99, 507–512. [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, et al. (1999). MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nature Medicine, 5, 1048–1051. [DOI] [PubMed] [Google Scholar]
- Schultz MJ, Wijnholds J, Peppelenbosch MP, Vervoodeldonk MJ, Speelman P, van Deventer SJ, et al. (2001). Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. Journal of Immunology, 166, 4059–4064. [DOI] [PubMed] [Google Scholar]
- Schuster VL. (2002). Prostaglandin transport. Prostaglandins & Other Lipid Mediators, 68–69, 633–647. [DOI] [PubMed] [Google Scholar]
- Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, & Gottesman MM. (2012). Drug resistance: Still a daunting challenge to the successful treatment of AML. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy, 15, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Chen ZS, & Ambudkar SV. (2012). Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy, 15, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Chufan EE, Singh S, Skoumbourdis AP, Kapoor K, Boxer MB, et al. (2014). Elucidation of the structural basis of interaction of the BCR-ABL kinase inhibitor, nilotinib (Tasigna) with the human ABC drug transporter P-glycoprotein. Leukemia, 28, 961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapati EK, Papadaki M, Kozaou Z, Rouka E, Michali E, Savvidou I, et al. (2011). Proliferation and bone marrow engraftment of AML blasts is dependent on beta-catenin signalling. British Journal of Haematology, 152, 164–174. [DOI] [PubMed] [Google Scholar]
- Sinha C, Ren A, Arora K, Moon CS, Yarlagadda S, Zhang W, et al. (2013). Multidrug resistance protein 4 (MRP4)-mediated regulation of fibroblast cell migration reflects a dichotomous role of intracellular cyclic nucleotides. The Journal of Biological Chemistry, 288, 3786–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Weissman IL, & Heimfeld S. (1991). Clonal analysis of hematopoietic stemcell differentiation in vivo. Proceedings of the National Academy of Sciences of the United States of America, 88, 2788–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille TC,&Cleary ML.(2006).Identificationandcharacterizationofleukemiastem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell, 10, 257–268. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, & Weissman IL. (1988). Purification and characterization of mouse hematopoietic stem cells. Science, 241, 58–62. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Gillet JP, Sauerbrey A, Gruhn B, Dawczynski K, Bertholet V, et al. (2006). ABCA3 as a possible cause of drug resistance in childhood acute myeloid leukemia. Clinical Cancer Research, 12, 4357–4363. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, & Sauerbrey A. (2002). BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia, 16, 1443–1447. [DOI] [PubMed] [Google Scholar]
- Stolarczyk EI,Reiling CJ,&Paumi CM.(2011).RegulationofABCtransporterfunction via phosphorylation by protein kinases. Current Pharmaceutical Biotechnology, 12, 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarczyk EI, Reiling CJ, Pickin KA, Coppage R, Knecht MR, & Paumi CM. (2012). Casein kinase 2alpha regulates multidrug resistance-associated protein 1 function via phosphorylation of Thr249. Molecular Pharmacology, 82, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, & Narumiya S. (2007). Prostaglandin E receptors. The Journal of Biological Chemistry, 282, 11613–11617. [DOI] [PubMed] [Google Scholar]
- Takada T, Suzuki H, Gotoh Y, & Sugiyama Y. (2005). Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metabolism and Disposition, 33, 905–909. [DOI] [PubMed] [Google Scholar]
- Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A, et al. (2005). BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leukemia Research, 29, 141–146. [DOI] [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, Knauf WU, Fey MF, Verhoef GE, et al. (2007). CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Annals of Hematology, 86, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk DM, Vellenga E, Scheffer GL, Muller M, Bates SE, Scheper RJ, et al. (2002). Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood, 99, 3763–3770. [DOI] [PubMed] [Google Scholar]
- Vasconcelles MJ, Jiang Y, McDaid K, Gilooly L, Wretzel S, Porter DL, et al. (2001). Identification and characterization of a low oxygen response element involved in the hypoxic induction of a family of Saccharomyces cerevisiae genes. Implications for the conservation of oxygen sensing in eukaryotes. The Journal of Biological Chemistry, 276, 14374–14384. [DOI] [PubMed] [Google Scholar]
- Vore SJ, Eling TE, Danilowicz M, Tucker AN, & Luster MI. (1989). Regulation of murine hematopoiesis by arachidonic acid metabolites. International Journal of Immunopharmacology, 11, 435–442. [DOI] [PubMed] [Google Scholar]
- Wang D, Mann JR, & DuBois RN. (2004). WNT and cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle, 3, 1512–1515. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, et al. (1997). Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nature Medicine, 3, 1275–1279. [DOI] [PubMed] [Google Scholar]
- Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R, et al. (2006). Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood, 108, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GG, Modlich S, Inagaki N, Reinhardt D, Schroers R, Griesinger F, et al. (2004). ABC transporter ABCA3 is expressed in acute myeloid leukemia blast cells and participates in vesicular transport. Haematologica, 89, 1395–1397. [PubMed] [Google Scholar]
- Xie Y, Xu K, Linn DE, Yang X, Guo Z, Shimelis H, et al. (2008). The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. The Journal of Biological Chemistry, 283, 3349–3356. [DOI] [PubMed] [Google Scholar]
- Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, & Sorrentino BP. (2002). Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proceedings of the National Academy of Sciences of the United States of America, 99, 12339–12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. (2001). The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature Medicine, 7, 1028–1034. [DOI] [PubMed] [Google Scholar]